Abstract
Background
This study aimed to determine if greater variability in body mass index (BMI) is associated with declines in physical functioning and incident disability in older adults.
Methods
Included were participants from the Health, Aging and Body Composition Study who had semi‐annual BMI data during the first 3 years of follow‐up. Participants were categorized into quintiles of BMI variability, using two methods. The first method used average successive variability, whereas the second method adjusted these values to remove the variability due to net change in BMI over the 3‐year period. Linear regression was used to assess the relationship between the two measures of BMI variability and net changes in BMI, fat mass index, appendicular lean mass index, and Health, Aging and Body Composition Physical Performance Score during the first 3 years of the study. Cox proportional hazard models were used to assess the relationship of BMI variability with the subsequent incidence of new disability, adjusting for confounding factors.
Results
Among 2121 participants, those in the highest BMI variability quintile were more likely to lose both body mass (β: −0.086 [95% confidence interval, CI: −0.133, −0.040], P < 0.01) and fat mass (β: −0.059 [95% CI: −0.117, −0.002], P = 0.04) and had greater declines in physical performance score (β: −0.094 [95% CI: −0.162, −0.026], P < 0.01) compared to participants with the least variability in BMI. Participants with high BMI variability also had higher rates of incident disability (hazard ratio: 1.36 [95% CI: 1.07, 1.72], P = 0.01), independent of net BMI change.
Conclusions
BMI variability in older adults is associated with decline in physical performance and incident disability. This relationship cannot be explained by net weight loss alone, supporting it as an independent feature of frailty.
Keywords: body composition, disability, weight fluctuation
Introduction
Frailty is a common syndrome associated with an increased risk of new‐onset disability and adverse health outcomes among older adults. It has been broadly defined as a systemic decline in physiological reserves and resilience associated with ageing and accelerated by disease. 1 Clinically, frailty presents as a combination of weakness, exhaustion, unintentional weight loss, slowness and/or low physical activity. A prominent feature is sarcopenia, or loss of muscle mass or function, which naturally occurs with ageing but can be heightened by nutritional deficits, disease and inflammation, inactivity, and hormonal and metabolic changes. Frailty affects a sizable proportion of older adults and increases the risk of incident physical disability, 2 , 3 cognitive decline, 4 falls and fractures, 5 heart failure 6 and overall mortality. 7 However, evidence suggests that early intervention can limit the progression of frailty to disability or even reverse the syndrome. 8 , 9 Therefore, identifying those at greatest risk of becoming frail and developing comorbidities is a worthwhile clinical objective.
Frailty is often associated with a long‐term decrease in body mass, and gains in weight over time may also increase the risk for poor physical function and disability. However, it is unclear how fluctuations in weight over time might be associated with the development of frailty, disability and other long‐term outcomes independent from the net overall changes in weight during the same period. Previous studies have defined body weight cycling in various ways and shown associations with adverse changes in body composition and adverse health effects. For example, Murphy et al. found that the presence of both weight gain and loss of 5% of weight in the first 4 years of the Health, Aging and Body Composition (Health ABC) Study was associated with an increased risk of disability, as was unidirectional weight loss. 3 More recently, Bangalore et al. showed that patients with cardiovascular disease who had average successive body weight fluctuations greater than one standard deviation above the mean had significantly increased risk of cardiovascular events and death. 10 Other studies have shown that weight fluctuation was associated with increased rates of cancer 11 in older women, as well as other types of incident disease (myocardial infarction, stroke, diabetes and hip fracture). 12 , 13 , 14
Given the strong association between frailty, body composition and metabolism, it is plausible that high body weight variability and related metabolic dysregulation could be an important marker of the risk of frailty among older adults. Because weight variability may occur prior to or independent of significant net weight loss, it is also of interest to investigate weight variability as a clinical predictor of adverse long‐term outcomes. The observation of fluctuating weight could be used to identify those at greatest risk and to target interventions early in the disease process. In addition to enabling early intervention, the investigation of body weight variability will help address the current knowledge gap surrounding the complex aetiology of frailty.
To elucidate the relationship between body weight variability and frailty, we conducted a novel analysis of an existing longitudinal cohort of older, community‐dwelling adults, from the Health ABC Study. We hypothesized that people who experience the greatest variability in body mass index (BMI) over time, after adjusting for net change in BMI, would be at the highest risk of incident disability. Specifically, we aimed to (1) determine if BMI variability over a 3‐year period is independently associated with a decline in physical functioning and/or declines in BMI, appendicular lean mass index (ALMI) and fat mass index (FMI) among older adults over the same period and (2) determine if older adults who have experienced more BMI variability are at greater risk of incident disability over the following decade. To accomplish these aims, we built upon prior observations by advancing the methods of quantifying BMI variability in order to assess prediction of incident disability independent of baseline BMI and net change in BMI change over the same time period.
Methods
Study setting
The study design is illustrated in Figure S1 . We analysed data from the Health ABC Study, a prospective observational study focused on decline of function and changes in body composition of well‐functioning older persons. The study enrolled 3075 community‐dwelling men and women aged 70–79 at baseline, beginning in 1997. The participants were recruited from a random sample of Medicare beneficiaries living within 1 h of two sites: Pittsburgh, PA (41%), and Memphis, TN (59%). The Black participant sample was augmented by recruitment from age‐eligible community‐dwelling older adults who were not Medicare recipients, resulting in a sample that was 33% Black and 67% White. All participants reported being able to walk a quarter of a mile, climb stairs and perform all activities of daily living without difficulty at baseline and did not have known life‐threatening cancer diagnoses. Annual clinical examinations were conducted for 6 years as well as follow‐up exams at Years 8 and 10. Additionally, semi‐annual phone calls to update health and functional status were conducted throughout and were continued for up to 16 years. Individuals who were followed at least to Year 4 and had semi‐annual assessments of weight were included in this analysis (N = 2121). We excluded participants if they were lost to follow‐up or missing any weight values (n = 952) in the first 3 years of the study. We required the presence of all seven BMI values because the participants whose BMI variabilities were calculated with fewer measures had systematically higher BMI variability than those with all values present. We also excluded participants with any recorded weight that appeared erroneous based on any single 6‐month change in BMI of >6.0 kg/m2 from that participant's nearest BMI value (n = 70).
Exposure: Body mass index variability
At annual in‐person visits, body weight was measured annually to the nearest 0.1 kg with participants wearing clinical gowns without shoes or heavy jewellery, using a calibrated balance beam scale. Additionally, self‐reported body weights were recorded semi‐annually during phone interviews. We calculated BMI throughout the study period using the height measured at the first clinic visit.
We used two methods to quantify BMI variability. In the first method, we calculated average successive variability (ASV) of BMI for each participant over the first 3 years of the study as described by Bangalore et al. and others. 10 , 15 , 16 , 17 Successive variability was defined as the absolute value of the difference between consecutive BMI values. Each 6‐month difference was then averaged over the first 3 years of the study. This method was used to capture all weight gain and loss that occurred over the 6‐month intervals of the exposure period. For example, a participant whose BMI changed between every measurement with recorded values of [30, 32, 30, 32, 30, 32, 30] would have an ASV of 2, whereas a participant with the same values whose BMI only changed once [30, 30, 30, 30, 32, 32, 32] would yield an ASV of 0.3. Participants were then sorted into quintiles from the lowest to highest BMI variability.
BMI variability is highly influenced by baseline BMI and net change in BMI over the same time period. We used a second method to isolate the effect of variability in the form of weight ‘cycling’, or oscillation around a stable mean, from variability due to net weight change. We used linear regression to estimate the amount of variability in BMI that was attributable to initial BMI and net change in BMI over the 3‐year period for each participant. In these models, BMI change was categorized as (1) loss of 10% or more, (2) loss of 5–10%, (3) <5% change (gain or loss), (4) gain of 5–10% or (5) gain of >10% of their initial BMI, measured over the whole 3‐year interval.
In this linear regression model, baseline BMI category (<18.5, 18.5–24.9, 25–29.9, 30–34.9 and ≥35 kg/m2) and BMI change category were independent variables, with ASV as the dependent variable. The predicted or expected ASV for each participant was derived from these models. We then calculated the observed − expected for each individual. This left us with residuals that represented the excess of BMI variability compared to what would be expected for an individual with a similar baseline BMI and similar net change in BMI at 3 years. Thus, this measure was independent of the expected effects of baseline BMI and overall changes in BMI over this time period. We sorted participants into quintiles based on these residual values, which we refer to as residual ASV (rASV). This method differs from Method 1 in that a participant who gains weight consistently every 6 months over the 3‐year period would be considered to have less variability than a participant who alternated gaining and losing weight. For example, a participant with BMI values of [30, 29, 28, 27, 26, 25, 24] would have an ASV of 1 but an rASV of 0. Tests for multicollinearity between BMI change or initial BMI and the rASV quintiles were not significant.
Outcomes: Body composition
Whole‐body dual‐energy X‐ray absorptiometry (DXA) was performed at both the Pittsburgh and the Memphis field centres (Hologic 4500A, Version 9.03; Hologic, Inc., Waltham, MA, USA) annually. In addition, bone mineral‐free appendicular lean mass and fat mass were derived from the whole‐body scan. ALMI and FMI were calculated by taking the estimated weight in kilograms of muscle and fat mass from whole‐body DXA and converting to an index to remove the influence of height by dividing by height squared, similar to the calculation of BMI. DXA quality‐assurance measurements were performed at both study sites to ensure scanner reliability, and identical patient scan protocols were used for all participants. For soft tissue, the coefficients of variation (CVs) were 1.0% and 2.1% for whole‐body lean mass and fat mass, respectively. Total FMI and ALMI were determined from whole‐body DXA and converted to age‐, sex‐ and race‐specific Z scores as previously described 18 using published nationally representative reference ranges from the National Health and Nutrition Examination Survey (NHANES 19 ). We determined changes in BMI, ALMI and FMI Z scores from Year 1 (baseline) to Year 4.
Outcomes: Physical functioning
We also investigated physical performance as measured by the Health ABC Physical Performance Score (Health ABC Score) during the same 3‐year period. Details of this validated measure have been previously described. 20 In brief, this battery includes five repeated chair stands, progressively more challenging tests of standing balance, a 6‐m walk to determine usual gait speed and a narrow walk in which participants are instructed to walk between lines of coloured tape 20 cm apart at their usual pace. Performance on each test is scored as a ratio to the maximum possible performance for older adults. These four scores are summed to generate a continuous scale ranging from 0 to 4, with a lower score indicating poorer function.
Outcomes: Incident disability
We also analysed adjudicated self‐report data on incident physical disability from interviewer‐administered questionnaires every 6 months. For incident disability, the outcome of interest was the time from baseline to any self‐reported disability at a subsequent visit, which was defined as severe difficulty or inability to walk 1/4 mile and/or climb 10 steps, needing equipment to ambulate or having any difficulty performing activities of daily living (i.e., getting in and out of bed or chairs, bathing or showering, and dressing). Incident disability was self‐reported in 6‐month intervals, and time was measured in days from Year 4 to the reported onset.
Statistical analysis
One‐way analysis of variance (ANOVA) and Kruskal–Wallis tests were used to compare the characteristics between participants in different quintiles of BMI variability (for both ASV and rASV methods). We analysed variance between potential confounding factors including age, race, sex, height, study site, income, education level, smoking status, alcohol use, height, time spent exercising per week, baseline BMI, FMI, ALMI, grip strength, walking speed, ability to walk 400 m, Health ABC Score and systolic blood pressure. We included those factors that were associated with BMI variability quintiles as covariates in each model. Grip strength was measured twice for each hand using a Jamar hydraulic dynamometer as has been previously described. 21 Maximum grip strength at each time point was used for analyses.
We used multivariable linear regression to assess the relationship between BMI variability and changes in body mass and composition over 3 years. Three models were used to determine associations between BMI variability and the net change in BMI, ALMI and FMI, adjusting for potential confounders. We adjusted for age, height, race, sex, study site, smoking, alcohol use, grip strength, family income, education level, baseline Health ABC Score and baseline ability to walk 400 m. Additionally, we adjusted for baseline BMI category in the BMI change model and both baseline ALMI and baseline FMI in the ALMI and FMI change models.
To assess the relationship between BMI variability and change in Health ABC Score over 3 years, we again used a multivariate linear regression and adjusted for age, height, race, sex, study site, smoking and drinking status, family income, education level, baseline Health ABC Score, baseline ability to walk 400 m and baseline BMI category. Additionally, we adjusted for net change in BMI from Year 1 to Year 4.
Lastly, we conducted a survival analysis using Cox proportional hazard models to investigate the relationship between BMI variability and incident disability adjusting for potential confounders identified in prior analyses.
All statistical analyses were performed using STATA 16.0 and were conducted for both methods of BMI variability quintiles.
Results
The details of the study population have been previously published. 22 We included 2121 of the 3075 participants, after applying the exclusions described, whose characteristics are shown in Table S1 . Of the 954 excluded participants, 190 died during the first 3 years of the study.
Factors associated with body mass index variability
Associations between baseline factors and BMI variability quintile are presented in Table S1 (ASV) and Table S2 (rASV). Those with higher variability, using either method, were observed to have higher Year 1 BMI, FMI and ALMI and lower education level, family income, and Health ABC Score and were more likely to report Black race and more likely to be unable to complete a 400‐m walk. Study site and grip strength were also associated with weight fluctuation measured by the rASV method, whereas drinking history was associated only with the ASV method.
Year 4 associations were similar to those observed at Year 1. Greater BMI variability was associated with greater Year 4 BMI, FMI and ALMI, lower Year 4 Health ABC Score and inability to complete a 400‐m walk at Year 4. Year 4 grip strength was not significantly associated with quintiles using either method.
Association of body mass index variability with changes in body composition
Associations between BMI variability quintile and changes in body composition over the same 3‐year period are presented in Table 1 . Participants in the highest quintile of BMI variability according to the ASV method had greater loss of body mass (β: −0.086 [95% confidence interval, CI: −0.133, −0.040], P < 0.01) and fat mass (β: −0.059 [95% CI: −0.117, −0.002], P = 0.04), on average, compared to those in the lowest quintile. As expected, variability measured by the rASV method, which adjusted for overall change in weight, was not significantly associated with the 3‐year change in BMI. There was also no association with change in FMI or ALMI Z scores.
Table 1.
Three‐year change in body composition and BMI variability quintile
| Quintile | ASV method | rASV method | |||
|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | ||
| ΔBMI Z score | 1 | 0 (reference) | — | 0 (reference) | — |
| 2 | 0.019 (−0.026, 0.054) | 0.41 | −0.011 (−0.055, 0.034) | 0.64 | |
| 3 | −0.009 (−0.054, 0.036) | 0.69 | −0.021 (−0.066, 0.024) | 0.35 | |
| 4 | −0.034 (−0.079, 0.011) | 0.14 | −0.008 (−0.053, 0.038) | 0.74 | |
| 5 | −0.086 (−0.133, −0.040) | <0.01 | −0.021 (−0.066, 0.024) | 0.35 | |
| ΔFMI Z score | 1 | 0 (reference) | — | 0 (reference) | — |
| 2 | 0.012 (−0.043, 0.066) | 0.68 | −0.017 (−0.070, 0.036) | 0.54 | |
| 3 | −0.002 (−0.057, 0.053) | 0.95 | −0.017 (−0.070, 0.037) | 0.54 | |
| 4 | 0.003 (−0.052, 0.058) | 0.91 | 0.023 (−0.031, 0.077) | 0.40 | |
| 5 | −0.059 (−0.117, −0.002) | 0.04 | −0.004 (−0.058, 0.050) | 0.89 | |
| ΔALMI Z score | 1 | 0 (reference) | — | 0 (reference) | — |
| 2 | 0.010 (−0.046, 0.065) | 0.74 | 0.003 (−0.053, 0.060) | 0.91 | |
| 3 | −0.006 (−0.046, 0.065) | 0.84 | −0.016 (−0.727, 0.042) | 0.46 | |
| 4 | −0.037 (−0.093, 0.019) | 0.20 | −0.004 (−0.061, 0.053) | 0.88 | |
| 5 | −0.044 (−0.102, 0.015) | 0.15 | 0.023 (−0.035, 0.080) | 0.44 | |
Note: Greater loss of BMI and FMI is observed among those with greater BMI variability by the ASV method, but not the rASV method. Abbreviations: ALMI, appendicular lean mass index; ASV, average successive variability; BMI, body mass index; CI, confidence interval; FMI, fat mass index; rASV, residual average successive variability.
Association of body mass index variability with changes in physical functioning
Of our sample of 2121 participants, 91 were missing Year 4 Health ABC scores and were excluded only from the physical functioning analysis (n = 2030).
Associations between BMI variability quintile and changes in Health ABC Score over the same 3‐year period are presented in Table 2 . Participants in the highest quintile of BMI variability had larger declines in physical functioning over the first 3 years of the study compared to those in the lowest quintile using the ASV method (β: −0.094 [95% CI: −0.16, −0.03], P < 0.01). Participants in the highest quintile had an unadjusted median change in Health ABC Score of −0.21 (inter‐quartile range [IQR]: −0.47 to 0.06), whereas those in the lowest variability quintile had a median change of −0.10 (IQR: −0.37 to 0.11) (P < 0.01).
Table 2.
Three‐year change in Health ABC Score by BMI variability quintile
| Quintile | ASV method | rASV method | ||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| 1 | 0 (reference) | — | 0 (reference) | — |
| 2 | −0.01 (−0.07, 0.05) | 0.73 | 0.018 (−0.044, 0.080) | 0.58 |
| 3 | 0.00 (−0.063, 0.063) | 0.99 | −0.038 (−0.101, 0.024) | 0.23 |
| 4 | −0.034 (−0.098, 0.030) | 0.29 | −0.016 (−0.079, 0.048) | 0.63 |
| 5 | −0.094 (−0.162, −0.026) | <0.01 | −0.067 (−0.132, −0.003) | 0.04 |
Note: Greater reductions in Health ABC Score are observed in the highest quintile of BMI variability. Abbreviations: ABC, Aging and Body Composition; ASV, average successive variability; BMI, body mass index; CI, confidence interval; rASV, residual average successive variability.
The results were similar, though more modest, using the rASV method, with participants in the highest quintile having significantly greater decline in Health ABC Score over 3 years (β: −0.0674 [95% CI: −0.13, 0.00], P = 0.04). Participants with the greatest BMI variability had an unadjusted median change in Health ABC Score of −0.20 (IQR: −0.44 to −0.07), whereas those in the lowest quintile had a median change of −0.10 (IQR: −0.41 to +0.10) (P = 0.07 for comparison of Quintile 1 to Quintile 5).
Of the 952 participants who met our exclusion criteria, 307 had Year 4 Health ABC scores recorded. Of these, there was no significant difference in median decline in Health ABC Score (−0.16, IQR: −0.51 to 0.08) compared to those that were included (−0.15, IQR: −0.42 to 0.09) with a P value of 0.06.
Association of body mass index variability with incident disability
Of the 2121 participants in our study, 731 had incidence of disability during the first 3 years and were excluded from the survival analysis. The excluded participants had greater BMI variability on average with median ASV of 0.75 (0.51, 1.04) and rASV of −0.03 (−0.22, 0.24) compared to median ASV of 0.62 (0.43, 0.90) and rASV of −0.11 (−0.28, 0.16) in those who remained disability free after 3 years.
Associations between BMI variability quintile and time to incident disability during the follow‐up period are presented in Table 3 . Greater BMI variability over 3 years was associated with a significantly higher risk of incident disability during the subsequent observation period. Using the ASV method, those in the highest quintile were at significantly greater risk than those in the lowest quintile (hazard ratio [HR]: 1.36 [95% CI: 1.07, 1.72], P = 0.01). The third and fourth quintiles were also associated with greater risk, with the greatest risk observed in the fourth quintile (HR: 1.56 [95% CI: 1.25, 1.94], P < 0.01).
Table 3.
Incident disability after Year 4 by BMI variability quintile
| Quintile | ASV method | rASV method | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| 1 | 1 (reference) | — | 1 (reference) | — |
| 2 | 1.20 (0.97, 1.47) | 0.09 | 1.19 (0.96, 1.47) | 0.11 |
| 3 | 1.25 (1.01, 1.5) | 0.04 | 1.17 (0.94, 1.45) | 0.17 |
| 4 | 1.56 (1.25, 1.939) | <0.01 | 1.23 (0.99, 1.53) | 0.06 |
| 5 | 1.36 (1.07, 1.72) | 0.01 | 1.38 (1.11, 1.72) | <0.01 |
Note: Those with higher BMI variability were more likely to experience incident disability by both the ASV and rASV methods. Abbreviations: ASV, average successive variability; BMI, body mass index; CI, confidence interval; HR, hazard ratio; rASV, residual average successive variability.
Using the rASV method, participants in the highest BMI variability also had a higher risk of incident disability compared to participants in the lowest quintile (HR: 1.38 [95% CI: 1.11, 1.72], P < 0.01). Kaplan–Meier survival curves for the ASV and rASV methods are shown in Figures 1 and 2 , respectively.
Figure 1.
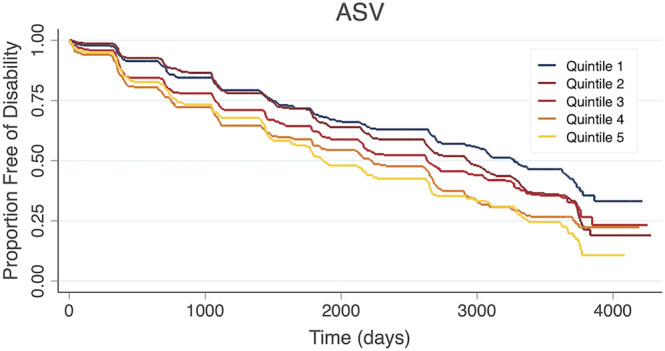
Kaplan–Meier curve showing the time to incident disability by body mass index variability quintile according to the average successive variability (ASV) method.
Figure 2.
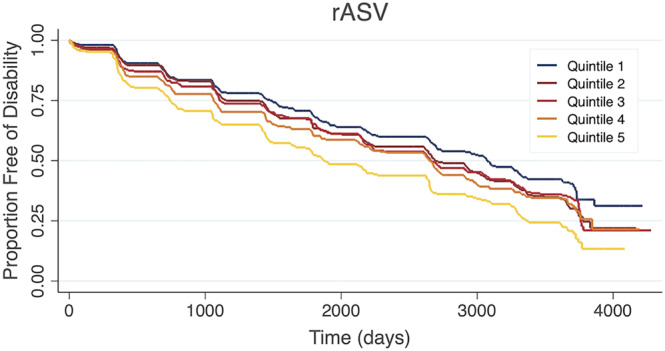
Kaplan–Meier curve showing the time to incident disability by body mass index variability quintile according to the residual average successive variability (rASV) method.
There was a significantly shorter median time to disability in participants who were excluded from our analysis due to BMI missing values (1473, IQR: 408 to 2978) compared to those that were included (2179, IQR: 1012 to 3617) with a P value <0.01.
Sensitivity analyses
We performed several sensitivity and subgroup analyses. There were no significant interactions by sex (not shown). For interactions by baseline (Year 4) BMI, a numerically greater association between BMI variability (rASV) and the risk of incident disability was observed in non‐obese participants, but this interaction was not statistically significant (Table S3 ).
Discussion
We quantified body weight variability over 3 years among older adults and demonstrated an association with declines in physical performance and incidence of physical disability. The data presented here support the hypothesis that body weight variability in older persons is associated with a concurrent decline in physical functioning and may be a biomarker of the metabolic processes that contribute to frailty. Importantly, the main advance of our study is to demonstrate that the association between weight variability and disability was independent net weight change and baseline weight.
These analyses show that participants with the greatest absolute BMI variability (as assessed by the ASV method) tend to also experience weight loss over the same time period. These results suggest that a primary driver of weight variability in older persons is weight loss, which is a known risk factor for frailty. We hypothesized that previous associations between weight variability and long‐term outcomes might be explained by an association between weight fluctuation and net weight loss. However, our novel rASV method of classifying participants' weight variability, which was designed to be independent of net BMI change and baseline BMI, still demonstrates a relationship with decline in physical performance and incident disability. This observation suggests that weight fluctuation can be informative even independent of the information provided by following an individual's simple BMI trajectory over time.
Our study is one of the few studies to quantify weight variability in order to evaluate dose‐dependent effects. It is also the first study to quantify weight variability independent of net weight change over the same period. The quantification of weight variability in this cohort builds upon previous work showing correlation between the presence of weight loss and re‐gain in the Health ABC population and decreased physical performance. 3 Although our study largely corroborates prior findings, it also builds on this prior work by utilizing previously defined approaches to the quantification of weight variability and by further considering net weight change and baseline weight in the analysis. Our results suggest that weight variability affects disability independent of baseline weight and net weight change, a novel observation. Additionally, the results of the rASV method suggest a dose‐dependent effect, though they do not prove it as we did statistically evaluate for trend.
Unintentional weight loss has been associated with frailty and adverse long‐term outcomes, perhaps by identifying patients with adverse metabolic changes related to comorbidity and poor health status. Weight fluctuations may similarly reflect underlying metabolic changes that have occurred that, themselves, predict adverse outcomes. However, some have hypothesized that weight fluctuation may also directly affect metabolism, adversely to long‐term health. Following periods of significant weight loss, fat mass reconstitution has been demonstrated to occur disproportionately faster than that of lean mass perhaps as the result of changes in cellular efficiency that are part of the physiological response to starvation. 23 However, these adaptive metabolic changes may become maladaptive, resulting in excess adiposity, insulin resistance and sympathetic hyperactivity over time. 23 , 24 More recently, immune cell accumulation and inflammation in adipose tissue has been implicated in metabolic dysfunction following weight fluctuation. 25 , 26 Excess adiposity, metabolic syndrome, insulin resistance and inflammation may contribute to metabolic breakdown of skeletal muscle and have each been associated with frailty and disability in older adults. 27 , 28
Although not statistically significant, there was a numerically greater association between weight fluctuation and the risk of disability among non‐obese participants. This may be intuitive, because we expect weight fluctuation to occur in parallel with features of frailty; however, these observations should be considered hypothesis generating and more work is needed in this area. A previous study in rheumatoid arthritis observed similar trends, with a greater association between weight fluctuation and cardiovascular events occurring among thinner participants. 29
Limitations of the current study include temporally constrained data and a reliance on self‐reported data for some BMI measures. In this study, self‐reported measures of weight were similar to in‐person measures; however, literature supports underestimation of weight by self‐report in some settings. 30 , 31 Our measures of weight variability could not identify changes in weight that may have occurred over periods of <6 months. Our results therefore may not be generalizable to variability measured between shorter or longer time points. As noted, self‐reported weight may underestimate directly assessed weight in some settings, though studies have suggested that these measures are generally highly accurate among older adults. 32 , 33 In addition, our analyses did not identify systematic differences between self‐reported values and in‐person weight measurements. It stands to reason that bias introduced through the use of self‐reported data would be non‐differential in nature. Lastly, the Health ABC Study population consisted of healthy older adults who may not be entirely representative of the general population. Weight variability could have a different relationship with physical performance in older persons with additional underlying health conditions or who are already limited by physical disability. Additionally, it was necessary to exclude participants who were missing any weight values during the first 3 years of the study, which we acknowledge may have been due to illness and introduced a selection bias. We cannot predict how this might affect the association of weight variability and our health outcomes, as we did not exhaustively assess the impact of comorbid conditions that may serve as important confounders or effect modifiers. However, we suspect that our sample was somewhat healthier than the underlying population, given the increased rate of incident disability in the excluded participants.
In summary, body weight variability in older persons is associated with decline in physical performance and incident disability, and this relationship cannot be explained by initial body weight or weight loss alone. Body weight variability may therefore represent a valuable biomarker of the underlying processes leading to frailty.
Conflict of interest statement
JFB has received consulting fees from Bristol‐Myers Squibb, Pfizer, CorEvitas, and Burns White LLC.
Supporting information
Figure S1. Illustration of study design.
Table S1. Characteristics of participants stratified by ASV method quintiles.
Table S2. Participant characteristics stratified by rASV quintile.
Table S3. BMI variability and incident disability stratified by year 4 BMI.
Acknowledgements
JFB has received support from a Veterans Affairs Clinical Science Research and Development Merit Award (I01 CX001703), a VA Rehabilitation Research and Development SPiRE Award (I21 CX003157) and a Rehabilitation Research and Development Merit Award (I01 RX003644). The opinions or assertions presented herein are the private views and opinions of the authors and do not represent the views of the Department of Veterans Affairs.
McMenamin KJ, Harris TB, Baker JF. (2023) Weight variability, physical functioning and incident disability in older adults, Journal of Cachexia, Sarcopenia and Muscle, 14, 1648–1656, 10.1002/jcsm.13239
References
- 1. Dent E, Morley JE, Cruz‐Jentoft AJ, Woodhouse L, Rodríguez‐Mañas L, Fried LP, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging 2019;23:771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community‐dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr 2011;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Newman AB, Koster A, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population‐based cohort study. J Am Geriatr Soc 2014;62:1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong JJ, Godin J, Launer LJ, White LR, Mitnitski A, Rockwood K, et al. Changes in frailty predict changes in cognition in older men: the Honolulu‐Asia Aging Study. J Alzheimers Dis 2016;53:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Vries OJ, Peeters GMEE, Lips P, Deeg DJH. Does frailty predict increased risk of falls and fractures? A prospective population‐based study. Osteoporos Int 2013;24:2397–2403. [DOI] [PubMed] [Google Scholar]
- 6. Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J 2013;166:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero‐Ortuno R. The Frailty Instrument for primary care of the Survey of Health, Ageing and Retirement in Europe predicts mortality similarly to a frailty index based on comprehensive geriatric assessment. Geriatr Gerontol Int 2013;13:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. Med Clin North Am 2011;95:427–438. [DOI] [PubMed] [Google Scholar]
- 10. Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body‐weight fluctuations and outcomes in coronary disease. N Engl J Med 2017;376:1332–1340. [DOI] [PubMed] [Google Scholar]
- 11. Welti LM, Beavers DP, Caan BJ, Sangi‐Haghpeykar H, Vitolins MZ, Beavers KM. Weight fluctuation and cancer risk in postmenopausal women: the Women's Health Initiative. Cancer Epidemiol Biomarkers Prev 2017;26:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim DH, Nam GE, Han K, Kim YH, Park KY, Hwang HS, et al. Variabilities in weight and waist circumference and risk of myocardial infarction, stroke, and mortality: a nationwide cohort study. Endocrinol Metab (Seoul) 2020;35:933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park KY, Hwang HS, Cho KH, Han K, Nam GE, Kim YH, et al. Body weight fluctuation as a risk factor for type 2 diabetes: results from a nationwide cohort study. J Clin Med 2019;8:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LeBlanc ES, Rizzo JH, Pedula KL, Yaffe K, Ensrud KE, Cauley JA, et al. Long‐term weight trajectory and risk of hip fracture, falls, impaired physical function, and death. J Am Geriatr Soc 2018;66:1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yeboah P, Hsu FC, Bertoni AG, Yeboah J. Body mass index, change in weight, body weight variability and outcomes in type 2 diabetes mellitus (from the ACCORD trial). Am J Cardiol 2019;123:576–581. [DOI] [PubMed] [Google Scholar]
- 16. Jung I, Koo DJ, Lee MY, Moon SJ, Kwon H, Park SE, et al. Increased risk of nonalcoholic fatty liver disease in individuals with high weight variability. Endocrinol Metab 2021;36:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Urso D, van Wamelen DJ, Batzu L, Leta V, Staunton J, Pineda‐Pardo JA, et al. Clinical trajectories and biomarkers for weight variability in early Parkinson's disease. npj Parkinsons Dis 2022;8:1, 95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker JF, Long J, Ibrahim S, Leonard MB, Katz P. Are men at greater risk of lean mass deficits in rheumatoid arthritis? Arthritis Care Res (Hoboken) 2015;67:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X‐ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, et al. Measuring higher level physical function in well‐functioning older adults: expanding familiar approaches in the Health ABC Study. J Gerontol A 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 21. National Institute on Aging . Health ABC operations manual volume III: grip strength hand‐held dynamometry. https://healthabc.nia.nih.gov/sites/default/files/grip.om1_0.pdf (2020). Accessed 14 Oct 2022.
- 22. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2004;52:1098–1104. [DOI] [PubMed] [Google Scholar]
- 23. Dulloo AG, Jacquet J, Montani JP. Pathways from weight fluctuations to metabolic diseases: focus on maladaptive thermogenesis during catch‐up fat. Int J Obes Relat Metab Disord 2002;26:S46–S57. [DOI] [PubMed] [Google Scholar]
- 24. Ernsberger P, Koletsky RJ, Kilani A, Viswan G, Bedol D. Effects of weight cycling on urinary catecholamines: sympathoadrenal role in refeeding hypertension. J Hypertens 1998;16:2001–2005. [DOI] [PubMed] [Google Scholar]
- 25. Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T‐cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes 2013;62:3180–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sougiannis AT, VanderVeen BN, Cranford TL, Enos RT, Velazquez KT, McDonald S, et al. Impact of weight loss and partial weight regain on immune cell and inflammatory markers in adipose tissue in male mice. J Appl Physiol (1985) 2020;129:909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pérez‐Tasigchana RF, León‐Muñoz LM, Lopez‐Garcia E, Gutierrez‐Fisac JL, Laclaustra M, Rodríguez‐Artalejo F, et al. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing 2017;46:807–812. [DOI] [PubMed] [Google Scholar]
- 28. Levine ME, Crimmins EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity 2012;20:2101–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker JF, Reed G, Kremer J. Weight fluctuation and the risk of cardiovascular events in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2022;74:229–235. [DOI] [PubMed] [Google Scholar]
- 30. Kovalchik S. Validity of adult lifetime self‐reported body weight. Public Health Nutr 2009;12:1072–1077. [DOI] [PubMed] [Google Scholar]
- 31. Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self‐reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc 2001;101:28–34. [DOI] [PubMed] [Google Scholar]
- 32. Fillenbaum GG, Kuchibhatla MN, Whitson HE, Batch BC, Svetkey LP, Pieper CF, et al. Accuracy of self‐reported height and weight in a community‐based sample of older African Americans and whites. J Gerontol A Biol Sci Med Sci 2010;65A:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yazawa A, Inoue Y, Kondo N, Miyaguni Y, Ojima T, Kondo K, et al. Accuracy of self‐reported weight, height and body mass index among older people in Japan. Geriatr Gerontol Int 2020;20:803–810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Illustration of study design.
Table S1. Characteristics of participants stratified by ASV method quintiles.
Table S2. Participant characteristics stratified by rASV quintile.
Table S3. BMI variability and incident disability stratified by year 4 BMI.


