Abstract
Physiological traits are often used for vulnerability assessments of organismal responses to climate change. Trait values can change dramatically over the life cycle of organisms but are typically assessed at a single developmental stage. Reconciling ontogenetic changes in physiological traits with vulnerability assessments often reveals early life-stage vulnerabilities. The degree to which ontogenetic changes in physiological traits are due to changes in body mass over development versus stage-specific responses determines the degree to which mass can be used as a proxy for vulnerability. Here, we use the painted lady butterfly, Vanessa cardui, to test ontogenetic changes in two physiological traits, the acute thermal sensitivity of routine metabolic rate (RMR Q10) and the critical thermal maximum (CTmax). RMR Q10 generally followed ontogenetic changes in body mass, with stages characterized by smaller body mass exhibiting lower acute thermal sensitivity. However, CTmax was largely decoupled from ontogenetic changes in body mass. In contrast with trends from other studies showing increasing vulnerability among progressively earlier developmental stages, our study revealed highly erratic patterns of vulnerability across ontogeny. Specifically, we found the lowest joint-trait vulnerability (both RMR Q10 and CTmax) in the earliest developmental stage we tested (3rd instar larvae), the highest vulnerabilities in the next two developmental stages (4th and 5th instar larvae), and reduced vulnerability into the pupal and adult stages. Our study supports growing evidence of mechanistic decoupling of physiology across developmental stages and suggests that body mass is not a universal proxy for all physiological trait indicators of climate vulnerability.
Keywords: Developmental trajectory, global change vulnerability, long-distance seasonal migration, thermal physiology, thermal sensitivity, thermal tolerance
Introduction
The responses of physiological tolerance and performance traits to variation in temperature are widely used as indicators of organismal vulnerabilities to global climate change (Huey et al., 2012; Kingsolver et al., 2013; Buckley and Huey, 2016). Organisms with the lowest vulnerability to temperature rise are those with a greater ability to survive exposure to extreme high temperatures or greater plasticity in sub-lethal performance traits such as metabolic rate that allow them to maintain homeostasis in the face of climatic warming (Sunday et al., 2014; Seebacher et al., 2015). Vulnerability assessments are often based on physiological trait values from single developmental stages and are especially biased towards mature stages (Klockmann and Fischer, 2017). Yet, owing to the fact that intra-specific variation in physiological trait values across ontogeny rivals the magnitude of trait differences between species, and can itself be quite variable across taxa (Dahlke et al., 2020, but see Pottier et al., 2022a), the use of single developmental stages for vulnerability assessments can lead to inaccurate estimates of resilience to climate change (Levy et al., 2015). It is increasingly recognized that organisms are only as resilient to climate change as their most vulnerable developmental stage (Klockmann and Fischer, 2017); however, there remain comparatively few tests of ontogenetic changes in physiological traits related to climate vulnerability.
Among ectothermic species, the dominant pattern of stage-dependent vulnerability based on heat tolerance that is emerging in the literature is one of high early-stage vulnerability, followed by reductions in vulnerability towards adolescence and/or metamorphic stages, and sometimes then followed by a resurgence of elevated vulnerability into adulthood. A synthesis of ontogenetic changes in heat tolerance across nearly 700 species of marine and freshwater fish (Dahlke et al., 2020) broadly supports this pattern. No such synthesis currently exists for terrestrial systems, though individual studies of ontogenetic changes in heat tolerance, for example, in the common frog (Ruthsatz et al., 2022), the mealworm beetle (Vorhees and Bradley, 2012), the sirex woodwasp (Li et al., 2019), and a tropical butterfly (Klockmann et al., 2017) provide support for early-stage vulnerability. Using a somewhat different approach, Levy et al. (2015) developed a life cycle model of population dynamics for North American lizards based on stage-specific estimates of thermal tolerance and microclimatic variation and found evidence of elevated vulnerability to climate change when including the embryo stage. In terrestrial systems, the pattern of early-stage vulnerability has, in some instances, been attributed to a positive association between body mass and heat tolerance (Chown and Nicolson, 2004; Klockmann et al., 2017).
In comparison to thermal tolerance, very different patterns are evident for thermal sensitivity of metabolic rate, another important physiological trait indicator of climate vulnerability that can change throughout ontogeny (Kingsolver and Buckley, 2020). Organisms with lower thermal sensitivity of metabolic rate are argued to be better able to compensate for changes in environmental temperature and therefore less vulnerable to climatic warming (Seebacher et al., 2015). However, ontogenetic changes in mean metabolic rate can be adaptive. For example, shifts from lower rates in early stages to higher rates in later stages confer benefits to total fitness in a marine bryozoan (Pettersen et al., 2016). Thus, ontogenetic changes in thermal sensitivity of metabolic rate, rather than mean trait values, might provide a more direct link with climate vulnerability (Magozzi and Calosi, 2015). Work in porcelain crabs (Leiva et al., 2018) and the budworm moth (Bawa et al., 2021) shows evidence of lower acute thermal sensitivity, and therefore lower vulnerability in earlier developmental stages compared with later stages. Relatedly, work in dung beetles (Carter and Sheldon, 2020) shows evidence of lower acclimation thermal sensitivity to different chronic temperatures in earlier developmental stages. However, Silva-Garay and Lowe (2021) describe the opposite pattern in stingrays, with juveniles exhibiting higher acclimation thermal sensitivity than adults. Yet caution must be exercised here, as acute and acclimation thermal sensitivities represent different processes (passive versus active plasticity, respectively) and thus cannot be directly compared (Havird et al., 2020). Furthermore, within-stage explorations of the relationship between mass and acute thermal sensitivity of metabolic rate in crickets revealed higher thermal sensitivity with increasing body mass (Nespolo et al., 2003) in certain environmental contexts, lending some support to the between-stage patterns observed among other taxa.
These contrasting patterns of the relationship between ontogeny and climate vulnerability based on heat tolerance versus thermal sensitivity of metabolic rate provide an opportunity to explore potential mechanisms underlying vulnerability across developmental stages. Specifically, they can be used to explore potential roles for: 1) intrinsic stage-dependent changes in physiology, shaped by stage-specific exposure to seasonal and microclimatic variation in temperature (Kingsolver et al., 2011), and reinforced by evidence of decoupling of physiological tolerance and performance mechanisms across ontogeny (Freda et al., 2017, 2022), 2) ontogenetic changes in body mass and downstream effects on physiology (Klockmann et al., 2017), and 3) stage-specific changes in activity driven by inherent mobility differences (e.g. relatively immobile pupae versus mobile adults), particularly for effects on metabolism (Carter and Sheldon, 2020). Here we used the painted lady butterfly, Vanessa cardui, to examine how high temperature tolerance and acute thermal sensitivity of metabolic rate change across ontogeny, from the 3rd larval instar through the 5th larval instar, and at the pupal and adult stages. If greater body mass is important for driving reduced vulnerability across ontogeny, we expected heat tolerance to increase with developmental stage from 3rd instar up to pupation and then decrease at the adult stage. By contrast, if lower body mass is important for driving reduced vulnerability across ontogeny, we expected acute thermal sensitivity of metabolic rate to increase with developmental stage (excepting the immobile pupal stage). Alternatively, if body mass is not responsible for driving vulnerability, we expected to find idiosyncratic changes in vulnerability across developmental stages. We further explored within-stage patterns of the relationship between mass and physiological traits, with the expectation that these would follow the between-stage patterns. Finally, we examined the effects of developmental acclimation temperature (20 versus 30°C) on between-stage and within-stage patterns to explore whether ontogenetic patterns were dependent on environmental context, as higher developmental acclimation temperatures can enhance the ability to physiologically resist acute thermal challenges (Angilletta, 2009; Havird et al., 2020).
Methods
Study system
The painted lady butterfly, V. cardui (Lepidoptera: Nymphalidae) is a holometabolous insect that goes through complete metamorphosis, with discrete developmental stages including five larval instars, and pupal and adult stages. This species has a nearly global distribution (excluding Antarctica and South America) and undergoes seasonal migration in temperate regions (Abbott, 1951; Stefanescu et al., 2013). Vanessa cardui uses long-distance movement to stay within suitable climatic and resource niches (Hu et al., 2021), making this species sensitive to changes in environmental temperature (Poston et al., 1977; Kelly and Debinski, 1999), despite its widespread geographic distribution.
Experimental design and laboratory rearing
We performed a laboratory experiment to quantify: 1) how two physiological indicator traits of vulnerability to warming change throughout ontogeny, and 2) the effects of developmental acclimation temperature on these ontogenetic changes in vulnerability. Our two physiological traits were the critical thermal maximum and the acute thermal sensitivity of metabolic rate.
To quantify responses across all developmental stages from the third larval instar to the adult stage, the experiment was conducted in two phases. Phase 1 included measurements on 3rd and 5th larval instars, pupae (acute thermal sensitivity of metabolic rate only), and adults (critical thermal maximum only) and ran from 11 March to 26 May 2021. Phase 2 included measurements on 3rd and 4th larval instars, and adults, and ran from 3 September to 22 November 2021. Measurements for 3rd instar larvae and adults were used to ensure congruence of results across phases 1 and 2 of the experiment.
We set up 32 individual larvae (3rd instar) of V. cardui (Carolina Biological) to establish the adult mating pairs whose offspring were measured in each of the two phases of the physiological trait experiment. Animals used to establish the mating pairs were held at a constant 25°C with a 14:10 L:D photoperiod (Percival Scientific growth chamber, growth chamber, 36-VL). Mating pairs were kept in flight cages (30cm each dimension: l × w × h, BugDorm) on benchtops in the laboratory near natural light. Mating pairs were provided with continuous access to food and water (10% sucrose solution) and a water-moistened paper towel as a substrate for oviposition.
The eggs produced from each mating pair (hereafter, ‘family’) were then split across two developmental acclimation temperature treatments (constant 20°C or constant 30°C, each on a 14:10 L:D photoperiod). Eggs were randomly assigned to the temperature treatments. Larvae were housed individually in small plastic cups (118 mL) and were provided with continuous access to an artificial diet (Carolina Biological painted lady butterfly culture medium). Larval molts were determined based on the presence of a shed head capsule and cuticle. Larvae were allowed to metamorphose in their larval rearing cups. Following pupation, animals were housed individually in larger plastic cups (500 mL) until adult eclosion.
At each developmental stage, beginning with the 3rd larval instar through the adult stage, we removed a subset of animals for physiological trait assessment. Animals were assigned a random number for physiological trait assessment in an effort to blind the researcher to the temperature treatment from which animals were taken. However, the effects of temperature on development time and an inability to mask the developmental stage of the animal during trait assessment prevented complete blinding of subject identity in our experiment. We first assessed metabolic rate at two test temperatures to quantify the acute thermal sensitivity of metabolic rate. Following metabolic rate measurements, we then assessed the critical thermal maximum on these same animals. Because the critical thermal maximum assay is often lethal, we do not have repeated assessments of the physiological traits across ontogeny for a single individual, but rather a sample of individuals (from the same family) selected for assessment at a particular developmental stage. We aimed to assess physiological traits (both the critical thermal maximum and acute thermal sensitivity of metabolic rate) for a minimum of 30 animals at each stage, comprising a median of 3 individuals per family (see Supplementary Material, Table S1 for a summary of sample sizes for physiological traits at each stage).
Metabolic rate
To assess the thermal sensitivity of metabolic rate, we measured the metabolic rate of individuals at two acute test temperatures, 20 and 30°C. Because we measured metabolic rate while the animal was permitted to engage in normal behaviours, we define our metabolic rate measure as ‘routine’ metabolic rate (RMR; sensu Metcalfe et al., 2016). To quantify metabolic rate at each test temperature, we used a CO2/H2O gas analyser (LI-7000, LI-COR Biosciences) in push mode that pushed air from the environment (scrubbed of CO2 and H2O with soda lime and Drierite, Sigma Aldrich) through two flow control meters (Alicat Scientific, MC-200SCCM for phase 1 of the experiment and MC-1SLPM for phase 2; this difference in flow control meters is due to the fact that adults, which require larger respirometry chambers, were only assessed for metabolic rate during phase 2 of the experiment) and then a respirometry flow multiplexer (RM-8, Sable Systems International). The respirometry equipment was held within a dark growth chamber (MIR 154, PHCbi) set to a constant 20 or 30°C. The flow controllers were calibrated (Gilian Gilibrator-2 Calibrator, Sensidyne LP) at both 20 and 30°C. Animals were tested at 20 and 30°C in a random order. Once placed inside the respirometry chambers, animals were allowed to acclimate at the given test temperature for 15 minutes prior to recording of metabolic rate.
We tested larvae (3rd to 5th instars) and pupae inside 30 mL glass chambers, and adults in 650 mL glass chambers (RC and RC-1; Sable Systems International). Eggs and very early stage larvae (1st and 2nd instars) were too small to reliably obtain respirometry recordings in preliminary trials, and so were omitted from the design. In the multiplexer, each animal chamber (n = 8 chambers maximum) contained one individual. CO2 was recorded for 10 minutes, with a 2-minute flush, at a constant flow rate, adjusted separately for each test temperature to achieve a volumetric flow rate of 100 mL min−1. For adults, the larger respirometry chambers required a higher flow rate, adjusted to achieve a target volumetric flow rate of 500 mL min−1, and a longer flush of 14 minutes. CO2 concentration (ppm) from the animal chambers was compared with the CO2 concentration (ppm) from the returning control line and recorded by the Licor-7000. All of the metabolic rate data were processed through a UI-3 data acquisition interface and ExpeData software (Sable Systems International). Once flow rate calibrations were complete, we then converted the raw CO2 values to the rate of CO2 production (VCO2 mL min−1) by dividing the outputted values by 1 000 000 to get the fractional CO2 value and multiplied this value by the flow rate. We considered the first 5 minutes as a settling-in period, and used the last 5 minutes of recording to compute the mean metabolic rate over this period. These butterflies breathe continuously and so the 5 minute interval is sufficient to get an estimation of metabolic rate, unlike species with discontinuous breathing (Winwood-Smith and White, 2018). We performed a complementary method to detect the flattest part of the trace using a rolling window analysis (each 5-minute interval possible over the 10 minutes of recording) and identifying the lowest slope value. Because calculations of metabolic rate based on this rolling window method were nearly identical to the last 5 minutes recording approach, we elected to use the latter method for simplicity. Finally, we standardized the change in metabolic rate across the two test temperatures as Q10 values, which describe the increase in metabolic rate for every 10°C increase in temperature (Birk, 2021), yielding our focal metric of acute thermal sensitivity of routine metabolic rate (RMR Q10).
For larvae, metabolic rate was assessed within 24 hours of the molt to the new instar. Larvae typically molted overnight, and metabolic rate was assessed the next day. During this period, the molting fluid was allowed to evaporate and the cuticle allowed to harden. On the morning of metabolic rate trials, post-molt larvae were sorted into cups without food prior to the assessment of metabolic rate. For adults, metabolic rate was assessed 24–48 hours after eclosion to allow the wings to expand and dry. Adults were kept at their respective developmental acclimation temperature during this period. Further, adults were not provided access to sugar solution prior to the assessment of metabolic rate, as the time since last feeding is especially influential for the metabolic rate of adult V. cardui (Woods Jr et al., 2010).
Critical thermal maximum
Following assessment of metabolic rate at both the 20 and 30°C test temperatures, individuals were allowed to recover for a 15-minute period on the laboratory bench (~23°C, 30–40% relative humidity, RH) prior to assessment of the critical thermal maximum (CTmax). The last test temperature experienced during the metabolic rate trial did not have a significant effect on CTmax (3rd instar: F1,78 = 0.510, P = 0.477; 4th instar: F1,54 = 1.26, P = 0.266; 5th instar: F1,46 = 0.378, P = 0.542; Adult: F1,49 = 0.763, P = 0.387, after accounting for the effects of developmental acclimation temperature and body mass), so we pooled data across those animals that experienced acute temperatures of 20°C versus 30°C most recently prior to the assessment of CTmax. The CTmax trials were performed using a water bath (A40 ARCTIC SC150, Thermo Fisher Scientific) with a dynamic temperature ramping protocol of 1°C min−1. The CTmax was designated as the temperature at which complete loss of movement occurred (i.e. when turning the containers over yielded no movement response from the organism), as this was a consistent diagnostic feature across all mobile developmental stages. We elected to use dynamically ramped assays for CTmax in an effort to limit the confounding effects of starvation, hydration, and thermal acclimation, particularly since these confounding effects might be sensitive to ontogenetic changes in mass (Terblanche et al., 2011). However, this assay approach relies on behaviour (loss of movement) and could not be applied to immobile stages of eggs and pupae, nor very early instar larvae (1st and 2nd instar) that were too small to reliably observe loss of movement. For the assessment of CTmax, larvae were housed in 12 mL plastic test tubes with a cotton plug. Adults were tested using 200 mL plastic containers plugged with a sponge. At the start of the trial, all individuals were placed in individual containers, and were allowed to acclimate to the starting water bath temperature of 35°C (starting RH being the same as room RH, ~ 30–40%) for 15 minutes.
Developmental trajectories, pre-trial body mass, and differences between sexes
We quantified developmental trajectories from the third larval instar through the adult stage by recording body mass and age at each developmental stage upon the molt to the new instar, pupation, or adult eclosion. Body mass was recorded to a precision of 0.0001 g (MSE124S-100-DA; Sartorius). These measurements also provided estimates of body mass prior to the assessment of physiological traits for the subset of animals removed for testing at each developmental stage. Note that because we recorded age and mass at each developmental stage prior to the given stage selected for physiological trait testing, we have comparatively greater sample sizes for developmental trajectory components (Supplementary Material, Tables S2, S3) than for the physiological trait measurements.
Sexual size dimorphism is generally marginal in V. cardui (O’Neill et al., 2008). We were able to determine the sex of most individuals at the pupal stage based on an abdominal suture that is present in females and absent in males (Genc, 2005). This diagnostic character was ambiguous for a small fraction of individuals that could not be sexed (12 out of 126 pupae that underwent physiological trials at the pupal stage and 22 out of 129 pupae that underwent trials at the adult stage). For those animals where sex could be determined, we were able to test for sexual size dimorphism in the pupal and adult stages. This also allowed us to directly model the effects of sex on physiological traits. However, for the larval instars, data were necessarily pooled across sex when examining the relationship between physiological traits and body mass.
Statistical analyses
We performed all statistical analyses using R version 4.2.1 (R Core Team, 2022). We present all results to 3 significant digits. To address our focal question of how climate vulnerability traits vary across developmental stage, we constructed a series of related models.
First, we examined developmental trajectories across ontogeny to establish a baseline expectation for how vulnerabilities should change with stage under an assumption of body mass as a main driver of stage-dependent vulnerability. To accomplish this, we constructed a linear mixed effects model using the lme function from the nlme library (Pinheiro et al., 2022) to quantify how body mass varies across ontogeny under different developmental acclimation temperatures. We considered body mass (natural-log transformed) as the response, and developmental stage, developmental acclimation temperature, and their interaction as the predictor variables. Family identity was included as a random intercept.
For our focal analyses, we then constructed several linear mixed effects models to examine how physiological traits vary across ontogeny. Either CTmax or RMR Q10 was considered as the response variable. We included developmental stage, developmental acclimation temperature, and their interaction as predictors. We performed two subsets of models that either additionally included or excluded body mass, taken prior to physiological trait testing and natural log transformed, as a covariate. The models that excluded body mass as a covariate (hereafter “mass-dependent” models of CTmax or RMR Q10) allowed us to assess the ecologically relevant patterns of vulnerability across developmental stages. The models that included body mass as a covariate (hereafter “mass-independent” models of CTmax or RMR Q10) allowed us to assess the degree to which stage-dependent changes in body mass were responsible for ontogenetic changes in physiological trait values and climate vulnerability (following Downs et al., 2013 for comparisons of mass-dependent and mass-independent models of physiological traits, and Nespolo et al., 2003 for inclusion of mass as a covariate in models of metabolic rate Q10). Statistical significance of the developmental stage term (and the associated pairwise contrasts between developmental stages) with body mass included as a covariate in the model would indicate an important role for factors other than mass in driving differences in vulnerability across developmental stages. We treated body mass as a simple covariate in these models, rather than interacting body mass with acclimation temperature and developmental stage, as here we were interested in the between-stage patterns. Family identity was included as a random intercept.
We used type III (marginal) F-tests to examine the statistical significance of model predictors. In the case where significant effects of developmental stage were found, we used pairwise post-hoc comparisons from the emmeans library (Lenth, 2022) to determine which stages were different from one another.
We also examined within-stage relationships between body mass and physiological traits. In this case, we performed models as above for comparisons across stages, but allowed the slope of the body mass-physiological trait relationship to vary by stage and developmental acclimation temperature (i.e. we included a 3-way interaction between mass, temperature, and stage). We then used the emtrends function from the emmeans library to examine the relationship between body mass and physiological trait values (either CTmax or RMR Q10) at a given developmental stage and for each of the two developmental acclimation temperatures.
For within-stage analyses, we were able to explore the effects of sex on physiological traits at the pupal and adult stages. We constructed models on subsets of the data for either pupal or adult stages. For adults, we examined both CTmax and RMR Q10 as functions of developmental acclimation temperature, mass, sex, and up to their three-way interaction. For pupae, we examined RMR Q10 only, but with this same set of predictor variables. Family identity was included in each of these models as a random intercept. When the sex term was not significant, we removed this term from the model to allow for more direct comparisons with larval within-stage model results.
We further used within-stage comparisons to explore the relationship between CTmax and RMR Q10. Owing to our experimental design with repeated measures of the two physiological traits on the same individual, we were able to explore potential patterns of covariation among these traits within a given developmental stage. To do so, we first computed the residuals from linear mixed effects models for each of the two physiological traits that accounted for the effects of body mass and developmental acclimation temperature and for family-level autocorrelation. We then examined the Spearman rank correlation between CTmax and RMR Q10 for each developmental stage.
Finally, because the experiment was conducted in two phases, we assessed whether there were differences between comparable developmental stage responses performed during both experiment phases. For the 3rd larval instar and adult stage, we developed a model of CTmax as the response, experiment phase (a two-level factor corresponding to phases 1 and 2 of the experiment) and developmental stage as predictors, and family identity as a random intercept.
Results
Our analyses of ontogenetic changes in body mass and age at each developmental stage of V. cardui revealed significant differences between each stage (Fig. 1A,B; Supplementary Material, Table S2; all pairwise contrasts between developmental stage for body mass and age at a given stage were significant, P < 0.0001). For both the 20 and 30°C developmental acclimation temperature treatments, the rank order of body mass (measured at the beginning of each stage) from smallest to largest was 3rd instar, 4th instar, 5th instar, adult and pupa (Fig. 1A,B; Supplementary Material, Table S3).
Figure 1.
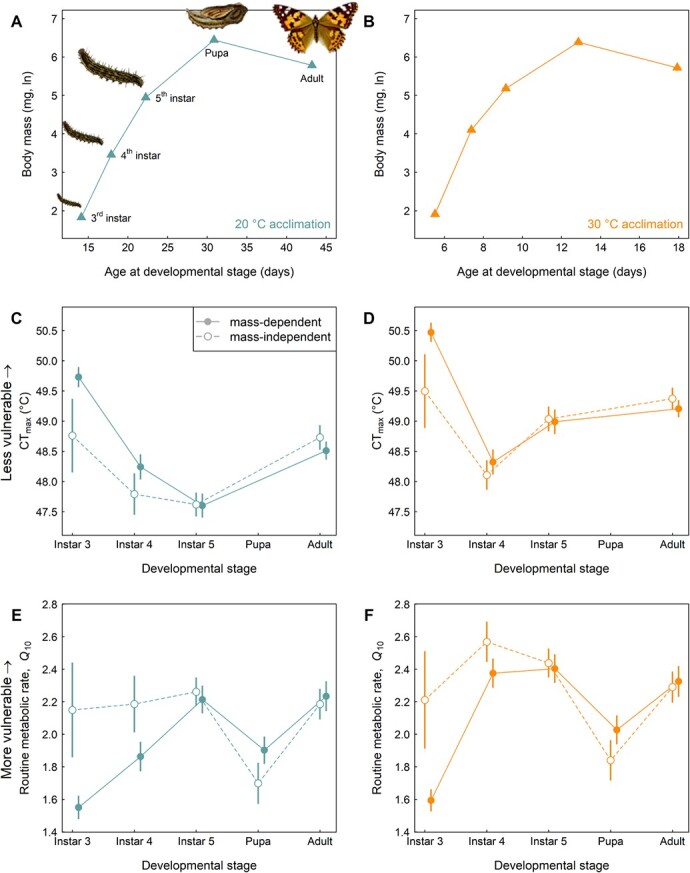
Developmental trajectories set expectations of mass-dependent vulnerability to temperature in the context of CTmax and RMR Q10. Trajectories (mean body mass ± 1 SE, natural log transformed, as a function of mean age at developmental stage ±1 SE; Supplementary Material, Table S3) are provided for the two developmental acclimation temperatures separately, including (A) 20°C and (B) 30°C. Note that SEs are largely not visible owing to their small amount relative to the point size depicting the mean values. Under the assumption of mass-dependent vulnerability, pupae and adults would be expected to exhibit the least vulnerability. Panels (C–F) show the actual stage-dependent results for physiological trait vulnerability estimates. Data are presented both as mass-dependent estimates (filled symbols, solid lines) and mass-independent estimates (open symbols, dashed lines). Predicted critical thermal maximum (CTmax) values ±1 SE from a linear mixed effects model of CTmax as a function of the interaction of developmental stage and developmental acclimation temperature, a random intercept for family identity, and, for the mass-independent estimates, a covariate of body mass, are plotted as a function of developmental stage. Note that CTmax values could not be obtained for the pupal stage. CTmax data are plotted separately for (C) 20°C and (D) 30°C developmental acclimation temperatures. Predicted routine metabolic rate (RMR) Q10 values ±1 SE from a linear mixed effects model of RMR Q10 as a function of the interaction of developmental stage and developmental acclimation temperature, a random intercept for family identity, and, for the mass-independent estimates, a covariate of body mass, are plotted as a function of developmental stage. RMR Q10 data are plotted separately for (E) 20°C and (F) 30°C developmental acclimation temperatures. Drawings of the developmental stages in panel A were obtained from William Buckler’s The larvæ of the British butterflies and moths, and Jacob Hübner’s Das kleine Schmetterlingsbuch (both public domain).
Between-stage patterns of vulnerability
Because we did not detect significant differences in physiological trait values (CTmax at 3rd instar and adult stages) between phases 1 and 2 of the experiment (F1,14 = 0.500, P = 0.493), we combined the two datasets for analysis. We found significant differences in vulnerability across ontogeny, though importantly, not all of these differences were attributable to ontogenetic changes in mass. Rather, some were due to stage-specific changes in physiological trait values independent of mass. That is, even with mass as a covariate in the models, significant differences in vulnerabilities between some developmental stages were detected (Table 1; Fig. 1C–F).
Table 1.
Statistical significance (test statistics and P-values) of predictors from linear mixed effects models of physiological traits, CTmax and RMR Q10, as a function of developmental stage, developmental acclimation temperature and their interaction
| Response | Model form | Term | F | P |
|---|---|---|---|---|
| CTmax | Mass-dependent | Acclimation | 12.8 | 0.000399 |
| Stage | 6.18 | 0.000423 | ||
| Acclimation × stage | 4.19 | 0.00623 | ||
| Mass-independent | Body mass | 2.82 | 0.094 | |
| Acclimation | 12.7 | 0.000419 | ||
| Stage | 4.06 | 0.00743 | ||
| Acclimation × stage | 3.14 | 0.0256 | ||
| RMR Q10 | Mass-dependent | Acclimation | 0.243 | 0.622 |
| Stage | 15.9 | <0.0001 | ||
| Acclimation × stage | 3.03 | 0.0179 | ||
| Mass-independent | Body mass | 4.62 | 0.0324 | |
| Acclimation | 0.488 | 0.485 | ||
| Stage | 6.60 | <0.0001 | ||
| Acclimation × stage | 1.09 | 0.363 |
Results presented separately for models where body mass was either excluded as a covariate (mass-dependent models) or included as a covariate (mass-independent models). Significant effects (P ≤ 0.05) are indicated with bolded P-values. For mass and acclimation temperature, ndf = 1; for stage and its interaction with acclimation temperature, ndf = 3 for CTmax models and ndf = 4 for RMR Q10 models. For CTmax, ddf = 336 for the mass-dependent model and ddf = 335 for the mass-independent model For RMR Q10, ddf = 325 for the mass-dependent model and ddf = 324 for the mass-independent model.
Overall, mass-dependent CTmax was highly erratic across ontogeny, and was influenced by developmental acclimation temperature (Fig. 1C,D; Supplementary Material, Table S4). Warmer developmental acclimation temperature increased CTmax, but acclimation temperature also interacted with developmental stage. In particular, all pairwise contrasts for CTmax under the 20°C developmental acclimation temperature were significant except for between 4th instar larvae and adults, and between 4th and 5th instar larvae. By comparison, under the 30°C acclimation temperature, while non-significant pairwise contrasts were again detected between 4th and 5th instar larvae, the difference between 5th instar larvae and adults was non-significant.
These patterns of interactive effects between acclimation temperature and developmental stage were even more pronounced for the mass-independent models (Fig. 2A; Supplementary Material, Table S5). While 4th instar larvae had significantly lower CTmax than 3rd instar larvae when reared under the 30°C acclimation temperature treatment, this effect was non-significant under the 20°C acclimation treatment. Further, while 4th instar larvae were significantly less tolerant than both 5th instar larvae and adults under the 30°C acclimation treatment, these contrasts were not significant under the 20°C acclimation treatment. Instead, under the 20°C acclimation treatment, 5th instar larvae were significantly less tolerant than adults.
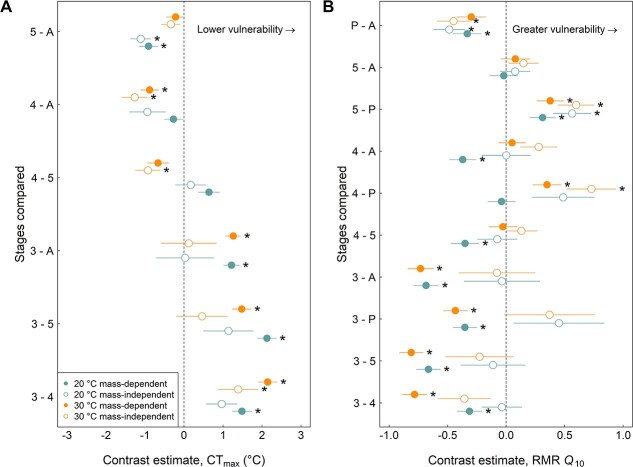
Figure 2.
Pairwise post-hoc comparisons for the differences in physiological traits between developmental stages for a given developmental acclimation temperature. Contrast estimates ±1 SE are shown for (A) CTmax and (B) RMR Q10, including results from mass-dependent (filled symbols) and mass-independent (open symbols) models and for 20°C and 30°C developmental acclimation temperature treatments (bottom two versus top two points, respectively). Contrasts are given such that the later developmental stage is subtracted from the earlier stage. Stages are abbreviated on the y-axis tick labels: 3, 4 and 5 correspond with their respective larval instars, P indicates pupae and A indicates adults. Asterisks to the right of each contrast indicate statistical significance (P ≤ 0.05).
While the overall trends for vulnerability across ontogeny were similar in the mass-independent models as the mass-dependent models, in mass-independent models the differences at later larval instars were more subtle, and the difference between 3rd instar larvae and adults was no longer significant. In the mass-independent models, CTmax was generally greatest during the 3rd larval instar, lowest during the final two instars (4th and 5th instar), and intermediate during the adult stage (Supplementary Material, Tables S2, S5; Fig. 1C,D). Both the fact that mass-independent models still indicate lower vulnerability based on CTmax for 3rd instar larvae, and that the general pattern of stages with smaller body mass (3rd instar) exhibiting higher CTmax, and stages with larger body mass (5th instar) exhibiting lower CTmax, indicates decoupling between stage-driven differences in mean body mass and CTmax.
For mass-dependent RMR Q10, vulnerability appeared to generally track ontogeny in the larval stages with 3rd instar being least vulnerable (i.e. the lowest acute thermal sensitivity of metabolic rate), followed by an increase in vulnerability in the 4th instar, and even greater vulnerability in the 5th instar (Fig. 1E, F). As with CTmax, there was evidence of an interaction between stage and developmental acclimation temperature for these larval stage comparisons, as 4th instar larvae were less vulnerable compared with 5th instar larvae under the 20°C acclimation temperature, but not under the 30°C acclimation temperature. At later developmental stages, RMR Q10 of pupae and adults was significantly elevated compared with 3rd instar larvae at both acclimation temperatures (Fig. 2B; Supplementary Material, Table S4).
As further evidence that mass was a major driver of ontogenetic variation in RMR Q10, mass-independent models did not indicate significant differences between any of the three larval instar stages (Fig. 2B; Supplementary Material, Table S5). Similarly, the significantly lower acute thermal sensitivity of metabolic rate in 3rd instar versus pupae and adults detected in the mass-dependent models became non-significant in the mass-independent models. For mass-independent models, only pupae (i.e. the only non-mobile stage) had consistently lower RMR Q10 values compared with adults and with 5th instar larvae across the two developmental acclimation temperatures. Pupae also had lower RMR Q10 than 4th instar larvae, but only at the 30°C acclimation temperature.
Within-stage relationships
The relationship between body mass and physiological traits appeared to differ strongly among stages when considering CTmax, but not RMR Q10. For RMR Q10, there was only one significant relationship between RMR Q10 and body mass within each developmental stage and for each developmental acclimation temperature (Table 2). By contrast, for CTmax, there were several significant relationships between CTmax and body mass, with qualitatively different relationships across the developmental stages (Fig. 3). In the 3rd larval instar, large body mass conferred significantly lower CTmax among individuals reared under the 20°C acclimation temperature. This negative relationship between mass and CTmax was also detected in the 4th instar, but the magnitude of the effect was weaker, and only significant among individuals reared under the 30°C acclimation temperature. By the 5th larval instar, the relationship between mass and CTmax was not significant among individuals reared at either of the two acclimation temperatures. At the adult stage, there was a significant positive relationship between mass and CTmax, but only among individuals reared under the 30°C acclimation temperature. We did not detect any significant effects of sex in the models of within-stage relationships between body mass and either of the two physiological traits (CTmax or RMR Q10) for the pupae or adults where sex could be determined (Supplementary Material, Table S6). We therefore dropped the term of sex from further consideration.
Table 2.
Estimates (slopes), standard errors, test statistics and P-values from post-hoc analyses of linear mixed effects models of within-stage patterns of the relationship between each of the two physiological traits, CTmax and RMR Q10 and body mass (natural log transformed)
| Response | Acclimation temperature | Stage | Slope estimate | SE | t | P |
|---|---|---|---|---|---|---|
| CTmax | 20°C | 3rd instar | −2.03 | 0.592 | −3.43 | 0.000678 |
| 4th instar | −0.810 | 0.973 | −0.833 | 0.406 | ||
| 5th instar | 0.828 | 0.818 | 1.01 | 0.312 | ||
| Adult | 0.155 | 0.644 | 0.240 | 0.810 | ||
| 30°C | 3rd instar | −0.326 | 0.296 | −1.10 | 0.271 | |
| 4th instar | −0.848 | 0.433 | −1.96 | 0.0508 | ||
| 5th instar | 0.00848 | 0.513 | 0.0173 | 0.987 | ||
| Adult | 1.05 | 0.524 | 2.00 | 0.0463 | ||
| RMR Q10 | 20°C | 3rd instar | 0.181 | 0.161 | 1.13 | 0.26 |
| 4th instar | −0.00946 | 0.436 | −0.0217 | 0.983 | ||
| 5th instar | 0.0807 | 0.351 | 0.23 | 0.818 | ||
| Pupa | −0.425 | 0.508 | −0.835 | 0.404 | ||
| Adult | 0.119 | 0.465 | 0.256 | 0.798 | ||
| 30°C | 3rd instar | 0.185 | 0.169 | 1.09 | 0.277 | |
| 4th instar | 0.495 | 0.191 | 2.6 | 0.00981 | ||
| 5th instar | 0.0927 | 0.21 | 0.442 | 0.659 | ||
| Pupa | −0.346 | 0.391 | −0.883 | 0.378 | ||
| Adult | 0.0695 | 0.51 | 0.136 | 0.892 |
Slopes that are significantly different from zero (P ≤ 0.05) have bolded P-values. For the CTmax post-hoc analyses, the ddf = 328; for RMR Q10 post-hoc analyses, the ddf = 315.
Figure 3.
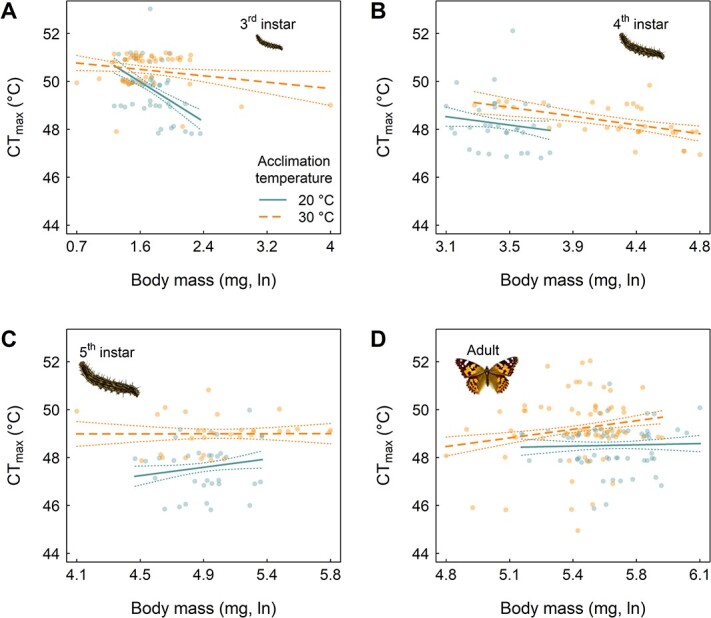
Within-stage patterns of the relationship between CTmax and body mass. Panels show results for each developmental stage for which CTmax was measured: (A) 3rd instar, (B) 4th instar, (C) 5th instar and (D) adult. Note the common y-axis range across panels for CTmax, but different x-axis ranges for body mass. Raw data points (actual x-values are shown, whereas the y-values being integers required a small amount of random jittering [in the y-direction only with a maximal shift of 0.2°C in either the positive or negative direction] to aid in visualization) are overplotted with predicted slopes (solid lines) ± 1 SE (dotted lines) from a linear model of CTmax (°C) as a function of developmental stage, developmental acclimation temperature, body mass (mg, natural log transformed), and up to their three-way interaction are presented. Raw, un-jittered data are provided online (see Data Availability statement). Results from the 20°C developmental acclimation temperature treatment are shown in solid lines, and from the 30°C developmental acclimation temperature treatment in dashed lines.
The repeated measures aspect of our study allowed us to examine the individual-level associations of CTmax and RMR Q10. We detected a significant negative relationship between CTmax and RMR Q10 early in development (3rd larval instar). That is, an individual with low vulnerability based on CTmax also had low vulnerability based on RMR Q10 within the 3rd instar (Table 3). However, this pattern weakened over development and became non-significant by the adult stage.
Table 3.
Correlations (Spearman’s rank correlation coefficient, test statistic, P-value, and sample size) between residual CTmax and RMR Q10 after accounting for the effects of body mass and developmental acclimation temperature. Correlations significantly different from zero (P ≤ 0.05) have bolded P-values
| Stage of comparison | n | rho | S | P |
|---|---|---|---|---|
| 3rd instar | 101 | −0.34 | 230 000 | 0.000504 |
| 4th instar | 63 | −0.201 | 50 000 | 0.114 |
| 5th instar | 64 | −0.199 | 52 400 | 0.114 |
| Adult | 58 | 0.0456 | 31 000 | 0.733 |
Discussion
Thermal physiological traits often change dramatically across ontogeny, causing different developmental stages to become more vulnerable to climatic warming than others. Yet, depending on the particular physiological traits used to assess vulnerability, contrasting predictions can be made for how body mass and developmental stage might influence physiological trait values. Heat tolerance (CTmax) might increase with gains in body mass over the life cycle, leading to reduced vulnerability. Acute thermal sensitivity of routine metabolic rate (RMR Q10) might also increase with gains in body mass over the life cycle, but with the effect of elevating vulnerability. We tested these contrasting predictions using the painted lady butterfly, V. cardui. We compared mass-dependent and mass-independent changes in vulnerability across developmental stage to understand how much of the variation in patterns of vulnerability across ontogeny was driven by changes in body mass versus stage-specific responses independent of mass. We found that while RMR Q10 was largely structured by ontogenetic changes in mass, CTmax was decoupled from ontogenetic changes in mass. The combination of expected ontogenetic changes for RMR Q10 and unexpected changes for CTmax contributed to erratic patterns of vulnerability across ontogeny. Our results suggest that body mass is not always a suitable proxy of stage-dependent physiological vulnerability to climate, and that assumptions of progressively increasing vulnerability with earlier developmental stages are not necessarily valid.
A rugged landscape of ontogenetic changes in vulnerability
Studies in terrestrial ectotherms provide evidence of elevated vulnerability to climate at earlier developmental stages based on heat tolerance (Vorhees and Bradley, 2012; Klockmann et al., 2017; Li et al., 2019; Ruthsatz et al., 2022) and thermal sensitivity of metabolic rate (Leiva et al., 2018; Carter and Sheldon, 2020; Bawa et al., 2021). The general consensus among these studies is that the rank-order vulnerability tends to continue to increase among progressively earlier developmental stages, and is in large part driven by ontogenetic changes in body mass (this pattern is most evident prior to metamorphosis, since post-metamorphic mass loss can generate a small increase in vulnerability; see especially Klockmann et al., 2017; Ruthsatz et al., 2022). By contrast, our results indicate much more erratic changes in vulnerability across ontogeny in the painted lady butterfly. We found the lowest joint-trait vulnerability (considering both CTmax and RMR Q10) in the 3rd larval instar, followed by the highest vulnerability in the 4th and 5th larval instars, and finally followed by a reduction in vulnerability (though not to the level of 3rd instar larvae) in the metamorphic pupal stage and final adult stage (Fig. 1C–F). Importantly, these patterns appeared to be underlain by different mechanisms for different traits and different developmental stages. Ontogenetic variation in RMR Q10 was driven by a combination of stage-dependent changes in body mass and activity levels. Specifically, larger body mass was generally associated with higher RMR Q10 values (Fig. 1A,B,E,F). Further, despite the large body mass of pupae, their immobility likely drove their relatively low RMR Q10 values (e.g. similar to Carter and Sheldon, 2020). By contrast, CTmax was largely independent of ontogenetic changes in body mass. Most notably, 3rd instar larvae had the smallest body mass, but highest CTmax values (Fig.l 1A–D).
Within-stage patterns for the relationship between body mass and CTmax lent further support to the interpretation of highly idiosyncratic changes in CTmax across ontogeny. In particular, individuals with large body mass exhibited a cost to CTmax at the 3rd larval instar (similar to many aquatic arthropods, e.g. Brans et al., 2017, Leiva et al., 2019, and some terrestrial arthropods, e.g. Youngblood et al., 2019). However, this pattern qualitatively changed by the adult stage, with individuals with large body mass exhibiting higher CTmax (consistent with many terrestrial arthropods, Chown and Nicolson, 2004; Fig. 3). Likewise, evidence of low vulnerability being reinforced across the two traits, i.e. individuals that exhibited both high CTmax and low RMR Q10 values, was only detected at the 3rd larval instar and no other developmental stage (Table 3).
While there is already a growing appreciation among vulnerability assessment approaches to consider multiple physiological traits (e.g. Shah et al., 2023), their variance and covariance across ontogeny are not well understood for many taxa, especially among terrestrial organisms (Klockmann and Fischer, 2017). Indeed, it remains to be seen whether the rugged landscape of ontogenetic changes in vulnerability that we found for the painted lady butterfly is rare or common among terrestrial ectotherms. Further, we do not yet have a complete picture of ontogenetic changes in vulnerability even for the painted lady butterfly, as our current methods were unable to assess vulnerability at the earliest developmental stages. We do not know if ontogenetic changes in vulnerability from the egg stage through the 2nd larval instar are also quite rugged as they were for the 3rd through 5th larval instars, or if they are consistent with patterns from other studies showing the most severe thermal bottlenecks at the earliest developmental stages (e.g. Levy et al., 2015). Even so, among the stages we were able to measure, the shift in vulnerability from the most to least vulnerable stages was of an ecologically relevant magnitude (e.g. compared with other intra- and inter-specific sources of variation in climate vulnerability traits, Diamond and Yilmaz, 2018). CTmax shifted by a magnitude of over 2°C, and RMR Q10 shifted by a factor of over 0.8 (Fig. 2).
Interestingly, we found that the rugged vulnerability landscape was fairly robust to changes in developmental acclimation temperature. Specifically, we found no effect of acclimation temperature on RMR Q10, consistent with the mixed support and lack of support for this relationship from other studies (Havird et al., 2020). For CTmax, although we found evidence of beneficial thermal acclimation consistent with findings from other ectothermic systems (Angilletta, 2009), acclimation temperature had little effect on the patterns of vulnerability across ontogeny. The one exception was that the 30°C treatment appeared to ameliorate the large drop in CTmax in the 5th instar compared with animals reared under the 20°C treatment (Fig. 1C,D). Thus, while our study suggests that vulnerability assessments might be complicated by erratic changes in vulnerability across ontogeny, developmental acclimation might not have appreciable interactive effects with these patterns. This could simplify the range of environmental conditions under which ontogenetic variation in vulnerability needs to be assessed.
Biological rather than methodological factors likely drive erratic ontogenetic changes in vulnerability
First, it is highly unlikely that the low vulnerability of 3rd instar larvae is driven by experimental artefacts of the time lag between air temperature and core body temperature. The assessment of RMR Q10 values had a 15-minute acclimation period within each acute test temperature prior to the start of respirometry recordings. Although the air-to-body temperature lag is undoubtedly present for the assessment of CTmax with a 1°C min−1 rate of temperature increase (Oyen and Dillon, 2018), the expectation would be that the small body mass of 3rd instar larvae would cause them to heat more quickly and thus have lower CTmax. However, 3rd instar larvae have the highest CTmax of any stage tested (Fig. 1C,D).
While RMR Q10 is low during the 3rd larval instar, essentially by default owing to small body mass at that stage (Fig. 1E,F), the fact that CTmax reaches its highest stage-specific value during the 3rd larval instar (and despite the small body mass of this stage; Fig. 1C,D) could reflect adaptive decoupling across developmental stages (Moore and Martin, 2019). Quantitative genetic, genomic association, and RNAi knockout studies (Freda et al., 2017, 2022) provide strong evidence for decoupling of thermal physiological traits across ontogeny, so the highly variable nature of CTmax across ontogeny in the painted lady butterfly is unsurprising. Yet the question remains regarding why 3rd instar larvae would need to be so heat tolerant. Here, it is worth considering the factors that ameliorate or elevate exposure to stressfully high temperatures across the life cycle in the painted lady butterfly. Eggs and early instar larvae can be protected by leaf boundary layer effects (Kingsolver et al., 2011), though we do not have heat tolerance data for these stages to assess potentially relaxed selection. Third instar larvae are sufficiently large to no longer be protected by those boundary effects (Woods, 2013), but are still small enough to potentially be limited in locomotor capacity and escape speed when seeking thermal refuge (Brackenbury, 1999). By contrast, 4th and 5th instar larvae are larger with potentially greater locomotor capacity, as are adults with the additional capability of flight and shifting their wing positioning to thermoregulate (Kingsolver, 1985).
Relevance for conservation of seasonal migrants
There are some data to suggest that seasonally migrating species exhibit reduced tolerances of thermal extremes compared with resident species, potentially owing to the avoidance of extreme temperatures through movement. Kimura and Beppu (1993), found that adults of Drosophila curviceps, a species that engages in seasonal migration upslope to avoid summer high temperatures in lowland sites, exhibited worse heat tolerance compared with non-migrating congeners (D. albomicans and D. immigrans) in lowland sites. Under climate change, while some migratory species are capable of shifting the timing and locations of their migration stops to keep within their historical niches (Sparks et al., 2005), migratory species that lack such flexibility could be more vulnerable than resident species owing to their reduced climatic tolerances. However, this expectation ignores ontogenetic variation in climatic vulnerability, and thus it remains an open question whether migratory species are more or less physiologically vulnerable to climate change compared with resident species.
Although we do not know whether rugged ontogenetic landscapes of vulnerability are typical of migratory species, data from one other migratory species support this hypothesis. The long-distance seasonally migrating monarch butterfly exhibited a similarly rugged landscape of vulnerability across ontogeny as to what we found with the painted lady butterfly. York and Oberhauser (2002) assessed mortality of chronic thermal stress (36°C) applied at each developmental stage from the 1st through the 5th larval instar and at the pupal stage. They found that while 1st, 3rd and 5th instar larvae and pupae that experienced chronic thermal stress had significantly higher mortality compared with larvae and pupae reared under control conditions (27°C), there were no significant differences between stressed and control organisms at the 2nd or 4th larval instars. Although these results describe ontogenetic variation in survival following thermal challenges rather than physiological indicator traits per se such as thermal tolerance or thermal sensitivity, they nonetheless support our findings of both bumpy and abrupt changes in vulnerability across the life cycle. Furthermore, for the imperilled monarch butterfly, this rugged landscape of ontogenetic vulnerability has important implications for understanding responses to climate change. Climate is a critical driver of population size in this species: models of monarch population size fluctuations based on long-term monitoring data revealed that breeding-season weather had substantially higher relative importance (the amount of explained variance attributable to particular factors) compared with milkweed host plant availability and migration between breeding and overwintering grounds (Zylstra et al., 2021). Linking these climate-population associations with ontogenetic changes in physiological traits could help to refine conservation plans for this species by identifying viability bottlenecks in the migration process that arise from the co-occurrence of unfavourable climatic conditions and stage-dependent climate vulnerability.
Another migratory species, the budworm, Helicoverpa punctigera, has also been studied for ontogenetic variation in heat tolerance and thermal sensitivity of metabolic rate (Bawa et al., 2021). However, because only 5th (final) instar larvae, pupae, and adults were measured, it is difficult to assess the ruggedness of changes over the life cycle (see also Régnier et al., 2023). In contrast to the migratory painted lady and monarch butterflies, the year-round resident squinting bush brown butterfly, Bicyclus anynana, exhibited increases in vulnerability (based on LT50, the temperature at which 50% of individuals died following exposure to acute heat stress) among progressively earlier pre-metamorphic stages (Klockmann et al., 2017). Across the entire life cycle (eggs, 1st through 5th instar larvae, pupae and adults), vulnerability closely tracked changes in body mass for this species.
It is unclear why ontogenetic vulnerability to climate might be especially rugged for migratory insect species. Given the relatively large number and diversity of long-distance and/or seasonally migrating insects including some species of butterflies, moths, dragonflies, and locusts (Chapman et al., 2015; Satterfield et al., 2020), there is an opportunity to use comparative approaches to explore the patterns and mechanisms underlying ontogenetic changes in physiological traits in this group. Yet regardless of whether rugged ontogenetic landscapes of vulnerability are more common for migratory species, the fact of their existence suggests that forecasting that ignores stage-specific trait variation might provide inaccurate estimates of vulnerability.
Study limitations and future directions
One limitation of our study involves the use of domesticated populations of painted lady butterflies. Inadvertent selection and evolution of faster development and larger body mass have been documented in other domesticated Lepidoptera species, including the tobacco hornworm, Manduca sexta (Kingsolver et al., 2009). Although this process can shift the overall distribution of body mass, this would not impact our focal comparisons of differences between developmental stages and the role of body mass in shaping ontogenetic shifts in physiology. Similarly, there might be direct effects of evolution in a constant thermal environment on physiological traits. However, these effects might be minimal, as work in zebrafish showed no evidence of loss of CTmax between wild and domesticated populations, nor of the duration of domestication on CTmax trait values (Morgan et al., 2019). Even in the case of domestication-caused evolution of physiological traits, the effect would likely be to dampen the differences between developmental stages, as all stages would experience the same thermal conditions. Our results would then be conservative underestimates of variation in among-stage responses. A second limitation of our study concerns the need to test the earliest developmental stages including the egg stage and the 1st and 2nd larval instars to be able to evaluate the hypothesis that thermal bottlenecks occur in the earliest developmental stages. These data could feasibly be collected using alternative techniques to ours such as static temperature treatments and fitting of thermal performance curves combined with more sensitive respirometry techniques (e.g. stop-flow) for small-bodied organisms (Lighton and Halsey, 2011; Jørgensen et al., 2021).
Another caveat, though one not particular to our study, concerns the ecological relevance of thermal vulnerability indices. Although indices such as CTmax and RMR Q10 provide very general estimates of relative differences in vulnerability, there are a number of ecological factors that can further shape thermal vulnerability indices themselves and environmental exposure of the organism to thermal stress (Clusella-Trullas et al., 2021). For example, in Drosophila melanogaster, while the adult stage has been shown to have the greatest basal heat resistance, the more sessile stages of larvae and pupae have relatively greater heat hardening capacity (Moghadam et al., 2019). Understanding the interaction between physiology and climate exposure across ontogeny in the wild (Pincebourde and Casas, 2015), and additionally, the potential for effects to carry over across developmental stages (Del Rio et al., 2021; Pottier et al., 2022b), therefore remain key future priorities.
Conclusions
Our study provides evidence of erratic changes in climate vulnerability across the life cycle of a long-distance seasonally migrating butterfly. Although we found that the acute thermal sensitivity of metabolic rate tracked ontogenetic changes in body mass, heat tolerance was decoupled from ontogenetic changes in body mass, indicating that body mass cannot safely be used as a proxy of ontogenetic variation in vulnerability for all physiological traits. Further, the very abrupt changes in vulnerability we observed between progressive developmental stages indicates that autocorrelation of sequentially adjacent stages cannot be assumed. In consequence, vulnerability assessments that rely on physiological traits but which fail to consider changes across the entire life cycle might run the risk of severely misestimating vulnerability.
Supplementary Material
Acknowledgments
We are grateful to Xuan Pu and Melina Gabel for assistance with animal husbandry. Gracie Bellino, Gideon Deme, Ryan Martin, Rogério Miranda, Eric Prileson, and anonymous reviewers provided helpful comments on a previous version of this manuscript.
Author Contributions
S.E.D. conceived the idea of the study and analysed data. S.E.D., C.R.B.d.S, O.A.M. and A.L., developed methods. O.A.M. and A.L. collected data in experiment phase 1. O.A.M. and R.A.M. collected data in experiment phase 2. O.A.M. wrote the first draft of the Methods section. S.E.D. wrote the first draft of the Introduction, Results and Discussion. All authors contributed to revisions.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding
This work was supported by the National Science Foundation [DEB-1845126 to S.E.D.].
Data Availability
Data are available at the Open Science Framework, DOI 10.17605/OSF.IO/UP5BJ.
Contributor Information
Osmary A Medina-Báez, Department of Biology, Case Western Reserve University, 2074 Adelbert Rd, Cleveland, OH 44106, USA.
Angie Lenard, Department of Biology, Case Western Reserve University, 2074 Adelbert Rd, Cleveland, OH 44106, USA.
Rut A Muzychuk, Department of Biology, Case Western Reserve University, 2074 Adelbert Rd, Cleveland, OH 44106, USA.
Carmen R B da Silva, Department of Biology, Case Western Reserve University, 2074 Adelbert Rd, Cleveland, OH 44106, USA; School of Biological Sciences, Monash University, 25 Rainforest Walk, Clayton 3800, Australia; College of Science and Engineering, Flinders University, Anchor Court, Bedford Park 5042, South Australia, Australia.
Sarah E Diamond, Department of Biology, Case Western Reserve University, 2074 Adelbert Rd, Cleveland, OH 44106, USA.
Supplementary Material
Supplementary material is available at Conservation Physiology online.
References
- Abbott CH (1951) A quantitative study of the migration of the painted lady butterfly, Vanessa cardui L. Ecology 32: 155–171. 10.2307/1930414. [DOI] [Google Scholar]
- Angilletta MJ (2009) Thermal Adaptation: A Theoretical and Empirical Synthesis. OUP Oxford, New York. [Google Scholar]
- Bawa SA, Gregg PC, Soccoro APD, Miller C, Andrew NR (2021) Estimating the differences in critical thermal maximum and metabolic rate of Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) across life stages. PeerJ 9: e12479. 10.7717/peerj.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk MA (2021) Respirometry: tools for conducting and analyzing respirometry experiments. R package version 1.3.0. https://CRAN.R-project.org/package=respirometry.
- Brackenbury J (1999) Fast locomotion in caterpillars. J Insect Physiol 45: 525–533. 10.1016/S0022-1910(98)00157-7. [DOI] [PubMed] [Google Scholar]
- Brans KI, Jansen M, Vanoverbeke J, Tüzün N, Stoks R, De Meester L (2017) The heat is on: genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob Chang Biol 23: 5218–5227. 10.1111/gcb.13784. [DOI] [PubMed] [Google Scholar]
- Buckley LB, Huey RB (2016) How extreme temperatures impact organisms and the evolution of their thermal tolerance. Integr Comp Biol 56: 98–109. 10.1093/icb/icw004. [DOI] [PubMed] [Google Scholar]
- Carter AW, Sheldon KS (2020) Life stages differ in plasticity to temperature fluctuations and uniquely contribute to adult phenotype in Onthophagus taurus dung beetles. J Exp Biol 223: jeb227884. 10.1242/jeb.227884. [DOI] [PubMed] [Google Scholar]
- Chapman JW, Reynolds DR, Wilson K (2015) Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol Lett 18: 287–302. 10.1111/ele.12407. [DOI] [PubMed] [Google Scholar]
- Chown SL, Nicolson S (2004) Insect Physiological Ecology: Mechanisms and Patterns. Oxford University Press, New York. [Google Scholar]
- Clusella-Trullas S, Garcia RA, Terblanche JS, Hoffmann AA (2021) How useful are thermal vulnerability indices? Trends Ecol Evol 36: 1000–1010. 10.1016/j.tree.2021.07.001. [DOI] [PubMed] [Google Scholar]
- Dahlke FT, Wohlrab S, Butzin M, Pörtner H-O (2020) Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369: 65–70. 10.1126/science.aaz3658. [DOI] [PubMed] [Google Scholar]
- Del Rio AM, Mukai GN, Martin BT, Johnson RC, Fangue NA, Israel JA, Todgham AE (2021) Differential sensitivity to warming and hypoxia during development and long-term effects of developmental exposure in early life stage Chinook salmon. Conserv Physiol 9: coab054. 10.1093/conphys/coab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond SE, Yilmaz AR (2018) The role of tolerance variation in vulnerability forecasting of insects. Curr Opin Insect Sci 29: 85–92. 10.1016/j.cois.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Downs CJ, Brown JL, Wone B, Donovan ER, Hunter K, Hayes JP (2013) Selection for increased mass-independent maximal metabolic rate suppresses innate but not adaptive immune function. Proc R Soc B Biol Sci 280: 20122636. 10.1098/rspb.2012.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freda PJ, Alex JT, Morgan TJ, Ragland GJ (2017) Genetic decoupling of thermal hardiness across metamorphosis in Drosophila melanogaster. Integr Comp Biol 57: 999–1009. 10.1093/icb/icx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freda PJ, Toxopeus J, Dowle EJ, Ali ZM, Heter N, Collier RL, Sower I, Tucker JC, Morgan TJ, Ragland GJ (2022) Transcriptomic and functional genetic evidence for distinct ecophysiological responses across complex life cycle stages. J Exp Biol 225: jeb244063. 10.1242/jeb.244063. [DOI] [PubMed] [Google Scholar]
- Genc H (2005) Determination of sex in pupae of Phyciodes phaon (Lepidoptera: Nymphalidae). Fla Entomol 88: 536–537. 10.1653/0015-4040(2005)88[536:DOSIPO]2.0.CO;2. [DOI] [Google Scholar]
- Havird JC, Neuwald JL, Shah AA, Mauro A, Marshall CA, Ghalambor CK (2020) Distinguishing between active plasticity due to thermal acclimation and passive plasticity due to Q10 effects: why methodology matters. Funct Ecol 34: 1015–1028. 10.1111/1365-2435.13534. [DOI] [Google Scholar]
- Hu G, Stefanescu C, Oliver TH, Roy DB, Brereton T, Van Swaay C, Reynolds DR, Chapman JW (2021) Environmental drivers of annual population fluctuations in a trans-Saharan insect migrant. Proc Natl Acad Sci U S A 118: e2102762118. 10.1073/pnas.2102762118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci 367: 1665–1679. 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen LB, Malte H, Ørsted M, Klahn NA, Overgaard J (2021) A unifying model to estimate thermal tolerance limits in ectotherms across static, dynamic and fluctuating exposures to thermal stress. Sci Rep 11: 12840. 10.1038/s41598-021-92004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L, Debinski DM (1999) Effects of larval food-limitation on Vanessa cardui Linnaeus (Lepidoptera: Nymphalidae). Am Midl Nat 141: 315–322. 10.1674/0003-0031(1999)141[0315:EOLFLO]2.0.CO;2. [DOI] [Google Scholar]
- Kimura MT, Beppu K (1993) Climatic adaptations in the Drosophila immigrans species group: seasonal migration and thermal tolerance. Ecol Entomol 18: 141–149. 10.1111/j.1365-2311.1993.tb01195.x. [DOI] [Google Scholar]
- Kingsolver JG (1985) Butterfly thermoregulation: organismic mechanisms and population consequences. J Res Lepid 24: 1–20. 10.5962/p.333812. [DOI] [Google Scholar]
- Kingsolver JG, Arthur Woods H, Buckley LB, Potter KA, MacLean HJ, Higgins JK (2011) Complex life cycles and the responses of insects to climate change. Integr Comp Biol 51: 719–732. 10.1093/icb/icr015. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Buckley LB (2020) Ontogenetic variation in thermal sensitivity shapes insect ecological responses to climate change. Curr Opin Insect Sci 41: 17–24. 10.1016/j.cois.2020.05.005. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Diamond SE, Buckley LB (2013) Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct Ecol 27: 1415–1423. 10.1111/1365-2435.12145. [DOI] [Google Scholar]
- Kingsolver JG, Ragland GJ, Diamond SE (2009) Evolution in a constant environment: thermal fluctuations and thermal sensitivity of laboratory and field populations of Manduca sexta. Evolution 63: 537–541. 10.1111/j.1558-5646.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- Klockmann M, Fischer K (2017) Effects of temperature and drought on early life stages in three species of butterflies: mortality of early life stages as a key determinant of vulnerability to climate change? Ecol Evol 7: 10871–10879. 10.1002/ece3.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann M, Günter F, Fischer K (2017) Heat resistance throughout ontogeny: body size constrains thermal tolerance. Glob Change Biol 23: 686–696. 10.1111/gcb.13407. [DOI] [PubMed] [Google Scholar]
- Leiva FP, Calosi P, Verberk WCEP (2019) Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water- and air-breathers. Philos Trans R Soc B 374: 20190035. 10.1098/rstb.2019.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva FP, Garcés C, Verberk WCEP, Care M, Paschke K, Gebauer P (2018) Differences in the respiratory response to temperature and hypoxia across four life-stages of the intertidal porcelain crab Petrolisthes laevigatus. Mar Biol 165: 146. 10.1007/s00227-018-3406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV (2022) Emmeans: estimated marginal means, aka least-squares means. R package version 1.6.2-1. https://CRAN.R-project.org/package=emmeans
- Levy O, Buckley LB, Keitt TH, Smith CD, Boateng KO, Kumar DS, Angilletta MJ (2015) Resolving the life cycle alters expected impacts of climate change. Proc R Soc B Biol Sci 282: 20150837. 10.1098/rspb.2015.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang L, Li J, Gao C, Luo Y, Ren L (2019) Thermal survival limits of larvae and adults of Sirex noctilio (Hymenoptera: Siricidae) in China. PLoS One 14: e0218888. 10.1371/journal.pone.0218888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton JRB, Halsey LG (2011) Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comp Biochem Physiol 158: 265–275. 10.1016/j.cbpa.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Magozzi S, Calosi P (2015) Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob Chang Biol 21: 181–194. 10.1111/gcb.12695. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Van Leeuwen TE, Killen SS (2016) Does individual variation in metabolic phenotype predict fish behaviour and performance? J Fish Biol 88: 298–321. 10.1111/jfb.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam NN, Ketola T, Pertoldi C, Bahrndorff S, Kristensen TN (2019) Heat hardening capacity in Drosophila melanogaster is life stage-specific and juveniles show the highest plasticity. Biol Lett 15: 20180628. 10.1098/rsbl.2018.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MP, Martin RA (2019) On the evolution of carry-over effects. J Anim Ecol 88: 1832–1844. 10.1111/1365-2656.13081. [DOI] [PubMed] [Google Scholar]
- Morgan R, Sundin J, Finnøen MH, Dresler G, Vendrell MM, Dey A, Sarkar K, Jutfelt F (2019) Are model organisms representative for climate change research? Testing thermal tolerance in wild and laboratory zebrafish populations. Conserv Physiol 7: coz036. 10.1093/conphys/coz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespolo RF, Lardies MA, Bozinovic F (2003) Intrapopulational variation in the standard metabolic rate of insects: repeatability, thermal dependence and sensitivity (Q10) of oxygen consumption in a cricket. J Exp Biol 206: 4309–4315. 10.1242/jeb.00687. [DOI] [PubMed] [Google Scholar]
- O’Neill BF, Zangerl AR, Casteel CL, Berenbaum M (2008) Larval development and mortality of the painted lady butterfly, Vanessa cardui (Lepidoptera: Nymphalidae), on foliage grown under elevated carbon dioxide. Gt Lakes Entomol 41: 103–110. [Google Scholar]
- Oyen KJ, Dillon ME (2018) Critical thermal limits of bumblebees (Bombus impatiens) are marked by stereotypical behaviors and are unchanged by acclimation, age or feeding status. J Exp Biol 221: jeb165589. 10.1242/jeb.165589. [DOI] [PubMed] [Google Scholar]
- Pettersen AK, White CR, Marshall DJ (2016) Metabolic rate covaries with fitness and the pace of the life history in the field. Proc R Soc B Biol Sci 283: 20160323. 10.1098/rspb.2016.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincebourde S, Casas J (2015) Warming tolerance across insect ontogeny: influence of joint shifts in microclimates and thermal limits. Ecology 96: 986–997. 10.1890/14-0744.1. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, R Core Team (2022) Nlme: linear and nonlinear mixed effects models. R package version 3.1-152. https://CRAN.R-project.org/package=nlme.
- Poston FL, Hammond RB, Pedigo LP (1977) Growth and development of the painted lady on soybeans (Lepidoptera: Nymphalidae). J Kans Entomol Soc 50: 31–36. [Google Scholar]
- Pottier P, Burke S, Drobniak SM, Nakagawa S (2022a) Methodological inconsistencies define thermal bottlenecks in fish life cycle: a comment on Dahlke et al., 2020. Evol Ecol 36: 287–292. 10.1007/s10682-022-10157-w. [DOI] [Google Scholar]
- Pottier P, Burke S, Zhang RY, Noble DWA, Schwanz LE, Drobniak SM, Nakagawa S (2022b) Developmental plasticity in thermal tolerance: ontogenetic variation, persistence, and future directions. Ecol Lett 25: 2245–2268. 10.1111/ele.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2022) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ [Google Scholar]
- Régnier B, Legrand J, Calatayud P-A, Rebaudo F (2023) Developmental differentiations of major maize stemborers due to global warming in temperate and tropical climates. Insects 14: 51. 10.3390/insects14010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthsatz K, Dausmann KH, Peck MA, Glos J (2022) Thermal tolerance and acclimation capacity in the European common frog (Rana temporaria) change throughout ontogeny. J Exp Zool A Ecol Integr Physiol 337: 477–490. 10.1002/jez.2582. [DOI] [PubMed] [Google Scholar]
- Satterfield DA, Sillett TS, Chapman JW, Altizer S, Marra PP (2020) Seasonal insect migrations: massive, influential, and overlooked. Front Ecol Environ 18: 335–344. 10.1002/fee.2217. [DOI] [Google Scholar]
- Seebacher F, White CR, Franklin CE (2015) Physiological plasticity increases resilience of ectothermic animals to climate change. Nat Clim Chang 5: 61–66. 10.1038/nclimate2457. [DOI] [Google Scholar]
- Shah AA, Hotaling S, Lapsansky AB, Malison RL, Birrell JH, Keeley T, Giersch JJ, Tronstad LM, Woods HA (2023) Warming undermines emergence success in a threatened alpine stonefly: a multi-trait perspective on vulnerability to climate change. Funct Ecol 37: 1033–1043. 10.1111/1365-2435.14284. [DOI] [Google Scholar]
- Silva-Garay L, Lowe CG (2021) Effects of temperature and body-mass on the standard metabolic rates of the round stingray, Urobatis halleri (Cooper, 1863). J Exp Mar Biol Ecol 540: 151564. 10.1016/j.jembe.2021.151564. [DOI] [Google Scholar]
- Sparks TH, Roy DB, Dennis RL (2005) The influence of temperature on migration of Lepidoptera into Britain. Glob Chang Biol 11: 507–514. 10.1111/j.1365-2486.2005.00910.x. [DOI] [Google Scholar]
- Stefanescu C, Páramo F, Åkesson S, Alarcón M, Ávila A, Brereton T, Carnicer J, Cassar LF, Fox R, Heliölä J et al. (2013) Multi-generational long-distance migration of insects: studying the painted lady butterfly in the Western Palaearctic. Ecography 36: 474–486. 10.1111/j.1600-0587.2012.07738.x. [DOI] [Google Scholar]
- Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Natl Acad Sci U S A 111: 5610–5615. 10.1073/pnas.1316145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL (2011) Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol 214: 3713–3725. 10.1242/jeb.061283. [DOI] [PubMed] [Google Scholar]
- Vorhees AS, Bradley TJ (2012) Differences in critical thermal maxima and mortality across life stages of the mealworm beetle Tenebrio molitor. J Exp Biol 215: 2319–2326. 10.1242/jeb.070342. [DOI] [PubMed] [Google Scholar]
- Winwood-Smith HS, White CR (2018) Short-duration respirometry underestimates metabolic rate for discontinuous breathers. J Exp Biol 221: jeb175752. 10.1242/jeb.175752. [DOI] [PubMed] [Google Scholar]
- Woods HA (2013) Ontogenetic changes in the body temperature of an insect herbivore. Funct Ecol 27: 1322–1331. 10.1111/1365-2435.12124. [DOI] [Google Scholar]
- Woods WA Jr, Wood CAL, Ebersole J, Stevenson RD (2010) Metabolic rate variation over adult lifetime in the butterfly Vanessa cardui (Nymphalidae: Nymphalinae): aging, feeding, and repeatability. Physiol Biochem Zool 83: 858–868. 10.1086/656216. [DOI] [PubMed] [Google Scholar]
- York HA, Oberhauser KS (2002) Effects of duration and timing of heat stress on monarch butterfly (Danaus plexippus) (Lepidoptera: Nymphalidae) development. J Kans Entomol Soc 75: 290–298. [Google Scholar]
- Youngblood JP, da Silva CRB, Angilletta MJ, VandenBrooks JM (2019) Oxygen limitation does not drive the decreasing heat tolerance of grasshoppers during development. Physiol Biochem Zool 92: 567–572. 10.1086/705439. [DOI] [PubMed] [Google Scholar]
- Zylstra ER, Ries L, Neupane N, Saunders SP, Ramírez MI, Rendón-Salinas E, Oberhauser KS, Farr MT, Zipkin EF (2021) Changes in climate drive recent monarch butterfly dynamics. Nat Ecol Evol 5: 1441–1452. 10.1038/s41559-021-01504-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.