Abstract
The replication and stable maintenance of latent Epstein-Barr virus (EBV) DNA episomes in human cells requires only one viral protein, Epstein-Barr nuclear antigen 1 (EBNA1). To gain insight into the mechanisms by which EBNA1 functions, we used a yeast two-hybrid screen to detect human proteins that interact with EBNA1. We describe here the isolation of a protein, EBP2 (EBNA1 binding protein 2), that specifically interacts with EBNA1. EBP2 was also shown to bind to DNA-bound EBNA1 in a one-hybrid system, and the EBP2-EBNA1 interaction was confirmed by coimmunoprecipitation from insect cells expressing these two proteins. EBP2 is a 35-kDa protein that is conserved in a variety of organisms and is predicted to form coiled-coil interactions. We have mapped the region of EBNA1 that binds EBP2 and generated internal deletion mutants of EBNA1 that are deficient in EBP2 interactions. Functional analyses of these EBNA1 mutants show that the ability to bind EBP2 correlates with the ability of EBNA1 to support the long-term maintenance in human cells of a plasmid containing the EBV origin, oriP. An EBNA1 mutant lacking amino acids 325 to 376 was defective for EBP2 binding and long-term oriP plasmid maintenance but supported the transient replication of oriP plasmids at wild-type levels. Thus, our results suggest that the EBNA1-EBP2 interaction is important for the stable segregation of EBV episomes during cell division but not for the replication of the episomes.
Epstein-Barr virus (EBV) is a gamma herpesvirus whose genome is maintained in the human host by latent infection of B lymphocytes (reviewed in references 30 and 43). During the latent mode of infection a small subset of the virally encoded proteins are expressed, and the viral genomes are maintained as low-copy-number DNA episomes in the cell nucleus. A cis-acting sequence, called oriP, has been shown to be important for the replication and segregation of the EBV episomes (59, 60). Plasmids containing oriP replicate once per cell cycle in EBV-infected cell lines and are stably maintained after many cell generations (1, 58, 60). oriP has been shown to contain two functional elements, termed the family of repeats (FR) and the dyad symmetry (DS) elements (14, 49). The DS element appears to contain the initiation site for DNA replication (23, 44, 56). The FR element acts as an enhancer, activating both transcription from viral promoters and DNA replication from the DS element, and is responsible for the stable segregation of the viral episomes (22, 40, 48, 49).
Replication and maintenance of oriP-containing episomes depends on one viral protein, Epstein-Barr nuclear antigen 1 (EBNA1) (60). EBNA1 binds to the multiple copies of its DNA recognition site present in the FR and DS elements of oriP, thereby activating DNA replication (47). EBNA1 binding to the FR element also enables plasmids containing this element to segregate stably and is required for the transactivation function of the FR (46, 57). Thus, EBNA1 functions as an origin DNA binding protein, a DNA segregation factor and a transcription factor. EBNA1 also likely plays a role in the oncogenic transformation of cells by EBV. Evidence for this comes from the observation that EBNA1 is the only viral protein expressed in all types of EBV-induced tumors, and the transgenic mice expressing EBNA1 develop B-cell lymphomas (33, 42, 55).
The mechanisms by which EBNA1 fulfills its functions are not well understood. EBNA1 does not appear to contain any intrinsic enzymatic activities but has been observed to participate in homotypic and heterotypic protein interactions (18, 20, 28, 31, 51–53, 61). The replication, segregation, and transactivation functions of EBNA1 all require a direct interaction of the EBNA1 DNA binding domain with oriP elements. This domain also mediates the dimerization of EBNA1 (2, 6, 7, 11). The structure and function of the EBNA1 DNA binding and dimerization region has been well defined (2, 6, 7, 11, 12, 52), but this region is not sufficient for EBNA1 function (32). Several regions of EBNA1 outside of the DNA binding and dimerization domains have been found to be important for EBNA1 replication, segregation, and transactivation function, but their precise contribution to these processes is unknown (31, 57).
The lack of enzymatic activities in EBNA1 and the fact that EBNA1 functions independently of other EBV gene products strongly suggests that specific interactions with cellular factors is an important part of the mechanism by which EBNA1 acts. To investigate the cellular protein partners of EBNA1, we used EBNA1 in a two-hybrid screen of a B-cell lymphoma library. Here we describe the isolation of a highly conserved protein that interacts with EBNA1 sequences that mediate the segregation function of EBNA1.
MATERIALS AND METHODS
Yeast strains.
The Y190 (MATa leu2-3,112 ura3-52 trp1-901 his3-Δ200 ade2-101 GAL4Δgal80Δ URA3 GAL-lacZ LYS GAL-HIS3 Cyhr) and Y187 (MATα GAL4 gal80 his3 trp1-901 ade2-101 ura3-52 leu2-3,112 URA3 GAL → lacZ Met−) strains containing pAS2-p53, pAS2-lamin, or pAS2-SNF1 are described in Harper et al. (26) and were kindly supplied by Brenda Andrews. Y190 contains integrated copies of both HIS3 and lacZ reporter genes under the control of GAL4 binding sites. The yeast strain used for the one-hybrid assays was constructed as follows. The FR element of oriP (EBV coordinates 7405 to 8071) was amplified by PCR by using pGEMoriP (21) as a template. The resulting fragment contained an engineered BamHI site at position 7405 and an EcoRI site at position 8071. The FR fragment was digested with BamHI and EcoRI and used to replace the BamHI/EcoRI fragment, containing GAL1 to 10 sequences, of his3-G25 (8). In the resulting construct (pFR-his3), the FR element is positioned upstream of the TATAAA box for the HIS3 reporter gene. his3-G25 contains the URA3 gene and sequences that mediate the integration of the HIS3 cassette in the leu2 locus of KY320 (13). pFR-his3 was linearized with XbaI and used to transform the KY320 to Ura protrophy. Ura+ isolates were grown on His− plates containing 5-fluoro-orotic acid to select for yeast cells that had integrated the allele (LF100).
Yeast expression plasmids.
To construct the EBNA1-expressing plasmid used in the two-hybrid screen (pAS2.EBNA1), the EBNA1 gene (amino acids 1 to 641 but lacking most of the Gly-Ala repeat) was PCR amplified from p205 (60) and cloned between the NdeI and BamHI sites of pAS2 (26), downstream of the GAL4 DNA binding domain (amino acids 1 to 147). Plasmids expressing EBNA1 amino acids 1 to 386 (pAS2.E1-386), 57 to 386 (pAS2.E57-386), and 452 to 641 (pAS2.E452-641) as a fusion with the GAL4 DNA binding domain and a hemagglutinin (HA) epitope were constructed by inserting the EBNA1 fragment between the NdeI and BamHI sites (for the fragments from 1 to 386 and from 452 to 641) or between the SmaI and BamHI sites (the 57-to-386 fragment) of pAS2. pAS2.EΔ325-376 and pAS2.EΔ41-376 express EBNA1 proteins lacking the Gly-Ala repeat and amino acids 325 to 376 or 41 to 376, respectively, fused to the GAL4 DNA binding domain. These EBNA1 mutants were previously constructed and cloned in pVL1392 (4). To generate the pAS2-based constructs, the EBNA1 mutants in pVL1392 were PCR amplified and cloned between the NdeI and BamHI sites of pAS2. pEBNA1 used in the one-hybrid assays was constructed by excising most of the GAL4 sequences in pAS2 with BamHI and SphI (partial digest) and replacing this fragment with the EBNA1 gene that had been PCR amplified from p205. The resulting construct expresses EBNA1 fused to the first 8 amino acids of GAL4 from the ADH promoter. pACT63 is a cDNA library construct isolated in the two-hybrid screen by using EBNA1 bait. It expresses EBP2 amino acids 21 to 306 fused to the GAL4 activation domain from the pACT vector (16).
Two-hybrid screen.
A λACT cDNA library, prepared from EBV-transformed human peripheral lymphocytes, was kindly provided by Stephen Elledge (16). pACT plasmids were excised from the λACT library as described in Durfee et al. (16). The pACT plasmids contain the LEU2 gene and express the cDNA library fused to the GAL4 activation domain from the ADH promoter. For the two-hybrid screen, Y190 was first transformed to Trp prototrophy with pAS2.GAL4-EBNA1 and then transformed to Leu prototrophy with the pACT human cDNA library. Transformants were plated on synthetic complete medium lacking Leu, Trp, and His (SC-Leu,Trp,His) containing 25 mM 3-aminotriazole (AT) to select for yeast in which the HIS3 gene was transactivated. Colonies were transferred to nitrocellulose filters and permeabilized by freezing in liquid nitrogen. After colonies were thawed, β-galactosidase activity assays were performed by overlaying the filters on Whatman 3MM paper soaked in Z buffer (60 mM Na2HPO4 · 7H2O, 40 mM NaHPO4 · H2O, 10 mM KCl, 1 mM MgSO4 · 7H2O, 35 mM β-mercaptoethanol) containing 1 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (9). The filters were incubated at 30°C for approximately 18 h to develop the color. Blue colonies were streaked for single colonies and retested for β-galactosidase activity. The specificity of the EBNA1 library protein interaction was tested by using the mating assay developed by Harper et al. (26). Positive colonies were grown on media containing cycloheximide to select for loss of the pAS2.EBNA1, and the resulting Trp− Leu+ strain (that retains the pACT-based library plasmid) was mated to Y187 strains that express lamin, p53, or SNF1 proteins fused to the GAL4 DNA binding domain. Trp+ Leu+ diploids from the matings were selected and screened for β-galactosidase activity. Library plasmids that only activated transcription in the presence of EBNA1 were recovered in Escherichia coli and retested for their ability to transactivate HIS3 and lacZ reporter genes in the presence of pAS2.EBNA1.
Two-hybrid assays of the EBNA1-EBP2 interaction.
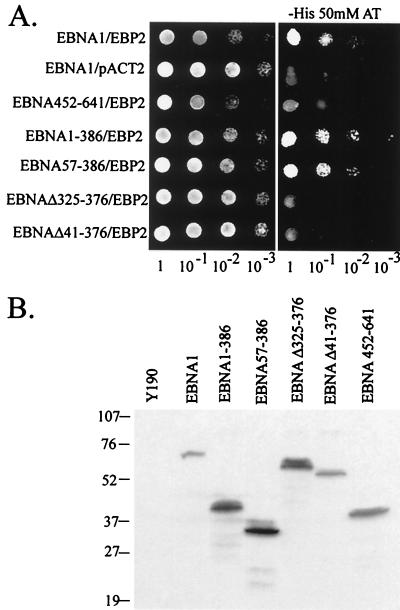
Y190 was transformed to Leu and Trp prototrophy either with pAS2.EBNA1 and pACT63, with pAS2.EBNA1 and pACT2 (39) (negative control), or with pSE1112 (SNF1 in pAS1 [16]) and pSE1111 (SNF4 in pACT [16]) (positive control). Negative control strains expressing EBNA1 binding protein 2 (EBP2) with lamin or SNF1 were generated by mating Y190 containing pACT63 with Y187 containing pAS2-lamin or pSE1112. All of the resulting strains were grown in SC-Trp,Leu to saturation and then diluted and grown to mid-log phase. Activation of the HIS3 reporter was then determined by spotting 10-fold serial dilutions of the cultures at an optical density at 600 nm (OD600) of 0.5 (5 μl/spot) onto SC plates either lacking Trp and Leu or lacking Trp, Leu, and His but containing 50 mM AT. The interaction of EBP2 with EBNA1 fragments was determined in an identical manner by cotransforming Y190 with pACT63 and pAS2.EBNA1, pAS2.E1-386, pAS2.E57-386, or pAS2.E452-641. In each case, the expression of the EBNA1 fragment was confirmed by Western blot analysis as follows. Mid-log-phase cultures of Y190 containing the EBNA1 expression plasmids were harvested, and approximately 8.5 × 107 cells were lysed by boiling in 120 μl of cracking buffer (100 mM Tris-HCl, pH 6.8; 200 mM dithiothreitol [DTT]; 20% glycerol; 4% sodium dodecyl sulfate [SDS]; 0.25% bromophenol blue). Insoluble material was pelleted by centrifugation, and 25 μl of the supernatant was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (12%) and Western blotted with antibodies against the HA epitope (Santa Cruz).
One-hybrid assay.
LF100 was transformed with both pEBNA1 and pACT63, and Trp and Leu prototrophs were selected. Three colonies from each yeast strain were grown to stationary phase in SC-Trp, Leu. The yeast cells were harvested, rinsed, and inoculated at OD600 of 0.2 in SC-Trp,Leu,His containing 5 mM AT. The growth of the cultures was followed by monitoring the OD600 for 48 h at 30°C, and the results were compared to the growth of cultures containing pEBNA1 with pACT2 (that expresses the GAL4 activation domain alone [39]) and pACT63 with pAS2.
Cloning of full-length cDNA for EBP2.
The full-length cDNA for EBP2 was isolated from a Human Leukocyte 5′-Stretch Plus cDNA library (Clontech) that was kindly supplied by Peter Whyte. The library was screened by using the EBP2 coding sequences from pACT63 as a probe according to the “Alternative Protocol for Hybridization in Aqueous Solution” in Current Protocols in Molecular Biology (3).
Baculoviruses.
To construct the baculovirus expressing EBP2, the full-length EBP2 cDNA was PCR amplified and cloned into the XhoI site of pET15b, downstream of the hexahistidine tag. Hexahistidine-tagged EBP2 was then excised from the pET15b backbone with XbaI and BamHI and cloned between the XbaI and BamHI sites of the pVL1392 baculovirus transfer vector (54) to generate pVLEBP2. A baculovirus expressing the tagged EBP2 was generated by homologous recombination of pVLEBP2 with Baculogold baculovirus DNA (Pharmingen, San Diego, Calif.) as previously described (4). Baculovirus expressing EBNA1 without the Gly-Ala repeat region (EBNA1 [21]), EBNA1 with the Gly-Ala repeat region (EBNA1GA [5]), or EBNA1 mutants lacking the Gly-Ala repeat plus amino acids 1 to 38 (EBNA39–641 [24]), 1 to 329 (EBNA330–641 [24]), 325 to 376 (EBNAΔ325–376 [4]), or 356 to 362 (EBNAΔ356–362 [37]) have been previously described. The baculovirus expressing EBNA1 lacking the Gly-Ala repeat and amino acids 367 to 376 (EBNAΔ367–376) was constructed by using two rounds of PCR, exactly as described for EBNAΔ356–362 except that in the first round of PCR EBNA1 codons 1 to 366 and 378 to 641 were amplified (4).
Coimmunoprecipitation assay.
Sf9 insect cells (107 cells/100-mm dish) were coinfected with baculoviruses expressing EBP2 and EBNA1 (or EBNA1 deletion mutants) and grown for 30 h (for 2-day infections) or 54 h (for 3-day infections) at 27°C in Grace’s medium supplemented with 0.33% yeastolate, 0.33% lactalbumin hydrolysate, and 10% fetal calf serum. The cells were then rinsed in phosphate-buffered saline (PBS) and incubated for 18 h at 27°C in methionine-free Grace’s medium containing 0.33% lactalbumin hydrolysate and 50 μCi of [35S]methionine. The cells from each dish were harvested and incubated in 0.7 ml of lysis buffer (50 mM Tris-HCl, pH 8.0; 250 mM NaCl; 1 mM DTT; 1% Nonidet P-40; 1 mM EDTA; 0.1 mM phenylmethyl sulfonyl fluoride) for 30 min at 4°C. Insoluble material was pelleted by a 10-min microfuge centrifugation, and the lysate supernatant was precleared with protein A-Sepharose CL-4B (7-μl bed volume; Pharmacia) for 15 min at 4°C with mixing. After removal of the Sepharose beads, the precleared lysate was incubated with 4 μl of anti-EBNA1 rabbit serum K67, which recognizes the DNA binding and dimerization domains of EBNA1 (kindly supplied by Jaap Middeldorp), and a 13-μl bed volume of protein A-Sepharose (equilibrated in lysis buffer plus 2 mg of bovine serum albumin per ml) for 2 h at 4°C with mixing. The Sepharose beads were then harvested and washed four times with 0.7 ml of lysis buffer. Protein bound to the beads was released by boiling the beads for 5 min in 50 μl of cracking buffer. Eluted proteins were analyzed by SDS–10% PAGE followed by autoradiography of the dried gels. To compare the expression levels of the various EBNA1 mutants, 5 μl of the above lysates prepared from insect cells coexpressing EBP2 and an EBNA1 protein were subjected to Western blot analysis with anti-EBNA1 rabbit serum.
To address the possibility that the EBNA1-EBP2 interaction was mediated by nucleic acid, coimmunoprecipitation assays were repeated as described above except that the Sepharose beads containing the EBNA1-EBP2 complexes were resuspended in 50 μl of RNase buffer (10 mM Tris-HCl, pH 7.5; 300 mM NaCl; 5 mM EDTA) containing or lacking 10 μg of RNase A (Boehringer Mannheim) or in 50 μl of DNase buffer (10 mM Tris-HCl, pH 7.0; 50 mM NaCl; 4 mM CaCl2) containing 10 μg of DNase I (Pharmacia) (36). Digestions were performed for 1 h at 30°C (RNase samples) or 37°C (DNase samples). The beads were then harvested, washed twice in lysis buffer, and boiled in cracking buffer to elute the bound proteins.
Mammalian expression plasmids.
The plasmids used for mammalian transfections were derived from pcDNA3 (Invitrogen, Carlsbad, Calif.) which contains the neomycin-resistance marker. This plasmid was first modified by the addition of EBV oriP DNA sequences. A DNA fragment encoding oriP was excised from pGEMoriP (21) by digestion with BamHI and RsaI and inserted between the BglII and NruI sites of pcDNA3 to generate pc3oriP. DNA fragments encoding EBNA1 or EBNA1 mutants lacking amino acids 325 to 376 or 41 to 376 were generated by PCR amplification from p205 (60), pVLEΔ325–376 (4), or pVLEΔ41–376 (4), respectively, by using a C-terminal primer containing a BamHI site and an N-terminal primer containing an NdeI or NcoI site. These DNA fragments were digested with NdeI or NcoI, filled in with DNA Klenow polymerase, and then digested with BamHI. DNA fragments containing the EBNA1 mutants lacking amino acids 356 to 362 or 367 to 376 were generated by digesting pVLEΔ356–362 (37) and pVLEΔ367–376 (described above) with EcoRI, filling in the 5′ overhang and then digesting with BamHI. All of the EBNA1 fragments (containing one blunt and one BamHI end) were ligated between the HindIII (made blunt by mung bean nuclease digestion) and BamHI sites of the multicloning site of pc3oriP, placing them under the control of the cytomegalovirus promoter, to generate pc3oriPE (containing EBNA1 lacking the Gly-Ala repeat), pc3oriPEΔ325–376, pc3oriPEΔ41–376, pc3oriPEΔ356–362, and pc3oriPEΔ367–376.
Transient replication assay.
C33A (human cervical carcinoma) cells were plated in 100-mm dishes at a density of 2.5 × 106 cells/dish in Dulbecco minimal essential medium with 10% fetal bovine serum and grown for 24 h (approximately 50% confluency) prior to transfection with plasmid DNA by the calcium phosphate coprecipitation method (25). Ten micrograms of pc3oriP plasmids containing EBNA1 or EBNA1 deletion mutants were combined with 10 μg of herring sperm DNA in 0.5 ml of 0.25 M CaCl2 and then added dropwise to 0.5 ml of 2× HBS (pH 6.95; 50 mM HEPES, 280 mM NaCl, 1.5 mM Na2HPO4) with vortexing. After 30 min at room temperature, the solution was added dropwise to the cells in 9 ml of medium, and the cells were incubated with the precipitate for 12 to 16 h at 37°C. The cells were then washed in PBS, replated in 150-mm plates, and grown for 72 h. Cells from each plate were harvested, counted, and lysed in 700 μl of 10 mM Tris (pH 7.5)–10 mM EDTA (pH 8.0)–0.6% SDS. High-molecular-weight DNA was precipitated by the addition of NaCl to 0.83 M and overnight incubation at 4°C (29). Low-molecular-weight DNA in the supernatant was extracted with phenol-chloroform (1:1), ethanol precipitated, and resuspended in TE buffer (pH 8.0). Half of each sample was linearized with XhoI, and 9/10 of the linearized samples were further digested with DpnI (2 to 4 U) for 2 h at 37°C. DNA fragments from the restriction digests were separated on a 1% agarose gel, transferred to GeneScreen Plus (NEN Research Products), and probed with pc3oriP that had been labelled with 32P by random primer extension. Radiolabelled bands were visualized by autoradiography and quantified by PhosphorImager analysis by using ImageQuant software (Molecular Dynamics).
Plasmid maintenance assay.
C33A cells in 100-mm dishes were transfected with 1 μg of the pc3oriPE plasmids (expressing EBNA1 or EBNA1 deletion mutants) and 19 μg of herring sperm DNA by calcium phosphate coprecipitation as described for the transient-replication assays. After 12 to 16 h of incubation with the precipitate, the cells were washed and replated in 150-mm plates in Dulbecco minimal essential medium containing 10% fetal bovine serum and 400 μg of G418 (Gibco BRL) per ml. Cells were grown under selection for 2 weeks and then harvested. Then, 5 × 106 cells from each plate were lysed by the method of Hirt (29). Low-molecular-weight DNA was cleaned, digested with XhoI and DpnI, and Southern blotted as described above for transient-replication assays. The linearized plasmid bands from the Southern blot were quantified with a PhosphorImager and compared to bands from known amounts of pc3oriPE markers in order to estimate the number of plasmids recovered per cell.
RESULTS
Two-hybrid screen.
We used the yeast two-hybrid system described in Harper et al. (26) and Durfee et al. (16) to discover cellular proteins that interact with EBNA1. EBNA1 lacking the nonessential Gly-Ala repeat was cloned as a fusion with the GAL4 DNA binding domain and expressed in yeast cells along with a human peripheral lymphocyte cDNA library fused to the GAL4 activation domain. Six million transformants were screened for transactivation of HIS3 and lacZ reporter genes under control of GAL4 binding sites, and 154 clones were initially found to activate both reporters. Of these clones, 63 were subsequently shown to interact specifically and reproducibly with EBNA1. These EBNA1 interaction clones were found to encode two different proteins. Fifty-nine clones encoded the previously identified SF2 associated protein p32 (35). p32 has been found to interact with a wide variety of proteins, and the cellular function of this protein is not yet clear (for a comprehensive summary, reference 53). The interaction of p32 with EBNA1 has been previously described (53) and will not be discussed here. The remaining four EBNA1-interacting clones consisted of an open reading frame that was not present in data banks at the time of isolation. The protein encoded by this cDNA will be referred to as EBP2 (for EBNA1 binding protein 2).
We investigated the specificity of the EBNA1-EBP2 interaction. The two-hybrid assay showed that activation of HIS3 and lacZ reporter genes occurred when EBNA1 and EBP2 fusion proteins were coexpressed but not when EBP2 was expressed with lamin or SNF1, nor when EBNA1 alone was expressed. These assays were conducted multiple times to ensure that the results were reproducible, and the results of a representative HIS3 activation assay are shown in Fig. 1.
FIG. 1.
Activation of the HIS3 reporter by EBNA1 and EBP2 in the two-hybrid system. Tenfold serial dilutions of log-phase cultures of Y190 strains expressing SNF1, EBNA1 or lamin as GAL4 DNA binding domain fusions and SNF4, EBP2, or nothing (pACT2) fused to the GAL4 activation domain were plated on SC-Trp,Leu (left panel) or SC-Trp,Leu,His plus 50 mM AT (right panel). The SNF1/SNF4 culture is a positive control for the two-hybrid interaction.
EBP2 interaction with DNA-bound EBNA1.
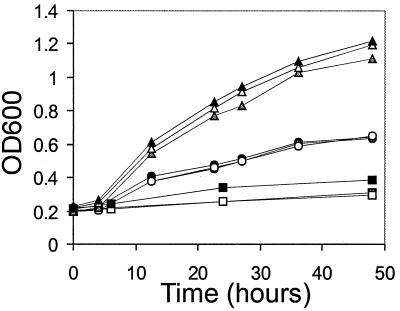
In order to fulfill its functions as an activator of latent-phase DNA replication and transcription and a mediator of DNA segregation, EBNA1 must bind to its recognition sites in oriP. Therefore, a cellular factor that mediates any of these EBNA1 activities is predicted to bind the DNA-bound form of EBNA1. We asked whether EBP2 could interact with EBNA1 bound directly to the FR element of oriP. This element contains 20 EBNA1 binding sites and is important for EBV replication, segregation, and transcription. We constructed a yeast strain containing an integrated copy of the HIS3 gene, which had been placed under control of the FR element, and expressed EBNA1 (without the GAL4 DNA binding domain) in this strain along with the EBP2-GAL4 activation domain fusion protein from the two-hybrid assay. Activation of the HIS3 reporter was measured by growth of the yeast in liquid culture lacking histidine and containing 5 mM AT (Fig. 2). We consistently found that the coexpression of EBNA1 and the EBP2 fusion protein activated the HIS3 reporter and that the expression of the EBP2 fusion protein alone did not. A small but measurable amount of transactivation was also detected when EBNA1 alone was expressed, but the level of transactivation was significantly less than that seen when both EBNA1 and EBP2 were expressed in the same cells. Therefore, the results indicate that EBP2 can interact with DNA-bound EBNA1.
FIG. 2.
Interaction of EBNA1 and EBP2 in the one-hybrid system. Triplicate cultures of LF100 expressing EBNA1 (from pEBNA1) and EBP2 fused to the GAL4 activation domain (from pACT63) were grown in liquid SC-Trp,Leu,His plus 5 mM AT, and the growth was monitored by measuring the OD600 (triangles). The growth of negative control cultures containing pEBNA1 with pACT2 (circles) or pACT63 with pAS2 (squares) is also shown.
EBP2 sequence.
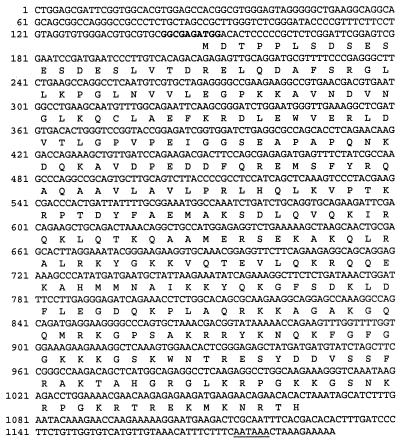
The two-hybrid and one-hybrid interaction assays suggested that the EBP2-EBNA1 interaction might be functional and was worthy of further study. We then cloned the full-length cDNA encoding EBP2. The cDNA molecule shown in Fig. 3 was isolated from a human leukocyte cDNA library by using the EBP2 cDNA fragment from the two-hybrid isolate as a probe. The cDNA isolate was found to contain the entire EBP2 two-hybrid fragment plus additional 5′ sequences. Twenty codons upstream of the 5′ end of the EBP2 two-hybrid fragment, an in-frame ATG (at position 148 in Fig. 3) was found embedded within a Kozak’s consensus sequence (34), and two in-frame stop codons were found upstream of this ATG. Thus, this ATG appears to be the start codon for EBP2. The first in-frame stop codon downstream of the start codon maps to position 1066 and is followed by a polyadenylation signal sequence at position 1174. The EBP2 open reading frame encodes a 35-kDa protein.
FIG. 3.
The EBP2 cDNA. The cDNA isolated from the 5′-Stretch Plus library is shown. The Kozak consensus sequence is in boldface and the polyadenylation sequence is underlined.
When the EBP2 protein sequence was used to search the data banks, we found an exact match with a recently entered human protein called nucleolar protein p40 (accession number U86602). Although the function of this protein is not known, Chatterjee et al. (10) had previously shown that nucleolar protein p40 is predominantly nucleolar and is associated with proliferating cells. Data bank searches also identified homolog of EBP2 in other organisms. Open reading frames encoding proteins with a high degree of homology to EBP2 exist in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Caenorhabditis elegans but have not been functionally characterized (Fig. 4). The sequences of all four of these proteins are highly conserved in the central and C-terminal portions of the proteins but diverge at the N terminus. While the human and Schizosaccharomyces pombe EBP2 proteins are very similar in length, the S. cerevisiae and C. elegans proteins are larger due to extensions at their N termini.
FIG. 4.
Alignment of human EBP2 with the S. cerevisiae (YKL172w), Schizosaccharomyces pombe (SPAC17H9), and C. elegans (C18A3.3) homologues. The alignment was performed by using the Clustal method in DNASTAR. Identical and highly conserved amino acids are shaded.
Databank searches also revealed homology of the conserved region of EBP2 with helical portions of proteins that participate in coiled-coil interactions. As suggested by the sequence homology to coiled-coil proteins, EBP2 is predicted to contain a great deal of helical character (47% α-helix), and the central region of the protein is predicted to participate in coiled-coil interactions (Fig. 5). EBP2 does not appear to contain any other previously defined functional motifs.
FIG. 5.
Predicted structure of human EBP2. The predicted secondary structure (2°) of EBP2 was determined by using PHDsec (EMBL, Heidelberg, Germany). L, loop; H, α-helix (no β-sheets were predicted). Predicted structures are only shown for residues with secondary structure reliability indices of 5 or more. The position of coiled coils (C-C) was determined by using PairCoil, and residues with a 40% or greater probability of forming coils are shown (C).
Coimmunoprecipitation of EBNA1 and EBP2.
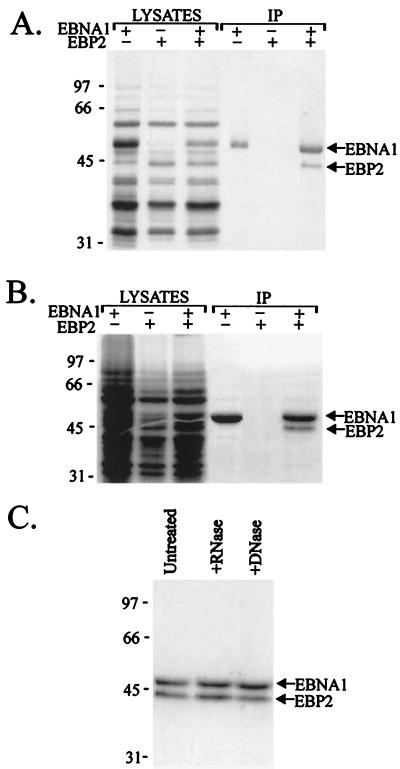
To verify the EBNA1-EBP2 interaction, we made a baculovirus expressing hexahistidine-tagged full-length EBP2 and coinfected insect cells with this virus and a second baculovirus expressing EBNA1 (21). The infected cells were labelled with [35S]methionine, and lysates were prepared 3 days postinfection. At this time, both EBNA1 and EBP2 can be seen as labelled bands in the lysates (Fig. 6A). EBNA1 and associated proteins were then precipitated from the lysates by using EBNA1 antisera. Immunoprecipitates from lysates expressing both EBNA1 and EBP2 contained two detectable labelled bands corresponding to the positions of the EBNA1 and EBP2 bands (Fig. 6A). The identity of the EBP2 band was further confirmed by Western blot with an antibody against the histidine tag (data not shown). The EBP2 band was not immunoprecipitated from lysates expressing EBNA1 alone nor from extracts expressing EBP2 alone.
FIG. 6.
Coimmunoprecipitation of EBNA1 and EBP2. Insect cells were infected with baculoviruses expressing EBNA1 or EBP2 alone or were coinfected with both baculoviruses. Lysates from metabolically labeled cells were prepared 3 days (A and C) or 2 days (B) postinfection and immunoprecipitated with anti-EBNA1 antibody (IP). (C) Immunoprecipitates from lysates coexpressing EBNA1 and EBP2 are shown before and after treatment with RNase A and DNase I.
To determine if EBNA1 and EBP2 would interact when the levels of the two proteins were decreased, we repeated the coimmunoprecipitation assay by using lysates collected 2 days postinfection (Fig. 6B). At this time, the lysates contained numerous labelled cellular proteins and levels of EBNA1 and EBP2 that were undetectable in the lysate. Immunoprecipitation of EBNA1 in these lysates again showed a specific association of EBNA1 with EBP2.
To test the possibility that the EBNA1-EBP2 interaction was mediated by nucleic acid, we repeated the coimmunoprecipitation assay (at 3 days postinfection) and compared the recovery of EBP2 before and after treatment of the EBNA1-EBP2 complexes with RNase A or DNase I (Fig. 6C). The digestion conditions used were previously shown to eliminate nucleic-acid-mediated interactions (36). The results showed that neither RNase nor DNase treatments had any detectable effect on the association of EBP2 with EBNA1, indicating that the EBNA1-EBP2 interaction is not mediated by nucleic acid.
The EBP2 interaction studies to this point were conducted with a functional version of EBNA1 that lacks most of the Gly-Ala repeat region involved in evasion of the host immune response (38). Since wild-type EBNA1 contains this sequence, we also tested whether EBP2 interacted with full-length EBNA1 containing the Gly-Ala repeat (EBNA1GA) by immunoprecipitation of EBNA1GA from insect cell lysates expressing EBNA1GA and EBP2. As shown in Fig. 7, EBNA1GA was found to specifically associate with EBP2.
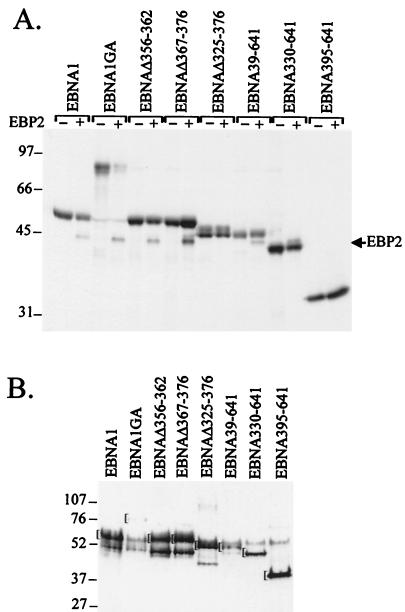
FIG. 7.
Coimmunoprecipitation of EBP2 with EBNA1 mutants. (A) Lysates from insect cells expressing EBNA1 proteins alone (−) or EBNA1 proteins with EBP2 (+) were prepared 2 days postinfection and immunoprecipitated with anti-EBNA1 antibody as in Fig. 6. Immunoprecipitated proteins are shown. (B) Insect cell lysates coexpressing EBNA1 proteins and EBP2 (1/140 the amount used in panel A) were analyzed by Western blotting with EBNA1 antisera. The position of the band corresponding to the full-length EBNA1 protein in question is marked by the bracket.
Mapping of the EBP2-interacting domain of EBNA1.
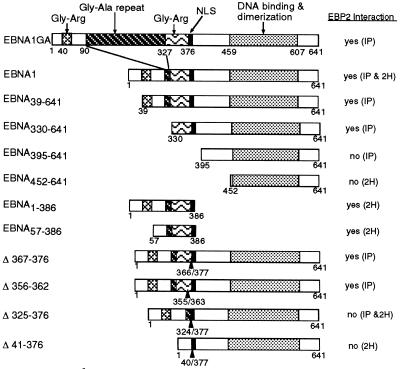
We next determined the region of EBNA1 that interacted with EBP2 by using a series of EBNA1 truncation mutants in the coimmunoprecipitation (Fig. 7) and two-hybrid (Fig. 8) assays. The results, summarized in Fig. 9, indicate that residues between amino acids 330 and 386 of EBNA1 mediate the EBP2 interaction. In keeping with this conclusion, an EBNA1 internal deletion mutant lacking the Gly-Arg-rich region between residues 325 and 376 (EBNAΔ325–376) did not detectably interact with EBP2 (Fig. 7A and 8A). The complete Gly-Arg-rich region was not required for the EBP2 interaction, however, as the small deletions present in EBNAΔ367–376 and EBNAΔ356–362 did not disrupt EBP2 binding (Fig. 7A). Differences in the abilities of EBNA1 mutants to bind EBP2 were not due to differences in the expression levels of the EBNA1 mutants; Western blot analyses of yeast and insect cell lysates indicated that there was no correlation between the amount of the EBNA1 protein expressed and its ability to bind EBP2 (Fig. 7B and 8B).
FIG. 8.
Interaction of EBNA1 mutants with EBP2 in the two-hybrid system. (A) Activation of the HIS3 reporter was determined as in Fig. 1. Tenfold serial dilutions of log-phase cultures of Y190 strains expressing EBNA1 or EBNA1 mutants as GAL4 DNA binding domain fusions and EBP2 or nothing (pACT2) fused to the GAL4 activation domain were plated on SC-Trp,Leu (left panel) or SC-Trp,Leu,His plus 50 mM AT (right panel). (B) Equal numbers of Y190 cells expressing the EBNA1 fusion proteins indicated were lysed and subjected to Western blot analysis with antibodies against the HA tag.
FIG. 9.
Summary of EBP2 interactions with EBNA1 fragments and mutants. A schematic representation of the EBNA1 polypeptides tested for EBP2 binding in co-immunoprecipitation (IP) and two-hybrid (2H) assays.
The EBP2-interacting domain of EBNA1 is required for plasmid maintenance.
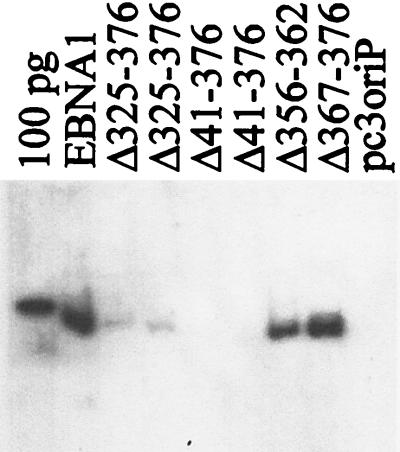
To gain insight into the functional significance of the EBNA1-EBP2 interaction, we tested the EBNA1 internal deletion mutants defective in EBP2 binding for their ability to maintain plasmids containing oriP in long-term culture. For these experiments, plasmids were constructed that contained oriP and a neomycin resistance marker and expressed either EBNA1 or an EBNA1 internal deletion mutant. These plasmids were used to transfect the C33A human cell line, and cells were grown in the presence of G418 to select for cells containing the plasmid. After 2 weeks the cells were harvested, and plasmid DNA was isolated and analyzed by Southern blotting. As shown in Fig. 10, the oriP-containing plasmid that expressed EBNA1 was maintained in the cells, while the control oriP plasmid lacking the EBNA1 gene was not maintained. EBNA1 mutants containing small deletions in the Gly-Arg region (EBNAΔ367–376 and EBNAΔ356–362) were found to maintain plasmids at a similar copy number as wild-type EBNA1 but deletions that removed all of the Gly-Arg region (EBNAΔ325–376 and EBNAΔ41–376) abrogated the plasmid maintenance function of EBNA1. A summary of the relative amounts of oriP plasmids recovered in multiple experiments is shown in Table 1. Thus, there is a correlation between the EBNA1 residues required for EBP2 binding and those required for plasmid maintenance.
FIG. 10.
Long-term plasmid maintenance ability of EBNA1 mutants. C33A cells were transfected with a plasmid containing oriP and expressing EBNA1, EBNAΔ325–376 (duplicate samples are shown), EBNAΔ41–376 (duplicate samples are shown), EBNAΔ367–376, EBNAΔ356–362, or no EBNA1 (pc3oriP) and maintained under selection for 14 days. Plasmid DNA from 5 × 106 cells was collected, digested with XhoI and DpnI, and analyzed by Southern blotting. A 100-pg linearized pc3oriPE marker is also shown.
TABLE 1.
Comparison of the ability of EBNA1 mutants to support transient DNA replication and long-term plasmid maintenance
| EBNA1 protein | % Plasmid maintenancea (SD) | % Transient replicationb (SD) |
|---|---|---|
| EBNA1 | 100 | 100 |
| EBNAΔ41–376 | 0 (0) | 47 (14) |
| EBNAΔ325–376 | 1 (1) | 95 (45) |
| EBNAΔ356–362 | 71 (32) | ND |
| EBNAΔ365–376 | 98 (27) | ND |
| None | 0 (0) | 7 (3) |
The amount of oriP plasmids (expressing EBNA1 proteins) recovered after 2 weeks of selection compared to the same plasmids expressing wild-type EBNA1. The percentages shown are the average of three different experiments. The standard deviations are shown in parentheses.
The amount of DpnI-resistant oriP plasmids (expressing EBNA1 proteins) recovered 3 days posttransfection compared to the same plasmids expressing wild-type EBNA1. The percentages shown are the average of four different experiments. The standard deviations are shown in parentheses. ND, not determined.
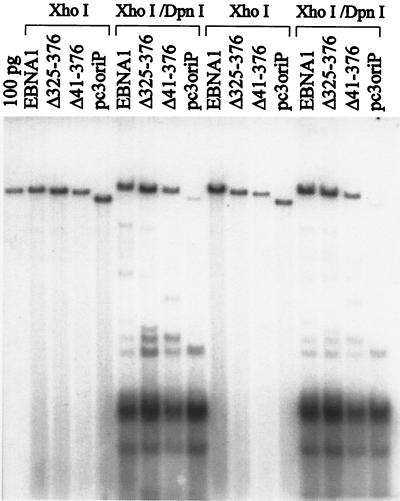
The maintenance of the oriP plasmids in the above assay depends both on the ability of the plasmids to replicate and on their ability to segregate during cell division. To determine which of these two functions was disrupted in EBNAΔ325–376 and EBNAΔ41–376, we tested the ability of these EBNA1 mutants to support the replication of the oriP-containing plasmids in transient-replication assays. In these assays, C33A cells were transfected with the same plasmids used in the plasmid maintenance assays but were grown without selection and were harvested 3 days posttransfection. The recovered plasmids were linearized, digested with DpnI to remove unreplicated plasmids, and analyzed by Southern blotting. As shown in Fig. 11 and Table 1, EBNAΔ325–376 was found to support the replication of oriP plasmids to the same degree as wild-type EBNA1, indicating that the plasmid maintenance defect of this mutant is not due to a defect in DNA replication but due to defective partitioning of oriP plasmids during cell division. EBNAΔ41–376 was also found to support transient replication of oriP plasmids, although somewhat less efficiently than wild-type EBNA1 (Table 1). Therefore, the ability of EBNA1 to bind EBP2 correlates with its ability to mediate plasmid segregation, suggesting that the EBNA1-EBP2 interaction might be important for this EBNA1 function.
FIG. 11.
Transient-replication assays of EBNA1 mutants. C33A cells were transfected with a plasmid containing oriP and expressing EBNA1, EBNAΔ325–376, EBNAΔ41–376, or no EBNA1 (pc3oriP) and grown without selection for 3 days. The plasmid DNA from each plate of cells was harvested; 1/10 was linearized with XhoI, and 9/10 was digested with both XhoI and DpnI prior to Southern blotting. The results from two experiments are shown.
DISCUSSION
We have isolated a protein, termed EBP2, that specifically interacts with EBNA1. The same protein has been previously shown to be a component of the nucleoli of proliferating human cells, but its cellular function is unknown (10). Homologues of EBP2 exist in the fission yeast Schizosaccharomyces pombe, the budding yeast S. cerevisiae, and C. elegans, but none of these proteins have been characterized. The C. elegans and S. cerevisiae proteins have N-terminal extensions not found in human or Schizosaccharomyces pombe EBP2. Despite the variation in length, we believe that the proteins are true homologues because we have shown (i) that the N-terminal extension of the S. cerevisiae protein is not required for its function in yeast cells and (ii) that the S. cerevisiae protein, like human EBP2, is predominantly nucleolar (17).
The EBP2-interacting region of EBNA1 maps to amino acids 330 to 386. This region contains a nuclear localization sequence (2) and a Gly-Arg rich region shown to mediate interactions between distant DNA-bound EBNA1 molecules (DNA looping or linking) (4, 19, 24, 37, 41). The Gly-Arg region is critical for the EBP2 interaction, since the deletion of this region abrogates EBP2 binding. The finding that small deletions in the Gly-Arg region do not disrupt EBP2 binding is in keeping with our previous results that show that this region is repetitive in both sequence and function (4, 37). Our functional studies have shown that EBNA1 mutants deficient in EBP2 binding can replicate oriP plasmids efficiently but are unable to maintain the plasmids after many cell generations. Therefore, EBNA1 residues that mediate EBP2 interactions are required for the partitioning function of EBNA1. Mutations in the Gly-Arg region that disrupt EBP2 binding also have profound effects on the DNA looping or linking activity of EBNA1 (4), and therefore presently we do not know whether the loss of the partition function is due to the abrogation of EBP2 binding or to loss of the DNA linking activity.
To gain insight into the cellular function of EBP2, we have begun to characterize the S. cerevisiae EBP2 homologue (yEBP2). We have shown that yEBP2 is an essential protein and that, like the human counterpart, it is predominantly nucleolar (17). Yeast strains expressing yEBP2 deletion mutants were shown to contain an increased population of large-budded cells in which the nucleus is stretched between the two cells (“cut” cells), and a strain expressing a conditional mutant of yEBP2 was shown to lose chromosomes at an increased rate at the permissive temperature relative to the wild-type strain. These phenotypes are indicative of a protein that functions in DNA segregation and therefore suggest that yEBP2 plays some role in the chromosomal segregation process.
Although much remains to be done to determine the precise function of EBP2, for the following reasons we believe that, like the yeast counterpart, human EBP2 plays a role in the DNA segregation pathway. First, the high degree of sequence similarity between the yeast and human EBP2s and the fact that it is the conserved portion of yEBP2 that is important for its essential function (17) strongly suggests that the proteins fulfill the same functional role in the two organisms. Second, as mentioned above, EBP2 interacts with EBNA1 residues that are important for the segregation function of EBNA1. Third, the nuclear localization, increased expression in proliferating cells (10) and conserved nature of EBP2 are all features that one would expect of a DNA segregation factor.
Little is known about the mechanism by which EBNA1 governs the partitioning of EBV episomes. Both EBNA1 and EBV genomes have been observed to associate with metaphase chromosomes, suggesting that EBNA1 mediates the attachment of EBV genomes to mitotic chromosomes, thereby ensuring that the EBV episomes segregate efficiently to the daughter cells (15, 27, 45). This hypothesis is also supported by the finding that the addition of oriP and the EBNA1 gene to yeast artificial chromosomes enables the stable maintenance of these chromosomes in human cells and causes them to associate with the host metaphase chromosomes (50). The component of mitotic chromosomes with which EBNA1 interacts has not been determined but we postulate that the EBNA1-chromosome interaction might be mediated by EBP2. It will thus be interesting to determine if EBP2 colocalizes with EBNA1 on metaphase chromosomes. Since the EBNA1-EBP2 interaction has not yet been studied in a pure system, we do not presently know if the interaction is direct or indirect. However, the fact that the interaction occurs in both yeast and insect cells suggests the former.
ACKNOWLEDGMENTS
We gratefully acknowledge Stephen Elledge for the λACT human lymphocyte cDNA library, two-hybrid plasmids, and protocols; Chris Brandl for the his3-G25 plasmid and KY320 yeast strain; Brenda Andrews for the Y190 and Y187 strains and two-hybrid plasmids; Peter Whyte for the C33A cells and the human leukocyte 5′-stretch cDNA library; and Jaap Middeldorp for the EBNA1 antiserum. We also thank Alexandra Laine for the EBNAΔ367–376 baculovirus and Carrie Rosenberger for technical assistance.
This work was supported by a grant from the Medical Research Council of Canada. L.F. was a Research Scientist of the National Cancer Institute of Canada throughout most of this work and is now a Medical Research Council of Canada Scientist.
REFERENCES
- 1.Adams A. Replication of latent Epstein-Barr virus genomes. J Virol. 1987;61:1743–1746. doi: 10.1128/jvi.61.5.1743-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambinder R F, Mullen M, Chang Y, Hayward G S, Hayward S D. Functional domains of Epstein-Barr nuclear antigen EBNA-1. J Virol. 1991;65:1466–1478. doi: 10.1128/jvi.65.3.1466-1478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley and Sons; 1991. [Google Scholar]
- 4.Avolio-Hunter T M, Frappier L. Mechanistic studies on the DNA linking activity of the Epstein-Barr nuclear antigen 1. Nucleic Acids Res. 1998;26:4462–4470. doi: 10.1093/nar/26.19.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake N, Lee S, Redchenko I, Thomas W, Steven N, Leese A, Steigerwald-Mullen P, Kurilla M G, Frappier L, Rickinson A. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (GLY-ALA) containing protein requires exogenous processing. Immunity. 1997;7:791–802. doi: 10.1016/s1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 6.Bochkarev A, Barwell J, Pfuetzner R, Bochkareva E, Frappier L, Edwards A M. Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin binding protein, EBNA1, bound to DNA. Cell. 1996;84:791–800. doi: 10.1016/s0092-8674(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 7.Bochkarev A, Barwell J, Pfuetzner R, Furey W, Edwards A, Frappier L. Crystal structure of the DNA binding domain of the Epstein-Barr virus origin binding protein EBNA1. Cell. 1995;83:39–46. doi: 10.1016/0092-8674(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 8.Brandl C J, Furlanetto A M, Martens J A, Hamilton K S. Characterization of NGG1, a novel yeast gene required for glucose repression of GAL4p-regulated transcription. EMBO J. 1993;12:5255–5265. doi: 10.1002/j.1460-2075.1993.tb06221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breeden L, Naysmith K. Regulation of the yeast HO gene. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee A, Freeman J W, Busch H. Identification and partial characterization of a Mr 40,000 nucleolar antigen associated with cell proliferation. Cancer Res. 1987;47:1123–1129. [PubMed] [Google Scholar]
- 11.Chen M-R, Middeldorp J M, Hayward S D. Separation of the complex DNA binding domain of EBNA-1 into DNA recognition and dimerization subdomains of novel structure. J Virol. 1993;67:4875–4885. doi: 10.1128/jvi.67.8.4875-4885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M-R, Zong J, Hayward S D. Delineation of a 16 amino acid sequence that forms a core DNA recognition motif in the Epstein-Barr virus EBNA-1 protein. Virology. 1994;205:486–495. doi: 10.1006/viro.1994.1669. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Struhl K. Saturation mutagenesis of a yeast his3 “TATA element”: genetic evidence for a specific TATA-binding protein. Proc Natl Acad Sci USA. 1988;85:2691–2695. doi: 10.1073/pnas.85.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chittenden T, Lupton S, Levine A J. Functional limits of oriP, the Epstein-Barr virus plasmid origin of replication. J Virol. 1989;63:3016–3025. doi: 10.1128/jvi.63.7.3016-3025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delecluse H-J, Bartnizke S, Hammerschmidt W, Bullerdiek J, Bornkamm G W. Episomal and integrated copies of Epstein-Barr virus coexist in Burkitt’s lymphoma cell lines. J Virol. 1993;67:1292–1299. doi: 10.1128/jvi.67.3.1292-1299.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn A E, Lee W-H, Elledge S J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 17.Dworet, J. H., M. Huber, K. Shire, L. Frappier, and M. A. McAlear. The budding yeast homolog of the human EBNA1-binding protein 2 (Ebp2p) is an essential nucleolar protein required for rRNA synthesis and ribosome assembly. Submitted for publication. [DOI] [PubMed]
- 18.Fischer N, Kremmer E, Lautscham G, Mueller-Lantzsch N, Grasser F A. Epstein-Barr virus nuclear antigen 1 forms a complex with the nuclear transporter karyopherin α2. J Biol Chem. 1997;272:3999–4005. doi: 10.1074/jbc.272.7.3999. [DOI] [PubMed] [Google Scholar]
- 19.Frappier L, Goldsmith K, Bendell L. Stabilization of the EBNA1 protein on the Epstein-Barr virus latent origin of DNA replication by a DNA looping mechanism. J Biol Chem. 1994;269:1057–1062. [PubMed] [Google Scholar]
- 20.Frappier L, O’Donnell M. Epstein-Barr nuclear antigen 1 mediates a DNA loop within the latent replication origin of Epstein-Barr virus. Proc Natl Acad Sci USA. 1991;88:10875–10879. doi: 10.1073/pnas.88.23.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frappier L, O’Donnell M. Overproduction, purification and characterization of EBNA1, the origin binding protein of Epstein-Barr virus. J Biol Chem. 1991;266:7819–7826. [PubMed] [Google Scholar]
- 22.Gahn T, Sugden B. An EBNA1-dependent enhancer acts from a distance of 10 kilobase pairs to increase expression of the Epstein-Barr virus LMP gene. J Virol. 1995;69:2633–2636. doi: 10.1128/jvi.69.4.2633-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gahn T A, Schildkraut C L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58:527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- 24.Goldsmith K, Bendell L, Frappier L. Identification of EBNA1 amino acid sequences required for the interaction of the functional elements of the Epstein-Barr virus latent origin of DNA replication. J Virol. 1993;67:3418–3426. doi: 10.1128/jvi.67.6.3418-3426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;5:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 26.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interaction protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 27.Harris A, Young B D, Griffin B E. Random association of Epstein-Barr virus genomes with host cell metaphase chromosomes in Burkitt’s lymphoma-derived cell lines. J Virol. 1985;56:328–332. doi: 10.1128/jvi.56.1.328-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison S, Fisenne K, Hearing J. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. J Virol. 1994;68:1913–1925. doi: 10.1128/jvi.68.3.1913-1925.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirt B. Selective extraction of polyoma DNA from infected mouse cell culture. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 30.Kieff E, Liebowitz D. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, editors. Virology. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1990. pp. 1889–1920. [Google Scholar]
- 31.Kim A L, Maher M, Hayman J B, Ozer J, Zerby D, Yates J L, Lieberman P M. An imperfect correlation between DNA replication activity of Epstein-Barr virus nuclear antigen 1 (EBNA1) and binding to the nuclear import receptor, Rch1/importin α. Virology. 1997;239:340–351. doi: 10.1006/viro.1997.8874. [DOI] [PubMed] [Google Scholar]
- 32.Kirchmaier A L, Sugden B. Dominant-negative inhibitors of EBNA1 of Epstein-Barr virus. J Virol. 1997;71:1766–1775. doi: 10.1128/jvi.71.3.1766-1775.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein G. Viral latency and transformation: the strategy of Epstein-Barr virus. Cell. 1989;58:5–8. doi: 10.1016/0092-8674(89)90394-2. [DOI] [PubMed] [Google Scholar]
- 34.Kozak M. An analysis of vertebrate mRNA sequencese: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krainer A R, Conway G C, Kozak D. Purification and characterization of pre-mRNA slicing factor SF2 from HeLa cells. Genes Dev. 1990;4:1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- 36.Lai J-S, Herr W. Ethidium bromide provides a simple tool for identifying genuine DNA-independent protein associations. Proc Natl Acad Sci USA. 1992;89:6958–6962. doi: 10.1073/pnas.89.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laine A, Frappier L. Identification of Epstein-Barr nuclear antigen 1 protein domains that direct interactions at a distance between DNA-bound proteins. J Biol Chem. 1995;270:30914–30918. doi: 10.1074/jbc.270.52.30914. [DOI] [PubMed] [Google Scholar]
- 38.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Elledge S J, Peterson C A, Bales E S, Legerski R J. Specific association between the human DNA repair proteins XPA and ERCC1. Proc Natl Acad Sci USA. 1994;91:5012–5016. doi: 10.1073/pnas.91.11.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupton S, Levine A J. Mapping of genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Mol Cell Biol. 1985;5:2533–2542. doi: 10.1128/mcb.5.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackey D, Middleton T, Sugden B. Multiple regions within EBNA1 can link DNAs. J Virol. 1995;69:6199–6208. doi: 10.1128/jvi.69.10.6199-6208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middleton T, Gahn T A, Martin J M, Sugden B. Immortalizing genes of Epstein-Barr virus. Adv Virus Res. 1991;40:19–55. doi: 10.1016/s0065-3527(08)60276-6. [DOI] [PubMed] [Google Scholar]
- 43.Miller G. Epstein-Barr virus. In: Fields B N, editor. Virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 1921–1958. [Google Scholar]
- 44.Niller H H, Glaser G, Knuchel R, Wolf H. Nucleoprotein complexes and DNA 5′-ends at oriP of Epstein-Barr virus. J Biol Chem. 1995;270:12864–12868. doi: 10.1074/jbc.270.21.12864. [DOI] [PubMed] [Google Scholar]
- 45.Petti L, Sample C, Kieff E. Subnuclear localization and phosphorylation or Epstein-Barr virus latent infection nuclear proteins. Virology. 1990;176:563–574. doi: 10.1016/0042-6822(90)90027-o. [DOI] [PubMed] [Google Scholar]
- 46.Polvino-Bodnar M, Schaffer P A. DNA binding activity is required for EBNA1-dependent transcriptional activation and DNA replication. Virology. 1992;187:591–603. doi: 10.1016/0042-6822(92)90461-w. [DOI] [PubMed] [Google Scholar]
- 47.Rawlins D R, Milman G, Hayward S D, Hayward G S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- 48.Reisman D, Sugden B. trans Activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986;6:3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reisman D, Yates J, Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985;5:1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson K, McGuigan A, Huxley C. Stable episomal maintenance of yeast artificial chromosomes in human cells. Mol Cell Biol. 1996;16:5117–5126. doi: 10.1128/mcb.16.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su W, Middleton T, Sugden B, Echols H. DNA looping between the origin of replication of Epstein-Barr virus and its enhancer site: stabilization of an origin complex with Epstein-Barr nuclear antigen 1. Proc Natl Acad Sci USA. 1991;88:10870–10874. doi: 10.1073/pnas.88.23.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Summers H, Barwell J A, Pfuetzner R A, Edwards A M, Frappier L. Cooperative assembly of EBNA1 on the Epstein-Barr virus latent origin of replication. J Virol. 1996;70:1228–1231. doi: 10.1128/jvi.70.2.1228-1231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Finan J E, Middeldorp J M, Hayward S D. P32/TAP, a cellular protein that interacts with EBNA-1 of Epstein-Barr virus. Virology. 1997;236:18–29. doi: 10.1006/viro.1997.8739. [DOI] [PubMed] [Google Scholar]
- 54.Webb N R, Summers M D. Expression of proteins using recombinant baculoviruses. Technique. 1990;2:173–188. [Google Scholar]
- 55.Wilson J B, Bell J L, Levine A J. Expression of Epstein-Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 1996;15:3117–3126. [PMC free article] [PubMed] [Google Scholar]
- 56.Wysokenski D A, Yates J L. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J Virol. 1989;63:2657–2666. doi: 10.1128/jvi.63.6.2657-2666.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yates J L, Camiolo S M. Dissection of DNA replication and enhancer activation functions of Epstein-Barr virus nuclear antigen 1. Cancer Cells. 1988;6:197–205. [Google Scholar]
- 58.Yates J L, Guan N. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J Virol. 1991;65:483–488. doi: 10.1128/jvi.65.1.483-488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yates J L, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USA. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yates J L, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Frappier L, Gibbs E, Hurwitz J, O’Donnell M. Human RPA (hSSB) interacts with EBNA1, the latent origin binding protein of Epstein-Barr virus. Nucleic Acids Res. 1998;26:631–637. doi: 10.1093/nar/26.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]