Abstract
The majority of emerging infectious diseases are zoonoses, most of which are classified as “neglected”. By affecting both humans and animals, zoonoses pose a dual burden. The disability-adjusted life year (DALY) metric quantifies human health burden since it combines mortality and morbidity. This review aims to describe and analyze the current state of evidence on neglected zoonotic diseases (NZDs) burden and start a discussion on the current understanding of the global burden of NZDs.
We identified 26 priority NZDs through consulting three international repositories for national prioritization exercises. A systematic review of global and national burden of disease (BoD) studies was conducted using pre-selected databases. Data on diseases, location and DALYs were extracted for each eligible study.
A total of 1887 records were screened, resulting in 74 eligible studies. The highest number of BoD was found for non-typhoidal salmonellosis (23), whereas no estimates were found for West Nile, Marburg and Lassa fever. Geographically, the highest number of studies was performed in the Netherlands (11), China (5) and Iran (4). The number of BoD retrieved mismatched the perceived importance in national prioritization exercises. For example, anthrax was considered a priority NZD in 65 countries; however, only one national study estimating BoD was retrieved. By summing the available global estimates, the selected NZDs caused at least 21 million DALYs per year, a similar order of magnitude to (but less than) the burden due to foodborne disease (included in the Foodborne Disease Burden Epidemiology Reference Group).
The global burden of disease landscape of NZDs remains scattered. There are several priority NZDs for which no burden estimates exist, and the number of BoD studies does not reflect national disease priorities. To have complete and consistent estimates of the global burden of NZDs, these diseases should be integrated in larger global burden of disease initiatives.
Keywords: Burden of disease, Neglected zoonotic diseases, Disability-adjusted life years, Years lived with disability, Years of life lost
Highlights
-
•
Identifying prioritised neglected zoonotic diseases by analysing different international repositories (WHO and CDC).
-
•
Providing state of the art among studies that estimate disability-adjusted life years for neglected zoonoses.
-
•
The number of studies retrieved mismatched the perceived importance of diseases in national prioritization exercises.
-
•
Expanding projects to cover more NZDs would enhance global comparability and discover the real burden.
1. Introduction
The World Health Organization (WHO) defines zoonoses as diseases and infections naturally transmitted between vertebrates and humans [1]. There are several thousand human infectious diseases documented in the literature [2], and it is estimated that 60% of them and 75% of emerging infections are zoonotic [3]. Zoonoses have high impacts on human and animal health and the ecosystem and are also responsible for enormous economic losses [[4], [5], [6], [7]].
Zoonotic diseases disproportionately affect vulnerable groups in low-and middle-income countries (LMICs) and are often “neglected” regarding the geopolitical attention and relative funding they receive for prevention and control initiatives and research [8,9]. In recent years, the WHO has established a roadmap to eliminate neglected tropical diseases (NTDs), focusing on 20 diseases, including six neglected zoonotic diseases (NZDs): echinococcosis, foodborne trematodiasis, human African trypanosomiasis (HAT), leishmaniosis, rabies, and taeniasis/cysticercosis [10].
The Foodborne Diseases Burden Epidemiology Reference Group (FERG) established by the WHO and Institute for Health Metrics and Evaluation (IHME) have carried out and set standards for Global Burden of Disease (GBD) studies and the global burden of foodborne diseases, quantifying population health losses associated with diseases [[25], [39]]. The metric central to these burden of disease (BoD) frameworks is the Disability-Adjusted Life Year (DALY) [11]. The DALY is a summary measure of public health that combines the effects of mortality (Years of Life Lost) and morbidity (Years Lived with Disability) into a single metric. This metric also integrates different health states defined by the disease model [12]. As a result, DALYs encompass all disease impacts of NZDs, including disabling acute and chronic outcomes, rather than just focusing on fatal outcomes [13]. Additionally, when the same methodology is used, the impacts of different diseases or injuries can be directly compared, which helps policymakers to set priorities and advocacy. There has also been development of methods to combine the human and animal health burdens caused by zoonoses that are consistent with DALYs, such as zDALY, which adds a time trade-off component for animal morbidity and mortality [14].
This review aims to describe and discuss the current state of evidence on the human burden of NZDs and the knowledge gaps that surround them. The results will help to quantify the current understanding of the public health impact of NZDs and provide a resource for more robust and comparable decision-making. The review considers only the burden that zoonoses pose on humans and does not address burden estimations for the livestock sector, as this will be covered by other areas of the Global Burden of Animal Diseases programme (GBADs), of which this review is part.
2. Methods
2.1. Selection of the neglected zoonotic diseases for the review
The zoonotic diseases investigated were selected based on the national prioritization exercises from the CDC One Health Zoonotic Disease Prioritization Process Overview [15] and the WHO Joint External Evaluation Mission Reports (JEE) [16], as these are considered authoritative sources. We compared our findings with the WHO document “Ending the Neglect to Attain the Sustainable Development Goals: a Roadmap for Neglected Tropical Disease 2021-2030” [10]. The data extraction from these exercises and reports was conducted in July 2021, when the JEE Mission Reports comprised 116 completed reports and the CDC One Health Zoonotic Disease Prioritization Process Overview page was updated on 18th May 2021. A complete list of countries considered by the CDC and the JEE can be found in the additional documents (supporting document pp. 1–4).
When extracting the data, we counted the number of times each disease was included as a priority by the country. We included the first ten diseases for each country in our calculation since most countries identified ten or fewer priority pathogens. This resulted in a list of 52 zoonotic diseases. For the scope of this review, only the first 25 priority NZDs were selected. Additionally, we included foodborne trematodiasis based on the WHO roadmap [10]. Table 1 shows the diseases included in the review, and specifies the number of times each disease was mentioned in a prioritization exercise and if it was included in the WHO roadmap.
Table 1.
Prioritization frequencies of neglected zoonotic diseases and status of inclusion in the WHO roadmap.
| Disease | Frequency⁎ | Included in the WHO roadmap |
|---|---|---|
| Rabies | 94 | X |
| Brucellosis | 75 | |
| Influenza a (h5n1 and h1n1) | 72 | |
| Anthrax | 65 | |
| Bovine tuberculosis | 47 | |
| Rift valley fever | 28 | |
| Non-typhoidal salmonellosis | 25 | |
| Ebola virus | 20 | |
| Leptospirosis | 20 | |
| Crimean/congo hemorrhagic fever | 17 | |
| Plague | 14 | |
| Yellow fever | 11 | |
| Alveolar echinococcosis | 9 | X |
| Cystic echinococcosis | 9 | X |
| Human african trypanosomiasis | 9 | X |
| Japanese encephalitis | 8 | |
| Lassa fever | 8 | |
| Marburg virus disease | 8 | |
| Cysticercosis | 7 | X |
| Q fever | 7 | |
| Toxoplasmosis | 7 | |
| Campylobacteriosis | 5 | |
| Glanders | 5 | |
| Leishmaniosis | 5 | X |
| West nile disease | 5 | |
| Foodborne trematodiases | / | X |
Number of times the disease was mentioned for a country in either the CDC one health zoonotic disease prioritization process or in the WHO joint external evaluation mission reports.
The prioritization exercises and the WHO roadmap had different degrees of detail in defining diseases (e.g., leishmaniosis, covering visceral and cutaneous ones). These differences were also visible in the literature review and prioritization exercises (supporting document pp. 5–6). The results acknowledge specific sub-groups or divisions of diseases, where applicable.
2.2. Searching and eligibility criteria
To identify the available evidence on the burden of the selected priority NZDs, a review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement (supporting document pp. 7–8) [17]. PubMed, Web of Science, and Embase were systematically searched for relevant articles using terms covering DALYs and pathogens. A complete list of the keywords used is presented in the supporting document (9–17). Only peer-reviewed articles published between January 1990 and November 2021 were included. Studies published before 1990 were omitted since the DALY metric was introduced in the mid-1990s [11]. Studies not focusing on humans were excluded. In addition, only studies primarily aimed to determine the burden with the DALY methodology were included. Hence, studies that presented DALY estimates, but not as the main objective, were excluded (e.g., cost-effectiveness, life-cycle assessment, and quantitative microbial risk assessment studies). Studies reporting on cause-specific subsets of GBD estimates were also excluded from the search since the most recent GBD data on these conditions were extracted regardless. Nevertheless, reference lists of the studies that used GBD data were screened to find additional peer-reviewed articles. No language restriction was applied. Publications with insufficiently detailed information, such as abstracts, editorials, or letters to editors, were excluded.
2.3. Data screening, selection and extraction
The authors used Rayyan, a tool developed to manage citations, screen abstracts and apply inclusion and exclusion criteria, in order to create a database of unique titles [18]. The articles screening was conducted by one researcher (CDB). The study supervisor (BD) provided support for articles difficult to categorise initially. For each eligible paper where the full text was available, information was extracted using a data extraction grid, and the reference list was searched for additional studies. The following information was extracted: study information, reference population, DALY result and total population (supporting document p. 18). CDB performed data extraction, and NV reviewed the information extracted. The results have been displayed using the R program and Drawio [[19], [20], [21]].
3. Results
3.1. Flowchart of selected studies
A total of 1887 entries were retrieved from the selected databases. After removing the duplicates and applying the eligibility assessment, 73 studies were included in the review. Additionally, the most recent estimates of GBD (2019) were identified and included [22]. Thus, 74 studies were included in the review (Fig. 1).
Fig. 1.
Flow chart of study selection.
3.2. Burden of disease studies by location and year
Of the 74 studies included, 60 provided national or sub-national burden estimates (supporting document pp. 19–25). Nine studies were global, including the estimates from the latest GBD version (nine diseases). We excluded previous versions of the GBD to avoid overrepresentation. Two BoD assessments focused on specific regions of the world, one on the European Union and one on Asia and Africa. The highest number of single-country BoD assessments were observed in the Netherlands (N = 11), followed by China (N = 5) and Iran (N = 4) (supporting document p. 26). The first BoD assessment was published in 2000 [26], excluding studies carried out by the GBD (which updates its estimates frequently). After 2008, the number of studies published for this disease set increased, reaching nine publications in 2017 alone (supporting document p.26).
3.3. Burden of disease studies by disease
The number of burden estimates varied significantly between the different diseases. Out of the 73 publications, 47 focused on a single disease. The most common group investigated was foodborne diseases (N = 14); within these publications, two are part of the FERG program [38,37]. Other sets of diseases examined together were grouped by the general label of infectious diseases (N = 4) [25,[40], [41], [42]], arboviral diseases (N = 1) [43], and parasitic zoonoses (N = 1) [44]. One study focused on specific sequelae, namely the post-infectious irritable bowel syndrome, and quantified the DALYs caused by each disease for these specific sequelae [46]. Finally, two studies looked at the general BoD (e.g., diseases, injuries and risk factors), one from the GBD and the second in Iran [22,47].
The diseases that had the highest number of BoD studies were non-typhoidal salmonellosis (N = 24), campylobacteriosis (N = 22), toxoplasmosis (N = 1), cystic echinococcosis (N = 13), cysticercosis (N = 13), rabies (N = 12), brucellosis (N = 11), and alveolar echinococcosis (N = 7) (supporting document p. 27). Five BoD studies were found for HAT; one focused exclusively on Rhodesian HAT, one addressed Gambian HAT, another estimated the burden for both (providing DALYs for each sub-type), and two did not distinguish between them. The same approach was found in the studies on leishmaniosis (N = 5). One study focused only on the cutaneous type, one estimated the independent burden for each kind (cutaneous and visceral), and three provided no distinctions. Four studies investigated the burden of leptospirosis. Three publications provided estimates for bovine tuberculosis and foodborne trematodiases, with one estimate quantifying only the burden for clonorchiasis. Two estimates were identified for Q fever, influenza A H5N1, Japanese encephalitis, and yellow fever. Only one BoD assessment was found for Ebola fever, anthrax, Rift Valley fever, and Crimean/Congo hemorrhagic fever. Finally, no estimates were found for West Nile disease, Influenza A subtype H1N1, Marburg virus disease, plague, Lassa fever, and glanders.
4. Discussion
This review aims to provide a comprehensive overview of the evidence on BoD studies for NZDs and to start a reflection on the current understanding of the global burden of NZDs. Seventy-four studies met our inclusion criteria, including the latest version of the GBD. Over two-thirds of the studies were national or sub-national. The highest number of BoD studies was found for the Netherlands, which mainly reported estimates for foodborne diseases but also on Influenza A N5H1 (N = 2) and Q fever (N = 2) [41,48]. This finding was somewhat surprising given that they were not part of the CDC and WHO prioritization exercises, which focused on LMICs and thus did not contribute to our priority list. A possible explanation would be that national BoD studies are well established as input for policymaking and conducted regularly in the Netherlands [49]. Furthermore, they do not only include diseases endemic to their own country but also a subset of the global priority list. No burden studies were found in South America or South Asia. Few were retrieved from Sub-Saharan Africa, even if most of the diseases selected are considered endemic to these regions. Over the period taken into consideration, we observed an increase in the number of BoD assessments for the selected diseases. This could reflect an increasing recognition of the DALY metric in decision-making. It could also indicate a rising effort of international communities and projects to acknowledge the public health impact of zoonoses. In 2005, for example, the WHO set up a series of meetings on neglected zoonotic diseases [50] and, in 2006, launched the initiative to estimate the Global Burden of Foodborne Diseases [51], which includes many NZDs.
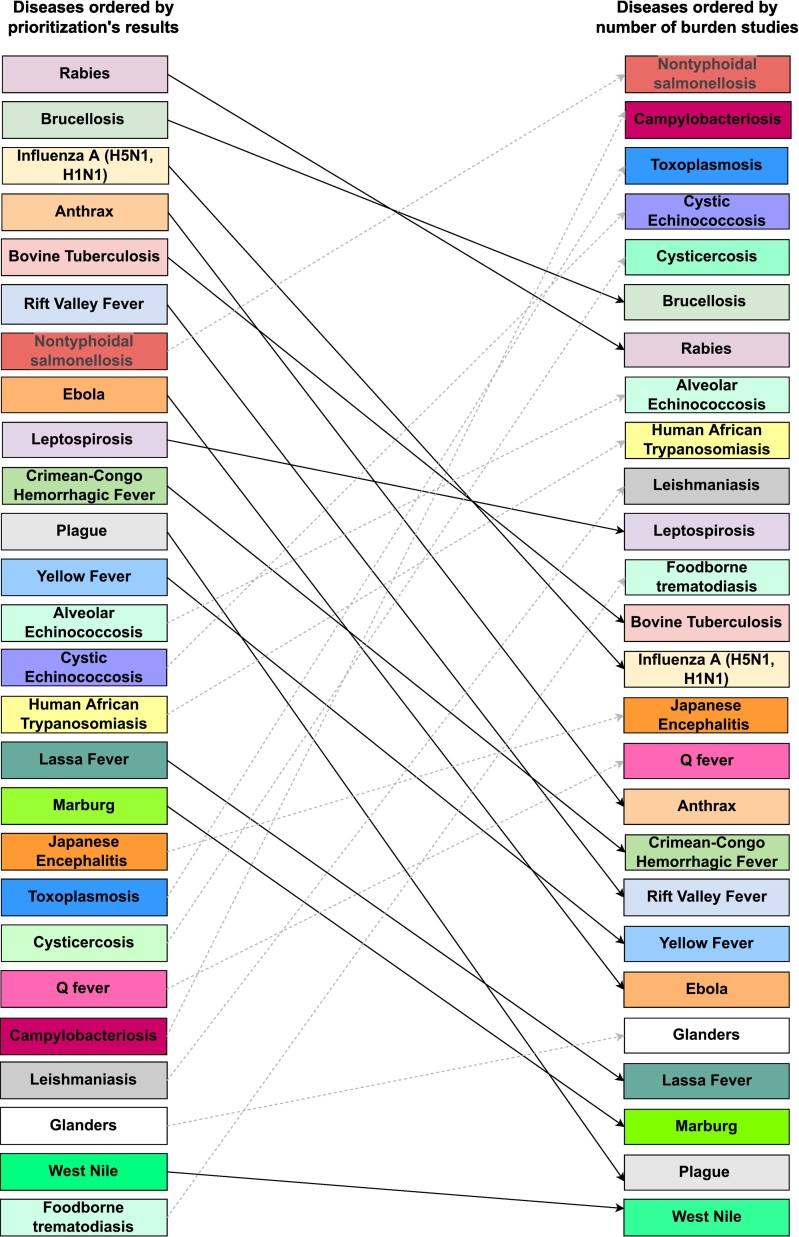
Differences were observed between the frequency of diseases listed in country prioritization exercises and the number of BoD estimates included in the review (Fig. 2). This could be caused by miscommunication between the different actors (e.g. policymakers, researchers, international organizations, etc.) due to their different aims. Some diseases frequently listed in the prioritization exercises had few BoD assessments. Anthrax, for example, was listed as a priority by 65 countries, but only one BoD study has been published. Notably, anthrax is not included in the GBD or the WHO roadmap, even though it is the only bacterial NZD included in the World Health Assembly resolution WHA 66.12 on NTDs [53]. For rabies, which had the highest frequency in the prioritization exercises (N = 94) and is included in the GBD and the WHO road map, only twelve BoD estimates were found.
Fig. 2.
Differences in the prioritization exercises from the WHO and CDC and the number of burden of disease studies found.
In this review, we included both (sub-)national and global estimates. Local and global estimates serve different purposes, and both have strengths and weaknesses. BoD assessments at the local level strengthen the local health information systems, improve the understanding of population health and define local priorities. In contrast, global estimates aggregate the disease burden worldwide and help establish priorities at the global level.
There were also diseases with a low frequency in prioritization exercises but a high number of BoD assessments, such as campylobacteriosis (N = 22) and toxoplasmosis (N = 16) (Fig. 2). The mismatch might suggest that some countries or academic groups may not use DALYs for their BoD assessments, or indicate a lacking capacity to carry on prioritization exercises to the next step. Furthermore, countries participating in prioritization exercises and the diseases that burden them most do not necessarily overlap with the countries carrying out BoD assessments and their priorities. Finally, factors such as missing data on duration or severity make the burden estimation for specific diseases through the standard BoD framework difficult and could also contribute to the mismatch.
A disease considered a disabler (e.g. low mortality rate), such as toxoplasmosis, might be overlooked in LMICs and gain more attention in high-income countries, where BoD studies are more common. Thus, some NZDs could be considered “neglected among the neglected” because they are overlooked in different contexts. Some of the prioritised NZDs are also part of other domains such as food safety, antimicrobial resistance, diarrheal disease, and maternal or neonatal health, which might contribute to their higher number of estimates.
When comparing diseases for which global burden estimates with the same methodology were available, the number of prioritization studies did not always align with the severity of the burden (Table 2). Brucellosis, for example, prioritised by 75 countries, has eleven estimates (national and global) and resulted in 264,073 (100540–6,187,148) DALYs globally in 2010 according to FERG. On the other hand, bovine TB caused 607,775 (458364–826,115) DALYs in 2010 but was only prioritised by 47 countries and had three burden estimates (one national, two FERG) [38,54].
Table 2.
DALYs per the selected diseases at the global level and the number of prioritisation exercises conducted for them⁎
| Disease | Source | DALYs | Result from the prioritization |
|---|---|---|---|
| Toxoplasmosis, acquired | FERG⁎⁎ | 153,779 (772676–1,733,114) |
7 |
| Cystic echinococcosis | FERG⁎⁎ | 183,573 (88082–1590 46) |
9 |
| Brucellosis | FERG⁎⁎ | 264,073 (100540–6,187,148) |
75 |
| Toxoplasmosis, congenital | FERG⁎⁎ | 526,515 (359756–835,537) |
7 |
| TB bovine | FERG⁎⁎ | 607,775 (458364–826,115) |
47 |
| Alveolar echinococcosis | FERG⁎⁎ | 687,823 (409190–1,106,320) |
9 |
| Foodborne trematodiasis | FERG⁎⁎ | 2,024,592 (1652243–2,483,514) |
– |
| Cysticercosis | FERG⁎⁎ | 2,788,426 (213763–3,606,582) |
9 |
| Campylobacteriosis | FERG⁎⁎ | 3,733,822 (2857037–5,273,652) |
5 |
| Non-typhoidal salmonellosis | FERG⁎⁎ | 4,377,930 (3242020–7,175,522) |
25 |
| African trypanosomiasis | GBD | 82,615 (37636–155,791) |
9 |
| Cystic echinococcosis | GBD | 122,457 (89244–168,556) |
9 |
| Ebola | GBD | 195,394 (230578–160,083) |
20 |
| Yellow fever | GBD | 290,137 (107073–597,713) |
11 |
| Leishmaniosis | GBD | 696,703 (375207–1,619,382) |
5 |
| Foodborne trematodiasis | GBD | 780,089 (385735–1,446,031) |
– |
| Rabies | GBD | 782,052 (320289–1,081,217) |
94 |
| Cysticercosis | GBD | 1,371,067 (874432–1,960,855) |
7 |
| Non-typhoidal salmonellosis (invasive) | GBD | 6,114,262 (3323425–9,705,738) |
25 |
| Rift Valley Fever | Labeaud et al. (2011) | 6156 (353–11,958) |
28 |
| Japanese encephalitis | Labeaud et al. (2011) | 1,062,474 (265778–1,859,170) |
8 |
| Leptospirosis | Torgerson et al. (2015) | 2,900,000 (1250000–4,540,000) |
20 |
This table only provides an overview of the different global estimates; it is important to note that they are not directly comparable since they come from different sources that applied different methodologies (as is visible, for example, from the estimates of non-typhoidal salmonella, for more information see [55]).
For FERG, the estimates are reported by all transmission routes, not just foodborne.
4.1. Disability Adjusted Life Years estimates for zoonoses
The most recent GBD only covered nine of the NZDs selected for this review, which accounted for approximately 10 million DALYs.24 Due to the limited number of diseases included, this number is, however, an underestimation of the total burden of NZDs.
When combining the estimates for all diseases with global BoD studies, using the most recent study or, whenever available, the estimate without age or time discounting, the total burden amounts to over 21·5 million DALYs. While this result only covers seventeen diseases (Table 2), it illustrates that the global burden of NZDs is substantial. To put it into perspective, the global DALY estimate due to infectious foodborne diseases calculated by FERG was 33 million in 2010, and the 2019 GBD estimate of the burden of enteric diseases was 96·8 million DALYs.
Our crude global NZD DALY estimate was calculated by manually adding (the medians of) estimates based on different methodological choices and assumptions and should be interpreted with great caution. To have complete and consistent estimates of the global burden of NZDs, these diseases would need to be integrated into larger global BoD initiatives. This will not only help to understand the real burden of these diseases and help define priorities based on evidence but could also inform policies aiming to eradicate these “neglected” diseases.
4.2. Zoonosis: where does its burden lie?
There is an ambiguity in what constitutes zoonoses and the role of animals as reservoirs. The WHO defines zoonoses as “diseases and infections naturally transmitted between vertebrates and humans” [56]. For this review, we interpreted this as the infection being maintained in an animal population, the reservoir, and a continuous source of human infection. Humans acquire zoonotic infections through direct contact with animals or indirect exposure routes such as vector-borne or environmental pathogens associated with the food system [57]. This first interpretation of zoonoses includes only diseases that are at stages two and three of the five-step framework for the evolution from animal to human diseases as proposed by Wolfe et al., where the animal is necessary and the pathogens can undergo only a few cycles of human-to-human transmission [59].
However, the zoonosis definition could also refer to zoonotic origins of the disease, but transmission irrespective of the animal reservoir. Examples of this would be COVID-19 or the human immunodeficiency virus (HIV), which started as an animal infection but spread to humans at some point (this phenomenon is called spillover) and later mutated into human-only strains, or stage five as described by Wolfe et al. [59,60].
Differentiating between diseases that may have originated in animals but independently persist in human populations and diseases that require a non-human animal host for pathogen transmission and survival enables more targeted and strategic initiatives for prevention and control. For instance, to tackle the spread of HIV, interventions do not focus on animals since the infection is mainly transmitted from human to human. On the other hand, for diseases such as rabies or brucellosis, interventions target the animal host because a permanent animal reservoir is needed to sustain the epidemic. Making this differentiation also helps determine where the disease burden lies (human or animal population). This helps understand changes in human morbidity and/or mortality as well as animal health/production and premature mortality due to disease and contributes to improving human health and animal productivity. In contrast, there are diseases like dengue where the role of the animal reservoirs is not yet clear, making it difficult to understand where the burden lies and determine the most effective initiatives [61]. Under the One Health approach, understanding where the burden lies will help assess the direct impact of the disease (on both animals and humans) and indirect ones, such as decreases in household incomes due to production losses, which may also affect health.
4.3. Disability-Adjusted Life Years as standard burden of disease metric
The use of DALYs implies multiple methodological choices and assumptions [62]. Thus, direct comparison between different estimates could lead to incorrect interpretation. For example, FERG and GBD estimates are structurally different, with the first producing an incident DALY and the second a prevalent DALY; consequently, although we presented them together (Table 2) to provide an overview, they should not be directly compared. Notably, there is no single or preferred way to estimate DALYs. Each methodology has its strengths and weaknesses. Qualitative or semi-quantitative prioritization exercises often reflect the notoriety or the perceived risk of a disease rather than the real threat or burden. Using DALYs to establish priorities sets up a more evidence-based and internally consistent framework for disease prioritization, limiting the participants' biases or specific interests in semi-quantitative prioritization exercises. Prioritization should consider both local concerns and DALY estimates.
Diseases that do not have DALY estimates or do not appear in the GBD will not receive the proper attention and probably be included in a category such as “others”. Indeed, it is interesting that some of the prioritised diseases (e.g., influenza A H1N1, plague and Lassa fever) are not acknowledged by international communities and do not have a burden estimate. This increases the chances that their importance is underestimated, especially if they mainly occur in LMICs.
5. Limitations
The review has several limitations. First, we could have missed out on some eligible and valid BoD studies, given that we did not consider ongoing estimates or BoD studies performed but not documented in peer-reviewed articles. Second, we acknowledge that the list of priority NZDs that we used is biased to a subset of global priorities and from the countries that took part in the exercises; however, it is informative since it is derived from established policy documentation. Third, we realize that using the number of identified BoD studies as an endpoint for determining whether disease prioritization aligns with the availability of burden estimates may lead to bias in different ways, which should be kept in mind when interpreting the results. Our choice to include only the most recent GBD study to avoid skewing the results influenced the number of BoD studies available for several diseases, especially for those who have been part of the GBD for a long time [63].
6. Future prospects
This review aimed to report the current state of BoD assessments for the set of selected diseases and to reflect on the current understanding of the public health impact of zoonoses. The findings can serve to improve the knowledge and advocacy of zoonoses. This is especially important for diseases that currently do not have BoD estimates and may be neglected by governments or policymakers. Consequently, future research should focus on acquiring the data needed to quantify the burden of these diseases, such as incidence or prevalence data, mortality, duration and disease models. Our findings highlight the need for more global BoD estimates for diseases such as anthrax, given their high frequencies in the prioritization exercises and their low representation in BoD assessments on a national or international level.
On the other hand, for diseases with sufficient estimates, such as brucellosis and toxoplasmosis, it could be impactful to include them in the GBD so that estimates could be compared to other diseases to provide a perspective on their relative impact. Indeed, this review suggests broadening the scope of GBD or FERG to include more diseases and increase the comparability between diseases at the global level. BoD assessments at the local level allow for a detailed look into data quality to strengthen local health information systems and better understand population health and will support local policymaking. Different efforts have been made in this field; for instance, FERG carried out different capacity-building activities and encouraged the use of information provided by BoD for evidence-informed policies. This review suggests that these efforts need to continue and stresses the importance of addressing methodological limitations inherent in the standard DALY approach. It is important to move towards a One Health approach to understand the full impact of zoonoses, develop a systematic methodology to describe the impact of animal diseases on society, including human health, and close the gap between human and animal health. This review is part of a broader initiative that aims to establish a systematic methodology for assessing the impact of animal diseases on society. The goal is to provide a comprehensive understanding of the burden of zoonotic diseases on both humans and animals. To achieve this, it is crucial to complement these estimates with similar studies focusing on the impact of these diseases on animals.
7. Conclusion
This review aimed to explore the current state of evidence on the BoD estimates for selected zoonoses and to reflect on understanding these diseases and their estimates. The results showed that not all of the diseases had BoD estimates and that the numbers of BoD estimates do not reflect the frequency of the diseases in prioritization exercises. This highlights the need for further research on zoonoses in order to have a better understanding of how each disease affects humans.
Declarations
This research is performed in the framework of the Global Burden of Animal Diseases (GBADs) programme which is led by the University of Liverpool and the World Organization for Animal Health (OIE) (https://animalhealthmetrics.org/). This research is supported through the Grant Agreement Investment ID INV-005366 with the Bill & Melinda Gates Foundation and the UK Foreign, Commonwealth and Development Office (FCDO). GBADs case studies receive additional funding from the following: European Commission, Australian Centre for International Agricultural Research (ACIAR), Brooke Foundation and the Food and Agriculture Organization of the United Nations (FAO). A full list of the GBADs collaborators can be accessed here: https://animalhealthmetrics.org/acknowledgements.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100595.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.World Health Organization, Zoonoses 2020. https://www.who.int/news-room/fact-sheets/detail/zoonoses
- 2.Wormser G.P., Dailey N.J., Fleischauer A.T. In: Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases. Keusch Gerald T., Pappaioanou Marguerite, Gonzalez Mila C., Scott Kimberly A., Tsai Peggy., editors. The National Academies Press; Washington, DC: 2009. (312 pp. 42.30paperback). 36 (E-book), (2010) [PubMed] [Google Scholar]
- 3.Institute of Medicine (U.S.) National Academies Press; Washington, DC: 2009. Committee on Achieving Sustainable Global Capacity for Surveillance and Response to Emerging Diseases of Zoonotic Origin., Gerald. Keusch, Sustaining Global Surveillance and Response to Emerging Zoonotic Diseases.http://site.ebrary.com/id/10367632 [PubMed] [Google Scholar]
- 4.Wellcome Trust Zoonotic Disease: Explained, Wellcome Trust. 2022. https://wellcome.org/news/zoonotic-disease-explained
- 5.Welburn S.C., Beange I., Ducrotoy M.J., Okello A.L. The neglected zoonoses—the case for integrated control and advocacy. Clin. Microbiol. Infect. 2015;21:433–443. doi: 10.1016/j.cmi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Narrod C., Zinsstag J., Tiongco M. A one health framework for estimating the economic costs of zoonotic diseases on society. EcoHealth. 2012;9:150–162. doi: 10.1007/s10393-012-0747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diao X., Alpuerto V., Nwafor M. HPAI Res. Brief; 2009. Economy wide impact of avian flu in Nigeria–A dynamic CGE model analysis.https://www.ifpri.org/publication/economywide-impact-avian-flu-nigeria-%E2%80%93-dynamic-cge-model-analysis [Google Scholar]
- 8.Welburn S.C., Beange I., Ducrotoy M.J., Okello A.L. The neglected zoonoses—the case for integrated control and advocacy. Clin. Microbiol. Infect. 2015;21:433–443. doi: 10.1016/j.cmi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Weld E.D., Waitt C., Barnes K., Garcia Bournissen F. Twice neglected? Neglected diseases in neglected populations. Br. J. Clin. Pharmacol. 2022;88:367–373. doi: 10.1111/bcp.15148. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030, n.d. https://www.who.int/publications-detail-redirect/9789240010352 (accessed June 3, 2021)
- 11.Murray C.J. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull. World Health Organ. 1994;72:429. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2486718/pdf/bullwho00414-0105.pdf [PMC free article] [PubMed] [Google Scholar]
- 12.Devleesschauwer B., Havelaar A.H., Maertens De Noordhout C., Haagsma J.A., Praet N., Dorny P., Duchateau L., Torgerson P.R., Van Oyen H., Speybroeck N. Calculating disability-adjusted life years to quantify burden of disease. Int. J. Public Health. 2014;59 doi: 10.1007/s00038-014-0552-z. [DOI] [PubMed] [Google Scholar]
- 13.Hotez P.J., Alvarado M., Basáñez M.-G., Bolliger I., Bourne R., Boussinesq M., Brooker S.J., Brown A.S., Buckle G., Budke C.M., Carabin H., Coffeng L.E., Fèvre E.M., Fürst T., Halasa Y.A., Jasrasaria R., Johns N.E., Keiser J., King C.H., Lozano R., Murdoch M.E., O’Hanlon S., Pion S.D.S., Pullan R.L., Ramaiah K.D., Roberts T., Shepard D.S., Smith J.L., Stolk W.A., Undurraga E.A., Utzinger J., Wang M., Murray C.J.L., Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torgerson P.R., Rüegg S., Devleesschauwer B., Abela-Ridder B., Havelaar A.H., Shaw A.P., Rushton J., Speybroeck N. zDALY: an adjusted indicator to estimate the burden of zoonotic diseases. One Health. 2018;5:40–45. doi: 10.1016/j.onehlt.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centre for Disease Control and Prevention One Health Zoonotic Disease Prioritization Process Overview, n.d. https://www.cdc.gov/onehealth/what-we-do/zoonotic-disease-prioritization/completed-workshops.html
- 16.World Health Organization Joint External Evaluation, n.d. https://extranet.who.int/sph/jee
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Rayyan n.d. https://www.rayyan.ai/
- 19.R Core Team R: A Language and Environment for Statistical Computing. 2022. https://www.r-project.org/
- 20.South Andy. Rnaturalearthdata: World Vector Map Data from Natural Earth Used in ’rnaturalearth’_. R package version 0.1.0. 2017. https://CRAN.R-project.org/package=rnaturalearthdata
- 21.Draw.io Draw.io, (n.d.) http://www.diagrams.net
- 22.Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., Abbasi-Kangevari M., Abbastabar H., Abd-Allah F., Abdelalim A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Lier E.A., Havelaar A.H., Nanda A. The burden of infectious diseases in Europe: a pilot study, Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2007;12:E3–E4. doi: 10.2807/esm.12.12.00751-en. [DOI] [PubMed] [Google Scholar]
- 26.Havelaar A.H., de Wit M.A., van Koningsveld R., van Kempen E. Health burden in the Netherlands due to infection with thermophilic Campylobacter spp. Epidemiol. Infect. 2000;125:505–522. doi: 10.1017/s0950268800004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F., Rokni M.B., Zhou X.-N., Fèvre E.M., Sripa B., Gargouri N., Fürst T., Budke C.M., Carabin H., Kirk M.D., Angulo F.J., Havelaar A., de Silva N. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Havelaar A.H., Angulo F.J. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., Speybroeck N., Cawthorne A., Mathers C., Stein C., Angulo F.J., Devleesschauwer B. World Health Organization foodborne disease burden epidemiology reference group, World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Lier A., McDonald S.A., Bouwknegt M., Van Der Sande M., Bijkerk P., Van Benthem B., Hahne S., Van Der Hoek W., Van Pelt W., Heijne J., Van Den Broek I., De Coul E.O., Van Der Maas N., Brandsema P., Slump E., Knol M., Friesema I., Havelaar A., Kretzschmar M., Mangen M.-J., Wallinga J., De Melker H.E., Brooke J., Haagsma J., De Wit A., Mangen M.-J.J., Havelaar A.H., Kramer A., Pinheiro P., Plas D., Fevre E., Gibbons C., Franco E., Longhi S., Ricciardi W., De Waure C., Jahn B., Muhlberger N., Siebert U., Lai T., Matsi A., Ruutel K., Cassini A., Colzani E., Kramarz P., Erkens C., Swaan C., Achterberg P., Land J. Disease burden of 32 infectious diseases in the Netherlands, 2007-2011. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brooke R., Van Lier A., Donker G., Van der Hoek W., Kretzschmar M., Brooke R.J., Van Lier A., Donker G.A., Van der Hoek W., Kretzschmar M.E.E. Comparing the impact of two concurrent infectious disease outbreaks on The Netherlands population, 2009, using disability-adjusted life years. Epidemiol. Infect. 2014;142:2412–2421. doi: 10.1017/S0950268813003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangen M.-J.J., Plass D., Havelaar A.H., Gibbons C.L., Cassini A., Mühlberger N., van Lier A., Haagsma J.A., Brooke R.J., Lai T., de Waure C., Kramarz P., Kretzschmar M.E.E., BCoDE consortium The pathogen- and incidence-based DALY approach: an appropriate [corrected] methodology for estimating the burden of infectious diseases. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labeaud A.D., Bashir F., King C.H. Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Popul. Health Metrics. 2011;9:1. doi: 10.1186/1478-7954-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devleesschauwer B., Ale A., Torgerson P., Praet N., Maertens de Noordhout C., Pandey B.D., Pun S.B., Lake R., Vercruysse J., Joshi D.D., Havelaar A.H., Duchateau L., Dorny P., Speybroeck N. The burden of parasitic zoonoses in Nepal: a systematic review. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haagsma J.A., Siersema P.D., De Wit N.J., Havelaar A.H. Disease burden of post-infectious irritable bowel syndrome in The Netherlands. Epidemiol. Infect. 2010;138:1650–1656. doi: 10.1017/S0950268810000531. [DOI] [PubMed] [Google Scholar]
- 47.Naghavi M., Abolhassani F., Pourmalek F., Lakeh M., Jafari N., Vaseghi S., Mahdavi Hezaveh N., Kazemeini H. The burden of disease and injury in Iran 2003. Popul. Health Metrics. 2009;7:9. doi: 10.1186/1478-7954-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wielders C.C.H., van Lier E.A., van ’t Klooster T.M., van Gageldonk-Lafeber A.B., van den Wijngaard C.C., Haagsma J.A., Donker G.A., Meijer A., van der Hoek W., Lugnér A.K., Kretzschmar M.E.E., van der Sande M.A.B. The burden of 2009 pandemic influenza A(H1N1) in the Netherlands. Eur. J. Pub. Health. 2012;22:150–157. doi: 10.1093/eurpub/ckq187. [DOI] [PubMed] [Google Scholar]
- 49.Hilderink H., Plasmans M.H., Poos M., Eysink P.E., Gijsen R. Dutch DALYs, current and future burden of disease in the Netherlands. Arch. Public Health. 2020;78:1–10. doi: 10.1186/s13690-020-00461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization . World Health Organization; 2006. The Control of Neglected Zoonotic Diseases: A Route to Poverty Alleviation.https://www.who.int/publications/i/item/9789241594301 [Google Scholar]
- 51.World Health Organization Estimating the burden of foodborne diseases: a practical handbook for countries: a guide for planning, implementing and reporting country-level burden of foodborne disease. 2021. https://www.who.int/publications/i/item/9789240012264
- 53.World Health Organization . World Health Organization; 2015. The Control of Neglected Zoonotic Diseases: From Advocacy to Action: Report of the Fourth International Meeting Held at WHO Headquarters, Geneva, Switzerland, 19–20 November 2014.https://apps.who.int/iris/handle/10665/183458 [Google Scholar]
- 54.Noguera Zayas L.P., Rüegg S., Torgerson P. The burden of zoonoses in Paraguay: a systematic review. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization . World Health Organization; 2015. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015.https://apps.who.int/iris/handle/10665/199350 [Google Scholar]
- 56.World Health Organization FAO expert committee on zoonoses. World Health Organ. Tech. Rep. Ser. 1959;58:1–84. [PubMed] [Google Scholar]
- 57.Haider N., Rothman-Ostrow P., Osman A.Y., Arruda L.B., Macfarlane-Berry L., Elton L., Thomason M.J., Yeboah-Manu D., Ansumana R., Kapata N., Mboera L., Rushton J., McHugh T.D., Heymann D.L., Zumla A., Kock R.A. COVID-19—zoonosis or emerging infectious disease? Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.596944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfe N.D., Dunavan C.P., Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization, Zoonoses 2020. https://www.who.int/news-room/fact-sheets/detail/zoonoses (accessed July 6, 2023)
- 61.Gwee S.X.W., St John A.L., Gray G.C., Pang J. Animals as potential reservoirs for dengue transmission: a systematic review. One Health. 2021;12 doi: 10.1016/j.onehlt.2021.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haagsma J.A., Polinder S., Stein C.E., Havelaar A.H. Systematic review of foodborne burden of disease studies: quality assessment of data and methodology. Int. J. Food Microbiol. 2013;166:34–47. doi: 10.1016/j.ijfoodmicro.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 63.Gwee Sylvia Xiao Wei, St Ashley L., John Gregory C., Gray Junxiong Pang. Animals as potential reservoirs for dengue transmission: a systematic review. One Health. 2021;12 doi: 10.1016/j.onehlt.2021.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.