Abstract
We present the case of a 71-year-old man who experienced congestive cardiac failure after transcatheter aortic valve replacement with a balloon-expandable transcatheter heart valve. Echocardiography and cardiac computed tomography demonstrated an aorto-right ventricular fistula, and successful percutaneous closure was performed with a vascular plug. (Level of Difficulty: Advanced.)
Key Words: aortic valve, computed tomography, echocardiography
Central Illustration
A 71-year-old man was transferred to our institution with heart failure after transcatheter aortic valve replacement (TAVR) with a balloon-expandable transcatheter heart valve (THV). The patient had severe aortic stenosis (mean aortic valve gradient 46 mm Hg) with preserved left ventricular systolic function and had reported NYHA functional class II symptoms. Preprocedural cardiac computed tomography (CT) demonstrated a heavily calcified trileaflet aortic valve (Figure 1). The aortic annulus area was 557 mm2.
Learning Objectives
-
•
To recognize the clinical presentation of aorto-right ventricular fistula so that this rare complication of transcatheter aortic valve replacement can be appropriately managed
-
•
To define the role that multimodality images plays in preprocedural planning, peri-procedural device deployment and post-procedural follow-up to ensure an optimal patient outcome
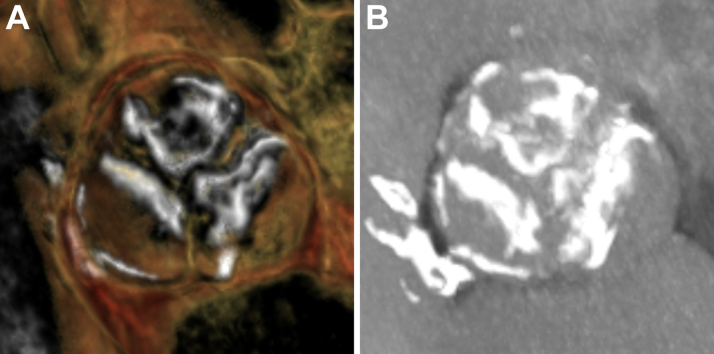
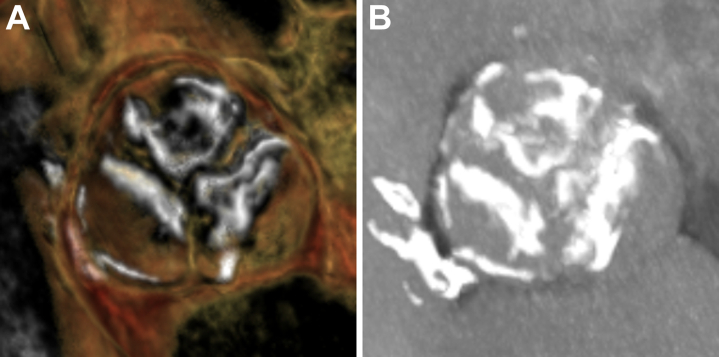
Figure 1.
Preprocedural Cardiac Computed Tomography Imaging
(A) 3-dimensional reconstructions and (B) maximum intensity projection imaging demonstrated a heavily calcified trileaflet aortic valve.
The patient underwent transfemoral TAVR with a 29-mm SAPIEN 3 THV (Edwards Lifesciences) with a nominal (33-mL) inflation volume, representing 19% oversizing by area. The valve implantation depth was approximately 70% aortic / 30% ventricular (Figure 2, Video 1). The procedure was complicated by completed heart block, and a dual-chamber permanent pacemaker was inserted.
Figure 2.
Transcatheter Aortic Valve Replacement Procedural Imaging
(A) Cineangiography revealed a heavily calcified aortic valve. (B) Final valve implantation depth.
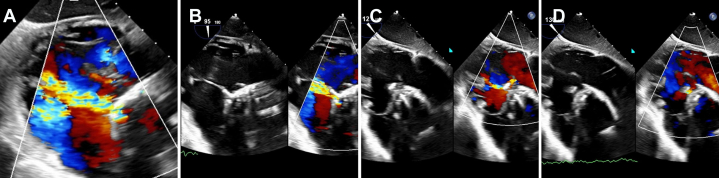
After the procedure, the patient reported worsening dyspnea. The result of clinical examination was notable for a grade 4/6 continuous cardiac murmur in the aortic region. Transthoracic echocardiography was performed the day after the procedure, demonstrating an aorto-right ventricular fistula with continuous left-to-right color Doppler flow and a peak gradient of 83 mm Hg (Figure 3, Video 2). Right ventricular size was moderately dilated with preserved systolic function, and there was moderate tricuspid regurgitation with an estimated right ventricular systolic pressure of 41 mm Hg.
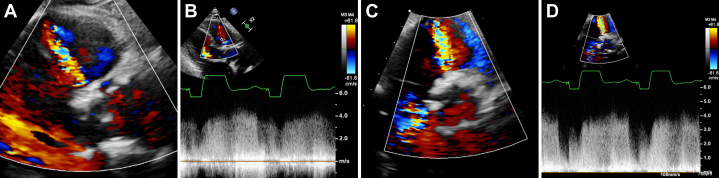
Figure 3.
Preprocedural Transthoracic Echocardiographic Imaging
(A, B) Parasternal long-axis imaging demonstrated an aorto-right ventricular fistula. (C, D) These findings were confirmed on short-axis imaging.
Medical History
The patient’s previous medical history included esophageal cancer, which had been treated with an esophagectomy. The patient had not received any prior chest radiotherapy or chemotherapy.
Differential Diagnosis
The differential diagnosis of this condition might include a doubly committed subarterial (supracristal) ventricular septal defect, which would be associated with a systolic murmur and systolic flow on Doppler assessment. Right ventricular enlargement was noted, and this can be associated with a left ventricular to right atrial communication (Gerbode defect). An aorto-right atrial fistula might also present with a continuous cardiac murmur and right ventricular volume overload. Significant paravalvular regurgitation might also present with heart failure symptoms. Damage to the tricuspid valve from a temporary pacing wire may also lead to significant tricuspid regurgitation and associated right ventricular volume overload.
Investigations
The patient underwent right heart catheterization, which demonstrated a saturation step-up within the right ventricle and a Qp:Qs of 1.63.
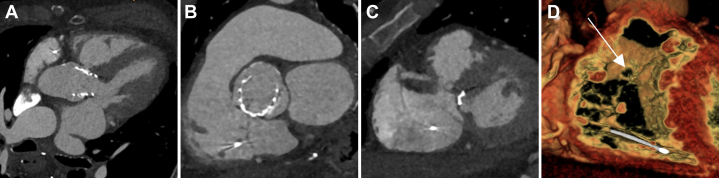
Cardiac CT revealed a defect in the aortic root connecting to the right ventricle, measuring 6.5 × 4 mm in width and 2 mm in depth, located a minimum of 3 mm above the aortic annulus (Figure 4).
Figure 4.
Preprocedural Cardiac Computed Tomography Imaging
(A to C) The defect was assessed in multiplanar and (D) 3-dimensional reconstructions.
A hemolysis screen was negative. Inflammatory markers did not suggest endocarditis.
Management
The case was discussed in the Heart Team meeting, and it was recommended that a transcatheter approach should be attempted in the first instance, because cardiac surgery, although feasible, could be challenging in the presence of a prior esophagectomy.
With the patient under general anesthesia, transesophageal echocardiography was performed to delineate the location of the defect and guide the procedure (Figure 5, Video 3).
Figure 5.
Periprocedural Transesophageal Echocardiography
Transgastric right ventricular inflow views were helpful in (A) identifying the defect, (B) confirming crossing of the guiding catheter, (C) positioning of the closure device, and (D) assessing for defect closure.
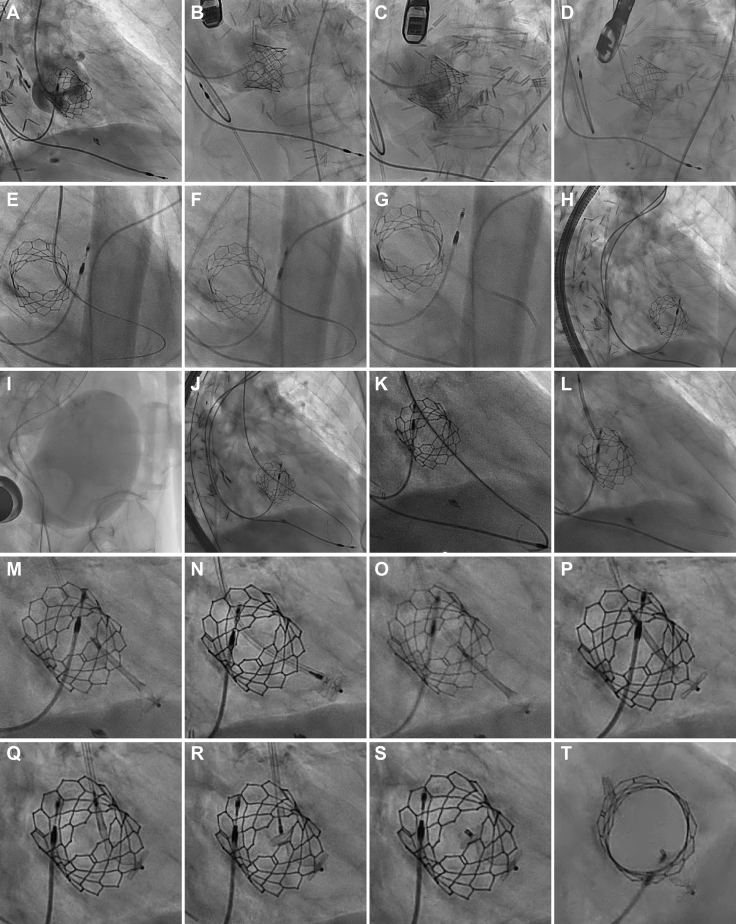
The key procedural steps are summarized in Figure 6 and Video 4. Femoral arterial axis was obtained, and cineangiography was performed to identify an optimal crossing angle. Using a 6-F Amplatz Left 0.75 guiding catheter, the defect was crossed with a straight hydrophilic coated guidewire. The catheter was slowly advanced into the right ventricle, and correct positioning was confirmed on cineangiography, hemodynamic tracings, and transesophageal echocardiography. The catheter would not progress farther, so advancement was performed using a mother-child technique with a 4-F catheter. A 10-mm Amplatzer vascular plug II (Abbott Laboratories) would not advance past the descending aorta because there was poor torque transmission resulting from significant tortuosity in the iliofemoral vasculature. A rigid guidewire was placed in the right ventricle, and the entire equipment was exchanged for a 7-F system, facilitating device delivery. Initial positioning of the device was entirely in the right ventricle, so the device was partially recaptured and deployed more proximally. Transesophageal echocardiographic imaging demonstrated almost complete elimination of flow on color Doppler and no significant interaction with the aortic valve or aortic regurgitation. There was stable device positioning upon release. Repeated cardiac catheterization revealed a Qp:Qs of 1.12.
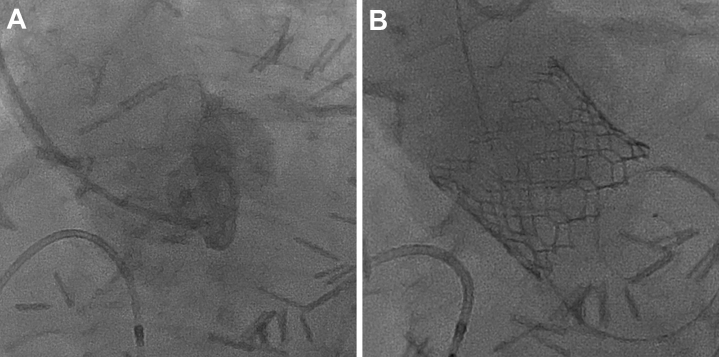
Figure 6.
Periprocedural Fluoroscopy
Cineangiography was performed in (A) right anterior oblique, (B) left anterior oblique cranial, and (C) left anterior oblique projections, identifying an optimal crossing angle. (D, E) The defect was crossed, and (F, G) the guiding catheter was advanced into the right ventricle using a mother-child technique. (H) A vascular plug would not advance through the guide because of (I) significant iliofemoral tortuosity and (J-L) upgrading of the equipment to a 7-F system. (M, N) The initial deployment was too distal, so (O) the device was partially recaptured and (P to R) positioned more proximally. (S, T) Final device positioning was satisfactory.
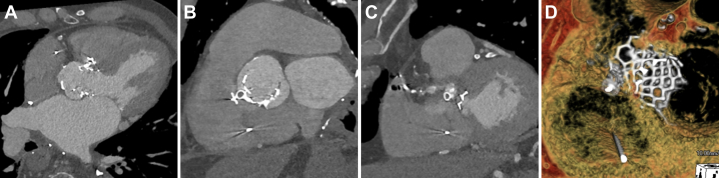
Immediate postprocedural cardiac CT demonstrated satisfactory device positioning and minimal residual shunt (Figure 7).
Figure 7.
Postprocedural Cardiac Computed Tomography Imaging
(A to C) There was only a minimal residual defect. (D) Three-dimensional reconstructions demonstrated satisfactory device positioning.
The patient made an uneventful postprocedural recovery and was discharged home on day 1 after the intervention.
Discussion
Aortic root trauma is a rare complication of TAVR, with a reported incidence of <1%.1 Risk factors for aortic root injury include subannular calcification, ≥20% area oversizing, and postdilatation.2 Aortic root trauma might potentially be minimized through the use of self-expanding THVs or incomplete inflation of balloon-expandable THVs to avoid excessive oversizing.3 Aorto-right ventricular fistula is an extremely uncommon mechanism of aortic root injury, typically arising after trauma to the right noninterleaflet triangle. The natural history of this condition in the setting of TAVR is unknown because of the scarcity of reported cases in the literature, but extrapolation from surgical literature suggests that heart failure will develop in the majority, that mortality is high in untreated patients, and that surgical or percutaneous intervention is associated with improved clinical outcomes.4 In the limited number of published case reports of aorto-right ventricular fistula after TAVR, treatment strategies that have been used include conservative treatment, which has been associated with a high incidence of mortality,5, 6, 7, 8, 9, 10, 11 and percutaneous intervention with either coils,12 septal occluders,13, 14, 15, 16 ductal occluders,17 or vascular plugs.18,19 In this case a vascular plug with a 3-lobe configuration was chosen, which facilitated occlusion of the fistula tunnel, and device sizing was selected to achieve 50% oversizing based on cardiac CT measurements.
The management of this condition ideally requires referral to a specialized center with a heart team experienced in the use of multimodality imaging and complex structural interventions. In this case we began by performing right heart catheterization to confirm a hemodynamically significant shunt, as had been suggested by both color Doppler assessment and the raised pulmonary pressures seen on transthoracic echocardiography. Cardiac CT imaging was critical for preprocedural planning, identifying the origin of the defect, its relationship to the TAVR prosthesis, and enabling accurate measurement of the defect dimensions, which were used for percutaneous closure device selection and sizing. Transesophageal echocardiography was vital to guide all procedural steps including crossing of the defect and device deployment. After the procedure, cardiac CT was again helpful in confirming a successful procedural outcome.
Follow-Up
At follow-up review, the patient reported an improvement in symptom status (NYHA functional class II symptoms). Echocardiography at 1 month demonstrated a small residual shunt, improvement in right ventricular size, and a reduction in right ventricular systolic pressure.
Conclusions
Aorto-right ventricular fistula is a rare complication after TAVR. The use of multimodality imaging is critical to ensuring a successful outcome from percutaneous closure techniques.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transcatheter Aortic Valve Replacement Imaging
Preprocedural Transthoracic Echocardiographic Imaging
Periprocedural Transoesophageal Echocardiography
Periprocedural Fluoroscopy
References
- 1.Walther T., Hamm C.W., Schuler G., et al. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data From the GARY registry. J Am Coll Cardiol. 2015;65:2173–2180. doi: 10.1016/j.jacc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Barbanti M., Yang T.H., Rodès Cabau J., et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation. 2013;128:244–253. doi: 10.1161/CIRCULATIONAHA.113.002947. [DOI] [PubMed] [Google Scholar]
- 3.Pasic M., Unbehaun A., Buz S., Drews T., Hetzer R. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. J Am Coll Cardiol Intv. 2015;8:1–9. doi: 10.1016/j.jcin.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Foster T.J., Amin A.H., Busu T., et al. Aorto-cardiac fistula etiology, presentation, and management: a systematic review. Heart Lung. 2020;49:317–323. doi: 10.1016/j.hrtlng.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz-García A.J., Rodríguez-Bailón I., Briales J.H., Navarro M.J., García J.M., de Teresa-Galván E. Aorto-right ventricular fistula after percutaneous aortic valve implantation of a CoreValve prosthesis. Tex Heart Inst J. 2011;38:728–729. [PMC free article] [PubMed] [Google Scholar]
- 6.Shakoor M.T., Islam A.M., Ayub S. Acquired aorto-right ventricular fistula following transcatheter aortic valve replacement. Case Rep Cardiol. 2015;2015 doi: 10.1155/2015/608539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leroux L., Dijos M., Peltan J., et al. Lethal aorto-right ventricular defect after transcatheter aortic valve implantation in a patient with radiation-induced porcelain aorta: notes of caution. Can J Cardiol. 2016;32:135.e9–135.e11. doi: 10.1016/j.cjca.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Leu H.B., Chang H.H., Wu M.H., Chen Y.H. Four-year follow-up of acquired aorto-right ventricular fistula after transcatheter aortic valve implantation. Eur Heart J. 2016;37(34):2679. doi: 10.1093/eurheartj/ehv716. [DOI] [PubMed] [Google Scholar]
- 9.Almanfi A., Qurie Am Strickman N. Aorto-right ventricular shunt after TAVR: rare complication of common procedure. Case Rep Cardiol. 2017;2017 doi: 10.1155/2017/1834394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagiwara K., Saito N., Yamazaki K., Kimura T. Aorto-right ventricular fistula following transcatheter aortic valve implantation using a 29 mm SAPIEN XT valve. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-219247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konda M.K., Kalavakunta J.K., Pratt J.W., Martin D., Gupta V. Aorto-right ventricular fistula following percutaneous transcatheter aortic valve replacement: case report and literature review. Heart Views. 2017;18:133–136. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_115_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilgrim T., Meier B., Wenaweser P. Aorto-right ventricular fistula after transfemoral aortic valve implantation. J Invasive Cardiol. 2010;22:E30–E31. [PubMed] [Google Scholar]
- 13.Nakamura K., Passeri J.J., Inglessis-Azuaje I. Percutaneous closure of acute aorto-right ventricular fistula following transcatheter bicuspid aortic valve replacement. Catheter Cardiovasc Interv. 2017;90:164–168. doi: 10.1002/ccd.26705. [DOI] [PubMed] [Google Scholar]
- 14.Damman P., de Jong K.H., de Geus K.F., de Winter R.J. Transcatheter closure of an iatrogenic aorta-right ventricular fistula after transfemoral aortic valve implantation. Eur Heart J Case Rep. 2017;1:ytx019. doi: 10.1093/ehjcr/ytx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamandi M., Potluri S. Aorto-right ventricular fistula closure after TAVR guided by intracardiac echocardiography. Structural Heart. 2019;3:252–254. [Google Scholar]
- 16.Vrettos A., Duncan A., Ahmed A., Heng E.L., Panoulas V. Successful percutaneous closure of aortic root-to-right ventricle fistula after transcatheter aortic valve implantation: a valuable option in high-risk surgical patients. Eur Heart J Case Rep. 2022;6:ytac094. doi: 10.1093/ehjcr/ytac094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niikura H., Schwartz J.G., Lin D., Lesser J., Sorajja P., Gössl M. Transcatheter closure of an aorto-right ventricular fistula after TAVR. Cardiovasc Interv Ther. 2019;34:290–292. doi: 10.1007/s12928-018-0549-2. [DOI] [PubMed] [Google Scholar]
- 18.Vainrib A.F., Ibrahim H., Hisamoto K., et al. Aorto-right ventricular fistula post-transcatheter aortic valve replacement: multimodality imaging of successful percutaneous closure. CASE (Phila) 2017;1:70–74. doi: 10.1016/j.case.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alabbady A.M., Sattur S., Bauch T.D., Harjai K.J. Aorto-right ventricular fistula and paravalvular leak after transcatheter aortic valve implantation. J Am Coll Cardiol Case Rep. 2019;1:859–864. doi: 10.1016/j.jaccas.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcatheter Aortic Valve Replacement Imaging
Preprocedural Transthoracic Echocardiographic Imaging
Periprocedural Transoesophageal Echocardiography
Periprocedural Fluoroscopy