Abstract
Through this systematic literature review, we assembled evidence to inform the EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus (SLE) and systemic sclerosis (SSc). We screened articles published between January 2000 and June 2021. Studies selected for data extraction (118 for SLE and 92 for SSc) were thematically categorised by the character of their intervention. Of 208 articles included, 51 were classified as robust in critical appraisal. Physical activity was the most studied management strategy and was found to be efficacious in both diseases. Patient education and self-management also constituted widely studied topics. Many studies on SLE found psychological interventions to improve quality of life. Studies on SSc found phototherapy and laser treatment to improve cutaneous disease manifestations. In summary, non-pharmacological management of SLE and SSc encompasses a wide range of interventions, which can be combined and provided either with or without adjunct pharmacological treatment but should not aim to substitute the latter when this is deemed required. While some management strategies i.e., physical exercise and patient education, are already established in current clinical practice in several centres, others e.g., phototherapy and laser treatment, show both feasibility and efficacy, yet require testing in more rigorous trials than those hitherto conducted.
Keywords: scleroderma, systemic; lupus erythematosus, systemic; autoimmune diseases; patient care team; patient reported outcome measures
WHAT IS ALREADY KNOWN ON THIS TOPIC
Non-pharmacological management of systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) is a helpful tool for patients and healthcare providers alike.
WHAT THIS STUDY ADDS
Physical activity and patient education comprise the most evidence-based non-pharmacological management strategies for these diseases.
In SLE, research focus has been placed on exploring psychosocial interventions.
High-quality randomised controlled trials studying the long-term efficacy of non-pharmacological management of SLE and SSc are needed.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The insights from this review can serve as an evidence base for European Alliance of Associations for Rheumatology recommendations for the non-pharmacological management of SLE and SSc.
Introduction
Systemic lupus erythematosus (SLE)1 and systemic sclerosis (SSc)2 belong to the rheumatic connective tissue diseases (CTDs) and are characterised by multiorgan involvement and a considerable morbidity burden, with a large proportion of the latter comprising comorbidities. Although advances in pharmacotherapy and non-pharmacological management have contributed to substantially improved patient outcomes during the last decades, sufferers from SLE and SSc still experience shorter life length compared with the general population and a severely impaired health-related quality of life (HRQoL).
Common organ systems that are afflicted in patients with SLE and SSc include the musculoskeletal, mucocutaneous, cardiopulmonary, vascular and nervous systems, resulting in activity limitations, pain, distress, skin eruptions and ulcers, shortness of breath, depressive symptoms and ultimately detrimental socioeconomic consequences.1 2
Accumulating evidence suggests that non-pharmacological management should constitute an integral part of the management of patients with CTDs.3–6 In patients with SLE and SSc in particular, physical activity and exercise have been shown to reduce fatigue and improve HRQoL.4 5 7 8 The appropriateness and efficacy of different delivery methods for non-pharmacological management have also been addressed in the literature, including e-health settings,9 which recently were urgently necessitated due to the pandemic caused by SARS-CoV-2.
Although many studies have been conducted on non-pharmacological interventions, evidence-driven recommendations are sparse. The systematic literature review (SLR) presented herein was conducted to inform the European Alliance of Associations for Rheumatology (EULAR) recommendations for the non-pharmacological management of SLE and SSc.10
Methods
Research questions
With the purpose of formulating recommendations for the non-pharmacological management of SLE and SSc, a task force was assembled in 2020 within EULAR, which comprised 25 experts across different healthcare professions and patient research partners. The literature search was steered by nine research questions (RQs) that were agreed on by the members of this EULAR task force. These RQs concerned the aims of non-pharmacological management (RQ1), types of interventions (RQ2), efficacy of interventions (RQ3), health-related domains or organ systems assessed (RQ4), outcome measures used (RQ5), time points of assessment (RQ6), patients’ needs, expectations and preferences (RQ7), educational needs for patients as well as healthcare providers (RQ8), and facilitators and barriers to the non-pharmacological management of SLE and SSc (RQ9). The precise formulations of the research questions are detailed in table 1.
Table 1.
Research questions formulated by the EULAR task force
| # | Research questions |
| 1 | What should non-pharmacological management aim for? |
| 2 | Which non-pharmacological interventions have been used? |
| 3 | Which non-pharmacological interventions have been shown to be efficacious? |
| 4 | Which instruments have been used to assess the outcome of non-pharmacological management? |
| 5 | When should the outcome of non-pharmacological management be assessed? |
| 6 | Within which health-related domains or organ systems should non-pharmacological management be assessed? |
| 7 | What are the patients’ needs, expectations and preferences with regard to non-pharmacological management of SLE and SSc? |
| 8 | What are the educational needs for healthcare professionals and patients regarding non-pharmacological management? |
| 9 | What are the facilitators and barriers to the use of non-pharmacological management? |
For all research questions, the populations of interest were adult patients with SLE or SSc.
EULAR, European Alliance of Associations for Rheumatology; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Search strategy and article selection
The search strategy was designed in collaboration with expert librarians from Karolinska Institutet. On 22 June 2021, the MEDLINE (Ovid), EMBASE (embase.com), Web of Science Core Collection and CINAHL (EBSCO) databases were searched for content published from January 2000 to June 2021. We conducted a two-block search, including the diagnoses of interest and a list of non-pharmacological management strategies, presented in full in online supplemental file 1. We excluded case series of less than five individuals, and articles in languages other than English, Spanish and Swedish. Due to the diverse nature of the questions, we did not exclude articles based on study design. Two independent reviewers (AG and JWC) screened the identified titles and abstracts. Disagreements between reviewers were solved through a consensus, together with two more investigators (IP and CB).
rmdopen-2023-003297supp001.pdf (37.2KB, pdf)
Data extraction
Using electronic forms customised for each RQ, two independent researchers per disease (AG and DP for SLE; AT and JWC for SSc) extracted information from full texts, including author, year of publication, country, study design, number of participants, demographic and clinical characteristics, intervention, health-related domain, outcome measure, time point of assessments and reported results. Considering the diversity on study designs included herein, and that one of the RQs concerned outcome measures used (RQ5), outcomes were summarised as reported by the authors, and comprised dichotomous and continuous data, as well as effect measures for dichotomous outcomes (risk ratios and ORs) and continuous outcomes (mean and standardised mean differences).
Discrepancies were discussed among the four researchers until a consensus was reached; when needed, IP and CB were consulted to resolve disagreements.
Critical appraisal
Risk of bias (RoB) of all included articles was conducted by the same investigator (AT) under the supervision of IP, using the critical appraisal (CA) tools (online supplemental checklists) by the Joanna Briggs Institute.11 Since study selection was not performed based on RoB, the overall appraisal terms “include”, “exclude” and “seek further info” were not applicable, and were thus replaced by “robust”, “weak” and “intermediate”, respectively. A study was rated as robust if it clearly fulfilled all, or all but no more than two checklist criteria, intermediate if it fulfilled all but three to five criteria, or weak if six or more checklist criteria were not clearly fulfilled.
rmdopen-2023-003297supp002.pdf (59.7KB, pdf)
Results
Study selection
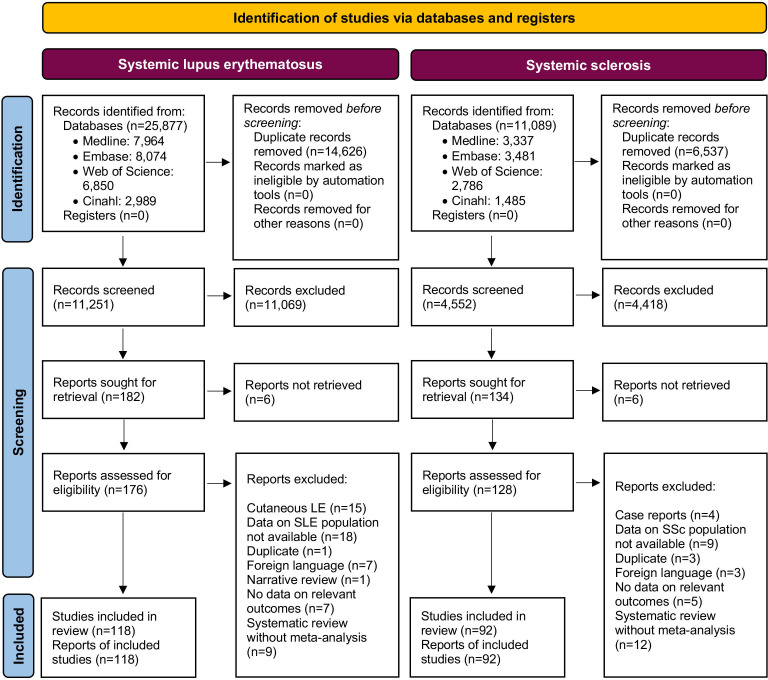
Stratified by diagnosis, the search resulted in 25 877 and 11 251 hits for SLE, and 11 089 and 4 552 hits for SSc before and after deduplication, respectively (figure 1 and online supplemental file 1). Of those 25 877 initial hits for articles on the non-pharmacological management of SLE, 118 articles were selected for full-text evaluation. Of the 11 089 initial hits for articles on the non-pharmacological management of SSc, 92 articles were selected for full-text evaluation (figure 1). Two studies, one cross-sectional study assessed as intermediate12 and one qualitative study assessed as robust13 in CA, included both patients with SLE and patients with SSc, and were therefore included in the analysis for both diseases.
Figure 1.
PRISMA flow diagram illustrating the steps followed for identification of studies. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
rmdopen-2023-003297supp003.pdf (392.5KB, pdf)
Study design and CA
For SLE, the largest study design categories were randomised controlled trials (RCTs; n=49), followed by quasi-experimental studies (n=38) and cross-sectional studies (n=12). For SSc, the largest study design categories were quasi-experimental studies (n=37), followed by RCTs (n=33) and qualitative studies (n=8). For SLE, 28 articles were assessed as robust in CA, 52 articles were assessed as intermediate and 38 were assessed as weak. For SSc, 24 articles were assessed as robust, 52 articles were assessed as intermediate and 16 articles were assessed as weak. A summary of the CA of the studies, sorted by disease and study design, is presented in table 2.
Table 2.
Critical appraisal of selected articles sorted by disease and study design
| Study design, disease | Robust | Intermediate | Weak | Total |
| Randomised controlled trials | ||||
| SLE | 0 | 16 | 33 | 49 |
| SSc | 2 | 18 | 13 | 33 |
| Quasi-experimental studies | ||||
| SLE | 8 | 29 | 1 | 38 |
| SSc | 13 | 23 | 1 | 37 |
| Cross-sectional studies | ||||
| SLE | 6 | 6 | 0 | 12 |
| SSc | 1 | 4 | 2 | 7 |
| Qualitative studies | ||||
| SLE | 6 | 0 | 2 | 8 |
| SSc | 7 | 1 | 0 | 8 |
| Case series | ||||
| SLE | 0 | 0 | 1 | 1 |
| SSc | 0 | 4 | 0 | 4 |
| Cohort studies | ||||
| SLE | 3 | 0 | 1 | 4 |
| SSc | 0 | 1 | 0 | 1 |
| Meta-analyses | ||||
| SLE | 5 | 0 | 0 | 5 |
| SSc | 0 | 0 | 0 | 0 |
| Case–control studies | ||||
| SLE | 0 | 1 | 0 | 1 |
| SSc | 1 | 1 | 0 | 2 |
SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Data extraction
Online supplemental tables 1–3 present the extracted data from all articles selected for data extraction, whereas online supplemental tables 4–11 detail the CA for each study, sorted by study design. Study design and overall appraisal for each study are also provided in online supplemental tables 1–3; in those tables, overall appraisal is colour coded to aid readability, with green denoting robust, yellow denoting intermediate and red denoting weak studies. The results (sorted by RQ) are summarised below.
Aims of management (RQ1)
For SLE, effects on HRQoL and disease activity emerged as major aims of non-pharmacological management, being addressed in 47 and 44 studies, respectively. Other prominent aims included improvements in fatigue (n=28), depression (n=24) and pain (n=15), as well as prevention of organ damage (n=11), increased self-efficacy (n=9) and improvements in aerobic capacity (n=6).
For SSc, many studies investigated improvements of hand mobility (n=22), HRQoL (n=19) and microstomia (n=11). Other aims of the non-pharmacological management of SSc included improvements in skin sclerosis other than perioral (n=10), gastrointestinal symptoms (n=7), skin ulcers (n=7), Raynaud’s phenomenon (n=5) and depression (n=4).
Categories of interventions (RQ2)
For SLE, the largest category of non-pharmacological management was physical exercise and physical activity (n=34),7 14–45 followed by patient education and self-management (n=21),13 31 46–64 psychological interventions (n=21),37 65–84 dietary therapy and nutrition (n=14),19 85–97 complementary medicine (n=5),98–102 photoprotection (n=5),103–107 healthcare models (n=4),108–111 laser treatment (n=2),112 113 social support (n=2)114 115 and others (n=6).116–121
For SSc, the largest category of non-pharmacological management was, as in SLE, physical exercise and physical activity (n=32),122–153 followed by patient education and self-management (n=12),13 144 148 154–162 bathing and thermal modalities (n=8),123 136 141–143 163–165 complementary medicine (n=8),146 163 166–171 manual therapy (n=8),126 127 129 136 139 172–174 dietary therapy and nutrition (n=6),175–180 phototherapy and laser treatment (n=6),181–186 shockwave therapy (n=4),187–190 healthcare models (n=3),155 191 192 hyperbaric oxygen or ozone therapy (n=3),165 193 194 oral hygiene (n=3)131 191 195 and others (n=4).196–199
Efficacy of interventions (RQ3)
Systemic lupus erythematosus
Physical exercise and physical activity
Three meta-analyses, all assessed as robust in CA, evaluated the effect of physical exercise and physical activity. The first meta-analysis, performed in 2017 on six RCTs and five quasi-experimental studies, found that exercise improved aerobic capacity (mean difference: 1.85; 95% CI: 1.12, 2.58; p<0.001) and decreased fatigue (mean difference: −0.61; 95% CI: −1.19 to –0.02; p=0.04) and depressive symptoms (mean difference: −0.40; 95% CI: −0.71 to –0.09; p=0.01) in patients with SLE, yet did not affect disease activity (mean difference: 0.01; 95% CI: −0.54, 0.56; p=0.97). The second meta-analysis was conducted the same year on two RCTs and one quasi-experimental study, and found that exercise reduced fatigue in patients with SLE (mean difference: −0.52; 95% CI: −0.91 to –0.13; p=0.009).8 The third meta-analysis, conducted in 2019, included two RCTs and found that physical activity improved physical functioning as measured by the 36-Item Short Form Survey (SF-36; mean difference: −9.20; 95% CI: –18.16, –0.23; p=0.04), but not vitality.37
Patient education and self-management
Ten RCTs, all assessed as weak in CA, examined patient education and self-management. Of these, five studies employed interventions aiming to improve medication adherence.50 51 53 57 60 The interventions that proved efficacious among those were patient information50 57 and targeted nursing (ie, tailored according to pathogenic condition and treatment period),53 whereas text-message reminders51 and electronic pill boxes60 did not show significant adherence improvement. Other RCTs (CA: weak) found web-based patient education to be efficacious in managing fatigue,61 self-efficacy,61 sleep disturbance63 and anxiety.63 Two non-RCTs in this category were assessed as robust in CA. These were both qualitative studies evaluating patient education programmes on disease management, where patients expressed satisfaction with their respective programmes.13 48
Psychological interventions
Three meta-analyses, all assessed as robust in CA, evaluated the effect of psychological interventions. The first meta-analysis, conducted in 2012 on six RCTs, found psychological interventions to be efficacious in managing anxiety (mean difference: −0.95; 95% CI: −1.57 to –0.34; p<0.001), depression (mean difference: −1.14; 95% CI: −1.84 to –0.44; p<0.001), disease activity (mean difference: −0.34; 95% CI: −0.57 to –0.11; p<0.001) and stress (mean difference: −0.63; 95% CI: −1.02 to –0.23; p<0.001).75 The second meta-analysis, conducted in 2014 on six RCTs, found psychological interventions efficacious in managing depression (mean difference: −0.44; 95% CI: −0.78, 0.10; p=0.01) and SF-36 physical component summary scores (mean difference: 8.85; 95% CI: 3.69, 14.0; p<0.001).77 The third meta-analysis, conducted in 2019 on two RCTs, found cognitive–behavioural therapy to improve HRQoL (mean difference: −17.7; 95% CI: –26.7, –8.63; p<0.001).37
Other categories
Studies on dietary therapy and nutrition that were assessed as robust in CA were all observational in nature86 90 97 and found that increased intake of vitamin B6 and vitamin C was associated with lower SLE disease activity,86 90 and that adherence to a Mediterranean diet was associated with a lower cardiovascular risk.97 One cross-sectional study on complementary medicine (CA: robust), defined as the use of any treatment not prescribed by an allopathic primary or specialist physician, was assessed as robust in CA and noted lower levels of organ damage and a better quality of life in patients who had used complementary therapies.100 One quasi-experimental study (CA: robust) showed that sunscreen protected against upregulation of ICAM-1 mRNA.103 Studies on healthcare models were few, non-robust in CA and diverse.108–111 Pulsed dye laser on discoid lesions was shown in one quasi-experimental study (CA: robust) to decrease mucocutaneous activity assessed using the Cutaneous Lupus Erythematosus Disease Area and Severity Index.112 Two cross-sectional studies (CA: robust) found that social support associated with better HRQoL, illness uncertainty and coping skills.114 115 One cross-sectional study (CA: robust) found that smoking cessation was associated with lower SLE disease activity,118 and one quasi-experimental study (CA: robust) found warm showers efficacious in improving fatigue.119
Systemic sclerosis
Physical exercise and physical activity
An RCT assessed as robust in CA evaluated the effect of a 4-week physical therapy programme followed by home exercise and found the intervention to be efficacious in managing microstomia, at a 12-month follow-up.137 RCTs assessed as intermediate in CA found physical exercise and physical activity to be efficacious in managing microstomia,132 149 aerobic capacity147 and hand function.151 Quasi-experimental studies assessed as robust in CA found physical exercise and physical activity to be efficacious in managing microstomia,122 aerobic capacity,130 pain144 and hand function assessed with the Hand Mobility in Scleroderma Instrument (HAMIS), but not functional impairment assessed with the Health Assessment Questionnaire-Disability Index (HAQ-DI).125
Patient education and self-management
The RCT rated highest in CA (CA: intermediate) evaluating patient education and self-management found face-to-face instruction combined with educational material more effective than educational material alone.161 Another RCT (CA: weak) found an internet-based self-management programme to improve quality of life.159 In quasi-experimental studies assessed as robust in CA, educating patients on self-management was found to improve self-efficacy,157 functional impairment158 and pain.144
Other categories
Two quasi-experimental studies (CA: robust) that investigated a combination of non-pharmacological interventions including bathing and thermal modalities found the interventions to be efficacious in improving hand function.136 141 A case–control study (CA: robust) found anorectal biofeedback therapy to ameliorate faecal incontinence.169 An RCT (CA: robust) on manual lymph drainage was also found to improve hand function.172 One RCT (CA: intermediate) and one quasi-experimental study (CA: intermediate) found that probiotics improved gastrointestinal symptoms.175 178 Two pilot studies, both quasi-experimental in nature (CA: robust), found light therapy to improve telangiectases182 and pain in digital ulcers,186 respectively. Four quasi-experimental studies on shockwave therapy (CA: intermediate) found the intervention to be efficacious in improving skin ulcers189 190 and skin sclerosis.187 188 A quasi-experimental study (CA: robust) on a multidisciplinary disease management programme found this to improve feelings of helplessness and acceptance of limitations.155 One case series (CA: intermediate) and one quasi-experimental study (CA: intermediate) found hyperbaric oxygen193 and ozone therapy165 aiding resolution of skin ulcers. Studies on oral hygiene interventions were few, dissimilar in design and not robust in CA.131 191 195 Individual studies have shown efficacy of autologous fat transplantation in improving mouth opening,196 of neuromuscular taping in improving hand mobility,197 of animal-assisted intervention (pet therapy) in alleviating anxiety198 and of application of amniotic membrane dressings in resolution of skin ulcers199; these studies were assessed as intermediate in CA.
Outcome measures (RQ4)
Numerous outcome measures have been used to determine the efficacy of non-pharmacological interventions of SLE and SSc. For detailed listings of which outcome measures were used for each study, see online supplemental table 3.
For SLE, the physician-assessed Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)200 and Systemic Lupus Activity Measure (SLAM)201 as well as the patient-reported Systemic Lupus Activity Questionnaire202 were used to determine disease activity. Exercise capacity was often assessed with the maximal oxygen uptake (VO2-max). Organ damage was mainly assessed through the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.203 Outcomes for the estimation of quality of life included the generic SF-36,204 EQ-5D205 and Patient-Reported Outcomes Measurement Information System (PROMIS),206 as well as the disease-specific Lupus Quality of Life questionnaire.207 Fatigue was mainly assessed with the Fatigue Severity Scale (FSS),208 Functional Assessment of Chronic Illness Therapy–Fatigue,209 Multidimensional Assessment of Fatigue210 and Visual Analogue Scales. Depression was assessed with the Center for Epidemiological Studies-Depression Scale,211 Hospital Anxiety and Depression Scale (HADS)212 and Beck Depression Inventory (BDI).213 Pain was mainly assessed through Visual Analogue Scales. Anxiety was mainly assessed with HADS and the State-Trait Anxiety Inventory (STAI).214 Body composition was mainly assessed through the body mass index.215 Self-efficacy was mainly assessed through self-efficacy scales.216 For a detailed overview of all outcome measures used in the included studies, see online supplemental table 3. The main inflammatory markers assessed were tumour necrosis factor alpha, C reactive protein, C3 and C4, interleukin (IL)-6 and IL-10.
For SSc, functional impairment was often measured with the HAQ-DI,217 and hand-specific outcomes such as HAMIS218 and Duruoz Hand Index.219 Skin sclerosis was mainly assessed using the modified Rodnan Skin Score,220 but also with light microscopy, as well as with subjective assessments by both patients and clinicians. Microstomia was virtually always assessed using length measurements, such as maximal mouth opening and interincisal distance. Skin ulcers were mainly assessed with qualitative scoring, and quantitatively with, for example, number of ulcers and time to ulcer resolution. Digestion was mainly assessed with the University of California Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument221 among other questionnaires. Oral hygiene was mainly assessed through subjective patient reporting, or with scoring instruments such as the Löe-Silness Gingival Index.222 Exercise capacity was often assessed with VO2-max and the 6 min walk test.223 Quality of life was mainly assessed using the SF-36 and PROMIS. Pain was mainly assessed using Visual Analogue Scales. Circulation was mainly assessed using laser Doppler imaging. The main biomarkers assessed were von Willebrand factor, vascular endothelial growth factor and IL-6.
Time points of assessment (RQ5)
Systemic lupus erythematosus
Studies on physical exercise and physical activity assessed outcomes at widely varying time points, from minutes after a single bout of exercise32 up to 36 months after baseline.45 Interventions employing patient education and self-management were assessed between 1 week (at the earliest)56 and 3 years (at the latest)55 from baseline. Psychological interventions were assessed at the earliest after 1 week from intervention initiation84 and at the latest after 15 months.73 Studies dealing with dietary therapy and nutrition assessed outcomes between 4 weeks and 5 years from baseline. Assessment time points for complementary therapies varied largely, ranging from 12 weeks to 2 years from baseline. The effect of photoprotection was assessed after 24 hours at the earliest to 9 weeks at the latest. Healthcare models were assessed between 12 weeks and several years after initiation of the intervention. Laser treatment was assessed at 4, 8 and 12 weeks from baseline. Studies on social support were of cross-sectional design and did not evaluate social support after a defined time point (see online supplemental table 3).
Systemic sclerosis
Studies examining the impact of physical exercise and physical activity in SSc assessed outcomes of the intervention from immediately after the intervention,136 with the study with the longest follow-up period in this category assessing participants yearly for 3 years.139 Studies on patient education and self-management assessed outcome measures between 4 weeks144 and 6 months158 from baseline. Assessment time points regarding bathing and thermal modalities varied widely, ranging from a few minutes to a full year after baseline. Complementary therapies were assessed between hours and 6 months from initiation of the intervention. The effects of manual therapy were assessed between 2 weeks and 3 years after baseline. Effects of dietary therapy and nutrition were assessed between 1 and 18 months after baseline. Studies on phototherapy and laser treatment assessed effects after as little as one bout of treatment to a year post-baseline. Outcomes of shockwave therapy were assessed at time points between one round of therapy and 9 weeks after baseline. Studies on healthcare models assessed outcomes after 6 weeks–12 months. Studies on hyperbaric oxygen or ozone therapy had set assessment time points at 20 and 40 days. Outcomes of oral hygiene interventions were evaluated between 10 min and 1 year after baseline.
Health-related domains or organ systems assessed (RQ6)
Systemic lupus erythematosus
In patients with SLE, non-pharmacological management strategies that were found to be efficacious within the mucocutaneous domain included laser treatment113 and photoprotection.105 Within the cardiopulmonary, vascular and musculoskeletal domains, exercise was found to improve aerobic capacity and neuromuscular responses, and lower the cardiovascular risk.16 42 86 Within the neuropsychiatric domain, anxiety and depression were efficaciously managed by cognitive–behavioural therapy.69
Systemic sclerosis
In patients with SSc, challenges within the mucocutaneous domain mainly consisted of skin sclerosis, calcinosis, skin ulcers and microstomia; these were shown to be efficaciously managed with, for example, physiotherapy,122 multidisciplinary care models,155 phototherapy186 and shockwave therapy.188 Within the cardiopulmonary and vascular domains, exercise improved VO2-max and cutaneous vascular conductance, respectively.128 145 Within the musculoskeletal domain, high-intensity interval training was found efficacious for improving inspiratory muscle and grip strength.150 Gastrointestinal manifestations, for example, bloating and distension, were efficaciously managed with probiotics.175 Within the neuropsychiatric domain, anxiety was efficaciously managed with, for example, animal-assisted interventions.198
Patients’ needs, expectations and preferences (RQ7)
Systemic lupus erythematosus
A qualitative study (CA: robust) assessing patients with SLE from medically underserved communities found that patients desired more education about their disease, as well as assistance in navigating the healthcare system.224 Patients also favoured peer support and the idea of a lupus health passport, that is, a notebook containing a personalised treatment plan, preventive health tips and health information.224 A thematic analysis of patient responses to open-ended questions posed online (CA: robust) identified increased visibility as a need of patients with SLE, both in social and healthcare settings.225
Systemic sclerosis
A qualitative study (CA: robust) aiming to characterise illness perception of patients with early SSc identified low personal control and concerns about the future as needs to be addressed.226 Another qualitative study (CA: robust) noted strong expectations from patients with SSc on the patient–physician relationship, including involvement in research and individualised treatment decisions.227
Educational needs (RQ8)
Systemic lupus erythematosus
An interview study including six participants (CA: robust) found poor communication and lack of validation to be shortcomings experienced by patients with SLE in interaction with their healthcare providers.228 This study concluded that there was a necessity to integrate physicians into social support interventions. To increase visibility and improve the care provided to patients with SLE, a study advocated that medical professionals might benefit from training of their skills in managing the psychosocial consequences of the disease.225
Systemic sclerosis
An observational study (CA: intermediate) employing open-ended questioning specifically addressing educational needs for healthcare professionals in the non-pharmacological management of SSc found that the educational needs were mainly oriented around the management of stiffness, pain and impaired hand function.229 A series of focus group interviews (CA: intermediate) found physical manifestations and disclosure of one’s disease to be central themes defining the experience of living with SSc, and concluded that healthcare professionals may stigmatise individuals due to ignorance of rare conditions.230
Facilitators and barriers (RQ9)
Systemic lupus erythematosus
Difficulty in navigating healthcare systems emerged as a barrier to disease management in patients with SLE.224 225 A qualitative analysis of the LUPUS UK online forum (CA: robust) found that diagnostic delays due to disbelief or dismissal of symptoms, along with medical miscommunications and misunderstandings, were perceived as barriers by patients.231 An interview study on smoking cessation (CA: weak) identified concerns for one’s health and concerns for others as facilitators, whereas enjoyment and using smoking as a coping mechanism emerged as barriers to quitting smoking.232
Systemic sclerosis
Two qualitative studies (CA: robust) found barriers of physical exercise in patients with SSc related to disease, such as shortness of breath and pain.233 234 Adjustments of duration, intensity and choice of physical activity were identified as facilitators for exercise.233 A study employing focus group discussions (CA: robust) found that for social support, careful choice of support source and honest communication could constitute facilitators, while aversion to speaking about one’s disease emerged as a barrier.235 This study also highlighted the importance of close relationships for enhanced social support.235
Discussion
In summary, this review examined current evidence on the non-pharmacological management of SLE and SSc. Physical exercise was the most studied management strategy in both diseases, and was found to be efficacious with regard to several outcomes, such as fatigue (in SLE) and hand function (in SSc) in studies assessed as robust in CA. Studies on patient education and self-management found improvements regarding self-efficacy, although these studies were of varying robustness in overall CA. Psychological interventions represented a prominent management strategy for depressive symptoms in SLE, while many studies on SSc pertained to phototherapy and laser treatment and found these interventions to be efficacious in improving cutaneous manifestations.
In several studies, interventions aimed to improve quality of life. In SSc, particular focus was also placed in functional impairment. Interventions identified during a systematic literature search were categorised thematically; key intervention categories included physical exercise, patient education and self-management. Non-pharmacological management of both SLE and SSc was mostly assessed for its effects within the mucocutaneous, cardiopulmonary, vascular, musculoskeletal and neuropsychiatric domains, whereas gastrointestinal manifestations were mainly assessed in SSc only.
The instruments for assessing outcomes of management were largely similar for both diseases in domains such as quality of life (SF-36), depression (HADS, BDI), anxiety (STAT-I) and fatigue (FSS). SLE disease activity was often assessed using SLAM or SLEDAI. In SSc, assessment of functional impairment using HAQ, the Mouth Handicap in Systemic Sclerosis scale (MHISS) or HAMIS appeared central. Within many intervention categories, outcomes were assessed at or around 12 weeks after baseline.
Patients’ needs, expectations and preferences involved the difficulty of navigating healthcare systems. This was in line with educational needs for healthcare professionals, which included an increased understanding of patients’ experience of their disease. Intervention-specific facilitators and barriers to non-pharmacological management related to social relationships of the patients, among other facets.
Within the SLE literature selected for analysis, studies on healthcare models were few, non-robust in CA and diverse; thus, no firm conclusions could be drawn regarding the efficacy of healthcare models.108–111 Likewise, studies on oral hygiene interventions within the selected SSc literature were few, dissimilar in design and non-robust in CA; thus, no firm conclusions could be drawn regarding their efficacy.131 191 195 Regarding organ systems for which interventions were evaluated, the gastrointestinal tract emerged as an important health-related domain that was often subject to investigation in SSc, but not in SLE. While gastrointestinal symptoms also constitute SLE manifestations, the higher number of studies addressing gastrointestinal symptoms in SSc may reflect that these symptoms comprise a larger area of concern in patients with SSc, or that efficacy of non-pharmacological management of the gastrointestinal domain within SLE has yet to be thoroughly explored.
Qualitative studies explicitly evaluating patients’ needs, expectations and preferences were not as intervention specific as the studies investigating facilitators and barriers to non-pharmacological management.232–234 Several studies that were selected for systematic data extraction were aimed at investigating tolerability and feasibility of different interventions.19 58 60 99 160 186
A subgroup of studies on patient education and self-management in SLE evaluated medication adherence,50 51 53 57 60 whereas a subgroup of studies within the same category in SSc evaluated self-administered rehabilitation programmes.144 148 161 These subcategories within educational interventions were unique for SLE or SSc, suggesting that certain issues addressed in studies are specific to each disease, presumably reflecting disease-specific patient needs. Furthermore, educational interventions were oftentimes managed and assessed by nurses, physiotherapists or occupational therapists.53 57 154 158 161 Since physicians have an overarching responsibility for the patients’ well-being, this poses to physicians the requirement of, at minimum, an understanding of the patient needs and patient education strategies.
Physical exercise was the most studied non-pharmacological management strategy in both SLE and SSc. Although many studies, especially on the management of SSc, examined combinations of different therapeutic modalities rather than only one intervention,126 129 131 135 136 143 none of the selected studies aimed at replacing pharmaceutical treatment with non-pharmacological alternatives.
The high degree of heterogeneity of the management strategies explored in literature limited us from performing in-depth analysis of individual strategies. Another shortcoming of the present SLR was that systematic cut-offs for overall appraisal with the CA tools from the Joanna Briggs Institute,11 conducted as described in the Methods section, have not been validated, and different ways to interpret the checklists could impact what studies were deemed as robust. A measure taken to assure quality in highly appraised studies was treating unclearly fulfilled criteria as unfulfilled, which may have led to a somewhat conservative selection of studies assessed as robust.
This review mapped a wide range of non-pharmacological interventions, as well as gaps in the current knowledge in the field of non-pharmacological management of SLE and SSc; most RCTs conducted on this subject lacked assessment time points after 1 year from baseline, and RCTs included in the review were often unclear about the blinding strategies followed for study participants, treatment providers and outcome assessors.
Apart from physical exercise, patient education and self-management, other large categories of interventions were distinct between SLE and SSc, that is, psychological interventions in SLE and phototherapy and laser treatment in SSc; laser treatment also emerged as an intervention category in SLE, although with fewer studies evaluating its efficacy.
All RCTs evaluating psychological interventions for the management of SLE were assessed as weak in CA.66 68 72–74 78 84 This may be due to the method followed for systematically evaluating overall appraisal, since RCTs are assessed by a higher amount of criteria compared with other study designs.11 Phototherapy and laser treatment,181–186 as well as shockwave therapy,187–190 were found to be efficacious for the management of SSc, and studies of these were all assessed as intermediate or robust in CA. However, none of these studies had an RCT design.
These findings underpin an encouragement for future studies of RCT design to clarify blinding strategies, and to assess outcomes in the long term, that is, a year or beyond. Also, while several quasi-experimental studies suggest that phototherapy and laser treatment strategies have favourable effects on skin sclerosis and wound healing in patients with SSc, future RCTs evaluating their efficacy are desirable.
The literature search was restricted to articles published between January 2000 and June 2021, which may be considered a limitation. This decision was made due to several reasons. First, since the SLR was conducted to inform the EULAR recommendations for the non-pharmacological management of SLE and SSc, it was important to ensure that the recommendation statements were not based on dated evidence. Second, major changes in the pharmacological management of rheumatic diseases were introduced in the beginning of the current century, for example, the introduction of biological agents. This had implications in the overall management of patients with rheumatic diseases; even though the first biological therapy for SLE was approved in 2011, off-label use of, for example, rituximab can be traced in literature back to the early 2000s. Lastly, important papers published earlier than January 2000 were captured through citations in more recent papers, including comprehensive reviews used for the background and discussion of the present SLR, as well as through experts’ awareness.
In conclusion, non-pharmacological management of SLE and SSc encompasses a wide range of interventions, which can be combined and provided either with or without adjunct pharmacological treatment, but should not aim to substitute the latter when this is deemed required. While some of the management strategies supported in this review, that is, physical exercise and patient education, are already established in current clinical practice in several centres, others, for example, phototherapy and laser treatment, show both feasibility and efficacy, yet require testing in more rigorous trials than those hitherto conducted.
rmdopen-2023-003297supp004.pdf (1.7MB, pdf)
Acknowledgments
The authors express gratitude to the members of the EULAR task force for recommendations of non-pharmacological management of SLE and SSc, as well as the EULAR Secretariat for the assistance. They would also like to thank Emma-Lotta Säätelä, librarian at the KI Library, for her help with the search strategy.
Footnotes
Twitter: @IoannisParodis parodislab, @gomezg_alvaro
Contributors: IP, AG and AT wrote the first draft of the manuscript. AG and JWC screened the identified titles and abstracts. Disagreements between reviewers were solved through a consensus, together with IP and CB. AG, AT, JWC and DP extracted data from selected articles. CG and TAS provided methodological expertise and guided the work process. All authors contributed to the interpretation of results, as well as read and approved the final version of the manuscript. IP and CB are responsible for the overall content as guarantors, controlled the decision to publish, and accept full responsibility for the finished work and conduct of the project.
Funding: This project was funded by the European Alliance of Associations for Rheumatology (EULAR) (ref: HPR046).
Competing interests: IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia, Elli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Otsuka and Roche. The other authors declare that they have no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. 10.1038/nrdp.2016.39 [DOI] [PubMed] [Google Scholar]
- 2.Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685–99. 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 3.Alexanderson H, Boström C. Exercise therapy in patients with idiopathic inflammatory Myopathies and systemic lupus erythematosus - A systematic literature review. Best Pract Res Clin Rheumatol 2020;34:S1521-6942(20)30064-4. 10.1016/j.berh.2020.101547 [DOI] [PubMed] [Google Scholar]
- 4.Fangtham M, Kasturi S, Bannuru RR, et al. Non-pharmacologic therapies for systemic lupus erythematosus. Lupus 2019;28:703–12. 10.1177/0961203319841435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liem SIE, Vliet Vlieland TPM, Schoones JW, et al. The effect and safety of exercise therapy in patients with systemic sclerosis: a systematic review. Rheumatol Adv Pract 2019;3:rkz044. 10.1093/rap/rkz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willems LM, Vriezekolk JE, Schouffoer AA, et al. Effectiveness of Nonpharmacologic interventions in systemic sclerosis: A systematic review. Arthritis Care Res (Hoboken) 2015;67:1426–39. 10.1002/acr.22595 [DOI] [PubMed] [Google Scholar]
- 7.O’Dwyer T, Durcan L, Wilson F. Exercise and physical activity in systemic lupus erythematosus: A systematic review with meta-analyses. Semin Arthritis Rheum 2017;47:204–15. 10.1016/j.semarthrit.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 8.Wu ML, Yu KH, Tsai JC. The effectiveness of exercise in adults with systemic lupus erythematosus: A systematic review and meta-analysis to guide evidence-based practice. Worldviews Evid Based Nurs 2017;14:306–15. 10.1111/wvn.12221 [DOI] [PubMed] [Google Scholar]
- 9.Ritschl V, Ferreira RJO, Santos EJF, et al. Suitability for E-health of non-pharmacological interventions in connective tissue diseases: Scoping review with a descriptive analysis. RMD Open 2021;7:e001710. 10.1136/rmdopen-2021-001710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parodis I, Girard-Guyonvarc’h C, Arnaud L, et al. EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus and systemic sclerosis. Ann Rheum Dis 2023:ard-2023-224416. 10.1136/ard-2023-224416 [DOI] [PubMed] [Google Scholar]
- 11.JBI manual for evidence synthesis. 2020. Available: https://synthesismanual.jbi.global
- 12.Arat S, Lenaerts JL, De Langhe E, et al. Illness representations of systemic lupus erythematosus and systemic sclerosis: a comparison of patients, their Rheumatologists and their general practitioners. Lupus Sci Med 2017;4:e000232. 10.1136/lupus-2017-000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown SJ, Somerset ME, McCabe CS, et al. The impact of group education on participants' management of their disease in lupus and scleroderma. Musculoskeletal Care 2004;2:207–17. 10.1002/msc.72 [DOI] [PubMed] [Google Scholar]
- 14.Ramsey-Goldman R, Schilling EM, Dunlop D, et al. A pilot study on the effects of exercise in patients with systemic lupus erythematosus. Arthritis Rheum 2000;13:262–9. [DOI] [PubMed] [Google Scholar]
- 15.Tench CM, McCarthy J, McCurdie I, et al. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford) 2003;42:1050–4. 10.1093/rheumatology/keg289 [DOI] [PubMed] [Google Scholar]
- 16.Carvalho MRP de, Sato EI, Tebexreni AS, et al. Effects of supervised cardiovascular training program on exercise tolerance, aerobic capacity, and quality of life in patients with systemic lupus erythematosus. Arthritis Rheum 2005;53:838–44. 10.1002/art.21605 Available: http://doi.wiley.com/10.1002/art.v53:6 [DOI] [PubMed] [Google Scholar]
- 17.Clarke-Jenssen AC, Fredriksen PM, Lilleby V, et al. Effects of supervised aerobic exercise in patients with systemic lupus erythematosus: a pilot study. Arthritis Rheum 2005;53:308–12. 10.1002/art.21082 Available: http://doi.wiley.com/10.1002/art.v53:2 [DOI] [PubMed] [Google Scholar]
- 18.do Prado DML, Gualano B, Miossi R, et al. Erratic control of breathing during exercise in patients with systemic lupus erythematosus: a pilot-study. Lupus 2011;20:1535–40. 10.1177/0961203311425525 [DOI] [PubMed] [Google Scholar]
- 19.Otto AD, Mishler AE, Shah N, et al. Feasibility of implementing a lifestyle intervention in overweight and obese patients with systemic lupus erythematosus. Med Sci Sport Exerc 2011;43:123. 10.1249/01.MSS.0000403040.26185.b7 [DOI] [Google Scholar]
- 20.Yuen HK, Holthaus K, Kamen DL, et al. Using WII fit to reduce fatigue among African American women with systemic lupus erythematosus: a pilot study. Lupus 2011;20:1293–9. 10.1177/0961203311412098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miossi R, Benatti FB, de Sá Pinto AL, et al. Using exercise training to counterbalance chronotropic incompetence and delayed heart rate recovery in systemic lupus erythematosus: a randomized trial. Arthritis Care Res 2012;64:n 10.1002/acr.21678 [DOI] [PubMed] [Google Scholar]
- 22.da Silva AE, dos Reis-Neto ET, da Silva NP, et al. The effect of acute physical exercise on cytokine levels in patients with systemic lupus erythematosus. Lupus 2013;22:1479–83. 10.1177/0961203313508832 [DOI] [PubMed] [Google Scholar]
- 23.dos Reis-Neto ET, da Silva AE, Monteiro CM de C, et al. Supervised physical exercise improves endothelial function in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:2187–95. 10.1093/rheumatology/ket283 [DOI] [PubMed] [Google Scholar]
- 24.Barnes JN, Nualnim N, Dhindsa M, et al. Macro- and Microvascular function in habitually exercising systemic lupus erythematosus patients. Scand J Rheumatol 2014;43:209–16. 10.3109/03009742.2013.846408 [DOI] [PubMed] [Google Scholar]
- 25.Perandini LA, Sales-de-Oliveira D, Mello SBV, et al. Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J Appl Physiol (1985) 2014;117:639–47. 10.1152/japplphysiol.00486.2014 [DOI] [PubMed] [Google Scholar]
- 26.Benatti FB, Miossi R, Passareli M, et al. The effects of exercise on lipid profile in systemic lupus erythematosus and healthy individuals: a randomized trial. Rheumatol Int 2015;35:61–9. 10.1007/s00296-014-3081-4 [DOI] [PubMed] [Google Scholar]
- 27.Bogdanovic G, Stojanovich L, Djokovic A, et al. Physical activity program is helpful for improving quality of life in patients with systemic lupus erythematosus. Tohoku J Exp Med 2015;237:193–9. 10.1620/tjem.237.193 [DOI] [PubMed] [Google Scholar]
- 28.Perandini LA, Sales-de-Oliveira D, Mello S, et al. Inflammatory cytokine Kinetics to single bouts of acute moderate and intense aerobic exercise in women with active and inactive systemic lupus erythematosus. Exerc Immunol Rev 2015;21:174–85. [PubMed] [Google Scholar]
- 29.Abrahão MI, Gomiero AB, Peccin MS, et al. Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: a randomized controlled trial. Scand J Rheumatol 2016;45:197–201. 10.3109/03009742.2015.1094126 [DOI] [PubMed] [Google Scholar]
- 30.Avaux M, Hoellinger P, Nieuwland-Husson S, et al. Effects of two different exercise programs on chronic fatigue in lupus patients. Acta Clin Belg 2016;71:403–6. 10.1080/17843286.2016.1200824 [DOI] [PubMed] [Google Scholar]
- 31.Boström C, Elfving B, Dupré B, et al. Effects of a one-year physical activity programme for women with systemic lupus erythematosus - a randomized controlled study. Lupus 2016;25:602–16. 10.1177/0961203315622817 [DOI] [PubMed] [Google Scholar]
- 32.Perandini LA, Sales-de-Oliveira D, Almeida DC, et al. Effects of acute aerobic exercise on Leukocyte inflammatory gene expression in systemic lupus erythematosus. Exerc Immunol Rev 2016;22:64–81. [PubMed] [Google Scholar]
- 33.Benatti FB, Miyake CNH, Dantas WS, et al. Exercise increases insulin sensitivity and Skeletal muscle AMPK expression in systemic lupus erythematosus: A randomized controlled trial. Front Immunol 2018;9:906. 10.3389/fimmu.2018.00906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton KR, Haaz Moonaz S, Hasni SA, et al. Yoga for systemic lupus erythematosus (SLE): clinician experiences and qualitative perspectives from students and yoga instructors living with SLE. Complement Ther Med 2018;41:111–7. 10.1016/j.ctim.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soriano-Maldonado A, Morillas-de-Laguno P, Sabio JM, et al. Effects of 12-week aerobic exercise on arterial stiffness, inflammation, and cardiorespiratory fitness in women with systemic LUPUS erythematosus: non-randomized controlled trial. J Clin Med 2018;7:477. 10.3390/jcm7120477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timóteo RP, Silva AF, Micheli DC, et al. Increased flexibility, pain reduction and unaltered levels of IL-10 and Cd11B + lymphocytes in patients with systemic lupus erythematosus were associated with Kinesiotherapy. Lupus 2018;27:1159–68. 10.1177/0961203318768880 [DOI] [PubMed] [Google Scholar]
- 37.da Hora TC, Lima K, Maciel R. The effect of therapies on the quality of life of patients with systemic lupus erythematosus: a meta-analysis of randomized trials. Adv Rheumatol 2019;59:34. 10.1186/s42358-019-0074-8 [DOI] [PubMed] [Google Scholar]
- 38.Sheikh SZ, Kaufman K, Gordon B-B, et al. Evaluation of the self-directed format of walk with ease in patients with systemic lupus erythematosus: the walk-SLE pilot study. Lupus 2019;28:764–70. 10.1177/0961203319846387 [DOI] [PubMed] [Google Scholar]
- 39.Wu ML, Tsai JC, Yu KH, et al. Effects of physical activity counselling in women with systemic lupus erythematosus: A randomized controlled trial. Int J Nurs Pract 2019;25:e12770. 10.1111/ijn.12770 [DOI] [PubMed] [Google Scholar]
- 40.Gavilan-Carrera B, Vargas-Hitos JA, Morillas-de-Laguno P, et al. Effects of 12-week aerobic exercise on patient-reported outcomes in women with systemic lupus erythematosus. Dis Rehabilit 2020;2020:1–9. 10.1136/annrheumdis-2019-eular.6789 [DOI] [PubMed] [Google Scholar]
- 41.Keramiotou K, Anagnostou C, Kataxaki E, et al. The impact of upper limb exercise on function, daily activities and quality of life in systemic lupus erythematosus: a pilot randomised controlled trial. RMD Open 2020;6:e001141. 10.1136/rmdopen-2019-001141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dionello CF, Souza PL, Rosa PV, et al. Acute neuromuscular responses to whole-body vibration of systemic lupus erythematosus individuals: A randomized pilot study. Applied Sciences 2021;11:138. 10.3390/app11010138 [DOI] [Google Scholar]
- 43.Kao VP, Wen HJ, Pan YJ, et al. Combined aerobic and resistance training improves physical and executive functions in women with systemic lupus erythematosus. Lupus 2021;30:946–55. 10.1177/0961203321998749 [DOI] [PubMed] [Google Scholar]
- 44.Lopes-Souza P, Dionello CF, Bernardes-Oliveira CL, et al. Effects of 12-week whole-body vibration exercise on fatigue, functional ability and quality of life in women with systemic lupus erythematosus: A randomized controlled trial. J Bodyw Mov Ther 2021;27:191–9. 10.1016/j.jbmt.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 45.Patterson SL, Trupin L, Yazdany J, et al. Physical inactivity independently predicts incident depression in a multi-racial/ethnic systemic lupus cohort. Arthritis Care Res 2021;9:09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohng KY. Effects of a self-management course for patients with systemic lupus erythematosus. J Adv Nurs 2003;42:479–86. 10.1046/j.1365-2648.2003.02647.x [DOI] [PubMed] [Google Scholar]
- 47.Harrison MJ, Morris KA, Horton R, et al. Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology 2005;65:1325–7. 10.1212/01.wnl.0000180938.69146.5e [DOI] [PubMed] [Google Scholar]
- 48.Miljeteig K, Graue M. Evaluation of a Multidisciplinary patient education program for people with systemic lupus erythematosus. J Nurs Healthc Chronic Illn 2009;1:87–95. 10.1111/j.1365-2702.2008.01010.x Available: http://blackwell-synergy.com/doi/abs/10.1111/jci.2009.1.issue-1 [DOI] [Google Scholar]
- 49.Drenkard C, Dunlop-Thomas C, Easley K, et al. Benefits of a self-management program in low-income African-American women with systemic lupus erythematosus: results of a pilot test. Lupus 2012;21:1586–93. 10.1177/0961203312458842 [DOI] [PubMed] [Google Scholar]
- 50.Ganachari MS, Almas SA. Evaluation of clinical pharmacist mediated education and counselling of systemic lupus erythematosus patients in tertiary care hospital. Indian J Rheumatol 2012;7:7–12. 10.1016/S0973-3698(12)60003-X [DOI] [Google Scholar]
- 51.Ting TV, Kudalkar D, Nelson S, et al. Usefulness of cellular text Messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. J Rheumatol 2012;39:174–9. 10.3899/jrheum.110771 [DOI] [PubMed] [Google Scholar]
- 52.Williams EM, Bruner L, Penfield M, et al. Stress and depression in relation to functional health behaviors in African American patients with systemic lupus erythematosus. Rheumatology (Sunnyvale) 2014;2014:005. 10.4172/2161-1149.S4-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Tian Y, Li J, et al. Effect of targeted nursing applied to SLE patients. Exp Ther Med 2016;11:2209–12. 10.3892/etm.2016.3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Riordan R, Doran M, Connolly D. Fatigue and activity management education for individuals with systemic lupus erythematosus. Occup Ther Int 2017;2017:4530104. 10.1155/2017/4530104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yelnik CM, Richey M, Haiduc V, et al. Cardiovascular disease prevention counseling program for systemic lupus erythematosus patients. Arthritis Care Res 2017;69:1209–16. 10.1002/acr.23128 Available: https://onlinelibrary.wiley.com/toc/21514658/69/8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kusnanto K, Sari NPWP, Harmayetty H, et al. Self-care model application to improve self-care agency, self-care activities, and quality of life in patients with systemic lupus erythematosus. J Taibah Univ Med Sci 2018;13:472–8. 10.1016/j.jtumed.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scalzi LV, Hollenbeak CS, Mascuilli E, et al. Improvement of medication adherence in adolescents and young adults with SLE using web-based education with and without a social media intervention, a pilot study. Pediatr Rheumatol Online J 2018;16:18. 10.1186/s12969-018-0232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams EM, Hyer JM, Viswanathan R, et al. Peer-to-peer mentoring for African American women with lupus: A feasibility pilot. Arthritis Care Res 2018;70:908–17. 10.1002/acr.23412 Available: http://doi.wiley.com/10.1002/acr.v70.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams EM, Dismuke CL, Faith TD, et al. Cost-effectiveness of a peer mentoring intervention to improve disease self-management practices and self-efficacy among African American women with systemic lupus erythematosus: analysis of the peer approaches to lupus self-management (PALS) pilot study. Lupus 2019;28:937–44. 10.1177/0961203319851559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harry O, Crosby LE, Mara C, et al. Feasibility and acceptability of an innovative adherence intervention for young adults with childhood-onset systemic lupus erythematosus. Pediatr Rheumatol Online J 2020;18:36. 10.1186/s12969-020-00430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kankaya H, Karadakovan A. Effects of web-based education and counselling for patients with systemic lupus erythematosus: self-efficacy, fatigue and assessment of care. Lupus 2020;29:884–91. 10.1177/0961203320928423 [DOI] [PubMed] [Google Scholar]
- 62.Khan F, Granville N, Malkani R, et al. Health-related quality of life improvements in systemic lupus erythematosus derived from a Digital therapeutic plus TELE-health coaching intervention: randomized controlled pilot trial. J Med Internet Res 2020;22:e23868. 10.2196/23868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allen KD, Beauchamp T, Rini C, et al. Pilot study of an Internet-based pain coping skills training program for patients with systemic lupus erythematosus. BMC Rheumatol 2021;5:20. 10.1186/s41927-021-00191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White AA, Ba A, Faith TD, et al. The care-coordination approach to learning lupus self-management: a patient navigator intervention for systemic lupus Inpatients. Lupus Sci Med 2021;8:e000482. 10.1136/lupus-2021-000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobkin PL, Da Costa D, Joseph L, et al. Counterbalancing patient demands with evidence: results from a pan-Canadian randomized clinical trial of brief supportive-expressive group psychotherapy for women with systemic lupus erythematosus. Ann Behav Med 2002;24:88–99. 10.1207/S15324796ABM2402_05 [DOI] [PubMed] [Google Scholar]
- 66.Edworthy SM, Dobkin PL, Clarke AE, et al. Group psychotherapy reduces illness Intrusiveness in systemic lupus erythematosus. J Rheumatol 2003;30:1011–6. [PubMed] [Google Scholar]
- 67.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis & Rheumatism 2004;51:625–34. 10.1002/art.20533 [DOI] [PubMed] [Google Scholar]
- 68.Karlson EW, Liang MH, Eaton H, et al. A randomized clinical trial of a Psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis & Rheumatism 2004;50:1832–41. 10.1002/art.20279 Available: http://doi.wiley.com/10.1002/art.v50:6 [DOI] [PubMed] [Google Scholar]
- 69.Goodman D, Morrissey S, Graham D, et al. The application of cognitive-behaviour therapy in altering illness representations of systemic lupus erythematosus. Behav Change 2005;22:156–71. 10.1375/bech.2005.22.3.156 [DOI] [Google Scholar]
- 70.Haupt M, Millen S, Jänner M, et al. Improvement of coping abilities in patients with systemic lupus erythematosus: a prospective study. Ann Rheum Dis 2005;64:1618–23. 10.1136/ard.2004.029926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng P, Chan W. Group Psychosocial program for enhancing psychological well-being of people with systemic lupus erythematosus. J Soc Work Disabil Rehabil 2007;6:75–87. 10.1300/J198v06n03_05 [DOI] [PubMed] [Google Scholar]
- 72.Navarrete-Navarrete N, Peralta-Ramírez MI, Sabio-Sánchez JM, et al. Efficacy of cognitive behavioural therapy for the treatment of chronic stress in patients with lupus erythematosus: a randomized controlled trial. Psychother Psychosom 2010;79:107–15. 10.1159/000276370 [DOI] [PubMed] [Google Scholar]
- 73.Navarrete-Navarrete N, Peralta-Ramírez MI, Sabio JM, et al. Quality-of-life Predictor factors in patients with SLE and their modification after cognitive behavioural therapy. Lupus 2010;19:1632–9. 10.1177/0961203310378413 [DOI] [PubMed] [Google Scholar]
- 74.Brown RT, Shaftman SR, Tilley BC, et al. The health education for lupus study: a randomized controlled cognitive-behavioral intervention targeting Psychosocial adjustment and quality of life in adolescent females with systemic lupus erythematosus. Am J Med Sci 2012;344:274–82. 10.1097/MAJ.0b013e3182449be9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Wei W, Wang CM. Effects of psychological interventions for patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus 2012;21:1077–87. 10.1177/0961203312447667 [DOI] [PubMed] [Google Scholar]
- 76.Jolly M, Peters KF, Mikolaitis R, et al. Body image intervention to improve health outcomes in lupus: a pilot study. J Clin Rheumatol 2014;20:403–10. 10.1097/RHU.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 77.Liang H, Tian X, Cao L-Y, et al. Effect of psychological intervention on Healthrelated quality of life in people with systemic lupus erythematosus: a systematic review. Int J Nurs Sci 2014;1:298–305. 10.1016/j.ijnss.2014.07.008 [DOI] [Google Scholar]
- 78.Williams EM, Penfield M, Kamen D, et al. An intervention to reduce Psychosocial and biological indicators of stress in African American lupus patients: the balancing lupus experiences with stress strategies study. Open J Prev Med 2014;4:22–31. 10.4236/ojpm.2014.41005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horesh D, Glick I, Taub R, et al. Mindfulness-based group therapy for systemic lupus erythematosus: A first exploration of a promising mind-body intervention. Complement Ther Clin Pract 2017;26:73–5. 10.1016/j.ctcp.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 80.Solati K, Mousavi M, Kheiri S, et al. The effectiveness of Mindfulness-based cognitive therapy on psychological symptoms and quality of life in systemic lupus erythematosus patients: A randomized controlled trial. Oman Med J 2017;32:378–85. 10.5001/omj.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conceição CTM, Meinão IM, Bombana JA, et al. Psychoanalytic psychotherapy improves quality of life, depression, anxiety and coping in patients with systemic lupus erythematosus: a controlled randomized clinical trial. Adv Rheumatol 2019;59:4. 10.1186/s42358-019-0047-y [DOI] [PubMed] [Google Scholar]
- 82.Kim H-A, Seo L, Jung J-Y, et al. Mindfulness-based cognitive therapy in Korean patients with systemic lupus erythematosus: A pilot study. Complement Ther Clin Pract 2019;35:18–21. 10.1016/j.ctcp.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 83.Sahebari M, Asghari Ebrahimabad MJ, Ahmadi Shoraketokanlo A, et al. Efficacy of acceptance and commitment therapy in reducing disappointment, psychological distress, and Psychasthenia among systemic lupus erythematosus (SLE) patients. Iran J Psychiatry 2019;14:130–6. [PMC free article] [PubMed] [Google Scholar]
- 84.Xu H, Teng Q, Zeng Y, et al. Psychoeducational intervention benefits the quality of life of patients with active systemic lupus erythematosus. J Nanomat 2021;2021:1–8. 10.1155/2021/9967676 [DOI] [Google Scholar]
- 85.Shah M, Kavanaugh A, Coyle Y, et al. Effect of a culturally sensitive cholesterol lowering diet program on lipid and lipoproteins, body weight, nutrient intakes, and quality of life in patients with systemic lupus erythematosus. J Rheumatol 2002;29:2122–8. [PubMed] [Google Scholar]
- 86.Minami Y, Sasaki T, Arai Y, et al. Diet and systemic lupus erythematosus: a 4 year prospective study of Japanese patients. J Rheumatol 2003;30:747–54. [PubMed] [Google Scholar]
- 87.Duffy EM, Meenagh GK, McMillan SA, et al. The clinical effect of dietary supplementation with Omega-3 fish oils and/or copper in systemic lupus erythematosus. J Rheumatol 2004;31:1551–6. [PubMed] [Google Scholar]
- 88.Shah M, Adams-Huet B, Kavanaugh A, et al. Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J Rheumatol 2004;31:71–5. [PubMed] [Google Scholar]
- 89.Aghdassi E, Morrison S, Landolt-marticorena C, et al. The use of Micronutrient supplements is not associated with better quality of life and disease activity in Canadian patients with systemic lupus erythematosus. J Rheumatol 2010;37:87–90. 10.3899/jrheum.090761 [DOI] [PubMed] [Google Scholar]
- 90.Minami Y, Hirabayashi Y, Nagata C, et al. Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: a prospective study of Japanese female patients. J Epidemiol 2011;21:246–54. 10.2188/jea.je20100157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davies RJ, Lomer MCE, Yeo SI, et al. Weight loss and improvements in fatigue in systemic lupus erythematosus: a controlled trial of a low Glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus 2012;21:649–55. 10.1177/0961203312436854 [DOI] [PubMed] [Google Scholar]
- 92.Elkan A-C, Anania C, Gustafsson T, et al. Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and Atherosclerosis. Lupus 2012;21:1405–11. 10.1177/0961203312458471 [DOI] [PubMed] [Google Scholar]
- 93.Khajehdehi P, Zanjaninejad B, Aflaki E, et al. Oral supplementation of Turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr 2012;22:50–7. 10.1053/j.jrn.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 94.Everett ST, Wolf R, Contento I, et al. Short-term patient-centered nutrition counseling impacts weight and nutrient intake in patients with systemic lupus erythematosus. Lupus 2015;24:1321–6. 10.1177/0961203315582284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shamekhi Z, Amani R, Habibagahi Z, et al. A randomized, double-blind, placebo-controlled clinical trial examining the effects of green tea extract on systemic lupus erythematosus disease activity and quality of life. Phytother Res 2017;31:1063–71. 10.1002/ptr.5827 [DOI] [PubMed] [Google Scholar]
- 96.Rothman D, Khan F, Rudin V. Individualized diet and lifestyle modifications reverse symptoms of systemic lupus erythematosus (preprint). Iproceedings [Preprint] 2018. 10.2196/preprints.11804 [DOI]
- 97.Pocovi-Gerardino G, Correa-Rodríguez M, Callejas-Rubio J-L, et al. Beneficial effect of Mediterranean diet on disease activity and cardiovascular risk in systemic lupus erythematosus patients: a cross-sectional study. Rheumatology (Oxford) 2021;60:160–9. 10.1093/rheumatology/keaa210 [DOI] [PubMed] [Google Scholar]
- 98.Wen C, Fan Y, Wang X, et al. Effect of detoxification, removing stasis and nourishing Yin method on corticosteroid-induced hyperlipidemia in patients with systemic lupus erythematosus. Chin J Integr Med 2007;13:180–4. 10.1007/s11655-007-0180-z [DOI] [PubMed] [Google Scholar]
- 99.Greco CM, Kao AH, Maksimowicz-McKinnon K, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus 2008;17:1108–16. 10.1177/0961203308093921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alvarez-Nemegyei J, Bautista-Botello A, Dávila-Velázquez J. Association of complementary or alternative medicine use with quality of life, functional status or Cumulated damage in chronic rheumatic diseases. Clin Rheumatol 2009;28:547–51. 10.1007/s10067-008-1082-y [DOI] [PubMed] [Google Scholar]
- 101.Liao Y-N, Liu C-S, Tsai T-R, et al. Preliminary study of a traditional Chinese medicine formula in systemic lupus erythematosus patients to taper steroid dose and prevent disease flare-up. Kaohsiung J Med Sci 2011;27:251–7. 10.1016/j.kjms.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 102.Zhong LLD, Bian ZX, Gu JH, et al. Chinese Herbal medicine (Zi Shen Qing) for mild-to-moderate systematic lupus erythematosus: A pilot prospective, single-blinded, randomized controlled study. Evid Based Complement Alternat Med 2013;2013:327245. 10.1155/2013/327245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stege H, Budde MA, Grether-Beck S, et al. Evaluation of the capacity of Sunscreens to Photoprotect lupus erythematosus patients by employing the Photoprovocation test. Photodermatol Photoimmunol Photomed 2000;16:256–9. 10.1034/j.1600-0781.2000.160604.x [DOI] [PubMed] [Google Scholar]
- 104.Herzinger T, Plewig G, Röcken M. Use of Sunscreens to protect against ultraviolet-induced lupus erythematosus. Arthritis & Rheumatism 2004;50:3045–6. 10.1002/art.20426 [DOI] [PubMed] [Google Scholar]
- 105.Szegedi A, Simics E, Aleksza M, et al. Ultraviolet-A1 Phototherapy modulates Th1/Th2 and Tc1/Tc2 balance in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:925–31. 10.1093/rheumatology/keh643 [DOI] [PubMed] [Google Scholar]
- 106.Zahn S, Graef M, Patsinakidis N, et al. Ultraviolet light protection by a Sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp Dermatol 2014;23:516–8. 10.1111/exd.12428 [DOI] [PubMed] [Google Scholar]
- 107.Abdul Kadir WD, Jamil A, Shaharir SS, et al. Photoprotection awareness and practices among patients with systemic lupus erythematosus and its association with disease activity and severity. Lupus 2018;27:1287–95. 10.1177/0961203318770016 [DOI] [PubMed] [Google Scholar]
- 108.Ward MM, Sundaramurthy S, Lotstein D, et al. Participatory patient-physician communication and morbidity in patients with systemic lupus erythematosus. Arthritis Rheum 2003;49:810–8. 10.1002/art.11467 [DOI] [PubMed] [Google Scholar]
- 109.Sahebalzamani M, Farahani H, Jamarani MT, et al. Effects of a continuous care model on patients' knowledge and health-related quality of life in systemic lupus erythematosus. Rehabil Nurs 2017;42:E9–18. 10.1002/rnj.283 [DOI] [PubMed] [Google Scholar]
- 110.Xie X, Song Y, Yang H, et al. Effects of transitional care on self-care, readmission rates, and quality of life in adult patients with systemic lupus erythematosus: a randomized controlled trial. Arthritis Res Ther 2018;20:184. 10.1186/s13075-018-1670-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L, Geng S, Qian L, et al. Multidisciplinary care in patients with systemic lupus erythematosus: a randomized controlled trial in China. Int J Clin Pharm 2019;41:1247–55. 10.1007/s11096-019-00870-y [DOI] [PubMed] [Google Scholar]
- 112.Erceg A, Bovenschen HJ, van de Kerkhof PCM, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol 2009;60:626–32. 10.1016/j.jaad.2008.11.904 [DOI] [PubMed] [Google Scholar]
- 113.Rerknimitr P, Tekacharin N, Panchaprateep R, et al. Pulsed-dye laser as an adjuvant treatment for discoid lupus erythematosus: a randomized, controlled trial. J Dermatolog Treat 2019;30:81–6. 10.1080/09546634.2018.1468063 [DOI] [PubMed] [Google Scholar]
- 114.Dorsey RR, Andresen EM, Moore TL. Health-related quality of life and support group attendance for patients with systemic lupus erythematosus. J Clin Rheumatol 2004;10:6–9. 10.1097/01.rhu.0000111311.38407.15 [DOI] [PubMed] [Google Scholar]
- 115.Li X, He L, Wang J, et al. Illness uncertainty, social support, and coping mode in hospitalized patients with systemic lupus erythematosus in a hospital in Shaanxi, China. PLoS One 2019;14:e0211313. 10.1371/journal.pone.0211313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bantornwan S, Watanapa WB, Hussarin P, et al. Role of meditation in reducing sympathetic hyperactivity and improving quality of life in lupus nephritis patients with chronic kidney disease. J Med Assoc Thai 2014;97:S101–7. [PubMed] [Google Scholar]
- 117.Squance ML, Reeves G, Attia J, et al. Self-reported lupus flare: association with everyday home and personal product exposure. Toxicol Rep 2015;2:880–8. 10.1016/j.toxrep.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu D, You X, Wang Z, et al. Chinese systemic lupus erythematosus treatment and research group Registry VI: effect of cigarette smoking on the clinical phenotype of Chinese patients with systemic lupus erythematosus. PLoS One 2015;10:e0134451. 10.1371/journal.pone.0134451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdelaziz SH, Elmetwaly OIA, Maged LA. Effect of using warm shower and warm water Footbath with and without adding Epsom salt on fatigue level in systemic lupus patients. Indian J Public Health Res Develop 2020;11:854–9. [Google Scholar]
- 120.Oliveira FAP de, Santos F de MMD, Dias AFM de P, et al. Cosmetic camouflage improves health-related quality of life in women with systemic lupus erythematosus and permanent skin damage: A controlled intervention study. Lupus 2020;29:1438–48. 10.1177/0961203320947802 [DOI] [PubMed] [Google Scholar]
- 121.Aranow C, Atish-Fregoso Y, Lesser M, et al. Transcutaneous Auricular Vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis 2021;80:203–8. 10.1136/annrheumdis-2020-217872 [DOI] [PubMed] [Google Scholar]
- 122.Pizzo G, Scardina GA, Messina P. Effects of a Nonsurgical exercise program on the decreased mouth opening in patients with systemic scleroderma. Clin Oral Investig 2003;7:175–8. 10.1007/s00784-003-0216-5 [DOI] [PubMed] [Google Scholar]
- 123.Sandqvist G, Akesson A, Eklund M. Evaluation of Paraffin Bath treatment in patients with systemic sclerosis. Disabil Rehabil 2004;26:981–7. 10.1080/09638280410001702405 [DOI] [PubMed] [Google Scholar]
- 124.Mugii N, Hasegawa M, Matsushita T, et al. The efficacy of self-administered stretching for finger joint motion in Japanese patients with systemic sclerosis. J Rheumatol 2006;33:1586–92. [PubMed] [Google Scholar]
- 125.Antonioli CM, Bua G, Frigè A, et al. An individualized rehabilitation program in patients with systemic sclerosis may improve quality of life and hand mobility. Clin Rheumatol 2009;28:159–65. 10.1007/s10067-008-1006-x [DOI] [PubMed] [Google Scholar]
- 126.Bongi SM, Del Rosso A, Galluccio F, et al. Efficacy of connective tissue massage and MC Mennell joint manipulation in the Rehabilitative treatment of the hands in systemic sclerosis. Clin Rheumatol 2009;28:1167–73. 10.1007/s10067-009-1216-x [DOI] [PubMed] [Google Scholar]
- 127.Maddali Bongi S, Del Rosso A, Galluccio F, et al. Efficacy of a tailored rehabilitation program for systemic sclerosis. Clin Exp Rheumatol 2009;27:44–50. [PubMed] [Google Scholar]
- 128.Oliveira NC, dos Santos Sabbag LM, de Sá Pinto AL, et al. Aerobic exercise is safe and effective in systemic sclerosis. Int J Sports Med 2009;30:728–32. 10.1055/s-0029-1224180 [DOI] [PubMed] [Google Scholar]
- 129.Maddali-Bongi S, Landi G, Galluccio F, et al. The rehabilitation of facial involvement in systemic sclerosis: efficacy of the combination of connective tissue massage, Kabat’s technique and Kinesitherapy: a randomized controlled trial. Rheumatol Int 2011;31:895–901. 10.1007/s00296-010-1382-9 [DOI] [PubMed] [Google Scholar]
- 130.Pinto ALS, Oliveira NC, Gualano B, et al. Efficacy and safety of concurrent training in systemic sclerosis. J Strength Cond Res 2011;25:1423–8. 10.1519/JSC.0b013e3181d6858b [DOI] [PubMed] [Google Scholar]
- 131.Yuen HK, Weng Y, Bandyopadhyay D, et al. Effect of a multi-Faceted intervention on Gingival health among adults with systemic sclerosis. Clin Exp Rheumatol 2011;29:S26–32. [PMC free article] [PubMed] [Google Scholar]
- 132.Yuen HK, Marlow NM, Reed SG, et al. Effect of orofacial exercises on oral aperture in adults with systemic sclerosis. Disabil Rehabil 2012;34:84–9. 10.3109/09638288.2011.587589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piga M, Tradori I, Pani D, et al. Telemedicine applied to Kinesiotherapy for hand dysfunction in patients with systemic sclerosis and rheumatoid arthritis: recovery of movement and Telemonitoring technology. J Rheumatol 2014;41:1324–33. 10.3899/jrheum.130912 [DOI] [PubMed] [Google Scholar]
- 134.Lima TRL, Guimarães FS, Carvalho MN, et al. Lower limb muscle strength is associated with functional performance and quality of life in patients with systemic sclerosis. Braz J Phys Ther 2015;19:129–36. 10.1590/bjpt-rbf.2014.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stefanantoni K, Sciarra I, Iannace N, et al. Occupational therapy integrated with a self-administered stretching program on systemic sclerosis patients with hand involvement. Clin Exp Rheumatol 2016;34:157–61. [PubMed] [Google Scholar]
- 136.Horváth J, Bálint Z, Szép E, et al. Efficacy of intensive hand physical therapy in patients with systemic sclerosis. Clin Exp Rheumatol 2017;35 Suppl 106:159–66. [PubMed] [Google Scholar]
- 137.Rannou F, Boutron I, Mouthon L, et al. Personalized physical therapy versus usual care for patients with systemic sclerosis: A randomized controlled trial. Arthritis Care & Research 2017;69:1050–9. 10.1002/acr.23098 Available: https://onlinelibrary.wiley.com/toc/21514658/69/7 [DOI] [PubMed] [Google Scholar]
- 138.Azar M, Rice DB, Kwakkenbos L, et al. Exercise habits and factors associated with exercise in systemic sclerosis: a scleroderma patient-centered intervention network (SPIN) cohort study. Disabil Rehabil 2018;40:1997–2003. 10.1080/09638288.2017.1323023 [DOI] [PubMed] [Google Scholar]
- 139.Brignoli E, Frassanito P, Vercesi E, et al. Rehabilitation in systemic sclerosis: proposed Personalised rehabilitation programme. G Ital Med Lav Ergon 2018;40:248–56. [PubMed] [Google Scholar]
- 140.Mitropoulos A, Gumber A, Crank H, et al. The effects of upper and lower limb exercise on the Microvascular reactivity in limited cutaneous systemic sclerosis patients. Arthritis Res Ther 2018;20:112. 10.1186/s13075-018-1605-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murphy SL, Barber MW, Homer K, et al. Occupational therapy treatment to improve upper extremity function in individuals with early systemic sclerosis: A pilot study. Arthritis Care Res 2018;70:1653–60. 10.1002/acr.23522 Available: https://onlinelibrary.wiley.com/toc/21514658/70/11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gregory WJ, Wilkinson J, Herrick AL. A randomised controlled trial of wax baths as an additive therapy to hand exercises in patients with systemic sclerosis. Physiotherapy 2019;105:370–7. 10.1016/j.physio.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 143.Kristensen LQ, Oestergaard LG, Bovbjerg K, et al. Use of Paraffin instead of lukewarm water prior to hand exercises had no additional effect on hand mobility in patients with systemic sclerosis: A randomized clinical trial. Hand Therapy 2019;24:13–21. 10.1177/1758998318824346 [DOI] [Google Scholar]
- 144.Landim SF, Bertolo MB, Marcatto de Abreu MF, et al. The evaluation of a home-based program for hands in patients with systemic sclerosis. J Hand Ther 2019;32:313–21. 10.1016/j.jht.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 145.Mitropoulos A, Gumber A, Akil M, et al. Exploring the Microcirculatory effects of an exercise programme including aerobic and resistance training in people with limited cutaneous systemic sclerosis. Microvasc Res 2019;125:S0026-2862(19)30116-5. 10.1016/j.mvr.2019.103887 [DOI] [PubMed] [Google Scholar]
- 146.Cetin SY, Calik BB, Ayan A. Investigation of the effectiveness of Tai Chi exercise program in patients with scleroderma: A randomized controlled study. Complement Ther Clin Pract 2020;40:101181. 10.1016/j.ctcp.2020.101181 [DOI] [PubMed] [Google Scholar]
- 147.Filippetti M, Cazzoletti L, Zamboni F, et al. Effect of a tailored home-based exercise program in patients with systemic sclerosis: A randomized controlled trial. Scand J Med Sci Sports 2020;30:1675–84. 10.1111/sms.13702 Available: https://onlinelibrary.wiley.com/toc/16000838/30/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.F. Landim S, B. Bertolo M, Del Rio AP, et al. Sustained efficacy of a concise self-management programme for hands in systemic sclerosis: a longitudinal case-control observational study. Rheumatology 2020;59:3330–9. 10.1093/rheumatology/keaa140 [DOI] [PubMed] [Google Scholar]
- 149.Cüzdan N, Türk İ, Çi̇ftçi̇ V, et al. The effect of a home-based orofacial exercise program on oral aperture of patients with systemic sclerosis: A single-blind prospective randomized controlled trial. Arch Rheumatol 2021;36:176–84. 10.46497/ArchRheumatol.2021.8295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Defi IR, Gultom C, Chorman MJ, et al. High-intensity interval training can improve hand grip strength, Inspiratory muscle, and quality of life in systemic sclerosis subjects. Reumatologia 2021;59:98–103. 10.5114/reum.2021.105454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gokcen N, Badak SO, Sarpel T, et al. The efficacy of a home-based, self-administered hand exercise program for patients with systemic sclerosis: A randomized controlled, Evaluator-blind, clinical trial. J Clin Rheumatol 2022;28:e422–9. 10.1097/RHU.0000000000001752 [DOI] [PubMed] [Google Scholar]
- 152.Maddali Bongi S, Passalacqua M, Landi G, et al. Rehabilitation of the face and Temporomandibular joint in systemic sclerosis. Ther Adv Musculoskelet Dis 2021;13:1759720X211020171. 10.1177/1759720X211020171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Murphy SL, Barber M, Huang S, et al. Intensive and App-delivered occupational therapy to improve upper extremity function in early diffuse cutaneous systemic sclerosis: a pilot two-arm trial. Rheumatology (Oxford) 2021;60:5002–11. 10.1093/rheumatology/keab339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Samuelson UK, Ahlmén EM. Development and evaluation of a patient education program for persons with systemic sclerosis (scleroderma). Arthritis Rheum 2000;13:141–8. Available: http://doi.wiley.com/10.1002/1529-0131(200006)13:3<>1.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 155.Kwakkenbos L, Bluyssen SJM, Vonk MC, et al. Addressing patient health care demands in systemic sclerosis: pre-post assessment of a psycho-educational group programme. Clin Exp Rheumatol 2011;29:S60–5. [PubMed] [Google Scholar]
- 156.Poole JL, Skipper B, Mendelson C. Evaluation of a mail-delivered, print-format, self-management program for persons with systemic sclerosis. Clin Rheumatol 2013;32:1393–8. 10.1007/s10067-013-2282-7 [DOI] [PubMed] [Google Scholar]
- 157.Poole JL, Mendelson C, Skipper B, et al. Taking charge of systemic sclerosis: a pilot study to assess the effectiveness of an Internet self-management program. Arthritis Care Res (Hoboken) 2014;66:778–82. 10.1002/acr.22192 [DOI] [PubMed] [Google Scholar]
- 158.Zanatta E, Rodeghiero F, Pigatto E, et al. Long-term improvement in activities of daily living in women with systemic sclerosis attending occupational therapy. Br J Occupat Therapy 2017;80:417–22. 10.1177/0308022617698167 [DOI] [Google Scholar]
- 159.Khanna D, Serrano J, Berrocal VJ, et al. Randomized controlled trial to evaluate an Internet-based self-management program in systemic sclerosis. Arthritis Care Res 2019;71:435–47. 10.1002/acr.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thombs BD, Dyas L, Pépin M, et al. Scleroderma patient-centered intervention network-scleroderma support group leader education (SPIN-SSLED) program: non-randomised feasibility trial. BMJ Open 2019;9:e029935. 10.1136/bmjopen-2019-029935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Uras C, Mastroeni S, Tabolli S, et al. A comparison between two educational methods in the rehabilitation of the Microstomia in systemic sclerosis: a randomized controlled trial. Clin Rehabil 2019;33:1747–56. 10.1177/0269215519858395 [DOI] [PubMed] [Google Scholar]
- 162.Thombs BD, Kwakkenbos L, Levis B, et al. Effects of a multi-Faceted education and support programme on anxiety symptoms among people with systemic sclerosis and anxiety during COVID-19 (SPIN-CHAT): a two-arm parallel, partially nested, randomised, controlled trial. Lancet Rheumatol 2021;3:e427–37. 10.1016/S2665-9913(21)00060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou J, Yang D, Zhou SH, et al. Clinical efficacy and safety of bathing with Chinese medicine Taohong Siwu decoction () for treatment of diffuse cutaneous systemic sclerosis: A randomized placebo-controlled trial. Chin J Integr Med 2018;24:185–92. 10.1007/s11655-017-2954-2 [DOI] [PubMed] [Google Scholar]
- 164.Lange U, Bogensperger S, Tarner IH, et al. The effects of a single carbon dioxide and hot water hand Bath on Acral perfusion in systemic sclerosis: A randomized, clinical study. J Scleroderma Relat Disord 2019;4:160–2. 10.1177/2397198318819919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nowicka D. Positive effect of Ozonotherapy on serum concentration of soluble Interleukin-2 receptor and Neopterin in patients with systemic sclerosis. Postepy Dermatol Alergol 2019;36:158–63. 10.5114/ada.2019.83651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sallam H, McNearney TA, Doshi D, et al. Transcutaneous electrical nerve stimulation (TENS) improves upper GI symptoms and balances the Sympathovagal activity in scleroderma patients. Dig Dis Sci 2007;52:1329–37. 10.1007/s10620-006-9257-3 [DOI] [PubMed] [Google Scholar]
- 167.Sporbeck B, Mathiske-Schmidt K, Jahr S, et al. Effect of Biofeedback and deep oscillation on Raynaud’s phenomenon secondary to systemic sclerosis: results of a controlled prospective randomized clinical trial. Rheumatol Int 2012;32:1469–73. 10.1007/s00296-011-1882-2 [DOI] [PubMed] [Google Scholar]
- 168.McNearney TA, Sallam HS, Hunnicutt SE, et al. Prolonged treatment with Transcutaneous electrical nerve stimulation (TENS) modulates neuro-gastric motility and plasma levels of vasoactive intestinal peptide (VIP), Motilin and Interleukin-6 (IL-6) in systemic sclerosis. Clin Exp Rheumatol 2013;31:140–50. [PubMed] [Google Scholar]
- 169.Collins J, Mazor Y, Jones M, et al. Efficacy of Anorectal Biofeedback in scleroderma patients with fecal Incontinence: a case-control study. Scand J Gastroenterol 2016;51:1433–8. 10.1080/00365521.2016.1218537 [DOI] [PubMed] [Google Scholar]
- 170.Dewi S, Isbagio H, Purwaningsih EH, et al. Randomized controlled trial of Ciplukan (Physalis Angulata LINN) extract on skin fibrosis, inflammatory, Immunology, and fibrosis biomarkers in scleroderma patients. Acta Med Indones 2019;51:303–10. [PubMed] [Google Scholar]
- 171.Giuggioli D, Lumetti F, Spinella A, et al. Use of Neem oil and Hypericum Perforatum for treatment of Calcinosis-related skin ulcers in systemic sclerosis. J Int Med Res 2020;48:0300060519882176. 10.1177/0300060519882176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bongi SM, Del Rosso A, Passalacqua M, et al. Manual lymph drainage improving upper extremity edema and hand function in patients with systemic sclerosis in edematous phase. Arthritis Care Res 2011;63:1134–41. 10.1002/acr.20487 [DOI] [PubMed] [Google Scholar]
- 173.Vannajak K, Boonprakob Y, Eungpinichpong W, et al. The short-term effect of Gloving in combination with traditional Thai massage, heat, and stretching exercise to improve hand mobility in scleroderma patients. J Ayurveda Integr Med 2014;5:50. 10.4103/0975-9476.128859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.O’Connor S, Durand M-J, Hudson M, et al. Effects of Osteopathic manipulative treatment on hand function, disease symptoms and functional status in systemic sclerosis: a series of single-case studies in working women. Int J Osteop Med 2016;22:21–32. 10.1016/j.ijosm.2016.06.002 [DOI] [Google Scholar]
- 175.Frech TM, Khanna D, Maranian P, et al. Probiotics for the treatment of systemic sclerosis-associated gastrointestinal Bloating/ Distention. Clin Exp Rheumatol 2011;29:S22–5. [PubMed] [Google Scholar]
- 176.Ortiz-Santamaria V, Puig C, Soldevillla C, et al. Nutritional support in patients with systemic sclerosis. Reumatol Clin 2014;10:283–7. 10.1016/j.reuma.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 177.Doerfler B, Allen TS, Southwood C, et al. Medical nutrition therapy for patients with advanced systemic sclerosis (MNT PASS): A pilot intervention study. JPEN J Parenter Enteral Nutr 2017;41:678–84. 10.1177/0148607115597883 [DOI] [PubMed] [Google Scholar]
- 178.Low AHL, Teng GG, Pettersson S, et al. A double-blind randomized placebo-controlled trial of Probiotics in systemic sclerosis associated gastrointestinal disease. Semin Arthritis Rheum 2019;49:411–9. 10.1016/j.semarthrit.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 179.Marighela TF, Arismendi MI, Marvulle V, et al. Effect of Probiotics on gastrointestinal symptoms and immune parameters in systemic sclerosis: a randomized placebo-controlled trial. Rheumatology (Oxford) 2019;58:1985–90. 10.1093/rheumatology/kez160 [DOI] [PubMed] [Google Scholar]
- 180.Fretheim H, Chung BK, Didriksen H, et al. Fecal Microbiota transplantation in systemic sclerosis: A double-blind, placebo-controlled randomized pilot trial. PLoS ONE 2020;15:e0232739. 10.1371/journal.pone.0232739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Foerster J, Fleischanderl S, Wittstock S, et al. Infrared-mediated hyperthermia is effective in the treatment of scleroderma-associated Raynaud’s phenomenon. J Invest Dermatol 2005;125:1313–6. 10.1111/j.0022-202X.2005.23938.x [DOI] [PubMed] [Google Scholar]
- 182.Murray AK, Moore TL, Richards H, et al. Pilot study of intense pulsed light for the treatment of systemic sclerosis-related Telangiectases. Br J Dermatol 2012;167:563–9. 10.1111/j.1365-2133.2012.11019.x [DOI] [PubMed] [Google Scholar]
- 183.Dinsdale G, Murray A, Moore T, et al. A comparison of intense pulsed light and laser treatment of Telangiectases in patients with systemic sclerosis: a within-subject randomized trial. Rheumatology 2014;53:1422–30. 10.1093/rheumatology/keu006 [DOI] [PubMed] [Google Scholar]
- 184.Burillo-Martinez S, Prieto-Barrios M, Velasco-Tamariz V, et al. Case series of pulsed dye laser treatment of telangiectasia in 23 patients with systemic sclerosis. Int J Dermatol 2017;56:e165–7. 10.1111/ijd.13595 Available: http://doi.wiley.com/10.1111/ijd.2017.56.issue-8 [DOI] [PubMed] [Google Scholar]
- 185.Rosholm Comstedt L, Svensson Å, Hesselstrand R, et al. Effects of intense pulsed light in Microstomia in patients with systemic sclerosis: A pilot study. J Cosmet Laser Ther 2017;19:143–8. 10.1080/14764172.2016.1262961 [DOI] [PubMed] [Google Scholar]
- 186.Hughes M, Moore T, Manning J, et al. A feasibility study of a novel low-level light therapy for Digital ulcers in systemic sclerosis. J Dermatolog Treat 2019;30:251–7. 10.1080/09546634.2018.1484875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tinazzi E, Amelio E, Marangoni E, et al. Effects of shock wave therapy in the skin of patients with progressive systemic sclerosis: a pilot study. Rheumatol Int 2011;31:651–6. 10.1007/s00296-009-1339-z [DOI] [PubMed] [Google Scholar]
- 188.Belloli L, Cugno M, D’Agostino MC, et al. Shock wave therapy for systemic sclerosis. Rheumatol Int 2013;33:1099–100. 10.1007/s00296-011-2277-0 [DOI] [PubMed] [Google Scholar]
- 189.Saito S, Ishii T, Ito K, et al. Extracorporeal shock wave therapy for Digital ulcers associated with systemic sclerosis. J Scleroderma Relat Dis 2016;1:181–5. 10.5301/jsrd.5000208 [DOI] [PubMed] [Google Scholar]
- 190.Saito S, Ishii T, Kamogawa Y, et al. Extracorporeal shock wave therapy for Digital ulcers of systemic sclerosis: A phase 2 pilot study. Tohoku J Exp Med 2016;238:39–47. 10.1620/tjem.238.39 [DOI] [PubMed] [Google Scholar]
- 191.Poole J, Conte C, Brewer C, et al. Oral hygiene in scleroderma: the effectiveness of a multi-disciplinary intervention program. Disability and Rehabilitation 2010;32:379–84. 10.3109/09638280903171527 [DOI] [PubMed] [Google Scholar]
- 192.Schouffoer AA, Ninaber MK, Beaart-van de Voorde LJJ, et al. Randomized comparison of a Multidisciplinary team care program with usual care in patients with systemic sclerosis. Arthritis Care Res 2011;63:909–17. 10.1002/acr.20448 Available: http://doi.wiley.com/10.1002/acr.v63.6 [DOI] [PubMed] [Google Scholar]
- 193.Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis - case series. Int J Dermatol 2017;56:636–40. 10.1111/ijd.13570 [DOI] [PubMed] [Google Scholar]
- 194.Hassanien M, Rashad S, Mohamed N, et al. Non-invasive oxygen-ozone therapy in treating Digital ulcers of patients with systemic sclerosis. Acta Reumatol Port 2018;43:210–6. [PubMed] [Google Scholar]
- 195.Yuen H, Westwater C, DeGarmo J, et al. Immediate effect of Xylitol chewing gum and mouth rinse on salivary levels of Mutans Streptococci in adults with systemic sclerosis: A pilot study. J Exp Integr Med 2012;2:89. 10.5455/jeim.221111.br.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Onesti MG, Fioramonti P, Carella S, et al. Improvement of mouth functional disability in systemic sclerosis patients over one year in a trial of fat transplantation versus Adipose-derived Stromal cells. Stem Cells Int 2016;2016:2416192. 10.1155/2016/2416192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parisi S, Celletti C, Scarati M, et al. Neuromuscular Taping enhances hand function in patients with systemic sclerosis: a pilot study. Clin Ter 2017;168:e371–5. 10.7417/T.2017.2036 [DOI] [PubMed] [Google Scholar]
- 198.Fiori G, Marzi T, Bartoli F, et al. The challenge of pet therapy in systemic sclerosis: evidence for an impact on pain, anxiety, Neuroticism and social interaction. Clin Exp Rheumatol 2018;36 Suppl 113:135–41. [PubMed] [Google Scholar]
- 199.Frech TM, McNeill C, Lebiedz-Odrobina D, et al. Amniotic membrane dressings: an effective therapy for SSC-related wounds. Rheumatology (Oxford) 2019;58:734–6. 10.1093/rheumatology/key410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bombardier C, Gladman DD, Urowitz MB, et al. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum 1992;35:630–40. 10.1002/art.1780350606 [DOI] [PubMed] [Google Scholar]
- 201.Liang MH, Socher SA, Larson MG, et al. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Care Res 1989;32:1107–18. 10.1002/anr.1780320909 Available: http://doi.wiley.com/10.1002/anr.v32:9 [DOI] [PubMed] [Google Scholar]
- 202.Karlson EW, Daltroy LH, Rivest C, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus 2003;12:280–6. 10.1191/0961203303lu332oa [DOI] [PubMed] [Google Scholar]
- 203.Griffiths B, Mosca M, Gordon C. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract Res Clin Rheumatol 2005;19:685–708. 10.1016/j.berh.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 204.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Medical Care 1992;30:473–83. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 205.EuroQol G. Euroqol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 206.Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care 2007;45:S3–11. 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McElhone K, Abbott J, Shelmerdine J, et al. Development and validation of a disease-specific health-related quality of life measure, the Lupusqol, for adults with systemic lupus erythematosus. Arthritis Rheum 2007;57:972–9. 10.1002/art.22881 [DOI] [PubMed] [Google Scholar]
- 208.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121–3. 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 209.Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol 1995;22:639–43. [PubMed] [Google Scholar]
- 211.Radloff LS. The CES-D scale:A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 212.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x Available: http://www.blackwell-synergy.com/toc/acp/67/6 [DOI] [PubMed] [Google Scholar]
- 213.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 214.Spielberger C, Gorsuch R, Lushene R, et al. Manual for the State-Trait Anxiety Inventory (Form Y1-Y2). 1983. [Google Scholar]
- 215.Keys A, Fidanza F, Karvonen MJ, et al. Indices of relative weight and obesity. J Chronic Dis 1972;25:329–43. 10.1016/0021-9681(72)90027-6 [DOI] [PubMed] [Google Scholar]
- 216.Tipton RM, Worthington EL. The measurement of generalized self-efficacy: a study of construct validity. J Pers Assess 1984;48:545–8. 10.1207/s15327752jpa4805_14 [DOI] [PubMed] [Google Scholar]
- 217.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 218.Sandqvist G, Eklund M. Hand mobility in scleroderma (HAMIS) test: the reliability of a novel hand function test. Arthritis Care Res 2000;13:369–74. [PubMed] [Google Scholar]
- 219.Duruöz MT, Poiraudeau S, Fermanian J, et al. Development and validation of a rheumatoid hand functional disability scale that assesses functional handicap. J Rheumatol 1996;23:1167–72. [PubMed] [Google Scholar]
- 220.Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in Progressive systemic sclerosis and localized scleroderma. Arthritis Rheum 1979;22:130–40. 10.1002/art.1780220205 [DOI] [PubMed] [Google Scholar]
- 221.Khanna D, Hays RD, Maranian P, et al. Reliability and validity of the University of California, Los Angeles scleroderma clinical trial consortium gastrointestinal tract instrument. Arthritis Rheum 2009;61:1257–63. 10.1002/art.24730 Available: http://doi.wiley.com/10.1002/art.v61%3A9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.LOE H, SILNESS J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand 1963;21:533–51. 10.3109/00016356309011240 [DOI] [PubMed] [Google Scholar]
- 223.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 224.Feldman CH, Bermas BL, Zibit M, et al. Designing an intervention for women with systemic lupus erythematosus from medically Underserved areas to improve care: a qualitative study. Lupus 2013;22:52–62. 10.1177/0961203312463979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brennan KAM, Creaven A-M. Living with invisible illness: social support experiences of individuals with systemic lupus erythematosus. Qual Life Res 2016;25:1227–35. 10.1007/s11136-015-1151-z [DOI] [PubMed] [Google Scholar]
- 226.van Leeuwen NM, Boonstra M, Huizinga TWJ, et al. Illness perceptions, risk perceptions and worries in patients with early systemic sclerosis: A focus group study. Musculoskeletal Care 2020;18:177–86. 10.1002/msc.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mouthon L, Alami S, Boisard AS, et al. Patients' views and needs about systemic sclerosis and its management: a qualitative interview study. BMC Musculoskelet Disord 2017;18:230. 10.1186/s12891-017-1603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Leung J, Ra J, Baker EA, et al. Not having the real support that we need": patients' experiences with ambiguity of systemic lupus erythematosus and erosion of social support. ACR Open Rheumatology 2019;1:135–44. 10.1002/acr2.1020 Available: https://onlinelibrary.wiley.com/toc/25785745/1/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Willems LM, Redmond AC, Stamm TA, et al. Content of non-pharmacological care for systemic sclerosis and educational needs of European health professionals: a Eushnet survey. Clin Exp Rheumatol 2015;33:S153–9. [PubMed] [Google Scholar]
- 230.Joachim G, Acorn S. Life with a rare chronic disease: the scleroderma experience. J Adv Nurs 2003;42:598–606. 10.1046/j.1365-2648.2003.02663.x [DOI] [PubMed] [Google Scholar]
- 231.Sloan M, Bosley M, Blane M, et al. But you don't look sick': a qualitative analysis of the LUPUS UK online forum. Rheumatol Int 2021;41:721–32. 10.1007/s00296-020-04726-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Terrell DR, Stewart LM, Tolma EL, et al. Barriers and Motivators for smoking cessation in patients with systemic lupus erythematosus (SLE). J Okla State Med Assoc 2015;108:492–9. [PubMed] [Google Scholar]
- 233.Harb S, Cumin J, Rice DB, et al. Identifying barriers and Facilitators to physical activity for people with scleroderma: a nominal group technique study. Disabil Rehabil 2021;43:3339–46. 10.1080/09638288.2020.1742391 [DOI] [PubMed] [Google Scholar]
- 234.Pettersson H, Nordin A, Svenungsson E, et al. Experiences of physical activity and exercise in individuals with systemic sclerosis: A qualitative study. Musculoskeletal Care 2020;18:150–60. 10.1002/msc.1447 [DOI] [PubMed] [Google Scholar]
- 235.Milette K, Thombs BD, Dewez S, et al. Scleroderma patient perspectives on social support from close social relationships. Disabil Rehabil 2020;42:1588–98. 10.1080/09638288.2018.1531151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003297supp001.pdf (37.2KB, pdf)
rmdopen-2023-003297supp002.pdf (59.7KB, pdf)
rmdopen-2023-003297supp003.pdf (392.5KB, pdf)
rmdopen-2023-003297supp004.pdf (1.7MB, pdf)