Abstract
Introduction
The dopamine D5 receptor (D5R) shows high expression in cortical regions, yet the role of the receptor in learning and memory remains poorly understood. This study evaluated the impact of prefrontal cortical (PFC) D5R knockdown in rats on learning and memory and assessed the role of the D5R in the regulation of neuronal oscillatory activity and glycogen synthase kinase‐3 (GSK‐3β), processes integral to cognitive function.
Materials and Methods
Using an adeno‐associated viral (AAV) vector, male rats were infused with shRNA to the D5R bilaterally into the PFC. Local field potential recordings were taken from freely moving animals and spectral power and coherence were evaluated in, and between, the PFC, orbitofrontal cortex (OFC), hippocampus (HIP), and thalamus. Animals were then assessed in object recognition, object location, and object in place tasks. The activity of PFC GSK‐3β, a downstream effector of the D5R, was evaluated.
Results
AAV‐mediated knockdown of the D5R in the PFC induced learning and memory deficits. These changes were accompanied by elevations in PFC, OFC, and HIP theta spectral power and PFC‐OFC coherence, reduced PFC‐thalamus gamma coherence, and increased PFC GSK‐3β activity.
Conclusion
This work demonstrates a role for PFC D5Rs in the regulation of neuronal oscillatory activity and learning and memory. As elevated GSK‐3β activity has been implicated in numerous disorders of cognitive dysfunction, this work also highlights the potential of the D5R as a novel therapeutic target via suppression of GSK‐3β.
Keywords: Dopamine D5 receptor, neuronal oscillations, prefrontal cortex, recognition memory, spatial memory
Reducing levels of the dopamine D5 receptor (D5R) in the prefrontal cortex (PFC) of rats altered brain wave patterns and increased activity of the protein GSK‐3, two processes tightly linked to learning and memory. D5R knockdown also induced memory deficits in a number of behavioral tasks.
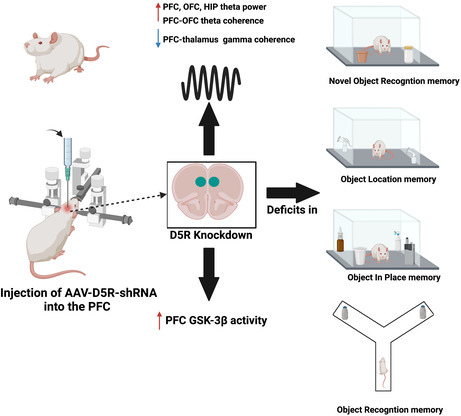
1. INTRODUCTION
Dopamine is a neurotransmitter in the central nervous system that has been shown to play an important role in motor function, 1 cognitive function, 2 emotion, 3 and reward. 4 Dopamine exerts its actions through dopamine receptors, which belong to the family of seven transmembrane G‐protein‐coupled receptors. Dopamine receptors are separated into two classes, the D1‐like class of receptors (D1R, D5R) and the D2‐like class (D2R, D3R, D4R). 5 , 6 , 7 , 8 The D1R and D5R are known to positively couple to adenylyl cyclase (AC) to generate cyclic adenosine monophosphate (cAMP), whereas the D2R, D3R, and D4R inhibit AC activity and cAMP production. 8 , 9 , 10 , 11
Dopamine D1‐like receptors have been shown to be involved in the regulation of cognitive function, 12 , 13 consistent with studies indicating that the receptors play an important role in long‐term potentiation (LTP). 14 , 15 , 16 , 17 For example, in humans, D1‐like receptor activation has been shown to impact motor cortex plasticity. 18 Additionally, animal studies have demonstrated the involvement of D1‐like receptors in mediating working memory in the T‐maze, 19 the radial arm maze, 20 and discrimination memory. 21 However, the relative contribution of the D1R and D5R in mediating these effects remains poorly understood due to the lack of subtype‐specific agonists and antagonists. This lack of subtype‐specific pharmacologics can be attributed to the high sequence homology exhibited by the two receptors, although there are distinguishing features in receptor affinity and localization. For example, the D5R exhibits a 10‐fold higher affinity for dopamine than the D1R and shows greater constitutive activation in the absence of an agonist. 22 Further, while the D1R and D5R both have high expression in cortical regions, 23 , 24 with D5R densities in the PFC even greater than D1R, 25 the expression of D1Rs in the striatum is much higher. 26 Therefore, given the critical role of the PFC in the regulation of cognitive function, 27 , 28 , 29 and the high expression of the D5R in this region, this suggests a potentially important role for the receptor in mediating PFC effects on cognitive processes. Indeed, this idea is supported by D5R knock‐out mice studies that revealed significant impairments in learning and memory tasks that included object location, object recognition, as well as spatial learning. 30 , 31
A link between PFC D5Rs specifically in learning and memory, and the signaling mechanisms that may underlie this link, remains for the most part unexplored. However, our past work has demonstrated a direct connection between upregulated glycogen synthase kinase‐3β (GSK‐3β) activity in the PFC or hippocampus (HIP), altered neuronal oscillatory function, and learning and memory deficits in rats. 32 Given the reported role of the D5R in the suppression of PFC GSK‐3β activity, 33 this suggests that the receptor may have a unique role in the regulation of neuronal oscillatory activity and learning and memory. The purpose of this study was to therefore evaluate the effects of PFC D5R knockdown in rats in tasks assessing learning and memory and to evaluate the impact on neuronal oscillatory function in the PFC, ventral HIP, orbitofrontal cortex (OFC), and thalamus, regions all implicated in cognitive function. 27 , 34 , 35 , 36 Finally, the effect of D5R knockdown on GSK‐3β in PFC was assessed.
2. MATERIALS AND METHODS
2.1. Animals
Sixteen male Sprague–Dawley rats (Charles River) aged 6 weeks and weighing approximately 350–400 g, were randomly assigned to two experimental groups. An additional six rats were used for in situ hybridization experiments. Animals were maintained in a 12‐h reverse light cycle and were pair‐housed in polypropylene cages until the surgery date with restricted access to food, receiving 15 g of 18% chow protein per day to maintain weights. Following surgery, rats were housed singly and were handled for 5 days, 5 min per day, before the beginning of the experiments. All procedures were carried out in accordance with the guidelines outlined in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993) and the Animal Care Committee at the University of Guelph.
2.2. Viral constructs
shRNA development and testing, and generation of the AAV8‐SYN‐drd5‐eGFP‐shRNAmir and AAV8‐SYN‐scrmb‐eGFP‐shRNAmir AAVs were generated by Vector Biolabs (Malvern, PA). The effect of four shRNAmir constructs on drd1 and drd5 gene expression was tested using luciferase reporter assay in HEK cells (Figure S1). The following sequence was chosen as it showed the greatest drd5 mRNA knockdown rate at 85%, with the lowest impact on DRD1 expression (24% reduction). The sequence of the shRNAmir was as follows: GCT GAAACCAGACGAATATGTCGAAGTTTTGGCCACTGACTGACTTCGACATTCGTCTGGTTT CAG.
2.3. Surgery
Animals underwent stereotaxic surgery to introduce either the AAV8‐SYN‐eGFP‐drd5‐shRNAmir or the control virus bilaterally into the prelimbic region of PFC using the following coordinates (AP + 3.24, ML 0.6, DV 3.5). Rats were anesthetized with isoflurane at 5% induction and 2.5% maintenance with body temperature maintained at 37°C using a thermostat‐regulated heating pad. 5 min prior to surgery, rats received a subcutaneous injection of 0.9% saline (3 mL) to ensure adequate hydration during surgeries, 5 mg/mL carprofen (0.4 mL, s.c.), as well as lidocaine/bupivacaine at the incision site. 1.8 μL/side of the virus was infused into the PFC at a rate of 0.3 μL/min and the syringe was left for 5 min post‐infusion before being slowly removed to avoid backflow. Animals were allowed 4 weeks to recover before undergoing a second surgery to implant unipolar electrodes bilaterally into the targeted four brain regions into the following coordinates: PFC (AP: +3.24, ML: ±0.6, DV: 3.5 mm), ventral HIP (AP: –5.5, ML: ±5.1, DV: 7.0 mm), OFC (AP: +3.24, ML: ±2.6, DV: 5.5 mm), and thalamus (AP: –3.24, ML: ±1.0, DV: 5.3 mm). Electrode placements were verified at the end of the study.
2.4. Electrophysiology
Animals were allowed 4 days to recover undisturbed after the electrode implantation surgery. Following that, rats were habituated to the transparent plexiglass recording chambers (45 cm × 45 cm × 45 cm) as well as open‐field arena for 4 days (5 min per day). After habituation, local field potential (LFP) recordings were collected for 30 min from freely moving rats utilizing a wireless W2100 system (MultiChannel Systems), at a sampling frequency of 1000 Hz. Chronux software package for MATLAB (MathWorks) was then used to analyze the spectral power in each region and the coherence between regions. The analysis was conducted on 5 min epochs, with each recording segmented, detrended, denoised, and low‐pass filtered to remove all frequencies higher than 100 Hz. Continuous multitaper spectral power, as well as coherence, were calculated for delta (1–4 Hz), theta (>4–12 Hz), beta (>12–32 Hz), low gamma (>32–60 Hz), and high gamma (>60–100 Hz).
2.5. Behavioral experiments
2.5.1. Novel Object Recognition (NOR)
To evaluate recognition memory, rats were tested in the NOR task as described. 36 During the acquisition phase, rats were allowed to explore two identical objects, placed in two corners of the arena, for 4 min. Following that, rats were given a 2 h delay period during which two clean objects were placed in the same locales as used during the acquisition phase, however, one of the objects was switched with a novel object. Animals were then allowed a 3‐min testing phase to explore the novel object. Object types were randomized between rats, and the positions of the objects in the acquisition phase and the test phase were counterbalanced between rats during the experiment. Exploration time was calculated, and the discrimination ratio [(novel object exploration–familiar object exploration)/total exploration time] was calculated.
2.5.2. Object Location (OL)
The OL task was used to evaluate spatial memory. 36 This task was composed of two phases, a 3‐min acquisition phase and a 3‐min test phase that were separated by a 5‐min delay period. During the acquisition phase, rats were allowed to explore two similar objects placed in two corners of the arena. During the delay period, objects were cleaned and placed back in the arena with one object relocated to the opposite corner from which it was originally placed. The object position was counterbalanced between animals and the discrimination ratio was calculated by subtracting the time spent exploring the novel objects from the time spent exploring the familiar object and dividing it by the total exploration time.
2.5.3. Object In Place (OiP)
The OiP task was used to assess associative object recognition memory and was performed as previously described. 32 , 37 , 38 The task was composed of two phases, a 5‐min acquisition phase as well as a 2‐min test phase separated by a 20‐min delay. During the acquisition phase, animals were allowed to explore four different objects placed in each corner of the arena. During the delay period, objects were cleaned and placed back in the arena with the position of two of the four objects switched (right or left counterbalanced) such that a “novel” side was created. Rats were then allowed 2‐min test phase to investigate the objects. Finally, to calculate the discrimination ratio, the time spent investigating the objects in the new location was subtracted from the time spent exploring the objects in the original location and divided by the total exploration time.
2.5.4. Object recognition memory
The Y‐maze apparatus was used to evaluate object recognition memory in the absence of contextual cues as described. 39 The task was comprised of a 10‐min acquisition phase followed by a 2‐min test phase separated by 5 min or 3 h delay to test for both short‐term and long‐term memory. Objects were made of either plastic or glass and they were approximately 26 cm in height and 11 cm wide. Rats were allowed to investigate two identical objects in the sample phase. During the delay period, one of the objects was replaced with a novel object and animals were then placed back in the apparatus for the test phase. Object positions were counterbalanced, and their types were randomized between animals to avoid any bias. The discrimination ratio was calculated as described above.
2.5.5. Immunohistochemistry
At the end of the behavioral tasks, rats were perfused using 4% paraformaldehyde and brain tissues were extracted, frozen, and stored at −80 degrees Celsius. Fluorescence immunohistochemistry was performed as done previously. 40 Brains were sectioned (30 μm), washed in TBS (60.5 mMTris, 87.6 mM NaCl ph 7.6), and then blocked for 2 h in blocking solution (10% goat serum, 1% BSA, 0.2% Triton‐X, 1X TBS). Subsequently, adjacent slices were incubated in primary antibodies rabbit anti‐pGSK‐3β (Ser9) (catalogue #ab9107166, 1:200, Abcam) or rabbit anti‐D5R (catalogue #ADR‐005, 1:200, Alomone Labs) for 60 h at 4°C. Brain sections were then washed in TBS and blocked (5% goat serum, 0.5%BSA, 0.01% Triton‐X, 1X TBS) before incubated in anti‐mouse Alexa 488 and/or anti‐rabbit Alexa 594 secondary antibodies for 2 h. Following that, slices were washed in TBS and mounted on slides using Prolong Gold (Thermo Fisher Scientific). Images were taken at 20X magnification using an Etaluma Fluorescence microscope.
2.6. RNAscope
Rats were anesthetized and their brains were removed and flash‐frozen. Coronal sections (20 μm) through the PFC were taken, dried for 1 h at −20°C, and stored at −80°C until use. For the detection of drd5 gene expression, we used the RNAscope 2.5 Duplex Assay (322,500; Advanced Cell Diagnostics) using diluent and only the red channel. We fixed the sections for 15 min in 4% PFA in 1x PBS at 4°C, followed by ethanol dehydration series on 50%, 70%, and 100%, 5 min each. The sections were incubated in H2O2 followed by protease IV (Advanced Cell Diagnostics). We hybridized the sections with the D5R mRNA probe (Cat #589931, Advanced Cell Diagnostics) for 2 h at 40°C then kept overnight in 5× SSC at room temperature. The following day, probes were amplified using RNAscope amplifiers as directed by the manufacturer. For brightfield microscopy, we detected the drd5 probe with the chromophore Fast Red, using a working solution of 2.5 ul of Red B and 150 ul of Red A. Sections were counterstained with 50% hematoxylin for 30 sec, washed, and then dried at 60°C for 30 min. Slides were cooled to room temperature and dipped in xylene and cover‐slipped using VectaMount mounting medium. Images were taken using an upright BX53 microscope (Olympus Canada Inc.) controlled by Neurolucida software (version 10, MBF Bioscience Williston). Individual images were captured using an Olympus 4× 0.16 N.A. UPlanSApo objective. Zoomed images for D5R shRNA‐treated rats were enhanced to improve visualization of weak signals.
2.7. Data analysis
All LFP data analyses were conducted using 5‐min epochs and are presented as normalized spectral power (to total power) or coherence. Quantification is representated as scatter graphs with means shown. Individual frequencies were extracted using Chronux and differences between the scrmb‐shRNA group and D5R‐shRNA group were evaluated using Student's t test. The frequency measures were presented as a percent change from the baseline. All behavior and IHC data analyses were performed using Student's t test. For all data, normality was evaluated using the Shapiro–Wilk test and equality of variance was assessed with the Levene's test. Data analyses were conducted using SPSS Statistical Package (IBM).
3. RESULTS
In this study, the effects of PFC knockdown on neuronal oscillatory activity and learning and memory were first evaluated. The experimental timeline is shown in Figure 1A with electrode placement sites shown in Figure 1B. There were relatively low levels of GFP dispersion (data not shown), an expected result given the design of the shRNA construct, with the shRNA insertion adjacent to the promotor and the GFP further downstream. However, using an antibody for the D5R, results showed an AAV‐induced reduction in PFC D5R expression (t (12) = 4.2, p = 0.001, Figure 1C (top panel)) in rats that received the shRNA, with a coincident reduction in GSK‐3β phosphorylation at Ser9 (t (12) = 3.9, p = 0.002, Figure 1C (bottom panel)), indicative of increased activation of this protein. AAV‐mediated drd5 gene expression was validated using RNAscope technology (Figure 1D). There was a robust expression of the drd5 mRNA in animals that received the control AAV, with expression found throughout the prelimbic region of the PFC and being the most sparse in layer I and most dense in layers II/III (Figure 1D, left panels). In line with the in vitro validation (Figure S1), animals that received D5R shRNA did not exhibit total drd5 mRNA knockdown but showed an approximate 82% reduction in the number of drd5 mRNA expressing cells, and a weaker overall signal (Figure 1D,E).
FIGURE 1.
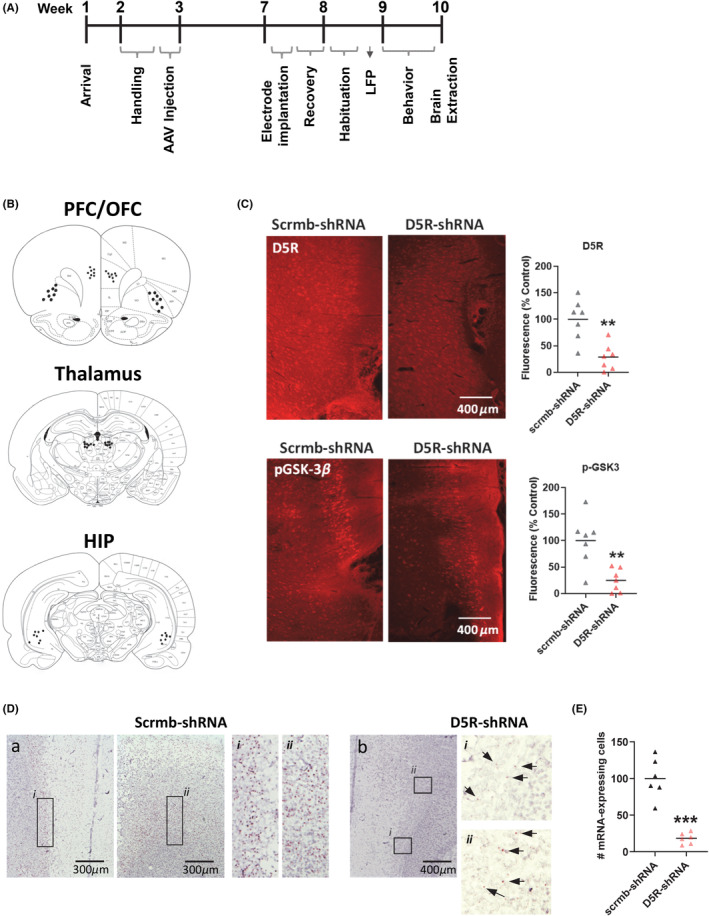
Knockdown of the D5R in PFC increases GSK‐3β activity. (A) The experimental timeline is shown. (B) Electrode placements in the PFC, OFC, thalamus, and HIP. (C) Representative images and quantification of fluorescence showing reduced PFC expression of the D5R (top panels) and GSK‐3β phosphorylation at Ser9 (bottom panels) following D5R‐shRNA induced knockdown. N = 7 rats/group (D) Images showing drd5 gene expression in control (left panels) animals or following drd5 mRNA knockdown (right panels). (E) Quantification of the number of drd5 mRNA expressing cells. N = 3 rats/group, 2 slices/rat. Quantified data are expressed as percent control. **p < 0.01, ***p < 0.001 Student's t test.
To analyze the effect of PFC D5R knockdown on system oscillatory function, LFP recordings were collected from freely moving animals from four different brain regions, PFC, OFC, HIP, and thalamus. Overall, reduced expression of the D5R in PFC resulted in regional changes selectively in low‐frequency spectral power, with no changes in beta or gamma power observed. Specifically, D5R knockdown increased PFC theta power (t (25) = −2.1, p = 0.046, Figure 2A) and OFC theta power (t (27) = −2.2, p = 0.031, Figure 2B), whereas in the HIP a reduction in delta power (t (27) = −2.4, p = 0.026) and an increase in theta power (t (27) = −2.2, p = 0.039, Figure 2C) was evident. In the thalamus, no significant changes in spectral power at any frequencies were observed (Figure 2D).
FIGURE 2.
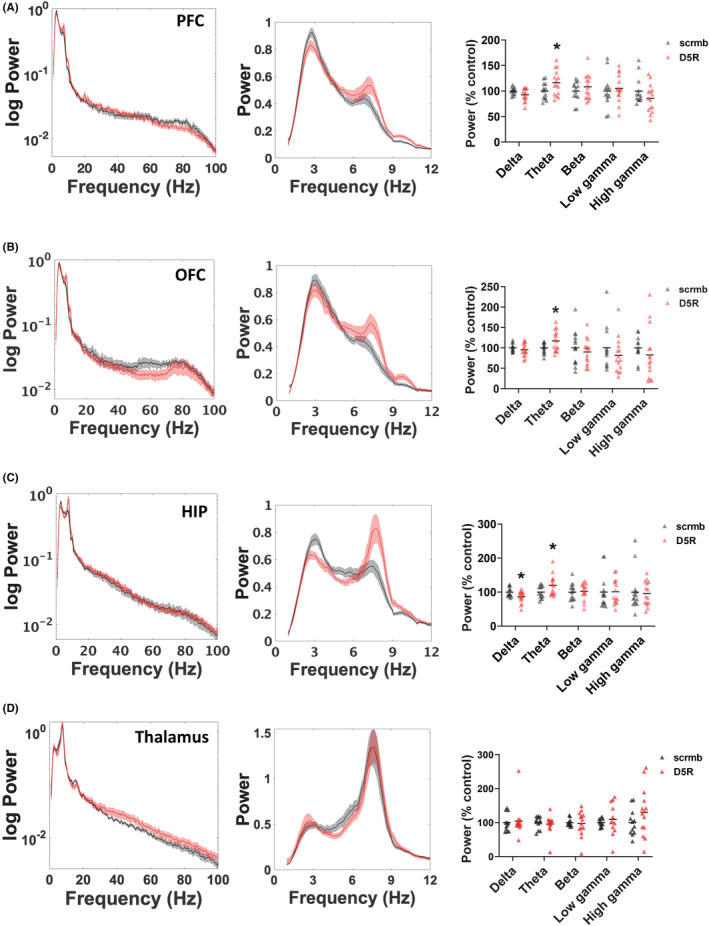
Effect of PFC D5R knockdown on oscillatory power activity in rats. Effect of PFC D5R knockdown on oscillatory power activity in rats. (A–D) Power spectra (left and center panels) and quantification of power (right panels) are shown. PFC D5R knockdown had the following regional effects on spectral power, (A) increased theta power in PFC, (B) increased theta power in OFC, and (C) increased theta power in HIP. (D) No effects of PFC D5R knockdown were evident in spectral power in the thalamus. Power curves are presented as normalized data with jackknife estimates of SEM shown as shaded areas. Quantified data are expressed as percent control. N = 7–8 rats/group, 1–2 electrodes/region/rat. *p < 0.05 Student's t test.
To determine the role of PFC D5Rs in interregional communication, coherence analysis was next performed. When the PFC connections were examined following D5R knockdown, an increase in PFC‐OFC theta coherence was observed (t (27) = −2.9, p = 0.007, Figure 3A). There was also a decrease in PFC‐thalamus high gamma coherence (t (21) = 3.3, p = 0.003, Figure 3B), with no effect on PFC‐HIP coherence (Figure 3C). There were no significant changes in coherence between any of the other regions in response to PFC D5R knockdown (Figure 3D–F).
FIGURE 3.
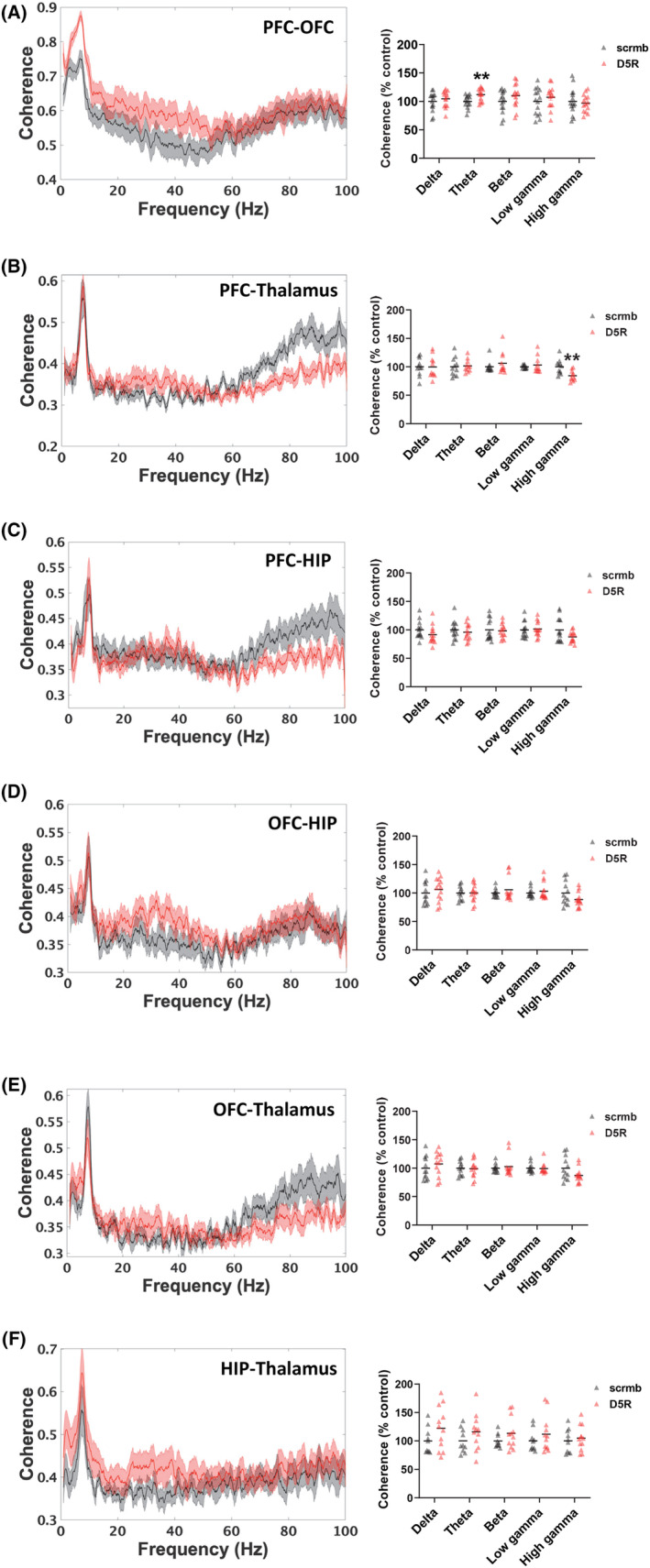
Effect of PFC D5R knockdown on oscillatory coherence in rats. (A–F) Coherence spectra (left panels) and quantification (right panels) are shown. (A) PFC D5R knockdown increased PFC‐OFC theta coherence, and (B) reduced PFC–thalamus high gamma coherence. (C–F) No significant effects on coherence were observed between PFC–HIP, OFC‐HIP, OFC‐thalamus, or thalamus‐HIP. Coherence curves are presented with jackknife estimates of SEM shown as shaded areas. Quantified data are expressed as percent control. N = 7–8 rats/group, 1–2 electrodes/region/rat. *p < 0.05, **p < 0.01 Student's t test.
The effect of PFC D5R knockdown on learning and memory was next assessed using various recognition and spatial memory tasks, including the NOR task, the OL task, the OiP task, and the Y‐maze. Knockdown of the D5R resulted in significant memory impairments. When recognition memory in the NOR task was assessed, the D5R knockdown group showed significant deficits, being unable to distinguish between the familiar object and the novel object (t (12) = 8.7, p = 0.0001, Figure 4A). Similarly, in the OL task, these animals were unable to distinguish between the stationary object and the moved object (t (12) = 4.8, p = 0.0001, Figure 4B). Rats were then evaluated in the OiP task to assess associative memory. In that task, PFC D5R knockdown resulted in rats being unable to link the object to the location where it was previously encountered (t (12) = 2.5, p = 0.026, Figure 4C). Finally, to assess short‐term (5‐min delay) and longer‐term (3 h delay) object recognition memory, rats were placed in the Y‐maze with minimal conceptual cues. Rats with PFC D5R knockdown showed no impairments in short‐term memory (Figure 4D (left panel)) but did show significant deficits in long‐term memory (t (14) = 3.5, p = 0.004, Figure 4D (right panel)).
FIGURE 4.
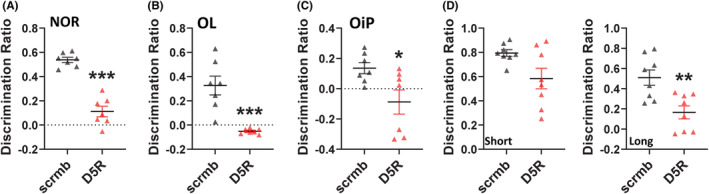
D5R knockdown in PFC induces deficits in learning and memory. (A) D5R knockdown in PFC induced deficits in object recognition memory in the NOR test. (B) Impaired spatial memory in the OL test was also evident. (C) D5R knockdown also induced impairment in associative recognition memory when tested in the OiP. (D) In the Y‐maze, D5R knockdown had no effects on short‐term recognition memory (left panel) but resulted in impairment on long‐term memory (right panel). N = 7–8 rats/group, *p < 0.05, **p < 0.01, ***p < 0.001 Student's t test.
4. DISCUSSION
The present study showed that reduced expression of the D5R in the PFC of rats induced learning and memory deficits, region‐specific alterations in neuronal oscillations, and an elevation in PFC GSK‐3β activity. Specifically, it was demonstrated that D5R knockdown in the PFC using an AAV‐mediated shRNA approach induced deficits in recognition, association, and spatial memory, signifying an important role for the receptor in the regulation of cognitive functions. These deficits were associated with increased PFC, OFC, and HIP theta power, an increase in PFC‐OFC theta coherence, and also with a reduction in PFC‐thalamus high gamma coherence. Reduced PFC D5R expression also increased activation of PFC GSK‐3β, a finding that may implicate the protein kinase as a mediator of these neurophysiological and/or behavioral effects given the recently reported relationship between PFC GSK‐3β, neuronal oscillatory function, and learning and memory. 32
The findings presented in this study indicated that reducing D5R expression in PFC impaired novel object recognition memory during the NOR task, with similar deficits in association and spatial memory also observed. Specifically, reduced expression of the D5R in PFC impaired spatial memory during the OL task and Y‐maze, as well as impaired associative memory during the OiP task. These findings agree with previous pharmacological studies that employed D1‐like receptor antagonists to evaluate receptor function in the cortex, but wherein the selective roles of the D1R and D5R were not delineated. For example, injection of a D1‐like receptor antagonist into the PFC of rats impaired performance during the oculomotor delayed‐response task, 13 working memory during the radial arm maze task in rats 41 as well as spatial memory learning. 42 In non‐human primates, injection of a D1‐like antagonist into the PFC impaired associative learning and decreased cognitive flexibility, 43 suggesting that optimal levels of D1‐like receptor activity are required for optimal frontal cortex performance during cognitive tasks. 44 , 45
The role of LTP in learning and memory is well documented. 46 , 47 , 48 Activation of D1‐like receptors stimulates LTP within the PFC, 18 , 49 , 50 , 51 as well as in the HIP. 52 , 53 , 54 In the PFC, pharmacological studies showed that activation of D1‐like receptors by agonists induced the maintenance of LTP, an effect that was abolished using a D1‐like receptor antagonist. 49 Further, exposure to novelty induces mesolimbic dopaminergic neuron activation 55 which, in turn, can elicit dopamine‐dependent LTP in the HIP via D1‐like receptors. 56 Additionally, activation of D1‐like receptors using the agonist SKF81297 infused into the PFC has been shown to enhance HIP‐PFC LTP. 57 This modulation of HIP‐PFC circuits by D1‐like receptors has been previously shown to play a crucial role in the working memory in rats. 41 Specifically, targeted unilateral injection of the D1‐like antagonist SCH23390 into the prelimbic region of the PFC, coupled with inactivation of the ventral HIP using lidocaine injection, severely impaired working memory in rats during the spatial‐win shift task in the radial arm maze, an effect that was absent in the vehicle group that received a saline injection in the ventral HIP. 41 Little is known regarding the relative contribution of the D1R or D5R on LTP. However, D5R deficiency in mice was shown to result in impaired LTP in HIP slices, 31 a finding also observed in HIP slices of D1R knockout mice, 58 and findings which together suggest similar effects of each receptor on synaptic plasticity.
The regulation of learning and memory processes are tightly coupled to neuronal oscillations, 32 , 59 , 60 and therefore the current study also evaluated the impact of PFC D5R knockdown on neuronal oscillatory activity in multiple regions. PFC D5R knockdown had wide‐reaching effects, impacting on low‐frequency oscillations not only within the PFC but also within the OFC and HIP. Further to this, increased PFC‐OFC theta coherence and reduced PFC‐thalamus gamma coherence were evident. The cortex, HIP, and thalamus, play significant roles in memory formation and cognitive function, 27 , 34 , 35 , 36 and oscillatory function in the theta and/or gamma frequencies in these regions have been linked to working memory and episodic memory. 32 , 61 , 62 , 63 , 64 , 65 Of relevance to the present findings, the theta frequency is often considered an event‐related activity triggered by novel components and is very abundant during memory retrieval and decision‐making tasks. 66 , 67 Further, electroencephalography (EEG) studies revealed that the slow rhythmic activity of theta often correlates with decision‐making and memory retrieval, indicative of successful working memory control. 67 , 68 Although the present study did not evaluate event‐related changes in oscillations, our findings showing learning and memory deficits do suggest that deficits in event‐related low‐frequency oscillations may also be present and are worth investigation. Deficits in theta oscillations, as well as in gamma, have also been demonstrated in cognitive dysfunction disorders such as schizophrenia and Alzheimer's disease 69 , 70 , 71 , 72 , 73 with studies having linked dysregulation of HIP theta and gamma oscillations to cognitive decline. 74 , 75
Information regarding the mechanisms by which the D5R may regulate neuronal oscillations is sparse, however here we demonstrated an upregulation in PFC GSK‐3 activity with D5R knockdown. A previous study by Perreault and colleagues (2013) attempted to understand the physiological function of D5R in the PFC by employing a multi‐species approach using rats, and mice gene‐deleted for the D5R or the D1R. Specifically, their findings showed that activation of the D5R enhanced the expression of brain‐derived neurotrophic factor (BDNF) and its receptor tropomyosin receptor kinase B in the PFC. BDNF has been linked to alterations in HIP LTP, 76 , 77 and is also an upstream regulator of GSK‐3 activity, via Akt. 33 , 78 Indeed, of relevance to the present findings, it was demonstrated that activation of the D5R induced an Akt‐mediated increase in PFC GSK‐3 phosphorylation (and its inactivation), a finding consistent with the present findings which demonstrated a reduction in Akt‐mediated phosphorylation of GSK‐3β that coincided with disruptions in learning and memory. Upregulated GSK‐3β activity has been demonstrated in multiple cognitive dysfunction disorders with a variety of therapeutics, such as lithium, known to inhibit the activity of the kinase. 79 , 80 GSK‐3 plays a crucial role in regulating synaptic plasticity via the modulation of LTP 81 , 82 and, indeed, a decrease in GSK‐3β activity with LTP has also been shown. 83 , 84 For example, transgenic mice with increased expression of GSK‐3β exhibited LTP deficits, an effect that was reversed via chronic treatment with the GSK‐3 inhibitor lithium. 83 LTP‐associated synapse impairment has also been shown to be increased upon the activation of GSK‐3β, an effect that was reversed with lithium. 85 Furthermore, persistent activation of GSK‐3β in either PFC or HIP of rats resulted in impaired cognitive function and disrupted oscillatory function in both regions. 32 In particular, the study showed that increased GSK‐3β levels in either PFC or HIP increased theta power in the PFC and/or HIP regions, an effect that was mimicked following PFC D5R knockdown in the current study. Together, these findings suggest that D5R‐mediated alterations in GSK‐3 activity may be involved in the regulation of neuronal oscillations that couple to learning and memory.
There were two significant limitations of the study that should be addressed. The first is that, as a result of the high sequence homology between the D5R and D1R, an shRNA could not be generated without some effect on D1R expression. Although we chose the one with the smallest impact on the D1R, with a significant proportion of D1R remaining functional, it cannot be stated conclusively that the small reduction in D1R expression had no effect. Second, this study focused on whether alterations of PFC D5R expression could drive changes in neuronal oscillatory function and behavior in male animals, however, it has yet to be explored whether there exist sex‐dependent variations in D5R function in vivo. This is an important consideration for future studies on the relationship between endogenous D5R function and cognition.
5. CONCLUSION
The findings reported in this study clearly demonstrate an important role for the D5R within the PFC in learning and memory responses, and further, suggest that these effects may be mediated by alterations in both neuronal oscillatory activity and GSK‐3β. Given the known role of GSK‐3β in disorders of cognitive dysfunction, and the circumscribed distribution of the D5R within cortical regions, this makes the receptor a potential drug target for focused pharmacological suppression of GSK‐3β activity, thus providing a critical advancement in the search for novel therapies to combat the cognitive symptoms inherent in many disorders.
AUTHOR CONTRIBUTIONS
AA and CN performed the experiments, analyzed the data, and wrote the manuscript. DR and CB assisted performing experiments. MP designed the study, assisted with the data analysis, and contributed to writing the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (#401359) and the Weston Family Foundation (#053440) (to MLP).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
CONSENT TO PARTICIPATE
Not applicable.
Supporting information
FigureS1
ACKNOWLEDGMENTS
We would like to acknowledge the land in Ontario, Canada on which this research was performed, the ancestral lands of the Attawandaron people and the treaty lands and territory of the Mississaugas of the Credit First Nation, and to offer our respect to the Indigenous peoples that reside here.
Albeely AM, Nolan CJ, Rasmussen DJ, Bailey CDC, Perreault ML. Cortical dopamine D5 receptors regulate neuronal circuit oscillatory activity and memory in rats. CNS Neurosci Ther. 2023;29:2469‐2480. doi: 10.1111/cns.14210
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available at the OSF repository osf.io/rpa6x.
REFERENCES
- 1. Mati J, Avital A, Hagai B. The dynamics of dopamine in control of motor behavior. Curr Opin Neurobiol. 2009;19(6):615‐620. doi: 10.1016/J.CONB.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 2. Ott T, Nieder A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn Sci. 2019;23(3):213‐234. doi: 10.1016/j.tics.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 3. Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33(4):971‐981. doi: 10.1093/schbul/sbm048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour–review of data from preclinical research. Acta Psychiatr Scand Suppl. 2005;111(427):14‐21. doi: 10.1111/j.1600-0447.2005.00540.x [DOI] [PubMed] [Google Scholar]
- 5. Carlsson A. A paradigm shift in brain research. Science. 2001;294(5544):1021‐1024. doi: 10.1126/SCIENCE.1066969 [DOI] [PubMed] [Google Scholar]
- 6. Kebabian JW, Petzold GL, Greengard P. Dopamine‐sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the dopamine receptor. Proc Natl Acad Sci U S A. 1972;69(8):2145‐2149. doi: 10.1073/PNAS.69.8.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277(5692):93‐96. doi: 10.1038/277093a0 [DOI] [PubMed] [Google Scholar]
- 8. Spano P, Govoni S, Trabucchi M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv Biochem Psychopharmacol. 1978;19:155‐165. [PubMed] [Google Scholar]
- 9. Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299‐231. doi: 10.1146/annurev.ne.16.030193.001503 [DOI] [PubMed] [Google Scholar]
- 10. Grandy DK, Zhang YA, Bouvier C, et al. Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proc Natl Acad Sci. 1991;88(20):9175‐9179. doi: 10.1073/PNAS.88.20.9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Missale C, Nash S, Robinson S, Jaber M, Caron M. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189‐225. doi: 10.1152/PHYSREV.1998.78.1.189 [DOI] [PubMed] [Google Scholar]
- 12. Herold C, Joshi I, Chehadi O, Hollmann M, Güntürkün O. Plasticity in D1‐like receptor expression is associated with different components of cognitive processes. PLoS One. 2012;7(5):e36484. doi: 10.1371/JOURNAL.PONE.0036484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sawaguchi T, Goldman‐Rakic PS. The role of D1‐dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed‐response task. J Neurophysiol. 1994;71(2):515‐528. doi: 10.1152/jn.1994.71.2.515 [DOI] [PubMed] [Google Scholar]
- 14. Kusuki T, Imahori Y, Ueda S, Inokuchi K. Dopaminergic modulation of LTP induction in the dentate gyrus of intact brain. Neuroreport. 1997;8(8):2037‐2040. doi: 10.1097/00001756-199705260-00046 [DOI] [PubMed] [Google Scholar]
- 15. Lemon N, Manahan‐Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long‐term potentiation and long‐term depression. J Neurosci. 2006;26(29):7723‐7729. doi: 10.1523/JNEUROSCI.1454-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swanson‐Park JL, Coussens CM, Mason‐Parker SE, et al. A double dissociation within the hippocampus of dopamine D1/D5 receptor and β‐adrenergic receptor contributions to the persistence of long‐ term potentiation. Neuroscience. 1999;92(2):485‐497. doi: 10.1016/S0306-4522(99)00010-X [DOI] [PubMed] [Google Scholar]
- 17. Fazio L, Pergola G, Papalino M, et al. Transcriptomic context of DRD1 is associated with prefrontal activity and behavior during working memory. Proc Natl Acad Sci U S A. 2018;115(21):5582‐5587. doi: 10.1073/PNAS.1717135115/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fresnoza S, Paulus W, Nitsche MA, Kuo MF. Nonlinear dose‐dependent impact of D1 receptor activation on motor cortex plasticity in humans. J Neurosci. 2014;34(7):2744‐2753. doi: 10.1523/JNEUROSCI.3655-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amico F, Spowart‐Manning L, Anwyl R, Rowan MJ. Performance‐ and task‐dependent effects of the dopamine D1/D5 receptor agonist SKF 38393 on learning and memory in the rat. Eur J Pharmacol. 2007;577(1–3):71‐77. doi: 10.1016/j.ejphar.2007.08.039 [DOI] [PubMed] [Google Scholar]
- 20. Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105(2):295‐306. doi: 10.1037/0735-7044.105.2.295 [DOI] [PubMed] [Google Scholar]
- 21. Schicknick H, Schott BH, Budinger E, et al. Dopaminergic modulation of auditory cortex‐dependent memory consolidation through mTOR. Cereb Cortex. 2008;18:2646‐2658. doi: 10.1093/cercor/bhn026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sunahara RK, Guan H‐C, O'Dowd BF, et al. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350(6319):614‐619. doi: 10.1038/350614a0 [DOI] [PubMed] [Google Scholar]
- 23. Bordelon‐Glausier JR, Khan ZU, Muly EC. Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: Coexpression in dendritic spines and axon terminals. J Comp Neurol. 2008;508(6):893‐905. doi: 10.1002/cne.21710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glausier JR, Khan ZU, Muly EC. Dopamine D1 and D5 receptors are localized to discrete populations of interneurons in primate prefrontal cortex. Cereb Cortex. 2009;19(8):1820‐1834. doi: 10.1093/cercor/bhn212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Díaz FC, Kramar CP, Hernandez MA, Medina JH. Activation of D1/5 dopamine receptors in the dorsal medial prefrontal cortex promotes incubated‐like aversive responses. Front Behav Neurosci. 2017;11:209. doi: 10.3389/fnbeh.2017.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luedtke RR, Griffin SA, Conroy SS, Jin X, Pinto A, Sesack SR. Immunoblot and immunohistochemical comparison of murine monoclonal antibodies specific for the rat D1a and D1b dopamine receptor subtypes. J Neuroimmunol. 1999;101(2):170‐187. doi: 10.1016/S0165-5728(99)00142-3 [DOI] [PubMed] [Google Scholar]
- 27. Friedman N, Robbins T. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2021;47:1‐18. doi: 10.1038/s41386-021-01132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Cogn Brain Res. 1996;5(1–2):175‐181. doi: 10.1016/S0926-6410(96)00054-7 [DOI] [PubMed] [Google Scholar]
- 29. Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399(6732):148‐151. doi: 10.1038/20178 [DOI] [PubMed] [Google Scholar]
- 30. Carr GV, Maltese F, Sibley DR, Weinberger DR, Papaleo F. The dopamine D5 receptor is involved in working memory. Front Pharmacol. 2017;8(OCT):666. doi: 10.3389/fphar.2017.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moraga‐Amaro R, González H, Ugalde V, et al. Dopamine receptor D5 deficiency results in a selective reduction of hippocampal NMDA receptor subunit NR2B expression and impaired memory. Neuropharmacology. 2016;103(103):222‐235. doi: 10.1016/j.neuropharm.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 32. Albeely AM, Williams OOF, Perreault ML. GSK‐3β disrupts neuronal oscillatory function to inhibit learning and memory in male rats. Cell Mol Neurobiol. 2022;42(5):1341‐1353. doi: 10.1007/s10571-020-01020-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perreault ML, Jones‐Tabah J, O'Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int J Neuropsychopharmacol. 2013;16(2):477‐483. doi: 10.1017/S1461145712000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferguson BR, Gao W‐J. Thalamic control of cognition and social behavior via regulation of GABAergic signaling and E/I balance in the medial prefrontal cortex. Biol Psychiatry. 2018;83(8):657‐669. doi: 10.1016/j.biopsych.2017.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jonker F, Jonker C, Scheltens P, Scherder E. The role of the orbitofrontal cortex in cognition and behavior. Rev Neurosci. 2015;26(1):1‐11. doi: 10.1515/REVNEURO-2014-0043 [DOI] [PubMed] [Google Scholar]
- 36. Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721‐10731. doi: 10.1523/JNEUROSCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitchnick KA, Creighton S, O'Hara M, Kalisch BE, Winters BD. Differential contributions of de novo and maintenance DNA methyltransferases to object memory processing in the rat hippocampus and perirhinal cortex–a double dissociation. Eur J Neurosci. 2015;41(6):773‐786. doi: 10.1111/ejn.12819 [DOI] [PubMed] [Google Scholar]
- 38. Mitchnick KA, Mendell AL, Wideman CE, et al. Dissociable involvement of estrogen receptors in perirhinal cortex‐mediated object‐place memory in male rats. Psychoneuroendocrinology. 2019;107:98‐108. doi: 10.1016/j.psyneuen.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 39. Creighton SD, Mendell AL, Palmer D, et al. Dissociable cognitive impairments in two strains of transgenic Alzheimer's disease mice revealed by a battery of object‐based tests. Sci Rep. 2019;9(1):1‐12. doi: 10.1038/s41598-018-37312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perreault ML, Hasbi A, Alijaniaram M, O'Dowd BF, George SR. Reduced striatal dopamine D1‐D2 receptor heteromer expression and behavioural subsensitivity in juvenile rats. Neuroscience. 2012;225:130‐139. doi: 10.1016/j.neuroscience.2012.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal‐prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18(4):1613‐1621. doi: 10.1523/jneurosci.18-04-01613.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rinaldi A, Mandillo S, Oliverio A, Mele A. D1 and D2 receptor antagonist injections in the prefrontal cortex selectively impair spatial learning in mice. Neuropsychopharmacology. 2007;32(2):309‐319. doi: 10.1038/sj.npp.1301176 [DOI] [PubMed] [Google Scholar]
- 43. Puig MV, Miller EK. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron. 2012;74(5):874‐886. doi: 10.1016/J.NEURON.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted‐U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10(3):376‐384. doi: 10.1038/nn1846 [DOI] [PubMed] [Google Scholar]
- 45. Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17(21):8528‐8535. doi: 10.1523/jneurosci.17-21-08528.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen Y, Avramoav S, Barkai E, Maroun M. Olfactory learning‐induced enhancement of the predisposition for LTP induction. Learn Mem. 2011;18(9):594‐597. doi: 10.1101/LM.2231911 [DOI] [PubMed] [Google Scholar]
- 47. Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long‐term potentiation in the hippocampus. Science. 2006;313(5790):1093‐1097. doi: 10.1126/science.1128134 [DOI] [PubMed] [Google Scholar]
- 48. Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141‐1444. doi: 10.1126/science.1128657 [DOI] [PubMed] [Google Scholar]
- 49. Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci U S A. 2004;101(9):3236‐3241. doi: 10.1073/pnas.0308280101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meunier CNJ, Callebert J, Cancela JM, Fossier P. Effect of dopaminergic D1 receptors on plasticity is dependent of serotoninergic 5‐HT1A receptors in L5‐pyramidal neurons of the prefrontal cortex. PLoS One. 2015;10(3):e0120286. doi: 10.1371/JOURNAL.PONE.0120286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25(32):7342‐7351. doi: 10.1523/JNEUROSCI.4603-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Papaleonidopoulos V, Kouvaros S, Papatheodoropoulos C. Effects of endogenous and exogenous D1/D5 dopamine receptor activation on LTP in ventral and dorsal CA1 hippocampal synapses. Synapse. 2018;72(8):e22033. doi: 10.1002/SYN.22033 [DOI] [PubMed] [Google Scholar]
- 53. Roggenhofer E, Fidzinski P, Bartsch J, Kurz F, Shor O, Behr J. Activation of dopamine D1/D5 receptors facilitates the induction of presynaptic long‐term potentiation at hippocampal output synapses. Eur J Neurosci. 2010;32(4):598‐605. doi: 10.1111/J.1460-9568.2010.07312.X [DOI] [PubMed] [Google Scholar]
- 54. Stramiello M, Wagner JJ. D1/5 receptor‐mediated enhancement of LTP requires PKA, Src family kinases, and NR2B‐containing NMDARs. Neuropharmacology. 2008;55(5):871‐877. doi: 10.1016/J.NEUROPHARM.2008.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67(1):145‐163. doi: 10.1152/JN.1992.67.1.145 [DOI] [PubMed] [Google Scholar]
- 56. Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine‐dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6(5):526‐531. doi: 10.1038/nn1049 [DOI] [PubMed] [Google Scholar]
- 57. Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor‐dependent long‐term potentiation at hippocampal‐prefrontal cortex synapses in vivo. J Neurosci. 2000;20(22):RC106. doi: 10.1523/jneurosci.20-22-j0003.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Granado N, Ortiz O, Suárez LM, et al. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP‐induced arc and zif268 expression in the hippocampus. Cereb Cortex. 2008;18(1):1‐12. doi: 10.1093/cercor/bhm026 [DOI] [PubMed] [Google Scholar]
- 59. Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24(1–2):61‐100. doi: 10.1016/S0167-8760(96)00057-8 [DOI] [PubMed] [Google Scholar]
- 60. Pina JE, Bodner M, Ermentrout B. Oscillations in working memory and neural binding: a mechanism for multiple memories and their interactions. PLoS Comput Biol. 2018;14(11):e1006517. doi: 10.1371/JOURNAL.PCBI.1006517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11(6):739‐744. doi: 10.1016/S0959-4388(01)00278-1 [DOI] [PubMed] [Google Scholar]
- 62. Kamiński J, Brzezicka A, Wróbel A. Short‐term memory capacity (7 ± 2) predicted by theta to gamma cycle length ratio. Neurobiol Learn Mem. 2011;95(1):19‐23. doi: 10.1016/J.NLM.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 63. Kragel JE, Vanhaerents S, Templer JW, et al. Hippocampal theta coordinates memory processing during visual exploration. Elife. 2020;9:e52108. doi: 10.7554/ELIFE.52108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory erika. Neurosci Biobehav Rev. 2010;34(7):1023‐1035. doi: 10.1016/j.neubiorev.2009.12.014.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tamura M, Spellman TJ, Rosen AM, Gogos JA, Gordon JA. Hippocampal‐prefrontal theta‐gamma coupling during performance of a spatial working memory task. Nat Commun. 2017;8(1):1‐9. doi: 10.1038/s41467-017-02108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414‐421. doi: 10.1016/J.TICS.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32(2):978‐987. doi: 10.1016/J.NEUROIMAGE.2006.02.018 [DOI] [PubMed] [Google Scholar]
- 68. Sirawaj I, Jan WR, Adam AR. Frontal theta is a signature of successful working memory manipulation. Exp Brain Res. 2013;224(2):255‐262. doi: 10.1007/S00221-012-3305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Barr MS, Farzan F, Tran LC, Chen R, Fitzgerald PB, Daskalakis ZJ. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res. 2010;121(1–3):146‐152. doi: 10.1016/j.schres.2010.05.023 [DOI] [PubMed] [Google Scholar]
- 70. Goodman MS, Kumar S, Zomorrodi R, et al. Theta‐gamma coupling and working memory in Alzheimer's dementia and mild cognitive impairment. Front Aging Neurosci. 2018;10:101. doi: 10.3389/fnagi.2018.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schmiedt C, Brand A, Hildebrandt H, Basar‐Eroglu C. Event‐related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25(3):936‐947. doi: 10.1016/j.cogbrainres.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 72. Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in schizophrenic patients. J Clin Neurophysiol. 2010;27(3):179‐190. doi: 10.1097/WNP.0B013E3181E0B20A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first‐episode and chronic schizophrenia. Psychophysiology. 1994;31(1):37‐43. doi: 10.1111/j.1469-8986.1994.tb01023.x [DOI] [PubMed] [Google Scholar]
- 74. McNaughton N, Ruan M, Woodnorth M‐A. Restoring theta‐like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16(12):1102‐1110. doi: 10.1002/HIPO.20235 [DOI] [PubMed] [Google Scholar]
- 75. Xiaxia X, Lei A, Xichao M, Zhang T. Impairment of cognitive function and synaptic plasticity associated with alteration of information flow in theta and gamma oscillations in melamine‐treated tats. PLoS One. 2013;8(10):e77796. doi: 10.1371/JOURNAL.PONE.0077796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Castrén E, Pitkänen M, Sirviö J, et al. The induction of LTP increases BDNF and NGF mRNA but decreases NT‐3 mRNA in the dentate gyrus. Neuroreport. 1993;4(7):895‐898. doi: 10.1097/00001756-199307000-00014 [DOI] [PubMed] [Google Scholar]
- 77. Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Brain‐derived neurotrophic factor expression after long‐term potentiation. Neurosci Lett. 1993;160(2):232‐236. doi: 10.1016/0304-3940(93)90420-P [DOI] [PubMed] [Google Scholar]
- 78. Mai L, Jope RS, Li X. BDNF‐mediated signal transduction is modulated by GSK3β and mood stabilizing agents. J Neurochem. 2002;82(1):75‐83. doi: 10.1046/j.1471-4159.2002.00939.x [DOI] [PubMed] [Google Scholar]
- 79. Beaulieu J. A role for Akt and glycogen synthase kinase‐3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2012;37(1):7‐16. doi: 10.1503/JPN.110011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1‐GSK3β signaling in schizophrenia. Nat Genet. 2004;36(2):131‐137. doi: 10.1038/ng1296 [DOI] [PubMed] [Google Scholar]
- 81. Fan X, Zhao Z, Wang D, Xiao J. Glycogen synthase kinase‐3 as a key regulator of cognitive function. Acta Biochim Biophys Sin (Shanghai). 2020;52(3):219‐230. doi: 10.1093/ABBS/GMZ156 [DOI] [PubMed] [Google Scholar]
- 82. Peineau S, Bradley C, Taghibiglou C, et al. The role of GSK‐3 in synaptic plasticity. Br J Pharmacol. 2008;153(Suppl 1):S428‐S437. doi: 10.1038/bjp.2008.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hooper C, Markevich V, Plattner F, et al. Glycogen synthase kinase‐3 inhibition is integral to long‐term potentiation. Eur J Neurosci. 2007;25(1):81‐86. doi: 10.1111/j.1460-9568.2006.05245.x [DOI] [PubMed] [Google Scholar]
- 84. Peineau S, Taghibiglou C, Bradley C, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53(5):703‐717. doi: 10.1016/j.neuron.2007.01.029 [DOI] [PubMed] [Google Scholar]
- 85. Zhu LQ, Wang SH, Liu D, et al. Activation of glycogen synthase kinase‐3 inhibits long‐term potentiation with synapse‐associated impairments. J Neurosci. 2007;27(45):12211‐12220. doi: 10.1523/JNEUROSCI.3321-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1
Data Availability Statement
The datasets used and/or analyzed during the current study are available at the OSF repository osf.io/rpa6x.


