Abstract
Pepsinogen, secreted from the gastric mucosa, is the precursor of pepsin. It is categorized as pepsinogen 1 and pepsinogen 2 based on its immunogenicity. The pepsinogen content that can enter the blood circulation through the capillaries of the gastric mucosa is approximately 1% and remains stable all the time. The pepsinogen content in serum will change with the pathological changes of gastric mucosa. Therefore, the level of pepsinogen in serum can play a role in serologic biopsy to reflect the function and morphology of different regions of gastric mucosa and serve as an indicator of gastric disease. This study conducts relevant research on serum pepsinogen 1, pepsinogen 2, and the ratio of pepsinogen 1 to pepsinogen 2, and reviews their important value in clinical diagnosis of Helicobacter pylori infection, gastric ulcer, and even gastric carcinoma, providing ideas for other researchers.
Keywords: Pepsinogen 1, Pepsinogen 2, Gastric diseases, Serological marker, Serological biopsy
Core Tip: Pepsinogen is the precursor of pepsin, including pepsinogen 1 and pepsinogen 2. Serum pepsinogen has certain clinical value in determining Helicobacter pylori (H. pylori) infection and its treatment monitoring, in identifying the location and extent of gastric mucosal lesions, and in diagnosing and screening ongoing gastric cancer and precancerous lesions. This work summarizes the clinical value of serum pepsinogen 1, pepsinogen 2, and the ratio of pepsinogen 1 to pepsinogen 2 in the diagnosis of H. pylori infection, atrophic gastritis, and gastric cancer to provide readers with research ideas.
INTRODUCTION
Pepsinogen is an inactive precursor of pepsin, a specific functional enzyme in gastric mucosa, which was initially reported in duodenal ulcer[1,2]. It is secreted by gastric mucosal cells. According to immunogenicity, it can be classified into two types: Pepsinogen 1 and pepsinogen 2. Pepsinogen 1 mainly comes from the secretion of the chief cells of the gastric fundus gland, which is the cervical mucus cells. Pepsinogen 2 can originate from the mucous cells of the cardiac and pyloric glands in the gastric antrum, as well as from the duodenal gland in the upper part of the duodenum. Please refer to Figure 1[3,4] for details. Pepsin is mainly stored in glandular cells as a proenzyme particle synthesized by the main cell and has no activity[5,6]. Under external or physiological chemical stimuli, these particles are secreted into the stomach[7]. Under acidic conditions in the stomach, pepsinogen can be hydrolyzed into active pepsin[8]. pepsinogen also can interact with existing active gastric proteases[8]. Pepsin can decompose most proteins in the human body, thereby completing food digestion[8]. Most of pepsinogen is secreted into the stomach, and a small portion is also secreted into the bloodstream[9]. When cells are stimulated, most pepsinogen is released into the glandular cavity under exocytosis. The pepsinogen that can enter the blood circulation is approximately 1%, and the content is always stable[10]. They participate in the circulation through the capillaries of gastric mucosa[10]. After blood circulation, about two-thirds of pepsinogen 1 is metabolized by the kidneys, whereas pepsinogen 2 is reabsorbed and completely metabolized by the kidneys[6]. The number of cells in gastric mucosa and the glands in it could be reflected by the level of pepsinogen 1/2 in the serum, not only that, but also the morphology of different regions of gastric mucosa and whether its secretion function is normal can be indirectly reflected by the level of pepsinogen 1/2[11,12]. If the patient’s gastric mucosa is diseased, the pepsinogen content in the serum will also be affected. One indicator of the function of gastric acid secreting gland cells is pepsinogen 1[13]. When gastric acid secretion increases, pepsinogen I increases and vice versa[1]. The secretory sites of pepsinogen II and its influencing factors are relatively numerous, and its rise is related to the atrophy of fundus gland, epithelial metaplasia, dysplasia, and various conditions[3]. Therefore, in order to detect the status of gastric mucosa, joint monitoring of pepsinogen 1 and pepsinogen 2 can be carried out. The relevant monitoring data of pepsinogen 1/pepsinogen 2 is also of significance for serological biopsy of gastric fundus gland mucosa. This study aims to investigate the clinical value of serum pepsinogen in the diagnosis and treatment of gastric diseases, with the hope of providing valuable insights for researchers.
Figure 1.
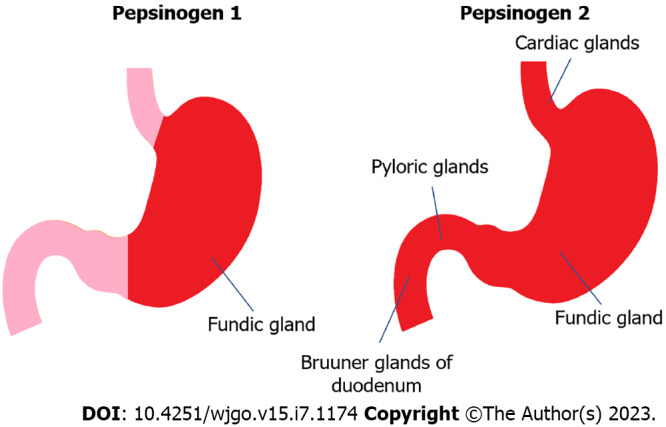
Expression of pepsinogen 1 and pepsinogen 2 in gastric mucosa.
PEPSINOGEN AND HELICOBACTER PYLORI INFECTION
Helicobacter pylori (H. Pylori) infection is a contributing factor to the development of gastric cancer[14,15]. In addition, it can induce atrophic gastritis in the antrum of the stomach[16,17]. Atrophic gastritis is widely acknowledged as a premalignant lesion of gastric cancer[18]. Lipopolysaccharides produced after H. pylori infection stimulate the chief cells of gastric mucosa, leading to increased pepsinogen 1 content in the serum[15]. It can also cause the release of INF-α, INF-γ, and other factors that promote cell apoptosis, thereby resulting in gastric gland atrophy and decreased gastric acid secretion[19,20]. H. pylori can promote the release of NF-κB and other cytokines, as well as gene transcription. Therefore, it will affect the chief cells and pepsinogen secretion. Some scholars believe that the increase of pepsinogen 2 content in serum is directly related to the changes of gastric mucosa caused by H. pylori infection, which is important in distinguishing normal gastric mucosa from abnormal gastric mucosa[3]. The pepsinogen contents of H. pylori-infected patients before and after sterilization treatment have been compared in some studies. The pepsinogen 1 content in the successful sterilization group is significantly lower than that prior to treatment, and the pepsinogen 2 content decreases more significantly. Accordingly, pepsinogen 1/pepsinogen 2 increases significantly. In the nonsterilization group, the pepsinogen content in serum does not significantly differ from that prior to treatment. Detecting the pepsinogen content has certain clinical value in evaluating whether H. pylori is eradicated. The development process from H. pylori infection to atrophic gastritis to gastric cancer is accompanied by changes in pepsinogen[21]. Extensive clinical research data have shown that H. pylori infection is very important in inducing gastric cancer. The World Health Origination classifies H. pylori infection as Class I carcinogen. Some studies have shown that H. pylori can promote AE1/p16 interaction by upregulating p16, thereby inducing gastric cancer. As shown in Figure 2, if H. pylori eradication treatment can be completed prior to “the point of no return” of gastric cancer, it can more effectively reduce the incidence rate of cancer. It can even prevent the occurrence of intestinal cancer. Until now, there have been main methods for eradicating H. pylori: Proton pump inhibitor (PPI) based triple therapy and PPI dual therapy administered through high-dose amoxicillin[22,23]. PPI has been approved by FDA and can be used for the treatment of H. pylori infection or other diseases, as it can effectively reduce the production of acids in the upper digestive system[7,24,25]. But new research suggests that if patients use PPI for a long time, it is likely to cause adverse reactions to the body[26,27]. Patients who receive long-term PPI treatment also have an increased risk of death[28]. Therefore, in the clinical practice of H. pylori eradication treatment, PPI drugs use should be used closely monitored, especially long-term medication.
Figure 2.
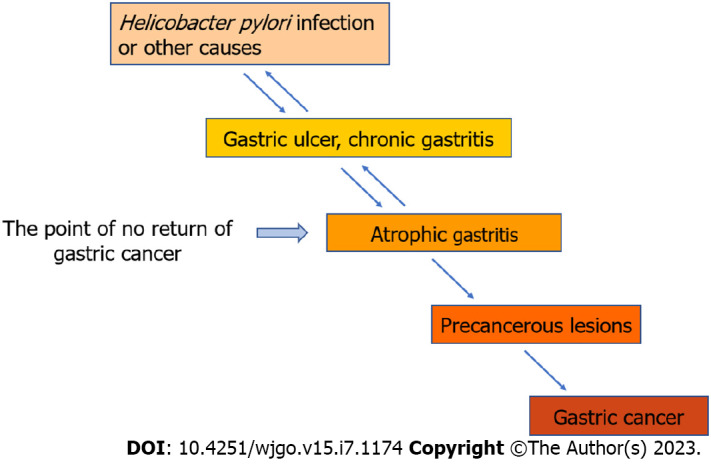
Development of gastric mucosal lesion.
In common cases, after H. pylori eradication, plasma pepsinogen 1 and pepsinogen 2 levels decrease and pepsinogen 2 decreases more significantly than pepsinogen 1[29]. Therefore, pepsinogen 1/pepsinogen 2 levels increase. Generally, pepsinogen 2 decreases after one month of H. pylori eradication, and the pepsinogen 1 level decreases within six months[30,31]. Currently, no unified view on whether pepsinogen can be used as a noninvasive serological indicator to evaluate the efficacy of H. pylori eradication. Gatta et al[32] suggested that the pepsinogen 2 level after eight weeks of H. pylori eradication is a reliable indicator of a successful procedure. The sensitivity and specificity of the optimum cutoff values (< 22.7 ng/mL) used to detect the complete eradication of H. pylori are 100.0% and 96.6%, respectively. No acceptable results are found with regard to other factors (pepsinogen 1 and gastrin-17) evaluated. However, according to Di Mario et al[3], pepsinogen 2 cannot be used as a successful marker for eradicating H. pylori. The biomarkers used to determine gastritis and H. pylori infection should be pepsinogen 2 serum level, pepsinogen 1 serum level, and pepsinogen 1/pepsinogen 2 ratio.
PEPSINOGEN, GASTRIC ULCER AND ATROPHIC GASTRITIS
Current research results on the relationship between gastric ulcer lesions and serum pepsinogen levels, there are differences in current research results. The differences would be related to the population, and also be affected by detection methods, and interference from dietary factors. In those patients who had gastric ulcer, the increase of pepsinogen 1 and pepsinogen 2 is related to the increase in gastric acid secretion, leading to an increase in serum pepsinogen secretion, and an increase in gastric mucosal permeability, with a large amount of pepsinogen entering the blood. Therefore, in clinical diagnosis, the serum level of pepsinogen can serve as a reliable biomarker for the diagnosis of gastric ulcers in patients. Atrophic gastritis is the first step in the reaction cascade leading to gastric adenocarcinoma, and it is an important precancerous lesion[33,34]. The gold standard for diagnosing atrophic gastritis remains gastroscopy and histological examination of biopsy specimens[35]. However, because the latter is conducted randomly and atrophic gastritis is distributed patchily, histological examination reflects only the state of mucosa, and the related detection is limited. Normal mucosa and gastric glands can secrete pepsinogen and gastric acid as required[36]. Pepsinogen can be activated to produce biologically active pepsin under acidic conditions, playing its physiological role[36]. When mucosal atrophy occurs, patients experience various degrees of atrophy of gastric glands[37]. Consequently, the gastric juice changes pH and the gastric juice decreases volume affecting the pepsinogen activity. The cells secreting pepsinogen 1 and pepsinogen 2 differ. When gastric mucosa atrophy occurs, the number of glandular cells and chief cells decreases, resulting in different changes in serum pepsinogen 1 and pepsinogen 2 contents[38,39]. Many studies have shown that the pepsinogen 1/pepsinogen 2 in serum and the pepsinogen 1 in it at low levels are indicators of atrophic gastritis[40]. Atrophic gastritis of the stomach is defined as serum pepsinogen 1/pepsinogen 2 < 3 and serum pepsinogen 1 level < 70 ng/mL[41]. Although a good correlation exists among endoscopy, histology, serology, and atrophic gastritis, the gastric atrophy presence is not always consistent with endoscopic findings[42]. The levels of pepsinogen 1 and the ratio of pepsinogen 1 to pepsinogen 2 in serum will decrease with the histological progression of atrophic gastritis. Moreover, when the stomach is severely atrophic, serum pepsinogen 1/pepsinogen 2 will also decrease accordingly[43,44]. For patients who was suffered severe atrophic gastritis, the levels of pepsinogen 1/pepsinogen 2 in their bodies would be lower than those mild atrophic gastritis patients[43,45].
The cutoff value of atrophic gastritis diagnosis and the diagnostic efficiency also vary due to different regions, genetic backgrounds of populations, and test kits[46]. Cha et al[47] used pepsinogen 1/ pepsinogen 2 = 4 as the cutoff value, and the sensitivity and specificity of detecting atrophic gastritis are 82.6% and 91.7%, respectively. According to Zoalfaghari A’s research, the specificity and sensitivity of pepsinogen 1/pepsinogen 2 in the diagnosis of atrophic gastritis were 71%, 71%, with the receiver operating characteristic curve area 0.639 (cut-off value = 8). Nguyen et al[48] conducted relevant studies on the Vietnamese population and found that serum pepsinogen 1 and pepsinogen 1/pepsinogen 2 ratios have diagnostic value for patients with moderate and severe atrophic gastritis. The specificity demonstrated by the research institute is 83.9%, the detection sensitivity is 73.0%, and the optimal critical value is pepsinogen 1/pepsinogen 2 ≤ 4.6; pepsinogen 1 ≤ 69.0[48]. Therefore, it is necessary to ensure the diagnosis of atrophic gastritis through serum pepsinogen testing, based on the premise of determining reasonable diagnostic parameters according to the actual situation.
PEPSINOGEN AND GASTRIC CANCER
The occurrence of gastric cancer is a multistep, multifactor, interactive process[49,50]. Gastric cancer develops from precancerous diseases, including atrophic gastritis, dysplasia and intestinal metaplasia[51,52]. In recent years, with the continuous deepening of clinical research, the status and function of gastric mucosa can be reflected effectively based on the serum pepsinogen level detection[16]. It can be used as a serological reference index for screening high-risk groups with precancerous lesions and early gastric cancer[53]. The significance of serum pepsinogen level change in the clinical screening of high-risk population with gastric cancer and the relationship between serum pepsinogen content and gastric cancer gradually become the research focus of scholars from various countries[6]. Persistent progression of precancerous lesions can lead to gastric cancer[54]. Therefore, the factors associated with the onset of gastric cancer are also related to the progression of precancerous lesions. The stomach is the only source of pepsinogen. Thus, the change in pepsinogen level can effectively reflect functional changes in the gastric mucosa[10]. Atrophic gastritis is an important background for mucosal gastric cancer, and pepsinogen 1/pepsinogen 2 levels continuously decrease with the continuous progression of gastric mucosal atrophy[14,55]. The atrophic degree of gastric mucosa of patients can be evaluated and judged through the detection of pepsinogen 1/pepsinogen 2 level[54].
According to existing research, there is a good correlation between changes in the morphology and function of the gastric mucosa in patients and serum levels of pepsinogen 1 and the ratio of pepsinogen 1 to pepsinogen 2. In the process of cancer screening, serum pepsinogen measurement is of great significance. According to research conducted by researchers on cancer patients and control groups, pepsinogen 1 ≤ 50 ng/mL and pepsinogen 1/pepsinogen 2 < 3 can be used for clinical diagnosis of cancer. The sensitivity, specificity, and accuracy values are 55%, 75%, and 72% respectively[11]. A 14-year cohort study based on a Japanese population shows that 97 of 2742 residents aged 40 and above develop symptomatic cancer. Among patients with early symptoms, approximately 62.8% (61 patients) showed pepsinogen 1/pepsinogen 2 levels < 3, while pepsinogen 1 levels < 70 ng/mL[56]. According to Mansour-Ghanaei et al[57], pepsinogen 1 serum levels and pepsinogen 1/pepsinogen 2 ratios can be used as screening serum biomarkers for gastric cancer screening. If the critical value of pepsinogen 1 is 70.95 μg/L, and the critical value of pepsinogen 1/pepsinogen 2 is 2.99, indicating excellent sensitivity and specificity in patients[57]. Therefore, it can be concluded that serum pepsinogen can be used to predict gastric cancer. However, this does not mean that large-scale cancer risk screening can be conducted through serum pepsinogen level detection, as there is a high likelihood of pepsinogen negative cancers represented by diffuse cancers[58-60]. Miki et al[58] created a set of “ABC classification” detection methods in 2011. This detection method can detect pepsinogen negative cancers[58,59]. Assess the cancer risk of patients by measuring their H. pylori antibody titers and serum pepsinogen levels, and then divide them into four groups: Group 1 [H. pylori antibody (-) pepsinogen (-)], Group 2 [H. pylori antibody (+) pepsinogen (-)], group 3 [H. pylori antibody (+) pepsinogen (+)], and group 4 [H. pylori antibody (-) pepsinogen (+)]. The risk of cancer gradually increases from group 1 to group 4[61-63]. In theory, the risk of cancer in Group 1 is the lowest. However, 2% to 10% of cancer patients are classified as Group 1[64]. Some patients were inaccurately classified as Group 1 due to receiving antibiotics to eradicate H. pylori and showing negative H. pylori antibodies[65]. From this perspective, when conducting risk assessment for gastric cancer, patients with high negative H. pylori antibodies should be distinguished and evaluated through other tests.
In addition, the joint detection of serum pepsinogen 1 and other gastric cancer-related tumor markers have very important diagnostic value. For the early diagnosis of gastric cancer, the joint detection of serum pepsinogen and serum tumor markers (such as CA125, CA199, CA242, CA724, and CEA) can play an important role. The research team led by the author of this article conducted joint testing on pepsinogen and serum soluble Tim-3[66]. The test results obtained indicate that the sensitivity for detecting cancer is 86.44%, while the specificity is 91.78%, significantly higher than those obtained by single detection[66]. For patients whose detection results indicate gradually decreased pepsinogen 1 and pepsinogen 1/ pepsinogen 2 levels, relevant endoscopic techniques can be used jointly. Some studies show that the joint detection of serum pepsinogen 1 and staining endoscopy improves the diagnostic rate of early gastric cancer, and the sensitivity of the joint detection is significantly higher than that of the individual approach[67].
CONCLUSION
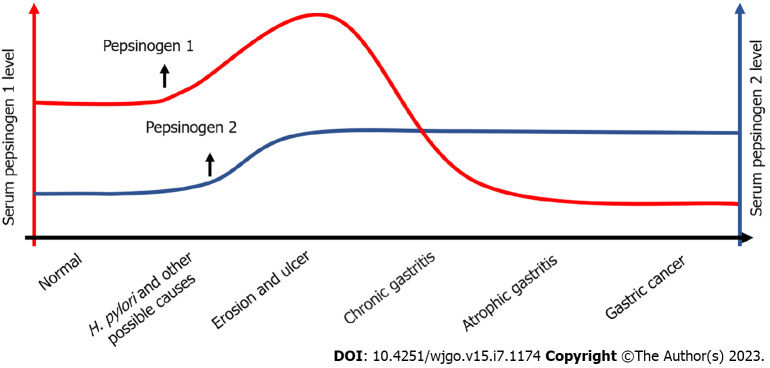
Pepsinogen is a serological marker whose subtypes are closely related to H. pylori and various gastrointestinal diseases[11]. Serum pepsinogen has certain clinical value in determining H. pylori infection and its treatment monitoring, in identifying the location and extent of gastric mucosal lesions, and in diagnosing and screening ongoing gastric cancer and precancerous lesions (Figure 3)[68]. Pepsinogen 1, pepsinogen 2, and pepsinogen 1/pepsinogen 2 are serum indicators of a certain gastric disease, and the changes in their levels in serum occur continuously throughout the process from inflammation to carcinogenesis in gastric tissue. Compared with the gastroscopy, serum pepsinogen detection is inexpensive, non-invasive, and simple. Thus, it is suitable for screening gastric diseases in large populations. The current research results on the diagnostic and therapeutic value of serum pepsinogen detection for gastric cancer are inconsistent, some shortcomings remain, and unified diagnostic standards cannot be used. However, with the establishment of screening strategies applicable to different populations and the deepening of related research, pepsinogen plays an important role in the prevention, screening, and diagnosis of cancer and atrophic gastritis. Moreover, it also performs well in predicting postoperative recurrence and metastasis of cancer. Moreover, developing novel sensitive and convenient detection methods is greatly important in further exerting the clinical value of serum pepsinogen detection.
Figure 3.
Relationship between serum pepsinogen level and gastric diseases. H. pylori: Helicobacter pylori.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 28, 2023
First decision: May 12, 2023
Article in press: June 14, 2023
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Caboclo JLF, Brazil; Haruma K, Japan; Kishikawa H, Japan S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
Contributor Information
Yuan Qin, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, Zhejiang Province, China.
Jia-Xin Geng, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, Zhejiang Province, China.
Biao Huang, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, Zhejiang Province, China. jswxhb@163.com.
References
- 1.Kageyama T. Pepsinogens, progastricsins, and prochymosins: structure, function, evolution, and development. Cell Mol Life Sci. 2002;59:288–306. doi: 10.1007/s00018-002-8423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rotter JI, Sones JQ, Samloff IM, Richardson CT, Gursky JM, Walsh JH, Rimoin DL. Duodenal-ulcer disease associated with elevated serum pepsinogen I: an inherited autosomal dominant disorder. N Engl J Med. 1979;300:63–66. doi: 10.1056/NEJM197901113000203. [DOI] [PubMed] [Google Scholar]
- 3.Di Mario F, Crafa P, Barchi A, Franzoni L, Franceschi M, Russo M, Bricca L, Brozzi L, Rodriguez Castro K, Rugge M. Pepsinogen II in gastritis and Helicobacter pylori infection. Helicobacter. 2022;27:e12872. doi: 10.1111/hel.12872. [DOI] [PubMed] [Google Scholar]
- 4.Hamashima C. Forthcoming Step in Gastric Cancer Prevention: How Can Risk Stratification Be Combined with Endoscopic Screening for Gastric Cancer? Gut Liver. 2022;16:811–824. doi: 10.5009/gnl210313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhu Z, Liu Z, Zhao Z, Xue X, Li X, Li P, Rong G, Ma Y. Diagnostic value of serum pepsinogen I, pepsinogen II, and gastrin-17 levels for population-based screening for early-stage gastric cancer. J Int Med Res. 2020;48:300060520914826. doi: 10.1177/0300060520914826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivanovic D, Plestina S, Honovic L, Dobrila-Dintinjana R, Vlasic Tanaskovic J, Vrbanec D. Gastric cancer detection using the serum pepsinogen test method. Tumori. 2022;108:386–391. doi: 10.1177/03008916211014961. [DOI] [PubMed] [Google Scholar]
- 7.Gritti I, Banfi G, Roi GS. Pepsinogens: physiology, pharmacology pathophysiology and exercise. Pharmacol Res. 2000;41:265–281. doi: 10.1006/phrs.1999.0586. [DOI] [PubMed] [Google Scholar]
- 8.Muller MJ, Defize J, Hunt RH. Control of pepsinogen synthesis and secretion. Gastroenterol Clin North Am. 1990;19:27–40. [PubMed] [Google Scholar]
- 9.Zhou MJ, Huang RJ. Catching Up with the World: Pepsinogen Screening for Gastric Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2022;31:1257–1258. doi: 10.1158/1055-9965.EPI-22-0372. [DOI] [PubMed] [Google Scholar]
- 10.Juan Cai W, Yin L, Kang Q, Chen Zeng Z, Liang Wang S, Cheng J. The Serum Pepsinogen Test as a Predictor of Kazakh Gastric Cancer. Sci Rep. 2017;7:43536. doi: 10.1038/srep43536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massarrat S, Haj-Sheykholeslami A. Increased Serum Pepsinogen II Level as a Marker of Pangastritis and Corpus-Predominant Gastritis in Gastric Cancer Prevention. Arch Iran Med. 2016;19:137–140. [PubMed] [Google Scholar]
- 12.Zhang J, Guo JZ, Xiao HL, Zhu L, Liu HY, Zhang Y, Huang B. Simultaneous detection of different serum pepsinogens and its primary application. World J Gastroenterol. 2010;16:3072–3077. doi: 10.3748/wjg.v16.i24.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabe K, Inoue K, Kamada T, Kato K, Kato M, Haruma K. Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig Endosc. 2022;34:412–419. doi: 10.1111/den.14063. [DOI] [PubMed] [Google Scholar]
- 14.Lahner E, Carabotti M, Annibale B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J Gastroenterol. 2018;24:2373–2380. doi: 10.3748/wjg.v24.i22.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Selgrad M, Bornschein J, Rokkas T, Malfertheiner P. Helicobacter pylori: gastric cancer and extragastric intestinal malignancies. Helicobacter. 2012;17 Suppl 1:30–35. doi: 10.1111/j.1523-5378.2012.00980.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheung KS, Leung WK. Risk of gastric cancer development after eradication of Helicobacter pylori. World J Gastrointest Oncol. 2018;10:115–123. doi: 10.4251/wjgo.v10.i5.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cave TR, Cave DR. Helicobacter pylori stimulates pepsin secretion from isolated rabbit gastric glands. Scand J Gastroenterol Suppl. 1991;181:9–14. doi: 10.3109/00365529109093202. [DOI] [PubMed] [Google Scholar]
- 19.Codolo G, Coletta S, D'Elios MM, de Bernard M. HP-NAP of Helicobacter pylori: The Power of the Immunomodulation. Front Immunol. 2022;13:944139. doi: 10.3389/fimmu.2022.944139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Chen T. An Insight into the Clinical Application of Gut Microbiota during Anticancer Therapy. Adv Gut & Microbiome Res. 2022;2022:8183993. [Google Scholar]
- 21.Ohkusa T, Takashimizu I, Fujiki K, Araki A, Honda K, Shimoi K, Sakurazawa T, Horiuchi T, Suzuki S, Ariake K, Ishii K. Changes in serum pepsinogen, gastrin, and immunoglobulin G antibody titers in helicobacter pylori-positive gastric ulcer after eradication of infection. J Clin Gastroenterol. 1997;25:317–322. doi: 10.1097/00004836-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Yun J, Wu Z, Qi G, Han T, Zhang D. The high-dose amoxicillin-proton pump inhibitor dual therapy in eradication of Helicobacter pylori infection. Expert Rev Gastroenterol Hepatol. 2021;15:149–157. doi: 10.1080/17474124.2021.1826306. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Wang Y, Shi J, Zhang C, Nie J, Li S, Zheng T. The status and progress of first-line treatment against Helicobacter pylori infection: a review. Therap Adv Gastroenterol. 2021;14:1756284821989177. doi: 10.1177/1756284821989177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Edinoff AN, Wu NW, Parker K, Dudossat E, Linquest L, Flanagan CJ, Dharani A, Patel H, Willett O, Cornett EM, Kaye AM, Kaye AD. Proton Pump Inhibitors, Kidney Damage, and Mortality: An Updated Narrative Review. Adv Ther. 2023;40:2693–2709. doi: 10.1007/s12325-023-02476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lespessailles E, Toumi H. Proton Pump Inhibitors and Bone Health: An Update Narrative Review. Int J Mol Sci. 2022;23 doi: 10.3390/ijms231810733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castellana C, Pecere S, Furnari M, Telese A, Matteo MV, Haidry R, Eusebi LH. Side effects of long-term use of proton pump inhibitors: practical considerations. Pol Arch Intern Med. 2021;131:541–549. doi: 10.20452/pamw.15997. [DOI] [PubMed] [Google Scholar]
- 28.Chinzon D, Domingues G, Tosetto N, Perrotti M. SAFETY OF LONG-TERM PROTON PUMP INHIBITORS: FACTS AND MYTHS. Arq Gastroenterol. 2022;59:219–225. doi: 10.1590/S0004-2803.202202000-40. [DOI] [PubMed] [Google Scholar]
- 29.Daugule I, Ruskule A, Moisejevs G, Rudzite D, Jonaitis L, Janciauskas D, Kiudelis G, Kupcinskas L, Leja M. Long-term dynamics of gastric biomarkers after eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2015;27:501–505. doi: 10.1097/MEG.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Paramo M, Albillos A, Calleja JL, Salas C, Marín MC, Marcos ML, Cacho G, Escartín P, Ortiz-Berrocal J. Changes in gastrin and serum pepsinogens in monitoring of Helicobacter pylori response to therapy. Dig Dis Sci. 1997;42:1734–1740. doi: 10.1023/a:1018873717985. [DOI] [PubMed] [Google Scholar]
- 31.Leja M, Lapina S, Polaka I, Rudzite D, Vilkoite I, Daugule I, Belkovets A, Pimanov S, Makarenko J, Tolmanis I, Lejnieks A, Boka V, Rumba-Rozenfelde I, Vikmanis U. Pepsinogen testing for evaluation of the success of Helicobacter pylori eradication at 4 weeks after completion of therapy. Medicina (Kaunas) 2014;50:8–13. doi: 10.1016/j.medici.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Gatta L, Di Mario F, Vaira D, Rugge M, Franzè A, Plebani M, Cavestro GM, Lucarini P, Lera M, Scarpignato C. Quantification of serum levels of pepsinogens and gastrin to assess eradication of Helicobacter pylori. Clin Gastroenterol Hepatol. 2011;9:440–442. doi: 10.1016/j.cgh.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Komoto K, Haruma K, Kamada T, Tanaka S, Yoshihara M, Sumii K, Kajiyama G, Talley NJ. Helicobacter pylori infection and gastric neoplasia: correlations with histological gastritis and tumor histology. Am J Gastroenterol. 1998;93:1271–1276. doi: 10.1111/j.1572-0241.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang YK, Yu JC, Kang WM, Ma ZQ, Ye X, Tian SB, Yan C. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0142080. doi: 10.1371/journal.pone.0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ MAPS Participants; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Virchows Arch. 2012;460:19–46. doi: 10.1007/s00428-011-1177-8. [DOI] [PubMed] [Google Scholar]
- 36.Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 37.Zagari RM, Rabitti S, Greenwood DC, Eusebi LH, Vestito A, Bazzoli F. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther. 2017;46:657–667. doi: 10.1111/apt.14248. [DOI] [PubMed] [Google Scholar]
- 38.Lee SY. Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection. Korean J Intern Med. 2016;31:835–844. doi: 10.3904/kjim.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiyohira K, Yoshihara M, Ito M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentration as a marker of Helicobacter pyloriinfection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol. 2003;38:332–338. doi: 10.1007/s005350300060. [DOI] [PubMed] [Google Scholar]
- 40.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 41.Kato M, Asaka M. Recent development of gastric cancer prevention. Jpn J Clin Oncol. 2012;42:987–994. doi: 10.1093/jjco/hys151. [DOI] [PubMed] [Google Scholar]
- 42.Kishikawa H, Nakamura K, Ojiro K, Katayama T, Arahata K, Takarabe S, Sasaki A, Miura S, Hayashi Y, Hoshi H, Kanai T, Nishida J. Relevance of pepsinogen, gastrin, and endoscopic atrophy in the diagnosis of autoimmune gastritis. Sci Rep. 2022;12:4202. doi: 10.1038/s41598-022-07947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada S, Matsuhisa T, Makonkawkeyoon L, Chaidatch S, Kato S, Matsukura N. Helicobacter pylori infection in combination with the serum pepsinogen I/II ratio and interleukin-1beta-511 polymorphisms are independent risk factors for gastric cancer in Thais. J Gastroenterol. 2006;41:1169–1177. doi: 10.1007/s00535-006-1951-6. [DOI] [PubMed] [Google Scholar]
- 44.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83:204–209. [PubMed] [Google Scholar]
- 45.Sitas F, Smallwood R, Jewell D, Millard PR, Newell DG, Meuwissen SG, Moses S, Zwiers A, Forman D. Serum anti-Helicobacter pylori IgG antibodies and pepsinogens A and C as serological markers of chronic atrophic gastritis. Cancer Epidemiol Biomarkers Prev. 1993;2:119–123. [PubMed] [Google Scholar]
- 46.Brenner H, Rothenbacher D, Weck MN. Epidemiologic findings on serologically defined chronic atrophic gastritis strongly depend on the choice of the cutoff-value. Int J Cancer. 2007;121:2782–2786. doi: 10.1002/ijc.22992. [DOI] [PubMed] [Google Scholar]
- 47.Cha JH, Jang JS. Clinical correlation between serum pepsinogen level and gastric atrophy in gastric neoplasm. Korean J Intern Med. 2020;35:550–558. doi: 10.3904/kjim.2018.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen CL, Dao TT, Phi TN, Nguyen TP, Pham VT, Vu TK. Serum pepsinogen: A potential non-invasive screening method for moderate and severe atrophic gastritis among an asian population. Ann Med Surg (Lond) 2022;78:103844. doi: 10.1016/j.amsu.2022.103844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang P, Yang M, Zhang Y, Xiao S, Lai X, Tan A, Du S, Li S. Dissecting the Single-Cell Transcriptome Network Underlying Gastric Premalignant Lesions and Early Gastric Cancer. Cell Rep. 2019;27:1934–1947.e5. doi: 10.1016/j.celrep.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 50.Venerito M, Vasapolli R, Rokkas T, Malfertheiner P. Gastric cancer: epidemiology, prevention, and therapy. Helicobacter. 2018;23 Suppl 1:e12518. doi: 10.1111/hel.12518. [DOI] [PubMed] [Google Scholar]
- 51.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 52.Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, Sipponen P, Shimosegawa T. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853–859. doi: 10.3748/wjg.15.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen XZ, Huang CZ, Hu WX, Liu Y, Yao XQ. Gastric Cancer Screening by Combined Determination of Serum Helicobacter pylori Antibody and Pepsinogen Concentrations: ABC Method for Gastric Cancer Screening. Chin Med J (Engl) 2018;131:1232–1239. doi: 10.4103/0366-6999.231512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho JH, Jin SY, Park S. Scoring model for discriminating gastric cancer risk in patients with negative serum pepsinogen and anti-Helicobacter pylori antibody results. J Gastroenterol Hepatol. 2021;36:3345–3353. doi: 10.1111/jgh.15630. [DOI] [PubMed] [Google Scholar]
- 55.Magris R, De Re V, Maiero S, Fornasarig M, Guarnieri G, Caggiari L, Mazzon C, Zanette G, Steffan A, Canzonieri V, Cannizzaro R. Low Pepsinogen I/II Ratio and High Gastrin-17 Levels Typify Chronic Atrophic Autoimmune Gastritis Patients With Gastric Neuroendocrine Tumors. Clin Transl Gastroenterol. 2020;11:e00238. doi: 10.14309/ctg.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oishi Y, Kiyohara Y, Kubo M, Tanaka K, Tanizaki Y, Ninomiya T, Doi Y, Shikata K, Yonemoto K, Shirota T, Matsumoto T, Iida M. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol. 2006;163:629–637. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 57.Mansour-Ghanaei F, Joukar F, Baghaee M, Sepehrimanesh M, Hojati A. Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer. Biomol Concepts. 2019;10:82–90. doi: 10.1515/bmc-2019-0010. [DOI] [PubMed] [Google Scholar]
- 58.Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - "ABC method". Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:405–414. doi: 10.2183/pjab.87.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furihata C. Human gastric cancer risk screening: From rat pepsinogen studies to the ABC method. Proc Jpn Acad Ser B Phys Biol Sci. 2021;97:462–478. doi: 10.2183/pjab.97.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leja M, Linē A. Early detection of gastric cancer beyond endoscopy - new methods. Best Pract Res Clin Gastroenterol. 2021;50-51:101731. doi: 10.1016/j.bpg.2021.101731. [DOI] [PubMed] [Google Scholar]
- 61.Park CH, Kim EH, Jung DH, Chung H, Park JC, Shin SK, Lee SK, Lee YC. The new modified ABCD method for gastric neoplasm screening. Gastric Cancer. 2016;19:128–135. doi: 10.1007/s10120-015-0473-4. [DOI] [PubMed] [Google Scholar]
- 62.Kishikawa H, Kimura K, Takarabe S, Kaida S, Nishida J. Helicobacter pylori Antibody Titer and Gastric Cancer Screening. Dis Markers. 2015;2015:156719. doi: 10.1155/2015/156719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizutani S, Takahashi Y, Shimamoto T, Nakagawa H, Hisada H, Oshio K, Kubota D, Mizutani H, Ohki D, Sakaguchi Y, Yakabi S, Niimi K, Kakushima N, Tsuji Y, Wada R, Yamamichi N, Fujishiro M. Performing the ABC Method Twice for Gastric Cancer Risk Stratification: A Retrospective Study Based on Data from a Large-Scale Screening Facility. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13071284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai KF, Liou JM, Chen MJ, Chen CC, Kuo SH, Lai IR, Yeh KH, Lin MT, Wang HP, Cheng AL, Lin JT, Shun CT, Wu MS Taiwan Gastrointestinal Disease and Helicobacter Consortium. Distinct Clinicopathological Features and Prognosis of Helicobacter pylori Negative Gastric Cancer. PLoS One. 2017;12:e0170942. doi: 10.1371/journal.pone.0170942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osborn JF, Cattaruzza MS, Ferri AM, De Angelis F, Renzi D, Marani A, Vaira D. How long will it take to reduce gastric cancer incidence by eradicating Helicobacter pylori infection? Cancer Prev Res (Phila) 2013;6:695–700. doi: 10.1158/1940-6207.CAPR-12-0428. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Hong J, Hu R, Yu X, Chen X, Zheng S, Qin Y, Zhou X, Wang Y, Zheng L, Fang H, Liu P, Huang B. Clinical Value of Combined Detection of Serum sTim-3 and Pepsinogen for Gastric Cancer Diagnosis. Cancer Manag Res. 2021;13:7759–7769. doi: 10.2147/CMAR.S328312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamei M, Yamashita S, Tokuishi K, Hashioto T, Moroga T, Suehiro S, Ono K, Miyawaki M, Takeno S, Yamamoto S, Kawahara K. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. 2010;30:4779–4783. [PubMed] [Google Scholar]
- 68.In H, Sarkar S, Ward J, Friedmann P, Parides M, Yang J, Epplein M. Serum Pepsinogen as a Biomarker for Gastric Cancer in the United States: A Nested Case-Control Study Using the PLCO Cancer Screening Trial Data. Cancer Epidemiol Biomarkers Prev. 2022;31:1426–1432. doi: 10.1158/1055-9965.EPI-21-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]