Abstract
Serum urate (SU) is the most common primary efficacy outcome in trials of urate-lowering therapies for gout. Despite this, it is not formally considered a validated surrogate outcome. In this paper we will outline the definitions of biomarkers and surrogate outcome measures, respectively as well as the available frameworks and challenges in the assessment of the validity of serum urate as a surrogate in gout (i.e. a reasonable replacement for gout symptoms).
Keywords: OMERACT, Gout, Serum urate, Biomarker, Surrogate, Patient Research Partners
Introduction
Surrogate endpoints are considered indispensable in clinical trials that establish a drug’s efficacy, particularly when the clinically important outcome of primary interest may take time to occur. Serum urate (SU) is the most common primary efficacy outcome in clinical trials of urate-lowering therapies (ULT) for the treatment of gout. While the U.S. Food and Drug Administration (FDA) accepts serum urate as a surrogate endpoint in clinical trials for drug approval [1–4], other stakeholders have questioned whether serum urate is a valid replacement for clinical outcomes such as gout flares and skepticism of serum urate as a surrogate endpoint in gout has been raised [5].
Serum urate has previously been endorsed by Outcome Measures in Rheumatology (OMERACT) as a “Core Outcome Domain” mandatory to collect and report in chronic gout trials [6, 7]. For OMERACT, core domains are considered the “what to measure” and there is an expectation that these core outcome domains will be collected and reported to allow the outcome of trials and other studies to be compared and combined as appropriate in evidence-based medicine [8]. The other core domains endorsed in chronic gout studies are flares, tophi burden/regression, health-related quality of life, activity limitation, pain, and patient global assessment [6]. Despite the deductively inferred argument that hyperuricemia is a critical component in the pathophysiology of gout, empirical validation of serum urate as an important biomarker (or as a potential surrogate outcome measure) has been challenging. In this paper, we outline some of the existing frameworks for biomarkers and the potential for these to qualify as surrogate outcomes, as well as the methods and challenges for confirming the validity of serum urate as a surrogate in gout trials (i.e., a reasonable replacement for gout flares to demonstrate therapeutic effectiveness).
Serum urate is on the causal pathway in gout
The clinical manifestations of gout, including flares and tophi occur because of the host inflammatory response to the presence of monosodium urate (MSU) crystals [9]. MSU crystals form in vitro at urate saturation concentrations (>0.41mmol/L, >6.8 mg/dL) at pH 7.0 and a temperature of 37°C, and (>0.364mmol/L, >6.0 mg/dL) at pH 7.0 at a temperature of 35°C. Thus, hyperuricemia defined as serum urate >0.41mmol/L is necessary for the crystal deposition needed for the development of gout. MSU crystal dissolution occurs at concentrations below the point of saturation and the velocity of crystal dissolution is dependent on the serum urate concentration [10, 11].
Definitions of biomarkers and surrogates
A biomarker is defined as a characteristic that is measured as an indicator of health or diseases, or responses to an exposure or intervention, including drug intervention[12, 13]. Biomarkers – a measure of pathophysiological manifestations - may be used to assist in diagnosis or prognosis of disease severity or outcome.
In trials, clinical endpoints of patient importance are considered the most important and reliable outcome measures. Clinical outcomes measure what is most important to people with the condition being studied and include how they feel, function, or survive [14]. A surrogate endpoint is defined as a laboratory measurement or physical sign used as a substitute (or potential replacement) for a clinically important outcome[14]. Inferring from surrogate endpoints usually allows for smaller sample sizes and shorter duration and therefore less expensive more feasible clinical trials. Examples of currently validated surrogate endpoints include systolic blood pressure for stroke, low density lipoprotein (LDL) cholesterol for myocardial infarction and HIV viral load for development of AIDS [15].
At the ‘Outcome Measures in Rheumatology’ (OMERACT) Serum Urate as a Clinically Valid Surrogate in Gout Working Group (https://omeract.org/working-groups/serum-urate/) virtual meeting (2020), which was attended by 2 patient research partners (PRPs) and 23 other participants from three continents (America, Australasia, and Europe) (see Figure 1), consideration was given to whether it was important to the OMERACT community, to distinguish biomarkers from surrogate endpoints with respect to serum urate in gout trials.
Figure 1.
Attendees at the OMERACT gout virtual special interest group meeting 2020.
Discussion centered on two issues: firstly, the overall purpose of validating serum urate as a surrogate and secondly the challenges surrounding the measurement of the most important clinical outcomes. OMERACT stakeholders questioned the overall need for validating serum urate as a surrogate endpoint in gout clinical trials because the FDA already identifies serum urate as a valid surrogate endpoint by accepting it as a primary outcome in ULT clinical trials. However, the controversy around serum urate as a surrogate measure and the need to validate it was emphasized, given the perspectives expressed in the 2017 American College of Physicians (ACP) Gout Guideline[5]. In comparison to the EULAR and ACR gout guidelines, the ACP Gout Guidelines did not recommend a treat-to-target serum urate strategy for managing gout, considering clinical outcomes to be much more relevant for practitioners and patients than serum urate biomarkers. Thus participants considered that endorsing serum urate as a valid surrogate endpoint for clinically relevant outcomes in gout is essential. The PRPs expressed value in being able to follow their serum urate, as well as the connection between serum urate and clinically relevant outcomes (flares, tophi, quality of life).
(PRP 1) “I had my latest flare in 2018, I have no pain, no swelling of my foot or fingers, and my tophi has almost disappeared. I have a good quality of life, and I can do what I want and never worry about a flare. My serum urate is normal, and I think it is nice to know, and to follow and connect the serum urate to my wellbeing”
PRP 2) “the feedback with serum urate which has been measured repeatedly has been really useful, allopurinol is the first long term urate-lowering drug I have been on and it is really motivating to see my serum urate drop”.
After the discussion, 19/20 (95%) participants agreed that it would be important to distinguish biomarkers from surrogate markers with respect to serum urate.
Validation of Biomarkers and Surrogates
Validation of a surrogate requires presenting a strong, independent and consistent association between the biomarker (potential surrogate) and the patient-important clinical outcome of interest that the surrogate could replace as the indicator of successful treatment. This is accomplished by providing evidence that the change in the measurement of a surrogate will (with high certainty) predict a patient-important clinically relevant outcome. Ideally the surrogate lies on the therapeutic (causal) pathway between the drug and the clinical outcome [15].
For gout, the questions remain as to how are we are able to establish a causal relationship and present an approach to critical appraisal of studies using a surrogate outcome (e.g., serum urate) substituting for the patient-important outcome (e.g., gout flares)? Since the results derived from any single trial will not enable us to inductively infer from “all trials”, we need to collect repeated study findings to assess the necessary consistency in order to evaluate it (based on evidence synthesis) to make the decision about the adequacy of a surrogate claim. Evaluation requires a comprehensive review of observational studies of the association between the biomarker (the potential surrogate outcome) and the target patient-important outcome, followed by conclusive evidence synthesis focusing on well-designed randomized trials that have evaluated treatment impact on both the biomarker of interest (potential surrogate) and the target outcome (the patient-important end point).
Validation of serum urate as a biomarker and/or surrogate
At OMERACT 8 a schema for validation of biomarkers was developed. Four domains were identified; target outcome, study design, statistical strength and penalties due to lack or no evidence [14]. At OMERACT 9, this was further refined with both validation criteria for soluble biomarkers in rheumatoid arthritis, psoriatic arthritis and spondyloarthritis (Table 1) [16] and levels of evidence for validation proposed [13]. The validation criteria for soluble biomarkers were adapted for use with serum urate in gout (Table 1). A previous systematic literature review revealed that serum urate fulfilled the criteria for a soluble biomarker with the exception of its effects on outcome measures [17]. On the basis of that review only 34% of voters at OMERACT 10 agreed that serum urate fulfilled the soluble biomarker criteria [18].
Table 1.
| OMERACT 9 draft validation criteria for a soluble biomarker to be regarded as a valid biomarker reflecting structural damage in RA, PsA, and SpA. [16] | OMERACT 9 soluble biomarker criteria adapted for use in chronic gout [17] | |
|---|---|---|
| Truth and discrimination - essential | The assay for measurement of the biomarker is reproducible according to reliability analysis. The effects of the following sources of variability on levels of the biomarker in appropriate controls are known: Age, sex, menopause, Physical activity, ethnicity, NSAIDs, Circadian rhythms, Comorbidity, Body mass index, Renal/hepatic function, Fasting/non-fasting The biomarker demonstrates independent association with the structural damage endpoint (van der Heijde modification of Sharp score for RA/PsA, mSASSS for AS) in patients at all stages of disease at the level of both absolute and relative change in prospective cohort studies and randomized controlled trials of adequate sample size, and followed for a sufficient duration to detect change in x-ray damage score. |
The assay for measurement of SU is reproducible according to reliability analysis. The effects of sources of variability on SU concentrations in appropriate controls are known for the following core variables — age, sex, ethnicity, circadian rhythms, body mass index, renal/hepatic function, fasting/non-fasting, medications and cardiovascular disease. SU demonstrates independent association with clinical and patient centred endpoints. The following key clinical/patient centred endpoints in chronic gout are: number of gout flares, tophus regression, dissolution of crystals, radiographic damage and patient function and quality of life [7]. |
| Truth - desirable but not essential |
A pre-clinical body of evidence that the biomarker reflects tissue remodelling in established animal models of disease (e.g. collagen arthritis for RA).
|
|
| Discrimination-desirable but not essential |
The metabolism, clearance, and half-life of the biomarker have been characterized in normal individuals and in patients with arthritis.
The biomarker demonstrates high sensitivity and specificity in comparisons of the disease population with age and sex matched healthy controls |
|
| Feasibility | The assay for measurement of the biomarker is internationally standardized (availability of reference standards), and is readily accessible if used for clinical practice. Stability of the biomarker at room temperature, in frozen specimen, after repeat freeze/thaw cycles, and after long-term storage has been documented. |
The assay for measurement of SU is internationally standardized (availability of reference standards), and is readily accessible if used for clinical practice. Stability of SU at room temperature, in frozen specimen, after repeat freeze/thaw cycles, and after long-term storage has been documented |
Serum urate as a surrogate for gout flares – what data are available?
The OMERACT Serum Urate as a Clinically Valid Surrogate in Gout Working Group has been developing the evidence for the validation of serum urate as a surrogate endpoint, rather than a biomarker, by different approaches.
Evidence synthesis
In a systematic review and meta-regression of urate-lowering therapy randomized controlled trials (RCTs), the strength of the relationship between serum urate and gout flares was evaluated in order to consolidate any causal link between serum urate and gout flares. Importantly, studies with different urate-lowering therapies (i.e., allopurinol, febuxostat, or pegloticase) were included. The analysis of clinical trial data available from the trial reports did not confirm the association between serum urate and gout flares, however in post hoc analyses there was an association between proportion of individuals achieving serum urate <6 mg/dL and the rate of flares (Figure 2) [19].
Figure 2.
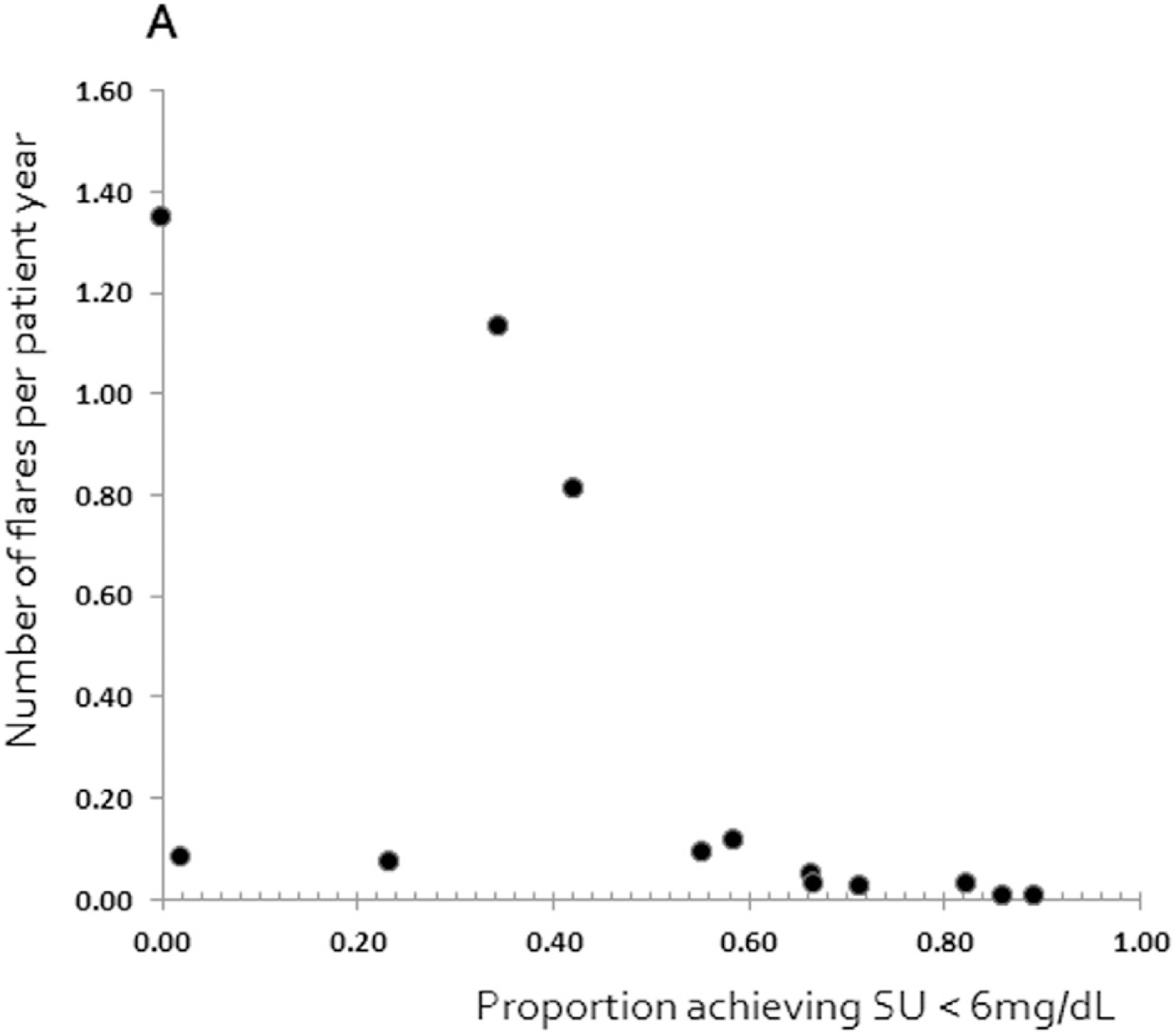
Association between SU and number of flares per patient year (Adapted from Stamp et al[19] with permission from Elsvier Inc License number 5130431327480).
There are a number of likely reasons why a relationship between serum urate and gout flares was not observed. Firstly, none of the trials were designed to determine the relationship between serum urate and flares per se. Secondly, there was variability in trial duration with many trials too short to determine the relationship between serum urate and flares. The time lag between achieving target serum urate (possible surrogate) and improving the patient relevant outcomes such as flare, quality of life, or function is challenged by the inherent paradox, that when initiating ULT, serum urate will be lowered while the flares may occur more frequently before gradually declining after several months [20]. Mobilization of total body store of urate are assumed to be responsible for the initial increased flare frequency observed during initiation of effective ULT. Hence the duration of the RCTs of ULT is an important factor to be considered since the effect of the drug (or drug class) will need a certain time to be evident. In addition, a sustained reduction in serum urate is required before clinical benefit is realized. In short-term trials it can be difficult to evaluate whether the drug is beneficial or harmful if the clinical outcome (flares) and the surrogate endpoint move in opposite directions, related to acute fluctuations in serum urate that are sometimes associated with more gout flares in the initial treatment period. This challenge would be overcome if it is acknowledged that serum urate is a surrogate for gout flares only over the longer-term (say, beyond 12 months of successful urate lowering). In view of this delayed clinical response to urate lowering, presumably due to slow clearance of total body urate, long-term studies of ULTs (2 years) are required. It would be of value for ongoing and future trials to include long term extensions after the randomized period, not only for flare assessment but also for assessing other patient relevant outcome measures.
Finally, there is considerable variation in how flare and serum urate are measured and reported which affects the ability to perform the appropriate analysis [15]. In a current systematic review exploring whether clinical trials are collecting and reporting data in accordance with the core domain set for clinical outcomes in studies of gout [21], we found that in 35 studies of ULT use in gout, while serum urate was reported in all 35 studies it was measured in 10 different ways. Flares were reported in 28 of 35 studies with eight different measurement/reporting methods (Table 2). The wide heterogeneity in reporting of serum urate and flares in clinical trials of ULT creates challenges when data is used in systematic reviews and meta-analysis.
Table 2.
Reporting of OMERACT chronic gout domains in 35 studies
| Domain (N= number of trials that reported on the domain) | Name of measurement | Count | Relative count (%) |
|---|---|---|---|
| Serum Urate (N=35) | SU <6 g/dL or 0.36 mmol/L | 23 | 66 |
| Mean % change SU | 16 | 44 | |
| SU <5 g/dL OR 0.30 mmol/L | 14 | 40 | |
| SU <4 g/ dL | 6 | 17 | |
| Mean change | 6 | 17 | |
| SU<3 g/ dL | 1 | 3 | |
| SU 0.31–0.36 mmol/L | 1 | 3 | |
| Baseline value | 34 | 94 | |
| Final visit value | 24 | 69 | |
| SU <8 g/dl | 1 | 3 | |
| Flares (N=28) | Flares pr. group (%) | 21 | 75 |
| Proportion of pt. per group with >1 flare | 5 | 18 | |
| Mean gout flare rate | 4 | 14 | |
| Number of gout flares per Group | 3 | 11 | |
| Proportion of pt. per group with 1 flare | 1 | 4 | |
| Proportion of pt. per group with ≥ 2 gout flares | 1 | 4 | |
| Proportion of pt. per group with > 2 gout flares | 1 | 4 | |
| Flares per patient year per group | 1 | 4 | |
| Average flare per patient per group | 1 | 4 | |
| Tophi (N=10) | % Complete Tophus resolution | 6 | 60 |
| % Reduction in tophus area | 2 | 20 | |
| Complete or partial tophus resolution of > 1 | 2 | 20 | |
| Change in number of tophi | 1 | 10 | |
| Mean % decrease in number of tophi | 1 | 10 | |
| Median change in number of tophi pr. Patient | 1 | 10 | |
| HR-QOL (N=1) | SF-36 physical component Summary score | 2 | 100 |
| Functional disability (N=2) | HAQ Baseline | 1 | 100 |
| Reduction in HAQ from baseline | 1 | 50 | |
| HAQ Final visit | 1 | 50 | |
| Pain (N=4) | Mean change (VAS 0–10, 0–100 or VAS not specified) | 2 | 50 |
| Mean pain score (VAS 0–10 or 0–100) | 2 | 50 | |
| Responder/non-responder (50% reduction) | 1 | 25 | |
| Patient global assessment (N=0) | None recorded | 0 | 0 |
Flares are notoriously difficult to measure in clinical trials due to their inherent episodic nature, a case definition that is difficult to assess outside a clinical setting and the absence of well-tested and proven tools/software to quantitate flares onset, severity and offset. A gout flare definition has been developed and validated [22, 23]. However, to date, this definition has not been routinely used in clinical trials and the relevance of implementing the validated gout flare definition in clinical trials must be emphasized. Tools that have been developed to capture flares suffer from technical challenges of not all patients having similar familiarity/access with/to various electronics, challenges with recall bias associated with infrequent data entry versus response extinction due to excessive prompting for data input, and proprietary interests of tool developers leading to lack of standardization and limited head-to-head comparisons.
Individual patient level data analysis
In an effort to overcome the difficulties with trial design as outlined above, an analysis of individual patient level data from two randomized controlled trials was undertaken [24]. Both of the trials included in the analysis were two years in duration allowing a period for achieving target urate, with assessment of gout flares at later time (Figure 3). For the purposes of this analysis we defined individuals who on average achieved target serum urate < 0.36mmol/L (6mg/dL) from the available serum urate data at 6, 9 and 12 months as “serum urate responders” and serum urate -non-responders were participants who had an average serum urate ≥0.36mmol/L (6mg/dL) at 6, 9 and 12 months (Figure 3).
Figure 3.
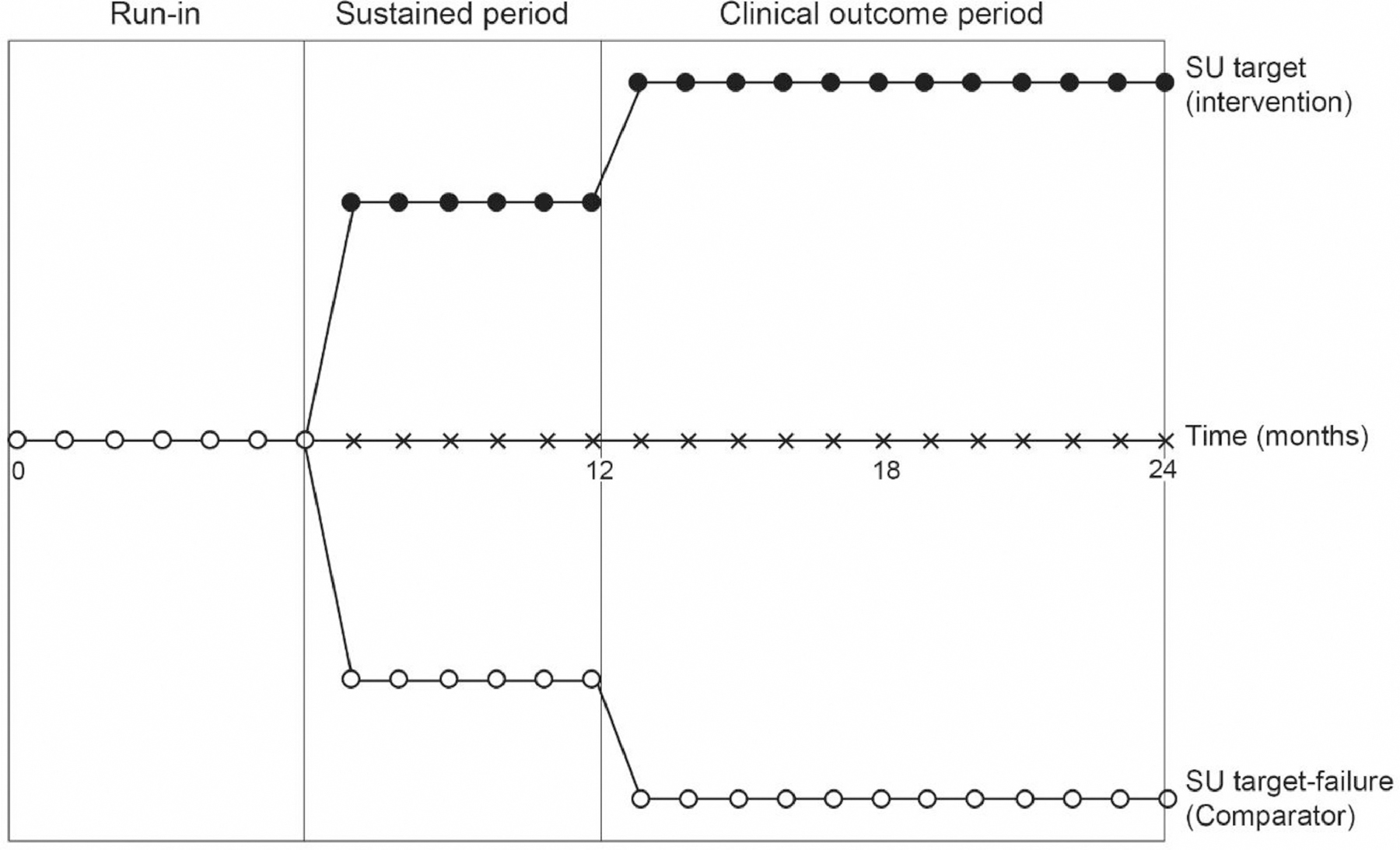
Study design [24]
Results of this analysis showed both a statistically and clinically significant relationship between serum urate responders and a reduced risk of gout flares between months 12 and 24 (unadjusted OR 0.20; 95%CI 0.15 to 0.29) and also that serum urate responders had fewer gout flares between months 12 and 24 (mean difference −1.64; 95%CI −1.85 to −1.44) [24]. Using data from the Nottingham study only [25], there was no effect of randomization on the relationship between serum urate response group and the presence or absence of gout flares. In addition, when the serum urate response group was included together with original random allocation in the regression model, only the response group showed a significant association with the presence of gout flares. In other words, the effect of the intervention appeared to be mediated through reduction in serum urate. Both of these results suggest that the reduction in serum urate is directly responsible for the absence of flares.
These data were presented at the OMERACT 2020 gout special interest group virtual meeting and 17/21 (81%) attendees – clearly a selected sample of stakeholders - believed that these data provided sufficient evidence for serum urate to qualify as a surrogate for gout flares. The group agreed that the presented evidence may not formally be acceptable in any of the existing statistical frameworks, although it is apparently already considered sufficient for the FDA for regulatory purposes. It was considered important to emphasize that serum urate could be considered a surrogate outcome in the context of adherence to ULT, and that measuring a surrogate should never imply that the other mandatory core outcome domains in gout [6] can be omitted in the reporting of clinical trials.
Figure 4.

Formal surrogate validation criteria – how does serum urate perform?
We have used two validation frameworks to assess serum urate as a surrogate. Neither of these frameworks were specifically developed for assessing serum urate as a surrogate.
The “Biomarker-Surrogate-Evaluation-Scheme-3” (BSES-3) framework includes four domains: study design; target outcome, statistical strength and generalizability [26]. Using this framework, serum urate did not fulfil the statistical strength domain and therefore did not fulfil the criteria for a biomarker/surrogate [19] (Table 3).
Table 3.
Performance of serum urate as a surrogate for gout flares according to the BSES 3 framework (S is the surrogate and T is the target outcome) [19].
| BIOMARKER-SURROGATE EVALUATION SCHEMA (BSES) 3 | SU score | ||
|---|---|---|---|
|
|
|||
| Biomarker-surrogate domains | Score | ||
| Study design | 0 | Biological plausibility & lower quality clinical studies e.g. cross-sectional observational studies | |
| 1 | Rank 0 and at least 2 good quality prospective observational cohort studies measuring S and T | ||
| 2 | Rank 1 and at least 2 high quality adequately powered RCTs measuring S and T | ||
| 3 | Rank 1 and all, and at least 5 high quality adequately powered, RCTs measuring S and T | X | |
| Target outcome | 0 | Target is reversible disease-centered biomarker of harm | |
| 1 | Target is irreversible disease-centered biomarker of harm | ||
| 2 | Target is patient-centered endpoint of reversible organ morbidity or clinical burden of disease or clinical harm | X | |
| 3 | Target is patient-centered endpoint of irreversible organ morbidity or clinical burden of disease or severe irreversible clinical harm or death | ||
| Statistical Evaluation of biosurrogate — Target (B-T) domai | 0 | Poor: Does not meet the criteria for Rank 1 | X |
| 1 | Fair: RCT R2trial≥0.2 AND STEP* ≥ 0.1 AND R2ind ≥ 0.2 OR cohort data R2ind ≥ 0.4 | ||
| 2 | Good: RCT R2trial ≥ 0.4 AND STEP ≥ 0.2 AND R2ind ≥ 0.4 | ||
| 3 | Excellent: RCT R2trial ≥ 0.6 AND STEP ≥ 0.3 AND R2ind ≥ 0.6 (without data subdivision)** | ||
| Generalizability | 0 | No clinical or pharmacologic evidence | |
| 1 | Clinical OR pharmacologic evidence | ||
| 2 | Clinical AND pharmacologic evidence | ||
| 3 | Consistent Clinical RCT AND pharmacologic RCT evidence | X | |
| TOTAL | |||
Score: Level A 12, Level B+, B, B− 11-9, Level C+, C, C−, D+, D, D− 8-6, Level D+, D, D−, E+, E, E− 5-3, Level E+, E, E−, F+, F, F-2-0
Bucher et al propose a framework for establishing a causal relationship. The authors note that surrogate outcomes will only be reliable if there is a causal relationship between a change in the surrogate and a change in the clinical outcome. Thus the surrogate must be on the causal pathway of the disease process and the intervention’s entire effect on the clinical outcome should be fully explained by a change in the surrogate [15]. The authors propose three core criteria when examining the evidence with regards surrogate markers:
Is there a strong, independent, consistent association between the surrogate and the clinical patient-important outcome?
Is there evidence from randomized trials in the same drug class that improvement in the surrogate end point has consistently led to improvement in the target outcome?
Is there evidence from randomized trials in other drug classes that improvement in the surrogate end point has consistently led to improvement?
The authors note the answers to questions two and three should be “yes” for there to be sufficient evidence to guide clinical practice. Assessment of serum urate as a surrogate for gout flares against these criteria is outlined in Table 4.
Table 4.
Performance of serum urate as a surrogate for gout flares according to the Bucher criteria [15]
| Criteria | Answer | Evidence |
|---|---|---|
| Is there a strong independent, consistent association between the surrogate endpoint and the clinical endpoint? | Yes | Recent IPD analysis from two allopurinol RCTs shows clear relationship between achieving urate <0.36mmol/l and reduction in flares [24] Trials of febuxostat and allopurinol and pegloticase consistently show reduction in flares [1, 25] |
| Is there evidence from randomized trials in the same drug class that improvement in the surrogate end point has consistently led to improvement in the target outcome? | Yes | Trials of febuxostat and allopurinol consistently show reduction in flares [1, 25] Recent IPD analysis from two allopurinol RCTs shows clear relationship between achieving urate <0.36mmol/l and reduction in flares [24] |
| Is there evidence from randomized trials in other drug classes that improvement in the surrogate end point has consistently led to improvement in the target outcome? | (Yes) | Trials of febuxostat and allopurinol and pegloticase consistently show reduction in flares [1, 25] |
Conclusion
Among gout-interested experts, it is axiomatic that serum urate is on the causal pathway for gout. Despite the reassuring deductive arguments, it has been challenging to provide unequivocal (empirical/inductive) evidence that serum urate is a surrogate for clinically important outcomes among patients with chronic gout. Our recent analysis of individual level patient data has for the first time confirmed that achieving target urate of <0.36mmol/L (<6mg/dL) appears to be causally linked with reduced risk and number of gout flares. Further analysis of urate-lowering therapies other than allopurinol, such as febuxostat, as well as drugs with a different mode of action for lowering urate lowering such as pegloticase, is required to definitively fulfil these criteria.
Acknowledgements
We would like to thank all who participated in our virtual SIG session (including but not limited to Kazuki Yoshida, Lara Maxwell, Brian LaMoreaux, Win Min Oo, David Grossberg, Niti Goel, Ade Adebajo), the OMERACT secretary, Shawna Grosskleg, for continuous support.
Funding
The Parker Institute is grateful for the financial support received from public and private foundations, companies, and private individuals over the years. The Parker Institute, Bispebjerg and Frederiksberg Hospital is supported by a core grant from the Oak Foundation (OCAY-18-774-OFIL); The Oak Foundation is a group of philanthropic organizations that, since its establishment in 1983, has given grants to not-for-profit organizations around the world. MBM has received PhD Scholarships from the Faculty of Health Sciences, University of Southern Denmark, The Region of southern Denmark, and The Danish Rheumatism Association. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data of the study and had final responsibility for the decision to submit for publication. Dr. Tedeschi receives support from the National Institutes of Health (K23 AR075070, L30 AR070514). Dr. Neogi receives support from NIH K24 AR070892 and P30 AR0702571.
Footnotes
Declaration of Competing Interest
MBM: No conflicts declared, RC: No conflicts declared, JAS: Has received consultant fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in TPT Global Tech, Vaxart pharmaceuticals and Charlotte’s Web Holdings, Inc. JAS previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JAS is on the speaker’s bureau of Simply Speaking. JAS is a member of the executive of Outcomes Measures in Rheumatology (OMERACT), an organization that develops outcome measures in rheumatology and receives arms-length funding from 12 companies. JAS serves on the FDA Arthritis Advisory Committee. JAS is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Criteria subcommittee. Director of the University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JAS previously served as a member of the following committees: member, the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee and the co-chair of the ACR Criteria and Response, ND: Reports consulting fees from AstraZeneca, Dyve BioSciences, Selecta, Horizon, and Arthrosi, speaker fees from Abbvie and Janssen, and research grants from Amgen and AstraZeneca, outside the submitted work. ORCID: 0000-0003-3485-0006, KS: Reports he is an Investigator for Horizon, Sobi, LG, Dyve and consulting fees from Horizon, WJT: No conflicts declared ORCID 0000-0001-6075-8479, TN: No conflicts declared. ORCID 000-002-9515-1711, MK: No conflicts declared ORCID 0000-0002-6445-8526, BMP: No conflicts declared, GMM: No conflicts declared, BJS: Is the senior Methodologist. ORCID 0000-0002-7686-2585, CDT: No conflicts declared. ORCID 0000-0001-6275-7699, SKT: No conflicts declared, RG: personal fees from Pfizer, Cornerstones, Jannsen, and Novartis. AA: No conflicts declared, ML: No conflicts declared, AG: No conflicts declared ORCID 0000-0001-7365-7212, LSS: Serves as a consultant to Horizon Therapeutics. He serves on the Executive of OMERACT and is Chair of the Finance Committee of OMERACT and Co-Chair of It’s Business Advisory Committee., AN: No conflicts declared, SMN: No conflicts declared, ML: No conflicts declared, PT: No conflicts declared ORCID 00000-0001-5062-0556, LKS: No conflicts declared ORCID 0000-0003-0138-2912
References
- [1].Becker M, Schumacher HR, Wortmann R, MacDonald P, Eustace D, Palo W, et al. Febuxostat compared with allopurinol in patients with hyperuricaemia and gout. N Engl J Med 2005;353:2450–61. [DOI] [PubMed] [Google Scholar]
- [2].Becker M, Fitz-Patrick D, Choi H, Dalbeth N, Storgarde C, Cravetsf M, et al. An open-label, 6-month study of allopurinol safety in gout: The LASSO study. Semin Arthritis Rheum 2015;2015(45):174–83. [DOI] [PubMed] [Google Scholar]
- [3].Sundy J, Baraf H, Yood R, Edwards N, Gutierrez-Urena S, Treadwell E, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011;306(7):711–20. [DOI] [PubMed] [Google Scholar]
- [4].Saag K, Fitz-Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, et al. Lesinurad Combined With Allopurinol: Randomized, Double-Blind, Placebo-Controlled Study in Gout Subjects With Inadequate Response to Standard of Care Allopurinol (A US-based Study). Arthritis Rheum 2017;69(1):203–12. [DOI] [PubMed] [Google Scholar]
- [5].Qaseem A, Harris R, Forciea M. of ftCGC, Physicians tACo. Management of Acute and Recurrent Gout: A Clinical Practice Guideline From the American College of Physicians. Ann Int Med 2017;166(1):58–68. [DOI] [PubMed] [Google Scholar]
- [6].Schumacher H, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. J Rheumatol 2009;36 (10):2342–5. [DOI] [PubMed] [Google Scholar]
- [7].Taylor W, Schumacher H, Baraf H, Chapman P, Stamp L, Doherty M, et al. A modified Delphi exercise to determine the extent of consensus with OMERACT outcome domains for studies of acute and chronic gout. Ann Rheum Dis 2008;67:888–91. [DOI] [PubMed] [Google Scholar]
- [8].The OMERACT Handbook [ Available from 2019https://omeracthandbook.org/handbook.
- [9].Narang RK, Dalbeth N. Pathophysiology of Gout. Semin Nephrol 2020;40(6):550–63. [DOI] [PubMed] [Google Scholar]
- [10].Lam-Erwin C, Nancollas GH. The crystallization and dissolution of sodium urate. J Cryst Growth 1981;53:215–23. [Google Scholar]
- [11].Perez-Ruiz F, Calabozo M, Pijoan J, Herrero-Beites A, Ruibal A. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Care Res 2002;47(4):356–60. [DOI] [PubMed] [Google Scholar]
- [12].Federal Drug Administration. FDA Facts about surrogates 2021. [Available from https://www.fda.gov/about-fda/innovation-fda/fda-facts-biomarkers-and-surrogate-endpoint.
- [13].Maksymowych W, Fitzgerald O, Wells G, Galdman D, Landewe R, Ostergaard M, et al. Proposal for levels of evidence schema for validation of a soluble biomarker reflecting damage endpoints in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, and recommendations for study design. J Rheumatol 2009;36:1792–9. [DOI] [PubMed] [Google Scholar]
- [14].Lassere M, Johnson K, Boers M, Tugwell P, Brooks P, Simon L, et al. Definitions and validation criteria for biomarkers and surrogate endpoints: Development and testing of a quantitative hierarchical levels of evidence schema. J Rheumatol 2007;34(3):607–15. [PubMed] [Google Scholar]
- [15].Bucher H, Guyatt G, Cook D, Holbrook A, McAlister F. Group ftE-BMW. Users’ Guides to the Medical Literature: XIX. Applying Clinical Trial Results A. How to Use an Article Measuring the Effect of an Intervention on Surrogate End Points. JAMA 1999;282(771–8). [DOI] [PubMed] [Google Scholar]
- [16].Maksymowych W, Landewe R, Tak P, Ritchlin C, Ostergaard M, Mease P, et al. Reappraisal of OMERACT 8 draft validation criteria for a soluble biomarker reflecting structural damage endpoints in rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis: The OMERACT 9 v2 criteria. Journal of Rheumatology 2009;36:1785–91. [DOI] [PubMed] [Google Scholar]
- [17].Stamp L, Zhu X, Dalbeth N, Jordan S, Edwards N, Taylor W. Serum urate as a soluble biomarker in chronic gout – evidence that serum urate fulfils the OMERACT validation criteria for soluble biomarkers. Semin Arthritis Rheum 2011;40:483–500. [DOI] [PubMed] [Google Scholar]
- [18].Stamp L, Khanna P, Dalbeth N, Boers M, Maksymowych W, Schumacher H, et al. Serum Urate in Chronic Gout — Will It Be the First Validated Soluble Biomarker in Rheumatology? J Rheumatol 2011;38:1642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stamp L, Morillon M, Taylor W, Dalbeth N, Singh J, Lassere M, et al. Serum urate as surrogate endpoint for flares in people with gout: a systematic review and meta-regression analysis. Semin Arthritis Rheum 2018;48(2):293–301. [DOI] [PubMed] [Google Scholar]
- [20].Dalbeth N, Merriman T, Gout Stamp L. Lancet 2016;388(2039–52). [DOI] [PubMed] [Google Scholar]
- [21].Morillon M, Noerup A, Smith K, Ellingsen T, Simon L, Singh J, et al. Outcome reporting in randomized trials in gout: protocol for a systematic review to inform the development of a core outcome measurement and reporting set. PROSPERO 2019. CRD42019151316 2019. [Google Scholar]
- [22].Gaffo A, Schumacher H, Saag K, Taylor W, Dinella J, Outman R, et al. Developing a provisional definition of a flare in patients with established gout. Arthritis Rheum 2012;64(5):1508–17. [DOI] [PubMed] [Google Scholar]
- [23].Gaffo A, Dalbeth N, Saag K, Singh J, Rahn E, Mudano A, et al. Validation of a Definition of Flare in Patients With Established Gout. Arthritis Rheumatol 2018;70 (3):462–7. [DOI] [PubMed] [Google Scholar]
- [24].Stamp L, Frampton C, Morillon M, Taylor W, Dalbeth N, Singh J, et al. Analysis of two randomized controlled trials to determine the association between serum urate and gout flares in people with gout - evidence for surrogate status. Lancet Rheumatol 2021. In press. [DOI] [PubMed] [Google Scholar]
- [25].Doherty M, Jenkins W, Richardson H, Sarmanova A, Abhishek A, Ashton D, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392(10156):1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lassere M, Johnson K, Schiff M, Rees D. Is blood pressure reduction a valid surrogate endpoint for stroke prevention? an analysis incorporating a systematic review of randomised controlled trials, a by-trial weighted errors-in-variables regression, the surrogate threshold effect (STE) and the biomarker-surrogacy (BioSurrogate) evaluation schema (BSES). BMC Medical Research Methodology 2012;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]


