Abstract
We have characterized sera from healthy volunteers immunized with a monomeric recombinant gp120 (rgp120) derived from a CCR5/CXCR4 (R5X4)-using subtype B isolate of human immunodeficiency virus type (HIV-1), HIV-1W61D, in comparison to sera from long-term HIV-1-infected individuals, using homologous reagents. Sera from vaccinees and HIV-1 positive subjects had similar binding titers to native monomeric rgp120W61D and showed a similar titer of antibodies inhibiting the binding of soluble CD4 (sCD4) to rgp120W61D. However, extensive peptide binding studies showed that the overall pattern of recognition of vaccinee and HIV-1-positive sera is different, with vaccinee sera displaying a wider and more potent recognition of linear V1/V2 and V3 domain epitopes. Neutralization of homologous HIV-1W61D or heterologous HIV-1M2424/4 peripheral blood mononuclear cell (PBMC)-derived virus lines by vaccinee sera could be achieved, but only after adaptation of the viruses to T-cell lines and was quickly lost on readaptation to growth in PBMC. Sera from HIV-positive individuals were able to neutralize both PBMC-grown and T-cell line-adapted viruses. Interestingly, rgp120W61D was recognized by monoclonal antibodies previously shown to neutralize primary HIV-1 isolates. The use of very potent adjuvants and R5X4 rgp120 led to an antibody response equivalent in binding activity and inhibition of binding of sCD4 to gp120 to that of HIV-positive individuals but did not lead to the induction of antibodies capable of neutralizing PBMC-grown virus.
In the absence of confirmed immunological correlates of protection, vaccine strategies have thus far attempted to induce human immunodeficiency virus type 1 (HIV-1)-specific broadly neutralizing antibodies and cytotoxic T-lymphocyte activity (16, 29). However, despite the generation of high-titer neutralizing antibodies to T-cell line-adapted (TCLA) strains in human trials using recombinant Env constructs derived from the prototype strains MN, IIIB, and SF2, the neutralization of heterologous primary isolates on mitogen-activated peripheral blood mononuclear cells (PBMC) in vitro has not been demonstrated (19). Moreover, these approaches do not appear to provide unequivocal protection from acquisition of HIV-1 infection in vivo (5, 6, 11). Here we investigate the antibody repertoire of vaccinee sera following immunization of healthy seronegative volunteers with a monomeric recombinant envelope glycoprotein (rgp120) derived from a CCR5/CXCR4 (R5X4)-using subtype B HIV-1 isolate, HIV-1W61D.
Sera were collected from 30 healthy HIV-1-negative volunteers over an 18-month period. The vaccinations took place at weeks 0, 4, and 28, and 23 individuals completed the schedule. rgp120W61D (200 μg) was given with alum, QS21–MPL-A, or QS21–MPL-A–emulsion (SmithKline Beecham Biologicals, Rixensart, Belgium) as adjuvants. The serological and neutralization responses of individuals from this trial (MRC V001) are documented elsewhere (38). In summary, antibody binding titers to rgp120W61D, V3MN, V3W61D, and the soluble CD4 (sCD4)/rgp120IIIB binding site and neutralizing antibody titers to the heterologous HIV-1MN strain were maximal following the third immunization and of the same order of magnitude as that seen in natural infection. However, these immunized individuals did not elicit neutralizing antibodies to a range (n = 5) of PBMC-grown HIV-1 isolates, including the homologous isolate HIV-1W61D, when assayed on mitogen-activated PBMC. In this present study, we contrast the serological and neutralization responses of sera from nine of these immunized individuals (eight of whom completed the vaccination schedule), who were selected for high antibody binding titers to the Env epitopes listed above and high neutralizing antibody titers to the heterologous HIV-1MN strain, with a panel of sera from HIV-1-infected individuals.
Sera from 28 HIV-1-infected individuals were subdivided into two groups based on the ability to neutralize the PBMC-grown isolate, HIV-1W61D, on MT2 cells. Briefly, 50 μl of virus stock (diluted to 25 50% tissue culture infectious doses per 50 μl in RPMI 1640 medium [Gibco] supplemented with 10% fetal calf serum [Gibco] and antibiotics [Sigma, UK]) was preincubated with serial twofold dilutions (50 μl) of serum for 1 h at 37°C, before the addition of 2.5 × 104 (100 μl) uninfected MT2 cells. The reciprocal of the final dilution of serum to reduce the formation of syncytia by 90% (RNT90%) compared to that of control wells was scored after 5 to 7 days. Those which failed to neutralize the PBMC-grown HIV-1W61D isolate (RNT90% of <10; n = 19) were termed W61D-nonneutralizing, while those which neutralized the HIV-1W61D isolate (RNT90% range of 40 to 320; n = 9) were termed W61D-neutralizing. Sera from HIV-1-negative immunized individuals, though selected on the basis of their high neutralizing activities to the heterologous HIV-1MN virus grown on a T-cell line, failed to neutralize the HIV-1W61D isolate on MT2 cells (RNT90% of <10; n = 9) when the stock of the infectious virus was generated by growth on PBMC.
In the first instance, the ability of sera from vaccinees and HIV-1-infected individuals to bind rgp120W61D, a 22-mer V3W61D peptide (TRKGIHIGPGRAFYAARKIIGD; Peptide and Protein Research), and inhibit the binding of sCD4 to rgp120W61D was investigated (Table 1). Serological responses to the V3W61D peptide were performed essentially as previously described (9), except that serial, rather than fixed, serum dilutions were used. For quantification of anti-gp120 antibodies, rgp120W61D was captured onto D7324 (Aalto Bioreagents, Dublin, Ireland)-coated enzyme-linked immunosorbent assay (ELISA) plates and the antibody binding titer of immune sera or HIV-1 positive sera was then determined as for the V3 peptide ELISA (9). Quantification of antibodies to the sCD4/rgp120W61D-binding site was determined by inhibition of sCD4 binding to D7324-immobilized rgp120W61D, according to published methodologies (22), except that binding was visualized with peroxidase-conjugated goat anti-human immunoglobulin G IgG (Sigma) and OPD substrate (Dako).
TABLE 1.
HIV-1-specific antibody titers in sera from vaccinees and infected individuals
| Seraa | Neutralizing antibody titer to HIV-1W61Dc | Antibody binding titers tod:
|
||
|---|---|---|---|---|
| V3W61D | rgp120W61D | sCD4/ rgp120W61D BS | ||
| Vaccinees (n = 9) | ||||
| Median | <10 | 800 | 12,800 | 80 |
| Range | <10 | 800–6,400 | 6,400–25,600 | 40–320 |
| W61D-Nonneutral-izing (n = 19) | ||||
| Median | <10 | 400 | 25,600 | 160 |
| Range | <10 | <100–1,600 | 3,200–102,400 | <20–640 |
| P valueb | NSe | 0.0104 | NS | NS |
| W61D-Neutral-izing (n = 9) | ||||
| Median | 40 | 100 | 25,600 | 160 |
| Range | 40–320 | <100–400 | 3,200–51,200 | 80–640 |
| P value | 0.0004 | 0.0004 | NS | NS |
Sera from rgp120W61D-immunized individuals and HIV-1-infected individuals. The latter are subdivided as W61D-nonneutralizing or W61D-neutralizing, according to the neutralization of the PBMC-derived isolate, HIV-1W61D, when assayed on MT2 cells.
P values, generated by the nonparametric unpaired Mann-Whitney U test, refer to differences between the vaccinee sera and the appropriate neutralization group.
Titers are expressed as the reciprocal of the highest dilution of serum which inhibited the formation of syncytia by 90% relative to that of controls.
Data are presented as the median and range of end point antibody binding titers to the V3W61D loop and to monomeric rgp120 derived from HIV-1W61D and 50% inhibition titers to the sCD4 binding site on monomeric rgp120W61D.
NS, P value of >0.05.
End point titers to the V3W61D peptide were significantly higher in vaccinee sera compared with sera from HIV-1-infected individuals, which may be due to fact that the V3W61D peptide represents the homologous V3 loop for vaccinees but not for HIV-1-infected individuals. That antibody binding titers to a linear V3 peptide were not associated with the ability to neutralize the homologous PBMC-grown virus is likely to be due to the overwhelming evidence that the V3 domain is relatively occluded on the intact oligomer and that neutralization of such isolates by V3-specific antibodies is, at best, weak (3, 7, 24, 32, 37). Notably, there were no significant differences between sera from immunized or naturally infected individuals in terms of total rgp120W61D binding antibody or the ability to inhibit the binding of sCD4 to rgp120W61D. Antibodies which compete with the binding of sCD4 for gp120 or which compete with monoclonal antibodies (MAbs) which have been mapped to this domain are highly prevalent in sera from infected individuals (17, 23, 25). The presence of antibodies inhibiting the binding of sCD4 to gp120 in vaccinee sera is not incompatible with the observation that these sera do not neutralize primary isolates, since only some of the MAbs mapped to the CD4 binding domain are able to neutralize primary HIV-1 isolates (24). Indeed, neutralizing and nonneutralizing CD4 binding site-directed MAbs form a single competition group for monomeric gp120 binding (28). Thus, conformational epitopes linked to the induction of antibodies capable of neutralizing primary isolates could be absent on monomeric rgp120W61D.
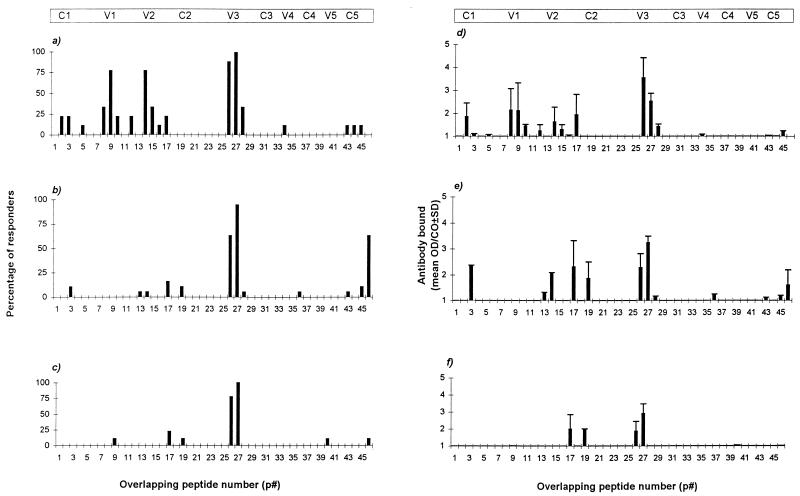
Next, we compared the ability of vaccinee sera and HIV-1-positive sera to bind a series of overlapping peptides (Fig. 1) spanning the C1 to C5 domains of gp120 from the HIV-1W61D isolate, using previously described methodologies (9). Peptides encompassing the V1/V2 and V3 domains were recognized by the majority of vaccinee sera (Fig. 2a). In contrast, while antibodies which bound the V3 domain peptides were found in the majority of sera from infected individuals, serological recognition of other linear peptides was poor (Fig. 2b and 2c). Of note, while there was a higher percentage of sera from these immunized individuals which bound peptides encompassing the V1 and V2 domains, the level of binding, represented as the mean optical density/assay cutoff (± standard deviation [SD]) of the positive responders, was similar to that observed with sera from HIV-1-infected individuals (Fig. 2d to f). Some recognition of the C1 and C5 domains was apparent in sera from both HIV-1-infected individuals and vaccinees, though as these domains are relatively inaccessible to MAb binding on the intact protein (26, 28), this is unlikely to be important in the context of primary isolate neutralization. Importantly, sera from the W61D-neutralizing group of individuals (Fig. 2c and 2f) bound few peptides outside the V3 domain, suggesting that the ability to neutralize the PBMC-grown HIV-1W61D isolate is not mediated by antibodies to linear epitopes. However, these differences in peptide recognition could be due to the fact that the W61D peptides represent homologous peptides for vaccinees but not for infected individuals.
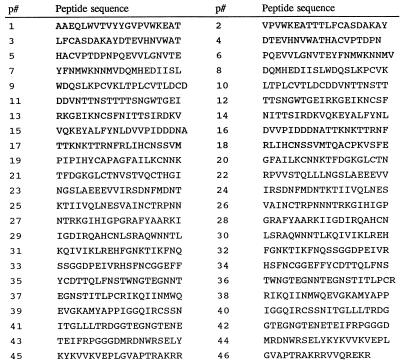
FIG. 1.
Amino acid sequences for a series of approximately 20-mer overlapping peptides spanning the C1 to C5 domains of gp120 derived from the isolate HIV-1W61D. The V1/V2 and V3 domains are located within peptides p9 to p18 and p25 to p29, respectively. Note that there was no available overlapping peptide to link the contiguous sequences represented by peptides p18-p19 and p21-p22.
FIG. 2.
Proportion and level of antibody binding to a series of overlapping peptides spanning the C1 to C5 domains of gp120 derived from the primary isolate HIV-1W61D. The percentage of sera samples which gave a positive reactivity (above the assay cutoff [CO] defined as the mean + 3 SD of a panel of HIV-1-negative serum controls) to each peptide is shown for vaccinee sera (a), W61D-nonneutralizing sera (b), or W61D-neutralizing sera (c). The level of antibody bound (expressed as the mean optical density [OD]/CO ± [SD for positively reactive sera) is shown for vaccinee sera (d), W61D-nonneutralizing sera (e), and W61D-neutralizing sera (f). Approximate positions of the conserved (C1 to C5) and variable (V1 to V5) domains are shown for clarity.
Direct sequencing of the PBMC-grown HIV-1W61D isolate (and the TCLA strain HIV-1W61D/SupT1) confirmed amino acid sequence identity between the virus-associated and linear peptide V1/V2 and V3 domains (data not shown). Several MAbs have been mapped to both linear and conformational epitopes within the V1/V2 domain (20, 27). The high proportion of vaccine sera with binding antibodies to the linear V1/V2 peptides compared to that of the HIV-1-positive sera, would appear to argue against V1/V2-specific antibodies playing a significant role in the neutralization of the PBMC-grown HIV-1W61D isolate. However, the presence of antibodies directed to conformational V1/V2 epitopes in the W61D-neutralizing sera, but not in the vaccinee or W61D-nonneutralizing sera, cannot be discounted.
Overall, these data appear to confirm and extend the observations of others (25, 36) that individuals immunized with monomeric gp120 predominantly elicit antibodies to linear epitopes in contrast to sera from infected individuals which predominantly contain antibodies to conformational epitopes and, where linear epitopes are recognized by sera from such individuals, these are directed against the V3 domain.
Following on from reports demonstrating that the propagation of PBMC-derived viruses in immortalized T-cell lines results in an increased neutralization sensitivity to antibodies generated by subunit immunization or natural infection (31, 39), we sought to compare the ability of vaccinee sera to neutralize the homologous HIV-1W61D isolate and a heterologous subtype B R5X4 HIV-1 isolate, HIV-1M2424/4 (3), before and after T-cell line adaptation (Table 2). Briefly, the HIV-1M2424/4 isolate was adapted by a series of stepwise acute infections of H9 cells and after nine such passages, readapted to growth in PBMC for a further five passages, while the HIV-1W61D isolate was adapted to growth in SupT1 cells after multiple passages (designated HIV-1W61D/SupT1). Neutralization of the PBMC-derived isolates and TCLA strains was performed in parallel on MT2 cells, as described above. Utilization of the HIV-1 coreceptors CCR5 and CXCR4 was determined by immunocytochemical staining of U87 cells stably expressing human CD4 and human CCR5 or CXCR4 4 to 5 days postchallenge, as described previously (12).
TABLE 2.
Neutralization of HIV-1 isolates on MT2 cells following passage in PBMC and T-cell lines
| MAbs, sCD4, and sera | Neutralizing antibody titer of indicated isolate toa:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| M2424/4
|
W61D
|
|||||||
| PBMC(p0)
|
H9(p1)
|
H9(p3)
|
H9(p9)
|
PBMC(p3)
|
PBMC(p5)
|
PBMC
|
SupT1
|
|
| R5X4 | ND | ND | R5X4 | ND | R5X4 | R5X4 | R5X4 | |
| MAbs and sCD4 | ||||||||
| 2F5 | 100 | >100 | 100 | 25 | 12.5 | 25 | >50 | 3.125 |
| 2G12 | >100 | 50 | 25 | 12.5 | 100 | 50 | 12.5 | 6.25 |
| 447-52D | 50 | 25 | 3.125 | 1.56 | 3.125 | 6.25 | >25 | 0.195 |
| sCD4 | 10 | 5 | 1.25 | 0.625 | 1.25 | 5 | ND | ND |
| IgG1b12 | ND | ND | ND | ND | ND | ND | >50 | 6.25 |
| HIV-1 positive sera | ||||||||
| 052 | 160 | 160 | 160 | 160 | 80 | 160 | 320 | 320 |
| 134 | 20 | 40 | 80 | 160 | 80 | 80 | ND | ND |
| FDA#2 | 20 | 20 | 40 | 80 | 40 | 40 | 10 | 320 |
| Vaccinee sera | ||||||||
| F55134 | — | — | 10 | 40 | — | — | — | 80 |
| F53860 | — | — | 10 | 10 | — | — | — | 40 |
| F53863 | — | — | — | 10 | — | — | — | 80 |
| M54662 | — | — | 10 | 20 | — | — | — | 80 |
| F53866 | — | — | 10 | 20 | — | — | — | 80 |
PBMC(p0) represents the primary HIV-1 isolate of M2424/4; H9(p1), H9(p3), and H9(p9) are the viral stocks obtained following the stated number of passages in H9 cells; PBMC(p3) and PBMC(p5) are virus stocks obtained following three or five passages of the H9(p9) stock in PBMC. The W61D virus stocks were obtained from PBMC or following multiple passages in SupT1 cells. Neutralization titers are shown as the reciprocal of the highest dilution of sera or the lowest concentration of MAb or sCD4 to inhibit the formation of syncytia in MT2 cells by 90%, and where no neutralization was observed with the highest concentration of MAb used, this is shown by >. R5X4 represents the ability to replicate in both U87-CD4-CCR5 and U87-CD4-CXCR4 cells. ND, Not done. —, neutralizing titer of <10.
In accordance with results of other studies (19, 39), neutralization sensitivity to vaccinee sera was only demonstrated in the T-cell line-grown viruses and, in the case of HIV-1M2424/4, was subsequently lost following readaptation to PBMC. Thus, neutralization sensitivity appeared to be determined by the cells on which the virus was amplified, rather than the cell substrate upon which neutralization was assayed. This acquired neutralization sensitivity also extended, to a greater or lesser extent, to diverse Env-specific MAbs and sera from HIV-1-infected individuals. Indeed, while the TCLA strains demonstrated an enhanced sensitivity to MAbs directed to diverse Env epitopes, by far the largest increase in susceptibility was to the V3-specific MAb, 447-52D, with a 32-fold increase between the HIV-1M2424/PBMC(p0) and HIV-1M2424/H9(p9) isolates and a >128-fold increase between the HIV-1W61D/PBMC and HIV-1W61D/SupT1 isolates. A similar but less striking increase in neutralization sensitivity was demonstrated with sCD4 and the CD4 binding site-directed MAb, IgG1b12. Thus, it is likely that the neutralization sensitivity of the TCLA strains to vaccine sera observed here is due, at least in part, to an increased exposure of the V3 domain and the CD4 binding site, especially as the V3 loop sequences of the PBMC-grown and T-cell line-grown isolates were identical throughout their passage history (data not shown). However, the contribution of differentially expressed host cell-acquired adhesion molecules (1, 2) on the surfaces of PBMC-grown and T cell line-grown viruses to this observed neutralization sensitivity, especially between sera from vaccinees and that of naturally infected individuals, cannot be excluded.
In addition, the ability of these HIV-1 isolates to utilize both CCR5 and CXCR4 coreceptors (designated R5X4 viruses [4]) was retained throughout their passage history, confirming recent observations that sensitivity to antibody-mediated neutralization of HIV-1 strains is independent of coreceptor usage (18, 21, 33).
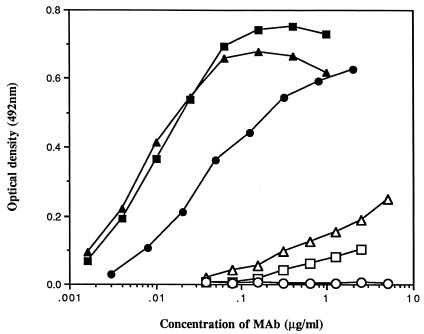
Finally, the ability of diverse MAbs to bind the monomeric rgp120W61D was investigated (Fig. 3) by following published methodologies (24, 25, 34), except that binding was visualized with peroxidase-conjugated goat anti-human IgG and OPD substrate. Three V3-specific MAbs (257-D, 268-D (15), and 447-52D (10)) bound rgp120W61D with estimated 50% maximal binding values of 0.006, 0.01, and 0.04 μg/ml, respectively. However, the finding that these MAbs only neutralized the homologous isolate following T-cell line adaptation (Table 2 and data not shown), despite potent antibody binding, only further underscores the dichotomy in epitope exposure between PBMC-grown and T-cell line-passaged strains. Importantly, two MAbs (IgG1b12 and 2G12 [8, 13, 35]) which recognize conformational/discontinuous epitopes and have been reported to efficiently neutralize diverse primary isolates did bind the monomeric rgp120W61D, albeit less potently than the V3-specific MAbs. Thus, their antibody binding epitopes are at least present on this protein and, as both the HIV-1M2424/4 and HIV-1W61D PBMC-grown isolates are relatively insensitive to neutralization by these two MAbs (Table 2), the presence of a low level of such antibodies in these vaccinee sera cannot be discounted. However, given that a disparity between antibody reactivity to monomeric gp120 and neutralization of the homologous isolate has been reported for these latter two MAbs (34) and the recent evidence that it is MAb reactivity to oligomeric, rather than monomeric, gp120 that is predictive of HIV-1 neutralization (14, 30), this appears to be unlikely.
FIG. 3.
Binding of human MAbs to immobilized monomeric rgp120 derived from the isolate HIV-1W61D. 447-52D (■), 257-D (▴), and 268-D (•) are directed to the V3 domain, while IgG1b12 (▵), 2G12 (□), and 2F5 (○) are directed to the CD4-binding domain, a discontinuous epitope on gp120 and a linear epitope in gp41, respectively.
In summary, we have shown that immunization of healthy volunteers with a monomeric rgp120 derived from an R5X4 subtype B HIV-1 isolate formulated in potent adjuvants elicits antibodies to a range of linear epitopes and, despite the generation of equivalent levels of antibodies to the sCD4/ gp120W61D binding site and monomeric rgp120W61D as those found in sera from HIV-1-infected individuals, these vaccinee sera do not neutralize PBMC-grown HIV-1 isolates in vitro. Neutralization could only be demonstrated following viral adaptation to immortalized T-cell lines and was quickly lost upon readaptation to PBMC. These results indicate that monomeric rgp120s per se are unlikely to be of use in the strategic development of a therapeutic HIV vaccine.
Acknowledgments
We thank the SmithKline Beecham Biologicals HIV Vaccine Project Team for production and characterization of the vaccine immunogen. We are also grateful to the V001 Steering Committee, who were responsible for the management of the vaccine trial, for advice and permission to conduct this study. We thank P. Easterbrook and M. Troop (Chelsea and Westminster Hospital, London, United Kingdom) for providing HIV-1-positive serum samples, D. Littman (Howard Hughes Medical Institute, New York University, New York, N.Y.) for providing the U87-CD4-CCR5 and U87-CD4-CXCR4 cells, S. Zolla-Pazner (New York University Medical Center, New York, N.Y.) for MAb 447-52D, and H. Holmes (Medical Research Council AIDS Reagent Programme, Potters Bar, United Kingdom) for the series of overlapping gp120 peptides and other reagents.
This work was supported by the Medical Research Council and the Wellcome Trust.
REFERENCES
- 1.Arthur L O, Bess J W, Jr, Sowder II R C, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 2.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Easterbrook P J, Simmonds P, Weber J. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol. 1998;79:77–82. doi: 10.1099/0022-1317-79-1-77. [DOI] [PubMed] [Google Scholar]
- 4.Berger E A, Doms R W, Fenyö E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 5.Berman P W, Gray A M, Wrin T, Vannari J C, Eastman D J, Nakamura G R, Francis D P, Gorse G, Schwartz D H. Genetic and immunologic characterization of viruses infecting MN-rgp120-vaccinated volunteers. J Infect Dis. 1997;176:384–397. doi: 10.1086/514055. [DOI] [PubMed] [Google Scholar]
- 6.Bolognesi D P, Matthews T J. Viral envelope fails to deliver? Nature. 1998;391:638–639. doi: 10.1038/35504. [DOI] [PubMed] [Google Scholar]
- 7.Bou Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W, Sawyer L S, Hendry R M, Dunlop N, Nara P L, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 9.Cheingsong-Popov R, Lister S, Callow D, Kaleebu P, Beddows S, Weber J. Serotyping HIV type 1 by antibody binding to the V3 loop: relationship to viral genotype. WHO Network for HIV Isolation and Characterization. AIDS Res Hum Retroviruses. 1994;10:1379–1386. doi: 10.1089/aid.1994.10.1379. [DOI] [PubMed] [Google Scholar]
- 10.Conley A J, Gorny M K, Kessler II J A, Boots L J, Ossorio Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor R I, Korber B T M, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmar M T, Simmons G, Hibbitts S, O’Hare M, Louisirirotchanakul S, Beddows S, Weber J, Clapham P R, Weiss R A. Langerhans cell tropism of human immunodeficiency virus type 1 subtype A through F isolates derived from different transmission groups. J Virol. 1997;71:8008–8013. doi: 10.1128/jvi.71.10.8008-8013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza M P, Livnat D, Bradac J A, Bridges S H the AIDS Clinical Trials Group Antibody Selection Working Group; Collaborating Investigators. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 14.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorny M K, Xu J Y, Gianakakos V, Karwowska S, Williams C, Sheppard H W, Hanson C V, Zolla-Pazner S. Production of site selected neutralizing human monoclonal antibodies against the third variable domain of HIV-1 envelope glycoprotein. Proc Natl Acad Sci USA. 1991;88:3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes B F. Scientific and social issues of human immunodeficiency virus vaccine development. Science. 1993;260:1279–1286. doi: 10.1126/science.8493572. [DOI] [PubMed] [Google Scholar]
- 17.Ho D D, McKeating J A, Ling Li X, Moudgil T, Daar E S, Sun N, Robinson J E. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol. 1991;65:489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Nunberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S NIAID AIDS Vaccine Evaluation Group. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 20.McKeating J A, Shotton C, Cordell J, Graham S, Balfe P, Sullivan N, Charles M, Page M, Bolmstedt A, Olofsson S, Kayman S C, Wu Z, Pinter A, Dean C, Sodroski J, Weiss R A. Characterization of neutralizing monoclonal antibodies to linear and conformational-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1993;67:4932–4944. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore J P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbant assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990;4:297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore J P, Sattentau Q J, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon S, Fung M S, Traincard F, Pinkus M, Robey G, Robinson J E, Ho D D, Sodroski J. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J Virol. 1993;67:6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salk J, Bretscher P A, Salk P L, Clerici M, Shearer G M. A strategy for prophylactic vaccination against HIV. Science. 1993;260:1270–1272. doi: 10.1126/science.8098553. [DOI] [PubMed] [Google Scholar]
- 30.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawyer L S W, Wrin M T, Crawford-Miksza L, Potts B, Wu Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatatos L, Cheng-Mayer C. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J Virol. 1995;69:6191–6198. doi: 10.1128/jvi.69.10.6191-6198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trkola A, Ketas T, Kewalramani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanCott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 37.VanCott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 38.Weber, J., and the MRC V001 Study. A comparative trial of rgp120 with novel adjuvants, p. 81–85. In M. Girard and B. Dodet (ed.), Onzième Colloque Des Cent Gardes: Retroviruses of human AIDS and related animal diseases, 27–29 October 1997. Marnes-la-Coquette, Paris, France.
- 39.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]