Abstract
This secondary analysis of a phase 2 clinical trial examines long-term survival for resectable cutaneous squamous cell carcinoma of the head and neck according to pathologic response.
In a pilot phase 2 trial, we observed pathologic responses in 15 of 20 patients (75%) with stage III/IV resectable cutaneous squamous cell carcinoma of the head and neck (CSCC-HN) treated with neoadjuvant cemiplimab.1 In this article, we report long-term survival according to pathologic response.
Methods
Study Design and Procedures
This single-arm, investigator-initiated phase 2 study included newly diagnosed or recurrent stage III/IV resectable CSCC-HN. The study procedures were previously described (Supplement 1).1 The study received institutional review board approval and participants provided written informed consent. Adjuvant radiotherapy (RT) was planned for all patients but reconsidered on a case-by-case basis according to pathologic response. Surveillance included cross-sectional imaging every 3 to 4 months for longer than 2 years.
Statistical Analysis
The data cutoff was October 14, 2022. The distribution of time-to-event end points was estimated using the Kaplan-Meier method. Patients with a pathologic complete response (pCR) or major pathologic response (MPR; ≤10% viable tumor) were classified as responders, and all other patients were classified as nonresponders. The hazard ratio (HR) and 95% CI was used to describe the magnitude of the difference in survival end points. Statistical analysis was conducted using R, version 4.2.3 (R Foundation).
Results
Patients and Surgery
Of 20 patients enrolled, 7 (35%) presented with recurrent disease and 12 (60%) were classified as having stage IV disease. All patients received 2 planned doses of neoadjuvant cemiplimab and underwent the proposed surgery according to the original extent of disease. There were no surgical delays.
Pathologic Responses and Adjuvant Therapy
Pathologic responses were observed in 15 patients (75%); 11 (55%) had a pCR and 4 (20%) had an MPR. Of these, 12 patients (80%) did not receive adjuvant RT. The remaining patients received adjuvant RT (n = 6) or concurrent chemoradiation (n = 2).
Survival Outcomes
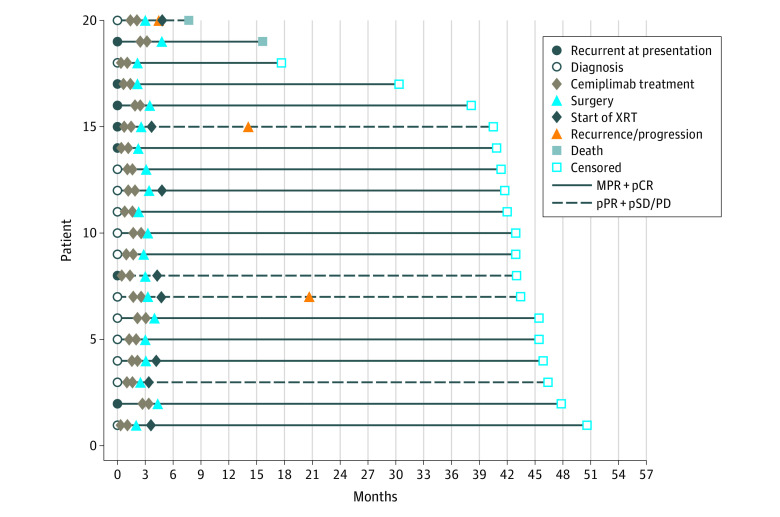
At a median follow-up of 42.3 months (range, 7.7-50.6), 17 patients (85%) remained disease free and 3 (15%) had recurrence (Figure 1). Of 15 patients (75%) who were responders (pCR or MPR), none had recurrence (1 patient died of other causes). Of 5 patients (25%) who were nonresponders, 3 developed local recurrences, including 2 patients who presented with substantial bone involvement.
Figure 1. Oncologic Outcomes Following Neoadjuvant Cemiplimab and Surgery.
Interval event chart aligned by diagnosis date indicating treatment procedures and patient outcomes following neoadjuvant cemiplimab and surgery. MPR indicates major pathologic response; pCR, pathologic complete response; pPR, partial pathologic response; pSD/PD, stable or progressive disease; XRT, radiotherapy.
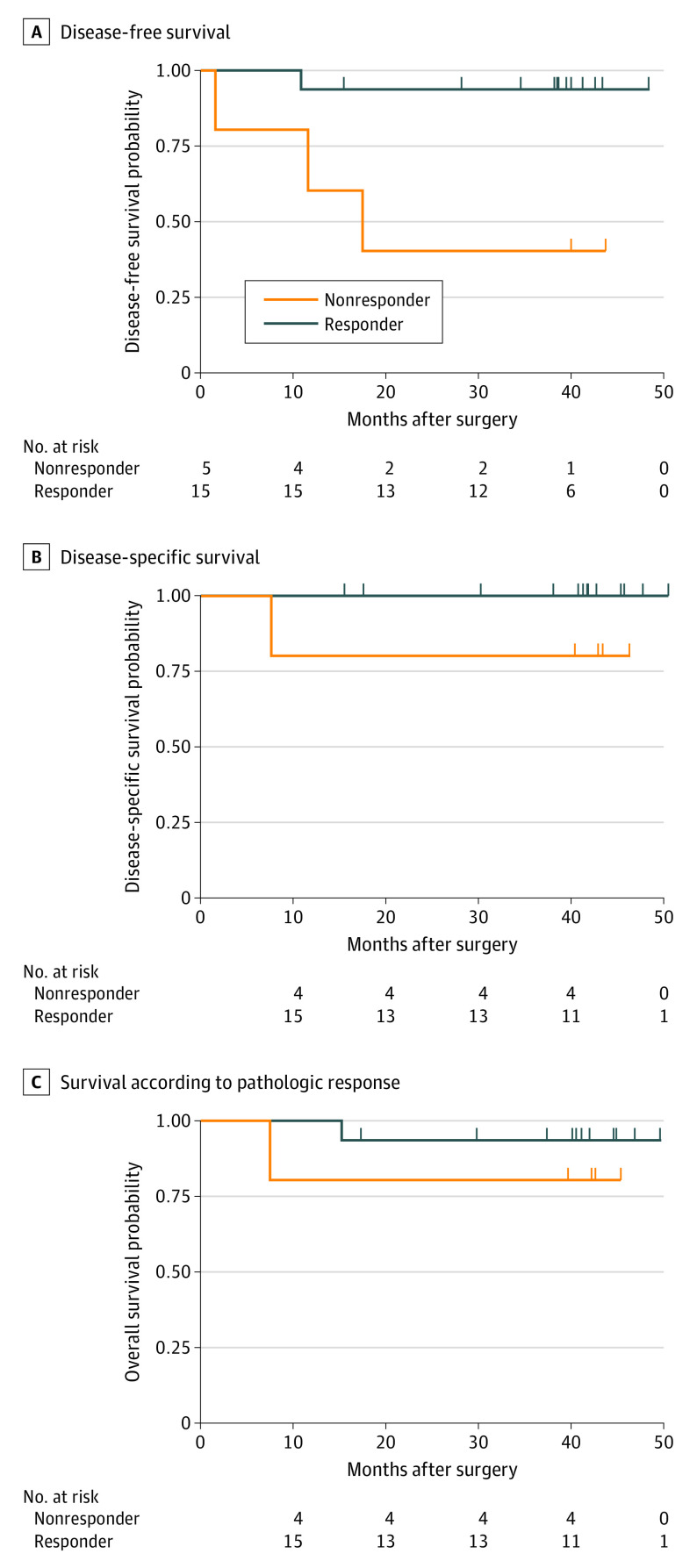
Two patients (10%) died, one due to progression and 1 of other causes. For the entire cohort, 3-year disease-free survival (DFS) and event-free survival was 83.9% (95% CI, 68.7%-100.0%), 3-year disease specific survival (DSS) was 95% (95% CI, 85.9%-100.0%), and 3-year overall survival was 90% (95% CI, 77.8%-100.0%). Pathologic responders demonstrated improved DFS (HR, 0.092; 95% CI, 0.010-0.886) but not DSS (HR, 0; 95% CI, 0 to infinity, convergence) or overall survival (HR, 0.298; 95% CI, 0.019-4.787) compared with nonresponders (Figure 2).
Figure 2. Survival According to Pathologic Response.
Kaplan-Meier estimates of disease-free survival (DFS) (A), disease-specific survival (DSS) (B), and overall survival (OS) (C) among 20 patients with stage III/IV cutaneous squamous cell carcinoma (CSCC) of the head and neck who were treated with 2 doses of neoadjuvant cemiplimab. Pathologic responders (pathologic complete response [pCR] or major pathologic response [MPR]) demonstrated improved DFS (hazard ratio [HR], 0.092; 95% CI, 0.010-0.886) but not DSS (HR, 0; 95%CI, 0 to infinity, convergence) or OS (HR, 0.298; 95% CI, 0.019-4.787) compared with nonresponders.
Discussion
The marked pathologic responses observed in the pilot trial were recently replicated in a phase 2, multicenter, confirmatory trial of neoadjuvant cemiplimab in stage II to IV resectable CSCC.2 Of 79 patients, a pCR was observed in 40 patients (51%) and an MPR in 10 (13%). However, the oncologic outcomes data from this study have yet to mature.
The survival outcomes reported in this secondary analysis compare favorably with prior published studies for advanced CSCC.3 With a median follow-up more than 42 months, none of the 15 patients who were pathologic responders experienced recurrence. This is particularly noteworthy given that 12 (80%) of these patients did not receive adjuvant RT. In contrast, 3 of the 5 patients who were nonresponders developed recurrence despite receiving adjuvant therapy. Limitations to this study included the small sample size, absence of a control group, and heterogeneity of adjuvant therapies applied.
Neoadjuvant immunotherapy has demonstrated favorable pathologic responses in multiple cancer types that have translated into improved survival.4,5 Checkpoint inhibition before surgery yields more antigen-specific T cells that can, in turn, better activate a systemic immune response.6
The use of neoadjuvant immunotherapy to allow function-preserving surgery and treatment de-escalation is of particular interest given that patients with CSCC-HN are often elderly with medical comorbidities. The proximity of disease to critical structures can yield substantial morbidity to patients from standard surgery and RT. The durable long-term DFS among pathologic responders after neoadjuvant immunotherapy may provide a strong rationale for the development of a randomized clinical trial.
Trial protocol
Data sharing statement
References
- 1.Ferrarotto R, Amit M, Nagarajan P, et al. Pilot phase II trial of neoadjuvant immunotherapy in locoregionally advanced, resectable cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res. 2021;27(16):4557-4565. doi: 10.1158/1078-0432.CCR-21-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross ND, Miller DM, Khushalani NI, et al. Neoadjuvant cemiplimab for stage II to IV cutaneous squamous-cell carcinoma. N Engl J Med. 2022;387(17):1557-1568. doi: 10.1056/NEJMoa2209813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porceddu SV, Bressel M, Poulsen MG, et al. Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: the randomized phase III TROG 05.01 trial. J Clin Oncol. 2018;36(13):1275-1283. doi: 10.1200/JCO.2017.77.0941 [DOI] [PubMed] [Google Scholar]
- 4.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976-1986. doi: 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021;27(2):301-309. doi: 10.1038/s41591-020-01188-3 [DOI] [PubMed] [Google Scholar]
- 6.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655-1661. doi: 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Data sharing statement