Abstract
Targeting of the human immunodeficiency virus type 1 (HIV-1) Gag precursor Pr55gag to the plasma membrane, the site of virus assembly, is primarily mediated by the N-terminal matrix (MA) domain. N-myristylation of MA is essential for the stable association of Pr55gag with membranes and for virus assembly. We now show that single amino acid substitutions near the N terminus of MA can dramatically impair assembly without compromising myristylation. Subcellular fractionation demonstrated that Gag membrane binding was compromised to a similar extent as in the absence of the myristyl acceptor site, indicating that the myristyl group was not available for membrane insertion. Remarkably, the effects of the N-terminal modifications could be completely suppressed by second-site mutations in the globular core of MA. The compensatory mutations enhanced Gag membrane binding and increased viral particle yields above wild-type levels, consistent with an increase in the exposure of the myristyl group. Our results support a model in which the compact globular core of MA sequesters the myristyl group to prevent aberrant binding to intracellular membranes, while the N terminus is critical to allow the controlled exposure of the myristyl group for insertion into the plasma membrane.
Human immunodeficiency virus type 1 (HIV-1) particle formation is driven by the Gag polyprotein Pr55gag, which is eventually cleaved by the viral protease (PR) to yield the mature structural proteins matrix (MA), capsid (CA), nucleocapsid (NC), and p6 (21, 26). MA, which forms the N-terminal domain of Pr55gag, is crucial for the targeting of the polyprotein to the plasma membrane, the site of virus assembly. The membrane targeting function of MA depends on the covalent attachment of myristic acid to a N-terminal Gly residue. Mutations which prevent the myristylation of MA invariably block particle assembly and virus replication (5, 23, 37). In addition to the N-terminal myristate, a conserved basic region within the MA domain is thought to contribute to the targeting of Pr55gag to the plasma membrane by forming electrostatic contacts with acidic phospholipids (52). The three-dimensional structure of HIV-1 MA reveals a single globular domain composed of five major helices (24, 33, 34). Interestingly, crystal structures indicate that MA forms a trimer with a putative membrane-binding surface on which basic residues concentrate (24).
The conserved basic region in MA has also been implicated in the nuclear import of the viral preintegration complex in nondividing cells (6, 20, 45). In contrast to oncoretroviruses, HIV-1 can productively infect nondividing cells because the preintegration complex is actively transported through the nucleopore (7, 30, 47). Substitutions in the conserved basic region of HIV-1 MA selectively affected virus replication in nondividing cells, consistent with a role in nuclear import (6, 45). However, others have reported contrasting results which indicate that the putative nuclear localization signal in MA is not specifically required for the productive infection of terminally differentiated cells (14, 15).
The globular core of HIV-1 MA has an essential role in the incorporation of the viral envelope (Env) glycoprotein complex into nascent particles (12, 51). The Env glycoproteins are synthesized in the form of a precursor designated gp160, which is cleaved by a cellular protease into an external surface subunit and a membrane-spanning subunit (TM) (16). The two subunits remain associated in an oligomeric complex that reaches the plasma membrane via the secretory pathway. The incorporation of the complex into nascent viral particles is frequently blocked by alterations in the globular core of MA (12, 51). However, second-site mutations which truncate TM distal to the membrane-spanning region can completely suppress the Env incorporation defect of MA mutants (17, 31). Moreover, removal of the cytoplasmic domain of TM can restore the ability of MA mutants to replicate in MT4 cells (31), a cell line which supports HIV-1 replication in the absence of the cytoplasmic domain of TM (49). Remarkably, efficient HIV-1 replication can be obtained even in the absence of the entire MA domain, provided that the cytoplasmic domain of TM is shortened (39). These results indicate that a major function of HIV-1 MA is to accommodate the unusually long cytoplasmic domain of TM.
The role of the globular core of MA in viral assembly remains ill defined. It has been reported that single amino acid substitutions in the α-helical core of MA can severely impair the production of extracellular particles and redirect particle formation to intracellular membrane compartments (18). A similar phenotype was observed for a mutant with a large in-frame deletion in MA (13). On the other hand, more extensive deletions which removed 80% or more of MA had relatively little effect on particle formation (29, 46). Intriguingly, it has also been shown that particle formation can be enhanced by deletions in α-helical regions of the MA core (12, 51) and even by deletions which remove most or all of the globular core of MA (39).
A possible explanation for these apparently contradictory results is offered by a myristyl switch model of Gag membrane binding. Such a model was originally proposed by Zhou and Resh to explain the relatively low affinity of mature MA for membranes, which may allow it to dissociate from the plasma membrane to participate in the nuclear import of the viral genome (53). However, a mechanism which modulates the exposure of the myristyl group could in principle also account for the selective targeting of Pr55gag to the plasma membrane. This concept is based on the examples of myristylated cellular proteins such as recoverin and ADP-ribosylation factor 1, for which the importance of regulated exposure of the myristyl group in membrane binding has been well documented (2, 3, 11, 38, 42, 54). In the case of MA, certain mutations may interfere with the exposure of the myristyl group and, as a consequence, block Gag membrane binding. In contrast, other alterations may interfere with the sequestration of the myristyl group by MA and thereby increase Gag membrane binding and particle assembly.
In support of this model, we show in the present study that substitutions at a conserved Leu residue between the myristyl anchor and the globular core of MA can dramatically impair Gag membrane binding and particle assembly without compromising Gag myristylation. Remarkably, these defects could be completely suppressed by single amino acid substitutions in the globular core of MA. We propose that mutations near the N terminus of MA reduced the availability of the myristyl group for membrane insertion, while the compensatory alterations in the globular core of MA prevented the sequestration of the myristyl group.
MATERIALS AND METHODS
Proviral constructs.
Site-directed mutagenesis was performed as described previously (23), using single-stranded DNA prepared from wild-type or mutant pSK + gag. The oligonucleotides used for mutagenesis were L8A (5′-TCCCCCGCTAGCTACTGACGCTC-3′), L8I (5′-TCTCCCCCGGATATCACTGACGCT-3′), W16A (5′-AATTTTTTCCGCGCGATCTAATTC-3′), K18A (5′-TAACCGAATGGCTTCCCATCGATC-3′), L21S (5′-CCCTGGTCTAGACCGAATTTTTTC-3′), and W36A (5′-CTCCCTGCTTGCCGCGACTATATG-3′). To generate full-length mutant proviral clones, 1.3-kb BssHII-ApaI fragments carrying the desired mutations were inserted into the infectious HXBH10 proviral construct (43), into HXBH10ΔCT (31), or into the subviral construct pGag/PR. HXBH10ΔCT is a variant of HXBH10 with a premature termination codon in place of codon 713 of env, and pGag/PR is a derivative of pHXBH10ΔenvCAT (44) that harbors a premature termination codon immediately after the PR coding sequence and an MscI-MscI deletion (nucleotides 2620 to 4552) in pol. The presence of mutations was confirmed by restriction endonuclease digestion and DNA sequencing.
Cell culture, transfection, and virus transmission.
HeLa cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. MT4 cells were maintained in RPMI 1640 medium with 10% fetal calf serum. HeLa cells (106) were seeded into 80-cm2 tissue culture flasks 24 h prior to transfection. The cells were transfected with 20 μg of plasmid DNA by a calcium phosphate precipitation technique (10). For virus replication studies, MT4 cells (5 × 106) were transfected by a DEAE-dextran procedure with 2 μg of proviral plasmid DNA. Viral replication was monitored by measuring particle-associated reverse transcriptase (RT) activity in the culture supernatants.
Viral protein analysis.
HeLa cell cultures were metabolically labeled for 12 h with [35S]cysteine (50 to 60 μCi/ml) or with [9,10-3H]myristic acid (100 μCi/ml) 48 h posttransfection. Labeled cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (140 mM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate [SDS]). Viral proteins were immunoprecipitated with serum from an individual infected with HIV-1 and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Viral particles released during the labeling period were pelleted through 20% sucrose cushions (in phosphate-buffered saline) for 2 h at 4°C and 27,000 rpm in a Beckman SW28 rotor. Pelleted virions were lysed in RIPA buffer, and viral proteins were either directly analyzed by SDS-PAGE or immunoprecipitated with patient serum prior to SDS-PAGE.
Subcellular fractionation.
Transfected and radiolabeled HeLa cells were suspended in ice-cold hypotonic buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA, 2 μg of antipain per ml, 2 μg of leupeptin per ml, 100 μg of phenylmethylsulfonyl fluoride per ml) and allowed to swell on ice for 15 min. Cells were disrupted on ice with 25 strokes of a Dounce homogenizer with a tight-fitting pestle. Homogenates were brought to 150 mM NaCl and 1 mM MgCl2, and nuclei, and cellular debris were removed by two successive low-speed centrifugations at 800 × g for 10 min at 4°C. The postnuclear supernatant was adjusted to 70% (wt/vol) sucrose in NTE buffer (100 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1 mM EDTA), placed at the bottom of a SW41 centrifuge tube, and overlaid with 65% (wt/vol) and 10% (wt/vol) sucrose. The flotation gradient was centrifuged in a Beckman SW41 rotor at 120,000 × g for 18 h at 4°C. Ten 1-ml fractions were taken from the top, and the density of each fraction was determined on an Abbe Mark II refractometer. The activity of 5′-nucleotidase, a marker enzyme for plasma membranes, was determined with a commercially available kit (Sigma Chemical Co., St. Louis, Mo.). After the addition of 5× RIPA buffer, viral proteins were immunoprecipitated from each fraction with patient serum and then subjected to SDS-PAGE.
Analysis of second-site revertant.
Total DNA from infected cells was purified with a QIAamp blood kit (Qiagen, Chatsworth, Calif.). A proviral segment encompassing the MA coding region was amplified by PCR and sequenced by using primers corresponding to HXBH10 nucleotide positions 685 to 702 (5′-GACGCAGGACTCGGCTTG-3′, sense) and 2438 to 2465 (5′-TAGCTTTATGTCCACAGATTTCTATGAG-3′, antisense).
RESULTS
Substitutions near the N terminus of MA that impair particle formation but not myristylation.
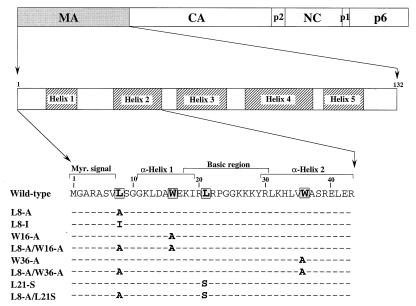
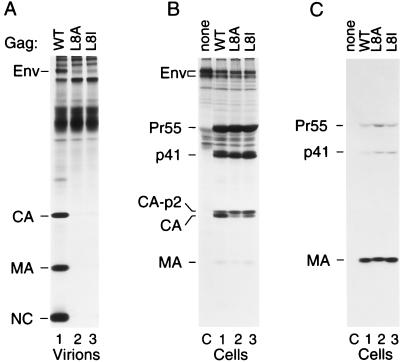
Leu8 of MA was selected for mutagenesis because this residue is situated between the N-terminal myristylation signal and the α-helical core of MA, a region that might be expected to be important for the positioning of the myristyl group (Fig. 1). Furthermore, Leu8 is invariant among primate lentiviruses (36), suggesting that it may be functionally important. When the codon for Leu8 was changed to a codon specifying Ala in the full-length HXBH10 proviral clone, virus replication in highly permissive CEMx174 cells was blocked, and a significant replication defect was observed even after the conservative substitution of Leu8 for Ile (data not shown). To examine whether virus morphogenesis was affected, the L8A and L8I mutant proviruses were transfected into HeLa cells, and viral particles released into the supernatant during 12 h of metabolic labeling were partially purified through 20% sucrose. Analysis of the particulate fraction by SDS-PAGE revealed that the L8A substitution essentially blocked viral particle production (Fig. 2A). Even the conservative L8I substitution caused a severe, albeit less pronounced, defect in particle production (Fig. 2A).
FIG. 1.
Schematic representation of MA substitution mutants. The domain organization of the HIV-1 Gag polyprotein is illustrated at the top. The shaded areas within the expanded view of the MA domain indicate the positions of α-helices (24). Changes made in the N-terminal third of MA are indicated below.
FIG. 2.
Substitutions of MA residue 8 impair particle production but not Gag myristylation. (A) HeLa cells were transfected with wild-type (WT) HXBH10 provirus or with the indicated mutants, followed by metabolic labeling with [35S]cysteine. Virions released during the 12-h labeling period were pelleted through 20% sucrose, and virion lysates were directly analyzed by SDS-PAGE. (B and C) HeLa cells were transfected with HXBH10-gag− (lane C), with wild-type HXBH10 (lane 1), or with the indicated mutants (lanes 2 and 3), and metabolically labeled for 12 h with [35S]methionine (B) or [3H]myristic acid (C). Viral proteins expressed by the transfected cells were immunoprecipitated with patient serum and separated by SDS-PAGE.
Because of the proximity of the myristic acid attachment site, it appeared possible that Leu8 is a critical component of the signal required for myristylation. To examine this possibility, HeLa cells transfected with the parental or mutant proviruses were metabolically labeled either with 35SExpress, which contains mainly [35S]methionine, or with [3H]myristic acid. Immunoprecipitation from the cultures labeled with 35SExpress showed that the defects in particle production were not due to low expression levels of the mutant Gag proteins (Fig. 2B). The L8A mutant Gag precursor in particular tended to accumulate intracellularly (see Fig. 9B), although to an extent that varied somewhat between experiments. Consistent with impaired assembly, a Gag processing defect was apparent from an increase in the ratio between the late processing intermediate CA-p2 (1) and fully processed CA (Fig. 2B). MA was only poorly labeled by 35SExpress because it lacks methionine residues. After metabolic labeling with [3H]myristic acid, MA was precipitated as a prominent band from cells transfected with the wild-type provirus (Fig. 2C, lane 2). As expected (23), weaker bands which corresponded to Pr55gag and to the Gag processing intermediate p41 (MA-CA-p2) were also visible. Interestingly, similar amounts of labeled myristic acid were incorporated into the corresponding Gag products of the mutant viruses (Fig. 2C, lanes 3 and 4), indicating that neither the L8A nor the L8I mutation interfered with Gag myristylation.
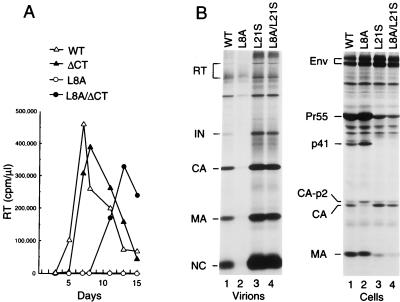
FIG. 9.
Emergence of a compensatory change in an infected culture which corrects the particle production defect of the L8A mutant. (A) An Env cytoplasmic tail truncation (ΔCT) allows replication of the L8A mutant. MT4 cells were transfected with the parental HXBH10 proviral construct (wild type [WT]) or with the indicated mutants, and viral replication was monitored by measuring RT activity in the culture supernatants. (B) Effect of the L21S compensatory change on particle production. Lysates of virions released from transfected HeLa cells during metabolic labeling with [35S]cysteine were directly analyzed by SDS-PAGE (left); cell-associated viral proteins were immunoprecipitated with patient serum (right).
The L8A mutation impairs Gag membrane binding.
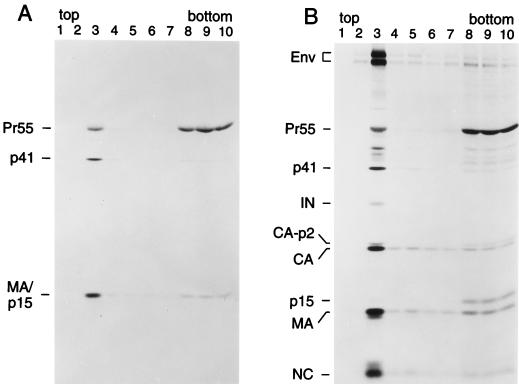
Having shown that the assembly defect of the L8A mutant is not due to inefficient Gag myristylation, we asked whether the plasma membrane targeting of Gag was affected despite apparently normal levels of myristylation. For subcellular fractionation studies, we used a subviral construct designated pGag/PR, which has the capacity to express Pr55gag as well as a truncated Gag-Pol precursor that provides a functional PR. The parental pGag/PR construct and the L8A mutant version were transfected into HeLa cells and then subjected to metabolic labeling for 12 h with [35S]cysteine. The labeled cells were disrupted by Dounce homogenization, and postnuclear supernatants were adjusted to 70% sucrose and placed at the bottom of a discontinuous sucrose gradient consisting of 65 and 10% sucrose. Following centrifugation to equilibrium, fractions were collected from the top of the gradient and analyzed individually by immunoprecipitation with patient serum. In parallel, we examined each fraction for the presence of 5′-nucleotidase activity, a conventional plasma membrane marker (4, 25).
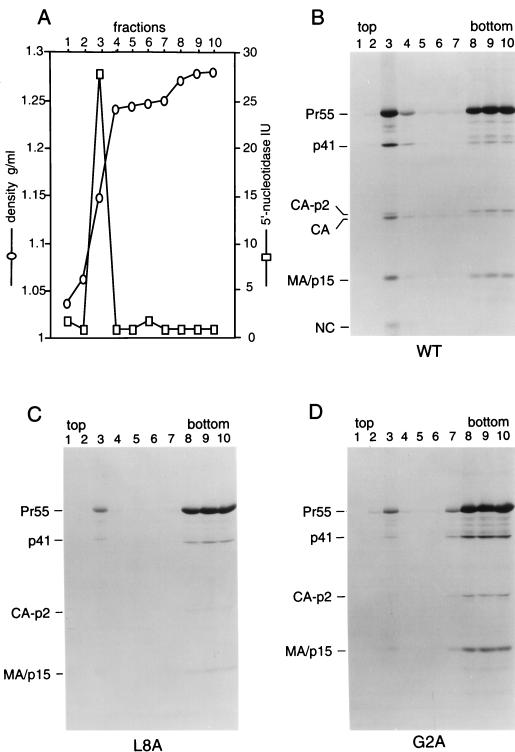
Figures 3A and B show representative results obtained with the parental pGag/PR construct. More than 90% of the total 5′-nucleotidase activity recovered from the gradient was detected in fraction 3, a region where the sucrose concentration rose sharply (Fig. 3A). This result, which was highly reproducible, indicates that plasma membranes had largely floated up to the 10%-65% interface and were essentially absent from the bottom of the gradient. While Pr55gag and several Gag cleavage products peaked sharply in fraction 3, a significant amount of Gag protein was also found near the bottom of the gradient (Fig. 3B). Quantitation by scanning densitometry indicated that not more than 40% of wild-type Pr55gag fractionated with plasma membranes. Interestingly, the L8A mutation caused a significant reduction in the amount of Gag protein that floated to the plasma membrane-containing fraction (Fig. 3C). About 95% of the mutant Gag protein was found in the plasma membrane-free bottom fractions as determined by scanning densitometry. The effect of the L8A mutation on Gag membrane binding was comparable to that of the G2A mutation (Fig. 3C and D), which blocks particle formation because it prevents the attachment of myristic acid to MA (23). Moreover, the L8A and G2A mutations both severely impaired the conversion of CA-p2 to mature CA, a late processing event which is likely to depend on a high local concentration of PR (48). Since the L8A mutation, in contrast to the G2A change, allowed the attachment of myristic acid, these results raised the possibility that the L8A mutation instead interfered with the availability of the myristic acid moiety for membrane insertion.
FIG. 3.
Effects of MA mutations on the subcellular distribution of Gag proteins. HeLa cells transfected with the parental (wild-type [WT]) Gag/PR construct (A and B) or with the indicated MA mutants (C and D) were metabolically labeled with [35S]cysteine for 12 h. Homogenates were prepared and fractionated by flotation in a discontinuous sucrose gradient as described in Materials and Methods. Fractions were collected from the top, and aliquots were analyzed for density and 5′-nucleotidase activity. HIV-1 Gag proteins were immunoprecipitated from each fraction with patient serum and resolved by SDS-PAGE.
The finding that the majority of wild-type Pr55gag failed to float to the plasma membrane-containing fraction was surprising, because previous subcellular fractionation studies had demonstrated that the Gag precursor of another retrovirus, Moloney murine leukemia virus, becomes rapidly and efficiently associated with the plasma membrane (41). We therefore examined whether the Pr55gag which remained in the bottom fractions was myristylated. While MA and the processing intermediate p41 (MA-CA-p2) were predominantly detected in the plasma membrane-containing fraction after metabolic labeling with [3H]myristic acid, the majority of labeled Pr55gag was again found in the bottom fractions (Fig. 4A). To examine whether the subviral Gag/PR construct lacked a viral factor required for the efficient membrane association of Gag, we also used the infectious proviral construct DFCI-HT, which is intact for all known HIV-1 gene functions (28). As shown in Fig. 4B, the viral envelope glycoproteins produced by this full-length construct became highly enriched at the 10%-65% sucrose interface, indicating that membranes were well separated from cytosolic material. Fully mature gag- and pol-encoded products were also clearly enriched in the plasma membrane-containing fraction. However, the majority of Pr55gag again remained in the bottom fractions. It is also noteworthy that at least 90% of the processing intermediate p41 (MA-CA-p2) floated up to the 10%-65% sucrose interface, while the corresponding C-terminal processing intermediate p15 (NC-p1-p6) remained in the membrane-free fractions. In contrast to the p15 species produced by the HXB2-derived Gag/PR, DFCI-HT p15 could be readily distinguished from MA, because a duplication in p6 reduces its electrophoretic mobility. Taken together, these results suggest that a significant fraction of myristylated Pr55gag exists in a membrane-free form or is readily dislodged from the membrane during cell fractionation.
FIG. 4.
Membrane-free Pr55gag is myristylated and is also detected when expressed from a complete provirus. HeLa cells were transfected with the Gag/PR subviral construct and labeled with [3H]myristic acid (A) or transfected with the full-length DFCI-HT proviral clone and labeled with [35S]cysteine (B). Subcellular fractionation on discontinuous sucrose gradients was then performed as described in the legend to Fig. 3.
The membrane binding and assembly defects of the L8A mutant can be corrected by single amino acid substitutions within the globular core of MA.
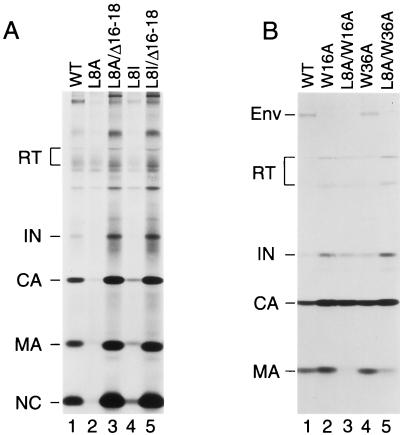
Spearman et al. (40) recently showed that deletions within α-helical regions of MA can dramatically increase its affinity for membranes, suggesting that the myristic acid moiety cannot be sequestered in the absence of an intact α-helical core. Consistent with this model, we previously reported that a small deletion termed Δ16-18, which removes a portion of helix 1 of MA, significantly increases particle assembly both at the plasma membrane and at intracellular membranes (12). Remarkably, the Δ16-18 deletion completely suppressed the effect of the L8A mutation on particle production (Fig. 5A, lanes 2 and 3). Similar to what was previously observed with a provirus that harbored only the Δ16-18 mutation (12), a double mutant which carried both the Δ16-18 and the L8A change yielded about fourfold more extracellular particles than the wild-type construct (Fig. 5A, lanes 1 and 3). Comparable results were obtained when the Δ16-18 deletion was combined with the L8I mutation (Fig. 5A, lanes 4 and 5). These results support the hypothesis that Leu8 is critical for the exposure of the myristic group in the context of the wild-type MA domain but is no longer required when the α-helical core of MA is disrupted.
FIG. 5.
Second-site mutations in the globular core of MA can rescue particle production by Leu8 mutants. HeLa cells were transfected with wild-type HXBH10 proviral DNA or with the indicated MA mutants and metabolically labeled with [35S]cysteine. Viral particles released during the 12-h labeling period were pelleted through sucrose cushions and directly analyzed by SDS-PAGE (A) or immunoprecipitated with patient serum prior to SDS-PAGE (B).
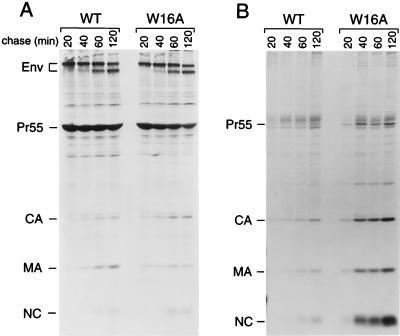
Apart from two charged residues, the Δ16-18 mutation removes a conserved tryptophan (Trp16) which projects into the hydrophobic core of MA. Because it is known that conserved aromatic residues contribute significantly to the myristyl-binding pocket of recoverin (42), we chose to individually replace both Trp16 in α-helix 1 and the highly conserved Trp36 in α-helix 2 of MA with Ala. Additionally, Lys18, one of the charged residues targeted by the Δ16-18 deletion, was individually changed to Ala. When introduced into the parental HXBH10 provirus, the W16A and W36A mutations both increased viral particle yields to a similar extent as the Δ16-18 deletion (Fig. 5B and data not shown). It is also noteworthy that the W16A mutation drastically reduced the incorporation of Env glycoprotein into viral particles, while the W36A mutation did not (Fig. 5B). Immunoprecipitation from the lysates of the transfected HeLa cells revealed that the W16A and W36A mutations markedly reduced the steady-state levels of cell-associated Pr55gag (data not shown). Consistent with this finding, the W16A mutation accelerated the loss of Pr55gag from the cell-associated fraction in a pulse-chase experiment (Fig. 6A). Concomitantly, the W16A mutation significantly accelerated the kinetics of extracellular viral particle release. It is evident from Fig. 6B that the amount of mutant particles at the 40-min time point already exceeded the amount of wild-type particles released within 2 h.
FIG. 6.
The W16A mutation in the globular core of MA markedly accelerates the kinetics of extracellular particle release. HeLa cells transfected with the wild-type (WT) HXBH10 provirus or with a variant carrying the W16A mutation were pulsed-labeled with [35S]cysteine for 30 min and chased for the times indicated. The cells were then lysed, and viral proteins were immunoprecipitated with patient serum (A). Particles released during the labeling period were pelleted through 20% sucrose, and their protein content was directly analyzed by SDS-PAGE (B).
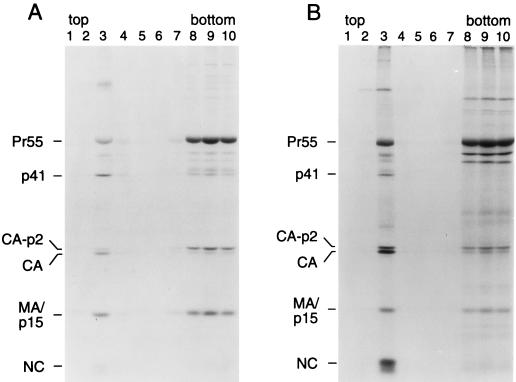
Remarkably, the W16A and W36A single amino acid substitutions were each sufficient to completely suppress the effect of the L8A mutation on particle formation (Fig. 5B). In contrast, the K18A mutation was unable to suppress the assembly defect of the L8A mutant and did not increase particle formation when introduced into the wild-type provirus (data not shown). Mutations in MA frequently affect its recognition by patient sera (12), which probably explains why little MA could be immunoprecipitated from L8A/W16A and L8A/W36A virions (Fig. 5B). Subcellular fractionation by flotation in a discontinuous sucrose gradient showed that the W16A mutation restored the ability of Leu8 mutant Gag to efficiently associate with membranes (Fig. 7). Quantitation by scanning densitometry of the autoradiographs shown in Fig. 7 indicated that the amount of membrane-associated L8A/W16A Gag relative to that in the plasma membrane-free fractions was about twofold increased in comparison to wild type.
FIG. 7.
The W16A second-site mutation neutralizes the membrane binding defect of the L8A mutant. HeLa cells transfected with the parental Gag/PR construct (A) or with the L8A/W16A double mutant variant (B) were metabolically labeled with [35S]cysteine for 12 h. Subcellular fractionation on discontinuous sucrose gradients was then performed as described in the legend to Fig. 3.
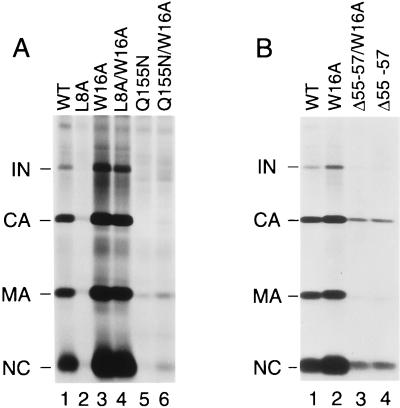
We also examined whether the W16A mutation alleviates the assembly defects of other Gag mutants. Interestingly, the previously documented assembly defect caused by the Q155N substitution in the major homology region of CA (32) was unaffected by the W16A mutation (Fig. 8A). Furthermore, the W16A mutation did not alleviate the relatively moderate assembly defect caused by a deletion (Δ55-57) in the central buried helix of MA (12). We conclude that the effect of the W16A mutation was selective and that the compensatory mutation specifically corrected the Gag membrane binding defect of the L8A mutant.
FIG. 8.
The W16A mutation does not generally alleviate the particle production defects of Gag mutants. HeLa cells were transfected with the parental HXBH10 proviral construct (wild type [WT]) or with mutant variants which harbor changes in MA (L8A, W16A, and Δ55-57) and/or CA (Q155N). Lysates of virions released during metabolic labeling with [35S]cysteine were directly analyzed by SDS-PAGE. Panels A and B show the results of independent experiments.
A second-site revertant of the L8A mutant.
L8A/W36A double-mutant particles contained significantly less Env than W36A particles (Fig. 5B, lanes 4 and 5), indicating that the L8A mutation interfered with Env incorporation. To examine whether an Env incorporation defect contributed to the replication defect of the L8A mutant, we constructed a variant which harbored the previously described ΔCT mutation in env (31, 49). This mutation truncates the long cytoplasmic tail of TM and thereby restores the ability of certain MA mutants to incorporate Env and to replicate in MT4 cells (31).
As shown in Fig. 9A, the ΔCT mutation partially corrected the replication defect of the L8A mutant. After transfection into MT4 cells, the L8A mutant did not replicate, but the L8A/ΔCT mutant yielded a rapid rise in RT activity after a delay of about 1 week relative to the wild-type virus or to a variant which carried only the ΔCT mutation (Fig. 9A). Since accelerated replication kinetics were observed after passage of the L8A/ΔCT virus, we extracted DNA from infected cells and amplified gag and env sequences. Sequence analysis of the PCR fragments showed that the putative revertant retained the L8A and ΔCT mutations, and in addition contained a base change resulting in a predicted substitution of Ser for Leu at position 21 of MA. A variant of the L8A/ΔCT mutant into which the L21S change was introduced by site-directed mutagenesis yielded similar virus replication kinetics in MT4 cells as the ΔCT mutant (data not shown), demonstrating that the second-site mutation in MA conferred a revertant phenotype.
Interestingly, Leu21 is invariant among primate lentiviruses (36). To examine whether the L21S substitution affected particle formation, the mutation was introduced into the parental HXBH10 proviral construct. Transfection into HeLa cells showed that the L21S change, in the context of an otherwise wild-type MA domain, clearly enhanced the yield of extracellular particles and concomitantly reduced the steady-state level of cell-associated Pr55gag (Fig. 9B, lanes 1 and 3). Importantly, the L21S mutation also effectively suppressed the assembly defect of the L8A mutant, improving particle production dramatically from the level observed in the presence of the L8A mutation alone (Fig. 9B, lanes 2 and 4). Thus, the L21S change, located within a highly conserved basic region that has previously been implicated in Gag membrane binding (52), had effects very similar to those of the above-described W16A and W36A substitutions in the α-helical core of MA.
DISCUSSION
The examples of recoverin and ADP-ribosylation factor 1 strongly suggest that the subcellular localization of myristylated proteins can be subject to regulation by a mechanism which controls the exposure of the myristyl group (2, 3). In the case of recoverin, where the extrusion of the myristyl group is triggered by Ca2+ binding (11, 54), the solution structures of the Ca2+-free and Ca2+-bound forms have both been determined (2, 42). In the Ca2+-free state, the myristyl group is buried in a deep pocket formed by five α-helices, where it is contacted by conserved aromatic and other nonpolar residues (42). Ca2+ binding causes a dramatic rearrangement of the helices surrounding the pocket and the unclamping of the myristyl group. N-terminal residues, which are disordered in the Ca2+-bound form, provide a flexible arm which allows the myristyl group to move out of its binding pocket (2).
Whether the α-helical core of HIV-1 MA can sequester the N-terminal myristyl group is unknown, because only the structures of nonmyristylated forms of MA have been reported to date (24, 33, 34). However, several findings lend support to the recent suggestion (53) that the membrane affinity of MA is regulated by a myristyl switch mechanism. Yu et al. (50) observed that phorbol ester treatment induced both the phosphorylation of MA and its rapid translocation to membranes. This behavior was attributed to a phosphorylation-induced conformational change which promoted the exposure of the myristyl group. Zhou and Resh (53) reported that the membrane affinity of HIV-1 MA increased after removal of the last α-helix (helix 5) and proposed that this helix regulates the exposure of the N-terminal myristate. More recently, Spearman et al. (40) showed that deletions which affect helices 1 to 4 can dramatically increase the in vivo association of MA with membranes, indicating that the entire α-helical core of MA is involved in the sequestration of the myristyl group.
The present study shows that significant amounts of myristylated Pr55gag can remain in a non-membrane-associated pool. We obtained comparable results when COS-7 rather than HeLa cells were used (data not shown), demonstrating that this phenomenon is not restricted to a single cell line. It was previously observed that HIV-1 Gag, when expressed alone, becomes less efficiently membrane associated than Moloney murine leukemia virus Gag, prompting the proposal that additional HIV-1 proteins are required (41). However, in our subcellular fractionation studies the majority of Pr55gag was not membrane bound even when expressed from a full-length provirus that contains all known viral genes. The relative amount of membrane-associated Pr55gag did not increase when cells were disrupted under isotonic conditions using nitrogen cavitation or if an isosmotic iodixanol density gradient was used for fractionation (data not shown). These observations indicated that myristylation per se does not ensure the stable association of Pr55gag with the plasma membrane, perhaps because the myristyl group is not always available for membrane insertion.
In support of a myristyl switch model of Gag membrane binding, we find that assembly defects caused by modification of the myristylated N terminus of MA can be completely suppressed by second-site mutations in its globular core. Substitutions at the highly conserved Leu8 of MA had no apparent effect on Gag myristylation but nevertheless caused a phenotype which resembled that of a mutant which lacked the myristic acid attachment site. Because previous studies have shown that complete inhibition of Gag myristylation is required to inhibit HIV-1 particle production (19, 35), the phenotypes of the Leu8 mutants could not be explained by subtle defects in myristylation. Interestingly, similar effects on Gag membrane binding and particle assembly were recently observed when Leu8 in the MA domain of a simian immunodeficiency virus was individually mutated (22). The latter mutant harbored a Leu8-to-Glu substitution, and it was speculated that the charged residue disrupted crucial hydrophobic interactions between the region surrounding Leu8 and the plasma membrane. However, in the present study, the conservative replacement of Leu8 of HIV-1 MA by Ile had a comparable effect on virus assembly. Also, Leu8 is not required for efficient HIV-1 particle assembly if the globular core of MA is deleted entirely (39), arguing against a direct role of Leu8 in membrane binding.
In the case of Mason-Pfizer monkey virus, myristylation is not required for the assembly of capsids in the cytoplasm, but is necessary for their membrane envelopment and release (26). Interestingly, certain substitutions in the MA domain of Mason-Pfizer monkey virus which introduce hydrophobic residues that point into its α-helical core prevent the membrane association of preassembled capsids, and it has been proposed that these changes stabilize the sequestered state of the myristyl group within MA (9). We therefore considered the possibility that the replacement of Leu8 of HIV-1 MA interfered with the availability of the myristyl group for membrane insertion. This hypothesis suggested that efficient particle assembly and release might be restored by second-site mutations which increase the exposure of the myristyl group. Accordingly, we targeted aromatic residues within α-helical regions, because such residues contribute to the sequestration of the myristyl group of recoverin (42). We find that even single amino acid substitutions at conserved aromatic positions in the globular core of MA are sufficient to completely neutralize both the membrane binding defects and the assembly defects of Leu8 mutants. Moreover, the second-site mutations increased Gag membrane binding as well as extracellular particle yields to levels which consistently exceeded those obtained with the wild-type construct. Because particle formation remained absolutely dependent on the presence of the myristyl acceptor Gly2 (data not shown), we consider it unlikely that the increases in membrane binding and particle yields were secondary to an increased exposure of hydrophobic residues in the MA core domain. Also, we previously observed a comparable increase in extracellular particle yield when the entire MA core domain was deleted and only the N-terminal myristylation signal was retained (39). This finding indicated that the myristylated N terminus, when exposed in the absence of the globular core of MA, is more efficient than the intact MA domain in anchoring the Gag polyprotein to membranes. Since the effects of the compensatory mutations in the globular core of MA mimicked those seen when only the myristyl anchor was retained, we propose that the single amino acid substitutions led to the constitutive exposure of the myristyl group.
Passage of a Leu8 mutant in highly permissive MT4 cells yielded another second-site change in MA which restored efficient particle assembly. Because the replacement of Leu8 with Ala impaired Env incorporation, it was necessary to remove the cytoplasmic domain of TM to obtain virus replication. A culture infected with this double mutant yielded a variant with an additional change predicted to replace the highly conserved Leu21 of MA with Ser. In the presence of this additional change, the Leu8-to-Ala substitution again no longer had any effect on assembly, and the production of extracellular particles exceeded wild-type levels. Interestingly, Leu21 is located within a highly conserved basic region of MA (Fig. 1). This conserved region adopts a β-strand structure which protrudes from the molecule and exposes several basic residues that are thought to contribute to the plasma membrane targeting of Pr55gag by binding to acidic phospholipids (24, 53). It has been shown that an N-terminal portion of MA which includes the conserved basic region can function as an autonomous membrane targeting domain (52). Furthermore, substitution of all the positively charged residues in the conserved basic region markedly impaired HIV-1 particle production (52), as expected if the basic residues are involved in membrane binding. Intriguingly, in good agreement with the present study, it was very recently reported that changes at Leu21 increase the binding of HIV-1 Gag to membranes and accelerate the kinetics of virion release (27). Perhaps the conserved hydrophobic residue is required to maintain a conformation which allows the sequestration of the myristyl group, and the adjacent basic residues have a role in the conformational change required for its exposure.
Collectively, the results of the present study support the hypothesis that the core domain of MA controls the exposure of the N-terminal myristate to ensure the targeting of Gag to a specific membrane compartment. It is noteworthy in this respect that mutations which affect the globular core of MA often cause indiscriminate assembly both at the plasma membrane and at intracellular membranes (8, 12, 13, 18, 39), a phenotype that might be expected if the myristyl group is constitutively available for membrane binding. Further insights into the function of the myristyl group are likely to come from structural studies on myristylated MA.
ACKNOWLEDGMENTS
We thank Fabrizio Mammano for an initial characterization of the L8A and L8I mutants.
This work was supported by National Institutes of Health grants AI42510 and AI28691 (Center for AIDS Research) and by a gift from the G. Harold and Leila Y. Mathers Charitable Foundation.
REFERENCES
- 1.Accola M, Höglund S, Göttlinger H G. A putative α-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. J Virol. 1998;72:2072–2078. doi: 10.1128/jvi.72.3.2072-2078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames J B, Ishima R, Tananka T, Gordon J I, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 3.Amor J C, Harrison D H, Kahn R A, Ringe D. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature. 1994;372:704–708. doi: 10.1038/372704a0. [DOI] [PubMed] [Google Scholar]
- 4.Avruch J, Wallach D F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971;233:334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- 5.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky M I, Haggerty S, Dempsy M P, Sarova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon P M, Matthews S, Clark N, Byles E D, Iourin O, Hockley D J, Kingsman S M, Kingsman A J. Structure-function studies of the human immunodeficiency virus type 1 matrix protein, p17. J Virol. 1997;71:3474–3483. doi: 10.1128/jvi.71.5.3474-3483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte M R, Klikova M, Hunter E, Ruml T, Matthews S. The three-dimensional structure of the matrix protein from the type D retrovirus, the Mason-Pfizer monkey virus, and implications for the morphology of retroviral assembly. EMBO J. 1997;16:5819–5826. doi: 10.1093/emboj/16.19.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen B R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 11.Dizhoor A M, Chen C-K, Olshevskaya E, Sinelnikova V V, Phillipov P, Hurley J B. Role of the acylated amino terminus of recoverin in CA2+-dependent membrane interaction. Science. 1993;259:829–832. doi: 10.1126/science.8430337. [DOI] [PubMed] [Google Scholar]
- 12.Dorfman T, Mammano F, Haseltine W A, Göttlinger H G. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1994;68:1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fäcke M, Janetzko A, Shoeman R L, Kräusslich H-G. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J Virol. 1993;67:4972–4980. doi: 10.1128/jvi.67.8.4972-4980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freed E O, Englund G, Martin M A. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed E O, Martin M A. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J Biol Chem. 1995;270:23883–23886. doi: 10.1074/jbc.270.41.23883. [DOI] [PubMed] [Google Scholar]
- 17.Freed E O, Martin M A. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific single amino acid substitutes in the human immunodeficiency virus type 1 matrix. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed E O, Orenstein J M, Buckler-White A J, Martin M A. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J Virol. 1994;68:5311–5320. doi: 10.1128/jvi.68.8.5311-5320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuishi K, Matsuoka H, Takama M, Takahashi I, Misumi S, Shoji S. Blockade of N-myristoylation of HIV-1 Gag induces the production of impotent progeny virus. Biochem Biophys Res Commun. 1997;237:504–511. doi: 10.1006/bbrc.1997.7178. [DOI] [PubMed] [Google Scholar]
- 20.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein as a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 21.Garnier L, Bowzard J B, Willis J. Recent advances and remaining problems in HIV assembly. AIDS. 1998;12:S5–S16. [PubMed] [Google Scholar]
- 22.González S A, Affranchino J L. Substitution of leucine 8 in the simian immunodeficiency virus matrix protein impairs particle formation without affecting N-myristylation of the Gag precursor. Virology. 1998;240:27–35. doi: 10.1006/viro.1997.8919. [DOI] [PubMed] [Google Scholar]
- 23.Göttlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill C P, Worthylake D, Bancroft D P, Christensen A M, Sundquist W I. Crystal structure of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc Natl Acad Sci USA. 1996;93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard A L, Wall D A, Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983;96:217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 27.Kiernan R E, Ono A, Englund G, Freed E O. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langhoff E, Terwilliger E F, Bos H J, Kalland K H, Posnansky M C, Bacon O M L, Haseltine W A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci USA. 1991;88:7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P P, Linial M L. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J Virol. 1994;68:6644–6654. doi: 10.1128/jvi.68.10.6644-6654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;8:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mammano F, Kondo E, Sodroski J, Bukovsky A, Göttlinger H G. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J Virol. 1995;69:3824–3830. doi: 10.1128/jvi.69.6.3824-3830.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mammano F, Öhagen A, Höglund S, Göttlinger H G. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J Virol. 1994;68:4927–4936. doi: 10.1128/jvi.68.8.4927-4936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massiah M A, Starich M R, Paschall C, Summers M F, Christensen A M, Sundquist W I. Three-dimensional structure of the human immunodeficiency virus type 1 matrix protein. J Mol Biol. 1994;244:198–223. doi: 10.1006/jmbi.1994.1719. [DOI] [PubMed] [Google Scholar]
- 34.Matthews S, Barlow P, Boyd J, Barton G, Russell R, Mills H, Cunningham M, Meyers N, Burns N, Clark N, Kingsman S, Kingsman A, Campbell I. Structural similarity between the p17 matrix protein of HIV-1 and interferon-γ. Nature. 1994;370:666–668. doi: 10.1038/370666a0. [DOI] [PubMed] [Google Scholar]
- 35.Morikawa Y, Hinata S, Tomoda H, Goto T, Nakai M, Aizawa C, Tanaka H, Omura S. Complete inhibition of human immunodeficiency virus Gag myristoylation is necessary for inhibition of particle budding. J Biol Chem. 1996;271:2868–2873. doi: 10.1074/jbc.271.5.2868. [DOI] [PubMed] [Google Scholar]
- 36.Myers G, Korber B, Wain-Hobson S, Smith R F, Pavlakis G N. Human retroviruses and AIDS 1993. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratory; 1993. [Google Scholar]
- 37.Pal R, Reitz M S, Tschachler E, Gallo R C, Sarngadharan M G, Veronese F D M. Myristoylation of gag proteins of HIV-1 plays an important role in virus assembly. AIDS Res Hum Retroviruses. 1990;6:721–730. doi: 10.1089/aid.1990.6.721. [DOI] [PubMed] [Google Scholar]
- 38.Randazzo P A, Terui T, Sturch S, Fales H M, Ferrige A G, Kahn R A. The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J Biol Chem. 1995;270:14809–14815. doi: 10.1074/jbc.270.24.14809. [DOI] [PubMed] [Google Scholar]
- 39.Reil H, Bukovsky A A, Gelderblom H R, Göttlinger H G. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 1998;17:2699–2708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suomalainen M, Hultenby K, Garoff H. Targeting of Moloney murine leukemia virus Gag precursor to the site of virus budding. J Cell Biol. 1996;135:1841–1852. doi: 10.1083/jcb.135.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, Ames J B, Harvey T S, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- 43.Terwilliger E T, Cohen E A, Lu Y, Sodroski J G, Haseltine W A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci USA. 1989;86:5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C, Sodroski J, Göttlinger H G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 45.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6292–6296. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C-T, Zhang Y, McDermott J, Barklis E. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J Virol. 1993;67:7067–7076. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinberg J B, Matthews T J, Cullen B R, Malim M H. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human cells. J Exp Med. 1991;174:1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiegers K, Rutter G, Kottler H, Tessmer U, Hohenberg H, Kräusslich H-G. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J Virol. 1998;72:2846–2854. doi: 10.1128/jvi.72.4.2846-2854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilk T, Pfeiffer T, Bosch V. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology. 1992;189:167–177. doi: 10.1016/0042-6822(92)90692-i. [DOI] [PubMed] [Google Scholar]
- 50.Yu G, Shen F S, Sturch S, Aquino A, Glazer R I, Felsted R L. Regulation of HIV-1 Gag protein subcellular targeting by protein kinase C. J Biol Chem. 1995;270:4792–4796. doi: 10.1074/jbc.270.9.4792. [DOI] [PubMed] [Google Scholar]
- 51.Y X, Xin Y, Matsuda Z, Lee T-H, Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou W, Parents L J, Willis J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou W, Resh M D. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci USA. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]