Abstract
Background
A common method for diagnosing sarcopenia involves estimating the muscle mass by computed tomography (CT) via measurements of the cross‐sectional muscle area (CSMA) of all muscles at the third lumbar vertebra (L3) level. Recently, single‐muscle measurements of the psoas major muscle at L3 have emerged as a surrogate for sarcopenia detection, but its reliability and accuracy remain to be demonstrated.
Methods
This prospective cross‐sectional study involved 29 healthcare establishments and recruited patients with metastatic cancers. The correlation between skeletal muscle index (SMI = CSMA of all muscles at L3/height2, cm2/m2) and psoas muscle index (PMI = CSMA of psoas at L3/height2, cm2/m2) was determined (Pearson's r). ROC curves were prepared based on SMI data from a development population (n = 488) to estimate suitable PMI thresholds. International low SMI cut‐offs according to gender were studied for males (<55cm2/m2) and for females (<39 cm2/m2). Youden's index (J) and Cohen's kappa (κ) were calculated to estimate the test's accuracy and reliability. PMI cut‐offs were validated in a validation population (n = 243) by estimating the percentage concordance of sarcopenia diagnoses with the SMI thresholds.
Results
Seven hundred and sixty‐six patients were analysed (mean age 65.0 ± 11.8 years, 50.1% female). Low SMI prevalence was 69.1%. Correlation between the SMI and PMI for the entire population was 0.69 (n = 731, P < 0.01). PMI cut‐offs for sarcopenia were estimated in the development population at <6.6cm2/m2 in males and at <4.8 cm2/m2 for females. The J and κ coefficients for PMI diagnostic tests were weak. The PMI cut‐offs were tested in the validation population where 33.3% of the PMI measurements were dichotomously discordant.
Conclusions
A diagnostic test employing single‐muscle measurements of the psoas major muscle as a surrogate for sarcopenia detection was evaluated but found to be unreliable. The CSMA of all muscles must be considered for evaluating cancer sarcopenia at L3.
Keywords: Cancer sarcopenia, Low muscle mass, Skeletal muscle index, CT scan, Psoas, L3
Background
Sarcopenia is defined as the loss of skeletal muscle mass and function 1 and is a common comorbidity in cancer. 2 It is associated with poor prognosis in cancer patients and an increased risk of postoperative complications, treatment intolerance, longer hospitalizations and diminishing quality of life. 3 , 4 , 5 , 6 , 7 Its impact becomes increasingly detrimental with increasing severity and delay in management, which warrants its diagnosis and treatment (via nutritional and/or physiotherapeutic interventions) at the earliest opportunity. A variety of methods exist for muscle mass estimation, which can be expressed as total body skeletal muscle mass, appendicular skeletal muscle mass or the cross‐sectional muscle area (CSMA) of selected muscle groups. 1
Computed tomography (CT) is an imaging technique being extensively investigated and implemented in the assessment of muscle mass for sarcopenia diagnosis. The third lumbar (L3) spinal segment is a primary site for CT muscle mass measurements as this area includes all muscles (including the psoas major, erector spinae, transverse abdominis, quadratus lumborum, the obliques and rectus abdominus 8 , 9 , 10 ). Furthermore, the CSMA at L3, normalized to patient height2 to obtain the skeletal muscle index ([SMI] = CSMA of all muscles at L3/height2, cm2/m2), has been shown to correlate strongly with total body muscle mass, thus making it a suitable surrogate for sarcopenia assessment and is recommended by many expert groups. 1 , 11 , 12 , 13 , 14 , 15 Despite the accuracy of this technique for muscle mass calculation, there remains a disparity in the reports of sarcopenia prevalence in the literature due to differences in the choice of cut‐off for low muscle mass. 9 , 12 , 16 , 17 , 18 , 19
Recently, single‐muscle measurements for sarcopenia diagnosis have emerged as an alternative to the total CSMA at the L3 level. The psoas major muscle is most frequently targeted to obtain the psoas muscle index (PMI = CSMA of psoas at L3/height2 in units of cm2/m2), a choice attributed to its function as the main hip flexor muscle that provides postural support of the spine and hip joints, its ease of identification and the relatively quick processing and analysis of CT images. 8 , 20 The conclusions regarding its suitability as a surrogate for cancer sarcopenia diagnosis are varied, and there is a lack of studies investigating and validating the use of this muscle for sarcopenia diagnosis in clinical practice. 8 Despite the uncertainty in its reliability and the lack of precise recommendations by any expert group, this method is being used by certain clinicians to evaluate sarcopenia and postoperative prognosis in oncology. 21 , 22 , 23 , 24 , 25 Recently, Westenberg et al. 26 emphasized the need to reach a consensus for the measurement of sarcopenia using CT. They proposed that future research and discussion should focus on establishing standard procedures and validating them.
The Sarcopenia Cancer and Nutrition (SCAN) study was conducted in France to assess the prevalence of sarcopenia in metastatic cancer patients in real‐life practice based on muscle mass measurements on CT. 27 Sarcopenia was determined by estimating low SMI in 766 patients. The data gathered from a large sample such as this provided an opportunity to investigate the relationship between the CSMA of psoas (PMI) and total body muscle mass (SMI) at L3 and in turn to explore the possibility of developing a reliable diagnostic test with a validated cut‐off for psoas muscles that can be applied to a wide range of patients in clinical practice.
Methods
Study design and patients
The SCAN study was a prospective cross‐sectional multicentre study conducted within 29 public and private oncology practices situated throughout France between September and October 2017. The primary objective of the study was to determine the prevalence of sarcopenia in metastatic cancer patients by estimating the SMI at the L3 cross‐sectional level. These results were published in a prior publication without data on psoas muscles. 27 Patients were men or women aged 18 years and above, were diagnosed with metastatic lung, kidney, colon, breast or prostate cancer and were undergoing chemotherapy, targeted therapy or immunotherapy at the time of inclusion. To be included in the study, patients had to have a CT scan comprising the cross section of the L3 segment, suitable for sarcopenia evaluation, performed between 6 weeks before and 4 weeks after the inclusion date. Informed consent was taken on the day of inclusion into the study.
The investigating pairs in each of the 29 centres consisted of an oncologist, who recorded patient data in a paper case report form (CRF), and a radiologist, who was trained in CSMA (cm2) measurement of the skeletal muscle via CT using a standardized approach. Data recorded during the visit included the patients' demographic characteristics, medical and treatment history, clinical characteristics, CSMA of all muscles at the L3 level and CSMA of the right and left psoas muscles at the L3 level. A centralized reading of 70 randomly selected patients was performed to assess inter‐observer and intra‐observer reproducibility. This blinded evaluation of all clinical and radiological data was performed by a different radiologist than those from the 29 centres.
Psoas muscle surface assessment
In this report, we present a post hoc analysis evaluating the reliability of using the psoas muscle as a surrogate marker of sarcopenia in lieu of the total lumbar muscle area at the L3 level (SMI) by determining and validating a suitable cut‐off value for the psoas muscle normalized to patient height2 (the psoas muscle index, [PMI] = CSMA of psoas at L3/height2 in units of cm2/m2). Following the SCAN study protocol, 27 pre‐established Hounsfield unit (HU) thresholds (−29 to +150 HU) were applied, and then the CSMA of psoas muscles were manually contoured from CT at the mid‐L3 level. The total CSMA of all muscles at the L3 level was also similarly determined and normalized to patient height2 to obtain the SMI. SMI cut‐offs for sarcopenia diagnosis from three studies are evaluated in this analysis: (i) Fearon et al., 12 where the patient is sarcopenic if L3 SMI is <55 cm2/m2 in males and <39 cm2/m2 in females (cut‐off 1) (Figure 3); (ii) Mourtzakis et al., 14 sarcopenic if L3 SMI is <52.4 cm2/m2 in males and <38.5 cm2/m2 in females (cut‐off 2); and (iii) Martin et al., 28 sarcopenic if L3 SMI is <43 cm2/m2 in males with BMI < 25.0 OR <53 cm2/m2 in males with BMI ≥ 25 and <41 cm2/m2 in females, irrespective of BMI (cut‐off 3). These cut‐offs were chosen, as they are most often used in clinical practice in oncology 29 , 30 , 31 and proposed by expert groups. 12 , 32 Data of cut‐offs 2 and 3 are presented on the supplemental data (Tables S1 and S2 and Figure S1).
Figure 3.
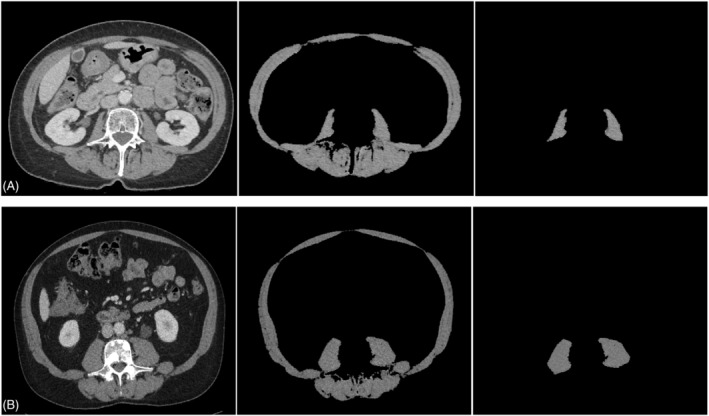
Abdominal CT scan images taken at mid‐L3 applied to quantify SMI and PMI with discordant evaluation. Panel (A) shows a metastatic lung cancer female patient with a high SMI measured at 39.5 cm2/m2 (>39 cm2/m2) 12 but a low PMI at 3.6 cm2/m2 (<4.8 cm2/m2). Panel (B) present a metastatic colon cancer male patient with a low SMI measured at 45.6 cm2/m2 (<55 cm2/m2) 12 but a high PMI at 8.2 cm2/m2 (>6.6 cm2/m2).
Statistical analyses
Statistical analyses and data management were performed by Cerner Enviza (Paris, France). Statistical analyses were performed using DAISIE (version 2.4.25 & 2.4.45) and R i386 3.6.0. The Pearson's r was estimated for correlation between continuous variables (SMI and PMI but also between the right and left psoas cross‐sections at mid‐L3). Fisher's z transformation was used to test the hypotheses on the value of the correlation coefficient r between the SMI and the PMI according to sex.
Receiver operating characteristic (ROC) curves were prepared to determine the most suitable PMI cut‐offs for males and females based on total L3 muscle SMIs from two‐thirds of the population (development population, n = 488)—this was done for all three SMI cut‐offs for sarcopenia, 12 , 14 , 28 cited in the previous sub‐section. Youden's indices (J) and Cohen's kappa coefficient (κ) were calculated to measure the accuracy of the cut‐off values in each case. The three PMI cut‐offs were then cross validated in the remaining third of the study population (validation population, n = 243) by estimating the percentage of concordance between both sarcopenia evaluation methods. Descriptive statistics [means, standard deviations (SD), median and range] are provided for continuous variables, and data are presented as percentages for categorical variables. The Z‐test or t‐test were used for comparisons between sarcopenic and non‐sarcopenic groups, with P < 0.05 taken to denote statistical significance.
For 70 randomly selected patients, inter‐observer and intra‐observer agreements were calculated using intraclass correlation coefficients (ICC) with 95% confidence intervals using a two‐way mixed single measures model. The agreement on low muscle mass assessment with SMI and PMI between observers was calculated using Cohen's kappa coefficients.
Results
From September to October 2017, 818 patient CRFs were received, of which 52 were excluded as they did not meet selection criteria or lacked essential information. Thus, 766 patients with metastatic cancer were included in the analysis. Patient characteristics were described in a previous publication. 27 The average age of patients was 65.0 ± 11.8 years and 50.1% were female. Cancer types were colon (37.1%), lung (25.5%), breast (22.6%), kidney (7.8%) and prostate (7.0%). Mean time since diagnosis of the primary tumour was 46.9 ± 60.7 months (median 23.2, range 0–400.0 months), and 40.5% were undergoing their first line of treatment. Low muscle mass prevalence was 69.1% (cut‐off 1 12 ), 62.5% (cut‐off 2, 14 ) and 58.6% (cut‐off 3 28 ) in cancer patients using three different cut‐offs for the L3 SMI. Among the 766 patients of the SCAN study, 746 patients with available measurements of the right psoas muscle, 733 of the left psoas muscle and 731 of both psoas muscles were included for this post hoc analysis.
Correlation between the skeletal muscle index and the psoas muscle index
A strong positive correlation was found between the right and left psoas area of each individual (r = 0.97, n = 731, P < 0.01). A moderately positive correlation was found between L3 SMI measurements and PMI measurements for the right, left and both psoas muscles together. The correlation coefficients of the entire population were as follows: with the right psoas PMI, r = 0.67, n = 746, P < 0.01; with the left psoas PMI, r = 0.65, n = 733, P < 0.01, with PMI derived from both psoas muscles, r = 0.69, n = 731, P < 0.01. The curves are presented in Figure 1.
Figure 1.
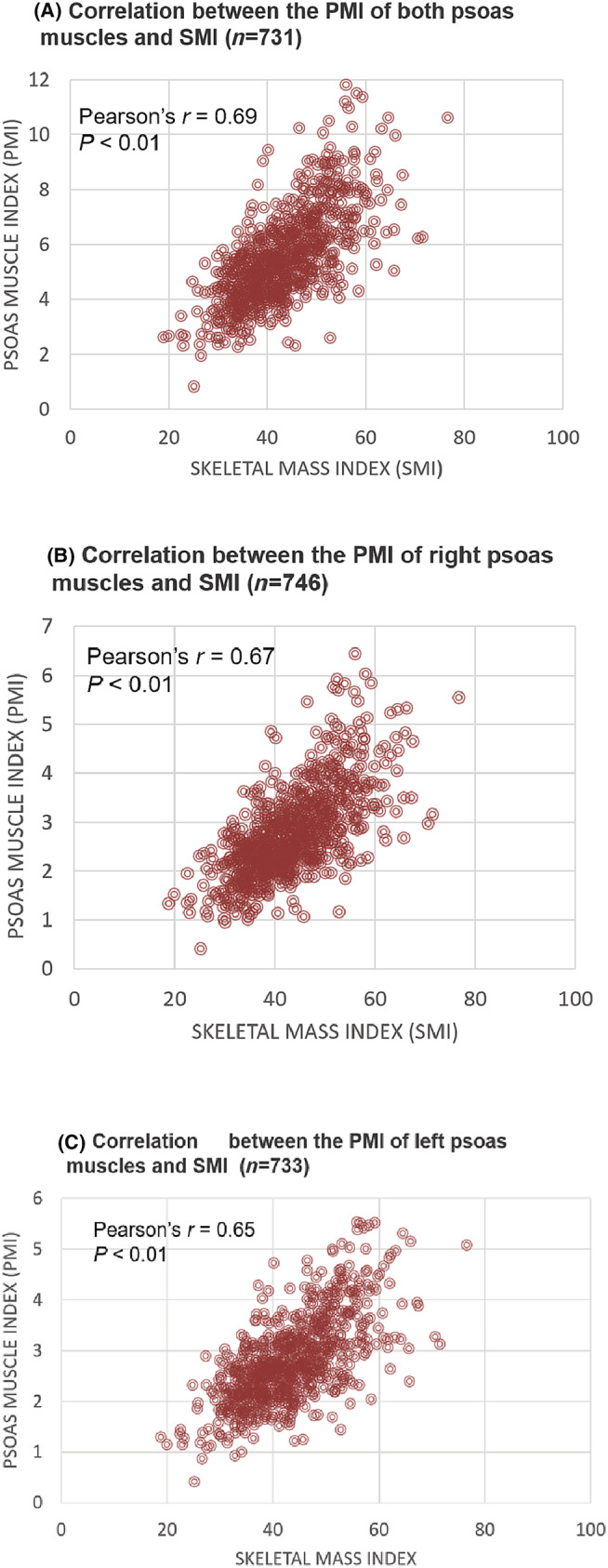
Pearson's r coefficients for the correlation between the skeletal muscle index (SMI) and psoas muscle index (PMI) of both (a), right (B) and left (C) psoas muscles.
The correlation coefficients with PMI derived from both psoas muscles according to sex were: r = 0,58, n = 371, P < 0.01 for males and r = 0,62, n = 360, P < 0.01 for females with no significant difference in Fischer z‐transformation, z = −0.8419, P = 0.3992.
We later proceeded to estimate optimal cut‐off points for low muscle mass evaluation using both the psoas muscles and against L3 SMI measurements.
Derivation and validation of a cut‐off for the psoas muscle index for sarcopenia
ROC curves were prepared from data derived from the development population (two‐thirds of the evaluable population, n = 488) to determine cut‐off values for PMI based on the total muscle SMI at the L3, according to each of the 3 SMI cut‐off values for low muscle mass diagnosis. 12 , 14 , 28 The curves for cut‐off 1 are presented in Figure 2. The corresponding values of sensitivity and specificity from which the PMI cut‐offs were determined for males and females are also presented in Table 1, together with the accuracy of the specific cut‐off, Youden's index, J (measure of the diagnostic efficacy of the test) and Cohen's Kappa coefficient, κ (measure of the level of agreement between the L3 and psoas results).
Figure 2.
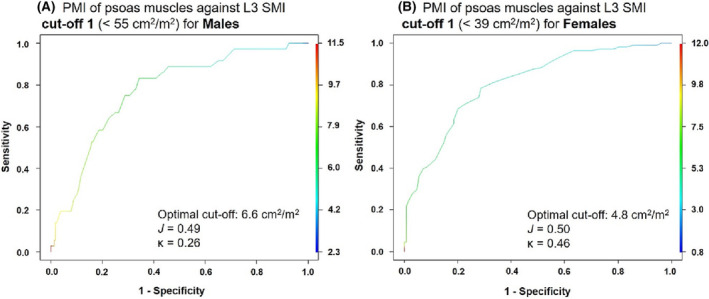
ROC curves based on SMI values of both psoas muscles together, for (A) males and (B) females with and without sarcopenia as per cut‐off 1. 12
Table 1.
Optimal cut‐off values for the detection of low muscle mass in males and females based on the psoas muscle index (PMI) of both psoas muscles on the development population
| Psoas cut‐off (cm 2 /m 2 ) | Sensitivity | Specificity | Accuracy | Youden's Index (J) | Cohen's Kappa co‐efficient (κ) | ||
|---|---|---|---|---|---|---|---|
| Cut‐off 1 a | Males (n = 248) | 6.6 | 0.83 | 0.66 | 0.29 | 0.49 | 0.26 |
| Females (n = 240) | 4.8 | 0.78 | 0.71 | 0.70 | 0.50 | 0.46 |
Cut‐off 1: Sarcopenic if total muscle SMI at L3 is <55 cm2/m2 in males and <39 cm2/m2 in females. 12
The PMI cut‐off values for the psoas muscles, below which the patient would be diagnosed as sarcopenic, were estimated at 6.6 cm2/m2 for males and 4.8 cm2/m2 for females, based on L3 SMI cut‐off 1. Based on these thresholds used as a diagnostic test for sarcopenia for males and females, PMI measurements had low accuracy and low level of concordance with SMI L3 values, as given by their corresponding Youden's index and Cohen's kappa coefficients, respectively.
These PMI cut‐offs were validated against the validation cohort (remaining third of the male and female population, n = 243) by calculating the percentage concordance between the PMI and SMI methods to diagnose cancer sarcopenia. A similar level of dichotomous concordance and discordance was observed of sarcopenia diagnoses were dichotomously concordant, whereas 33.3% were discordant (Table 2).
Table 2.
Concordance between the two sarcopenia evaluation methods on the validation population: According to the PMI of the two psoas muscles and the total muscle SMI at the L3
| Patients (n = 243) | % | ||
|---|---|---|---|
| Cut‐off 1 12 | Concordant | 162 | 66.7% |
| Sarcopenic according to both evaluation methods | 121 | 49.8% | |
| Non‐sarcopenic according to both evaluation methods | 41 | 16.9% | |
| Discordant | 81 | 33.3% | |
| Sarcopenic as per the SMI at L3 and non‐sarcopenic as per the psoas SMI | 51 | 21.0% | |
| Non‐sarcopenic as per the SMI at L3 and sarcopenic as per the psoas SMI | 30 | 12.3% |
Using SMI cut‐offs 2 and 3 shows the same range of results. Data are presented in Tables S1 and S2 and Figure S1.
Inter‐observer and intra‐observer variability of SMI and PMI
The inter‐observer and intra‐observer ICCs of SMI and PMI were excellent even though SMI was more reproducible.
Excellent inter‐observer agreement was obtained for both SMI and PMI intraclass correlation coefficient (ICC) of 0.995 (95% CI 0.99; 0.997) and 0.97 (0.944; 0.984), respectively.
Excellent intra‐observer agreement was obtained for both SMI and PMI with ICC of 1.0 (95% CI 1.0; 1.0) and 0.996 (95% CI 0.993; 0.998), respectively.
Discussion
This study consisted of the largest, multicentre samples of metastatic cancer patients to date in which a diagnostic test was performed to assess the suitability of using the psoas muscles to evaluate low SMI in oncology. Sarcopenia and low muscle mass have been assessed from the CSMA at the L3 vertebral level via CT in numerous studies, thus attesting to the reliability and popularity of this method. 10 , 12 , 14 , 16 , 17 , 18 , 19 , 28 , 30 , 31 , 33
It is expected that this segmentation be performed automatically in the near future, and thus total muscle measurements could be performed quicker. 34 , 35 , 36 However, muscle tissue segmentation from CT scans are often still performed manually or semi‐automatically, which is why oncologists, surgeons and radiologists may prefer single‐muscle, rather than total muscle measurements at the L3 level, for a faster turnaround of diagnostic results. 8 , 22 Due to this reason, the reliability of measurements of the psoas muscle for muscle mass evaluation has been the subject of numerous investigations, 8 , 20 , 21 , 22 , 24 , 25 the results of which are highly heterogenous as to the diagnostic value of this method. Any trade‐off between convenience and diagnostic accuracy in this instance seems unacceptable.
As part of our study, we created ROC curves based on data from two‐thirds of the total analysable population (development population, n = 488) to assess the diagnostic ability of the PMI against the total SMI at the L3 level (Figures 2 and S1). We were able to demonstrate that the psoas muscle alone cannot be used for a reliable assessment of low SMI recommended by many expert groups. 1 , 11 , 12 , 13 , 14 , 15 To be thorough, we even performed these diagnostic tests using the three aforementioned sets of SMI cut‐offs for cancer sarcopenia, that are most commonly encountered in the literature; the reliability of the PMI was found to be weak for all three cut‐offs (Tables 1 and S1).
Rutten et al. 20 performed a retrospective study involving 150 ovarian cancer patients, where they confirm our conclusions. In their quantitative study, they found a poor correlation and poor kappa agreement between total lumbar area and psoas area. They explain that the psoas muscles are not representative of the total L3 musculature in the context of cancer sarcopenia assessment, due to their higher propensity for increased degradation from degenerative disorders of the lumbar spine, meaning that psoas muscle loss may not specifically be related to the patient's cancer in certain cases. Additionally, because degenerative diseases of the lumbar spine are more prevalent in older patients, using psoas‐only measurements for sarcopenia diagnosis would not be reliable in this patient population. Furthermore, the psoas muscle is prone to high measurement error, owing to its irregular and very small size, in relation to the total L3 musculature. 8 , 20 There have been studies where a relationship between psoas muscle area and the occurrence of post‐operative complications has been shown 21 , 22 , 24 ; however, none of them demonstrated a relationship between low psoas muscle area and long‐term survival.
These problems are not limited to patients with malignancies. Ebadi et al. 37 show a moderate concordance with low PMI and SMI on a population of 353 patients with cirrhosis who were candidates for liver transplantation. Overall, 104 patients (29%) were misclassified between SMI and PMI categories. They also found poor performance of the PMI compared with SMI for identification of patients to predict liver transplant waitlist mortality. Low PMI identifies an incomplete subset of patients at increased risk of mortality indicated by low SMI.
In our study, when the derived PMI cut‐offs from each of the SMI cut‐offs were validated in the remaining third of the population (validation population, n = 243), a high level of discordance was also found between the PMI method and SMI method of sarcopenia diagnosis. Using the PMI threshold derived from cut‐off 1, 12 sarcopenia diagnosis was missed in 21% of patients (Table 2 and Figure 3), and in 15.6% and 22.3% of patients under cut‐off 2 14 and cut‐off 3 28 (Table S2, Supporting Information), respectively. In oncology, an underestimation in the range of 20% for a condition that could impair a patient's prognosis and quality of life, if not managed or treated as early as possible, is not acceptable. As discussed by Baracos, 8 it is difficult to claim that any one muscle is representative of human skeletal muscle mass.
Limitations
As this study was performed across 29 centres, to determine the SMI and PMI at the middle of L3 level, the inter‐centre accuracy of CT readings needs to be addressed. However, the excellent inter‐observer and intra‐observer agreement was obtained for both SMI and PMI limits the impact of this potential bias.
Moreover, the design of our study does not allow us to assess and compare the impact of low SMI or PMI on clinical outcomes (such as overall survival) in our patient population, an objective beyond the scope of our study. Even if the PMI measurement does not reliably identify low SMI patients, it might be interesting in future research to investigate the contribution of PMI in association to SMI but not as a sole marker as proposed in most studies. Indeed, all the physiological characteristics specific to the psoas muscle developed in the discussion could have a prognostic impact independent of the total muscle area in specific populations.
Lastly, ethnicity has an impact on the choice of thresholds for low SMI. 38 Studies from Asia do not use the same cut off values, and it is for this very reason that we chose to use thresholds that were validated using a Western population. 12 , 14 , 28 Regrettably, local French laws and regulations do not authorize the analysis of race or ethnicity in our clinical studies. We cannot therefore show the impact of ethnicity on the relationship between psoas cross‐sectional area and total L3 muscles.
Conclusions
CT scan muscle mass measurements need to be standardized. Our diagnostic ROC curves quantitatively show that the PMI is not a reliable surrogate for SMI measurements in the context of cancer sarcopenia diagnosis. The only advantage it seems to offer is an increased ease and rapidity of segmenting CT images at the L3 level, but this would be offset by the loss in accuracy, and the potential to under‐estimate sarcopenia prevalence in patients who may require urgent nutritional management. In clinical practice, this point may be solved in the near future by the contribution by artificial intelligence to organ segmentation in CT images. This would allow total muscle segmentation which would be more representative than a single sentinel muscle for diagnosing sarcopenia.
Conflict of interest
All authors were remunerated for their participation as investigators in the SCAN study.
Funding
This study was funded by Fresenius Kabi France.
Supporting information
Data S1. Supporting Information
Acknowledgements
We thank Cerner Enviza France for undertaking the data management and statistical analyses for this study and Saannya Sequeira of ClinSearch, France, for assisting in the preparation of this manuscript.
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle. 39 This study has been performed in adherence to local laws and the Declaration of Helsinki. All persons provided their informed consent prior to enrolment in the study. The protocol was in line with the French data protection committee (CNIL) regulations and was approved by the French ethical research committee (CPP) on the 6th of July 2017 (number 2017‐A01648‐45).
Pigneur F., Di Palma M., Raynard B., Guibal A., Cohen F., Daidj N., et al (2023) Psoas muscle index is not representative of skeletal muscle index for evaluating cancer sarcopenia, Journal of Cachexia, Sarcopenia and Muscle, 14, 1613–1620, 10.1002/jcsm.13230
References
- 1. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract 2017;32:30–39. [DOI] [PubMed] [Google Scholar]
- 3. Chemama S, Raynard B, Antoun S. Impact of cancer muscle mass loss on anticancer treatment toxicities. Bull Cancer 2016;103:786–793. [DOI] [PubMed] [Google Scholar]
- 4. Huang DD, Zhou CJ, Wang SL, Mao ST, Zhou XY, Lou N, et al. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery 2017;161:680–693. [DOI] [PubMed] [Google Scholar]
- 5. Nipp RD, Fuchs G, el‐Jawahri A, Mario J, Troschel FM, Greer JA, et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist 2018;23:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane‐based chemotherapy. Clin Cancer Res 2017;23:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen Y, Hao Q, Zhou J, Dong B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta‐analysis. BMC Geriatr 2017;17:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 2018;8:11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 11. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr 2014;33:737–748. [DOI] [PubMed] [Google Scholar]
- 12. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 13. Holt DQ, Strauss BJ, Lau KK, Moore GT. Body composition analysis using abdominal scans from routine clinical care in patients with Crohn's disease. Scand J Gastroenterol 2016;51:842–847. [DOI] [PubMed] [Google Scholar]
- 14. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 15. Schweitzer L, Geisler C, Pourhassan M, Braun W, Glüer CC, Bosy‐Westphal A, et al. What is the best reference site for a single MRI slice to assess whole‐body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 2015;102:58–65. [DOI] [PubMed] [Google Scholar]
- 16. Cortellini A, Palumbo P, Porzio G, Verna L, Giordano AV, Masciocchi C, et al. Single‐institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non‐small cell lung cancer patients treated with first‐line chemotherapy. Thorac Cancer 2018;9:1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rier HN, Jager A, Sleijfer S, Maier AB, Levin MD. The prevalence and prognostic value of low muscle mass in cancer patients: a review of the Literature. Oncologist 2016;21:1396–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Werf A, Langius JAE, de van der Schueren MAE, Nurmohamed SA, van der Pant K, Blauwhoff‐Buskermolen S, et al. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr 2018;72:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016;7:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutten IJG, Ubachs J, Kruitwagen R, Beets‐Tan RGH, Olde Damink SWM, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanaoka M, Yasuno M, Ishiguro M, Yamauchi S, Kikuchi A, Tokura M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis 2017;32:847–856. [DOI] [PubMed] [Google Scholar]
- 22. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross‐sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015;17:O20–O26. [DOI] [PubMed] [Google Scholar]
- 23. Lu J, Zheng ZF, Li P, Xie JW, Wang JB, Lin JX, et al. A novel preoperative skeletal muscle measure as a predictor of postoperative complications, long‐term survival and tumor recurrence for patients with gastric cancer after radical gastrectomy. Ann Surg Oncol 2018;25:439–448. [DOI] [PubMed] [Google Scholar]
- 24. Womer AL, Brady JT, Kalisz K, Patel ND, Paspulati RM, Reynolds HL, et al. Do psoas muscle area and volume correlate with postoperative complications in patients undergoing rectal cancer resection? Am J Surg 2018;215:503–506. [DOI] [PubMed] [Google Scholar]
- 25. Yaguchi Y, Kumata Y, Horikawa M, Kiyokawa T, Iinuma H, Inaba T, et al. Clinical significance of area of psoas major muscle on computed tomography after gastrectomy in gastric cancer patients. Ann Nutr Metab 2017;71:145–149. [DOI] [PubMed] [Google Scholar]
- 26. Westenberg LB, Zorgdrager M, Viddeleer AR, Pol RA. Defining sarcopenia and myosteatosis: the necessity for consensus on a technical standard and standardized cut‐off values. J Cachexia Sarcopenia Muscle 2022;13:1429–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raynard B, Pigneur F, Di Palma M, Deluche E, Goldwasser F. The prevalence of CT‐defined low skeletal muscle mass in patients with metastatic cancer: a cross‐sectional multicenter French study (the SCAN study). Support Care Cancer 2022;30:3119–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 29. Cortellini APP, Porzio G, Verna L, Giordano AV, Masciocchi C, Parisi A, et al. Single‐institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non‐small cell lung cancer patients treated with first‐line chemotherapy. Thorac Cancer 2018;9:1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elliott JA, Doyle SL, Murphy CF, King S, Guinan EM, Beddy P, et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg 2017;266:822–830. [DOI] [PubMed] [Google Scholar]
- 31. Sierzega M, Chrzan R, Wiktorowicz M, Kolodziejczyk P, Richter P. Prognostic and predictive implications of sarcopenia in Western patients undergoing gastric resections for carcinoma of the stomach. J Surg Oncol 2019;120:473–482. [DOI] [PubMed] [Google Scholar]
- 32. Haute Autorité de Santé ‐ Diagnostic de la dénutrition de l'enfant et de l'adulte. [https://www.has‐sante.fr/jcms/p_3118872/fr/diagnostic‐de‐la‐denutrition‐de‐l‐enfant‐et‐de‐l‐adulte]
- 33. Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose‐limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 2016;5:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cespedes Feliciano EM, Popuri K, Cobzas D, Baracos VE, Beg MF, Khan AD, et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle 2020;11:1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Popuri K, Cobzas D, Esfandiari N, Baracos V, Jägersand M. Body composition assessment in axial CT images using FEM‐based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging 2016;35:512–520. [DOI] [PubMed] [Google Scholar]
- 36. Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 2019;290:669–679. [DOI] [PubMed] [Google Scholar]
- 37. Ebadi M, Wang CW, Lai JC, Dasarathy S, Kappus MR, Dunn MA, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 39. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


