Abstract
Independent studies have demonstrated different cell tropisms for molecular clones of feline immunodeficiency virus (FIV). In this report, we examined three clones, FIV-pF34, FIV-14, and FIV-pPPR, for replication in Crandell feline kidney (CrFK) cells, feline peripheral blood mononuclear cells (PBMC), and feline macrophage cultures. Importantly, cell tropism for these three clones was also examined in vivo. FIV-pF34 replication was efficient in CrFK cells but severely restricted in PBMC, whereas replication of FIV-pPPR was vigorous in PBMC but severely restricted in CrFK cells. FIV-14 replication was productive in both CrFK cells and PBMC. Interestingly, all three molecular clones replicated with similar efficiencies in primary feline monocyte-derived macrophages. In vivo, FIV-pF34 proved least efficient for establishing persistent infection, and proviral DNA when detectable, was localized predominately to nonlymphoid cell populations (macrophages). FIV-pPPR proved most efficient for induction of a persistent viremia in vivo, and proviral DNA was localized predominately in CD4+ and CD8+ lymphocyte subsets. FIV-14 inoculation of cats resulted in an infection characterized by seroconversion and localization of proviral DNA in CD4+ lymphocytes only. Results of this study on diverse FIV molecular clones revealed that in vitro replication efficiency of an FIV isolate in PBMC directly correlated with replication efficiency in vivo, whereas proficiency for replication in macrophages in vitro was not predictive for replication potential in vivo. Also, infection of both CD4+ and CD8+ lymphocyte subsets was associated with higher virus load in vivo. Results of the studies on these three FIV clones, which exhibited differential cell tropism, indicated a correlation between in vitro and in vivo cell tropism and virus replication.
The feline immunodeficiency virus (FIV) is a member of the lentivirus subfamily of retroviruses and a causative agent of AIDS in domestic cats (24, 43). Similar to other immunodeficiency-inducing lentiviruses such as human immunodeficiency virus (HIV) and simian immunodeficiency virus, strains of FIV exhibit a tropism for T lymphocytes and macrophages in vitro and in vivo (2–4, 12, 14, 16, 36, 40). Both natural and experimental infections of cats with FIV result in CD4+ T-cell depletion as well as other immunologic disorders (1, 21, 35). As with T-cell line-tropic isolates of HIV, specific FIV variants have been reported to utilize the α-chemokine receptor CXCR4 as a principal coreceptor (19, 41). Thus, FIV infection of cats has emerged as an important animal model for HIV/AIDS pathogenesis in humans.
Cell tropism has been hypothesized to influence lentiviral pathogenesis in the infected host (6, 9, 15, 20, 33, 44). Previous reports compared replication of various biological and molecularly cloned FIV isolates in vitro in primary feline peripheral blood mononuclear cells (PBMC), primary feline macrophages, feline T-cell lines, or feline adherent cell lines. Although observations from earlier studies revealed FIV molecular clones FIV-pF34 (FIV 34TF10) (32), FIV-pPPR (PPR) (25), and FIV-14 (23) to be minimally pathogenic or nonpathogenic, these cloned isolates of FIV exhibited unique in vitro growth properties and replication efficiencies in vivo (19, 23, 25, 27, 29, 30, 40, 41). To examine possible correlations of in vitro cell tropism properties with virus replication efficiency and cell tropism in vivo, the present study compared infection and replication of molecular clones FIV-pF34, FIV-pPPR, and FIV-14 in different cell culture systems and in specific host cell populations following experimental infection of specific-pathogen-free (SPF) cats. In vitro replication efficiency of an FIV isolate in PBMC directly correlated with replication efficiency in vivo, whereas positive growth properties in a feline adherent cell line inversely correlated with in vivo virus replication. Proficiency for replication in monocyte-derived macrophages (MDM) in vitro was not predictive for replication potential in vivo. Infection of multiple lymphocyte subsets including CD4+, CD8+, and CD21+ lymphocytes was associated with a higher virus load in vivo. Taken together, these studies indicated that virus load induced by a cloned FIV isolate was related to the cell tropism specific to that isolate.
MATERIALS AND METHODS
Feline cells and virus stocks.
A feline adherent cell line (Crandell feline kidney [CrFK] cells; ATCC CCL 94) and primary feline PBMC were cultured as described previously (31) and used for short-term passage (14 days or less) of virus stocks. FIV-pPPR virus stocks were generated by transfection with plasmid construct FIV-pPPR (25) or an infectious FIV-PPR provirus construct (pSV-pPPR) encoding a hybrid 5′ long terminal repeat (LTR) composed of the simian virus 40 early enhancer region and TATA box, a deleted U3 (bp −1 to −10), and full-length R and U5 (30). Titered virus stocks derived from either FIV-pPPR or FIV-14 plasmids (23) were generated by transfection of CrFK cells and cocultivation with feline PBMC as previously described (30). FIV-pF34-derived virus stocks were produced by transfection with FIV-pF34 plasmid (25) and cultivation in CrFK cells.
For cultivation and infection of feline MDM, PBMC preparations were resuspended in feline macrophage differentiation medium consisting of RPMI 1640, 10% heat-inactivated fetal calf serum, 10% heat-inactivated pooled SPF cat serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (Fungizone; 25 μg/ml) and plated at a density of 4 × 106 to 8 × 106 cells per well in 24-well tissue culture plates. Cultures were washed with Hanks’ buffered salt solution to remove nonadherent cells 24 h after plating and fed fresh medium every 3 to 4 days. Using growth conditions identical to those described for cultivation in 24-well plates, feline MDM cultures plated on Thermanox slides were found to contain 99% or more macrophages based on immunocytochemical analysis (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.) using a mouse monoclonal antibody against human lysozyme (a macrophage marker) (Dako, Carpenteria, Calif.) and a mouse monoclonal against human CD3 (a pan T-cell marker) (Dako) under conditions recommended by the manufacturer (data not shown).
Replication of FIV molecular clones in vitro.
For in vitro replication studies, wells in a six-well microtiter plate were seeded with either 2 × 105 CrFK cells or 2 × 106 feline PBMC. For CrFK replication studies, duplicate wells were inoculated either with 103 50% tissue culture infective doses (TCID50) of a virus stock described above or with uninfected tissue culture medium (mock infection control). Virus inocula containing either 102, 500, or 103 TCID50 per well were tested in PBMC replication studies. For macrophage infection studies, each well of a 24-well plate (approximately 105 MDM) was inoculated with 103 TCID50 of a virus stock or were mock infected. Cells were incubated with virus inocula overnight, washed with Hanks’ buffered salt solution the following day, and fed fresh tissue culture media. Supernatant was collected from all inoculated cell cultures every 3 to 4 days up to 2 weeks postinoculation (p.i.) for detection of FIV Gag (p24), using either a FIV p24 antigen capture enzyme-linked immunosorbent assay (ELISA) previously described (10) or a commercial FIV p24 antigen capture ELISA (Idexx Corp., Westbrook, Maine).
Inoculation of cats.
Twenty-two juvenile (7- to 9-month-old) SPF cats were obtained from the SPF cat colony of J. G. Morris and Q. R. Rogers (University of California, Davis) and housed in infectious disease isolation facilities provided by Animal Resources Services, University of California, Davis. Cats were randomly assigned to one of four experimental groups. Group 1 consisted of four animals sham inoculated with saline as a negative control. Groups 2 to 4 consisted of six animals each and were inoculated by the intraperitoneal route with 1 ml of Hanks’ buffered salt solution containing 64 to 102 TCID50 of a virus stock derived from either FIV-pPPR, FIV-pF34, or FIV-14 titered as described above in feline cells and virus stocks. Prior to inoculation, blood was collected from cats for serological and hematological assays, and PBMC were prepared and frozen to later assess for proviral DNA by PCR amplification. After inoculation, blood samples were collected for serological and hematological assays and for virus detection assays including virus isolation and PCR amplification of proviral DNA. Lymphoid tissues (including lymph node and spleen) were biopsied from cats at either early time points or final time points to sort lymphocyte preparations into single subsets to be assayed for viral DNA.
Hematology and lymphocyte phenotype analysis.
Complete blood counts and differentials were determined by standard methods (1). CD4+ and CD8+ T-lymphocyte percentages were determined in peripheral blood by flow cytometry using a FACScan (Becton Dickenson, San Jose, Calif.) as previously described (11).
Serology.
Serum was tested for antibody against FIV p24 Gag with an ELISA using recombinant FIV p24 (18). Sera found positive by ELISA at dilutions of 1:100 or greater were considered antibody positive and confirmed by immunoblot analysis with whole virus as previously described (43).
Virus detection.
PBMC (106) prepared from whole blood by Ficoll centrifugation were cocultured with PBMC from an SPF donor cat as previously described (31), and PBMC culture supernatants were monitored weekly for viral p24 by ELISA for up to 6 weeks. PBMC pellets prepared from heparinized blood after inoculation were stored at −70°C for later genomic DNA extraction and PCR amplification for proviral DNA. Lymphocyte subsets were sorted from splenic tissue samples harvested by biopsy from one cat from each group between 6 and 7 weeks p.i. and again from a second cat within both FIV-14 or FIV-pPPR-inoculated groups at 11 weeks p.i. Lymphocyte subsets were also sorted from mesenteric lymph nodes sampled postmortem at 23 weeks p.i. and stored for later proviral DNA assessment. Peritoneal macrophages harvested by peritoneal lavage at 22.5 weeks p.i. and hemolymphatic tissues sampled just prior to euthanasia (23 weeks p.i.) were also assayed for proviral DNA.
PCR and reverse transcription-PCR.
DNA was extracted from tissues or PBMC by using a commercial kit (QIAamp tissue kit; Qiagen Corp., Chatsworth, Calif.) and was evaluated for integrity by gel electrophoresis through 0.8% agarose. Nested PCR was performed on 1 μg of DNA with primers specific for the LTR sequence of each molecular clone (see below) (32). Each round of PCR was performed in a BioOven III thermal cycler (BioTherm Corp., Fairfax, Va.) and consisted of 35 cycles (30 s of template denaturation at 94°C, 30 s of primer annealing at 55°C, and 45 s of primer extension at 72°C). Round 1 forward primers used were clone specific: LTR3.19 (5′ TGGGATGAGTATTGGGACC; FIV-PPR-specific), NP 143.19 (5′ TGGGATGAGTACTGGA ACC; FIV-pF34-specific); and NP 142.19 (5′ TGGGATGAGTATTGGAACC; FIV14-specific). The round 1 reverse primer used for all clones, LTR4.19 5′ (TGCGAAGTTCTCGGCCCGG), yielded a 356-bp product. Second-round primers, LTR 5.19 (5′ CATGACTCATAGT TAAAGCGCTAGCAGCTG; forward) and LTR-2 5′ (GTTCTCGGCCCGGATTCCGAGACC TCACAGG; reverse), were used for all clones and yielded a 261-bp product. Each sample was tested three times by PCR amplification before a final result was recorded. The nested PCR is sensitive to less than 10 proviral copies/μg of genomic DNA.
Open reading frame orf-A (also referred to as orf 2) encoded by molecular clone FIV-pF34 is truncated due to a single base pair change resulting in a premature stop codon (32). To assess retention of this truncation within FIV-pF34 virus stocks, orf-A was amplified by a single round of PCR from genomic DNA extracted from FIVp-34-infected CrFK cells, using PCR primers pF34U5957 (5′ CCCCCAGC TGGAACCCTGTTATGG; forward) and pF34L6303 (5′ CCTATCCATTGTCTATTGGCTGC; reverse). PCR products containing orf-A sequences were then sequenced directly with an ABI automated sequencer (Perkin-Elmer/ABI, Foster City, Calif.).
For reverse transcription-PCR, viral RNA was prepared from 1-ml aliquots of virus stocks by using TRI reagent (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer’s directions and treated with DNase as previously described (13). RNA (0.4 μg) was reverse transcribed with a reverse transcription kit (Promega Biotech, Madison, Wis.), using an FIV env-specific primer (Fenv6). Reverse transcription products were amplified by nested PCR using nested primer sets specific for FIV env described below for the heteroduplex mobility assay (HMA).
Magnetic cell sorting.
Lymph node or spleen was dissociated into a single-cell suspension by using a Cellector (Bellco Glass Inc., Vineland, N.J.) from which viable mononuclear cells were harvested by density gradient centrifugation through Ficoll-Hypaque (density, 1.077 g/ml; Sigma, St. Louis, Mo.). Mononuclear cells were labeled with either fluorescein isothiocyanate-conjugated mouse monoclonal antibody specific for CD4 (Fel.7B12; generously provided by P. F. Moore, University of California, Davis), CD8 (fT2; Southern Biotechnology Associates, Inc., Birmingham, Ala.), or CD21 (CA2.1D6; generously provided by P. F. Moore) and prepared with rat anti-mouse immunoglobulin G1 microbeads (Miltenyi Biotec, Auburn, Calif.) for sorting as previously described (13). Labeled cells were harvested by magnetic cell sorting using a Mini-MACS (Miltenyi Biotec) as instructed by the manufacturer. The purity of cells was confirmed by flow cytometry using a FACScan (Becton Dickenson).
HMA.
Nested PCR was performed as described above on genomic DNA from mesenteric lymph node or spleen. First-round primers (Fenv1 [5′ GCTATTGTACAGACCCATTAC; forward] and Fenv6 [5′ GTACAATTACAATTCATATACCC; CC, reverse]) amplified a 699-bp fragment, and second-round primers (Fenv2 [5′ TCCCACTGATCAATTATACATTTGG; forward] and Fenv3 [5′ GTCATCTACCTTCATAGTAAACCCG; reverse]) amplified a 562-bp fragment. The second-round product included the V3-V4 region of the envelope gene. Heteroduplexes were formed between PCR products from tissues and PCR products generated from DNA reverse transcribed from the original viral inoculum by melting combined DNA at 94°C and reannealing them by rapid cooling on ice. Reannealed samples were then separated by electrophoresis on 5% polyacrylamide gel and stained with ethidium bromide.
RESULTS
Virus replication in vitro in PBMC and MDM.
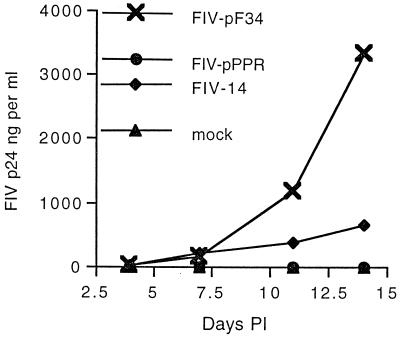
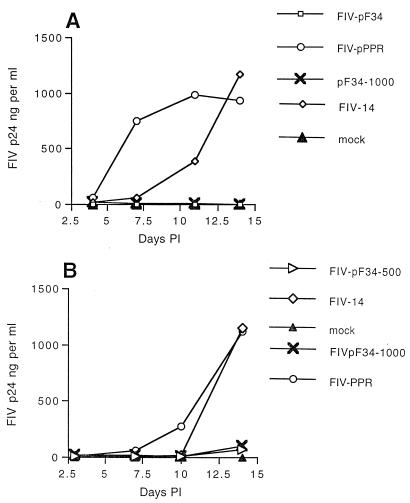
To evaluate the significance of cell tropism for viral replication and pathogenesis in cats, we first examined replication of molecular clones FIV-pF34, FIV-pPPR, and FIV-14 in primary feline PBMC and MDM and in CrFK cells, a feline adherent cell line. DNA sequence analysis indicated that FIV-pF34 virus stocks retained the single base pair change observed in the molecular cloned provirus which encodes a truncation of viral gene orf-A (data not shown). Data presented in Fig. 1 to 3 represent findings consistent with three or more experiments. In agreement with previous reports, FIV-pF34 achieved the most efficient replication in CrFK cells, compared to the modest replication observed with FIV-14 and negligible replication of FIV-pPPR (Fig. 1) (25, 40). In contrast, supernatants harvested from PBMC inoculated with either FIV-pPPR or FIV-14 contained markedly higher concentrations of viral antigen by 14 days p.i. compared to FIV-pF34-infected cultures (Fig. 2). Although virus production levels observed with FIV-14 and FIV-pPPR were similar by 14 days p.i., FIV-14 replication in PBMC was frequently delayed compared to that of FIV-pPPR. Replication of FIV-pF34 was severely restricted in PBMC cultures regardless of infectious titer of the virus inoculum (102 to 103 TCID50), similar to our previous observations (29). Although infectious titers measured for FIV-pF34 on CrFK cells and FIV-14 virus stocks on feline PBMC were frequently lower (60 to 200 TCID50 per ml) than titers found for FIV-pPPR (103 TCID50 or greater per ml), FIV-p24 concentration (data not shown) and/or reverse transcriptase activity per milliliter (29) were comparable to values measured for FIV-pPPR. FIV-pF34 inocula based on infectious titer potentially contained higher virus concentrations than FIV-pPPR. Therefore, the reduced infectivity of FIV-pF34 for PBMC is unlikely due to a bias introduced by titering of FIV-pF34 on CrFK cells.
FIG. 1.
Replication of FIV molecular clones in CrFK cells. CrFK cells were inoculated with 103 TCID50 of a virus stock and sampled as described in Materials and Methods. Results shown are representative of three or more experiments.
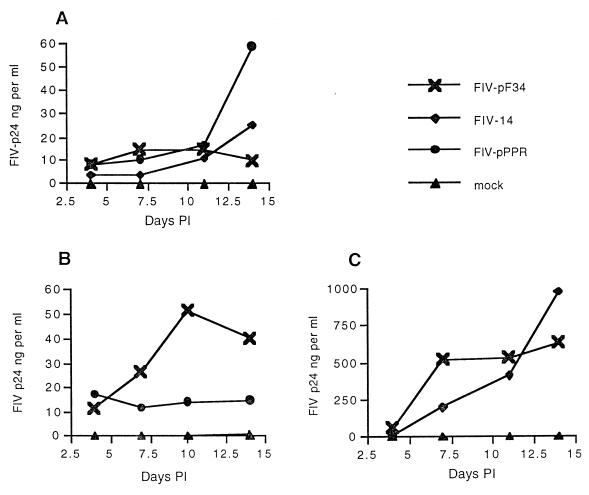
FIG. 3.
Replication of FIV molecular clones in primary feline MDM. Feline MDM were inoculated with 103 TCID50 of a virus stock or with 1 ml of uninfected tissue culture supernatant (mock). The three panels show results (representative of four or more experiments) of three separate experiments testing replication in MDM prepared from three different SPF donor cats.
FIG. 2.
Replication of FIV molecular clones in primary feline PBMC. Dose inocula for all infections was 102 TCID50 except where indicated for FIV-pF34 infections using either 500 or 1,000 TCID50. The two panels show results (representative of three or more experiments) of two separate experiments testing replication in feline PBMC prepared from two different SPF donor cats.
Replication kinetics and efficiency observed with the different molecular clones of FIV in primary feline MDM were somewhat more variable, a finding possibly associated with variation in MDM preparations from different SPF cats (Fig. 3) (22). In general, all three molecular clones exhibited a modest and comparable efficiency for replication in feline MDM. Virus production observed with FIV-pPPR and FIV-14 infection of primary MDM, as measured by a FIV p24 antigen capture ELISA, was usually 10-fold or more lower than in either PBMC or CrFK infection studies testing these same viruses (Fig. 3A and B). Virus production more similar to that observed from PBMC was observed in less than a third of the macrophage infection studies (Fig. 3C). Compared to the virus inocula used for PBMC infection studies (102 TCID50), a greater concentration of infectious virus inoculum (103 TCID50) was required to consistently establish a detectable virus infection of primary feline MDM (data not shown). Although severely restricted in primary feline PBMC, replication of FIV-pF34 in primary feline MDM was efficient and similar to that of FIV-pPPR and FIV-14.
Virologic evaluation in cats.
Previous experiments demonstrated a differential ability of molecular clones FIV-pF34 and FIV-pPPR to replicate in cats (29). To address the possibility that differences in vivo replication of the two molecular clones resulted from variation in infectious titer of the viral inocula used in the previous study, inoculation dose of all three molecular clones was standardized for this study. Furthermore, a rigorous sampling schedule was used to reduce the possibility that transient virologic events would be missed.
Clinical and hematological manifestations.
All cats were evaluated clinically for fever, lymphadenopathy, lethargy, inappetance, and weight loss. No significant clinical abnormalities were observed in any cat throughout the study period. Furthermore, no significant hematologic abnormalities or alterations in lymphocyte phenotype percentages (CD4+/CD8+ lymphocyte ratios) were observed (data not shown).
FIV-pF34-inoculated cats.
All FIV-pF34-inoculated cats were virus negative by virus isolation at all time points tested (Table 1). Three cats, 5, 6, and 7, were antiviral antibody positive by 9 weeks p.i. and viral DNA positive in PBMC for one or more time points and in one or more tissues collected postmortem. Cat 8 was seronegative as well as virus isolation and viral DNA negative in peripheral blood; however, provirus was detected in three tissues harvested 23 weeks p.i. (final time point). Cat 10 was viral antibody and DNA negative in all lymphoid tissues tested but was viral DNA positive in PBMC at a single point (12 weeks p.i.). Cat 9 was negative by all assays at all time points tested. The absence of detectable replicating virus in peripheral blood is indicative of severely restricted replication in lymphocytes in vivo and may be directly correlated to the restricted replication of this molecular clone in PBMC in vitro. Interestingly, no cats in this group were viral DNA positive in thymic tissue, while four cats were positive in at least one of the other tissues tested.
TABLE 1.
Virologic evaluation of peripheral blood and tissues of cats inoculated with FIV molecular clones FIV-pF34, FIV-14, and FIV-pPPRa
| Clone used for infection | Cat no. | Result at:
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 wpi
|
6 wpi
|
9 wpi
|
12 wpi
|
15 wpi
|
23 wpi
|
Terminal PCR analysis
|
||||||||||||||||||
| Ab | PCR | VI | Ab | PCR | VI | Ab | PCR | VI | Ab | PCR | VI | Ab | PCR | VI | Ab | PCR | VI | CLN | Thym | Spl | MLN | BM | ||
| FIV-pF34 | 5 | − | − | − | − | − | − | + | + | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − |
| 6 | − | − | − | − | − | − | + | − | − | + | − | − | + | + | − | + | − | − | − | − | − | + | − | |
| 7 | − | − | − | + | + | − | + | − | − | + | − | − | + | − | − | + | + | − | − | − | + | + | + | |
| 8 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | + | |
| 9 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 10 | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | |
| FIV-14 | 11 | − | − | − | + | + | − | + | + | − | + | + | + | + | + | + | + | + | + | + | − | + | − | − |
| 12 | − | − | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | − | + | − | − | |
| 13 | − | − | + | − | + | + | + | + | + | + | − | − | + | − | − | + | − | − | + | + | + | + | + | |
| 14 | − | − | − | − | + | − | − | − | − | + | + | − | + | − | − | + | − | − | + | + | + | − | + | |
| 15 | − | − | − | + | − | − | + | + | − | + | + | − | + | + | − | + | + | − | + | − | + | − | − | |
| 16 | − | − | − | − | − | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | + | − | − | |
| FIV-pPPR | 17 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 18 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | |
| 19 | − | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 20 | − | − | − | − | − | − | + | − | − | + | + | − | − | − | − | + | + | − | − | − | + | + | − | |
| 21 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 22 | + | − | − | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | − | |
Abbreviations: wpi, weeks p.i.; Ab, anti-FIV antibody detected by ELISA; PCR, clone-specific nested PCR for FIV LTR; VI, virus isolation; CLN, cervical lymph node; Thym, thymus; MLN, mesenteric lymph node; BM, bone marrow.
FIV-14-inoculated cats.
All six cats were positive for antiviral antibody by 12 weeks p.i. and for viral DNA in PBMC at two or more time points (Table 1). Of the two cats found to be virus isolation positive, one was positive at later time points (12, 15, and 23 weeks p.i.) only, whereas the other was virus isolation positive at early time points (3, 6, and 9 weeks p.i.). Cervical lymph node and spleen harvested at the terminal time point were viral DNA positive for all six cats, whereas detection of viral DNA was variable in other lymphoid tissues tested.
FIV-pPPR-inoculated cats.
Four of six cats were persistently virus isolation positive by 6 weeks p.i., and five cats were antiviral antibody positive by 9 weeks p.i. Five of six cats were viral DNA positive in PBMC at two or more time points (Table 1). Cat 18 was never positive by any assay in peripheral blood and remained seronegative but was positive for provirus in spleen and mesenteric lymph node harvested at 23 weeks p.i.
In vivo cell tropism of molecular clones.
While FIV-pPPR and FIV-14 proved to be tropic for both primary PBMC and macrophages, FIV-pF34 was found in these studies to be primarily macrophagetropic in vitro. To characterize and compare in vivo cell tropism of these three molecular clones, lymphocyte subpopulations were purified from lymphoid tissues of inoculated cats and analyzed for viral DNA by nested PCR. A partial splenectomy was performed as soon as PBMC were provirus positive by PCR in five cats (between 9 and 14 weeks p.i.). Lymphocyte populations were fractionated and analyzed. Similarly, mesenteric lymph node was evaluated in three cats from each group at the terminal time point. Macrophages harvested later in infection from three cats per group by peritoneal lavage were also tested for viral DNA by nested PCR. Only one FIV-pF34-inoculated cat was evaluated at an early time point, and proviral DNA was localized to CD4+ and CD21+ lymphocytes (Table 2). At 23 weeks p.i., viral DNA was not detected in any lymphocyte subpopulation in the three FIV-pF34-inoculated cats evaluated, although viral DNA was found in unfractionated lymph node mononuclear cells. This finding suggested that a nonlymphoid population may be a reservoir for virus in FIV-pF34-infected cats. Although we did not evaluate fractionated macrophages and dendritic cells from the mesenteric lymph node, macrophages harvested from the peritoneal cavity were tested and found to be provirus positive in all three FIV-pF34-inoculated cats tested. These data suggested FIV-pF34 may transiently infect lymphocytes early in infection, but persistence of infection is maintained in tissue macrophages of the host.
TABLE 2.
In vivo tropism of FIV molecular clones
| Group | Cat no. | Result at:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9–14 wk p.i.a
|
23 wk p.i.
|
|||||||||
| CD4 | CD8 | CD21 | Totalb | CD4 | CD8 | CD21 | Total | Macrophagesc | ||
| pF34 | 5 | + | − | + | + | − | − | − | + | + |
| 6 | NDd | ND | ND | ND | − | − | − | + | + | |
| 7 | ND | ND | ND | ND | − | − | − | + | + | |
| FIV-14 | 11 | + | − | + | + | + | − | − | + | + |
| 13 | ND | ND | ND | ND | + | − | − | + | − | |
| 16 | + | − | − | + | − | − | − | + | − | |
| pPPR | 17 | + | + | − | + | + | + | − | + | ND |
| 19 | ND | ND | ND | ND | + | − | − | + | − | |
| 21 | ND | ND | ND | ND | + | + | + | + | ND | |
| 22 | + | + | + | + | ND | ND | ND | ND | − | |
PCR performed on mononuclear cells from mesenteric lymph node for cats 5 and 6 and mononuclear cells from spleen for all other cats.
Unfractionated mononuclear cells.
Obtained by peritoneal lavage.
ND, not determined.
Early in infection, viral DNA was detected in splenic CD4+ T cells harvested from both FIV-14-infected cats tested, while CD8+ T cells from both cats were found negative for viral DNA (Table 2); CD21+ lymphocytes (B cells) harvested from one of these two cats were also viral DNA positive. At 23 weeks p.i., two of three FIV-14-infected cats tested harbored provirus in CD4+ cells, while all three cats were negative for viral DNA in either CD8+ or CD21+ cells. Provirus in peritoneal macrophages was observed from only one of three FIV-14-infected cats sampled. These observations suggested that FIV-14 may be localized primarily in CD4+ cells in vivo.
Of two FIV-pPPR-inoculated cats evaluated early in infection, both harbored provirus in splenic CD4+ and CD8+ T cells whereas only one was viral DNA positive in CD21+ cells (Table 2). Results were similar at 23 weeks p.i.; however, one of three cats was positive only in CD4+ cells. Viral DNA could not be detected in peritoneal macrophages harvested from either of two FIV-pPPR-infected cats tested. These observations suggested that FIV-pPPR may be primarily lymphocytetropic in vivo and specifically for CD4+ and CD8+ cells.
Emergence of viral variants.
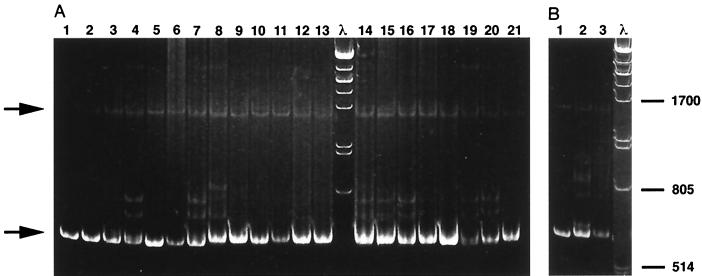
To determine the fidelity of the in vitro-propagated virus preparations used for cat inoculation and to reveal virus variants emerging in vivo after inoculation with FIV clones, the HMA was performed. A retrospective comparison of each virus inoculum to the original molecular clone used to generate the virus stock revealed no detectable variants in FIV-pPPR or FIV-14 inocula used for these studies, whereas variants were observed in FIV-pF34 inocula (Fig. 4). Conditions for preparation of virus stocks for all molecular clones were very similar as far as transfection of provirus plasmid into CrFK cells and duration of virus passage (10 to 14 days). However, FIV-14 and FIV-pPPR virus stocks were passaged in PBMC cocultured with transfected CrFK cells, whereas FIV-pF34 was cultivated in transfected CrFK cells. HMA analysis of the FIV-pF34 virus stock suggests that even relatively short passage in a highly permissive cell line such as CrFK cells may generate env-specific variants of molecular clone FIV-pF34.
FIG. 4.
HMA to identify emergence of viral variants in virus inoculum and FIV molecular-clone-infected cats. (A) PCR amplification products of the FIV env region from cats infected with molecular clone FIV-pPPR (lanes 1 to 7), FIV-14 (lanes 8 to 13), or FIV-pF34 (lanes 14 to 18) were annealed with amplification products from the respective original virus inoculum. The annealed products were separated by electrophoresis on a 5% polyacrylamide gel and stained with ethidium bromide. Lanes 19 to 21 show annealed PCR products from the amplification of FIV plasmid clones PPR and 14, PPR and F34, and 14 and F34, respectively. (B) Lanes 1 to 3 show annealed PCR products from the amplification of the plasmid virus clone used for cell transfections and the virus inoculum used for infection of cats (FIV-pPPR, FIV-pF34, and FIV-14, respectively). Molecular weight markers are in lanes marked λ; positions are indicated in bases. The lower arrow indicates homoduplexes, the upper arrow indicates single-stranded DNA, and heteroduplexes can be seen between the arrows in lanes 4, 7, 8, 14, 15, 16, 19, and of panel A 20 and lane 2 of panel B. The specific cat and tissue evaluated for each lane: lane 1, cat 17, spleen; lane 2, cat 19, spleen; lane 3, cat 22, thymus; lane 4, cat 18, mesenteric lymph node; lane 5, cat 20, mesenteric lymph node; lane 6, cat 21, mesenteric lymph node; lane 7, cat 22, mesenteric lymph node; lane 8, cat 12, cervical lymph node; lane 9, cat 11, spleen; lane 10, cat 13, spleen; lane 11, cat 14, spleen; lane 12, cat 15, spleen; lane 13, cat 16, spleen; lane 14, cat 7, bone marrow; lane 15, cat 5, cervical lymph node; lane 16, cat 6, cervical lymph node; lane 17, cat 7, spleen; lane 18, cat 8, spleen.
Viral DNA from lymphoid tissues, including mesenteric lymph node, spleen, and bone marrow, that yielded the greatest signal for viral DNA at 23 weeks p.i. by PCR was compared to the proviral genome of the virus inoculum given to that group of cats. Variants were found in bone marrow harvested from the three FIV-pF34-inoculated cats that seroconverted and were viral DNA positive in peripheral blood. This was not surprising considering the presence of variants in the inoculum. Variants in lymphoid tissues were found in one (cat 12) of six and two (cats 18 and 22) of six FIV-14- and FIV-pPPR-inoculated cats, respectively. The variant observed with either FIV-pPPR or FIV-14 inoculation was not associated with the status of the inoculated cat with respect to either virus isolation or seroconversion.
DISCUSSION
Previous studies assessing FIV-pF34, FIV-14, and FIV-pPPR have shown these viruses to be unique for both in vitro growth properties and replicative efficiency in vivo (23, 25, 27, 29, 30). However, experimental studies comparing virus replication and host cell tropism of all three cloned isolates in primary cell culture systems, as well as in cats, have not been reported. For in vitro studies, replication of these molecular clones was compared in CrFK cells, a feline adherent cell line, primary feline PBMC, and primary feline MDM. CrFK cells were permissive for both FIV-14 and FIV-pF34 and nonpermissive for FIV-pPPR. Primary feline PBMC were permissive for FIV-pPPR and FIV-14, whereas replication of FIV-pF34 was either severely restricted or absent. These observations are in agreement with previous reports (23, 25, 32, 40) and can be explained by the origin of the viruses and known tropism determinants (34, 38, 39).
FIV-pF34 was originally isolated from CrFK cells chronically infected with CrFK cell-adapted FIV-Petaluma (FIV-PetalumaCrFK) and encodes a premature stop codon within orf-A, a viral determinant reported to be essential for efficient replication in feline primary PBMC (34, 40). In addition, amino acid sequence of the third variable (V3) region of the FIV-pF34 env gene includes an E→K change at position 407 and R at position 397, determinants previously reported for CrFK tropism (39). In contrast, the FIV-pPPR genome was cloned from virus-infected primary feline PBMC, shows 91% nucleotide homology with FIV-pF34 (32), does not encode the E→K change at position 407 within the V3 domain of env, and encodes a full-length orf-A. Similar to FIV-pF34, the FIV-14 genome was molecularly cloned from CrFK cells infected with FIV-PetalumaCrFK and has 99% nucleotide homology with FIV-pF34. FIV-14, however, is unique in its ability to replicate moderately well in both CrFK cells and PBMC. Although FIV-14 encodes a full length orf-A, FIV-14 replication is delayed compared to that of FIV-pPPR in PBMC and modest compared to that of FIV-pF34 in CrFK cells. That FIV-14 does not encode either of two env determinants reported to generate FIV tropism for CrFK cells (E→K at position 407; M→T at position 751) (38, 39) suggests that additional viral determinants for PBMC and CrFK tropism have yet to be characterized.
Surprisingly, all three molecular clones replicated with similar efficiencies in primary feline MDM. Our findings for FIV-pF34 replication in MDM differ from those of a previous report showing that replication of FIV-pF34 in MDM was significantly restricted (40) as a result of the premature truncation encoded within orf-A. Nucleotide sequence analysis of the FIV-pF34 virus stocks used for our studies revealed that the stop codon within orf-A was maintained; thus, reversion to the wild-type sequence was not responsible for our findings of FIV-pF34 replication in macrophages. Based on observations from macrophage infection studies in other lentivirus systems, possible explanations for these conflicting observations include variation in susceptibility to infection of macrophages isolated from different cat donors, different cultivation conditions for primary feline MDM, and/or variable differentiation states of macrophages at time of infection (5, 17, 22, 26, 37).
Virus production from infected macrophages observed with these in vitro studies was usually 10-fold lower than virus production from PBMC. Similar differences in virus replication from infected human macrophages and PBMC have been reported for macrophagetropic isolates of HIV (7, 8, 28). Virus production observed from MDM infected with molecularly cloned FIV isolates contrasts with the severely restricted replication in peritoneal macrophages previously reported for a CrFK cell line-adapted preparation of uncloned FIV-Petaluma (4). The issue of macrophage tropism and virus variation has not been well characterized for FIV, and identification of FIV-encoded molecular determinants specific for macrophage tropism will require well-characterized molecular clones deficient for macrophage replication as well as macrophagetropic isolates.
Inoculation studies with various pathogenic biologic isolates of FIV, including FIV-PetalumaPBMC and FIV-NCSU1, reported detection of proviral DNA and viral RNA in CD4+ T cells, CD8+ T cells, and B lymphocytes by PCR amplification (13, 16, 42), viral RNA in tissue macrophages by in situ hybridization (2), and viral antigen in CD4+ T cells and follicular dendritic cells of lymph nodes (36). Thus, these FIV isolates were shown to possess a broad tropism for different lymphocyte subsets as well as macrophages in vivo. In agreement with previous reports, inoculation of SPF cats with any one of the three FIV clones tested in this study resulted in a minimally pathogenic infection (27, 29, 30) despite differences in ability to induce a persistent viremia.
FIV-pF34 proved least efficient for establishing viremia; only three of six cats seroconverted, and none of the seropositive cats were virus isolation positive at any time point tested. Although mesenteric lymph node sampled from seropositive cats were positive for viral DNA, PBMC were rarely positive for provirus. Proviral DNA was found in CD4+ T-cell and CD21+ lymphocyte subsets in one FIV-pF34-inoculated cat assessed during the acute phase of infection; however, viral DNA was absent from all lymphocyte subsets (CD4+, CD8+, and CD21+) obtained from either mesenteric lymph node or spleen harvested 23 weeks p.i. from each of the three seropositive cats. The presence of viral DNA in peritoneal macrophages harvested from these seropositive cats, and in unfractionated lymph node mononuclear cells as well, also suggested that a nonlymphoid cell population, such as tissue macrophages, may provide a reservoir for virus in FIV-pF34-inoculated cats. These observations of FIV-pF34 infection of macrophages in vivo correlated well with positive growth properties in macrophage cultures demonstrated by this virus. The restricted replication of FIV-pF34 in primary feline PBMC in vitro also correlated well with the absence of replicating virus and proviral DNA in peripheral blood in infected cats.
Of the three clones tested, FIV-pPPR proved most efficient at inducing a detectable virus load, as four of six cats inoculated with this isolate were persistently viremic by virus isolation from peripheral blood throughout the study, and five of six cats seroconverted. All FIV-pPPR-infected cats were viral DNA positive in multiple lymphoid tissues. For those FIV-pPPR-inoculated cats tested either early or late in infection, viral DNA was most consistently detected in the CD4+ and CD8+ lymphocyte subsets and less frequently in the CD21+ lymphoid cells. The frequency of virus isolation from PBMC, viral DNA detection in PBMC, and viral DNA in multiple lymphocyte subsets in FIV-pPPR-inoculated cats correlated directly with the proficiency of this molecular clone to replicate in primary feline PBMC in vitro.
In comparison to FIV-pPPR, FIV-14 was moderately efficient for establishing viremia, as only two of six inoculated cats were virus isolation positive for two or more time points. All six FIV-14-inoculated cats however, seroconverted and were frequently positive for viral DNA in PBMC and multiple lymphoid tissues. The modest efficiency of FIV-14 for inducing a viremia in peripheral blood correlated with the moderate proficiency of this clone for replication in primary feline PBMC in vitro and differed with in vitro and in vivo replication observed for closely related FIV clone FIV-pF34. In contrast to FIV-pPPR infection of cats, viral DNA was not observed in fractionated CD8+ T cells obtained from lymphoid tissues either early or later in FIV-14 infection, although viral DNA was consistently observed in CD4+ T cells and rarely found in CD21+ lymphocytes. Absence of viral DNA in CD8+ T cells sorted from lymphoid tissues differentiated FIV-14 infection from that of FIV-pPPR, which is a clone more proficient for inducing higher virus loads in cats.
The absence of viral DNA in peritoneal macrophages harvested from two FIV-pPPR-inoculated cats was surprising, considering the replication efficiency of FIV-pPPR in MDM in vitro. Similar to FIV-pPPR infection, viral DNA was detected in peritoneal macrophages harvested from only one of three FIV-14-inoculated cats sampled. Considering the small sample number (two to three cats for one time point only), strong conclusions cannot be made regarding the negative findings for FIV-pPPR and FIV-14 macrophage tropism in vivo. Future studies assessing macrophages obtained from lymphoid tissues at multiple time points will be necessary to confirm the absence of either FIV-14 or FIV-pPPR infection of this cell population in vivo.
Comparing replicative capacity in vitro and in vivo with host cell populations targeted by cloned FIV isolates has not been previously reported. Contrasting observations for these minimally pathogenic molecular clones with previous observations reported for pathogenic biological isolates of FIV will help to elucidate viral determinants of pathogenicity. Observations from this study revealed a direct correlation between viral replication efficiency in PBMC in vitro and virus replication in vivo. Proficiency for replication in a feline adherent cell line inversely correlated with in vivo growth properties, whereas positive growth properties in primary feline macrophages in vitro was not predictive for induction of virus load in vivo.
Findings from these studies also indicated that infection of multiple lymphocyte subsets, including both CD4+ and CD8+ T cells, as observed with FIV-pPPR infection may be necessary to establish higher virus loads. Data for distribution of virus in tissue macrophages that were generated from these inoculation studies were too limited to allow us to draw general conclusions but suggested that macrophages may be a primary target for FIV-pF34 infection in vivo. Observations from this study also suggested that macrophages as well as CD21+ lymphocytes (B cells) were not major target cells for infection in cats inoculated with FIV-pPPR and FIV-14. These findings directly contrast with previous observations describing infection of B cells as well as T cells in cats chronically infected with pathogenic isolates of FIV (12, 16) and tissue macrophages as the predominant infected cell in tissues of cats clinically ill with FIV-PetalumaPBMC (2). Restriction in cell tropism in vivo may at least partially account for the lack of pathogenicity of these two FIV cloned viruses, and particularly FIV-pPPR, which was nonpathogenic in the face of a persistent viremia. Future in vivo studies assessing tropism of pathogenic and nonpathogenic FIV clones for tissue macrophages in particular, as well as other cell populations (dendritic cells and lymphocyte subsets), will be crucial for characterizing the role of virus host cell tropism in FIV pathogenicity.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of Jeff Carlson, Kim Floyd-Hawkins, Joanne Higgins, Amy Poland, Harry Louie, Carol Oxford, Alora LaVoy, May Chien, and Steve Ramirez.
This study was supported by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis, and by NIAID grants AI01262 (G.A.D.) and AI34776 (E.E.S.).
REFERENCES
- 1.Barlough J E, Ackley C D, George J W, Levy N, Acevedo R, Moore P F, Rideout B A, Cooper M D, Pedersen N C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquired Immune Defic Syndr. 1991;4:219–227. [PubMed] [Google Scholar]
- 2.Beebe A M, Dua N, Faith T G, Moore P F, Pedersen N C, Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J Virol. 1994;68:3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown W C, Bissey L, Logan K S, Pedersen N C, Elder J H, Collisson E W. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991;65:3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner D, Pedersen N C. Infection of peritoneal macrophages in vitro and in vivo with feline immunodeficiency virus. J Virol. 1989;63:5483–5488. doi: 10.1128/jvi.63.12.5483-5488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang J, Naif H M, Li S, Sullivan J S, Randle C M, Cunningham A L. Twin studies demonstrate a host cell genetic effect on productive human immunodeficiency virus infection of human monocytes and macrophages in vitro. J Virol. 1996;70:7792–7803. doi: 10.1128/jvi.70.11.7792-7803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 7.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collman R, Godfrey B, Cutilli J, Rhodes A, Hassan N F, Sweet R, Douglas S D, Friedman H, Nathanson N, Gonzalez S F. Macrophage-tropic strains of human immunodeficiency virus type 1 utilize the CD4 receptor. J Virol. 1990;64:4468–4476. doi: 10.1128/jvi.64.9.4468-4476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandekar S, Beebe A M, Barlough J, Phillips T, Elder J, Torten M, Pedersen N. Detection of feline immunodeficiency virus (FIV) nucleic acids in FIV-seronegative cats. J Virol. 1992;66:4040–4049. doi: 10.1128/jvi.66.7.4040-4049.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean G A, Quackenbush S L, Ackley C D, Cooper M D, Hoover E A. Flow cytometric analysis of T-lymphocyte subsets in cats. Vet Immunol Immunopathol. 1991;28:327–335. doi: 10.1016/0165-2427(91)90124-u. [DOI] [PubMed] [Google Scholar]
- 12.Dean G A, Reubel G H, Moore P F, Pedersen N C. Proviral burden and infection kinetics of feline immunodeficiency virus in lymphocyte subsets of blood and lymph node. J Virol. 1996;70:5165–5169. doi: 10.1128/jvi.70.8.5165-5169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean G A, Reubel G H, Pedersen N C. Simian immunodeficiency virus infection of CD8+ lymphocytes in vivo. J Virol. 1996;70:5646–5650. doi: 10.1128/jvi.70.8.5646-5650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dow S W, Dreitz M J, Hoover E A. Feline immunodeficiency virus neurotropism: evidence that astrocytes and microglia are the primary target cells. Vet Immunol Immunopathol. 1992;35:23–35. doi: 10.1016/0165-2427(92)90118-a. [DOI] [PubMed] [Google Scholar]
- 15.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D L, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.English R V, Johnson C M, Gebhard D H, Tompkins M B. In vivo lymphocyte tropism of feline immunodeficiency virus. J Virol. 1993;67:5175–5186. doi: 10.1128/jvi.67.9.5175-5186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gendelman H E, Narayan O, Kennedy S S, Kennedy P G, Ghotbi Z, Clements J E, Stanley J, Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986;58:67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George J W, Pedersen N C, Higgins J. The effect of age on the course of experimental feline immunodeficiency virus infection in cats. AIDS Res Hum Retroviruses. 1993;9:897–905. doi: 10.1089/aid.1993.9.897. [DOI] [PubMed] [Google Scholar]
- 19.Hosie M J, Broere N, Hesselgesser J, Turner J D, Hoxie J A, Neil J C, Willett B J. Modulation of feline immunodeficiency virus infection by stromal cell-derived factor. J Virol. 1998;72:2097–2104. doi: 10.1128/jvi.72.3.2097-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael N L, Louie L G, Rohrbaugh A L, Schultz K A, Dayhoff D E, Wang C E, Sheppard H W. The role of CCR5 and CCR2 polymorphisms in HIV-1 transmission and disease progression. Nat Med. 1997;3:1160–1162. doi: 10.1038/nm1097-1160. [DOI] [PubMed] [Google Scholar]
- 21.Novotney C, English R V, Housman J, Davidson M G, Nasisse M P, Jeng C R, Davis W C, Tompkins M B. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990;4:1213–1218. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Olafsson K, Smith M S, Marshburn P, Carter S G, Haskill S. Variation of HIV infectibility of macrophages as a function of donor, stage of differentiation, and site of origin. J Acquired Immune Defic Syndr. 1991;4:154–164. [PubMed] [Google Scholar]
- 23.Olmsted R A, Barnes A K, Yamamoto J K, Hirsch V M, Purcell R H, Johnson P R. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:2448–2452. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 25.Phillips T R, Talbott R L, Lamont C, Muir S, Lovelace K, Elder J H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990;64:4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich E A, Chen I S, Zack J A, Leonard M L, O’Brien W A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Investig. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigby M A, Hosie M J, Willett B J, Mackay N, McDonald M, Cannon C, Dunsford T, Jarrett O, Neil J C. Comparative efficiency of feline immunodeficiency virus infection by DNA inoculation. AIDS Res Hum Retroviruses. 1997;13:405–412. doi: 10.1089/aid.1997.13.405. [DOI] [PubMed] [Google Scholar]
- 28.Schmidtmayerova H, Bolmont C, Baghdiguian S, Hirsch I, Chermann J C. Distinctive pattern of infection and replication of HIV1 strains in blood-derived macrophages. Virology. 1992;190:124–133. doi: 10.1016/0042-6822(92)91198-4. [DOI] [PubMed] [Google Scholar]
- 29.Sparger E E, Beebe A M, Dua N, Himathongkam S, Elder J H, Torten M, Higgins J. Infection of cats with molecularly cloned and biological isolates of the feline immunodeficiency virus. Virology. 1994;205:546–553. doi: 10.1006/viro.1994.1677. [DOI] [PubMed] [Google Scholar]
- 30.Sparger E E, Louie H, Ziomeck A M, Luciw P A. Infection of cats by injection with DNA of a feline immunodeficiency virus molecular clone. Virology. 1997;238:157–160. doi: 10.1006/viro.1997.8787. [DOI] [PubMed] [Google Scholar]
- 31.Sparger E E, Shacklett B L, Renshaw G L, Barry P A, Pedersen N C, Elder J H, Luciw P A. Regulation of gene expression directed by the long terminal repeat of the feline immunodeficiency virus. Virology. 1992;187:165–177. doi: 10.1016/0042-6822(92)90305-9. [DOI] [PubMed] [Google Scholar]
- 32.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tersmette M, Gruters R A, de Wolf W F, de Goede R, Lange J M, Schellekens P T, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomonaga K, Miyazawa T, Sakuragi J, Mori T, Adachi A, Mikami T. The feline immunodeficiency virus ORF-A gene facilitates efficient viral replication in established T-cell lines and peripheral blood lymphocytes. J Virol. 1993;67:5889–5895. doi: 10.1128/jvi.67.10.5889-5895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torten M, Franchini M, Barlough J E, George J W, Mozes E, Lutz H, Pedersen N C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991;65:2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyosaki T, Miyazawa T, Furuya T, Tomonaga K, Shin Y S, Okita M, Kawaguchi Y, Kai C, Mori S, Mikami T. Localization of the viral antigen of feline immunodeficiency virus in the lymph nodes of cats at the early stage of infection. Arch Virol. 1993;131:335–347. doi: 10.1007/BF01378636. [DOI] [PubMed] [Google Scholar]
- 37.Tuttle D L, Harrison J K, Anders C, Sleasman J W, Goodenow M M. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–4969. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahlenkamp T W, Verschoor E J, Schuurman N N, van Vliet A L, Horzinek M C, Egberink H F, de Ronde A. A single amino acid substitution in the transmembrane envelope glycoprotein of feline immunodeficiency virus alters cellular tropism. J Virol. 1997;71:7132–7135. doi: 10.1128/jvi.71.9.7132-7135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verschoor E J, Boven L A, Blaak H, van Vliet A L, Horzinek M C, de Ronde A. A single mutation within the V3 envelope neutralization domain of feline immunodeficiency virus determines its tropism for CRFK cells. J Virol. 1995;69:4752–4752. doi: 10.1128/jvi.69.8.4752-4757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters A K, De P A, Lerner D L, Neil J C, Thompson F J, Elder J H. Influence of ORF2 on host cell tropism of feline immunodeficiency virus. Virology. 1996;215:10–16. doi: 10.1006/viro.1996.0002. [DOI] [PubMed] [Google Scholar]
- 41.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo J C, Dean G A, Pedersen N C, Moore P F. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J Virol. 1997;71:8632–8641. doi: 10.1128/jvi.71.11.8632-8641.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto J K, Sparger E, Ho E W, Andersen P R, O’Connor T P, Mandell C P, Lowenstine L, Munn R, Pedersen N C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1258. [PubMed] [Google Scholar]
- 44.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]