Significance
Estrogens very rapidly affect social memory formation and synaptic plasticity, but the mechanisms underlying these effects are unknown. Here, we explored mechanisms through which estradiol facilitates short-term social memory through actions in the dorsal hippocampus, a region involved in social memory formation, in female mice. We identified extracellular signal-regulated protein kinase (ERK) and phosphoinositide 3-kinase (PI3K) signaling as key mechanisms necessary for estradiol’s rapid facilitation of short-term social memory and increased glutamatergic synapse number. Remarkably, estradiol decreased glutamatergic synapse number in mice that did not perform the social memory task, with this reduction blocked by ERK antagonism only. This demonstrates previously unknown, bidirectional, rapid actions of estradiol on brain and behavior, underscoring the importance of estrogen signaling to social behavior.
Keywords: social behavior, steroid hormones, neuroplasticity
Abstract
Social memory is essential to the functioning of a social animal within a group. Estrogens can affect social memory too quickly for classical genomic mechanisms. Previously, 17β-estradiol (E2) rapidly facilitated short-term social memory and increased nascent synapse formation, these synapses being potentiated following neuronal activity. However, what mechanisms underlie and coordinate the rapid facilitation of social memory and synaptogenesis are unclear. Here, the necessity of extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K) signaling for rapid facilitation of short-term social memory and synaptogenesis was tested. Mice performed a short-term social memory task or were used as task-naïve controls. ERK and PI3K pathway inhibitors were infused intradorsal hippocampally 5 min before E2 infusion. Forty minutes following intrahippocampal E2 or vehicle administration, tissues were collected for quantification of glutamatergic synapse number in the CA1. Dorsal hippocampal E2 rapid facilitation of short-term social memory depended upon ERK and PI3K pathways. E2 increased glutamatergic synapse number (bassoon puncta positive for GluA1) in task-performing mice but decreased synapse number in task-naïve mice. Critically, ERK signaling was required for synapse formation/elimination in task-performing and task-naïve mice, whereas PI3K inhibition blocked synapse formation only in task-performing mice. While ERK and PI3K are both required for E2 facilitation of short-term social memory and synapse formation, only ERK is required for synapse elimination. This demonstrates previously unknown, bidirectional, rapid actions of E2 on brain and behavior and underscores the importance of estrogen signaling in the brain to social behavior.
To behave appropriately within their social groups, social species require specialized cognitive abilities. Perhaps paramount of these is social recognition – the ability to recognize a conspecific or distinguish between conspecifics (1, 2). Without social recognition, an animal can display maladaptive social behaviors due to inability to distinguish, for example, groupmates from intruders (3). Social behaviors, including social recognition, are processed through a “social brain network” including the medial extended amygdala, lateral septum, and certain hypothalamic nuclei (4, 5). Importantly, while not thought of as a classically “social” brain region, the dorsal hippocampus is essential to the formation of social memories via inputs from social brain regions, and its disruption results in impairments in social memory (6–9). While much is understood with regard to hippocampal contributions to nonsocial memory (particularly spatial memory) (10), the integration of social information that occurs within the hippocampus requires further investigation.
Being reproductively active has been linked with social memory. Female mice show improved performance on a social recognition task during proestrus, the high estrogen and progesterone phase of the estrous cycle when females are sexually receptive (11). Ovariectomy results in impairments in social memory that can be rescued through administration of estrogens (12, 13). Estrogens have long been known to elicit their effects by binding to intracellular estrogen receptors that then dimerize, translocate to the nucleus, and directly affect gene transcription and protein expression (14). In addition to these delayed “classical” effects, estrogens affect molecular (15, 16), cellular (17–19), systems (20, 21), and behavioral (22–26) processes very rapidly (minutes) through intracellular mechanisms including activation of cell signaling cascades, such as the extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K) cascades.
Previous research discovered and richly characterized rapid effects of estrogens on short-term (27–29) and long-term memory in various tasks, such as spontaneous object recognition and object placement spatial memory tasks (25), as well as social memory (7, 27–30). Given preacquisition in a 40-min social recognition task, systemic (27), intradorsal hippocampal (7, 28), and intramedial amygdalar (30) administration of 17β-estradiol (E2), the most abundant and bioactive estrogen in adult mammals, facilitates short-term social memory in ovariectomized (OVX) female mice. The same doses of E2 increase hippocampal dendritic spine density in task-naïve mice (7, 27) and in ex vivo hippocampal slices (28) within the same timeframe, consistent with other findings (19, 31, 32). However, the necessity of dendritic spine increases to behavior has been questioned (33). While an increase in dendritic spine number suggests an increase in synapse number, evidence indicates the relationship is more complex. For instance, frequency of AMPA miniature postsynaptic currents in ex vivo CA1 is reduced following the same treatment of E2, which increases CA1 dendritic spine density (28). Importantly, neuronal activity is needed for the formation of stable synapses from “silent” or nascent synapses (34, 35). Ergo, synapse formation by estrogens, and not dendritic spine formation alone, may be a more salient and parsimonious mechanism through which estrogens affect behavior. Synapse formation via priming by E2 and subsequent activation during learning may underlie the previously observed effects of E2 on short-term social memory.
Previous investigations into the mechanisms underlying the rapid effects of estrogens on memory have explored consolidation and long-term memory of nonsocial memories (25). However, short-term memory is necessary for the dynamic modulation of ongoing behaviors and needs to be processed in short periods of time (36). While some short-term memories will be consolidated into long-term memories, most information is not needed long-term, and the brain is selective in what memories are stored long-term. For instance, social information obtained during a party that adaptively modulates social responses for the duration of the party may become irrelevant in the days and weeks following. In view of their differing behavioral significance, it is perhaps unsurprising that the molecular underpinnings of short-term and long-term memory do not fully overlap (e.g., refs. 37 and 38, but see refs. 39 and 40 for discussion). The rapid and transient creation by estrogens of neuronal substrate for memory encoding provides a highly dynamic mechanism for short-term memory processing, especially in information-rich, dynamic environments such as social interactions.
Estrogen-induced increases in synapse density observed both in vivo (19) and in vitro (17, 34) suggest that presynaptic input to estrogen-treated neurons may be required for estrogen-mediated memory enhancements, as depicted in the concept of “two-step wiring plasticity”. This model posits that estrogens first transiently increase dendritic spine density, creating the neuronal substrate for learning to occur (34). Following activation (for instance, through memory task performance), these spines mature into active synapses (35). These estrogen-driven neurophysiological changes depend upon activity of cell signaling cascades, including ERK (16, 17, 34, 41) and PI3K (17, 41) pathways, which are also required for enhancement of object and spatial long-term memory consolidation by estrogens in female mice (32, 42–46). While ERK is known to be necessary for the rapid increase in CA1 dendritic spine density following E2 (32), the contributions of this kinase and the PI3K pathway to synapse formation in vivo are unknown. Additionally, two-step wiring plasticity suggests that the effects of estrogens on memory may depend upon activity (i.e., experience), leading to the hypothesis that learning events are drivers or modifiers of estrogen-mediated synaptic plasticity. Notably, recent ex vivo evidence suggests that E2-induced potentiation requires synaptic activity in female mice (47). However, whereas rapid effects of estrogens in dorsal hippocampus spine and synapse formation have been consistently reported (33), many underlying mechanisms remain unknown, especially in relation to their established beneficial roles in short-term social memory (7, 28).
The present investigation explores the cellular mechanisms involved in E2 rapid facilitation of short-term social memory. We investigate whether the ERK or PI3K cell signaling cascades are required for the facilitating effects of E2 in the dorsal hippocampus on short-term social memory. Additionally, we investigate interplay between the rapid effects of E2 and short-term social memory task performance on CA1 glutamatergic synapse formation.
Results
ERK and PI3K Inhibition in the Dorsal Hippocampus Blocked Short-Term Social Memory in a Dose-Dependent Manner.
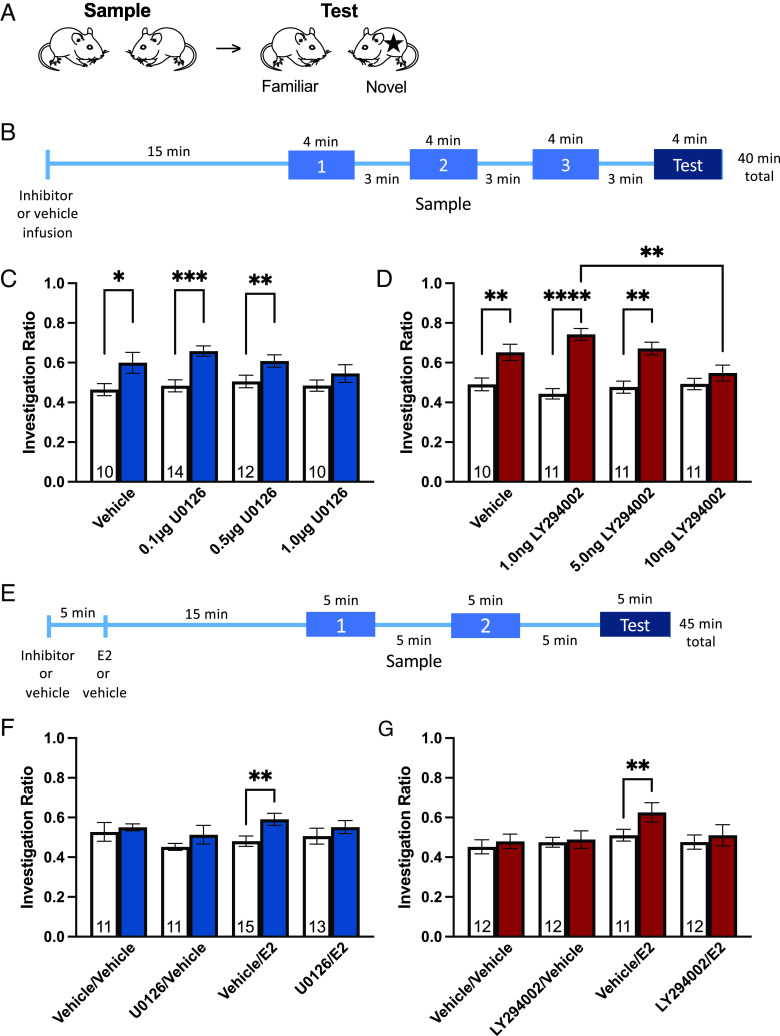
To evaluate the necessity of the cell signaling cascades to E2-facilitated short-term social memory, we first determined whether and at what doses intradorsal hippocampal ERK or PI3K inhibition blocked short-term social memory in an “easy” task where control animals can discriminate familiar from novel conspecifics. This task was designed such that vehicle-treated OVX CD1 mice show social memory by preferentially investigating a novel stimulus over a previously encountered stimulus at test (29). Mice were exposed to two novel OVX stimulus mice for three 4-min sample phases (Fig. 1B). Upon test, two social stimuli were reintroduced into the cage for a 4-min test phase: one novel stimulus and one previously encountered stimulus from the sample phases (Fig. 1B). This “easy” task is designed such that it can show impairing effects of treatment on social memory.
Fig. 1.
Rapid effects of 17β-estradiol on short-term social recognition are blocked by ERK and PI3K pathway inhibition. (A) Schematic of overall short-term social recognition testing. (B) Timeline of treatment and “easy” short-term social recognition testing. (C) Intradorsal hippocampal U0126 (ERK pathway inhibitor, 1.0 µg/side) blocks short-term social recognition memory. (D) Intradorsal hippocampal LY294002 (PI3K pathway inhibitor, 10 ng/side) blocks short-term social recognition memory. (E) Timeline of treatment and “difficult” short-term social recognition testing. (F) Intradorsal hippocampal U0126 blocks the facilitating effects of E2 on short-term social recognition memory. (G) Intradorsal hippocampal LY294002 blocks the facilitating effects of E2 on short-term social recognition memory. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. White bars indicate average sample phase investigation ratios, shaded bars indicate test phase investigation ratios. Sample size for each group depicted in white bars. Data presented as mean ± SEM.
In both experiments, our main model revealed significant main effects of paradigm session [i.e., sample and test; F(1, 42) = 37.41, η2 = 0.201, P < 0.0001, (Fig. 1C); F(1, 39) = 82.68, η2 = 0.375, P < 0.0001, (Fig. 1D)], but not treatment, with a significant session by treatment interaction in the LY294002 experiment [F(3, 39) = 6.79, η2 = 0.0922, P = 0.0009, (Fig. 1D)]. As indicated by a significant difference in planned comparisons between sample and test investigation ratios, vehicle-treated control mice (t = 2.56, df = 9, Cohen’s d = 0.991, P = 0.031), as well as 0.1 µg/side (t = 4.72, df = 14, Cohen’s d = 1.66, P = 0.0004) and 0.5 µg/side (t = 3.49, df = 12, Cohen’s d = 0.937, P = 0.0051) U0126-treated mice had significant short-term social memory, whereas 1.0 µg/side U0126 impaired short-term social memory [P = 0.0932, (Fig. 1C)]. Similarly, vehicle- (t = 4.64, df = 10, Cohen’s d = 1.40, P = 0.0012), 1.0 ng/side (t = 6.72, df = 10, Cohen’s d = 3.21, P < 0.0001), and 5.0 ng/side (t = 4.57, df = 10, Cohen’s d = 1.89, P = 0.001) LY294002-treated mice had significant short-term social memory, whereas 10 ng/side LY294002-treated mice did not (P = 0.118; main effect of session: F(1, 39) = 82.68, η2 = 0.375, P < 0.0001; session by treatment interaction: F(3, 39) = 6.79, η2 = 0.0922, P = 0.0009) (Fig. 1D). Additionally, there was a main effect of LY294002 treatment on IRTest (F(3, 39) = 5.21, η2 = 0.286, P = 0.004), with 10 ng/side significantly lower than 1.0 ng/side LY294002 (P = 0.002) (Fig. 1D). These effects are consistent with and comparable to effects seen on long-term object recognition memory (42, 43). Total social investigation time was unaffected between groups (SI Appendix, Figs. S1 and S2). Therefore, short-term social memory is impaired by either ERK or PI3K pathway inhibition at higher doses of pathway inhibitors, implicating these pathways as necessary for short-term social memory.
Dorsal Hippocampal ERK or PI3K Inhibition Block E2-Facilitated Short-Term Social Memory.
Having determined the doses at which ERK and PI3K pathway inhibitors block short-term social memory, we determined whether intradorsal hippocampal E2 requires ERK and/or PI3K signaling to facilitate short-term social memory. Therefore, the highest doses of U0126 (0.5 µg/side) and LY294002 (5 ng/side) that did not block social memory in the “easy” social recognition task were used in conjunction with E2 (6.81 pg/side), a dose known to facilitated short-term social memory in the ‘difficult’ task (7, 28). It should be noted that these doses are low compared to doses most often used in vivo (e.g., refs. 48–54) and equal to many studies that use similar experimental design (42, 44, 55, 56). This paradigm is similar to the “easy” version except that there are two 5-min sample phases and one 5-min test (Fig. 1E). The decreased number of exposures to the stimulus mice makes this task more difficult than the “easy” paradigm, and vehicle-treated OVX CD1 mice do not show short-term social memory, whereas those who receive treatments that facilitate memory for the social stimulus (e.g., E2) do (7, 27–30). This “difficult” task is designed such that it can show improving/facilitating effects of treatment on social memory. It cannot, however, demonstrate impairments.
In both experiments, our main model revealed significant main effects of paradigm session [Wilcoxon matched-pairs signed rank test: W = 617, Cohen’s d = 0.532, P = 0.0024 (Fig. 1F); main effect of session (F(1, 43) = 5.85, η2 = 0.0294, P = 0.020) (Fig. 1G)], but not treatment. A priori binary mean comparisons revealed E2-treated mice exhibited significant short-term social memory [Wilcoxon matched-pairs signed rank test: W = 102, n = 15, Cohen’s d = 1.01, P = 0.002 (Fig. 1F); t = 3.31, df = 11, Cohen’s d = 0.858, P = 0.0079 (Fig. 1G)], replicating previous findings (7, 28), whereas vehicle and inhibitor-only controls did not [ps > 0.261, (Fig. 1F); ps > 0.512, (Fig. 1G)]. Critically, the facilitating effects of E2 were blocked by ERK (P = 0.258) or PI3K inhibitor infusion (P = 0.42) (Fig. 1 F and G). E2-treated mice in the ERK-pathway experiment showed greater social investigation than vehicle controls [P = 0.0363; F(3, 46) = 3.05, η2 = 0.111, P = 0.0379) (SI Appendix, Fig. S2). However, we do not see a similar effect in the PI3K-pathway experiment (SI Appendix, Fig. S4) nor in previous investigations (7, 28). Furthermore, neither the E2- nor vehicle-treated groups differed from either U0126-treated group (ps > 0.102), suggesting that this increase in social investigation by E2 does not explain treatment effects on short-term social recognition memory. Overall, these data show that both the ERK and PI3K pathways are required for the rapid facilitation of short-term social memory by E2.
E2 Increased Glutamatergic Synapses in Task-Performing Mice in an ERK- and PI3K-Dependent Manner.
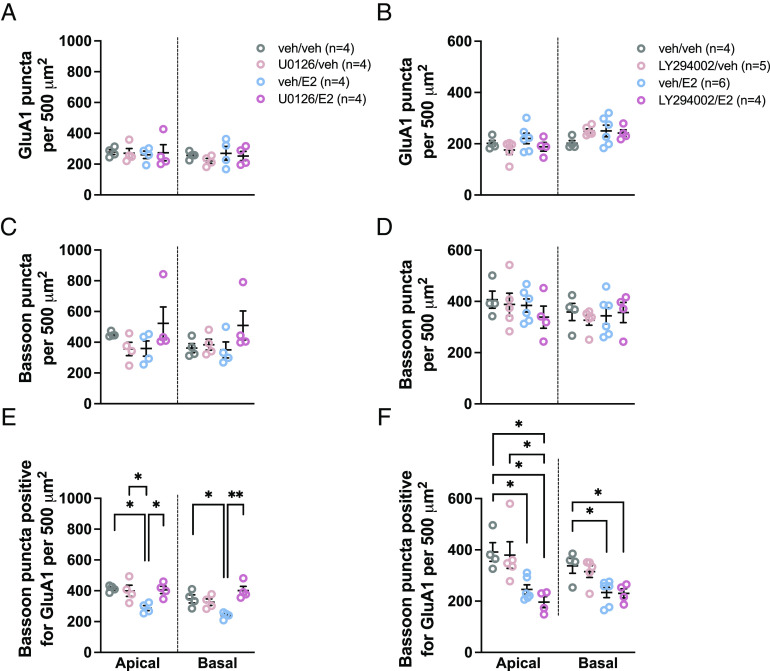
As E2 required both the ERK and PI3K pathways to facilitate social memory, and knowing that E2 rapidly inhibits glutamatergic transmission in CA1 dorsal hippocampal pyramidal neurons of task-naïve mice (28) we investigated the effect of dorsal hippocampal E2 with or without pretreatment with ERK or PI3K inhibitors on glutamatergic synapse formation following social recognition training. In these experiments, we defined a glutamatergic synapse as a presynaptic marker (bassoon) being positive for overlap with a postsynaptic maker (GluA1 subunit of AMPA receptors). E2 and/or inhibitor treatment did not affect GluA1 or bassoon expression in either apical or basal subfields in task-performing mice [no main effects of treatment, ps > 0.223 and ps > 0.0525, respectively (Figs. 2 C–F and 3 A–D)]. However, E2-treated mice had a higher density of synaptic puncta (bassoon puncta positive for GluA1) than vehicle-treated mice [apical – Cohen’s d = 1.74, P = 0.039, main effect of treatment (F(3, 19) = 5.00, η2 = 0.441, P = 0.0101]; basal – Cohen’s d = 2.08, P = 0.0476, main effect of treatment (F(3, 19) = 5.51, η2 = 0.461, P = 0.0073) (Fig. 2G); apical – Cohen’s d = 1.84, P = 0.0306, main effect of treatment (F(3, 18) = 6.53, η2 = 0.521, P = 0.0035); basal – Cohen’s d = 2.58, P = 0.0127, main effect of treatment (F(3, 18) = 7.59, η2 = 0.559, P = 0.0017) (Fig. 2H)]. The effect of E2 on synaptic puncta in trained animals was blocked by pretreatment with either U0126 (ps < 0.0132) or LY294002 (ps < 0.0175) (Fig. 2 G and H). Conversely, treatment with these inhibitors alone had no effect on synaptic puncta (U0126: ps > 0.993; LY294002: ps > 0.806) (Fig. 2 G and H). When E2-treated mice were pooled across experiments and their synaptic puncta densities compared to task performance, there were significant positive correlations between investigation time during the test phase and synapse puncta density (apical – r = 0.603, P = 0.0497; basal – r = 0.711, P = 0.030; SI Appendix, Table S2). No other meaningful or consistent correlations between task performance and synaptic puncta were observed (SI Appendix, Table S2). Together, these data show that E2 administration in task-performing mice induces a change in the number of bassoon puncta that overlapped with GluA1, suggesting an increase in glutamatergic synapse number, and that this effect requires ERK and PI3K signaling.
Fig. 2.
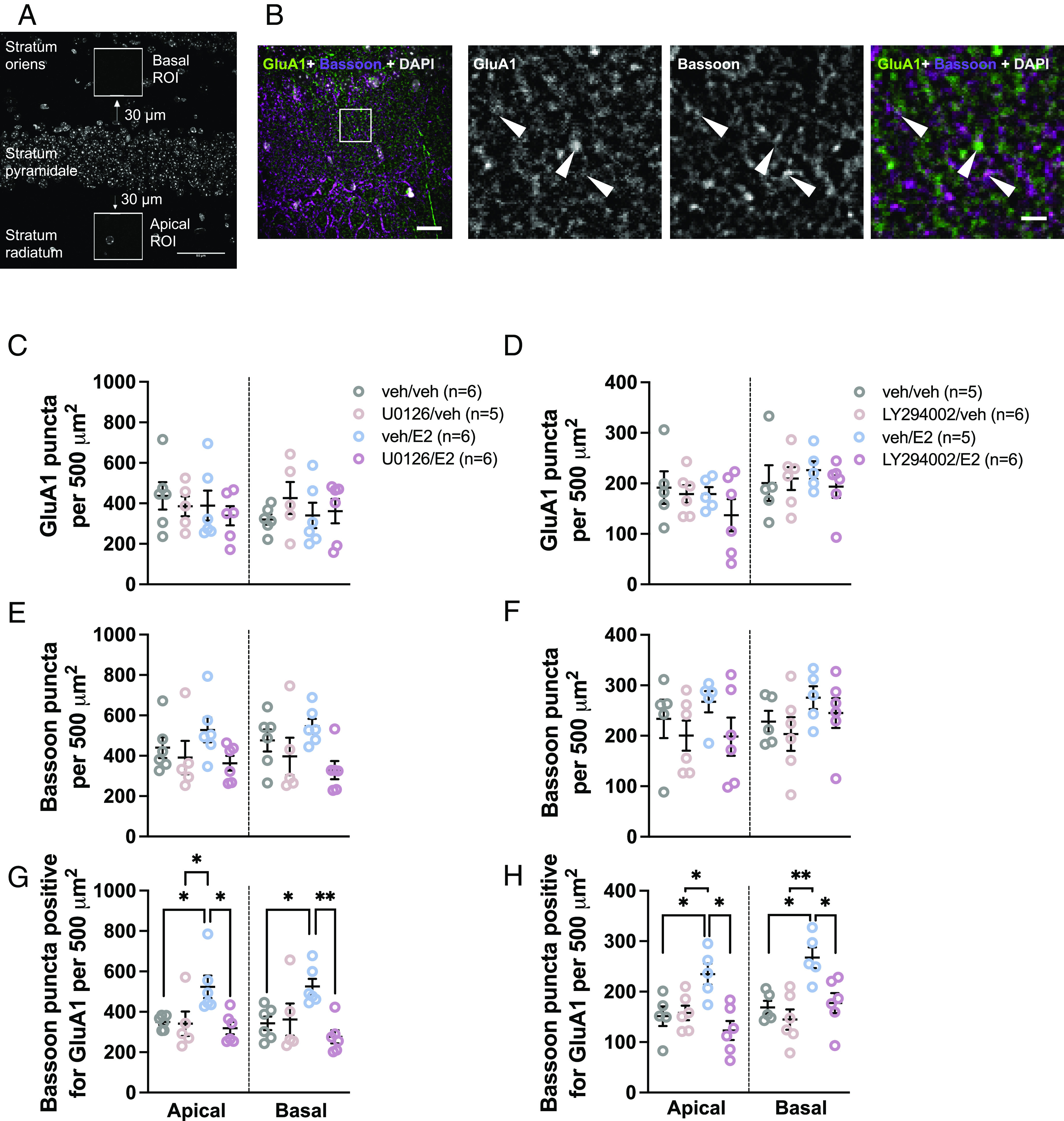
17β-estradiol rapidly increases glutamatergic synapse number in CA1 pyramidal neurons in short-term social recognition task-performing mice. (A) Schematic depicting apical and basal ROIs in dorsal CA1 slices. (B) Representative images of GluA1 puncta, bassoon puncta, and bassoon puncta positive for GluA1. (Scale bars, 20 µm Right and 5 µm Left.) (C and D) GluA1 puncta density is unaffected by intradorsal hippocampal E2 or inhibitor [U0126 (C) and LY294002 (D)] treatment. (E and F) Bassoon puncta density is unaffected by intradorsal hippocampal E2 or inhibitor [U0126 (E) and LY294002 (F)] treatment. (G) E2 increases glutamatergic synapse number in task-performing mice. ERK pathway inhibition blocks this effect. (H) E2 increases glutamatergic synapse number in task-performing mice. LY294002 pathway inhibition blocks this effect. *P < 0.05, **P < 0.01. Data presented as mean ± SEM.
Fig. 3.
17β-estradiol rapidly decreases glutamatergic synapse number in CA1 pyramidal neurons in task-naïve mice. (A and B) GluA1 puncta density is unaffected by intradorsal hippocampal E2 or inhibitor [U0126 (A) and LY294002 (B)] treatment. (C and D) Bassoon puncta density is unaffected by intradorsal hippocampal E2 or inhibitor [U0126 (C) and LY294002 (D)] treatment. (E) E2 decreases glutamatergic synapse number (bassoon puncta positive for GluA1) in task-naïve mice. ERK pathway inhibition blocks this effect. (F) E2 decreases glutamatergic synapse number in task-naïve mice. LY294002 pathway inhibition does not block this effect. *P < 0.05, **P < 0.01. Data presented as mean ± SEM.
E2 Decreased Glutamatergic Synapse Number in Task-Naïve Mice in an ERK-, but Not PI3K- Dependent Manner.
To evaluate whether task-performance affects the effects of E2 on number of synaptic puncta, task-naïve mice receiving the same treatments as task-performing mice were killed following the same delay but without performing the social recognition task. Analysis of the number of bassoon puncta positive for overlap with GluA1 in the CA1 of these mice revealed that E2 decreased synaptic puncta in both basal and apical dendrites of pyramidal neurons [apical – Cohen’s d = 4.67, P = 0.0121, main effect of treatment (F(3, 12) = 6.40, η2 = 0.616, P = 0.0078); basal – Cohen’s d = 2.70, P = 0.0233, main effect of treatment (F(3, 12) = 8.98, η2 = 0.692, P = 0.0022) (Fig. 3E); apical – Cohen’s d = 2.42, P = 0.0397, main effect of treatment (F(3, 15) = 7.40, η2 = 0.597, P = 0.0029); basal; Cohen’s d = 1.92, P = 0.0250, main effect of treatment (F(3, 15) = 5.70, η2 = 0.533, P = 0.0083) (Fig. 3F)]. This occurred without an effect on the total expression of either synaptic protein (apical – ps > 0.237, basal – ps > 0.208) (Fig. 3 A–D). This decrease in bassoon puncta positive for GluA1 was blocked by U0126 microinfusion prior to E2 (Cohen’s ds > 2.92, ps < 0.0183), but not by LY294002 (ps > 0.746) (Fig. 3 E and F). Synaptic puncta in mice that received LY294002 and E2 did not differ from the E2-only group, but was significantly reduced compared to vehicle controls (Cohen’s ds > 2.23, ps < 0.035) (Fig. 3F). This suggests that E2-induced changes in the number of bassoon puncta that overlapped with GluA1, indicating a decrease in glutamatergic synapse number, in task-naïve mice requires ERK, but not PI3K, signaling.
Discussion
Social cognition is essential for adaptive behaviors in social species for which rapid modification of behavior is integral to responding to dynamic changes in the social environment. Critical to this is the ability to recognize and distinguish between conspecifics. It is now recognized that estrogens act as potent modulators of brain and behavior, including social memory (7, 27, 28, 30), within minutes (22, 25). Recent evidence provides potential neuronal substrate for these effects: 17β-estradiol rapidly increases dendritic spine density (7, 27, 28), suggesting an increase in synapse formation, and modulates glutamatergic signaling (17, 28, 34), an effect that appears dependent upon synaptic plasticity. The exact molecular mechanisms of E2’s rapid effects on brain and behavior have yet to be established. As such, we asked the questions 1) what intracellular processes are required for E2 facilitation of social memory? 2) does E2 modulate synapse number in both social memory task-performing and task-naïve animals? and 3) do the same intracellular processes required for E2’s modulation of social memory also coordinate E2-mediated synapse formation? As predicted by the two-step wiring plasticity model, the present results demonstrate rapid and dynamic modulation of short-term social memory and hippocampal glutamatergic synapse number by 17β-estradiol is dependent upon ERK and PI3K signaling and task-performance.
ERK and PI3K pathways in the dorsal hippocampus were both found to be required for short-term social memory per se and its facilitation by E2, similarly to long-term object and spatial memory (32, 42–45). This finding suggests that similar intracellular mechanisms may be at play in short- and long-term memory, the parsimonious explanation for which being that the dorsal hippocampus, in both regards, is functioning as a crucial memory processing center. While often regarded as a hub of spatial memory formation (10), accumulating evidence implicates the dorsal hippocampus in the processing of social information in interplay with upstream and downstream regions of the social brain (6–9, 28), and here we show that the ERK and PI3K pathways are critical to social memory in this region. Interestingly, recent evidence suggests that the dorsal CA1 is not necessary for social memory, whereas the ventral CA1 and its connections to the nucleus accumbens are (57). Here, however, we demonstrate that E2 in the CA1 is sufficient to improve short-term social memory and rely on ERK and PI3K signaling.
Estrogens modulate social cognition (2, 58), including social memory. We have previously shown that systemic or intradorsal hippocampal administration of E2 rapidly facilitates short-term social memory and that these same treatments also increase dendritic spine density in CA1 pyramidal neurons of task-naïve mice (7, 27, 28). Here, we have elucidated key intracellular mechanisms underlying the rapid facilitation of short-term social memory by E2. ERK and PI3K were previously found to be rapidly activated by estrogens (16, 42, 43) and necessary for long-term memory enhancements by E2 (32, 42–44, 46). Our results suggest that these pathways are also necessary for the rapid enhancement of short-term social memory, thus underlying the adaptive dynamic modulation of social interactions by estrogens at times of changing social milieu. We cannot rule out the possibility that our doses of ERK and PI3K pathway inhibitors could impair social memory to such an extent that E2 would be unable to restore it. However, the low doses used herein did not impair social memory in the “easy” task nor did they on their own affect any measured parameter in the difficult task. Our results are further in line with studies using similar experimental approaches (and similar inhibitor doses) in long-term memory (42–45). Additionally, there is a convergence of data showing that the doses used in this study do not outright block plasticity. Our results that U0126 but not LY294002 was capable of blocking E2-induced changes in plasticity in task-naïve mice demonstrate that the dose of the inhibitor used here is insufficient to completely block plasticity. Furthermore, in studies using a similar design, western blot analyses demonstrate that doses of both inhibitors do not reduce the level of the phosphorylated signaling proteins below the baseline of a vehicle injected group (see e.g., refs. 42, 44, 55, and 56). Hence, the most parsimonious explanation for our results is that these pathways are necessary for rapid E2-facilitated short-term social memory.
Converging evidence suggests significant de novo production of E2 from testosterone plays a role in rapid modulation of E2 signaling in the hippocampus (59, 60). Importantly, forebrain-neuron-specific knockout of aromatase, the enzyme responsible for converting testosterone to E2, in mice resulted in hippocampal memory and plasticity deficits and reduction in long-term potentiation (LTP) amplitude (41). These effects were rescued by exogenous E2, with the exception being that LTP was not rescued when an ERK pathway inhibitor was coadministered (41). Localized estrogen synthesis thus appears to be an effective and rapid method by which short-term social memory could be modulated; however, its necessity has yet to be demonstrated.
While the rapid effects of E2 on short-term social memory and synapse formation occur within the same timeframe, the causal link previously remained elusive. Here, E2 in the dorsal hippocampus rapidly facilitated short-term social memory and increased glutamatergic synapse number [defined as the number of bassoon puncta (presynaptic marker) with positive overlap for GluA1 (postsynaptic marker)] in the same task-performing animals. Furthermore, when facilitation of social memory by E2 was blocked by ERK or PI3K pathway inhibition, so too was synapse formation. This suggests that glutamatergic synapse formation drives the rapid facilitating effects of E2 on social memory and does so in an ERK- and PI3K-dependent manner. Remarkably, in task-naïve animals, E2 decreased synaptic puncta – that is, in the absence of task-performance, E2 decreases glutamatergic synapse number. It is of note that owing to the diffraction limit of traditional light microscopes and the tightly packed nature of synapses in the hippocampus that the number of synapses could be greater than what we have measured. Nevertheless, consistent with our current findings, we have previously shown that E2 induced the formation of silent synapses (28) that can be potentiated by synaptic stimulation (34). There is a high degree of functional relevance to these findings. Memory formation requires formation of novel synapses, but unchecked synapse formation is likely maladaptive and would lead to interference between memory traces (61, 62). Here, we have shown E2 to reduce existing synapses in a paucity of stimulation (i.e., lack of task performance) and increase synapse number when a learning event occurs (i.e., performance of social recognition task), consistent with two-step wiring plasticity mechanism of estrogen action (35). This fine-tuning of estrogens’ rapid synaptic effects by task performance may sharpen the signal of relevant memory traces by increasing task relevant synapses and decreasing irrelevant synapses, thereby increasing the signal-to-noise ratio. Furthermore, the data in this study demonstrate that the bidirectional effects of synapse density require signaling through the ERK pathway – ERK inhibition blocked both E2-induced increases and decreases in GluA1/bassoon puncta in task-performing and task-naïve mice, respectively. Conversely, PI3K appears to have a more specific role – inhibition of this pathway blocked E2-mediated increases in synaptic puncta in task-performing mice but did not reverse E2’s ability to reduce synaptic density in task-naïve mice. While it remains possible that the reductions in synaptic puncta are a result of social isolation of the task-naïve mice during the testing window (when task-performing mice are exposed to conspecifics), that these reductions only occur within the E2-treated group suggests E2 involvement in this rapid neuroplastic event. Through these previously unknown bidirectional effects, E2 is capable of both providing plasticity and modifying its use.
Estrogens are potent neuromodulators in a variety of behaviors (23, 26, 63) and in learning and memory (22, 25, 64–66). Throughout the lifespan, estrogens allow for adaptive responses to the challenges brought on by a dynamic world (58), including dynamic social environments. Foundational to this are the rapid effects of E2 on short-term social memory and modulation of hippocampal glutamatergic synapse number described here. That there are bidirectional effects of E2 on synapse number dependent upon task-performance introduces a previously undescribed dynamic neuromodulation of brain and behavior by estrogens, which warrants further future investigation.
Materials and Methods
Detailed methods can be found in SI Appendix.
Subjects.
Young adult (2-mo-old), experimentally naïve female CD1 mice (Mus musculus) were used (Charles River, Kingston, NY, USA). Following surgeries, experimental mice were single-housed for 10 to 15 d prior to experiments. Stimulus mice were single-housed for 7 d postsurgery then pair-housed for a minimum of 7 d prior to participating in behavioral testing. All behavioral tests were run during the dark cycle (between 09:00 h and 19:00 h) in the experimental animals’ home cages under red light. All procedures were approved by the University of Guelph Animal Care and Use Committee and followed the guidelines of the Canadian Council on Animal Care.
Surgeries.
All mice were OVX to minimize gonadal hormone levels and fluctuations. Within the same surgical session, experimental mice were further implanted with bilateral guide cannulae directed at the dorsal hippocampus. Stimulus mice were OVX to ensure that their hormone status would not affect investigative behaviors by experimental mice.
Rapid Social-Recognition Paradigms.
Two rapid social-recognition paradigms of short-term social memory were used (Fig. 1A). The first, “easy” paradigm was designed such that vehicle-treated OVX mice show social recognition by preferentially investigating a novel over a previously encountered social stimulus at test (29). OVX mice receiving treatment that impairs social recognition will show no preference between the two stimuli. In this “easy” paradigm, experimental mice were exposed to two novel OVX stimulus mice for three 4-min sample phases (Fig. 1B). Sample phases were separated by 3-min rest periods in which no stimuli were present in the cage. After the final sample phase and 3-min memory retention period, two stimuli were reintroduced into the cage for a 4-min test phase: one novel stimulus and one previously encountered stimulus from the sample phases (Fig. 1B).
To show facilitating effects of treatment, a “difficult” rapid social recognition paradigm, similar to the “easy” version except that there are two 5-min sample phases and one 5-min test each separated by 5-min rests (Fig. 1E), was used (29). The decreased number of exposures to the stimulus mice makes this task more difficult than the “easy” paradigm and vehicle-treated OVX CD1 mice do not exhibit short-term social memory, whereas those who receive treatments that facilitate memory for the social stimulus (e.g. E2) do (7, 27–30, 67, 68).
Treatment Administration.
Effects of ERK or PI3K pathway inhibition on social recognition.
OVX mice were bilaterally microinfused (0.5 μL/side at 0.2 μL/min) with 0.1, 0.5, or 1.0 μg/side of MEK inhibitor 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126; Promega, Madison, WI); 0.5, 1.0, 5.0, or 10 ng/side of PI3K inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002; Santa Cruz Biotechnology, Dallas, TX); or vehicle (50% dimethyl sulfoxide in 0.9% NaCl solution) and then tested on the “easy” social recognition paradigm 15 min following the beginning of the infusion (Fig. 1B). These drugs and doses were chosen based on previous literature showing high efficacy in pathway inhibition (17, 42–44). In all experiments, infusers were left in place for an additional minute following each infusion to ensure the full dose was administered and to prevent back-flow.
Effects of ERK or PI3K pathway inhibition on estradiol-facilitated social recognition.
OVX mice were bilaterally microinfused first with 0.5 µg/side U0126, 5.0 ng/side LY294002, or vehicle (0.25 μL/side at 0.2 μL/min) 5 min before 6.81 pg/side 17β-estradiol (E2; Sigma-Aldrich, Oakville, ON, Canada) or vehicle (0.25 μL/side at 0.2 μL/min) and then tested on the “difficult” social recognition paradigm 15 min following the beginning of the infusion (Fig. 1E). This dose of E2 previously facilitated social recognition in OVX female mice in the “difficult” paradigm (7, 28). These doses of U0126 and LY294002 did not impair social recognition in OVX female mice in the “easy” paradigm (Fig. 1 C and D).
Effects of estradiol and cell signaling inhibition in task-naïve OVX mice.
OVX mice were bilaterally microinfused with the same treatments as in estradiol-facilitated social recognition experiments above (vehicle, 0.5 μg/side U0126, or 5.0 ng/side LY294002 followed by vehicle or 6.81 pg/side E2) and left undisturbed for 40 min before tissue collection to determine the effects of treatment on synapse number in the dorsal CA1 of task-naïve mice.
Immunohistochemistry, Confocal Imaging, and Analysis.
Coronally sectioned hippocampi from task-performing and task-naïve groups (4 to 6 mice/treatment, selected randomly from the overall cohort) were used for immunohistochemistry. Procedures and data collection were performed with experimenters blinded to treatment. Briefly, sections were permeabilized in PBS supplemented with 0.05% Triton-X100; blocked in 10% Normal Goat Serum, 1.5% BSA, 0.3% Triton-X100 in PBS. for 3 to 4 h > Primary antibodies: Rabbit-α-GluA1 (Sigma-Aldrich AB1504, 1:300) and Mouse-α-bassoon (Abcam ab82958, 1:200) were incubated overnight in blocking solution. Sections were then incubated in secondary antibody diluted in blocking solution (1:1,000 Goat-α-rabbit AlexaFluor488; 1:1,000 Goat-α-mouse AlexaFluor568) for 2 h, before coverslipping with mounting medium containing DAPI (Prolong Gold; Thermofisher). For antigen retrieval, sections were incubated for 10 to 15 min in 10mM sodium citrate (pH 6.2) at RT, followed by a 15-min incubation with 10mM sodium citrate (pH 6.2) at 78 °C before permeabilization.
Confocal images of the CA1 region were taken using an Inverted Spinning Disk confocal microscope (Nikon, Japan) and 60× oil immersion lens objective (NA 1.4). Exposure time was kept constant for the entire dataset. Images were acquired as a stack spanning 6 to 10 μm, at an interval of 0.3 μm. Three 3 noncontinuous slices were imaged and analyzed from each animal, with data averaged to a single datum for each animal. Synaptic puncta were analyzed in ImageJ (https://imagej.net/Welcome), using a previously published pipeline (69). Analysis of synaptic puncta was performed in the strata oriens and radiatum – corresponding to the basal and apical dendritic regions, respectively, of CA1 pyramidal neurons (Fig. 2A) and limited to 50 × 100 μm Region of interest (ROI) 20 µm either side of the stratum pyramidale to limit synaptic analysis to secondary and higher dendritic branching (Fig. 2A). following parameters described in ref. 69. In all sections, GluA1 or bassoon expression was first determined. Synaptic puncta were determined by first defining ROIs around bassoon puncta. Bassoon ROIs were then overlaid in the GluA1 channel and intensity of GluA1 staining measured. The presence of GluA1 was defined as bassoon ROIs containing GluA1 staining that was greater than background staining – calculated for each image by averaging the background intensity of five random background areas plus 2× SD. The number of puncta for each assessment [total GluA1, total bassoon, bassoon puncta positive for GluA1 (synaptic puncta)] area’s puncta were expressed as per unit area.
Behavior Data Analysis.
Videos were collected from all sample and test phases and analyzed (The Observer, Noldus Information Technology, Wageningen, The Netherlands) for both social (sniffing stimuli, digging/burying near stimuli, etc.) and nonsocial (horizontal movement, vertical noninvestigative behavior, grooming, etc.) behaviors (7, 27–30, 67, 68) (SI Appendix, Table S1). Active sniffing within 1 to 2 mm of a stimulus mouse-containing cylinder was considered social investigative behavior. An investigation ratio was calculated; IR = N/(N+F), in which N is the time spent investigating the novel (in sample phases, N is the stimulus that will be replaced) and F is the time spent investigating the familiar stimulus. Investigation ratios for sample phases were averaged for analysis.
Statistical Analysis.
The investigation ratios were analyzed with a mixed-design repeated measures ANOVA with treatment as the main factor and paradigm session (average sample and test) as the repeated-measure-dependent variable. When normality was violated, main effects were assessed using Kruskal–Wallis ANOVAs (treatment) and Wilcoxon matched-pairs signed rank tests (paradigm session). To reduce type I errors, specific mean comparisons were planned a priori to assess differences between IRSam and IRTest within each treatment group. One-way ANOVAs were used to assess treatment effects on IRTest, followed by Tukey post hoc tests. The durations of the other behaviors (SI Appendix, Table S1) were analyzed using a mixed-design repeated measures ANOVA with treatment as the main factor and paradigm session (each of the sample phases and test) as the repeated-measures-dependent variable, followed by Tukey post hoc tests. One-way ANOVAs with Tukey post hoc tests were used to determine effects of treatment and task performance on GluA1 and bassoon puncta and GluA1/bassoon colocalization. GraphPad Prism (v9.3.0) was used for all statistical analyses. Cohen’s d and eta-squared effect sizes are provided where appropriate. Two-tailed statistical significance was set at P < 0.05.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
Work performed at the University of Guelph was funded by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2018-04699 and RGPAS-522695-2018) and the Canada Foundation for Innovation (Grant No. 9585) to (E.C.). Work performed at King’s College London was supported by the Medical Research Council (MRC) Centre grant (MR/N026063/1). D.P.S. also acknowledges funding from the MRC (MR/L021064/1) and Royal Society UK (Grant RG130856). He is also a recipient of an Independent Investigators award from the Brain and Behavior Foundation (formally National Alliance for Research on Schizophrenia and Depression; Grant No. 25957). We thank Jenna Ashley and Marika Gummieny-Matsuo for help performing behavioral experiments and the Wohl Cellular Imaging Centre at the IoPPN, King’s College, London, for help with microscopy.
Author contributions
P.A.S.S., D.P.S., and E.C. designed research; P.A.S.S., D.C., A.L., D.V., and M.C.S.D. performed research; P.A.S.S., D.C., and D.P.S. analyzed data; and P.A.S.S., D.P.S., and E.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix, with full behavior and microscopy datasets available on Borealis: https://doi.org/10.5683/SP3/3U1ZFL (70).
Supporting Information
References
- 1.Choleris E., Kavaliers M., Pfaff D. W., Functional genomics of social recognition. J. Neuroendocrinol. 16, 383–389 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Sheppard P. A. S., Kuun T., Paletta P., Choleris E., “Who are you and what do you know? Estrogenic regulation of social recognition and social learning” in Estrogens and Memory: Basic Research and Clinical Implications (Oxford University Press, 2020), pp. 170–183. [Google Scholar]
- 3.Tumulty J. P., Sheehan M. J., What drives diversity in social recognition mechanisms? Front. Ecol. Evol. 7, 00517 (2020). [Google Scholar]
- 4.Newman S. W., The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 877, 242–257 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Prounis G. S., Ophir A. G., One cranium, two brains not yet introduced: Distinct but complimentary views of the social brain. Neurosci. Biobehav. Rev. 108, 231–245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maaswinkel H., Baars A. M., Gispen W. H., Spruijt B. M., Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol. Behav. 60, 55–63 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Sheppard P. A. S., et al. , Protein synthesis and actin polymerization in the rapid effects of 17β-estradiol on short-term social memory and dendritic spine dynamics in female mice. Psychoneuroendocrinology 128, 105232 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Kogan J. H., Frankland P. W., Silva A. J., Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Hitti F. L., Siegelbaum S. A., The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird C. M., Burgess N., The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 9, 182–194 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Andrade G., James B. M., Kendrick K. M., Neural encoding of olfactory recognition memory. J. Reprod. Dev. 51, 547–558 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Hlinák Z., Social recognition in ovariectomized and estradiol-treated female rats. Horm. Behav. 27, 159–166 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Spiteri T., Agmo A., Ovarian hormones modulate social recognition in female rats. Physiol. Behav. 98, 247–250 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Nilsson S., et al. , Mechanisms of estrogen action. Physiol. Rev. 81, 1535–1565 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Sellers K., Raval P., Srivastava D. P., Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front. Neuroendocrinol. 36, 72–89 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Sheppard P. A. S., Puri T. A., Galea L. A. M., Sex differences and estradiol effects in MAPK and Akt cell signaling across subregions of the hippocampus. Neuroendocrinology 112, 621–635 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Sellers K. J., et al. , Rapid modulation of synaptogenesis and spinogenesis by 17β-estradiol in primary cortical neurons. Front. Cell. Neurosci. 9, 137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avila J. A., et al. , Estradiol rapidly increases GluA2-mushroom spines and decreases GluA2-filopodia spines in hippocampus CA1. Hippocampus 27, 1224–1229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLusky N. J., Luine V. N., Hajszan T., Leranth C., The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146, 287–293 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Barth C., Villringer A., Sacher J., Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 9, 37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonn Eisinger K. R., Larson E. B., Boulware M. I., Thomas M. J., Mermelstein P. G., Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 133, 53–59 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paletta P., Sheppard P. A. S., Matta R., Ervin K. S. J., Choleris E., Rapid effects of estrogens on short-term memory: Possible mechanisms. Horm. Behav. 104, 88–99 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Laredo S. A., Villalon Landeros R., Trainor B. C., Rapid effects of estrogens on behavior environmental modulation and molecular mechanisms. Front. Neuroendocrinol. 35, 447–458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luine V., Serrano P., Frankfurt M., Rapid effects on memory consolidation and spine morphology by estradiol in female and male rodents. Horm. Behav. 104, 111–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taxier L. R., Gross K. S., Frick K. M., Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci. 21, 535–550 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornil C. A., Ball G. F., Balthazart J., The dual action of estrogens hypothesis. Trends Neurosci. 38, 408–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phan A., et al. , Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37, 2299–2309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan A., et al. , Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc. Natl. Acad. Sci. U.S.A. 112, 16018–16023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan A., Lancaster K. E., Armstrong J. N., MacLusky N. J., Choleris E., Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology 152, 1492–1502 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Lymer J. M., et al. , Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 89, 30–38 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Jacome L. F., et al. , Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 157, 1357–1362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuscher J. J., Luine V., Frankfurt M., Frick K. M., Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J. Neurosci. 36, 1483–1489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheppard P. A. S., Choleris E., Galea L. A. M., Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain 12, 22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava D. P., et al. , Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc. Natl. Acad. Sci. U. S. A. 105, 14650–14655 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava D. P., Two-step wiring plasticity–a mechanism for estrogen-induced rewiring of cortical circuits. J. Steroid Biochem. Mol. Biol. 131, 17–23 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Camina E., Güell F., The neuroanatomical, neurophysiological and psychological basis of memory: Current models and their origins. Front. Pharmacol. 8, 438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer D., et al. , Mice deficient for striatal vesicular acetylcholine transporter (VAChT) display impaired short-term but normal long-term object recognition memory. Behav. Brain Res. 311, 267–278 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Pereira L. M., et al. , Estradiol effect on short-term object memory under hypocholinergic condition. Brain Res. Bull. 140, 411–417 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Cowan N., Short-term memory based on activated long-term memory: A review in response to Norris (2017). Psychol. Bull. 145, 822–847 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norris D., Short-term memory and long-term memory are still different. Psychol. Bull. 143, 992–1009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y., et al. , Neuron-derived estrogen regulates synaptic plasticity and memory. J. Neurosci. 39, 2792–2809 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan L., et al. , Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci. 30, 4390–4400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez S. M., et al. , Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci. 28, 8660–8667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortress A. M., Fan L., Orr P. T., Zhao Z., Frick K. M., Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem. 20, 147–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koss W. A., Haertel J. M., Philippi S. M., Frick K. M., Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-estradiol. eNeuro 5, ENEURO.0267-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Z., Fan L., Frick K. M., Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 107, 5605–5610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain A., Woolley C. S., Mechanisms that underlie expression of estradiol-induced excitatory synaptic potentiation in the hippocampus differ between males and females. J. Neurosci. 43, 1298–1309 (2023). 10.1523/JNEUROSCI.2080-19.2023 (February 1, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sananbenesi F., Fischer A., Schrick C., Spiess J., Radulovic J., Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: A possible link between stress and fear memory. J. Neurosci. 23, 11436–11443 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly Á., Laroche S., Davis S., Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 23, 5354–5360 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., et al. , PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat. Neurosci. 8, 925–931 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Han X., et al. , Activation of TREM2 attenuates neuroinflammation via PI3K/Akt signaling pathway to improve postoperative cognitive dysfunction in mice. Neuropharmacology 219, 109231 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Fabbrin S. B., et al. , Spermidine-induced improvement of memory consolidation involves PI3K/Akt signaling pathway. Brain Res. Bull. 164, 208–213 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Roesler R., et al. , Phosphoinositide 3-kinase is required for bombesin-induced enhancement of fear memory consolidation in the hippocampus. Peptides 30, 1192–1196 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Lee J. L. C., Hynds R. E., Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus 23, 233–244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortress A. M., Heisler J. D., Frick K. M., The mTOR and canonical Wnt signaling pathways mediate the mnemonic effects of progesterone in the dorsal hippocampus. Hippocampus 25, 616–629 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Orr P. T., Rubin A. J., Fan L., Kent B. A., Frick K. M., The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm. Behav. 61, 487–495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okuyama T., Kitamura T., Roy D. S., Itohara S., Tonegawa S., Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Been L. E., Sheppard P. A. S., Galea L. A. M., Glasper E. R., Hormones and neuroplasticity: A lifetime of adaptive responses. Neurosci. Biobehav. Rev. 132, 679–690 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Fester L., Prange-Kiel J., Jarry H., Rune G. M., Estrogen synthesis in the hippocampus. Cell Tissue Res. 345, 285–294 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Spool J. A., Bergan J. F., Remage-Healey L., A neural circuit perspective on brain aromatase. Front. Neuroendocrinol. 65, 100973 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang G., et al. , Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83, 1131–1143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valnegri P., et al. , RNF8/UBC13 ubiquitin signaling suppresses synapse formation in the mammalian brain. Nat. Commun. 8, 1271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balthazart J., Ball G. F., Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 29, 241–249 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Frick K. M., Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav. 74, 4–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luine V. N., Estradiol and cognitive function: Past, present and future. Horm. Behav. 66, 602–618 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheppard P. A. S., Koss W. A., Frick K. M., Choleris E., Rapid actions of oestrogens and their receptors on memory acquisition and consolidation in females. J. Neuroendocrinol. 30, e12485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabor C., Lymer J., Phan A., Choleris E., Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol. Behav. 149, 53–60 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Lymer J., Robinson A., Winters B. D., Choleris E., Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology 77, 131–140 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Halff E. F., et al. , Effects of chronic exposure to haloperidol, olanzapine or lithium on SV2A and NLGN synaptic puncta in the rat frontal cortex. Behav. Brain Res. 405, 113203 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Sheppard P., Dataset for: Social memory in female mice is rapidly modulated by 17β-estradiol through ERK and Akt modulation of synapse formation. Borealis. 10.5683/SP3/3U1ZFL. Deposited 11 July 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix, with full behavior and microscopy datasets available on Borealis: https://doi.org/10.5683/SP3/3U1ZFL (70).