Abstract
Cycloartenyl ferulate (CF) is abundant in brown rice with multiple biologic functions. It has been reported to possess antitumor activity; however, the related mechanism of action of CF has not been clarified. Herein, we unexpectedly uncover the immunological regulation effects of CF and its molecular mechanism. We discovered that CF directly enhanced the killing capacity of natural killer (NK) cells for various cancer cells in vitro. In vivo, CF also improved cancer surveillance in mouse models of lymphoma clearance and metastatic melanoma dependent on NK cells. In addition, CF promoted anticancer efficacy of the anti-PD1 antibody with improvement of tumor immune microenvironment. Mechanistically, we first unveiled that CF acted on the canonical JAK1/2-STAT1 signaling pathway to enhance the immunity of the NK cells by selectively binding to interferon γ receptor 1. Collectively, our results indicate that CF is a promising immunoregulation agent worthy of attention in clinical application in the future. Due to broad biological significance of interferon γ, our findings also provide a capability to understand the diverse functions of CF.
Keywords: cycloartenyl ferulate, NK cell, cancer, immunology, IFNγ receptor, signaling pathway
The development of malignancies attributed to escaping these host-protecting processes through various immune-evading mechanisms, including the production of inadequate T cells and NK cells activation within the tumor microenvironment (TME), recruitment of regulatory T cells (Tregs), myeloid-derived suppressor cells, and so on, leading eventually to the disease progression (1, 2). In the past decade, therapeutic advances in immunotherapy have revolutionized the treatment of multiple cancers (3, 4). Interests in harnessing natural killer (NK) cells for cancer immunotherapy are rapidly growing (5, 6). NK cells are distinguished cytotoxic lymphocytes of the innate immune system, which can recognize a wide array of tumor cells across all cancer types and contribute to their elimination by direct cytotoxicity and by shaping a multicellular protective immune response via their secretion of cytokines and chemokines (7, 8, 9). Therefore, theoretically a broader spectrum of cancers might respond to NK cellular therapy (10). However, the efficacy of NK cell-based immunotherapy remains limited in most trials (11). Strategies to augment the killing efficacy of NK cells are thus much needed.
To augment the therapeutic efficacy of NK cells, most current studies revolve around two focal points: optimizing the source of NK cells and improving their functionality in vivo (11, 12). Various strategies have been developed to restore NK cell function, including adoptive cell transfer, cytokine therapies, and monoclonal antibodies targeting activating and inhibitory receptors and the TME (13, 14, 15). However, these methods have several limitations. Adoptive transfer of ex vivo expanded autologous NK cells has been tested in early clinical trials to treat patients with renal cell carcinoma (16), lymphoma (17), breast (18), digestive (19), colon (20), and lung cancer (20). Although some clinical trials have reported responses in one out of three to one out of two patients, the efficacy of adoptive NK transfer remains unsatisfying and limited in most trials. Cytokines used on NK cells during in vivo expansion generate various side effects. Monoclonal antibodies are generally well tolerated in humans, but there are still some serious side effects (21). Therefore, there is an urgent need to develop novel and safe strategies to enhance NK cell activity for targeted cancer therapy.
With the research and development of drugs, many compounds of natural origin have been found to have unique physiological activities. Indeed, many natural products have been studied extensively and utilized to regulate immune cell function with the goal of cancer treatment and prevention (22, 23, 24). Cycloartenyl ferulate (CF) is one of the typical triterpene alcohols present in and unique to rice bran oil, which has been approved as a pharmaceutical in Japan (Fig. S1). CF exhibits several biological activities including antioxidative activity, blood cholesterol-lowering activity, and anti-tumorigenicity (25, 26, 27). It has been reported that the oryzanol-supplemented diet is able to inhibit colorectal adenocarcinoma (28). Additionally, in the two-stage carcinogenesis model in mouse skin, CF inhibited tumor promotion (29). Despite CF diverse therapeutic potential against cancer cells, the function of CF in regulating innate immune responses for cancer treatment has not been explored, and the action mechanism in cancer treatment has not been very well clarified.
In the present study, we unexpectedly found that CF isolated from rice bran oil (MCE, HY-125938) directly promoted the activation and cytotoxicity of NK cells from NK92 cell lines, peripheral blood mononuclear cells (PBMCs) of normal people or the pleural effusion of lung cancer patients in vitro, and CF effectively activated NK cells to suppress tumor metastasis and growth in vivo. CF was revealed to selectively bind to IFNγ receptor 1 (IFNγR1) to directly enhance the cytotoxicity of NK cells to cancer cells. Moreover, treatment of CF in advance of programmed death receptor 1 antibody delays carcinogenesis in the xenograft model of lung cancer. This research provides a clue to understanding the potent anticancer immunity effect of CF.
Results
CF activates NK cells from different sources to lyse various cancer cells
To investigate whether CF is involved in the regulation of NK cell function, NK92 cells were treated with CF for 72 h and then cocultured with K562, A2058, or A549 cells (the major histocompatibility complex (MHC) class I low, NK cell-sensitive target cells) and tested by lactate dehydrogenase analysis. Remarkably, the CF-treated NK92 cells increased cytolytic activities to these cancer cells in a dose-dependent manner in comparison to control groups (Fig. 1A). CF did not impair the vitalities of the above cancer cells and NK92 cells (Fig. S2). The population of NK92 cells bearing CD107a (a membrane marker of NK cell degranulation) was found to be significantly augmented correspondingly (Fig. 1B). Western blot analysis revealed a marked-up-regulation of activation receptors including NKG2D, NKp30, and NKp44 after CF treatment (Fig. 1C). Furthermore, as shown in Figure 1D, the evident release increases of granzyme B and perforin expression as well as IFNγ determined by ELISA. Taken together, our results indicate that CF can trigger NK92 activation directly and promotes its cytotoxicity to target cells.
Figure 1.
CF activates NK92 cells and NK cells from healthy people and lung cancer patients and enhances their cytolysis toward various cancer cells. A, NK92 cell cytotoxicity toward various cancer cells. The NK92 cells were incubated with CF (0, 0.1, 1, and 10 μM) for 72 h and placed in the culture with various cancer cells at the different effector-target ratios for 6 h. The supernatant was measured by an LDH release assay. B, the degranulation level of NK92 treated by CF. The NK92 cells were treated with different concentrations of CF for 72 h and incubated with K562 cells for 4 h at 37 °C. Flow cytometry was used to measure the quantity of cytotoxic degranulation (CD107a+) of NK92 cells. C, expression of the cell-activated receptor on NK92 treated by CF. The cells were treated with different concentrations of CF for 24 h and measured the expressions of NKG2D, NKp30, and NKp44 by Western blot analysis. D, the releases of perforin, granzyme B, and IFNγ were evaluated by ELISA analysis. E–G, the cytotoxicity effect and degranulation level of NK cells from healthy donors (n = 8). Isolated lymphocytes were pretreated with the CF and mixed with CFSE-stained K562, A2058, and A549 cells. Treatment and determination as above referred. H–J, the cytotoxicity effect and degranulation level of NK cells from the pleural effusion of lung cancer patients (n = 7). Treatment and determination as above referred. Data represent the mean ± SD. The data shown represent at least three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA with the Dunnett’s test. CF, cycloartenyl ferulate; CFSE, carboxyfluoroscein succinimidyl ester; LDH, lactate dehydrogenase; NK, natural killer.
We next investigated whether CF boosts the cytolytic activity of NK cells from healthy people and patients. The primary NK cells were obtained from eight healthy donors' peripheral blood and seven lung cancer patients' pleural effusion. We similarly detected cytotoxicity of NK cells and CD107 expression by flow cytometry analysis with carboxyfluoroscein succinimidyl ester (CFSE)/ propidium iodide (PI) staining. The results showed that the cytolytic abilities and CD107a expressions of NK cells from normal peripheral blood (PBNK) to the three cancer cells were enhanced in a dose-dependent manner, with more than 2 to 3 folds increase at 10 μM of CF than the untreated groups, respectively (Fig. 1, E–G). Similar results were obtained in the NK cells from pleural effusion (PE-NK), approximately four folds increase at 10 μM of CF (Fig. 1, H–J). Altogether, these data indicate that CF can activate NK cells and promotes its immune lysis functions toward cancer cells.
CF enhances NK cell-mediated lymphoma clearance
Numerous studies have shown the ability of NK cells to kill abnormal cells lacking MHC class-I molecules in vivo (30). Here, we used a syngeneic tumor clearance model to determine whether CF enhances NK cell function in vivo. For ease of identification, the mouse lymphoma cells with normal and defective expression of MHC class-I molecules (referred to as RMA and RMAS, respectively) were labeled at different concentrations of CFSE, and then equal amounts of the RMA and RMAS cells were i.p. co-injected into the mice after 3 days of CF intragastric administration (i.g.) pretreatment (Fig. 2A). NK cells preferentially kill MHC class-I-deficient RMAS cells rather than their parent RMA (23, 24). As expected, the clearance of RMAS cells with CF pretreatment was remarkably increased in a dose-dependent fashion (Fig. 2, B and C). The mice were depleted of NK cells by injecting 200 μg of anti-NK1.1 antibodies i.p before CF treatment for 2 days. Flow cytometry analyses revealed the frequencies of NK cells (CD3- NK1.1+) in the spleen of mice, which were remarkably decreased compared to the control group mice (Fig. 2, D and E). We found that the capability of CF on peritoneal clearance of RMAS cells was dramatically diminished after anti-NK1.1-mediated depletion of NK cells (Fig. 2, F and G). These results suggest that CF enhances NK cell-mediated immune surveillance of cancer cells in vivo. On the other hand, we noticed an appreciable elimination of the RMA cells that were pretreated with CF compared with the control (Fig. 2, F and G, right panels), implying that other effector cells should also be subjected to activation by CF.
Figure 2.
CF improves NK cell-mediated lymphoma clearance in mice. A, the experimental scheme of CF pretreatment in lymphoma clearance assay. C57BL/6J mice (n = 3) received increasing doses of CF (i.g.) for 72 h before an i.p. injection of CFSE-stained RMAS (0.3 μM CFSE) and CFSE-stained RMA (6 μM CFSE) cells at a ratio of 1:1. After 12 h post challenge, the mice were sacrificed. B and C, NK cell-sensitive RMAS cells relative to NK cell-resistant RMA cells that remained in the peritoneal cavity were assessed using flow cytometry. D and E, elimination of NK cells in mice. C57BL/6J mice (n = 3) received either CF (50 mg/kg; i.g.) or vehicle with or without anti-NK1.1 (200 μg; i.p.) before an i.p. injection of CFSE-stained RMAS (0.3 μM CFSE) and CFSE-stained RMA (6 μM CFSE) at a ratio of 1:1. Mice were sacrificed and the frequencies of NK cells (CD3−NK1.1+) in the spleen of each group are shown as representative flow cytometry profiles and a summary graph of statistical bar charts. F and G, after eliminating NK cells in mice, it received CF (i.g.) for 72 h before an i.p. injection of RMAS and RMA cells. Then, the RMAS and RMA cells that remained in the peritoneal cavity were analyzed using flow cytometry. Representative results and a summary graph of statistical bar charts. Rejection (%) to RMAS or RMA cells = [(Area (50 mg/kg) – Area (Vehicle)]/Area (Vehicle). The data shown are representative of three experiments. Data represent the mean ± SD. n.s., nonsignificant; ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA with the Dunnett’s test. CF, cycloartenyl ferulate; CFSE, carboxyfluoroscein succinimidyl ester; NK, natural killer.
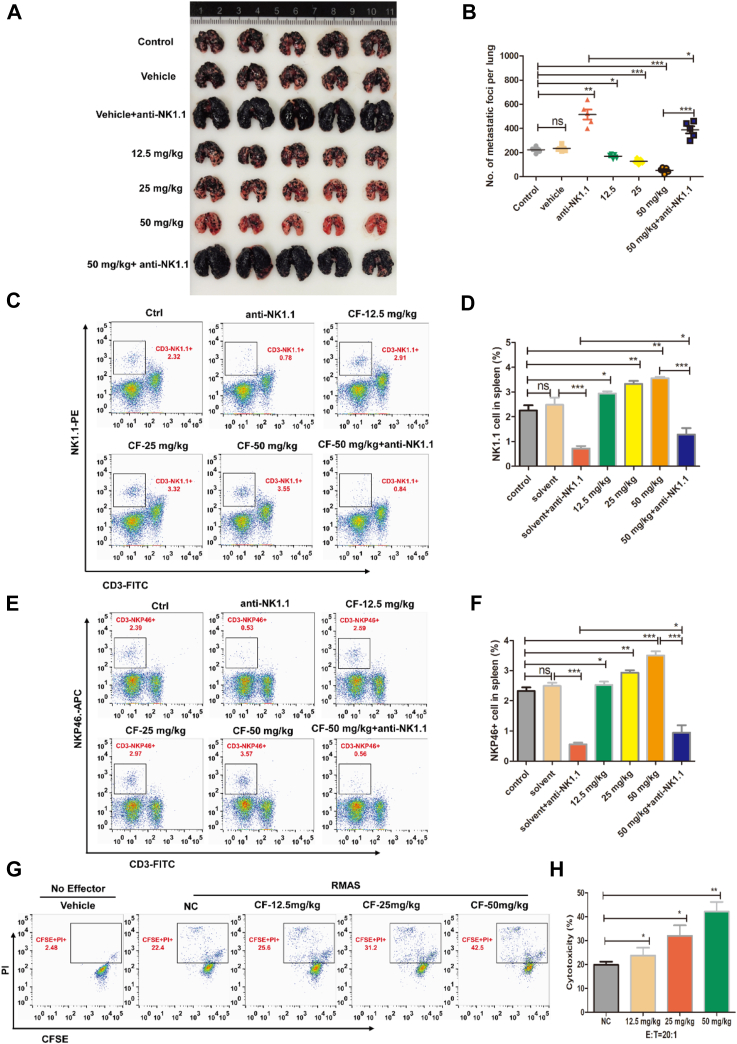
CF boosts NK cell activation to inhibit B16-F10 lung metastasis
We continued to test the effect of CF on the development of metastatic tumors with NK cells, given the crucial role of NK cells in blocking cancer metastasis (31). To this end, we established the B16-F10 melanoma xenograft model that is commonly used in the study of NK cell function against cancer metastasis. B16-F10 melanoma cells were injected into mice i.v. and assessed the effect of CF on pulmonary metastatic growth. As expected, CF effectively prevented the formation of dark melanoma nodules in lung tissue, which was partly reversed by the anti-NK1.1 antibody (Fig. 3, A and B).
Figure 3.
CF increases immunity activity and frequency of NK cells to overcome B16-F10 melanoma lung metastasis in mice. C57BL/6J mice were injected with 2 × 105 B16-F10 melanoma cells (i.v.) and treated with increasing doses of CF (i.g.). After 21 days, the mice were euthanized. A and B, representative images of mouse lungs at the time of harvest. C–F, the number and activity of NK cells were detected in mouse spleen tissues of each group. Representative FACS dot plots depict the frequencies of CD45+CD3-NK1.1+ (C and D) and CD45+CD3-NKp46+ (E and F) cells were detected by flow cytometry. G and H, the cytotoxicity effect of NK cells which were isolated from splenic organ after CF pretreatment. The cells were incubated with CFSE-stained RMAS (2 μM CFSE) for 4 h at 37 °C. Flow cytometry was used to assess the CFSE/PI-stained RMAS. The experiments were conducted with five mice per group. The data shown are representative of three experiments. Data are means ± SD. n.s., nonsignificant; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 by one-way ANOVA with the Dunnett’s test. CF, cycloartenyl ferulate; CFSE, carboxyfluoroscein succinimidyl ester; FACS, fluorescence-activated cell sorting; NK, natural killer; PI, propidium iodide.
We then measured the populations of CD3- NK1.1+ (PanNK) and CD3- NKp46+ (i.e., activated NK cells) in the spleen by fluorescence-activated cell sorting analysis. Compared with the vehicle control, CF remarkably increased the percentage of CD3- NK1.1+ (Fig. 3, C and D) and CD3- NKp46+ (Fig. 3, E and F) cells, and anti-NK1.1 antibody effectively but not totally resisted the process induced by CF. There remained some proportions of CD3- NK1.1+ and CD3- NKp46+ cells in the combination group. Furthermore, we isolated the NK cells from the spleen and had them incubated with the RMAS target cells. We observed a marked, dose-dependent increase in the cytolytic activity of the splenic NK cells (Fig. 3, G and H). Taken together, these results suggest that CF enhances NK cell immunity against the pulmonary colonization of B16-F10 melanoma cells.
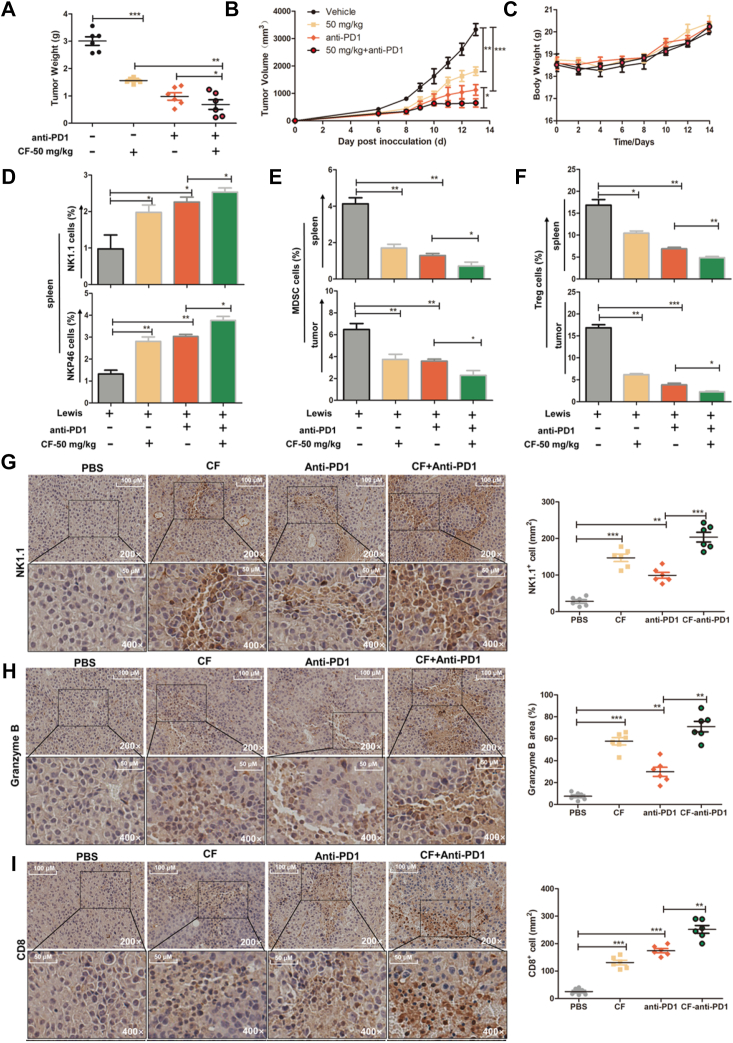
CF combined with anti-PD1 antibody delays carcinogenesis by increasing tumor infiltration of T and NK cell
Immune checkpoint blockade has proven to be a promising strategy for cancer treatment (32, 33). However, the cancer control efficacy of checkpoint inhibitors (such as anti-PDl antibody) needs to be further improved because of the established immunosuppressive milieu and a large tumor burden that prevent optimal immune activation and antitumor efficacy (34). We here tested the combined effect of treatment of CF and anti-PDl antibody on the activation of antitumor immunity. Subsequently, the murine Lewis tumor cells were injected into C57BL/6J mice subcutaneously. They were randomly assigned into four groups and treated with CF and anti-PDl. As expected, CF or anti-PD1 alone obviously inhibited tumor growth compared to control, while combination treatment of CF and anti-PD1 exhibited a significant delay in tumor growth compared to the anti-PD1 treatment group (Fig. 4, A and B). Mice in all groups which received treatment had no significant change in body weight (Fig. 4C).
Figure 4.
The combination of CF with anti-PD1 delays carcinogenesis on Lewis lung xenograft by activation and infiltration increases of NK and T cells. C57BL/6J mice were injected with 5 × 105 Lewis cells (s.c.) and treated with CF (i.g.). After 19 days, the mice were euthanized. A and B, tumor weight and volume were isolated from different groups. C, body weight was calculated every day. D, the number and activity of NK cells were detected in mouse spleen tissues of each group. The quantification of the frequency of CD3-NK1.1+ and CD3-NKp46+ cells in the spleen tissues were detected by flow cytometry. E and F, flow cytometry analysis of the frequencies of MDSC (CD45+CD11B+LY6G+) and Treg (CD3+CD4+CD25+Foxp3+) cells in the spleen and tumor. G–I, the summarized data and representative results of NK (NK1.1) and T (CD8) cells recruitment to the tumor microenvironment and release of granzyme B in tumors by IHC. All images were obtained at × 200 and × 400 magnification. The experiments were conducted with six mice per group. The data shown are representative of three experiments. Data are means ± SD. n.s., nonsignificant; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 by one-way ANOVA with the Dunnett’s test. CF, cycloartenyl ferulate; FCM, flow cytometry; IHC, immunohistochemistry; MDSC, myeloid-derived suppressor cell; NK, natural killer.
To verify the effect of combination treatment on the immune response in vivo, we used flow cytometry to profile immunological cells of the spleen and tumor tissue. Compared with the control, CF or anti-PD1 remarkably increased the percentages of both CD3- NK1.1+ and CD3- NKp46+ cells. Combination treatment exhibited a significantly increased activity compared to anti-PD1 treatment alone (Fig. 4D). Furthermore, there were significant reductions of myeloid-derived suppressor cells and Foxp3+ Treg population both in the spleen and tumor tissue after CF or anti-PD1 treatment, and combination group was more potent than single treatment of anti-PD1 (Fig. 4, E and F).
We further detected the infiltration of NK and T cells in the tumor tissue by immunohistochemical staining analysis. The expressions of the NK cells were apparently increased in CF and anti-PD1 treated group compared with tumors in the control group, and the presence of infiltrating NK cells in combination group was two folds more than anti-PD1 treatment (Fig. 4G). Similar results were obtained when we quantified the frequency of granzyme B in the tumor (Fig. 4H). Additionally, CF or anti-PD1 treatment led to an evident increase of CD8+ T cells in the tumor tissue than the vehicle treatment, and their combination treatment led to a 1.6 fold increase of CD8+ T cells in the tumor tissue than the anti-PD1 alone (Fig. 4I). Taken together, these indicate that CF treatment ahead of anti-PD1 further delays murine lung tumor growth by improving the tumor immune microenvironment in vivo.
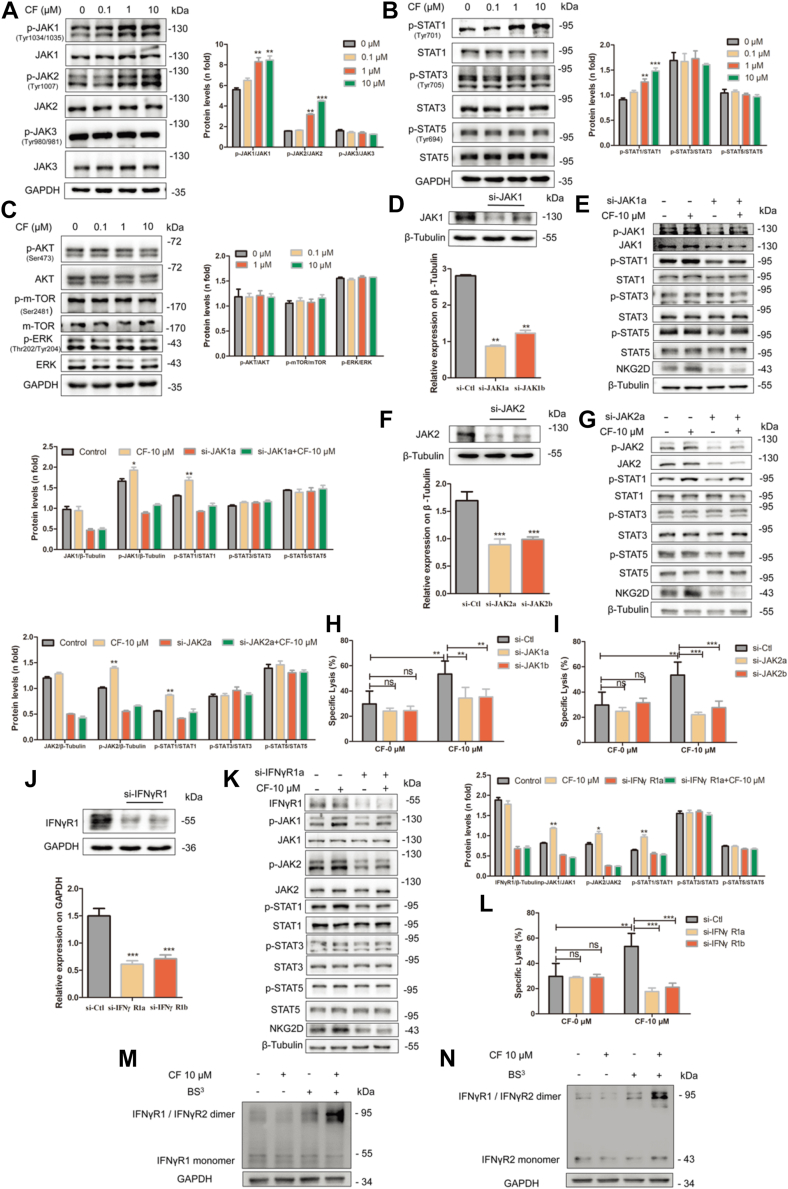
CF activates NK cells through the IFNγR-JAK-STAT pathway
Having demonstrated the role of CF in boosting NK cell immunity to cancer cells, we turned to clarify the specific signaling pathway(s) that CF might engage to exert its pharmacological effect. Herein, we focused on the signaling changes of JAK/STAT, and AKT/mTOR pathways, which are two canonical pathways involved in NK cell immune activation (35, 36). With treatment of CF, the NK92 cells presented an increase in the levels of phosphorylated JAK1, JAK2 (Fig. 5A), and STAT1 (Fig. 5B), whereas the phosphorylation levels of JAK3, STAT3, STAT5, mTOR, AKT, and ERK (i.e., extracellular signal-regulated kinase) were not altered under the same conditions (Fig. 5, A–C). Moreover, we used si-JAK1 and si-JAK2 to silence the expression of JAK1 and JAK2 in the NK92 cells, respectively. As a result, CF was unable to effectively induce the upregulation of p-STAT1 and the expression of the activation receptor NKG2D (Fig. 5, D–G). Consistent with this finding, the cytotoxicity of the NK92 cells to the target K562 cells was essentially deprived (Fig. 5, H and I). Thus, it is likely that CF functions by enhancing the signal transduction of the JAK1/2-STAT1 pathway.
Figure 5.
CF activates NK cells via the IFNγR-JAK1/2-STAT1-dependent signaling pathway. A–C, the molecules involved in NK92 cell activation were detected. The expression levels of the total and phosphorylated JAK1/2/3, STAT1/3/5, and mTOR/AKT in CF-treated (0, 0.1, 1, and 10 μM) cells for 24 h before lysis were compared with those in untreated cells by Western blot. D, F, and J, JAK1, JAK2, and INFγR1 knockdown (KD) efficiencies ere confirmed by Western blot assay. Quantification of Western blot was presented in the bar graphs. E and G, NK92 cells were pretreated with si-JAK1a/JAK2a treated with CF and then analyzed JAK1, p-JAK1, JAK2, p-JAK2, p-STAT1, STAT1, and NKG2D in NK92 cells by Western blot. K, effect of interfering IFNγR1 on JAK-STAT of NK92 cells. The cells were pretreated with si-IFNγR1a for 48 h. Then, they were treated with CF (10 μM) for another 24 h. Western blot analysis revealed the expression levels of p-JAK1, p-JAK2, IFNγR1, JAK1, JAK2, p-STAT1, STAT1, and NKG2D in NK92 cells by Western blot. H, I, and L after interfering with JAK1/JAK2/IFNγR1, the NK92 cells were treated with CF (10 μM) for another 24 h and co-cultured with K562 for 6 h. The supernatant was measured by an LDH release assay. M and N effects of CF on IFNγR1/IFNγR2 protein aggregation. NK92 cells were incubated with CF (10 μM) for 24 h and treated with the BS3 before lysis. Protein band densities were quantified by normalizing to GAPDH. The data shown are representative of three experiments. Data are means ± SD. n.s., nonsignificant; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 by one-way ANOVA with the Dunnett’s test. BS3, bis (sulfosuccinimidyl) suberate; CF, cycloartenyl ferulate; IFNγR, IFNγ receptor; LDH, lactate dehydrogenase; NK, natural killer.
As the JAK1/2-STAT1 canonical signaling pathway is known to be activated by IFNγ binding to IFNγR1 and receptor 2 (IFNγR2) (37), we then investigated the effect of CF on the IFNγR-mediated activation of the JAK1/2-STAT1 pathway. We treated NK92 cells with si-IFNγR1a, and the cells exhibited a significant reduction in the phosphorylation levels of JAK1, JAK2, and STAT1 as well as in the expression of NKG2D in the presence of CF (Fig. 5, J and K), following dramatically decreased cytotoxicity to the target cells (Fig. 5L). We further examined the assembly of IFNγR1 and IFNγR2 in the NK92 cells. Specifically, the NK92 cells here were pretreated with the bis (sulfosuccinimidyl) suberate protein crosslinker before lysis to facilitate the identification of the weak interaction of native IFNγR1 and IFNγR2. At 10 μM level, we found CF significantly favored the formation of the heterodimeric IFNγR1/IFNγR2 complex (Fig. 5, M and N). Grounded on our results, we reason that the IFNγR1/2-JAK1/2-STAT pathway is the one that CF participates in to enhance NK cell immunity.
CF enhances NK cell immunity by binding to IFNγR1
We next sought to explore the molecular mechanism by which CF acts on the IFNγR-JAK-STAT1 signaling pathway. We first conducted the cellular thermal shift assay (CETSA) (38) to probe the potential interaction of CF to IFNγR1 and IFNγR2 in the NK92 cells. When treated with CF, the thermal stability of IFNγR1 was significantly increased over a range of temperatures tested (Fig. 6A), which is in marked contrast with that observed for IFNγR2 (Fig. S3). This finding indicates that IFNγR1 might be a binding target of CF. To test this hypothesis, we incubated the NK92 lysates with a biologically active CF-biotin conjugate (Fig. S4, A and B) and then performed affinity pull-down analysis. IFNγR1 was found to undergo precipitation with streptavidin rather than IFNγR2 (Figs. 6B and S4C), verifying the engagement CF to IFNγR1.
Figure 6.
CF stimulates JAK1/JAK2-STAT1 signaling by binding to IFNγR1.A, cellular thermal shift assay for IFNγR1 in NK92 cells. The cells were treated with CF (10 μM) for 3 h before being heated at different temperatures. Immunoblotting analyses were performed with the indicated IFNγR1 antibodies. Protein band densities were quantified by normalizing to GAPDH. B, the combination of IFNγR1 and CF was analyzed by affinity pull-down assay. The NK92 cell lysates were incubated with free biotin or CF-linked biotin and the precipitated proteins were separated by Western blot. C–E, surface plasmon resonance sensorgrams of CF binding to IFNγR1 (C), IFNγ binding to IFNγR1 (D), and IFNγ binding to IFNγR1 (E). F–G computational insights into the binding modes of CF to the IFNγR1 alone (F) and the IFNγ-IFNγR1 complex (G). The IFNγ-binding region of IFNγR1 is highlighted in slate. The two IFNγ monomers are represented as orange and yellow surfaces, respectively, and IFNγR1 as a gray surface. CF is depicted in green sticks and its interaction details with IFNγR1 and/or IFNγ are illustrated in the insets. The black dashed lines indicate hydrogen bonds. H and I, Western blot analysis (H) and quantification (I) of the phosphorylation levels of STAT1 in NK92 cells treated with indicated concentrations of IFNγ and CF. Data are means ± SD. n.s., nonsignificant; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 by one-way ANOVA with the Dunnett’s test. CF, cycloartenyl ferulate; IFNγR, IFNγ receptor; NK, natural killer.
Additionally, the surface plasmon resonance (SPR) technique was used to qualify the binding affinities of CF and IFNγ for IFNγR1. The data showed CF is a relatively weak binder with submicromolar affinity (Kd = 0.5 μM) binding to IFNγR1 as compared to IFNγ with nanomolar affinity (Kd = 0.0028 μM) (Fig. 6, C and D). The difference is not unexpected as the protein-protein binding interface is generally far more extensive than the protein-small molecule interface. In the presence of CF, however, IFNγ exhibited a slightly reduced affinity for IFNγR1 (Kd = 0.0045 μM, Fig. 6E), implying that CF could compete for binding to a part of the IFNγ-binding region on IFNγR1.
To test this hypothesis, we resorted to molecular docking and molecular dynamics simulations to search for the CF-binding site on IFNγR1. Excluding IFNγ, we were able to identify a CF-binding site that partly overlaps with the broad IFNγ-binding patch on IFNγR1 (Fig. 6F). Interestingly, the IFNγ-IFNγR1 complex presents a unique crevice for anchoring CF, which is instead mostly lined by the residues from the IFNγ dimer (Fig. 6G). This CF-binding region is spatially adjacent to but shares no common residues with the above one identified from IFNγR1 alone (Fig. 6F). Based on this observation, we reasoned that IFNγ must first squeeze out the bound CF before complete occupation of its native binding interface on IFNγR1. This notion is apparently supported by our SPR experiments showing a decrease in the affinity constant between IFNγ and IFNγR1 when tested in the presence of CF.
Remarkably, our free energy calculations suggested an enhanced binding affinity between IFNγ and IFNγR1 in the preformed complex with CF relative to that without CF bound (Table S4). A close inspection of the simulation trajectories revealed that the strengthened interaction of IFNγ and IFNγR1 in the ternary assembly mainly stems from multiple hydrogen bonds and salt bridges newly formed at their binding interfaces (Table S5). As a result, the buried area between IFNγ and IFNγR1 had widened by ∼54 Å2 upon CF anchoring.
Based on the above computational results, we further investigated the combined effect of IFNγ and CF on the IFNγR-JAK-STAT1 signaling in the NK92 cells. The combination treatment with IFNγ and CF led to an increased level of p-STAT1 (relative to the control) that is higher than that from either separate treatments (Fig. 6, H and I). Taken together, our results unveil that CF exerts a synergistic effect on the IFNγ-activated IFNγR-JAK-STAT1 signaling pathway by reinforcing the interaction between IFNγ and IFNγR1.
Discussion
NK cells are considered to be an important part of the innate immune system and the first line of defense of antitumor immunity, which has the function of immune surveillance. In this report, our studies reveal that CF is able to promote the activation of NK cells to lyse cancer cells in vitro and in vivo and synergizes with anti-PD1 antibody to delay tumor growth.
We first found that CF enhanced the immune cytotoxic functions of NK92 cells accompanied by the expressions of activation receptors and releases of cytokines such as perforin, granzyme B, and IFNγ. In many cases, a good immune system is essential for complete remission during tumor therapy. Deficiency or decreased activity of immune cells has affected the efficacy of conventional tumor therapy. Therefore, we study whether CF boosts the cytolytic activity of NK cells in healthy people and patients. Similarly, PBNK or PE-NK also exhibits remarkable cytotoxicity in cancer cells. These results suggest that CF may exert anticancer effects in vivo through the activation of NK cells.
NK cells can recognize and kill tumor cells with the loss of human leukocyte antigen (HLA-I) (39). Therefore, we used a syngeneic tumor clearance model and found that CF significantly enhanced the clearance of RMAS cells (loss of HLA-I) dependent on NK cells because we found that the capability of CF on peritoneal clearance of RMAS cells was dramatically diminished after depletion of NK cells by NK1.1 antibody in mice. However, we noticed a significant elimination of the RMA (HLA-I expression) cells after being treated with CF compared with the control, implying that other effector cells may be subjected to activation by CF. Similarly, we found that metastasis and growth cancer was remarkably inhibited in the B16-F10 melanoma xenograft model dependent on NK cells, although these effects are not completely blocked by the anti-NK1.1 body.
IFNγ signals through the receptor mediate downstream signaling events. IFNγ induces a rapid response via the JAK1/JAK2-STAT1 canonical pathway (40, 41), and activation of different kinases such as ERK1/ERK2 or GSK3β noncanonical signaling independent of cell context (42). In our efforts to explore the mechanism underlying the activation of NK of CF, we found that CF upregulated the phosphorylation levels of JAK1, JAK2, and STAT1 rather than JAK3, STAT3, and STAT5 and did not induce the protein activations of ERK, AKT, and mTOR. These results suggest that CF promotes NK cell immunity with an action pattern similar to IFNγ. With various experiment methods including molecular docking, CETSA, and pull-down experiments, and so on, we prove that CF is capable of interacting directly with IFNγR1, promoting the formation of a complex IFNγR1 and IFNγR2 to stimulate the downstream JAK1/2-STAT1 signaling pathway. To our best knowledge, it is for the first time to identify the molecular target of CF.
T cells also can be activated by IFNγ. We verify that CF can also heighten the activation and cytotoxic function of T cells through the canonical JAK1/2-STAT1 signaling pathway of IFNγR activation in vitro (Fig. S5, A–E). In addition, NK cells have further functions beyond cytotoxicity and are important activators of the adaptive immune response. They produce numerous antitumor cytokines upon activation, such as IFNγ, which is crucial for the functioning of all immune cells and multilevel regulation of both innate and adaptive immune responses, especially in the TME (43). Together, these results can explain the in vivo results that there were significant anticancer effects after the depletion of NK cells.
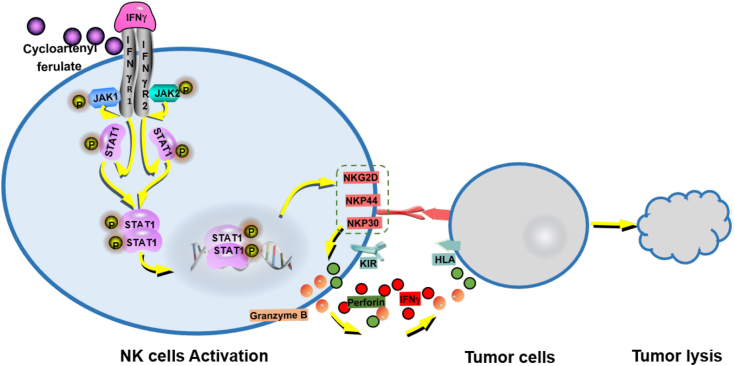
In sum, we first prove that CF selectively binds to the IFNγ receptor which in turn promotes activation and cytolytic activity of NK cells, and improves the TME, thereby preventing cancer from metastasis and growth in vivo (Fig. 7). Our research may pave a new avenue to understanding the potent anticancer immunity effect of CF.
Figure 7.
A schematic working model of CF based on our findings. CF, cycloartenyl ferulate.
Experimental procedures
Mice and human samples
The C57BL/6J female mice (Beijing Vital River Laboratory Animal Technology Co, Ltd), with 6 to 8 weeks of age and 18 to 20 g of weight, were used. All animal work was performed following the Institutional Animal Care and was approved by the Ethics for Animal Experiments committee of the Ocean University of China (OUC-SMP-2020–08–02). Human blood samples from eight normal healthy donors and pleural effusion from seven lung cancer patients were used for research purposes under a protocol approved by the Institutional Review Board of the Ocean University of China (OUC-HM-2022–011). Informed consent was obtained from all patients and normal healthy donors.
Cell culture and reagents
Human NK92 cells were cultured in alpha minimum essential medium supplemented with 12.5% horse serum (Solarbio) and 12.5% fetal bovine serum (FBS) (Gibco), 100 to 200U/ml recombinant IL-2 (PeproTech). A549, K562, and B16-F10 were cultured in RPMI-1640 medium. A2058 was cultured in Dulbecco’s modified Eagle’s medium. Mouse Lewis, RMA (murine T-cell lymphoma), RMAS (an RMA variant with decreased cell surface expression of MHC class I molecules) were constructed and conserved by our laboratory. All the cancer cells were obtained from the Cell Bank of the Chinese Academy of Sciences and cultured at 37 °C with 5% CO2 under fully humidified conditions with the above media (HyClone) supplemented with 10% FBS (Gibco), 100 U/ml penicillin (Solarbio), and 100 μg/ml streptomycin (Solarbio).
In vitro killing assay
Target cells (K562, A2058, and A549) seeded at 1 × 104 cells/well in a 96-well plate were co-incubated with effector cells at indicated effector to target (E/T) ratios in complete media for 6 h. Supernatants were harvested and determined by the lactate dehydrogenase assay (Beyotime Institute of Biotechnology, Cat. Number: C0508). For flow cytometric analysis, target cells were labeled with CFSE (Invitrogen, Cat. Number: C34554) and incubated with effector cells at various effector-to-target ratios for 6 h at 37 °C. Then 4 μl of 100 μg/ml PI solution was added to each sample before flow cytometric analysis. Dead target cells were identified as CFSE and PI double positive. Data were analyzed using FACSDiva software (BD Biosciences; https://www.bdbiosciences.com/).
Cell isolation and activation
PBMCs were isolated from human blood samples and pleural effusion by density gradient centrifugation using lymphocyte separation medium (Solarbio). Then, the cells were cultured in RPMI-1640 with 20% FBS, 10 ng/ml IL-2, and 20 ng/ml IL-15 (PeproTech) for at least 1 week for PBNK and PE-NK cells activation and expansion before use. To acquire activated T cells, PBMCs were cultured in RPMI-1640 with 20% FBS and 10 ng/ml IL-2 for at least 1 week before use, following addition of the human CD3/CD28 T Cell activation beads (Thermo Fisher Scientific, Cat. Number: 11161D).
Cell counting kit-8 assay
The cells (K562, A549, A2058, and NK92 cells) were treated with CF (0, 0.1, 1, and 10 μM) for 48 h and measured using Cell Counting Kit-8 assay kit (Beyotime Institute of Biotechnology) following the manufacturer’s general protocol. The absorbance at a wavelength of 450 nm was read by a microplate reader (Thermo Fisher Scientific).
Cytokine release assays
NK92 cells treated with various concentrations of CF or not were cultured in 24-well plates (2 × 105/ml) for 72 h. K562 cells (5 × 103) were plated in 96-cell plates, effector cells were added to the plates at E/T ratios of 5:1 and incubated for 12 h at 37 °C, and then the supernatants were collected for ELISA according to the manufacturer’s instructions. ELISA kits for the detection of human IFNγ (Cat. Number: 1,110,002), granzyme B (Cat. Number: 1,118,302), and perforin (Cat. Number: 1,118,302) were from Dakewe.
Western blot analysis
NK92 cells treated with various concentrations of CF or not were cultured in 24-well plates (2 × 105/ml) for 24 h. Cells were lysed with radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail at 4 °C for 30 min. Then, equal amounts of protein per sample were fractionated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was blocked for 1 h with 3% bovine serum albumin. Membranes were probed with primary antibodies overnight at 4 °C and followed by incubation with secondary antibodies for 1 h at room temperature. Blots were developed using enhanced chemiluminescence reagents (Thermo Fisher Scientific). The antibodies used are summarized in Table S1. The relative densities of the protein bands were analyzed with NIH ImageJ software (https://imagej.net/).
Flow cytometry analysis (for subsets of immune cells)
Harvested cells (5 × 105) were washed with PBS (pH 7.2, containing 1% FBS). The cells were stained with the antibody from BD Biosciences for 30 min at 4 °C. The antibodies used are summarized in Table S2. For the staining of intracellular cells, the cells were stained with surface staining. Then, these cells were stimulated with Factor Fixation/Permeabilization (Invitrogen, Cat. Number: 00–5521–00) for 30 min and 0.1% Triton100 for 5 min. Finally, the intracellular staining was carried out with anti-Mouse Foxp3 for the last 1 h. Then cells were filtrated through 100 mM nylon. The fluorescence intensity was detected by flow cytometry (Calibur, BD Biosciences). The results of the flow cytometry are presented as percentages of the positive fluorescent cells. The data were analyzed using FlowJo7.6.1 analysis software (https://www.bdbiosciences.com/).
In vivo lymphoma clearance assay
Lymphoma cells expressing MHC class-I (RMA) were labeled with a high CFSE concentration (6 μM), whereas those with defective expression of MHC class-I (RMAS) were labeled with a low CFSE concentration (0.3 μM). The cells were mixed in a 1:1 ratio (1 × 106 cells per cell type) and injected i.p. into C57BL/6J mice for 12 h. To deplete the NK cells, i.p. injections of rabbit anti-NK1.1 (200 μl) or hamster IgG isotype control (Bio X Cell, Cat. Number: 2A3) were performed before injection of CFSE-stained RMA and RMAS cells. Rejection of NK cell-sensitive RMAS relative to NK cell-resistant RMA cells in the peritoneal cavity was calculated as follows: ([CFSE high] control - [CFSE high] experimental group) × 100%.
In vivo pulmonary metastasis assay
For metastasis assays, B16-F10 melanoma cells (2 × 105 cells/200 μl PBS) were injected into the C57BL/6J mice via tail vein. In vivo, due to the high concentration and low solubility of CF, the intragastric CF delivery method was chosen. We also consulted some references (44, 45). Then, the mice were i.g. injected with CF, or vehicle-injected (5% carboxymethylcellulose sodium) daily. To deplete the NK cells, i.p. injections of rabbit anti-NK1.1 (200 μl) or hamster IgG isotype control were performed before 2 days. After 22 days of tumor implantation, the mice were sacrificed and the lungs were harvested for examination of tumor cell colonization.
In vivo subcutaneous xenograft growth studies
C57BL/6J mice were inoculated subcutaneously on their right flank with 2 × 106 Lewis lung cells on day 1, and the mice were subsequently randomly separated into groups. After mice developed palpable tumors, mice were i.g. injected with CF (50 mg/kg) or vehicle-injected daily and started on either 0.2 mg anti-PD1 (Bio X cell, Cat. Number: RPM1-14) or hamster IgG isotype control (Bio X cell, Cat. Number: 2A3) every three days intraperitoneally. The mice were sacrificed on day 19 after implantation, and then tumors were excised, weighed, and photographed. The spleen was isolated to prepare splenocytes. Tumor growth was measured by caliper measurements, tumor volume was calculated using the following formula: V = 1/2 × a × b2, where V = tumor volume, a = maximum tumor diameter, and b= minimum tumor diameter.
Immunohistochemistry analysis
The tumor tissues in mice were fixed with formalin for subsequent immunohistochemistry analysis to detect protein expression. The antibodies used are summarized in Table S2. Then, tumor tissues were fixed in 4% paraformaldehyde; the paraffin-embedded tissue sections (4 μm) were deparaffinized, dehydrated, and treated with 3% hydrogen peroxide to block endogenous peroxidase. The images were acquired at 20 × magnification using a ZEISS Axioskop 2 plus advanced positive microscope.
Cell transfection
The short interfering RNAs that targeted JAK1 (si-JAK1a/b), JAK2 (si-JAK2a/b), IFNγR1 (si-IFNγR1a/b), and corresponding siRNA negative controls (si-NC) were purchased from Shanghai GenePharma Co, Ltd. NK92 cells were seeded into 24-well plates, and then transiently transfected with si-RNA or negative control using Lipofectamine 3000 (Invitrogen, Cat. Number: L3000–015) according to the manufacturer’s instructions. Sequences of siRNA are provided in Table S3.
Cellular thermal shift assay
NK92 cells were treated with dimethyl sulfoxide or CF for 3 h. The cells were then resuspended in PBS and divided into several aliquots. The samples were heated at different temperatures by Biometra TOne PCR (Analytik Jena). The heated cells were freeze-thawed 3 times with liquid nitrogen and then centrifuged at 20, 000 g for 20 min at 4 °C. The supernatants were collected and loading buffer was added before boiling. Protein levels were analyzed by Western blot.
Streptavidin-biotin affinity pull-down assay
First of all, standard coupling of CF with Biotin using EDCI/HOBt in CH2Cl2 at 25 °C cleanly afforded compound CF-biotin, which was obtained after flash chromatography and pure (98%) enough to be used for the pull-down assay. Then, NK92 cell lysates were harvested and incubated with free biotin, CF, or CF-biotin at 4 °C with gentle rotation. Recombinant streptavidin agarose beads (BEAVER, 22309–1) were subsequently added to pull-down proteins interacting with biotin or biotin-labeled CF. Protein levels were analyzed by Western blot.
Surface plasmon resonance
SPR was determined using a Biacore X-100 plus instrument (GE HealthCare). IFNγR1 proteins were immobilized on the sensor chip (CM5) using the amine-coupling method according to standard protocols. CF or IFNγ was diluted by PBS-P buffer. To estimate the affinity, the binding assay was examined at 25 °C at a flow rate of 30 μl min-1 using PBS-P buffer. The affinity constants of binding were obtained using the 1:1 Langmuir binding model via BIA evaluation software (https://www.cytivalifesciences.com/).
Molecular docking and molecular dynamics simulation
The crystal structure of human IFNγ-IFNγR1 (Protein Data Bank code: 1FG9) at 2.9 Å resolution was chosen for molecular docking of CF. The docking program AutoDock Vina (46) was used to perform global search of CF binding sites on the IFNγR1 monomer. The resultant complexes with predicted lowest binding free energies were taken for ensuing MD simulations using the AMBER22 engine. The small molecule CF was parametrized following the standard protocol as previously described (47). The protein and water molecules were treated by the AMBER ff19SB force field (48) and Optimal Point Charges model (49), respectively. Each complex was solvated in a truncated octahedral water box with a minimum thickness of 13.5 Å from each side, with Na+ and Cl- ions added to attain 150 mM physiological concentration. The production simulations were performed at 310.15 K and 1 bar, extending to 100 ns. The last 20 ns of trajectories were extracted for analysis.
Statistical analyses
All data are presented as mean value ± SD from at least three independent experiments. Statistical analysis was performed using GraphPad Prism 5.0 (https://www.graphpad.com/). Two-way ANOVA with Tukey’s multiple comparisons was performed to assess the differences in tumor burden (tumor volume and tumor weight). n.s., nonsignificant, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 were considered statistically significant.
Data availability
All data are contained within the manuscript.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 82273847 and 32000885), the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (No. 2021CXLH0012), the Shandong Provincial Natural Science Foundation (major basic research projects) (No. ZR2021ZD28). The computations in this paper were performed on the cluster supported by the Center for High Performance Computing at Shanghai University of Engineering Science.
Author contributions
M. L., A. Z., and Y. W. methodology; M. L. writing–original draft; J. C. software; X. Q. validation; Y. M., C. H., Y. X., X. Z., and W. X. formal analysis; D. L. and Y. L. data curation; Z. Z. and J. L. conceptualization; J. L. funding acquisition.
Reviewed by members of the JBC Editorial Board. Edited by Qi-Qun Tang
Contributor Information
Zhicheng Zuo, Email: ZhichengZuo@sues.edu.cn.
Jing Li, Email: lijing_ouc@ouc.edu.cn.
Supporting information
References
- 1.Heintzman D.R., Fisher E.L., Rathmell J.C. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell Mol. Immunol. 2022;19:316–326. doi: 10.1038/s41423-021-00833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santiago-Sanchez G.S., Hodge J.W., Fabian K.P. Tipping the scales: immunotherapeutic strategies that disrupt immunosuppression and promote immune activation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.993624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow A., Perica K., Klebanoff C.A., Wolchok J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022;19:775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narni-Mancinelli E., Vivier E. Advancing natural killer therapies against cancer. Cell. 2022;185:1451–1454. doi: 10.1016/j.cell.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Pan R., Ryan J., Pan D., Wucherpfennig K.W., Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185:1521–1538.e1518. doi: 10.1016/j.cell.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng C., Zhong Q., Song W., Yi K., Kong H., Wang H., et al. Membrane-fusion-mediated multiplex engineering of tumor cell surface glycans for enhanced NK cell therapy. Adv. Mater. 2022;35 doi: 10.1002/adma.202206989. [DOI] [PubMed] [Google Scholar]
- 7.Demaria O., Gauthier L., Debroas G., Vivier E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur. J. Immunol. 2021;51:1934–1942. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 8.Kucuksezer U.C., Aktas Cetin E., Esen F., Tahrali I., Akdeniz N., Gelmez M.Y., et al. The role of natural killer cells in Autoimmune diseases. Front. Immunol. 2021;12:622306. doi: 10.3389/fimmu.2021.622306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dani?Lle K., Marianne H., Kuppen P.J.K. The role of natural killer T cells in cancer—A phenotypical and functional approach. Front. Immunol. 2018;9:367. doi: 10.3389/fimmu.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers J.A., Miller J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 12.Kundu S., Gurney M., O'Dwyer M. Generating natural killer cells for adoptive transfer: expanding horizons. Cytotherapy. 2021;23:559–566. doi: 10.1016/j.jcyt.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Suen C.W., Lee Y.W., Leung K.T., Pan X.H., Li G. Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Invest. 2018;36:431–457. doi: 10.1080/07357907.2018.1515315. [DOI] [PubMed] [Google Scholar]
- 14.Propper D.J., Balkwill F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022;19:237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 15.Munari E., Quatrini L., Ciancaglini C., Eccher A., Bogina G., Moretta L., et al. Immunotherapy targeting inhibitory checkpoints: the role of NK and other innate lymphoid cells. Semin. Immunol. 2022;61-64 doi: 10.1016/j.smim.2022.101660. [DOI] [PubMed] [Google Scholar]
- 16.Hercend T., Farace F., Baume D., Charpentier F., Droz J.P., Triebel F., et al. Immunotherapy with lymphokine-activated natural killer cells and recombinant interleukin-2: a feasibility trial in metastatic renal cell carcinoma. J. Biol. Response Mod. 1990;9:546–555. [PubMed] [Google Scholar]
- 17.Burns L.J., Weisdorf D.J., Defor T.E., Vesole D.H., Repka T.L., Blazar B.R., et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I|[sol]|II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 18.Liang S., Xu K., Niu L., Wang X., Liang Y., Zhang M., et al. Comparison of autogeneic and allogeneic natural killer cells immunotherapy on the clinical outcome of recurrent breast cancer. Onco Targets Ther. 2017;10:4273–4281. doi: 10.2147/OTT.S139986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto N., Ishikawa T., Kokura S., Okayama T., Yoshikawa T., et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J. Transl. Med. 2015;13:277. doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause S.W., Gastpar R., Andreesen R., Gross C., Ullrich H., Thonigs G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin. Cancer Res. 2004;10:3699–3707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- 21.Rajewsky K. The advent and rise of monoclonal antibodies. Nature. 2019;575:47–49. doi: 10.1038/d41586-019-02840-w. [DOI] [PubMed] [Google Scholar]
- 22.Kwon H.J., L H., Choi G.E., Kwon S.J., Song A.Y., Kim S.J., et al. Ginsenoside F1 promotes cytotoxic activity of NK cells via insulin-like growth factor-1-dependent mechanism. Front. Immunol. 2018;9:2785. doi: 10.3389/fimmu.2018.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia W., Qi X., Li M., Wu Y., Sun L., Fan X., et al. Metformin promotes anticancer activity of NK cells in a p38 MAPK dependent manner. Oncoimmunology. 2021;10 doi: 10.1080/2162402X.2021.1995999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Dong W., Nalin A.P., Wang Y., Pan P., Xu B., et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1431085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneda I., Kubo F., Sakurai H. Relationship between trace metal concentration and antioxidative activity of ancient rice bran (red and black rice) and present-day rice bran (Koshihikari) J. Trace Elem. Med. Biol. 2007;21:43–51. doi: 10.1016/j.jtemb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Nagasaka R., Chotimarkorn C., Shafiqul I.M., Hori M., Ozaki H., Ushio H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007;358:615–619. doi: 10.1016/j.bbrc.2007.04.178. [DOI] [PubMed] [Google Scholar]
- 27.Choi S.P., Kim S.P., Nam S.H., Friedman M. Antitumor effects of dietary black and brown rice brans in tumor-bearing mice: relationship to composition. Mol. Nutr. Food Res. 2013;57:390–400. doi: 10.1002/mnfr.201200515. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.P., Kang M.Y., Nam S.H., Friedman M. Dietary rice bran component gamma-oryzanol inhibits tumor growth in tumor-bearing mice. Mol. Nutr. Food Res. 2012;56:935–944. doi: 10.1002/mnfr.201200057. [DOI] [PubMed] [Google Scholar]
- 29.Yasukawa K., Akihisa T., Kimura Y., Tamura T., Takido M.J.B., Bulletin P. Inhibitory effect of cycloartenol ferulate, a component of rice bran, on tumor promotion in two-stage carcinogenesis in mouse skin. Biol. Pharm. Bull. 1998;21:1072–1076. doi: 10.1248/bpb.21.1072. [DOI] [PubMed] [Google Scholar]
- 30.Debska-Zielkowska J., Moszkowska G., Zielinski M., Zielinska H., Dukat-Mazurek A., Trzonkowski P., et al. KIR receptors as key regulators of NK cells activity in health and disease. Cells. 2021;10:1777. doi: 10.3390/cells10071777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merzoug L.B., Marie S., Satoh-Takayama N., Lesjean S., Albanesi M., Luche H., et al. Conditional ablation of NKp46+ cells using a novel Ncr1(greenCre) mouse strain: NK cells are essential for protection against pulmonary B16 metastases. Eur. J. Immunol. 2014;44:3380–3391. doi: 10.1002/eji.201444643. [DOI] [PubMed] [Google Scholar]
- 32.Le Calvez B., Moreau P., Touzeau C. Immune checkpoint inhibitors for the treatment of myeloma: novel investigational options. Expert Opin. Investig. Drugs. 2021;30:965–973. doi: 10.1080/13543784.2021.1955103. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Chen Z., Li Y., Zhao W., Wu J., Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.731798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowell D., Yoo S.K., Valero C., Pastore A., Krishna C., Lee M., et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat. Biotechnol. 2022;40:499–506. doi: 10.1038/s41587-021-01070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotthardt D., Trifinopoulos J., Sexl V., Putz E.M. JAK/STAT cytokine signaling at the crossroad of NK cell development and maturation. Front. Immunol. 2019;10:2590. doi: 10.3389/fimmu.2019.02590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali A.K., Nandagopal N., Lee S.H. IL-15-PI3K-AKT-mTOR: a critical pathway in the life journey of natural killer cells. Front. Immunol. 2015;6:355. doi: 10.3389/fimmu.2015.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach E.A., Tanner J.W., Marsters S., Ashkenazi A., Aguet M., Shaw A.S., et al. Ligand-induced assembly and activation of the gamma interferon receptor in intact cells. Mol. Cell Biol. 1996;16:3214–3221. doi: 10.1128/mcb.16.6.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez Molina D., Jafari R., Ignatushchenko M., Seki T., Larsson E.A., Dan C., et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 39.Laskowski T.J., Biederstadt A., Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer. 2022;22:557–575. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blouin C.M., Hamon Y., Gonnord P., Boularan C., Kagan J., Viaris de Lesegno C., et al. Glycosylation-dependent IFN-gammaR partitioning in lipid and actin nanodomains is critical for JAK activation. Cell. 2016;166:920–934. doi: 10.1016/j.cell.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Randal M K.A. The structure and activity of a monomeric interferon-γ:α-chain receptor signaling complex. Structure. 2001;9:155–163. doi: 10.1016/s0969-2126(01)00567-6. [DOI] [PubMed] [Google Scholar]
- 42.Ramana C.V., Gil M.P., Han Y., Ransohoff R.M., Schreiber R.D., Stark G.R. Stat1-independent regulation of gene expression in response to IFN-gamma. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6674–6679. doi: 10.1073/pnas.111164198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn G.P., Ikeda H., Bruce A.T., Koebel C., Uppaluri R., Bui J., et al. Interferon-gamma and cancer immunoediting. Immunol. Res. 2005;32:231–245. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 44.Islam M.S., Murata T., Fujisawa M., Nagasaka R., Ushio H., Bari A.M., et al. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2010;154:812–824. doi: 10.1038/bjp.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SP K.M., Nam S.H., Friedman M. Dietary rice bran component γ-oryzanol inhibits tumor growth in tumor-bearing mice. J. Mol. Nutr. Food Res. 2012;56:935–944. doi: 10.1002/mnfr.201200057. [DOI] [PubMed] [Google Scholar]
- 46.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He X., Man V.H., Yang W., Lee T.S., Wang J. A fast and high-quality charge model for the next generation general AMBER force field. J. Chem. Phys. 2020;153 doi: 10.1063/5.0019056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian C., Kasavajhala K., Belfon K.A.A., Raguette L., Huang H., Migues A.N., et al. ff19SB: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theor. Comput. 2020;16:528–552. doi: 10.1021/acs.jctc.9b00591. [DOI] [PubMed] [Google Scholar]
- 49.Izadi S., Anandakrishnan R., Onufriev A.V. Building water models: a different approach. J. Phys. Chem. Lett. 2014;5:3863–3871. doi: 10.1021/jz501780a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.