Abstract
Background
Carotid artery stenosis is narrowing of the carotid arteries. Asymptomatic carotid stenosis is when this narrowing occurs in people without a history or symptoms of this disease. It is caused by atherosclerosis; that is, the build‐up of fats, cholesterol, and other substances in and on the artery walls. Atherosclerosis is more likely to occur in people with several risk factors, such as diabetes, hypertension, hyperlipidaemia, and smoking. As this damage can develop without symptoms, the first symptom can be a fatal or disabling stroke, known as ischaemic stroke. Carotid stenosis leading to ischaemic stroke is most common in men older than 70 years. Ischaemic stroke is a worldwide public health problem.
Objectives
To assess the effects of pharmacological interventions for the treatment of asymptomatic carotid stenosis in preventing neurological impairment, ipsilateral major or disabling stroke, death, major bleeding, and other outcomes.
Search methods
We searched the Cochrane Stroke Group trials register, CENTRAL, MEDLINE, Embase, two other databases, and three trials registers from their inception to 9 August 2022. We also checked the reference lists of any relevant systematic reviews identified and contacted specialists in the field for additional references to trials.
Selection criteria
We included all randomised controlled trials (RCTs), irrespective of publication status and language, comparing a pharmacological intervention to placebo, no treatment, or another pharmacological intervention for asymptomatic carotid stenosis.
Data collection and analysis
We used standard Cochrane methodological procedures. Two review authors independently extracted the data and assessed the risk of bias of the trials. A third author resolved disagreements when necessary. We assessed the evidence certainty for key outcomes using GRADE.
Main results
We included 34 RCTs with 11,571 participants. Data for meta‐analysis were available from only 22 studies with 6887 participants. The mean follow‐up period was 2.5 years. None of the 34 included studies assessed neurological impairment and quality of life.
Antiplatelet agent (acetylsalicylic acid) versus placebo
Acetylsalicylic acid (1 study, 372 participants) may result in little to no difference in ipsilateral major or disabling stroke (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.47 to 2.47), stroke‐related mortality (RR 1.40, 95% CI 0.54 to 3.59), progression of carotid stenosis (RR 1.16, 95% CI 0.79 to 1.71), and adverse events (RR 0.81, 95% CI 0.41 to 1.59), compared to placebo (all low‐certainty evidence). The effect of acetylsalicylic acid on major bleeding is very uncertain (RR 0.98, 95% CI 0.06 to 15.53; very low‐certainty evidence). The study did not measure neurological impairment or quality of life.
Antihypertensive agents (metoprolol and chlorthalidone) versus placebo
The antihypertensive agent, metoprolol, may result in no difference in ipsilateral major or disabling stroke (RR 0.14, 95% CI 0.02 to1.16; 1 study, 793 participants) and stroke‐related mortality (RR 0.57, 95% CI 0.17 to 1.94; 1 study, 793 participants) compared to placebo (both low‐certainty evidence). However, chlorthalidone may slow the progression of carotid stenosis (RR 0.45, 95% CI 0.23 to 0.91; 1 study, 129 participants; low‐certainty evidence) compared to placebo. Neither study measured neurological impairment, major bleeding, adverse events, or quality of life.
Anticoagulant agent (warfarin) versus placebo
The evidence is very uncertain about the effects of warfarin (1 study, 919 participants) on major bleeding (RR 1.19, 95% CI 0.97 to 1.46; very low‐certainty evidence), but it may reduce adverse events (RR 0.89, 95% CI 0.81 to 0.99; low‐certainty evidence) compared to placebo. The study did not measure neurological impairment, ipsilateral major or disabling stroke, stroke‐related mortality, progression of carotid stenosis, or quality of life.
Lipid‐lowering agents (atorvastatin, fluvastatin, lovastatin, pravastatin, probucol, and rosuvastatin) versus placebo or no treatment
Lipid‐lowering agents may result in little to no difference in ipsilateral major or disabling stroke (atorvastatin, lovastatin, pravastatin, and rosuvastatin; RR 0.36, 95% CI 0.09 to 1.53; 5 studies, 2235 participants) stroke‐related mortality (lovastatin and pravastatin; RR 0.25, 95% CI 0.03 to 2.29; 2 studies, 1366 participants), and adverse events (fluvastatin, lovastatin, pravastatin, probucol, and rosuvastatin; RR 0.76, 95% CI 0.53 to1.10; 7 studies, 3726 participants) compared to placebo or no treatment (all low‐certainty evidence). The studies did not measure neurological impairment, major bleeding, progression of carotid stenosis, or quality of life.
Authors' conclusions
Although there is no high‐certainty evidence to support pharmacological intervention, this does not mean that pharmacological treatments are ineffective in preventing ischaemic cerebral events, morbidity, and mortality. High‐quality RCTs are needed to better inform the best medical treatment that may reduce the burden of carotid stenosis. In the interim, clinicians will have to use other sources of information.
Keywords: Humans, Aspirin, Aspirin/adverse effects, Atherosclerosis, Atherosclerosis/complications, Atorvastatin, Carotid Stenosis, Carotid Stenosis/complications, Carotid Stenosis/drug therapy, Chlorthalidone, Fluvastatin, Hemorrhage, Ischemic Stroke, Ischemic Stroke/complications, Metoprolol, Pravastatin, Probucol, Rosuvastatin Calcium, Stroke, Stroke/etiology, Stroke/prevention & control, Warfarin
Plain language summary
What medicines are best for people with narrowing of the carotid arteries (blood vessels that deliver oxygen‐rich blood from the heart to the brain)?
Key messages
Compared to placebo (an inactive medicine): ‐ warfarin, an anticoagulant (blood‐thinning medicine), may reduce the risk of side effects by 11%; ‐ chlorthalidone, an antihypertensive (medicine for lowering high blood pressure), may slow the progression of carotid stenosis (narrowing of the carotid arteries) by 55%.
Studies with more participants and with long‐term follow‐up are needed to define the best medical treatment for modifiable risk factors in people with no symptoms of carotid narrowing.
What is asymptomatic carotid stenosis?
Carotid artery stenosis is narrowing of the carotid arteries, the major blood vessels that provide the brain's blood supply. 'Asymptomatic carotid stenosis' is when this narrowing occurs in people without symptoms of this disease. It is caused by atherosclerosis: the buildup of fats, cholesterol (high blood fats), and other substances in and on the blood vessel walls. Narrowing of the carotid arteries can develop without symptoms, so the first symptom can be a fatal or disabling stroke.
How is asymptomatic carotid stenosis treated?
The risk of having a stroke might be reduced by controlling modifiable, atherosclerosis risk factors, such as high blood pressure, smoking, cholesterol, and diabetes. There are a range of medicines used for these purposes, including:
‐ antihypertensive medicines (which lower high blood pressure); ‐ cholesterol‐ or lipid‐lowering medicines (drugs that lower high cholesterol levels); ‐ anticoagulants (also called 'blood thinners'); or ‐ antiplatelet medicines (drugs that prevent blood clots from forming).
What did we want to find out?
We wanted to find out which medicines for asymptomatic carotid stenosis are best for preventing: damage to the brain, stroke, death, major bleeding, and progression of the carotid arteries' narrowing.
We also wanted to find out if these medicines make any difference to people's quality of life and whether they are associated with any unwanted or harmful effects.
What did we do?
We searched for studies that compared one type of medicine with another type of medicine, placebo (an inactive medicine), or no treatment, in people of any age with asymptomatic carotid narrowing.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 34 studies that examined the medicines we were interested in. The studies involved a total of 11,571 people with asymptomatic carotid stenosis. The participants' average age was 61 years (range = 18 to 100 years old), and nearly two‐thirds of participants were male. The studies were carried out in outpatient medical settings around the world. The average follow‐up period was under three years.
Of these 34 studies, only 22 assessed our outcomes of interest and were included in our analyses. These 22 studies involved a total of 6887 people with asymptomatic carotid stenosis.
None of the studies assessed participants for neurological (i.e. brain) damage, and none measured changes in people's quality of life.
Main results
Antiplatelets (aspirin) compared to placebo
Aspirin (1 study; 372 participants) may not prevent stroke, stroke‐related death, progression of carotid narrowing, or increase side effects compared to placebo. We are very uncertain about the effect of aspirin on large bleeding events.
Antihypertensive drugs (metoprolol and chlorthalidone) compared to placebo
It is uncertain if metoprolol (1 study, 793 participants) may prevent stroke or stroke‐related death. However, chlorthalidone (1 study, 129 participants) may slow the progression of carotid narrowing compared to placebo. Neither study measured large bleeding events or side effects.
Anticoagulant drug (warfarin) compared to placebo
It is uncertain whether warfarin (1 study, 919 participants) increases large bleeding events compared to placebo. However, it may lead to side effects compared to placebo. The study did not measure stroke, stroke‐related death, or progression of carotid stenosis.
Cholesterol‐lowering drugs (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and probucol) compared to placebo or no treatment
It is unclear if cholesterol‐lowering drugs prevent stroke (5 studies, 2235 participants), stroke‐related death (2 studies, 1366 participants), or increase side effects (7 studies, 3726 participants) compared to placebo or no treatment. The studies did not measure large bleeding events or progression of carotid stenosis.
What are the limitations of the evidence?
We have limited confidence in the evidence for prevention of stroke, death, progression of carotid narrowing, side effects, and major bleeding events. Some studies had methodological problems or study designs that were not well reported. Overall, there is limited evidence to inform decision‐making about the use of medicines for asymptomatic carotid artery stenosis.
How up to date is this evidence?
The evidence is up to date to August 2022.
Summary of findings
Summary of findings 1. Antiplatelet agent versus placebo for asymptomatic carotid stenosis.
| Antiplatelet agent compared to placeboa for asymptomatic carotid stenosis | |||||
| Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: antiplatelet agent Comparison: placebo | |||||
| Outcomes (measurement) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with antiplatelet agent | ||||
| Neurological impairment | The included study did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke (CT scan or MRI) Follow‐up: 2.3 years |
372 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 1.08 (0.47 to 2.47) | Study population | |
| 54 per 1000 | 4 more per 1000 (29 fewer to 80 more) | ||||
| Stroke‐related mortality (CT scan or MRI) Follow‐up: 2.3 years |
372 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 1.40 (0.54 to 3.59) | Study population | |
| 38 per 1000 | 15 more per 1000 (17 fewer to 99 more) | ||||
| Major bleeding (not reported) Follow‐up: 2.3 years |
372 (1 RCT)b | ⊕⊝⊝⊝ Very lowc,d | RR 0.98 (0.06 to 15.53) | Study population | |
| 5 per 1000 | 0 fewer per 1000 (5 fewer to 79 more) | ||||
| Progression of carotid stenosis (DUS/every 6 months) Follow‐up: 2.3 years |
372 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 1.16 (0.79 to 1.71) | Study population | |
| 201 per 1000 | 32 more per 1000 (42 fewer to 143 more) | ||||
| Adverse events (not reported) Follow‐up: 2.3 years |
372 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 0.81 (0.41 to 1.59) | Study population | |
| 92 per 1000 | 16 fewer per 1000 (52 fewer to 47 more) | ||||
| Quality of life | The included study did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CT scan: computerised tomography scan; DUS: duplex ultrasonography; MRI: magnetic resonance imaging; №: number; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aOne study included in this comparison bAcetylsalicylic acid cDowngraded two levels due to imprecision: few events, one study, and 95% CI consistent with possible benefit and possible harm dDowngraded one level due to indirectness: unexplained major bleeding definition
Summary of findings 2. Antihypertensive agent versus placebo for asymptomatic carotid stenosis.
| Antihypertensive agent compared to placeboa for asymptomatic carotid stenosis | |||||
| Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: antihypertensive agent Comparison: placebo | |||||
| Outcomes (measurement/time point) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with antihypertensive agent | ||||
| Neurological impairment | Neither included study measured this outcome. | ||||
| Ipsilateral major or disabling stroke (not reported) Follow‐up: 3 years |
793 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 0.14 (0.02 to 1.16) | Study population | |
| 18 per 1000 | 15 fewer per 1000 (17 fewer to 3 more) | ||||
| Stroke‐related mortality (not reported) Follow‐up: 3 years |
793 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 0.57 (0.17 to 1.94) | Study population | |
| 18 per 1000 | 8 fewer per 1000 (15 fewer to 17 more) | ||||
| Major bleeding | Neither included study measured this outcome. | ||||
| Progression of carotid stenosis (DUS/at beginning and end) Follow‐up: 2 years |
129 (1 RCT)d | ⊕⊕⊝⊝ Lowc | RR 0.45 (0.23 to 0.91) | Study population | |
| 310 per 1000 | 171 fewer per 1000 (239 fewer to 28 fewer) | ||||
| Adverse events | Neither included study measured this outcome. | ||||
| Quality of life | Neither included study measured this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CT scan: computerised tomography scan; DUS: duplex ultrasonography; MRI: magnetic resonance imaging; №: number; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aTwo studies included in this comparison bMetoprolol cDowngraded two levels due to imprecision: few events, few studies, and 95% CI consistent with possible benefit and possible harm dChlorthalidone
Summary of findings 3. Anticoagulant agent versus placebo for asymptomatic carotid stenosis.
| Anticoagulant agent compared to placeboa for asymptomatic carotid stenosis | |||||
| Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: anticoagulant agent Comparison: placebo | |||||
| Outcomes (measurement/time point) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo | Risk difference with anticoagulant agent | ||||
| Neurological impairment | The included study did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke | The included study did not measure this outcome. | ||||
| Stroke‐related mortality | The included study did not measure this outcome. | ||||
| Major bleeding (hospital records/every 6 weeks) Follow‐up: 2.8 years |
919 (1 RCT)b | ⊕⊝⊝⊝ Very lowc,d | RR 1.19 (0.97 to 1.46) | Study population | |
| 260 per 1000 | 49 more per 1000 (8 fewer to 120 more) | ||||
| Progression of carotid stenosis | The included study did not measure this outcome. | ||||
| Adverse events (hospital records/every 6 weeks) Follow‐up: 2.8 years |
919 (1 RCT)b | ⊕⊕⊝⊝ Lowc | RR 0.89 (0.81 to 0.99) | Study population | |
| 644 per 1000 | 71 fewer per 1000 (122 fewer to 6 fewer) | ||||
| Quality of life | The included study did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aOne study included in this comparison b Warfarin cDowngraded two levels due to imprecision: few events, one study, and 95% CI consistent with possible benefit and possible harm cDowngraded one level due to indirectness: unexplained major bleeding definition
Summary of findings 4. Lipid‐lowering agent compared to placebo or no treatment for asymptomatic carotid stenosis.
| Lipid‐lowering agent compared to placeboa or no treatment for asymptomatic carotid stenosis | |||||
| Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: lipid‐lowering agent Comparison: placebo or no treatment | |||||
| Outcomes (measurement/time point) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo or no treatment | Risk difference with lipid‐lowering agent | ||||
| Neurological impairment | The included studies did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke (only reported for two studies: one used CT scan, MRI and hospital records/every 6 weeksb; the other used physical examination/at beginning and 10 days after the endc) Follow‐up: 3.1 years |
2235 (5 RCTs)d | ⊕⊕⊝⊝ Lowe | RR 0.36 (0.09 to 1.53) | Study population | |
| 18 per 1000 | 11 fewer per 1000 (16 fewer to 10 more) | ||||
| Stroke‐related mortality (only reported for one study: CT scan, MRI and hospital records/every 6 weeksb) Follow‐up: 4 years |
1366 (2 RCTs)f | ⊕⊕⊝⊝ Lowe | RR 0.25 (0.03 to 2.29) | Study population | |
| 4 per 1000 | 3 fewer per 1000 (4 fewer to 6 more) | ||||
| Major bleeding | The included studies did not measure this outcome. | ||||
| Progression of carotid stenosis | The included studies did not measure this outcome. | ||||
| Adverse events (only reported for two studies: one study used CT scan, MRI and hospital records/every 6 weeksb; the other used physical examination/at beginning and 10 days after the endc) Follow‐up: 3.3 years |
3726 (7 RCTs)g | ⊕⊕⊝⊝ Lowe | RR 0.76 (0.53 to 1.10) | Study population | |
| 86 per 1000 | 21 fewer per 1000 (41 fewer to 9 more) | ||||
| Quality of life | The included studies did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CT scan: computerised tomography scan; MRI: magnetic resonance imaging; №: number; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aNine studies included in this comparison bFurberg 1994 cZheng 2022 dLovastatin, pravastatin, rosuvastatin, and atorvastatin eDowngraded two levels due to imprecision: few events, one study, and 95% CI consistent with possible benefit and possible harm fLovastatin and pravastatin gFluvastatin, rosuvastatin, lovastatin, pravastatin, and probucol
Background
See Table 5 for a glossary of terms.
1. Glossary of terms.
| Term | Definition |
| Amaurosis fugax | Transient monocular visual loss associated with vascular thromboembolic events arising from the internal carotid arterial system |
| Anticoagulants | Drugs that suppress, delay, or prevent blood clots |
| Antiplatelet agents | Drugs which prevent blood clots by inhibiting platelet function |
| Atherosclerosis | A disease characterised by a build‐up of abnormal fat, cholesterol and platelet deposits on the inner wall of the arteries |
| Atheromatous plaques | A fatty deposit in the inner lining (intima) of an artery, resulting from atherosclerosis |
| Atherosclerotic debris | Pieces of atheromatous plaque that can break off and be carried by the bloodstream |
| Body mass index (BMI) | Body mass divided by the square of the body height, universally expressed in units of kg/m2 |
| Computed tomography angiography (CTA) | Computed tomography scanning that uses an injection of contrast material into the blood vessels to help diagnose and evaluate blood vessel disease or related conditions |
| Digital subtraction angiography (DSA) | Fluoroscopy technique used in interventional radiology to clearly visualise blood vessels in a bony or dense soft tissue environment |
| Direct thrombin inhibitors | A drug that acts as an anticoagulant by directly inhibiting the enzyme thrombin (factor IIa) |
| Duplex ultrasound | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices |
| Dyslipidaemia | Abnormal concentration of fats (lipids or lipoproteins) in the blood |
| Embolism | Obstruction of an artery or vein, typically by a clot of blood or an air bubble |
| Fator Xa inhibitors | A type of anticoagulant that works by selectively and reversibly blocking the activity of clotting factor Xa, preventing clot formation |
| Heparin | A drug which is used to prevent blood clotting (anticoagulant, blood thinner) |
| Ipsilateral encephalic territories | The same side of the brain |
| Low molecular weight heparin | A drug which is used to prevent blood clotting (anticoagulant) |
| Magnetic resonance angiography (MRA) | A group of techniques based on magnetic resonance imaging (MRI) to image blood vessels |
| Obesity | A condition where the amount of body fat is beyond healthy conditions (BMI greater than 30 kg/m2) |
| Oedema | Excess watery fluid which collects in tissues of the body, causing swelling when fluid leaks out of the body's vessels |
| Overweight | Where body fat is over that of the average population, but less than unhealthy conditions (BMI between 25 kg/m2 and 30 kg/m2) |
| Placebo | Substance or treatment with no active effect, like a sugar pill |
| Randomised controlled trial (RCT) | A study in which the participants are divided randomly into separate groups to compare different treatments |
| Stroke | Neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause, persisting ≥ 24 hours or until death |
| Thrombosis | Local coagulation of blood (clot) in a part of the circulatory system |
| Transient ischaemic attack (TIA) | A transient episode (less than 24 hours) of neurological dysfunction caused by focal brain, spinal cord, or retinal ischaemia without acute infarction |
| Unfractionated heparin (UFH) | A mixture of heparins obtained from animals which is used to prevent blood coagulation. Used to prevent and treat clotting disorders |
| Vascular | Relating to blood vessels (arteries and veins) |
| Vitamin K antagonists (VKAs) | Substances that reduce blood clotting by reducing the action of vitamin K |
Description of the condition
Strokes, characterised by brain tissue injury due to stenosis or arterial occlusion, can cause death or permanent neurological disability, and approximately 90% of strokes are ischaemic. This largely occurs as a result of carotid stenosis, hypertension, or cardiac arrhythmia (Brott 2013; Flumignan 2017; Mozaffarian 2016). Carotid artery stenosis (narrowing of the carotid arteries) is an important cause of cerebrovascular disease and transient ischaemic attack (TIA), underlying almost 15% of strokes (Easton 2009). The cumulative risk of stroke related to severe carotid stenosis is nearly 12% in the first year (approximately 15% to 18% in one year and 26% over two years (Barnett 1991)), and approximately 30% over five years (Barnett 1991; Moore 1995). Significant stenosis (of more than 50% of vessel diameter) is usually responsible for 8% of all strokes, and increases the risk of recurrence after the first episode to 16% over five years (Hillen 2003), mostly due to cerebral embolisms caused by biological changes to the atherosclerotic plaque (Flaherty 2013).
Ischaemic stroke is the second most common cause of death and a major global public health problem (Naylor 2023; Feigin 2021). Each year, more than 7.6 million new strokes are recorded and about 3.3 million people die from ischaemic stroke (Feigin 2021).
Furthermore, stroke is a significant cause of permanent neurological disability in Europe: out of approximately 1.2 million stroke survivors in the UK (De Waard 2017), 60% are discharged with some impairment (CDC 2001; NICE 2019; Strong 2007).
The direct costs of stroke alone amounted to approximately USD 28 billion (USD 28,000 million) between the years 2014 and 2015 in the USA, and this cost is expected to more than double in the next 20 years (Benjamin 2019; Feigin 2016; Gorelick 1999). By 2020, it was expected that there would be 80 million strokes worldwide, with 12 million deaths (an increase of 50% compared with 2012), and 200 million disability‐adjusted life years lost worldwide (Benjamin 2019; Feigin 2021).
Extracranial carotid stenosis may be asymptomatic or symptomatic. The embolisation of atherosclerotic debris or thrombotic material from plaques of arterial stenoses are most frequently associated with cerebrovascular symptoms such as stroke, TIA in the ipsilateral encephalic territories, and amaurosis fugax. People with asymptomatic carotid stenosis (ACS) are at risk not only of stroke and related symptoms, but also of other cardiovascular episodes, such as myocardial infarction (heart attack) and peripheral artery disease (Divya 2015; Flumignan 2017).
Asymptomatic carotid stenosis is a common condition in clinical practice, affecting about 3% to 7% of the general population. It is more prevalent in older people (over 60 years of age), and can evolve into a stroke in 0.3% to 2% of patients each year (De Weerd 2010; Park 2019). An atherosclerotic lesion, a diffuse and degenerative disease of the arteries, usually provokes ACS, which narrows the vessel wall. A sudden rupture of atheromatous plaques from significant asymptomatic stenosis of the carotid artery can lead to thromboembolism, which causes 10% to 15% of all strokes (Bulbulia 2017). Thus, for people with extracranial carotid disease, it is important to identify risk factors, the degree of stenosis of the artery, and the characteristics of the plaque, such as ulcerations, intra‐plaque haemorrhage, and lipid content, that may increase the likelihood of a cerebrovascular event (De Waard 2017; Derdeyn 2007; Naylor 2023; Ricotta 2011).
The modifiable risk factors associated with ACS — such as hypertension, smoking, dyslipidaemia, diabetes, obesity, a sedentary lifestyle, alcoholism, inadequate diet quality, and psychosocial factors — can vary in importance according to region, ethnic group, gender, age, and family history. However, together these factors consistently contribute towards increasing the risk of cerebrovascular disease, making them targets for general approaches to preventing cerebrovascular events worldwide (Arnett 2019; Guzik 2017; O'Donnel 2016).
In order to diagnose and classify ACS, there are some complementary imaging tests: duplex ultrasound (DUS) and angiography by magnetic resonance imaging (MRI), computed tomography angiography (CTA), or digital subtraction angiography (DSA) (Naylor 2023). DSA was discontinued in practice at the end of the 20th century as a diagnostic method, especially in asymptomatic patients, as it is associated with a 1.2% risk of neurological events (Walker 1995; Wardlaw 2006). On the other hand, DUS is affordable and non‐invasive. It also does not bring the additional risks associated with DSA, magnetic resonance angiography (MRA), and CTA, such as the use of iodinated or paramagnetic contrast, X‐ray exposure, and embolisation risks (Cassola 2022). Thus, DUS is widely used as the first diagnostic method for detecting carotid stenosis in both symptomatic patients and those with risk factors for asymptomatic stenosis (Daolio 2019; Ricotta 2011).
The European Carotid Surgery Trial (ECST 1998) and the North American Symptomatic Carotid Endarterectomy Trial (NASCET; Barnett 1991) applied different techniques to measure the percentage of stenosis in DSA (Figure 1), and identified those patients who would benefit from revascularisation. Whilst the ECST used residual lumen diameter as a denominator, the NASCET used disease‐free diameter in a segment of the carotid artery above the stenosis. Using NASCET measurement standards, other studies (namely, the Asymptomatic Carotid Atherosclerosis Study (ACAS; Walker 1995) and the Asymptomatic Carotid Surgery Trial 1 (ACST‐1)) have shown that surgical intervention would also benefit some asymptomatic patients with carotid stenosis greater than 60% of diameter on DSA (Halliday 2004; Naylor 2023; Ricotta 2011).
1.
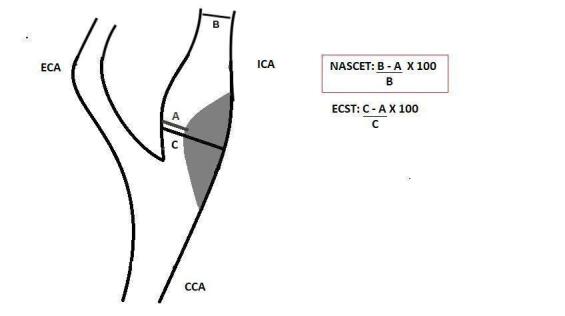
Longitudinal view of carotid bifurcation with methods of measuring carotid stenosis at angiography
A: narrowest ICA diameter B: normal distal cervical ICA diameter C: estimated original diameter at the site of the most stenosis
CCA: common carotid artery ECA: external carotid artery ECST: European Carotid Surgery Trial ICA: internal carotid artery NASCET: North American Symptomatic Carotid Endarterectomy Trial
Description of the intervention
It is important to ensure that people with ACS receive the best therapeutic option to avoid cerebral ischaemias. These options include: the control of hypertension; the use of lipid‐lowering drugs to reduce cholesterol levels in order to regress plaque(s), decrease the risk of plaque accident, and for anti‐inflammatory purposes; the use of hypoglycaemic drugs; and the use of antiplatelet and anticoagulant agents.
Antihypertensive therapy
High blood pressure is one of the most powerful risk factors, and its decrease seems to be directly related to a lower incidence of stroke. A reduction of 5 mmHg to 10 mmHg blood pressure is associated with a 30% to 40% reduced risk of stroke compared with placebo (Lawes 2004). Despite a lack of randomised controlled trials (RCTs) assessing the effects of antihypertensives in people with ACS, the European Society for Vascular Surgery (ESVS) recommends a target blood pressure for people with ACS below 140/90 mmHg (Naylor 2023; Ricotta 2011). More radically, in two guidelines, the American Heart Association (AHA) lowered these ideal blood pressure levels to close to 130/80 mmHg, with diastolic blood pressure less than 85 mmHg for people with diabetes (Arnett 2019; Brott 2013).
Maintaining blood pressure may reduce stenosis and prevent lesion progression. Calcium channel blockers and angiotensin‐converting enzyme inhibitors are associated with plaque reduction to a greater extent than diuretics and beta‐blockers (Arnett 2019; Naylor 2023; Ricotta 2011).
Lipid‐lowering drugs
At the start of the 21st century, there was an increase in statin use as studies showed a decrease in cardiovascular events in symptomatic patients by more than one‐third when low‐density lipoprotein (LDL) cholesterol levels were below 70 mg/dL (Amarenco 2006; Ricotta 2011; Taylor 2002). Systematic reviews observed a significant reduction in cardiovascular mortality (including stroke) when statins, mainly atorvastatin 80 mg daily, were used in primary prevention; for instance, in people with ACS (Brott 2013; Naylor 2023; Taylor 2013). However, ezetimibe or proprotein convertase subtilisin/kexin type (9PCSK9) inhibitors may be an alternative treatment for high‐risk patients who cannot tolerate statins (Wilson 2019; Zhan 2018).
Management of diabetes
Diabetes mellitus is an independent predictor of moderate and severe carotid stenosis, and can contribute to doubling the chances of stroke (Holman 2014). Medications used for glycaemic control include oral hypoglycaemic agents (metformin or sulphonylureas, or both), insulin therapy, or the new glucose‐lowering medications, such as the analogue of human glucagon‐like peptide 1, dipeptidyl peptidase 4 inhibitors, sodium‐glucose cotransporter 2 inhibitors, and thiazolidine (Holman 2014). Strong control of glycaemic levels is not directly related to a decreased risk of stroke, but glycosylated haemoglobin levels lower than 7% may contribute to a reduction in other related events, such as microangiopathy (Zhang 2013). Meanwhile, systematic reviews indicated that strict control in people with a body mass index above 30 kg/m2 was effective in reducing the risk of cerebrovascular disease (Naylor 2023; Ricotta 2011).
Antiplatelet drugs
There is weak evidence for the use of antiplatelet drugs in people with ACS for reducing the risk of stroke, but there is more robust evidence for their use in secondary prevention (Murphy 2019). However, the use of aspirin at doses between 75 mg and 325 mg (or clopidogrel 75 mg when aspirin is intolerable) is recommended in asymptomatic patients to prevent other cardiovascular events (Naylor 2023; Ricotta 2011).
Anticoagulant agents
Anticoagulant therapy is known to prevent stroke in people with atrial fibrillation, but warfarin has not been shown to be more effective compared to antiplatelet therapy for secondary prevention in people without atrial fibrillation (Ricotta 2011). However, recent studies have indicated that the use of low‐dose rivaroxaban together with aspirin may decrease the risk of stroke in both symptomatic and asymptomatic patients (Sharma 2019).
How the intervention might work
As carotid atherosclerosis is an important aspect in stroke pathophysiology, proper management of the diseases that lead to its increase may correspond to key targets for stroke prevention. The approaches discussed above work together to control the risk factors that increase atherosclerosis, avoiding irregular and ulcerated plaques and microembolic particles, and preventing carotid artery disease from progressing (Naylor 2023).
The ACAS and ACST‐1 studies used an initial pharmacological therapy which has significantly changed in recent decades. For instance, only around 10% to 20% of ACAS and ACST‐1 participants regularly used lipid‐lowering drugs (Walker 1995). There was a decline in annual stroke rates of approximately 60% between 1995 and 2004, which strongly correlates with improved pharmacological treatment associated with the increased use of aspirin, antihypertensive drugs, and statins, in that decade (Naylor 2023). Control of hypertension can reduce the risk of stroke by up to 30%, while control of cholesterol can reduce this risk by 15% (Ricotta 2011). In addition, people with diabetes who, associated with glycaemic control, were taking statins, antiplatelet, and antihypertensive drugs, showed a 60% reduction in the risk of cardiovascular disease and death (Halliday 2004; Ricotta 2011).
Why it is important to do this review
Some RCTs have evaluated the use of pharmacological interventions, and topical guidelines currently recommend triple medical therapy (e.g. antiplatelet agents, antihypertensive therapy, and statins) in addition to lifestyle interventions to reduce the risk of stroke (Naylor 2023). Routine carotid endarterectomy or stenting is not reasonable in asymptomatic patients, except in particular high‐risk patients on medical therapy (Naylor 2023). However, the optimal therapeutic management strategy remains unclear (Raman 2013). Additionally, recent studies suggest that direct oral anticoagulants plus antiplatelet agents may be more effective than antiplatelet agents alone for decreasing the risk of major vascular events (Abbott 2007; Sharma 2019).
Stroke continues to be the main cause of permanent disability and one of the most important causes of death in the world. Its impact leads to considerable socioeconomic impairment, not only to the individual and their family, but also to society as a whole. In this context, pursuing the best pharmacological strategies may be useful in decreasing ACS‐related mortality and permanent neurological disability (Naylor 2023).
Objectives
To assess the effects of pharmacological interventions for the treatment of asymptomatic carotid stenosis in preventing neurological impairment, ipsilateral major or disabling stroke, death, major bleeding, and other outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs with parallel (e.g. cluster or individual) or cross‐over design. We planned to only use data from the first phase of cross‐over studies to avoid the risk of carry‐over effects, as described in Section 23.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). We included studies reported in full texts, as abstracts only, and unpublished data. We did not include quasi‐randomised trials (i.e. studies in which participants are allocated to intervention groups based on methods that are not truly random, such as hospital number or date of birth).
Types of participants
We considered for inclusion participants of any gender and any age with ACS. Carotid stenosis was defined as a narrowing of the internal or common carotid artery (or both), diagnosed by at least one valid objective test (e.g. DUS or angiography by tomography, magnetic resonance, or digital subtraction). We used the classification of carotid stenosis with the use of ultrasound, as defined by Grant 2003, for participant classification (Table 6). We used the Mannheim Consensus to distinguish between augmented intima‐media thickness (IMT) and carotid stenosis, as described by Touboul 2012, where the latter refers to plaque with an intima‐media thickness greater than 1.3 mm, from the media‐adventitia interface to the intima‐lumen interface. We considered participants as asymptomatic if they were without ipsilateral neurological symptoms (e.g. amaurosis fugax, TIA, or stroke) in the previous six months (Naylor 2023). We considered all trials involving participants with ACS, irrespective of the degree of stenosis or the method of determining the degree of stenosis.
2. DUS criteria for internal carotid stenosis.
| Consensus panel based on Grant 2003 | ||||
| Degree of stenosis (%) | Primary parameters | Additional parameters | ||
| ICA PSV (cm/sec) | Plaque estimate (%)* | ICA/CCA PSV ratio | ICA EDV (cm/sec) | |
| Normal | < 125 | None | < 2.0 | < 40 |
| < 50% | < 125 | < 50 | < 2.0 | < 40 |
| 50% to 69% | 125 to 230 | ≥ 50 | 2.0 to 4.0 | 40 to 100 |
| ≥ 70% but less than near occlusion | > 230 | ≥ 50 | > 4.0 | > 100 |
| Near occlusion | High, low or undetectable | Visible | Variable | Variable |
| Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
| *Plaque estimate (diameter reduction) based on DUS B‐mode and on additional colour mode ultrasound. | ||||
CCA: common carotid artery DUS: duplex ultrasound EDV: end diastolic velocity ICA: internal carotid artery PSV: peak systolic velocity
If we found studies with mixed populations, and only a subset of the participants met our inclusion criteria, we attempted to obtain data for the subgroup of interest from the trialists in order to include the study. For studies with mixed populations where we could not obtain data on the subgroup of interest, but at least 50% of the study population was of interest, we included all participants in our analysis. We explored the effect of this decision in sensitivity analyses. We excluded studies in which less than 50% of the population were of interest and data on the subgroup of interest were not available.
Types of interventions
We included trials comparing one pharmacological intervention (agent or drug) with placebo, no treatment, or another pharmacological intervention. We included trials of any combination of interventions, providing co‐treatments were balanced between the treatment and control arms for the ACS treatment. We considered interventions such as fish oil and diet as no treatment. We also included studies that compared different doses of drugs.
We considered the following interventions:
anticoagulants (unfractionated heparin (UFH) and low molecular weight heparins (LMWHs); vitamin K antagonists (VKAs); direct oral anticoagulants (DOACs), factor Xa inhibitors and direct thrombin inhibitors; pentasaccharides);
antiplatelet agents (e.g. aspirin, clopidogrel);
antihypertensive drugs (e.g. angiotensin‐converting enzyme inhibitors, beta‐blockers);
glycaemic‐lowering agents (e.g. biguanides, sulphonylureas); and
lipid‐lowering agents (e.g. statins).
The possible comparisons were:
anticoagulants plus antiplatelet agents versus antiplatelet agents;
one antiplatelet drug versus a combination of antiplatelets from two drugs;
one antiplatelet drug versus another antiplatelet drug;
anticoagulants versus antiplatelet drugs;
one lipid‐lowering drug versus another lipid‐lowering drug;
one antihypertensive drug versus another antihypertensive drug;
one glycaemic‐lowering drug versus another glycaemic‐lowering drug; and
any combination of the above treatments versus any combination, with or without placebo.
Types of outcome measures
Primary outcomes
Neurological impairment, assessed using clinical outcome measures or any validated international scales (e.g. the National Institutes of Health Stroke Scale (NIHSS), the modified Rankin Scale (mRS), the Barthel Index (BI)). If we identified both dichotomous and continuous variables related to neurological impairment, we reported them separately as independent outcomes.
Ipsilateral major or disabling stroke, related to the extracranial carotid stenosis and confirmed by any objective additional test (e.g. computerised tomography, angiography) other than clinical examination only.
Secondary outcomes
Stroke‐related mortality
Major bleeding: defined by a haemoglobin concentration decrease of 2 g/dL or more, a retroperitoneal or intracranial bleed, a transfusion of two or more units of blood, or fatal haemorrhagic events, as defined by the International Society on Thrombosis and Haemostasis (Schulman 2010). We also considered the definition stipulated by the included study.
Progression of carotid stenosis (any increase in extracranial carotid stenosis), evaluated by change in range of stenoses; that is, less than 50%, 50% to 69%, 70% or more, near occlusion or occlusion. We considered the carotid stenosis if it was evaluated by any valid objective method (e.g. duplex ultrasound (Grant 2003), or angiography by tomography, magnetic resonance, or digital subtraction (Barnett 1991)).
Adverse events, such as all‐cause mortality, gastrointestinal events, allergic reaction, renal failure, or minor bleeding.
Quality of life, analysed by any validated questionnaire (e.g. SF‐36 (Ware 1992)) or participants' subjective perception of improvement (yes or no) as reported by the study authors. If we were unable to pool data on quality of life due to the use of different measurements, we planned to extract data on improvement.
We presented the outcomes at the following two time points after the start of the intervention, if data were available:
early outcomes (at six months or less after the start of the intervention); and
long‐term outcomes (more than six months after the start of the intervention).
Search methods for identification of studies
We searched for trials in all languages and arranged for the translation of relevant articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group trials register and the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, issue 8) in the Cochrane Library (searched 9 August 2022);
MEDLINE Ovid (from 1946 to 9 August 2022);
Embase Ovid (from 1974 to 9 August 2022);
Literatura Latino‐Americana e do Caribe em Ciências da Saúde (LILACS) (from 1982 to 9 August 2022), via Virtual Health Library); and
Indice Bibliográfico Español de Ciencias de la Salud (IBECS), via Virtual Health Library (searched 9 August 2022).
We modelled the subject strategies for databases on the search strategy designed for MEDLINE by the Cochrane Stroke Group's Information Specialist. We opted to write a highly‐sensitive search strategy and eliminated the pharmacological interventions component of the search entirely. The reasons for this are as follows. The problem component 'asymptomatic carotid stenosis' is already well‐defined and, when combined with Cochrane's verified RCT filter, retrieved a low number of results during test searches in MEDLINE Ovid. Pharmacological interventions search blocks can help improve recall when included in search strategies. However, because the initial test search recall was relatively low, as suggested above, we elected not to include them in the enclosed search, but we selected the relevant interventions manually. We combined all search strategies deployed with subject strategy adaptations of the highly‐sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2022).
We searched the following ongoing trials registers:
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov/; searched 9 August 2022); and
World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/ictrp/en/; searched 9 August 2022).
The most recent searches were carried out on 9 August 2022. The search strategies are reported in Appendix 1.
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials, we:
checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials, and searched Google Scholar to forward‐track relevant references (scholar.google.co.uk/);
contacted original trial authors for clarification and further data if trial reports were unclear;
where necessary, contacted experts/trialists/organisations in the field to obtain additional information on relevant trials, using a standard letter template (Appendix 2); and
-
conducted a search of various grey literature sources, dissertation and theses databases, and databases of conference abstracts, including:
Repositório UNIFESP (thesis repository of Universidade Federal de São Paulo, Brazil; searched 9 August 2022; Appendix 1);
British Library EThOS (UK E‐Theses Online Service; searched 9 August 2022; Appendix 1);
ProQuest Dissertation and Theses Global (searched 9 August 2022; Appendix 1).
Data collection and analysis
Selection of studies
Two review authors (CNBC, NC) independently screened titles and abstracts of the references obtained as a result of our searching activities, and excluded obviously irrelevant reports using the Covidence tool. We retrieved the full‐text articles for the remaining references and two review authors (CNBC, NC) independently screened these, to identify studies for inclusion and to record reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, when required, we consulted a third review author (RLGF). We collated multiple reports of the same study so that each study, not each reference, was the unit of interest in the review. We recorded the selection process and complete a PRISMA flow diagram (Page 2021).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we piloted on at least one study in the review. Two review authors (CNBC, NC) independently extracted data from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting and date of study.
Participants: number randomised, number lost to follow‐up/withdrawn, number analysed, number of interest, mean age, age range, gender, severity of condition, diagnostic criteria, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
We resolved disagreements by consensus or by involving a third review author (RLGF). One review author (CNBC) transferred data into Review Manager (Review Manager 2020). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data extraction form. A second review author (NC) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (CNBC, NC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by involving another review author (RLGF). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other bias.
We graded each potential source of bias as high, low, or unclear and provide a quote from the study report, together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Clezar 2020), and reported any deviations from it in the Differences between protocol and review section of the review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs).
Unit of analysis issues
Individuals were our unit of analysis. If trials included multiple intervention arms, we considered only the arms relevant to the scope of our review. Where a study included multiple intervention groups, we combined groups to create a single pair‐wise comparison. Where a study included repeated observations, we followed recommendations in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data (e.g. when we identified a study as an abstract only). Where possible, we used the Review Manager calculator to calculate missing standard deviations using other data from the trial, such as confidence intervals. Where this was not possible, and we thought the missing data introduced serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. For all outcomes, we followed intention‐to‐treat (ITT) principles to the greatest degree possible: that is, we analysed participants in their randomised group regardless of what intervention they actually received. We used available‐case data for the denominator if ITT data were not available.
We presented study‐level data so that missing and unclear data were clearly indicated and to make available any unpublished data acquired from investigators.
Assessment of heterogeneity
We inspected studies for clinical (variation in population, interventions, and outcomes) and methodological (variation in study design, outcome measurement, or risk of bias) heterogeneity.
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We used the I2 statistic to measure heterogeneity amongst the trials in each analysis; we acknowledge that there is substantial uncertainty in the value of I2 when there are few studies. If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis.
As strict thresholds for interpretation of I2 are not recommended, we followed the guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity; or
75% to 100%: considerable heterogeneity.
When the I2 value lies in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions amongst the trials contributing data to the analysis (Deeks 2019).
Assessment of reporting biases
We did not use funnel plots to investigate reporting biases because we did not identify 10 or more studies in one comparison.
Data synthesis
We synthesised the data using Review Manager 5.4 (Review Manager 2020). We undertook meta‐analysis only where this was meaningful; that is, if the treatments, participants, and the underlying clinical question were similar enough for pooling to be appropriate.
If we were confident that trials were estimating the same underlying treatment effect — that is, the included studies were homogenous (considering population, interventions, comparators, and outcome characteristics) — we used a fixed‐effect meta‐analysis. If clinical differences were sufficient to expect that underlying treatment effects differed between trials or if we identified at least substantial heterogeneity, we used a random‐effects meta‐analysis. If there was substantial clinical, methodological, or statistical heterogeneity across trials that prevented the pooling of data, we used a narrative approach to data synthesis (Deeks 2019).
We addressed all outcomes listed in Types of outcome measures in the Effects of interventions section of the review, presenting the outcomes in the order in which they are shown in Types of outcome measures. In addition, we presented one summary of findings table for each comparison, in which we summarised the main outcomes. We included the results of individual studies and any statistical summary of these in Data and analyses tables in the review.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct our preplanned subgroup analyses (Clezar 2020), due to insufficient data.
Sensitivity analysis
We were only able to conduct one of our preplanned sensitivity analyses (Clezar 2020), comparing a fixed‐effect versus random‐effects model for the 'ipsilateral major or disabling stroke' outcome.
Summary of findings and assessment of the certainty of the evidence
We created tables for each of our 10 comparisons, and from these, selected the four most clinically relevant to present as our core summary of findings tables. We have presented the remaining comparisons as additional tables.
We present the following outcomes in all tables:
neurological impairment;
ipsilateral major or disabling stroke;
stroke‐related mortality;
major bleeding;
progression of carotid stenosis;
adverse events; and
quality of life.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes (GRADE 2004). We used methods and recommendations described in Chapter 15 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022), and GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Two review authors (CNBC, NC), working independently, made judgements about the certainty of the evidence, with disagreements resolved by discussion or involving a third review author (RLGF). We justified, documented, and incorporated judgements into the reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables, and prepared our summary of findings tables before writing the results and conclusions of our review.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 10,368 records through the database searches and removed 1959 duplicate records. Two review authors (CNBC and NC) screened 8409 records and eliminated 8344 irrelevant records. We screened the remaining records against our inclusion criteria and analysed the full texts of 65 studies. We included 34 studies in qualitative analysis; 22 of these studies contributed to the quantitative analysis. Three included studies were multi‐armed (Furberg 1994; Hedblad 2001; Sawayama 2002). We excluded 30 studies (see Excluded studies). We identified one ongoing trial (Aranzulla 2021). See Figure 2 for the study flow diagram (Liberati 2009).
2.
Study flow diagram
Included studies
We included 34 studies that met our prespecified inclusion criteria (Anderssen 2005; Applegate 1991; Blanco‐Colio 2004; ELSA 2002; Bots 2007; Corti 2005; Côté 1995; Crouse 2007; Furberg 1994; Hedblad 2001; Hu 2009; Ikeda 2013; Kadoglou 2010; VHAS 1998; Meaney 2009; Mercuri 1996; Nohara 2012; Norris 1990; Reid 2005; Salonen 1995; Sawayama 2002; Semplicini 2000; Shinoda‐Tagawa 2002; Stumpe 2007; Sutton‐Tyrrell 1994; Tang 2009; Terpstra 2004; Underhill 2008; Yamada 2009; Yamamoto 2011; Zanchetti 2004; Zeng 2004; Zheng 2022). All 34 included studies were individually randomised, parallel RCTS. We identified no eligible cluster‐RCTs or cross‐over studies.
Three of the included studies were multi‐armed (Furberg 1994; Hedblad 2001; Sawayama 2002). Participants in Furberg 1994 and Hedblad 2001 were randomly assigned to four groups; participants in Sawayama 2002 were randomly assigned to three groups.
Of these included studies, 14 were conducted in Europe (Italy, Finland, England, Ireland, Poland, the Netherlands, Czech Republic, Germany, Austria, Greece, Spain, Norway, Sweden, and France), 10 in Asia (four in China and six in Japan), seven in North America (five in the USA, one in Canada, and one in Mexico), and three were conducted in different continents at the same time (two in North America and Europe ‐ including Belgium ‐ and one in North America, Europe, and Oceania ‐ Australia).
Only one study was performed in the last decade (Zheng 2022). Twenty‐one studies were conducted in the 2000s, 10 were conducted in the 1990s (ELSA 2002; Côté 1995; Furberg 1994; VHAS 1998; Mercuri 1996; Mercuri 1996; Salonen 1995; Sawayama 2002; Sutton‐Tyrrell 1994; Zanchetti 2004), and two were performed in the 1980s (Applegate 1991; Norris 1990)
The length of follow‐up for these participants ranged from 30 days to six years, with more than half of the studies lasting between two and three years. The run‐in phase was only mentioned in 17 of the included studies, lasting between two and eight weeks, with placebo washouts generally being performed.
Twenty‐one studies mentioned their sponsor. Of these, 15 were sponsored exclusively by pharmaceutical companies, five studies received government funds, and seven obtained sponsorship from both. Two studies were self‐sponsored. The funding resources were not mentioned in five studies. Only 13 studies mentioned conflicts of interest of the authors.
Amongst the included studies, only 22 had the outcomes prespecified in our protocol (Anderssen 2005; Applegate 1991; Bots 2007; Côté 1995; Crouse 2007; ELSA 2002; Furberg 1994; Hedblad 2001; Ikeda 2013; Mercuri 1996; Nohara 2012; Salonen 1995; Sawayama 2002; Stumpe 2007; Sutton‐Tyrrell 1994; Tang 2009; Terpstra 2004; Yamada 2009; Zanchetti 2004; Zeng 2004; Zheng 2022; Zhu 2006). In the remaining 12 studies, despite meeting the inclusion criteria proposed in our protocol, none assessed any of our prespecified outcomes of interest (Blanco‐Colio 2004; Corti 2005; Hu 2009; Kadoglou 2010; VHAS 1998; Meaney 2009; Norris 1990; Reid 2005; Semplicini 2000; Shinoda‐Tagawa 2002; Underhill 2008; Yamamoto 2011).
Full descriptions of the included studies are presented in the Characteristics of included studies table.
Population
The included studies involved a total of 11,571 outpatient participants with asymptomatic carotid stenosis. The 22 studies available for quantitative analysis had a total of 6887 participants. Two studies did not provide any demographic details of their participants (Norris 1990; Zeng 2004). The age of participants ranged from 18 to 100 years old (mean age of 61 years old), and the proportion of men was about 61% of included participants. We could not find smoking data in 11 of the 34 included studies. In the remaining 23 studies, nearly 23% of participants were smokers during the course of the trial.
Sample size
The studies' sample size ranged from 14 to 2035. Twelve studies had fewer than 100 participants (Blanco‐Colio 2004; Corti 2005; Hu 2009; Kadoglou 2010; Meaney 2009; Norris 1990; Reid 2005; Semplicini 2000; Tang 2009; Underhill 2008; Yamada 2009; Yamamoto 2011), and nine had at least 500 participants (Anderssen 2005; Applegate 1991; ELSA 2002; Bots 2007; Crouse 2007; Furberg 1994; Hedblad 2001; Zanchetti 2004; Zheng 2022).
Interventions and comparators
All but one type of intervention (glycaemic‐lowering agents) that we set out to investigate could be found in the included studies. Twenty‐two studies explored lipid‐lowering agents (Anderssen 2005; Blanco‐Colio 2004; Bots 2007; Corti 2005; Crouse 2007; Furberg 1994; Hu 2009; Ikeda 2013; Kadoglou 2010; Meaney 2009; Mercuri 1996; Nohara 2012; Reid 2005; Salonen 1995; Sawayama 2002; Tang 2009; Underhill 2008; Yamada 2009; Zanchetti 2004; Zeng 2004; Zheng 2022; Zhu 2006). Fourteen studies addressed other interventions, such as anticoagulants (Furberg 1994; Shinoda‐Tagawa 2002), antiplatelet agents (Côté 1995), and antihypertensive drugs (Applegate 1991; ELSA 2002; Hedblad 2001; VHAS 1998; Norris 1990; Semplicini 2000; Sutton‐Tyrrell 1994; Stumpe 2007; Terpstra 2004; Yamamoto 2011; Zanchetti 2004).
Fifteen included studies compared an intervention with placebo. Other studies used varied comparators, including: different doses of the same lipid‐lowering agent; one class of lipid‐lowering agent versus another class of lipid‐lowering agent; one class of antihypertensive agent versus another class of antihypertensive agent; anticoagulant agent versus antiplatelet agent, or no treatment.
We performed quantitative analysis in 10 comparisons for which we could extract numerical data (Table 1; Table 2; Table 3; Table 4; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12). Additionally, we could conduct meta‐analysis for: three outcomes when comparing lipid‐lowering agents to placebo (Analysis 5.1; Analysis 5.2; Analysis 5.3); one outcome when comparing one class of lipid‐lowering agent to another class of lipid‐lowering agent (Analysis 7.2); and two outcomes when comparing one class of antihypertensive agent to another class of antihypertensive agent (Analysis 9.1; Analysis 9.2).
3. Additional SoF table: one antihypertensive agent plus lipid‐lowering agent compared to another antihypertensive agent plus lipid‐lowering agent for asymptomatic carotid stenosis.
| One antihypertensive agent plus lipid‐lowering agent compared to another antihypertensive agent plus lipid‐lowering agenta for asymptomatic carotid stenosis | |||||
|
Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: one antihypertensive agent plus lipid‐lowering agent Comparison: another antihypertensive agent plus lipid‐lowering agent | |||||
| Outcomes (measurement/time point) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with another antihypertensive agent plus lipid‐lowering agent | Risk difference with one antihypertensive agent plus lipid‐lowering agent | ||||
| Neurological impairment | The included study did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke (not reported) |
254 (1 RCT)b | ⨁⨁◯◯ Lowc | RR 0.34 (0.01 to 8.23) | 8 per 1000 | 5 fewer per 1000 (8 fewer to 56 more) |
| Stroke‐related mortality | The included study did not measure this outcome. | ||||
| Major bleeding | The included study did not measure this outcome. | ||||
| Progression of carotid stenosis | The included study did not measure this outcome. | ||||
| Adverse events | The included study did not measure this outcome. | ||||
| Quality of life | The included study did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RR: risk ratio; SoF: summary of findings | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | |||||
aOne study included in this comparison bHydrochlorthiazide + pravastatin versus fosinopril + pravastatin cDowngraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm
4. Additional SoF table: lipid‐lowering agent plus antihypertensive agent compared to antihypertensive agent for asymptomatic carotid stenosis.
| Lipid‐lowering agent plus antihypertensive agent compared to antihypertensive agenta for asymptomatic carotid stenosis | |||||
|
Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: lipid‐lowering agent plus antihypertensive agent Comparison: antihypertensive agent | |||||
| Outcomes (measurement/time point) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with antihypertensive agent | Risk difference with lipid‐lowering agent plus antihypertensive agent | ||||
| Neurological impairment | The included study did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke (physical examination, CT scan) | 225 (1 RCT)b | ⨁◯◯◯ Very lowc,d | RR 0.64 (0.27 to 1.50) | 109 per 1000 | 39 fewer per 1000 (80 fewer to 55 more) |
| Stroke‐related mortality | The included study did not measure this outcome. | ||||
| Major bleeding | The included study did not measure this outcome. | ||||
| Progression of carotid stenosis | The included study did not measure this outcome. | ||||
| Adverse events (not reported) | 225 (1 RCT)b | ⨁◯◯◯ Very lowc,d | RR 20.09 (1.19 to 338.84) | 0 per 1000 | 0 fewer per 1000 (0 fewer to 0 fewer) |
| Quality of life | The included study did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RR: risk ratio; SoF: summary of findings | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aOne study in this comparison bFenofibrate + benazepril and/or amlodipine versus benazepril and/or amlodipine cDowngraded one level due to high risk of bias for blinding of participants and personnel (open‐label study) dDowngraded two levels due to imprecision: few participants, few studies, and 95% CI consistent with possible benefit and possible harm
5. Additional SoF table: one lipid‐lowering agent compared to another lipid‐lowering agent for asymptomatic carotid stenosis.
| One lipid‐lowering agent compared to another lipid‐lowering agenta for asymptomatic carotid stenosis | |||||
|
Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: one lipid‐lowering agent Comparison: another lipid‐lowering agent | |||||
| Outcomes (measurement/time point) | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with another lipid‐lowering agent | Risk difference with one lipid‐lowering agent | ||||
| Neurological impairment | Neither included study measured this outcome. | ||||
| Ipsilateral major or disabling stroke (not reported) | 332 (1 RCT)b | ⨁◯◯◯ Very lowc,d | RR 2.96 (0.12 to 72.24) | 0 per 1000 | 0 fewer per 1000 (0 fewer to 0 fewer) |
| Stroke‐related mortality | Neither included study measured this outcome. | ||||
| Major bleeding | Neither included study measured this outcome. | ||||
| Progression of carotid stenosis | Neither included study measured this outcome. | ||||
| Adverse events (laboratory measurement/1, 2, 4, 6, 12, 18, and 24 months) | 497 (2 RCTs)e | ⨁◯◯◯ Very lowc,d | RR 0.92 (0.30 to 2.86) | 298 per 1000 | 24 fewer per 1000 (209 fewer to 555 more) |
| Quality of life | Neither included study measured this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RCT: randomised controlled trial; RR: risk ratio; SoF: summary of findings | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aTwo studies included in this comparison bRosuvastatin versus pravastatin cDowngraded one level due to high risk of bias (blinding) dDowngraded two levels due to imprecision: few events, few studies, and 95% CI consistent with possible benefit and possible harm eRosuvastatin versus pravastatin; probucol versus pravastatin
6. Additional SoF table: two lipid‐lowering agents compared to one lipid‐lowering agent for asymptomatic carotid stenosis.
| Two lipid‐lowering agents compared to one lipid‐lowering agenta for asymptomatic carotid stenosis | |||||
|
Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: two lipid‐lowering agents Comparison: one lipid‐lowering agent | |||||
|
Outcomes (measurement/time point) |
№ of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with one lipid‐lowering agent | Risk difference with two lipid‐lowering agents | ||||
| Neurological impairment | The included study did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke (not reported) | 683 (1 RCT)b | ⨁⨁◯◯ Lowc | RR 3.04 (0.12 to 74.46) | 0 per 1000 | 0 fewer per 1000 (0 fewer to 0 fewer) |
| Stroke‐related mortality | The included study did not measure this outcome. | ||||
| Major bleeding | The included study did not measure this outcome. | ||||
| Progression of carotid stenosis | The included study did not measure this outcome. | ||||
| Adverse events (not reported) | 683 (1 RCT)b | ⨁⨁◯◯ Lowc | RR 1.25 (0.61 to 2.56) | 38 per 1000 | 9 more per 1000 (15 fewer to 59 more) |
| Quality of life | The included study did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RCT: randomised controlled trial; RR: risk ratio; SoF: summary of findings | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aOne study included in this comparison bTorcetrapib plus atorvastatin versus atorvastatin alone cDowngraded two levels due to imprecision: few events, one study, and 95% CI consistent with possible benefit and possible harm
7. Additional SoF table: one antihypertensive agent compared to another antihypertensive agent for asymptomatic carotid stenosis.
| One antihypertensive agent compared to another antihypertensive agenta for asymptomatic carotid stenosis | |||||
|
Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: one antihypertensive agent Comparison: another antihypertensive agent | |||||
|
Outcomes (measurement/time point) |
№ of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with another antihypertensive agent | Risk difference with one antihypertensive agent | ||||
| Neurological impairment | The included studies did not measure this outcome. | ||||
| Ipsilateral major or disabling stroke (review meeting/semi‐annualb; review meeting/3 timesc) | 2918 (2 RCTs)d | ⨁⨁◯◯ Lowe | RR 0.99 (0.34 to 2.87) | 12 per 1000 | 0 fewer per 1000 (8 fewer to 22 more) |
| Stroke‐related mortality | The included studies did not measure this outcome. | ||||
| Major bleeding | The included studies did not measure this outcome. | ||||
| Progression of carotid stenosis | The included studies did not measure this outcome. | ||||
| Adverse events (only reported for two studies: one used review meeting/semi‐annualb; the other used review meeting/3 timesc) | 3239 (4 RCTs)f | ⨁⨁◯◯ Lowe | RR 1.00 (0.82 to 1.21) | 136 per 1000 | 0 fewer per 1000 (24 fewer to 29 more) |
| Quality of life | The included studies did not measure this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RCT: randomised controlled trial;RR: risk ratio; SoF: summary of findings | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aFour studies included in this comparison bApplegate 1991 cELSA 2002 dIsrapidin versus hydrochlorothiazide; lacidipine versus atenolol eDowngraded two levels due to imprecision: few events, few studies, and 95% CI consistent with possible benefit and possible harm fIsrapidin versus hydrochlorothiazide; lacidipine versus atenolol; olmesartan versus atenolol; amlodipine versus lisinopril
8. Additional SoF table: higher dose of lipid‐lowering agent compared to lower dose of the same lipid‐lowering agent for asymptomatic carotid stenosis.
| Higher dose of lipid‐lowering agent compared to lower dose of the same lipid‐lowering agenta for asymptomatic carotid stenosis | |||||
|
Patient or population: asymptomatic carotid stenosis Setting: outpatients Intervention: higher dose of lipid‐lowering agent Comparison: lower dose of the same lipid‐lowering agent | |||||
|
Outcomes (measurement/time point) |
№ of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with lower dose of the same lipid‐lowering agent | Risk difference with higher dose of lipid‐lowering agent | ||||
| Neurological impairment | Neither included study measured this outcome. | ||||
| Ipsilateral major or disabling stroke (not reported) |
40 (1 RCT)b | ⨁⨁◯◯ Lowc | RR 0.33 (0.01 to 7.72) | 50 per 1000 | 33 fewer per 1000 (50 fewer to 336 more) |
| Stroke‐related mortality | Neither included study measured this outcome. | ||||
| Major bleeding | Neither included study measured this outcome. | ||||
| Progression of carotid stenosis | Neither included study measured this outcome. | ||||
| Adverse events (laboratory measurements/baseline and 12 months) | 278 (1 RCT)d | ⨁◯◯◯ Very lowc,e | RR 1.57 (0.66 to 3.71) | 56 per 1000 | 32 more per 1000 (19 fewer to 153 more) |
| Quality of life | Neither included study measured this outcome. | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; №: number; RCT: randomised controlled trial;RR: risk ratio; SoF: summary of findings | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aTwo studies included in this comparison bPitavastatin cDowngraded two levels due to imprecision: few events, few studies, and 95% CI consistent with possible benefit and possible harm dAtorvastatin eDowngraded one level due to high risk of bias (blinding)
5.1. Analysis.

Comparison 5: Lipid‐lowering agent versus placebo or no treatment, Outcome 1: Ipsilateral major or disabling stroke
5.2. Analysis.

Comparison 5: Lipid‐lowering agent versus placebo or no treatment, Outcome 2: Stroke‐related mortality
5.3. Analysis.

Comparison 5: Lipid‐lowering agent versus placebo or no treatment, Outcome 3: Adverse events
7.2. Analysis.

Comparison 7: One lipid‐lowering agent versus another lipid‐lowering agent, Outcome 2: Adverse events
9.1. Analysis.

Comparison 9: One antihypertensive agent versus another antihypertensive agent, Outcome 1: Ipsilateral major or disabling stroke
9.2. Analysis.

Comparison 9: One antihypertensive agent versus another antihypertensive agent, Outcome 2: Adverse events
Outcomes
Although we included 34 studies, as noted above, only 22 had the outcomes of interest prespecified in our protocol (Clezar 2020).
Of the primary outcomes, we found data on ipsilateral major or disabling stroke in 14 studies (Applegate 1991; ELSA 2002; Bots 2007; Côté 1995; Furberg 1994; Hedblad 2001; Nohara 2012; Salonen 1995; Tang 2009; Yamada 2009; Zanchetti 2004; Zeng 2004; Zheng 2022; Zhu 2006), but we could not extract information on neurological impairment from any of the included studies.
Of the secondary outcomes, we found data for stroke‐related mortality in four studies (Côté 1995; Furberg 1994; Hedblad 2001; Salonen 1995), major bleeding in two studies (Côté 1995; Furberg 1994), progression of carotid stenosis in two studies (Côté 1995; Sutton‐Tyrrell 1994), and adverse events in 16 studies (Anderssen 2005; Applegate 1991; ELSA 2002; Bots 2007; Côté 1995; Crouse 2007; Furberg 1994; Ikeda 2013; Mercuri 1996; Nohara 2012; Sawayama 2002; Stumpe 2007; Terpstra 2004; Salonen 1995; Zheng 2022; Zhu 2006). We did not find information in the included studies about quality of life in people with asymptomatic carotid stenosis undergoing pharmacological treatment.
Excluded studies
We excluded 30 studies in total (Anand 2018; Bondjers 2000; Davidson 2012; Duman 2007; Esposito 2004; Fayad 2011; Hosomi 2001; Huang 2006; Ichihara 2006; Igase 2012; Ito 2004; Koeijvoets 2005; Laurora 1998; Ludwig 2002; Mazzone 2006; Meuwese 2009; Mizuguchi 2008; Mok 2010; Mortsell 2007; Oyama 2008; Persson 1996; Pontremoli 2001; Saremi 2013; Stanton 2001; Stumpe 1994; Tasić 2006; Vukusich 2010; Yamasaki 2010; Yilmaz 2004; Yokoyama 2005). In every case, the general reason for exclusion was an ineligible study population. In 24 of the excluded studies, participants had an intima‐media thickness (IMT) test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. Three of the excluded studies included participants with an IMT test value of greater than 1.3 mm (Ito 2004; Oyama 2008; Vukusich 2010). However, these studies did not subgroup participants by IMT test value, and we were unable to extract data specific to our population of interest. We excluded one study, Anand 2018, because less than 50% of the population was of interest and data on the subgroup of interest were unavailable. We excluded one study, Fayad 2011, because it did not evaluate plaque but rather the decrease in blood flow by volume per time (mL/minute). We excluded the final study, Stumpe 1994, because its exclusion criteria effectively meant that it excluded people with carotid stenosis.
Risk of bias in included studies
We provide information on risk of bias in the included studies in the Characteristics of included studies table, and summarise this information in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation (selection bias)
The randomisation of participants was adequate in 20 studies, and we assessed these as having a low risk of bias (Applegate 1991; ELSA 2002; Bots 2007; Côté 1995; Crouse 2007; Furberg 1994; Hedblad 2001; Ikeda 2013; Mercuri 1996; Nohara 2012; Salonen 1995; Sawayama 2002; Shinoda‐Tagawa 2002; Stumpe 2007; Sutton‐Tyrrell 1994; Terpstra 2004; Zheng 2022; Yamada 2009; Zanchetti 2004; Zeng 2004). However, the remaining 14 studies did not report the precise methodology of sequence generation, and we assessed these as having an unclear risk of bias in this domain.
Allocation
We assessed 17 of the included RCTs as having a low risk of bias with adequate allocation and concealment (Applegate 1991; ELSA 2002; Bots 2007; Côté 1995; Crouse 2007; Furberg 1994; Hedblad 2001; Ikeda 2013; Meaney 2009; Mercuri 1996; Nohara 2012; Salonen 1995; Shinoda‐Tagawa 2002; Stumpe 2007; Sutton‐Tyrrell 1994; Terpstra 2004; Zheng 2022).
The remaining 17 studies provided insufficient details for determining adequacy of the allocation process or its concealment; thus, we assessed them as having an unclear risk of bias (Anderssen 2005; Blanco‐Colio 2004; Corti 2005; Hu 2009; Kadoglou 2010; VHAS 1998; Norris 1990; Reid 2005; Sawayama 2002; Semplicini 2000; Tang 2009; Underhill 2008; Yamada 2009; Yamamoto 2011; Zanchetti 2004; Zeng 2004; Zhu 2006).
Blinding
Participant blinding (performance bias)
In 20 studies, both the participants and personnel were double‐blinded, so we assessed these studies as having a low risk of bias (Applegate 1991; ELSA 2002; Blanco‐Colio 2004; Bots 2007; Côté 1995; Crouse 2007; Furberg 1994; Hedblad 2001; Mercuri 1996; Norris 1990; Reid 2005; Salonen 1995; Semplicini 2000; Stumpe 2007; Sutton‐Tyrrell 1994; Tang 2009;Terpstra 2004; Underhill 2008; Zheng 2022; Zanchetti 2004). Only one study was single‐blinded (Anderssen 2005), and we assessed it as having a high risk of bias. A further eight studies were open‐label and, consequently, we also judged these to have a high risk of bias in this domain (Ikeda 2013; Kadoglou 2010; VHAS 1998; Meaney 2009; Nohara 2012; Yamada 2009; Yamamoto 2011; Zhu 2006).
We assessed five studies as having an unclear risk of performance bias because these studies did not report on blinding of participants and personnel (Corti 2005; Hu 2009; Sawayama 2002; Shinoda‐Tagawa 2002; Zeng 2004).
Investigator blinding (detection bias)
Thirty‐one of the 34 studies described blinded outcome assessment; we judged these studies to be at low risk of bias. Two studies did not report a blinded assessor (Blanco‐Colio 2004; Zeng 2004); we judged these to be at an unclear risk of bias. After six months of double‐blinding, participants in the VHAS 1998 study continued with treatment under an open‐label trial design; we thus assessed it as having a high risk of bias.
Incomplete outcome data
For 27 of the included RCTs, there were no serious issues relating to attrition at the end of the intervention, and we assessed these as having a low risk of bias arising from incomplete outcome data. We assessed the remaining seven studies to be at an unclear risk of bias due to incomplete outcome data as they did not report follow‐up participant data (Hu 2009; Norris 1990; Reid 2005; Semplicini 2000; Shinoda‐Tagawa 2002; Zeng 2004; Zhu 2006).
Selective reporting
For 30 of the 34 studies, there were no serious issues relating to reporting biases, and we judged these to be at low risk of bias. Three other studies did not report details about outcomes, and we assessed these as having an unclear risk of bias (Norris 1990; Shinoda‐Tagawa 2002; Zeng 2004). We assessed the one remaining study, Sutton‐Tyrrell 1994, to be at a high risk of bias. A weakness of this study (also known as the SHEP trial) was that the duplex scans were not obtained earlier in the study, before treatment. Unfortunately, the SHEP trial ended before all participants had completed their follow‐up scans.
Other potential sources of bias
We judged 33 studies to be at low risk of other potential sources of bias. However, we assessed the Zeng 2004 study as having an unclear risk of bias, as the study method was not reported.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
From the 34 studies identified for this review, we included 22 in the quantitative analysis. In addition, we performed a sensitivity analysis comparing a fixed‐effect versus random‐effects model for the outcome of 'ipsilateral major or disabling stroke' for the following comparisons only: 'lipid‐lowering agent versus placebo or no treatment' and 'one antihypertensive agent compared to another antihypertensive agent'.
1. Antiplatelet agent versus placebo
We identified one study for this comparison: Côté 1995, a Canadian trial from the early 1990s, compared the antiplatelet, acetylsalicylic acid (enteric‐coated aspirin), 325 mg per day, to placebo in 372 participants. It reported outcomes at six‐month intervals throughout the six‐year period. We assessed the overall risk of bias for Côté 1995 as low. This study did not measure two of our prespecified outcomes: the primary outcome of neurological impairment, and the secondary outcome of quality of life. See Table 1.
Primary outcomes
Ipsilateral major or disabling stroke
Acetylsalicylic acid may result in no difference in ipsilateral major or disabling stroke when compared to placebo (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.47 to 2.47; P = 0.86; 372 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Antiplatelet agent versus placebo, Outcome 1: Ipsilateral major or disabling stroke
Secondary outcomes
Stroke‐related mortality
Acetylsalicylic acid may result in no difference in stroke‐related mortality when compared to placebo (RR 1.40, 95% CI 0.54 to 3.59; P = 0.49; 372 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Antiplatelet agent versus placebo, Outcome 2: Stroke‐related mortality
Major bleeding
The effect of acetylsalicylic acid on major bleeding when compared to placebo is very uncertain (RR 0.98, 95% CI 0.06 to 15.53; P = 0.99; 372 participants; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Antiplatelet agent versus placebo, Outcome 3: Major bleeding
Progression of carotid stenosis
Acetylsalicylic acid may result in no difference in progression of carotid stenosis when compared to placebo (RR 1.16, 95% CI 0.79 to 1.71; P = 0.44; 372 participants; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Antiplatelet agent versus placebo, Outcome 4: Progression of carotid stenosis
Adverse events
Acetylsalicylic acid may result in no difference in adverse events when compared to placebo (RR 0.81, 95% CI 0.41 to 1.59, P = 0.53; 372 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Antiplatelet agent versus placebo, Outcome 5: Adverse events
2. Antihypertensive agent versus placebo
We included two studies in this comparison (Hedblad 2001; Sutton‐Tyrrell 1994), both conducted in the 1990s. Sutton‐Tyrrell 1994 (129 participants) compared chlorthalidone 12.5 mg daily to placebo, and obtained two serial duplex scans of the carotid arteries separated by two years. We assessed the overall risk of bias for Sutton‐Tyrrell 1994 as low. The Hedblad 2001 study randomised participants to placebo or 25 mg of metoprolol CR/XL (metoprolol succinate extended‐release tablets) once daily and measured changes in mean intima‐media thickness (IMT) in the common carotid artery. Also, Hedblad 2001 monitored adverse events, laboratory findings, mortality, and incidence of myocardial infarction and stroke for three years. We assessed the overall risk of bias for Hedblad 2001 as low. Neither included study measured four of our prespecified outcomes: the primary outcome of neurological impairment, and the secondary outcomes of major bleeding, adverse events, and quality of life. We were unable to perform a meta‐analysis or sensitivity analysis on this comparison because the studies reported different outcomes. See Table 2.
Primary outcomes
Ipsilateral major or disabling stroke
One study, Hedblad 2001, found that metoprolol may result in no difference in ipsilateral major or disabling stroke when compared to placebo (RR 0.14, 95% CI 0.02 to 1.16; P = 0.07; 793 participants; low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Antihypertensive agent versus placebo, Outcome 1: Ipsilateral major or disabling stroke
Secondary outcomes
Stroke‐related mortality
One study, Hedblad 2001, found that metoprolol may result in no difference in stroke‐related mortality when compared to placebo (RR 0.57, 95% CI 0.17 to 1.94; P = 0.37; 793 participants; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Antihypertensive agent versus placebo, Outcome 2: Stroke‐related mortality
Progression of carotid stenosis
One study, Sutton‐Tyrrell 1994, found that chlorthalidone may prevent progression of carotid stenosis when compared to placebo (RR 0.45, 95% CI 0.23 to 0.91; P = 0.02; 129 participants; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Antihypertensive agent versus placebo, Outcome 3: Progression of carotid stenosis
3. One antihypertensive agent plus lipid‐lowering agent versus another antihypertensive agent plus lipid‐lowering agent
We found one study for this comparison: Zanchetti 2004, with 254 participants in Italy, compared hydrochlorothiazide 25 mg per day versus fosinopril 20 mg per day, plus pravastatin 40 mg per day, concomitantly with open‐label nifedipine GITS (gastrointestinal therapeutic system), 30 to 60 mg daily. A complete carotid ultrasound examination was performed every six months for three years to assess changes in mean maximum IMT. The study evaluated changes in the clinic and ambulatory blood pressure and changes in serum total, low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) cholesterol, and other laboratory variables. We assessed the overall risk of bias for Zanchetti 2004 as low. This study did not measure six of our prespecified outcomes (namely, the primary outcome of neurological impairment, and the five secondary outcomes). See Table 7.
Primary outcomes
Ipsilateral major or disabling stroke
One antihypertensive agent plus lipid‐lowering agent (hydrochlorothiazide plus pravastatin) may result in little to no difference in ipsilateral major or disabling stroke when compared to another antihypertensive agent plus lipid‐lowering agent (fosinopril plus pravastatin) (RR 0.34, 95% CI 0.01 to 8.23; P = 0.51; 254 participants; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: One antihypertensive agent plus lipid‐lowering agent versus another antihypertensive agent plus lipid‐lowering agent, Outcome 1: Ipsilateral major or disabling stroke
4. Anticoagulant agent versus placebo
We included one study for this comparison. Furberg 1994 compared warfarin, administered at a fixed 1 mg daily, to placebo in 919 participants from the USA in the 1990s with a mean follow‐up of three years. Regular clinic visits were scheduled every six weeks for the first 15 months and quarterly thereafter to permit safety monitoring. The study reported all outcomes at six‐month intervals throughout the six‐year period. Trialists conducted B‐mode ultrasonography semi‐annually and alanine aminotransferase (ALT) and urine tests at every visit. Drug adherence was assessed by pill count and participant report of usage. The annual visits involved a brief physical examination and dietary assessment. We assessed the overall risk of bias for Furberg 1994 as low. This study did not measure five of our prespecified outcomes: neither of the primary outcomes, and the secondary outcomes of stroke‐related mortality, progression of carotid stenosis, and quality of life. See Table 3.
Secondary outcomes
Major bleeding
The effect of warfarin on major bleeding when compared to placebo is uncertain (RR 1.19, 95% CI 0.97 to 1.46; P = 0.10; 919 participants; very low‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Anticoagulant agent versus placebo, Outcome 1: Major bleeding
Adverse events
Warfarin may reduce adverse events when compared to placebo (RR 0.89, 95% CI 0.81 to 0.99; P = 0.04; 919 participants; low‐certainty evidence; Analysis 4.2).
4.2. Analysis.

Comparison 4: Anticoagulant agent versus placebo, Outcome 2: Adverse events
5. Lipid‐lowering agent versus placebo or no treatment
We identified nine studies for this comparison (Anderssen 2005; Crouse 2007; Furberg 1994; Mercuri 1996; Salonen 1995; Sawayama 2002; Yamada 2009; Zeng 2004; Zheng 2022). Six different lipid‐lowering agents were investigated by these studies: fluvastatin, rosuvastatin, lovastatin, atorvastatin, probucol, and pravastatin. They provided data for short‐ and long‐term outcomes (ranging from six months to six years after the beginning of the intervention) for 3916 participants from Japan, China, USA, and Europe (Norway, Italy, and Finland) in the 1990s, 2000s, and 2010s. The studies ranged in duration from two to six years. They assessed a wide range of physiological, biochemical, and clinical outcomes. We assessed seven studies as having a low overall risk of bias, one as having an unclear risk of bias (Zeng 2004), and the remaining study as having an overall high risk of bias (Yamada 2009). None of these included studies measured our prespecified primary outcome of neurological impairment, and three of our secondary outcomes (major bleeding, progression of carotid stenosis, and quality of life). See the Characteristics of included studies table for details of individual studies and Table 4.
Primary outcomes
Ipsilateral major or disabling stroke
Five studies assessed this outcome (Furberg 1994; Salonen 1995; Yamada 2009; Zeng 2004; Zheng 2022). Lipid‐lowering agents (lovastatin, pravastatin, atorvastatin, rosuvastatin) may result in no difference in ipsilateral major or disabling stroke when compared to placebo or no treatment (RR 0.36, 95% CI 0.09 to 1.53; P = 0.13, I2 = 44%; 5 studies, 2235 participants; low‐certainty evidence; Analysis 5.1). A sensitivity analysis using a fixed‐effect model changed the effect estimate substantially (RR 0.39, 95% CI 0.18 to 0.87; Figure 5).
5.
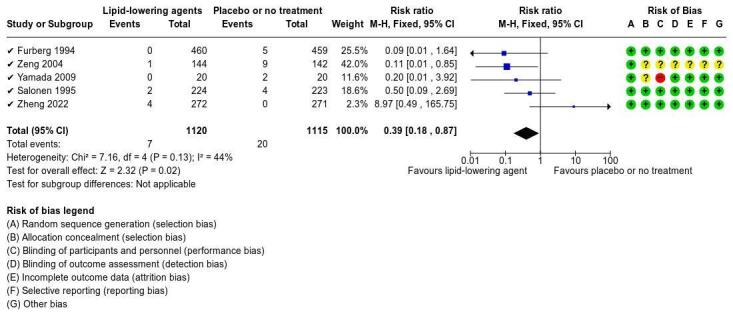
Sensitivity analysis (Ipsilateral major or disabling stroke): fixed effect.
Secondary outcomes
Stroke‐related mortality
Two studies assessed this outcome (Furberg 1994; Salonen 1995). Lipid‐lowering agents (lovastatin and pravastatin) may result in no difference in stroke‐related mortality when compared to placebo or no treatment (RR 0.25, 95% CI 0.03 to 2.29; P = 0.82; 2 studies, 1366 participants; low‐certainty evidence; Analysis 5.2).
Adverse events
Seven studies assess this outcome (Anderssen 2005; Crouse 2007; Furberg 1994; Mercuri 1996; Salonen 1995; Sawayama 2002; Zheng 2022). Lipid‐lowering agents (probucol, pravastatin, lovastatin, fluvastatin, rosuvastatin) may result in no difference in adverse events when compared to placebo or no treatment (RR 0.76, 95% CI 0.53 to 1.10; P = 0.04, I2 = 54%; 7 studies, 3726 participants; low‐certainty evidence; Analysis 5.3).
6. Lipid‐lowering agent plus antihypertensive agent versus antihypertensive agent
We included one study in this comparison. Zhu 2006 compared 160 mg of micronised fenofibrate daily plus antihypertensive drug therapy (benazepril 10 to 20 mg/day and/or amlodipine 5 to 10 mg/day) to only antihypertensive drug therapy (benazepril 10 to 20 mg/day and/or amlodipine 5 to 10 mg/day). The study reported all outcomes at the end of the observation period (two years). This study did not measure five of our prespecified outcomes: the primary outcome of neurological impairment, and the secondary outcomes of stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life. We assessed the overall risk of bias as high. See Table 8.
Primary outcomes
Ipsilateral major or disabling stroke
It is uncertain whether fenofibrate plus benazepril and/or amlodipine prevent ipsilateral major or disabling stroke when compared to benazepril and/or amlodipine alone (RR 0.64, 95% CI 0.27 to 1.50; P = 0.30; 225 participants; very low‐certainty evidence; Analysis 6.1).
6.1. Analysis.

Comparison 6: Lipid‐lowering agent plus antihypertensive agent versus antihypertensive agent, Outcome 1: Ipsilateral major or disabling stroke
Secondary outcomes
Adverse events
It is uncertain whether fenofibrate plus benazepril and/or amlodipine increase adverse events when compared to benazepril and/or amlodipine alone (RR 20.09, 95% CI 1.19 to 338.84; P = 0.04; 225 participants; very low‐certainty evidence; Analysis 6.2).
6.2. Analysis.

Comparison 6: Lipid‐lowering agent plus antihypertensive agent versus antihypertensive agent, Outcome 2: Adverse events
7. One lipid‐lowering agent versus another lipid‐lowering agent
We included two studies in this comparison (Nohara 2012; Sawayama 2002). Nohara 2012 compared 5 mg rosuvastatin once daily to 10 mg pravastatin once daily. It was an open‐label study, with blinded end‐point evaluation, and we assessed it at high risk of bias. Sawayama 2002 compared probucol 500 mg twice daily to pravastatin 10 mg/day, and we assessed the overall risk of bias as low. They provided data for long‐term outcomes in 650 participants from Japan and Mexico for one to two years, during the 1990s and 2000s. Both studies assessed a wide range of biochemical and clinical outcomes. Neither of the included studies for this comparison measured our primary outcome (i.e. neurological impairment) or four of our secondary outcomes (stroke‐related mortality, major bleeding, progression of carotid stenosis, or quality of life). We were unable to perform a meta‐analysis or sensitivity analysis on the primary outcome ipsilateral major or disabling stroke because only one of the two studies measured this outcome. See Table 9.
Primary outcomes
Ipsilateral major or disabling stroke
One study, Nohara 2012, measured this outcome. It is uncertain whether rosuvastatin results in any difference in ipsilateral major or disabling stroke when compared to pravastatin (RR 2.96, 95% CI 0.12 to 72.24, P = 0.50; 332 participants; very low‐certainty evidence; Analysis 7.1).
7.1. Analysis.

Comparison 7: One lipid‐lowering agent versus another lipid‐lowering agent, Outcome 1: Ipsilateral major or disabling stroke
Secondary outcomes
Adverse events
It is uncertain whether rosuvastatin or probucol results in any difference in adverse events when compared to pravastatin (RR 0.92, 95% CI 0.30 to 2.86; P = 0.03, I2 = 80%; 2 studies, 497 participants; very low‐certainty evidence; Analysis 7.2).
8. Two lipid‐lowering agents compared to one lipid‐lowering agent
We found one study for this comparison. Bots 2007 compared torcetrapib 60 mg plus atorvastatin 10, 20, 40, or 80 mg per day to atorvastatin 10, 20, 40, or 80 mg per day in 683 participants in 64 centres in North America and Europe (Canada, USA, Czech Republic, Finland, France and the Netherlands) in the 2000s. This study was prematurely terminated as all torcetrapib clinical trials were stopped. Therefore, 48 participants who were still receiving torcetrapib were contacted and instructed to discontinue treatment immediately and return for final evaluation that same month. This study did not measure five of our prespecified outcomes: the primary outcome of neurological impairment, and four of the secondary outcomes (stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life). See Table 10.
Primary outcomes
Ipsilateral major or disabling stroke
Two lipid‐lowering agents (torcetrapib plus atorvastatin) may result in no difference in ipsilateral major or disabling stroke when compared to one lipid‐lowering agent (atorvastatin) (RR 3.04, 95% CI 0.12 to 74.46; P = 0.49; 683 participants; low‐certainty evidence; Analysis 8.1).
8.1. Analysis.

Comparison 8: Two lipid‐lowering agents versus one lipid‐lowering agent, Outcome 1: Ipsilateral major or disabling stroke
Secondary outcomes
Adverse events
Two lipid‐lowering agents (torcetrapib plus atorvastatin) may result in no difference in adverse events when compared to one lipid‐lowering agent (atorvastatin) (RR 1.25, 95% CI 0.61 to 2.56; P = 0.54; 683 participants; low‐certainty evidence; Analysis 8.2).
8.2. Analysis.

Comparison 8: Two lipid‐lowering agents versus one lipid‐lowering agent, Outcome 2: Adverse events
9. One antihypertensive agent compared to another antihypertensive agent
We identified four studies for this comparison (Applegate 1991; ELSA 2002; Stumpe 2007; Terpstra 2004). These studies ranged in duration from two to four years, and in participant numbers from 165 to 2035. Applegate 1991 compared 2.5 mg or 5 mg isradipine twice daily to 12.5 mg or 25 mg hydrochlorothiazide twice daily. ELSA 2002 compared lacidipine 4 mg once daily to atenolol 50 mg once daily. Stumpe 2007 compared olmesartan 20 mg once a day to atenolol 50 mg daily. Terpstra 2004 compared amlodipine 5 mg to lisinopril 10 mg. We assessed the overall risk of bias for the four studies as low. These studies assessed a wide range of imaging and clinical outcomes. However, none measured five of our prespecified outcomes: the primary outcome of neurological impairment, and the secondary outcomes of stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life. See Table 11.
Primary outcomes
Ipsilateral major or disabling stroke
Two studies measured this outcome (Applegate 1991; ELSA 2002). One antihypertensive agent (isradipine or lacidipine) may result in little to no difference in ipsilateral major or disabling stroke when compared to another antihypertensive agent (hydrochlorothiazide or atenolol) (RR 0.99, 95% CI 0.34 to 2.87; P = 0.17, I2 = 46%; 2 studies, 2918 participants; low‐certainty evidence; Analysis 9.1). A sensitivity analysis using a fixed‐effect model did not change the effect estimate substantially (RR 0.88, 95% CI 0.43 to 1.79; Figure 6).
6.
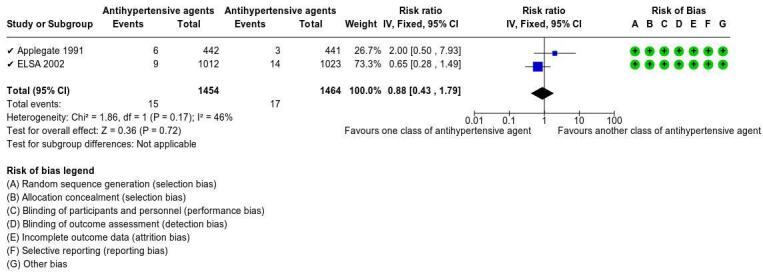
Sensitivity analysis (Ipsilateral major or disabling stroke): fixed effect.
Secondary outcomes
Adverse events
One antihypertensive agent (isradipine, lacidipine, olmesartan, or amlodipine) may result in little to no difference in adverse events when compared to another antihypertensive agent (hydrochlorothiazide, atenolol, or lisinopril) (RR 1.00, 95% CI 0.82 to 1.21; P = 0.38, I2 = 3%; 4 studies, 3239 participants; low‐certainty evidence; Analysis 9.2).
10. Higher dose of lipid‐lowering agent compared to low dose of the same lipid‐lowering agent
We found two studies for this comparison (Ikeda 2013;Tang 2009). Ikeda 2013 compared pitavastatin at different doses. Outcomes were measured after 12 months. This was an open‐label study, with a blinded end‐point evaluation, and we assessed the performance bias domain as high risk of bias. Tang 2009 compared 80 mg atorvastatin once daily to 10 mg atorvastatin once daily. We assessed the overall risk of bias for as low. These studies evaluated 573 participants from the USA, Japan, Greece, and the UK in the 2000s with four months to two years of follow‐up. The two studies assessed a wide range of imaging and clinical outcomes. Neither included study measured five of our prespecified outcomes: the primary outcome of neurological impairment, and four of the secondary outcomes (stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life). We were unable to perform a meta‐analysis or sensitivity analysis on this comparison because the studies reported different outcomes. See Table 12.
Primary outcomes
Ipsilateral major or disabling stroke
One study measured this outcome (Tang 2009). A higher dose of a lipid‐lowering agent (atorvastatin 80 mg) may result in no difference in ipsilateral major or disabling stroke when compared to a lower dose of the same lipid‐lowering agent (atorvastatin 10 mg) (RR 0.33, 95% CI 0.01 to 7.72; P = 0.49; 40 participants; low‐certainty evidence; Analysis 10.1).
10.1. Analysis.

Comparison 10: Higher dose of lipid‐lowering agent versus lower dose of the same lipid‐lowering agent, Outcome 1: Ipsilateral major or disabling stroke
Secondary outcomes
Adverse events
One study measured this outcome (Ikeda 2013). It is uncertain whether a higher dose of a lipid‐lowering agent (pitavastatin 3 (± 1.2) mg) results in any difference in adverse events when compared to a lower dose of the same lipid‐lowering agent (pitavastatin 1.9 (± 0.8) mg) (RR 1.57, 95% CI 0.66 to 3.71; P = 0.31; 278 participants; very low‐certainty evidence; Analysis 10.2).
10.2. Analysis.

Comparison 10: Higher dose of lipid‐lowering agent versus lower dose of the same lipid‐lowering agent, Outcome 2: Adverse events
Discussion
This review aimed to assess the effects of pharmacological interventions on preventing neurological impairment, ipsilateral major or disabling stroke, death, major bleeding, and other outcomes in people with asymptomatic carotid stenosis.
Summary of main results
We included 34 randomised controlled trials (RCTs) in total in the review; of these, we included 22 in the quantitative analysis. These studies compared different pharmacological interventions, such as antiplatelet agents, anticoagulant agents, lipid‐lowering agents, and antihypertensive agents. Three of the included studies were multi‐armed trials (Furberg 1994; Hedblad 2001; Sawayama 2002). We identified one ongoing study (Aranzulla 2021).
Of the included studies, 12 did not assess any of our prespecified outcomes (Clezar 2020). The other 22 studies provided data for 10 different comparisons. However, these studies did not assess all of our outcomes of interest, including neurological impairment and quality of life.
A sensitivity analysis comparing fixed‐effect versus random‐effects models was only possible for the outcome of 'ipsilateral major or disabling stroke', in just two comparisons: 'lipid‐lowering agent versus placebo or no treatment' and 'one class of antihypertensive agent compared to another class of antihypertensive agent'.
Antiplatelets agents
One included Canadian study from the early 1990s (Côté 1995), which compared an antiplatelet agent versus placebo in people with asymptomatic carotid stenosis, provided data for our protocol‐proposed outcomes. This study showed that antiplatelet agents may result in no difference for ipsilateral major or disabling stroke, stroke‐related mortality, progression of carotid stenosis, and adverse events (all low‐certainty evidence). The effect of antiplatelet agents on major bleeding when compared to placebo was very uncertain and the certainty of the evidence was very low (Table 1). There were no data regarding neurological impairment or quality of life.
Lipid‐lowering agents
We found five different comparisons of lipid‐lowering agents, involving 23 studies, 15 of which measured outcomes predefined in our protocol (Clezar 2020).
The most common comparison in studies in lipid‐lowering agents was with placebo or no treatment (ranging from six weeks to five years after the beginning of the intervention) (Anderssen 2005; Blanco‐Colio 2004; Crouse 2007; Hu 2009; Furberg 1994; Hedblad 2001; Mercuri 1996; Reid 2005; Salonen 1995; Sawayama 2002; Yamada 2009; Zheng 2022; Zeng 2004). Data from nine studies showed that lipid‐lowering agents may result in no difference in ipsilateral major or disabling stroke, stroke‐related mortality, and adverse events when compared to placebo or no treatment (all low‐certainty evidence; Table 4). A sensitivity analysis using the fixed‐effect model changed the effect estimate substantially for ipsilateral major or disabling stroke (Figure 5). Neurological impairment, major bleeding, progression of carotid stenosis, and quality of life were not reported.
Another five studies compared two different doses of the same lipid‐lowering agent (Corti 2005; Ikeda 2013; Kadoglou 2010; Tang 2009; Underhill 2008), of which only two assessed our outcomes of interest. Tang 2009 showed that a higher dose of a lipid‐lowering agent may result in no difference in ipsilateral major or disabling stroke when compared to a lower dose of a lipid‐lowering agent (low‐certainty evidence). Ikeda 2013 suggested that it is uncertain whether a higher dose of lipid‐lowering agents results in any difference in adverse events when compared to a lower dose of the same lipid‐lowering agent (very low‐certainty evidence; Table 12). Neurological impairment, stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life outcomes were not reported in either trial. The three other studies did not assess any of the outcomes prespecified in our protocol.
Three studies compared different lipid‐lowering agents (Meaney 2009; Nohara 2012; Sawayama 2002). All three studies administered pravastatin. However, Meaney 2009 did not assess any of our prespecified outcomes, and thus was not included in the quantitative analysis. It is uncertain whether one lipid‐lowering agent results in any difference in ipsilateral major or disabling stroke or an increase in adverse events when compared to another lipid‐lowering agent. In both cases, the certainty of the evidence was very low (Table 9). Neither study assessed neurological impairment, stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life.
One study compared two lipid‐lowering agents to one lipid‐lowering agent (Bots 2007). The findings from this study indicated that two lipid‐lowering agent may result in no difference in ipsilateral major or disabling stroke and adverse events when compared to one lipid‐lowering agent (both low‐certainty evidence; Table 10). Bots 2007 did not assess neurological impairment, stroke‐related mortality, major bleeding, progression of carotid stenosis, and quality of life.
The last comparison involving lipid‐lowering agents was in the Zhu 2006 study. Based on this study's results, it is uncertain whether a lipid‐lowering agent plus antihypertensive agent prevent ipsilateral major or disabling stroke or increase adverse events when compared to an antihypertensive agent alone; the certainty of the evidence was very low for both outcomes (Table 8). No other outcome of interest was reported in this comparison.
Anticoagulant agents
Furberg 1994 compared warfarin to placebo. An anticoagulant agent may reduce adverse events (low‐certainty evidence), but the effect of anticoagulants on major bleeding when compared to placebo is uncertain and the certainty of the evidence was very low (Table 3). This study did not assess neurological impairment, ipsilateral major or disabling stroke, stroke‐related mortality, progression of carotid stenosis, and quality of life.
Another trial that compared anticoagulant agents was Shinoda‐Tagawa 2002. It compared cilostazol (100 to 200 mg daily) to no treatment in 89 Japanese participants for three years. This study did not report any of our prespecified outcomes.
Antihypertensive agents
Eleven included trials studied an antihypertensive agent, accounting for four different comparisons outlined below: (1) antihypertensive agent versus placebo; (2) one antihypertensive agent versus another antihypertensive agent; (3) one antihypertensive agent plus a lipid‐lowering agent versus another antihypertensive agent; and (4) an antihypertensive agent (metoprolol) plus aspirin versus placebo.
Two studies compared an antihypertensive agent to placebo (Hedblad 2001; Sutton‐Tyrrell 1994). Hedblad 2001 assessed two of our prespecified outcomes (ipsilateral major or disabling stroke; stroke‐related mortality), and Sutton‐Tyrrell 1994 assessed only progression of carotid stenosis. Based on data from these studies, an antihypertensive agent may result in no difference in ipsilateral major or disabling stroke and stroke‐related mortality, but may prevent the progression of carotid stenosis when compared to placebo (all low‐certainty evidence; Table 2). These studies did not assess neurological impairment, major bleeding, adverse events, and quality of life.
Seven studies compared two different antihypertensive agents (Applegate 1991; ELSA 2002; Semplicini 2000; Stumpe 2007; Terpstra 2004; VHAS 1998; Yamamoto 2011). However, only four of these assessed any of our prespecified outcomes (Applegate 1991; ELSA 2002; Stumpe 2007; Terpstra 2004). Applegate 1991 and ELSA 2002 reported data on ipsilateral major or disabling stroke; all four studies presented data on adverse events (Stumpe 2007; Terpstra 2004). We were thus able to perform meta‐analysis for two prespecified outcomes. Antihypertensive agents may result in no difference in ipsilateral major or disabling stroke and adverse events when compared to another antihypertensive agent (both low‐certainty evidence; Table 11). A sensitivity analysis using a fixed‐effect model did not change the effect estimate substantially for ipsilateral major or disabling stroke (Figure 6).
Only one included study, with 254 participants, compared an antihypertensive agent plus a lipid‐lowering agent to another antihypertensive agent plus a lipid‐lowering agent (Zanchetti 2004); it reported one of our outcomes of interest. An antihypertensive agent plus a lipid‐lowering agent may result in little to no difference in ipsilateral major or disabling stroke when compared to another antihypertensive agent plus a lipid‐lowering agent (low‐certainty evidence; Table 7).
The remaining study compared an antihypertensive agent (metoprolol) plus aspirin to placebo in 162 participants (Norris 1990). We could not extract any usable data from this study and our attempt to obtain raw data directly from the trial authors was unsuccessful.
Overall completeness and applicability of evidence
In this systematic review, we focused on people of any age with asymptomatic carotid stenosis, to provide information about the effects of different classes of drugs in cardiovascular outcomes, including the prevention of neurological impairment, stroke, adverse effects, major bleeding, and quality of life. We included only RCTs.
Study design
Our extensive search for RCTs investigating pharmacological interventions for asymptomatic carotid stenosis identified only 34 studies with our predefined interventions.
Although all studies were RCTs, most did not provide complete and clear information about their methodology or data. As a result, it was difficult to perform quantitative analyses and assess the risk of bias for many outcomes in some studies. Furthermore, there were only one to nine studies in each comparison, and most comparisons had only one or two of the included studies.
Population
The randomised population ranged between 18 and 100 years of age, with the mean age in the 60‐year age group. Most participants were men. Both of these features are consistent with the epidemiology of the disease.
Most of our included studies had relatively low participant numbers: 25 studies had up to 500 participants, 12 of which had fewer than 100 participants; seven studies had between 500 and 1000 participants; and just two studies had more than 1000 participants.
Intervention
In two of our five interventions of interest – namely, lipid‐lowering and antihypertensive agents – there was considerable variation in the use of the intervention (e.g. dosages, different agents, association with other agents). In two other interventions – antiplatelet agents and anticoagulant agents – there were fewer studies and the intervention was limited to the standard dosage of the agent or was associated with another agent. Notably, we did not include any studies with one of our interventions of interest: glycaemic‐lowering agents.
Setting
The studies included in this review were carried out in 21 different countries, with most (90%) being high‐income countries. Three of the included studies were multicentric.
It should be remembered that various factors, such as socioeconomic conditions, access to physical activity, type of food and cuisine, and culture of the population of each country, may interfere with the acceptability and effectiveness of pharmacological treatments of asymptomatic carotid stenosis. Hence, the external validity of the general evidence presented in this review should be considered with caution.
Outcomes
None of the included studies reported our primary outcome of neurological impairment. Only 14 of 34 studies reported ipsilateral major or disabling stroke in the different comparisons. Of our secondary outcomes, four studies reported stroke‐related mortality, two other studies detailed major bleeding, two reported progression of carotid stenosis, and 16 reported adverse events. However, we found no studies that evaluated the impact of pharmacological interventions on quality of life.
Certainty of the evidence
The evidence for this review came from RCTs, but some studies had methodological problems, poorly‐reported study designs, or both. Randomisation and allocation were adequately reported in almost half of the trials; we judged the remaining as having an unclear risk of bias in these domains. We judged nine open‐label studies as having a high risk of bias due to not blinding participants and personnel, and another five studies as unclear. However, 20 trials were blinded to participants and personnel, avoided performance bias as much as possible, and were adequately reported. Furthermore, only two RCTs did not report the blinding of outcome assessment; we assessed these as having an unclear risk of bias for this domain. Also, we considered seven studies as having an unclear risk of attrition bias with incomplete outcomes, and three as having an unclear risk of reporting bias. We assessed only one trial as having a high risk of selective reporting bias.
The certainty of the evidence for our outcomes ranged from low to very low. We downgraded the certainty of the evidence due to the risk of bias in four RCTs, mainly regarding the blinding of participants and personnel, as these studies were open‐label. Also, we downgraded the certainty of the evidence for all outcomes for imprecision because of the small number of participants in the trials, the few studies in each comparison, and large confidence intervals. Moreover, two trials were imprecise in their definitions of bleeding, which led to downgrading the evidence certainty of the major bleeding outcome.
There are numerous clinical guidelines on and RCTs investigating treatments for asymptomatic carotid stenosis. However, there is still no high‐certainty evidence about the best pharmacological treatment for people with asymptomatic carotid stenosis.
Potential biases in the review process
We performed an unrestricted literature search and followed guidance in the Cochrane Handbook for Systematic Reviews of Interventions in our selection of studies (Lefebvre 2022). We believe that we identified all relevant studies meeting our inclusion criteria. However, there is the possibility that some studies may have been missed, especially in the grey literature.
We designed and published our protocol for studying pharmacological interventions in asymptomatic carotid stenosis prior to data collection and analysis (Clezar 2020), and we adhered to the prespecified inclusion and exclusion criteria in the protocol to limit subjectivity. We did not include non‐randomised studies due to their high vulnerability to error and bias. Also, we attempted to contact study authors in order to obtain additional relevant data but were unable to do so with the included studies. If we are able to collect supplemental data, we will consider it in future updates.
Agreements and disagreements with other studies or reviews
Systematic reviews of interventions
To the best of our knowledge, there are no systematic reviews that compare pharmacological treatments with placebo, no treatment, or another pharmacological intervention for asymptomatic carotid stenosis. However, there are three systematic reviews comparing pharmacological treatments with surgical treatment in people with carotid artery stenosis (Gasior 2023; Müller 2021; Raman 2013). Gasior 2023 compared the effects of pharmacological treatment with invasive carotid endarterectomy and carotid artery stenting in people with asymptomatic carotid artery stenosis. They found evidence that contemporary pharmacological treatment shows similar reductions in stroke and carotid endarterectomy mortality. Furthermore, pharmacological treatment has the potential to reduce the need for surgical intervention in people with asymptomatic carotid stenosis. Müller 2021 reviewed available evidence from randomised clinical trials comparing pharmacological treatment with surgical treatment (both carotid artery stenting and carotid endarterectomy) in people with symptomatic and asymptomatic carotid stenosis. They found that carotid artery stenting may slightly increase the risk of stroke or death up to 30 days after treatment compared with carotid endarterectomy in asymptomatic patients. Raman 2013 reviewed RCT and non‐randomised study evidence for three different treatment strategies for asymptomatic carotid stenosis: pharmacological therapy alone, carotid endarterectomy plus pharmacological therapy, and carotid artery stenting plus pharmacological therapy. They also examined single‐group prospective cohort studies of pharmacological therapy to measure stroke incidence. They found evidence from three studies that carotid endarterectomy reduces the risk of ipsilateral stroke when compared to pharmacological treatment, but cautioned that these results may no longer be applicable to current clinical practice as they are from older studies. No study in their review compared carotid artery stenting with pharmacological therapy.
Clinical guidelines and systematic reviews of clinical guidelines
There are some systematic reviews of guidelines for the primary and secondary prevention of stroke, which encompass both surgical (carotid endarterectomy and carotid angioplasty/stenting) and pharmacological treatment.
Abbott 2015 systematically searched for guidelines with recommendations on carotid endarterectomy and carotid angioplasty/stenting between January 2008 and 2015, published in any language. This review highlighted limitations in terms of the clarity, accessibility, organisation, and consistency of the recommendations, and also in terms of the currency of the scientific evidence used in these guidelines and protocols. The literature was outdated, as the studied therapies have undergone several modifications over the last 30 years (Abbott 2015). As we observed in our review, most of the studies that evaluated pharmacological treatment in asymptomatic carotid stenosis are from the 1990s and 2000s, with a lot of emphasis on lipid‐lowering agents and less emphasis on antihypertensive agents. We also found few studies that assessed anticoagulants and antiplatelet drugs and no studies on hypoglycaemic agents and how diabetes management can impact these patients.
All protocols and guidelines regarding the treatment of asymptomatic carotid stenosis are informed by clinical trials on carotid endarterectomy, carotid angioplasty/stenting, and pharmacological treatment, most of which were conducted 20 to 40 years ago. Many of these studies, including the Veterans Affairs Cooperative Study Group, the Asymptomatic Carotid Atherosclerosis Study (ACAS) study, and the ACST‐1 (Asymptomatic Carotid Surgery Trial) study, were not supportive of pharmacological treatment because they were conducted at a time when a minority of participants were using lipid‐lowering agents and blood pressure targets were not as low as they are today. However, our review shows that antihypertensive drugs can reduce the risk of progression of carotid stenosis and lipid‐lowering drugs can reduce the risk of major or disabling stroke.
Consequently, new RCTs are required to legitimise current guidelines. At present, there are a few ongoing studies for asymptomatic carotid stenosis involving the use of carotid endarterectomy/carotid artery stenting and pharmacological treatment, including the Carotid Revascularization Endarterectomy versus Stent Trial 2 (Howard 2017).
Authors' conclusions
Implications for practice.
There is limited evidence to inform decision‐making about the use of pharmacological interventions in asymptomatic carotid artery stenosis. There is no evidence currently available from randomised controlled trials about the effects of pharmacological interventions on neurological impairment and quality of life.
Antiplatelets, lipid‐lowering drugs, and the antihypertensive drug, metoprolol, may have little to no effect on stroke and stroke‐related death.
Antiplatelets and lipid‐lowering medications may have little to no effect on side effects, and antiplatelets may have little to no effect on the progression of carotid narrowing.
Anticoagulants in people with asymptomatic carotid stenosis may decrease the risk of adverse events by 11% compared to placebo.
Chlorthalidone – an antihypertensive drug – may decrease the risk of progression of carotid stenosis by 55% compared to placebo.
The evidence of the effects of antiplatelets and anticoagulants on major bleeding is very uncertain.
Therefore, this restricted evidence should not be interpreted as demonstrating the ineffectiveness of pharmacological treatment for asymptomatic carotid stenosis, but rather highlights a need for more trials. In the interim, clinicians will have to use information from other prevention trials to help guide decision‐making.
Implications for research.
There is no high‐quality evidence on pharmacological interventions to prevent stroke and its sequelae.
Given the lack of evidence, randomised controlled trials involving more participants (at least 4000 in total) and with a minimum follow‐up of two years are needed to assess cardiovascular changes and events over the long term in people with atherosclerosis. Studies should focus on the following outcomes: neurological impairment, mortality, and changes in quality of life.
Adherence to pharmacological interventions remains an issue, even in high‐income countries and even when people are participating in randomised controlled trials (Haley 2021). Researchers should thus anticipate and try to address this problem when developing new trial protocols.
Most data in our review come from high‐income countries. Data from under‐represented continents, particularly Africa, and from participants with different social and economic characteristics are warranted, to enhance external validity and translate evidence into practice.
History
Protocol first published: Issue 4, 2020
Acknowledgements
We thank Cochrane Stroke, Cochrane Brazil, and the Division of Vascular and Endovascular Surgery of Universidade Federal de São Paulo, Brazil, for their methodological support.
The following people conducted the editorial process:
Sign‐off Editor (final editorial decision): Peter Langhorne, University of Glasgow
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article, conducted editorial policy checks, and supported editorial team): Hazel Fraser, Cochrane Stroke.
Peer reviewers (provided comments and recommended an editorial decision): Peter Langhorne, University of Glasgow (clinical/content review and methods review), Dr Amanda Barugh, Associate Editor Cochrane Stroke (clinical/content review), Aryelly Rodriguez, Edinburgh Clinical trials unit (ECTU) at the University of Edinburgh (statistical review).
Two further peer reviewers provided comments on the review but chose not to be publicly acknowledged.
Parts of the methods section of this review are based on a standard template established by Cochrane.
Appendices
Appendix 1. Search strategies
CENTRAL search strategy
Cochrane Central Register of Controlled Trials (Issue 4 of 9, August 2022; last searched 9 August 2022); n = 758
#1MeSH descriptor: [Carotid Artery Diseases] this term only #2MeSH descriptor: [Carotid Artery Thrombosis] this term only #3MeSH descriptor: [Carotid Stenosis] this term only #4MeSH descriptor: [Carotid Arteries] this term only #5MeSH descriptor: [Carotid Artery, Common] this term only #6MeSH descriptor: [Carotid Artery, External] this term only #7MeSH descriptor: [Carotid Artery, Internal] this term only #8{or #1‐#7} #9MeSH descriptor: [Asymptomatic Diseases] explode all trees #10(asymptomatic):ti,ab,kw #11#9 or #10 #12#8 AND #11
MEDLINE (Ovid) search strategy
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to August 9, 2022; last searched 9 August 2022); n = 1901
1. carotid artery diseases/ or carotid artery thrombosis/ or carotid stenosis/ 2. carotid arteries/ or carotid artery, common/ or carotid artery, external/ or carotid artery, internal/ 3. (carotid adj5 (stenosis or thrombo$ or disease$ or narrow$ or plaque$ or arterioscler$ or atheroscler$)).tw. 4. or/1‐3 5. exp Asymptomatic Diseases/ 6. asymptomatic.tw. 7. 5 or 6 8. 4 and 7 9. randomized controlled trial.pt. 10. controlled clinical trial.pt. 11. randomized.ab. 12. placebo.ab. 13. randomly.ab. 14. trial.ab. 15. groups.ab. 16. or/9‐15 17. 8 and 16
Embase (Ovid) search strategy
Embase 1980 to 2022 Week 32 (last searched 9 August 2022); n = 4922
1. carotid artery disease/ or carotid atherosclerosis/ or exp carotid artery thrombosis/ 2. carotid artery/ or carotid sinus/ or exp common carotid artery/ or external carotid artery/ or internal carotid artery/ 3. (carotid adj5 (stenosis or thrombo$ or disease$ or narrow$ or plaque$ or arterioscler$ or atheroscler$)).tw. 4. or/1‐3 5. asymptomatic disease/ 6. asymptomatic.tw. 7. 5 or 6 8. 4 and 7 9. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 10. Randomization/ 11. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 12. control group/ or controlled study/ 13. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 14. crossover procedure/ 15. single blind procedure/ or double blind procedure/ or triple blind procedure/ 16. placebo/ or placebo effect/ 17. (random$ or RCT or RCTs).tw. 18. (controlled adj5 (trial$ or stud$)).tw. 19. (clinical$ adj5 trial$).tw. 20. clinical trial registration.ab. 21. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 22. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 23. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 24. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 25. (cross‐over or cross over or crossover).tw. 26. (placebo$ or sham).tw. 27. trial.ti. 28. (assign$ or allocat$).tw. 29. controls.tw. 30. or/9‐29 31. 8 and 30
LILACS/IBECS search strategy
1 mh: (carotid artery diseases) or mh: (Enfermedades de las Arterias Carótidas) or mh: (Doenças das Artérias Carótidas) or (Arterial Disease* Carotid) or (Arterial Disease* Common Carotid) or (Arterial Disease* External Carotid) or (Arterial Disease* Internal Carotid) or (Artery Disease* Carotid) or (Artery Disorder* Carotid) or (Atherosclerotic Disease* Carotid) or (Carotid Arterial Disease*) or (Carotid Atheroscleros*) or (Carotid Atherosclerotic Disease*) or (Common Carotid Artery Disease*) or (External Carotid Artery Disease*) or (Internal Carotid Artery Disease*) or C10.228.140.300.200 or C14.907.253.123 or (Aterosclerosis de la Carótida) or (Aterosclerose Carotídea) or (Aterosclerose da Carótida) 2 mh: (carotid arteries) or mh: (Arterias Carótidas) or mh: (Artérias Carótidas) or (Arteries Carotid) or (Artery Carotid) 3 mh: (Carotid Stenosis) or mh: (Estenosis Carotídea) or mh: (Estenose das Carótidas) or (Artery Narrowing* Carotid) or (Artery Plaque* Carotid) or (Artery Stenoses Carotid) or (Artery Stenosis Carotid) or (Carotid Artery Narrowing*) or (Carotid Artery Plaque*) or (Carotid Artery Stenoses) or (Carotid Artery Stenosis) or (Carotid Artery Ulcerating Plaque) or (Carotid Stenoses) or (Carotid Ulcer*) or (Common Carotid Artery Stenosis) or (External Carotid Artery Stenosis) or (Internal Carotid Artery Stenosis) or (Plaque Carotid Artery) or (Stenosis Carotid) or (Stenosis Carotid Artery) or (Stenosis Common Carotid Artery) or (Stenosis External Carotid Artery) or (Ulcerating Plaque Carotid Artery) or (Estrechamiento de la Arteria Carótida) or (Úlcera de la Carótida) or C10.228.140.300.200.360 or C14.907.137.230 or C14.907.253.123.360 or (Estenose Carotídea) or (Estreitamento das Artérias Carótidas) or (Úlcera Carotídea) 4 mh: Atherosclerosis or mh: Aterosclerosis or mh: Aterosclerose or Atherogenesis or Atheroscleroses or Ateroesclerosis or Aterogénesis or Aterogênese 5 or/1‐4 mh: (carotid artery diseases) or mh: (Enfermedades de las Arterias Carótidas) or mh: (Doenças das Artérias Carótidas) or (Arterial Disease* Carotid) or (Arterial Disease* Common Carotid) or (Arterial Disease* External Carotid) or (Arterial Disease* Internal Carotid) or (Artery Disease* Carotid) or (Artery Disorder* Carotid) or (Atherosclerotic Disease* Carotid) or (Carotid Arterial Disease*) or (Carotid Atheroscleros*) or (Carotid Atherosclerotic Disease*) or (Common Carotid Artery Disease*) or (External Carotid Artery Disease*) or (Internal Carotid Artery Disease*) or C10.228.140.300.200 or C14.907.253.123 or (Aterosclerosis de la Carótida) or (Aterosclerose Carotídea) or (Aterosclerose da Carótida) or mh: (carotid arteries) or mh: (Arterias Carótidas) or mh: (Artérias Carótidas) or (Arteries Carotid) or (Artery Carotid) or mh: (Carotid Stenosis) or mh: (Estenosis Carotídea) or mh: (Estenose das Carótidas) or (Artery Narrowing* Carotid) or (Artery Plaque* Carotid) or (Artery Stenoses Carotid) or (Artery Stenosis Carotid) or (Carotid Artery Narrowing*) or (Carotid Artery Plaque*) or (Carotid Artery Stenoses) or (Carotid Artery Stenosis) or (Carotid Artery Ulcerating Plaque) or (Carotid Stenoses) or (Carotid Ulcer*) or (Common Carotid Artery Stenosis) or (External Carotid Artery Stenosis) or (Internal Carotid Artery Stenosis) or (Plaque Carotid Artery) or (Stenosis Carotid) or (Stenosis Carotid Artery) or (Stenosis Common Carotid Artery) or (Stenosis External Carotid Artery) or (Ulcerating Plaque Carotid Artery) or (Estrechamiento de la Arteria Carótida) or (Úlcera de la Carótida) or C10.228.140.300.200.360 or C14.907.137.230 or C14.907.253.123.360 or (Estenose Carotídea) or (Estreitamento das Artérias Carótidas) or (Úlcera Carotídea) or mh: Atherosclerosis or mh: Aterosclerosis or mh: Aterosclerose or Atherogenesis or Atheroscleroses or Ateroesclerosis or Aterogénesis or Aterogênese 6 mh: (Asymptomatic Diseases) or mh: (Enfermedades Asintomáticas) or mh: (Doenças Assintomáticas) or (Asymptomatic Condition*) or (Asymptomatic Disease*) or (Asymptomatic State*) or (Disease* Pre‐Symptomatic) or (Disease* Presymptomatic) 7 5 and 6 (mh: (carotid artery diseases) or mh: (Enfermedades de las Arterias Carótidas) or mh: (Doenças das Artérias Carótidas) or (Arterial Disease* Carotid) or (Arterial Disease* Common Carotid) or (Arterial Disease* External Carotid) or (Arterial Disease* Internal Carotid) or (Artery Disease* Carotid) or (Artery Disorder* Carotid) or (Atherosclerotic Disease* Carotid) or (Carotid Arterial Disease*) or (Carotid Atheroscleros*) or (Carotid Atherosclerotic Disease*) or (Common Carotid Artery Disease*) or (External Carotid Artery Disease*) or (Internal Carotid Artery Disease*) or C10.228.140.300.200 or C14.907.253.123 or (Aterosclerosis de la Carótida) or (Aterosclerose Carotídea) or (Aterosclerose da Carótida) or mh: (carotid arteries) or mh: (Arterias Carótidas) or mh: (Artérias Carótidas) or (Arteries Carotid) or (Artery Carotid) or mh: (Carotid Stenosis) or mh: (Estenosis Carotídea) or mh: (Estenose das Carótidas) or (Artery Narrowing* Carotid) or (Artery Plaque* Carotid) or (Artery Stenoses Carotid) or (Artery Stenosis Carotid) or (Carotid Artery Narrowing*) or (Carotid Artery Plaque*) or (Carotid Artery Stenoses) or (Carotid Artery Stenosis) or (Carotid Artery Ulcerating Plaque) or (Carotid Stenoses) or (Carotid Ulcer*) or (Common Carotid Artery Stenosis) or (External Carotid Artery Stenosis) or (Internal Carotid Artery Stenosis) or (Plaque Carotid Artery) or (Stenosis Carotid) or (Stenosis Carotid Artery) or (Stenosis Common Carotid Artery) or (Stenosis External Carotid Artery) or (Ulcerating Plaque Carotid Artery) or (Estrechamiento de la Arteria Carótida) or (Úlcera de la Carótida) or C10.228.140.300.200.360 or C14.907.137.230 or C14.907.253.123.360 or (Estenose Carotídea) or (Estreitamento das Artérias Carótidas) or (Úlcera Carotídea) or mh: Atherosclerosis or mh: Aterosclerosis or mh: Aterosclerose or Atherogenesis or Atheroscleroses or Ateroesclerosis or Aterogénesis or Aterogênese) and (mh: (Asymptomatic Diseases) or mh: (Enfermedades Asintomáticas) or mh: (Doenças Assintomáticas) or (Asymptomatic Condition*) or (Asymptomatic Disease*) or (Asymptomatic State*) or (Disease* Pre‐Symptomatic) or (Disease* Presymptomatic)) 8 7 and lilacs and ibecs tw:((mh: (carotid artery diseases) OR mh: (enfermedades de las arterias carótidas) OR mh: (doenças das artérias carótidas) OR (arterial disease* carotid) OR (arterial disease* common carotid) OR (arterial disease* external carotid) OR (arterial disease* internal carotid) OR (artery disease* carotid) OR (artery disorder* carotid) OR (atherosclerotic disease* carotid) OR (carotid arterial disease*) OR (carotid atheroscleros*) OR (carotid atherosclerotic disease*) OR (common carotid artery disease*) OR (external carotid artery disease*) OR (internal carotid artery disease*) OR c10.228.140.300.200 OR c14.907.253.123 OR (aterosclerosis de la carótida) OR (aterosclerose carotídea) OR (aterosclerose da carótida) OR mh: (carotid arteries) OR mh: (arterias carótidas) OR mh: (artérias carótidas) OR (arteries carotid) OR (artery carotid) OR mh: (carotid stenosis) OR mh: (estenosis carotídea) OR mh: (estenose das carótidas) OR (artery narrowing* carotid) OR (artery plaque* carotid) OR (artery stenoses carotid) OR (artery stenosis carotid) OR (carotid artery narrowing*) OR (carotid artery plaque*) OR (carotid artery stenoses) OR (carotid artery stenosis) OR (carotid artery ulcerating plaque) OR (carotid stenoses) OR (carotid ulcer*) OR (common carotid artery stenosis) OR (external carotid artery stenosis) OR (internal carotid artery stenosis) OR (plaque carotid artery) OR (stenosis carotid) OR (stenosis carotid artery) OR (stenosis common carotid artery) OR (stenosis external carotid artery) OR (ulcerating plaque carotid artery) OR (estrechamiento de la arteria carótida) OR (úlcera de la carótida) OR c10.228.140.300.200.360 OR c14.907.137.230 OR c14.907.253.123.360 OR (estenose carotídea) OR (estreitamento das artérias carótidas) OR (úlcera carotídea) OR mh: atherosclerosis OR mh: aterosclerosis OR mh: aterosclerose OR atherogenesis OR atheroscleroses OR ateroesclerosis OR aterogénesis OR aterogênese) AND (mh: (asymptomatic diseases) OR mh: (enfermedades asintomáticas) OR mh: (doenças assintomáticas) OR (asymptomatic condition*) OR (asymptomatic disease*) OR (asymptomatic state*) OR (disease* pre‐symptomatic) OR (disease* presymptomatic))) AND ( db:("LILACS" OR "IBECS")) 205
US National Institutes of Health Ongoing Trials Register
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; last searched 14 April 2020); n = 24
AREA[StudyType] EXPAND[Term] COVER[FullMatch] "Interventional" AND AREA[ConditionSearch] asymptomatic carotid stenosis
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
Basic search: asymptomatic carotid stenosis Phases are: ALL
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en) (last searched 9 August 2022) n = 58
UNIFESP search strategy
#1 carotid artery 451 #2 1 and asymptomatic 1 #3 carotid artery diseases 14686 #4 3 and asymptomatic 6 #5 carotid stenosis 781 #6 5 and asymptomatic 0 #7 Atherosclerosis 684 #8 7 and asymptomatic 0 #9 or/2,4,6,8 6
British Library EthOS search strategy
"asymptomatic carotid stenosis" 21
ProQuest search strategy
"noft(asymptomatic carotid stenosis)" 63
Appendix 2. Enquiry letter
Dear Doctor
I am currently conducting a systematic review entitled 'Pharmacological interventions for asymptomatic carotid stenosis' with the Cochrane Stroke Group based in the University of Edinburgh. To ensure that the results are valid, it is essential that all relevant trials are included.
Cochrane was established to ensure all forms of health care will be subject to critical evaluation using standard criteria and specialised software.
As a [manufacturer/expert/trialist] of [drug/intervention name], it is possible that a trial of this or a similar agent has been conducted in patients with asymptomatic carotid stenosis. If so, we would be grateful if you could supply us with copies of any relevant protocols, reports or publications in the first instance; later it may become necessary to obtain the raw data. If the trial is eligible for inclusion in the review, [Pharmaceutical company/specialist name] will be cited in the final report which will be published electronically within the Cochrane Database of Systematic Reviews, and in standard medical journals.
I would be grateful if you could fill in the accompanying form, and forward any information which you feel may be appropriate.
Thank you for your help.
Yours faithfully
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Form for reply from Pharmaceutical Company/Trialist/Expert
Trials that fulfil the following criteria will be eligible for inclusion in the review:
Types of participants:
Treatment regimen:
A valid randomisation method:
For example: a centralised scheme, e.g. by telephone or scheme controlled by pharmacy, e.g. pre‐coded or numbered containers or on‐site computer system where allocations are in a locked unreadable file or assignment envelopes ‐ sequentially numbered, sealed and opaque or other combinations which provide assurance of adequate concealment. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Name of Pharmaceutical Company/Trialist/Expert
Name (person to whom any future correspondence should be addressed):
Trials fulfilling the above criteria:
Have not been conducted ( ) Are currently underway * ( ) Have been conducted in the past * ( )
* Please enclose relevant protocols, citations, reports or other publications
Thank you for your valuable help.
Please complete and return to:
Dr Caroline NB Clezar, MD Department of Surgery, Division of Vascular and Endovascular Surgery Universidade Federal de São Paulo Rua Borges Lagoa, 754 São Paulo Brazil
e‐mail: caroline.bessa@gmail.com
Data and analyses
Comparison 1. Antiplatelet agent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Stroke‐related mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Major bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Progression of carotid stenosis | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Antihypertensive agent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Stroke‐related mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Progression of carotid stenosis | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. One antihypertensive agent plus lipid‐lowering agent versus another antihypertensive agent plus lipid‐lowering agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Anticoagulant agent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Major bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Adverse events | 1 | 919 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.81, 0.99] |
Comparison 5. Lipid‐lowering agent versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Ipsilateral major or disabling stroke | 5 | 2235 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.09, 1.53] |
| 5.2 Stroke‐related mortality | 2 | 1366 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.03, 2.29] |
| 5.3 Adverse events | 7 | 3726 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.10] |
Comparison 6. Lipid‐lowering agent plus antihypertensive agent versus antihypertensive agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. One lipid‐lowering agent versus another lipid‐lowering agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.2 Adverse events | 2 | 497 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.30, 2.86] |
Comparison 8. Two lipid‐lowering agents versus one lipid‐lowering agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.2 Adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 9. One antihypertensive agent versus another antihypertensive agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Ipsilateral major or disabling stroke | 2 | 2918 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.34, 2.87] |
| 9.2 Adverse events | 4 | 3239 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.82, 1.21] |
Comparison 10. Higher dose of lipid‐lowering agent versus lower dose of the same lipid‐lowering agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Ipsilateral major or disabling stroke | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.2 Adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderssen 2005.
| Study characteristics | ||
| Methods |
Study design: randomised, placebo‐controlled, 2x2 factorial trial Total duration of study: 4 years Details of any 'run‐in' period: no details given Number of study centres and location: no details given, Norway Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 568 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 568 participants Number of interest: 568 participants Mean age: fluvastatin alone group: 56.8 ± 8.6 years; placebo alone group: 57.5 ± 8.2 years; fluvastatin and lifestyle group: 57.9 ± 8.7 years; placebo and lifestyle group: 56.4 ± 9.1 years Age range: 40 to 74 years Gender: 568 men Severity of condition: hypertension Diagnostic criteria: total cholesterol 4.5–8.0 mmol/L, triglycerides < 4.5 mmol/L, body mass index 25–35 kg/m2, and a sedentary lifestyle (< 1 hour per week of regular exercise) Smoking history: current smokers: 104 participants, former smokers: 227 participants Inclusion criteria: "men aged 40 to 74 years receiving drug treatment for hypertension were recruited, and were eligible for enrolment if they exhibited total cholesterol 4.5–8.0 mmol/L, triglycerides < 4.5 mmol/L, body mass index 25–35 kg/m2, and a sedentary lifestyle (< 1 hour per week of regular exercise)" Exclusion criteria: "main exclusion criteria included any symptomatic cardiovascular disease (MI, angina pectoris, stroke), congestive heart failure, type 1 diabetes mellitus, history of coronary intervention, need for treatment with lipid‐lowering medications other than the study drug, known or suspected impaired hepatic or renal function or malignancy, history of alcohol and/or drug abuse, vegetarian diet or diet comprising a high omega‐3 fatty acid intake, and inability to perform physical exercise" |
|
| Interventions |
Intervention: fluvastatin, 40 mg daily Comparison: placebo, and either intensive lifestyle intervention or usual care Concomitant medications: calcium antagonists, beta‐blockers, diuretics, ACE inhibitors Excluded medications: no details given |
|
| Outcomes |
Primary outcome: change in carotid IMT from baseline to study end point Secondary outcomes: LV mass; cardiovascular disease events Time points reported
|
|
| Notes |
Funding for trial: HYRIM was supported by grants from Novartis Pharma AG, Ulleva °l University Hospital, Norwegian University of Physical Education and the Throne Holst Legacy Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The statin arm was double blind, whereas the lifestyle arm was single blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The sonographer and operators carrying out off‐line analyses were masked to all patient information, the randomization group, and to the results of previous examinations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | Mostly, but adverse events and cholesterol level at baseline and at 4‐year follow‐up not provided |
| Other bias | Low risk | No evidence of other bias |
Applegate 1991.
| Study characteristics | ||
| Methods |
Study design: multicenter, randomised, double‐blind, active‐control, parallel‐group trial Total duration of study: 3 years. Details of any 'run‐in' period: "eligibility was determined during an initial screening period of three to eight weeks' duration. Participants who had been taking antihypertensive medications at the beginning of the screening period underwent a short wash‐out period. All participants then underwent a three‐ to eight‐week placebo period during which the blood pressure entry criteria were evaluated." Number of study centres and location: 9 clinical centres located across the USA Study setting and date of study: outpatients; 9 July 1988 to 12 December 1989 |
|
| Participants |
Number randomised: 883 participants Number lost to follow‐up/withdrawn: "20% of those on isradipine treatment and 18% of those on hydrochlorothiazide (HCTZ) treatment had withdrawn from their respective study medications" Number analysed: 883 participants Number of interest: 883 participants Mean age: 58.7 years Age range: 40 years and older Gender: 687 men and 196 women Severity of condition: no details given Diagnostic criteria: "only diastolic BP (DBP) was used to determine the presence of hypertension. Hypertension was defined as an average DBP of from 90 mmHg to 115 mmHg" Smoking history: 340 former smokers and 176 current smokers Inclusion criteria "1) Men and women over the age of 40 years; 2) average sitting diastolic blood pressure greater than 90 mmHg and less than 115 mmHg on each of the last three visits of the placebo run‐in period; 3) presence of one or more atherosclerotic lesions in the extracranial carotid artery, demonstrated by quantitative B‐mode ultrasound scanning at baseline with a maximum plaque thickness of between 1.3 mm and 3.5 mm; 4) total serum cholesterol and triglyceride levels within a week prior to randomisation" Exclusion criteria "1) Determination that the patient was considered unlikely to complete the 3‐year treatment period; 2) presence of any form of secondary hypertension; 3) presence of malignant or accelerated hypertension; 4) presence of symptomatic orthostatic hypotension; 5) an average sitting diastolic blood pressure < 115 mmHg at any visit during the screening or placebo wash‐out period; 6) presence of unstable or poorly controlled angina pectoris; 7) history of a cerebrovascular accident, MI, or TIA within the past three months; 8) previous carotid endarterectomy on the side of the qualifying plaque; 9) potential need for diuretic therapy over a 3‐year period, including a history of mild heart failure; 10) presence of cardiac arrhythmias of sufficient severity as to place the patient at risk for an adverse outcome during the course of the study; 11) presence of insulin‐dependent diabetes mellitus; 12) presence of any severe disease or use of any medication that might confound the study results or interfere with completion of the study." |
|
| Interventions |
Intervention: 2.5 mg or 5 mg isradipine twice daily Comparison: 12.5 mg or 25 mg HCTZ twice daily Concomitant medications: "the small proportion of participants who did not demonstrate adequate blood pressure control with dose‐doubling were given open‐label enalapril in doses ranging from 2.5 mg to 10 mg twice daily." Excluded medications: no details given |
|
| Outcomes |
Primary outcome: reducing the rate of progression of early extracranial carotid artery atherosclerosis Secondary outcomes: "defined specifically for the purpose of identifying the effect, if any, on the specific segments of carotid artery. These end points were rate of progression in IMT of the following: (1) "normal" arterial walls, defined as the mean of those walls with IMTs less than 1.0 mm at baseline; (2) "borderline" walls with mean IMTs between 1.0 and 1.3 mm at baseline; (3) "diseased" walls with mean IMTs between 1.3 and 3.5 mm at baseline; (4) the 4 walls of the common carotid artery; (5) the 4walls of the carotid bifurcation; (6) the 4 far walls of the common and bifurcation combined; (7) the single wall with the greatest maximum IMT at baseline; and (8) the single wall with the greatest maximum increase." Time points reported "Follow‐up visits every 2 months during the first year and every 3 months during the remaining 2 years. B‐mode ultrasonography of carotid arteries was performed twice at baseline, twice at the final visit, and once every 6 months in the interim." |
|
| Notes |
Funding for trial: MIDAS is sponsored by Sandoz Pharmaceuticals Corporation Notable conflicts of interest of trial authors: "Although the Sandoz Research Institute is responsible for centralizing data entry and editing, all data analysis will be conducted by the Operations/Analysis Center at the Bowman Gray School of Medicine. The scientific direction for the study rests with the Investigators' Committee. A Policy and Data Monitoring Committee, with no voting member from Sandoz or any of the participating institutions, is charged with monitoring the trial for safety and efficacy, and with approving the final report of the trial results." Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization process was stratified and blocked by clinic to provide equal probability of assignment to either treatment group throughout the study." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization process was stratified and blocked by clinic to provide equal probability of assignment to either treatment group throughout the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Qualifying participants were randomized at the baseline visit and began a 36‐week double‐blind drug treatment period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All reported clinical events were reviewed, adjudicated, and classified by the MIDAS Investigators' Morbidity and Mortality Committee, consisting of 6 clinicians, each from a different clinical center; all were blinded to the randomization assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Blanco‐Colio 2004.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blinded, multicentre study Total duration of study: 4 to 6 weeks Details of any 'run‐in' period: 6‐week run‐in period of placebo treatment Number of study centres and location: 1 centre, Hospital Clínico San Carlos, Madrid, Spain Study setting and date of study: outpatients; date of study was not reported |
|
| Participants |
Number randomised: 14 participants Number lost to follow‐up/withdrawn: no detail given Number analysed: 14 participants Number of interest: 14 participants Mean age: 71.5 +/‐ 6 years in atorvastatin group and 68.6 +/‐ 9 years in "no treatment" group Age range: 18 to 80 years Gender: 10 men and 4 women Severity of condition: "carotid atherosclerosis (carotid stenosis > 70%, as diagnosed by Doppler echocardiography)" Diagnostic criteria: "normocholesterolemic patients with carotid atherosclerosis (carotid stenosis > 70%, as diagnosed by Doppler echocardiography) and without previous statin therapy" Smoking history: 1 smoker Inclusion criteria: "participants were included in the trial if, after discontinuation of any lipid‐regulating drug, formal dietary counselling, good compliance with the prescribed diet, and a six‐week run‐in period of placebo treatment, they had a mean (of 2 consecutive analyses at weeks 4 and 2) triglyceride level of < 500 and > 200 mg/dL, respectively, in addition to LDL cholesterol < 250 and > 190, 180, 160, or 135 mg/dL, depending on the global risk status (low, moderate, high, or presence of coronary heart disease, respectively), according to the European Atherosclerosis Society (EAS) recommendations" Exclusion criteria: "people were excluded from the trial if they were pregnant or nursing, had an inflammatory disease or tumour, or had been treated with hypolipaemic or anti‐inflammatory drugs (except aspirin < 325 mg/day) during the year preceding the study. Patients must not have had a myocardial infarction, angioplasty, severe or unstable angina pectoris, or any other cardiovascular event resulting in hospitalisations during the six months preceding the study." |
|
| Interventions |
Intervention: 80 mg/day atorvastatin Comparison: no treatment Concomitant medications: no details given Excluded medications: hypolipaemic or anti‐inflammatory drugs |
|
| Outcomes |
Primary outcome: sFasL levels in participants with clinical atherosclerosis without marked hyperlipidaemia Secondary outcome: adverse events Time points reported: no details given |
|
| Notes |
Funding for trial: this study was supported by grants from the Ministerio de Ciencia y Tecnología (SAF 2001‐0717), the Spanish Cardiovascular Network (03/01), the Fundación Ramón Areces, and Pfizer, Madrid, Spain. Notable conflicts of interest of trial authors: "Josep M Sol, Cristina Díaz, and Gonzalo Hernández are employees of Pfizer. They were engaged in the design and recruitment of patients included in the ATOMIX study (Atorvastatin versus Bezafibrate in Mixed Hyperlipidaemia: Randomised Clinical Trial of Efficacy and Safety) from which we took the samples. Therefore, although they are employees of Pfizer, they have no particular conflict of interest with the content of this paper. Drs. Blanco‐Colio and Martín‐Ventura contributed equally to this work." Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The lipid values of randomized patients were kept unknown to both the patient and the investigator until the end of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Bots 2007.
| Study characteristics | ||
| Methods |
Study design: phase 3, multicentre, double‐blind, randomised, parallel group Total duration of study: 3 years Details of any 'run‐in' period: "4‐week washout phase prior to screening during which lipid‐lowering therapy was discontinued and counselling given on lifestyle changes. Eligible participants commenced a 10 mg daily atorvastatin only during the run‐in period." Number of study centres and location: 64 centres in North America and Europe: Canada, USA, Czech Republic, Finland, France, and the Netherlands Study setting and date of study: outpatients; 1 December 2003 to 27 December 2006 |
|
| Participants |
Number randomised: 752 participants Number lost to follow‐up/withdrawn: "69 discontinued intervention: 28 adverse events related to study drug, 10 adverse events not related to study drug, 23 defaulted, 8 moved away or lost to follow‐up." Number analysed: 683 participants Number of interest: 683 participants Mean age: 56.5 (8.2) years old in atorvastatin monotherapy group and 57.9 (8.1) years old in atorvastatin plus torcetrapib group Age range: 18 to 70 years old Gender: 482 men and 270 women Severity of condition: hyperlipidaemia Diagnostic criteria: "triglycerides of greater than 1.7 mmol/L and a concurrent LDL cholesterol concentration that was high enough to qualify for statin treatment according to the guidelines of the US National Cholesterol Education Programme (NCEP) Adult Treatment Panel." Smoking history: 121 current smokers Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: torcetrapib 60 mg plus atorvastatin 10, 20, 40, or 80 mg Comparison: atorvastatin 10, 20, 40, or 80 mg Concomitant medications: aspirin, beta‐blocker, angiotensin‐converting enzyme inhibitor, or angiotensin receptor blocker Excluded medications: other statins or other concomitant therapy with known lipid altering effects on LDL and HDL including fibrates and nicotinic acid (high doses) |
|
| Outcomes |
Primary outcome: change in intima‐media thickness as measured by carotid ultrasound Secondary outcomes: changes in levels of lipids and other biomarkers Time points reported: replicated scans at baseline and at each participant’s final visit, and scans at visits at 6, 12, and 18 months, to give a maximum of seven scans for each participant |
|
| Notes |
Funding for trial: the study sponsor, Pfizer, collaborated with academic investigators in design of the study, and monitored the study. The study data were analysed independently by the sponsor, the core laboratories, and the principal investigators. The sponsor reviewed the manuscript and provided editorial comments to the lead authors. The corresponding author had full access to all the data in the study. The corresponding author made the final decision to submit for publication in collaboration with co‐authors Notable conflicts of interest of trial authors: MLB has received grants for studies on carotid intima‐media thickness, honoraria for professional input regarding issues on carotid intima‐media thickness, or both, from Astra‐Zeneca, Icelandic Heart Foundation, Organon, Pfizer, Netherlands Heart Foundation, Netherlands Organisation for Health Research and Development, Servier, and Unilever. FLJV has received research grants from Merck, and Netherlands Organisation for Health Research and Development. GWE has received honoraria, consulting fees, and grant support for professional input on CIMT issues from Astra‐Zeneca, Organon, and Pfizer. WAR has received research contracts from Astra‐Zeneca, Organon, and Pfizer. DEG has received grant support from, and delivered lectures for, Pfizer, Astra‐Zeneca, Organon, Servier, and Merck. JJPK has received research grant support from Pfizer. RMV has had a contract as a study investigator with Pfizer, and has periodically received honoraria from Pfizer for lectures. CHT has no conflicts of interest. JHR, CLS, and WTD are employees of, and CLS and WTD are shareholders of, Pfizer Note: all torcetrapib–atorvastatin clinical trials were stopped on 2 December 2006, when an independent data safety and monitoring board for another study of torcetrapib and atorvastatin recommended that it be terminated because of an increase in deaths in the treatment group. Participants who were still receiving treatment on that date were asked to discontinue treatment immediately and to return for final visits in that month as originally planned. Protocol: NCT00134238 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by use of a central scheme with a computer‐generated permuted block design, and a block size of four." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised by use of a central scheme with a computer‐generated permuted block design, and a block size of four." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants and study personnel were unaware of treatment assignment, laboratory measurements, and carotid imaging findings" and "The placebo tablets were identical in appearance to active torcetrapib tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Readers were unaware of the interventions assigned to patients, and of previous measurements." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Corti 2005.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind trial Total duration of study: 3 years Details of any 'run‐in' period: no details given Number of study centres and location: 1 centre, Mount Sinai School of Medicine, New York Study setting and date of study: outpatients; March 1999 to 2002 |
|
| Participants |
Number randomised: 51 participants Number lost to follow‐up/withdrawn: "one patient was lost to follow‐up during the first 6 months: a 52‐year‐old man with no previous episode of angina died suddenly during exercise 3 weeks after starting in the conventional treatment group." Number analysed: 51 participants Number of interest: 51 participants Mean age: 62 years Age range: 41.4 to 82.9 years Gender: 31 men and 20 women Severity of condition: clinically asymptomatic patients Diagnostic criteria: "hypercholesteraemic (LDL 130 mg/dL and triglycerides 445 mg/dL)" Smoking history: 16 previous smokers and 15 current smokers Inclusion criteria: "based on the pre‐existence of atherosclerotic plaques (thoracic aortic wall 4.0 mm and/or carotid wall 2.0 mm thick) detected by carotid B‐mode ultrasound, echocardiography, or MRI" Exclusion criteria: "heart failure, renal or hepatic disease, significant carotid disease, or a clinically significant medical or surgical event within 3 months before study entry" |
|
| Interventions |
Intervention: simvastatin 80 mg Comparison: simvastatin 20 mg Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "change in vessel wall area (VWA) as a surrogate for atherosclerotic burden" Secondary outcomes: no details given Time points reported: "clinical follow‐up was done at 6, 12, 24, and 48 weeks and blood samples were drawn at baseline, 6, 12, 24, 48, 72, and 96 weeks to determine lipid levels and safety parameters." |
|
| Notes |
Funding for trial: "this study was supported by grants from the National Institutes of Health (HL54469, Drs Fuster and Badimon); the National Heart, Lung, and Blood Institute (HL61801, Dr Fuster); the Swiss National Research Foundation (Dr Corti); the National Heart Foundation of Australia (Dr Worthley); the French Federation of Cardiology (Dr Helft); and Merck and Co, Inc. Merck and Co. was partially responsible for the funding of the project." Notable conflicts of interest of trial authors: Mount Sinai authors are fully responsible for data acquisition, evaluation, and writing the manuscript without any interference from the funding sources Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A Prospective, Randomized, Double‐Blind Trial" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The measurements were performed blinded to the patient’s identity and image order." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Crouse 2007.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled, international, multicentre, parallel‐group study Total duration of study: 2 years Details of any 'run‐in' period: 6‐week run‐in period that included three clinic visits Number of study centres and location: 61 primary care centres in the USA and Europe Study setting and date of study: outpatients; August 2002 to May 2006 |
|
| Participants |
Number randomised: 984 participants Number lost to follow‐up/withdrawn: "3 patients did not receive rosuvastatin as assigned and withdrew consent, and 105 discontinued study prior to any follow‐up: 55 adverse events, 4 non‐adherence, 33 withdrew consent, 5 lost to follow‐up, 1 investigator’s decision, 11 other reasons." Number analysed: 876 participants were included in primary efficacy analysis and 781 participants were included in safety analysis Number of interest: 876 participants Mean age: 57 years old Age range: 45 to 70 years old Gender: 588 men and 396 women Severity of condition: low‐risk patients Diagnostic criteria: "10‐year Framingham risk < 10% with modest C‐IMT (C‐IMT > 1.2 mm and < 3.5 mm) and elevated LDL." Smoking history: 38 smokers Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: rosuvastatin 40 mg once daily Comparison: placebo Concomitant medications: "a bile acid sequestrant was added to the treatment regimen under the following circumstances: placebo group, if LDL levels are ≥ 190 mg/dL (4.9 mmol/L) on two consecutive visits (in participants with only age as a risk factor) or if LDL levels are ≥ 160 mg/dL (4.1 mmol/L) on two consecutive visits (in participants with a < 10% risk of CHD over 10 years); rosuvastatin group, if LDL levels are ≥ 100 mg/dL (2.56 mmol/L) on two consecutive visits" Excluded medications: potent immunosuppressants not permitted |
|
| Outcomes |
Primary outcome: "change from baseline (visit 4) to the end of treatment (visit 13) in the mean of the maximum (MeanMax) IMT" Secondary outcomes: "change from baseline to end of treatment in the MeanMax IMT of the right and left CCA, carotid bifurcation and ICA independently, and the mean IMT of the near and far walls of the right and left CCA; change from baseline to study end in LDL, total cholesterol, high‐density lipoprotein cholesterol (HDL) and non‐HDL components, non‐HDL:HDL ratio, triglyceride, apolipoprotein A‐I or Apo B levels and the Apo B:Apo A‐I ratio: change in C‐reactive protein level from baseline to study end also measured." Time points reported: during the study, participants visited the clinic nine further times |
|
| Notes |
Funding for trial: the METEOR study was funded by AstraZeneca Notable conflicts of interest of trial authors: "Dr Crouse reported receiving grant or salary support from Merck, Merck‐Schering Plough, Pfizer, AstraZeneca, and Kos Pharmaceuticals; and giving lectures for Merck, Merck‐Schering Plough, Pfizer, AstraZeneca, Abbott, and Kos Pharmaceuticals. Dr Raichlen reported being an employee of AstraZeneca. Dr Riley reported receiving research contracts from AstraZeneca, Organon, and Pfizer. Mr Evans reported receiving grant support and honoraria from AstraZeneca, Organon, and Pfizer; and being a consultant to AstraZeneca and Pfizer. Dr Palmer reported being an employee of AstraZeneca. Dr O’Leary reported being on data and safety monitoring boards for Pfizer and AstraZeneca; being a consultant to Pfizer, Sankyo Pharma, Sanofi‐Aventis, GlaxoSmithKline, Eli Lilly, Schering‐Plough, Esperion Therapeutics, and Merck; and being an equity partner in Imagepace LLC. Dr Grobbee reported receiving grant support from and delivering lectures for Pfizer, AstraZeneca, Organon, Servier, and Merck. Dr Bots reported receiving study grants for studies on carotid intima‐media thickness and/or honoraria for professional input on carotid intima‐media thickness issues from AstraZeneca, Icelandic Heart Foundation, Organon, Pfizer, the Netherlands Heart Foundation, the Netherlands Organisation for Health Research and Development, Servier, and Unilever." Protocol: NTC00225589 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible participants were randomised to either the placebo or rosuvastatin groups in blocks of seven (five rosuvastatin, two placebo) at each clinical site" |
| Allocation concealment (selection bias) | Low risk | Quote: "This random allocation means that any regression to the mean occurring within the study affects both treatment groups equally and that estimates of treatment effect within quartiles of baseline C‐IMT are unbiased" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinded study medication was supplied in individual numbered bottles prepared prior to the clinic visits and eligible individuals were allocated study medication sequentially" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators checked adherence but were unaware of treatment allocations for the duration of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes described in the methods section reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Côté 1995.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Total duration of study: 6 years Details of any 'run‐in' period: no details given Number of study centres and location: five centres in Montreal and Quebec City, Quebec Study setting and date of study: outpatients; May 1988 to May 1994 |
|
| Participants |
Number randomised: 372 participants Number lost to follow‐up/withdrawn: "only two patients (both in the placebo group) were lost to follow‐up, after 1.5 and 2.7 years in the study, respectively." Number analysed: 372 participants Number of interest: 372 participants Mean age: 65 years old Age range: 23 to 91 years old Gender: 175 men and 197 women Severity of condition: neurologically asymptomatic patients Diagnostic criteria: "audible cervical bruit in whom duplex ultrasonography indicated the presence, in at least one artery, of a carotid lesion that reduced the diameter of the artery by at least 50%." Smoking history: 273 smokers Inclusion criteria: "people with a cervical bruit audible to a study physician were eligible." Exclusion criteria: "people were excluded if they had a history of symptomatic ischaemic cerebrovascular disease, valvular heart disease other than mitral valve prolapse, nonvalvular atrial fibrillation, recent (< 3 months before study entry) MI or unstable angina, previous carotid endarterectomy, medically necessary use of aspirin or regular use of nonsteroidal anti‐inflammatory drugs, use of anticoagulant agents, life expectancy of less than 5 years, and allergy to or intolerance of aspirin compounds." |
|
| Interventions |
Intervention: enteric‐coated aspirin, 325 mg/day Comparison: placebo Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcomes: "the first event in the composite end point, which consisted of TIA, stroke, MI, unstable angina, or death." Secondary outcomes: combinations of outcomes: "1) TIA, stroke, MI, unstable angina, and death from vascular causes; 2) stroke, MI, and death from vascular causes; 3) TIA and stroke; 4) stroke and death from vascular causes; and 5) MI, unstable angina, and death from vascular causes." Time points reported: "clinical evaluations by a study physician and nurse coordinator, as well as duplex ultrasonography, were repeated for all participants at 6‐month intervals throughout the 6‐year period." |
|
| Notes |
Funding for trial: "the study medication and placebo were provided by Merck‐Frosst Canada Inc., Kirkland, Quebec, and secretarial assistance was provided by Sandy Lavigne." Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible participants were allocated to receive either one aspirin or placebo tablet per day on the basis of a centrally determined blocked randomisation arrangement." |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment codes were only available centrally to the monitoring committee and locally to the pharmacist‐in‐chief of each institution." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Aspirin was supplied as 325 mg enteric‐coated tablets in plastic bottles that contained enough tablets for 6 months (approximately 200 tablets). The placebo tablets were identical in appearance and packaging." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The central adjudication committee verified participant eligibility and conducted blinded review of all outcome events reported in the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions reported with reasons and by study group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
ELSA 2002.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind, multinational trial Total duration of study: 4 years Details of any 'run‐in' period: 4‐week placebo wash‐out period Number of study centres and location: 410 clinical units in France, Germany, Greece, Italy, Spain, Sweden, and the UK Study setting and date of study: outpatients; June 1994 to November 1995 |
|
| Participants |
Number randomised: 2334 participants Number lost to follow‐up/withdrawn: 43 atenolol and 49 lacidipine participants lost to follow‐up Number analysed: 2035 participants Number of interest: 2035 participants Mean age: "mean age of patients was 55.9 years in atenolol group and 56.1 years in lacidipine group." Age range: 45 to 75 years old Gender: 1115 men and 920 women Severity of condition: no details given Diagnostic criteria: "sitting systolic blood pressure (SBP) of 150 to 210 mmHg and diastolic blood pressure (DBP) of 95 to 115 mmHg." Smoking history: 417 current smokers Inclusion criteria: "both sexes, aged 45 to 75 years, with sitting systolic blood pressure (SBP) 150 to 210 mmHg and diastolic blood pressure (DBP) 95 to 115 mmHg, fasting serum total cholesterol concentration < 320 mg/dL, fasting serum triglyceride concentration < 300 mg/dL, serum creatinine concentration < 1.7 mg/dL and a readable ultrasound carotid artery scan with maximum intima–media thickness (IMT) no greater than 4.0 mm" Exclusion criteria: "the main exclusion criteria were a recent MI or stroke and insulin‐dependent diabetes mellitus" |
|
| Interventions |
Intervention: lacidipine 4 mg once daily Comparison: atenolol 50 mg once daily Concomitant medications: open‐label hydrochlorothiazide added (12.5 mg daily month 3 and 25 mg daily month 6) Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "the change in mean maximum IMT of the 4 far walls in the distal common carotids and carotid bifurcations bilaterally (CBMmax) during 4 years." Secondary outcomes: "increase or decrease in plaque number (focal IMT of 1.3 mm) at study‐end, and incidence of fatal and nonfatal cardiovascular events and total mortality." Time points reported: "duplicate carotid scans were performed by certified sonographers at 23 referral centres between beginning of run‐in and randomisation, and subsequently at yearly intervals; scans were performed 4 years after randomisation in participants who withdrew prematurely." |
|
| Notes |
Funding for trial: "ELSA was an investigator‐generated trial, sponsored by GlaxoSmithKline Italy, Verona and Boehringer Ingelheim International GmbH, Ingelheim am Rhein." Notable conflicts of interest of trial authors: "all authors have received research grants and lecture honoraria from either Boehringer Ingelheim or GlaxoSmithKline. Dr Eckes is an employee of Boehringer Ingelheim. Dr Rizzini is an employee of GlaxoSmithKline." Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated, using separate lists for each referral center with a block size of 4." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was computer‐generated, using separate lists for each referral center with a block size of 4." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and study personnel, excluding the Safety Committee, were blinded to treatment assignment for the study duration." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Scans of any individual patient were assigned to the same reader, but the scan time‐sequence was randomized so that the reader was blind to the time of recording." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Furberg 1994.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Total duration of study: 5 years Details of any 'run‐in' period: "at the completion of visit 3, eligible participants received lovastatin placebo pills and open‐labelled warfarin pills (1 mg). Participants were masked to the identity of the placebo and were told to take one of each kind of pill daily for 21 to 28 days (until the next [baseline] visit) to rule out any reaction to either medication." Number of study centres and location: clinical centres at four academic institutions (Bowman Gray School of Medicine and the Universities of Iowa, Kentucky, and Tennessee) Study setting and date of study: outpatients of community clinics; May 1988 to June 1993 |
|
| Participants |
Number randomised: 919 participants Number lost to follow‐up/withdrawn: "lovastatin/LP or warfarin/WP were prematurely discontinued in 118 and 116 participants, respectively; 94 people stopped both medications. Blind breaks occurred in 11 people." Number analysed: 919 participants Number of interest: 919 participants Mean age: "mean age 61.7 years in lovastatin plus warfarin group, 61.9 years in lovastatin plus warfarin placebo, 62 years in lovastatin placebo plus warfarin and 61.3 years in lovastatin placebo plus warfarin placebo." Age range: 40 to 79 years old Gender: 474 men and 445 women Severity of condition: free of a history of MI, severe angina, stroke, or TIA Diagnostic criteria: "low‐density lipoprotein cholesterol values ranging from either 130 to 159 mg/dL (regardless of the number of coronary risk factors) or 160 to 189 mg/dL (with 1 coronary risk factor) with at least one carotid artery intima‐medial wall thickening > 1.5 mm (common or internal carotid artery) or > 1.6 mm (bifurcation) and less than 3.5 mm (common, internal, or bifurcation)" Smoking history: current smokers: 109, former smokers: 408 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: "warfarin was administered in a fixed 1 mg daily dose. The initially assigned dose of lovastatin was 20 mg per day. The goal was to lower the LDL cholesterol to a value of 90 to 110 mg/dL (2.31 to 2.85 mmol/L). The dosage of lovastatin was doubled if serum levels were above that range after an average 4.5 months of treatment." Comparison: placebo Concomitant medications: "all participants were encouraged to use open‐label aspirin (81 mg/day) unless there was a contraindication for its use." |
|
| Outcomes |
Primary outcome: "change over time (i.e. the slope) during the course of treatment in the mean of maximum IMT across up to 12 preselected segments in the carotid arteries." Secondary outcome: "progression of the single maximum IMT measurement among the same preselected carotid artery segments." Time points reported: "regular clinic visits were scheduled every 6 weeks for the first 15 months and quarterly thereafter to permit safety monitoring. Fasting lipid profiles were obtained during follow‐up at 1.5, 3, 6, and 12 months and then annually. B‐mode ultrasonography was conducted semiannually. ALT and urine were examined at every visit. Drug adherence was assessed by pill count and participant report of usage. The annual visits involved a brief physical examination and dietary assessment." |
|
| Notes |
Funding for trial: "this study was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md (R01‐HL‐38194); Merck, Sharp and Dohme Research Laboratories, West Point, PA; and DuPont Pharmaceuticals, Wilmington, DE. Drugs were supplied by Merck, Sharp and Dohme (lovastatin), Du Pont Pharmaceuticals (warfarin), and Sterling Drug Company, NewYork, NY (aspirin)." Notable conflicts of interest of trial authors: no details given Protocol: NCT00000469 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The principal components of the randomization system were the computerised randomization list (devised in randomized blocks of 4 and 8) and the randomization program that confirmed participant eligibility and assigned the next identification number, which represented one of the four treatment groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "All data collection and adjudication was done by investigators who were unaware of treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The medications were formulated to maintain blinding of the participants and investigators." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All data collection and adjudication was done by investigators who were unaware of treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions reported with reasons and by study group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Hedblad 2001.
| Study characteristics | ||
| Methods |
Study design: primary‐prevention, randomised, double‐blind, placebo‐controlled study with factorial design Total duration of study: 3 years Details of any 'run‐in' period: no details given Number of study centres and location: single centre, Sweden Study setting and date of study: outpatients; November 1994 to February 1999 |
|
| Participants |
Number randomised: 793 participants Number lost to follow‐up/withdrawn: "168 participants were not included due to GSM protocol violation (i.e. 30, 46, 52 and 40 participants, respectively, in the four treatment groups). The reason for exclusion was withdrawal (n = 68), did not attend all visits (n = 53) or missing 36‐month follow‐up ultrasound examination (n = 47)." Number analysed: 793 participants Number of interest: 793 participants Mean age: 61.8 +/‐5.3 years Age range: 49 to 70 years Gender: 361 men and 432 women Severity of condition: no symptoms of carotid artery disease Diagnostic criteria: "plaque in right carotid artery, plaque > 10 mm2 at baseline and after 36‐month follow‐up, feasible for measurement of GSM." Smoking history: 244 smokers Inclusion criteria: "plaque in the right carotid artery but with no symptoms of carotid artery disease" Exclusion criteria: "history of MI, angina pectoris, or stroke within the preceding 3 months; history of surgical intervention in the right carotid artery; regular use of beta‐blockers or statins; blood pressure 160 (systolic) or 95 (diastolic) mmHg; total cholesterol 8.0 mmol/L; hyperglycaemia suspected to require insulin treatment; and conditions that in the opinion of the investigator rendered the person unsuitable for the trial." |
|
| Interventions |
Intervention: metoprolol CR/XL (25 mg once daily)/fluvastatin (40 mg once daily) Comparison: placebo/placebo, metoprolol CR/XL (25 mg once daily)/placebo, fluvastatin (40 mg once daily)/placebo Concomitant medications: lipid‐lowering therapy Excluded medications: no details given |
|
| Outcomes |
Primary outcomes: "change in mean IMT (IMTmean) in the common carotid artery (10‐mm long section) and change in maximum IMT (IMTmax) in the carotid bulb." Secondary outcomes: "adverse events, laboratory findings, mortality, and incidence of myocardial infarction and stroke." Time points reported: "During the first year, visits occurred after 1, 3, 6, and 12 months and every 6 months thereafter. Weight was measured every 6 months, and a fasting lipid profile (total cholesterol, LDL lipoprotein, HDL lipoprotein, and triglycerides) was determined every year. Liver transaminases (AST, ALT) and creatine kinase were obtained at every visit during the first year and then every year thereafter. AST or ALT values 3 times and creatine kinase values 10 times the upper limit of normal were considered elevated during the study. Carotid ultrasound investigation was performed at baseline and after 18 and 36 months of treatment." |
|
| Notes |
Funding for trial: "this study was supported by grants from AstraZeneca Pharmaceuticals, Mölndal, Sweden." Notable conflicts of interest of trial authors: "John Wikstrand was a former senior medical adviser at AstraZeneca, at present professor emeritus at the Wallenberg Laboratory for Cardiovascular Research at Sahlgrenska Academy at Gothenburg University, Sweden. There are no other conflicts of interest." Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly allocated to 1 of 4 treatment groups according to a factorial design." |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were randomly allocated to 1 of 4 treatment groups according to a factorial design." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "BCAPS was a randomized, double blind, placebo‐controlled, single center clinical trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The Data and Safety Monitoring Board, consisting of independent scientists with expertise in fields relevant to BCAPS, regularly monitored toxicity and blinded outcome data" and "Each image was analysed without knowledge of the subject’s randomization group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions reported with reasons |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Hu 2009.
| Study characteristics | ||
| Methods |
Study design: randomised Total duration of study: 12 weeks Details of any 'run‐in' period: no details given Number of study centres and location: 1 centre, Nanjing University Drum Tower Hospital, Nanjing, China Study setting and date of study: outpatients; 2006 to 2007 |
|
| Participants |
Number randomised: 43 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 43 participants Number of interest: 43 participants Mean age: 57.0 ± 1.4 Age range: no details given Gender: 23 men and 20 women Severity of condition: Type 2 diabetic patients Diagnostic criteria: "participants with significant carotid plaques were defined as carotid IMT > 1.2 mm. IMT ≥ 0.9 mm with or without carotid plaques were defined as having carotid atherosclerosis" Smoking history: no details given Inclusion criteria: "Type 2 diabetes was diagnosed based on diagnostic criteria of the American Diabetes Association." Exclusion criteria: "all participants had no history of heart, liver, kidney, and lung diseases, and had no overt acute or chronic infection, trauma, or surgery during the follow‐up period." |
|
| Interventions |
Intervention: 40 mg simvastatin Comparison: control group without simvastatin treatment Concomitant medications: routine medication (e.g. insulin, metformin, sulfonylurea) for glucose control Excluded medications: no details given |
|
| Outcomes |
Primary outcome: changes in adipokines and inflammation markers measurements Secondary outcomes: "Lipids in plasma and fractionated lipoproteins were analysed" Time points reported: "monthly during the three‐month study period" |
|
| Notes |
Funding for trial: "the study was partially supported by Chinese Natural Science Fund #30671004, Jiangsu Natural Science Fund #BK2006006, Nanjing Targeted Science and Technology Development Fund #ZKX06014." Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a single trained operator blind to the study group." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost to follow‐up/withdrawn not reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Ikeda 2013.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, open‐label, blinded end points, two‐arm, parallel treatment group Total duration of study: 1 year Details of any 'run‐in' period: no details given Number of study centres and location: 15 centres in Japan Study setting and date of study: outpatients; August 2007 to September 2009 |
|
| Participants |
Number randomised: 303 participants Number lost to follow‐up/withdrawn: "80 lost to follow‐up, withdrew consent, did not receive study drug, did not complete end point assessment or IMT was not performed or analysable" Number analysed: 223 participants Number of interest: 223 participants Mean age: 66.3 years Age range: 20 to 80 years Gender: 174 men and 129 women Severity of condition: no details given Diagnostic criteria: "LDL‐C at the time of enrolment was no less than 100 and common carotid IMT was 1.1 mm and over." Smoking history: 32 current smokers Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: pitavastatin, starting at 4 mg daily Comparison: pitavastatin, starting at 2 mg daily Concomitant medications: aspirin, ticlopidine, clopidogrel, beta‐blocker, RA inhibitor, PPAR‐g agonist, sulfonylurea, a‐GI, BG, insulin, calcium blocker, nitrate, diuretic, aldosterone blocker, warfarin, antiarrhythmic agent Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "absolute changes in carotid intima‐media thickness" Secondary outcomes:
Time points reported: 12 months |
|
| Notes |
Funding for trial: self‐funding Notable conflicts of interest of trial authors: no details given Protocol: UMIN000001229 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled patients were randomly assigned to intensive or moderate therapy in a 1:1 ratio." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was computer‐generated by a central randomization facility using a stratified randomization for prognostic factors including gender, presence or absence of diabetes mellitus (DM), age and history of coronary artery disease (CAD)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The data of carotid ultrasonography were all sent to a core center (Saiseikai Shiga Prefecture Hospital) and analyzed by one sonographer blinded to the randomization and all clinical information." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Kadoglou 2010.
| Study characteristics | ||
| Methods |
Study design: open‐label, randomised, prospective study Total duration of study: 12 months Details of any 'run‐in' period: no details given Number of study centres and location: 6 centres, Thessaloniki and Athens, Greece Study setting and date of study: "internal medicine due to cerebrovascular ischaemic events or individuals visiting our outpatient department with more than two cardiovascular risk factors" ; no details given |
|
| Participants |
Number randomised: 140 participants Number lost to follow‐up/withdrawn: "9 participants did not complete all measurements. 2 of them experienced TIA (group A), 1 had a heart attack (group A), 2 participants underwent coronary angioplasty (group B), 2 participants (1 in group A and 1 in group B) discontinued therapy due to liver enzymes elevation associated with atorvastatin usage and 2 participants from both groups were lost to follow‐up." Number analysed: 131 participants Number of interest: 90 participants (41 symptomatic participants excluded) Mean age: "64.76 +/‐ 7.31 in moderate lipid‐lowering therapy group (atorvastatin 10 mg) and 63.26 +/‐ 6.76 in aggressive lipid‐lowering therapy (atorvastatin 80 mg)." Age range: 50 to 75 years old Gender: 60 men and 71 women Severity of condition: carotid stenosis of at least one internal carotid artery Diagnostic criteria: "symptomatic subgroup had recently, within 10 days, experienced cerebrovascular event (non‐disabling ischaemic stroke, TIA, amaurosis fugax). After the co‐evaluation of medical history, neurological signs, and brain computed tomography and/or magnetic resonance imaging findings that event had been attributed to ipsilateral carotid stenosis. On the other hand, the absence of focal neurological symptoms and ischaemic lesions in CT and/or MRI scan characterised asymptomatic patients with carotid stenosis." Smoking history: 22 smokers Inclusion criteria: "people with carotid stenosis of at least one internal carotid artery (ICA), but without indications for carotid revascularisation." Exclusion criteria: "autoimmune or life‐threatening diseases, absence of discrete carotid plaques, indications for carotid revascularisation, recently diagnosed/untreated hypothyroidism, osteoporosis, coronary artery disease, overt cardiac‐origin symptoms, liver (ALT > 2.5 times higher than the upper normal limit) or renal (creatinine levels > 2.0 mg/dL) impairment, ongoing use of lipid‐lowering medications, and contraindications to the use of statins." |
|
| Interventions |
Intervention: atorvastatin (10 mg/day or 20 mg/day) to target LDL < 100 mg/dL Comparison: atorvastatin (80 mg/day) to target LDL < 70 mg/dL Concomitant medications: "an antiplatelet regimen (acetylsalicylic acid 100 mg/day or clopidogrel 75 mg/day) was prescribed to all participants. Concomitant antihypertensive and hypoglycaemic medications remained unaltered, unless it was considered medically necessary." Excluded medications: no details given |
|
| Outcomes |
Primary outcomes: measurement of the carotid plaque echogenicity, assessed by Gray‐Scale Median (GSM) score and measurement of the serum OPN and OPG levels Secondary outcomes: measurement of blood pressure, lipid and glycaemic indexes; hs‐CRP Time points reported: "ultrasound of both carotids was performed at baseline and at the end of the study. Blood samples were obtained after an overnight fast at baseline and at the end of the study." |
|
| Notes |
Funding for trial: no details given Notable conflicts of interest of trial authors: "the editors and reviewers of this article have no relevant financial relationships to disclose." Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Image acquisition and GSM measurements were performed by a single, experienced, operator blinded to patients’ history and assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Meaney 2009.
| Study characteristics | ||
| Methods |
Study design: randomised, comparative, open‐label trial Total duration of study: 12 months Details of any 'run‐in' period: no details given Number of study centres and location: 2 centres in Mexico Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 90 participants Number lost to follow‐up/withdrawn: 26 participants were removed from the study Number analysed: 90 participants Number of interest: 90 participants Mean age: "59 +/‐ 7 in group A, 57 +/‐ 8 in group B and 58 +/‐ 9 in group C." Age range: 40 to 72 years Gender: 44 men and 53 women Severity of condition: high‐risk coronary patients Diagnostic criteria: "10‐year absolute risk for coronary death or myocardial infarction > 20 according to the ATP III recommendations." Smoking history: no details given Inclusion criteria: "any gender, aged 40 to 72 years, with a 10‐year absolute risk for coronary death or MI > 20 according to the ATP III recommendations. None of the participants had received ezetimibe previously, but the vast majority of them had received statins, generally at low or very low doses." Exclusion criteria: "people with severe systemic diseases, including liver diseases, chronic renal failure, heart failure, malignancies, autoimmune diseases, AIDS, or a history of alcohol or other drug abuse, pregnant or fertile women without a totally reliable contraception method or breastfeeding mothers." |
|
| Interventions |
Intervention and comparison: Group A: pravastatin 40 mg once daily Group B: simvastatin 40 mg once daily Group C: combination of 20 mg of simvastatin and 10 mg of ezetimibe Concomitant medications: "if the therapeutic goals were not attained (< 100 mg/dL of low‐density lipoprotein cholesterol for type C and < 70 mg for type D), participants in group A received pravastatin 40 mg and ezetimibe 10 mg, group B received simvastatin 80 mg, and group C received simvastatin 40 mg and ezetimibe 10 mg." Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "change of IMT over the course of 1 year." Secondary outcomes: "changes in LDL and in high sensitive C‐reactive protein (CRPhs)" Time points reported: "the participants were evaluated every 2 months clinically and for the detection of secondary effects. Lipids were analysed at 2 months and 6 months after randomisation for titration purposes, as well as at the end of the trial 1 year later. Vascular ultrasounds and C‐reactive proteins (CRP) were conducted and measured, respectively, at the beginning and at the end of the trial." |
|
| Notes |
Funding for trial: "we acknowledge our gratitude to the following institutions that gave us unrestricted research grants: Merck Sharp & Dohme, Mexico; the Mexican Association for the Prevention of Atherosclerosis and its Complications (AMPAC); and the National Association of Cardiologists serving the State Employees (ANCISSSTE)" Notable conflicts of interest of trial authors: "the design of the study, the conduct of the trial, and the analysis of the data were done only by the investigators" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Ninety patients were randomly allocated to 1 of 3 groups of 30 patients each." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Each group was assigned a different open‐label treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Carotid IMT was measured by a trained ultrasonographer who was blinded to all clinical and treatment information." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Mercuri 1996.
| Study characteristics | ||
| Methods |
Study design: multicentre, parallel group, randomised, placebo‐controlled, double‐blind clinical trial Total duration of study: 3 years Details of any 'run‐in' period: "6 weeks single‐blind run‐in period in which they were treated with placebo and advised to follow a low‐fat diet meeting the recommendations of the European Atherosclerosis Society." Number of study centres and location: seven Lipid Clinics of Academic Medical Centres (Universities of Milan, Padua, Trieste, Bologna, Perugia, Rome and Naples), Italy Study setting and date of study: outpatients; March 1991 to June 1995 |
|
| Participants |
Number randomised: 305 participants Number lost to follow‐up/withdrawn: "12 of 42 dropouts suffered a serious adverse event: 5 of 12 events (4 MIs and 1 angina requiring coronary revascularisation) were of cardiovascular origin with 3 occurring in the pravastatin‐treated group. Cancer was detected in 7 participants (3 in the pravastatin group and 4 in the placebo group)." Number analysed: 305 participants Number of interest: 305 participants Mean age: 55 years old Age range: 45 to 65 years Gender: 162 men and 143 women Severity of condition: hypercholesterolaemia Diagnostic criteria: "LDL cholesterol levels between 3.88 and 6.47 mmol/L and triglycerides level < 2.82 mmol/L." Smoking history: 73 smokers Inclusion criteria: "male and female outpatients from the seven participating centres, without symptoms, signs or clinical history of CHD were screened (people with controlled hypertension, taking ACE‐inhibitors were eligible). Eligibility required ultrasonographic evidence of at least one uncomplicated carotid atherosclerotic lesion (clinically asymptomatic) in which the IMT ranges between 1.3 and 3.5 mm. The selected participants had, on at least 3 baseline determinations, a low‐density lipoprotein (LDL) cholesterol level, calculated according to Friedewald formula, between 150 and 250 mg/dL." Exclusion criteria: "plasma triglycerides > 250 mg/dL; uncontrolled hypertension with diastolic BP > 95 mmHg; history of myocardial infarction, angina pectoris on chronic treatment, stroke, TIA, or intermittent claudication; regular use of lipid‐lowering agents, anticoagulants or calcium channel blockers; persistent liver function abnormalities; history of allergies or intolerance to HMG CoA reductase inhibitors; other serious medical conditions (cancer, Type I or II diabetes), endocrine disorders, excessive ethanol consumption (> 50 g/day); chronic smoking (> 10 cigarettes/day)." |
|
| Interventions |
Intervention: 40 mg pravastatin Comparison: placebo Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "progression of early uncomplicated carotid lesions." Secondary outcome: "assessment of the drug safety, the evaluation of the effects of treatments on blood lipids, and to monitor morbid and fatal events." Time points reported: "all participants were seen every 3 months at their respective referral clinical centres." |
|
| Notes |
Funding for trial: "Bristol‐Myers Squibb S.p.A. Italy, and in part by a grant from the Italian National Research Council." Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Independent co‐ordinating centre controlled allocation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Independent co‐ordinating centre controlled allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients were double blindly randomized to either pravastatin (40 mg once daily) or its placebo manufactured to exactly resemble the pravastatin tablets." and "Double‐blind: participants and personnel." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The video‐recorded examinations were interpreted centrally by readers masked to patient information using image processing workstations (PC with 286 microprocessors, image processing)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported Quote: "ITT used, 13% dropped out" |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Nohara 2012.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, open‐label, blinded end‐point evaluation, multicentre, parallel‐group, comparative study Total duration of study: 1 year Details of any 'run‐in' period: no details given Number of study centres and location: 1 centre in Japan Study setting and date of study: outpatients; June 2008 to April 2011 |
|
| Participants |
Number randomised: 348 participants Number lost to follow‐up/withdrawn: 50 lost to follow‐up Number analysed: 314 participants Number of interest: 314 participants Mean age: mean age of participants was 63.9 +/‐ 8.9 years in rosuvastatin group and 63.3 +/‐ 9.1 years in pravastatin group Age range: 20 years and older Gender: 155 men and 159 women Severity of condition: no details given Diagnostic criteria: "hypercholesterolaemia and a maximum IMT ≥ 1.1 mm as measured with B‐mode ultrasound at the posterior wall of the common carotid artery." Smoking history: 61 current smokers Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: "5 mg rosuvastatin orally administered once daily for 2 years Target LDL‐C levels were 80 mg/dL for primary prevention, and 70 mg/dL for secondary prevention. If these levels were not achieved, doses were gradually increased (e.g. rosuvastatin (10 mg/day), rosuvastatin (10 mg/day) + another hypolipidemic drug)." Comparison: "10 mg pravastatin orally administered once daily for 2 years. Target LDL‐C levels were in compliance with JASGL (Japan Atherosclerosis Society Guidelines for Lipids) 2007. If these levels were not achieved, doses were gradually increased (e.g. pravastatin (20 mg/day), pravastatin (20 mg/day) + another hypolipidemic drug)." Concomitant medications: "the investigator in charge was allowed to administer combination therapy with anion‐exchange resin, probucol or EPA, if the increased dose of each test drug failed to reduce the target LDL‐C level." Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "the percent changes from baseline in mean‐IMT at the end of 24 months." Secondary outcomes:
Time points reported "Follow‐up visits were scheduled at 1, 2, 4, 6, 12, 18, and 24 months. At each visit, serum levels of lipids (LDL‐C, HDL‐C, and TG) were measured. Treatment compliance was also investigated at each follow‐up visit. Laboratory tests were performed at 1, 4, 6, 12, and 24 months. Laboratory data were analysed at the central laboratory. Systolic and diastolic blood pressure were measured at 0 (baseline), 12, and 24 months. Participants were scheduled to undergo ultrasonographic examinations at 0 (within 3 months before enrollment), 12, and 24 months." |
|
| Notes |
Funding for trial: a Japan Heart Foundation Research Grant supported this study Notable conflicts of interest of trial authors: no details given Protocol: UMIN000001174 "Trial terminated early because intensive therapy arm showed superiority to conventional therapy arm." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was computer‐generated by a central randomization facility using a dynamic allocation method with balancing factors of maximum IMT, serum LDL‐C level, presence/ absence of DM (including impaired glucose tolerance), and center." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was computer‐generated by a central randomization facility using a dynamic allocation method with balancing factors of maximum IMT, serum LDL‐C level, presence/ absence of DM (including impaired glucose tolerance), and center." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A single observer who was blinded to the treatment assignments measured the meanIMT in the core laboratory using Intimascope®" and "Open ‐ but assessor(s) are blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Norris 1990.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Total duration of study: 2 years Details of any 'run‐in' period: no details given Number of study centres and location: 3 centres, Universities of Toronto, Ottawa and London (Canada); Linkoping (Sweden) and Melbourne (Australia) Study setting and date of study: no details given |
|
| Participants |
Number randomised: 162 participants Number lost to follow‐up/withdrawn: 17 lost to follow‐up Number analysed: 145 participants Number of interest: no details given Mean age: no details given Age range: no details given Gender: no details given Severity of condition: asymptomatic carotid stenosis Diagnostic criteria: no details given Smoking history: no details given Inclusion criteria: no details given Exclusion criteria: no details given |
|
| Interventions |
Intervention: metoprolol and aspirin Comparison: placebo Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: evaluate carotid Doppler and clinical data Secondary outcome: no details given Time points reported: 18 months |
|
| Notes |
Funding for trial: no details given Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We conducted a randomised double‐blind placebo‐controlled trial of metoprolol and aspirin ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "We conducted a randomised double‐blind placebo‐controlled trial of metoprolol and aspirin ..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Low risk | No details given |
Reid 2005.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind trial Total duration of study: no details given Details of any 'run‐in' period: no details given Number of study centres and location: Vascular Surgery Unit of Belfast City Hospital Study setting and date of study: outpatients; August 2001 to February 2003 |
|
| Participants |
Number randomised: 28 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 28 participants Number of interest: 28 participants Mean age: 70 (1.5) years in placebo group and 71 (1.3) years in pravastatin group Age range: no details given Gender: no details given Severity of condition: carotid artery disease Diagnostic criteria: no details given Smoking history: 35 smokers Inclusion criteria: "people with carotid artery disease not undergoing surgery and with cholesterol concentration less than 5.5 mmol/L." Exclusion criteria: "patients were excluded if they were already on a cholesterol‐lowering drug or had previous carotid endarterectomy." |
|
| Interventions |
Intervention: pravastatin 40 mg daily Comparison: placebo Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: combined measure of IMT of the right and left CCAs Secondary outcome: serological measurements of cholesterol concentration Time points reported: 3, 6, and 9 months following randomisation |
|
| Notes |
Funding for trial: Bristol‐Myers Squibb and Northern Ireland Chest Heart and Stroke Association Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly assigned in blocks of four but not described how it was done |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were randomly assigned in blocks of four in a blinded fashion to receive treatment with either pravastatin 40 mg daily or placebo"; and "One operator, blinded to patient treatment or randomisation performed all the scans." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The IMT was calculated using a computer program removing any observer bias." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of lost to follow‐up/withdrawn not reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Salonen 1995.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐masked, placebo‐controlled, single centre study Total duration of study: 3 years Details of any 'run‐in' period: 2 month placebo lead‐in period Number of study centres and location: 1 centre, Research Institute of Public Health, University of Kuopio, Finland Study setting and date of study: outpatients; January 1990 to 1993 |
|
| Participants |
Number randomised: 447 participants Number lost to follow‐up/withdrawn: "during the study, 39 participants discontinued study medication: 16 in the pravastatin group and 23 in the placebo group. Of these discontinuations, 20 were due to adverse events (pravastatin 8, placebo 12); six participants died, 3 in each group; 5 participants discontinued at their own request (pravastatin 3, placebo 2); 2 participants in the placebo group were lost to follow‐up; 4 participants, 2 in each group, were discontinued because of poor compliance with the protocol, and 2 participants in the placebo group were discontinued because they received prohibited lipid‐lowering medication." Number analysed: 424 participants Number of interest: 424 participants Mean age: 57.3 years Age range: 44 to 65 years Gender: 424 men Severity of condition: hypercholesteraemic men Diagnostic criteria: "LDL levels of 4.25 mmol/L or more and body mass index of 32 kg/m2 or less." Smoking history: 117 current smokers and 196 former smokers Inclusion criteria: "serum LDL > 4.25 mmol/L, serum total cholesterol < 8.0 mmol/L, body mass index < 32 kg/m2, and liver enzymes (ALT and ASAT) not exceeding 1.5‐fold the laboratory upper normal limit." Exclusion criteria: no details given |
|
| Interventions |
Intervention: pravastatin 40 mg once daily at bedtime Comparison: placebo Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "rate of carotid atherosclerotic progression" Secondary outcomes: "rate of atherosclerotic progression in the far walls of the common carotid artery, bulb and femoral artery individually, and the combined outcome of the carotid and femoral arteries." Time points reported: "the participants visited the study centre at 3‐month intervals." |
|
| Notes |
Funding for trial: "this study was supported by grants from the Academy of Finland and the Bristol‐Myers Squibb Pharmaceutical Research Institute, Princeton, NJ." Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified to obtain equal distribution over the treatment groups and to enable statistical tests of effect modification" and "Regular smokers (at least 10 cigarettes/d) and nonsmokers (for the purpose of stratified randomization defined as less than 10 cigarettes/d) and subjects with and without atherosclerotic lesions at their baseline ultrasound examination were randomized separately" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization scheme was generated by a KAPS biostatistician." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All subjects were entered into the double‐masked phase. Double‐masked treatment units were prepared at the Bristol‐Myers Squibb Pharmaceutical Research Institute, Moreton, UK, which also provided the drug supplies. Placebo and pravastatin tablets looked identical." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To assure the masking of the investigators and other staff, the lipid values were kept in a data register, to which there was no access for investigators other than the chief lipid chemist (KN)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Sawayama 2002.
| Study characteristics | ||
| Methods |
Study design: randomised Total duration of study: 2 years Details of any 'run‐in' period: no details given Number of study centres and location: 1 centre, Fukuoka, Japan Study setting and date of study: outpatient; February 1996 to February 2000 |
|
| Participants |
Number randomised: 246 participants Number lost to follow‐up/withdrawn: "34 (21%) of the 165 participants in the intent‐to‐treat population did not complete the study." Number analysed: 246 participants Number of interest: 246 participants Mean age: 66 years Age range: 30 to 89 years old Gender: 77 men and 169 women Severity of condition: asymptomatic hypercholesteraemic patients Diagnostic criteria: serum total cholesterol level of at least 220 mg/dL Smoking history: 146 smokers Inclusion criteria: "1) primary hypercholesterolaemia (defined as a serum total cholesterol level of at least 220 mg/dL); and 2) treatment with either probucol or pravastatin" Exclusion criteria: "exclusion criteria included a serum triglyceride level > 350 mg/dL; uncontrolled heart failure; recent (< 6 months) MI; severe or unstable angina pectoris; hypothyroidism/hyperthyroidism or other endocrine diseases; secondary hyperlipidaemia; uncontrolled diabetes mellitus; uncontrolled hypertension; heavy drinking; obese patients on weight reduction programs; diseases that might interfere with drug absorption; any severe illness; and treatment with certain drugs, including corticosteroids, other lipid‐lowering agents or antacids containing aluminium salts." |
|
| Interventions |
Intervention and comparison:
Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "rate of progression of carotid atherosclerosis" Secondary outcome: "incidence of major atherosclerotic events, as effected by each treatment." Time points reported: "ultrasonography was performed at enrolment and then every six months for the next 24 months." |
|
| Notes |
Funding for trial: Japanese Ministry of Education, Science, and Culture, Tokyo, Japan Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by the minimization method, controlling for the following four factors: total cholesterol level, age, gender and IMT." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were randomly assigned to one of the following three groups: 1) a probucol group (n 82, age 41 to 80 years) that received probucol at 500 mg twice daily after meals; 2) a pravastatin group (n 83, age 41 to 89 years) that received pravastatin at 10 mg/day after the evening meal; and 3) a control group (n 81, age 30 to 89 years) that was on diet alone." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All examinations were performed by one trained physician who had no knowledge of the clinical history and risk factor profile of the subjects." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Semplicini 2000.
| Study characteristics | ||
| Methods |
Study design: double‐blind, randomised, parallel study Total duration of study: 3 months Details of any 'run‐in' period: 4‐week single‐blind placebo period Number of study centres and location: no details given, Italy Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 15 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 15 participants Number of interest: 15 participants Mean age: no details given Age range: 55 to 75 years Gender: 13 men and 2 women Severity of condition: essential hypertension Diagnostic criteria: "at least one stenosis (50% to 70%) of an internal carotid artery." Smoking history: no details given Inclusion criteria: "essential hypertensive were selected from the outpatient clinic database because of the presence of at least one moderate (30% to 60%) stenosis of the internal carotid arteries at echocolor Doppler examination." Exclusion criteria: "secondary hypertension was excluded by means of standard biochemical and radiological imaging tests, all had a negative history of cerebrovascular diseases." |
|
| Interventions |
Intervention: lacidipine (4 to 6 mg once daily, orally) Comparison: hydrochlorothiazide (HCTZ, 25 to 50 mg once daily orally) Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "measure of mean relative perfusion (MRP) in the cortical and subcortical areas (thalami and basal ganglia)." Secondary outcome: clinical (blood pressure) measurement Time points reported: regional cerebral perfusion was assessed at baseline and at the end of the treatment period with HMPAO‐SPECT (12 weeks) |
|
| Notes |
Funding for trial: "the study was made possible by a research grant from GlaxoWellcome." Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients were enrolled for a double‐blind, parallel study" and "The examination was carried out by the same sonographer who was not aware of the patient’s clinical data and treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "CT scans were examined twice by a single observer (C.C.) unaware of the patient identity." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up/withdrawn not reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Shinoda‐Tagawa 2002.
| Study characteristics | ||
| Methods |
Study design: randomised, single‐blind, controlled trial Total duration of study: 3 years Details of any 'run‐in' period: no details given Number of study centres and location: 2 centres in Japan Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 91 participants Number lost to follow‐up/withdrawn: 2 lost to follow‐up or withdrew consent Number analysed: 89 participants Number of interest: 89 participants Mean age: "mean age of patients was 61.0 +/‐7.2 years in control group and 60.3 +/‐ 7.9 years in cilostazol group." Age range: 41 to 75 years old Gender: 44 men and 45 women Severity of condition: no details given Diagnostic criteria: Type II diabetes Smoking history: no details given Inclusion criteria: "no episodes of ketoacidosis and absence of ketonuria; diagnosis of diabetes after 30 years of age; insulin therapy (if any) started after duration of diabetes for at least 5 years; absence of overt diabetic nephropathy or other renal tract disease; and absence of active diabetic proliferative retinopathy." Exclusion criteria: no details given |
|
| Interventions |
Intervention: cilostazol 100±200 mg/day Comparison: no treatment Concomitant medications: "oral hypoglycaemic agents, insulin, diuretics, beta‐blockers, alpha‐blockers, Ca‐channel blockers, and angiotensin converting enzyme inhibitors, clofibrates, probucol, and 3‐hydroxy‐3‐methylglutaryl coenzyme reductase inhibitors." Excluded medications: no details given |
|
| Outcomes |
Primary outcome: number of brain lesions and measure of IMT Secondary outcomes: clinical (blood pressure and API) and biochemical analysis Time points reported: "during the observation period of 3.2 +/‐ 0.5 years, the lipid profile, blood pressure, IMT and API were determined every year. Brain MRI was taken at the beginning and end of the study period." |
|
| Notes |
Funding for trial: no details given Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were allocated at random into two groups with and without cilostazol." |
| Allocation concealment (selection bias) | Low risk | Quote: "The subjects were allocated at random into two groups with and without cilostazol." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All scans were conducted by physicians who were unaware of the clinical characteristics of the subjects" and "The physicians evaluating MRI findings were unaware of patients' characteristics and IMT evaluation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary or secondary outcomes not reported |
| Selective reporting (reporting bias) | Unclear risk | Primary or secondary outcomes not reported |
| Other bias | Low risk | No other source of bias detected |
Stumpe 2007.
| Study characteristics | ||
| Methods |
Study design: multicentre, double‐blind, randomised Total duration of study: 104 weeks Details of any 'run‐in' period: initial 2‐week tapering‐off period Number of study centres and location: 31 clinical centres throughout Austria, the Czech Republic, Germany, Italy, and Poland Study setting and date of study: outpatients; November 2001 to February 2006 |
|
| Participants |
Number randomised: 165 participants Number lost to follow‐up/withdrawn: "discontinued (n = 35): adverse event (n = 6), lack of efficacy (n = 1), withdrawal of consent (n = 11), concomitant medication usage (n = 1), other reasons (n = 16)." Number analysed: analysed (n = 155): failed to provide efficacy data (n = 10) Number of interest: 155 participants Mean age: "62.1 +/‐ 6.6 years old in atenolol group and 62.3 +/‐ 7,4 years old in olmesartan group." Age range: 35 to 75 years old Gender: 95 men and 60 women Severity of condition: hypertensive patients Diagnostic criteria: "seated systolic blood pressure of 140 to 180 mmHg and seated diastolic blood pressure of 90 to 105 mmHg, an increased common carotid artery IMT of between 0.8 and 1.6 mm, at least one plaque in the CCA or the carotid bulb (plaque volume: 4 to 500 µl), and ≥ 1 of the following predefined risk factors: smoking, diabetes mellitus, dyslipidaemia (high‐density lipoprotein (HDL)‐cholesterol < 0.9 or low‐density lipoprotein (LDL)‐cholesterol > 2.6 or triglycerides > 1.7 mmol/L), left ventricular hypertrophy and history of cardiovascular disease, or complications of cardiovascular disease." Smoking history: 53 current smokers Inclusion criteria
Exclusion criteria:
|
|
| Interventions |
Intervention: olmesartan 20 mg Comparison: atenolol 50 mg Concomitant medications: "patients with uncontrolled BP (DBP > 90 mmHg and/or SBP > 140 mmHg) at these dose levels after 4 weeks of treatment were titrated to olmesartan 40 mg or atenolol 100 mg once daily. Hydrochlorothiazide at a dose of 12.5 mg with up‐titration to 25 mg after another 4 and 8 weeks, respectively, was added if BP remained uncontrolled." Excluded medications: no details given |
|
| Outcomes |
Primary outcome: change of intima media thickness of the common carotid artery on the leading side of the neck Secondary outcomes:
Time points reported: "at screening, participants underwent a complete physical examination, ultrasound measurements of IMT and PV were made and assessments of BP, and routine laboratory parameters were carried out. After randomisation, participants made 10 further visits to the study centres. Visits to ultrasound centres for measurements of IMT and PV were scheduled at screening, weeks 28, 52 and 104." |
|
| Notes |
Funding for trial: Sankyo Pharma Gmbh Notable conflicts of interest of trial authors: no details given Protocol: NCT00185185 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation list was prepared centrally by PRA International, Mannheim, Germany, using appropriate blocks and guaranteeing that in study centres patients were assigned to one of the treatment groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated randomisation list was prepared centrally by PRA International, Mannheim, Germany, using appropriate blocks and guaranteeing that in study centres patients were assigned to one of the treatment groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study medication was provided in externally indistinguishable capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded ultrasound readings and quality assessment evaluations were carried out using a specifically designed 2D and 3D Post Processing Image Analysis System (PPAS) with an option for re‐performing measurements on the MODs." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Sutton‐Tyrrell 1994.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled, stepped‐care treatment programme Total duration of study: 2 years Details of any 'run‐in' period: "participants were monitored at multiple drug evaluation visits during a 2‐ to 8‐week period to determine blood pressure eligibility off medication" Number of study centres and location: 1 centre, University of Pittsburgh Centre Study setting and date of study: outpatients; June 1984 to October 1996 |
|
| Participants |
Number randomised: 129 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 129 participants Number of interest: 129 participants Mean age: 75 years old Age range: 60 to 100 years old Gender: 49 men and 80 women Severity of condition: isolated systolic hypertension Diagnostic criteria: SBP 160 to 219 mmHg and DBP < 90 mmHg Smoking history: 48 smokers Inclusion criteria
Exclusion criteria: "persons were excluded on the basis of history and/or signs of specified major cardiovascular diseases. Other major diseases (e.g. cancer, alcoholic liver disease, established renal dysfunction), with competing risk for the SHEP (Systolic Hypertension in the Elderly Program) primary end point or the presence of medical management problems, were also exclusions" |
|
| Interventions |
Intervention: chlorthalidone 12.5 mg daily Comparison: placebo Concomitant medications: "drug dosage was doubled (including matching placebo) for participants failing to achieve the SBP goal at follow‐up visits. If the SBP goal was not reached at the maximal dose of step 1 medication, atenolol, 25 mg/d, or matching placebo was added as the usual step 2 drug. When atenolol was contraindicated, reserpine, 0.05 mg/d, or matching placebo could be substituted. When required to reach the blood pressure goal, the dosage of the step 2 drug could be doubled. Potassium supplements were given to all participants who had serum potassium concentrations below 3.5 mmol/L at two consecutive visits" Excluded medications: no details given |
|
| Outcomes |
Primary outcome: total stroke Secondary outcomes: sudden cardiac death, rapid cardiac death, nonfatal MI, fatal MI, left ventricular failure, other cardiovascular death—presumed myocardial infarction that did not meet diagnostic criteria, or other cardiovascular causes, TIA, coronary artery therapeutic procedures, renal dysfunction Ancillary study outcomes: "determine progression of carotid artery stenosis" Time points reported: "2 serial duplex scans of the carotid arteries separated by 2 years were obtained" |
|
| Notes |
Funding for trial: "SHEP trial was supported by contracts with the National Heart, Lung, and Blood Institute and the National Institute on Aging. Drugs were supplied by the Lemmon Co, Sellersville, Pa; Wyeth Laboratories/Ayerst Laboratories, AH Robins Co, Richmond, Va; and Stuart Pharmaceuticals, Wilmington, Del" "This ancillary study was supported by National Institutes of Health grant HL‐39871" Notable conflicts of interest of trial authors: no details given Protocol: NCT00000514 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "screeners were randomly allocated by the coordinating centre to one of two treatment groups. Randomization was stratified by clinical centre and by anti hypertensive medication status at initial contact." |
| Allocation concealment (selection bias) | Low risk | Quote: "screeners were randomly allocated by the coordinating centre to one of two treatment groups. Randomization was stratified by clinical centre and by anti hypertensive medication status at initial contact." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were to be randomized at each centre to either chlorthalidone or matching placebo in a double‐blind manner." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Scans were recorded on videotape for later scoring. A reader assigned a grade from 0 to 3 to each of seven segments in the carotid system based on the number and size of lesions present." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants apparently completed the study. No treatment withdrawals, no losses to follow‐up, no trial group changes and no major adverse events were reported |
| Selective reporting (reporting bias) | High risk | One weakness of this study is that the duplex scans were not obtained earlier in the study, before treatment. Unfortunately, the SHEP trial ended before all participants had completed their follow‐up scans. At the beginning of the study, a decision was made that progression of disease would include all areas of the carotid system, not just the ICA. Before analysis of the data, changes in the blood flow velocity and velocity ratios were used to ascertain progression |
| Other bias | Low risk | No other source of bias detected |
Tang 2009.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, single‐centre, double‐blind clinical trial Total duration of study: 12 weeks Details of any 'run‐in' period: no details given Number of study centres and location: single‐centre, GSK Investigational Site, Cambridge, Cambridgeshire, UK Study setting and date of study: outpatients; July 2006 to August 2007 |
|
| Participants |
Number randomised: 47 participants Number lost to follow‐up/withdrawn: "7 patients did not complete the study because of an adverse event (n = 2; both were in the high‐dose group and had deranged liver function tests during the study that were outside the limits of acceptability from the protocol), withdrawn consent (n = 1), or other reasons not associated with this specific study (n = 4)." Number analysed: 40 participants Number of interest: 40 participants Mean age: 67.6 +/‐ 7.7 years Age range: 18 years to 80 years Gender: 36 men and 4 women Severity of condition: "clinically documented atherosclerotic carotid disease." Diagnostic criteria: "clinically documented atherosclerotic carotid disease and had demonstrated the presence of inflammation within their carotid lesions on USPIO‐enhanced MRI regardless of symptomatic status." Smoking history: 30 current or former smokers Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: 80 mg atorvastatin once daily Comparison: 10 mg atorvastatin once daily Concomitant medications: antiplatelets Excluded medications: no details given |
|
| Outcomes |
Primary outcomes: "changes from baseline in USPIO‐enhanced MRI signal in carotid plaques at 6 weeks and 12 weeks in low‐ and high‐dose atorvastatin groups (within‐groups comparison) Secondary outcomes: "baseline corrected changes in USPIO‐enhanced MRI signal in carotid plaques. Changes from baseline in tensile stress, micro‐emboli counts, soluble plasma biomarker at 12 weeks in low‐ and high‐dose atorvastatin groups " Time points reported: "the USPIO‐enhanced MRI was performed at baseline (i.e. before randomisation) and at 6 and 12 weeks." |
|
| Notes |
Funding for trial: GlaxoSmithKline Notable conflicts of interest of trial authors: none Protocol: NCT00368589 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients in the ATHEROMA trial were randomized (1:1) to receive low‐ (10 mg) and high‐ (80 mg) dose atorvastatin for 12 weeks." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Unblinding of the treatment assignment occurred only after this had happened to avoid bias, and permitted independent confirmation of the analyses." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The readers were blind to the patients’ demographic data and statin dose" and "The spectra from all saved ES (emboli signal) were recorded onto the hard drive of the computer, and all signals were later reviewed offline in consensus by 2 experienced observers in ES detection who were blinded to the patients’ demographic and lipid profiles." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Terpstra 2004.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind, single‐centre trial Total duration of study: 2 years Details of any 'run‐in' period: "participants with hypertension and aged between 60 and 75 years were selected for the study and advised to restrict their salt intake (low‐salt diet). After another period of 4 weeks, blood pressure was measured for the fifth time and hypertensive patients who met the inclusion criteria received placebo treatment for 2 weeks. If blood pressure remained stable during this run‐in period, the patients were randomly assigned to the double‐blind treatment phase." Number of study centres and location: 1 centre in the Netherlands Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 166 participants Number lost to follow‐up/withdrawn: "reasons for not completing the study in the amlodipine group (n = 24) were: adverse events (14), withdrawal of informed consent (6), violation of procedure (2), death (1), and other (1). Reasons for not completing the study in the lisinopril group (n = 22) were: adverse events (11), withdrawal of informed consent (4), violation of procedure (4), and other (3)" Number analysed: 166 participants Number of interest: 166 participants Mean age: 67+/‐4 years Age range: 60 to 75 years old Gender: 92 men and 74 women Severity of condition: untreated mild to moderate hypertension Diagnostic criteria: "four measurements of DBP were between 95 and 115 mmHg or SBP was between 160 and 220 mmHg (or both), derived from several measurements made on three occasions over a period of 4 weeks" Smoking history: 68 current smokers Inclusion criteria
Exclusion criteria:
|
|
| Interventions |
Intervention: amlodipine 5 to 10 mg Comparison: lisinopril 10 to 20 mg Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: change from baseline of the combined mean maximum far wall IMT of carotid and femoral arteries Secondary outcome: changes in maximum far wall IMT of the common carotid artery and the common femoral artery Time points reported: "before and after 1 and 2 years of treatment, IMT was measured in three carotid and two femoral arterial sites by B‐mode ultrasound" |
|
| Notes |
Funding for trial: "the study was sponsored by an unrestricted grant of Pfizer BV" Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were randomly assigned to the double‐blind treatment phase." |
| Allocation concealment (selection bias) | Low risk | Quote: "166 patients were allocated randomly to groups to receive amlodipine or lisinopril." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patients were randomly assigned to the double‐blind treatment phase." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All images were saved on S‐VHS tape and analysed off‐line throughout the study by an analyst who was unaware of the patients’ characteristics." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Underhill 2008.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, multicentre trial Total duration of study: 2 years Details of any 'run‐in' period: "all cholesterol‐lowering drugs were discontinued during a 6‐week dietary lead‐in period, after which baseline serum lipid values were obtained." Number of study centres and location: 2 centres, University of Washington, Seattle, WA, and the University of Utah, Salt Lake City, Utah Study setting and date of study: outpatients; 6 January 2000 (first participant enrolled), to 15 August 2004 (last participant completed) |
|
| Participants |
Number randomised: 43 participants Number lost to follow‐up/withdrawn: "4 of 43 patients did not complete the study because of an adverse event (n = 2), withdrawn consent (n = 1), or other reasons (n = 1). Of the 39 participants who completed the study, all remained asymptomatic and 33 (n low = 13, n high = 20) had matched baseline and 2‐year scans of sufficient image quality for identification of the vessel boundaries and automated compositional analysis." Number analysed: 33 participants Number of interest: 33 participants Mean age: 65.2 years Age range: 18 years and older Gender: 21 men and 12 women Severity of condition: neurologically asymptomatic patients Diagnostic criteria: "fasting low‐density lipoprotein cholesterol ≥ 100 and b250 mg/dL and 16% to 79% carotid stenosis by duplex ultrasound." Smoking history: 7 current smokers Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: rosuvastatin low dose (5 mg) Comparison: rosuvastatin high dose (40/80 mg/d) Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: changes in carotid wall volume as measured by MRI scan Secondary outcomes:
Time points reported
|
|
| Notes |
Funding for trial: "this research was supported by AstraZeneca, London, UK, and the National Institutes of Health, Bethesda, MD (T‐32, HL07838)" Notable conflicts of interest of trial authors: no details given Protocol: NCT00654394 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Study participants were randomized to receive rosuvastatin low dose (5 mg) or high dose (40/80 mg/d) for 2 years." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The randomized, double‐blind ORION trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "33 patients had matched serial MRI scans to compare by reviewers blinded to clinical data, dosage, and temporal sequence of scans." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
VHAS 1998.
| Study characteristics | ||
| Methods |
Study design: prospective, multicentre, randomised, parallel‐group, clinical trial Total duration of study: 4 years Details of any 'run‐in' period: "all eligible participants entered a placebo run‐in period of 3 weeks after discontinuation of any previous antihypertensive therapy" Number of study centres and location: 8 Italian centres Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 1414 participants Number lost to follow‐up/withdrawn: "in total, 1099 participants completed the 2‐year treatment period; 315 dropped out (21.6% of the verapamil group and 22.9% of the chlorthalidone group)" Number analysed: 1414 participants Number of interest: 183 participants Mean age: mean age of participants was 54.2 years Age range: 40 to 65 years Gender: 693 men and 721 women Severity of condition: no details given Diagnostic criteria: "essential hypertension (sitting systolic blood pressure > 160 mmHg and diastolic blood pressure > 95 mmHg)" Smoking history: 256 current smokers Inclusion criteria: "essential hypertension defined as a systolic blood pressure when seated equal to or greater than 160 mmHg and a diastolic blood pressure equal to or greater than 95 mmHg (Korotkoff phase V) measured at the end of a placebo run‐in period of 3 weeks; aged 40–65 years; either sex; gave informed consent to participate in the study" Exclusion criteria: "major exclusion criteria were all forms of secondary hypertension, a recent history (less than 6 months ago) of cerebrovascular events (TIA, strokes) or MI, unstable angina requiring continuous drug treatment, severe peripheral artery disease (grades III and IV of Fontaine’s classification), severe bradycardia (a heart rate < 50 beats/min), sick sinus syndrome, atrioventricular blockage of degrees II and III, heart failure (New York Heart Association classes II–IV), clinically significant renal insufficiency (a serum creatinine level > 1.7 mg/dL), hepatic insufficiency (serum aspartate (AST) and alanine (ALT) aminotransferase levels greater than twice the upper normal limit, an albumin:globulin ratio < 1, a total serum bilirubin level > 2 mg/dL), hyperuricaemia (> 7 mg/dL), hypokalaemia (< 3.8 mmol/L), type I diabetes mellitus and uncontrolled type II diabetes mellitus, familial dyslipidaemia, any serious concomitant disease or condition or medication that might have interfered with the study (patients being administered antihypertensive agents, antiarrhythmic drugs, nitrates, steroidal and nonsteroidal anti‐inflammatory agents and analgesics in chronic administration were excluded), known intolerance to calcium antagonists, diuretics or angiotensin converting enzyme inhibitors." |
|
| Interventions |
Intervention: verapamil slow‐release (240 mg once a day) Comparison: chlorthalidone (25 mg once a day) Concomitant medications: captopril 25 mg once or twice a day Excluded medications: "other antihypertensive agents, antiarrhythmic drugs, nitrates, steroidal and nonsteroidal anti‐inflammatory agents and analgesics in chronic administration" |
|
| Outcomes |
Primary outcomes: clinical assessment (blood pressure and heart rate measurement) and safety assessment: 12‐lead electrocardiogram and laboratory evaluations (determinations of serum glucose, creatinine, total and high‐density lipoprotein (HDL) cholesterol, triglycerides, urate, blood urea nitrogen, AST, ALT, sodium, and potassium levels) Secondary outcomes: "determine the prevalence of carotid thickenings and atherosclerotic lesions" Time points reported: "B‐mode ultrasound scan was performed according to a standardized procedure at baseline and after 3, 12, 24, 36 and 48 months of treatment." |
|
| Notes |
Funding for trial: no details given Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the end of the 3 weeks’ placebo run‐in period, eligible patients were randomly assigned either to verapamil at 240 mg or chlorthalidone at 25 mg once a day." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The VHAS (The Verapamil‐Hypertension Atherosclerosis Study) was a multicentre randomized double‐blind (for the first 6 months, open subsequently)." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The VHAS (The Verapamil‐Hypertension Atherosclerosis Study) was a multicentre randomized double‐blind (for the first 6 months, open subsequently)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All prespecified outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures have been reported in the results section |
| Other bias | Low risk | No other source of bias detected |
Yamada 2009.
| Study characteristics | ||
| Methods |
Study design: open‐label, single‐centre, prospective randomised study Total duration of study: 6 months Details of any 'run‐in' period: no details given Number of study centres and location: 1 centre, Gifu University Graduate School of Medicine, Japan Study setting and date of study: outpatients; April 2008 to September 2013 |
|
| Participants |
Number randomised: 40 participants Number lost to follow‐up/withdrawn: all 40 participants completed the study Number analysed: 40 participants Number of interest: 40 participants Mean age: 72 +/‐ 7.1 years old Age range: 50 to 84 years old Gender: 31 men and 9 women Severity of condition: "asymptomatic carotid artery stenosis (30% to 60%)" Diagnostic criteria: non‐ or slight hypercholesterolaemia (total cholesterol < 240 mg/dL) Smoking history: 10 smokers Inclusion criteria: "non‐ or slight hypercholesterolaemia (total cholesterol < 240 mg/dL), asymptomatic carotid artery stenosis (30% to 60%) based on carotid ultrasonography and magnetic resonance (MR) angiography." Exclusion criteria: "patients with carotid stenosis > 60% were excluded because the Asymptomatic Carotid Atherosclerosis Study recommended performing carotid endarterectomy in patients with asymptomatic carotid artery stenosis > 60%. Patients with carotid stenosis < 30% were also excluded because tissue characterisation of carotid plaques by IBS ultrasound was not available due to the relatively large size of the region of interest." |
|
| Interventions |
Intervention: atorvastatin 20 mg/day Comparison: diet Concomitant medications: aspirin, ticlopidine, cilostazol, diuretic, calcium channel blockers, beta‐blockers, ACE inhibitors, ARBs Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "the property change in carotid artery plaque after three and six months using IB (integrate backscatter) echo and Black Blood MRI" Secondary outcomes: "1) the change of the serum lipid metabolism in six months: Serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides; 2) the change of the inflammatory marker in six months: serum high sensitivity CRP; 3) ischaemic attack in the territory of the internal carotid artery on the ipsilateral side." Time points reported: "IBS values of carotid artery plaques and maximum intima media thickness were measured at baseline and after 6 months of either diet or statin therapy." |
|
| Notes |
Funding for trial: self funding Notable conflicts of interest of trial authors: no details given Protocol: UMIN000001114 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomized to a statin (atorvastatin 20 mg/day) treatment group (n = 20) or a diet group (n = 20)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A single well‐trained operator performed all carotid scans without having any information on the clinical characteristics of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other source of bias detected |
Yamamoto 2011.
| Study characteristics | ||
| Methods |
Study design: multicentre, prospective, randomised, open, blinded end‐point trial Total duration of study: 18 months Details of any 'run‐in' period: no details given Number of study centres and location: no details given, Japan Study setting and date of study: outpatients; February 2006 to 2011 |
|
| Participants |
Number randomised: 57 participants Number lost to follow‐up/withdrawn: "of 29 patients in the losartan‐based treatment group, 3 were lost to follow‐up: death due to infection (n = 1) and withdrawal of informed consent (n = 2). In the amlodipine‐based treatment group, 5 of 29 patients were lost to follow‐up: sudden death (n = 1), withdrawal of informed consent (n = 1) and other (n = 3)" Number analysed: 57 participants Number of interest: 57 participants Mean age: "61±13 in losartan group and 61±9 in amlodipine group" Age range: 20 years and above Gender: 45 men and 12 women Severity of condition: "mild‐to‐moderate hypertension, LV hypertrophy, diastolic dysfunction and preserved systolic function" Diagnostic criteria: "hypertensive patients with LV hypertrophy and diastolic dysfunction." Smoking history: no details given Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention: losartan 50 mg once daily Comparison: amlodipine 2.5 mg once daily Concomitant medications: thiazide diuretics or alpha‐blockers. Other medications: statins and antiplatelet agents Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "assess LV diastolic function and atherosclerosis of the carotid artery" Secondary outcomes: effects in blood pressure, measurement of laboratory blood samplings (creatinine, uric acid, PIIIP, CITP, brain natriuretic peptide and high‐sensitivity C‐reactive protein). Time points reported: "Doppler echocardiography and blood sampling will be conducted at study entry and every 6 months after randomisation. Carotid ultrasonography will be conducted at study entry and 12 and 18 months after randomisation." |
|
| Notes |
Funding for trial: "this study is supported by grants and endowments from Banyu Pharmaceutical through the Osaka Heart Club." Notable conflicts of interest of trial authors: no details given Protocol: UMIN Clinical Trials Registry: C000000319 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "assessor(s) are blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Zanchetti 2004.
| Study characteristics | ||
| Methods |
Study design: multicentre, longitudinal, randomly allocated, double‐blind, double‐dummy study, with a factorial structure (2x2) and four treatment groups Total duration of study: 3 years Details of any 'run‐in' period: "6‐week washout under triple placebo and American Heart Association low‐lipid diet." Number of study centres and location: 13 Italian hospitals Study setting and date of study: outpatients; March 1995 to June 2000 |
|
| Participants |
Number randomised: 508 participants Number lost to follow‐up/withdrawn: "93 patients had baseline data that did not exactly fulfill all entry criteria (with sitting DBP < 95 mmHg, with LDL cholesterol < 4.14 mmol/L < 160 mm/dL, and with IMTmax < 1.3 mm)." Number analysed: 508 participants Number of interest: 508 participants Mean age: 58.4 ± 6.7 years Age range: 45 to 70 years old Gender: 204 men and 304 women Severity of condition: "untreated or uncontrolled hypertension, hypercholesterolaemia, asymptomatic carotid atherosclerosis." Diagnostic criteria: "systolic blood pressure 150 to 210 mmHg, diastolic blood pressure 95 to 115 mmHg, serum low‐density lipoprotein cholesterol 4.14 to 5.17 mmol/L (160 to 200 mg/dL), and triglycerides < 3.39 mmol/L (< 300 mg/dL) and maximum carotid IMT, Tmax, 1.3 to 4.0 mm." Smoking history: 83 smokers Inclusion criteria: "men and women, aged 45 to 70 years, with a seated diastolic blood pressure of 95 to 115 mm Hg, serum LDL cholesterol between 160 and 200 mg/dL, and at least one uncomplicated atherosclerotic lesion in the carotid arteries with an intima–media thickness of between 1.3 mm and 4.0 mm." Exclusion criteria: no details given |
|
| Interventions |
Intervention and comparison
Concomitant medications: open‐label nifedipine GITS, 30 to 60 mg daily Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "rate of change in mean maximum IMT of the 8 far and near walls in distal common carotids and bifurcations bilaterally." Secondary outcomes:
Time points reported: "a complete carotid ultrasound examination were performed every 6 months." |
|
| Notes |
Funding for trial: Bristol Myers Squibb Italy, Rome, and Menarini, Florence Notable conflicts of interest of trial authors: "all authors have received research grants or lecture honoraria from the sponsors." Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer generated with a block size of 4." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and study personnel were blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Duplicate scans were read blindly during study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 20% dropouts reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other source of bias detected |
Zeng 2004.
| Study characteristics | ||
| Methods |
Study design: randomised, double controlled study Total duration of study: 36 months Details of any 'run‐in' period: washout period of 2 weeks Number of study centres and location: Chengdu No 2 Hosp, Chengdu, China Study setting and date of study: outpatients; no details given |
|
| Participants |
Number randomised: 286 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 286 participants Number of interest: 286 participants Mean age: no details given Age range: no details given Gender: no details given Severity of condition: hypercholesterolaemia Diagnostic criteria: no details given Smoking history: no details given Inclusion criteria: no details given Exclusion criteria: no details given |
|
| Interventions |
Intervention: pravastatin 20 to 40 mg/day Comparison: fish oil 9 g/day Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: measure of carotid plaque Secondary outcome: no details given Time points reported: "follow‐up 36 months and checked by B‐ultrasonography" |
|
| Notes |
Funding for trial: no details given Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two hundred eighty six patients with carotid plaques and hypercholesterolemia were assigned to a randomized double controlled study." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Zheng 2022.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled, multicentre parallel trial Total duration of study: 4 years Details of any 'run‐in' period: no details given Number of study centres and location: 25 centres, China Study setting and date of study: outpatients; 17 September 2015 to 29 January 2019 |
|
| Participants |
Number randomised: 543 participants Number lost to follow‐up/withdrawn: "126 of 543 patients did not complete the study: eligibility criteria not fulfilled (1), withdraw by subject (69), adverse event (28), patient specific reasons (3), lost to follow‐up (5), non‐compliance with study drug (4), met withdraw criteria (16)." Number analysed: 543 participants Number of interest: 543 participants Mean age: 59.4 years Age range: "Males aged ≥ 45 and < 70 years or females aged ≥ 55 and < 70 years." Gender: 239 men and 304 women Severity of condition: neurologically asymptomatic patients Diagnostic criteria: "Chinese adults (men aged ≥ 45 and < 70 years or women aged ≥ 55 and < 70 years) with subclinical atherosclerosis." Smoking history: 92 current smokers Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: 20 mg rosuvastatin tablets, orally once daily Comparison: placebo Concomitant medications: no details given Excluded medications: no details given |
|
| Outcomes |
Primary outcome: "annualized rate of change in mean of the maximum (MeanMax) CIMT measurements from each of the 12 carotid artery sites" Secondary outcomes: "(1) the annualized rate of change in the MeanMax CIMT of the near and far walls of the right and left CCA, carotid bulb, or ICA; (2) the annualized rate of change in the mean of the mean CIMT of the near and far walls of the right and left CCA; and (3) the percentage change from baseline in LDL‐C (low‐density lipoprotein cholesterol), total cholesterol, HDL‐C (high‐density lipoprotein cholesterol), triglycerides, non–HDL‐C, apoB, apo AI, non–HDL‐C/HDL‐C, and apoB/apo AI." Time points reported
|
|
| Notes |
Funding for trial: "this research was supported by AstraZeneca, London, UK, and the National Institutes of Health, Bethesda, MD (T‐32, HL07838)." Notable conflicts of interest of trial authors: "Michiel L. Bots declares no conflicts of interest, apart from being paid for his services by the organization that received the METEOR‐China grant from AstraZeneca to run the study. The payment went to UMC Utrecht. Drs Karlson, Zhao, Wei, and Meng are employees of AstraZeneca. The other authors report no conflicts." Protocol: NCT02546323 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was 1:1 using block size 4, stratified by ischemic CVD risk (<5% or 5%–<10%)" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was completed sequentially via an interactive web/voice‐response system" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects, investigators, study site personnel, sonographers, ultrasound image readers, and sponsor personnel involved with data review and analysis will remain blinded to the study treatment throughout the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects, investigators, study site personnel, sonographers, ultrasound image readers, and sponsor personnel involved with data review and analysis will remain blinded to the study treatment throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome measures have been reported in the results section |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other source of bias detected |
Zhu 2006.
| Study characteristics | ||
| Methods |
Study design: randomised, single‐centre, open‐label trial Total duration of study: 2 years Details of any 'run in' period: "Participants underwent 2‐week washout period during which they received 160 mg of micronized fenofibrate daily in combination with hypotensive agents (Benazepril 10 –20 mg/day and/or Amlodipine 5–10 mg/day) in an attempt to bring the blood pressure to < 140/90 mm Hg or achieve a 15% reduction of baseline blood pressure." Number of study centres and location: Clinical Medical College of Shandong University. Study setting and date of study: outpatients, September 2001 to June 2003 |
|
| Participants |
Number randomised: 225 participants Number lost to follow‐up/withdrawn: no details given Number analysed: 225 participants Number of interest: 225 participants Mean age: 61.1 (10.8) in control group and 60.3 (11.9) years in treatment group Age range: no details given Gender: 139 men and 86 women Severity of condition: essential hypertension Diagnostic criteria: blood pressure >140/90 mm Hg Smoking historxy: no details given Inclusion criteria: "The major inclusion criteria were total cholesterol > 5.20 mmol/L, LDL‐cholesterol > 3.40 mmol/L, or triglyceride > 2.30 mmol/L, carotid IMT > 1.0 mm, or atherosclerotic plaque > grade 1." Exclusion criteria: "Patients with diabetes mellitus, coronary artery disease, previous stroke, renal dysfunction, peripheral vascular disease, chronic inflammatory diseases, or malignant disease were excluded from the study. During the 2‐week washout period, participants unable to tolerate the medication or those with poor compliance or blood pressure control were excluded from the study." |
|
| Interventions |
Intervention: "160 mg of micronized fenofibrate daily + antihypertensive drug therapy (Benazepril 10 –20 mg/day and/ or Amlodipine 5–10 mg/day)." Comparison: "only antihypertensive drug therapy (Benazepril 10 –20 mg/day and/ or Amlodipine 5–10 mg/day)." |
|
| Outcomes |
Primary outcome: evaluation of carotid atherosclerosis Secondary outcomes: biochemical assays, incidence of stroke and adverse events Time points reported: at baseline and at the end of the observation period (24 months) |
|
| Notes |
Funding for trial: "This study was supported in part by Jinan Science and Technology Research Foundation, Jinan, China." Notable conflicts of interest of trial authors: no details given Protocol: no details given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quoted "Participants randomly assigned to the treatment group by research investigators" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quoted "To minimise the variation of sonography imaging, 2 sonographers, under blinded conditions, performed measurements, and the values of IMT and D were taken as the means of 10 measurements." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported lost to follow‐up/withdrawn |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | All prespecified outcomes reported. |
a‐GI: alpha‐glucosidase inhibitor;ACE: angiotensin‐converting‐enzyme; API: ankle pressure index; ALT: alanine aminotransferase; ARBs: angiotensin receptor blockers; AST: aspartate transaminase; ATP: Adult Treatment Panel; BG: biguanide; BP: blood pressure; CCA: common carotid artery; CCB: calcium‐channel blockers; CCr: clearance of creatinine; CHD: coronary heart disease; C‐IMT: carotid intima‐media thickness; CITP: carboxy‐terminal telopeptide of collagen type I; CK; creatine kinase; CPK: creatine phosphokinase; Cr: creatinine; CR/XL: controlled release/extended release; CVD: cardiovascular disease; DBP: diastolic blood pressure; ECG: electrocardiogram; EPA: ethyl icosapentate; ESRD: end‐stage renal disease; GITS: gastrointestinal therapeutic system; GSM: Gray‐Scale Median; HCTZ: hydrochlorothiazide ; HDL: high‐density lipoprotein ; HDL‐C: high‐density lipoprotein cholesterol; HMG‐CoA: hydroxymethylglutaryl‐coenzyme A reductase inhibitor; HMPAO‐SPECT: Technetium‐99m hexamethyl propyleneimine oxime; HPAQ: Habitual Physical Activity Questionnaire; hs‐CRP: high‐sensitivity C‐reactive protein; HYRIM: Hypertension High Risk Management trial; IBS: integrated backscatter; ICA: internal carotid artery; IL‐6: Interleukin 6; IMT: intima‐media thickness;IMT‐Cmax: maximum common carotid artery IMT;IMT‐Bmax: maximum carotid bulb IMT; INR: international normalised ratio; ITT: intention‐to‐treat; JASGL: Japan Atherosclerosis Society Guidelines for Lipids; LDL: low‐density lipoprotein; LDL‐C: low‐density lipoprotein cholesterol; LV: left ventricular; MACE: major adverse clinical events; max: maximum; MI: myocardial infarction; MRI: magnetic resonance imaging; NYHA: New York Heart Association; OPN: osteopontin; OPG: osteoprotegerin; PPAR‐g agonist: peroxisome proliferator‐activated receptor; PIIIP: carboxy‐terminal of procollagen type III; PV: plaque volume; RA inhibitor: renin–angiotensin inhibitor; RLP‐C: remnant‐like particles‐cholesterol; SBP: systolic blood pressure; sFasL: solubilised Fas ligand; TIA: transient ischaemic attack; TG: triglycerides; USPIO: ultra‐small superparamagnetic particles of iron oxide ; VHAS: The Verapamil‐Hypertension Atherosclerosis Study; VWA: vessel wall area
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anand 2018 | Ineligible population. Less than 50% of the population was of interest and data on the subgroup of interest were unavailable |
| Bondjers 2000 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Davidson 2012 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Duman 2007 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Esposito 2004 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Fayad 2011 | Ineligible population. The study did not evaluate carotid stenosis. Instead, it assessed arterial inflammation, defined as an arterial tissue‐to‐blood ratio (TBR) of 1.6 or higher. TBR was assessed as 18F‐FDG (F‐fluorodeoxyglucose) uptake, measured by PET/CT (positron emission tomography‐computed tomography) scan. It has been suggested that 18F‐FDG‐PET/CT could be used to measure inflammation within atherosclerosis plaque and potentially track its change with appropriate therapies. |
| Hosomi 2001 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Huang 2006 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Ichihara 2006 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Igase 2012 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Ito 2004 | Ineligible population. This study did not subgroup participants by IMT test value, and we were unable to extract data specific to our population of interest. |
| Koeijvoets 2005 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Laurora 1998 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Ludwig 2002 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Mazzone 2006 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Meuwese 2009 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Mizuguchi 2008 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Mok 2010 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Mortsell 2007 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Oyama 2008 | Ineligible population. This study did not subgroup participants by IMT test value, and we were unable to extract data specific to our population of interest. |
| Persson 1996 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Pontremoli 2001 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Saremi 2013 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Stanton 2001 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Stumpe 1994 | Ineligible population. The study excluded people with stenosis or plaques of the common carotid arteries and of the internal carotid arteries of 70% of luminal diameter. |
| Tasić 2006 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Vukusich 2010 | Ineligible population. This study did not subgroup participants by IMT test value, and we were unable to extract data specific to our population of interest. |
| Yamasaki 2010 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Yilmaz 2004 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
| Yokoyama 2005 | Ineligible population. The study’s participants had an IMT test value of less than 1.3 mm, meaning they did not have carotid stenosis according to our definition. |
IMT: intima‐media thickness
Characteristics of ongoing studies [ordered by study ID]
Aranzulla 2021.
| Study name | Carotid plaque stabilisation and regression with evolocumab (CARUSO) |
| Methods |
Study design: randomised, pre‐controlled, parallel‐assignment, single‐blinded (investigator, outcomes assessor) Number of study centres and location: 1 centre, Italy |
| Participants |
Number of participants: 130 Age: 18 to 80 years old Gender: all sexes Inclusion criteria: asymptomatic patients with uni‐ or bilateral carotid artery stenosis ≥ 50% and low‐density lipoprotein cholesterol (LDL‐C) values ≥ 100 mg/dL despite ongoing lipid‐lowering therapy Exclusion criteria
|
| Interventions |
Intervention: subcutaneous evolocumab 140 mg will be administered every 2 weeks on top of optimal lipid‐lowering therapy Comparison: no further treatment besides optimal lipid‐lowering therapy will be administered |
| Outcomes | Primary outcome measures: "(a) carotid plaque morphological stabilization at 6‐month follow‐up, defined as the disappearance of ulcerations and fluffy components, and achievement of a regular plaque morphology with prevalence of fibrous atheroma (type III or IV), estimated by DUS and/or MRI, or CT; and/or (b) carotid plaque regression at 12 months, defined as reduction of the entity of the stenosis and/or PSV by at least 5%, as compared with baseline" Secondary outcome measures: "absolute and percentage changes of LDL‐C values; HDL‐C [high‐density lipoprotein cholesterol], total cholesterol, triglicerides, Lp(a), and apoB will be also analyzed; collect data on adverse cerebrovascular and cardiac events (all‐cause mortality, cardiovascular mortality, stroke, myocardial infarction, any cardiac or peripheral revascularization)" |
| Starting date | 1 March 2021 |
| Contact information | Tiziana Claudia Aranzulla, MD, +390115085038, taranzulla@mauriziano.it |
| Notes | Funding for trial: Azienda Ospedaliera Ordine Mauriziano di Torino |
Differences between protocol and review
Objectives
Aiming to better reflect our intentions, we changed our objectives from “To assess the effects of pharmacological interventions for the treatment of asymptomatic carotid stenosis, to prevent neurological impairment, stroke, disability, death, and other complications” in the protocol (Clezar 2020), to “To assess the effects of pharmacological interventions for the treatment of asymptomatic carotid stenosis in preventing neurological impairment, ipsilateral major or disabling stroke, death, major bleeding, and other outcomes” in the review.
Types of interventions
We clarified that alternative comparators, such as fish oil and diet, were also eligible for inclusion and would be considered as 'no treatment'.
Assessment of risk of bias in included studies
We did not encounter any cluster‐RCTs. Should we find eligible cluster‐RCTs in future updates of this review, we will consider additional biases specific to these types of studies, as recommended in section 8.15.1.1 of the Cochrane Handbook for Systematic Reviews of Interventions: 1) recruitment bias; 2) baseline imbalance; 3) loss of clusters; 4) incorrect analysis; and 5) comparability with individually randomised trials (Higgins 2017).
Measures of treatment effects
There were no continuous data in the included studies. Should we find such data in future updates of this review, we will analyse them using either the mean difference (MD) when the same scale/score is used, or the standardised mean difference (SMD) when different scales/scores are used, with 95% CIs. We will enter data presented as a scale with a consistent direction of effect.
In future updates, should we find skewed data reported as medians and interquartile ranges, we will describe it narratively.
Unit of analysis issues
We did not identify any eligible cluster‐ or cross‐over RCTs. In future updates of this review, if we identify any such studies, we will manage them using these methods:
for cross‐over trials: we will only use data from the first phase in order to avoid the risk of carry‐over effects, as described in Section 23.2.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022);
for cluster‐randomised trials: we will include cluster‐RCTs in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in Section 23.1.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both types of trials if there is little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Assessment of reporting bias
We did not use funnel plots to investigate reporting biases because we did not identify 10 or more studies in one comparison. In future review updates, if possible, we will follow the recommendations in Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), when including 10 or more studies in one comparison.
Subgroup analysis and investigation of heterogeneity
We had insufficient data to conduct subgroup analyses. In future review updates, if possible, we will perform subgroup analyses for each of the following factors on our primary outcomes (neurological impairment and ipsilateral major or disabling stroke) only.
-
Participant characteristics:
age (e.g. adults (18 years to 74 years) and older people (75 years and over));
ethnicity;
comorbidities (e.g. tobacco addiction); and
degree of baseline stenosis, as defined by Grant 2003 and available in Table 6.
-
Intervention characteristics:
doses of drugs;
types of drugs (e.g. unfractionated heparins (UFHs), low molecular weight heparins (LMWHs), vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs) amongst anticoagulants; aspirin, clopidogrel amongst antiplatelet agents);
route of administration (e.g. oral, intravenous, subcutaneous); and
prespecified target achieved (e.g. low‐density lipoprotein level below 70 mg/dL).
We will use the formal test for subgroup differences in Review Manager 5.4 (Review Manager 2020) and base our interpretation on this.
Sensitivity analysis
We had insufficient data to conduct all our preplanned sensitivity analyses. Should we have such data in future, we will conduct the following sensitivity analyses to test whether key methodological factors or decisions have affected the main results for our primary outcomes (i.e. neurological impairment and ipsilateral major or disabling stroke).
Only including studies with a low risk of bias. We will consider a study to have a low risk of bias overall if there is no high‐risk judgement in any of the four main domains (random sequence generation, allocation concealment, incomplete outcome data, and selective reporting).
If we identify studies with missing data that are unobtainable, we will repeat analyses excluding these studies to determine their impact on the primary analyses.
If possible, we will group analyses according to study design (individual, cross‐over, or cluster).
Contributions of authors
CNBC conceived the review; designed the review; co‐ordinated the review; searched and selected studies for inclusion in the review; collected data for the review; assessed the risk of bias in the included studies; analysed the data; assessed the certainty in the body of evidence; interpreted the data; and wrote the review. NC conceived the review; designed the review; searched and selected studies for inclusion in the review; collected data for the review; assessed the risk of bias in the included studies; assessed the certainty in the body of evidence; and wrote the review. CDQF conceived the review; designed the review; co‐ordinated the review; analysed the data; interpreted the data; and wrote the review. LCUN conceived the review; designed the review; and wrote the review. VFMT conceived the review; designed the review; and co‐ordinated the review. RLGF conceived the review; designed the review; co‐ordinated the review; resolved differences in opinions regarding study selection, data extraction, risk of bias assessment and ratings in the certainty of the evidence; analysed the data; interpreted the data; and wrote the review.
All authors reviewed and approved the review content prior to submission.
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Department of Surgery, Brazil
Non‐financial support
External sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil
This study was financed in part by CAPES, finance code 001.
Declarations of interest
CNBC: none known. NC: none known. CDQF: none known. LCUN: none known. VFMT: none known. RLGF: none known.
New
References
References to studies included in this review
Anderssen 2005 {published data only (unpublished sought but not used)}
- Anderssen SA, Hjelstuen AK, Hjermann I, Bjerkan K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis 2005;178:387-97. [DOI: 10.1016/j.atherosclerosis.2004.08.033] [DOI] [PubMed] [Google Scholar]
Applegate 1991 {published data only (unpublished sought but not used)}
- Applegate WB, Byington RP, on behalf of the MIDAS Research Group. MIDAS, the multicenter isradipine/diuretic atherosclerosis study. Design features and baseline data. American Journal of Hypertension 1991;4(2):114S-7S. [DOI: 10.1093/ajh/4.2.114s] [DOI] [PubMed] [Google Scholar]
- Bond MG, Mercuri M, Borhani NO. The multicenter isradipine/diuretic atherosclerosis study (MIDAS): effect of a calcium antagonist on carotid arteries. Atherosclerosis 1994;109(1-2):294. [Google Scholar]
- Borhani NO, Bond MG, Sowers JR, Canossa-Terris M, Buckalew V, Gibbons ME, et al. The multicenter isradipine/diuretic atherosclerosis study: a study of the antiatherogenic properties of isradipine in hypertensive patients. Journal of Cardiovascular Pharmacology 1991;18 Suppl 3:S15-9. [PubMed] [Google Scholar]
- Borhani NO, Brugger SB, Byington RP, on behalf of the US MIDAS Research Group. Multicenter study with isradipine and diuretics against atherosclerosis. Journal of Cardiovascular Pharmacology 1990;15 Suppl 1:S23-9. [PubMed] [Google Scholar]
- Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa-terris M, Carr AA, et al. Final outcome results of the multicenter isradipine diuretic atherosclerosis study (MIDAS). JAMA 1996;276(10):785-91. [DOI: 10.1001/jama.1996.03540100029024] [DOI] [PubMed] [Google Scholar]
- Byington RP, Craven TE, Furberg CD, Pahor M. Isradipine, raised glycosylated haemoglobin, and risk of cardiovascular events. Lancet 1997;350:1075-6. [DOI: 10.1016/S0140-6736(05)70455-4] [DOI] [PubMed] [Google Scholar]
- Furberg CD, Borhani NO, Byington RP, Gibbons ME, Sowers JR. Calcium antagonists and atherosclerosis. The multicenter isradipine/diuretic atherosclerosis study. American Journal of Hypertension 1993;6:24S-9S. [DOI] [PubMed] [Google Scholar]
- Furberg CD, Byington RP, Borhani NO The MIDAS Research Group. Multicenter isradipine diuretic atherosclerosis study (MIDAS). American Journal of Medicine 1989;86(Suppl 4A):37-9. [DOI] [PubMed] [Google Scholar]
- Grimm RH, Flack JM, Byington R, Bond G, Brugger S, The MIDAS Research Group. A comparison of antihypertensive drug effects on the progression of extracranial carotid atherosclerosis. The multicenter isradipine diuretic atherosclerosis study (MIDAS). Drugs 1990;40 Suppl 2:38-43. [DOI] [PubMed] [Google Scholar]
- Prisant LM, Zemel PC, Nichols FT, Zemel MB, Sowers JR, Carr AA, et al. Carotid plaque associations among hypertensive patients. Archives of Internal Medicine 1993;153(4):501-6. [PubMed] [Google Scholar]
- Schnaper HW, Applegate WB, Buckalew VM, Canossa-Terris M, Lee M, Borhani N, the MIDAS Investigator Group. The Multiple Isradipine/Diuretic Atherosclerosis Study: adverse events over 3 years of antihypertensive therapy with isradipine vs hydrochlorothiazide. Atherosclerosis 1994;109(1-2):157. [Google Scholar]
- The Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS) Research Group. Effect of isradipine and diurectics on early carotid atherosclerosis in hypertension. Journal of Hypertension 1994;12 Suppl 3:S67. [Google Scholar]
Blanco‐Colio 2004 {published data only (unpublished sought but not used)}
- Blanco-Colio LM, Martin-Ventura JL, Sol JM, Diaz C, Hernandez G, Egido J. Decreased circulating fas ligand in patients with familial combined hyperlipidemia or carotid atherosclerosis. Journal of the American College of Cardiology 2004;43(7):1188-94. [DOI: 10.1016/j.jacc.2003.10.046] [DOI] [PubMed] [Google Scholar]
- Gómez-Gerique JA, Ros E, Oliván J, Mostaza JM, Vilardell M, Pintó X, et al. Effect of atorvastatin and bezafibrate on plasma levels of C-reactive protein in combined (mixed) hyperlipidemia. Atherosclerosis 2002;162(2):245–51. [DOI: 10.1016/s0021-9150(01)00708-0] [DOI] [PubMed] [Google Scholar]
Bots 2007 {published data only (unpublished sought but not used)}
- Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 2007;379(9582):153-60. [DOI: 10.1016/S0140-6736(07)61088-5] [DOI] [PubMed] [Google Scholar]
- Dogan S, Duivenvoorden R, Grobbee DE, Kastelein JJ, Shear CL, Evans GW, et al. Completeness of carotid intima media thickness measurements depends on body composition: the RADIANCE 1 and 2 trials. Journal of Atherosclerosis and Thrombosis 2010;17(5):526-35. [DOI: 10.5551/jat.3269] [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Bots ML, Riley WA, Evans GW, Meijer R, Revkin JH, et al. Design of a study comparing torcetrapib/atorvastatin with atorvastatin alone on atherosclerosis in patients with familial hypercholesterolemia. Atherosclerosis. Supplements 2005;6(1):51-2. [DOI: 10.1016/S1567-5688(05)80205-3] [DOI] [Google Scholar]
- Kastelein JJ, Van Leuven SI, Burgess L, Evans GW, Kuivenhoven JA, Barter PJ, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. New England Journal of Medicine 2007;356(16):1620-30. [DOI: 10.1056/NEJMoa071359] [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Van Leuven SI, Evans GW, Riley WA, Revkin JH, Shear CL, et al. Designs of RADIANCE 1 and 2: carotid ultrasound studies comparing the effects of torcetrapib/atorvastatin with atorvastatin alone on atherosclerosis. Current Medical Research and Opinion 2007;23(4):885-94. [DOI: 10.1185/030079907X182121] [DOI] [PubMed] [Google Scholar]
- NCT00134238. Carotid B-mode Ultrasound Study to compare anti-atherosclerotic effect of torcetrapib/atorvastatin to atorvastatin (RADIANCE 2). clinicaltrials.gov/ct2/show/NCT00134264 (first received 22 August 2005). [CLINICAL TRIALS REGISTER: NCT00134238]
Corti 2005 {published data only}
- Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Chaplin WF, et al. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. Journal of the American College of Cardiology 2005;46(1):106-12. [DOI: 10.1016/j.jacc.2005.03.054] [DOI] [PubMed] [Google Scholar]
Côté 1995 {published data only (unpublished sought but not used)}
- Côté R, Battista R, Langlois Y. Asymptomatic cervical bruit study (ACBS). Stroke 1995;26:349. [DOI] [PubMed] [Google Scholar]
- Côté R, Battista RN, Abrahamowicz M, Langlois Y, Bourque F, Mackey A. Lack of effect of aspirin in asymptomatic patients with carotid bruits and substantial carotid narrowing. The Asymptomatic Cervical Bruit Study Group. Annals of Internal Medicine 1995;123(9):649‐55. [DOI: 10.7326/0003-4819-123-9-199511010-00002] [DOI] [PubMed] [Google Scholar]
- Côté R, the Asymptomatic Cervical Bruit Study Group. The Asymptomatic Cervical Bruit Study: progress report. Canadian Journal of Neurological Sciences 1994;21 Suppl 2:S7. [Google Scholar]
- The Asymptomatic Cervical Bruit Study Group. Natural history and effectiveness of aspirin in asymptomatic patient with cervical bruits. The Asymptomatic Cervical Bruit Study Group. Archives of Neurology 1991;48(7):683-6. [DOI: 10.1001/archneur.1991.00530190029010] [DOI] [PubMed] [Google Scholar]
Crouse 2007 {published data only (unpublished sought but not used)}
- Bots ML, Palmer MK, Dogan S, Plantinga Y, Raichlen JS, Evans GW, et al. Intensive lipid lowering may reduce progression of carotid atherosclerosis within 12 months of treatment: the METEOR study. Journal of Internal Medicine 2009;265:698-707. [DOI: 10.1111/j.1365-2796.2009.02073.x] [DOI] [PubMed] [Google Scholar]
- Crouse JR 3rd, Grobbee DE, O'Leary DH, Bots ML, Evans GW, Palmer MK, et al. Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis - the rationale and methodology of the METEOR Study. Cardiovascular Drugs and Therapy 2004;18(3):231-8. [DOI: 10.1023/B:CARD.0000033645.55138.3d] [DOI] [PubMed] [Google Scholar]
- Crouse JR, Bots ML, Evans GW, Palmer MK, O'Leary DH, Grobbee DE, et al. Does baseline carotid intima-media thickness modify the effect of rosuvastatin when compared with placebo on carotid intima-media thickness progression? The METEOR study. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17:223-9. [DOI: 10.1097/HJR.0b013e3283359c38] [DOI] [PubMed] [Google Scholar]
- Crouse JR, Grobbee DE, O'Leary DH, Bots ML, Evans GW, Palmer MK, et al. Rosuvastatin arrests progression of carotid intima media thickness in low-risk individuals: main results of the METEOR study. Journal of the American College of Cardiology 2007;49(9):392A-3A. [DOI: 10.1016/j.jacc.2007.01.043] [DOI] [Google Scholar]
- Crouse JR, Grobbee DE, O'Leary DH, Kastelein JJ, Bots ML, Evans GW, et al. Measuring effects on intima media thickness: an evaluation of rosuvastatin - the METEOR Study. Atherosclerosis. Supplements 2002;3(2):94. [DOI] [PubMed] [Google Scholar]
- Crouse JR, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA 2007;297(12):1344-53. [DOI: 10.1001/ jama.297.12.1344] [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Wiegman A, Groot E. Surrogate markers of atherosclerosis: impact of statins. Atherosclerosis. Supplements 2003;4:31-6. [DOI: 10.1016/S1567-5688(03)00007-2] [DOI] [PubMed] [Google Scholar]
- NCT00225589. A study measuring effects on intima media thickness: an evaluation of rosuvastatin 40 mg (METEOR). clinicaltrials.gov/ct2/show/NCT00225589 (first received 22 September 2005). [CLINICAL TRIAL: NCT00225589]
- Paraskevas KI, Wierzbicki AS, Mikhailidis DP. METEOR: aiming at the stars for asymptomatic carotid artery stenosis? International Journal of Clinical Practice 2007;61(8):1239-50. [DOI: 10.1111/j.1742-1241.2007.01483.x] [DOI] [PubMed] [Google Scholar]
- Peters SA, Dogan S, Meijer R, Palmer MK, Grobbee DE, Crouse JR 3rd, et al. The use of plaque score measurements to assess changes in atherosclerotic plaque burden induced by lipid-lowering therapy over time: the METEOR study. Journal of Atherosclerosis and Thrombosis 2011;18(9):784-95. [DOI: 10.5551/jat.8169] [DOI] [PubMed] [Google Scholar]
- Peters SA, Palmer MK, Grobbee DE, Crouse JR, O'Leary DH, Raichlen JS, et al. C-reactive protein lowering with rosuvastatin in the METEOR study. Journal of Internal Medicine 2010;268:155-61. [DOI: 10.1111/j.1365-2796.2010.02230.x] [DOI] [PubMed] [Google Scholar]
ELSA 2002 {published data only}
- Bond G, Dal Paly C, Hansson L, Magnani B, Mancia G, Neiss A, et al. The ELSA trial: protocol of a randomized trial to explore the differential effect of antihypertensive drugs on atherosclerosis in hypertension. Journal of Cardiovascular Pharmacology 1994;23 Suppl 5:S85-7. [PubMed] [Google Scholar]
- Bond MG, Mercuri M, the ELSA Research Group. Potential modification of plaque behavior through the European Lacidipine Study on Atherosclerosis. Journal of Cardiovascular Pharmacology 1995;25 Suppl 3:S11-6. [PubMed] [Google Scholar]
- Giannattasio C, Failla M, Hennig M, Hollweck R, Laurent S, Mallion JM, et al. Different relation between 24-h blood pressure and distensibility at different peripheral arteries. Data from the European Lacidipine Study on Atherosclerosis (ELSA). Journal of Hypertension 2005;23(3):557-62. [DOI: 10.1097/01.hjh.0000160212.33232.3e] [DOI] [PubMed] [Google Scholar]
- Leonetti G. Preliminary clinical results of the ELSA study. Annali Italiani di Medicina Interna 1995;10 Suppl:74S-7S. [PubMed] [Google Scholar]
- Rahn KH, on behalf of the ELSA International Steering Committee and Investigators. The European Lacidipine Study on Atherosclerosis (ELSA): study design and results. Deutsche Medizinische Wochenschrift 2001;126 Suppl 3:S15. [Google Scholar]
- Rahn KH, on behalf of the ELSA Investigators. The European Lacipidine Study on Atherosclerosis (ELSA): prevalence of baseline carotid lesions and correlations with risk factors. Journal of Hypertension 1998;16 Suppl 9:S31-3. [Google Scholar]
- Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Palu CD, et al. Calcium antagonist lacidipine slows down progression asymptomatic carotid atherosclerosis. Principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002;106:2422-7. [DOI: 10.1161/01.cir.0000039288.86470.dd] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Palù CD, et al. Risk factors associated with alterations in carotid intima—media thickness in hypertension. Journal of Hypertension 1998;16(7):949-61. [DOI: 10.1097/00004872-199816070-00008] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Bond MG, Hennig M, Tang R, Hollweck R, Mancia G, et al. Absolute and relative changes in carotid intima media thickness and atherosclerotic plaques during long-term antihypertensive treatment. Journal of Hypertension 2004;22(6):1201-12. [DOI: 10.1097/00004872-200406000-00022] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Hennig M, Baurecht H, Tang R, Cuspidi C, Carugo S, et al. Prevalence and incidence of the metabolic syndrome in the European Lacidipine Study on Atherosclerosis (ELSA) and its relation with carotid intima-media thickness. Journal of Hypertension 2007;25(12):2463-70. [DOI: 10.1097/hjh.0b013e3282f063d5] [DOI] [PubMed] [Google Scholar]
- Zanchetti A. Evaluating the benefits of an antihypertensive agent using trials based on event and organ damage: the Systolic Hypertension in the Elderly Long-term Lacidipine (SHELL) trial and the European Lacidipine Study on Atherosclerosis (ELSA). Journal of Hypertension. Supplement 1995;13 Suppl 4:S35-9. [DOI: 10.1097/00004872-199512002-00006] [DOI] [PubMed] [Google Scholar]
Furberg 1994 {published data only (unpublished sought but not used)}
- Adams HP, Byington RP, Hoen H, Dempsey R, Furberg CD. Effect of cholesterol-lowering medications on progression of mild atherosclerotic lesions of the carotid arteries and on the risk of stroke. Cerebrovascular Diseases 1995;5:171-7. [DOI: 10.1159/000107847] [DOI] [Google Scholar]
- Anonymous. Lovastatin reverses carotid atherosclerosis. Primary Cardiology 1994;20(8):10. [Google Scholar]
- Byington RP, Evans GW, Espeland MA, Applegate WB, Hunninghake DB, Probstfield J, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999;100(3):e14-17. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Dolecek TA, Bradham KH, Espeland MA, Margitic SE, Byington RP, Hoen H, et al. Maximising recruitment efforts in a drug lipid-lowering trial with dietary intervention to lower LDL cholesterol. Controlled Clinical Trials 1996;17:33-45. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Craven TE, Riley WA, Corson J, Romont A, Furberg CD. Reliability of longitudinal ultrasonographic measurements of carotid intimal-medial thicknesses. Asymptomatic Carotid Artery Progression Study Research Group. Stroke 1996;27(3):480-5. [DOI: 10.1161/01.str.27.3.480] [DOI] [PubMed] [Google Scholar]
- Furberg CD, Adams HP, Applegate WB, Byington RP, Espeland MA, Hartwell T, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Circulation 1994;90(4):1679-87. [DOI: 10.1161/01.cir.90.4.1679] [DOI] [PubMed] [Google Scholar]
- Furberg CD, Byington RP, on behalf of the ACAPS Group. ACAPS: effects of lovastatin on progression of carotid atherosclerosis and clinical events. Circulation 1993;88:I-386. [Google Scholar]
- Furberg CD, Hoen HM, Espeland MA, Applegate WB, Bendixen BH. Effect of lovastatin on early carotid atherosclerosis in women. Atherosclerosis 1994;109 (1-2):158. [Google Scholar]
- Furberg CD, on behalf of the ACAPS Research Group. Lovastatin, early carotid atherosclerosis and cardiovascular events: the results of ACAPS. Atherosclerosis 1994;109(1-2):259. [DOI] [PubMed] [Google Scholar]
- Hoen H, Espeland M, Riley W, Furberg C, on behalf of the ACAPS Investigators. Summarizing carotid intimal-medial thickness in the asymptomatic carotid artery plaque study. Controlled Clinical Trials 1993;14(5):433. [Google Scholar]
- Margitic SE. Logistics of closeout in the asymptomatic carotid artery plaque study (ACAPS). Controlled Clinical Trials 1993;14(5):415. [Google Scholar]
- Margitić SE, Byington RP, Espeland MA, Furberg CD, Hunninghake DB, Probstfield JL. Rationale and design for the Asymptomatic Carotid Artery Plaque Study (ACAPS). The ACAPS Group. Controlled Clinical Trials 1992;13(4):293-314. [DOI] [PubMed] [Google Scholar]
- NCT00000469. Asymptomatic Carotid Artery Plaque Study (ACAPS). clinicaltrials.gov/ct2/show/study/NCT00000469 (first received 27 October 1999). [CLINICAL TRIAL: NCT00000469]
- Probstfield JL, Margitic SE, Byington RP, Espeland MA, Furberg CD. Results of the primary outcome measure and clinical events from the asymptomatic carotid artery progression study. American Journal of Cardiology 1995;76:47c-53c. [DOI] [PubMed] [Google Scholar]
Hedblad 2001 {published data only (unpublished sought but not used)}
- BCAPS. BCAPS: Beta-blocker Cholesterol-lowering Asymptomatic Plaque Study. Cardio-Vascular Clinical Trials Forum: Registry of Recent and On-Going Clinical Trials (BioMedNet) 1998.
- Berglund G, Wikstrand J, Janzon L, Wedel H, Hedblad B. Low dose metoprolol and fluvastatin slow progression of atherosclerosis: main results from BCAPS. Atherosclerosis 2000;151(1):4. [Google Scholar]
- Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness. Main results from a B-blocker Cholesterol-lowering Asymtomatic Plaque Study (BCAPS). Circulation 2001;103:1721-6. [DOI: 10.1161/01.CIR.103.13.1721] [DOI] [PubMed] [Google Scholar]
- Ostling G, Goncalves I, Wikstrand J, Berglund G, Nilsson J, Hedblad B. Long-term treatment with low-dose metoprolol CR/XL is associated with increased plaque echogenicity: the Beta-blocker Cholesterol-lowering Asymptomatic Plaque Study (BCAPS). Atherosclerosis Supplements 2011;12(1):67. [DOI] [PubMed] [Google Scholar]
- Ostling G, Gonçalves I, Wikstrand J, Berglund G, Nilsson J, Hedblad B. Long-term treatment with low-dose metoprolol CR/XL is associated with increased plaque echogenicity: the Beta-blocker Cholesterol-lowering Asymptomatic Plaque Study (BCAPS). Atherosclerosis 2011;215(2):440-5. [DOI: 10.1016/j.atherosclerosis.2010.12.031] [DOI] [PubMed] [Google Scholar]
- Wikstrand J, Berglund G, Hedblad B, Hulthe J. Antiatherosclerotic effects of beta-blockers. American Journal of Cardiology 2003;91(12 Suppl 1):25H-9H. [DOI: 10.1016/S0002-9149(03)00431-4] [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only (unpublished sought but not used)}
- Hu Y, Tong G, Xu W, Pan J, Ryan K, Yang R, et al. Anti-inflammatory effects of simvastatin on adipokines in type 2 diabetic patients with carotid atherosclerosis. Diabetes and Vascular Disease Research 2009;6(4):262-8. [DOI: 10.1177/1479164109339966] [DOI] [PubMed] [Google Scholar]
Ikeda 2013 {published data only (unpublished sought but not used)}
- Koji I, Tomosaburo T, Hiroyuki Y, Kiyoaki M, Takahisa S, Takashi M, et al. Effect of intensive statin therapy on regression of carotid intima-media thickness in patients with subclinical carotid atherosclerosis (a prospective, randomized trial: PEACE (Pitavastatin Evaluation of Atherosclerosis Regression by Intensive Cholesterol-lowering Therapy) study). European Journal of Preventive Cardiology 2013;20(6):1069-79. [CLINICALTRIALS.GOV IDENTIFIER:: NCT00711919] [DOI: 10.1177/2047487312451539] [UNIQUE ID ISSUED BY UMIN: UMIN000001229] [DOI] [PubMed] [Google Scholar]
- NCT00711919. Pitavastatin on carotid intima-media thickness (PEACE). clinicaltrials.gov/ct2/show/record/NCT00711919 (first received 7 July 2008). [CLINICAL TRIALS REGISTER: NCT00711919]
Kadoglou 2010 {published data only (unpublished sought but not used)}
- Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Gerasimidis T, Liapis CD. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. Journal of Vascular Surgery 2010;51:114-21. [DOI: 10.1016/j.jvs.2009.07.119] [DOI] [PubMed] [Google Scholar]
- Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou AT, Fotiadis G, Vitta I, et al. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. Journal of Vascular Surgery 2009;49(5 suppl 1):4S-5S. [DOI] [PubMed] [Google Scholar]
Meaney 2009 {published data only (unpublished sought but not used)}
- Meaney A, Ceballos G, Asbun J, Solache G, Mendoza E, Vela A, et al. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. Journal of Clinical Pharmacology 2009;49:838-47. [DOI: 10.1177/0091270009337011] [DOI] [PubMed] [Google Scholar]
Mercuri 1996 {published data only (unpublished sought but not used)}
- Baldassarre D, Veglia F, Gobbi C, Gallus G, Ventura A, Crepaldi G, et al. Intima-media thickness after pravastatin stabilizes also in patients with moderate to no reduction in LDL-cholesterol levels: the carotid atherosclerosis Italian ultrasound study. Atherosclerosis 2000;151(2):575-83. [DOI: 10.1016/s0021-9150(99)00434-7] [DOI] [PubMed] [Google Scholar]
- Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic Mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study. American Journal of Medicine 1996;101(6):627-34. [DOI: 10.1016/s0002-9343(96)00333-6] [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Bianchi G, Bond MG, D’Alo’ G, Gallus G, Liberatore S, et al. Pravastatin intervention trial on carotid artery atherosclerosis in patients with mild hypercholesterolemia: the CAIUS study. International Journal of Cardiovascular Imaging 1995;11(S2):119-24. [DOI: 10.1007/bf01419825] [DOI] [Google Scholar]
Nohara 2012 {published data only (unpublished sought but not used)}000001174
- Kurabayashi M, Sakuma I, Kawamori R, Daida H, Yamazaki T, Yoshida M, et al. Can intensive lipid-lowering therapy with statins ameliorate atherosclerosis in Japanese patients? Journal of Atherosclerosis and Thrombosis 2009;17(4):416-22. [DOI: 10.5551/jat.2899] [UMIN ID: UMIN000001174] [DOI] [PubMed] [Google Scholar]
- Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Effect of intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness in Japanese patients - Justification for Atherosclerosis Regression Treatment (JART) study. Circulation Journal 2012;76:221-9. [DOI: 10.1253/circj.CJ-11-0887] [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Nohara R, Daida H, Hata M, Kaku K, Kawamori R, et al. Intensive lipid-lowering therapy for slowing progression as well as inducing regression of atherosclerosis in Japanese patients: subanalysis of the JART study. International Heart Journal 2013;54:33-9. [DOI: 10.1536/ihj.54.33] [DOI] [PubMed] [Google Scholar]
Norris 1990 {published data only (unpublished sought but not used)}
- Carotid Stenosis Study Group. Failure of metoprolol and aspirin to regress carotid stenosis. Stroke 1990;21:169. [DOI: 10.1161/01.STR.21.1.156] [DOI] [Google Scholar]
- Norris JW, Ziliotto C, Taylor DW, Chambers BR, Bornstein NM, D'Alton JG, et al. Failure of metoprolol and aspirin to regress carotid stenosis. Neurology 1990;40 Suppl 1:415. [DOI: 10.1212/WNL.40.4_Suppl_1.1] [DOI] [Google Scholar]
Reid 2005 {published data only (unpublished sought but not used)}
- Reid JA, Wolsley C, Lau LL, Hannon RJ, Lee B, Young IS, et al. The effect of pravastatin on intima media thickness of the carotid artery in patients with normal cholesterol. European Journal of Vascular and Endovascular Surgery 2005;30:464-8. [DOI: 10.1016/j.ejvs.2005.05.007] [DOI] [PubMed] [Google Scholar]
Salonen 1995 {published data only (unpublished sought but not used)}
- Salonen R, Nyyssonen K, Porkkal-sarataho E, Salonen JT. The Kuopio Atherosclerosis Prevention Study (KAPS): effect of pravastatin treatment on lipids, oxidation resistance of lipoproteins, and atherosclerotic progression. American Journal of Cardiology 1995;76:34C-39C. [DOI: 10.1016/s0002-9149(99)80468-8] [DOI] [PubMed] [Google Scholar]
- Salonen R, Nyyssonen K, Porkkala E, Rummukainen J, Belder R, Park J-S, et al. Kuopio atherosclerosis prevention study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92(7):1758-64. [DOI: 10.1161/01.CIR.92.7.1758] [DOI] [PubMed] [Google Scholar]
- Salonen R, Nyyssonen K, Porkkata E, Rummukainen J, Salonen JT. KAPS: the effect of pravastatin on atherosclerotic progression in carotid and femoral arteries. Circulation 1994;90:I-127. [DOI] [PubMed] [Google Scholar]
Sawayama 2002 {published data only (unpublished sought but not used)}
- Sawayama Y, Hayashi J, Maeda N, Shimizu C, Tanaka Y, Kashiwagi S. The therapeutic effects of probucol and pravastatin on common carotid intima-media thickness in asymptomatic hypercholesterolemic Japanese patients. Atherosclerosis 2000;151(1):130. [Google Scholar]
- Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucal and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka atherosclerosis trial (FAST). Journal of the American College of Cardiology 2002;39(4):610-6. [DOI: 10.1016/s0735-1097(01)01783-1] [DOI] [PubMed] [Google Scholar]
- Wada N, Sekiguchi M, Yoshioka N, Toyoshima H, Matsumoto A, Nomura M, et al. Effect of probucol and pravastatin on common-carotid atherosclerosis in diabetic patients with hypercholesterolemia. Journal of the Japan Diabetes Society 2005;48(1):53-6. [Google Scholar]
Semplicini 2000 {published data only (unpublished sought but not used)}
- Semplicini A, Maresca A, Simonella C, Chierichetti F, Pauletto P, Meneghetti G, et al. Cerebral perfusion in hypertensives with carotid artery stenosis: a comparative study of lacidipine and hydrochlorothiazide. Blood Pressure 2000;9:34-9. [DOI: 10.1080/080370500439407 · S] [PubMed] [Google Scholar]
- Semplicini A, Simonella C, Meneghetti G, Chierichetti F, Santipolo N, Pauletto P, et al. Lacidipine and cerebral perfusion in uncomplicated essential hypertensives with mild to moderate carotid artery stenosis. Journal of Hypertension 1998;16 Suppl 2:S243. [Google Scholar]
Shinoda‐Tagawa 2002 {published data only}
- Shinoda-Tagawa T, Yamasaki Y, Yoshida S, Kajimoto Y, Tsujino T, Hakui N, et al. A phosphodiesterase inhibitor, cilostazol, prevents the onset of silent brain infarction in Japanese subjects with type II diabetes. Diabetologia 2002;45(2):188-94. [DOI: 10.1007/s00125-001-0740-2] [DOI] [PubMed] [Google Scholar]
Stumpe 2007 {published data only (unpublished sought but not used)}
- Ludwig M, Stumpe KO, Agabiti-Rosei E, Scholze J, Stumpe I, Zielinski T. Quantification of antihypertensive treatment effects on carotid atherosclerosis by 3-dimensional ultrasound: first report from the MORE trial. Stroke 2006;37(2):664. [Google Scholar]
- NCT00185185. Olmesartan Medoxomil in Atherosclerosis. clinicaltrials.gov/ct2/show/NCT00185185 (first received 12 September 2005). [CLINICALTRIALS.GOV IDENTIFIER:: NCT00185185]
- Stumpe KO, Agabiti-Rosei E, Zielinski T, Schremmer D, Scholze J, Laeis P, et al. Carotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Therapeutic Advances in Cardiovascular Disease 2007;1(2):97-106. [DOI: 10.1177/ 1753944707085982] [DOI] [PubMed] [Google Scholar]
Sutton‐Tyrrell 1994 {published data only (unpublished sought but not used)}
- Davis BR, Vogt T, Frost PH, Burlando A, Cohen J, Wilson A, et al. Risk factors for stroke and type of stroke in persons with isolated systolic hypertension. Stroke 1998;29:1333-40. [DOI] [PubMed] [Google Scholar]
- NCT00000514. Systolic Hypertension in the Elderly Program (SHEP). clinicaltrials.gov/ct2/show/NCT00000514 (first received 27 October 1999). [CLINICALTRIALS.GOV IDENTIFIER: ClinicalTNCT00000514]
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265(24):3255-64. [DOI: 10.1001/jama.1991.03460240051027] [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Alcorn HG, Herzog H, Kelsey SF, Kuller LH. Morbidity, mortality, and antihypertensive treatment effects by extent of atherosclerosis in older adults with isolated systolic hypertension. Stroke 1995;26:1319-24. [DOI: 10.1161/01.STR.26.8.1319] [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Kuller LH, Wolfson SK. Blood pressure treatment slows the rate of progression of carotid stenosis in patients with isolated systolic hypertension. Journal of the American College of Cardiology 1993;21:70A. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Wolfson SK, Kuller LW. Blood pressure treatment slows the progression of carotid stenosis in patients with isolated systolic hypertension. Stroke 1994;25:44-50. [DOI: 10.1161/01.STR.25.1.44] [DOI] [PubMed] [Google Scholar]
- The Systolic Hypertension in the Elderly Program (SHEP) Cooperative Research Group. Rationale and design of a randomized clinical trial on prevention of stroke in isolated systolic hypertension. Journal of Clinical Epidemiology 1988;41(12):1197-208. [DOI: 10.1016/0895-4356(88)90024-8] [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only (unpublished sought but not used)}
- ISRCTN64894118. A 12-week, randomised, double-blind study evaluating the effects of low-dose (10 mg) and high-dose (80 mg) atorvastatin on macrophage activity and carotid plaque inflammation as determined by ultra small super-paramagnetic iron oxide (USPIO) enhanced carotid magnetic resonance imaging (MRI). www.isrctn.com/ISRCTN64894118 (first applied 3 March 2006). [DOI: 10.1186/ISRCTN64894118] [DOI]
- Li Z-Y, Tang T, Gillard J. Aggressive atorvastatin decreases wall shear stress in the carotid artery. Stroke 2012;43 Suppl 1:A2790. [Google Scholar]
- Li ZY, Tang TY, Jiang F, Zhang Y, Gillard JH. Reduction in arterial wall strain with aggressive lipid-lowering therapy in patients with carotid artery disease. Circulation Journal 2011;75(6):1486-92. [DOI: 10.1253/circj.cj-10-1210] [DOI] [PubMed] [Google Scholar]
- NCT00368589. Effects of atorvastatin on macrophage activity and plaque inflammation using magnetic resonance imaging. clinicaltrials.gov/ct2/show/NCT00368589 (first received 22 August 2006). [CLINICAL TRIALS REGISTER:: NCT00368589]
- Sadat U, Howarth SP, Usman A, Taviani V, Tang TY, Graves MJ, et al. Effect of low- and high-dose atorvastatin on carotid artery distensibility using carotid magnetic resonance imaging - A post-hoc sub group analysis of ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) study. Journal of Atherosclerosis and Thrombosis 2013;20(1):46-56. [DOI: 10.5551/jat.12633] [DOI] [PubMed] [Google Scholar]
- Tang TY, Howarth SPS, Miller SR, Graves MJ, Patterson AJ, U-King-Im J-M, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Journal of the American College of Cardiology 2009;53(22):2039-50. [DOI: 10.1016/j.jacc.2009.03.018] [CLINICALTRIALS.GOV IDENTIFIER: NCT00368589] [DOI] [PubMed] [Google Scholar]
Terpstra 2004 {published data only}
- Terpstra WF, May JF, Smit AJ, De Graeff PA, Meyboom-de Jong B, Crijns HJ. Effects of amlodipine and lisinopril on intima-media thickness in previously untreated, elderly hypertensive patients (the ELVERA trial). Journal of Hypertension 2004;22:1309-16. [DOI: 10.1097/01.hjh.0000125412.50839.b5] [DOI] [PubMed] [Google Scholar]
Underhill 2008 {published data only (unpublished sought but not used)}
- Du R, Cai J, Ping Y, Wang Q, Liu D, Wu H. Effect of low dose rosuvastatin therapy on regression of carotid plaque and vasa vasorums in Chinese population: a prospective clinical trial by MRI. Journal of the American College of Cardiology 2013;61(10 Suppl 1):E938. [Google Scholar]
- Hatsukami TS, Zhao XQ, Yuan C, Tessier JJ, Miller E, Pears JS. Study design for a randomized, double-blind trial to assess the effect of 24 months of dosing with rosuvastatin on progression of carotid artery atheroma in moderately hypercholesterolemic patients with asymptomatic carotid stenosis. Atherosclerosis. Supplements 2001;2:47-8. [DOI: 10.1016/s1567-5688(01)80057-x] [DOI] [Google Scholar]
- NCT00654394. Progression of carotid artery atheroma in moderately hypercholesterolemic subjects. clinicaltrials.gov/ct2/show/NCT00654394 (first received 3 April 2008). [CLINICAL TRIALS REGISTER: NCT00654394]
- Underhill HR, Yuan C, Zhao XQ, Kraiss LW, Parker DL, Saam T, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. American Heart Journal 2008;155(3):584.e1–584.e8. [DOI: 10.1016/j.ahj.2007.11.018] [CLINICALTRIALS.GOV IDENTIFIER: NCT00654394] [DOI] [PubMed] [Google Scholar]
VHAS 1998 {published data only}
- Magnani B, Dal Palu C, Zanchetti A. Preliminary clinical experience with calcium antagonists in atherosclerosis. Drugs 1992;44 Suppl 1:128-33. [DOI] [PubMed] [Google Scholar]
- Rosei EA, Dal Palù C, Leonetti G, Magnani B, Pessina A, Zanchetti A, VHAS Investigators. Clinical results of the Verapamil in Hypertension and Atherosclerosis Study. Journal of Hypertension 1997 Nov;15(11):1337-44. [DOI: 10.1097/00004872-199715110-00019] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Magnani B, Dal Palu C, on behalf of the VHAS Investigators. Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of ultrasonography evaluations. Journal of Hypertension 1997;15 Suppl 4:S91. [Google Scholar]
- Zanchetti A, Magnani B, Dal Palu C, on behalf of the Verapamil-Hypertension Atherosclerosis Study (VHAS) Investigators. Atherosclerosis and calcium antagonists: the VHAS. Journal of Human Hypertension 1992;6 Suppl 2:S45-8. [PubMed] [Google Scholar]
- Zanchetti A, Rosei EA, Dal Palu C, Leonetti G, Magnani B, Pessina A, et al. The Verapamil in Hypertension and Atherosclerosis Study (VHAS): results of long-term randomized treatment with either verapamil or chlorthalidone on carotid intima-media thickness. Journal of Hypertension 1998;16:1667-76. [DOI] [PubMed] [Google Scholar]
- Zanchetti A. VHAS: Verapamil in Hypertension and Atherosclerosis Study. Cardio-Vascular Clinical Trials Forum: Registry of Recent and On-Going Clinical Trials (BioMedNet) 1998.
Yamada 2009 {published data only (unpublished sought but not used)}UMIN000001114
- UMIN000001114. Effects of lipid lowering by atorvastatin on carotid atherosclerotic plaque (ACAP) study: a randomized trial for analysis of qualitative change in carotid atherosclerrotic plaque with IB echo and Black Blood MRI. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000001347 (first received 01 June 2006). [UNIQUE ID ISSUED BY UMIN: UMIN000001114]
- Yamada K, Yoshimura S, Kawasaki M, Enomoto Y, Asano T, Minatoguchi S, et al. Effects of atorvastatin on carotid atherosclerotic plaques: a randomized trial for quantitative tissue characterization of carotid atherosclerotic plaques with integrated backscatter ultrasound. Cerebrovascular Diseases 2009;28:417-24. [DOI: 10.1159/000235746] [DOI] [PubMed] [Google Scholar]
Yamamoto 2011 {published data only (unpublished sought but not used)}C000000319
- C000000319. The effect of losartan and amlodipine on left ventricular diastolic function in patients with mild-to-moderate hypertension. upload.umin.ac.jp (first received 01 February 2006). [UMIN-CTR CLINICAL TRIAL REGISTER:: C000000319]
- Hori M. The effect of losartan and amlodipine on left ventricular diastolic function in patients with mild-to-moderate hypertension. Circulation Journal 2006;70(1):124-8. [DOI: 10.1253/circj.70.124] [DOI] [PubMed] [Google Scholar]
- The J-ELAN Investigators. The effect of losartan and amlodipine on left ventricular diastolic function in patients with mild-to-moderate hypertension (J-ELAN): rationale and design. Circulation Journal 2006;70:124-8. [DOI: 10.1253/circj.70.124] [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ozaki H, Takayasu K, Akehi N, Fukui S, Sakai A, et al. The effect of losartan and amlodipine on left ventricular diastolic function and atherosclerosis in Japanese patients with mild-to-moderate hypertension (J-ELAN) study. Hypertension Research 2011;34:325-30. [DOI: 10.1038/hr.2010.237] [UMIN CLINICAL TRIALS REGISTRY: C000000319] [DOI] [PubMed] [Google Scholar]
Zanchetti 2004 {published data only (unpublished sought but not used)}
- The PHYLLIS Project Group. Plaque hypertension lipid-lowering Italian Study (PHYLLIS): a protocol for non-invasive evaluation of carotid atherosclerosis in hypercholesterolaemic hypertensive subjects. Journal of Hypertension 1993;11 Suppl 5:S314-5. [PubMed] [Google Scholar]
- Zanchetti A, Crepaldi G, Bond G, Gallus G, Veglia F, Mancia G, et al. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis. Principal results of PHYLLIS - a randomized double-blind trial. Stroke 2004;35:2807-12. [DOI: 10.1161/01.STR.0000147041.00840.59] [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Crepaldi G, Bond G, Gallus G, Veglia M, Mancia G, et al. Effects of fosinopril and pravastatin on progression of asymptomatic carotid atherosclerosis in hypertension: results of the Plaque Hypertension Lipid Lowering Italian Study (PHYLLIS). Journal of Hypertension 2003;21 Suppl 4:S346. [Google Scholar]
- Zanchetti A, Crepaldi G, Bond MG, Gallus GV, Veglia F, Ventura A, et al. Systolic and pulse blood pressures (but not diastolic blood pressure and serum cholesterol) are associated with alterations in carotid intima–media thickness in the moderately hypercholesterolemic hypertensive patients of the Plaque Hypertension Lipid Lowering Italian Study. Journal of Hypertension 2001;19(1):79-88. [DOI: 10.1097/00004872-200101000-00011] [DOI] [PubMed] [Google Scholar]
- Zanchetti A. The hypertensive patient with multiple risk factors. Is treatment really so difficult? American Journal of Hypertension 1997;10(10 Pt 2):223S-9S. [DOI: 10.1016/s0895-7061(97)00327-0] [DOI] [PubMed] [Google Scholar]
Zeng 2004 {published data only (unpublished sought but not used)}
- Zeng X, Zeng X Sr, Li Y, Zeng Y II. Effects of pravastatin on carotid plaques and preventing stroke in patients with hypercholesterolemia. Stroke 2004;35(1):257. [DOI: ] [Google Scholar]
Zheng 2022 {published and unpublished data}
- Wang Y, Wang A, Li H, Li Z, Hu B, Li X, et al. Measuring effects on intima-media thickness: an evaluation of rosuvastatin in Chinese subjects with subclinical atherosclerosis - design, rationale, and methodology of the METEOR-China study. Trials 2020;21(1):921. [DOI: 10.1186/s13063-020-04741-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li H, Wang Y, Li Z, Hu B, Li X, et al. Rosuvastatin slows progression of carotid intima-media thickness: the METEOR-China randomized controlled study. Stroke 2022;53(10):3004-13. [DOI: 10.1161/STROKEAHA.120.031877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2006 {published data only (unpublished sought but not used)}
- Zhu S, Su G, Meng QH. Inhibitory effects of micronized fenofibrate on carotid atherosclerosis in patients with essential hypertension. Clinical Chemistry 2006;52(11):2036-42. [DOI: 10.1373/clinchem.2006.074724] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anand 2018 {published data only (unpublished sought but not used)}
- Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391(10117):219-29. [DOI: 10.1016/ S0140-6736(17)32409-1] [DOI] [PubMed] [Google Scholar]
- Bhagirath VC, Eikelboom JW, Anand SS. Low-dose rivaroxaban plus aspirin for the prevention of cardiovascular events: an evaluation of COMPASS. Future Cardiology 2018;14(6):443-53. [DOI: 10.2217/fca-2018-0059] [DOI] [PubMed] [Google Scholar]
- Bhatt DL. Setting a new direction in CAD and PAD - the COMPASS trial. Cardiology 2018;140 Suppl 1:200. [Google Scholar]
- Bosch J, Eikelboom JW, Connolly SJ, Bruns NC, Lanius V, Yuan F, et al. Rationale, design and baseline characteristics of participants in the cardiovascular outcomes for people using anticoagulation strategies (COMPASS) trial. Canadian Journal of Cardiology 2017;33(8):1027-35. [DOI: 10.1016/j.cjca.2017.06.001] [DOI] [PubMed] [Google Scholar]
- Darmon A, Sorbets E, Ducrocq G, Elbez Y, Abtan J, Popovic B, et al. Identifying higher risk patients among the COMPASS-eligible population: an analysis from the REduction of Atherothrombosis for Continued Health (REACH) Registry. European Heart Journal 2018;39 Suppl 1:1084. [DOI: 10.1016/j.acvdsp.2018.10.007] [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. New England Journal of Medicine 2017;377(14):1319-30. [DOI: 10.1056/NEJMoa1709118] [DOI] [PubMed] [Google Scholar]
- Hussain MA, Wheatcroft M, Nault P, Lindsay TF, Bhatt DL, Anand SS, et al. COMPASS for vascular surgeons: practical considerations. Current Opinion in Cardiology 2019;34(2):178-84. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Lamy A, Eikelboom J, Connolly S, Bosch J, Fox KA, Tong W, et al. Costs impact rivaroxaban plus aspirin versus aspirin in the COMPASS trial. Circulation 2017;136:e456-7. [Google Scholar]
- NCT01776424. Rivaroxaban for the prevention of major cardiovascular events in coronary or peripheral artery disease (COMPASS). clinicaltrials.gov/ct2/show/NCT01776424 (first received 24 January 2013). [CLINICAL TRIALS REGISTER: NCT01776424]
- Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KK, et al. Stroke outcomes in the COMPASS trial. Circulation 2019;139(9):1134-45. [DOI: 10.1161/CIRCULATIONAHA.118.035864] [DOI] [PubMed] [Google Scholar]
Bondjers 2000 {published data only (unpublished sought but not used)}
- Bondjers G, Wiklund O, Hulthe J, Schmidt C, Olofsson SO, Wikstrand J. The effect of metoprolol CR/XL on atherosclerosis. Atherosclerosis 2000;151(1):92. [DOI: 10.1016/s0021-9150(00)80419-0] [DOI] [Google Scholar]
Davidson 2012 {published data only}
- Davidson M, Rosenson RS, Maki KC, Nicholls SJ, Ballantyne CM, Setze C, et al. Study design, rationale, and baseline characteristics: evaluation of fenofibric acid on carotid intima-media thickness in patients with type IIb dyslipidemia with residual risk in addition to atorvastatin therapy (FIRST) trial. Cardiovascular Drugs and Therapy 2012;26:349-58. [CLINICALTRIALS.GOV: NCT00616772] [DOI: 10.1007/s10557-012-6395-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duman 2007 {published data only (unpublished sought but not used)}
- Duman D, Demirtunc R, Sahin S, Esertas K. The effects of simvastatin and levothyroxine on intima-media thickness of the carotid artery in female normolipemic patients with subclinical hypothyroidism: a prospective, randomized-controlled study. Journal of Cardiovascular Medicine 2007;8:1007-11. [DOI] [PubMed] [Google Scholar]
Esposito 2004 {published data only}
- Esposito K, Giugliano D, Nappo F, Marfella R, on behalf of the Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004;110:214-9. [DOI: 10.1161/01.CIR.0000134501.57864.66] [DOI] [PubMed] [Google Scholar]
Fayad 2011 {published data only}
- Calcagno C, Ramachandran S, Izquierdo-Garcia D, Mani V, Millon A, Rosenbaum D. The complementary roles of dynamic contrast-enhanced MRI and 18F-fluorodeoxyglucose PET/CT for imaging of carotid atherosclerosis. European Journal of Nuclear Medicine and Molecular Imaging 2013;40(12):1884-93. [DOI: 10.1007/s00259-013-2518-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivenvoorden R, Mani V, Woodward M, Kallend D, Suchankova G, Fuster V. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study. JACC: Cardiovascular Imaging 2013;6(10):1087-94. [DOI: 10.1016/j.jcmg.2013.03.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad ZA, Mani V, Woodward M, Kallend D, Bansilal S, Pozza J, et al. Rationale and design of dal-PLAQUE: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computer tomography. American Heart Journal 2011;162:214-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad ZA, Mani V, Woodward M, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378(9802):1547-59. [DOI: 10.1016/S0140-6736(11)61383-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosomi 2001 {published data only}
- Hosomi N, Mizushige K, Ohyama H, Hatanaka Y, Matsuo H, Koziol JA. ACE inhibition with enalapril slows progressive intima-media thickening of common carotid artery in NIDDM patients. Circulation 2000;102(18 Suppl 2):II-869. [DOI] [PubMed] [Google Scholar]
- Hosomi N, Mizushige K, Ohyama H, Takahashi T, Kitadai M, Hatanaka Y, et al. Angiotensin-converting enzyme inhibition with enalapril slows progressive intima-media thickening of the common carotid artery in patients with non-insulin-dependent diabetes mellitus. Stroke 2001;32:1539-45. [DOI: 10.1161/01.str.32.7.1539] [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only (unpublished sought but not used)}
- Huang Z, Lei MX, Liu L, Tang QB. Effects of rosiglitazone on the IMTc and serum MMP-9 levels in newly diagnosed Type 2 diabetic patients. Zhong Nan da Xue Xue Bao Yi Xue Ban (Journal of Central South University - Medical Sciences) 2006;31(3):367-72. [PubMed] [Google Scholar]
Ichihara 2006 {published data only}
- Ichihara A, Kaneshiro Y, Sakoda M, Takemitsu T, Itoh H. Add-on amlodipine improves arterial function and structure in hypertensive patients treated with an angiotensin receptor blocker. Journal of Cardiovascular Pharmacology 2007;49(3):161-6. [DOI: 10.1097/FJC.0b013e31803104e5] [DOI] [PubMed] [Google Scholar]
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M. Effects of amlodipine and valsartan on vascular damage and ambulatory blood pressure in untreated hypertensive patients. Journal of Human Hypertension 2006;20:787-94. [DOI: ] [DOI] [PubMed] [Google Scholar]
Igase 2012 {published data only (unpublished sought but not used)}
- Igase M, Kohara K, Tabara Y, Nagai T, Ochi N, Kido T, et al. Low-dose rosuvastatin improves the functional and morphological markers of atherosclerosis in asymptomatic postmenopausal women with dyslipidemia. Menopause 2012;19(12):1294-9. [DOI: 10.1097/gme.0b013e318259c04e] [DOI] [PubMed] [Google Scholar]
Ito 2004 {published data only (unpublished sought but not used)}
- Ito Y, Kawasaki M, Yokoyama H, Okubo M, Sano K, Arai M, et al. Different effects of pravastatin and cerivastatin on the media of the carotid arteries as assessed by integrated backscatter ultrasound. Circulation Journal 2004;68(8):784-90. [DOI: 10.1253/circj.68.784] [DOI] [PubMed] [Google Scholar]
Koeijvoets 2005 {published data only (unpublished sought but not used)}
- Koeijvoets KC, Rodenburg J, Hutten BA, Wiegman A, Kastelein JJ, Sijbrands EJ. Low-density lipoprotein receptor genotype and response to pravastatin in children with familial hypercholesterolemia. Substudy of an Intima-Media Thickness Trial. Circulation 2005;112:3168-73. [DOI: 10.1161/CIRCULATIONAHA.105.565507] [DOI] [PubMed] [Google Scholar]
Laurora 1998 {published data only}
- Laurora G, Cesarone MR, Belcaro G, Sanctis MT, Pomante P, Incandela L, et al. Control of arteriosclerosis progression in high risk subjects treated with mesoglycan. Evaluation of intima-media thickness. Minerva Cardioangiologica 1998;46(3):41-8. [PubMed] [Google Scholar]
Ludwig 2002 {published data only}
- Ludwig M, Stapff M, Ribeiro A, Fritschka E, Tholl U, Smith RD, et al. Comparison of the effects of losartan and atenolol on common carotid artery intima-media thickness in patients with hypertension: results of a 2-year, double-blind, randomized, controlled study. Clinical Therapeutics 2002;24(7):1175-93. [DOI: 10.1016/s0149-2918(02)80028-5] [DOI] [PubMed] [Google Scholar]
Mazzone 2006 {published data only}
- Betteridge DJ. CHICAGO, PERISCOPE and PROactive: CV risk modification in diabetes with pioglitazone. Fundamental & Clinical Pharmacology 2009;23(6):675-9. [DOI: 10.1111/j.1472-8206.2009.00741.x] [DOI] [PubMed] [Google Scholar]
- Davidson M, Meyer PM, Haffner S, Feinstein S, D'Agostino R, Kondos GT, et al. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with Type 2 Diabetes Mellitus. Circulation 2008;117(16):2123-30. [DOI: 10.1161/CIRCULATIONAHA.107.746610] [DOI] [PubMed] [Google Scholar]
- Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D'Agostino RB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 2006;296(21):2572-81. [CLINICALTRIALS.GOV IDENTIFIER:: NCT00225264] [DOI: 10.1001/jama.296.21.joc60158] [DOI] [PubMed] [Google Scholar]
- NCT00225264. Efficacy study of pioglitazone and glimepiride on the rate of progression of atherosclerotic disease (CHICAGO). www.clinicaltrials.gov/ct2/show/NCT00225264 (first received 23 September 2005). [CLINICALTRIALS.GOV: NCT00225264]
Meuwese 2009 {published data only}
- Meuwese MC, De Groot E, Duivenvoorden R, Trip MD, Ose L, Maritz FJ, et al. ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: the CAPTIVATE randomized trial. JAMA 2009;301(11):1131-9. [DOI: 10.1001/jama.301.11.1131] [DOI] [PubMed] [Google Scholar]
- NCT00151788. Efficacy and safety of the ACAT Inhibitor CS-505 (pactimibe) for reducing the progression of carotid artery disease. (CAPTIVATE study). www.clinicaltrials.gov/ct2/show/NCT00151788 (first received 7 September 2005). [CLINICALTRIALS.GOV IDENTIFIER: NCT00151788]
Mizuguchi 2008 {published data only (unpublished sought but not used)}
- Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circulation Journal 2008;72:538-44. [DOI: 10.1253/circj.72.538] [DOI] [PubMed] [Google Scholar]
Mok 2010 {published data only (unpublished sought but not used)}
- Mok CC, Lai J, Wong CK, Lam CS. Effect of rosuvastatin on homocysteine, hsCRP and endothelial markers in systemic lupus erythematosus (SLE): a randomized controlled trial. Lupus 2010;19 Suppl 1:5. [DOI: 10.1177/09612033100190010101] [DOI] [Google Scholar]
Mortsell 2007 {published data only (unpublished sought but not used)}
- Mortsell D, Malmqvist K, Held C, Kahan T. Irbesartan reduces common carotid artery intima-media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study. Journal of Internal Medicine 2007;261:472-9. [DOI: 10.1111/j.1365-2796.2007.01775.x] [DOI] [PubMed] [Google Scholar]
Oyama 2008 {published data only (unpublished sought but not used)}
- Oyama T, Saiki A, Endoh K, Ban N, Nagayama D, Ohhira M, et al. Effect of acarbose, an alpha-glucosidase inhibitor, on serum lipoprotein lipase mass levels and common carotid artery intima-media thickness in type 2 diabetes mellitus treated by sulfonylurea. Journal of Atherosclerosis and Thrombosis 2008;15(3):154-9. [DOI: 10.5551/jat.e549] [DOI] [PubMed] [Google Scholar]
Persson 1996 {published data only}
- Persson J, Israelsson B, Stavenow L, Holmstrom E, Berglund G. Progression of atherosclerosis in middle-aged men: effects of multifactorial intervention. Journal of Internal Medicine 1996;239(5):425-33. [DOI: 10.1046/j.1365-2796.1996.476814000.x] [DOI] [PubMed] [Google Scholar]
Pontremoli 2001 {published data only (unpublished sought but not used)}
- Pontremoli R, Viazzi F, Ravera M, Leoncini G, Berruti V, Bezante GP, et al. Long term effect of nifedipine GITS and lisinopril on subclinical organ damage in patients with essential hypertension. Journal of Nephrology 2001;14(1):19-26. [PubMed] [Google Scholar]
Saremi 2013 {published data only (unpublished sought but not used)}
- Saremi A, Schwenke DC, Buchanan TA, Hodis HN, Mack WJ, Banerji M, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Atherosclerosis, Thrombosis and Vascular Biology 2013;33:393-9. [DOI: 10.1161/ATVBAHA.112.300346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2001 {published data only}
- Stanton AV, Chapman JN, Mayet J, Sever PS, Poulter NR, Hughes AD, et al. Effects of blood pressure lowering with amlodipine or lisinopril on vascular structure of the common carotid artery. Clinical Science 2001;101(5):455-64. [DOI: ] [PubMed] [Google Scholar]
- Stanton AV, Chapman JN, Sever PS, Poulter NR, Hughes AD, Thom SA. Greater regression of early atherosclerosis by calcium channel blockade than by angiotensin converting enzyme inhibition. Hypertension 1998;32(4):795. [Google Scholar]
Stumpe 1994 {published data only}
- Stumpe KO, Ludwig M, Heagerty AM, Kolloch RE, Mancia G, Safar M, et al. Vascular wall thickness in hypertension: the Perindopril Regression of Vascular Thickening European Community Trial: PROTECT. American Journal of Cardiology 1995;76:50E-4E. [DOI] [PubMed] [Google Scholar]
- Stumpe KO, Ludwig M, Heagerty AM, Kolloch RE, Mancia G, Safar M. Effect of antihypertensive therapy on increased vascular wall thickness: objectives and design of the PROTECT Study. Journal of Hypertension 1994;12 Suppl:149. [Google Scholar]
Tasić 2006 {published data only}
- Tasić IS, Mijalković D, Djordjević D, Lović B, Janković D, Miladinović-Tasić N, et al. Effect of fosinopril on progression of the asymptomatic carotid atherosclerosis and left ventricular hypertrophy in hypertensive patients. Srpski Arhiv Za Celokupno Lekarstvo 2006;134(3-4):106-13. [DOI: 10.2298/sarh0604106t] [DOI] [PubMed] [Google Scholar]
Vukusich 2010 {published data only (unpublished sought but not used)}
- Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clinical Journal of the American Society of Nephrology 2010;5(8):1380-7. [DOI: 10.2215/CJN.09421209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamasaki 2010 {published data only}
- Yamasaki Y, Katakami N, Furukado S, Kitagawa K, Nagatsuka K, Kashiwagi A, et al. Long-term effects of pioglitazone on carotid atherosclerosis in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. Journal of Atherosclerosis and Thrombosis 2010;17:1132-40. [DOI: 10.5551/jat.4663] [DOI] [PubMed] [Google Scholar]
Yilmaz 2004 {published data only (unpublished sought but not used)}
- Yilmaz R, Altun B, Kahraman S, Ozer N, Akinci D, Turgan C. Impact of amlodipine or ramipril treatment on left ventricular mass and carotid intima-media thickness in nondiabetic hemodialysis patients. Renal Failure 2010;32(8):903-12. [DOI: 10.3109/0886022X.2010.502276] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Yilmaz R, Altun B, Kahraman S, Ozer N, Akinci D, Turgan C. Impact of amlodipine or ramipril treatment on left ventricular mass and carotid intima-media thickness in nondiabetic hemodialysis patients. Renal Failure 2010;32:903-12. [DOI: 10.3109/0886022X.2010.502276] [DOI] [PubMed] [Google Scholar]
Yokoyama 2005 {published data only (unpublished sought but not used)}
- Yokoyama H, Kawasaki M, Ito Y, Minatoguchi S, Fujiwara H. Effects of fluvastatin on the carotid arterial media as assessed by integrated backscatter ultrasound compared with pulse-wave velocity. Journal of the American College of Cardiology 2005;46(11):2031-7. [DOI: 10.1016/j.jacc.2005.06.084] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Aranzulla 2021 {published data only (unpublished sought but not used)}
- Aranzulla TC, Piazza S, Ricotti A, Musumeci G, Gaggiano A. Carotid plaque stabilization and regression with evolocumab: rationale and design of the CARUSO study. Catheter Cardiovascular Intervention 2021;98(1):E115-21. [DOI: 10.1002/ccd.29743] [DOI] [PubMed] [Google Scholar]
Additional references
Abbott 2007
- Abbott AL, Bladin CF, Levi CR, Chambers BR. What should we do with asymptomatic carotid stenosis? International Journal of Stroke 2007;2(1):27-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Abbott 2015
- Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke 2015;46(11):3288-301. [DOI: 10.1161/STROKEAHA.115.003390] [PMID: ] [DOI] [PubMed] [Google Scholar]
Amarenco 2006
- Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. New England Journal of Medicine 2006;355(6):549-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arnett 2019
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596-646. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnett 1991
- Barnett HJ, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. New England Journal of Medicine 1991;325(7):445-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Benjamin 2019
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics - 2019 update: a report from the American Heart Association. Circulation 2019;139(10):e56-e528. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brott 2013
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography. Catheterization and Cardiovascular Interventions 2013;81(1):E76-123. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bulbulia 2017
- Bulbulia R, Halliday A. The Asymptomatic Carotid Surgery Trial-2 (ACST-2): an ongoing randomised controlled trial comparing carotid endarterectomy with carotid artery stenting to prevent stroke. Health Technology Assessment 2017;21(57):1-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassola 2022
- Cassola N, Baptista-Silva JC, Nakano LC, Flumignan CD, Sesso R, Vasconcelos V et al. Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments. Cochrane Database of Systematic Reviews 2022, Issue 7. Art. No: CD013172. [DOI: 10.1002/14651858.CD013172.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2001
- Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults - United States, 1999. Morbidity and Mortality Weekly Report 2001;50(7):120-5. [PMID: ] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 25 March 2020. Available at www.covidence.org.
Daolio 2019
- Daolio RM, Cassola N, Flumignan C, Nakano L, Guedes H, Amorim J, et al. PC126. Accuracy of vascular ultrasound compared with computed tomography angiography for extracranial carotid stenosis imaging. Journal of Vascular Surgery 2019;69(6):e239-40. [DOI: 10.1016/j.jvs.2019.04.356] [DOI] [Google Scholar]
De Waard 2017
- De Waard DD, Morris D, De Borst GJ, Bulbulia R, Halliday A. Asymptomatic carotid artery stenosis: who should be screened, who should be treated and how should we treat them? Journal of Cardiovascular Surgery 2017;58(1):3-12. [DOI: 10.23736/S0021-9509.16.09770-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Weerd 2010
- De Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke 2010;41(6):1294-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Derdeyn 2007
- Derdeyn CP. Carotid stenting for asymptomatic carotid stenosis: trial it. Stroke 2007;38(2 Suppl):715-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Divya 2015
- Divya KP, Sandeep N, Sarma S, Sylaja PN. Risk of stroke and cardiac events in medically treated asymptomatic carotid stenosis. Journal of Stroke and Cerebrovascular Diseases 2015;24(9):2149-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Easton 2009
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40(6):2276-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
ECST 1998
- European Carotid Surgery Trialists' Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351(9113):1379-87. [PMID: ] [PubMed] [Google Scholar]
Feigin 2016
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurology 2016;15(9):913-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Feigin 2021
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Neurology 2021;20(10):795-820. [DOI: 10.1016/s1474-4422(21)00252-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flaherty 2013
- Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013;40(1):36-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2017
- Flumignan CD, Flumignan RL, Navarro TP. Extracranial carotid stenosis: evidence based review [Estenose de carotida extracraniana: revisao baseada em evidencias]. Revista do Colegio Brasileiro de Cirurgioes 2017;44(3):293-301. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gasior 2023
- Gasior SA, O'Donnell JP, Davey M, Clarke J, Jalali A, Ryan É, et al. Optimal management of asymptomatic carotid artery stenosis: a systematic review and network meta-analysis. European Journal of Vascular and Endovascular Surgery 2023;65(5):660-9. [DOI: 10.1016/j.ejvs.2023.01.020] [DOI] [PubMed] [Google Scholar]
Gorelick 1999
- Gorelick PB, Sacco RL, Smith DB, Alberts M, Mustone-Alexander L, Rader D, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA 1999;281(12):1112-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group, Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grant 2003
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis - Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229(2):340-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guzik 2017
- Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum 2017;23(1):15-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haley 2021
- Haley W, Shawl F, Sternbergh WC, Turan TN, Barrett K, Voeks J et al. Non-adherence to antihypertensive guidelines in patients with asymptomatic carotid stenosis. Journal of Stroke and Cerebrovascular Diseases 2021;30(8):105918. [PMID: 10.1016/j.jstrokecerebrovasdis.2021.105918] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halliday 2004
- Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363(9420):1491-502. [DOI: 10.1016/S0140-6736(04)16146-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hillen 2003
- Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke 2003;34(6):1457-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Holman 2014
- Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet 2014;383(9933):2008-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Howard 2017
- Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD Jr, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials. Int J Stroke 2017 Oct;12(7):770-8. [DOI: 10.1177/1747493017706238] [CLINICALTRIALS.GOV: NCT02089217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawes 2004
- Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004;35(3):776-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1995
- Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation 1995;91(2):566-79. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mozaffarian 2016
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics - 2016 update: a report from the American Heart Association. Circulation 2016;133(4):e38-360. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murphy 2019
- Murphy SJ, Naylor AR, Ricco JB, Sillesen H, Kakkos S, Halliday A, et al. Optimal antiplatelet therapy in moderate to severe asymptomatic and symptomatic carotid stenosis: a comprehensive review of the literature. European Journal of Vascular and Endovascular Surgery 2019;57(2):199-211. [DOI: 10.1016/j.ejvs.2018.09.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Müller 2021
- Müller MD, Bonati LH. Carotid artery stenosis – Current evidence and treatment recommendations. Clinical and Translational Neuroscience 2021;5(1):1-8. [DOI: 10.1177/2514183X211001654] [DOI] [Google Scholar]
Naylor 2023
- Naylor R, Rantner B, Ancetti S, Borst GJ, Carlo M, Halliday A, et al. Editor's Choice – European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. European Journal of Vascular and Endovascular Surgery 2023;65(1):7-111. [DOI: 10.1016/j.ejvs.2022.04.011] [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Clinical Excellence (NICE). Impact stroke. NICE practice guidelines 2019. Available from www.nice.org.uk/Media/Default/About/what-we-do/Into-practice/measuring-uptake/NICE-Impact-stroke.pdf (accessed 09 July 2019).
O'Donnel 2016
- O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang X, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;20(388):761-75. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2019
- Park MS, Kwon S, Lee MJ, Kim KH, Jeon P, Park YJ, et al. Identification of high risk carotid artery stenosis: a multimodal vascular and perfusion imaging study. Frontiers in Neurology 2019;10:765. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raman 2013
- Raman G, Moorthy D, Hadar N, Dahabreh IJ, O'Donnell TF, Thaler DE, et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Annals of Internal Medicine 2013 May 7;158(9):676-85. [DOI: 10.7326/0003-4819-158-9-201305070-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Ricotta 2011
- Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. Journal of Vascular Surgery 2011;54(3):e1-31. [DOI: 10.1016/j.jvs.2011.07.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulman 2010
- Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis 2010;8(1):202-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sharma 2019
- Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KK, et al. Stroke outcomes in the COMPASS Trial. Circulation 2019;139(9):1134-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strong 2007
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurology 2007;6(2):182-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2002
- Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: ARterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 2002;106(16):2055-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2013
- Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD004816. [DOI: 10.1002/14651858.CD004816.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Touboul 2012
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011): an update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovascular Diseases 2012;34(4):290-6. [DOI: 10.1159/000343145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1995
- Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, et al. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273(18):1421-8. [PMID: ] [PubMed] [Google Scholar]
Wardlaw 2006
- Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al. Accurate, practical and cost-effective assessment of carotid stenosis in the UK. Health Technology Assessment 2006;10(30):iii-iv, ix-x, 1-182. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36-item Short-form Health Survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Wilson 2019
- Wilson PW, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139(25):e1144-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhan 2018
- Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD012502. [DOI: 10.1002/14651858.CD012502.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang C, Zhou YH, Xu CL, Chi FL, Ju HN. Efficacy of intensive control of glucose in stroke prevention: a meta-analysis of data from 59,197 participants in 9 randomized controlled trials. PLoS One 2013;8(1):e54465. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Clezar 2020
- Clezar CN, Cassola N, Flumignan CD, Nakano LC, Trevisani VF, Flumignan RL. Pharmacological interventions for asymptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013573. [DOI: 10.1002/14651858.CD013573] [DOI] [PMC free article] [PubMed] [Google Scholar]


