Abstract
Background
Adverse childhood experiences (ACEs) have a profound negative impact on health. However, the strength of the association between ACEs and pregnancy complications and adverse pregnancy outcomes is not well quantified or understood.
Objective
To conduct a systematic review and meta-analysis of the association between ACEs and risk of pregnancy complications and adverse pregnancy outcomes.
Search strategy
A comprehensive search was conducted using PubMed, Embase, CINAHL, PsycINFO, ClinicalTrials.gov and Google scholar up to July 2022.
Data collection and analysis
Two reviewers independently conducted the screening and quality appraisal using a validated tool. Meta-analysis using the quality-effects model on the reported odds ratio (OR) was conducted. Heterogeneity and inconsistency were examined using the I2 statistics.
Results
32 studies from 1508 met a priori inclusion criteria for systematic review, with 21 included in the meta-analysis. Pooled analyses showed that exposure to ACEs increased the risk of pregnancy complications (OR 1.37, 95% CI 1.20 to 1.57) and adverse pregnancy outcomes (OR 1.31, 95% CI 1.17 to 1.47). In sub-group analysis, maternal ACEs were associated with gestational diabetes mellitus (OR 1.39, 95% CI 1.11 to 1.74), antenatal depression (OR 1.59, 95% CI 1.15 to 2.20), low offspring birth weight (OR 1.27, 95% CI 1.02 to 1.47), and preterm delivery (OR 1.41, 95% CI 1.16 to 1.71).
Conclusion
The results suggest that exposure to ACEs increases the risk of pregnancy complications and adverse pregnancy outcomes. Preventive strategies, screening and trauma-informed care need to be examined to improve maternal and child health.
Keywords: epidemiology, epidemiology, adverse events, diabetes in pregnancy, prenatal diagnosis
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Maternal adverse childhood experiences (ACEs) were associated with an increased risk of pregnancy complications, including gestational diabetes mellitus, hypertensive disorder of pregnancy, excess gestational weight gain, and depression/anxiety during pregnancy.
ACE exposure showed a significant association with any adverse pregnancy outcome.
Most of the included studies are from high-income western countries. Due to the lack of data, we could not conduct the ACEs item-specific analysis.
The dose-response relationship in all studies could not be assessed as different studies use different screening tools and cut-off values.
Introduction
Adverse childhood experiences (ACEs)1 are psychosocial stressors and traumas experienced by an individual before 18 years of age2 3 The pioneering study by Fellitti and colleagues in 1998 demonstrated that exposure to ACEs is common, ACEs co-occur, and that exposure to multiple ACEs are associated with an increased risk of health risk behaviours and illnesses.4 Subsequently, a growing body of research has continued to provide consistent evidence that ACEs are a major public health issue due to their high prevalence and harmful effects that ACEs have on human health throughout life.5 6
Early life experiences are recognised as essential determinants for health outcomes later in life, especially in pregnant women and their children.7 Adverse health outcomes in pregnancy can then result in intergenerational transmission of adverse health outcomes. Perhaps this occurs because women who have experienced ACEs may be a vulnerable group for the development of health risk behaviours, including smoking, drug and alcohol use and sedentary lifestyle, along with consequences of trauma such as poor sleep.5 These behaviours increase the risk of pregnancy complications including gestational diabetes mellitus (GDM), hypertensive disorder of pregnancy (HDP), excess gestational weight gain (GWG), depression/anxiety during pregnancy8 and adverse pregnancy outcomes including low birth weight and preterm birth.9–11 Systematic reviews have reported that women who had experienced child maltreatment are more likely to have pregnancy complications and that physical abuse and household substance abuse were associated with greater risk of GDM,12 13 resulting in intergenerational transmission of adverse health outcomes. Overall, those reporting exposure to multiple ACEs (mostly four or more) have an increased risk of physical, mental, and substance use disorders.14
There is little information about ACEs and the associated risk of pregnancy complications and adverse birth outcomes. A longitudinal study in Australia reported that women exposed to three or more ACEs had an elevated GDM risk.15 In contrast, a longitudinal study from the USA reported no significant association between ACEs (for each score change and reported four or more ACEs) and GDM.16 A systematic review suggests that total ACEs (score in continuous scale) are associated with preterm birth, although this finding needs to be confirmed in other studies to explore the associations between ACEs and preterm birth using appropriate and valid instruments.17 Another systematic review and meta-analysis reported that maternal history of abuse before pregnancy was significantly associated with preterm delivery and low birth weight.18 No systematic review and meta-analysis has investigated the association of ACEs and the risk of pregnancy complications including GDM, HDP, GWG, depression/anxiety during pregnancy and adverse pregnancy outcomes. This study aims to systematically review and meta-analyse existing studies to establish the extent of association between ACEs and pregnancy complications and adverse birth outcomes. Understanding these associations will inform maternal clinical care and support for offspring of those women exposed to ACEs.
Methods
In this systematic review and meta-analysis, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines19 and the Meta-Analysis of Observational Studies in Epidemiology protocol20 to ensure all necessary steps were followed. In accordance with the guidelines, the systematic review and meta-analysis protocol was registered in PROSPERO (CRD42021278030).
Literature search strategy
Our search included studies published to July 2022 using PubMed, Embase, CINAHL, PsycINFO, ClinicalTrials.gov and Google scholar. The search strategy employed with PubMed is: ‘adverse childhood experiences’ OR ‘childhood adversities’ OR ‘childhood abuse’ OR ‘childhood maltreatment’ OR ‘child trauma’ OR ‘adverse childhood events’ OR ‘childhood sexual abuse’ OR ‘childhood physical abuse’ OR ‘childhood mental abuse’ OR ‘childhood trauma’ OR ‘childhood violence’ OR ‘childhood hardship’ OR ‘childhood suffering’ OR ‘childhood stress’ AND ‘pregnancy complications’ OR ‘depression’ OR ‘anxiety’ OR ‘prenatal depression’ OR ‘depressive symptoms’ OR ‘antenatal depression’ OR ‘mental health problem’ OR ‘gestational diabetes mellitus’ OR ‘GDM’ OR ‘hypertensive disorder of pregnancy’ OR ‘HDP’ OR ‘preeclampsia’ OR ‘maternal body weight’ OR ‘excess weight gain’ OR ‘abnormal fetal growth’ OR ‘intrauterine growth restriction’ OR ‘low birth weight’ OR ‘LBW’ OR ‘IUGR’ OR ‘stillbirth’ OR ‘small for gestational age’ OR ‘preterm birth’. These search details are presented in a online supplemental table S1.
bmjopen-2022-063826supp001.pdf (239.6KB, pdf)
Inclusion criteria
Studies were included if the full text was published in English, the population was pregnant women, if they reported any ACEs including childhood maltreatment (childhood physical, emotional and sexual abuse, childhood physical and emotional neglect, and exposure to parental intimate partner violence), childhood trauma or childhood hardship/suffering, and if studies reported any pregnancy-related complications according to National Institutes of Health (NIH)21 (GDM, HDP, GWG, depression/anxiety during pregnancy) and adverse birth outcomes such as low birth weight, intrauterine growth restriction (IUGR), preterm birth, and stillbirth. Studies were excluded if: (1) they were published in languages other than English; (2) they included the general population (not pregnant); (3) they reported reviews, qualitative studies, editorials, abstracts, case reports and letters to the editor; and (4) they explored violence during pregnancy.
Data extraction
Two independent reviewers (TB and AAM) carried out the data extraction. If AAM and TB did not reach agreement, a small group (AAM, TB, LC and JS) discussed discrepancies to reach a consensus. A similar approach was used for title/abstract and full text reviews. We excluded study protocol, systematic review, and qualitative study during the title screening phase. During the abstract screening phase, we excluded articles that did not present any association between ACEs and pregnancy complications and outcomes (figure 1). Relevant data from each of the selected studies were extracted, including: first author; study title; country of study; sample size; study design; types of ACEs; measurement scale; and outcomes (both risk of pregnancy complications and adverse pregnancy outcomes), and were recorded on an Excel spreadsheet.
Figure 1.
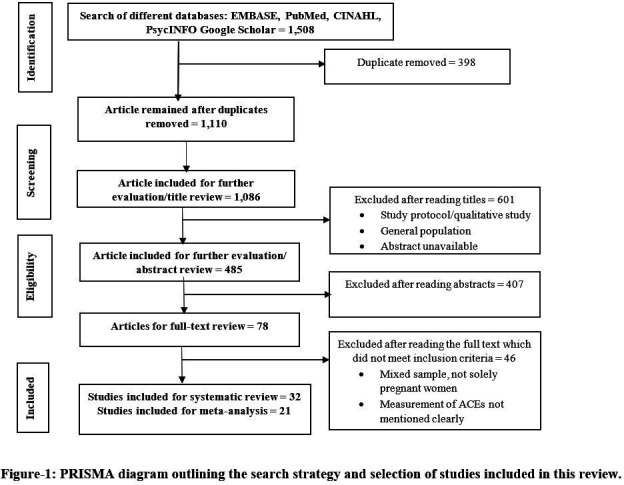
PRISMA diagram outlining the search strategy and selection of studies included in this review. ACEs, adverse childhood experiences; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Quality assessment
Fifteen-point scale quality assessment tools were used to assess the quality and risk of bias of the studies. We adapted a quality assessment tool from the NIH ‘Quality Assessment Tool for Observational Cohort and Cross-sectional studies’.22 This tool allowed assessment of the question, population, participation, inclusion/exclusion criteria, sample size, exposures, timeframe, levels of exposure, independent variables, longitudinal/repeated ACEs, dependent variable, objectively measured independent variables, objectively measured dependent variables, lost to follow-up and confounders (online supplemental table S2). Overall quality score was considered as a continuous variable for bias adjustment in the pooled estimates. However, we have also categorised the overall quality score into three groups: 13–15 as high; 10–12 as moderate; and <10 as low.
The results of the quality assessment are presented in online supplemental table S3.
Data analysis
Meta-analysis was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. Analyses focused on the overall association between ACEs and risk of pregnancy complications and adverse birth outcomes. Subgroup data synthesis was performed only when three or more studies were available with the estimates for a similar type of ACE exposures. ACE scores were considered on the continuous scale (for each unit change) and three categories: (1) none versus one ACE; (2) two to three ACEs (low ACEs); and (3) four or more ACEs (high ACEs). Although most of the studies reported the odds ratio (OR) as the measurement of association between exposures and outcomes, two studies reported relative risk (RR) and one study reported hazard ratio (HR). We converted all measures of associations into ORs using conversion methods reported elsewhere.23 In the meta-analysis, we used the quality effects model (QE)24 for bias adjustment. The advantage of the QE model is that the between-study variability is adjusted based on the relative quality rank of the studies instead of on random variables assigned by the random effect model. The heterogeneity of the studies was reported by the I2 value that measures the proportion of total variance between studies beyond random error.24 We checked for publication bias through visualisation by funnel plot and Doi plot.25 All the analyses were conducted using the MetaXL software version 5.3.26
Results
The literature search resulted in 1508 records, which were screened for duplication (n=398), review of titles (n=1086) and further abstract evaluation (n=485). Finally, 32 studies met our inclusion criteria for systematic review, and 21 were included in the meta-analysis (figure 1). Seventy-five percent of the studies were cohort studies and the remainder were either cross sectional or case–control studies. The majority of the studies were conducted in the USA (n=19), with fewer studies from Canada (n=3), Europe (n=6) and other regions (n=5). The study sample sizes varied from 48 to 11 556. The publication year ranged from 1994 to 2022. Thirteen studies used the 10-item ACEs questionnaire,8 16 27–37 three used the WHO ACE-IQ questionnaires,38–40 one study used 8-items41 and two studies used 19-items questionnaire,42 43 and 14 studies used other measures35 44–55 (table 1).
Table 1.
Characteristics of studies included in the systematic review and meta-analysis
| SI# | First author/pub date | Country | Study design | Sample size | Measurement scale |
| 1 | Christiaens et al, 201534 | Canada | Case–control | 622 | 10-item self-report tool by Felliti et al |
| 2 | Grimstad et al, 199944 | Norway | Case–control | 174 | Were asked about the character of the experience(s): genital touch; forced to touch the other person’s genitals; attempted coitus; penile vaginal coitus |
| 3 | Noll et al, 200745 | USA | Cohort | 186 | Childhood sexual abuse |
| 4 | Leeners et al, 201446 | Switzerland | Cohort | 255 | Childhood sexual abuse experiences were additionally explored using questions modified by Wyatt |
| 5 | Selk et al, 201647 | USA | Case–control | 51 434 | The measure of physical abuse included items from the Revised Conflict Tactics Scale (CTS); the sexual abuse measure was derived from the survey by Finkelhor et al |
| 6 | Harville et al, 201048 | UK | Cohort | 4865 | The phrase ‘childhood hardship’ is used herein to refer to a number of adverse situations in childhood:
|
| 7 | Appleton et al, 201937 | USA | Cohort study | 126 | 10-item self-report tool by Felliti et al |
| 8 | Versteegen et al, 202116 | USA | Cohort | 30 | 10-item self-report tool by Felliti et al |
| 9 | Stanhope et al, 20208 | USA | Cohort | 2319 | 10-item self-report tool by Felliti et al |
| 10 | Schoenaker et al, 201915 | Australia | Cohort | 11 556 | 10-item self-report tool by Felliti et al |
| 11 | Miller et al, 201749 | USA | Prospective study | 744 | Asked women a series of questions about their family’s conditions during childhood |
| 12 | Mersky et al, 201942 | USA | Longitudinal | 1848 | 19-item assessment that has demonstrated good internal consistency |
| 13 | Mason et al, 201635 | USA | Cohort | 45 550 | Physical abuse and sexual abuse |
| 14 | Cammack et al, 201850 | USA | Cohort | 230 | Childhood Trauma Questionnaire Short-Form (CTQ) |
| 15 | Bala et al, 202051 | Rhode Island | Population-based survey | 3350 | 7-item questionnaire |
| 16 | Ben Salah et al, 201938 | Tunisia | Prospective follow-up study | 593 | ACE-International Questionnaire (ACE-IQ) |
| 17 | Bhengu et al, 202039 | South Africa | Cross-sectional | 223 | WHO-ACE IQ |
| 18 | Gillespie et al,52 2017 | USA | Prospective observational design | 89 | The Stress and Adversity Inventory (STRAIN) |
| 19 | Leeners et al, 201446 | Switzerland | Cohort | 225 | Using questions modified from a questionnaire developed by Wyatt |
| 20 | McDonnell et al, 201436 | USA | Cohort | 398 | 10-item self-report tool by Felliti et al |
| 21 | Shaikh et al, 201940 | Pakistan | Cohort | 300 | WHO 31-item ACEs |
| 22 | Smith et al, 201653 | USA | Cohort | 2303 | The main modification of the instrument was to collapse the sexual events before the age of 18 questions into one question asking about childhood sexual abuse before age 18 |
| 23 | Ranchod et al, 201654 | USA | Longitudinal study | 2873 | 4-item questionnaire |
| 24 | Fredriksen et al, 201727 | Norway | Cohort | 762 | 10-item self-report tool by Felliti et al |
| 25 | Hantsoo et al, 201928 | USA | Observational study | 48 | 10-item self-report tool by Felliti et al |
| 26 | Howell et al, 201929 | USA | Observational study | 101 | 10-item self-report tool by Felliti et al |
| 27 | Letourneau et al, 201930 | Canada | Cohort | 907 | 10-item self-report tool by Felliti et al |
| 28 | Narayan et al, 201831 | USA | Cohort | 101 | 10-item self-report tool by Felliti et al |
| 29 | Racine et al, 202032 | Canada | Cohort | 1994 | 10-item self-report tool by Felliti et al |
| 30 | Young-Wolff et al, 201933 | USA | Cohort | 355 | 10-item self-report tool by Felliti et al |
| 31 | Barrios et al, 201541 | USA | Cohort | 1521 | 8 questions from CDC |
| 32 | Hardcastle et al, 202255 | UK | Cross sectional | 865 | 10-item self-report tool by Felliti et al |
ACE, adverse childhood experience; CDC, Centers for Disease Control and Prevention.
In total, 32 studies were included for quality assessment. Eleven studies (34.38%) were assessed as high quality, 12 studies (37.50%) were assessed as moderate quality, and nine studies (28.13%) were assessed as poor quality (online supplemental table S3).
ACEs and risk of pregnancy complications
ACEs and GDM
Six studies8 15 16 35 36 51 described an association between ACEs and GDM and only one study reported (table 2) there was no association between ACEs and GDM.42 A large epidemiological study in Australia15 reported that, in pregnant women, exposure to any three ACEs (adjusted RR (aRR) 1.73, 95% CI 1.0 to 3.0) or four or more ACEs (aRR 1.70, 95% CI 1.00 to 2.90) was associated with elevated GDM risk after adjusting for preconception body mass index, unhealthy diet, parity, and maternal age. Another study in the USA35 reported that both moderate (adjusted OR (aOR) 1.08, 95% CI 0.96 to 1.22) and severe (aOR 1.42, 95% CI 1.21 to 1.66) childhood physical abuse was associated with an increased risk of GDM. This study also reported that forced sexual activity during childhood was associated with an increased risk of GDM (aOR 1.30, 95% CI 1.14 to 1.49).
Table 2.
Summary of published measures of effect
| 1 | Appleton et al, 201937 | Depression | ACEs score (continuous) | Pearson’s correlation coefficients (0.37) |
| 2 | Versteegen et al, 202116 | GDM | ACEs total | 1.05 (0.98 to 1.14) |
| ACEs binary | 2.85 (1.15 to 7.06) | |||
| 3 | Stanhope et al, 20208 | GDM | ACEs 4+ | 1.03 (0.71 to 1.49) |
| Continuous ACE score | 0.96 (0.88 to 1.04) | |||
| HDP | ACEs 4+ | 1.03 (0.71 to 1.49) | ||
| Continuous ACE score | 1.03 (0.71 to 1.49) | |||
| 4 | Schoenaker et al, 2019 | GDM | 3 ACEs | 1.73 (1.02 to 3.01) |
| ≥4 ACEs | 1.76 (1.04 to 2.99) | |||
| 5 | Mason et al, 201635 | GDM | Mild physical abuse | 1.08 (0.96 to 1.22) |
| Moderate physical abuse | 11.16 (1.04 to 1.29) | |||
| Severe physical abuse | 1.42 (1.21 to 1.66). | |||
| Forced sexual activity | 1.30 (1.14 to 1.49) | |||
| Combined | 1.42 (1.21 to 1.66) | |||
| 6 | Bala et al, 202051 | GDM | ≥3 ACEs | 1.24 (0.81 to 1.90) |
| 1–2 ACEs | 1.18 (0.90 to 1.55) | |||
| 7 | McDonnell et al, 201436 | GDM | GDM not correlated with ACE indicators | |
| 8 | Ranchod et al, 201654 | GWG | Physical abuse | 1.2 (1.1 to 1.4) |
| Household alcohol abuse | 1.2 (1.1 to 1.3) | |||
| Household mental illness | 1.1 (0.9 to 1.2). | |||
| 9 | Fredriksen et al, 201715 | Depression | ACEs continuous | 1.3 (0.92 to 1.82) |
| 10 | Hantsoo et al,201928 | Depression | <2 ACES | EPDS (median (IQR)): 5 (3–6) |
| ≥2 ACES | EPDS (median (IQR)): 3 (1.5–6.0) | |||
| 11 | Howell et al, 202029 | Depression | ACEs continuous | Adverse childhood experiences had a direct effect on depression, B=1.11, SE=0.44, p=0.01 |
| 12 | Letourneau et al, 201930 | Depression | ACEs continuous | Maternal ACEs were associated with symptoms of anxiety and depression during pregnancy |
| 13 | Narayan et al, 201831 | Depression | ACEs continuous | Maternal ACEs were associated with depression during pregnancy (β=0.32, p<0.01) |
| 14 | Racine et al, 202032 | Depression | ACEs continuous | 1.26 (1.12 to 1.43) |
| 15 | Young-Wolff et al, 201933 | Depression | 3+ ACEs | 3.08 (1.12 to 7.39) |
| 1–2 ACEs | 2.42 (1.09 to 5.41) | |||
| 16 | Barrios et al, 201541 | Depression | 2.07 (1.58 to 2.71) |
ACEs, adverse childhood experiences; EPSD, Edinburgh Postnatal Depression Scale; GDM, gestational diabetes mellitus; GWG, gestational weight gain; HDP, hypertensive disorder of pregnancy.
ACEs, GWG and HDP
Only one study by Ranchod et al 54 examined the association between ACEs and GWG. They found that exposure to physical abuse and household alcohol abuse were independently associated with a 20% increase in the risk of excessive GWG. A study by Stanhope et al 8 found that for each ACEs score, there was a slight increase in the HDP risk (aOR 1.03, 95% CI 0.71 to 1.49), although it was not statistically significant. However, they found that physical abuse (aOR 1.22, 95% CI 1.10 to 1.42) and household alcohol abuse (aOR 1.21, 95% CI 1.11 to 1.32) were associated with a significant increase in the risk of excessive GWG (table 2).
ACEs and depression/anxiety
Nine studies27–33 37 41 examined the association between ACEs and depression/anxiety, with almost all studies reporting a significant positive association during pregnancy(table 2). For example, a large cohort study in Canada32 reported that ACEs were associated with depressive symptoms in pregnancy (aOR 1.26, 95% CI 1.12 to 1.43). Another study30 reported that for each maternal ACE, there was an increased risk of symptoms of anxiety and depression during pregnancy. An observational study in the USA by Hantsoo et al 28 29 reported that ACEs directly affected depression (B=1.1, SE=0.44, p=0.01).
Meta-analytic results for maternal ACEs and risk of pregnancy complications
A total of 11 studies (72 889 participants) were available for the quality-effect meta-analysis, which produced an association between maternal any ACEs and risk of any adverse pregnancy complications (OR 1.37, 95% CI 1.20 to 1.57) (figure 2). In risk factor-specific sub-analysis, five studies (7116 participants) were available for meta-analysis, which produced a moderate association between maternal ACEs and risk of GDM (OR 1.39, 95% CI 1.11 to 1.74). For depression/anxiety during pregnancy, four studies (6116 participants) were available for this meta-analysis, which produced an association between maternal ACEs and risk of depression/anxiety during pregnancy (OR 1.5, 95% CI 1.15 to 2.2). Both low (OR 1.30, 95% CI 1.10 to 1.50) and high (OR 1.41, 95% CI 1.02 to 1.90) numbers of ACEs were associated with pregnancy complications (online supplemental figure S1.1 and 1.2).
Figure 2.
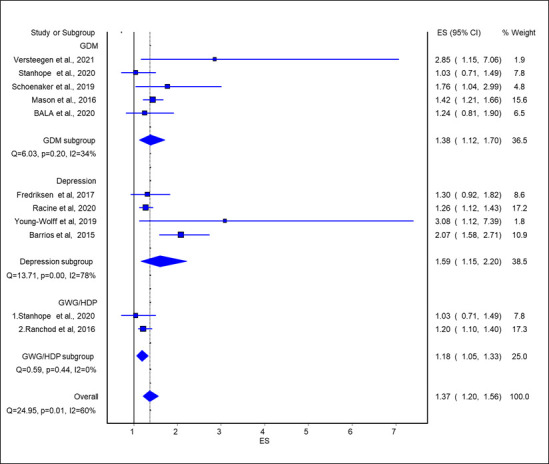
Association of any ACE exposure with risk of pregnancy complications. ACE, adverse childhood experience; ES, effect size; GDM, gestational diabetes mellitus; GWG, gestational weight gain; HDP, hypertensive disorder of pregnancy.
ACEs and adverse pregnancy outcomes
ACEs and preterm birth
Out of 31 studies, 1234 38–40 42–48 50 55 reported the association between ACEs and preterm birth (table 3). A study in Tunisia by Ben Salah et al 38 reported that after adjustment for high-risk pregnancies, environmental tobacco smoke, and intra-familial ACEs, the risk of premature birth was significantly associated with exposure to collective violence (p<0.001) and witnessing community violence (p<0.05). In another study, Harville et al 48 reported that violence exposure during childhood was associated with a 44% increased risk of preterm birth (aRR 1.40, 95% CI 1.00 to 1.90). They also found the family mental health issues increased by 24%, and there was a 25% increase in the risk of preterm birth. A case–control study in the USA by Selk et al 47 reported that women exposed to forced sex during childhood had a 22% greater risk of preterm birth (aRR 1.2, 95% CI 1.10 to 1.30) than those in the no exposure group. Furthermore, exposure to physical and sexual abuse during childhood was associated with a 35% greater risk of preterm birth (aRR 1.30, 95% CI 1.10 to 1.60). A study by Miller et al reported that mothers’ childhood economic hardship was independently associated with multiple adverse birth outcomes.49 A study by Gillespie et al reported that maternal childhood abuse was associated with birth timing (birth timing was operationalised as a day’s gestation at birth continuous variable and calculated according to the obstetric estimate of date of delivery and actual date of delivery extracted from the prenatal and labour and delivery records).52
Table 3.
Summary of published measures of effect
| SI# | First author/pub date | Outcomes | Types of ACEs and analytical unit | Findings (OR, 95% CI) |
| 1 | Christiaens et al, 201534 | Preterm birth | High ACE score (≥2 ACE) | 2.09 (1.10 to 3.98) |
| ACEs score (continuous) | 1.18 (0.99 to 1.40) | |||
| 2 | Grimstad et al,199944 | Preterm birth | Sexual abuse | 1.03 (0.44 to 2.4) |
| Low birth weight | Sexual abuse | 1.21 (0.5 to 2.93) | ||
| 3 | Noll et al, 200745 | Preterm birth | Sexual abuse | 2.16 (0.77 to 6.06) |
| 4 | Leeners et al, 201446 | Preterm birth | Sexual abuse | 2.47 (1.11 to 5.51) |
| 5 | Selk et al, 201647 | Preterm birth | Severe physical only | 1.02 (0.88 to 0.17) |
| Forced sex only | 1.22 (1.1 to 1.35) | |||
| Experienced both severe abuse types | 1.35 (1.13 to 1.62) | |||
| 6 | Harville et al, 201048 | Preterm birth | Financial/structural hardship | 1.20 (0.90 to 1.60) |
| No interest in education | 1.17 (0.93 to 1.48) | |||
| Family dysfunction | 1.20 (0.94 to 1.52) | |||
| Lack of supportive caregiving | 0.98 (0.81 to 1.19) | |||
| Violence/mental health issues | 1.24 (0.94 to 1.63) | |||
| Issues of family structure | 1.25 (1.02 to 1.54) | |||
| No. of hardships (≥4) | 1.45 (1.09 to 1.93) | |||
| Low birth weight | Financial/structural hardship | 1.18 (0.88 to 1.60) | ||
| No interest in education | 1.18 (0.88 to 1.60) | |||
| Family dysfunction | 1.18 (0.88 to 1.60) | |||
| Lack of supportive caregiving | 1.18 (0.88 to 1.60) | |||
| Violence/mental health issues | 1.48 (1.12 to 1.96) | |||
| Issues of family structure | 1.48 (1.12 to 1.96) | |||
| No. of hardships (≥4) | 1.48 (1.12 to 1.96) | |||
| 11 | Miller et al, 201749 | Birth outcomes | Childhood economic hardship | Mother’s hardship independently associated with multiple adverse birth outcomes |
| 12 | Mersky et al, 201942 | Preterm birth | ACE scores (continuous) | 1.07 (1.01 to 1.12) |
| 1 or 2 ACEs | 1.22 (0.79 to 1.89) | |||
| 3 or 4 ACEs | 1.29 (0.82 to 2.02) | |||
| 5 or more ACEs | 1.46 (0.95 to 2.26) | |||
| Low birth weight | ACE scores (continuous) | 1.08 (1.03 to 1.15) | ||
| 1 or 2 ACEs | 0.98 (0.62 to 1.56) | |||
| 3 or 4 ACEs | 1.22 (0.76 to 1.96) | |||
| 5 or more ACEs | 1.39 (0.88 to 2.19) | |||
| Pregnancy loss | ACE scores (continuous) | 1.12 (1.08 to 1.17) | ||
| 1 or 2 ACEs | 0.93 (0.66 to 1.31) | |||
| 3 or 4 ACEs | 1.27 (0.89 to 1.80) | |||
| 5 or more ACEs | 1.27 (0.89 to 1.80) | |||
| 14 | Cammack et al, 201850 | Low birth weight | Emotional abuse | 0.88 (0.66 to 1.00) Cohen’s kappas (95% CI) |
| Physical abuse | 0.50 (0.01 to 0.99) | |||
| Sexual abuse | 0.75 (0.43 to 1.00) | |||
| Emotional neglect | 0.59 (0.18 to 1.00) | |||
| Physical neglect | 0.28 (−0.16 to 0.73) | |||
| Preterm birth | Emotional abuse | 0.78 (0.55 to 1.00) | ||
| Physical abuse | 0.69 (0.36 to 1.00) | |||
| Sexual abuse | 0.78 (0.55 to 1.00) | |||
| Emotional neglect | 0.44 (0.12 to 0.77) | |||
| Physical neglect | 0.39 (−0.03 to 0.81) | |||
| NICU admission | Emotional abuse | 0.58 (0.25 to 0.91) | ||
| Physical abuse | 0.28 (−0.15 to 0.71) | |||
| Sexual abuse | 0.73 (0.45 to 1.00) | |||
| Emotional neglect | 0.55 (0.20 to 0.90) | |||
| Physical neglect | 0.55 (0.20 to 0.90) | |||
| 16 | Ben Salah et al, 201938 | Preterm birth low birth weight | ACEs continuous | After adjustment for high-risk pregnancies, environmental tobacco smoke, and intra-familial ACEs, the risk of premature birth was significantly associated with exposure to collective violence (p<0.001) and witnessing community violence (p<0.05) |
| 17 | Bhengu et al, 201939 | Preterm birth | ACEs continuous | 1.21 (1.03 to 1.43) |
| 18 | Gillespie et al. 201752 | Birth timing | ACEs continuous | Cumulative childhood stress predicted birth timing (p=0.01) |
| 19 | Leeners et al, 201446 | Preterm birth | CSA, physical abuse as well as other ACEs were associated with an increased risk for premature delivery | |
| 21 | Shaikh et al, 201940 | Preterm birth | ACEs continuous | We found no association between ACE and preterm birth |
| 22 | Smith et al, 2016 | Birth weight and shorter gestational age | ACEs continuous | Each additional ACE decreased birth weight by 16.33 g and decreased gestational age by 0.063 |
| 32 | Hardcastle et al, 202255 | Preterm birth | 1 ACE | 0.80 (0.32 to 2.00) |
| 2–3 ACEs | 1.17 (0.46 to 2.97) | |||
| ≥4 ACEs | 2.67 (1.14 to 6.23) |
ACEs, adverse childhood experiences; CSA, child sexual abuse; NICU, neonatal intensive care unit.
ACEs and low birth weight
Out of 31 studies, six38 42 44 48 50 53 reported an association between ACEs and low birth weight (table 3).
Harville et al reported that violence exposure during childhood was associated with an increased risk of low birth weight (aOR 1.5, 95% CI 1.1 to 2.0). They also found that violence/mental health issues (aOR 1.4, 95% CI 1.1 to 1.9) and issues of family structure increased the risk of low birth weight (aOR 1.4, 95% CI 1.1 to 1.9). A study by Smith et al reported that each additional ACE decreased gestational age at birth as well as birth weight.53
Meta-analytic results for maternal ACEs and adverse pregnancy outcomes
A total of 12 studies were available for this quality-effects meta-analysis, which produced an association between maternal ACEs and any adverse pregnancy outcomes (OR 1.31, 95% CI 1.17 to 1.47). In a sub-analysis of eight studies (59 607 participants), the quality-effects meta-analysis showed an association between maternal ACEs and preterm birth (OR 1.41, 95% CI 1.16 to 1.71). On the other hand, three studies (7014 participants) were available for the quality-effects meta-analysis for low birth weight, which showed an association between maternal ACEs and low birth weight (OR 1.27, 95% CI 1.17 to 1.47) (figure 3). In low (one to three ACEs) and high (four+) ACEs specific analysis, five studies reported low ACEs exposure and nine studies reported high ACEs exposure. Both low (OR 1.27, 95% CI 1.05 to 1.54) and high (OR 1.41, 95% CI 1.20 to 1.65) ACE exposure showed a significant association with any adverse pregnancy outcome. For each additional unit increase in the number of ACEs, the odds of adverse pregnancy outcomes increased 1.10 times (OR 1.10, 95% CI 1.05 to 1.15) (online supplemental figure S2.1 and 2.2).
Figure 3.
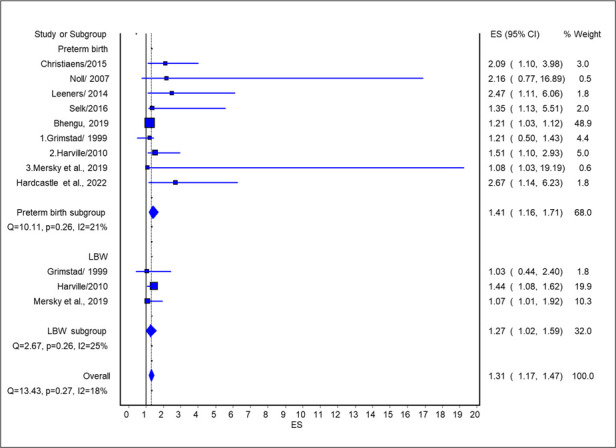
Association of any ACE exposure and adverse pregnancy outcomes. ACE, adverse childhood experience; ES, ???; LBW, low birth weight.
Discussion
This systematic review and meta-analysis found that maternal ACEs were associated with an increased risk of pregnancy complications including GDM, HDP, GWG and mental health during pregnancy. Similarly, this study also found that maternal ACEs were associated with an increased risk of adverse pregnancy outcomes including preterm birth and low birth weight. All these associations were stronger for four or more compared with less than four ACEs. There was a dose-response association between ACEs and adverse pregnancy outcome. Overall, findings of this study suggest there is a robust association between ACEs and pregnancy complications and adverse pregnancy outcomes. Early prevention of ACEs might reduce the risk of pregnancy complications and adverse outcomes.
To our knowledge, this is the first systematic review and meta-analysis to assess the association between ACEs and pregnancy complications and adverse pregnancy outcomes. A recent systematic review and meta-analysis reported an association between ACEs and maternal depression and/or anxiety in the perinatal period (pregnancy to 1 year postpartum),22 though the results of our study are not directly comparable to this study because outcomes were considered at different perinatal windows and results were presented differently (eg, effect size vs OR). Our results on maternal ACEs and increased risk of adverse pregnancy outcomes are more comprehensive than previous systematic reviews18 56 57 due to the availability of 12 recent primary studies. Overall, the direction and strength of the associations in our study are similar to these earlier studies.18 56 57
There could be several potential direct and indirect pathways to explain the relationship between ACEs and pregnancy complications and adverse pregnancy outcomes. Direct mechanisms may include altering the regulation of stress-signalling pathways58 and immune system function59; changing brain structure and function; and changing the expression of DNA and by accelerating cellular ageing.60 For example, abuse or neglect might directly lead to malnutrition. Similarly, stress can directly lead to dysregulation of the hypothalamic-pituitary-adrenal axis and associated neuroendocrine-immune61 as well as epigenetic effects.62 Results from animal models63 64 and longitudinal human studies such as the Nurses’ Health Study35 have proposed that a strong history of ACEs may alter the hypothalamic-pituitary-adrenal axis as reflected by elevated cortisol levels that in turn alter glucose metabolism and body weight regulation. Brain development begins in fetal life and continues into early adulthood. Early life maternal ACEs may alter the structure and function of the brain.65 66 These neurodevelopmental alterations may result in neuroendocrine disruption of cortisol regulation, linked to glucose metabolism.67 68 The experience of ACEs increased the risk of physical or sexual abuse during pregnancy and is associated with placental damage, uterine contractions, premature rupture of membranes, and genitourinary infections which ultimately increase the risk of preterm birth and low birth weight.69 Exposure to ACEs is also associated with an increased risk of health risk behaviours including substance use, physical inactivity and unhealthy diet.4 Previous research has shown that ACEs are associated with pre-pregnancy obesity.70 In addition, it is also established that socioeconomic status and cumulative disadvantage produces health disparities across the life course.71 Any of these mechanisms could explain the transgenerational nature of obesity and diabetes in families affected by maternal ACEs. Chronic inflammation, unhealthy behaviours, poor sleep and altered stress regulatory pathways are risk factors for adverse pregnancy complications, including GDM, HDP and depression/anxiety.72 73 The interplay of these different pathways remains largely unclear.
According to our findings and other systematic review evidence, it may be valuable to assess the role of routine ACEs screening during pregnancy to improve maternal and child health. Trauma-informed care is not well incorporated into clinical practice guidelines. Much of the emphasis in maternity care is on individual behaviour change, including advice about diet, exercise, smoking cessation and uptake of clinical care. Approaches that do not incorporate the personal experiences of trauma by women attending antenatal services may inadvertently cause iatrogenic harm. For many years, there has been an interest in improving pregnancy outcomes by focusing on a limited set of physical parameters that can easily be measured such as gestational weight gain, without attention to the underlying mechanisms.74 75 Overall, studies of diet and exercise in pregnancy to reduce GDM, HDP and other adverse pregnancy outcomes have been disappointing.76
A recent scoping review by Tran et al 77 found that healthcare providers perceive that they are not being trained to screen for ACEs in their undergraduate training programme or in their professional training in clinical settings. In addition, healthcare workers already have a high demand on their time and limited capacity to incorporate new practices without additional resources. There is some controversy about whether screening for ACEs is a safe and ethical practice, especially if the consequences of discussing ACEs (eg, effects on mental health) cannot be readily addressed.78 79 These identified barriers are similar to those reported by healthcare providers in relation to ACE screening in general clinical settings.80 Healthcare providers may appreciate the importance of asking about ACEs to help raise issues that otherwise would be unknown and unaddressed.77 Furthermore, Mishra et al 81 found that ACEs screening did not excessively disrupt clinic workflow. and was both acceptable for the patient and feasible for the provider. However, to determine if screening for ACEs is worthwhile, studies need to assess whether trauma-informed clinical care translates to improved clinical outcomes for mother and offspring.82 Beyond screening for ACEs, our findings emphasise the importance of preventing ACEs in children to reduce immediate impacts as well as intergenerational transmission of ACEs. As well as supporting clinicians and providing services to address ACEs, there is growing awareness of the crucial role of upstream policy- and community-level interventions to improve and support positive family and social environments and a need for wide-scale testing of the effectiveness of such interventions.83 84
There are some limitations to the current study, which reduce the generalisability of the findings. First, most of the included studies are from high-income western countries. Second, due to the lack of data, we could not conduct the ACEs item-specific analysis. Thirdly, the dose-response relationship in all studies could not be assessed as different studies use different screening tools and cut-off values. Only five studies exploring pregnancy complications and five studies investigating adverse pregnancy outcomes could be assessed for a dose-response relationship. Lastly, as we considered various types of ACE exposures in a single review, we expected much heterogeneity in the study methodologies, populations, exposures, and outcome identification. To address these limitations, the Quality Effect model, which incorporates the heterogeneity of effects across the studies and reduces the risk-of-bias assessment, was used in the meta-analysis. Nevertheless, our study has several strengths considering the comprehensive nature of the inclusion criteria, including relevant studies published up to July 2021. In addition, we assessed the methodological quality of studies using standard tools appropriate for observational cohort and cross-sectional studies.
Conclusion
This systematic review and meta-analysis found that exposure to ACEs increases the risk of pregnancy complications and adverse pregnancy outcomes. The identification of women exposed to ACEs and personalising their care may provide opportunities to improve maternal and child mental and physical health.
Supplementary Material
Footnotes
Contributors: AM and TB contributed towards the literature search, data analysis and interpretation, figures and tables, and writing of the manuscript. AM, TB, LKC, JS, PDS contributed towards the drafting of the protocol, review of the study design, data collection and interpretation and provided a critical review of the manuscript. AM and SD contributed towards the data management and analysis plan and provided oversight and interpretation of the analyses. AM, DM, KT, FMB, NMD, MM, KM, AK, LH contributed towards the study design and editing. AM and LC contributed towards the design of the manuscript, development of the protocol, and critical evaluation and interpretation of the results and critical review of the manuscript. AM is responsible for the overall content as the guarantor.
Funding: This research was partially supported by the Australian Research Council Centre of Excellence for Children and Families over the Life Course (CE200100025).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Atzl VM, Narayan AJ, Rivera LM, et al. Adverse childhood experiences and prenatal mental health: type of ACEs and age of maltreatment onset. J Fam Psychol 2019;33:304–14. 10.1037/fam0000510 [DOI] [PubMed] [Google Scholar]
- 2. Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017;2:e356–66. 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- 3. Wickramasinghe YM, Raman S, Garg P, et al. Burden of adverse childhood experiences in children attending paediatric clinics in South Western Sydney, Australia: a retrospective audit. BMJ Paediatr Open 2019;3:e000330. 10.1136/bmjpo-2018-000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) study. Am J Prev Med 1998;14:245–58. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 5. Petruccelli K, Davis J, Berman T. Adverse childhood experiences and associated health outcomes: A systematic review and meta-analysis. Child Abuse Negl 2019;97:104127. 10.1016/j.chiabu.2019.104127 [DOI] [PubMed] [Google Scholar]
- 6. Schroeder K, Schuler BR, Kobulsky JM, et al. The association between adverse childhood experiences and childhood obesity: a systematic review. Obes Rev 2021;22:e13204. 10.1111/obr.13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuh D, Ben-Shlomo Y, Lynch J, et al. Life course epidemiology. J Epidemiol Community Health 2003;57:778–83. 10.1136/jech.57.10.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stanhope KK, Cammack AL, Perreira KM, et al. Adverse childhood experiences and lifetime adverse maternal outcomes (gestational diabetes and hypertensive disorders of pregnancy) in the Hispanic community health study/study of Latinos. Ann Epidemiol 2020;50:1–6. 10.1016/j.annepidem.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry 2012;25:141–8. 10.1097/YCO.0b013e3283503680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsen JM. Integrative review of pregnancy health risks and outcomes associated with adverse childhood experiences. J Obstet Gynecol Neonatal Nurs 2018;47:783–94. 10.1016/j.jogn.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 11. Dachew BA, Mamun A, Maravilla JC, et al. Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: a systematic review and meta-analysis. Psychiatry Res 2018;260:458–67. 10.1016/j.psychres.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 12. Montgomery E, Pope C, Rogers J. A feminist narrative study of the maternity care experiences of women who were sexually abused in childhood. Midwifery 2015;31:54–60. 10.1016/j.midw.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 13. Montgomery E, Pope C, Rogers J. The re-enactment of childhood sexual abuse in maternity care: a qualitative study. BMC Pregnancy Childbirth 2015;15:194. 10.1186/s12884-015-0626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lê-Scherban F, Wang X, Boyle-Steed KH, et al. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics 2018;141:e20174274. 10.1542/peds.2017-4274 [DOI] [PubMed] [Google Scholar]
- 15. Schoenaker D, Callaway LK, Mishra GD. The role of childhood adversity in the development of gestational diabetes. Am J Prev Med 2019;57:302–10. 10.1016/j.amepre.2019.04.028 [DOI] [PubMed] [Google Scholar]
- 16. Versteegen M, Bozlak CT, Larkin H, et al. Maternal depression, adverse childhood experiences, and social support in relation to gestational diabetes risk: results from the Albany Infant and Mother Study (AIMS). BMC Pregnancy Childbirth 2021;21:335. 10.1186/s12884-021-03814-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sulaiman S, Premji SS, Tavangar F, et al. Total adverse childhood experiences and preterm birth: a systematic review. Matern Child Health J 2021;25:1581–94. 10.1007/s10995-021-03176-6 [DOI] [PubMed] [Google Scholar]
- 18. Nesari M, Olson JK, Vandermeer B, et al. Does a maternal history of abuse before pregnancy affect pregnancy outcomes? A systematic review with meta-analysis. BMC Pregnancy Childbirth 2018;18:404. 10.1186/s12884-018-2030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 20. Stroup DF. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 21. Pregnancy. n.d. Available: https://www.nichd.nih.gov/health/topics/pregnancy
- 22. Racine N, Devereaux C, Cooke JE, et al. Adverse childhood experiences and maternal anxiety and depression: a meta-analysis. BMC Psychiatry 2021;21:28. 10.1186/s12888-020-03017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shor E, Roelfs D, Vang ZM. The "Hispanic mortality paradox" revisited: meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med 2017;186:20–33. 10.1016/j.socscimed.2017.05.049 [DOI] [PubMed] [Google Scholar]
- 24. Borenstein M, et al. Basics of meta‐analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 2017;8:5–18. 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- 25. Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc 2018;16:195–203. 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 26. Barendregt JJ, Doi SA. MetaXL user guide version 5.3. EpiGear International Pty Ltd, 2016. [Google Scholar]
- 27. Fredriksen E, von Soest T, Smith L, et al. Patterns of pregnancy and postpartum depressive symptoms: latent class trajectories and predictors. J Abnorm Psychol 2017;126:173–83. 10.1037/abn0000246 [DOI] [PubMed] [Google Scholar]
- 28. Hantsoo L, Jašarević E, Criniti S, et al. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav Immun 2019;75:240–50. 10.1016/j.bbi.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Howell KH, Miller-Graff LE, Schaefer LM, et al. Relational resilience as a potential mediator between adverse childhood experiences and prenatal depression. J Health Psychol 2020;25:545–57. 10.1177/1359105317723450 [DOI] [PubMed] [Google Scholar]
- 30. Letourneau N, Dewey D, Kaplan BJ, et al. Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. J Dev Orig Health Dis 2019;10:88–99. 10.1017/S2040174418000648 [DOI] [PubMed] [Google Scholar]
- 31. Narayan AJ, Rivera LM, Bernstein RE, et al. Positive childhood experiences predict less psychopathology and stress in pregnant women with childhood adversity: a pilot study of the benevolent childhood experiences (BCEs) scale. Child Abuse Negl 2018;78:19–30. 10.1016/j.chiabu.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 32. Racine N, Zumwalt K, McDonald S, et al. Perinatal depression: the role of maternal adverse childhood experiences and social support. J Affect Disord 2020;263:576–81. 10.1016/j.jad.2019.11.030 [DOI] [PubMed] [Google Scholar]
- 33. Young-Wolff KC, Alabaster A, McCaw B, et al. Adverse childhood experiences and mental and behavioral health conditions during pregnancy: the role of resilience. J Womens Health (Larchmt) 2019;28:452–61. 10.1089/jwh.2018.7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christiaens I, Hegadoren K, Olson DM. Adverse childhood experiences are associated with spontaneous Preterm birth: a case-control study. BMC Med 2015;13:124. 10.1186/s12916-015-0353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mason SM, Tobias DK, Clark CJ, et al. Abuse in childhood or adolescence and gestational diabetes: a retrospective cohort study. Am J Prev Med 2016;50:436–44. 10.1016/j.amepre.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McDonnell CG, Valentino K. Intergenerational effects of childhood trauma: evaluating pathways among maternal aces, perinatal depressive symptoms, and infant outcomes. Child Maltreat 2016;21:317–26. 10.1177/1077559516659556 [DOI] [PubMed] [Google Scholar]
- 37. Appleton AA, Kiley K, Holdsworth EA, et al. Social support during pregnancy modifies the association between maternal adverse childhood experiences and infant birth size. Matern Child Health J 2019;23:408–15. 10.1007/s10995-018-02706-z [DOI] [PubMed] [Google Scholar]
- 38. Ben Salah A, Lemieux A, Mlouki I, et al. Impact of social violence and childhood adversities on pregnancy outcomes: a longitudinal study in Tunisia. J Glob Health 2019;9:020435. 10.7189/jogh.09.020435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhengu BS, Tomita A, Mashaphu S, et al. The role of adverse childhood experiences on perinatal substance use behaviour in Kwazulu-Natal province, South Africa. AIDS Behav 2020;24:1643–52. 10.1007/s10461-019-02661-y [DOI] [PubMed] [Google Scholar]
- 40. Shaikh K, Premji SS, Lalani S, et al. Ethnic disparity and exposure to supplements rather than adverse childhood experiences linked to preterm birth in Pakistani women. J Affect Disord 2020;267:49–56. 10.1016/j.jad.2020.01.180 [DOI] [PubMed] [Google Scholar]
- 41. Barrios YV, Gelaye B, Zhong Q, et al. Association of childhood physical and sexual abuse with intimate partner violence, poor general health and depressive symptoms among pregnant women. PLoS One 2015;10:e0116609. 10.1371/journal.pone.0116609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mersky JP, Lee CP. Adverse childhood experiences and poor birth outcomes in a diverse, low-income sample. BMC Pregnancy Childbirth 2019;19:387. 10.1186/s12884-019-2560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Do HP, Baker PRA, Van Vo T, et al. Intergenerational effects of violence on women's perinatal wellbeing and infant health outcomes: evidence from a birth cohort study in central Vietnam. BMC Pregnancy Childbirth 2021;21:648. 10.1186/s12884-021-04097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grimstad H, Schei B. Pregnancy and delivery for women with a history of child sexual abuse. Child Abuse Negl 1999;23:81–90. 10.1016/S0145-2134(98)00113-6 [DOI] [PubMed] [Google Scholar]
- 45. Noll JG, Schulkin J, Trickett PK, et al. Differential pathways to preterm delivery for sexually abused and comparison women. J Pediatr Psychol 2007;32:1238–48. 10.1093/jpepsy/jsm046 [DOI] [PubMed] [Google Scholar]
- 46. Leeners B, Rath W, Block E, et al. Risk factors for unfavorable pregnancy outcome in women with adverse childhood experiences. J Perinat Med 2014;42:171–8. 10.1515/jpm-2013-0003 [DOI] [PubMed] [Google Scholar]
- 47. Selk SC, Rich-Edwards JW, Koenen K, et al. An observational study of type, timing, and severity of childhood maltreatment and preterm birth. J Epidemiol Community Health 2016;70:589–95. 10.1136/jech-2015-206304 [DOI] [PubMed] [Google Scholar]
- 48. Harville EW, Boynton-Jarrett R, Power C, et al. Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study. Arch Pediatr Adolesc Med 2010;164:533–9. 10.1001/archpediatrics.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller GE, Culhane J, Grobman W, et al. Mothers' childhood hardship forecasts adverse pregnancy outcomes: role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav Immun 2017;65:11–9. 10.1016/j.bbi.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cammack AL, Hogue CJ, Drews-Botsch CD, et al. An exploratory study of whether pregnancy outcomes influence maternal self-reported history of child maltreatment. Child Abuse Negl 2018;85:145–55. 10.1016/j.chiabu.2018.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bala K. The association between adverse childhood experiences and diabetes status during pregnancy among women in Rhode Island, 2016-2018. R I Med J 2020;103:52–5. [PubMed] [Google Scholar]
- 52. Gillespie SL, Christian LM, Alston AD, et al. Childhood stress and birth timing among African American women: Cortisol as biological mediator. Psychoneuroendocrinology 2017;84:32–41. 10.1016/j.psyneuen.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J 2016;20:790–8. 10.1007/s10995-015-1909-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ranchod YK, Headen IE, Petito LC, et al. Maternal childhood adversity, prepregnancy obesity, and gestational weight gain. Am J Prev Med 2016;50:463–9. 10.1016/j.amepre.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hardcastle K, Ford K, Bellis MA. Maternal adverse childhood experiences and their association with preterm birth: secondary analysis of data from universal health visiting. BMC Pregnancy Childbirth 2022;22:129. 10.1186/s12884-022-04454-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leeners B, Richter-Appelt H, Imthurn B, et al. Influence of childhood sexual abuse on pregnancy, delivery, and the early postpartum period in adult women. J Psychosom Res 2006;61:139–51. 10.1016/j.jpsychores.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 57. Wosu AC, Gelaye B, Williams MA. Maternal history of childhood sexual abuse and preterm birth: an epidemiologic review. BMC Pregnancy Childbirth 2015;15:174. 10.1186/s12884-015-0606-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elwenspoek MMC, Kuehn A, Muller CP, et al. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017;82:140–54. 10.1016/j.psyneuen.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 59. Bick J, Zhu T, Stamoulis C, et al. Effect of early Institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr 2015;169:211–9. 10.1001/jamapediatrics.2014.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marini S, Davis KA, Soare TW, et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 2020;113:104484. 10.1016/j.psyneuen.2019.104484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Danese A. The hidden wounds of childhood trauma: psychoneuroimmunology of early stress and the impact on mental health. Brain Neurosci Adv 2017;1:345–6. [Google Scholar]
- 62. Thaler L, Steiger H. Eating disorders and epigenetics. Adv Exp Med Biol 2017;978:93–103. 10.1007/978-3-319-53889-1_5 [DOI] [PubMed] [Google Scholar]
- 63. Sadeghimahalli F, Zardooz H, Golchoobian R. Early postnatal hypothalamic-pituitary-adrenal axis activity and reduced insulin sensitivity in adult rats. Endocr Regul 2019;53:213–20. 10.2478/enr-2019-0021 [DOI] [PubMed] [Google Scholar]
- 64. Chen Y-T, Hu Y, Yang Q-Y, et al. Excessive glucocorticoids during pregnancy impair fetal brown fat development and predispose offspring to metabolic dysfunctions. Diabetes 2020;69:1662–74. 10.2337/db20-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology 2016;41:177–96. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kornmeier J, Bach M. Ambiguous figures–what happens in the brain when perception changes but not the stimulus. Front Hum Neurosci 2012;6:51. 10.3389/fnhum.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry 2016;80:23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 2012;106:29–39. 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- 69. Adcock A, Bate A, Woodhouse J. Effect of social media on the mental health of young people. 2016. Available: http://researchbriefings.parliament.uk/ResearchBriefing/Summary/CDP2016-0196
- 70. Hollingsworth K, Callaway L, Duhig M, et al. The association between maltreatment in childhood and pre-pregnancy obesity in women attending an antenatal clinic in Australia. PLoS One 2012;7:e51868. 10.1371/journal.pone.0051868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seabrook JA, Avison WR. Socioeconomic status and cumulative disadvantage processes across the life course: implications for health outcomes. Can Rev Sociol 2012;49:50–68. 10.1111/j.1755-618x.2011.01280.x [DOI] [PubMed] [Google Scholar]
- 72. Cornelius DC, Cottrell J, Amaral LM, et al. Inflammatory mediators: a causal link to hypertension during preeclampsia. Br J Pharmacol 2019;176:1914–21. 10.1111/bph.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rodrigo N, Glastras SJ. The emerging role of biomarkers in the diagnosis of gestational diabetes mellitus. J Clin Med 2018;7:120. 10.3390/jcm7060120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dodd JM, Turnbull DA, McPhee AJ, et al. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: the LIMIT randomised controlled trial. BMC Preg Child 2011;11:79. 10.1186/1471-2393-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767–77. 10.1016/S2213-8587(15)00227-2 [DOI] [PubMed] [Google Scholar]
- 76. International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017:358. 10.1136/bmj.j3119 [DOI]
- 77. Tran N, Callaway L, Shen S, et al. Screening for adverse childhood experiences in antenatal care settings: a scoping review. Aust N Z J Obstet Gynaecol 2022;62:626–34. 10.1111/ajo.13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gillespie RJ. Screening for adverse childhood experiences in pediatric primary care: pitfalls and possibilities. Pediatr Ann 2019;48:e257–61. 10.3928/19382359-20190610-02 [DOI] [PubMed] [Google Scholar]
- 79. Campbell TL. Screening for adverse childhood experiences (ACEs) in primary care: a cautionary note. JAMA 2020;323:2379–80. 10.1001/jama.2020.4365 [DOI] [PubMed] [Google Scholar]
- 80. Whitney E-O, Nicole R, Sheri M. Asking about adverse childhood experiences (ACEs) in prenatal and pediatric primary care: a narrative review and critique. 2020. [Google Scholar]
- 81. Mishra K. Screening for adverse childhood experiences in preventive medicine settings: a scoping review. J Public Health (Bangkok) 2021:1–10. [Google Scholar]
- 82. Gentry SV, Paterson BA. Does screening or routine enquiry for adverse childhood experiences (ACEs) meet criteria for a screening programme? A rapid evidence summary. J Public Health (Bangkok) 2022;44:810–22. 10.1093/pubmed/fdab238 [DOI] [PubMed] [Google Scholar]
- 83. Courtin E, Allchin E, Ding AJ, et al. The role of socioeconomic interventions in reducing exposure to adverse childhood experiences: a systematic review. Curr Epidemiol Rep 2019;6:423–41. 10.1007/s40471-019-00216-2 [DOI] [Google Scholar]
- 84. Gervin DW, Holland KM, Ottley PG, et al. Centers for disease control and prevention investments in adverse childhood experience prevention efforts. Am J Prev Med 2022;62:S1–5. 10.1016/j.amepre.2021.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063826supp001.pdf (239.6KB, pdf)
Data Availability Statement
No data are available.


