Abstract
Objective
Developing and validating a risk assessment tool aiming to identify older adults (≥65 years) at increased risk of possibly medication-related readmission to hospital within 30 days of discharge.
Design
Retrospective cohort study.
Setting
The risk score was developed using data from a hospital in southern Sweden and validated using data from four hospitals in the mid-eastern part of Sweden.
Participants
The development cohort (n=720) was admitted to hospital during 2017, whereas the validation cohort (n=892) was admitted during 2017–2018.
Measures
The risk assessment tool aims to predict possibly medication-related readmission to hospital within 30 days of discharge. Variables known at first admission and individually associated with possibly medication-related readmission were used in development. The included variables were assigned points, and Youden’s index was used to decide a threshold score. The risk score was calculated for all individuals in both cohorts. Area under the receiver operating characteristic (ROC) curve (c-index) was used to measure the discrimination of the developed risk score. Sensitivity, specificity and positive and negative predictive values were calculated using cross-tabulation.
Results
The developed risk assessment tool, the Hospitalisations, Own home, Medications, and Emergency admission (HOME) Score, had a c-index of 0.69 in the development cohort and 0.65 in the validation cohort. It showed sensitivity 76%, specificity 54%, positive predictive value 29% and negative predictive value 90% at the threshold score in the development cohort.
Conclusion
The HOME Score can be used to identify older adults at increased risk of possibly medication-related readmission within 30 days of discharge. The tool is easy to use and includes variables available in electronic health records at admission, thus making it possible to implement risk-reducing activities during the hospital stay as well as at discharge and in transitions of care. Further studies are needed to investigate the clinical usefulness of the HOME Score as well as the benefits of implemented activities.
Keywords: risk management, geriatric medicine, health & safety, quality in health care
STRENGTHS AND LIMITATIONS OF THIS STUDY.
In this study, a risk assessment tool—the Hospitalisations, Own home, Medications, and Emergency admission (HOME) Score—aiming to identify older adults (≥65 years) at increased risk of possibly medication-related readmission to hospital within 30 days of discharge was developed and externally validated.
Only variables available in the electronic health records at admission to hospital were included in the risk assessment tool.
Possibly medication-related readmissions were identified using the same tool, Assessment Tool for identifying Hospital Admissions Related to Medication (AT-HARM10), in both the development cohort and the validation cohort.
Further validations of the HOME Score are needed in order to establish its clinical usefulness in different departments as well as in other countries.
Introduction
Readmission to hospital is common, especially in older adults, where almost 20% of discharges result in a readmission within 30 days.1–3 In older adults, hospitalisation can be associated with a risk of complications such as exposure to infections, a rise in adverse events, episodes of confusion and accidental injury through falls.4 5 As readmissions are not only a risk for the individual patient but also for the health economy,3 many countries have set goals to decrease the frequency of readmission within 30 days of discharge.3 6 7
According to previous research,8–10 a relatively large proportion of readmissions to hospital, in older adults, is medication related. However, the amount differs greatly between studies as shown in a systematic review by El Morabet et al.8 In this study, the amount of medication-related readmission reported was 3%–64% with a median of 21% (IQR 14%–23%). These differences are due to a number of factors, one being the use of different definitions of ‘medication related’ between studies.8 While some studies measure readmissions related to adverse drug reactions, adverse drug events or drug–drug reactions others measure readmissions related to medication-related problems, thus including all the above-mentioned problems.8
Many medication-related readmissions may be possible to prevent, even though the proportion deemed preventable also differs between studies,8 again, due to differences in methods used. According to previous research, preventive measures should aim to improve medication use as well as transitions of care11 12 and are best performed by combining several minor activities into concepts.12 13 These activities should preferably include interdisciplinary actions during the hospital stay and at discharge12 as well as collaboration between hospital, primary and municipal care in transitions of care.14
To effectively implement interventions, healthcare personnel need to be able to identify patients at increased risk of medication-related readmission. This could preferably be done by using a risk assessment tool or risk score.15 Some risk assessment tools linked to medication-related readmission have been developed.16 17 The Prospective study to develop a model to stratify the RIsk of Medication-related harm in hospitalised Elderly patients (PRIME) tool, developed by Parekh et al,16 identifies older adults at increased risk of medication-related harm requiring healthcare use within 8 weeks of discharge, while the decision support tool developed by Olson et al17 predicts the risk of readmission in older adults using high-risk medication regimens. None of these tools have been validated in an external population or tested in a setting other than the one where it was developed.
To our knowledge, there is no risk assessment tool available that specifically aims to identify older adults at increased risk of possibly medication-related readmission to hospital within 30 days of discharge. If such a tool was available, interventions aiming to prevent readmission could be implemented based on the risk in the individual patient.15 This could make it possible to not only increase patient safety but also relocate some resources to other areas within healthcare.
Objective
The aim of this study was to develop and validate a risk assessment tool that can be used to identify older adults (≥65 years) at increased risk of possibly medication-related readmission to hospital within 30 days of discharge.
Methods
This study is reported according to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement.15
Setting
Sweden is divided into 21 regions and 290 municipalities.18 Primary and hospital care is provided by the regions, while nursing care, in the community or in nursing homes, is provided by the local municipalities. When it comes to planning patient care after hospital discharge, hospital and municipal care are expected to collaborate.19
According to Swedish directives and general advice,20 a medication reconciliation should be performed by the attending physician when patients aged 75 years and older using five medications or more are admitted to hospital. In performing the medication reconciliation, the attending physician can be supported by other healthcare personnel, for example, a clinical pharmacist.
If medication-related problems are present, the medication reconciliation should be followed by a medication review which could or could not be performed interdisciplinary (ie, involving a geriatrician or a clinical pharmacist). Unfortunately, adherence to these directives seems generally low21 with only about 15% of patients aged 75 years and older receiving a medication reconciliation and/or medication review during their hospital stay.21
Patient and public involvement
Patients or the public were not involved in this study.
Development of the risk assessment tool
The risk assessment tool was developed using anonymised data and results from our previously published retrospective studies,10 22 where further details on the population and methods of data collection can be found.
Study sample and procedure
The study was conducted at Kristianstad hospital, which is an emergency hospital with 255 beds situated in Skåne county in the south of Sweden. The study population, which is further referred to as the development cohort, consisted of randomly selected patients (n=720), aged 65 years and older, who had been admitted to Kristianstad hospital for at least 24 hours in 2017. Patients were admitted to one of the following departments: internal medicine, infectious disease, general surgery, orthopaedics or ear/nose/throat. The study group (n=360) was readmitted to any department in the hospital, for at least 24 hours, within 30 days of discharge, while the comparison group (n=360) was not. Variables were collected from electronic health records in an unblinded yet standardised and objective manner, as previously described.22
In total, 143 of 360 readmissions (39.7%) were assessed as being possibly medication related.10 Assessments were made using the Assessment Tool for identifying Hospital Admissions Related to Medication (AT-HARM10), a validated tool to distinguish between admissions that are possibly and unlikely medication related.23 With AT-HARM10 a possibly medication-related (re)admission is defined as being either caused by or significantly contributed to by a medication-related problem and a medication-related problem is defined according to Strand et al,24 that is, as an ‘undesirable patient experience that involves medication therapy and that actually or potentially interferes with desired patient outcomes’.23 This means that medication-related problems involve not only adverse drug reactions or adverse drug events but also problems such as inappropriate prescribing, non-compliance and problems related to over-the-counter medications.23 For further details on AT-HARM10, see online supplemental appendix 1.
bmjopen-2022-070559supp001.pdf (168.4KB, pdf)
Preliminary assessments, made by the first author in an unblinded fashion, were reviewed, revised and finalised by an experienced geriatrician. For further details on the assessment process, see our previous publication.10
Through multiple logistic regression analysis (stepwise backward), individual risk factors associated with all-cause readmission, possibly medication-related readmission, and unlikely medication-related readmission within 30 days of discharge were identified, as described in our previous publications.10 22
Variables included
The risk assessment tool was developed using variables identified by comparing patients with a possibly medication-related readmission (n=143) with those that did not have a possibly medication-related readmission (n=577) (ie, patients with an unlikely medication-related readmission (n=217) and patients not readmitted (n=360)). Only variables known at first admission to hospital were included in the development of the risk assessment tool.
Variables shown to be associated with possibly medication-related readmission, through multiple logistic regression analysis, were chosen to be included in the final risk assessment tool. For continuous variables, categorical variables were created based on comparisons between groups.
Data analysis
Based on the odds ratios (ORs) of the individual variables in the final multiple logistic regression model, suitable weighting and scoring were decided on for each of the included variables. A risk score, which summarised the points assigned to each of the variables included, was calculated for all the included individuals. Finally, a new logistic regression analysis was performed with possibly medication-related readmission as the dependent variable and the risk score as the test variable, saving the probabilities for further analysis. To estimate the quality of the model Hosmer-Lemeshow goodness of fit was calculated as well as Nagelkerke R2.
A ROC curve was plotted using the saved probabilities and the area under the ROC curve (c-index) was calculated giving a measure of how well the tool predicts possibly medication-related readmission.
To decide on a suitable threshold value in the risk assessment tool Youden’s index (J=sensitivity+specificity–1) was calculated for all steps in the risk score. Cross-tabulation was used to calculate sensitivity, specificity and positive and negative predictive values as well as to identify the number of correctly predicted patients.
Statistical analyses were performed using IBM SPSS Statistics V.27.
External validation of the risk score
To check the predictive ability of the risk score, as well as its precision and usefulness in other populations, we performed an external validation using data from the Medication Reviews Bridging Healthcare (MedBridge) trial.25 26
Study sample and procedure
The MedBridge trial25 26 was a randomised clinical trial conducted at four hospitals (Uppsala, Gävle, Västerås and Enköping) in the mid-eastern part of Sweden. The aim of the trial was to study the effects of hospital-based medication reviews including postdischarge follow-up on the use of healthcare resources in older adults (≥65 years), compared with hospital-based reviews and usual care only.
Included participants were admitted to a medical ward at one of the four included hospitals for at least 24 hours within the time frame 6 February 2017 to 19 October 2018. Out of the 2637 patients included in the trial, 1745 were included in 1 of the 2 medication review groups, and 892 patients were included in the group receiving usual care. Outcomes measured in the trial included readmission to hospital within 30 days of discharge and possibly medication-related readmission, as assessed with AT-HARM10.24 For further details on the population and methods of data collection used in the MedBridge trial, see Kempen et al’s study.25
To make sure the medication review interventions in the MedBridge trial could not affect the result of the validation, the MedBridge control group, that is, the 892 patients receiving usual care, was chosen to create the validation cohort in which the developed risk assessment tool was validated. In the validation cohort (n=892), 132 patients were readmitted within 30 days of discharge and 54 of these readmissions (40.9%) were assessed as being possibly medication related.
Data analysis
A multiple logistic regression analysis with the variables included in the risk assessment tool was performed in the validation cohort, comparing patients with a possibly medication-related readmission (n=54) and those that did not have a possibly medication-related readmission (n=838) (ie, those with an unlikely medication-related readmission (n=78) and those that were not readmitted within 30 days of discharge (n=760)). To estimate the quality of the model, Hosmer-Lemeshow goodness of fit was calculated as well as Nagelkerke R2.
The risk score was calculated for each of the individuals in the validation cohort, and a new logistic regression analysis was performed with possibly medication-related readmission as the dependent variable and the risk score as the test variable. Probabilities were saved and used to plot a ROC curve where the c-index was calculated giving an estimate of the predictive ability of the risk assessment tool in this external population.
Cross-tabulation was used at each of the steps in the risk score to calculate sensitivity, specificity and positive and negative predictive values. Furthermore, the number of correctly predicted patients was identified.
Statistical analysis was performed using IBM SPSS Statistics V.27.
Results
Development of the risk assessment tool
Variables included
The following variables were shown to be individually associated with possibly medication-related readmission and chosen to be included in the risk assessment tool: number of hospitalisations within the last 12 months, living in own home with home care, living in own home alone, number of medications at admission and emergency admission.
For the continuous variables, number of hospitalisations within the last 12 months and number of medications at admission, categorical variables were created based on comparisons of means and medians between groups.
The mean number of hospitalisations in patients with a possibly medication-related readmission was 1.94 and the median was 2. The mean number in the comparison group (including patients not readmitted and those with a readmission unlikely related to medications) was 1.67 and the median was 1. Hence, the categorical variable was set as hospitalisations within the last 12 months≥2.
The mean number of medications at first admission to hospital in patients with a possibly medication-related readmission and in the comparison group (ie, patients not readmitted and those with a readmission unlikely related to medications) was 10.30 and 8.09, respectively, and the median was 10 and 7, respectively. Both the categorical variable, number of medications at admission≥5 and number of medications at admission≥10, were tested in the multiple logistic regression model. Both variables showed similar odds ratios (2.20 with number of medications≥5 and 1.99 with number of medications≥10) and both had significant p values (0.005 with number of medications≥5 and <0.001 with number of medications≥10). Finally, we chose to use the categorical variable, number of medications at admission≥5, in the final model (table 1).
Table 1.
Final multiple logistic regression model from the model development dataset with possibly medication-related readmission within 30 days of discharge as the outcome variable*
| Variable | OR | 95% CI for OR | P value |
| Age | 1.00 | 0.98 to 1.03 | 0.986 |
| Sex | 1.02 | 0.69 to 1.50 | 0.939 |
| Emergency admission | 4.03 | 1.42 to 11.45 | 0.009 |
| Hospitalisations in the last 12 months≥2 | 1.53 | 1.04 to 2.27 | 0.033 |
| Medications at admission≥5 | 2.20 | 1.27 to 3.80 | 0.005 |
| Living in own home with home care | 1.84 | 1.17 to 2.89 | 0.009 |
| Living in own home alone | 1.59 | 1.06 to 2.39 | 0.026 |
Hosmer-Lemeshow goodness-of-fit test p value: 0.802. Nagelkerke R2: 0.113.
Significant p values are indicated in bold.
*Adjusted for gender and age.
Developing the risk score
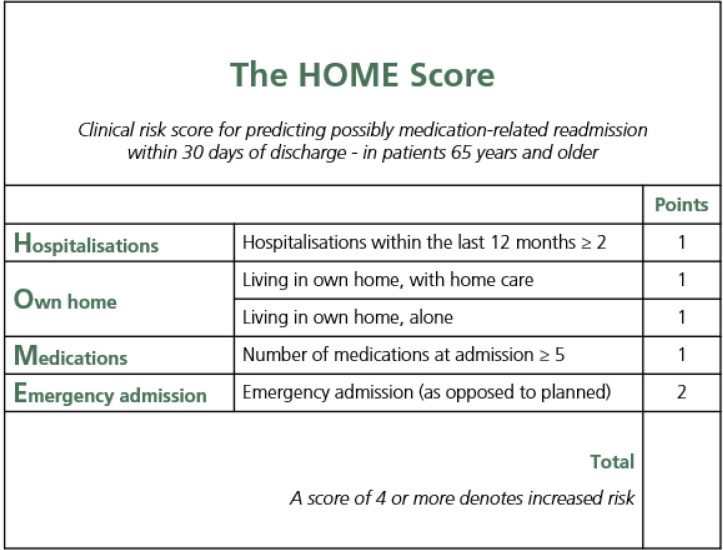
The ORs of the variables that were individually associated with possibly medication-related readmission were used for assigning points to each of the included variables. Hence, since the OR for emergency admission was about double the size of the other included variables, emergency admission was assigned two points whereas the other variables were assigned one point each, giving a maximum score of six points. The resultant 0–6 points risk score, shown in figure 1, was named the Hospitalisations, Own home, Medications, and Emergency admission (HOME) Score.
Figure 1.
The Hospitalisations, Own home, Medications, and Emergency admission (HOME) Score to be used at admission to hospital in order to identify older adults at increased risk of possibly medication-related readmission within 30 days of discharge. Hospitalisations within the last 12 months and living in own home, alone and/or with home care, refer to events and conditions prior to the admission in question.
The model showed fair calibration with a Hosmer-Lemeshow goodness-of-fit p value of 1.000 and Nagelkerke R2 of 0.117. The calculated area under the risk score ROC curve (c-index) was 0.69 (95% CI 0.64 to 0.74).
Youden’s Index was calculated for each step in the risk score using the coordinates in the ROC curve (table 2). A suitable threshold value would be where Youden’s Index is closest to 1, in this case at a score of 4 or 5.
Table 2.
Youden’s Index calculated for each step in the risk score in order to find a suitable threshold value
| Score | Sensitivity | 1−Specificity | Specificity | Youden’s Index |
| 0 | 1.000 | 1.000 | 0.000 | 0.000 |
| 1 | 1.000 | 0.974 | 0.026 | 0.026 |
| 2 | 0.951 | 0.827 | 0.173 | 0.124 |
| 3 | 0.937 | 0.794 | 0.206 | 0.143 |
| 4 | 0.755 | 0.466 | 0.534 | 0.289 |
| 5 | 0.413 | 0.170 | 0.830 | 0.243 |
| 6 | 0.147 | 0.055 | 0.945 | 0.092 |
A score of ≥4 points was finally chosen as the threshold score. The choice was based on the desire to identify as many patients at increased risk of possibly medication-related readmission as possible, that is, sensitivity rather than specificity should be as high as possible. At the threshold score (≥4 points), sensitivity was 76%, specificity 53%, positive predictive value 29% and negative predictive value 90% (table 3). The number of correctly predicted patients was 108 (out of 143).
Table 3.
Diagnostic testing of the HOME Score in the development and validation cohorts
| Development cohort | Validation cohort | |
| Sample size | 720 | 892 |
| Readmission within 30 days of discharge, n (%) | 360 (50) | 132 (15) |
| Possibly medication-related readmission, n (%) | 143 (40) | 54 (41) |
| Unlikely medication-related readmission, n (%) | 217 (60) | 78 (59) |
| Area under ROC curve (SE) | 0.69 (0.02) | 0.65 (0.04) |
| 95% CI | 0.64 to 0.74 | 0.57 to 0.72 |
| Patient distribution | ||
| HOME Score<4, n (%) | 343 (48) | 443 (50) |
| HOME Score≥4, n (%) | 377 (52) | 447 (50) |
| Patients with possibly medication-related readmission | ||
| HOME Score<4, n (%) | 35 (10) | 20 (5) |
| HOME Score≥4, n (%) | 108 (29) | 34 (8) |
| At HOME Score≥4: | ||
| Sensitivity, % | 76 | 63 |
| Specificity, % | 53 | 51 |
| Positive predictive value, % | 29 | 8 |
| Negative predictive value, % | 90 | 96 |
| Number of correctly predicted patients, n | 108 | 34 |
| At HOME Score≥5 | ||
| Sensitivity, % | 41 | 43 |
| Specificity, % | 83 | 80 |
| Positive predictive value, % | 38 | 12 |
| Negative predictive value, % | 85 | 96 |
| Number of correctly predicted patients, n | 59 | 23 |
HOME, Hospitalisations, Own home, Medications, and Emergency admission; ROC, receiver operating characteristic.
External validation of the risk assessment tool
In the validation cohort only the variable hospitalisations within the last 12 months≥2 was shown to be individually associated with possibly medication-related readmission (table 4).
Table 4.
Comparison* of variables between groups in the development and validation cohort
| Predictor | Development cohort | Validation cohort | ||||
| PMRR (n=143) | Comparison group† (n=577) | P value | PMRR (n=54) | Comparison group† (n=838) | P value | |
| Hospitalisations within the last 12 months≥2, % | 52 | 36 | <0.001 | 30 | 17 | 0.018 |
| Living in own home, with home care, % | 37 | 18 | <0.001 | 35 | 24 | 0.058 |
| Living in own home, alone, % | 53 | 37 | <0.001 | 54 | 45 | 0.213 |
| Number of medications at admission≥5, % | 87 | 71 | <0.001 | 91 | 81 | 0.077 |
| Emergency admission, % | 97 | 89 | 0.002 | 100 | 96 | 0.150 |
Significant p values (p<0.05) are indicated in bold.
*A χ2 test was used for analysis in all cases.
†Comparison group=patients not readmitted and patients with an unlikely medication-related readmission.
PMRR, possibly medication-related readmission.
Logistic regression analysis in the validation cohort, with possibly medication-related readmission as the dependent variable and HOME Score as the test variable, showed fair calibration with a Hosmer-Lemeshow goodness-of-fit p value of 1.000 and Nagelkerke R2 of 0.051.
The c-index of the HOME Score was 0.65 (95% CI 0.57 to 0.72, p value<0.001) in the validation cohort. The risk score, with the cut-off point set at ≥4 points, showed a non-significant difference between groups (p value 0.051). At this threshold score (≥4), sensitivity was 63%, specificity 51%, positive predictive value 8% and negative predictive value 96% (table 3). The number of correctly predicted patients was 34 (out of 54). With the cut-off point set at ≥5 points, there was a significant difference between groups (p value<0.001). Sensitivity was 43%, specificity 80%, positive predictive value 12% and negative predictive value 96%. The number of correctly predicted patients was 23 (out of 54) (table 3).
Discussion
The risk assessment tool developed in this study, the HOME Score, is the first externally validated risk assessment tool that can be used to identify older adults (≥65 years) at increased risk of possibly medication-related readmission to hospital within 30 days of discharge. The HOME Score was fairly discriminative of possibly medication-related readmission and showed fair calibration in development as well as in external validation. The tool is easy to use and includes variables that should be readily available in the electronic health records at admission, thus making it possible to implement risk-reducing activities during the hospital stay as well as at discharge and in transitions of care.
Comparisons to other studies
There have not yet, to our knowledge, been any risk assessment tools developed that are directly comparable to the HOME Score. However, there are several tools that can be used to identify patients at increased risk of all-cause readmission to hospital within 30 days of discharge, such as the Hemoglobin at discharge, discharge from an Oncology service, Sodium level at discharge, Procedure during the index admission (any ICD-9-CM-coded procedure), Index Type of admission (nonelective vs elective), number of Admissions during the last 12 months and Length of stay (HOSPITAL) Score,27 the Length of stay, Acuity of the admission, Comorbidity of the patient (the total Charlson Comorbidity Index) and Emergency department attendances in the last 6 months (LACE) Index28 and the Potentially Avoidable Readmission-Risk (PAR-Risk) Score.29 Even though the PAR-Risk Score focuses on medications as a risk factor for potentially avoidable hospital readmissions, it does not specifically predict medication-related readmissions. There are, however, a few risk assessment tools related to medication-related healthcare use after discharge, such as the PRIME tool16 and the decision support tool developed by Olson et al.17 None of the above-mentioned tools solely includes factors that are known already at admission as does the HOME Score.
The PRIME tool, developed by Parekh et al,16 identifies older patients (≥65 years) at increased risk of medication-related harm requiring healthcare use within 8 weeks of discharge from hospital. The tool was derived in a multicentre, prospective cohort study in the UK. In total, 818 patients discharged from 5 UK teaching hospitals between 2013 and 2015 were included. The PRIME tool was internally validated using bootstrapping and the c-index was 0.69 before and 0.66 after validation. Hence, compared with the PRIME tool, the HOME Score has a similar predictive ability with a c-index of 0.69 in the development cohort and 0.65 in the validation cohort.
With the PRIME tool,16 healthcare use after discharge includes not only hospital readmissions but also other healthcare use such as visits to the emergency department, in-person or telephone consultations with a general practitioner, or visits to outpatient clinics. This means that the PRIME tool predicts healthcare use in broader sense than does the HOME Score. Further, medication-related harm in the PRIME tool is defined as adverse drug reactions and harm arising from non-adherence only while the HOME Score defines medication-related problems more broadly, also including problems such as inappropriate prescribing and problems related to over-the-counter medications (see online supplemental appendix 1).23
Variables included in the model
The variables included in the HOME Score were identified in our previous studies10 22 where we identified risk factors for all-cause readmission, possibly medication-related readmission and unlikely medication-related readmission within 30 days of discharge, in patients 65 years and older. We chose to solely include variables known already at admission since research suggests that the successful reduction of possibly medication-related readmission demands the implementation of actions during the hospital stay30 as well as at discharge12 and in transitions of care.14 In order to do this, patients at increased risk of possibly medication-related readmission need to be identified already at admission. Hence, the HOME Score has an advantage compared with previously developed tools such as the PRIME tool,16 which include factors not known until discharge.
Hospitalisations within the last 12 months ≥2
The number of previous hospitalisations is a measure of disease burden and the fact that readmitted patients are more ill does not really come as a surprise since this has been shown previously.2 22 28 In a Swedish study from 2022, Naseer et al31 showed that emergency department visits in older adults are significantly associated with several variables indicating disease burden, such as number of chronic diseases, number of primary care visits, number of emergency department visits, polypharmacy and receipt of home care.
Naseer et al32 have also shown that prior healthcare use is associated with emergency department revisits within 30 days, in older adults. Similarly, we have identified previous healthcare use as a risk factor of possibly medication-related readmissions within 30 days of discharge10 which is why this factor was included in the HOME Score. Prior healthcare use has also been indicated as a risk factor for all-cause readmission22 27 28 and the factor is included, in some form, in the HOSPITAL Score,27 the LACE Index28 and the PAR-Risk Score.29
Living in own home with home care and/or alone
Living in your own home alone is included as a variable in the HOME Score as well as in the PRIME tool.16 Living arrangements have been previously indicated as risk factors for readmission in several studies. In 2016, Olson et al33 identified an increased risk of readmission in older men living in their own home with only their adult children as caregivers. Further, Gruneir et al34 have shown that patients using high-risk medications have an 80% increased risk of readmission within 30 days if discharged to their own home as opposed to a nursing home. However, Naseer et al32 did not find living alone to be explanatory of emergency department revisits in older adults. They did, on the other hand, find the receipt of home care to be significantly associated with emergency department revisits in one of the two Swedish regions studied. Similarly, Dahlberg et al35 have shown that living at home with home care is significantly associated with unplanned (emergency) admission to hospital.
When it comes to readmission to hospital, we have previously shown that living in the community with home care is a risk factor for all-cause readmission22 and in this study, further analyses showed that it is also associated with possibly medication-related readmission. This factor is not, to our knowledge, found in other assessment tools aiming to identify all-cause readmission or possibly medication-related readmission. However, it is part of several comprehensive geriatric assessment tools aiming to identify vulnerability and frailty.36–38 Such comprehensive geriatric assessment tools have also been shown to be predictive of all-cause readmission to hospital within 30 days36 and 60 days37 of discharge, in older adults.
Number of medications at admission ≥5
Polypharmacy is a commonly indicated risk factor for medication-related problems in older adults.39 Polypharmacy, in itself, is not necessarily a bad thing, but with age comes physiological changes that affect the pharmacokinetics and pharmacodynamics of medications. This leads to increased sensitivity39 40 which, in turn, leads to an increased risk of medication-related problems.39 The presence of polypharmacy32 41 42 and medication-related problems8 43 can lead to increased healthcare use and, as shown in this study, to possibly medication-related readmissions. Hence, polypharmacy was included in the HOME Score. Similarly, the number of medications used is included as a risk factor in the PRIME tool16 as well as in the decision support tool predicting elderly patients’ risk of readmission based on their high-risk medication regimens, developed by Olson et al.17
Emergency admission
Emergency admission, as opposed to planned admission, has been indicated as a risk factor for 30-day readmission in several studies, including ours,10 22 and the factor is included in both the HOSPITAL Score27 and the LACE Index.28
In the study by Dahlberg et al,35 the only social factor significantly associated with unplanned hospital admission was living at home with home care. Furthermore, in our previous study, we showed that older adults with a possibly medication-related readmission who lived alone were more often readmitted due to an unsustainable home situation than those living with someone.10 Since living with home care and living alone are also indicated as risk factors for all-cause and possibly medication-related readmissions, this indicates that these readmitted older adults need closer supervision after discharge. At the very least, they need better planning before discharge. To achieve this, the collaboration among hospital, primary and municipal care needs to improve.12 14
Implications for clinical use
Healthcare involving multimorbid older adults is complex and integrating care across disciplines, as well as working together in interdisciplinary teams, is important to achieve safe and effective healthcare.11 12 14 44 Improving medication use as well as transitions of care has been shown to be important factors when aiming to reduce the frequency of medication-related readmissions.11 12 Including clinical pharmacists in the interdisciplinary team, to help with medication reconciliation and medication review as well as information transfer and follow-up regarding medications and medication changes, can support this.12 45–47 The HOME Score can be used to find the patients in most need of this support.
Even though the positive predictive value of the HOME Score is quite low (29% in the development cohort and 8% in the validation cohort), it could be useful in clinical practice, especially considering the negative predictive value. Among the 50% of older adults identified as at low risk of medication-related readmission, 90% of patients in the development cohort and 96% in the validation cohort were indeed not readmitted due to medication-related problems. Hence, using the HOME Score, healthcare personnel can easily rule out 50% of patients 65 years and older who are not at increased risk of medication-related readmission. This can be done already at admission to hospital, and in doing so, the efficiency and effectiveness of preventive actions aiming to improve medication use and transitions of care can probably improve. This can possibly, in turn, lead to an increase in patient safety as well as benefits to the health economy. Further studies are needed to test these hypotheses.
Strengths and limitations
According to the TRIPOD statement15 an internal validation should always be performed when developing a prediction model, which was not done in this study. This choice was based on the fact that an external validation, using a geographically separate population, was performed. We considered this to be sufficient as clinical prediction models are always in need of further validation studies, as performance differs between locations, settings and over time.48 Hence, this is just a first edition of the HOME Score and further studies are needed to test its clinical usefulness and to keep it up to date.
The HOME Score was developed using data from a retrospective study performed in a population admitted to a single Swedish hospital. This could limit its generalisability, which is why an external validation was carried out using data from four other hospitals in another part of Sweden. The tool’s predictive ability was withstanding, suggesting that it can be used when aiming to identify patients at increased risk of possibly medication-related readmission in Sweden. However, further studies are needed to assess the international validity of the HOME Score as well as its validity in other populations within Sweden. As stated previously, this is merely a first edition of the HOME Score and further studies are needed to test its clinical usefulness and to keep it updated.
We chose to include the categorical variable number of medications at admission≥5 in the final risk score even though the mean number of medications was 10.30 in patients with a possibly medication-related readmission and 8.09 in the comparison group. This choice was based on the Swedish directives and general advice20 stating that a medication reconciliation should be performed in admitted patients taking five medications or more, but it may have weakened the prediction model. This is one of the aspects that should be examined when further validating the HOME Score and investigating its clinical usefulness.
The population used in developing the HOME Score was tailored for the identification of risk factors for all-cause readmission and possibly medication-related readmission.10 22 This led to a larger proportion of readmitted patients in the development cohort (50%) compared with the proportion in the validation cohort (15%), the proportion of 30-day readmissions in the validation cohort being closer to that reported in previous studies.1–3 This could be considered a weakness.
The tool AT-HARM1023 was used by clinical pharmacists in both the development10 and validation cohort25 26 in order to assess whether 30-day readmissions were possibly or unlikely medication-related. This is a strength as the same definition of medication-related readmission was used in both populations. However, even though the tool has been validated,23 the assessments are implicit, and the result depends on the person conducting them. This could be considered a weakness. The fact that the amount of possibly medication-related readmissions was almost the same in the development and validation cohort (40% in the development cohort and 41% in the validation cohort) indicates that this may not be a big issue.
In the development cohort, included patients were admitted to medical as well as surgical departments whereas patients in the validation cohort were admitted solely to medical wards. This could have affected the results and further validations of the HOME Score are needed in order to establish its clinical usefulness in different departments as well as in other countries.
Conclusion
The HOME Score can be used to identify older adults at increased risk of possibly medication-related readmission within 30 days of discharge. The tool is easy to use and includes variables that should be readily available in electronic health records at admission, thus making it possible to implement risk-reducing activities during the hospital stay as well as at discharge and in transitions of care. These activities could possibly help increase patient safety as well as be beneficial to the health economy but further studies are needed to investigate the clinical usefulness of the HOME Score as well as the benefits of implemented activities.
Supplementary Material
Acknowledgments
We want to thank Patrick O’Reilly for his expertise and advice in editing the manuscript. Furthermore, we want to thank Anton Hedman for fast and reliable help with extracting data from the MedBridge trial database. This study was accomplished within the context of the Swedish National Graduate School on Ageing and Health.
Footnotes
Contributors: All authors have contributed to the design of this study. MG collected, interpreted and analysed the data with the support of the other authors. The first draft of the manuscript was completed by MG after which it was critically read and commented on by the other authors. All authors have read and approved the final manuscript. MG is responsible for the overall content as the guarantor.
Funding: This study was supported by a grant from the ALF funding from Region Skåne, awarded to PM. The study was further supported by doctoral grants from the Research and Development Committee at Kristianstad-Hässleholm Hospitals and the Southern Medical Care Region, awarded to MG. The funding body had no role in the design of the study, the collection, analysis or interpretation of data or in writing the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. In accordance with the Swedish Public Access to Information and Secrecy Act,49 Swedish authorities restrict public access to the datasets analysed during the current study. However, data can be made available for research after a special review including approval of the research project by an ethics committee as well as the authorities’ data safety committees. Queries regarding data access are referred to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval was applied for and approved by the Swedish Ethics Review Authority (Dnr 2021-06612-01).
References
- 1.Swedish Association of Local Authorities and Regions . Health care in numbers. Swedish Association of Local Authorities and Regions, 2022. Available: https://www.vardenisiffror.se [Google Scholar]
- 2.Pedersen MK, Meyer G, Uhrenfeldt L. Risk factors for acute care hospital readmission in older persons in Western countries: a systematic review. JBI Database System Rev Implement Rep 2017;15:454–85. 10.11124/JBISRIR-2016-003267 [DOI] [PubMed] [Google Scholar]
- 3.Bailey MK, Weiss AJ, Barrett ML, et al. Characteristics of 30-day all-cause hospital Readmissions, 2010–2016. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [online]. Statistical Brief #248. Rockville, MD: Agency for Healthcare Research and Quality (US), 2019. [Google Scholar]
- 4.Mudge AM, McRae P, Hubbard RE, et al. Hospital-associated complications of older people: a proposed multicomponent outcome for acute care. J Am Geriatr Soc 2019;67:352–6. 10.1111/jgs.15662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219–23. 10.7326/0003-4819-118-3-199302010-00011 [DOI] [PubMed] [Google Scholar]
- 6.Kristensen SR, Bech M, Quentin W. A roadmap for comparing readmission policies with application to Denmark, England, Germany and the United States. Health Policy 2015;119:264–73. 10.1016/j.healthpol.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 7.Swedish Association of Local Authorities and Regions . Indicators for consistent health and social care. language: Swedish. 2018. Available: https://skr.se/download/18.42336a32177c8ab158d3fd70/1615301657651/Indikatorer%20fo%CC%88r%20sammanha%CC%8Allen%20va%CC%8Ard%20-%20en%20va%CC%88gledning.pdf
- 8.El Morabet N, Uitvlugt EB, van den Bemt BJF, et al. Prevalence and preventability of drug-related hospital readmissions: a systematic review. J Am Geriatr Soc 2018;66:602–8. 10.1111/jgs.15244 [DOI] [PubMed] [Google Scholar]
- 9.Linkens AEMJH, Milosevic V, van der Kuy PHM, et al. Medication-related hospital admissions and readmissions in older patients: an overview of literature. Int J Clin Pharm 2020;42:1243–51. 10.1007/s11096-020-01040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glans M, Kragh Ekstam A, Jakobsson U, et al. Medication-related hospital readmissions within 30 days of discharge-a retrospective study of risk factors in older adults. PLoS One 2021;16:e0253024. 10.1371/journal.pone.0253024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med 2016;176:484–93. 10.1001/jamainternmed.2015.7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uitvlugt EB, Janssen MJA, Siegert CEH, et al. Medication-related hospital readmissions within 30 days of discharge: prevalence, preventability, type of medication errors and risk factors. Front Pharmacol 2021;12:567424. 10.3389/fphar.2021.567424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med 2012;157:417–28. 10.7326/0003-4819-157-6-201209180-00006 [DOI] [PubMed] [Google Scholar]
- 14.Blachman NL, Blaum CS. Integrating care across disciplines. Clin Geriatr Med 2016;32:373–83. 10.1016/j.cger.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. 10.1136/bmj.g7594 [DOI] [PubMed] [Google Scholar]
- 16.Parekh N, Ali K, Davies JG, et al. Medication-related harm in older adults following hospital discharge: development and validation of a prediction tool. BMJ Qual Saf 2020;29:142–53. 10.1136/bmjqs-2019-009587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson CH, Dierich M, Adam T, et al. Optimization of decision support tool using medication regimens to assess rehospitalization risks. Appl Clin Inform 2014;5:773–88. 10.4338/ACI-2014-04-RA-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swedish Association of Local Authorities and Regions . Municipalities and regions 2022. language: Swedish. Available: https://skr.se/skr/englishpages/municipalitiesandregions.1088.html
- 19.Swedish Government Offices . Law (2017:612) on collaboration at discharge from hospital Healthcare. 2018. Available: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/lag-2017612-om-samverkan-vid-utskrivning-fran_sfs-2017-612
- 20.Swedish National Board of Health and Welfare . Directives and general advice (HSLF-FS 2017:37) on prescription and handling of medicines in health care. Swedish National Board of Health and Welfare, 2017. Available: https://patientsakerhet.socialstyrelsen.se/lagar-och-foreskrifter/foreskrifter-och-handbocker/hslf-fs-201737 [Google Scholar]
- 21.Swedish National Board of Health and Welfare . Medication reviews - A follow-up and evaluation of the National board of health and welfare’s regulations about medication reviews in Chapter 11 HSLF-FS 2017. 2019. Available: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2019-2-22.pdf
- 22.Glans M, Kragh Ekstam A, Jakobsson U, et al. Risk factors for hospital readmission in older adults within 30 days of discharge - a comparative retrospective study. BMC Geriatr 2020;20:467. 10.1186/s12877-020-01867-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempen TGH, Hedström M, Olsson H, et al. Assessment tool for hospital admissions related to medications: development and validation in older patients. Int J Clin Pharm 2019;41:198–206. 10.1007/s11096-018-0768-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strand LM, Morley PC, Cipolle RJ, et al. Drug-related problems: their structure and function. DICP 1990;24:1093–7. 10.1177/106002809002401114 [DOI] [PubMed] [Google Scholar]
- 25.Kempen TGH, Bertilsson M, Hadziosmanovic N, et al. Effects of hospital-based comprehensive medication reviews including postdischarge follow-up on older patients' use of health care: a cluster randomized clinical trial. JAMA Netw Open 2021;4:e216303. 10.1001/jamanetworkopen.2021.6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kempen TGH, Bertilsson M, Lindner K-J, et al. Medication reviews bridging healthcare (Medbridge): study protocol for a pragmatic cluster-randomised crossover trial. Contemp Clin Trials 2017;61:126–32. 10.1016/j.cct.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 27.Donzé J, Aujesky D, Williams D, et al. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med 2013;173:632–8. 10.1001/jamainternmed.2013.3023 [DOI] [PubMed] [Google Scholar]
- 28.van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ 2010;182:551–7. 10.1503/cmaj.091117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanc A-L, Fumeaux T, Stirnemann J, et al. Development of a predictive score for potentially avoidable hospital readmissions for general internal medicine patients. PLoS One 2019;14:e0219348. 10.1371/journal.pone.0219348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustafsson M, Sjölander M, Pfister B, et al. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. Eur J Clin Pharmacol 2017;73:827–35. 10.1007/s00228-017-2249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naseer M, J McKee K, Ehrenberg A, et al. Individual and contextual predictors of emergency department visits among community-living older adults: a register-based prospective cohort study. BMJ Open 2022;12:e055484. 10.1136/bmjopen-2021-055484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naseer M, Agerholm J, Fastbom J, et al. Factors associated with emergency Department Revisits among older adults in two Swedish regions: a prospective cohort study. Arch Gerontol Geriatr 2020;86:103960. 10.1016/j.archger.2019.103960 [DOI] [PubMed] [Google Scholar]
- 33.Olson CH, Dey S, Kumar V, et al. Clustering of elderly patient subgroups to identify medication-related readmission risks. Int J Med Inform 2016;85:43–52. 10.1016/j.ijmedinf.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 34.Gruneir A, Fung K, Fischer HD, et al. Care setting and 30-day hospital readmissions among older adults: a population-based cohort study. CMAJ 2018;190:E1124–33. 10.1503/cmaj.180290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahlberg L, Agahi N, Schön P, et al. Planned and unplanned hospital admissions and their relationship with social factors: findings from a national, prospective study of people aged 76 years or older. Health Serv Res 2018;53:4248–67. 10.1111/1475-6773.13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregersen M, Hansen TK, Jørgensen BB, et al. Frailty is associated with hospital readmission in geriatric patients: a prognostic study. Eur Geriatr Med 2020;11:783–92. 10.1007/s41999-020-00335-w [DOI] [PubMed] [Google Scholar]
- 37.Stillman GR, Stillman AN, Beecher MS. Frailty is associated with early hospital readmission in older medical patients. J Appl Gerontol 2021;40:38–46. 10.1177/0733464819894926 [DOI] [PubMed] [Google Scholar]
- 38.Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011;59:2129–38. 10.1111/j.1532-5415.2011.03597.x [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Parish AL. Polypharmacy and medication management in older adults. Nurs Clin North Am 2017;52:457–68. 10.1016/j.cnur.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 40.Corsonello A, Pedone C, Incalzi RA. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem 2010;17:571–84. 10.2174/092986710790416326 [DOI] [PubMed] [Google Scholar]
- 41.Picker D, Heard K, Bailey TC, et al. The number of discharge medications predicts thirty-day hospital readmission: a cohort study. BMC Health Serv Res 2015;15:282. 10.1186/s12913-015-0950-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basnet S, Zhang M, Lesser M, et al. Thirty-day hospital readmission rate amongst older adults correlates with an increased number of medications, but not with beers medications. Geriatr Gerontol Int 2018;18:1513–8. 10.1111/ggi.13518 [DOI] [PubMed] [Google Scholar]
- 43.Ekerstad N, Bylin K, Karlson BW. Early rehospitalizations of frail elderly patients - the role of medications: a clinical, prospective, observational trial. Drug Healthc Patient Saf 2017;9:77–88. 10.2147/DHPS.S139237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekdahl AW. How to promote better care of elderly patients with multi-morbidity in Europe: a Swedish example. European Geriatric Medicine 2012;3:103–6. 10.1016/j.eurger.2011.10.002 [DOI] [Google Scholar]
- 45.Daliri S, Hugtenburg JG, Ter Riet G, et al. The effect of a Pharmacy-led transitional care program on medication-related problems post-discharge: a before-after prospective study. PLoS One 2019;14:e0213593. 10.1371/journal.pone.0213593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med 2007;2:314–23. 10.1002/jhm.228 [DOI] [PubMed] [Google Scholar]
- 47.Glans M, Midlöv P, Kragh Ekstam A, et al. Obstacles and opportunities in information transfer regarding medications at discharge - a focus group study with hospital physicians. Drug Healthc Patient Saf 2022;14:61–73. 10.2147/DHPS.S362189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Calster B, Steyerberg EW, Wynants L, et al. There is no such thing as a validated prediction model. BMC Med 2023;21:70. 10.1186/s12916-023-02779-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Government Offices of Sweden . The public access to information and secrecy act 2009. 2022. Available: https://www.government.se/information-material/2009/09/public-access-to-information-and-secrecy-act
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-070559supp001.pdf (168.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. In accordance with the Swedish Public Access to Information and Secrecy Act,49 Swedish authorities restrict public access to the datasets analysed during the current study. However, data can be made available for research after a special review including approval of the research project by an ethics committee as well as the authorities’ data safety committees. Queries regarding data access are referred to the corresponding author.