Abstract
Gluconeogenesis is an endogenous process of glucose production from non-carbohydrate carbon substrates. Both the liver and kidneys express the key enzymes necessary for endogenous glucose production and its export into circulation. We would be remiss to add that more recently gluconeogenesis has been described in the small intestine, especially under high-protein, low-carbohydrate diets. The contribution of the liver glucose release, the net glucose flux, towards systemic glucose is already well known. The liver is, in most instances, the primary bulk contributor due to the sheer size of the organ (on average, over 1 kg). The contribution of the kidney (at just over 100 g each) to endogenous glucose production is often under-appreciated, especially on a weight basis. Glucose is released from the liver through the process of glycogenolysis and gluconeogenesis. Renal glucose release is almost exclusively due to gluconeogenesis, which occurs in only a fraction of the cells in that organ (proximal tubule cells). Thus, the efficiency of glucose production from other carbon sources may be superior in the kidney relative to the liver or at least on the level. In both these tissues, gluconeogenesis regulation is under tight hormonal control and depends on the availability of substrates. Liver and renal gluconeogenesis are differentially regulated under various pathological conditions. The impact of one source vs the other changes, based on post-prandial state, acid-base balance, hormonal status, and other less understood factors. Which organ has the oar (is more influential) in driving systemic glucose homeostasis is still in-conclusive and likely changes with the daily rhythms of life. We reviewed the literature on the differences in gluconeogenesis regulation between the kidneys and the liver to gain an insight into who drives the systemic glucose levels under various physiological and pathological conditions.
Keywords: Gluconeogenesis in the kidney and liver, Diabetes, Hormonal regulation, Metabolic acidosis, Insulin resistance, Net glucose metabolism
Core Tip: The liver and kidneys have an essential role in regulating glucose homeostasis through gluconeogenesis. However, the two tissues prefer different substrates. The contribution of kidney vs liver gluconeogenesis may vary under certain physiological and pathological conditions. However, increased gluconeogenesis in the liver and kidneys contributes to hyperglycemia in the pathogenic stage of type 2 diabetes mellitus. While in the case of metabolic acidosis, which develops in response to diabetes, gluconeogenesis induction occurs exclusively in the kidneys. Nevertheless, the two organs often compensate for each other by inter-organ coordination to maintain glucose and energy homeostasis.
INTRODUCTION
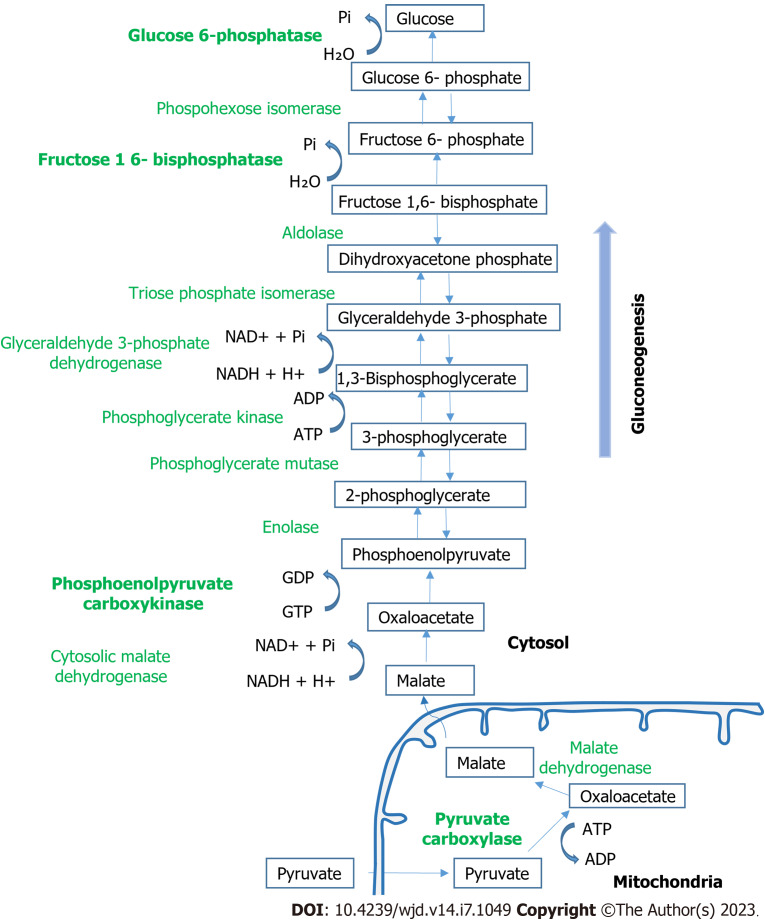
Glucose is the primary or even requisite source of energy for many tissues, including the brain, kidney medulla, and red blood cells. Blood glucose levels are maintained within a very narrow range between 3.9-7.1 mmol/L. In addition to dietary glucose, glucose produced through the process of glycogenolysis and gluconeogenesis results in the release of additional glucose into the circulation when blood levels drop. Glycogenolysis involves the breakdown of glycogen to glucose-6-phosphate and its subsequent hydrolysis by glucose-6-phosphatase (G6PC) to free glucose. Gluconeogenesis involves the formation of glucose-6-phosphate from non-carbohydrate carbon substrates such as lactate, glycerol, and amino acids with its subsequent hydrolysis by G6PC to free glucose. The process requires several enzymatic steps and counters the glycolytic breakdown of glucose. The key enzymes in the gluconeogenesis pathway are pyruvate car-boxylase, phosphoenolpyruvate carboxykinase (PEPCK), fructose 1,6-bisphosphatase, and G6PC[1]. There are three rate-limiting, unidirectional steps in gluconeogenesis, which all occur in the cytosol. The first is the phosphorylation of decarboxylated oxaloacetate to form phosphoenolpyruvic acid, which is catalyzed by PEPCK[2]. The phosphoenolpyruvic acid is converted into fructose 1,6-bisphosphate through a series of reactions, which is hydrolyzed to fructose 6-phosphate in the second rate-limiting step via the fructose 1,6-bisphosphatase enzyme. Glucose phosphate isomerase converts fructose 6-phosphate to glucose 6-phosphate. Finally, in the third rate-limiting step, glucose 6-phosphatase dephosphorylates glucose 6-phosphate to release glucose into the bloodstream (Figure 1).
Figure 1.
Overview of gluconeogenesis metabolic pathways. NADH: Nicotinamide adenine dinucleotide.
GLUCONEOGENESIS IN THE LIVER AND KIDNEYS
The liver and kidneys are the primary organs that can synthesize glucose through the process of gluconeogenesis and can also export the synthesized glucose into the bloodstream.
WHO USES WHAT?
Lactate, glycerol, and certain glucogenic amino acids, e.g., alanine and glutamine, are the primary substrates accounting for 90% of overall gluconeogenesis[3,4]. For liver gluconeogenesis, lactate, which is produced during anaerobic glycolysis, is the primary substrate. In the kidney, glutamine appears to be the major substrate. Although a few studies have suggested lactate as the main substrate, the renal conversion of lactate to glucose was found to be less than that of glutamine (50% vs 70% of its overall systemic gluconeogenesis)[3,5]. Moreover, in the post-absorptive phase, glutamine contributes 73% toward renal gluconeogenesis, while alanine contributes only 4%. It is the opposite for the liver, where alanine majorly contributes to gluconeogenesis. Moreover, hepatic gluconeogenesis from lactate and alanine is an endergonic process that consumes energy, while renal gluconeogenesis by utilizing glutamine is an exergonic process that produces four ATP/mole of synthesized glucose[6]. The transport systems for gluconeogenic amino acids also vary between the liver and kidneys. In renal tubular cells, glutamine transport depends on the A amino acid transport system, while in hepatocytes the transport depends on the N system. Nevertheless, the differences in glucogenic amino acid substrates would indicate differences in the regulatory mechanisms of glucose production in the two organs.
WHO IS MORE SENSITIVE TO HORMONAL REGULATION?
Insulin, glucagon, and catecholamines regulate plasma glucose levels within minutes through their acute glucoregulatory actions on the liver and kidney gluconeogenesis. The effects of growth hormone, thyroid hormone, and cortisol take a long time either by altering the sensitivity of the liver towards the acute regulatory hormones or by affecting the glycogen stores regulating enzyme activity and gluconeogenic precursor availability[7]. Moreover, most of these studies have been conducted in animals and their effect on renal glucose release in humans is largely unknown.
Insulin is by far the most well-known negative regulator of gluconeogenesis in both the liver and kidneys. Insulin can act by directly activating or deactivating the rate-limiting enzymes for gluconeogenic substrate availability or by acting on gluconeogenic activators. The insulin-dependent transcriptional control of gluconeogenic gene expression involves the FOXO family of transcription factors, which act through the IRS1/Akt2/mTORC1/2 and IRS/PI3k/Akt/FOXO1 pathways[8-12]. Recent studies suggest that the kidney may be more sensitive than the liver to hormonal downregulation of gluconeogenesis[13]. Moreover, insulin receptor-specific signaling may be necessary for the downregulation of renal gluconeogenesis. Proximal tubule-targeted insulin receptor deletion in mice resulted in an elevation in fasting blood glucose and increased renal protein and mRNA expression of G6PC[14]. Also, in proximal tubule cell culture, knockdown of the insulin receptor, but not the insulin-like growth factor type 1 receptor abrogated the inhibitory effects of insulin on glucose production[15].
Unlike the liver, where glucagon increases gluconeogenesis[16], the regulation of gluconeogenesis in the kidneys by glucagon is still controversial. Upregulation in PEPCK, IRS2, and PGC1α expression and glucose production by human proximal tubule cells, independent of the action of insulin, was observed upon glucagon stimulation[17]. Similar gluconeogenic effects of elevated glucagon levels were also reported in type 2 diabetes mellitus (T2DM) subjects[18]. Catecholamines also affect glucose release by the two organs by increasing the availability of gluconeogenic substrates and by decreasing insulin secretion[19,20]. In addition, both glucagon and catecholamines may positively regulate hepatic gluconeogenesis through cyclic AMP-dependent activation of protein kinase A[21,22] and acutely by the phosphorylation of the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 at Ser36[23].
WHO DRIVES SYSTEMIC GLUCOSE RELEASE DURING STARVATION?
After overnight fasting, endogenous glucose production is approximately 10-11 µmol/kg/min in humans[3]. The liver contributes to systemic glucose production through both glycogenolysis and gluconeogenesis, while the kidney produces glucose only through renal gluconeogenesis as it does not store glycogen in a healthy state. In the first hour of fasting, hepatic glycogen stores break down to glucose to meet the energy demand. Thus, in the liver, glycogenolysis is considered the primary (approximately 75%) source of glucose production in the early phase of the post-absorptive period while gluconeogenesis contributes approximately 25%[24]. It was suggested that only upon the depletion of glycogen stores, hepatic gluconeogenesis take over glucose production. However, other studies reported the contribution of gluconeogenesis at approximately 50% of hepatic glucose production, even in the early post-absorptive period when liver glycogen stores were maximal[25]. At the other extreme, Landau et al[26] reported a 54% contribution of gluconeogenesis after 14 h of fasting, and the percentage rose to 64% after 22 h of fast. Thus, the length of fasting gluconeogenesis and glycogenolysis in the liver were considered to contribute equally toward glucose production. These assumptions were made as the net organ balance studies suggested the liver as the primary site for glucose production, as kidneys showed little or no net glucose production in healthy humans during starvation[27-29].
A breakthrough in determining the role of kidney gluconeogenesis in whole-body glucose homeostasis came from the studies of Mutel et al[30]. They showed using liver-specific deletion of the G6pc gene (L-G6pc-/-mice) that the absence of hepatic glucose release had no major effect on the control of fasting plasma glucose concentration. The authors also suggested that in early fasting an induction of gluconeogenesis in the kidneys sustained endogenous glucose production and maintained euglycemia. Re-evaluation of the renal contribution to glucose release during starvation using net renal glucose balance together with a deuterated glucose dilution method suggested that renal glucose production handled approximately 20% of whole-body glucose release[24]. In prolonged fasting, renal gluconeogenesis increased and accounted for about 40% of the total systemic gluconeogenesis. New methodologies demonstrated that the kidneys not only produce glucose in the cortex but also utilize glucose for energy in the medullary region, thus the net organ balance of glucose may not truly reflect renal glucose production. This paradigm-shifting set of studies brought into effect new thinking that de novo systemic glucose production is likely provided equally by glycogenolysis (in the liver) and gluconeogenesis (approximately 30% by the liver and 20% by the kidney) during periods of extended fasting.
Overall, it has been realized that the contribution of the kidneys and liver towards endogenous glucose production changes under various nutritional situations, including long-term fasting. This repartition seems necessary for the body to maintain constant plasma glucose and simultaneously preserve the energetic status of the body for anabolic purposes. However, the predominant mechanism for glucose release into the circulation by the two organs varies in the fed state. In the kidneys, two mechanisms are in operation for the net release of glucose: The high energy-consuming gluconeogenesis and a relatively lower energy-driven glucose reabsorption process[12]. Whereas in the liver, glucose release occurs solely through gluconeogenesis. In the fasting state, however, the inability to reabsorb sufficient glucose, together with inactivated insulin signaling, promotes ATP-consuming gluconeogenesis. The role of the insulin receptor in the fast-fed regulation of gluconeogenesis in the human proximal tubule with insulin receptor substrates as direct effectors has recently been described[17].
WHO DRIVES HYPERGLYCEMIA IN DIABETES?
Increased liver as well as renal glucose release have been reported in T2DM[31-33,34-36]. The liver was commonly believed to be the primary source for this increased release of glucose into the circulation in humans with T2DM. Although renal glucose release has only been assessed in a handful of studies in humans with T2DM, the absolute increase in renal glucose release seems to be comparable to the liver by the combined isotopic-net renal glucose balance technique[36-38]. Unlike the liver, where glycogenolysis also contributes to the release of new glucose into the circulation, the increased release of new glucose by the kidney into the circulation is exclusively a result of the rise in gluconeogenesis.
In humans with or without diabetes, renal glucose release into the circulation increased for 2-3 h after a 75-g oral glucose load, whereas hepatic glucose release was reduced throughout the entire postprandial period[39]. However, the average rate of postprandial glucose release was roughly twice as high in diabetic patients as it was in non-diabetic subjects, and renal glucose release accounted for nearly 49% of the overall glucose release. This was predominantly due to defective endogenous glucose release regulation and to a lesser extent, decreased initial ingested glucose splanchnic sequestration. This effect is expected in patients with diabetes having lower postprandial insulin release or insulin resistance[9].
“Carryover” of the elevated renal gluconeogenesis observed in the post-absorptive state may have also contributed to endogenous glucose release[36], in addition to the higher availability of free fatty acid[40] and gluconeogenic precursors observed in T2DM patients[41]. Nevertheless, increased gluconeogenesis (both liver and kidneys) contributes to hyperglycemia in T2DM. However, in the kidneys enhanced glucose reabsorption via sodium-glucose cotransporters (SGLT1 and SGLT2) may also sustain hyperglycemia in T2DM. Inhibiting SGLT2 lowers blood glucose levels in T2DM[42]. Two distinct mechanisms have been indicated to improve glycemic control and reduce the plasma glucose levels by SGLT2 inhibitors: (1) By increasing the removal of plasma glucose; and (2) By ameliorating glucotoxicity, which leads to improved insulin sensitivity in peripheral tissues and enhanced β cell function[43].
Paradoxically, SGLT2 inhibitors also increased the hepatic gluconeogenic response while decreasing plasma insulin and offset by approximately 50% the increase in urinary glucose excretion[43-45]. The increase in endogenous glucose production by SGLT2 inhibitors corroborated well with the observed increase in plasma glucagon concentration[44]. Glucagon is a powerful stimulator of hepatic gluconeogenesis as already discussed in the previous section.
Glucosuria-induced glucagon secretion by SGLT2 inhibitors is beyond the scope of this review. However, glucosuria through neural reflex might activate the kidney-liver axis directly or through neuronal centers in the central nervous system[43]. Nevertheless, there are studies to suggest SGLT2 inhibitors might enhance gluconeogenesis predominantly in the kidney[44,46]. Moreover, the influence of diet intake control on the metabolic effects of SGLT2 inhibitors, including gluconeogenesis, has been observed[47].
The increase in gluconeogenesis in diabetes has been attributed to impaired insulin suppression of PEPCK and other gluconeogenic enzyme activities[31,48-50]. Elevated gluconeogenic gene expression in the kidneys was reported in proximal tubule-specific IRS1/2 double-knockout (KO) mice. These mice also exhibited attenuated phosphorylation of insulin signaling molecules including Akt and FOXO1[12]. Similarly, proximal tubule-specific insulin receptor KO increased fasting glucose concentration and renal G6pc mRNA in KO mice[14]. Moreover, studies conducted in a rat model of T2DM[51] and T2DM patients[52] also demonstrated the downregulation of insulin receptor subunit protein levels, the activation of glycogen synthase kinase 3 beta kinase, and increased gluconeogenic enzymes in proximal tubules.
Another mechanism by which insulin resistance can enhance gluconeogenesis is through impaired insulin-induced suppression of lipolysis. Accelerated lipolysis in insulin resistance or insulin deficiency releases free fatty acids and glycerol into the circulation, demonstrating a role for adipose tissue as another source of increased substrate supply for gluconeogenesis[3,53]. The rates of glycerol turnover and gluconeogenesis from glycerol increase in overnight fasted T2DM patients[54,55]. In renal tissues of human diabetes patients, an increase in plasma concentrations of alanine, glycerol, and lactate were detected demonstrating the role of increasing substrate availability enabling the possibility of enhanced gluconeogenesis[56,57]. In diabetic rats, the elevated renal Nicotinamide adenine dinucleotide phosphate oxidase activity and oxidative stress were suggested to upregulate PEPCK expression via CREB and the ERK1/2 pathway leading to accelerated renal gluconeogenesis[48,58].
Unlike diabetes where gluconeogenesis is regulated in both the liver and kidney, metabolic acidosis, such as what occurs in T2DM, regulation is primarily in the kidneys[59,60]. To counterbalance acidosis, the kidney generates ammonia, mainly from glutamine deamination, which forms α-ketoglutarate and NH4+ via the ammonia genesis pathway[61]. The proximal tubule imports glutamine and catalyzes it into glutamate, freeing up NH4+ to secrete into the lumen to eliminate acid equivalents and reabsorbs basolaterally bicarbonate to normalize blood pH. Glutamate in the proximal tubules is then converted to α-ketoglutarate, which is a substrate for gluconeogenesis[62]. It is the transcription of the PEPCK-C gene in the kidney cortex by metabolic acidosis that is unique to the kidney, whereas the transcription of PEPCK-C in the liver does not respond to changes in pH[63].
REPARTITIONING ENDOGENOUS GLUCOSE PRODUCTION AMONG ABLE ORGANS
Inter-organ coordination among the liver, kidneys, and potentially intestine may be expected if glucose and energy homeostasis is to be maintained[30]. A similar regulation may be expected during the anhepatic phase of liver transplantation in humans. In mice with liver-specific deletion of the G6PC gene, the absence of hepatic glucose production, glucagon was suggested to account for the basal induction of the renal G6PC gene[30]. Moreover, glucose production was suggested to counter-regulate insulin-induced hypoglycemia in humans during increased glucagon and cortisol secretions[64]. These studies highlight the important role of the kidney in endogenous glucose production. Similarly, the liver is also expected to compensate for hypoglycemia due to renal insufficiencies. However, it does not appear to always be the case as patients with renal failure are prone to hypoglycemia[65,66]. Underlying hepatic issues in such patients could be a possibility in individuals with reduced hepatic glycogen stores or less available gluconeogenic substrates[67]. Moreover, acidosis would limit the ability of the liver to compensate via hepatorenal reciprocity[68].
In this vein, renal gluconeogenesis diminution was shown to promote the repartition of endogenous glucose production in intestinal gluconeogenesis leading to the sparing of glycogen stores in the liver in mice lacking kidney-specific G6pc[69]. Thus intestine-liver crosstalk might take place in the situations of deficient renal glucose production, such as chronic kidney disease. However, studies are warranted to determine the contribution of intestinal gluconeogenesis to systemic glucose release and to confirm that the repartition of endogenous glucose production takes place and contributes to a glycemic reduction in chronic kidney disease with reduced renal gluconeogenesis. More studies are needed to understand the relative role of the liver vis-à-vis extrahepatic gluconeogenic organs in glucose homeostasis.
CONCLUSION
Gluconeogenesis in the liver as well as kidneys is now considered important in maintaining glucose homeostasis. The difference in the preference for gluconeogenic substrates by the liver and kidneys and the hormonal regulation of the process in the two organs would imply that the regulatory mechanisms of glucose production are not the same in the two organs. Moreover, the contribution of kidney vs liver gluconeogenesis may vary under certain physiological and pathological conditions. For example, in the early phase of fasting as the hepatic glycogen gets depleted, the systemic glucose production was considered equally by glycogenolysis (in the liver), and gluconeogenesis (approximately 30% by the liver and 20% by renal gluconeogenesis). In prolonged fasting, renal gluconeogenesis increases and accounts for about 40% of the total systemic gluconeogenesis. In the pathological state of T2DM, increased gluconeogenesis in both the liver and kidneys contributes towards hyperglycemia. In metabolic acidosis in response to diabetes, gluconeogenesis induction exclusively occurs in the kidneys, and liver gluconeogenesis remains unaffected. Similarly, differential effects of SGLT2 inhibitors on renal and liver gluconeogenesis have been reported in the liver and kidneys. In addition, the two organs can compensate, at least partially, for the impaired glucose release due to renal or liver insufficiency suggesting an inter-organ coordination to maintain glucose and energy homeostasis. For translational implications, more studies in the area are needed to know the real driver of systemic glucose production under pathological states, such as in patients with liver or renal insufficiency.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 16, 2023
First decision: February 8, 2023
Article in press: April 11, 2023
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jovandaric MZ, Serbia; Shalaby MN, Egypt; Horowitz M, Australia S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YX
Contributor Information
Biswajit Sahoo, Department of Molecular Medicine and Biotechnology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014, India.
Medha Srivastava, Department of Molecular Medicine and Biotechnology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014, India.
Arpit Katiyar, Department of Molecular Medicine and Biotechnology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014, India.
Carolyn Ecelbarger, Department of Medicine, Georgetown University, Washington, DC 20057, United States.
Swasti Tiwari, Department of Molecular Medicine and Biotechnology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014, India. tiwari.pgi@gmail.com.
References
- 1.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rognstad R. Rate-limiting steps in metabolic pathways. J Biol Chem. 1979;254:1875–1878. [PubMed] [Google Scholar]
- 3.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382–391. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE. Control of glycaemia. Baillieres Clin Endocrinol Metab. 1993;7:551–586. doi: 10.1016/s0950-351x(05)80207-1. [DOI] [PubMed] [Google Scholar]
- 5.Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab. 2002;282:E428–E434. doi: 10.1152/ajpendo.00116.2001. [DOI] [PubMed] [Google Scholar]
- 6.Chung ST, Chacko SK, Sunehag AL, Haymond MW. Measurements of Gluconeogenesis and Glycogenolysis: A Methodological Review. Diabetes. 2015;64:3996–4010. doi: 10.2337/db15-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345–350. doi: 10.1046/j.1463-1326.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 8.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer C, Dostou J, Nadkarni V, Gerich J. Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am J Physiol. 1998;275:F915–F921. doi: 10.1152/ajprenal.1998.275.6.F915. [DOI] [PubMed] [Google Scholar]
- 10.Cersosimo E, Garlick P, Ferretti J. Insulin regulation of renal glucose metabolism in humans. Am J Physiol. 1999;276:E78–E84. doi: 10.1152/ajpendo.1999.276.1.E78. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Tsukada H, Seki G, Satoh N, Mizuno T, Fujii W, Horita S, Moriya K, Sato Y, Kume H, Nangaku M, Suzuki M. Insulin promotes sodium transport but suppresses gluconeogenesis via distinct cellular pathways in human and rat renal proximal tubules. Kidney Int. 2020;97:316–326. doi: 10.1016/j.kint.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki M, Sasako T, Kubota N, Sakurai Y, Takamoto I, Kubota T, Inagi R, Seki G, Goto M, Ueki K, Nangaku M, Jomori T, Kadowaki T. Dual Regulation of Gluconeogenesis by Insulin and Glucose in the Proximal Tubules of the Kidney. Diabetes. 2017;66:2339–2350. doi: 10.2337/db16-1602. [DOI] [PubMed] [Google Scholar]
- 13.Cano N. Inter-relationships between renal metabolism (both in physiology and renal dysfunction) and the liver. Curr Opin Clin Nutr Metab Care. 2001;4:279–285. doi: 10.1097/00075197-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari S, Singh RS, Li L, Tsukerman S, Godbole M, Pandey G, Ecelbarger CM. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J Am Soc Nephrol. 2013;24:1209–1214. doi: 10.1681/ASN.2012060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey G, Shankar K, Makhija E, Gaikwad A, Ecelbarger C, Mandhani A, Srivastava A, Tiwari S. Reduced Insulin Receptor Expression Enhances Proximal Tubule Gluconeogenesis. J Cell Biochem. 2017;118:276–285. doi: 10.1002/jcb.25632. [DOI] [PubMed] [Google Scholar]
- 16.Stumvoll M, Meyer C, Kreider M, Perriello G, Gerich J. Effects of glucagon on renal and hepatic glutamine gluconeogenesis in normal postabsorptive humans. Metabolism. 1998;47:1227–1232. doi: 10.1016/s0026-0495(98)90328-6. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R, Sahoo B, Srivastava A, Tiwari S. Reduced insulin signaling and high glucagon in early insulin resistance impaired fast-fed regulation of renal gluconeogenesis via insulin receptor substrate. J Cell Biochem. 2022;123:1327–1339. doi: 10.1002/jcb.30294. [DOI] [PubMed] [Google Scholar]
- 18.Bankir L, Bouby N, Blondeau B, Crambert G. Glucagon actions on the kidney revisited: possible role in potassium homeostasis. Am J Physiol Renal Physiol. 2016;311:F469–F486. doi: 10.1152/ajprenal.00560.2015. [DOI] [PubMed] [Google Scholar]
- 19.Meyer C, Stumvoll M, Welle S, Woerle HJ, Haymond M, Gerich J. Relative importance of liver, kidney, and substrates in epinephrine-induced increased gluconeogenesis in humans. Am J Physiol Endocrinol Metab. 2003;285:E819–E826. doi: 10.1152/ajpendo.00145.2003. [DOI] [PubMed] [Google Scholar]
- 20.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest. 1995;96:2528–2533. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Exton JH, Park CR. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3',5'-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968;243:4189–4196. [PubMed] [Google Scholar]
- 22.Blair JB, Cimbala MA, Foster JL, Morgan RA. Hepatic pyruvate kinase. Regulation by glucagon, cyclic adenosine 3'-5'-monophosphate, and insulin in the perfused rat liver. J Biol Chem. 1976;251:3756–3762. [PubMed] [Google Scholar]
- 23.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerich JE, Campbell PJ. Overview of counterregulation and its abnormalities in diabetes mellitus and other conditions. Diabetes Metab Rev. 1988;4:93–111. doi: 10.1002/dmr.5610040202. [DOI] [PubMed] [Google Scholar]
- 25.Petersen KF, Price T, Cline GW, Rothman DL, Shulman GI. Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. Am J Physiol. 1996;270:E186–E191. doi: 10.1152/ajpendo.1996.270.1.E186. [DOI] [PubMed] [Google Scholar]
- 26.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378–385. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol. 1997;273:E1209–E1215. doi: 10.1152/ajpendo.1997.273.6.E1209. [DOI] [PubMed] [Google Scholar]
- 28.Brundin T, Wahren J. Renal oxygen consumption, thermogenesis, and amino acid utilization during i.v. infusion of amino acids in man. Am J Physiol. 1994;267:E648–E655. doi: 10.1152/ajpendo.1994.267.5.E648. [DOI] [PubMed] [Google Scholar]
- 29.Björkman O, Gunnarsson R, Hagström E, Felig P, Wahren J. Splanchnic and renal exchange of infused fructose in insulin-deficient type 1 diabetic patients and healthy controls. J Clin Invest. 1989;83:52–59. doi: 10.1172/JCI113884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutel E, Gautier-Stein A, Abdul-Wahed A, Amigó-Correig M, Zitoun C, Stefanutti A, Houberdon I, Tourette JA, Mithieux G, Rajas F. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes. 2011;60:3121–3131. doi: 10.2337/db11-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mithieux G, Vidal H, Zitoun C, Bruni N, Daniele N, Minassian C. Glucose-6-phosphatase mRNA and activity are increased to the same extent in kidney and liver of diabetic rats. Diabetes. 1996;45:891–896. doi: 10.2337/diab.45.7.891. [DOI] [PubMed] [Google Scholar]
- 32.Bearn AG, Billing BH, Sherlock S. Hepatic glucose output and hepatic insulin sensitivity in diabetes mellitus. Lancet. 1951;2:698–701. doi: 10.1016/s0140-6736(51)91476-6. [DOI] [PubMed] [Google Scholar]
- 33.Carlsten A, Hallgren B, Jagenburg R, Svanborg A, Werkö L. Arterio-hepatic venous differences of free fatty acids and amino acids. Studies in patients with diabetes or essential hypercholesterolemia, and in healthy individuals. Acta Med Scand. 1967;181:199–207. doi: 10.1111/j.0954-6820.1967.tb07246.x. [DOI] [PubMed] [Google Scholar]
- 34.Felig P, Wahren J, Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes. 1978;27:121–126. doi: 10.2337/diab.27.2.121. [DOI] [PubMed] [Google Scholar]
- 35.Waldhäusl W, Bratusch-Marrain P, Gasić S, Korn A, Nowotny P. Insulin production rate, hepatic insulin retention and splanchnic carbohydrate metabolism after oral glucose ingestion in hyperinsulinaemic Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1982;23:6–15. doi: 10.1007/BF00257722. [DOI] [PubMed] [Google Scholar]
- 36.Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest. 1998;102:619–624. doi: 10.1172/JCI2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer C, Tolias A, Platanisiotis D, Stumvoll M, Vlachos L, Mitrakou A. Increased renal glucose metabolism in Type 1 diabetes mellitus. Diabet Med. 2005;22:453–459. doi: 10.1111/j.1464-5491.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- 38.Moller N, Jensen MD, Rizza RA, Andrews JC, Nair KS. Renal amino acid, fat and glucose metabolism in type 1 diabetic and non-diabetic humans: effects of acute insulin withdrawal. Diabetologia. 2006;49:1901–1908. doi: 10.1007/s00125-006-0287-3. [DOI] [PubMed] [Google Scholar]
- 39.Meyer C, Woerle HJ, Dostou JM, Welle SL, Gerich JE. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1049–E1056. doi: 10.1152/ajpendo.00041.2004. [DOI] [PubMed] [Google Scholar]
- 40.Krebs HA, Speake RN, Hems R. Acceleration of renal gluconeogenesis by ketone bodies and fatty acids. Biochem J. 1965;94:712–720. doi: 10.1042/bj0940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer C, Dostou JM, Welle SL, Gerich JE. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;282:E419–E427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- 42.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 44.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cefalu WT. Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition. J Clin Invest. 2014;124:485–487. doi: 10.1172/JCI74297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atageldiyeva K, Fujita Y, Yanagimachi T, Mizumoto K, Takeda Y, Honjo J, Takiyama Y, Abiko A, Makino Y, Haneda M. Sodium-Glucose Cotransporter 2 Inhibitor and a Low Carbohydrate Diet Affect Gluconeogenesis and Glycogen Content Differently in the Kidney and the Liver of Non-Diabetic Mice. PLoS One. 2016;11:e0157672. doi: 10.1371/journal.pone.0157672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashiuchi E, Watanabe H, Kimura K, Matsumoto M, Inoue H, Inaba Y. Diet intake control is indispensable for the gluconeogenic response to sodium-glucose cotransporter 2 inhibition in male mice. J Diabetes Investig. 2021;12:35–47. doi: 10.1111/jdi.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winiarska K, Jarzyna R, Dzik JM, Jagielski AK, Grabowski M, Nowosielska A, Focht D, Sierakowski B. ERK1/2 pathway is involved in renal gluconeogenesis inhibition under conditions of lowered NADPH oxidase activity. Free Radic Biol Med. 2015;81:13–21. doi: 10.1016/j.freeradbiomed.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Lemieux G, Aranda MR, Fournel P, Lemieux C. Renal enzymes during experimental diabetes mellitus in the rat. Role of insulin, carbohydrate metabolism, and ketoacidosis. Can J Physiol Pharmacol. 1984;62:70–75. doi: 10.1139/y84-010. [DOI] [PubMed] [Google Scholar]
- 50.Weber G, Lea MA, Convery HJ, Stamm NB. Regulation of gluconeogenesis and glycolysis: studies of mechanisms controlling enzyme activity. Adv Enzyme Regul. 1967;5:257–300. doi: 10.1016/0065-2571(67)90020-9. [DOI] [PubMed] [Google Scholar]
- 51.Wen Y, Lin N, Yan HT, Luo H, Chen GY, Cui JF, Shi L, Chen T, Wang T, Tang LJ. Down-Regulation of Renal Gluconeogenesis in Type II Diabetic Rats Following Roux-en-Y Gastric Bypass Surgery: A Potential Mechanism in Hypoglycemic Effect. Obes Facts. 2015;8:110–124. doi: 10.1159/000381163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gatica R, Bertinat R, Silva P, Carpio D, Ramírez MJ, Slebe JC, San Martín R, Nualart F, Campistol JM, Caelles C, Yáñez AJ. Altered expression and localization of insulin receptor in proximal tubule cells from human and rat diabetic kidney. J Cell Biochem. 2013;114:639–649. doi: 10.1002/jcb.24406. [DOI] [PubMed] [Google Scholar]
- 53.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nurjhan N, Consoli A, Gerich J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:169–175. doi: 10.1172/JCI115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puhakainen I, Koivisto VA, Yki-Järvinen H. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1992;75:789–794. doi: 10.1210/jcem.75.3.1517368. [DOI] [PubMed] [Google Scholar]
- 56.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38:550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- 57.Jansson PA, Larsson A, Smith U, Lönnroth P. Lactate release from the subcutaneous tissue in lean and obese men. J Clin Invest. 1994;93:240–246. doi: 10.1172/JCI116951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winiarska K, Focht D, Sierakowski B, Lewandowski K, Orlowska M, Usarek M. NADPH oxidase inhibitor, apocynin, improves renal glutathione status in Zucker diabetic fatty rats: a comparison with melatonin. Chem Biol Interact. 2014;218:12–19. doi: 10.1016/j.cbi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Kamm DE, Fuisz RE, Goodman AD, Cahill GF Jr. Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest. 1967;46:1172–1177. doi: 10.1172/JCI105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma R, Kumari M, Prakash P, Gupta S, Tiwari S. Phosphoenolpyruvate carboxykinase in urine exosomes reflect impairment in renal gluconeogenesis in early insulin resistance and diabetes. Am J Physiol Renal Physiol. 2020;318:F720–F731. doi: 10.1152/ajprenal.00507.2019. [DOI] [PubMed] [Google Scholar]
- 61.Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol. 2013;3:201–220. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002;6:322–326. doi: 10.1186/cc1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor L, Curthoys NP. Glutamine metabolism: Role in acid-base balance*. Biochem Mol Biol Educ. 2004;32:291–304. doi: 10.1002/bmb.2004.494032050388. [DOI] [PubMed] [Google Scholar]
- 64.Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9:463–73; quiz 474. [PMC free article] [PubMed] [Google Scholar]
- 65.Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am. 1989;18:103–121. [PubMed] [Google Scholar]
- 66.Rubenfeld S, Garber AJ. Abnormal carbohydrate metabolism in chronic renal failure. The potential role of accelerated glucose production, increased gluconeogenesis, and impaired glucose disposal. J Clin Invest. 1978;62:20–28. doi: 10.1172/JCI109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woerle HJ, Meyer C, Popa EM, Cryer PE, Gerich JE. Renal compensation for impaired hepatic glucose release during hypoglycemia in type 2 diabetes: further evidence for hepatorenal reciprocity. Diabetes. 2003;52:1386–1392. doi: 10.2337/diabetes.52.6.1386. [DOI] [PubMed] [Google Scholar]
- 68.Bolli GB, Tsalikian E, Haymond MW, Cryer PE, Gerich JE. Defective glucose counterregulation after subcutaneous insulin in noninsulin-dependent diabetes mellitus. Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions. J Clin Invest. 1984;73:1532–1541. doi: 10.1172/JCI111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko K, Soty M, Zitoun C, Duchampt A, Silva M, Philippe E, Gautier-Stein A, Rajas F, Mithieux G. The role of kidney in the inter-organ coordination of endogenous glucose production during fasting. Mol Metab. 2018;16:203–212. doi: 10.1016/j.molmet.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]