Abstract
Background
Prostaglandins are naturally occurring lipids that are synthesised from arachidonic acid. Multiple studies have evaluated the benefits of prostaglandins in reducing ischaemia reperfusion injury after liver transplantation. New studies have been published since the previous review, and hence it was important to update the evidence for this intervention.
Objectives
To evaluate the benefits and harms of prostaglandins in adults undergoing liver transplantation compared with placebo or standard care.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 27 December 2022.
Selection criteria
We included randomised clinical trials evaluating prostaglandins initiated in the perioperative period compared with placebo or standard care for adults undergoing liver transplantation. We included trials irrespective of reported outcomes.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. all‐cause mortality, 2. serious adverse events, and 3. health‐related quality of life. Our secondary outcomes were 4. liver retransplantation, 5. early allograft dysfunction, 6. primary non‐function of the allograft, 7. acute kidney failure, 8. length of hospital stay, and 9. adverse events considered non‐serious. We used GRADE to assess certainty of evidence.
Main results
We included 11 randomised clinical trials with 771 adult liver transplant recipients (mean age 47.31 years, male 61.48%), of whom 378 people were randomised to receive prostaglandins and 393 people were randomised to either placebo (272 participants) or standard care (121 participants). All trials were published between 1993 and 2016. Ten trials were conducted in high‐ and upper‐middle‐income countries.
Prostaglandins may reduce all‐cause mortality up to one month (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.61 to 1.23; risk difference (RD) 21 fewer per 1000, 95% CI 63 fewer to 36 more; 11 trials, 771 participants; low‐certainty evidence). Prostaglandins may result in little to no difference in serious adverse events (RR 0.92, 95% CI 0.60 to 1.40; RD 81 fewer per 1000, 95% CI 148 fewer to 18 more; 6 trials, 568 participants; low‐certainty evidence). None of the included trials reported health‐related quality of life. Prostaglandins may result in little to no difference in liver retransplantation (RR 0.98, 95% CI 0.49 to 1.96; RD 1 fewer per 1000, 95% CI 33 fewer to 62 more; 6 trials, 468 participants; low‐certainty evidence); early allograft dysfunction (RR 0.62, 95% CI 0.33 to 1.18; RD 137 fewer per 1000, 95% CI 241 fewer to 47 more; 1 trial, 99 participants; low‐certainty evidence); primary non‐function of the allograft (RR 0.58, 95% CI 0.26 to 1.32; RD 23 fewer per 1000, 95% CI 40 fewer to 16 more; 7 trials, 624 participants; low‐certainty evidence); and length of hospital stay (mean difference (MD) −1.15 days, 95% CI −5.44 to 3.14; 4 trials, 369 participants; low‐certainty evidence). Prostaglandins may result in a large reduction in the development of acute kidney failure requiring dialysis (RR 0.42, 95% CI 0.24 to 0.73; RD 100 fewer per 1000, 95% CI 132 fewer to 49 fewer; 5 trials, 477 participants; low‐certainty evidence). The evidence is very uncertain about the effect of prostaglandins on adverse events considered non‐serious (RR 1.19, 95% CI 0.42 to 3.36; RD 225 fewer per 1000, 95% CI 294 fewer to 65 fewer; 4 trials, 329 participants; very low‐certainty evidence).
Two trials reported receiving funding; one of these was with vested interests.
We found one registered ongoing trial.
Authors' conclusions
Eleven trials evaluated prostaglandins in adult liver transplanted recipients. Based on low‐certainty evidence, prostaglandins may reduce all‐cause mortality up to one month; may cause little to no difference in serious adverse events, liver retransplantation, early allograft dysfunction, primary non‐function of the allograft, and length of hospital stay; and may have a large reduction in the development of acute kidney injury requiring dialysis. We do not know the effect of prostaglandins on adverse events considered non‐serious. We lack adequately powered, high‐quality trials evaluating the effects of prostaglandins for people undergoing liver transplantation.
Keywords: Adult, Humans, Male, Middle Aged, Liver, Prostaglandins, Prostaglandins/therapeutic use, Quality of Life, Randomized Controlled Trials as Topic
Plain language summary
Prostaglandins to treat adults who have undergone liver transplantation
Are prostaglandins (a drug given to protect the liver) a useful treatment in adults who have had liver transplantation surgery?
Key messages
In adults who have had liver transplantation surgery, prostaglandins may reduce death from any cause, up to one month after surgery, compared with placebo (sham treatment) or standard care. Prostaglandins may result in a large reduction in the development of acute kidney failure requiring dialysis.
Prostaglandins may result in little to no difference in serious adverse events (side effects), and we do not know the effect of prostaglandins on adverse events considered non‐serious.
Further updates of this review, based on future studies, may help in reaching more certain conclusions about prostaglandins.
What is liver transplantation?
The treatment for advanced liver disease and liver failure is liver transplantation. It involves replacing a diseased liver with a new, healthy one.
What are prostaglandins?
Prostaglandins are medicines that could help in prompt functioning of the new liver. Prostaglandins are also produced by the body and increase blood supply to the liver and kidneys.
What did we want to find out?
We wanted to know if prostaglandins are a useful treatment after liver transplantation and if they caused any side effects when compared to placebo or usual care.
We looked at the following outcomes: deaths from any cause up to one month after treatment; any side effects; effects on quality of life; whether the need for retransplantation was decreased; whether initial poor or non‐function of the liver was decreased; whether early kidney injury needing dialysis was decreased; and whether length of hospital stay was decreased.
What did we do?
We searched for studies on prostaglandins used to treat adults who had received a liver transplant compared with placebo or standard care. Participants could be of any sex or ethnicity.
We compared the results of the studies and rated them, based on factors such as study methods and sizes.
What did we find?
We found 11 studies with 771 participants. Of these, 378 participants were given prostaglandins. Apart from one study, all other studies took place in high‐ and upper‐middle‐income countries.
Main results
Death from any cause
Prostaglandins may reduce death from any cause (11 studies, 771 people). In 1000 people, we may expect that 21 fewer people would die with prostaglandins compared with standard care or placebo.
Did people get better with prostaglandins?
– Prostaglandins may result in little to no difference in need for retransplantation (6 studies, 468 participants).
– Prostaglandins may result in little to no difference in initial poor function of the liver (1 study, 99 participants).
– Prostaglandins may result in little to no difference in initial non‐function of the liver (7 studies, 624 participants).
– Prostaglandins may result in a large reduction in the development of acute kidney injury needing dialysis (5 studies, 477 participants).
Did people get worse with prostaglandins?
We did not find any information to suggest that prostaglandins cause harm.
Quality of life
None of the studies reported quality of life.
Unwanted effects
We found no information to suggest that prostaglandins cause harm.
What are the limitations of the evidence?
Our evidence is limited because studies used different methods to measure and record their results, and we did not find studies for some of our outcomes of interest. In addition, our confidence in the evidence was low for all outcomes except for serious adverse events considered non‐serious, for which our confidence in the evidence was very low. Low and very low confidence in the evidence means that the obtained results are uncertain, and when further studies are performed and data are added, results will change further.
How up‐to‐date is this evidence?
This evidence is current to 27 December 2022.
Summary of findings
Summary of findings 1. Prostaglandin compared with placebo or standard care for adult liver transplanted recipients.
| Prostaglandins for adult liver transplanted recipients | ||||||
|
Patient or population: adult liver transplanted recipients Setting: perioperative Experimental intervention: prostaglandins Control intervention: placebo or standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
№ of participants (RCTs) |
Certainty of the evidence (GRADE) |
Comments | |
|
Assumed risk with placebo or standard care |
Risk difference with prostaglandin | |||||
|
All‐cause mortality (follow‐up: up to 1‐month; range not reported to 24 months; 1 month (median)) |
150 per 1000 | 129 per 1000 (87 to 186) |
RR 0.86 (0.61 to 1.23) |
771 (11 RCTs) |
⊕⊕⊖⊖ Lowa,b | Note: 4 trials reported all‐cause mortality but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 7 trials rather than 11. |
|
Serious adverse eventsc (follow‐up: 1–6 months; median 6 months; range 1–6 months; 6 months (median)) |
260 per 1000 | 180 per 1000 (112 to 279) | RR 0.92 (0.60 to 1.40) | 568 (6 RCTs) | ⊕⊕⊖⊖ Lowa,b | The most often occurring serious adverse events were respiratory complications, haemodynamic instability, bleeding, and infections. |
| Health‐related quality of life | — | — | — | — | — | None of the included trials reported quality of life. |
|
Liver retransplantation (follow‐up: 1–12 months; median 9 months) |
63 per 1000 | 62 per 1000 (30 to 124) |
RR 0.98 (0.49 to 1.96) |
468 (6 RCTs) |
⊕⊕⊖⊖ Lowa,b | Note: 1 trial reported liver retransplantation but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 5 trials rather than 6. |
|
Primary non‐function of the allograft (at up to 1 month) |
53 per 1000 | 30 per 1000 (13 to 69) |
RR 0.58 (0.26 to 1.32) |
624 (7 RCTs) |
⊕⊕⊖⊖ Lowa,b | Note: 2 trials reported primary non‐function of the allograft but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 5 trials rather than 7. |
|
Acute kidney failure requiring dialysis (follow‐up: 1–6 months; median 6 months) |
167 per 1000 | 67 per 1000 (35 to 119) |
RR 0.42 (0.24 to 0.73) |
477 (6 RCTs) |
⊕⊕⊖⊖ Lowa,d | Note: 1 trial reported acute kidney failure requiring dialysis but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 5 trials rather than 6. |
|
Adverse events considered non‐serious (follow‐up: 4–24 months; median 9 months) |
341 per 1000 | 116 per 1000 (48 to 276) | RR 1.19 (0.42 to 3.36) | 329 (4 RCTs) | ⊕⊖⊖⊖ Very lowa,b,e | Dose‐related adverse effects related to prostaglandins included hypotension, diarrhoea, flushing, and headache. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (unclear blinding of outcome assessment, and bias in incomplete outcome data and selective outcome reporting). bDowngraded one level for imprecision (the optimal information size was not met (i.e. sample size fewer than 1000), wide CIs in the result, and the 95% CI included both benefits and harms). cSerious adverse events are those that the study authors clearly stated to be due to the experimental or control intervention and if it fulfilled the definition of serious adverse events of the International Council for Harmonisation Guidelines (ICH‐GCP 2016), that is, any event that led to death; was life‐threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability, congenital birth or anomaly; and any important medical event which may have jeopardised the person or required intervention to prevent it (ICH‐GCP 2016). dDowngraded one level for imprecision. The optimal information size was not met (i.e. sample size fewer than 1000). eDowngraded one level for inconsistency: there was considerable heterogeneity with an I2 value of 81% (four RCTs contributed to the analysis: one trial favoured the intervention, two trials favoured the control, one trial found no difference).
Background
Orthotopic liver transplantation is the treatment of choice for people with decompensated cirrhosis, acute liver failure, selected hepatocellular carcinomas, and certain inherited metabolic disorders. In the US, the number of liver transplantations per year increased from 2690 in 1990 to 9236 in 2021, while in Europe the number increased from 2124 in 1990 and peaked at 7614 in 2018 (ELTR 2021; OPTN 2022). As a result of the advances made in surgical and organ preservation techniques, immunosuppression, and intensive care, one‐year survival after liver transplantation exceeds 90% (OPTN 2022). Despite the overall progress made, complications such as early allograft dysfunction, primary non‐function of the allograft, and acute kidney failure requiring dialysis continue to affect patient outcomes.
Description of the condition
After orthotopic liver transplantation, a degree of clinical and biochemical dysfunction almost invariably occurs, the severity of which is related to the degree of hepatic ischaemic‐reperfusion injury. The most severe of this presentation is known as primary non‐function of the allograft, which affects 0.9% to 8.5% of liver transplantation recipients (Hartog 2022). It usually requires emergency retransplantation and significantly increases the risk of death of the recipient (Masior 2022).
Description of the intervention
A major reason for primary non‐function of the allograft is ischaemia‐reperfusion injury. Although the proinflammatory properties of individual prostaglandins during an acute inflammatory phase are known, it has been shown in multiple studies that administration of prostaglandins, mainly prostaglandin E1, could reduce the ischaemia‐reperfusion injury (Ricciotti 2011). The exact timing, dose, route, and duration of prostaglandins to prevent primary non‐function is not standardised in medical literature (Hossain 2006; Takaya 1995).
How the intervention might work
The ratio of prostacyclin (PGI2) to thromboxane (TxA2) is decreased in ischaemia‐reperfusion injury, thereby, promoting local leukocyte adhesion and platelet aggregation. The administration of prostacyclin was found to reduce ischaemia‐reperfusion injury after liver transplantation and increase hepatic oxygen supply by inducing vasodilation and reducing thrombocyte aggregation (Lironi 2017; Smith 1981). Other proposed mechanisms by which prostacyclin could improve liver function include improved hepatic microcirculation, decreased cell‐mediated cytotoxicity, and enhanced DNA synthesis by increasing cyclic adenosine monophosphate levels (Neumann 2000).
Acute kidney injury is another complication following liver transplantation. A reduction in the synthesis of vasodilator prostaglandins was proposed as having an important role in the pathogenesis of kidney insufficiency associated with hepatic dysfunction. The administration of prostaglandins is also postulated to prevent the occurrence of acute renal (kidney) failure (Ricciotti 2011).
Administration of prostaglandins can cause reversible, dose‐related adverse effects such as hypotension, diarrhoea, flushing, and headache. Although prostaglandins inhibit platelet aggregation, there is no evidence of increased risk of bleeding (Cavalcanti 2011; Hunt 1981).
Why it is important to do this review
Even though the incidence of primary graft non‐function has reduced over the years, problems such as early allograft dysfunction and acute kidney injury do occur. This is an update of a Cochrane Review first published in 2011, which found no evidence to suggest that prostaglandins reduced overall mortality, need for liver retransplantation, primary non‐function of the allograft, or acute kidney injury (Cavalcanti 2011). However, a few clinical trials with more robust methodology have subsequently been published, and therefore, we decided to update the evidence for perioperative administration of prostaglandins in liver transplantation and its effect on overall mortality, early allograft dysfunction, primary non‐function of the allograft, and acute renal failure.
Objectives
To evaluate the benefits and harms of prostaglandins in adults undergoing liver transplantation compared with placebo or standard care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials with a parallel group design, irrespective of publication type, publication status, language, blinding, size, duration of follow‐up, objectives, or reported outcomes. We assessed the benefits or harms, or both, of intravenous prostaglandins in adults undergoing liver transplantation. Though unlikely, we planned to include cross‐over and cluster randomised trials, if found. We excluded pseudo‐randomised studies (i.e. quasi‐randomised studies) as the method of allocation to the study groups is not truly random.
Types of participants
Adults, aged 18 years and older, undergoing liver transplantation. The previous review authors selected the cut‐off of 18 years (Cavalcanti 2011).
Types of interventions
Experimental interventions
Prostaglandin E1 or E2, initiated during the perioperative period, irrespective of the dose, duration of infusion, and route of administration. We defined the perioperative period as the period between the beginning of liver surgery and up to 96 hours after liver transplantation.
Control interventions
Placebo or standard care. Standard care included what was reported as "standard" or "routine" by the study authors. This included postoperative organ support, antibiotics, nutrition, routine nursing care, and rehabilitation. When the control group received an added infusion in the form of saline, then this was termed "placebo" for the purpose of this review.
We allowed any co‐interventions if they were administered equally to the trial participants in the experimental and control groups.
Types of outcome measures
We included the trials regardless of outcomes reported.
We assessed the following dichotomous and continuous outcomes at the longest follow‐up, and we used these data for our primary analyses.
Primary outcomes
All‐cause mortality.
Serious adverse events: proportion of participants with serious adverse events. We considered an event as a serious adverse event if the trial authors clearly stated that it was due to the experimental or control intervention and if it fulfilled the definition of serious adverse events of the International Council for Harmonisation Guidelines (ICH‐GCP 2016), that is, any event that led to death; was life‐threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability, congenital birth or anomaly; and any important medical event that may have jeopardised the person or required intervention to prevent it (ICH‐GCP 2016).
Health‐related quality of life: we planned to use the Liver Disease Quality of Life Instrument (LDQOL 1.0) as it is a well‐validated score with a high internal consistency (Gralnek 2000).
Secondary outcomes
Liver retransplantation.
Early allograft dysfunction defined as one or more of the following: serum bilirubin 10 mg/dL or greater on postoperative day seven; international normalised ratio (INR) 1.6 or greater on postoperative day seven; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level greater than 2000 IU/L on postoperative day seven (Olthoff 2010).
-
Primary non‐function of the allograft, as defined in the original trials, except when a trial did not define or report this outcome. Whenever it happened, we asked the author of the trial to provide data according to the following definition proposed by the Organ Procurement and Transplantation Network (OPTN 2017). Primary non‐function is defined (in the absence of vascular thrombosis)
for a transplanted whole liver, as AST 3000 U/L or greater and at least one of the following: 1. INR 2.5 or greater; 2. arterial pH 7.30 or less; 3. venous pH 7.25 or less; 4. lactate 4 mmol/L or greater, within seven days of transplant, OR
for a transplanted liver segment from a deceased or living donor, to have at least one of the following: 1. INR 2.5 or greater; 2. arterial pH 7.30 or less; 3. venous pH 7.25 or less; 4. lactate 4 mmol/L or greater, within 7 days of transplant (OPTN 2017).
Acute kidney failure requiring dialysis defined as any of the following: increase in serum creatinine by 0.3 mg/dL (26.5 μmol/L) within 48 hours; or increase in serum creatinine to 1.5 times baseline, which is known or presumed to have occurred within the prior seven days; or urine volume of 0.5 mL/kg/hour for six hours; or need for renal replacement (KDIGO 2012).
Length of hospital stay.
Adverse events considered non‐serious, that is events that are not included in the above definition of serious adverse events or as defined by trial authors. Dose‐related adverse effects related to prostaglandins include hypotension, diarrhoea, flushing, and headache.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group (CHBG) Controlled Trials Register (searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web; 27 December 2022), the Cochrane Central Register of Controlled Trials (2022, Issue 12) in the Cochrane Library, MEDLINE Ovid (1946 to 27 December 2022), Embase Ovid (1974 to 27 December 2022), LILACS (Bireme; 1982 to 27 December 2022), Science Citation Index Expanded (1900 to 27 December 2022), and Conference Proceedings Citation Index – Science (1990 to 27 December 2022). The latter two were searched simultaneously through Web of Science.
Appendix 1 gives the search strategies with the date range of the searches.
We did not apply any language, date, or publication status restrictions.
We did not perform a systematic search for quasi‐randomised and other observational studies reporting on harms which is a limitation as adverse events are rarely reported in randomised trials.
Searching other resources
We identified other potentially eligible studies or ancillary publications by searching the reference lists of all included studies and of relevant meta‐analyses identified through the electronic searches. We contacted investigators of the included trials to obtain additional information on the retrieved studies.
We also searched the online trial registries ClinicalTrial.gov (clinicaltrials.gov/), European Medicines Agency (EMA; www.ema.europa.eu/ema/), World Health Organization International Clinical Trial Registry Platform (www.who.int/ictrp), and the Food and Drug Administration (FDA; www.fda.gov), as well as pharmaceutical company sources for ongoing or unpublished trials to December 2022.
Data collection and analysis
We performed the review following Cochrane recommendations for review preparation (Higgins 2011a; Higgins 2021), and instructions on the CHBG website (CHBG Information for authors). When data were missing in a published report, we contacted the corresponding author of the trial report. We collected data from unpublished studies by writing to authors of previously published studies (email, and by post when no email address was provided). We performed the analyses using Review Manager 5 (Review Manager 2020) and Review Manager Web (Review Manager Web 2023), and we used Trial Sequential Analysis as sensitivity analysis of imprecision (TSA 2017). We used Trial Sequential Analysis to calculate the required information size (i.e. optimal informational size in GRADE).
For information, see Characteristics of included studies and Characteristics of excluded studies tables.
Selection of studies
Pairs of review authors (ZUM and CTV, LKK and DB, or UG and RCN) independently identified trials for inclusion by screening the titles and abstracts of records identified by the literature search and sought full‐text versions of any records identified by at least one review author for potential inclusion. We selected trials for inclusion based on the full‐text versions. We identified and excluded duplicates and collated multiple reports of the same trial, so that each trial, rather than each report, was the unit of interest in the review. We included trials even if they did not report on the outcomes of interest to our review. We resolved any discrepancies through discussion. As recommended in the PRISMA statement (Moher 2009), we documented the trial selection process in a flow chart showing the total number of retrieved references and the numbers of included and excluded studies (Figure 1). We listed the records that we excluded and the reasons for their exclusion in the Characteristics of excluded studies table.
1.
Study flow diagram
Date of last search 27 December 2022
Data extraction and management
We conducted data extraction according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We used a piloted data extraction form (tested on a few trials) and entered data information in Review Manager Web (Review Manager Web 2023). Pairs of review authors (ZUM and CTV, LKK and DB, or UG and RCN) independently assessed the eligibility of the trials. In case of disagreements, the review authors discussed the reasons for their decisions. When a disagreement was not solved during the process, a third review author served as arbitrator (SS).
In case of any doubts about the trial design, ZUM and CTV contacted the authors of the publication. ZUM and CTV checked the selected trials for multiplicity of publication. When we identified a group of publications for which we were uncertain whether they were reports on the same trial, we contacted the authors of the publication. Whenever we received a confirmation from a trial author, we included the reference of the report below the main reference of the included trial. If we did not receive a response, we reached a consensus regarding the identified report and categorised the study accordingly.
One review author (CTV) transferred data into Review Manager 5 (Review Manager 2020), and a second review author (ZUM) checked trial characteristics for accuracy against the trial report.
We extracted the following information, where reported.
General information: author, title, source, publication date, trial registration, ethics committee approval, country, language, duplicate publications.
Study characteristics: trial design, setting, and dates, inclusion/exclusion criteria, comparability of groups, treatment cross‐overs, compliance with assigned treatment, length of follow‐up.
Participant characteristics: age, sex, ethnicity, number of participants recruited/allocated/evaluated.
Experimental interventions: dosage, frequency, timing, duration, route of administration, setting, duration of follow‐up.
Control interventions (details on placebo or standard care alone): dosage, frequency, timing, duration, route of administration, setting, duration of follow‐up.
Outcomes in the single trials. We recorded whether a trial measured adverse events as number of participants with an adverse event or measured multiple adverse events on the same participant.
Risk of bias assessment: randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result.
Funding sources.
Conflicts of interests of trial authors.
Assessment of risk of bias in included studies
Pairs of review authors (ZUM and LKK; CTV and DB) independently assessed risk of bias in the included trials. We assessed risk of bias according to the Cochrane RoB 1 tool (Higgins 2011b; Higgins 2011c; Sterne 2011), and methodological studies (Kjaergard 2001; Lundh 2017; Moher 1998; Savović 2012a; Savović 2012b; Savović 2018; Schulz 1995; Wood 2008), using the following definitions within domains.
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random numbers table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random or was quasi‐random. We planned to include such studies only for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. whether the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation, so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that investigators who assigned the participants knew the allocation sequence. We planned to include such studies only for assessment of harms.
Blinding of participants and personnel
Low risk of bias: either of the following: blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken; or rarely, no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding, such as mortality.
Unclear risk of bias: either of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the study did not address this outcome.
High risk of bias: either of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinding of outcome assessment
Low risk of bias: either of the following: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; or rarely, no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding such as mortality.
Unclear risk of bias: either of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the study did not address this outcome.
High risk of bias: either of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: information was insufficient for assessment of whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, serious adverse events, and early allograft dysfunction or primary non‐function of allograft. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.ClinicalTrials.gov), the outcomes sought were those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all predefined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded.
High risk of bias: one or more predefined outcomes were not reported.
Other bias
Low risk of bias: the study appeared free of other factors that could have put it at risk of bias (e.g. intention‐to‐treat, baseline characteristics including number of acute liver failures in the study and control group, documented sample size calculation).
Unclear risk of bias: the study may or may not have been free of other factors that could have put it at risk of bias.
High risk of bias: other factors in the study could have put it at risk of bias such as rerandomisation into the study for retransplantation, premature stopping of the trial, and publication of interim analyses.
Overall risk of bias
Low risk of bias: if all the bias domains described above were classified at low risk of bias.
High risk of bias: if any of the bias domains described above were classified at unclear or high risk of bias.
Measures of treatment effect
To analyse dichotomous data (i.e. number of events and number of people assessed in the intervention and comparison groups), we used risk ratio (RR) or risk difference (RD) with their 95% confidence intervals (CIs).
To analyse continuous data, we used the mean, standard deviation, and number of participants assessed for both the experimental intervention and control groups to calculate the mean difference (MD) between treatment arms with a 95% CI. If the MD was reported without individual group data, we used this MD to report the study results. Data from studies that reported median and range or median and interquartile range were addressed using standardised methods (Higgins 2022). We reported RR as relative effect measures are, on average, more consistent than absolute measures (Deeks 2022).
We estimated the 'overall effect' for all outcomes.
We did not have outcomes with time‐to‐event data.
Unit of analysis issues
The unit of analysis was the participant undergoing liver transplantation according to the experimental or the control intervention groups to which the participant was randomly assigned. In a clinical trial with a simple parallel group design, we collected and analysed a single measurement for each outcome from each group.
Where the number of events appeared to be equal to the number of participants, we treated the events as the unit of analysis (Higgins 2021).
Dealing with missing data
We extracted outcome data on all randomised participants in order to allow intention‐to‐treat analysis. If data for intention‐to‐treat analysis were lacking, we attempted to contact trial authors to obtain missing data, or we used the data that were available to us. If after this, data were still missing, we had to make explicit assumptions of any methods the included trials used. Authors of only one trial provided additional information on trial participants (Bharathan 2016). We also performed sensitivity analysis on the outcome 'all‐cause mortality', imputing incomplete or missing data of participants according to best‐worst and worst‐best scenarios (see Sensitivity analysis).
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using the Chi2 test with a significance level of P < 0.1. We used the I2 statistic (Higgins 2003), and visual examination of the forest plot, to assess possible heterogeneity (I2 > 30% to signify moderate heterogeneity, and I2 > 75% to signify considerable heterogeneity) (Deeks 2021). We planned that if the I2 was above 80%, we would explore possible causes of heterogeneity through heterogeneity analyses.
Assessment of reporting biases
We entered information on the included trials in the Characteristics of included studies table. We compared the methods section of a trial to its results section to identify any potential selective reporting (e.g. outcome reporting bias). For trials published after July 2005, we also searched ClinicalTrial.gov (clinicaltrials.gov/), EMA (www.ema.europa.eu/ema/), and World Health Organization International Clinical Trial Registry Platform (www.who.int/ictrp) to identify the protocols of our included trials to identify possible presence of selective reporting of outcomes. We planned to create and examine a funnel plot to explore possible small‐study biases if we had at least 10 trials in a meta‐analysis. In interpreting funnel plots, we would have examined the different possible reasons for funnel plot asymmetry as outlined in Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions and relate this to the results of the review (Page 2021). In future updates of this review, if we have at least 10 trials per meta‐analysed outcome, we will perform formal statistical tests to investigate funnel plot asymmetry and will follow the recommendations in Section 13.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Page 2021).
Data synthesis
We included in our primary analysis all eligible trials, no matter the risk of bias. If the clinical and methodological characteristics of individual trials were sufficiently homogeneous, we meta‐analysed the data. We performed analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021; Deeks 2022). We treated placebo and standard care as the same intervention, as well as standard care at different institutions and time points. We performed the analyses in Review Manager 5 (Review Manager 2020), Review Manager Web (Review Manager Web 2023), and using Trial Sequential Analysis (TSA 2017). One review author (ZUM) entered the data into the software, and a second review author (CTV) checked the data for accuracy. We used the random‐effects model for our primary analyses, and the fixed‐effect model as a sensitivity analysis.
When a meta‐analysis was not possible, we commented on the result narratively.
For binary outcomes, we based the estimation of the between‐study variance using the Mantel‐Haenszel method (Deeks 2021).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Trials at 'low' risk of bias compared to trials at 'high' risk of bias as trials at high risk of bias may overestimate beneficial intervention effects or underestimate harmful intervention effects (Higgins 2021; Kjaergard 2001).
Trials with placebo in the control group compared to trials with standard care in the control group as the use of different comparators may cause heterogeneity in the results in the control groups.
Trials with intention‐to‐treat compared to trials with per‐protocol analysis as the type of analysis may cause heterogeneity amongst the trial results.
Trials at risk of for‐profit support (funded trials) compared to trials without for‐profit support (non‐funded trials) as trials with for‐profit support may overestimate beneficial intervention effects or underestimate harmful intervention effects (Lundh 2017).
Sensitivity analysis
We performed the following sensitivity analyses.
Conducting the analyses of all outcomes using the fixed‐effect model.
Assessing imprecision with Trial Sequential Analysis (see below).
As 'all‐cause mortality' was the only primary outcome with data from all trials, we performed an extreme‐case analysis on this outcome. Extreme‐case analysis favouring the experimental intervention ('best‐worst' case scenario): none of the dropouts/participants lost from the experimental arm, but all the dropouts/participants lost from the control arm will be assumed to have experienced the outcome, including all randomised participants in the denominator. Extreme‐case analysis favouring the control intervention ('worst‐best' case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm, will be assumed to have experienced the outcome, including all randomised participants in the denominator.
We compared our assessments of imprecision in the included trials, performed by GRADE and Trial Sequential Analysis, for each of the Primary outcomes and Secondary outcomes (Castellini 2018; Gartlehner 2019).
Trial Sequence Analysis
We conducted a Trial Sequential Analysis because cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Imberger 2016; Thorlund 2017; TSA 2017; Wetterslev 2008; Wetterslev 2017). To minimise random errors, we calculated the diversity‐adjusted required information size (DARIS; i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Jakobsen 2014; Wetterslev 2008; Wetterslev 2017). The DARIS calculation also accounts for the diversity present in the meta‐analysis (Jakobsen 2014; Wetterslev 2008; Wetterslev 2009; Wetterslev 2017). In our meta‐analysis, we based the required information size on the event proportion in the control group; assumption of a plausible relative risk reduction of 15%; a risk of type I error of 2.5% because of our three primary outcomes and 1.40% because of our six secondary outcomes, a risk of type II error of 10% (Castellini 2018); and the assumed diversity of the meta‐analysis (Wetterslev 2009). We used the software version 0.9.5.10 Beta to perform the Trial Sequential Analysis using the random‐effects model. We added the trials according to the year of publication. If more than one trial was published during the same year, we added the trials alphabetically, according to the last name of the first study author irrespective of bias risk. On the basis of the required information size, we constructed trial sequential monitoring boundaries (Thorlund 2017; Wetterslev 2008; Wetterslev 2017). These boundaries determined the statistical inference we drew regarding the cumulative meta‐analysis that had not reached the required information size; if the trial sequential monitoring boundary for benefit or harm was crossed before the required information size was reached, firm evidence perhaps was established, and further trials may turn out to be superfluous. In contrast, if the boundaries were not surpassed, it is most probably necessary to continue adding trials in order to detect or reject a certain intervention effect. That can be determined by assessing whether the cumulative Z‐curve crosses the trial sequential monitoring boundary for futility. We reported the Trial Sequential Analysis adjusted CI if the cumulative Z‐curve did not pass through any of the trial sequential monitoring boundaries for harm, benefit, or futility. Trial Sequential Analysis can also assist in assessing imprecision for GRADE (Castellini 2018; Gartlehner 2019). We made a comparison between our Trial Sequential Analysis and the GRADE assessment of imprecision as a sensitivity analysis. We downgraded our assessment of imprecision in GRADE two levels if the accrued number of participants was below 50% of the DARIS, and one level if between 50% and 100% of the DARIS (Jakobsen 2014). We did not downgrade if the cumulative Z‐curve reaches futility or the DARIS.
See also Dealing with missing data.
Summary of findings and assessment of the certainty of the evidence
We created Table 1 comparing prostaglandins with placebo or standard care including the outcomes all‐cause mortality, serious adverse events, health‐related quality of life, liver retransplantation, primary non‐function of the allograft, acute kidney failure requiring dialysis, and adverse events considered non‐serious. We chose to present the results of these outcomes as we consider them most clinically important. We provided the longest follow‐up and range, with median for each outcome. We used GRADEpro GDT software and evaluated the certainty of the evidence using the GRADE approach for intervention reviews based on randomised clinical trials (GRADEpro GDT). The GRADE system appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. There are five GRADE domains that are used to assess the certainty of the evidence of the trials, that is, risk of bias (i.e. overall risk of bias judgement), consistency of effect, imprecision, indirectness, and publication bias.
The certainty of the evidence is downgraded for:
serious (−1) or very serious (−2) risk of bias;
serious (−1) or very serious (−2) inconsistency;
serious (−1) or very serious (−2) uncertainty about directness;
serious (−1) or very serious (−2) imprecise or sparse data;
serious (−1) or very serious (−2) probability of reporting bias.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021a). We used the overall risk of bias judgement, derived from the RoB 1 tool, to inform our decision on downgrading the certainty of the evidence for risk of bias. We phrased the findings and certainty of the evidence as suggested in the informative statement guidance (Santesso 2020).
We conducted the review according to the prespecified protocol (Vasconcelos 2006), and we reported any deviations from it in the Differences between protocol and review section of the systematic review.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
The first database search performed on 9 February 2022 identified 543 records and an additional eight records through other resources. Updates of the electronic searches on 20 July 2022 identified an additional 100 records and on 27 December 2022 an additional 235 records. After removing duplicates, we screened 394 records and excluded 341 records based on title and abstract. We assessed the full‐texts of the remaining 53 records for eligibility and excluded 38 records (including three studies that had three published records each (Almazroo 2021; Ghonem 2011; Greig 1989), and one study that had two published records (Tancharoen 1992)) (see Characteristics of excluded studies table). One study was ongoing (see Characteristics of ongoing studies table) and the remaining 15 records, referring to 11 trials, were included (see Characteristics of included studies table). We recorded the selection process in sufficient detail which allowed us to complete a PRISMA flow diagram (Figure 1).
Included studies
Eleven randomised trials were eligible for this systematic review (Alevizacos 1993; Bärthel 2012; Bharathan 2016; Bosch 2000; Henley 1995; Hidalgo 2002; Himmelreich 1993; Ismail 1995; Klein 1996; Manasia 1996; Neumann 2000). None of the trials provided data on quality of life, and, therefore, quantitative synthesis could not be performed. None of the trials reported on ethical approval for using the grafts for transplantation.
All trials were published between 1993 and 2016 and enrolled a total of 771 adult liver transplant recipients (mean age 47.31 years, male 61.48%) (Table 2). The median sample size was 79 participants, ranging from 20 to 160. Ten trials were conducted in high‐ and upper‐middle‐income countries. The remaining trial was conducted in India (Bharathan 2016). Only Bharathan 2016 evaluated recipients with living donor liver grafts. All except Klein 1996 were single‐centre trials. All trials were published as full‐text articles except one which was published as an abstract (Bosch 2000), and another was published as an abstract and a doctoral thesis (Hidalgo 2002). Only two trials received funding from external sources (Henley 1995; Ismail 1995). While Henley 1995 received funding from a neutral source, Ismail 1995 received funding from an industrial organisation that would benefit from the results of the trial.
1. Characteristics of randomised trials evaluating prostaglandins for adult liver transplanted recipients.
|
Study (number of participants) |
Prostaglandin regimen |
Risk of bias | ||||||
|
Adequate sequence generation |
Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | ||
|
Alevizacos 1993 (58) |
Prostaglandin E1, 0.1 μg/kg/hour up to 0.5 μg/kg/hour. From anaesthesia induction to 3rd postoperative day. | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
|
Bärthel 2012 (80) |
Prostaglandin I2 analogue, iloprost, administered immediately after admission to intensive care unit after liver transplantation by continuous intravenous infusion at a rate of 1 ng/kg bodyweight/minute for 7 days. | Low | Unclear | High | Low | Low | Unclear | Unclear |
|
Bharathan 2016 (99) |
PGE1 (alprostadil) 0.25 μg/kg/hour, starting 1 hour after portal venous reperfusion and continued for 96 hours. | Low | Low | Low | Low | Low | Low | Unclear |
|
Bosch 2000 (25) |
Prostaglandin E1 0.6 μg/kg/hour during the first 90 minutes after graft reperfusion through a catheter placed in portal vein. | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
|
Henley 1995 (160) |
Prostaglandin E1, 10–40 μg/hour. From anhepatic phase up to the 21st postoperative day. | Low | Low | Unclear | Low | Low | Unclear | High |
|
Hidalgo 2002 (90) |
Prostaglandin E1 0.6 μg/kg/hour intraportal followed by 100–200 μg/hour intravenously. From reperfusion up to 4 or 5 days. | Low | Unclear | High | High | High | Unclear | Unclear |
|
Himmelreich 1993 (20) |
Prostaglandin E1 10–40 μg/hour. From the beginning of surgery to 3rd postoperative day. | Unclear | Unclear | High | Low | Low | Unclear | Unclear |
|
Ismail 1995 (81) |
Enisoprost, 100 μg 3 times daily orally for 12 weeks. Started within 24 hours continued for 3 months. | Low | Low | Low | Unclear | Low | Unclear | Low |
|
Klein 1996 (118) |
Prostaglandin E1 0.25–1 μg/kg/hour. From reperfusion to the 7th postoperative day. | Low | Low | Low | Low | Low | Unclear | High |
|
Manasia 1996 (21) |
Prostaglandin E1 0.6 μg/kg/hour. Started 12 hours after surgery continued for 5 hours. | Low | Low | Low | Low | High | Unclear | High |
|
Neumann 2000 (30) |
Prostacyclin, 0.5–4 μg/kg/minute. From reperfusion to the 7th postoperative day. | Unclear | Unclear | Unclear | Low | High | Unclear | Unclear |
Study design and Intervention
All included trials used a parallel‐group design. There were no trials with more than two groups, cluster‐randomised trials, or cross‐over trials.
Setting
Ten trials were from single centres (Germany four trials, USA two trials, Spain two trials, UK one trial, and India one trial). Klein 1996 was a multicentre trial in the US.
Participants
All participants were adults aged at least 18 years.
Experimental intervention
Eight trials assessed intravenous prostaglandin E1 (Alevizacos 1993; Bharathan 2016; Bosch 2000; Henley 1995; Hidalgo 2002; Himmelreich 1993; Klein 1996; Manasia 1996), one trial used oral/enteral enisoprost (a prostaglandin E1 analogue) (Ismail 1995), one trial used iloprost (Bärthel 2012), and one trial used prostacyclin (Neumann 2000). The intervention was started immediately before or during surgery in most trials, or within the first 24 hours postoperatively in two trials (Ismail 1995; Manasia 1996).
Control intervention (comparison)
Four trials received standard treatment with no mention of a placebo in the control arm (Alevizacos 1993; Bärthel 2012; Hidalgo 2002; Himmelreich 1993). The remaining seven trials administered placebo (in the form of saline) in addition to standard treatment in the control arm (Bharathan 2016; Bosch 2000; Henley 1995; Ismail 1995; Klein 1996; Manasia 1996; Neumann 2000).
Co‐interventions
There were no trials with co‐intervention or multiple interventions.
Outcomes
Only two trials used graft dysfunction as a primary outcome – primary graft dysfunction by Bärthel 2012 and early allograft dysfunction by Bharathan 2016. The remaining nine trials did not clearly mention a primary outcome.
Follow‐up
The duration of follow‐up for the outcomes in our review varied between trials. The duration of follow‐up was one month for Bharathan 2016 and Klein 1996; four months for Ismail 1995; six months for Bärthel 2012, Henley 1995, and Manasia 1996; 12 months for Hidalgo 2002 and Neumann 2000; and 24 months for Alevizacos 1993. Information on follow‐up duration was not available for Bosch 2000 and Himmelreich 1993.
Amongst the 771 participants who were randomised, 48 (6.2%) dropped out after randomisation. Of these, 21 were from the prostaglandin group and 24 from the control group. Group allocation was not clear for three participants. None of the trials provided information on participants lost to follow‐up.
Excluded studies
We excluded 30 studies (37 records, of which 22 were not randomised trials, 12 did not have a population of interest, and three did not have an adequate comparator) (see Characteristics of excluded studies table). One of these trials was included in the previous version of the review (but not in the analyses as we were unable to obtained clarification from the trial authors), as we obtained no further information we excluded the trial (Neumann 1998).
Ongoing trials
We identified one registered, open‐label randomised trial, which, as of 4 September 2022, had not started recruiting (Shin 2021).
Risk of bias in included studies
We assessed risk of bias and presented our assessments in Figure 2 and Figure 3. All the trials were at overall high risk of bias either due to high risk of bias in at least one domain (Bärthel 2012; Henley 1995; Hidalgo 2002; Himmelreich 1993; Klein 1996; Manasia 1996; Neumann 2000), or due to unclear reporting in one (Bharathan 2016) or more domains (Alevizacos 1993; Bosch 2000; Ismail 1995).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We assessed random sequence generation at low risk of bias in seven trials (Bärthel 2012; Bharathan 2016; Henley 1995; Hidalgo 2002; Ismail 1995; Klein 1996; Manasia 1996), and unclear in four trials because the method of sequence generation was not specified (Alevizacos 1993; Bosch 2000; Himmelreich 1993; Neumann 2000).
Allocation concealment
We assessed allocation concealment at low risk of bias in five trials (Bharathan 2016; Henley 1995; Ismail 1995; Klein 1996; Manasia 1996), and unclear in six trials because there was no information provided (Alevizacos 1993; Bärthel 2012; Bosch 2000; Hidalgo 2002; Himmelreich 1993; Neumann 2000).
Blinding
Blinding of participants and healthcare personnel
We assessed the blinding of participants and healthcare personnel at low risk of bias in four trials (Bharathan 2016; Ismail 1995; Klein 1996; Manasia 1996), and unclear in four trials because there was no information provided (Alevizacos 1993; Bosch 2000; Henley 1995; Neumann 2000). We assessed the remaining three trials at high risk of bias because they were not blinded (Bärthel 2012; Hidalgo 2002; Himmelreich 1993).
Blinding of outcome assessors
We assessed the blinding of outcome assessor at low risk of bias in seven trials because the outcomes reported were objective and not involving judgement by the observer (Bärthel 2012; Bharathan 2016; Henley 1995; Himmelreich 1993; Klein 1996; Manasia 1996; Neumann 2000). We assessed the blinding of outcome assessment to be unclear in three trials because the reported outcomes were not objective (Alevizacos 1993; Bosch 2000; Ismail 1995). We assessed the remaining trial at high risk of bias because of the intraportal route of drug administration in the experimental group (Hidalgo 2002).
Incomplete outcome data
We assessed eight trials at low risk of bias because they provided outcomes of all randomised participants (Alevizacos 1993; Bärthel 2012; Bharathan 2016; Bosch 2000; Henley 1995; Himmelreich 1993; Ismail 1995; Klein 1996). We assessed three trials at high risk of bias because they excluded randomised participants from analysis (Hidalgo 2002; Manasia 1996; Neumann 2000).
Selective reporting
We assessed one trial at low risk of bias due to selective outcome reporting because the study protocol was published and available, and we identified no discrepancies in the reported and planned outcomes (Bharathan 2016). We assessed 10 trials at unclear risk of selective outcome reporting because there was no published study protocol (Alevizacos 1993; Bärthel 2012; Bosch 2000; Henley 1995; Hidalgo 2002; Himmelreich 1993; Ismail 1995; Klein 1996; Manasia 1996; Neumann 2000).
Other potential sources of bias
We assessed the risk of intention‐to‐treat bias to be low in six trials (Alevizacos 1993; Bärthel 2012; Bosch 2000; Himmelreich 1993; Ismail 1995; Klein 1996). We assessed the risk of bias due to early stopping to be low in three trials (Bharathan 2016; Henley 1995; Ismail 1995), unclear in seven trials (Alevizacos 1993; Bärthel 2012; Bosch 2000; Hidalgo 2002; Himmelreich 1993; Klein 1996; Neumann 2000), and high in one trial (Manasia 1996). We assessed the imbalance in baseline characteristics leading to unclear risk of bias to be present in four trials with two trials reporting imbalance (Bärthel 2012; Bharathan 2016), and two trials not reporting baseline characteristics (Bosch 2000; Himmelreich 1993). We assessed the imbalance in baseline characteristics to be absent leading to low risk of bias in seven trials (Alevizacos 1993; Henley 1995; Hidalgo 2002; Ismail 1995; Klein 1996; Manasia 1996; Neumann 2000).
We assessed the overall risk of other bias to be low for one trial (Ismail 1995), unclear for seven trials (Alevizacos 1993; Bärthel 2012; Bharathan 2016; Bosch 2000; Hidalgo 2002; Himmelreich 1993; Neumann 2000), and high for three trials (Henley 1995; Klein 1996; Manasia 1996).
Overall risk of bias
The overall risk of bias was high for all trials, either due to high risk in at least one of the domains (Bärthel 2012; Henley 1995; Hidalgo 2002; Himmelreich 1993; Klein 1996; Manasia 1996; Neumann 2000), or due to unclear risk in one (Bharathan 2016) or more (Alevizacos 1993; Bosch 2000; Ismail 1995) of the domains.
Effects of interventions
See: Table 1
We constructed Table 1 in which we present our assessments of the certainty of evidence for the comparison 'Prostaglandins compared with placebo or standard care for adult liver transplant trial recipients'.
Prostaglandins compared with placebo or standard care
Primary outcomes
All‐cause mortality
Eleven trials reported all‐cause mortality (Alevizacos 1993; Bärthel 2012; Bharathan 2016; Bosch 2000; Henley 1995; Hidalgo 2002; Himmelreich 1993; Ismail 1995; Klein 1996; Manasia 1996; Neumann 2000). Prostaglandins may reduce all‐cause mortality up to one month (RR 0.86, 95% CI 0.61 to 1.23; RD 21 fewer per 1000, 95% CI 63 fewer to 36 more; 11 trials, 771 participants; low‐certainty evidence; Analysis 1.1; note: 4 trials reported all‐cause mortality but recorded 0 events in both groups. Thus, the RR, RD, and CIs were calculated from 7 trials rather than 11). Our reasons for downgrading were risk of bias (unclear blinding of outcome assessment, and bias in incomplete outcome data and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000), wide CIs, and the 95% CIs included both benefits and harms.
1.1. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 1: All‐cause mortality
Serious adverse events
Three trials reported serious adverse events with follow‐up data between one and six months (median six months) (Bärthel 2012; Bharathan 2016; Henley 1995). Prostaglandins may result in little to no difference in serious adverse events (RR 0.92, 95% CI 0.60 to 1.40; RD 81 fewer per 1000, 95% CI 148 fewer to 18 more; 6 trials, 568 participants; low‐certainty evidence; Analysis 1.2). Our reasons for downgrading were risk of bias (unclear blinding of outcome assessment, and bias in incomplete outcome data and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000), wide CIs, and that the 95% CIs included both benefits and harms.
1.2. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 2: Serious adverse events
Health‐related quality of life
No trial measured or provided data on health‐related quality of life.
Secondary outcomes
Liver retransplantation
Six trials reported liver retransplantation with follow‐up data between one and 12 months (median nine months) (Bärthel 2012; Bharathan 2016; Henley 1995; Hidalgo 2002; Himmelreich 1993; Neumann 2000). Prostaglandins may result in little to no difference in liver retransplantation (RR 0.98, 95% CI 0.49 to 1.96; RD 1 fewer per 1000, 95% CI 33 fewer to 62 more; 6 trials, 468 participants; low‐certainty evidence; Analysis 1.3; note: 1 trial reported liver retransplantation but recorded 0 events in both groups. Thus, the RR, RD, and CIs were calculated from 5 trials rather than 6). Our reasons for downgrading were risk of bias (unclear blinding of outcome assessment, and bias in incomplete outcome data and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000), wide CIs, and that the 95% CIs included both benefits and harms.
1.3. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 3: Liver retransplantation
Early allograft dysfunction
Only one trial reported early allograft dysfunction with follow‐up data of one month (Bharathan 2016). Prostaglandins may result in little to no difference in early allograft dysfunction after liver transplantation (RR 0.62, 95% CI 0.33 to 1.18; RD 137 fewer per 1000, 95% CI 241 fewer to 47 more; 1 trial, 99 participants; low‐certainty evidence; Analysis 1.4). Our reasons for downgrading were risk of bias (unclear blinding of outcome assessment, and bias in incomplete outcome data and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000), wide CIs, and that the 95% CIs included both benefits and harms.
1.4. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 4: Early allograft dysfunction
Primary non‐function of the allograft
Seven trials reported on primary non‐function of the allograft, which by definition should occur within seven days of transplantation (Alevizacos 1993; Bärthel 2012; Bharathan 2016; Henley 1995; Hidalgo 2002; Klein 1996; Neumann 2000). Prostaglandins may result in little to no difference in primary non‐function of the allograft (RR 0.58, 95% CI 0.26 to 1.32; RD 23 fewer per 1000, 95% CI 40 fewer to 16 more; 7 trials, 624 participants; low‐certainty evidence; Analysis 1.5; note: 2 trials reported primary non‐function of the allograft but recorded 0 events in both groups. Thus, the RR, RD, and CIs were calculated from 5 trials rather than 7). Our reasons for downgrading were risk of bias (unclear blinding of outcome assessment, bias in incomplete outcome data, and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000), wide CIs, and that the 95% CIs included both benefits and harms.
1.5. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 5: Primary non‐function of the allograft
Acute kidney failure requiring dialysis
Five trials reported acute kidney failure with follow‐up data between one and six months (median six months) (Bharathan 2016; Henley 1995; Hidalgo 2002; Klein 1996; Manasia 1996). Prostaglandins may result in a large reduction in the development of acute kidney failure requiring dialysis (RR 0.42 95% CI 0.24 to 0.73; RD 100 fewer per 1000, 95% CI 132 fewer to 49 fewer; 6 trials, 477 participants; low‐certainty evidence; Analysis 1.6; note: 1 trial reported acute kidney failure requiring dialysis but recorded 0 events in both groups. Thus, the RR, RD, and CIs were calculated from 5 trials rather than 6). Our reasons for downgrading were serious risk of bias (unclear blinding of outcome assessment, bias in incomplete outcome data, and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000).
1.6. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 6: Acute kidney failure requiring dialysis
Length of hospital stay
Four trials reported length of hospital stay with follow‐up data between one and 12 months (median six months) (Bärthel 2012; Bharathan 2016; Henley 1995; Hidalgo 2002). Prostaglandins may result in little to no difference on the length of hospital stay (MD −1.15 days, 95% CI −5.44 to 3.14; 4 trials, 369 participants; low‐certainty evidence; Analysis 1.7). Our reasons for downgrading were risk of bias (most trials had no blinding of participants, personnel, or outcome assessors, or it was not clear from the reports whether these groups were blinded), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000) and wide CIs.
1.7. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 7: Length of hospital stay (days)
Adverse events considered non‐serious
Four trials reported on adverse events considered non‐serious with follow‐up data between four and 24 months (median nine months) (Alevizacos 1993; Henley 1995; Ismail 1995; Neumann 2000). The evidence is very uncertain about the effect of prostaglandins on adverse events considered non‐serious (RR 1.19, 95% CI 0.42 to 3.36; RD 225 fewer per 1000, 95% CI 294 fewer to 65 fewer; 4 trials, 329 participants; very low‐certainty evidence; Analysis 1.8). Our reasons for downgrading were risk of bias (unclear blinding of outcome assessment, and bias in incomplete outcome data and selective outcome reporting), and imprecision because the optimal information size was not met (i.e. sample size fewer than 1000) and heterogeneity.
1.8. Analysis.

Comparison 1: Prostaglandins versus placebo/standard care, Outcome 8: Adverse events considered non‐serious
The most commonly reported outcome was 'all‐cause mortality'. However, even for this outcome, at least one data point was available in only seven trials. Hence, we did not create a funnel plot to explore possible small‐study biases.
Subgroup analyses
We did not perform subgroup analysis between trials at overall low risk of bias and trials at overall high risk of bias as all the trials had high risk of bias.
We performed a subgroup analysis based on whether placebo or standard care was used as the control group. There were no subgroup differences in the outcome variables of all‐cause mortality, liver retransplantation, or primary non‐function in either subgroup (Analysis 2.1; Analysis 2.2; Analysis 2.3). There was a protective effect of prostaglandins in preventing acute kidney failure requiring dialysis when compared with placebo, but not with standard care (Analysis 2.4).
2.1. Analysis.

Comparison 2: Prostaglandins compared with placebo/standard care: subgroup analyses, Outcome 1: All‐cause mortality
2.2. Analysis.

Comparison 2: Prostaglandins compared with placebo/standard care: subgroup analyses, Outcome 2: Liver retransplantation
2.3. Analysis.

Comparison 2: Prostaglandins compared with placebo/standard care: subgroup analyses, Outcome 3: Primary non‐function
2.4. Analysis.

Comparison 2: Prostaglandins compared with placebo/standard care: subgroup analyses, Outcome 4: Acute kidney failure requiring dialysis
We performed a subgroup analysis based on whether trial analysis was by intention‐to‐treat or per protocol. There were no subgroup differences in the outcome variables of all‐cause mortality, liver retransplantation, or primary non‐function in either subgroup (Analysis 3.1; Analysis 3.2; Analysis 3.3). There was a protective effect of prostaglandins in preventing acute kidney failure requiring dialysis when using a per‐protocol analysis but not an intention‐to‐treat analysis (Analysis 3.4).
3.1. Analysis.

Comparison 3: Intention‐to‐treat analysis compared with per‐protocol analysis: subgroup analyses, Outcome 1: All‐cause mortality
3.2. Analysis.

Comparison 3: Intention‐to‐treat analysis compared with per‐protocol analysis: subgroup analyses, Outcome 2: Liver retransplantation
3.3. Analysis.

Comparison 3: Intention‐to‐treat analysis compared with per‐protocol analysis: subgroup analyses, Outcome 3: Primary non‐function
3.4. Analysis.

Comparison 3: Intention‐to‐treat analysis compared with per‐protocol analysis: subgroup analyses, Outcome 4: Acute kidney failure requiring dialysis
We performed a subgroup analysis based on whether the trials were funded with vested interests or not. There were no subgroup differences in the outcome variable of all‐cause mortality (Analysis 4.1).
4.1. Analysis.

Comparison 4: Trials funded for‐profit compared to trials without for‐profit support: subgroup analyses, Outcome 1: All‐cause mortality
Sensitivity analysis
Risk of bias assessment components
We performed a sensitivity analysis for the outcome 'all‐cause mortality' using the fixed‐effect model. We found no difference in the results between the random‐effects and the fixed‐effect model.
The 'best‐worst' case scenario analysis on all‐cause mortality favoured prostaglandin (RR 0.64, 95% CI 0.46 to 0.89; RD 63 fewer per 1000, 95% CI 99 fewer to 11 fewer; 11 trials, 786 participants; I2 = 0%; Analysis 5.1). The 'worst‐best' case scenario analysis on all‐cause mortality favoured control (RR 1.10, 95% CI 0.80 to 1.51; RD 15 more per 1000, 95% CI 30 fewer to 77 more; 11 trials, 786 participants; I2 = 0%; Analysis 5.1). The total number of participants changed when postrandomisation dropout participants were added to the total number of participants, especially in trials that presented per‐protocol analysis (Analysis 3.1).
5.1. Analysis.

Comparison 5: Sensitivity analysis, Outcome 1: All‐cause mortality
The I2 was 81% in adverse events considered non‐serious. We rechecked and verified the data. This persisted with random‐effects and fixed‐effect models.This could be because non‐serious adverse events have no universally accepted definition and, therefore, there is no uniform scale for effect measure. There seems to be a qualitative interaction with adverse events considered non‐serious being more in the prostaglandins group in Alevizacos 1993 and Ismail 1995, while that being more in the control group in Henley 1995 and Neumann 2000. The heterogeneity of 74% persisted when Ismail 1995 was removed, as this was the only trial that provided oral prostaglandins, as opposed to intravenous prostaglandins by the other groups. Ismail 1995 administered prostaglandins for over one month while the other three trials administered prostaglandins for less than one month (Alevizacos 1993; Henley 1995; Neumann 2000). Similarly, when non‐German trials were excluded (Henley 1995; Ismail 1995), the I2 was 73%.
Assessment of imprecision with Trial Sequential Analysis
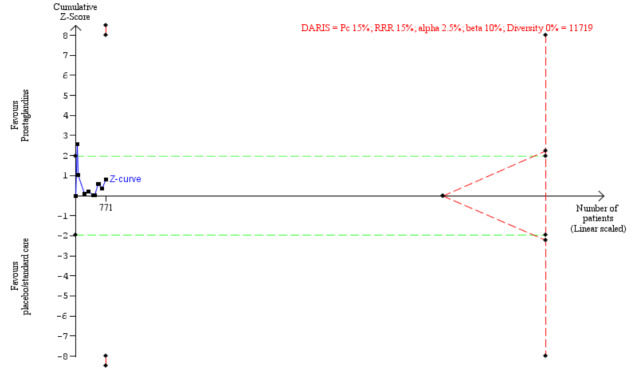
The Trial Sequential Analysis results on all‐cause mortality showed that the DARIS for detecting an intervention effect was 11,719 participants, calculated based on the proportion of events 15% in the control group; a relative risk reduction of 15%; an alpha of 2.5%; a beta of 10%, and a diversity of 0%. The cumulative Z‐curve crossed the conventional boundary after the one trial but fell back to the boundary and neither crossed the boundaries of benefit nor harm, suggesting that the current evidence, with a sample size of 771 after seven trials is insufficient, and that further trials are required to determine the effect of prostaglandins on mortality (Figure 4). The Trial Sequential Analysis adjusted 95% CI of RR, using the random‐effects model, was 0.87 (95% CI 0.21 to 3.59). Based on the DARIS, imprecision was downgraded two levels.
4.
All‐cause mortality.
The Trial Sequential Analysis results showed that the Trial Sequential Analysis adjusted 95% CI of risk ratio (RR), using the random‐effects model, was 0.87 (95% CI 0.21 to 3.59), and the diversity‐adjusted required information size (DARIS) for detecting an intervention effect was 11,719 participants. The blue line (Z‐curve) shows the cumulative z value (771 participants). The horizontal green dotted lines show the threshold for significance in conventional meta‐analysis, at 1.96 of the Z‐value, corresponding to the P‐value of 0.05. The red lines, at the left top and bottom corners, show the trial sequential boundaries for benefit or harm, representing the threshold for statistical significance in the TSA. The red dotted triangular shape to the right shows the futility boundaries and futility area of the TSA.
For the rest of the primary and secondary outcomes, the sample size was less than 5% of the DARIS, and, therefore, no figure on their Trial Sequential Analysis' is provided.
Discussion
Summary of main results
The aim of this systematic review was to evaluate the effects of prostaglandins compared with placebo or standard care alone in adults undergoing liver transplantation. This is the second version of this systematic review. We included 11 randomised clinical trials with 771 participants of whom 378 received prostaglandins. Seven hundred and seventy‐one participants contributed data on one or more outcomes.
Prostaglandins may reduce all‐cause mortality up to one month (RR 0.86, 95% CI 0.61 to 1.23; RD 21 fewer per 1000, 95% CI 63 fewer to 36 more; 11 trials, 771 participants; low‐certainty evidence; note: 4 trials reported all‐cause mortality but recorded 0 events in both groups. Thus, the RR, RD, and CIs were calculated from 7 trials rather than 11). As the 95% CIs were wide, with values compatible with either a clinically relevant harmful or beneficial effect, it is possible that further trials might change this conclusion.
Prostaglandins may result in little to no difference on other outcomes such as serious adverse events, liver retransplantation, early allograft dysfunction, primary non‐function of the allograft, and length of hospital stay. Prostaglandins may result in a large reduction in the development of acute kidney failure requiring dialysis. The evidence is very uncertain about the effect of prostaglandins on adverse events considered non‐serious. Only one trial had a vested interest. We cannot know whether a difference would be seen if more trials with vested interests are conducted. There are no data on the impact of prostaglandins on quality of life after liver transplantation in adults. As we did not demonstrate benefits from using prostaglandins for adult liver transplanted recipients, we did not plan to perform a systematic review of harms of prostaglandins.
Overall completeness and applicability of evidence
The identified 11 randomised clinical trials were conducted mainly in high‐ and upper‐middle‐income countries, and they investigated the effects of prostaglandins in adults undergoing liver transplantation. The results of this systematic review are applicable to most recipients of liver transplants, although it is relevant to mention that we found only one trial including recipients of living donor grafts (Bharathan 2016).
Almost all trials provided data on an outcome that we had prespecified in our review, but these data were insufficient to reach robust conclusions. All 11 trials reported all‐cause mortality, but only seven provided data that could be used to calculate the RR and CIs as four studies reported zero events in both groups. This scenario was worse for all the secondary outcomes. Although six trials reported retransplantation rates, only four contributed data as two trials reported zero events in both groups; one trial reported early allograft dysfunction, seven trials reported primary non‐function of the allograft but only five contributed data as two reported zero events in both groups; six trials reported acute kidney failure but only five contributed data as one trial reported zero events in both groups; four trials reported length of hospital stay, and four trials reported data on adverse events considered non‐serious. This raises the risk of selective outcome reporting bias, in particular because those outcomes are easy to assess (Schünemann 2021b). The total of 771 participants in this meta‐analysis is less than the optimal information size of 6442 participants required for detecting an intervention effect.
Quality of the evidence
We included data from 11 randomised clinical trials to assess the effects of prostaglandins in adult liver transplant recipients when compared with placebo or standard care alone. We evaluated the certainty of the evidence using the GRADE approach, with any downgrading substantiated (see Table 1). The evidence for most studied outcomes was low certainty and was very low for one outcome. We downgraded the certainty of evidence to low because of risk of bias (by one level) and imprecision (by one level) for the outcomes: all‐cause mortality, serious adverse events, liver retransplantation, early allograft dysfunction, primary non‐function of the allograft, acute kidney failure requiring dialysis, and length of hospital stay. We downgraded the certainty of evidence to very low because of risk of bias (one level), imprecision (one level), and inconsistency (heterogeneity) (one level) for the outcome, adverse events considered non‐serious. No studies reported health‐related quality of life.
Potential biases in the review process
In order to ensure a high degree of internal and external validity, we followed a systematic approach for trial identification, trial selection, data abstraction, and analysis. We searched for all relevant trials using sensitive and validated search strategies in several bibliographic databases. We included trials regardless of publication status or language in our review. One trial that was not published in scientific periodicals and not in English language was identified and included in this review, providing evidence of our efforts to minimise the risk of publication bias (Hidalgo 2002). We are confident that we identified all relevant studies, and we will monitor ongoing studies as well as full publication of preprints closely after the publication of this review. We contacted original investigators and previous Cochrane Review authors, although only Dr Bharathan and Dr Cavalcanti were able to contribute additional information.
We did not specifically search for observational studies reporting on harms, which is a limitation of this Cochrane Review.
Agreements and disagreements with other studies or reviews
This current review is an update of the 2011 Cochrane Review on the same topic with a different set of authors (Cavalcanti 2011). The following are the differences in methodology between the two reviews.
We defined the perioperative period as the period between the beginning of liver surgery and up to 96 hours after liver transplantation, while Cavalcanti and colleagues defined it as 48 hours (Cavalcanti 2011). This was done in an attempt to increase possible eligible studies. However, there were no additional studies that were identified due to this particular change in protocol.
The previous review had only one primary outcome (i.e. all‐cause mortality), while we had three clinically and patient‐relevant primary outcomes, namely all‐cause mortality, serious adverse events, and health‐related quality of life (Cavalcanti 2011).
While number of people requiring liver retransplantation, number of people with primary non‐function of the allograft, and number of people with acute renal failure requiring dialysis were common secondary outcomes in both the reviews, we added number of people with early allograft dysfunction and adverse events considered non‐serious as new secondary outcomes (Cavalcanti 2011).
Risk of bias assessment. While Cavalcanti used 'Blinding (performance bias and detection bias) – all outcomes', we split that assessment into two parts called 'Blinding of participants and personnel' and 'Blinding of outcome assessment'. This has led to some discrepancy in the assessment of blinding (Henley 1995; Himmelreich 1993; Ismail 1995; Neumann 2000). While Cavalcanti 2011 gave low risk for bias in 'other bias', for Alevizacos 1993, we have given unclear risk, as we do not know if the study was terminated early, as no information on sample size calculation was available. We gave low risk of bias for selective reporting bias. Our risk of bias assessment (random sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting) of Bärthel 2012 is different to Cavalcanti 2011 as we had the full manuscript that was published subsequently. While Cavalcanti 2011 gave low risk for bias in selective reporting, for Henley 1995, we have given unclear risk, as the trial protocol was not available. We gave high risk of other bias, as trial participants were not analysed under the groups in which they were randomised. While Cavalcanti 2011 gave unclear risk for other bias for Klein 1996, we have given high risk, as people did not necessarily receive treatment based on the groups in which they were randomised.
We performed a Trial Sequence Analysis of outcomes, which was not performed in the previous review.
We were able to identify more records with the initial search and included two new trials in the analysis. One was a new trial published after the last review and the other was a full trial of an abstract included in the previous review. We excluded one trial that was included in the previous version of the review (but not in the analyses as we were unable to obtained clarification from the trial authors), as we obtained no further information (Neumann 1998). We also identified one new registered trial that has not yet started recruiting (ongoing study).
Authors' conclusions
Implications for practice.
This review summarises the best available evidence to date on the effects of prostaglandin administration for people undergoing liver transplantation. The overall certainty of the evidence across all comparisons is low to very low using the GRADE system (GRADEpro GDT). We found low‐certainty evidence that prostaglandins may reduce all‐cause mortality up to one month. We found low‐certainty evidence suggesting that serious adverse events, liver retransplantation, early allograft dysfunction, primary non‐function, or length of hospital stay may not be affected by prostaglandins. We found low‐certainty evidence suggesting that prostaglandins may result in a large reduction in the development of acute kidney failure requiring dialysis. The evidence regarding the effect of prostaglandin is very uncertain regarding adverse events considered non‐serious.
Implications for research.
In this second version of a systematic review on the effects of prostaglandin in adult liver transplant recipients, we included data from 11 randomised clinical trials. Trials generally reported different primary outcomes. Furthermore, they reported different secondary outcomes, and safety data reporting were incomplete. These aspects lower the certainty of the evidence and make it difficult to draw valid conclusions. Therefore, there is still a need for adequately powered trials with low risk of bias to evaluate the effects of prostaglandins for people undergoing liver transplantation.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2023 | New search has been performed | We added 2 new trials (Bharathan 2016; Bosch 2000), and obtained further information from an abstract that the previous authors used (as the full text was published subsequently, Bärthel 2012). Cavalcanti 2011 had used data from 9 trials for their meta‐analysis (10 trials for this systematic review). We identified 1 ongoing trial that has not started recruiting. |
| 4 August 2023 | New citation required and conclusions have changed | Apart from confirming the findings of Cavalcanti 2011, after adding more participant data, we also found uncertain effects on early allograft dysfunction and adverse events (serious and non‐serious). Our methodology was more robust, including a Trial Sequential Analysis, and we provided a diversity‐adjusted required information size for the question explored. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 11, 2011
| Date | Event | Description |
|---|---|---|
| 11 January 2018 | New citation required and major changes | The whole protocol was updated to reflect improvements in methodology |
| 21 September 2017 | Amended | New authors' team |
Acknowledgements
We are grateful to VK Bharathan for providing additional data.
The Cochrane Hepato‐Biliary Group (CHBG) supported the authors in the development of this review.
The following people from the Editorial Team office of the CHBG conducted the editorial process for this review.
Sign‐off Editor (final editorial decision): Christian Gluud, Hepato‐Biliary Group, Denmark
Contact editor: Stefano Trastulli, Italy
Statistical Editor: Giovanni Casazza, Italy
Managing Editor (selected peer reviewers, provided comments, provided editorial guidance to authors, edited the article): Dimitrinka Nikolova, Hepato‐Biliary Group, Denmark
Information Specialist (developing search strategies and trial search): Sarah Louise Klingenberg, Hepato‐Biliary Group, Denmark
External peer review.
Peer reviewers (provided expert comments): Ib Christian Rasmussen, Sweden; Emmanuel Weiss, France
Peer reviewer (search strategies): Ina Monsef, Germany
Peer reviewer (Trial Sequential Analysis): Mark Aninakwah Asante, Denmark
The following people from the Cochrane Central Production Service supported the production of this review.
Evidence Synthesis Development Editor: Leslie Choi, UK, Evidence Production and Methods Department, Cochrane, UK
Copy Editor (copy‐editing and production): Anne Lawson, Cochrane Central Production Service
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, the Capital Region, Rigshospitalet, Copenhagen, Denmark. Disclaimer: the views and opinions expressed in this review are those of the review authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Search strategies
| Database | Time of search | Search strategies |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | 27 December 2022 | (prostaglandin* or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or pge) and ((liver or hepat*) and (transplant* or graft*)) |
| Cochrane Central Register of Controlled Trials in the Cochrane Library | Issue 12, 2022 | #1 MeSH descriptor: [Prostaglandins] explode all trees #2 (prostaglandin* or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or pge) #3 #1 or #2 #4 MeSH descriptor: [Liver Transplantation] explode all trees #5 ((liver or hepat*) and (transplant* or graft*)) #6 #4 or #5 #7 #3 and #6 |
| MEDLINE Ovid | 1946 to 27 December 2022 | 1. exp Prostaglandins/ 2. (prostaglandin* or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or PGE).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 3. 1 or 2 4. exp Liver Transplantation/ 5. ((liver or hepat*) and (transplant* or graft*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 6. 4 or 5 7. 3 and 6 8. (randomized controlled trial or controlled clinical trial or retracted publication or retraction of publication).pt. 9. clinical trials as topic.sh. 10. (random* or placebo*).ab. or trial.ti. 11. 8 or 9 or 10 12. exp animals/ not humans.sh. 13. 11 not 12 14. 7 and 13 |
| Embase Ovid | 1974 to 27 December 2022 | 1. exp prostaglandin/ 2. (prostaglandin* or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or pge).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] 3. 1 or 2 4. exp liver transplantation/ 5. ((liver or hepat*) and (transplant* or graft*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] 6. 4 or 5 7. 3 and 6 8. Randomized controlled trial/ or Controlled clinical study/ or randomization/ or intermethod comparison/ or double blind procedure/ or human experiment/ or retracted article/ 9. (random$ or placebo or parallel group$1 or crossover or cross over or assigned or allocated or volunteer or volunteers).ti,ab. 10. (compare or compared or comparison or trial).ti. 11. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 12. (open adj label).ti,ab. 13. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (controlled adj7 (study or design or trial)).ti,ab. 16. (erratum or tombstone).pt. or yes.ne. 17. or/8‐16 18. (random$ adj sampl$ adj7 ('cross section$' or questionnaire$ or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.) 19. Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.) 20. (((case adj control$) and random$) not randomi?ed controlled).ti,ab. 21. (Systematic review not (trial or study)).ti. 22. (nonrandom$ not random$).ti,ab. 23. 'Random field$'.ti,ab. 24. (random cluster adj3 sampl$).ti,ab. 25. (review.ab. and review.pt.) not trial.ti. 26. 'we searched'.ab. and (review.ti. or review.pt.) 27. 'update review'.ab. 28. (databases adj4 searched).ab. 29. (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/ 30. Animal experiment/ not (human experiment/ or human/) 31. or/18‐30 32. 17 not 31 33. 7 and 32 |
| LILACS (Bireme) | 1982 to 27 December 2022 | (prostaglandin$ or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or pge) [Words] and ((liver or hepat$) and (transplant$ or graft$)) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to 27 December 2022 | #5 #4 AND #3 #4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=((liver or hepat*) and (transplant* or graft*)) #1 TS=(prostaglandin* or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or pge) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to 27 December 2022 | #5 #4 AND #3 #4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=((liver or hepat*) and (transplant* or graft*)) #1 TS=(prostaglandin* or prostacyclin or alprostadil or epoprostenol or prostin or enisoprost or pge) |
Data and analyses
Comparison 1. Prostaglandins versus placebo/standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 11 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.61, 1.23] |
| 1.2 Serious adverse events | 6 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.60, 1.40] |
| 1.3 Liver retransplantation | 6 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.49, 1.96] |
| 1.4 Early allograft dysfunction | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.18] |
| 1.5 Primary non‐function of the allograft | 7 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.32] |
| 1.6 Acute kidney failure requiring dialysis | 6 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.73] |
| 1.7 Length of hospital stay (days) | 4 | 369 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐5.44, 3.14] |
| 1.8 Adverse events considered non‐serious | 4 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.42, 3.36] |
Comparison 2. Prostaglandins compared with placebo/standard care: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Prostaglandins versus placebo | 7 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.61, 1.40] |
| 2.1.2 Prostaglandins versus standard care | 4 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.29, 1.87] |
| 2.2 Liver retransplantation | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Prostaglandins versus placebo | 3 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.49, 2.62] |
| 2.2.2 Prostaglandins versus standard care | 3 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.20, 2.46] |
| 2.3 Primary non‐function | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Prostaglandins versus placebo | 4 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.29, 1.70] |
| 2.3.2 Prostaglandins versus standard care | 3 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.64] |
| 2.4 Acute kidney failure requiring dialysis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Prostaglandins versus placebo | 4 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.81] |
| 2.4.2 Prostaglandins versus standard care | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.11, 1.29] |
Comparison 3. Intention‐to‐treat analysis compared with per‐protocol analysis: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Intention‐to‐treat analysis | 6 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.55, 1.98] |
| 3.1.2 Per‐protocol analysis | 5 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| 3.2 Liver retransplantation | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Intention‐to‐treat analysis | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.10, 2.58] |
| 3.2.2 Per‐protocol analysis | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.53, 2.44] |
| 3.3 Primary non‐function | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.3.1 Intention‐to‐treat analysis | 3 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.06, 4.20] |
| 3.3.2 Per‐protocol analysis | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.17, 1.51] |
| 3.4 Acute kidney failure requiring dialysis | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.4.1 Intention‐to‐treat analysis | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.24, 1.12] |
| 3.4.2 Per‐protocol analysis | 4 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.75] |
Comparison 4. Trials funded for‐profit compared to trials without for‐profit support: subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause mortality | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Funded trials | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.28, 2.58] |
| 4.1.2 Non‐funded trials | 10 | 690 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.25] |
Comparison 5. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All‐cause mortality | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 'Best–worst' case scenario | 11 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
| 5.1.2 'Worst–best' case scenario | 11 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.80, 1.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alevizacos 1993.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: Germany Single centre Period of recruitment: August 1990 to July 1991 Number randomised: 58 (1:1) Postrandomisation dropouts: 0 (0%) Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: retransplantation |
|
| Participants | Experimental: 29 (17 men and 12 women) Control: 29 (18 men and 11 women) Mean age (years): 42 Women: 23 (39.7%) |
|
| Interventions | Experimental: intravenous PGE1 0.1–0.5 μg/kg/hour from induction to 3rd postoperative day, beginning at induction of anaesthesia Control: standard care with no added intervention |
|
| Outcomes | The trial reported on parameters of reperfusion injury after cold preservation of liver grafts
|
|
| Notes | Source of funding: not reported, but the authors' affiliation was a University Clinic Rudolf Virchow, Berlin, Germany Trial name/trial registry number: not reported Attempted to contact the authors in December 2020; received no reply It is unclear if all the authors of the publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not specify the method of sequence generation. Quote: "58 adult patients were randomized." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided, but most likely, blinding not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided, but most likely no blinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Unclear risk | Baseline characteristics similar between groups. It is unclear whether trial was stopped early or not because sample size calculation was not published. Intention‐to‐treat analysis performed. |
Bharathan 2016.
| Study characteristics | ||
| Methods | Randomised, double‐blind, clinical trial Country: India Single centre Period of recruitment: January 2012 to December 2013 Number randomised: 100 (1:1) Postrandomisation dropouts: 1 (1%) from prostaglandin group Lost to follow‐up: not reported Haemodynamic instability Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: ABO‐incompatible liver transplantation, diseased donor liver transplantation, auxiliary partial orthotopic liver transplantation, and people with acute liver failure who were already receiving prostaglandin infusion at time of transplantation |
|
| Participants | Experimental: 49 (41 men and 8 women) Control: 50 (47 men and 3 women) Mean age (years): 43 Women: 11 (11.1%) The authors reported per‐protocol analysis. However, when we added the postrandomisation dropout, for the sensitivity analysis, the total number of participants in the experimental group was 50. |
|
| Interventions | Experimental: intravenous PGE1 (alprostadil) 0.25 μg/kg/hour, starting 1 hour after portal venous reperfusion, and continued for 96 hours Control: placebo (normal saline) |
|
| Outcomes | Primary outcome
Secondary outcomes
Follow‐up (months): 1 |
|
| Notes | Source of funding: not reported Trial name/trial registry number: Clinical Trials Registry of India (Registry No. CTRI/2013/13/09/003991) Contacted Dr Bharathan via email viju505@gmail.com. Unpublished data on number of participants retransplanted and role of individual authors within the study were sought and provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using a computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "… sealed opaque envelopes, which were opened on the morning of surgery by the unblinded study coordinator." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "… senior staff nurse who prepared the infusion … delivered to the operating room …' After starting the infusion, the participants were initially monitored by the initiating anaesthetist, and subsequently monitored in the Transplant Intensive Care Unit by the blinded critical care specialist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All primary end points and most secondary end points were observer‐reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Low risk | Published protocol available. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5798&EncHid=&userName=003991 |
| Other bias | Unclear risk | Baseline imbalance present as the number of participants undergoing emergency living donor liver transplantation for acute liver failure was higher in the study arm (7 participants in the experimental arm versus 3 participants in control arm). Trial was stopped when the planned sample size was reached. Per‐protocol analysis performed. |
Bosch 2000.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: Spain Single centre Period of recruitment: information not available Number randomised: 25 Postrandomisation dropouts: 0 (0%) Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: not reported |
|
| Participants | Experimental: 12 Control: 13 Mean age (years): not reported Women: not reported |
|
| Interventions | Experimental: intravenous PGE1 0.6 μg/kg/hour during the first 90 minutes after graft reperfusion through a catheter placed in portal vein Control: placebo |
|
| Outcomes |
Follow‐up (months): not reported |
|
| Notes | Source of funding: not reported Trial name/trial registry number: not reported Attempted to contact the authors in December 2020; received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided "randomised." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "There was no need to stop or slow down the infusion in any case." Quote: "High dose intraportal PGE1 infusion administered by portal vein after reperfusion is well tolerated …" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There was no need to stop or slow down the infusion in any case.' Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Unclear risk | Only abstract available. No data of baseline parameters available. It was unclear whether trial was stopped early or not because sample size calculation was not published. Intention‐to‐treat analysis used. |
Bärthel 2012.
| Study characteristics | ||
| Methods | Randomised, open label, pilot clinical trial Country: Germany Single centre Period of recruitment: September 2006 to October 2008 Number randomised: 80 (1:1) Postrandomisation dropouts: 5 (6.25%). 2 from the prostaglandin group and 3 from the control group Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: living‐related and split‐liver transplants, retransplantations, known intolerance to the study medication, severe circulatory instability after graft reperfusion with prolonged noradrenaline dosage ≥ 0.5 μg/kg bodyweight/minute. |
|
| Participants | Experimental: 40 (29 men and 11 women) Control: 40 (30 men and 10 women) Mean age (years): 54 Women: 21 (26.3%) |
|
| Interventions | Experimental: prostaglandin I2 analogue, iloprost, administered immediately after admission to intensive care unit by continuous intravenous infusion at a rate of 1 ng/kg bodyweight/minute for 7 days Control: standard care with no added intervention All participants were treated with a comparable calcineurin inhibitor‐based quadruple induction immunosuppressive regimen. |
|
| Outcomes | Primary outcome
Note: trial authors changed the definition of graft dysfunction in a post hoc analysis. Secondary outcomes
However, the authors reported on more outcomes, such as biliary complications (in 8 participants in the experimental and 8 participants in the control group). Complications in the prostaglandin group included: respiratory insufficiency 6; pleural effusion 8; acute renal failure 5; pneumonia 4; peritonitis 2; sepsis/multiple organ failure 2; opportunistic (i.e. cytomegalovirus) 6; cardiac 2; gastrointestinal tract 5; relaparotomy 9; wound healing impairment 3; new‐onset cancer 1 Complications in the control group included: blood/lymphatic 2; respiratory insufficiency 7; pleural effusion 3; acute renal failure 8; pneumonia 3; urinary tract 2; peritonitis 1; sepsis/multiple organ failure 2; opportunistic (i.e. cytomegalovirus) 6; cardiac 3; gastrointestinal tract 6; relaparotomy 8; wound healing impairment 2 Follow‐up (months): 6 |
|
| Notes | Source of funding: not reported Trial name/trial registry number: not reported Quote: "The procedures abide by Good Clinical Practice and the ethical principles described in the current (at that time) revision of Declaration of Helsinki." Emailed erik.baerthel@med.uni‐jena.de in May 2019, December 2020, and March 2022. Received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomised (1:1) in blocks of 2 up to 6 participants to the iloprost or control group using a random allocation software. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary end points were mainly observer‐reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Unclear risk | Baseline imbalance present as the authors stated "… higher proportion of patients with severe liver dysfunction in the treatment group." Higher proportion of extended criteria donors (which are donors with certain risk factors such as donor age, steatosis, donation after cardiac death, and donors with increased risk of disease transmission) in the control group. It is unclear whether trial was stopped early or not because sample size calculation was performed based on feasibility. Quote: "A sample size of 80 patients was chosen for reasons of feasibility for the pilot study to prove the treatment concept and to obtain preliminary estimates of the treatment effect." Intention‐to‐treat analysis and per‐protocol analyses were performed. |
Henley 1995.
| Study characteristics | ||
| Methods | Randomised, double‐blind clinical trial Country: US Single centre Period of recruitment: April 1990 to November 1992 Number randomised: 165 Postrandomisation dropouts: 0 (0%) Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: retransplantation (if performed within 180 days) |
|
| Participants | Experimental: 78 (41 men and 37 women) Control: 82 (40 men and 42 women) Mean age (years): 44 Women: 79 (49%) |
|
| Interventions | Experimental: intravenous PGE1 infusion started at 10 μg/hour before the anhepatic phase and increased by 10 μg/hour every 30 minutes until a maintenance level of 40 μg/hour was reached. A fresh infusion prepared every 24 hours, and infusions continued for up to 21 days Control: placebo Normal post‐transplantation care routine was maintained during the study. All participants were treated with a comparable steroid‐based quadruple induction immunosuppressive regimen. |
|
| Outcomes | Primary outcomes
Secondary outcome
However, the authors reported on more outcomes, such as renal support, rejection, and other complications. Complications in prostaglandin group included: tissue integrity 5; ventilatory issues 1; infection 2; haemorrhage 2; laparotomy 2 Complications in control group included: tissue integrity 13; ventilatory issues 8; infection 8; haemorrhage 9; laparotomy 4 Follow‐up (months): 6 |
|
| Notes | Source of funding: supported in part by the General Clinical Research Center (MOI‐RR00042 of NIH) Trial name/trial registry number: not reported Attempted to contact Dr Henley via post in December 2020 and March 2022; received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: '"… randomization code generated …" |
| Allocation concealment (selection bias) | Low risk | Quote: "… randomization code generated by the University of Michigan School of Public Health." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "… a physician who was not an investigator under this protocol had access to this randomization code." No details of randomisation provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mostly observer reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | High risk | Baseline characteristics similar between groups. Trial was stopped when the planned sample size was reached. Per‐protocol analysis performed. An additional 9 participants were excluded from analyses because they underwent retransplantation and were rerandomised, and retreated within 180 days. 4 participants underwent retransplantation and were rerandomised into the study after completing 180 days of follow‐up; these participants were included as 8 participants in the analyses. There was potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomised. |
Hidalgo 2002.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: Spain Single centre Period of recruitment: January 1996 to July 1997 Number randomised: 90 Postrandomisation dropouts: 11 (12.2%). 8 from the prostaglandin group and 3 from the control group. Reasons for dropouts were unclear from the text. Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: not reported |
|
| Participants | Experimental: 37 (21 men and 16 women) Control: 42 (31 men and 11 women) Mean age (years): 50 Women: 58 (64%) The authors reported per‐protocol analysis. However, when we added the postrandomisation dropout, for the sensitivity analysis, the total number of participants in the experimental and control group became 45 each, with 8 dropouts from experimental group and 3 dropouts from control group |
|
| Interventions | Experimental: PGE1 0.6 μg/kg/hour intraportal followed by PGE1 100–200 μg/hour intravenously administration of PGE1. From reperfusion up to 4 or 5 days Control: standard care with no added intervention |
|
| Outcomes |
Other outcomes reported in the study: systemic and hepatic haemodynamics; peak transaminases; incidence of graft rejection; acute kidney injury Follow‐up (months): 12 |
|
| Notes | Source of funding: not reported Trial name/trial registry number: not reported Attempted to contact Dr Hidalgo between August and December 2020; received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Probably adequate. Quote: "Randomisation was conducted with sealed envelopes which were opened before transplant surgery." |
| Allocation concealment (selection bias) | Unclear risk | Use of sealed envelopes described, although it is unclear whether the envelopes were sequentially numbered and opaque. Quote: "Randomisation was conducted with sealed envelopes which were opened before transplant surgery." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. Quote: "Two groups of 45 patients each were formed by randomisation. One received intra‐portal prostaglandin E1 versus the control group, which received no drug at all." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. Quote: "Two groups of 45 patients each were formed by randomisation. One received intra‐portal prostaglandin E1 versus the control group, which received no drug at all." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/90 participants were excluded from analyses. Quote: "… 11 patients were excluded from the analysis because of technical difficulties for measuring intra‐operative flows." |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Unclear risk | Baseline characteristics similar between groups. It was unclear whether trial was stopped early or not because sample size calculation was not published. Per‐protocol analysis performed. |
Himmelreich 1993.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: Germany Single centre Period of recruitment: not reported Number randomised: 20 Postrandomisation dropouts: 0 (0%) Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: not reported |
|
| Participants | Experimental: 10 Control: 10 Mean age (years): not reported Women (%): not reported |
|
| Interventions | Experimental: continuous intravenous infusion of PGE1 with a starting dose of 10 µg/hour administered at beginning of surgery and increased by 10 µg/hour to a maximal dose of 40 µg/hour Control: standard care with no added intervention |
|
| Outcomes |
Follow‐up (months): not reported |
|
| Notes | Source of funding: not reported, but the authors' affiliation include Departments of Internal Medicine, Surgery, and Anesthesiology, University Clinic Rudolf Virchow, Berlin, Germany Trial name/trial registry number: not reported Attempted to contact Dr Himmelreich via post in December 2020; received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Quote: "… prospective, randomised, and open study …" |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Quote: "… prospective, randomised, and open study …" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | End points were observer‐reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Unclear risk | Baseline data according to treatment group not reported, so large imbalances were possible. It was unclear whether trial was stopped early or not because sample size calculation was not published. No mention on the type of analysis performed. Most probably it was intention to treat. |
Ismail 1995.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: UK Single centre Period of recruitment: May 1989 to August 1990 Number randomised: 81 Postrandomisation dropouts: 15 (18.51%). 6 from prostaglandin group (1 bleeding and 5 death) and 9 from control group (1 cytomegalovirus infection, 1 abdominal pain, 1 diarrhoea, and 6 deaths) did not complete study. Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: intrinsic renal disease |
|
| Participants | Experimental: 40 (10 men and 30 women) Control: 41 (9 men and 32 women) Mean age (years): 54 Women: 62 (76.5%) |
|
| Interventions | Experimental: enisoprost 100 μg 3 times daily orally for 12 weeks, commenced within 24 hours of liver transplantation Control: standard care with no added intervention All participants were treated with a comparable calcineurin inhibitor‐based immunosuppressive regimen. |
|
| Outcomes |
Creatinine clearance: 33.1 (SD 14.9) mL/min for prostaglandin group and 37 (SD 12.4) mL/min for control group Glomerular filtration rate: 73.7 (SD 29.2) mL/min for prostaglandin group and 80.1 (SD 30.4) mL/min for control group Graft rejection: 29 for prostaglandin group and 25 for control group Follow‐up (months): 4 |
|
| Notes | Source of funding: trial grant from GD Searle Co Ltd, High Wycombe, UK. The authors' affiliation was The Liver Unit, Queen Elizabeth Hospital, Birmingham, UK. Trial name/trial registry number: not reported Contacted Dr Ismail via email in December 2020. However, he was unable to provide further information. It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… randomly assigned in the order of enrollment …" |
| Allocation concealment (selection bias) | Low risk | Quote: "… none of the investigators had access to the code until the study had been completed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "… double‐blind …" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | End points were observer‐reported outcomes involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Low risk | Baseline characteristics similar between groups. Trial was stopped when the planned sample size was reached. Intention‐to‐treat analysis was performed. |
Klein 1996.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: US Multicentre Period of recruitment: May 1992 to April 1994 Number randomised: 118 Postrandomisation dropouts: 10 (8.4%). 3 from the prostaglandin group and 7 from the control group Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: not reported |
|
| Participants | Experimental: 58 (27 men and 31 women) Control: 60 (37 men and 23 women) Mean age (years): 45 Women: 54 (46%) |
|
| Interventions | Experimental: prostaglandin E1 infusion rate of 0.25 μg/kg/hour increased in increments of 0.25 μg/kg/hour every 10 minutes up to a maximum 1 μg/kg/hour. Infusion of study drug continued for 7 days or until the participant transferred out of intensive care unit, whichever occurred first. Maximum dose 80 μg/hour. Control: placebo (normal saline or 5% dextrose) |
|
| Outcomes |
Creatinine: 1.4 (SD 1.0) mg/dL for prostaglandin group and 2.0 (SD 1.0) mg/dL for control group Blood urea nitrogen: 41.9 (SD 23.7) mg/dL for prostaglandin group and 59.8 (SD 30.5) mg/dL for control group Ammonia: 36.6 (SD 19.4) mmol/L for prostaglandin group and 40.5 (SD 19.9) mmol/L for control group Aspartate aminotransferase: 1152 (SD 1357) IU/L for prostaglandin group and 1574 (SD 2755) IU/L for control group Alanine aminotransferase: 855 (SD 911) IU/L for prostaglandin group and 1170 (SD 1466) IU/L for control group Alkaline phosphatase: 130 (SD 75) IU/L for prostaglandin group and 144 (SD 142) IU/L for control group Bile output: 214 (SD 108) mL/day for prostaglandin group and 234 (SD 141) mL/day for control group Follow‐up (months): 1 |
|
| Notes | Source of funding: not reported, but the authors' affiliations included Departments of Surgery and Medicine, The Johns Hopkins University School of Medicine, Maryland, US; Departments of Surgery and Pharmacy, Medical University of South Carolina, South Carolina, US; and Departments of Surgery and Pharmacy, University of Virginia Health Sciences Center, Virginia, US. Trial name/trial registry number: not reported. Attempted to contact the authors in December 2020; received no reply. It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… randomization was performed centrally by the staff …" |
| Allocation concealment (selection bias) | Low risk | Quote: "… who distributed code sealed envelopes to the pharmacies at each participating transplant center." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding procedures clearly described. Quote: "Control solutions consisted of vehicle (normal saline or 5% dextrose in water) only. Individual solutions were labelled only with respect to the nature of the crystalloid vehicle, with no indication whether they contained prostaglandin E1." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | End points were mostly observer‐reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all randomised participants provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | High risk | Baseline characteristics similar between groups. It was unclear whether trial was stopped early or not because sample size calculation was not published. Intention‐to‐treat analysis was performed. Quote: "At the discretion of the principal investigator, patient with evidence of profoundly deteriorating liver function could either be discontinued from the study or could be discontinued and have open administration started as compassionate therapy." Comment: this causes a high risk of bias and further details of this treatment strategy is not provided in the results. |
Manasia 1996.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: US Single centre Period of recruitment: October 1992 to January 1994 Number randomised: 24 Postrandomisation dropouts: 3 (12.5%). Primary non‐function, drug error, and haemodynamic instability in 1 participant each. Text did not mention which groups each belonged to. Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: chronic renal failure, hepatorenal syndrome, renal transplantation, or malignancy |
|
| Participants | Experimental: 10 (7 men and 3 women) Control: 11 (9 men and 2 women) Mean age (years): 53 Women: 10 (41.6%) The authors reported per‐protocol analysis. However, when we add the postrandomisation dropouts, for the sensitivity analysis, the total number of participants became 24. The authors did not mention which groups each belonged to. |
|
| Interventions | Experimental: intravenous PGE1 (alprostadil) 0.6 μg/kg/hour started within 12 hours of patient reaching intensive care unit and continued for 5 days Control: placebo (normal saline) |
|
| Outcomes | Primary outcome
Secondary outcomes
Mortality at 30 days: 0 Acute kidney failure requiring dialysis: 1 participant in each group Follow‐up (months): 6 |
|
| Notes | Source of funding: not reported, but the authors' affiliation included Departments of Surgery, Anesthesiology and Nuclear Medicine, The Mount Sinai Medical Centre, New York, US. Prostaglandin (Alprostadil) was supplied free of charge by The Upjohn Company, Kalamazoo, Michigan, US. Trial name/trial registry number: not reported Attempted to contact the authors in December 2020; received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… assigned based on a table of random numbers …" |
| Allocation concealment (selection bias) | Low risk | Quote: "… allocation decided from pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "… physician and personnel did not have access to the master code …" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | End points were mostly observer‐reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/24 participants were excluded from the analysis. 1 because of primary non‐function of the graft, 1 because of need of vasopressor, 1 because of erroneous discontinuation of the drug. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | High risk | Baseline characteristics similar between groups. The trial was stopped halfway, as interim analysis demonstrated lack of statistical significance on the main outcome. Per‐protocol analysis performed. |
Neumann 2000.
| Study characteristics | ||
| Methods | Randomised clinical trial Country: Germany Single centre Period of recruitment: not reported Number randomised: 30 Postrandomisation dropouts: 3 (10%). 1 in prostaglandin group (bleeding complications, which were not attributed to the prostaglandin) and 2 in control group (1 because of a bleeding complication and 1 due to a dislocation of the hepatic venous catheter) Lost to follow‐up: not reported Inclusion criteria: adults undergoing liver transplantation Exclusion criteria: not reported |
|
| Participants | Experimental: 15 (8 men and 7 women) Control: 15 (10 men and 5 women) Mean age (years): 50 Women: 12 (40%) |
|
| Interventions | Experimental: initial intravenous PGI2 0.5 ng/kg/minute increased in increments of 0.5 ng/kg/minute every 10 minutes up to 4 ng/minute/kg bodyweight. Infusion of PGI2 continued until the 7th day after liver transplantation Control: standard care with no added intervention |
|
| Outcomes |
Cold ischaemia time: 9 (SD 3) hours for prostaglandin group and 10 (SD 3) hours for control group Warm ischaemia time: 56 (SD 21) minutes for prostaglandin group and 61 (SD 8) minutes for control group Intraoperative transfusions of packed red cells: 5 (SD 3) units for prostaglandin group and 8.7 (SD 3.4) units for control group Aspartate aminotransferase: 418 (SD 99) IU/L for prostaglandin group and 638 (SD 156) IU/L for control group Alanine aminotransferase: 503 (SD 164) IU/L for prostaglandin group and 545 (SD 165) IU/L for control group Mean arterial pressure, cardiac output and central venous pressure were not significantly different between groups. Mortality at 12 months: 0 Initial poor function: 0 in prostaglandin group and 2 in control group Follow‐up (months): 12 |
|
| Notes | Source of funding: not reported, but the authors' affiliation was Humboldt Universita, Berlin, Germany Trial name/trial registry number: not reported Attempted to contact the authors in December 2020 and March 2021; received no reply It is unclear if all the authors of publication participated in the trial as information was lacking. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… following randomisation, patients were assigned to receive either PGI2 … or placebo …" |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | End points were mostly observer‐reported outcomes not involving judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/30 participants were excluded from the analysis: 2 because of bleeding and 1 because of dislocation of catheter. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. There was insufficient information to judge this domain. |
| Other bias | Unclear risk | Baseline characteristics similar between groups. It was unclear whether trial was stopped early or not because sample size calculation was not published. Per‐protocol analysis performed. Number of participants analysed in the prostaglandin group was unclear. |
ALT: alanine transaminase; AST: aspartate transaminase; PGE1: prostaglandin E1; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almazroo 2021 | Not a randomised trial. |
| Briegel 1992 | Not a randomised trial. |
| Bucuvalas 2001 | Included population not of interest for this review. |
| Garcia‐Valdecasas 1994 | Not a randomised trial. |
| Ghonem 2011 | Included population not of interest for this review. |
| Giostra 1997 | Not a randomised trial. |
| Grazi 1994 | Included population not of interest for this review. |
| Greig 1989 | Not a randomised trial. |
| Hommann 2007 | Not a randomised trial. |
| Hsieh 2014 | Included population not of interest for this review. |
| Kaisers 1996 | Comparison not of interest for this review. |
| Klein 1999 | Included population not of interest for this review. |
| Kornberg 2004 | Not a randomised trial. |
| Maeda 1998 | Included population not of interest for this review. |
| Natori 1997 | Included population not of interest for this review. |
| Neumann 1998 | Comparison not of interest for this review. |
| Neumann 1999 | Comparison not of interest for this review. |
| Onoe 2013 | Not a randomised trial. |
| Radojkovic 2017 | Included population not of interest for this review. |
| Ruwart 1996 | Not a randomised trial. |
| Sato 2003 | Not a randomised trial. |
| Sheiner 1995 | Not a randomised trial. |
| Shin 2012 | Not a randomised trial. |
| Smith 1996 | Not a randomised trial. |
| Suehiro 2005 | Not a randomised trial. |
| Suzuki 1991 | Included population not of interest for this review. |
| Takaya 1993 | Not a randomised trial. |
| Takaya 1995 | Not a randomised trial. |
| Tancharoen 1992 | Not a randomised trial. |
| Totsuka 1998 | Included population not of interest for this review. |
Characteristics of ongoing studies [ordered by study ID]
Shin 2021.
| Study name | |
| Methods | Interventional, parallel group, open label, randomised controlled trial |
| Participants | Adults aged 19–79 years |
| Interventions | Prostaglandin (alprostadil) for 2 or 3 weeks |
| Outcomes | Primary outcome
To compare the frequency of antibody‐mediated rejection (multiple hepatic biliary stenosis) within 1 year after administration of the investigational drug after administration of the investigational drug in patients with ABO blood type incompatible in vivo liver transplantation. Secondary outcome
|
| Starting date | 1 May 2021 |
| Contact information | 88 Olympic‐Ro 43‐Gil, Songpa‐Gu, Seoul 05505, Korea Telephone: +82‐2‐3010‐7182 Email: asancrc0516@hanmail.net Affiliation: Asan Medical Center |
| Notes | CRIS KCT0006115 |
Differences between protocol and review
We have changed the title of the review from 'Prostaglandins for adult liver transplanted patients' to 'Prostaglandins for adult liver transplanted recipients'.
We added adverse events considered non‐serious as a secondary outcome.
We removed hypotension, which was in the protocol as an exploratory outcome.
We did not intend to use the Review Manager Web for the review while preparing the protocol as it was not available during the protocol stage (Review Manager Web 2023).
As anticipated intervention effects for the primary outcomes in the Trial Sequential Analysis, a relative risk reduction (RRR) of 10% was planned in the protocol. However, we used an RRR of 15% in the review as the required information size was too large with a 10% RRR. Because of the change in the number of secondary outcomes from four to five between the protocol and the review, the alpha was changed from 2% to 1.67%.
Although we planned to assess the risk of bias of 'Selective outcome reporting' domain per outcome, we have not done it as the current Review Manager tool is developed to correspond with RoB 2 tool and not with RoB 1 tool.
In general, the whole protocol part was updated before we resumed our work on the review in 2019.
Contributions of authors
ZUM: acquiring trial reports, study selection, data extraction, data analysis, data interpretation, writing the review, and future review updates.
CTV: acquiring trial reports, study selection, data extraction, data analysis, data interpretation, writing the review, and future review updates.
AS: guided regarding statistical analyses, review drafting, providing advice on the review.
LK: review draft, providing advice on the review.
UG: review draft, providing advice on the review.
DB: review draft, providing advice on the review.
RN: review draft, providing advice on the review.
SS: co‐ordinating the review, providing advice on the review.
All authors approved of the final review for publication.
Sources of support
Internal sources
-
None, India
The authors did not receive any external financial support to conduct this review.
-
None, Saudi Arabia
The authors did not receive any external financial support to conduct this review.
External sources
-
None, India
The authors did not receive any external financial support for conducting this review.
-
None, Saudi Arabia
The authors did not receive any external financial support to conduct this review.
-
Cochrane Hepato‐Biliary Group Editorial team, Denmark
Provided guidance to authors and ran the peer review process.
Declarations of interest
ZUM: none.
CTV: none.
AS: none.
LK: none.
UG: none.
DB: none.
RN: none.
SS: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alevizacos 1993 {published data only}
- Alevizacos P, Bechstein WO, Rossaint R, Neuhaus P. Failure of PGE1 to prevent allograft reperfusion injury in a prospective randomized trial. Transplantation Proceedings 1993;25:2545-6. [PubMed] [Google Scholar]
Bärthel 2012 {published data only (unpublished sought but not used)}
- Bärthel E, Rauchfus F, Habrecht O, Koch A, Klinzing S, Marxs G, et al. Impact of iloprost on early graft viability after liver transplantation – results of a prospective randomized controlled clinical trial. Liver Transplantation 2008;14(S1):S137. [Google Scholar]
- Bärthel E, Rauchfuß F, Hoyer H, Habrecht O, Jandt K, Gotz M, et al. Impact of stable PGI2 analog iloprost on early graft viability after liver transplantation: a pilot study. Clinical Transplantation 2012;26(1):E38-47. [DOI: 10.1111/j.1399-0012.2011.01516.x] [DOI] [PubMed] [Google Scholar]
Bharathan 2016 {published and unpublished data}
- Bharathan VK, Chandran B, Gopalakrishnan U, Varghese CT, Menon RC, Balakrishnan D, et al. Perioperative prostaglandin E1 infusion in living donor liver transplantation: a double-blind, placebo-controlled randomized trial. Liver Transplantation 2016;22(8):1067-74. [DOI] [PubMed] [Google Scholar]
Bosch 2000 {published data only}
- Bosch C, Mora A, Roigé J, Hidalgo E, Lazaro JL, Margarit C, et al. Haemodynamic tolerance of intraportal prostaglandin E1 in liver transplantation. European Journal of Anaesthesiology 2000;17:45. [Google Scholar]
Henley 1995 {published data only}
- Henley KS, Lucey MR, Normolle DP, Merion RM, McLaren ID, Crider BA, et al. A double-blind, randomized, placebo controlled trial of prostaglandin E1 in liver transplantation. Hepatology 1995;21:366-72. [PubMed] [Google Scholar]
Hidalgo 2002 {published data only (unpublished sought but not used)}
- Hidalgo EL, Margaritt CC. Hemodynamic Study of Liver Transplantation: Ischemia-Reperfusion Injury Analysis and Evaluation of Intra-Portal Prostaglandin E1 Administration in the Reperfusion Phase [Estudiohemodinámico del Transplante Hepático: Análisis dela Lésion de Isquemia–reperfusión y Valoración de Laadministración Intraportal de Prostaglandina E1 en la Fasede Revascularización] [Doctoral Thesis]. Barcelona: Universitat Autonoma De Barcelona, 2002. [Google Scholar]
- Margarit C, Hidalgo E, Bilbao I, Mora A, Murio E, Lazaro JL, et al. Intraportal prostaglandin E1 (PGE1) infusion after liver graft reperfusion: a randomized control trial. Liver Transplantation 2003;9:C84. [Google Scholar]
Himmelreich 1993 {published data only}
- Himmelreich G, Hundt K, Bechstein WO, Rossaint R, Neuhaus P, Riess H. Influence of prostaglandin E1 infusion on hemostasis in orthotopic liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):273-8. [DOI] [PubMed] [Google Scholar]
- Himmelreich G, Hundt K, Neuhaus P, Bechstein WO, Roissant R, Riess H. Evidence that intraoperative prostaglandin E1 infusion reduces impaired platelet aggregation after reperfusion in orthotopic liver transplantation. Transplantation 1993;55(4):819-26. [DOI] [PubMed] [Google Scholar]
Ismail 1995 {published data only}
- Ismail T, Ayres R, Kiff P, McMaster P, Neuberger J. Enisoprost in liver transplantation. Transplantation 1995;59:1298-301. [PubMed] [Google Scholar]
Klein 1996 {published data only}
- Chavin KD, Uber L, Baliga P, Rajagopalan P, Cofer J. The effects of prostaglandin E1 on hepatic allograft vascular inflow: a prospective randomized double-blind study. American Surgeon 1996;62(3):184-7. [PubMed] [Google Scholar]
- Klein AS, Cofer JB, Pruett TL, Thuluvath PJ, McGory R, Uber L, et al. Prostaglandin E1 administration following orthotopic liver transplantation: a randomized prospective multicenter trial. Gastroenterology 1996;111:710-5. [DOI] [PubMed] [Google Scholar]
Manasia 1996 {published data only}
- Manasia AR, Leibowitz AB, Miller CM, Silverstein JH, Schwartz M, Delgiudice R, et al. Postoperative intravenous infusion of alprostadil (PGE1) does not improve renal function in hepatic transplant recipients. Journal of the American College of Surgeons 1996;182(4):347-52. [PubMed] [Google Scholar]
Neumann 2000 {published data only}
- Neumann UP, Kaisers U, Langrehr JM, Glanemann M, Muller AR, Lang M, et al. Administration of prostacyclin after liver transplantation: a placebo controlled randomized trial. Clinical Transplantation 2000;14(1):70-4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almazroo 2021 {published data only}
- Almazroo O, Miah M, Pillai V, Humar A, Tevar A, Hughes C, et al. Clinical evaluation of the safety and preliminary efficacy of continuous infusion of treprostinil to prevent ischemia and reperfusion injury in adult orthotopic liver transplant recipients. American Journal of Transplantation 2017;17(Suppl 3):295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazroo OA, Miah MK, Pillai VC, Shaik IH, Xu R, Dharmayan S, et al. An evaluation of the safety and preliminary efficacy of peri- and post-operative treprostinil in preventing ischemia and reperfusion injury in adult orthotopic liver transplant recipients. Clinical Transplantation 2021;35(6):e14298. [DOI: 10.1111/ctr.14298] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramanan R, Shaik IH, Pillai VC, Almazroo OA, Miah MK, Xu R, et al. Treprostinil is safe and potentially useful in preventing ischemia and reperfusion injury in adult orthotopic liver transplant recipients. American Journal of Transplantation 2020;20(Suppl 3):1054-5. [Google Scholar]
Briegel 1992 {published data only}
- Briegel J, Haller M, Zulke C, Kilger E, Pratschke E, Jauch KW, et al. Primary graft nonfunction following orthotopic liver transplantation: treatment with prostacyclin. Transplantation Proceedings 1992;24(6):2693–5. [PubMed] [Google Scholar]
Bucuvalas 2001 {published data only}
- Bucuvalas JC, Ryckman FC, Krug S, Alonso MH, Balistreri WF, Kotagal U. Effect of treatment with prostaglandin E1 and N-acetylcysteine on pediatric liver transplant recipients: a single-center study. Pediatric Transplantation 2001;5(4):274-8. [DOI] [PubMed] [Google Scholar]
Garcia‐Valdecasas 1994 {published data only}
- Garcia-Valdecasas JC, Rull R, Grande L, Fuster J, Rimola A, Lacy AM, et al. Prostaglandin and lipoperoxide levels and postoperative liver function in human liver transplantation. Transplantation Proceedings 1994;26(6):3633-4. [PubMed] [Google Scholar]
Ghonem 2011 {published data only}
- Ghonem N, Yoshida J, Stolz DB, Humar A, Starzl TE, Murase N, et al. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation. American Journal of Transplantation 2011;11:2508-16. [DOI] [PubMed] [Google Scholar]
- Ghonem NS, Zhao W, Zhang S, Murase N, Yoshida J, Stolz DB, et al. Hepatic ischemia-reperfusion injury impairs drug metabolism and treprostinil, a prostacyclin analog, improves the functional capacity of the liver graft post-transplantation. Drug Metabolism Reviews 2010;42(Suppl 1):81-2. [DOI: 10.3109/03602532.2010.506057] [DOI] [Google Scholar]
- Ghonem NS. Treprostinil for Protection of Liver Grafts Against Ischemia and Reperfusion Injury During Orthotopic Liver Transplantation – a Translational Study [Doctoral dissertation]. Pittsburgh: University of Pittsburgh, 2004. [Google Scholar]
Giostra 1997 {published data only}
- Giostra E, Chen H, Deng H, Buhler L, Romand JA, Hadengue A, et al. Prophylactic administration of prostaglandin E1 in liver transplantation: results of a pilot trial. Transplantation Proceedings 1997;29(5):2381-4. [DOI] [PubMed] [Google Scholar]
Grazi 1994 {published data only}
- Grazi GL, Mazziotti A, Jovine E, Stefanini GF, Frena A, Ercolani G, et al. Prostaglandin therapy in primary liver graft nonfunction after orthotopic transplantation. Transplantation Proceedings 1994;26(6):3651-2. [PubMed] [Google Scholar]
Greig 1989 {published data only}
- Greig PD, Woolf GM, Abecassis M, Forster J, Strasberg SM, Taylor BR, et al. Prostaglandin E1 for primary nonfunction following liver transplantation. Transplantation Proceedings 1989;21(2):3360-1. [PubMed] [Google Scholar]
- Greig PD, Woolf GM, Abecassis M, Strasberg SM, Taylor B, Superina RA, et al. Treatment of primary liver graft nonfunction with prostaglandin E1 results in increased graft and patient survival. Transplantation Proceedings 1989;21(1 Pt 2):2385-8. [PubMed] [Google Scholar]
- Greig PD, Woolf GM, Sinclair SB, Abecassis M, Strasberg AM, Taylor BR, et al. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation 1989;48:447-53. [DOI] [PubMed] [Google Scholar]
Hommann 2007 {published data only}
- Hommann M, Kammerer D, Lehmann G, Kornberg A, Kupper B, Daffner W, et al. Prevention of early loss of bone mineral density after liver transplantation by prostaglandin E1. Transplantation Proceedings 2007;39:540-3. [DOI] [PubMed] [Google Scholar]
Hsieh 2014 {published data only}
- Hsieh C, Hsieh S, Chiu WJ. Protective effects of n-acetylcysteine and a prostaglandin E1 analog, alprostadil, against hepatic ischemia: reperfusion injury in rats. Journal of Traditional and Complementary Medicine 2014;4(1):64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaisers 1996 {published data only}
- Kaisers U, Neumann U, Kuhlen R, Sprenger M, Neuhaus P, Rossaint R. Nitroglycerin versus epoprostenol: effects on hemodynamics, oxygen delivery, and hepatic venous oxygenation after liver transplantation. Liver Transplantation and Surgery 1996;2:455-60. [DOI] [PubMed] [Google Scholar]
Klein 1999 {published data only}
- Klein M, Geoghegan J, Wangemann R, Böckler D, Schmidt K, Scheele J. Preconditioning of donor livers with prostaglandin I2 before retrieval decreases hepatocellular ischemia-reperfusion injury. Transplantation 1999;67:1128-32. [DOI] [PubMed] [Google Scholar]
Kornberg 2004 {published data only}
- Kornberg A, Schotte U, Kupper B, Hommann M, Scheele J. Impact of selective prostaglandin E1 treatment on graft perfusion and function after liver transplantation. Hepato-gastroenterology 2004;51(56):526-31. [PubMed] [Google Scholar]
Maeda 1998 {published data only}
- Maeda T, Murase N, Subbotin V, Sakamoto T, Yamada T, Terakura M, et al. Analogs of cyclic nucleotides in rat liver preservation. Transplantation 1998;66(7):844-51. [DOI] [PubMed] [Google Scholar]
Natori 1997 {published data only}
- Natori S, Fujii Y, Kurosawa H, Nakano A, Shimada H. Prostaglandin E1 protects against ischemia-reperfusion injury of the liver by inhibition of neutrophil adherence to endothelial cells. Transplantation 1997;64(11):1514-20. [DOI] [PubMed] [Google Scholar]
Neumann 1998 {published data only (unpublished sought but not used)}
- Neumann UP, Kaisers U, Langrehr JM, Lang M, Glanemann M, Raakow R, et al. Treatment with PGE1 in patients after liver transplantation. Transplantation Proceedings 1998;30:1869-70. [DOI] [PubMed] [Google Scholar]
Neumann 1999 {published data only (unpublished sought but not used)}
- Neumann UP, Kaisers U, Langrehr JM, Glanemann M, Muller AR, Lang M, et al. Reduction of reperfusion injury with prostacyclin I2 after liver transplantation. Transplantation Proceedings 1999;31:1029-30. [DOI] [PubMed] [Google Scholar]
Onoe 2013 {published data only}
- Onoe T, Tanaka Y, Ide K, Ishiyama K, Oshita A, Kobayashi T, et al. Attenuation of portal hypertension by continuous portal infusion of PGE1 and immunologic impact in adult-to-adult living-donor liver transplantation. Transplantation 2013;95:1521-7. [DOI] [PubMed] [Google Scholar]
Radojkovic 2017 {published data only}
- Radojkovic M, Stojanovic M, Stanojevic G, Radojkovic D, Gligorijevic J, Ilic I, et al. Ischemic preconditioning vs adenosine vs prostaglandin E1 for protection against liver ischemia/reperfusion injury. Brazilian Journal of Medical and Biological Research 2017;50(8):e6185. [DOI: 10.1590/1414-431X20176185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruwart 1996 {published data only}
- Ruwart MJ, Henley KS. Prostaglandins in liver disease and liver transplantation. Gastroenterology Clinics of North America 1996;25(2):397-407. [DOI] [PubMed] [Google Scholar]
Sato 2003 {published data only}
- Sato Y, Kurosaki I, Yamamoto S, Nakatsuka H, Oya H, Shirai Y, et al. Postoperative management for donor safety in living related donor liver transplantation. Hepato-gastroenterology 2003;50(49):196-200. [PubMed] [Google Scholar]
Sheiner 1995 {published data only}
- Sheiner PA. Prostaglandins in liver transplantation. Hepatology 1995;21(2):592-3. [PubMed] [Google Scholar]
Shin 2012 {published data only}
- Shin M, Song SH, Kim JM, Kim SJ, Joh JW, Lee SK, et al. Effectiveness of intraportal prostaglandin E1 administration after liver transplantation. Transplantation Proceedings 2012;44:500-4. [DOI] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Smith DG, Henley KS, Remmert CS, Hass SL, Campbell DA, McLaren ID. A cost analysis of alprostadil in liver transplantation. Pharmacoeconomics 1996;9(6):517-24. [DOI] [PubMed] [Google Scholar]
Suehiro 2005 {published data only}
- Suehiro T, Shimada M, Kishikawa K, Shimura T, Soejima Y, Yoshizumi T, et al. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transplant International 2005;18:923-8. [DOI: 10.1111/j.1432-2277.2005.00159.x] [DOI] [PubMed] [Google Scholar]
Suzuki 1991 {published data only}
- Suzuki S, Nakamura S, Koizumi T, Sakaguchi S, Baba S, Muro H, et al. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation 1991;52(6):979-83. [DOI: 10.1097/00007890-199112000-00008] [DOI] [PubMed] [Google Scholar]
Takaya 1993 {published data only}
- Takaya S, Bronsther O, Bu-Elmagd K, Ramos H, Fung JJ, Todo S, et al. Use of prostaglandin E1 in crossmatch negative liver transplant recipients treated with FK 506. Transplantation Proceedings 1993;25(3):2381-5. [PMC free article] [PubMed] [Google Scholar]
Takaya 1995 {published data only}
- Takaya S, Doyle H, Todo S, Irish W, Fung JJ, Starzl TE. Reduction of primary nonfunction with prostaglandin E1 after clinical liver transplantation. Transplantation Proceedings 1995;27(2):1862-7. [PMC free article] [PubMed] [Google Scholar]
Tancharoen 1992 {published data only}
- Tancharoen S, Jones RM, Angus PW, Hardy KJ. Beneficial effect of prostaglandin E1 on hepatic allograft rejection following orthotopic liver transplantation. Transplantation Proceedings 1993;25(5):2890-1. [PubMed] [Google Scholar]
- Tancharoen S, Jones RM, Angus PW, Michell ID, McNicol L, Hardy KJ. Prostaglandin E1 therapy in orthotopic liver transplantation recipients: indications and outcome. Transplantation Proceedings 1992;24(5):2248-9. [PubMed] [Google Scholar]
Totsuka 1998 {published data only}
- Totsuka E, Todo S, Zhu Y, Ishizaki N, Kawashima Y, Jin MB, et al. Attenuation of ischemic liver injury by prostaglandin E1 analogue, misoprostol, and prostaglandin I2 analogue, OP-41483. Journal of the American College of Surgeons 1998;187:276-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Shin 2021 {published data only}
- Shin YU, Song GW. Prospective, randomized, open-label, single center, case control study to evaluate impact of Eglandin® (Alprostadil) on incidence of antibody-mediated rejection according to length of use in ABO-incompatible living donor liver transplant patient. www.cris.nih.go.kr/cris/search/detailSearchEn.do?seq=18959 (accessed 4 September 2022).
Additional references
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(110):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
ELTR 2021
- European Liver Transplant Registry. Evolution of liver transplants in Europe. www.eltr.org/Evolution-of-LTs-in-Europe.html (accessed 15 August 2021).
Gartlehner 2019
- Gartlehner G, Nussbaumer-Streit B, Wagner G, Patel S, Swinson-Evans T, Dobrescuc A, et al. Increased risks for random errors are common in outcomes graded as high certainty of evidence. Journal of Clinical Epidemiology 2019;106:50-9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 April 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), October 2013. Available at gradepro.org.
Gralnek 2000
- Gralnek IM, Hays RD, Kilbourne A, Rosen HR, Keeffe EB, Artinian L, et al. Development and evaluation of the liver disease quality of life instrument in persons with advanced, chronic liver disease – The LDQOL 1.0. American Journal of Gastroenterology 2000;95(12):3552-65. [DOI] [PubMed] [Google Scholar]
Hartog 2022
- Hartog H, Hann A, Perera MT. Primary nonfunction of the liver allograft. Transplantation 2022;106(1):117-28. [DOI: 10.1097/TP.0000000000003682] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011c
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2022
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hossain 2006
- Hossain MA, Wakabayashi H, Izuishi K, Okano K, Yachida S, Maeta H. The role of prostaglandins in liver ischemia-reperfusion injury. Current Pharmaceutical Design 2006;12(23):2935-51. [DOI: 10.2174/138161206777947678] [DOI] [PubMed] [Google Scholar]
Hunt 1981
- Hunt JN, Franz DR. Effect of prostaglandin E2 on gastric mucosal bleeding caused by aspirin. Digestive Diseases and Sciences 1981;26(4):301-5. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 2016
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 4 September 2022).
Imberger 2016
- Imberger G, Thorlund K, Gluud C, Wetterslev J. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review. BMJ Open 2016;6(8):e011890. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO 2012
- Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for acute kidney injury. Kidney International 2012;2(Suppl 1):1-138. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135:982-9. [DOI] [PubMed] [Google Scholar]
Lironi 2017
- Lironi C, McLin VA, Wildhaber BE. The effect and safety of prostaglandin administration in pediatric liver transplantation. Transplant Direct 2017;3(6):e163. [DOI: 10.1097/TXD.0000000000000682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: MR000033. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masior 2022
- Masior Ł, Grąt M. Primary nonfunction and early allograft dysfunction after liver transplantation. Digestive Diseases 2022;40:766-76. [DOI: 10.1159/000522052] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olthoff 2010
- Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplantation 2010;16(8):943-9. [DOI] [PubMed] [Google Scholar]
OPTN 2017
- Organ Procurement and Transplantation Network (OPTN). Policy 9: allocation of livers and liver-intestines. optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (accessed 22 November 2022).
OPTN 2022
- Organ Procurement and Transplantation Network (OPTN). National data. optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (accessed 19 May 2022).
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Review Manager Web 2023 [Computer program]
- Review Manager Web (RevMan Web). Version 4.27.0. Copenhagen: The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Ricciotti 2011
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis Thrombosis and Vascular Biology 2011;31(5):986-1000. [DOI: 10.1161/ATVBAHA.110.207449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane Reviews: the ROBES Meta-Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
Schünemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Schünemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Smith 1981
- Smith JB. Prostaglandins and platelet aggregation. Journal of Internal Medicine 1981;210(S651):91-8. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Sutton A, Loannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Thorlund 2017
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA); 2nd edition. Copenhagen Trial Unit, 2017. Available from ctu.dk/tsa/learn-more (accessed 19 September 2022).
TSA 2017 [Computer program]
- TSA – Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2017. ctu.dk/tsa/downloads/.
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cavalcanti 2011
- Cavalcanti AB, De Vasconcelos CP, Perroni de Oliveira M, Rother ET, Ferraz LJR. Prostaglandins for adult liver transplanted patients. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD006006. [DOI: 10.1002/14651858.CD006006.pub2] [DOI] [PubMed] [Google Scholar]
Vasconcelos 2006
- Vasconcelos CP, Ferraz LJR, Rother ET, Cavalcanti AB. Prostaglandins for adult liver transplanted patients. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD006006. [DOI: 10.1002/14651858.CD006006] [DOI] [PubMed] [Google Scholar]