Abstract
Parkinson’s disease (PD), characterized by loss of nigrostriatal dopaminergic neurons, is one of the most predominant neurodegenerative diseases affecting the elderly population worldwide. The concept of stem cell therapy in managing neurodegenerative diseases has evolved over the years and has recently rapidly progressed. Neural stem cells (NSCs) have a few key features, including self-renewal, proliferation, and multipotency, which make them a promising agent targeting neurodegeneration. It is generally agreed that challenges for NSC-based therapy are present at every stage of the transplantation process, including preoperative cell preparation and quality control, perioperative procedures, and postoperative graft preservation, adherence, and overall therapy success. In this review, we provided a comprehensive, careful, and critical discussion of experimental and clinical data alongside the pros and cons of NSC-based therapy in PD. Given the state-of-the-art accomplishments of stem cell therapy, gene therapy, and nanotechnology, we shed light on the perspective of complementing the advantages of each process by developing nano-stem cell therapy, which is currently a research hotspot. Although various obstacles and challenges remain, nano-stem cell therapy holds promise to cure PD, however, continuous improvement and development from the stage of laboratory experiments to the clinical application are necessary.
Keywords: Parkinson’s disease, Synuclein, Neural stem cells, Nanomaterials, Nano-stem cell therapy
Core tip: Neural stem cell (NSC) transplantation is a groundbreaking therapy with therapeutic effects for many neurodegenerative diseases. Parkinson’s disease (PD) is the second most common neurodegenerative movement disorder. The ability of NSCs for neural differentiation has been suggested as an effective mechanism in PD subjected to cell transplantation. Here, the potential therapeutic mechanisms, challenges, nanobased support, and manifestations of NSCs in PD are discussed, along with examples of preclinical and clinical studies.
INTRODUCTION
Parkinson’s disease (PD), with a global prevalence of > 6 million, is the second most common neurodegenerative movement disorder. Currently, no disease-modifying cure is available, and occurrence of PD has been projected to double over the next generation[1]. PD is an age-related disorder affecting about 5% of the population by the age of over 85 years.
The primary pathological property of PD is the presence of abnormal α-synuclein (ASN) in the form of neural inclusions called Lewy bodies, whose toxic effect is hypothesized to contribute to progressive degeneration and cell loss[2,3]. Dopaminergic (DAergic) neurons in the substantia nigra pars compacta specifically undergo degeneration leading to dopamine (DA) and several other biochemical deficits in the nigrostriatal system, resulting in the development of motor symptoms, including bradykinesia, resting tremor, muscular rigidity, and postural instability[4]. Moreover, loss of other types of neurons and the presence of Lewy pathology in many parts of the central, enteric, and autonomic nervous systems have been demonstrated that probably contribute to non-motor manifestations, such as autonomic dysfunction, olfactory impairment, and mood, cognitive or sleep disturbances present in PD patients[5,6].
Dopamine replacement therapy (DRT) is the gold standard treatment for alleviating PD symptoms. However, as the disease progresses, compensatory mechanisms for denervation affect the duration and delay the alleviating effect of DRT in a nonlinear manner[7]. Surgical procedures are also used, including stereotactic ablations or deep brain stimulation. Nevertheless, these approaches only partially manage the severe motor symptoms and alleviate DAergic desensitization supporting DRT in advanced PD patients[8,9]. Aggregation of ASN, besides the gain-of-toxic function, can also result in loss of function, thereby decreasing its functions[10]. Since ASN exerts physiological activity towards newly formed neurons by promoting the development and maturation of dendrites and the spine, depending on the expression level[11], the intraneuronal inclusions of ASN contribute to the PD pathogenesis by also affecting neurogenesis[12]. Therefore, stimulation of endogenous neurogenesis or cell-replacement therapy is considered a therapeutical option against PD neurodegeneration[13]. Accordingly, strategies aiming at rebuilding the pathway and reshaping the brain have been developing[14].
Endogenous neural stem cells (NSCs) exist throughout life and are found in specific areas of the human brain. NSCs exhibiting abilities to self-renew and differentiate into neurons, astrocytes, or oligodendroglia are responsible for restoring brain function under normal circumstances. The regeneration of DAergic neurons from stem cells is considered an alternative treatment for PD[15].
In the early phase of preclinical and clinical studies, foetal ventral midbrain tissues were grafted into the striatum of rats[16,17] and PD patients[18-23]. The foetal DAergic neuron transplants survived, released DA, and improved behavioural deficits in animal models of PD[16,17] and individuals[20,21]. However, apart from these benefits, in some patients, dyskinesia developed[22,23]. Moreover, independent postmortem examinations revealed that the healthy neurons grafted into the brains of PD patients acquired Lewy bodies several years after transplantation[18,19] due to a prion-like mechanism with permissive templating of synucleinopathy progression[1,6]. In this context, DA neuron-based transplant approaches are not curative; nevertheless, they offer a competitive therapeutic effect[5]. The rapid development of cell- and molecular-based research opens new perspectives for cell therapy approaches in managing PD[5]. During the last decade, cumulative research on using different types of stem cells as starting material supports the potential of stem-cell-based DAergic neuron replacement therapy for PD[5]. The considered candidates are NSCs, embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs)[24]. Importantly, translation into the clinic of the stem cells transplantation approach requires avoidance of adverse immunological response and proliferation and related risk of tumorigenesis[5].
This review focuses on the therapeutic potential of NSCs in PD. Accordingly, we give an overview of their use in experimental studies and clinical trials and discuss challenges related to their application, alongside the pros and cons of NSC-based therapy in PD. Given the state-of-the-art accomplishments of stem cell therapy, gene therapy, and nanotechnology, we shed light on the perspective of complementing the advantages of each process by developing nano-stem cell therapy, also using genetically engineered NSCs.
EMERGENCE OF NSCS IN ADULT BRAIN AND ALTERNATIVE SOURCES OF NSCS
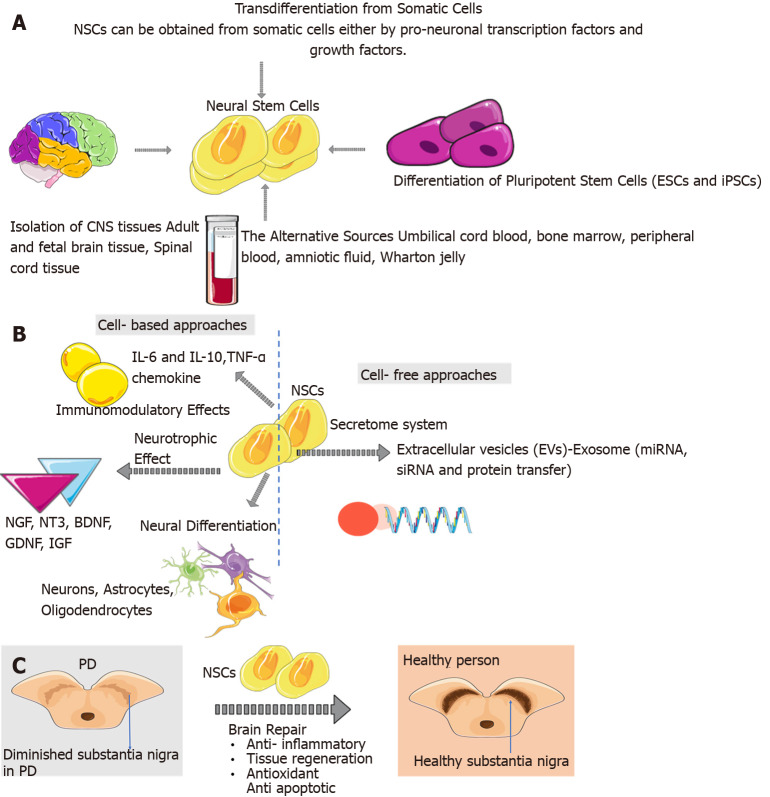
NSCs are an important central nervous system (CNS) element. NSCs exist in the embryonic brain and adult nervous system during human life and are found in two specific brain regions (Figure 1A). The subgranular region of the dentate gyrus and the lateral wall of the subventricular zone both contain a significant number of adult endogenous NSCs[25]. These cells have a few key features, including self-renewal, proliferation, and multipotency, which make them a major component in the CNS, maintaining the cell pool of nervous tissues[15]. NSCs can differentiate into neurons, astrocytes, or oligodendrocytes and may replace the loss of DAergic neurons in PD[13]. Moreover, NSCs can encourage endogenous repair mechanisms as they can migrate near the site of damage to increase neuroblasts and promote tissue repair. NSCs can have immunomodulatory effects such as cytokine, chemokines, and chemokine receptors secretion and T cell proliferation inhibition[26,27] (Figure 1B). Neurotransmitters, neuropeptides, cytokines, metabolites, extracellular matrix proteins, and accessory cells control NSCs proliferation[13].
Figure 1.
Emergence of neural stem cells in the adult brain and alternative sources of neural stem cells. A: The sources of neural stem cells (NSCs) are embryonic stem cells and induced pluripotent stem cells, adult/foetal brain tissue and spinal cord tissue. Also, NSCs can be obtained from somatic cells either by transcription factors and growth factors. The alternative sources of NSCs are foetal and adult nervous systems, umbilical cord blood, bone marrow, peripheral blood, amniotic fluid, Wharton jelly; B: NSCs can have immunomodulatory effects such as cytokine, chemokines, and chemokine receptors secretion and T cell proliferation inhibition. NSCs can have neurotrophic effects such as nerve growth factor, neurotrophin-3, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor. NSCs can target secretome and paracrine effects via extracellular vesicles like exosomes. Also, NSCs play a role in neural differentiation; C: NSCs after their transplantation, could migrate, survive, and proliferate in specific brain sites. And NSCs play a role in brain repair mechanisms such as antiapoptotic, anti-inflammatory, and antioxidant. CNS: Central nervous system; BDNF: Brain-derived neurotrophic factor; ESCs: Embryonic stem cells; EVs: Extracellular vesicles; GDNF: Glial cell line-derived neurotrophic factor; iPSCs: Induced pluripotent stem cells; NGF: Nerve growth factor; NT-3: Neurotrophin-3; NSCs: Neural stem cells; TFs: Transcription factors; PD: Parkinson’s disease; TGF: Transforming growth factor. Citation: The parts of the figures were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/Licenses/by/3.0/).
The alternative sources of NSCs are foetal and adult nervous system tissues, umbilical cord blood, bone marrow, peripheral blood, amniotic fluid, Wharton jelly, skin- or adipose-derived MSCs, or iPSCs[28] (Figure 1A). In the transplantation procedure, these stem cells are either in vitro pre-differentiated or, according to the concept that signals in the injured tissue will recruit the stem cells and induce their differentiation in situ, NSCs are applied directly in the undifferentiated state[28]. Recently, trans-differentiation, especially chemically induced, whereby somatic cells are reprogrammed into patient-specific neuronal cells by skipping an intermediate proliferative pluripotent stem cell stage, offers perspectives on alternative autologous cell therapeutic strategies for PD[29]. By producing the desired type of CNS cells, these sources have all been used to demonstrate their potential for treating various neurodegenerative diseases[30].
EXPERIMENTAL APPROACHES TO NSC TRANSPLANTATION
Several preclinical studies have shown that different types of human NSCs, after their transplantation, could migrate, survive, and proliferate in specific brain sites. Immunohistochemical examinations support evidence that the transplanted human NSCs could transform into functional neurons and integrate into neural circuits[31]. In in vivo studies, it has been demonstrated that NSCs transplantation was an efficient therapeutic method for PD (Table 1). Yang et al[32] have shown that the clonal line C17.2 of NSCs grown in vitro under control conditions and transplanted into intact or 6-hydroxydopamine (6-OHDA)-lesioned rats spontaneously developed into neuronal-like cells, expressed traits of a DA phenotype, and integrated into the host brain[32]. Svendsen et al[33] have demonstrated that expanded populations of human CNS progenitor cells maintained in a proliferative state in culture and can migrate and differentiate into both neurons and astrocytes following intracerebral grafting into the striatum of adult rats with unilateral DAergic lesions[33]. NSCs, secreting in vitro nerve growth factor and neurotrophin-3, engrafted into the 6-OHDA lesioned striatum of rats, have been reported to survive and partially differentiate into tyrosine hydroxylase (TH)-positive cells. A significant rotational behaviour improvement accompanied these effects[33].
Table 1.
Experimental studies on the therapeutic role of neural stem cells in Parkinson’s disease models
|
Cells type
|
Model
|
Effects
|
Ref.
|
| Undifferentiated NSCs line C17.2 | 6-OHDA rat model | Striatum deposition | Yang et al[32] |
| ↑ Expression of β-tubulin III, NSE, NeuN | |||
| ↑ TH- and AADC-positive cells | |||
| ↓ Motor behaviour deficits | |||
| hCNSPCs | 6-OHDA rat model | Striatum deposition | Svendsen et al[33] |
| ↑ Expression of β-tubulin III | |||
| ↑ TH- and hGFAP-positive cells | |||
| ↓ Motor behavior deficits | |||
| hNSCs | 6-OHDA mice model | Striatum deposition | Zuo et al[35] |
| ↓ IL-1 β, IL-2, and TNF-a | |||
| ↑ IL-10 | |||
| ↓ Motor behavior deficits | |||
| hfNSC line (HB1.F3) | 6-OHDA rat model | Striatum deposition | Yasuhara et al[37] |
| ↑ Expression of nestin, HuD, β-tubulin III-positive, MAP2-positive, and NeuN positive and HuC negative | |||
| ↑ TH-positive cells | |||
| ↓ Motor behavior deficits | |||
| hNFG-GFP-OBNSCs | 6-OHDA rat model | Striatum deposition normal histoarchitecture of the striatum | Marei et al[38] |
| ↑ Striatal neurons | |||
| ↓ Necrotic cells | |||
| ↓ Motor behavior deficits | |||
| human dental papilla-derived stem cells and hbNSCs | 6-OHDA rat model | Striatum deposition | Yoon et al[40] |
| ↑ MAP2- positive cells | |||
| ↑ TH- and GIRK2-positive cells | |||
| ↔ No differences behavioral amelioration | |||
| hbNSCs | 6-OHDA rat model | Striatum deposition | Shin et al[41] |
| ↑ TH- and human β2 microglobulin-positive cells | |||
| ↔ No differences behavioral amelioration | |||
| hNSCs | MPTP-induced monkeys | Striatum deposition | Redmond et al[43] |
| ↑ TH- and DAT- positive cells | |||
| ↑ DA level | |||
| ↓ Motor behavior deficits | |||
| hpNSCs | MPTP-induced monkeys | Striatum deposition | Gonzalez et al[44] |
| ↑ Striatal DA concentration, TH- positive cells, TH fiber innervation | |||
| ↓ Behavior deficit |
6-OHDA: 6-hydroxydopamine; AADC: Aromatic L-amino decarboxylase; DA: Dopamine; DAergic: Dopaminergic; DAT: Dopamine transporter; GIRK2: G Protein-activated inward rectifier potassium channel 2; hNSCs: Human NSCs; hbNSCs: Human brain-derived NSCs; hfNSCs: Human fetal-derived NSCs; hGFAP: Human glial fibrillary acidic protein; hNFG-GFP-OBNSCs: Olfactory bulb neural stem cells (OBNSCs) genetically engineered to express hNGF and GFP; hpNSCs: Human parthenogenetic stem cell-derived NSCs; HuC: Very early neuronal marker; HuD: Postmitotic neuronal marker; MAP2: Microtubule-associated protein 2; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NSE: Neuron-specific enolase; NeuN: Matured neuronal marker; NPCs: Neural progenitor cells; TH: Tyrosine hydroxylase; TNF-α: Tumor necrosis factor- α.
The ability of NSCs for neural differentiation and to exert a trophic effect by secreting nerve growth factor and neurotrophin-3 on dying DA neurons in the striatum has been suggested as an effective mechanism against behavioural deficits in PD rats subjected to cell transplantation[34]. A high-throughput quantitative proteomic study revealed that the protein profile of the subventricular zone severely distorted in mice by 6-OHDA injection was normalized and striatal astrocytes were activated after human NSC transplantation accompanied by an increase in neurotrophic factors. In addition, the grafted mice exhibited improved behavioural performance. The changes in the proteome have been suggested to result from the stimulation of host nigral DAergic neurons and their nigrostriatal projections rather than their replacement[35]. To increase trophic factor secretion, genetically engineered human NSCs were developed[36]. Stereotaxic transplantation of human NSCs (HB1.F3 clone) secreting stem cell factor into the 6-OHDA-lesioned striatum of rats has been demonstrated to result in functional improvements and ameliorated Parkinsonian behavioural symptoms. It was accompanied by the activation of endogenous neurogenesis in the subventricular zone, alongside the preservation of TH-positive cells of the nigrostriatal pathway[37]. Similarly, human olfactory bulb NSCs genetically engineered to express human nerve growth factor ameliorated the cognitive deficits associated with 6-OHDA-induced lesions in PD model rats. Transplanted cells exhibiting enhanced survival and differentiation rate migrated to damaged areas to promote repair or neuroprotection through cell replacement, integration, and/or neuroprotection[38]. Foetus-derived human NSCs (hVM1 clone 32) transplanted in their undifferentiated state protected against motor and nonmotor deficits in middle-aged parkinsonian mice, although degeneration of the nigrostriatal pathway was not significantly improved. Thus, the multifactoriality of NSCs was suggested[39]. Yoon et al[40] have reported that although the human-brain-derived NSCs injected into the striatum of rats with the 6-OHDA-induced lesion of the nigrostriatal pathway differentiated into DAergic neurons, no functional or behavioural improvements were observed[40]. Transplantation of human-brain-derived NSCs in the form of cell aggregates with a pre-established cell network into the striatum of 6-OHDA-injected rats appeared to be more effective than single-cell transplantations. The transplanted cells not only survived but also exerted a therapeutic effect attributed to secreting DA[41].
Nonhuman primate models of PD represent the highest clinical translational value due to close-to-human similarities in physiology, anatomy, and immunology and are used to validate therapeutical strategies at preclinical stages[42]. Successful transplantation of undifferentiated human NSCs into the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-challenged primates promoted homeostatic adjustments attributed to normalization of endogenous neuronal number-to-size ratios and protection of host nigrostriatal circuitry that resulted in behavioural improvement. In addition, the percentage of cells containing ASN inclusion dropped from 80% to 20% upon human NSC transplantation. Importantly, no tumours, overgrowth, or inappropriate stem cell migration in the transplanted primates were observed[43]. Gonzalez et al[44] evaluated the safety and efficacy of two doses of human parthenogenetic stem-cell-derived NSCs injected in the striatum and substantia nigra of MPTP-lesioned monkeys with moderate to severe PD symptoms. They found that human parthenogenetic NSCs engrafts promoted behavioural recovery accompanied by raised striatal DA concentration, fibre innervation, and several DA neurons. Increased expression of genes and pathways downregulated in PD has been observed. Importantly, the 12 mo post-transplantation of human parthenogenetic NSCs was safe and well tolerated by the animals with no serious adverse events[44]. This safety aspect is significant as the potential for inappropriate stem cell migration and tumour formation must be minimized before cell transplantation[5].
Stem cell therapeutic strategies for PD not only deal with the direct replacement of damaged or lost cells but also target secretome and paracrine effects on neurotrophic and growth factors and endogenous neural precursor cell activity[45,46] (Figure 1B). The heterogeneous population of membrane-bound exosomes and extracellular vesicles (EVs) is a part of the secretome system[45]. Nanosized EVs derived from human iPSC (hiPSC)-NSCs have been suggested as a safer alternative to NSCs. Upadhya et al[47] demonstrated that EVs exhibited similar neuroreparative properties as NSCs, and they could be administered noninvasively as an autologous or allogeneic off-the-shelf product. EVs comprising miRNAs and/or proteins promoting synaptogenesis, synaptic plasticity, and better cognitive function have been demonstrated to incorporate well into neurons, microglia, and astrocytes of rodents after intranasal treatment, resulting in enhancement of hippocampal neurogenesis and an anti-inflammatory effect[47]. The results hold the promise of using stem-/progenitor-cell-derived EVs, or EVs loaded with bioactive molecules such as DA, catalase, curcumin, and siRNAs, to alleviate parkinsonian symptoms[48]. Specifically, exosomes biologically active EVs, with high miR-133b, which are lacking in PD patients, can promote the growth of neurites[49]. Lee et al[50] have demonstrated that NSC-derived EVs via a paracrine signalling-based mechanism exerted antioxidant, antiapoptotic, and anti-inflammatory effects, thereby protecting against loss of neuronal population and function of target cells, both in vitro and in vivo PD models[50]. Nevertheless, isolation, characterization, and testing of the biological properties of EVs retain critical issues for translation[47]. An emerging alternative to the cell-based approach is the nanotechnology approach for the targeted delivery of growth factors aiming to support and expand resident CNS stem cells for endogenous repair[51].
PROS AND CONS OF THE USE OF NSCs IN PD
Injected neural progenitor cells (NPCs) accumulate in the brain and integrate with the injured zone; however, in only a small percentage, most of the cells remained undifferentiated. The promotion of tissue survival is associated with anti-inflammatory and antiapoptotic effects at both mRNA and protein levels and increased secretion of growth factors[52] (Figure 1C). Additionally, NPCs cooperate with nearby immune and resident cells of the CNS to modulate the focal release of stem cell regulators and aid in the functional recovery from CNS injuries[53,54]. As Marsh and Blurton-Jones[55] have reviewed, there is strong evidence that NSC-mediated changes in neurotrophies resulting in increased synaptic plasticity, long-term potentiation, and neuronal survival contribute to the improvement of cognitive and motor performance observed in vivo. Thus, NSCs providing neurotrophic support to affected neuronal populations and synapses is a promising approach for treating neurodegenerative diseases such as PD[55]. Safety, including a potential tumorigenic effect, raised concerns. Amariglio et al[56] (2009) have reported a donor-derived brain tumour in a boy with ataxia telangiectasia following intracerebellar and intrathecal injection of human foetal NSCs[56]. Moreover, the complex issue of the immune rejection of transplanted cells in the brain has been demonstrated[5]. Therefore, the development of stem-cell-derived neuronal transplant therapies in PD requires consideration of this safety aspect alongside the effectiveness ones, which can be managed by addressing issues related to type and source of cells, their reproducible generation, accurate zone of transplantation and maintenance of function and viability of these grafted cells should be considered[5].
Considering transplantation safety, protocol standardisation, and the ethical aspect, approaches with the use of ESCs and IPSCs for making DAergic neurons are being developed[5,14].
ESCs, undifferentiated pluripotent cells derived from mammalian blastocysts, can make all types of cells in the body. Also, iPSCs, laboratory-generated counterparts of ESCs, that are generated from adult somatic cells, possess similar properties and can differentiate into any other cell. ESCs and iPSCs are used in many clinical trials are going on globally and have wide applications in regenerative medicine for specific diseases and conditions. The results of these trials do not, however, reach the general population because of the long and complex time frame needed to complete the clinical trials and publish the data obtained[57].
iPSCs have some advantages, such as obtaining the reprogrammed cells directly from the patients, thus potentially reducing the risk of transmissible infections and immune reactions. Additionally, they can change into any type of cell approach to treating PD[58]. Using iPSCs for treating PD is ethically permissible because patients’ somatic cells can be differentiated into a pluripotent state to produce DAergic neurons implanted into the brain. Several investigations elucidated the promising role of iPSCs in regenerating DAergic neurons for treating PD. Transplantation of iPSCs ameliorated PD symptoms[58]. iPSCs have some disadvantages, such as powerful pluripotency; hence the risk of tumour development of iPSCs may be greater than other stem cells. The heterogeneity of iPSCs for clinical therapy could lead to potential tumorigenicity risks in vivo. Additionally, iPSCs made from autologous PD patients may contain pathogenic gene mutations that impact the outcome of cell replacement therapy[59]. Although grafted autologous iPSCs cells are considered immune-privileged, they can still be recognized as foreign (allogeneic) by the patient’s immune system in some situations. Cell preparation protocol and transplantation site have been suggested to influence iPSC-derived cell immunogenicity[60]. Hence, decreasing the heterogeneity and increasing the controllability of iPSCs and cell preparation through isolation, purification, amplification, and modification of stem cells is necessary to decrease the tumorigenicity and immunogenicity potentials[61].
Although these scalable and traceable sources with well-established protocols and the efficacy in preclinical PD models have made ESCs and iPSCs promising candidates for cell-based therapy (Table 2), the risks of tumour development and immunosuppression have not been eliminated. These sources are still not ethically neutral, especially ESCs, as the embryo is used in the persevering protocol[5,14].
Table 2.
Experimental studies on the therapeutic role of alternative stem cell sources in Parkinson’s disease models
|
Stem cells source
|
Model
|
Effects
|
Ref.
|
| hPESC line | MPTP-induced monkeys | Striatum deposition | Wang et al[93] |
| Q-CTS-hESC-1 | ↑ DA level | ||
| ↑ Expression of TH-, hNCAM and GIRK2-positive cells | |||
| ↑ Behavioral improvement | |||
| NPCs | 6-OHDA rat model | ↑ DA level | Song et al[94] |
| ↑ Nurr1 and Foxa2 transcription factors | |||
| Improvement in behavior is not mentioned | |||
| hiPSC lines | 6-OHDA rat model | Striatum deposition | Song et al[95] |
| ↑ DAergic neurons density | |||
| ↑ Behavioral amelioration | |||
| MSCs | 6-OHDA rat model | ↑ TH-positive fibers in the striatum and TH-positive neurons in the SNpc | Wang et al[96] |
| ↑ Therapeutic effects of SDF-1α | |||
| ↑ Behavioral amelioration | |||
| hMSCs | 6-OHDA rat model | striatum deposition | Cova et al[97] |
| ↑ DAergic neuron density | |||
| ↑ Ki67/ PCNA – positive cells level | |||
| ↑ Dcx expression | |||
| None of the grafted cells were TH- or DAT-positive | |||
| Improvement in behavior is not mentioned |
6-OHDA: 6-hydroxydopamine; DA: Dopamine; DAergic: Dopaminergic; DAT: Dopamine transporter; Dcx: Double cortin; GIRK2: G protein-activated inward rectifier potassium channel 2; hMSCs: Human adult MSCs; hiPSC: Human iPSC; hPESC: Human parthenogenetic ESCs; hNCAM: Human specific neural cell adhesion molecule; PCNA: Proliferating cell nuclear antigen; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NPCs: Neural progenitor cells; Ki67: Proliferating cell nuclear antigen, SDF-1α: Stromal cell-derived factor 1α; SNpc: Substantia nigra pars compacta; TH: Tyrosine hydroxylase.
Despite these great efforts, there is still a gap between experimental therapeutic approaches and their translation into clinical practice. Although several clinical trials (Table 3) have been studied using cell-based treatments for PD (Clinical Trials. gov; NCT03119636[62], NCT05635409[63], NCT02452723[64], NCT03815071[65], UMIN000033564[66], NCT03128450[67], NCT03309514[68], NCT01898390[69], NCT02795052[70], NCT00976430[71], NCT03724136[72], NCT03684122[73]) these days, the main obstacles restricting the clinical use of stem cells refer mainly to ethical concerns, immune response, tumorigenesis, and toxicity[31].
Table 3.
Clinical trials for cell-based therapy in Parkinson’s disease
|
Study identifier
|
Cell type
|
Aim of the study
|
| NCT03119636[62] | hESC-NPCs | Safety and efficacy evaluation of intracerebral transplantation |
| NCT05635409[63] | hESC-DA neurons | Safety and tolerability evaluation of intraputamenal transplantation |
| NCT02452723[64] | ISC-hpNSC® | Safety evaluation of intracerebral transplantation |
| NCT03815071[65] | iPS-NSCs | Safety and efficacy evaluation |
| UMIN000033564[66] | iPSC-derived dopaminergic progenitors | Safety and efficacy of transplantation into the corpus striatum |
| NCT03128450[67] | hNSCs | Safety and efficacy evaluation of nasal delivery |
| NCT03309514[68] | NSC-derived neurons | Safety and efficacy evaluation of intracerebral injection |
| NCT01898390[69] | hVMT | Safety and efficacy evaluation |
| NCT02795052[70] | BMSC | Efficacy evaluation of intravenous and intranasal delivery |
| NCT00976430[71] | BMSC | Safety and efficacy evaluation |
| NCT03724136[72] | BMSC | Efficacy evaluation of intravenous and intranasal delivery on cognitive impairment |
| NCT03684122[73] | Umbilical cord derived MSCs differentiated into NSCs | Safety evaluation of intrathecal and intravenous injections |
BMSC: Bone-marrow-derived stem cell; iPS-NSC cells: Pluripotent stem-cell-derived neural stem cells; ISC-hpNSC®: Human parthenogenetic stem-cell-derived neural stem cells developed by international stem cell corporation; hNSCs: Human neural stem cells; hVMT: Human ventral mesencephalon tissue; hESC-NPCs: Human embryonic stem cells-derived neural precursor cells.
PROSPECTS FOR NSC-BASED THERAPY
From the short review above, several challenges for NSC-based therapy emerge, which can be identified at every stage of the neurosurgical procedure, including preoperative cell preparation and quality control, perioperative procedures, and postoperative graft preservation, adherence, and overall success of the therapy[74].
Strict quality control and safety assessments of the relevant cells must be in place at the preoperative stage. To achieve the optimal number of DAergic neurons, the required number of implanted cells in the final graft should be achieved[74]. The DA neurons can be derived from different stem cells or directly from NSCs. The use of foetus-derived NSCs has shown outstanding potential in rodent models of PD, and the possibility for tumorigenicity is minimal[75]. Safety issues related to the risk of tumorigenesis by grafted stem cells need to be evaluated for the long-term survival and phenotype stability of stem-cell-derived neurons in the graft following transplantation to identify the appropriate stem cell type. In addition, cell dose, case selection, and delivery route are also necessary as a precondition for the clinical use of cell-based therapies[76]. Patients receiving treatment with NSCs products are at risk for safety issues related to delivery methods, immune and allergic reactions to the medication, tumour development, heterotopic neuronal differentiation with functional disruption, and viral and microbial contamination[26]. Therefore, the fundamental concern of cell-based therapies is that before entering the clinic, transplantable cell populations must be differentiated and characterized according to good manufacturing practice regulations both in vitro and in vivo[77]. This, in turn, requires access to a large pool of differentiated cells stored in a tissue bank. However, providing sufficient in vitro differentiation of neurons from NSCs is challenging[78]. Cell origin is a significant problem that must be considered in treating PD by NSC transplantation as the high concentration of NSCs is needed to treat diseases[79].
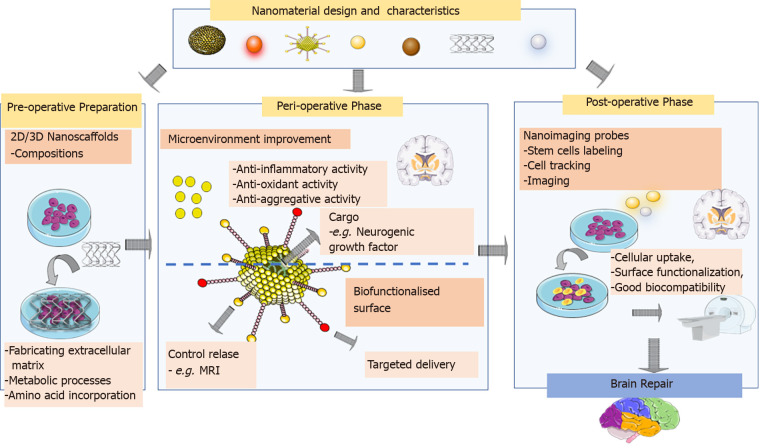
For the promotion of the behaviour of NSCs, the fabrication of culture surfaces is developed. In this context, nanomaterials, with their nano size, unique physicochemical properties, and capability for surface functionalization, offer the potential for designed manipulation of cell behaviour. Fabricating the extracellular matrix with biocompatible nanomaterial-based scaffolds with topology simulating the microenvironment in vivo seems to be a promising strategy. Pandanaboina et al[78] demonstrated successful differentiation of embryonic rat NSCs on the nanocellulose coupled with lysine scaffold as 87% of the cells attached to the surface, and more than half of the NSCs had differentiated into functional neurons[78]. Previously they found that plasmonic gold nanorods integrated into growth surfaces tuneable stimulated and modulated embryonic rat NSCs[80]. A robust scaffold for culturing NSCs in vitro is three-dimensional graphene (3DG) foam, which behind supporting their growth, maintains cells in an active proliferative state through the modulation of metabolism and several metabolic processes. 3DG-stimulated pathways are involved in amino acid incorporation and glucose metabolism[81] (Figure 2).
Figure 2.
Application of nanomaterials in stem cell therapy of Parkinson’s disease. Preoperative preparation: Nanomaterials, with their small size, unique physicochemical properties, and capability for surface functionalisation, offer the potential for designed manipulating cell behaviour. A promising strategy is that fabricating the extracellular matrix with biocompatible nanomaterial-based scaffolds with topology simulating the microenvironment. Preoperative phase: nanomaterials capable of suppressing oxidative stress, neuroinflammation, and toxic protein aggregation can act as bioactive nanomedicines addressing the limitations of cell-based therapy for Parkinson’s disease. Moreover, “intelligent” nano-drug delivery systems functionalised by targeting ligands with the controlled release of loaded molecules like growth factor aims to support and expand stem cells for brain repair. At the postoperative stage, nanomaterials, with their unique optical properties, cellular uptake, surface functionalisation, and good biocompatibility, are promising candidates for cell tracking and imaging. MRI: Magnetic resonance imaging. Citation: The parts of the figures were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/Licenses/by/3.0/).
As NSCs are regulated by a specialized vasculature during adult neurogenesis, transplanted, they may be importantly affected by the adjoining vasculature and endothelial cells[76]. Chou et al[82] have shown the importance of establishing an appropriate neurovascular microenvironment to deliver NSCs to replace lost DA neurons. This study indicated the molecular and cellular signalling involved in forming vasculature-like structures and increased neuronal differentiation through endothelial signalling[82]. The ability to properly locate and track transplanted NSCs is necessary to develop the efficacy of NSC therapies and ensure the treatment's safety[25].
A critical step for efficient NSCs therapy is the survival and integration of the grafted DAergic neurons, due to the lack of both stromal and growth factor support of the cells and the risk of ischaemic stress and an innate immune response[51]. Prion-like transmission synucleinopathy to the healthy neurons grafted into the brains of PD patients should be considered[18,19,21]. Nanomaterials capable of suppressing oxidative stress[83], neuroinflammation[84], or even toxic protein aggregation[85] can act as bioactive nanomedicines addressing the limitations of cell-based therapy for PD. The strategy of combining nano-enabled therapy with stem-cell-based therapy shows potential for the treatment of PD[46]. Accordingly, NSCs isolated from human olfactory bulb human coengrafted with carbon nanotubes (CNTs) restored cognitive deficits and neurodegenerative changes in the trimethyl-induced rat model of neurodegeneration. Moreover, the CNTs supported engrafted NSCs by stimulating differentiation into neurons rather than glial cells[86]. To support individual cells over the first few weeks at the postgrafting stage, attached growth-factor-loaded nanoparticles (NPs) are directly to the cell before grafting has been developed[51]. Nanomaterial carriers can be designed into “intelligent” nanodrug delivery systems functionalised with targeting ligands with the controlled release of loaded drugs/genes/cells for effective transport of them to inaccessible and specific brain areas[46]. Preclinical data have supported the safety and efficacy of the surrogate NPs stroma as no adverse effects after injection of stroma/cell constructs into the brain were evident, and human foetal ventral mesencephalic cell survival in vivo was increased fourfold[87]. At the postoperative stage, monitoring the injected/transplanted cells is critical for the optimisation of the neurosurgical procedure. Nanomaterials, with their unique optical properties, cellular uptake, surface functionalisation, and good biocompatibility, are promising candidates for cell tracking and imaging[46] (Figure 2).
Advances in the development of stem cell and gene therapies create new opportunities. The combination of stem cell and gene therapy could be a technical breakthrough that increases the therapeutic effectiveness of stem cells. Engineered cells overexpressing genes involved in DA synthesis or neurotrophic factors might increase their functional capability and solve differentiation and survival issues, thus improving gene therapy's efficacy[88]. Taking into account the safety of cell-based therapy the cells can be reprogrammed to avoid as many adverse effects as possible, including immune reactions and tumours[89].
CHALLENGES FOR NANO-STEM CELL THERAPY
Nano-stem cell therapy can face many challenges, mainly related to the safety profile of NPs, including potential cytotoxicity and unknown impact on stem cell differentiation and biodegradability. There is ample scope for further research for understanding mechanisms by which cells interact with nanomaterials, the biotransformation of NPs, and their influence on cell functions, making it challenging to control the safety and effectiveness of nano-cell therapy[46,90]. Since the activity of NPs strongly depends on their sizes, structures, shapes, and surface chemistry and is cell type dependent[91], several problems related to methods for the synthesis, bio-functionalisation, characterisation, and tailoring of 3D nanostructures in tissue engineering should be addressed[46,90].
Given the challenges and state-of-art accomplishments of nanotechnology, it can be expected to lead to the development of nano-stem cell therapy for PD. It is supported by recently merged multifunctional nanotheragnostic approaches for effective stem cell therapy in managing brain cancers[92].
CONCLUSION
PD significantly affects society because it carries a mounting socioeconomic burden, while no cure is currently available. The studies discussed in this review have shown the potential of NSC-based therapy for PD (Figure 1C). Although NSCs present major promise in treating this neurodegenerative disease, they raise significant concerns. They may form a tumour, which is a more devastating condition than PD. Moreover, immunological rejection, ethical issues, limited cell sources, and complications related to skill to maturation into desired neuronal cell types and proper differentiation are important reasons for further research to facilitate NSC therapy in PD.
Currently, combining stem cell therapy, including genetically engineered cells, with nanotechnology approaches to complement each other’s advantages provides new insights into the improving the therapy protocol and efficacy. The safety profile and the efficacy of nano-stem cell therapy in treating neurodegenerative diseases is a research hotspot. It is believed that future advancements in new nanomaterials will surely benefit NSCs-based therapy from this technology.
In conclusion, although various obstacles and challenges remain, nano-stem cell therapy holds promise to cure PD. Clearly, its continuous improvement and development from the stage of laboratory experiments to the clinical application are necessary.
ACKNOWLEDGMENTS
We would like to thank their appreciation to all the scientists whose previous work contributed to this review article.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Royal Society of Chemistry, 719492; IEEE, 97680059; FENS, FENS23081; Polish Neuroscience Society, PL90031; Eurotox/Polish Society of Toxicology.
Peer-review started: February 28, 2023
First decision: April 19, 2023
Article in press: May 16, 2023
Specialty type: Cell and tissue engineering
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Toledano A, Spain; Wang G, China S-Editor: Li L L-Editor: Kerr C P-Editor: Li L
Contributor Information
Tuba Oz, Department of Toxicology, Poznan University of Medical Sciences, Poznan 60-631, Poland.
Ajeet Kaushik, NanoBioTech Laboratory, Health System Engineering, Department of Environmental Engineering, Florida Polytechnic University, Lakeland, FL 33805, United States; School of Engineering, University of Petroleum and Energy Studies, Dehradun 248007, India.
Małgorzata Kujawska, Department of Toxicology, Poznan University of Medical Sciences, Poznan 60-631, Poland. kujawska@ump.edu.pl.
References
- 1.Tolosa E, Garrido A, Scholz SW, Poewe W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol . 2021;20:385–397. doi: 10.1016/S1474-4422(21)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerri S, Mus L, Blandini F. Parkinson's Disease in Women and Men: What's the Difference? J Parkinsons Dis . 2019;9:501–515. doi: 10.3233/JPD-191683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman MU, Bilal M, Shah JA, Kaushik A, Teissedre PL, Kujawska M. CRISPR-Cas9-Based Technology and Its Relevance to Gene Editing in Parkinson's Disease. Pharmaceutics . 2022;14 doi: 10.3390/pharmaceutics14061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kujawska M, Jourdes M, Witucki Ł, Karaźniewicz-Łada M, Szulc M, Górska A, Mikołajczak PŁ, Teissedre PL, Jodynis-Liebert J. Pomegranate Juice Ameliorates Dopamine Release and Behavioral Deficits in a Rat Model of Parkinson's Disease. Brain Sci . 2021;11 doi: 10.3390/brainsci11091127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbuti PA, Barker RA, Brundin P, Przedborski S, Papa SM, Kalia LV, Mochizuki H MDS Scientific Issues Committee. Recent Advances in the Development of Stem-Cell-Derived Dopaminergic Neuronal Transplant Therapies for Parkinson's Disease. Mov Disord . 2021;36:1772–1780. doi: 10.1002/mds.28628. [DOI] [PubMed] [Google Scholar]
- 6.Kujawska M, Jodynis-Liebert J. What is the Evidence That Parkinson's Disease is a Prion Disorder, Which Originates in the Gut? Int J Mol Sci . 2018;19 doi: 10.3390/ijms19113573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kujawska M, Bhardwaj SK, Mishra YK, Kaushik A. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson's Disease Diagnostics. Biosensors (Basel) . 2021;11 doi: 10.3390/bios11110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss D, Volkmann J, Fasano A, Kühn A, Krack P, Deuschl G. Changing Gears - DBS For Dopaminergic Desensitization in Parkinson's Disease? Ann Neurol . 2021;90:699–710. doi: 10.1002/ana.26164. [DOI] [PubMed] [Google Scholar]
- 9.Kujawska M, Kaushik A. Exploring magneto-electric nanoparticles (MENPs): a platform for implanted deep brain stimulation. Neural Regen Res . 2023;18:129–130. doi: 10.4103/1673-5374.340411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma M, Burré J. α-Synuclein in synaptic function and dysfunction. Trends Neurosci. 46:153–166. doi: 10.1016/j.tins.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziewczapolski G, Lie DC, Ray J, Gage FH, Shults CW. Survival and differentiation of adult rat-derived neural progenitor cells transplanted to the striatum of hemiparkinsonian rats. Exp Neurol . 2003;183:653–664. doi: 10.1016/s0014-4886(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 12.Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol . 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- 13.Salmina AB, Kapkaeva MR, Vetchinova AS, Illarioshkin SN. Novel Approaches Used to Examine and Control Neurogenesis in Parkinson's Disease. Int J Mol Sci . 2021;22 doi: 10.3390/ijms22179608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Tang L, Tang X. Current Developments in Cell Replacement Therapy for Parkinson's Disease. Neuroscience . 2021;463:370–382. doi: 10.1016/j.neuroscience.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Suksuphew S, Noisa P. Neural stem cells could serve as a therapeutic material for age-related neurodegenerative diseases. World J Stem Cells . 2015;7:502–511. doi: 10.4252/wjsc.v7.i2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brundin P, Nilsson OG, Strecker RE, Lindvall O, Astedt B, Björklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson's disease. Exp Brain Res . 1986;65:235–240. doi: 10.1007/BF00243848. [DOI] [PubMed] [Google Scholar]
- 17.Brundin P, Strecker RE, Widner H, Clarke DJ, Nilsson OG, Astedt B, Lindvall O, Björklund A. Human fetal dopamine neurons grafted in a rat model of Parkinson's disease: immunological aspects, spontaneous and drug-induced behaviour, and dopamine release. Exp Brain Res . 1988;70:192–208. doi: 10.1007/BF00271860. [DOI] [PubMed] [Google Scholar]
- 18.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med . 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 19.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord . 2008;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 20.Peschanski M, Defer G, N'Guyen JP, Ricolfi F, Monfort JC, Remy P, Geny C, Samson Y, Hantraye P, Jeny R. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson's disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain . 1994;117 (Pt 3):487–499. doi: 10.1093/brain/117.3.487. [DOI] [PubMed] [Google Scholar]
- 21.Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, Hauser RA, Smith DA, Nauert GM, Perl DP. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med . 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 22.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol . 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 23.Politis M, Oertel WH, Wu K, Quinn NP, Pogarell O, Brooks DJ, Bjorklund A, Lindvall O, Piccini P. Graft-induced dyskinesias in Parkinson's disease: High striatal serotonin/dopamine transporter ratio. Mov Disord . 2011;26:1997–2003. doi: 10.1002/mds.23743. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Z, Lei T, Liu Y, Yang Y, Bi W, Du H. The potential therapy with dental tissue-derived mesenchymal stem cells in Parkinson's disease. Stem Cell Res Ther . 2021;12:5. doi: 10.1186/s13287-020-01957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue CR, Wang K, Zhang MZ, Wang Z, Song YY, Yu HJ, Hao Y, Guan YT. Tracking Neural Stem Cells in vivo: Achievements and Limitations. Stem Cell Rev Rep . 2022;18:1774–1788. doi: 10.1007/s12015-022-10333-z. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Muñoz B, Garcia-Delgado AB, Arribas-Arribas B, Sanchez-Pernaute R. Human Neural Stem Cells for Cell-Based Medicinal Products. Cells . 2021;10 doi: 10.3390/cells10092377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminska A, Radoszkiewicz K, Rybkowska P, Wedzinska A, Sarnowska A. Interaction of Neural Stem Cells (NSCs) and Mesenchymal Stem Cells (MSCs) as a Promising Approach in Brain Study and Nerve Regeneration. Cells . 2022;11 doi: 10.3390/cells11091464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jablonska A, Kozlowska H, Markiewicz I, Domanska-Janik K, Lukomska B. Transplantation of neural stem cells derived from human cord blood to the brain of adult and neonatal rats. Acta Neurobiol Exp (Wars) . 2010;70:337–350. doi: 10.55782/ane-2010-1806. [DOI] [PubMed] [Google Scholar]
- 29.Mollinari C, Zhao J, Lupacchini L, Garaci E, Merlo D, Pei G. Transdifferentiation: a new promise for neurodegenerative diseases. Cell Death Dis . 2018;9:830. doi: 10.1038/s41419-018-0891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vishwakarma SK, Bardia A, Tiwari SK, Paspala SA, Khan AA. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J Adv Res . 2014;5:277–294. doi: 10.1016/j.jare.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Chen W, Tan S, Lin T. Stem Cells for Modeling and Therapy of Parkinson's Disease. Hum Gene Ther . 2017;28:85–98. doi: 10.1089/hum.2016.116. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Stull ND, Berk MA, Snyder EY, Iacovitti L. Neural stem cells spontaneously express dopaminergic traits after transplantation into the intact or 6-hydroxydopamine-lesioned rat. Exp Neurol . 2002;177:50–60. doi: 10.1006/exnr.2002.7989. [DOI] [PubMed] [Google Scholar]
- 33.Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol . 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 34.Wei P, Liu J, Zhou HL, Han ZT, Wu QY, Pang JX, Liu S, Wang TH. Effects of engrafted neural stem cells derived from GFP transgenic mice in Parkinson's diseases rats. Neurosci Lett . 2007;419:49–54. doi: 10.1016/j.neulet.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 35.Zuo F, Xiong F, Wang X, Li X, Wang R, Ge W, Bao X. Intrastriatal Transplantation of Human Neural Stem Cells Restores the Impaired Subventricular Zone in Parkinsonian Mice. Stem Cells . 2017;35:1519–1531. doi: 10.1002/stem.2616. [DOI] [PubMed] [Google Scholar]
- 36.Tuncer Z, Dereli Can G, Dönmez Keklikoğlu H, Eren FA, Yülek F, Deniz O. The Relationship between Visual-Evoked Potential and Optic Coherence Tomography and Clinical Findings in Parkinson Patients. Parkinsons Dis . 2023;2023:7739944. doi: 10.1155/2023/7739944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci . 2006;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marei HE, Lashen S, Farag A, Althani A, Afifi N, A AE, Rezk S, Pallini R, Casalbore P, Cenciarelli C. Human olfactory bulb neural stem cells mitigate movement disorders in a rat model of Parkinson's disease. J Cell Physiol . 2015;230:1614–1629. doi: 10.1002/jcp.24909. [DOI] [PubMed] [Google Scholar]
- 39.Nelke A, García-López S, Martínez-Serrano A, Pereira MP. Multifactoriality of Parkinson's Disease as Explored Through Human Neural Stem Cells and Their Transplantation in Middle-Aged Parkinsonian Mice. Front Pharmacol . 2021;12:773925. doi: 10.3389/fphar.2021.773925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon HH, Min J, Shin N, Kim YH, Kim JM, Hwang YS, Suh JK, Hwang O, Jeon SR. Are human dental papilla-derived stem cell and human brain-derived neural stem cell transplantations suitable for treatment of Parkinson's disease? Neural Regen Res . 2013;8:1190–1200. doi: 10.3969/j.issn.1673-5374.2013.13.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin ES, Hwang O, Hwang YS, Suh JK, Chun YI, Jeon SR. Enhanced efficacy of human brain-derived neural stem cells by transplantation of cell aggregates in a rat model of Parkinson's disease. J Korean Neurosurg Soc . 2014;56:383–389. doi: 10.3340/jkns.2014.56.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teil M, Arotcarena ML, Dehay B. A New Rise of Non-Human Primate Models of Synucleinopathies. Biomedicines . 2021;9 doi: 10.3390/biomedicines9030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redmond DE Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci U S A . 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez R, Garitaonandia I, Poustovoitov M, Abramihina T, McEntire C, Culp B, Attwood J, Noskov A, Christiansen-Weber T, Khater M, Mora-Castilla S, To C, Crain A, Sherman G, Semechkin A, Laurent LC, Elsworth JD, Sladek J, Snyder EY, Redmond DE Jr, Kern RA. Neural Stem Cells Derived from Human Parthenogenetic Stem Cells Engraft and Promote Recovery in a Nonhuman Primate Model of Parkinson's Disease. Cell Transplant . 2016;25:1945–1966. doi: 10.3727/096368916X691682. [DOI] [PubMed] [Google Scholar]
- 45.Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, Kenyon SM, Carver SR, Benthem SD, Stimmell AC, Moseley SC, Hike D, Grant SC, Wilber AA, Olcese JM, Meckes DG Jr. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics . 2021;11:8129–8142. doi: 10.7150/thno.62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei M, Yang Z, Li S, Le W. Nanotherapeutic and Stem Cell Therapeutic Strategies in Neurodegenerative Diseases: A Promising Therapeutic Approach. Int J Nanomedicine . 2023;18:611–626. doi: 10.2147/IJN.S395010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upadhya R, Madhu LN, Attaluri S, Gitaí DLG, Pinson MR, Kodali M, Shetty G, Zanirati G, Kumar S, Shuai B, Weintraub ST, Shetty AK. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J Extracell Vesicles . 2020;9:1809064. doi: 10.1080/20013078.2020.1809064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Upadhya R, Shetty AK. Extracellular Vesicles for the Diagnosis and Treatment of Parkinson's Disease. Aging Dis . 2021;12:1438–1450. doi: 10.14336/AD.2021.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells . 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee EJ, Choi Y, Lee HJ, Hwang DW, Lee DS. Human neural stem cell-derived extracellular vesicles protect against Parkinson's disease pathologies. J Nanobiotechnology . 2022;20:198. doi: 10.1186/s12951-022-01356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metcalfe SM, Bickerton S, Fahmy T. Neurodegenerative Disease: A Perspective on Cell-Based Therapy in the New Era of Cell-Free Nano-Therapy. Curr Pharm Des . 2017;23:776–783. doi: 10.2174/1381612822666161206141744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain . 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 53.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature . 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 54.Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci U S A . 2006;103:13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsh SE, Blurton-Jones M. Neural stem cell therapy for neurodegenerative disorders: The role of neurotrophic support. Neurochem Int . 2017;106:94–100. doi: 10.1016/j.neuint.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med . 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pendse S, Vaidya A, Kale V. Clinical applications of pluripotent stem cells and their derivatives: current status and future perspectives. Regen Med . 2022;17:677–690. doi: 10.2217/rme-2022-0045. [DOI] [PubMed] [Google Scholar]
- 58.Goodarzi P, Aghayan HR, Larijani B, Soleimani M, Dehpour AR, Sahebjam M, Ghaderi F, Arjmand B. Stem cell-based approach for the treatment of parkinson's disease. Med J Islam Repub Iran . 2015;29:168. [PMC free article] [PubMed] [Google Scholar]
- 59.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature . 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 60.Scheiner ZS, Talib S, Feigal EG. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J Biol Chem . 2014;289:4571–4577. doi: 10.1074/jbc.R113.509588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong C, Liu M, Pan X, Zhu H. Tumorigenicity risk of iPSCs in vivo: nip it in the bud. Precis Clin Med . 2022;5:pbac004. doi: 10.1093/pcmedi/pbac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Q. Safety and Efficacy Study of Human ESC-derived Neural Precursor Cells in the Treatment of Parkinson's Disease. [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03119636. ClinicalTrials.gov Identifier: NCT03119636.
- 63.Skane R. A Trial to Determine the Safety and Tolerability of Transplanted Stem Cell Derived Dopamine Neurons to the Brains of Individuals With Parkinson's Disease (STEM-PD). [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05635409. ClinicalTrials.gov Identifier: NCT05635409.
- 64.Cyto Therapeutics Pty Limited. A Study to Evaluate the Safety of Neural Stem Cells in Patients With Parkinson's Disease [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02452723. ClinicalTrials.gov Identifier: NCT02452723.
- 65. Allife Medical Science and Technology Co, Ltd. A Study on the Treatment of Parkinson's Disease With Autologous Neural Stem Cells [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03815071. ClinicalTrials.gov Identifier: NCT03815071.
- 66.Takahashi R. Kyoto Trial to Evaluate the Safety and Efficacy of iPSC-derived dopaminergic progenitors in the treatment of Parkinson's Disease [accessed 2023 May 9]. In: UMIN Clinical Trials Registry [Internet]. Available from: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000038278. Unique ID issued by UMIN UMIN000033564.
- 67.Liu CF. A Study To Evaluate the Safety and Efficacy of Human Neural Stem Cells for Parkinson's Disease Patient (hNSCPD) [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03128450. ClinicalTrials.gov Identifier: NCT03128450.
- 68.Levesque MF. Transplantation of Neural Stem Cell-Derived Neurons for Parkinson's Disease [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03309514. ClinicalTrials.gov Identifier: NCT03309514.
- 69. TRANSEURO Open Label Transplant Study in Parkinson's Disease (TRANSEURO) [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT01898390. ClinicalTrials.gov Identifier: NCT01898390.
- 70.MD Stem Cells. Neurologic Stem Cell Treatment Study (NEST) [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02795052. ClinicalTrials.gov Identifier: NCT02795052.
- 71.Doshi P, Jaslok Hospital and Research Centre. Autologous Mesenchymal Stem Cell Transplant for Parkinson's Disease [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT00976430. ClinicalTrials.gov Identifier: NCT00976430.
- 72.MD Stem Cells. Alzheimer's Autism and Cognitive Impairment Stem Cell Treatment Study (ACIST) [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03724136. ClinicalTrials.gov Identifier: NCT03724136.
- 73.Jamali F, University of Jordan. Use of Mesenchymal Stem Cells (MSCs) Differentiated Into Neural Stem Cells (NSCs) in People With Parkinson's (PD) [accessed 2023 May 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03684122. ClinicalTrials.gov Identifier: NCT03684122.
- 74.Xue J, Wu Y, Bao Y, Zhao M, Li F, Sun J, Sun Y, Wang J, Chen L, Mao Y, Schweitzer JS, Song B. Clinical considerations in Parkinson's disease cell therapy. Ageing Res Rev . 2023;83:101792. doi: 10.1016/j.arr.2022.101792. [DOI] [PubMed] [Google Scholar]
- 75.Sanberg PR. Neural stem cells for Parkinson's disease: to protect and repair. Proc Natl Acad Sci USA . 2007;104:11869–11870. doi: 10.1073/pnas.0704704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chou CH, Fan HC, Hueng DY. Potential of Neural Stem Cell-Based Therapy for Parkinson's Disease. Parkinsons Dis . 2015;2015:571475. doi: 10.1155/2015/571475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sundberg M, Isacson O. Advances in stem-cell--generated transplantation therapy for Parkinson's disease. Expert Opin Biol Ther . 2014;14:437–453. doi: 10.1517/14712598.2014.876986. [DOI] [PubMed] [Google Scholar]
- 78.Pandanaboina SC, RanguMagar AB, Sharma KD, Chhetri BP, Parnell CM, Xie JY, Srivatsan M, Ghosh A. Functionalized Nanocellulose Drives Neural Stem Cells toward Neuronal Differentiation. J Funct Biomater . 2021;12 doi: 10.3390/jfb12040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen W. Neural stem cells therapy to treat neurodegenerative diseases. E3S Web of Conferences . 2021;271:03076. [Google Scholar]
- 80.Pandanaboina SC, Alghazali KM, Nima ZA, Alawajji RA, Sharma KD, Watanabe F, Saini V, Biris AS, Srivatsan M. Plasmonic nano surface for neuronal differentiation and manipulation. Nanomedicine . 2019;21:102048. doi: 10.1016/j.nano.2019.102048. [DOI] [PubMed] [Google Scholar]
- 81.Fang Q, Zhang Y, Chen X, Li H, Cheng L, Zhu W, Zhang Z, Tang M, Liu W, Wang H, Wang T, Shen T, Chai R. Three-Dimensional Graphene Enhances Neural Stem Cell Proliferation Through Metabolic Regulation. Front Bioeng Biotechnol . 2019;7:436. doi: 10.3389/fbioe.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chou CH, Sinden JD, Couraud PO, Modo M. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PLoS One . 2014;9:e106346. doi: 10.1371/journal.pone.0106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeCoteau W, Heckman KL, Estevez AY, Reed KJ, Costanzo W, Sandford D, Studlack P, Clauss J, Nichols E, Lipps J, Parker M, Hays-Erlichman B, Leiter JC, Erlichman JS. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine . 2016;12:2311–2320. doi: 10.1016/j.nano.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Zhu FD, Hu YJ, Yu L, Zhou XG, Wu JM, Tang Y, Qin DL, Fan QZ, Wu AG. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front Pharmacol . 2021;12:683935. doi: 10.3389/fphar.2021.683935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Debnath K, Pradhan N, Singh BK, Jana NR. Poly(trehalose) Nanoparticles Prevent Amyloid Aggregation and Suppress Polyglutamine Aggregation in a Huntington's Disease Model Mouse. ACS Appl Mater Interfaces . 2017;9:24126–24139. doi: 10.1021/acsami.7b06510. [DOI] [PubMed] [Google Scholar]
- 86.Marei HE, Elnegiry AA, Zaghloul A, Althani A, Afifi N, Abd-Elmaksoud A, Farag A, Lashen S, Rezk S, Shouman Z, Cenciarelli C, Hasan A. Nanotubes impregnated human olfactory bulb neural stem cells promote neuronal differentiation in Trimethyltin-induced neurodegeneration rat model. J Cell Physiol . 2017;232:3586–3597. doi: 10.1002/jcp.25826. [DOI] [PubMed] [Google Scholar]
- 87.Zhao JW, Dyson SC, Kriegel C, Tyers P, He X, Fahmy TM, Metcalfe SM, Barker RA. Modelling of a targeted nanotherapeutic 'stroma' to deliver the cytokine LIF, or XAV939, a potent inhibitor of Wnt-β-catenin signalling, for use in human fetal dopaminergic grafts in Parkinson's disease. Dis Model Mech . 2014;7:1193–1203. doi: 10.1242/dmm.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang J-Y, Won J-S, Nam H, Lee HW, Joo KM. Current advances in combining stem cell and gene therapy for neurodegenerative diseases. Precision and Future Medicine . 2018;2:53–65. [Google Scholar]
- 89.Sancho-Bielsa FJ. Parkinson's disease: Present and future of cell therapy. Neurology Perspectives . 2022;2:S58–S68. [Google Scholar]
- 90.Dong Y, Wu X, Chen X, Zhou P, Xu F, Liang W. Nanotechnology shaping stem cell therapy: Recent advances, application, challenges, and future outlook. Biomed Pharmacother . 2021;137:111236. doi: 10.1016/j.biopha.2021.111236. [DOI] [PubMed] [Google Scholar]
- 91.Tadyszak K, Wychowaniec JK, Litowczenko J. Biomedical Applications of Graphene-Based Structures. Nanomaterials (Basel) . 2018;8 doi: 10.3390/nano8110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nehra M, Uthappa UT, Kumar V, Kumar R, Dixit C, Dilbaghi N, Mishra YK, Kumar S, Kaushik A. Nanobiotechnology-assisted therapies to manage brain cancer in personalized manner. J Control Release . 2021;338:224–243. doi: 10.1016/j.jconrel.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 93.Wang YK, Zhu WW, Wu MH, Wu YH, Liu ZX, Liang LM, Sheng C, Hao J, Wang L, Li W, Zhou Q, Hu BY. Human Clinical-Grade Parthenogenetic ESC-Derived Dopaminergic Neurons Recover Locomotive Defects of Nonhuman Primate Models of Parkinson's Disease. Stem Cell Reports . 2018;11:171–182. doi: 10.1016/j.stemcr.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song JJ, Oh SM, Kwon OC, Wulansari N, Lee HS, Chang MY, Lee E, Sun W, Lee SE, Chang S, An H, Lee CJ, Lee SH. Cografting astrocytes improves cell therapeutic outcomes in a Parkinson's disease model. J Clin Invest . 2018;128:463–482. doi: 10.1172/JCI93924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH, Bolshakov VY, Kong SW, Schweitzer JS, Kim KS. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models. J Clin Invest . 2020;130:904–920. doi: 10.1172/JCI130767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, Kondo A, Kadota T, Baba T, Tayra JT, Kikuchi Y, Miyoshi Y, Date I. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci . 2010;11:52. doi: 10.1186/1471-2202-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res . 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]