Abstract
BACKGROUND
The global spread of severe acute respiratory syndrome coronavirus 2, responsible for coronavirus disease 2019 (COVID-19), poses a significant risk to public health. Beyond the respiratory issues initially associated with the condition, severe cases of COVID-19 can also lead to complications in other organs, including the liver. Patients with severe COVID-19 may exhibit various clinical signs of liver dysfunction, ranging from minor elevations in liver enzymes without symptoms to more serious cases of impaired liver function. Liver damage is more commonly observed in patients with severe or critical forms of the disease.
AIM
To present the research landscape on COVID-19 and liver dysfunction while also offering valuable insights into the prominent areas of interest within this particular domain.
METHODS
On 18 February 2023, Scopus was utilised to conduct a comprehensive exploration of the relationship between COVID-19 and the liver dysfunction. The investigation encompassed the period from 1 January 2020 to 31 December 2022. Primary sources were meticulously examined and organised in a Microsoft Excel 2013 spreadsheet, categorised by journal, institution, funding agency, country and citation type. VOSviewer version 1.6.18 was employed to explore the prominent topics and knowledge network related to the subject.
RESULTS
There were 2336 publications on COVID-19 and liver dysfunction analysed in this study, of which 558 were published in 2020, 891 in 2021 and 887 in 2022. Researchers from 111 different countries participated in the retrieved documents. The United States contributed the most studies, with 497 documents, representing 21.28% of the total, followed by China with 393 documents (16.82%) and Italy with 255 documents (10.92%). In the context of research related to COVID-19 and the liver, co-occurrence analysis identified three distinct clusters of topics: (1) ‘COVID-19 vaccines in liver transplant recipients’; (2) ‘liver function tests as a predictor of the severity and clinical outcomes in hospitalised patients’; and (3) ‘care of patients with liver disease during the COVID-19 pandemic’.
CONCLUSION
This bibliometric study provides a comprehensive overview of liver-related publications in COVID-19 research over the past 3 years. This study highlights the significant contributions of high-income nations, particularly the United States, China, and Italy, to the production of liver-related scholarly literature in this field. Most of the articles focused on liver dysfunction in patients with COVID-19 and the implications of the virus for gastroenterologists and hepatologists.
Keywords: COVID-19, Bibliometric, Scopus, Vosviewer, Liver
Core Tip: Severe cases of coronavirus disease 2019 (COVID-19) can lead to liver dysfunction, and this study provides a comprehensive overview of liver-related publications in COVID-19 research. The findings highlight the significant contributions of high-income countries, such as the United States, China, and Italy, to the production of liver-related scholarly literature in this field. The research clusters identified in the study focus on COVID-19 vaccines in liver transplant recipients, liver function tests as predictors of severity and clinical outcomes in hospitalized patients, and the care of patients with liver disease during the COVID-19 pandemic.
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) has had a marked global impact. As of 3 May 2023, there have been over 765 million confirmed cases and nearly 6.92 million fatalities[1]. COVID-19 symptoms range from modest respiratory difficulties to severe respiratory distress syndrome, which can lead to organ failure and death. Older people with preexisting health issues are especially vulnerable. It is crucial to mention that in addition to its effects on the lungs, COVID-19 can cause liver damage[2-6]. COVID-19-related liver injury is defined as any liver damage that occurs in people who have COVID-19, regardless of whether they have a preexisting liver ailment[7-11]. Severe COVID-19 infections can cause liver damage through a variety of mechanisms, including immune-mediated damage, ischemic hepatitis caused by a systemic inflammatory response, drug-induced liver injury, reactivation of preexisting chronic liver disease, and direct cytopathic effects from the virus replicating in hepatocytes[12-15].
COVID-19-induced liver dysfunction has a variable prognosis and symptoms[16-20]. Because there is no cure for COVID-19-induced liver damage, treatment is only supportive. Preexisting liver diseases and nutritional support can help identify and treat liver disorders[21]. Research on COVID-19 and liver dysfunction is evolving, but there are not enough quantitative and visual studies based on bibliometrics. This chasm has stifled research in this field. Hence, this bibliometric analysis provides a view of the available COVID-19 and liver dysfunction research that is indexed in the Scopus database. This analysis has identified research hotspots for COVID-19 and liver dysfunction, as well as historical trends and future directions.
MATERIALS AND METHODS
Study design
This cross-sectional study was conducted on 18 February 2023 using a bibliometric methodology.
Bibliographic database
The Scopus database was chosen as the source of data for this study for various reasons. First, Scopus stands out as the largest scientific database compared with alternatives such as Web of Science. Second, it offers convenient options for exporting and analysing data, and it is compatible with Microsoft Excel and visualisation tools such as VOSviewer. Third, Scopus is a comprehensive database that encompasses citations from diverse fields, including social and health disciplines. Consequently, Scopus emerged as the most appropriate choice for conducting the present study[22-24].
Search strategies
The search was limited to publications between 1 January 2020 and 31 December 2022. The search strategy involved three steps.
In the first step, terms related to COVID-19 were selected from the Medical Subject Headings (MeSH) of PubMed and previous studies related to COVID-19[25-29]. Next, the retrieved terms were entered into the Scopus search engine as ‘Article Title/Abstract’ to retrieve publications related to COVID-19.
In the second step, the publications obtained in the first step were filtered to include only those with "liver and related words" in their title. Keywords relevant to the liver were selected from the previous liver and COVID-19 meta-analyses[30-34] and were entered into the Scopus engine to retrieve publications related to the liver.
In the third step, publications that were published as an erratum were excluded.
The final search query used for data extraction from Scopus looked like this: (TITLE-ABS ("COVID 19") OR TITLE-ABS ("2019 novel coronavirus") OR TITLE-ABS ("novel coronavirus") OR TITLE-ABS ("coronavirus 2019") OR TITLE-ABS ("*novel CoV") OR TITLE-ABS ("coronavirus disease 2019") OR TITLE-ABS ("2019-novel CoV") OR TITLE-ABS ("Wuhan coronavirus") OR TITLE-ABS ("2019 ncov") OR TITLE-ABS ("Wuhan pneumonia") OR TITLE-ABS ("COVID 2019") OR TITLE-ABS (COVID19) OR TITLE-ABS (*COVID*) OR TITLE-ABS (nCoV) OR TITLE-ABS ("corona virus 2019") OR TITLE-ABS ("nCoV-2019") OR TITLE-ABS (nCoV2019) OR TITLE-ABS ("nCoV 2019") OR TITLE-ABS (2019-ncov) OR TITLE-ABS (COVID-19) OR TITLE-ABS ("Severe acute respiratory syndrome coronavirus 2") OR TITLE-ABS ("SARS Coronavirus 2") OR TITLE-ABS ("SARS-CoV-2") OR TITLE-ABS ("SARS-CoV 2")) AND (TITLE (liver) OR TITLE (Hepati*) OR TITLE (aminotransferase) OR TITLE (bilirubin) OR TITLE (prothrombin) OR TITLE (ALT) OR TITLE (AST) OR TITLE (Hepato*) OR TITLE (Cirrhosis)) AND (LIMIT-TO (PUBYEAR,2022) OR LIMIT-TO (PUBYEAR,2021) OR LIMIT-TO (PUBYEAR,2020) ) AND (EXCLUDE (DOCTYPE,"er" ) )
During every stage of the search process, quotation marks were employed to accurately retrieve the specific phrase. Moreover, the use of asterisk truncation acted as a flexible wildcard, enabling the retrieval of any possible term.
Validation of the search strategy
Limiting the search to the publication titles in the Scopus database can improve the accuracy of retrieved data by reducing the number of false positive results. By focusing the search on the title, the search algorithm will only retrieve articles with ‘liver’ in their titles, meaning that irrelevant articles that may mention ‘liver’ in their abstracts or full texts will not be retrieved. As mentioned, this approach may slightly reduce the level of sensitivity, meaning that some relevant articles that do not have a ‘liver’ in their titles may be missed[35,36]. This study employed a validated research approach to ensure dependable and precise findings. To minimise the risk of false positive results, every 15th document (15, 30, 45, 60, etc.) up to the end of the retrieved document list was carefully assessed by evaluating its title and abstract. The research strategy underwent continuous refinement until an entirely accurate collection of randomly selected outcomes was obtained. To confirm the absence of false-negative results or missed findings, the research productivity of 20 active authors in the field was analysed. A Spearman correlation test was utilised to compare the results derived from the research strategy with those from the authors. The study revealed a strong significant correlation (r = 0.953, P < 0.001) between the two sets of findings, underscoring the research strategy’s high level of validity. Importantly, Sweileh WM[37,38], and Zyoud SH[39] had previously employed this validation approach.
Bibliometric analysis
The data collected included the following bibliometric parameters: the types of documents (e.g. articles, books, or conference proceedings), the year of publication, the number of publications, the citation count (which indicates how many times other works have cited the publication), the country where the publication originated, the institution or organization that produced the publication, and the journals where the publications appeared. The Impact Index Per Article displayed represents the top 10 most-cited papers; it is derived from the Reference Citation Analysis (RCA, https://www.referencecitationanalysis.com/) database. RCA is an open citation analysis database covering various fields and is owned by Baishideng Publishing Group Inc., situated in Pleasanton, CA 94566, United States[40-42].
Visualize analysis
VOSviewer (version 1.6.18, Leiden University, Leiden, the Netherlands) was used for bibliometric visualisation[43]. In scientific research, the use of VOSviewer software for bibliometric visualisation and term co-occurrence analysis is widespread. By identifying patterns of international collaboration and analysing the co-occurrence of terms in the titles and abstracts of publications, researchers can gain insight into the hotspots in a particular field and track scientific progress[43]. Using VOSviewer software, a network of terms illustrates the relationship between terms according to the number of publications in which they appear together. This enables researchers to identify clusters of related terms that represent particular research areas or trending topics. As a result, researchers can better understand the current state of research in a particular field and identify areas for future study by identifying hotspots[44,45]. These data can be used to guide funding decisions, to identify possible collaborators and to inform policy decisions.
RESULTS
General characteristics of the retrieved articles
A total of 2336 publications on COVID-19 and liver dysfunction were analysed in this study. Among them, 558 were published in 2020, 891 in 2021, and 887 in 2022. Regarding the types of publications, 1438 (61.56%) were articles, 417 (17.85%) were letters, 357 (15.28%) were reviews, and 124 (5.31%) fell under other categories, such as editorials and notes.
Top 10 active countries
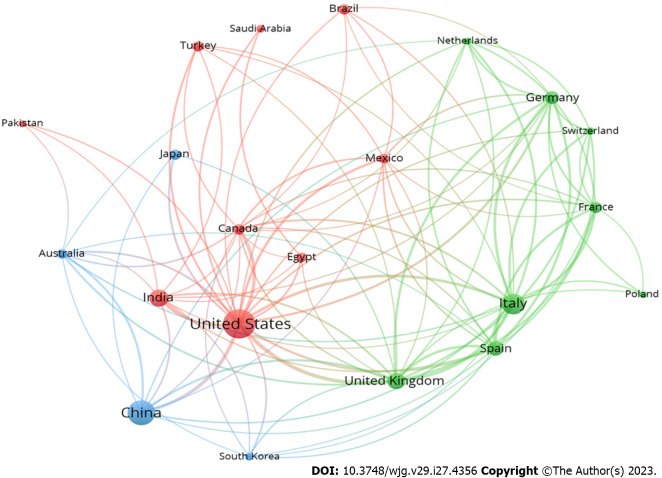
Researchers from 111 different countries participated in the retrieved documents. The United States contributed the most studies, with 497 documents, representing 21.28% of the total. China followed closely behind with 393 documents, representing 16.82% of the total, and Italy came in third with 255 documents, representing 10.92% of the total publications on research related to COVID-19 and liver dysfunction. Table 1 lists the top 10 active countries, which together represented 84.63% of all articles published. Figure 1 is a network visualisation map showing the collaboration between countries regarding co-authorship. The minimum inclusion threshold was set at 30 documents per country; a total of 22 countries met this threshold. In the visualisation, each country is represented by a circle, and the size of the circle indicates the level of contribution the country has made in terms of co-authorship. Lines represent the links between countries, and the thickness of the line indicates the strength of collaboration between the two countries. According to the centrality measures used in the map, the United States appears to be the most central country in terms of collaborations, followed by China and Italy.
Table 1.
The top 10 countries contributing to research related to coronavirus disease 2019 and liver
|
Ranking
|
Country
|
No. of documents
|
%
|
| 1st | United States | 497 | 21.28 |
| 2nd | China | 393 | 16.82 |
| 3rd | Italy | 255 | 10.92 |
| 4th | India | 185 | 7.92 |
| 5th | United Kingdom | 170 | 7.28 |
| 6th | Spain | 125 | 5.35 |
| 7th | Germany | 107 | 4.58 |
| 8th | Iran | 97 | 4.15 |
| 9th | France | 74 | 3.17 |
| 9th | Turkey | 74 | 3.17 |
Figure 1.
Network visualization map of country collaboration. A minimum of 30 documents per country was set as a threshold, and 22 countries met the threshold. Countries can be represented as nodes in a network visualization made with VOSviewer, and lines can be created between the nodes to show correlations between co-occurrences. The strength of the co-occurrence link between the countries can be determined by the line's thickness or weight. Thicker lines are frequently used to depict stronger links.
Analysis of institutions
Eight thousand six hundred forty institutions participated in research in this field, of which the top 10 institutions represented 15.97% of all published articles. INSERM contributed the most articles (n = 26, 5.49%), followed by Imperial College London (n = 23, 4.85%) and Sorbonne Université (n = 21, 4.43%) (Table 2).
Table 2.
The top 10 institutions contributing to research related to coronavirus disease 2019 and liver
|
Ranking
|
Institute
|
Country
|
No. of documents
|
%
|
| 1st | Huazhong University of Science and Technology | China | 55 | 2.35 |
| 2nd | Tongji Medical College | China | 53 | 2.27 |
| 3rd | Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas | Spain | 48 | 2.05 |
| 4th | INSERM | France | 36 | 1.54 |
| 4th | University of Pennsylvania | United States | 36 | 1.54 |
| 6th | Yale School of Medicine | United States | 31 | 1.33 |
| 7th | Hospital Clinic Barcelona | Spain | 30 | 1.28 |
| 8th | AP-HP Assistance Publique - Hopitaux de Paris | France | 29 | 1.24 |
| 8th | Zhejiang University School of Medicine | China | 29 | 1.24 |
| 10th | Universitat de Barcelona | Spain | 26 | 1.11 |
Journal analysis
The top 10 most productive journals in research related to COVID-19 and the liver are shown in Table 3. Approximately 19.91% of the articles were published on this list. The Journal of Hepatology published the most articles (n = 85, 3.64%), followed by Liver International (n = 64, 2.74%) and the World Journal of Gastroenterology (n = 62, 2.65%).
Table 3.
Top 10 active journals publishing research related to coronavirus disease 2019 and liver
|
Ranking
|
Journal/source title
|
No. of documents
|
%
|
IF1
|
| 1st | Journal of Hepatology | 85 | 3.64 | 30.083 |
| 2nd | Liver International | 64 | 2.74 | 8.754 |
| 3rd | World Journal of Gastroenterology | 62 | 2.65 | 5.374 |
| 4th | Hepatology | 50 | 2.14 | 17.298 |
| 5th | Hepatology Communications | 37 | 1.58 | 5.701 |
| 6th | Clinical Gastroenterology and Hepatology | 36 | 1.54 | 13.576 |
| 7th | Liver Transplantation | 34 | 1.46 | 6.112 |
| 7th | World Journal of Hepatology | 34 | 1.46 | NA |
| 9th | World Journal of Clinical Cases | 32 | 1.37 | 1.534 |
| 10th | Journal of Clinical and Experimental Hepatology | 31 | 1.33 | NA |
Impact factors were retrieved from the 2021 Journal Citation Reports (Clarivate Analytics).
Analysis of citations
The retrieved documents had a total of 30,766 citations, a mean of 13.17, and an h-index of 75. A total of 638 (27.3%) documents had no citations, while 55 had 100 or more citations. The top 10 articles, ranked by the number of citations, collectively received 4,758 citations. The citations for these publications varied from 283 to 1,126 in total (Table 4) [13,46-54]. Among the top 10 most-cited articles, the Impact Index Per Article ranged from 88.7 to 394.7.
Table 4.
Top 10 articles on total citations for research related to coronavirus disease 2019 and liver from 2020 to 2022
|
Ranking
|
Ref.
|
Title
|
Year
|
Source title
|
Cited by
|
Impact index per article1
|
| 1st | Zhang et al[13] | Liver injury in COVID-19: Management and challenges | 2020 | The Lancet Gastroenterology and Hepatology | 1126 | 394.7 |
| 2nd | Mao et al[51] | Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis | 2020 | The Lancet Gastroenterology and Hepatology | 549 | 181 |
| 3rd | Cai et al[48] | COVID-19: Abnormal liver function tests | 2020 | Journal of Hepatology | 542 | 177 |
| 4th | Xu et al[53] | Liver injury during highly pathogenic human coronavirus infections | 2020 | Liver International | 522 | 186 |
| 5th | Fan et al[49] | Clinical Features of COVID-19-Related Liver Functional Abnormality | 2020 | Clinical Gastroenterology and Hepatology | 460 | 156.7 |
| 6th | Wang et al[52] | SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19 | 2020 | Journal of Hepatology | 347 | 117.7 |
| 7th | Boettler et al[47] | Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper | 2020 | JHEP Reports | 326 | 88.7 |
| 8th | Ji et al[50] | Nonalcoholic fatty liver diseases in patients with COVID-19: A retrospective study | 2020 | Journal of Hepatology | 314 | 112 |
| 9th | Zhang et al[54] | Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single center in Wuhan city, China | 2020 | Liver International | 289 | 97.7 |
| 10th | Bangash et al[46] | COVID-19 and the liver: Little cause for concern | 2020 | The Lancet Gastroenterology and Hepatology | 283 | 90.7 |
The Impact Index Per Article is presented based on Reference Citation Analysis (Source: Baishideng Publishing Group Inc. Pleasanton, CA 94566, United States). COVID-19: Coronavirus disease 2019.
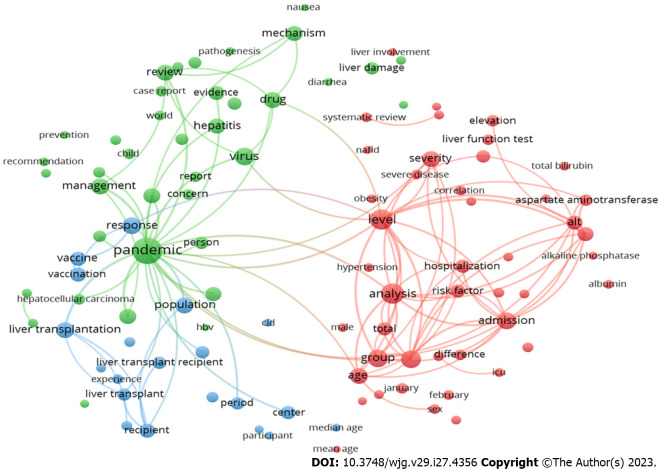
Term co-occurrence cluster analysis of research hotspots
In the context of COVID-19 and liver research, co-occurrence analysis was used to identify the most commonly used terms in the titles and abstracts of the relevant articles. In this case, VOSviewer was used to create a bubble map, which represents each term as a bubble, and the bubble size indicates the frequency of occurrence of that term. The analysis identified 172 terms that occurred at least 50 times in the titles and abstracts of the included publications (Figure 2). The bubbles are grouped into clusters based on the similarity of their co-occurrence patterns. There are three different groups: (1) The blue cluster, which is named ‘COVID-19 vaccines in liver transplant recipients’; (2) the red group, which is titled ‘liver function tests as a predictor of COVID-19 severity and clinical outcomes of COVID-19 in hospitalised patients’; and (3) the green group, which is titled ‘care of patients with liver disease during the COVID-19 pandemic’.
Figure 2.
Research topics clustered by mapping the co-occurrences of terms in the title/abstract of publications on research related to coronavirus disease 2019 and liver. Of the 26722 terms, 172 terms occurred at least 50 times. The largest set of connected terms was stratified into three clusters. VOSviewer may produce a network visualization in which terms are represented as nodes and co-occurrence connections are depicted by lines connecting the nodes. The strength of the association between the terms' co-occurrence can be determined by the line's thickness or weight. Thicker lines are frequently used to depict stronger links.
DISCUSSION
The correlation between liver injury and COVID-19 is associated with the severity and mortality of the disease. These findings strongly indicate a relationship between liver injury and COVID-19. Despite the observation that severe and fatal cases of the disease primarily affect older adults with liver injury, the specific underlying mechanisms remain unclear[55].
The findings indicate that the United States, China and Italy have been actively involved in COVID-19 and liver dysfunction research and have produced many publications on the subject. Numerous studies on the productivity of COVID-19 research in various fields[56-60], as measured by publications, have found that the United States, China and Italy have been the leading producers of COVID-19 publications. The United States and China have dominated research output in numerous fields, including the health sciences[61,62]. According to a report released by Japan's Science and Technology Ministry, China has emerged as the global frontrunner in scientific research output, surpassing the United States in both overall volume and the number of impactful studies. The report, published by Japan's National Institute of Science and Technology Policy, revealed that China now leads the world in annual scientific research paper publications, followed by the United States and Germany[63]. There are several factors that contribute to both the United States and China being leaders in scientific research. These factors include the size of their economies, the significant amount of money invested in research and development, and the large number of researchers working across various fields[64,65]. Notably, both countries have allocated significant funds to healthcare and biotechnology[61,65-67], ensuring that researchers have the resources they need to conduct thorough studies that yield reliable results. Furthermore, both countries have large and diverse scientific communities that encourage interdisciplinary research and expert collaboration. Funding also plays a pivotal role in driving research output, as researchers studying COVID-19 and liver dysfunction in both countries can seek substantial grants. These grants provide the necessary resources to pursue ambitious research projects, and thus attract top talent, and address critical health issues. Notable organizations such as the National Natural Science Foundation of China[68-70], the National Institutes of Health[71,72], and Gilead Sciences[47,73] offer sizable grants for researchers studying COVID-19 and liver dysfunction in the United States and China.
One of the key hotspots in the current study is ‘COVID-19 vaccines in liver transplant recipients’. Solid organ transplant recipients face a significant risk of death from COVID-19, with mortality rates ranging from 13% to 39%[74,75]. As a result, many countries have given priority to vaccinating this vulnerable group using messenger RNA (mRNA) vaccines. Nonetheless, there is limited information available regarding their response to vaccination and its effectiveness[76]. It is critical that liver transplant recipients receive the COVID-19 vaccine to avoid hospitalisation and serious illness. COVID-19 vaccines are safe and effective in people who have had liver transplants. It should be noted, however, that these vaccines may not elicit as strong an immune response as in healthy individuals[77]. As a result, liver transplant recipients should consult their health care providers about the best time to get vaccinated, considering their specific immunosuppression regimens and recent transplant surgeries[78]. Patients who have had a liver transplant should talk to their doctors about whether they need more vaccine doses or a different vaccine to maximise their immune response[79].
‘Liver function tests as a predictor of COVID-19 severity and clinical outcomes in hospitalised patients’ is another study topic. Liver function tests (LFTs) are regularly conducted in hospitalised patients with COVID-19 to assess disease severity and the likelihood of poor clinical outcomes[80]. COVID-19 causes liver inflammation and abnormal LFT results[81]. In 2022, the World Journal of Gastroenterology reported that abnormal levels of aspartate aminotransferase (AST) and total bilirubin (T-Bil) are associated with higher mortality rates than other liver damage indicators during hospitalisation. Vasopressor medications and mechanical ventilation are linked to abnormal AST, T-Bil, and alkaline phosphatase (ALP) levels. Thus, patients with COVID-19 often have abnormal LFT results upon hospital admission, which can predict their severity and prognosis. Health care practitioners can assess risk and predict the need for advanced treatment for these individuals by measuring alanine transaminase (ALT), AST, ALP and T-Bil[82]. Patients with COVID-19 and abnormal LFT results may also have prior liver disease, medication toxicity or bacterial or viral coinfection. Thus, LFT results should be included with other clinical and laboratory data when predicting severity and clinical outcomes[83,84].
The treatment of liver disease has become increasingly important during the COVID-19 pandemic. Individuals with liver disease are more vulnerable to COVID-19-related severe illness and mortality due to factors such as a compromised immune system, advanced age and underlying health conditions[85]. Given this scenario, patients must carefully prioritise pandemic preparedness measures. Any deviation from their treatment plan – missed appointments or delayed therapy – can aggravate their liver disease and increase their COVID-19 risks[86]. Therefore, individuals with liver disease should take COVID-19 precautions such as frequent and thorough handwashing, avoid large crowds and wear face masks[87]. Adopting a healthy lifestyle is also important for strengthening their immune systems and preventing COVID-19 complications. This includes regular exercise, a balanced diet, abstaining from alcohol and smoking, and getting enough sleep[88,89].
Strengths and limitations
Although this is the first bibliometric analysis to assess the worldwide research output in the field of COVID-19 and liver dysfunction, according to previous similar studies, this study had several limitations, and it is essential to acknowledge them to ensure transparency and to promote further research in the field. First, Scopus is a widely used database for bibliometric analyses, and its coverage of peer-reviewed literature is generally comprehensive. However, the bibliometric analysis may not capture all the relevant COVID-19 and liver research publications because not all journals are indexed in Scopus, and some of the relevant publications may have been published in non-indexed or non-English-language journals. Second, searching for publications based on MeSH terms in the title is a common practice in bibliometric analysis to ensure the accuracy and relevance of the search results. However, including some publications that use related terms in other parts of the document may be a mistake. Therefore, it is recommended to use a combination of search terms, including title, abstract and keywords, to increase the sensitivity of the search. If false-negative results had occurred in the current study, they would have had little effect on the overall results. Third, the analysis may not account for the quality of the publications, as it only counts the number of publications without assessing their impact, significance or credibility. Furthermore, the ranking of publications based on total citations rather than annual citation averages could have excluded some recently published high-quality studies. However, this does not undermine the importance or contribution of these publications to the field. It is essential to note that the limitations identified in this bibliometric analysis could affect the precision and completeness of the obtained results. However, these limitations do not significantly compromise the study’s validity.
CONCLUSION
This bibliometric study provides a comprehensive overview of liver-related publications in COVID-19 research over the past 3 years. It highlights the significant contributions of high-income nations, particularly the United States, China and Italy, to the scholarly liver-related literature in this field. Most of the articles focus on liver dysfunction in patients with COVID-19 and the implications of the virus for gastroenterologists and hepatologists. Although the study acknowledges the importance of abnormal LFTs in COVID-19, it also identifies knowledge gaps regarding their pathophysiological and prognostic value. This study indicates that well-designed prospective studies with specific research questions can be used to fill in these gaps and further investigate the topic. This bibliometric analysis provides a solid foundation for future research on liver injury and treatment for COVID-19. The findings of this study can be used by decision-makers or researchers to prioritise research areas and allocate resources to enhance our understanding of the impact of COVID-19 on liver function.
ARTICLE HIGHLIGHTS
Research background
Abnormal liver chemistry findings are commonly observed in individuals with coronavirus disease 2019 (COVID-19). As a result, there is a significant body of literature comprising published clinical studies that specifically examine the implications of hepatic involvement in COVID-19.
Research motivation
Understanding the current state of research and hotspots in the field of COVID-19 and liver dysfunction is crucial for identifying knowledge gaps and informing future research directions.
Research objectives
The purpose of this study is to present a comprehensive overview of the existing research on COVID-19 and liver dysfunction through the utilization of bibliometric analysis.
Research methods
A thorough and validated search query was conducted using the SciVerse Scopus database to identify relevant publications on the topic of COVID-19 and liver dysfunction. Subsequently, the research hotspots in related fields were examined using VOSviewer software.
Research results
Three distinct clusters of topics were identified: COVID-19 vaccines in liver transplant recipients, liver function tests as predictors of severity and clinical outcomes in hospitalized patients, and the care of patients with liver disease during the COVID-19 pandemic.
Research conclusions
The first bibliometric analysis is presented here, which is represented by the study and offers valuable insights into the research landscape on COVID-19 and liver dysfunction. Valuable reference can be derived from the findings in this field by scholars, as a comprehensive summary is provided and the frontiers of research related to COVID-19 and liver dysfunction are identified.
Research perspectives
The goal of this study is to have current areas of focus within the field of COVID-19 and liver dysfunction identified, which can help future research be guided and clinical practice be informed. By using bibliometric analysis, a comprehensive overview of the literature on this topic is provided by the study, and the latest developments in the field can be kept up-to-date by researchers and clinicians.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 24, 2023
First decision: May 23, 2023
Article in press: June 27, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Palestine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Teles RHG, Brazil; Xie C, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. [cited 3 May 2023]. Available from: https://covid19.who.int/
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, Ho RC. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Zhu R, Bai T, Han P, He Q, Jing M, Xiong X, Zhao X, Quan R, Chen C, Zhang Y, Tao M, Yi J, Tian D, Yan W. Clinical Features of Patients Infected With Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study. Hepatology. 2021;73:1509–1520. doi: 10.1002/hep.31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao SR, Zhang SY, Lian JS, Jin X, Ye CY, Cai H, Zhang XL, Hu JH, Zheng L, Zhang YM, Jia HY, Yu GD, Wang XY, Gu JQ, Lu YF, Yu XP, Yu L, Xiang DR, Jin CL, Qiu YQ, Li LJ, Sheng JF, Liang TB, Yang YD. Liver Enzyme Elevation in Coronavirus Disease 2019: A Multicenter, Retrospective, Cross-Sectional Study. Am J Gastroenterol. 2020;115:1075–1083. doi: 10.14309/ajg.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol. 2021;33:114–115. doi: 10.1097/MEG.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924–935. doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627–634. doi: 10.1016/j.aohep.2020.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha L, Vij S, Rawat K. Liver injury induced by COVID 19 treatment - what do we know? World J Gastroenterol. 2022;28:6314–6327. doi: 10.3748/wjg.v28.i45.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naeem M, Bano N, Manzoor S, Ahmad A, Munawar N, Razak SIA, Lee TY, Devaraj S, Hazafa A. Pathogenetic Mechanisms of Liver-Associated Injuries, Management, and Current Challenges in COVID-19 Patients. Biomolecules. 2023;13 doi: 10.3390/biom13010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bzeizi K, Abdulla M, Mohammed N, Alqamish J, Jamshidi N, Broering D. Effect of COVID-19 on liver abnormalities: a systematic review and meta-analysis. Sci Rep. 2021;11:10599. doi: 10.1038/s41598-021-89513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072–13088. doi: 10.26355/eurrev_202012_24215. [DOI] [PubMed] [Google Scholar]
- 18.Abdulla S, Hussain A, Azim D, Abduallah EH, Elawamy H, Nasim S, Kumar S, Naveed H. COVID-19-Induced Hepatic Injury: A Systematic Review and Meta-Analysis. Cureus. 2020;12:e10923. doi: 10.7759/cureus.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puli S, Baig M, Walayat S. Gastrointestinal Symptoms and Elevation in Liver Enzymes in COVID-19 Infection: A Systematic Review and Meta-Analysis. Cureus. 2020;12:e9999. doi: 10.7759/cureus.9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shokri Afra H, Amiri-Dashatan N, Ghorbani F, Maleki I, Rezaei-Tavirani M. Positive association between severity of COVID-19 infection and liver damage: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13:292–304. [PMC free article] [PubMed] [Google Scholar]
- 21.Vargas-Mendoza N, García-Machorro J, Angeles-Valencia M, Martínez-Archundia M, Madrigal-Santillán EO, Morales-González Á, Anguiano-Robledo L, Morales-González JA. Liver disorders in COVID-19, nutritional approaches and the use of phytochemicals. World J Gastroenterol. 2021;27:5630–5665. doi: 10.3748/wjg.v27.i34.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni AV, Aziz B, Shams I, Busse JW. Comparisons of citations in Web of Science, Scopus, and Google Scholar for articles published in general medical journals. JAMA. 2009;302:1092–1096. doi: 10.1001/jama.2009.1307. [DOI] [PubMed] [Google Scholar]
- 23.De Groote SL, Raszewski R. Coverage of Google Scholar, Scopus, and Web of Science: a case study of the h-index in nursing. Nurs Outlook. 2012;60:391–400. doi: 10.1016/j.outlook.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Powell KR, Peterson SR. Coverage and quality: A comparison of Web of Science and Scopus databases for reporting faculty nursing publication metrics. Nurs Outlook. 2017;65:572–578. doi: 10.1016/j.outlook.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Zyoud SH, Al-Jabi SW, Shahwan MJ, Jairoun AA. Global research production pertaining to gastrointestinal involvement in COVID-19: A bibliometric and visualised study. World J Gastrointest Surg. 2022;14:494–505. doi: 10.4240/wjgs.v14.i5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zyoud SH, Shakhshir M, Abushanab AS, Koni A, Shahwan M, Jairoun AA, Al-Jabi SW. Mapping the output of the global literature on the links between gut microbiota and COVID-19. J Health Popul Nutr. 2023;42:3. doi: 10.1186/s41043-023-00346-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xavier-Santos D, Padilha M, Fabiano GA, Vinderola G, Gomes Cruz A, Sivieri K, Costa Antunes AE. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci Technol. 2022;120:174–192. doi: 10.1016/j.tifs.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Jabi SW. Current global research landscape on COVID-19 and depressive disorders: Bibliometric and visualization analysis. World J Psychiatry. 2021;11:253–264. doi: 10.5498/wjp.v11.i6.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tantengco OAG. Investigating the evolution of COVID-19 research trends and collaborations in Southeast Asia: A bibliometric analysis. Diabetes Metab Syndr. 2021;15:102325. doi: 10.1016/j.dsx.2021.102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung KS, Mok CH, Mao X, Zhang R, Hung IF, Seto WK, Yuen MF. COVID-19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: A meta-analysis. Clin Mol Hepatol. 2022;28:890–911. doi: 10.3350/cmh.2022.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong X, Zeng DY, Xing QQ, Hong MZ, Pan JS. Liver chemistries in severe or non-severe cases of COVID-19: A systematic review and meta-analysis. World J Hepatol. 2022;14:2012–2024. doi: 10.4254/wjh.v14.i12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. doi: 10.5888/pcd19.210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang R, Feng J, Wan H, Zeng X, Ji P, Zhang J. Liver injury associated with the severity of COVID-19: A meta-analysis. Front Public Health. 2023;11:1003352. doi: 10.3389/fpubh.2023.1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo JJ, Yon DK, Lee SW, Shin JI, Kim BK. Humoral Immunogenicity to SARS-CoV-2 Vaccination in Liver Transplant Recipients: A Systematic Review and Meta-Analysis. Int J Biol Sci. 2022;18:5849–5857. doi: 10.7150/ijbs.77030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweileh WM. A bibliometric analysis of global research output on health and human rights (1900-2017) Glob Health Res Policy. 2018;3:30. doi: 10.1186/s41256-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweileh WM, Al-Jabi SW, Zyoud SH, Shraim NY, Anayah FMA, Sawalha AF, AbuTaha AS. Bibliometric analysis of global publications in medication adherence (1900-2017) Int J Pharm Pract. 2019;27:112–120. doi: 10.1111/ijpp.12471. [DOI] [PubMed] [Google Scholar]
- 37.Sweileh WM. Substandard and falsified medical products: bibliometric analysis and mapping of scientific research. Global Health. 2021;17:114. doi: 10.1186/s12992-021-00766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweileh WM. Global research activity on mathematical modeling of transmission and control of 23 selected infectious disease outbreak. Global Health. 2022;18:4. doi: 10.1186/s12992-022-00803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zyoud SH. The state of current research on COVID-19 and antibiotic use: global implications for antimicrobial resistance. J Health Popul Nutr. 2023;42:42. doi: 10.1186/s41043-023-00386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JL, Ma YJ, Ma L, Ma N, Guo DM, Ma LS. Baishideng's Reference Citation Analysis database announces the first Article Influence Index of multidisciplinary scholars. World J Clin Cases. 2022;10:10391–10398. doi: 10.12998/wjcc.v10.i29.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JL, Ma YJ, Ma L, Ma N, Guo DM, Ma LS. Baishideng's Reference Citation Analysis database announces the first Journal Article Influence Index of 101 core journals and a list of high-quality academic journals in gastroenterology and hepatology. World J Gastroenterol. 2022;28:5383–5394. doi: 10.3748/wjg.v28.i37.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JL, Ma YJ, Ma L, Ma N, Guo DM, Ma LS. Baishideng's Reference Citation Analysis database announces the first Journal Article Influence Index of 104 core journals and a list of high-quality academic journals in orthopedics. World J Orthop. 2022;13:891–902. doi: 10.5312/wjo.v13.i10.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flis I, van Eck NJ. Framing psychology as a discipline (1950-1999): A large-scale term co-occurrence analysis of scientific literature in psychology. Hist Psychol. 2018;21:334–362. doi: 10.1037/hop0000067. [DOI] [PubMed] [Google Scholar]
- 45.van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. 2017;111:1053–1070. doi: 10.1007/s11192-017-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 55.Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zyoud SH, Shakhshir M, Koni A, Shahwan M, Jairoun AA, Al-Jabi SW. Olfactory and Gustatory Dysfunction in COVID-19: A Global Bibliometric and Visualized Analysis. Ann Otol Rhinol Laryngol. 2023;132:164–172. doi: 10.1177/00034894221082735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doskaliuk B, Yatsyshyn R, Klishch I, Zimba O. COVID-19 from a rheumatology perspective: bibliometric and altmetric analysis. Rheumatol Int. 2021;41:2091–2103. doi: 10.1007/s00296-021-04987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei N, Xu Y, Wang H, Jia Q, Shou X, Zhang X, Zhang N, Li Y, Zhai H, Hu Y. Bibliometric and visual analysis of cardiovascular diseases and COVID-19 research. Front Public Health. 2022;10:1022810. doi: 10.3389/fpubh.2022.1022810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu SC, Zhao XY, Xing HP, Wu W, Zhang SY. Cardiac Involvement in COVID-19: A Global Bibliometric and Visualized Analysis. Front Cardiovasc Med. 2022;9:955237. doi: 10.3389/fcvm.2022.955237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao J, Zhu J, Huang C, Zhu X, Zhu Z, Wu Q, Yuan R. Uncovering the information immunology journals transmitted for COVID-19: A bibliometric and visualization analysis. Front Immunol. 2022;13:1035151. doi: 10.3389/fimmu.2022.1035151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Y, Zhang C, Lai Q. China's rise as a major contributor to science and technology. Proc Natl Acad Sci U S A. 2014;111:9437–9442. doi: 10.1073/pnas.1407709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li M, Liu X, Zhang L. Health sciences journals: an overview of outputs by Chinese authors. Health Info Libr J. 2015;32:255–264. doi: 10.1111/hir.12112. [DOI] [PubMed] [Google Scholar]
- 63.The Guardian. China overtakes the US in scientific research output. 2022. [Cited 10 June 2023] Available from: https://www.theguardian.com/world/2022/aug/11/china-overtakes-the-us-in-scientific-research-output .
- 64. Optimizing the Nation's Investment in Academic Research: A New Regulatory Framework for the 21st Century. Washington (DC): National Academies Press (US); 2016-Jul-27 . [PubMed] [Google Scholar]
- 65.Momtazmanesh S, Saghazadeh A, Becerra JCA, Aramesh K, Barba FJ, Bella F, Blakney A, Capaccioli M, Castagna R, Crisanti U, Davtyan T, Dorigo T, Ealy J, Farokhnia M, Grancini G, Gupta M, Harbi A, Krysztofiak W, Kulasinghe A, Lam CM, Leemans A, Lighthill B, Limongelli V, Lopreiato P, Luongo L, Maboloc CR, Malekzadeh R, Gomes OC, Milosevic M, Nouwen J, Ortega-Sánchez D, Pawelek J, Pramanik S, Ramakrishna S, Renn O, Sanseviero S, Sauter D, Schreiber M, Sellke FW, Shahbazi MA, Shelkovaya N, Slater WH, Snoeck D, Sztajer S, Uddin LQ, Veramendi-Espinoza L, Vinuesa R, Willett WC, Wu D, Żyniewicz K, Rezaei N. International Scientific Collaboration Is Needed to Bridge Science to Society: USERN2020 Consensus Statement. SN Compr Clin Med. 2021;3:1699–1703. doi: 10.1007/s42399-021-00896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie Q, Freeman RB. Bigger Than You Thought: China's Contribution to Scientific Publications and Its Impact on the Global Economy. China & World Economy. 2019;27:1–27. [Google Scholar]
- 67.Kwiek M. Internationalists and locals: international research collaboration in a resource-poor system. Scientometrics. 2020;124:57–105. [Google Scholar]
- 68.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 71.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881–883. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. 2021;105:37–55. doi: 10.1097/TP.0000000000003523. [DOI] [PubMed] [Google Scholar]
- 75.Dumortier J, Duvoux C, Roux O, Altieri M, Barraud H, Besch C, Caillard S, Coilly A, Conti F, Dharancy S, Durand F, Francoz C, Garaix F, Houssel-Debry P, Kounis I, Lassailly G, Laverdure N, Leroy V, Mallet M, Mazzola A, Meunier L, Radenne S, Richardet JP, Vanlemmens C, Hazzan M, Saliba F French Solid Organ Transplant COVID Registry; Groupe de Recherche Français en Greffe de Foie (GReF²) Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45:101639. doi: 10.1016/j.clinre.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tu ZH, Jin PB, Chen DY, Chen ZY, Li ZW, Wu J, Lou B, Zhang BS, Zhang L, Zhang W, Liang TB. Evaluating the Response and Safety of Inactivated COVID-19 Vaccines in Liver Transplant Recipients. Infect Drug Resist. 2022;15:2469–2474. doi: 10.2147/IDR.S359919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozaka S, Kobayashi T, Mizukami K, Murakami K. COVID-19 vaccination and liver disease. World J Gastroenterol. 2022;28:6791–6810. doi: 10.3748/wjg.v28.i48.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alqahtani SA, Barry M, Memish Z, Hashim A, Alfares MA, Alghamdi SA, Al-Hamoudi WK, Al-Judaibi B, Alhazzani W, Al-Tawfiq JA, Abaalkhail F. Use of COVID-19 vaccines in patients with liver disease and post-liver transplantation: Position statement of the Saudi association for the study of liver diseases and transplantation. Saudi J Gastroenterol. 2021;27:201–207. doi: 10.4103/sjg.sjg_223_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luxenburger H, Reeg DB, Lang-Meli J, Reinscheid M, Eisner M, Bettinger D, Oberhardt V, Salimi Alizei E, Wild K, Graeser A, Karl V, Sagar, Emmerich F, Klein F, Panning M, Huzly D, Bengsch B, Boettler T, Elling R, Thimme R, Hofmann M, Neumann-Haefelin C. Boosting compromised SARS-CoV-2-specific immunity with mRNA vaccination in liver transplant recipients. J Hepatol. 2023;78:1017–1027. doi: 10.1016/j.jhep.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdelrahman MM, Abdel-Baset AA, Younis MA, Mahmoud MG, Shafik NS. Liver function test abnormalities in COVID-19 patients and factors affecting them - a retrospective study. Clin Exp Hepatol. 2021;7:297–304. doi: 10.5114/ceh.2021.109225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Przekop D, Gruszewska E, Chrostek L. Liver function in COVID-19 infection. World J Hepatol. 2021;13:1909–1918. doi: 10.4254/wjh.v13.i12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krishnan A, Prichett L, Tao X, Alqahtani SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH, Woreta TA. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol. 2022;28:570–587. doi: 10.3748/wjg.v28.i5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivandzadeh GR, Askari H, Safarpour AR, Ejtehadi F, Raeis-Abdollahi E, Vaez Lari A, Abazari MF, Tarkesh F, Bagheri Lankarani K. COVID-19 infection and liver injury: Clinical features, biomarkers, potential mechanisms, treatment, and management challenges. World J Clin Cases. 2021;9:6178–6200. doi: 10.12998/wjcc.v9.i22.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kayaaslan B, Guner R. COVID-19 and the liver: A brief and core review. World J Hepatol. 2021;13:2013–2023. doi: 10.4254/wjh.v13.i12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022;106:1026–1041. doi: 10.4269/ajtmh.21-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu X, Sun L, Guo Z, Wu C, Yu X, Li J. Management of COVID-19 patients with chronic liver diseases and liver transplants. Ann Hepatol. 2022;27:100653. doi: 10.1016/j.aohep.2021.100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Güner R, Hasanoğlu I, Aktaş F. COVID-19: Prevention and control measures in community. Turk J Med Sci. 2020;50:571–577. doi: 10.3906/sag-2004-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monye I, Adelowo AB. Strengthening immunity through healthy lifestyle practices: Recommendations for lifestyle interventions in the management of COVID‐19. Lifestyle Medicine 2020; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veljković M, Pavlović DR, Stojanović NM, Džopalić T, Popović Dragonjić L. Behavioral and Dietary Habits That Could Influence Both COVID-19 and Non-Communicable Civilization Disease Prevention-What Have We Learned Up to Now? Medicina (Kaunas) 2022;58 doi: 10.3390/medicina58111686. [DOI] [PMC free article] [PubMed] [Google Scholar]