Abstract
Impairments in the function of the hypothalamic-pituitary-adrenal (HPA) axis and enhanced glucocorticoid receptor (GR) activity in the central amygdala (CeA) are critical mechanisms in the pathogenesis of alcohol use disorder (AUD). The GR antagonist mifepristone attenuates craving in AUD patients, alcohol consumption in AUD models, and decreases CeA γ-aminobutyric acid (GABA) transmission in alcohol-dependent rats. Previous studies suggest elevated GR activity in the CeA of male alcohol-preferring Marchigian-Sardinian (msP) rats, but its contribution to heightened CeA GABA transmission driving their characteristic post-dependent phenotype is largely unknown.
We determined Nr3c1 (the gene encoding GR) gene transcription in the CeA in male and female msP and Wistar rats using in situ hybridization and studied acute effects of mifepristone (10 μM) and its interaction with ethanol (44 mM) on pharmacologically isolated spontaneous inhibitory postsynaptic currents (sIPSCs) and electrically evoked inhibitory postsynaptic potentials (eIPSPs) in the CeA using ex vivo slice electrophysiology.
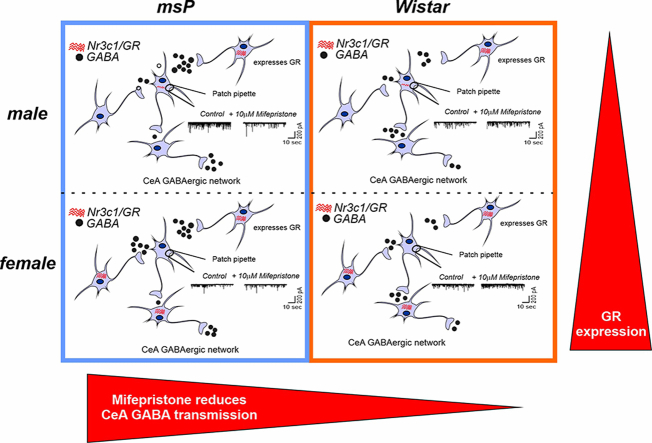
Female rats of both genotypes expressed more CeA GRs than males, suggesting a sexually dimorphic GR regulation of CeA activity. Mifepristone reduced sIPSC frequencies (GABA release) and eIPSP amplitudes in msP rats of both sexes, but not in their Wistar counterparts; however, it did not prevent acute ethanol-induced increase in CeA GABA transmission in male rats.
In msP rats, GR regulates CeA GABAergic signaling under basal conditions, indicative of intrinsically active GR. Thus, enhanced GR function in the CeA represents a key mechanism contributing to maladaptive behaviors associated with AUD.
Graphical abstract
1. Introduction
Alcohol use disorder (AUD) is a chronic, relapsing disease (Carvalho et al., 2019; Heilig et al., 2021; Koob, 2021) that is characterized by a compulsion to seek and consume alcohol, accompanied by both the loss of control over intake and the emergence of negative affect when access to alcohol is not possible (Koob, 2015; Koob and Schulkin, 2019). Importantly, 5.3% of all deaths, translating to approximately 3 million premature deaths per year, are caused by harmful alcohol consumption (Glantz et al., 2020).
Dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis is an integral mechanism in the transition to, and maintenance of, AUD. Consequently, the corticotropin-releasing factor (CRF)/CRF1 receptor system (Koob, 2021, 2022) and further downstream systems, such as glucocorticoids and their congener receptors (i.e., glucocorticoid receptors [GRs] and mineralocorticoid receptors [MRs] (Meijer et al., 2022) have potential as therapeutic targets for the treatment of AUD. Recent studies identified elevations of GR phosphorylation, which is indicative of enhanced GR function, in the central nucleus of the amygdala (CeA) of alcohol-dependent rats (Vendruscolo et al., 2015). The CeA is a critical brain region processing negative emotional states that are associated with AUD (Roberto et al., 2021). The GR antagonist mifepristone and more selective GR modulators (e.g., CORT113176, CORT118335, CORT122928, and CORT125134) decreased alcohol consumption in alcohol-dependent and in genetically selected alcohol preferring rats (Benvenuti et al., 2021; McGinn et al., 2021; Vendruscolo et al., 2012, 2015). Mifepristone also prevented stress-induced reinstatement of ethanol seeking in nondependent rats (Simms et al., 2012) and importantly reduced cue-induced craving and alcohol consumption in AUD patients (Vendruscolo et al., 2015). At the synaptic level, we reported that mifepristone and CORT118335 decreased CeA γ-aminobutyric acid (GABA) transmission more efficiently in male alcohol-dependent rats than in alcohol-naive controls (Khom et al., 2022), indicating GR recruitment in response to the stress that is associated with chronic intermittent alcohol exposure (CIE) and withdrawal (Koob, 2022; Koob and Schulkin, 2019).
Unlike in rodent models in which alcohol dependence is induced (e.g., by CIE), genetically selected Marchigian-Sardinian alcohol-preferring (msP) rats consume large amounts of ethanol, and they exhibit innately high levels of anxiety-like, stress-like, and depression-like symptoms. A causal role has been attributed to polymorphisms of the Crhr1 gene (Borruto et al., 2021; Ciccocioppo et al., 2006; Cippitelli et al., 2015; Hansson et al., 2006). A common phenomenon that is observed in alcohol dependence, induced by chronic intermittent ethanol consumption, across species, including rats (Khom et al., 2020a, 2020b, 2022; Roberto et al., 2004; Varodayan et al., 2017), mice (Herman et al., 2016a; Patel et al., 2021), macaques (Patel et al., 2022), and humans (Augier et al., 2018), is the elevated activity of CeA GABAergic synapses. Indeed, CeA GABAergic (Herman et al., 2013) and glutamatergic (Herman et al., 2016b; Natividad et al., 2017, p. 201) transmission in male msP rats is elevated compared to Wistar rats. Additionally, in msP rats, CeA CRF1 receptor sensitivity to CRF, accompanied by higher levels of phosphorylated GRs [indicative of more pronounced GR control over CeA activity (Natividad et al., 2021)], mimics features of a post-dependent phenotype. Moreover, behavioral studies show that selective GR antagonism reduces alcohol self-administration in female but not in male msP rats (Benvenuti et al., 2021) suggesting sex differences in response to GR antagonism. These findings prompted us to further investigate the role of GRs in regulating CeA GABAergic activity in male and female msP rats compared with their non genetically-selected Wistar counterparts. We employed in situ hybridization (ISH) to investigate CeA GR gene expression (Nr3c1) and its colocalization with either GABAergic or glutamatergic neurons. We used mifepristone as a pharmacological tool to dissect potential differences in the GR regulation of CeA GABA transmission in msP vs. Wistar rats using ex vivo slice electrophysiology.
2. Materials and methods
2.1. Animals
All procedures were approved by the Scripps Research Institutional Animal Care and Use Committee (protocol no. 09-0006). All animal experiments were conducted in accordance with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the results are reported according to ARRIVE guidelines. All efforts were made to minimize animal suffering and reduce the number of animals used.
We used 65 adult (22 male msP, 14 female msP, 18 male Wistar, 11 female Wistar) rats. Wistar rats were either purchased from Charles River (Raleigh, NC, USA), weighing 225–250 g upon arrival, or taken from our breeding colony at Scripps Research. msP rats were taken from our breeding colony at Scripps Research. All rats were group-housed (2–3/cage) in standard plastic cages in a temperature- and humidity-controlled room under a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. Females were allowed to freely cycle.
2.2. Chronic ethanol vapor inhalation
We used the standard chronic intermittent ethanol exposure (CIE) paradigm at Scripps Research to induce alcohol dependence in eight male Wistar rats in two separate cohorts. The animals were daily exposed to ethanol vapor in their home cages (14 h vapor on/10 h vapor off) for 5–7 weeks (Khom et al., 2020a; Roberto et al., 2004; Varodayan et al., 2022). We determined blood alcohol levels (BALs) from tail vein blood samples 1–2 times weekly. Average BALs from all animals were 223 ± 29 mg/dl.
2.3. In situ hybridization
A total of 12 rats (n = 3/sex and genotype; 2 CeA sections from each rat) were deeply anesthetized with 3–5% isoflurane and decapitated. Brains were rapidly removed, flash frozen in prechilled isopentane on dry ice, and stored at −80 °C. Brains were sliced on a cryostat in 20 μm-thick sections. The sections were mounted on SuperFrost Plus slides (Fisher Scientific, catalog no. 1255015) and stored at −80 °C for further processing. We then performed ISH using the RNAscope fluorescent multiplex kit (ACD, catalog no. 320850), including a target retrieval protocol under RNase-free conditions as outlined in the manufacturer's manual (ACD, catalog no. 320513 and 320293). The slides were fixed in cold phosphate-buffered saline/Z-fix (Anathec, catalog no. 6480) for 15 min at 4 °C and then dehydrated at room temperature in 50%, 70%, and 100% ethanol for 5 min at each concentration. After pretreatment, protease IV (ACD, catalog no. 322336) was applied to the slides for 30 min at room temperature. The slides were incubated with the following probes: Nr3c1 (ACD, catalog no. 466991-C1; encoding GR), Slc17a7 (ACD, catalog no. 317001-C2; encoding vesicular glutamate transporter type 1 [Vglut1]), and Gad2 (ACD, catalog no. 435801-C3; encoding glutamate decarboxylase) for 2 h at 40 °C. A negative control (ACD, catalog no. 320871) was run in tandem. We amplified the signal to detect RNA transcripts for a total of 90 min at 40 °C according to the manufacturer's instructions. Finally, the slides were mounted with DAPI-containing Vectashield (Fisher Scientific, catalog no. NC9029229).
2.4. Imaging and analysis
We took multiple (6) images from each CeA at similar bregma points to control for variation using a Zeiss LSM 780 laser scanning confocal microscope (40 × oil immersion, 1024 × 1024, tile scanning of CeA 5 μm z-stacks) and kept all microscope settings the same between the experiments during image acquisition. Analysis and quantification were performed as previously described (Khom et al., 2020b; Varodayan et al., 2022). Briefly, we identified and counted nuclei in the CeA based on their DAPI staining using Fiji. Nuclei were considered positive for the probe if the corresponding fluorescent signal was present after background (negative control) subtraction. The percentage of positive nuclei for labeled cells was calculated. Next, the percentage of Nr3c1+ nuclei that expressed the marker of interest was determined by dividing the number of co-labeled nuclei by the total number of Nr3c1+ nuclei.
2.5. Ex vivo slice electrophysiology
We prepared brain slices and performed electrophysiological recordings as previously described (Demoret et al., 2020; Khom et al., 2021; Roberto et al., 2004, 2010; Steinman et al., 2021; Suárez et al., 2020; Varodayan et al., 2018). We decapitated deeply anesthetized rats (3–5% isoflurane anesthesia) and quickly removed their brains in ice-cold oxygenated cutting solution (composition: 206 mM sucrose, 2.5 mM KCl, 0.5 mM CaCl2, 7 mM MgCl2, 1.2 mM NaH2PO4, 26 mM NaHCO3, 5 mM glucose, and 5 mM HEPES). We cut 300–400 μm thick coronal slices that contained the medial subdivision of the CeA using a Leica VT 1000S or Leica VT 12000S. Notably, dependent rats were euthanized in the last hour of their daily ethanol vapor exposure and were still intoxicated at the time of euthanasia. However, we did not supplement any solution (slicing, incubation, or bath solution) with ethanol; thus, the slices underwent acute in vitro ethanol withdrawal (1–8 h; (Khom et al., 2022; Khom et al., 2020a; Khom et al., 2020b; Kirson et al., 2021; Varodayan et al., 2022).
2.5.1. Intracellular recordings of evoked responses
CeA slices were incubated in an interface configuration for 15–20 min and then completely submerged and continuously superfused (flow rate of 2–4 ml/min) with 95% O2/5% CO2 equilibrated artificial cerebrospinal fluid (aCSF) of the following composition: 130 mM NaCl, 3.5 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgSO4•7H2O, 2.0 mM CaCl2, 24 mM NaHCO3, and 10 mM glucose. We recorded from 56 CeA (medial subdivision) neurons using sharp micropipettes that were filled with 3M KCl in discontinuous current-clamp mode (Roberto et al., 2004, 2010), holding them near their resting membrane potential (−82.4 ± 0.8 mV). We acquired data with an Axoclamp-2A amplifier (Molecular Devices, Foster City, CA, USA) and stored them for offline analysis using pClamp 10.2 software (Molecular Devices, Foster City, CA, USA). Hyperpolarizing and depolarizing current steps (200 pA increments, 750 ms duration) were applied to generate I–V curves to assess cellular health. Recordings were obtained randomly from all neuronal cell types that are commonly found in the CeA. We evoked GABAA receptor-mediated inhibitory postsynaptic potentials (eIPSPs) by stimulating locally within the CeA through a bipolar stimulating electrode and superfusing the slices with aCSF that contained blockers of glutamate-mediated synaptic transmission (20 μM 6,7-dinitroquinoxaline-2,3-dione [DNQX] and 30 μM DL-2-amino-5-phosphonovalerate [DL-AP-5]) and GABAB receptors (1 μM CGP55845A). To determine synaptic response parameters for each cell, we performed an input/output (I/O) protocol (Roberto et al., 2003, 2004, 2010) that consisted of a range of five current stimulations (50–250 nA, 0.125 Hz), starting at the minimum current that was required to elicit an IPSP up to the strength that is required to elicit the maximum subthreshold amplitude. These stimulus strengths were maintained throughout the entire duration of the experiment. We examined paired-pulse facilitation (PPF) in each neuron using paired stimuli at 100 ms interstimulus intervals (Roberto et al., 2004). The stimulus strength was adjusted such that the amplitude of the first eIPSP was ∼50% of the maximal amplitude that was determined by the I/O protocol. PPF was calculated as the amplitude of the second eIPSP over the first eIPSP. Drug-induced changes in the PPF generally reflect presynaptic effects, such that an increase in the PPF suggests a decrease in neurotransmitter (e.g., GABA) release (Roberto et al., 2004). All measures were taken before drug superfusion (control) and during drug superfusion.
2.5.2. Patch clamp recordings
We recorded from 88 neurons from the medial subdivision of the CeA using the whole-cell voltage clamp configuration as previously described (Khom et al., 2020a, 2021; Varodayan et al., 2018). We visualized neurons using infrared differential interference contrast optics and either 40 × or 60 × water-immersion objectives (Olympus BX51WI) and charge-coupled device cameras (EXi Aqua, QImaging). We recorded in gap-free acquisition mode with a 20 kHz sampling rate and 10 kHz low-pass filtering using a MultiClamp700B amplifier, Digidata 1440A, and pClamp 10 software (MolecularDevices).
We pulled patch pipettes (average resistance, 3–5 MΩ) from borosilicate glass (King Precision) and filled them with a KCl-based internal solution that was composed of 135 mM KCl, 5 mM EGTA, 5 mM MgCl2, 10 mM HEPES, 2 mM Mg-ATP, and 0.2 mM Na-GTP (pH 7.2–7.4, adjusted with 1M KOH, 290–300 mOsm). All neurons were held at −60 mV during the entire recording, and pharmacologically isolated action potential-dependent GABAA receptor-mediated spontaneous postsynaptic inhibitory currents (sIPSCs) were isolated by adding antagonists of glutamate-mediated transmission and GABAB receptors to the bath solution as described above. Data were derived from neurons with access resistance (Ra) ≤ 15 MΩ and a maximum change of Ra ≤ 20% during the recording as monitored by 10 mV pulses that were applied approximately every 1 min.
2.6. Drugs
Mifepristone was purchased from Cayman Chemical (Ann Arbor, MI, USA). Ethanol was obtained from Remet (La Mirada, CA, USA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). We prepared stock solutions of DL-AP-5 and CGP55845A in distilled water. DNQX and mifepristone were dissolved in 100% dimethylsulfoxide. All drugs, including ethanol, were added to the bath solution to achieve the indicated concentrations.
2.7. Data and statistical analysis
Data throughout the manuscript are presented as the mean ± standard error of the mean (SEM) of either raw values or normalized data. Sample sizes are based on experience from our laboratory for similar studies. All datasets were derived from ≥3 rats from each sex and genotype and are indicated for each experiment in the figure legend; n denotes the total number of recorded cells. Data were pooled per genotype and sex but not by estrous cycle stage. To avoid pseudo-replication by collecting multiple recordings from one individual animal, we set a cutoff ≤3 data points for all datasets from single animals. The criterion for statistical significance for all experiments was set to p < 0.05. We used a two-way analysis of variance (ANOVA) to determine statistically significant changes in the Nr3c1 expression with sex and genotype as factors shown in Fig. 1. sIPSC characteristics were determined in Mini Analysis 5.1 software (Synaptosoft, Leonia, NJ, USA). sIPSCs with amplitudes >5 pA were counted, and each event was visually confirmed. We analyzed sIPSC frequencies, amplitudes, rise and decay times combining them in 3-min bins, except for baseline for which we used 3–5 min bins. Intracellular recordings were analyzed using Clampfit 10.2 software (Molecular Devices). We used Prism 9.5 software (GraphPad Software, San Diego, CA, USA) to plot the results and perform the statistical analysis. For the electrophysiological data, we used the Kolmogorov-Smirnov test to probe for a Gaussian data distribution, one-sample t-tests to determine per se effects of drugs (percent changes from baseline), two-tailed unpaired t-tests to compare two independent groups, and two-way ANOVAs to test for differences between multiple groups, with sex and genotype as factors, followed by the Tukey post hoc test when appropriate.
Fig. 1.
Elevated GR expression in the female CeA in both msP and Wistar rats. (A) Representative images of the medial subdivision of the CeA after in situ hybridization for Gad2 (yellow signal) as a marker of GABAergic neurons, Slc17a7 (red signal) as a marker of glutamatergic neurons, Nr3c1 (green signal) for expression of GR, and DAPI to label cell bodies. The data are expressed as the mean ± SEM of normalized cell counts of GAD2-positive (B), Slc17a7-positive (C), and Nr3c1-positive (D) neurons in the CeA in rats of the indicated sexes and genotypes. The data are expressed as the mean ± SEM of the number of neurons that co-expressed either Nr3c1 and GAD2 (E) or Nr3c1 and SLc17a7 (F). Main effects of sex and genotype and a sex × genotype interaction were determined by two-way ANOVA ($p < 0.05, main effect of sex; %p < 0.05, main effect of genotype). Two sections from 3 animals for each sex and genotype were imaged and analyzed. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Results
3.1. Female CeA neurons express more Nr3c1 compared with males
We first performed ISH using RNAscope technology to determine potential sex- and genotype-dependent differences in CeA GR (Nr3c1) expression and its co-localization with glutamic acid decarboxylase (GAD2) as a marker for GABAergic neurons and vesicular glutamate transporter subtype 1 (Slc17a7) as a marker for glutamatergic neurons (Wolfe et al., 2019). As shown in Fig. 1A and B, the majority of CeA cells in both genotypes and sexes expressed GAD2, which is consistent with the primarily GABAergic composition of the CeA. Notably, the two-way ANOVA identified a main effect of genotype on GAD2 expression (F1,20 = 5.352, p = 0.0315) but no main effect of sex (F1,20 = 1.373, p = 0.2551) and no sex × genotype interaction (F1,20 = 1.20, p = 0.1178), suggesting that the CeA in msP rats comprises more GABAergic neurons compared with their Wistar counterparts (Fig. 1A). We also found a main effect of sex on Slc17a7 expression (F1,20 = 11.80, p = 0.0026) but no effect of genotype (F1,20 = 0.5616, p = 0.4623) and no sex × genotype interaction (F1,8 = 1.010, p = 0.3270), suggesting that females have more glutamatergic neurons than males, regardless of genotype (Fig. 1C). Likewise, the two-way ANOVA revealed a significant main effect of sex on Nr3c1 expression (F1,20 = 47.55, p < 0.0001) but no main effect of genotype (F1,20 = 0.8345, p = 0.3719) and no sex × genotype interaction (F1,20 = 0.3007, p = 0.5895), indicating overall broader GR expression in the female CeA (Fig. 1D).
Importantly, the vast majority (75–85%) of Nr3c1 puncta were detected on GAD2-positive neurons. The two-way ANOVA did not identify any main effects of sex (F1,20 = 0.1875, p = 0.6695) or genotype (F1,20 = 3.541, p = 0.0745) or a sex × genotype interaction (F1,20 = 2.310, p = 0.1442), supporting a critical role for GRs in the regulation of CeA GABA activity in both genotypes and sexes (Fig. 1E). Notably, we found a significant main effect of genotype (F1,20 = 0.141, p = 0.0067) on Nr3c1 co-expression with Slc17a7, suggesting potentially more pronounced GR control of glutamatergic activity in msP rats (Fig. 1F), but no main effect of sex (F1,20 = 0.0524, p = 0.8212) and no sex × genotype interaction (F1,20 = 1.034, p = 0.3214).
3.2. Both sex and genotype shape the activity of CeA GABAergic synapses
Previous studies reported elevated GABA signaling in the CeA in male msP rats (Herman et al., 2013) to a similar extent as in alcohol-dependent rats (Roberto et al., 2004). Specifically, both electrically evoked GABA transmission and vesicular GABA release in male msP rats are significantly enhanced compared with their male Wistar counterparts (Herman et al., 2013). To our knowledge, sex differences in CeA GABA signaling in msP and Wistar rats have not been previously studied. In the present study, we recorded action-potential dependent sIPSCs reflecting synaptic network activity and evoked IPSPs from the medial subdivision of the CeA, the major output of the amygdala, in both sexes of both genotypes. We found significant differences in baseline action potential-dependent pre- and postsynaptic GABA signaling between both genotypes and sexes (Table 1). Specifically, the two-way ANOVA of sIPSC frequencies revealed a significant genotype × sex interaction (F1,80 = 4.42, p = 0.0387), with no main effect of genotype (F1,80 = 1.450, p = 0.2321) or sex (F1,80 = 0.22, p = 0.6403). The Tukey post hoc test did not reveal any further differences between groups. We did not find main effects of either sex or genotype or a sex × genotype interaction for sIPSC amplitudes (two-way ANOVA; genotype: F1,80 = 1.003, p = 0.3196; sex: F1,80 = 0.2386, p = 0.6266; sex × genotype interaction: F1,80 = 1.133, p = 0.2903) or rise times (genotype: F1,80 = 0.3446, p = 0.5589; sex: F1,80 = 9.4282, p = 0.5147; sex × genotype interaction: F1,80 = 0.9494, p = 0.3328). We found a significant main effect of genotype on sIPSC decay times (F1,80 = 5.192, p = 0.0378) but no main effect of sex (F1,80 = 0.5107, p = 0.4769) and no sex × genotype interaction (F1,80 = 1.034, p = 0.3123), driven by longer decay times in Wistar rats compared with msP rats.
Table 1.
Summary of sIPSC and eIPSP characteristics of all recorded CeA neurons.
| Spontaneous inhibitory postsynaptic currents | |||||
|---|---|---|---|---|---|
| Frequency (Hz) | Amplitude (pA) | Rise time (ms) | Decay time (ms) | n | |
| Male msP | 2.12 ± 0.26$ | 73.55 ± 3.65 | 2.48 ± 0.07 | 8.00 ± 0.41& | 25 |
| Female msP | 1.60 ± 0.18$ | 68.57 ± 4.48 | 2.57 ± 0.06 | 7.08 ± 0.44& | 25 |
| Male Wistar | 1.23 ± 0.12$ | 63.87 ± 3.00 | 2.60 ± 0.07 | 8.62 ± 0.53& | 18 |
| Female Wistar |
1.92 ± 0.39$ |
71.15 ± 6.23 |
2.48 ± 0.10 |
8.79 ± 0.81& |
20 |
| Evoked inhibitory postsynaptic potentials | Paired-pulse facilitation | ||||
|
Amplitude (mV) |
n |
Ratio |
n |
||
| Male msP | 9.89 ± 0.42$ | 21 | 0.74 ± 0.07 | 11 | |
| Female msP | 6.74 ± 0.63$ | 8 | 0.86 ± 0.14 | 7 | |
| Male Wistar | 9.11 ± 1.20$ | 14 | 0.95 ± 0.11 | 9 | |
| Female Wistar | 10.18 ± 0.93$ | 11 | 0.88 ± 0.07 | 8 | |
Differences between groups were determined using two-way ANOVA. $Significant genotype × sex interaction for the indicated parameter. &Significant effect of genotype on the indicated parameter. The number of cells (n) is indicated for each group.
Similarly, the two-way ANOVA identified a significant sex × genotype interaction for evoked IPSP amplitudes (F1,50 = 5.856, p = 0.0192) but no main effects of sex (F1,50 = 1.427, p = 0.2379) or genotype (F1,50 = 2.325, p = 0.1336). The two-way ANOVA did not identify main effects of sex (F1,31 = 0.03272, p = 0.8576) or genotype (F1,31 = 1.368, p = 0.2511) on the PPF or a sex × genotype interaction (F1,31 = 0.8263, p = 0.3703; Table 1).
3.3. Glucocorticoid receptors regulate CeA GABA transmission in msP rats
Our previous electrophysiological studies of the GR regulation of CeA GABA transmission revealed significantly stronger effects of the GR antagonist mifepristone in male alcohol-dependent Sprague-Dawley rats compared with naive controls (Khom et al., 2022), indicating recruitment of the GR system in regulating CeA GABA activity after chronic intermittent ethanol exposure. In the present study, we tested the effect of acute mifepristone (10 μM) on CeA sIPSCs in both genotypes and sexes. The two-way ANOVA identified a significant main effect of genotype on sIPSC frequencies (F1,46 = 4.361, p = 0.0423) but no effect of sex (F1,46 = 0.2312, p = 0.6329) and no sex × genotype interaction (F1,46 = 0.6824, p = 0.4130; Fig. 2A and B). One-sample t-tests revealed that mifepristone decreased sIPSC frequencies (reflecting decrease in GABA release) in both male (t = 3.506, df = 17, p = 0.0027) and female (t = 2.663, df = 8, p = 0.0287) msP rats but it had no significant effect on sIPSC frequencies in Wistar rats (male: t = 1.578, df = 12, p = 0.1405; female: t = 0.09201, df = 9, p = 0.9287; Fig. 2A–D), indicating GR regulation of CeA GABA transmission in msP rats under basal conditions but not in Wistar controls. The two-way ANOVA did not reveal any significant main effects of sex or genotype on sIPSC amplitudes or rise times in the presence of mifepristone or a sex × genotype interaction, but one-sample t-tests identified significantly larger sIPSC amplitudes (t = 2.511, df = 17, p = 0.0225) and rise times (t = 2.414, df = 17, p = 0.274) during mifepristone application compared with baseline in male msP rats, suggesting an effect on postsynaptic GABAA receptor functions. Lastly, we found a significant main effect of sex (F1,46 = 4.273, p = 0.0444) on sIPSC decay times in the presence of mifepristone but no main effect of genotype (F1,46 = 0.0032, p = 0.9554) and no sex × genotype interaction (F1,46 = 2.102, p = 0.1538), mainly driven by significantly larger sIPSC decay times in the presence of mifepristone in male msP rats (one-sample t-test: t = 3.550, df = 17, p = 0.0025), whereas sIPSC decay times did not differ from baseline in the other groups (see insets in Fig. 2A for scaled sIPSC averages and Fig. 2F).
Fig. 2.
Glucocorticoid receptors regulate CeA GABA activity in msP rats, but not in Wistar rats. (A) Representative sIPSC traces from CeA neurons from the indicated genotype and sex before (control, left panel) and during 10 μM mifepristone application (right panel). Color-coded scaled sIPSC averages of each trace (black line: control conditions; red line: in the presence of mifepristone) are shown in the adjacent boxes. (B) Cumulative frequency plots for the indicated animal groups. Scatter dot plots depict means ± SEM of sIPSC (C) frequencies, (D) amplitudes, (E) rise times, and (F) decay times in the presence of mifepristone from 9 to 19 different neurons. Differences between sexes and genotypes were determined using two-way ANOVA ($p = 0.05). Simple drug effects (i.e., differences from baseline) were calculated using one-sample t-tests (*p = 0.05, **p = 0.01). Means ± SEM were derived from at least four different animals per group (9 male and 5 female msP rats; 9 male and 4 female Wistar rats). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
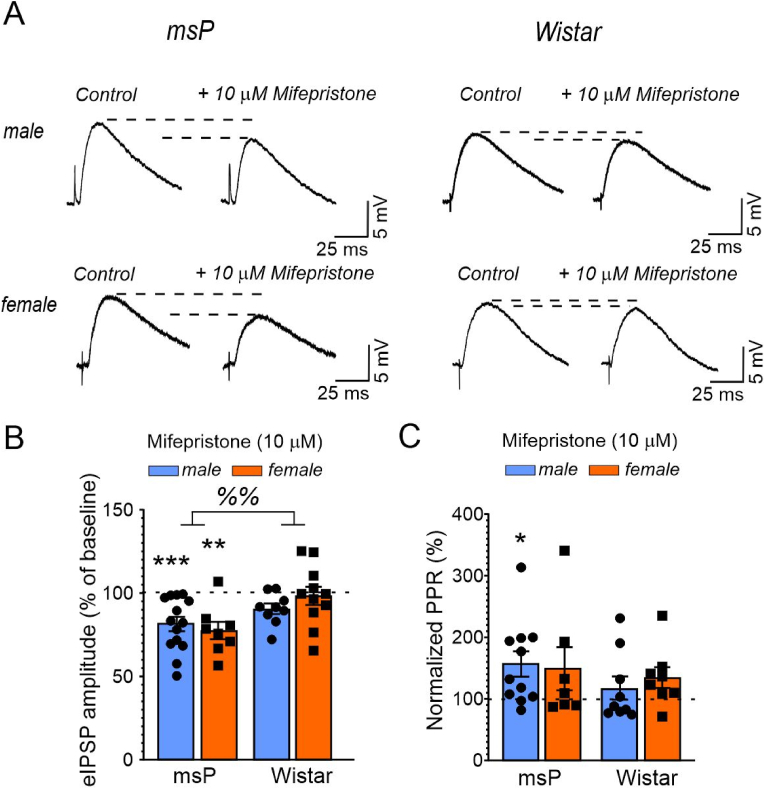
Similarly, we found a significant main effect of genotype on the effects of mifepristone on CeA evoked IPSP amplitudes (two-way ANOVA: F1,38 = 9.586, p = 0.0037) but no main effect of sex (F1,38 = 0.1582, p = 0.6931) and no sex × genotype interaction (F1,38 = 1.449, p = 0.2361), indicating that mifepristone decreased evoked GABA transmission only in the CeA in msP rats and not in Wistar controls (Fig. 3A and B). We found that mifepristone significantly decreased evoked IPSP amplitudes in male (t = 4.310, df = 13, p = 0.008) and female (t = 4.308, df = 7, p = 0.0035, both one-sample t-test) msP rats but not in male or female Wistar rats. Lastly, mifepristone significantly increased PPF only in male msP rats (t = 2.753, df = 10, p = 0.0204; Fig. 3C) and not in any other group. The two-way ANOVA did not reveal any significant effects of genotype or sex on the effects of mifepristone on PPF or a sex × genotype interaction.
Fig. 3.
Mifepristone decreases evoked CeA IPSP amplitudes in msP rats, but not in Wistar rats. (A) Representative CeA eIPSP traces during baseline control (left side) and during 10 μM mifepristone superfusion in the indicated animal groups. Scatter dot plots illustrate means ± SEM of the effects of mifepristone on (B) eIPSP amplitudes and (C) the PPF compared with baseline control (indicated as dashed line). Differences between groups were determined using two-way ANOVA (main effects: %%p < 0.01). Differences from baseline control (dashed line) were determined using one-sample t-tests (*p < 0.05, **p < 0.01, ***p < 0.001). Means ± SEM were derived from 8 to 14 individual cells from 12 male, 8 female msP rats, 8 male and 7 female Wistar rats, respectively.
3.4. Acute ethanol increases GABA transmission only in the CeA of male rats
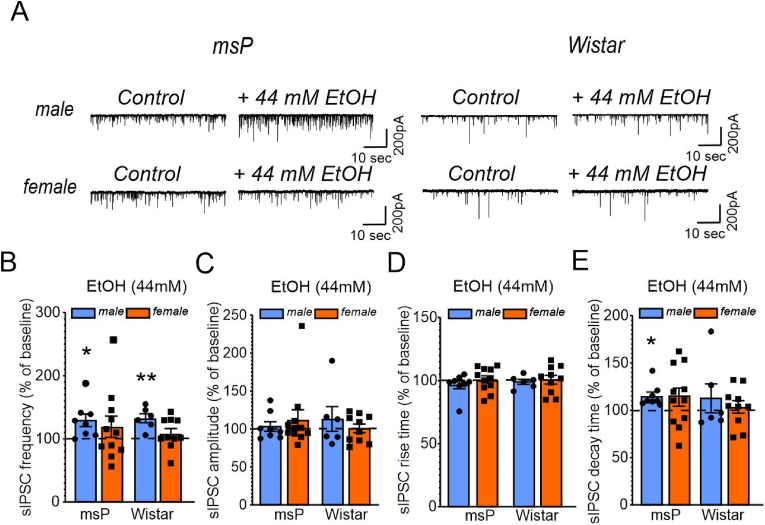
In male rats (Sprague-Dawley and Wistar), both acute slice application of 44 mM ethanol and in vivo CIE significantly and reproducibly elevate CeA GABA transmission (Khom et al., 2020a, 2020b; Roberto et al., 2003, 2004; Varodayan et al., 2017). These phenomena are not observed in female Sprague-Dawley rats, where CIE leads to acute ethanol sensitivity of the CeA GABAergic synaptic network without affecting baseline functions (Kirson et al., 2021). Notably, despite the already elevated CeA GABA tone in naïve male msP compared to Wistar rats, acute ethanol still facilitates its vesicular GABA release (Herman et al., 2013) Ethanol effects on CeA GABAergic synapses in female msP rats remain unknown. Thus, to determine how sex and genotype impact CeA sensitivity towards the acute effects of ethanol, we investigated the effect of a single ethanol concentration (44 mM) on network-dependent CeA GABAergic synaptic transmission in msP and Wistar rats (Fig. 4A). As shown in Fig. 4A and B 44 mM ethanol significantly increased sIPSC frequencies in both male msP rats (t = 2.931, df = 7, p = 0.022) and male Wistar rats (t = 4.597, df = 5, p = 0.0059), but did not have an overall effect in female msP rats (t = 1.142, df = 10, p = 0.2800) or female Wistar rats (t = 1.136, df = 9, p = 0.2851). The two-way ANOVA did not indicate any significant main effects of genotype (F1,32 = 0.3618, p = 0.5518) or sex (F1,32 = 0.6415, p = 0.4291) on the acute effect of ethanol on sIPSC frequencies or a sex × genotype interaction (F1,32 = 0.6847, p = 0.4141). Acute ethanol did not significantly alter sIPSC amplitudes (Fig. 4C) or rise times (Fig. 4D) in any group but increased sIPSC decay times in male msP rats (t = 3.619, df = 7, p = 0.0085). The two-way ANOVA did not indicate any significant main effects of sex (F1,27 = 0.0006, p = 0.9808) or genotype (F1,27 = 1.266, p = 0.2705) on the effects of ethanol on sIPSC decay times or a sex × genotype interaction (F1,27 = 0.769, p = 0.3883; Fig. 4E). These data suggest that acute ethanol enhances GABA signaling at both pre- and postsynaptic sites in male rats but does not significantly alter GABA transmission in female rats.
Fig. 4.
Acute ethanol enhances CeA GABA transmission only in male rats. (A) Representative traces of CeA sIPSCs from the indicated genotypes and sexes during baseline control (left trace) and during 44 mM ethanol application (right trace). Bar graphs summarize effects of ethanol on sIPSC (B) frequencies and (C) amplitudes and (D) rise and (E) decay times. The data are expressed as the mean ± SEM from 7 to 10 cells, derived from at least three different animals per group (6 male and 6 female msP rats; 3 male and 3 female Wistar rats). Differences from the baseline control (dashed line) were calculated using one-sample t-tests (*p = 0.05, **p = 0.01). Differences between groups were determined using two-way ANOVA.
3.5. Mifepristone does not prevent the ethanol-induced elevation of CeA GABA transmission in male rats
Our recent electrophysiological studies of GR function revealed that mifepristone blunted acute ethanol effects on CeA GABA release in male alcohol-dependent Sprague-Dawley rats but not in ethanol-naive controls (Khom et al., 2022). These findings support sensitized CeA GR signaling that stems from CIE and suggest that GR antagonism counteracts chronic ethanol effects on GABA transmission and prevents its acute effects (Khom et al., 2022). As shown in Fig. 5A and B, in the continued presence of mifepristone, 44 mM EtOH significantly increased sIPSC frequencies in both male msP rats (t = 2.509, df = 7, p = 0.0404) and Wistar rats (t = 2.501, df = 8, p = 0.0369), suggesting that unlike in alcohol-dependent rats, GRs might not be involved in mediating acute effects of ethanol on CeA GABA release in msP rats. Importantly, mifepristone did not alter the relative lack of sensitivity of the female CeA to the effects of acute ethanol (Wistar: t = 1.219, df = 7, p = 0.2624; msP: t = 1.476, df = 7, p = 0.1834). The two-way ANOVA did not identify any significant main effects of sex (F1,29 = 0.1457, p = 0.7054) or genotype (F1,29 = 0.0047, p = 0.9458) on sIPSC frequency or a sex × genotype interaction (F1,29 = 0.01753, p = 0.8956). Ethanol in the presence of mifepristone did not significantly alter sIPSC amplitudes (Fig. 5C) or rise times (Fig. 5D) in any group but increased sIPSC decay times in both male msP (t = 2.583, df = 7, p = 0.0363) and Wistar rats (t = 3.195, df = 9, p = 0.0127) but not in female Wistar (t = 1.562, df = 7, p = 0.1622) or female msP rats (t = 0.5270, df = 7, p = 0.6145; Fig. 5E). The two-way ANOVA identified a main effect of sex (F1,29 = 4.331, p = 0.0464) on the effects of ethanol in the presence of mifepristone on sIPSC decay times but no main effect of genotype (F1,29 = 0.2474, p = 0.6227) and no sex × genotype interaction (F1,29 = 2.585, p = 0.1187), indicating that mifepristone does not counteract the ethanol-induced facilitation of CeA GABA transmission at both pre- and postsynaptic sites in male rats.
Fig. 5.
Mifepristone does not block acute effects of ethanol on CeA GABA transmission in male rats. (A) Representative sIPSC recordings from CeA neurons during baseline control conditions (left panel), during 10 μM mifepristone (middle panel), and during 44 mM ethanol in the continued presence of mifepristone (right panel) from the indicated rat groups. Effects of ethanol were normalized to the last 3 min of mifepristone application (indicated as dashed line). Scatter dot plots depict means ± SEM of effects of ethanol in the presence of mifepristone from 8 to 9 individual cells on sIPSC (B) frequencies and (C) amplitudes and (D) rise and (E) decay times. Differences between groups were calculated using two-way ANOVA ($p > 0.05). Differences from pre-ethanol baseline were calculated using one-sample t-tests (*p < 0.05). Data were derived from at least four different animals per group (4 male and 5 female msP rats; 5 male and 4 female Wistar rats).
3.6. Comparable effects of mifepristone on CeA GABA activity in male alcohol-naive msP rats and alcohol-dependent Wistar rats
Given that effects of mifepristone on CeA GABA transmission in alcohol-naive msP rats strongly resembled those that were previously observed in male alcohol-dependent Sprague-Dawley rats (Khom et al., 2022), we used the chronic intermittent ethanol vapor inhalation paradigm to induce alcohol dependence in male Wistar rats and compared effects of mifepristone in those dependent animals to their alcohol-preferring but alcohol-naive msP counterparts. As shown in Fig. 6A–C, mifepristone significantly decreased sIPSC frequencies (t = 2.749, df = 5, p = 0.0404) and increased sIPSC amplitudes (t = 2.899, df = 5, p = 0.0338) and decay times (t = 4.269, df = 5, p = 0.0079), but did not have a significant effect on sIPSC rise times (t = 2.075, df = 5, p = 0.0926) in male alcohol-dependent Wistar rats. Two-tailed unpaired t-tests that compared effects of mifepristone in dependent Wistar to msP rats further revealed that effects of mifepristone on sIPSC frequencies (t = 0.0090, df = 22, p = 0.9929), amplitudes (t = 0.6844, df = 22, p = 0.5008), and decay times (t = 0.1143, df = 22, p = 0.91) did not differ significantly, whereas effects of mifepristone on sIPSC rise times were distinct (t = 2.124, df = 22, p = 0.0452).
Fig. 6.
Similar regulation of CeA GABA transmission in male alcohol-dependent Wistar and msP rats. (A) Representative CeA sIPSC traces from a male, alcohol-dependent Wistar rat during baseline control conditions (upper trace) and in the presence of mifepristone (lower trace). Bars represent means ± SEM of sIPSC (B) frequencies and (C) postsynaptic measures in the presence of mifepristone from the indicated treatment group compared with baseline control conditions (dashed line) from 6 and 18 cells, respectively. (D) Representative CeA eIPSP traces from a male, alcohol-dependent Wistar rat before and during mifepristone application. Bars represent means ± SEM of (E) eIPSP amplitudes and (F) PPF during mifepristone application compared with baseline control (dashed line) from 11 to 16 cells. All data for dependent Wistar rats were derived from 8 animals. Differences from baseline control were determined using one-sample t-tests, and differences between groups were determined using two-tailed unpaired t-tests (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). All data for msP rats in these graphs were taken from Fig. 2.
Similarly, we found that mifepristone significantly decreased CeA evoked IPSP amplitudes (t = 7.997, df = 11, p < 0.0001; Fig. 6D and E) and increased the PPF (t = 3.253, df = 9, p = 0.0099, both one-sample t-test; Fig. 6E) in dependent Wistar male rats, indicating a decrease in CeA GABA release. Effects of mifepristone on evoked CeA GABA transmission in dependent Wistar rats also did not differ from those in male msP rats. Specifically, two-tailed unpaired t-tests indicated comparable effects of mifepristone on evoked IPSP amplitudes (t = 0.3523, df = 24, p = 0.7275) and the PPF (t = 0.7252, df = 19, p = 0.4772) in male msP and dependent Wistar rats.
Collectively, these data indicate a very similar regulation of CeA GABA transmission by GRs in male dependent Wistar and msP rats and provide further critical evidence that male msP rats emulate several aspects of the post-dependent phenotype.
4. Discussion
The sensitization of stress/anti-reward systems and attenuated reward circuitry activity represent fundamental aspects of AUD pathogenesis (Koob and Schulkin, 2019; Tunstall et al., 2017). Central to the transition to and maintenance of AUD is the dysfunction of the HPA axis, allowing several possible pharmacological therapeutic approaches to treat AUD, such as targeting the CRF/CRF1 receptor system (Koob, 2022; Roberto et al., 2010), 11-β-hydroxylase (which is involved in the biosynthesis of cortisol and aldosterone) (Sanna et al., 2016), and the glucocorticoid/GR/MR system further downstream (Vendruscolo et al., 2012, 2015). Interestingly, despite the crucial role of CRF in AUD pathogenesis, in which CRF1 receptor antagonists effectively reduce ethanol consumption in preclinical models of AUD, CRF1 receptor antagonist administration does not ameliorate AUD symptoms in patients (Kwako et al., 2015; Schwandt et al., 2016). Dampening glucocorticoid effects is an alternative approach to tackle HPA dysfunction, given that GR activation enhances CeA CRH transcription (Makino et al., 1994). Moreover, recent studies using the GR antagonist mifepristone obtained promising results in abstinent AUD patients by reducing craving and alcohol consumption during treatment (Vendruscolo et al., 2015).
The CeA is a key brain site to mediate glucocorticoid effects associated with harmful ethanol consumption. Specifically, intra-CeA application of mifepristone reduced both alcohol-consumption in alcohol-dependent rats (Vendruscolo et al., 2015), and also prevented yohimbine-induced reinstatement of alcohol seeking (Simms et al., 2012). Furthermore, our recent mechanistic studies revealed a stronger GR-mediated reduction of GABA transmission in male, alcohol-dependent rats compared with naïve rats, suggesting alcohol dependence-induced recruitment of the GR system that controls CeA activity (Khom et al., 2022).
The present study investigated CeA GR function in AUD using genetically selected alcohol-preferring msP rats. msP rats have Crhr1 polymorphisms that play a role in shaping multiple behaviors that are commonly observed in alcohol-dependent animals, including extensive alcohol consumption to alleviate heightened levels of anxiety and stress. Thus, msP rats are well-suited to study HPA-related aspects of AUD (Borruto et al., 2021; Ciccocioppo et al., 2006; Hansson et al., 2006). GABAergic activity in the CeA is upregulated in male msP rats compared to Wistar rats (Herman et al., 2013), and accompanied by higher levels of phosphorylated CeA GRs (Natividad et al., 2021). Of note, enhanced GR phosphorylation leading to increased transcriptional GR activity (including Crh transcription), is associated with sensitized GR signaling and represents a key component of addictive disorders (Carmack et al., 2022; Vendruscolo et al., 2015). Greater efficacy of CRF1 antagonists and CRF in modulating CeA GABA transmission (Roberto et al., 2010) and increased Crh expression in the CeA (Sommer et al., 2008) have been previously reported in alcohol-dependent rats. Similarly, in msP rats, increased expression of CeA CRF1 is associated with increased sensitivity to stress and to CRF1 antagonists (Hansson et al., 2008). These findings further support the resemblance of male msP rats to male alcohol-dependent Wistar rats at both the behavioral and cellular/molecular levels (Roberto et al., 2004; Vendruscolo et al., 2015).
Given evidence of sexually dimorphic HPA axis function and substantial differences in AUD pathologies and manifestations between sexes (Finn, 2020; Leistner and Menke, 2020), we also studied female rats. We used ISH to look at CeA GR gene transcription and electrophysiology to determine the role of GRs in regulating CeA GABA transmission in both genotypes. We found higher Nr3c1 mRNA levels in female rats compared with males, independent of genotype suggesting a prominent role for GR in modulation of CeA activity in female rats. Yet, the GR antagonist mifepristone did not significantly alter synaptic GABA transmission in female Wistar rats. Thus, although our data do not directly support the hypothesis that females are more susceptible to glucocorticoid-induced stress (Fox and Sinha, 2009; Peltier et al., 2019), they provide additional evidence for a more widespread expression of GR on CeA neurons in females capable of regulating synaptic activity. Of note, we are cognizant that GR expression does not necessarily correlate with protein abundance or function; thus, future studies will address mechanistically potential sex-specific GR function in neuronal and synaptic control. We also speculate that GR modulation of synaptic signaling depends on posttranslational modifications, subcellular localization of GR (membrane-bound vs. cytosolic), interaction with co-factors, genomic vs. non-genomic signaling, ligand-independent (i.e., intrinsic activity) and ligand-dependent activities including corticosterone release (Chrousos and Kino, 2005; de Kloet et al., 2005; de Kloet and Joëls, 2023; Meijer et al., 2022). This is further supported by the fact the Nr3c1 levels in male msP rats also did not differ from male Wistar controls, but mifepristone decreased CeA GABA transmission only in msP rats. Thus, despite similar gene transcription and neuronal expression patterns, GRs in male msP rats may be intrinsically (more) active because of their enhanced phosphorylation status (Natividad et al., 2021). We found that 75–85% of Nr3c1 puncta were localized on GABAergic neurons, which is consistent with the tight control of CeA GABA activity by GRs. Moreover, our data indicated the co-localization of GRs with markers of glutamatergic neurons in msP rats. Although the CeA is mainly GABAergic, this putatively enhanced GR control of a small subpopulation of CeA glutamate neurons in msP rats might have profound effects on the activity of the entire synaptic network. Indeed, we recently reported enhanced CeA glutamate transmission in male msP rats (Herman et al., 2016b; Natividad et al., 2017). Furthermore, corticosterone, like ethanol, decreases electrically evoked glutamate transmission in the entire CeA of male, alcohol-naive rats, while its effect on the female CeA is drastically diminished. Specifically, corticosterone decreases the activity of glutamatergic synapses in the lateral subdivision of the CeA – although less efficiently than in males - while it does not alter glutamate signaling in the medial subdivision (Logrip et al., 2017). Thus, future studies will need to further elucidate the circuitry-specific contribution of GRs in regulating CeA glutamate signaling in AUD.
At the synaptic level, we found that mifepristone decreased GABA transmission in naive msP rats (both evoked and spontaneous) but did not significantly alter it in Wistar rats. These findings resemble data from our previous studies with naïve and alcohol-dependent Sprague-Dawley rats (Khom et al., 2022) and further substantiate the critical role of GRs in regulating GABA transmission in msP rats under physiological conditions. Mifepristone also had postsynaptic effects in male msP rats, specifically increasing sIPSC amplitudes and prolonging both rise and decay times, thereby enhancing postsynaptic inhibition, which in turn might counteract its presynaptic effects (i.e., decreasing GABA release). Thus, effects of mifepristone on CeA GABA transmission in male msP rats may neutralize each other, possibly explaining why mifepristone did not reduce ethanol self-administration in male msP rats (Benvenuti et al., 2021), or anxiety-like behaviors (Vozella et al., 2021). Notably, when comparing synaptic effects of mifepristone in male naïve msP rats to alcohol-dependent Wistar rats, we found that both pre- and postsynaptic effects of mifepristone in msP rats vs. dependent Wistar rats were comparable. We also found that mifepristone did not block acute effects of ethanol [i.e., augmentation of GABA release (Roberto et al., 2003)] in male rats, suggesting that other systems (e.g., CRF) mediate the acute ethanol-induced facilitation of CeA GABA release (Roberto et al., 2010).
These overlapping cellular phenotypes support the hypothesis that msP rats phenocopy several aspects of alcohol dependence, such as increases in ethanol drinking and anxiety, and recapitulate multiple, but not all, behaviors of alcohol-dependent rats and selected CeA neuroadaptations that are induced by chronic intermittent ethanol vapor exposure. Interestingly, we also found that CeA GABAergic synapses of female msP rats did not respond to the acute effects of alcohol, resembling female alcohol-dependent Sprague Dawley rats (Kirson et al., 2021). Of note, the msP rats used in this study did not consume any alcohol and we previously reported that despite the heightened GABAergic tone in the CeA under basal conditions, both acute and chronic alcohol elevate CeA GABA signaling in male rats (Herman et al., 2013). For these reasons, future studies will need to determine the neurobiological and mechanistic effects of acute and chronic ethanol/withdrawal on CeA neurons of both male and female msP rats.
Heightened GABAergic activity in the CeA is a well-established mechanism that drives multiple behaviors that are associated with AUD and both anxiety- and stress-related disorders. Thus, normalizing CeA GABA transmission is a critical molecular mechanism of several clinical and preclinical AUD therapeutics (Roberto et al., 2021). Mifepristone reduces CeA GABAergic activity in models of AUD and is among the drugs that have been intensively investigated in clinical trials in recent years (Burnette et al., 2022; Ray et al., 2021; Vendruscolo et al., 2015). Nonetheless, it has shown mixed behavioral effects. For example, mifepristone decreased ethanol self-administration in male alcohol-dependent (CIE) rats (McGinn et al., 2021; Repunte-Canonigo et al., 2015; Somkuwar et al., 2017; Vendruscolo et al., 2012, 2015), decreased ethanol intake in High Drinking in the Dark (HDID-1) mice (Savarese et al., 2020), and blocked yohimbine (stress)-induced reinstatement of ethanol seeking in rats (Simms et al., 2012). In nonhuman primates, chronic mifepristone treatment decreased voluntary ethanol consumption but did not prevent relapse (Jimenez et al., 2020). Mifepristone administration prior to restraint stress prevented escalation of alcohol consumption (Ostroumov et al., 2016) and attenuated the severity of withdrawal symptoms in rats (Sharrett-Field et al., 2013). However, mifepristone did not prevent ethanol consumption in Wistar rats with a history of footshock stress (Logrip and Gainey, 2020), did not alter ethanol self-administration in baboons (Holtyn and Weerts, 2019), did not alter binge-drinking in C57BL/6J mice (Lowery et al., 2010) and had mixed effects on ethanol drinking in a mouse model of social defeat (i.e. decrease in drinking in animals with continuous access to alcohol but increase in drinking in animals with intermittent access to alcohol) (Newman et al., 2018). These divergent effects of mifepristone on ethanol seeking and intake are not completely understood, but evidence suggests that the efficacy of mifepristone increases with stress intensity (e.g., powerful stressors, such as yohimbine) and in CIE paradigms where animals have BALs ≥150 mg/dl over several weeks and thus experience bouts of heavy intoxication and withdrawal daily. Additionally, mifepristone does not bind selectively to GRs; it also binds to progesterone receptors, which may also contribute to differential outcomes (Karena et al., 2022).
Collectively, our data show that chronic ethanol exposure induces neuroadaptations in the CeA GABAergic synaptic network in non-genetically selected rats which are innately present in alcohol-naïve msP rats. Thus, the present study further broadens our understanding of CeA synaptic function and activity in msP rats, demonstrating the GR regulation of CeA GABAergic synapses in msP rats under basal conditions (i.e., in absence of ethanol consumption) that presumably contributes to maladaptive behaviors that are commonly associated with this genotype.
Author contribution
Sophia Khom: Conceptualization; Data curation, Formal analysis, Investigation, Project administration, Writing – original draft.Vittoria Borgonetti: Data curation, Formal analysis, Investigation, Writing – review & editing.Valentina Vozella: Data curation, Formal analysis, Investigation, Writing – review & editing.Dean Kirson: Data curation, Formal analysis, Investigation, Writing – review & editing.Larry Rodriguez:Data curation, Formal analysis, Investigation, Writing – review & editing.Pauravi Gandhi:Data curation, Formal analysis, Investigation, Writing – review & editing.Paula Cristina Bianchi:Data curation, Formal analysis, Investigation, Writing – review & editing.Angela Snyder:Data curation, Formal analysis, Investigation, Writing – review & editing.Roman Vlkolinsky:Data curation, Formal analysis, Investigation, Writing – review & editing.Michal Bajo:Data curation, Formal analysis, Investigation, Writing – review & editing.Christopher S. Oleata:Data curation, Formal analysis, Investigation, Writing – review & editing.Roberto Ciccocioppo:Writing – review & editing, Project administration, Funding acquisition.MarisaRoberto:Conceptualization; Data curation, Formal analysis, Investigation, Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This is manuscript number 30222 from The Scripps Research Institute. This study was supported by National Institutes of Health grants AA017447 (MR and RC), AA013498 (MR), AA027700 (MR), AA021491 (MR), AA006420 (MR), AA029841 (MR), AA007456 (MR) and AA026638 (DK), The Schimmel Family Chair, The Pearson Center for Alcoholism Addiction Research and a FAPESP postdoctoral fellowship 2021/12978-1 (PCB). The authors thank Dr. Sarah Wolfe and Shannon D'Ambrosio for technical assistance with the ISH analysis and Michael Arends for editing the manuscript. The authors declare no competing financial interests.
Handling Editor: Prof. R. Lawrence Reagan
Contributor Information
Sophia Khom, Email: sophia.khom@univie.ac.at.
Vittoria Borgonetti, Email: vborgonetti@scripps.edu.
Valentina Vozella, Email: vvozella@scripps.edu.
Dean Kirson, Email: dkirson@uthsc.edu.
Larry Rodriguez, Email: larryrod@usc.edu.
Pauravi Gandhi, Email: pgandhi@scripps.edu.
Paula Cristina Bianchi, Email: pbianchi@scripps.edu.
Angela Snyder, Email: aesnyder414@gmail.com.
Roman Vlkolinsky, Email: vlkolins@scripps.edu.
Michal Bajo, Email: mbajo@scripps.edu.
Christopher S. Oleata, Email: coleata@scripps.edu.
Roberto Ciccocioppo, Email: roberto.ciccocioppo@unicam.it.
Marisa Roberto, Email: mroberto@scripps.edu.
Data availability
Data will be made available on request.
References
- Augier E., Barbier E., Dulman R.S., Licheri V., Augier G., Domi E., Barchiesi R., Farris S., Nätt D., Mayfield R.D., Adermark L., Heilig M. A molecular mechanism for choosing alcohol over an alternative reward. Science. 2018;360:1321–1326. doi: 10.1126/science.aao1157. [DOI] [PubMed] [Google Scholar]
- Benvenuti F., Cannella N., Stopponi S., Soverchia L., Ubaldi M., Lunerti V., Vozella V., Cruz B., Roberto M., Ciccocioppo R. Effect of glucocorticoid receptor antagonism on alcohol self-administration in genetically-selected Marchigian Sardinian alcohol-preferring and non-preferring Wistar rats. Int. J. Mol. Sci. 2021;22:4184. doi: 10.3390/ijms22084184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto A.M., Stopponi S., Li H., Weiss F., Roberto M., Ciccocioppo R. Genetically selected alcohol-preferring msP rats to study alcohol use disorder: anything lost in translation? Neuropharmacology. 2021;186 doi: 10.1016/j.neuropharm.2020.108446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette E.M., Nieto S.J., Grodin E.N., Meredith L.R., Hurley B., Miotto K., Gillis A.J., Ray L.A. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82:251–274. doi: 10.1007/s40265-021-01670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmack S.A., Vendruscolo J.C.M., Adrienne McGinn M., Miranda-Barrientos J., Repunte-Canonigo V., Bosse G.D., Mercatelli D., Giorgi F.M., Fu Y., Hinrich A.J., Jodelka F.M., Ling K., Messing R.O., Peterson R.T., Rigo F., Edwards S., Sanna P.P., Morales M., Hastings M.L., Koob G.F., Vendruscolo L.F. Corticosteroid sensitization drives opioid addiction. Mol. Psychiatr. 2022;27:2492–2501. doi: 10.1038/s41380-022-01501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.F., Heilig M., Perez A., Probst C., Rehm J. Alcohol use disorders. Lancet. 2019;394:781–792. doi: 10.1016/S0140-6736(19)31775-1. [DOI] [PubMed] [Google Scholar]
- Chrousos G.P., Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci. STKE. 2005;2005:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R., Economidou D., Cippitelli A., Cucculelli M., Ubaldi M., Soverchia L., Lourdusamy A., Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict. Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A., Ayanwuyi L.O., Barbier E., Domi E., Lerma-Cabrera J.M., Carvajal F., Scuppa G., Li H., Ubaldi M., Heilig M., Roberto M., Ciccocioppo R. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology. 2015;232:1083–1093. doi: 10.1007/s00213-014-3743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M. The cortisol switch between vulnerability and resilience. Mol. Psychiatr. 2023 doi: 10.1038/s41380-022-01934-8. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Demoret R.M., Baker M.A., Ohtawa M., Chen S., Lam C.C., Khom S., Roberto M., Forli S., Houk K.N., Shenvi R.A. Synthetic, mechanistic, and biological interrogation of Ginkgo biloba chemical space En Route to (-)-Bilobalide. J. Am. Chem. Soc. 2020;142:18599–18618. doi: 10.1021/jacs.0c08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn D.A. The Endocrine system and alcohol drinking in females. Alcohol Res. 2020;40:2. doi: 10.35946/arcr.v40.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H.C., Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv. Rev. Psychiatr. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz M.D., Bharat C., Degenhardt L., Sampson N.A., Scott K.M., Lim C.C.W., Al-Hamzawi A., Alonso J., Andrade L.H., Cardoso G., De Girolamo G., Gureje O., He Y., Hinkov H., Karam E.G., Karam G., Kovess-Masfety V., Lasebikan V., Lee S., Levinson D., McGrath J., Medina-Mora M.-E., Mihaescu-Pintia C., Mneimneh Z., Moskalewicz J., Navarro-Mateu F., Posada-Villa J., Rapsey C., Stagnaro J.C., Tachimori H., Ten Have M., Tintle N., Torres Y., Williams D.R., Ziv Y., Kessler R.C., WHO World Mental Health Survey Collaborators The epidemiology of alcohol use disorders cross-nationally: findings from the World Mental Health Surveys. Addict. Behav. 2020;102 doi: 10.1016/j.addbeh.2019.106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A.C., Cippitelli A., Sommer W.H., Fedeli A., Björk K., Soverchia L., Terasmaa A., Massi M., Heilig M., Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A.C., Rimondini R., Neznanova O., Sommer W.H., Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur. J. Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M., MacKillop J., Martinez D., Rehm J., Leggio L., Vanderschuren L.J.M.J. Addiction as a brain disease revised: why it still matters, and the need for consilience. Neuropsychopharmacology. 2021;46:1715–1723. doi: 10.1038/s41386-020-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M.A., Contet C., Roberto M. A functional switch in tonic GABA currents alters the output of central amygdala corticotropin releasing factor receptor-1 neurons following chronic ethanol exposure. J. Neurosci. 2016;36:10729–10741. doi: 10.1523/JNEUROSCI.1267-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M.A., Kallupi M., Luu G., Oleata C.S., Heilig M., Koob G.F., Ciccocioppo R., Roberto M. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M.A., Varodayan F.P., Oleata C.S., Luu G., Kirson D., Heilig M., Ciccocioppo R., Roberto M. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: alcohol and CRF effects. Neuropharmacology. 2016;102:21–31. doi: 10.1016/j.neuropharm.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn A.F., Weerts E.M. Evaluation of mifepristone effects on alcohol-seeking and self-administration in baboons. Exp. Clin. Psychopharmacol. 2019;27:227–235. doi: 10.1037/pha0000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez V.A., Walter N.A.R., Shnitko T.A., Newman N., Diem K., Vanderhooft L., Hunt H., Grant K.A. Mifepristone decreases chronic voluntary ethanol consumption in rhesus macaques. J. Pharmacol. Exp. Therapeut. 2020;375:258–267. doi: 10.1124/jpet.120.000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karena Z.V., Shah H., Vaghela H., Chauhan K., Desai P.K., Chitalwala A.R. Clinical utility of mifepristone: apprising the Expanding horizons. Cureus. 2022;14 doi: 10.7759/cureus.28318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S., Nguyen J.D., Vandewater S.A., Grant Y., Roberto M., Taffe M.A. Self-administration of Entactogen psychostimulants dysregulates gamma-aminobutyric acid (GABA) and kappa opioid receptor signaling in the central nucleus of the amygdala of female Wistar rats. Front. Behav. Neurosci. 2021;15 doi: 10.3389/fnbeh.2021.780500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S., Rodriguez L., Gandhi P., Kirson D., Bajo M., Oleata C.S., Vendruscolo L.F., Mason B.J., Roberto M. Alcohol dependence and withdrawal increase sensitivity of central amygdalar GABAergic synapses to the glucocorticoid receptor antagonist mifepristone in male rats. Neurobiol. Dis. 2022;164 doi: 10.1016/j.nbd.2022.105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S., Steinkellner T., Hnasko T.S., Roberto M. Alcohol dependence potentiates substance P/neurokinin-1 receptor signaling in the rat central nucleus of amygdala. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz1050. eaaz1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S., Wolfe S.A., Patel R.R., Kirson D., Hedges D.M., Varodayan F.P., Bajo M., Roberto M. Alcohol dependence and withdrawal impair serotonergic regulation of GABA transmission in the rat central nucleus of the amygdala. J. Neurosci. 2020;40:6842–6853. doi: 10.1523/JNEUROSCI.0733-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D., Khom S., Rodriguez L., Wolfe S.A., Varodayan F.P., Gandhi P.J., Patel R.R., Vlkolinsky R., Bajo M., Roberto M. Sex differences in acute alcohol sensitivity of naïve and alcohol dependent central amygdala GABA synapses. Alcohol Alcohol. 2021;56:581–588. doi: 10.1093/alcalc/agab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Anhedonia, hyperkatifeia, and negative Reinforcement in substance use disorders. Curr. Top. Behav. Neurosci. 2022;58:147–165. doi: 10.1007/7854_2021_288. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Drug addiction: hyperkatifeia/negative Reinforcement as a framework for Medications development. Pharmacol. Rev. 2021;73:163–201. doi: 10.1124/pharmrev.120.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. The dark side of emotion: the addiction perspective. Eur. J. Pharmacol. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Schulkin J. Addiction and stress: an allostatic view. Neurosci. Biobehav. Rev. 2019;106:245–262. doi: 10.1016/j.neubiorev.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Kwako L.E., Spagnolo P.A., Schwandt M.L., Thorsell A., George D.T., Momenan R., Rio D.E., Huestis M., Anizan S., Concheiro M., Sinha R., Heilig M. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistner C., Menke A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020;175:55–64. doi: 10.1016/B978-0-444-64123-6.00004-7. [DOI] [PubMed] [Google Scholar]
- Logrip M.L., Gainey S.C. Sex differences in the long-term effects of past stress on alcohol self-administration, glucocorticoid sensitivity and phosphodiesterase 10A expression. Neuropharmacology. 2020;164 doi: 10.1016/j.neuropharm.2019.107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip M.L., Oleata C., Roberto M. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology. 2017;114:123–134. doi: 10.1016/j.neuropharm.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery E.G., Spanos M., Navarro M., Lyons A.M., Hodge C.W., Thiele T.E. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Gold P.W., Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- McGinn M.A., Tunstall B.J., Schlosburg J.E., Gregory-Flores A., George O., de Guglielmo G., Mason B.J., Hunt H.J., Koob G.F., Vendruscolo L.F. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology. 2021;188 doi: 10.1016/j.neuropharm.2021.108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer O.C., Buurstede J.C., Viho E.M.G., Amaya J.M., Koning A.-S.C.A.M., van der Meulen M., van Weert L.T.C.M., Paul S.N., Kroon J., Koorneef L.L. Transcriptional glucocorticoid effects in the brain: finding the relevant target genes. J. Neuroendocrinol. 2022 doi: 10.1111/jne.13213. [DOI] [PubMed] [Google Scholar]
- Natividad L.A., Buczynski M.W., Herman M.A., Kirson D., Oleata C.S., Irimia C., Polis I., Ciccocioppo R., Roberto M., Parsons L.H. Constitutive increases in amygdalar corticotropin-releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol. Psychiatr. 2017;82:500–510. doi: 10.1016/j.biopsych.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad L.A., Steinman M.Q., McGinn M.A., Sureshchandra S., Kerr T.M., Ciccocioppo R., Messaoudi I., Edwards S., Roberto M. Impaired hypothalamic feedback dysregulates brain glucocorticoid signaling in genetically-selected Marchigian Sardinian alcohol-preferring rats. Addict. Biol. 2021;26 doi: 10.1111/adb.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E.L., Albrechet-Souza L., Andrew P.M., Auld J.G., Burk K.C., Hwa L.S., Zhang E.Y., DeBold J.F., Miczek K.A. Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress: suppression by CRF-R1 antagonism. Psychopharmacology. 2018;235:1807–1820. doi: 10.1007/s00213-018-4905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A., Thomas A.M., Kimmey B.A., Karsch J.S., Doyon W.M., Dani J.A. Stress increases ethanol self-administration via a shift toward Excitatory GABA signaling in the ventral tegmental area. Neuron. 2016;92:493–504. doi: 10.1016/j.neuron.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.R., Varodayan F.P., Herman M.A., Jimenez V., Agnore R., Gao L., Bajo M., Cuzon Carlson V.C., Walter N.A., Fei S.S., Grant K.A., Roberto M. Synaptic effects of IL-1β and CRF in the central amygdala after protracted alcohol abstinence in male rhesus macaques. Neuropsychopharmacology. 2022;47:847–856. doi: 10.1038/s41386-021-01231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.R., Wolfe S.A., Bajo M., Abeynaike S., Pahng A., Borgonetti V., D'Ambrosio S., Nikzad R., Edwards S., Paust S., Roberts A.J., Roberto M. IL-10 normalizes aberrant amygdala GABA transmission and reverses anxiety-like behavior and dependence-induced escalation of alcohol intake. Prog. Neurobiol. 2021;199 doi: 10.1016/j.pneurobio.2020.101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier M.R., Verplaetse T.L., Mineur Y.S., Petrakis I.L., Cosgrove K.P., Picciotto M.R., McKee S.A. Sex differences in stress-related alcohol use. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L.A., Grodin E.N., Leggio L., Bechtholt A.J., Becker H., Feldstein Ewing S.W., Jentsch J.D., King A.C., Mason B.J., O'Malley S., MacKillop J., Heilig M., Koob G.F. The future of translational research on alcohol use disorder. Addict. Biol. 2021;26 doi: 10.1111/adb.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V., Shin W., Vendruscolo L.F., Lefebvre C., van der Stap L., Kawamura T., Schlosburg J.E., Alvarez M., Koob G.F., Califano A., Sanna P.P. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol. 2015;16:68. doi: 10.1186/s13059-015-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Cruz M.T., Gilpin N.W., Sabino V., Schweitzer P., Bajo M., Cottone P., Madamba S.G., Stouffer D.G., Zorrilla E.P., Koob G.F., Siggins G.R., Parsons L.H. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol. Psychiatr. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Kirson D., Khom S. The role of the central amygdala in alcohol dependence. Cold Spring Harb. Perspect. Med. 2021;11:a039339. doi: 10.1101/cshperspect.a039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Madamba S.G., Moore S.D., Tallent M.K., Siggins G.R. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Madamba S.G., Stouffer D.G., Parsons L.H., Siggins G.R. Increased GABA release in the central amygdala of ethanol-dependent rats. J. Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna P.P., Kawamura T., Chen J., Koob G.F., Roberts A.J., Vendruscolo L.F., Repunte-Canonigo V. 11β-hydroxysteroid dehydrogenase inhibition as a new potential therapeutic target for alcohol abuse. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese A.M., Ozburn A.R., Metten P., Schlumbohm J.P., Hack W.R., LeMoine K., Hunt H., Hausch F., Bauder M., Crabbe J.C. Targeting the glucocorticoid receptor reduces binge-like drinking in high drinking in the dark (HDID-1) mice. Alcohol Clin. Exp. Res. 2020;44:1025–1036. doi: 10.1111/acer.14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt M.L., Cortes C.R., Kwako L.E., George D.T., Momenan R., Sinha R., Grigoriadis D.E., Pich E.M., Leggio L., Heilig M. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrett-Field L., Butler T.R., Berry J.N., Reynolds A.R., Prendergast M.A. Mifepristone pretreatment reduces ethanol withdrawal severity in vivo. Alcohol Clin. Exp. Res. 2013;37:1417–1423. doi: 10.1111/acer.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms J.A., Haass-Koffler C.L., Bito-Onon J., Li R., Bartlett S.E. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar S.S., Vendruscolo L.F., Fannon M.J., Schmeichel B.E., Nguyen T.B., Guevara J., Sidhu H., Contet C., Zorrilla E.P., Mandyam C.D. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. doi: 10.1016/j.psyneuen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer W.H., Rimondini R., Hansson A.C., Hipskind P.A., Gehlert D.R., Barr C.S., Heilig M.A. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatr. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Steinman M.Q., Kirson D., Wolfe S.A., Khom S., D'Ambrosio S.R., Spierling Bagsic S.R., Bajo M., Vlkolinský R., Hoang N.K., Singhal A., Sureshchandra S., Oleata C.S., Messaoudi I., Zorrilla E.P., Roberto M. Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol. Psychiatr. 2021;26:3093–3107. doi: 10.1038/s41380-020-00920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez J., Khom S., Alén F., Natividad L.A., Varodayan F.P., Patel R.R., Kirson D., Arco R., Ballesta A., Bajo M., Rubio L., Martin-Fardon R., Rodríguez de Fonseca F., Roberto M. Cessation of fluoxetine treatment increases alcohol seeking during relapse and dysregulates endocannabinoid and glutamatergic signaling in the central amygdala. Addict. Biol. 2020;25 doi: 10.1111/adb.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall B.J., Carmack S.A., Koob G.F., Vendruscolo L.F. Dysregulation of brain stress systems mediates compulsive alcohol drinking. Curr. Opin. Behav. Sci. 2017;13:85–90. doi: 10.1016/j.cobeha.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan F.P., de Guglielmo G., Logrip M.L., George O., Roberto M. Alcohol dependence disrupts amygdalar L-type voltage-Gated calcium channel mechanisms. J. Neurosci. 2017;37:4593–4603. doi: 10.1523/JNEUROSCI.3721-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan F.P., Khom S., Patel R.R., Steinman M.Q., Hedges D.M., Oleata C.S., Homanics G.E., Roberto M., Bajo M. Role of TLR4 in the modulation of central amygdala GABA transmission by CRF following restraint stress. Alcohol Alcohol. 2018 doi: 10.1093/alcalc/agx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan F.P., Patel R.R., Matzeu A., Wolfe S.A., Curley D.E., Khom S., Gandhi P.J., Rodriguez L., Bajo M., D'Ambrosio S., Sun H., Kerr T.M., Gonzales R.A., Leggio L., Natividad L.A., Haass-Koffler C.L., Martin-Fardon R., Roberto M. The amygdala noradrenergic system is compromised with alcohol use disorder. Biol. Psychiatr. 2022;91:1008–1018. doi: 10.1016/j.biopsych.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L.F., Barbier E., Schlosburg J.E., Misra K.K., Whitfield T.W., Logrip M.L., Rivier C., Repunte-Canonigo V., Zorrilla E.P., Sanna P.P., Heilig M., Koob G.F. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo L.F., Estey D., Goodell V., Macshane L.G., Logrip M.L., Schlosburg J.E., McGinn M.A., Zamora-Martinez E.R., Belanoff J.K., Hunt H.J., Sanna P.P., George O., Koob G.F., Edwards S., Mason B.J. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozella V., Cruz B., Natividad L.A., Benvenuti F., Cannella N., Edwards S., Zorrilla E.P., Ciccocioppo R., Roberto M. Glucocorticoid receptor antagonist mifepristone does not alter innate anxiety-like behavior in genetically-selected Marchigian Sardinian (msP) rats. Int. J. Mol. Sci. 2021;22:3095. doi: 10.3390/ijms22063095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S.A., Sidhu H., Patel R.R., Kreifeldt M., D'Ambrosio S.R., Contet C., Roberto M. Molecular, Morphological, and functional characterization of corticotropin-releasing factor receptor 1-Expressing neurons in the central nucleus of the amygdala. eNeuro. 2019;6 doi: 10.1523/ENEURO.0087-19.2019. ENEURO.0087-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.