Abstract
BACKGROUND
Using rat stomach perforation as a prototypic direct lesion applied in cytoprotection research, we focused on the first demonstration of the severe occlusion/ occlusion-like syndrome induced by stomach perforation. The revealed stomach-induced occlusion/occlusion-like syndrome corresponds to the previously described occlusion/occlusion-like syndromes in rats suffering multicausal pathology and shared severe vascular and multiorgan failure. This general point was particularly reviewed. As in all the described occlusion/occlusion-like syndromes with permanent occlusion of major vessels, peripheral and central, and other similar noxious procedures that severely affect endothelium function, the stable gastric pentadecapeptide BPC 157 was resolving therapy.
AIM
To reveal the stomach perforation-induced general occlusion/occlusion-like syndrome and BPC 157 therapy effect.
METHODS
The procedure included deeply anesthetized rats, complete calvariectomy, laparotomy at 15 min thereafter, and stomach perforation to rapidly induce vascular and multiorgan failure occlusion/occlusion-like syndrome. At 5 min post-perforation time, rats received therapy [BPC 157 (10 µg or 10 ng/kg) or saline (5 mL/kg, 1 mL/rat) (controls)] into the perforated defect in the stomach). Sacrifice was at 15 min or 60 min post-perforation time. Assessment (gross and microscopy; volume) included: Brain swelling, peripheral vessels (azygos vein, superior mesenteric vein, portal vein, inferior caval vein) and heart, other organs lesions (i.e., stomach, defect closing or widening); superior sagittal sinus, and peripherally the portal vein, inferior caval vein, and abdominal aorta blood pressures and clots; electrocardiograms; and bleeding time from the perforation(s).
RESULTS
BPC 157 beneficial effects accord with those noted before in the healing of the perforated defect (raised vessel presentation; less bleeding, defect contraction) and occlusion/occlusion-like syndromes counteraction. BPC 157 therapy (into the perforated defect), induced immediate shrinking and contraction of the whole stomach (unlike considerable enlargement by saline application). Accordingly, BPC 157 therapy induced direct blood delivery via the azygos vein, and attenuated/eliminated the intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension. Thrombosis, peripherally (inferior caval vein, portal vein, abdominal aorta) and centrally (superior sagittal sinus) BPC 157 therapy markedly reduced/annihilated. Severe lesions in the brain (swelling, hemorrhage), heart (congestion and arrhythmias), lung (hemorrhage and congestion), and marked congestion in the liver, kidney, and gastrointestinal tract were markedly reduced.
CONCLUSION
We revealed stomach perforation as a severe occlusion/occlusion-like syndrome, peripherally and centrally, and rapid counteraction by BPC 157 therapy. Thereby, further BPC 157 therapy may be warranted.
Keywords: Stomach perforation, General occlusion/occlusion-like syndrome, Stable gastric pentadecapeptide BPC 157, Cytoprotection, Therapy, Rats
Core Tip: Rats with perforated stomachs exhibited the rapidly emerging severe occlusion/occlusion-like syndrome, an innate general vascular and multiorgan failure, peripherally and centrally. The pentadecapeptide BPC 157 application into stomach defects was efficacious therapy. With an activated azygos vein-rescuing pathway, there was cause-consequence counteraction of lesions in the brain (swelling, hemorrhage), heart (congestion), lung (hemorrhage), and congestion in the liver, kidney, and gastrointestinal tract. Whole occlusion/occlusion-like syndrome, arrhythmias, blood pressure disturbances (intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension), major vessel failure, and widespread thrombosis, Virchow triad circumstances peripherally and centrally were all attenuated/eliminated by BPC 157 therapy.
INTRODUCTION
As a novel approach with the beneficial effect of the stable gastric pentadecapeptide BPC 157[1-6] to the stomach perforation therapy, we intend to better implement in practice Robert’s and Szabo’s cytoprotection concept[7-9]. Conceptually, this might improve the original acceptance of basic cytoprotection maxim, the direct epithelium (Robert)[7,8], endothelium (Szabo)[9] protection/maintenance in the stomach vs direct agent’s noxious effect (i.e., intragastric alcohol[7]; stomach perforation[10]). To resolve this issue, along with the recently reported stomach perforation in the rats[10], this report describes the rats with a perforated stomach rapidly emerging severe general syndrome occlusion/occlusion-like syndrome recently reviewed[1-4,11-24] as an innate general vascular and multiorgan failure and its therapy with the stable pentadecapeptide BPC 157 application (activation of the collateral pathways)[1,2,11-24].
In the rats with perforated stomachs, noxious course progression, and general detrimental syndrome peripherally and centrally, the BPC 157 therapy outcome might be similar to the beneficial effect that occurs peripherally and centrally during major vessel occlusion[11-19] and other similar noxious procedure application[20-24]. To specifically link the occlusion/occlusion-like syndrome and cytoprotection issue we should mention BPC 157 as a novel cytoprotection mediator maintaining gastrointestinal mucosa integrity, used in ulcerative colitis trials, lethal dose (LD1) not achieved in toxicology studies, native to and stable in human gastric juice, and thereby, easy applicable cytoprotective capabilities and also permitting per-oral application[1-6]. Further, this occlusion/occlusion-like syndrome[11-24] includes, in particular, the absolute alcohol intragastric instillation as a challenge, the prototype commonly used in cytoprotection studies[7,8,24]. Given the previous therapy effects in the occlusion/occlusion-like syndrome[11-24] and in the cytoprotective studies (for review see[25-31]), including perforated defects[10,32], the stable gastric pentadecapeptide BPC 157, might be the resolving therapy (for additional review see[1-6]) equally acting peripherally and centrally. These might be the novel particular shared pathology and shared therapy indication. This might be due to the BPC 157 therapy advantages, its particular capabilities, cytoprotection[1] as a particular vascular effect[2], wound healing[5], and neuroprotection[6,33]. These implemented its particular additional cytoprotective capabilities[1], and thereby, in the severe vessel and multiorgan failure occlusion/occlusion-like syndrome[2], the prompt particular activation of the collateral pathways (i.e., azygos vein direct blood flow delivery) rapidly occurred as resolving key, and recovery of the muscle disturbances, striated, smooth, and heart failure recovery as whole[3,4].
As mentioned, very recently, in the surgically perforated stomach, the stable gastric pentadecapeptide BPC 157 therapy, which locally immediately restored vessels, counteracted bleeding and contracted perforated defect, and eventually, leads to complete healing (day 7), and fewer adhesions, unlike the failed effect of the standard anti-ulcer agents[10]. Now, given the complexity of the stomach perforation lesions, given the general syndrome simultaneously progressing peripherally and centrally, some additional particular parallel effects of its therapy might be further demonstrated[1-6]. Considering the stomach perforation as the part or cause of the occlusion/occlusion-like syndrome of the vascular and multiorgan failure[11-24] might better define the whole emergency made by perforation injury. Previously, in the occlusion/occlusion-like syndrome studies[11-24], with consistently evidenced BPC 157 therapy resolving effect[11-24] due to particular activation of collateral pathways, reported were the counteractions of the severe lesions in the brain and intracerebral and intraventricular hemorrhage, heart (congestion, infarction, and arrhythmias), hemorrhage and congestion in the lung, liver, kidney and gastrointestinal tract. BPC 157 therapy effect included the attenuation/elimination of the blood pressure disturbances, (intracranial superior sagittal sinus), portal and caval hypertension, and aortal hypotension). Arterial and venous thrombosis peripherally and centrally were almost annihilated; congested inferior caval and superior mesenteric vein, collapsed azygos vein as failed major vessels were recovered to rescuing pathways organization[11-24]. Together, on the one hand, these were summarized as rapidly acting Virchow triad circumstances, and on the other hand, as rapidly acting, “key bypassing” therapy with BPC 157 application[11-24], and likely, they might occur rapidly also in the rats after stomach perforation[10]. As particular damaging points appeared the variety of the major vessel occlusion (inferior caval vein, infrarenal and suprahepatic[11,12], portal vein and hepatic artery[13], superior mesenteric vein[14,15] and artery[15,16], superior sagittal sinus[17], both carotid arteries[18], and cauterized episcleral veins[19]). A variety of other similar severe procedures appeared as additional particular points, i.e., myocardial infarction and re-infarction induced by isoprenaline[21], bile duct occlusion acute pancreatitis[23], severe intra-abdominal hypertension mechanically maintained[20], and the intoxication with endothelium damaging agents, alcohol[24] or lithium[22] prototype agent in bipolar disorders, in particular, all as occlusion/occlusion-like syndrome[20-24].
As an important conceptual point to combine all these lesions and to resolve the shared therapy, the prototype ulcerogenic procedure in cytoprotection studies, the absolute alcohol intragastric instillation[24], was one of the innate injury models readily combined with full occlusion/occlusion-like syndrome[11-24]. Thereby, the resolution of the prime lesion and the resolution of the whole syndrome might be evidence of the full cytoprotective effect of the BPC 157 therapy. As the perforated defect is direct damage analogous to the intragastric alcohol-induced direct damage[10,24] (Robert’s lesion by direct contact[7]), it represents an analogous prototype lesion and an essential hallmark in cytoprotection studies[10]. Thereby, the therapy for the perforated stomach defect mandated the resolution of the full occlusion/occlusion-like syndrome to be achieved as in the previous studies[11-24]. Thus, in cytoprotection terms, in the perforated stomach defect[10], the advantageous effect of the BPC 157 therapy, to the same wide extent, might likely occur. Nevertheless, the resolving intragastric alcohol stomach lesions and full occlusion/occlusion-like syndrome might be the successful therapy effects[10] (i.e., activated azygos vein to provide direct blood delivery and inferior caval vein-superior caval vein rescuing pathway[2]) suited for generalization[11-24] of the activated collateral pathways occurring as resolving key.
Conceptually, within the standard view and standard cytoprotection agents, the recovery of the perforated stomach defect[10] may be still outside of the cytoprotection concept although born in the stomach[2]. Cytoprotection has essential theoretical and practical points (i.e., holding direct epithelial cell protection[7,8], beneficial effect on other organs[7] (cytoprotection→organoprotection)[34]; direct endothelium cell protection[9], and endothelial maintenance→epithelial maintenance[7-9]). However, these could be hardly assumed to recover a perforated stomach[10] within the standard cytoprotective agents[1,2] as limited more to the prophylaxis, not therapy effect[7-9]. On the other hand, BPC 157 therapy, based on the noted effects[1-6], thereby, may be proof of the corresponding significance (i.e., the counteraction of the leaky gut syndrome[35], and free radical scavenging activity in the various tissues[35-37]). It may be that the rapid upgrading of the minor vessel to take over and compensate for the function of the failed major vessels, and the specific activation of the collateral pathways, are highlights of the innate cytoprotection function[1-6] as an effective cytoprotection mediator. As native and stable in human gastric juice, it may take the continuous maintenance of the stomach and gastrointestinal mucosa integrity, thereby, the pleiotropic beneficial effect occurred[1-6] (including the recovery of the muscle disturbances, striated, smooth, and heart failure recovery as a whole[3,4]). As such, it might be easily applicable as therapy and might override limitations known for the standard cytoprotective agents[1-9] and contribute to the recovery of stomach perforation as both local and general (occlusion/occlusion-like syndrome) disturbance. These might be particular effects in the vascular studies[11,13-16,21,22,26,35-37], given its special interaction with nitric oxide (NO)-system[29,38-40], thrombocytes[40-42] and many molecular pathways[43-52] (i.e., the counteraction of tumor-induced muscle cachexia and the signaling process implicated in cancer cachexia)[49].
On the other hand, whatever the cause, there were all organ lesions, progressing venous/arterial thrombosis/stasis, peripherally and centrally, all counteracted by BPC 157 therapy application. As additional proof of the concept, the ECG disturbances, intracranial (superior sagittal sinus) hypertension, portal and caval hypertension, and aortal hypotension were eliminated/attenuated[11-24]. Accordingly, given the considerable blood volume that might be trapped with(in) failed vessels[11-24], there might be also a comparable syndrome that might be also induced rapidly with the stomach perforation as the initial injury[10]. Likewise, in the rats with stomach perforation, along with the therapy effect realized locally in the perforated stomach[10], BPC 157 therapy’s particular beneficial effect might be realized with the rapid upgrading of the minor vessel to take over and compensate the function of the failed major vessels, making possible the specific activation of the collateral pathways, as before[11-24]. Thus, the activated azygos vein (and thereby, direct blood flow delivery from the inferior to the superior caval vein) might serve as a “bypassing vascular key” taking general significance (for review see[11-24]) to recover otherwise imminent occlusion/occlusion-like syndrome also in rats with the perforated stomachs.
For this purpose, given its cytoprotective stomach capability to counteract the whole imminent occlusion/occlusion-like syndrome, and brain lesions, in particular[11-24], we used the calvariectomized rats, for direct monitoring, and the BPC 157 therapy was given directly into the perforated stomach defect. We focused on the subsequent presentation of the stomach and the presentation of the brain swelling and all organ lesions. Then, progressing venous/arterial thrombosis/stasis, peripherally and centrally, were all counteracted by BPC 157 therapy application. These were along with attenuation/elimination of the arrhythmias, intracranial (superior sagittal sinus) hypertension, portal and caval hypertension, and aortal hypotension. Thus, full therapy effects were determined during the essential early period.
MATERIALS AND METHODS
Animals
Male Albino Wistar rats, 200 g bw, kept under standard laboratory conditions, were randomly assigned at 6 rats/group/interval for all experiments, approved by the Local Ethic Committee, carried out under a blind protocol, and the effects evaluated by the examiners who were unaware of the applied protocol.
Drugs
Stable gastric pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, molecular weight 1419; Diagen, Slovenia), was used in dose and application regimens described previously (i.e., without the use of a carrier or peptidase inhibitor) (for review see[1-6]).
Experimental protocol
In deep anesthesia [40 mg/kg thiopental (Rotexmedica, Germany) and 10 mg/kg diazepam (Apaurin; Krka, Slovenia) intraperitoneally] rats underwent complete calvariectomy[17], and laparotomy at 15 min thereafter, and to induce rapid vascular failure and concomitant general and peripheral occlusion/occlusion-like syndrome, stomach perforation was performed as described before[10,17]. Sacrifice was at 15 min or 60 min post-perforation time.
At 5 min post-perforation time, rats received therapy [BPC 157 (10 µg or 10 ng/kg) or saline (5 mL/kg, 1 mL/rat) (controls)] into the perforated defect in the stomach.
Before the procedure, after perforation, after therapy application, and before sacrifice, recordings [camera attached to a VMS-004 Discovery Deluxe USB microscope (Veho, United States)] of brain swelling, corresponding presentation of the peripheral vessels (azygos vein, superior mesenteric vein, portal vein, and inferior caval vein), and organ lesions, were as in our vascular studies[11-24], and assessed as before[11-24]. At all the indicated time points until the end of the 15-min period stomach defect closing or widening (both as % of the presentation immediately before therapy) was assessed as described[10].
In a separate group of animals, the stomach perforation-induced bleeding as bleeding time from the perforation(s) was assessed throughout the 15 min, given the quite short time after perforation available as described[10]. Immediately after perforation induction, rats received BPC 157 (10 µg or 10 ng/kg) or saline (5 mL/kg, 1 mL/rat) (controls) as therapy applied to the perforated defect in the stomach.
Superior sagittal sinus, portal, caval vein, and abdominal aorta pressure recording
The recording procedure in rats was described in detail in our previous vascular studies[11-24]. At 15 min and 60 min post-perforation time, under deep anesthesia, a cannula (BD Neoflon™ Cannula) connected to a pressure transducer (78534C MONITOR/ TERMINAL; Hewlett Packard, United States), inserted into the portal vein, inferior caval vein and superior sagittal sinus, as well as the abdominal aorta at the level of the bifurcation.
Accordingly[11-24], -24 mmHg to -27 mmHg values of superior sagittal sinus pressure were in healthy rats. The 3-5 mmHg values were considered normal in portal pressure in healthy rats. Similar to that were in the inferior vena cava, though with values at least 1 mmHg higher in the portal vein. Abdominal aorta blood pressure values of 100-120 mmHg at the bifurcation level were considered normal in healthy rats.
ECG recording
In rats under deep anesthesia, before the procedure, and in post-perforation time (i.e., after stomach perforation, after therapy application, and before sacrifice), precise recordings, measurements, and analysis of ECG parameters (PQ intervals, QTc, heart frequency) as specifically described[11-24] were with ECGs recorded continuously for all three main leads, by positioning stainless steel electrodes on all four limbs using an ECG monitor with a 2090 programmer (Medtronic, United States) connected to a Waverunner LT342 digital oscilloscope (LeCroy, United States)[11-24].
Thrombus assessment
After rats’ sacrifice, the clots were weighed when the superior sagittal sinus, and peripherally the portal vein, inferior caval vein, and abdominal aorta were removed[11-24].
Brain volume, heart, and vessel volume presentation
We applied the procedure used in our previous vascular studies[11-24]. Brain volume and vessel volume and heart volume or stomach volume were proportional to the change in the brain or vessel or heart or stomach surface area. The presentation of the brain and peripheral vessels (superior mesenteric vein, inferior caval vein, and azygos vein) was recorded in deeply anesthetized rats, with a camera attached to a VMS-004 Discovery Deluxe USB microscope (Veho, United States). The border of the brain (or vessels, or heart or stomach) in the image was marked using ImageJ software and then the surface area of the brain (or veins, or heart or stomach) was measured. This was done with brain (or veins or stomach) images for healthy rats, and then/or for both the control (saline) group and treated (BPC 157) group of rats at the same intervals after the application and at the time of sacrifice. The arithmetic mean of the surface areas was calculated for both groups. Then, the ratio of these two areas was calculated as (Acon/Abpc), where Acon is the arithmetic mean brain (or veins) area of the control group and Abpc is the arithmetic mean brain (or veins or heart or stomach) area of the treated group. Starting from the square-cube law equations[1,2], an equation for the change in brain (or veins, or heart, or stomach) volume proportional to the change in brain surface area (or veins, or heart, or stomach)[6] was derived. In expressions[1-5], “l” is defined as any arbitrary one-dimensional length of the brain (for example rostro-caudal length of the brain) (or veins or heart or stomach), used only for defining the one-dimensional proportion (l2/l1) between two observed brains (or veins or heart or stomach) and as an inter-factor (and because of that not measured[6]) for deriving final expression[6].
The procedure was as follows: (1) A2 = A1 × (l2/l1)2 (square-cube law); (2) V2 = V1 × (l2/l1)3 (square-cube law); (3) A2/A1 = (l2/l1)2 [from (1), after dividing both sides by A1]; (4) l2/l1 = (A2/A1)1/2 [from (3), after taking the square root of both sides]; (5) V2/V1 = (l2/l1)3 [from (2), after dividing both sides by V1]; and (6) V2/V1 = [(A2/A1)1/2]3 [after incorporating expression (4) into equation (5)].
Gross assessment of gastrointestinal lesions
In rats laparatomized under anesthesia, gross lesions in the gastrointestinal tract and in the stomach (sum of the longest diameters, mm) assessment was with the camera attached to a VMS-004 Discovery Deluxe USB microscope (Veho, United States) before sacrifice as described before[11-24].
Microscopy
As described in the previous studies[11-24], evaluation was done by light microscopy using an Olympus 71 digital camera and an Olympus BX51 microscope (OLYMPUS Europa SE & CO. KG). Digital images were saved as uncompressed 24-bit RGB TIFF files using the software program AnalySIS (Olympus Soft Imaging System GmbH, Munster, Germany). The field size was measured and marked with a manufacturer's default scale bar in the software program AnalySIS (Olympus Soft Imaging System GmbH, Munster, Germany). Representative tissue specimens (i.e., the brain, liver, kidney, stomach, small and large intestine, lungs, and heart taken at the end of the experiment, fixed in 10% neutral buffered formalin (pH 7.4) at room temperature for 24 h) were embedded in paraffin, sectioned at 4 μm, stained with hemalaun and eosin.
Brain histology: As described in the previous studies[11-24], the brain was dissected according to NTP-7, at levels 3 and 6 with neuroanatomic subsites presented in certain brain sections using coronal sections with three mandatory sections. We used a semiquantitative neuropathological scoring system, and the sum of analyzed affected areas (0-4) (i) and karyopyknotic cells in the brain areas (0-4) (ii) making (i)+(ii) a combined score (0-8), as follows: (1) Specifically affected brain areas [cerebral (NTP-7, level 3), cerebellar cortex (NTP-7, level 6), hippocampus, thalamus, and hypothalamus (NTP-7, level 3)] were scored (0-4), (score 0 indicates no histopathologic change), as follows. Small, patchy, complete, or incomplete infarcts (≤ 10% of the area affected) represented score 1. Partly confluent or incomplete infarcts (20%-30% of the area affected) represented score 2. Large confluent complete infarcts (40%-60% of the area affected) represented score 3. In the cortex total disintegration of the tissue, in the hypothalamus, thalamus, and hippocampus large complete infarcts (> 75% of the area affected) represented score 4; and (2) Analyzed were karyopyknotic cells in the affected brain areas (0-4) (score 0 indicates no change), cerebral (NTP-7, level 3), cerebellar cortex (NTP-7, level 6), hippocampus, thalamus, and hypothalamus (NTP-7, level 3) as follows: A few karyopyknotic of neuronal cells (≤ 20%, score 1); patchy areas of karyopyknotic cells (50%, score 2); more extensive karyopyknotic areas (75%, score 3); complete infarction (100%, score 4). Brain tissue hemorrhage was obtained estimating a percentage of affected areas. Intraventricular hemorrhage was noted as present or absent.
We also assessed the neuronal pathological changes in acquired digital images saved as uncompressed 24-bit RGB TIFF files in the software program AnalySIS (Olympus Soft Imaging System GmbH, Munster, Germany) performing quantitative analysis of neuronal damage in the karyopyknotic areas. The neurons of the cortical cerebral, cerebellar region, hippocampus, and hypothalamus were counted in 10 different high-powered fields (HPF, 400 ×) and 3 to 5 serial sections of each sample were used to do the count as described[53]. The field size was 0.24 μm2.
We used four criteria for the estimation of the edema: Pale myelin, the sieve-like appearance of myelinated areas, dilation of perivascular and pericellular spaces, and vacuolar appearance of the neuropil of gray matter. Edema was graded as heavy, moderate, slight, or no edema (score 0-3)[54].
Lung histology: The same scoring system as in the previous studies[11-24] was used to grade the degree of lung injury in lung tissue analysis. Each of the features (i.e., focal thickening of the alveolar membranes, congestion, pulmonary edema, intra-alveolar hemorrhage, interstitial neutrophil infiltration, and intra-alveolar neutrophil infiltration) was scored (0-3) as absent (0) or present a mild (1), moderate (2), or severe (3) degree and a final histology score were determined.
Renal, liver, and heart histology: The same scoring system as in the previous studies[11-24] was used to grade renal (i.e., the degeneration of Bowman’s space and glomeruli, degeneration of the proximal and distal tubules, vascular congestion, and interstitial edema), liver (i.e., vacuolization of hepatocytes and pyknotic hepatocyte nuclei, activation of Kupffer cells, and enlargement of sinusoids) and heart (i.e., dilatation and congestion of blood vessels within the myocardium and coronary arteries) histology. Each specimen was scored using a scale ranging from 0-3 (0: none, 1: mild, 2: moderate, and 3: severe) for each criterion, and a final histology score was determined (0: none, 1: mild, 2: moderate, and 3: severe).
Gastrointestinal histology: As in previous studies[11-24], we used a histologic scoring scale adapted from Chui and coworkers[55] for the stomach tissue damage scoring 0-5 (normal to severe) in three categories (mucosal injury, inflammation, hyperemia/hemorrhage) for a total score of 0 to 15, as described by Lane and coworkers[56]. Illustratively, the assessment included morphologic features of mucosal injury (i.e., different grades of epithelial lifting, villi denudation, and necrosis), inflammation (i.e., focal to diffuse according to lamina propria infiltration or subendothelial infiltration), and hyperemia/hemorrhage (i.e., focal to diffuse according to lamina propria or subendothelial localization).
Statistical analysis
Statistical analysis was performed by parametric one-way analysis of variance (ANOVA), with the Newman-Keuls post-hoc test or the non-parametric Kruskal-Wallis test and subsequently the Mann-Whitney U test to compare groups. Values are presented as the mean ± SD and as the minimum/median/maximum. To compare the frequency difference between groups, the chi-squared test or Fischer’s exact test was used. P < 0.05 was considered statistically significant.
RESULTS
Along with the recently described stomach perforation course notated in the injured stomach, particular vascular failure (vessels “disappear”/empty), prolonged bleeding, debilitated defect large widening[10], stomach perforation consistently induced the vascular and multiorgan failure, progressing thrombosis, intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension. These were fully compatible with the general occlusion/ occlusion-like syndrome occurring peripherally and centrally. There were also cause-consequence lesions in the brain (swelling, hemorrhage), heart (congestion), lung (hemorrhage), congestion in the liver, kidney, and gastrointestinal tract, arrhythmias, and vessel failure, peripherally and centrally. Noteworthy, the vascular failure studies confirmed similar occlusion/occlusion-like syndromes induced by major vessel occlusion or other similar noxious procedures such as alcohol or lithium intoxication, myocardial infarction, acute pancreatitis, intra-abdominal hypertension that all severely affect endothelium function[11-24].
With counteraction by BPC 157 therapy, beneficial effects were along with those noted before in the healing of the perforated defect (raised vessel presentation; less bleeding, defect contraction) comparable to the previous BPC 157 therapy of the mentioned occlusion/occlusion-like syndromes. A common key finding to recovering rats with perforated stomachs that might break instantly the injurious circle might be the prompt and sustained activation of the azygos vein direct blood flow delivery as the particular effect of the stable gastric pentadecapeptide BPC 157 therapy application. Consistently attenuated/counteracted intracranial (superior sagittal sinus) hypertension and aortal hypotension, major arrhythmias, progressing arterial and vein thrombosis, lesions in the brain, heart, lungs, liver, kidneys, and gastrointestinal tract)[11-24] all prove counteraction of whole occlusion/occlusion-like syndrome.
A perilous syndrome occurred peripherally and centrally
Blood pressure disturbances: In the rats with perforated stomachs, the rapidly presented, portal and caval hypertension, and even more the intracranial (superior sagittal sinus) hypertension (gross brain swelling) as well as the aortal hypotension were markedly attenuated by BPC 157 application. This might be a cause-consequence relation relevant to the effectiveness of the therapy application as BPC 157 reduced consistently blood pressure disturbances that were otherwise induced rapidly by similar occlusion/occlusion-like syndromes. This includes a peripheral presentation (portal and caval hypertension, aortal hypotension) as well an even more central presentation (superior sagittal sinus hypertension, Table 1).
Table 1.
Blood pressures and thrombosis in rats at 15 min and 60 min following stomach perforation
|
Application of the 1 mL/rat through the perforated defect in the injured stomach at 5 min following perforation
|
Blood pressures and thrombosis in rats at 15 min and 60 min following stomach perforation
|
|
|
15 min
|
60 min
|
|
| Superior sagittal sinus pressure, mmHg, mean ± SD | ||
| Control | 9 ± 1 | 8 ± 1 |
| BPC 157 10 μg/kg | -3 ± 1a | -1 ± 1a |
| BPC 157 10 ng/kg | -1 ± 1a | -2 ± 1a |
| Portal pressure, mmHg, mean ± SD | ||
| Control | 16 ± 2 | 17 ± 1 |
| BPC 157 10 μg/kg | 9 ± 1a | 9 ± 1a |
| BPC 157 10 ng/kg | 9 ± 1a | 8 ± 1a |
| Caval pressure, mmHg, mean ± SD | ||
| Control | 15 ± 1 | 15 ± 1 |
| BPC 157 10 μg/kg | 8 ± 1 | 9 ± 1a |
| BPC 157 10 ng/kg | 7 ± 1 | 9 ± 1a |
| Aortal pressure, mmHg, mean ± SD | ||
| Control | 42 ± 5 | 45 ± 7 |
| BPC 157 10 μg/kg | 75 ± 4a | 72 ± 5a |
| BPC 157 10 ng/kg | 80 ± 5a | 78 ± 7a |
| Superior sagittal sinus, thrombus mass, g, mean ± SD | ||
| Control | 0.0012 ± 0.0005 | 0.0022 ± 0.0009 |
| BPC 157 10 μg/kg | 0 ± 0a | 0 ± 0a |
| BPC 157 10 ng/kg | 0 ± 0a | 0 ± 0a |
| Portal vein, thrombus mass, g, mean ± SD | ||
| Control | 0.0034 ± 0.0006 | 0.0056 ± 0.0008 |
| BPC 157 10 μg/kg | 0.0010 ± 0.0005a | 0.0020 ± 0.0005a |
| BPC 157 10 ng/kg | 0.0010 ± 0.0007a | 0.0018 ± 0.0006a |
| Inferior caval vein, thrombus mass, g, mean ± SD | ||
| Control | 0.0039 ± 0.0007 | 0.0061 ± 0.0008 |
| BPC 157 10 μg/kg | 0.0002 ± 0.0001a | 0.0003 ± 0.0001a |
| BPC 157 10 ng/kg | 0.0008 ± 0.0001a | 0.0005 ± 0.0002a |
| Abdominal aorta, thrombus mass, g, mean ± SD | ||
| Control | 0.0044 ± 0.0007 | 0.0063 ± 0.0009 |
| BPC 157 10 μg/kg | 0 ± 0a | 0 ± 0a |
| BPC 157 10 ng/kg | 0 ± 0a | 0 ± 0a |
P < 0.05, at least vs control.
Blood pressures (intracranial (superior sagittal sinus), portal and caval hypertension and aortal hypotension, mmHg), and thrombosis (thrombus mass, g) in rats at 15 min and 60 min following perforation of the stomach. Saline (5 mL/kg) (control) or BPC 157 (10 μg/kg and 10 ng/kg) were given at 5 min thereafter.
Thrombosis
After perforation of the stomach, as a shared effect with other occlusion/occlusion-like syndromes, in the rats with perforated stomachs, thrombosis was rapidly presented, progressing peripherally in veins (i.e., portal and inferior caval vein) as well as in arteries (i.e., abdominal aorta) and centrally (i.e., superior sagittal sinus). Contrary, with BPC 157 therapy thrombosis was promptly reduced both peripherally and centrally (Table 1) as the effective cause-consequence course of the therapy both peripherally and centrally.
Stomach presentation
As before with the application of the BPC 157 as a bath in the abdominal cavity, which might beneath the damaged stomach[10], the application of the BPC 157 directly into the injured stomach through the perforated defect, might rapidly reduce the defect size. Illustratively, given the perforation defect size ratio (%) after therapy/before therapy, considerable widening of the perforated defect occurred in the controls (235 ± 56 and 255 ± 66, at 15 min and 60 min after perforation, respectively). Contrarily, there was a marked shrinking of the defect after BPC 157 therapy (75 ± 8, 65 ± 8, BPC 157 10 μg at 15 min and 60 min perforation-time; 80 ± 8, 75 ± 8, BPC 157 10 ng at 15 min and 60 min perforation-time, P < 0.05, at least vs control). Likewise, accordingly with previous findings[10], the bleeding time (sec) was also reduced in BPC 157 treated rats (i.e., 100 ± 8 in controls vs 50 ± 8 BPC 157 10 μg and 55 ± 7 BPC 157 10 ng, P < 0.05, at least vs control). In addition, with BPC 157 therapy into the perforated defect, upon volume application instead of volume-induced dilatation as regular finding in the rats with the perforated stomach, the whole stomach reacted with marked shrinking (Table 2, Figure 1).
Table 2.
Relative volume of the stomach of the heart, azygos vein, in rats at 5 min and 15 min following stomach perforation
|
0 min
|
5 min
|
5 min
|
15 min
|
| Relative volume (healthy/perforation, %) of the stomach in rats immediately after perforation | Application of the 1 mL/rat through the perforated defect in the injured stomach at 5 min following perforation | Relative volume (healthy/perforation, healthy/perforation + therapy, %) of the stomach, in rats at 5 min and 15 min following stomach perforation | |
| Relative volume (healthy/perforation, %) of the stomach, mean ± SD | Medication | Relative volume (healthy/perforation + therapy, %) of the stomach, mean ± SD | |
| 100 ± 3 | Saline 5 mL/kg (control) | 114 ± 2a,b | 118 ± 2a,b |
| 100 ± 2 | BPC 157 10 μg/kg | 87 ± 5a,b,c | 76 ± 8a,b,c |
| 101 ± 2 | BPC 157 10 ng/kg | 81 ± 7a,b,c | 79 ± 9a,b,c |
P < 0.05, at least vs healthy.
P < 0.05, at least vs immediately after perforation.
P < 0.05, at least vs corresponding control at the time of the assessment.
Relative volume (healthy/perforation, healthy/perforation + therapy, %) of the stomach of the heart, azygos vein, in rats at 5 min and 15 min following stomach perforation. Saline (5 mL/kg, control) or BPC 157 (10 μg/kg and 10 ng/kg) were given at 5 min after perforation.
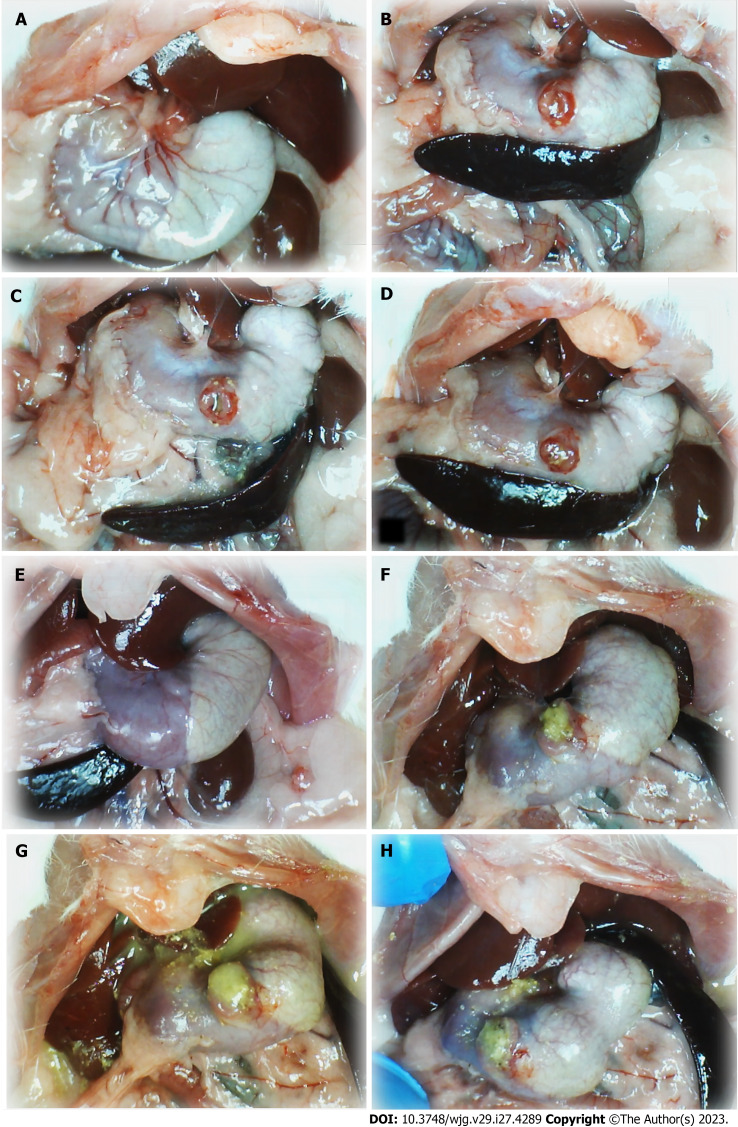
Figure 1.
Stomach illustrative presentation. A and E: Before procedure; B and F: After perforation before therapy; C and D: Course after given therapy into the perforated defect, saline; G and H: BPC 157. Control rats: (1) Before procedure (normal rats, A); (2) after perforation before therapy application (B); (3) after saline application (1 mL/rat into the perforated defect in the stomach) (C and D), immediately thereafter (C); and (4) later (at 5 min following medication, D). BPC 157 rats: (1) Stomach before perforation (normal rats, E); (2) after perforation, but before BPC 157 therapy application (F), and (3) specifically after BPC 157 application (10 µg or 10 ng/kg, 1 mL/rat into the perforated defect in the stomach, G and H), immediately thereafter (G), and later (at 5 min following medication, H).
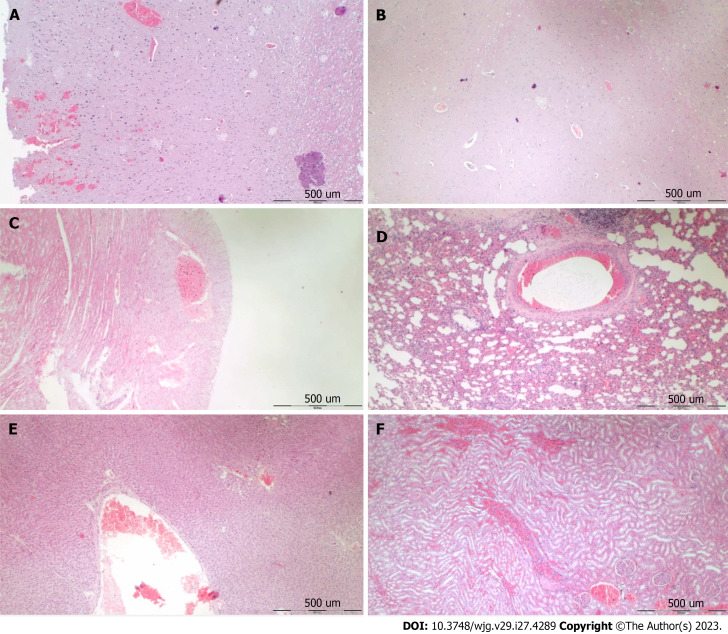
Collateral pathways, blood vessels, and brain gross presentation: All rats with perforated stomachs without therapy converge to the similar failed effects consistently noted with other occlusion/occlusion-like syndromes. Indicatively for a not functioning common clue (lack of activation of collateral pathways) might be the failed azygos vein, superior mesenteric vein, inferior caval vein, and abdominal aorta. We used their disturbed presentation as indicative illustrations (Figure 2) to envisage the failed collateral pathways presentation.
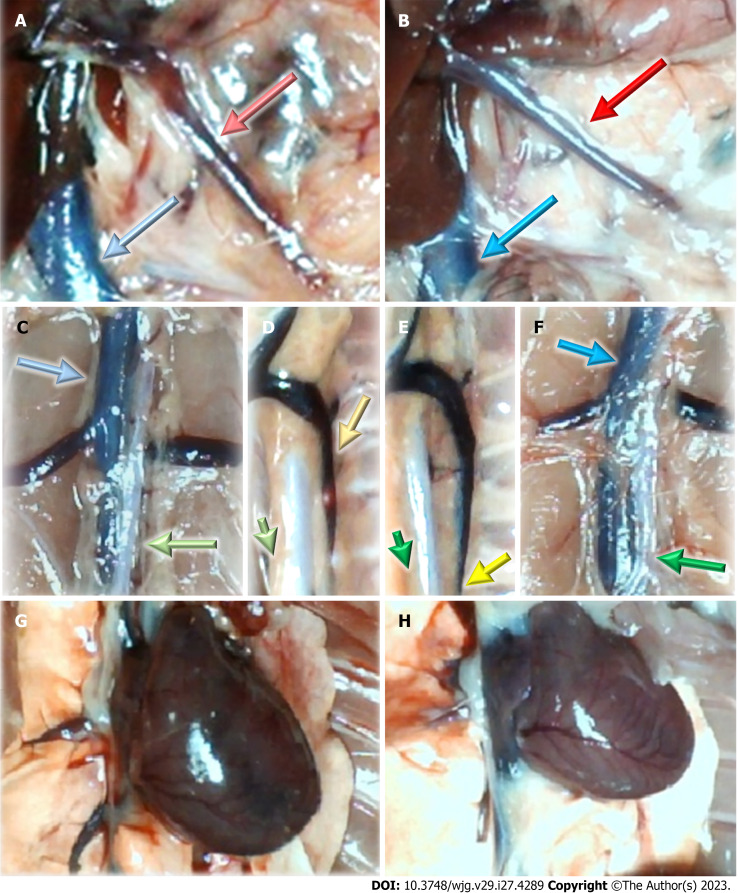
Figure 2.
Vessels and heart illustrative presentation. A, C, D, G: In the control rats (light color arrows); B, E, F, H: In the BPC 157 rats (dark color arrows); superior mesenteric vein (red arrows), inferior caval vein (blue arrows), aorta (green arrows), azygos vein (yellow arrows), and heart (G and H).
On the other hand, the attenuation of blood pressure disturbances, peripherally and centrally, we perceived as an effect of BPC 157 along with progressing thrombosis fully counteracted in all vessels investigated, veins and arteries, peripherally and centrally (Table 1). Thus, the major vessel failure and blood stasis, the particular vessels recruitment might compensate. Peripherally, note the rescued presentation of the superior mesenteric vein and inferior caval vein as well as the activated azygos vein, and thereby the heart as well (Figure 2), and centrally, note brain swelling rapidly disappeared (Figure 3). Thus, with BPC 157 therapy, advanced collateral pathways presentation occurred as a key therapy effect.
Figure 3.
Brain illustrative presentation in the rats with perforated stomach. A, C, E, G: Control rats before perforation (A); after perforation (C, E, G), before saline medication (C), specifically after saline application (5 mL/kg, 1 mL/rat into the perforated defect in the stomach; E and G), immediately thereafter (E), and later after sacrifice (G); B, D, F, H: BPC 157 rats before perforation (B); after perforation (D, F, H), before BPC 157 medication (D), specifically after BPC 157 application (10 µg or 10 ng/kg, 1 mL/rat into the perforated defect in the stomach, capitals; F, H), immediately thereafter (F), and later after sacrifice (H).
The relative volume of the brain, heart, azygos vein, superior mesenteric vein, and inferior caval vein might summarize the harmful event of the stomach perforation and subsequent deleterious worsening (swollen brain, congested superior mesenteric vein and inferior caval vein for the congested lung, liver, kidney and gastrointestinal tract and heart failure) (Table 3, Figures 2 and 3). On the other hand, vice versa, relative volume change might clearly indicate the strong therapy effect of the BPC 157 application, and reversal toward the normal vessels (presentation close to normal of the superior mesenteric vein and inferior caval vein that had been over-congested), likely due to the activation of the azygos vein (direct blood delivery) that had been failed. Heart dilatation was reversed. Likewise, the brain swelling was rapidly reversed. Note, upon sacrifice, ex vivo, the control/treated brain volume ratio (%) reached for BPC 157 10 µg or 10 ng/kg (P < 0.05, at least vs control) 120 ± 4 and 122 ± 4 at 15 min and 123 ± 4 and 121 ± 4 at 60 min after perforation.
Table 3.
Relative volume of the brain, inferior caval vein, and superior mesenteric vein, and relative volume of the heart, azygos vein, in rats at 15 min and 60 min following stomach perforation
|
0 min
|
5 min
|
15 min
|
60 min
|
| Relative volume (healthy/perforation, %) of the brain, heart, azygos vein, inferior caval vein, superior mesenteric vein, and abdominal aorta in rats immediately after perforation | Application of the 1 mL/rat through the perforated defect in the injured stomach at 5 min following perforation | Relative volume (healthy/perforation, healthy/perforation + therapy, %) of the brain, inferior caval vein, and superior mesenteric vein, and relative volume (control/treated, %) of the heart, azygos vein, in rats at 15 min and 60 min following stomach perforation | |
| Relative volume (healthy/perforation) (%) of the brain, mean ± SD | Medication | Relative volume (healthy/perforation + therapy, %) of the brain, mean ± SD | |
| 116 ± 3a | Saline 5 mL/kg (control) | 124 ± 2a,b | 124 ± 2a,b |
| 120 ± 2a | BPC 157 10 μg/kg | 100 ± 1b,c | 99 ± 2b,c |
| 121 ± 2a | BPC 157 10 ng/kg | 101 ± 3b,c | 100 ± 1b,c |
| - | Relative volume (control/treated, %) of the heart, mean ± SD | ||
| BPC 157 10 μg/kg | 137 ± 4c | 141 ± 5c | |
| BPC 157 10 ng/kg | 136 ± 3c | 138 ± 4c | |
| Relative volume (control/treated, %) of the azygos vein, mean ± SD | |||
| BPC 157 10 μg/kg | 66 ± 6c | 58 ± 7c | |
| BPC 157 10 ng/kg | 64 ± 4c | 54 ± 8c | |
| Relative volume (healthy/perforation + therapy, %) of the inferior caval vein, mean ± SD | |||
| 130 ± 3a | Saline 5 mL/kg (control) | 164 ± 8a,b | 174 ± 9a,b |
| 128 ± 4a | BPC 157 10 μg/kg | 98 ± 2b,c | 100 ± 2b,c |
| 126 ± 4a | BPC 157 10 ng/kg | 100 ± 3b,c | 99 ± 1b,c |
| Relative volume (healthy/perforation + therapy, %) of the superior mesenteric vein, mean ± SD | |||
| 180 ± 5a | Saline 5 mL/kg (control) | 204 ± 8a,b | 212 ± 9a,b |
| 188 ± 9a | BPC 157 10 μg/kg | 96 ± 6b,c | 99 ± 7b,c |
| 186 ± 8a | BPC 157 10 ng/kg | 98 ± 6b,c | 101 ± 1b,c |
P < 0.05, at least vs healthy.
P < 0.05, at least vs immediately after perforation.
P < 0.05, at least vs corresponding control at the time of the assessment.
Relative volume (healthy/perforation, healthy/perforation + therapy, %) of the brain, inferior caval vein, and superior mesenteric vein, and relative volume (control/treated, %) of the heart, azygos vein, in rats at 15 min and 60 min following stomach perforation. Saline (5 mL/kg) (control) or BPC 157 (10 μg/kg and 10 ng/kg) were given at 5 min after perforation.
Heart and ECG disturbances: Commonly, in the procedure with the stomach perforation, BPC 157 therapy counteracted the whole noxious chain of events (i.e., continuous tachycardia along with prolonged PQ prolonged and QTc intervals) (Table 4). Tachycardia, QTc intervals or PQ intervals disturbances were regularly attenuated or absent in all BPC 157-treated rats.
Table 4.
ECG changes, PQ interval, QTc interval, msec, heart frequency, beats/min, in rats at 15 min and 60 min following stomach perforation
| Application of the 1 mL/rat through the perforated defect in the injured stomach at 5 min following perforation |
ECG changes in rats at 15 min and 60 min following stomach perforation
|
|
|
15 min
|
60 min
|
|
| PQ interval, msec mean ± SD | ||
| Control | 64 ± 5 | 67 ± 6 |
| BPC 157 10 μg/kg | 50 ± 5a | 50 ± 5a |
| BPC 157 10 ng/kg | 50 ± 5a | 50 ± 5a |
| QTc interval, msec, mean ± SD | ||
| Control | 312 ± 10 | 347 ± 10 |
| BPC 157 10 μg/kg | 180 ± 10a | 188 ± 10a |
| BPC 157 10 ng/kg | 185 ± 10a | 183 ± 10a |
| Heart frequency, beats/min, mean ± SD | ||
| Control | 530 ± 30 | 550 ± 30 |
| BPC 157 10 μg/kg | 375 ± 10 a | 360 ± 10a |
| BPC 157 10 ng/kg | 385 ± 10 a | 365 ± 10a |
P < 0.05, at least vs control.
ECG changes, PQ interval, QTc interval, msec, heart frequency, beats/min, in rats at 15 min and 60 min following stomach perforation. Saline (5 mL/kg, control) or BPC 157 (10 μg/kg and 10 ng/kg) were given at 5 min thereafter.
A perilous syndrome occurred peripherally
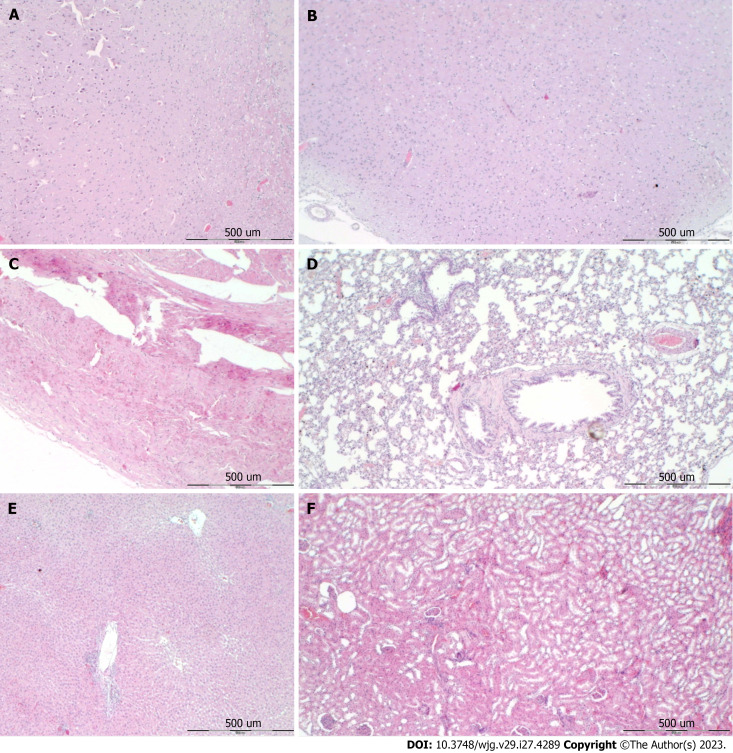
Gastrointestinal, lung, liver, kidney, and heart lesions: In the untreated rats, all of the post-perforation intervals consistently expressed the particular lesion severity evidenced in the affected organs (Figures 4-7, Table 5). Also, the evidenced intracranial (superior sagittal sinus), portal, and caval hypertension, aortal hypotension, progressed thrombosis, peripherally and centrally, failed collateral recruitment, and ECG disturbances may together indicate a common clue that might be failed. Thereby, there is the immediate impact of the activated collateral pathway that the reduced severity of lesions by BPC 157 therapy evidently occurred along with the reduced intracranial (superior sagittal sinus), portal, and caval hypertension, and reduced aortal hypotension, counteracted ECG disturbances. Thus, BPC 157 therapy appeared as part of the cause-consequence therapeutic course.
Figure 4.
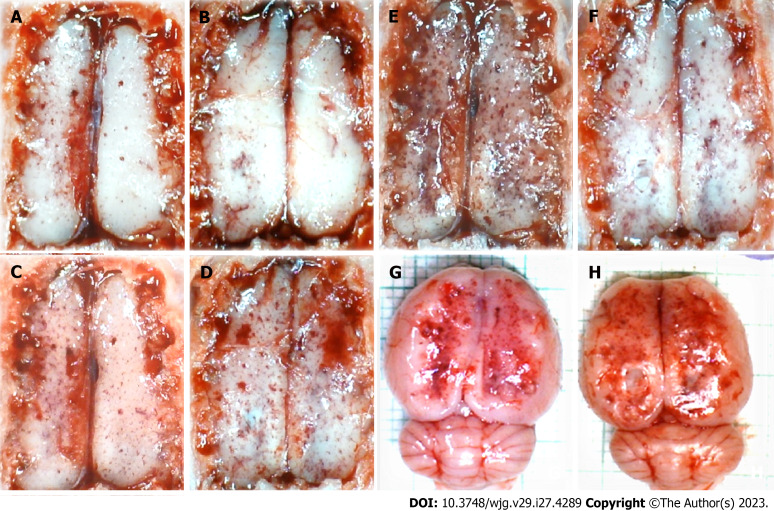
Stomach histology and control. A-F: Margins of the stomach perforation showed mucosal congestion. Transmural hyperemia of the stomach wall at the margins of the stomach perforation and in the rest of the stomach wall (A, E). HE staining: Magnification 20 × (A, B), magnification 100 × (C-E), magnification 200 × (F).
Figure 7.
Following stomach perforation, illustrative microscopy presentation of the organs lesion in BPC 157 treated rats. A and B: Cerebral cortex, no edema and congestion in the cerebral cortex. Small cerebral hemorrhage of the neocortex; C: Heart, no changes; D: Lung, only mild congestion in the lung parenchyma; E: Liver, no changes; F: Kidney, no changes. HE staining: Magnification 40 ×.
Table 5.
Lesions scored microscopically (heart, lung, liver, kidney, stomach), or macroscopically (stomach, in rats at 15 min and 60 min following stomach perforation
| Application of the 1 mL/rat through the perforated defect in the injured stomach at 5 min following perforation |
Lesions scored microscopically (heart, lung, liver, kidney, stomach, small and large intestine), or macroscopically (stomach), in rats at 15 min and 60 min following stomach perforation
|
|
|
15 min
|
60 min
|
|
| Heart (scored 0-3, min/med/max) | ||
| Control | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0a | 0/0/0a |
| BPC 157 10 ng/kg | 0/0/0a | 0/0/0a |
| Lung (scored 0-3, min/med/max) | ||
| Control | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 1/1/1a | 1/1/1a |
| BPC 157 10 ng/kg | 1/1/1a | 1/1/1a |
| Liver (scored 0-3, min/med/max) | ||
| Control | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0a | 0/0/0a |
| BPC 157 10 ng/kg | 0/0/0a | 0/0/0a |
| Kidney (scored 0-3, min/med/max) | ||
| Control | 3/3/3 | 3/3/3 |
| BPC 157 10 μg/kg | 0/0/0a | 0/0/0a |
| BPC 157 10 ng/kg | 0/0/0a | 0/0/0a |
| Stomach (scored 0-15, min/med/max) | ||
| Control | 5/5/5 | 4/5/5 |
| BPC 157 10 μg/kg | 1/1/1a | 1/1/1a |
| BPC 157 10 ng/kg | 1/1/1a | 1/1/1a |
P < 0.05, at least vs control.
Lesions scored microscopically (heart, lung, liver, kidney, and stomach), or macroscopically (stomach, in rats at 15 min and 60 min following stomach perforation. Saline (5 mL/kg, control) or BPC 157 (10 μg/kg and 10 ng/kg) were given at 5 min thereafter.
Gastrointestinal lesion: Transmural hyperemia of the stomach wall in the control group with marked mucosal congestion at perforation’s margins (Figure 4, Table 5). No congestion of the stomach wall in the BPC 157 group, and only mild mucosal congestion was at the stomach perforation’s margins (Figure 5, Table 5).
Figure 5.
Stomach histology, BPC 157. A-F: Margins of the stomach perforation showed only mild mucosal congestion. No congestion was found in the rest of the stomach wall (A and E). HE staining: Magnification 20 × (A and B), magnification 40 × (C-E), and magnification 100 × (F).
Heart: Severe myocardial congestion was commonly noted in all the control rats. Contrarily, in rats treated with BPC 157, these lesions were completely annihilated (Figures 6 and 7, Table 5). These findings were along with counteracted heart dilatation and counteracted ECG disturbances (Tables 3 and 4, Figure 2).
Figure 6.
Following stomach perforation, illustrative microscopy presentation of the organ lesion in saline-treated rats (control). A and B: Cerebral cortex, increased edema and congestion in the cerebral cortex. Severe cerebral hemorrhage of the neocortex; C: Heart, severe congestion within the myocardium; D: Lung, focal thickening of the alveolar membrane, lung congestion, pulmonary edema, and severe intra-alveolar hemorrhage; E: Liver, marked congestion of liver parenchyma with dilatation of blood vessels in portal tracts, central veins, and sinusoids; F: Kidney, a renal parenchyma showed severe vascular congestion and mild interstitial edema. HE staining: Magnification 40 ×.
Lung: Without therapy, focal thickening of the alveolar membranes occurred, lung congestion, pulmonary edema, and severe intra-alveolar hemorrhage. Contrarily, BPC 157 rats exhibited only mild congestion in lung parenchyma (Figures 6 and 7, Table 5).
Liver: Severe enlargement of sinusoids with liver congestion occurred in all control rats, while BPC 157 exhibited no changes in liver parenchyma (Figures 6 and 7, Table 5).
Kidney: Severe vascular congestion and mild interstitial edema occurred in all control rats. Contrarily, no changes were found in renal parenchyma in BPC 157 treated rats (Figures 6 and 7, Table 5).
A perilous syndrome occurred centrally
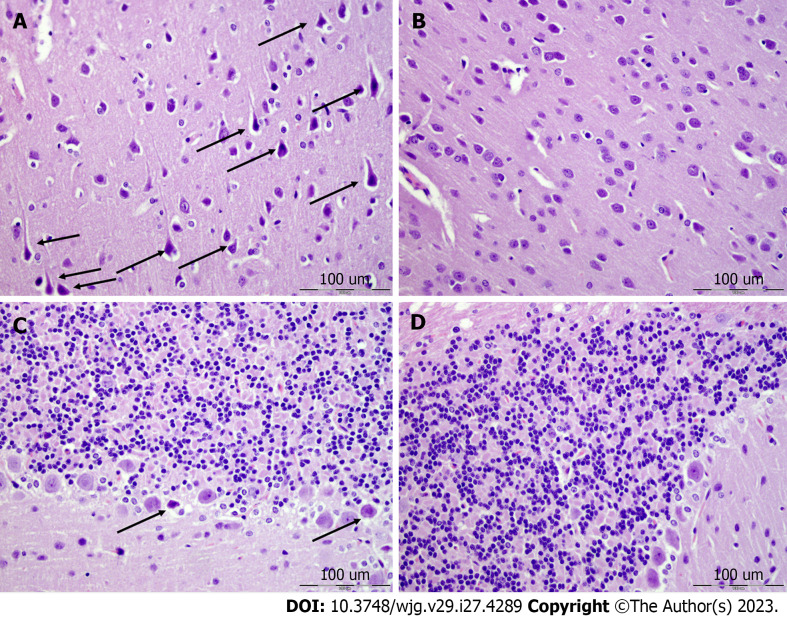
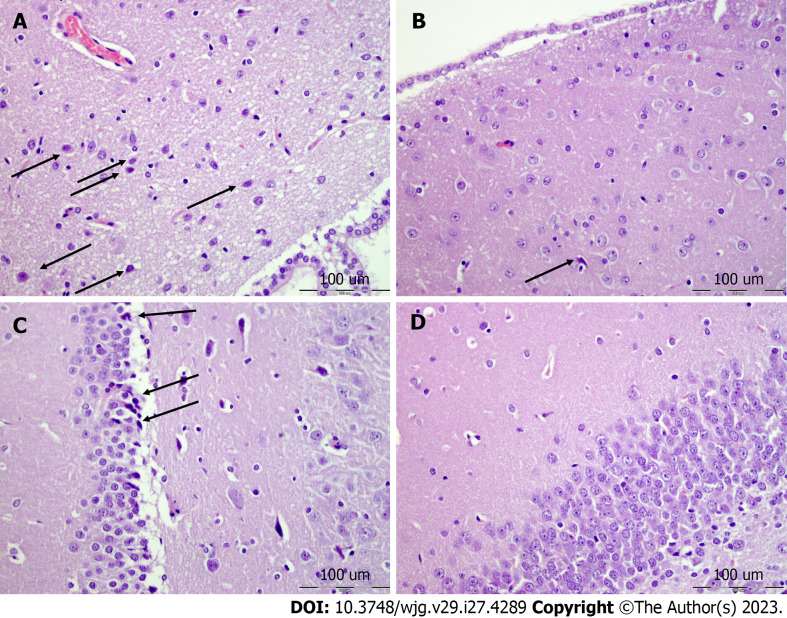
Brain lesions, cerebral and cerebellar cortex, hypothalamus/thalamus, and hippocampus: All of the control rats with perforated stomachs converge to a similar brain lesion (Figures 7 and 8, Table 6) indicating a common clue that might be failed. Namely, these occurred along with intracranial (superior sagittal sinus), portal, and caval hypertension, aortal hypotension, progressed thrombosis, peripherally and centrally, failed collateral recruitment, disturbed ECG presentation, and peripheral organs lesion. The brain swell (Figure 3) occurred with the increased intracranial (superior sagittal sinus) hypertension in the immediate and prolonged post-application-period. These rats with perforated stomachs exhibited marked edema and congestion in neocortical brain tissue with an increased number of karyopycnotic nuclei of cortical neurons, marked edema, and an increased number of karyopycnotic nuclei of cerebellar Purkinje cells. Marked edema and congestion in the hypothalamic area appeared with an increased number of karyopycnotic cells. Marked edema and an increased number of karyopycnotic nuclei occurred also in the hippocampal area.
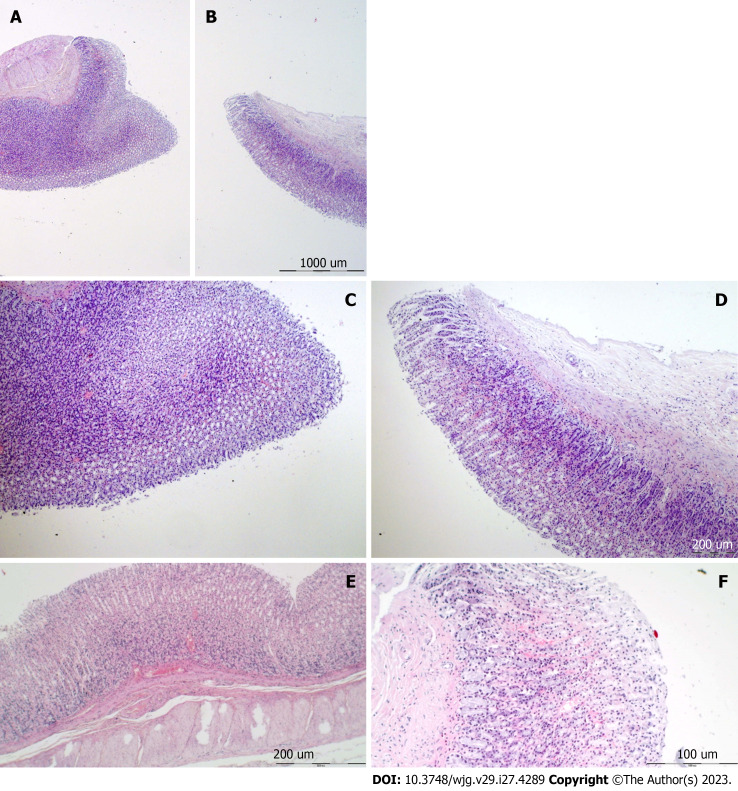
Figure 8.
Neocortex, cerebellum, and neuropathological changes. A: In the control group marked edema and congestion in the neocortical brain tissue of the control animal with an increased number of karyopycnotic nuclei of cortical neurons; B: Only mild edema was found in the neocortex in BPC 157 treated animals; C: Edema and an increased number of karyopycnotic nuclei of the cerebellar Purkinje cells in the control animals; D: No changes were found in BPC 157 treated animals. HE staining: Magnification 400 ×.
Table 6.
Lesions were assessed microscopically (cerebrum, cerebellum, hypothalamus, hippocampus), in rats at 15 min and 60 min following stomach perforation
|
Application of the 1 ml/rat through the perforated defect in the injured stomach at 5 min following perforation
|
Lesions scored microscopically cerebrum, cerebellum, hypothalamus, and hippocampus in rats at 15 min and 60 min following stomach perforation
|
|
|
15 min
|
60 min
|
|
| Cerebrum (scored 0-8, min/med/max); percentage of karyopycnotic cells (%), mean ± SD | ||
| Control | 2/2/2; 30 ± 5 | 2/2/2, 35 ± 5 |
| BPC 157 10 μg/kg | 0/1/1a, 5 ± 5a | 0/1/1a, 5 ± 5a |
| BPC 157 10 ng/kg | 0/1/1a, 5 ± 5a | 0/1/1a, 5 ± 5a |
| Neuronal damage in the karyopyknotic areas, %, mean ± SD (10 HPF, 400 ×) | ||
| Control | 39 ± 5 | 42 ± 4 |
| BPC 157 10 μg/kg | 2 ± 2a | 3 ± 2a |
| BPC 157 10 ng/kg | 2 ± 2a | 2 ± 2a |
| Hemorrhage (% of total area), mean ± SD | ||
| Control | 30 ± 2 | 25 ± 2 |
| BPC 157 10 μg/kg | 5 ± 2a | 5 ± 2a |
| BPC 157 10 ng/kg | ||
| Edema (scored 0-3, min/med/max) | ||
| Control | 37683 | 37683 |
| BPC 157 10 μg/kg | 1/1/1a | 1/1/1a |
| BPC 157 10 ng/kg | 1/1/1a | 1/1/1a |
| Cerebellum (scored 0-8, min/med/max); percentage of karyopycnotic cells (%), mean ± SD | ||
| Control | 1/2/2, 13 ± 5 | 1/1/1, 15 ± 5 |
| BPC 157 10 μg/kg | 0/0/1a, 5 ± 5a | 0/0/1a, 5 ± 5a |
| BPC 157 10 ng/kg | 0/0/1a, 5 ± 5a | 0/0/1a, 5 ± 5a |
| Neuronal damage in the karyopyknotic areas (%), mean ± SD (10 HPF, 400 ×) | ||
| Control | 27 ± 4 | 23 ± 5 |
| BPC 157 10 μg/kg | 0 ± 0a | 0 ± 0a |
| BPC 157 10 ng/kg | 0 ± 0a | 0 ± 0a |
| Hemorrhage (% of total area), mean ± SD | ||
| Control | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 |
| Edema (scored 0-3, min/med/max) | ||
| Control | 37683 | 37318 |
| BPC 157 10 μg/kg | 0/0/1a | 0/0/1a |
| BPC 157 10 ng/kg | 0/0/1a | 0/0/1a |
| Hippocampus (scored 0-8, min/med/max); percentage of karyopycnotic cells (%), mean ± SD | ||
| Control | 37289 | 36924 |
| 25 ± 3 | 28 ± 2 | |
| BPC 157 10 μg/kg | 0/0/0a, 0 ± 0a | 0/0/0a, 0 ± 0a |
| BPC 157 10 ng/kg | 0/0/0a, 0 ± 0a | 0/0/0a, 0 ± 0a |
| Neuronal damage in the karyopyknotic areas (%), mean ± SD (10 HPF, 400 ×) | ||
| Control | 23 ± 2 | 24 ± 3 |
| BPC 157 10 μg/kg | 0 ± 0a | 0 ± 0a |
| BPC 157 10 ng/kg | 0 ± 0a | 0 ± 0a |
| Hemorrhage (% of total area), mean ± SD | ||
| Control | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 |
| Edema (scored 0-3, min/med/max) | ||
| Control | 37683 | 37318 |
| BPC 157 10 μg/kg | 0/0/0a | 0/0/0a |
| BPC 157 10 ng/kg | 0/0/0a | 0/0/0a |
| Hypothalamus (scored 0-8, min/med/max); percentage of karyopycnotic cells (%), mean ± SD | ||
| Control | 2/2/2, 31 ± 3 | 1/1/1, 29 ± 4 |
| BPC 157 10 μg/kg | 0/0/1a, 5 ± 5a | 0/0/1a, 5 ± 5a |
| BPC 157 10 ng/kg | 0/0/1a, 5 ± 5a | 0/0/1a, 5 ± 5a |
| Neuronal damage in the karyopyknotic areas (%), mean ± SD (10 HPF, 400 ×) | ||
| Control | 24 ± 3 | 28 ± 5 |
| BPC 157 10 μg/kg | 5 ± 2a | 6 ± 3a |
| BPC 157 10 ng/kg | 5 ± 2a | 6 ± 3a |
| Hemorrhage (% of total area), mean ± SD | ||
| Control | 0 ± 0 | 0 ± 0 |
| BPC 157 10 μg/kg | 0 ± 0 | 0 ± 0 |
| BPC 157 10 ng/kg | 0 ± 0 | 0 ± 0 |
| Edema (scored 0-3, min/med/max) | ||
| Control | 37683 | 37318 |
| BPC 157 10 μg/kg | 0/1/1a | 0/1/1a |
| BPC 157 10 ng/kg | 0/1/1a | 0/1/1a |
P < 0.05, at least vs control.
Lesions were assessed microscopically (cerebrum, cerebellum, hypothalamus, and hippocampus), in rats at 15 min and 60 min following stomach perforation. Saline (5 mL/kg, control) or BPC 157 (10 μg/kg and 10 ng/kg) were given at 5 min thereafter.
Contrarily, with BPC 157 therapy, there was a marked reduction of intracranial (superior sagittal sinus) hypertension (and elimination of portal and caval hypertension). These went along with the counteracted brain swelling (Figure 3). BPC 157-treated rats had only mild edema in the neocortex, no change in cerebellar Purkinje cells, only a few karyopycnotic cells in the hypothalamic area, and no change in the hippocampal area (Figures 8 and 9, Table 6).
Figure 9.
Hypothalamus, hippocampus, neuropathological changes. A: In the control group marked edema and congestion in the hypothalamic area of control animals with brain tissue of control animal with an increased number of karyopycnotic cells; B: Only a few karyopycnotic cells in BPC 157 treated animals; C: Edema and increased number of karyopycnotic nuclei of the hippocampal area were noted in the control animals; D: While no changes were found in BPC 157 treated animals. HE staining, magnification 400 ×.
In summary, in rats with stomach perforation after BPC 157 therapy, in either of the regimens (µg, ng, and intragastrically), there was an immediate effect on the perforated stomach (i.e., decreased bleeding, shrinking of the defect and whole stomach). At the same time, rats rapidly exhibited markedly attenuated portal and caval hypertension, and superior sagittal sinus hypertension, ameliorated aortal hypotension, and had no ECG disturbance, almost annihilated venous and arterial thrombosis, both peripherally and centrally. They had reduced or eliminated central and peripheral organ lesions, in the brain, heart, lung, liver, kidney, and gastrointestinal tract. Gross assessment (i.e., rapid counteraction of the brain swelling, counteraction of the heart dilatation), and microscopic assessment (i.e., myocardial congestion completely annihilated) support each other. Thus, BPC 157 therapy suggests that the consistent antagonization exerted probably takes place as a network of interrelated beneficial effects. The reversal of the failed vessel presentation (i.e., inferior caval and superior mesenteric vein severely congested; azygos vein collapsed) might be the key finding of an activated particular collateral pathway responsible for the noted beneficial effects. The rescued and activated azygos vein may combine in rats the inferior caval vein and the left superior caval vein, and may effectively reorganize blood flow.
DISCUSSION
In the rats with surgically perforated stomachs, Robert’s direct injury[10] and occlusion/occlusion-like syndrome[11-24] occur as a particular model for cytoprotection studies[1,2]. Thus, the success of the BPC 157 therapy given directly into the perforated defect might likely resolve several cytoprotection issues.
As expected, we evidenced complete correspondence between the perforated stomach-induced occlusion/occlusion-like syndrome and the intragastric alcohol-induced stomach injuries and whole occlusion/occlusion-like syndrome equally presented in rats[24]. Thereby, the correspondence between the BPC 157 therapy resolving the immediate course after alcohol intragastric application and BPC 157 therapy resolving the immediate course after stomach perforation in the rats, might support each other effect. These findings (i.e., effectively applied directly into the perforated defect) are consistent with BPC 157 as a novel cytoprotection mediator native to and stable in human gastric juice rapidly acting to maintain gastrointestinal mucosa integrity, capable to induce a general beneficial effect as well[1-6]. In the rats with perforated stomachs, there was the counteraction of the immediate triad, particular vascular failure (vessels “disappear”/empty), prolonged bleeding, and a large widening of the debilitated defect[10]. Without therapy, these might fairly extend an unresolved local cytoprotection stomach issue to the rapidly emerging severe widespread syndrome occlusion/occlusion-like syndrome as the general cytoprotection failure[10-24]. As a follow-up, the therapy essentially represents the resolution of the perforated stomach defect as the innate vascular and multiorgan failure like that which might occur during the major vessel occlusion and other alike noxious procedure application, occlusion/occlusion like-syndrome[11-24]. Consequently, this implies a wide range of the therapy effect as with the other occlusion/occlusion-like syndromes[11-24], as the perforation of the stomach in the occlusion/occlusion-like syndrome course might be rapidly complicated with the severe lesions. These lesions could be widespread in the brain with intracerebral and intraventricular hemorrhage, heart (congestion, arrhythmias), hemorrhage and congestion in the lung, liver, kidney and gastrointestinal tract. As proof of the therapy concept might be the attenuated/eliminated blood pressure disturbances[10-24]. Otherwise, the blood pressure disturbances occurred instantly (intracranial (superior sagittal sinus) hypertension (grossly, immediate brain swelling), portal and caval hypertension, and aortal hypotension). These might mean the perpetuating noxious circle, initiated peripherally/centrally hampered healing, but successfully reversed by the therapy effects. This might be illustrative, as the stomach perforation combines the arterial and venous thrombosis, peripherally and centrally, failed major vessels (i.e., congested inferior caval and superior mesenteric vein, collapsed azygos vein). Consequently, as in other occlusion/occlusion-like studies[11-24], the almost annihilated thrombosis, vessels presentation close to normal (inferior caval vein, superior mesenteric vein), and activated azygos vein (direct blood flow delivery) (thus stasis eliminated) might verify that Virchow triad circumstances are resolved (and thereby, attenuated/eliminated bleeding/attenuated/eliminated thrombosis). Thus, such therapy might provide an adequate resolution at both local and general levels as the perforation regularly goes with the failed spontaneous cytoprotection maxim endothelial maintenance→epithelial maintenance[7-9], failed upgrading of the minor vessels as a whole, and the widespread rapidly acting Virchow triad circumstances[10-24].
Previously, in the surgically perforated stomach, BPC 157 therapy might have particular effects[10]. Illustrating these particular effects, BPC 157 reversed to the normal healthy values in the stomach tissue surrounding the defect the otherwise increased malondialdehyde- and decreased NO-values. Likewise, already in very early post-perforation-time, BPC 157 may act beneficially in the perforated stomach lesion throughout NO- and prostaglandinds-system[10], two of the essential cytoprotection systems[1-6], as shown in the findings of the mRNA expression studies (Cox2, VEGFa, Nos1, Nos 2, Nos3, Nkap (NF-kappa-B activating protein gene)), done at that very early post-perforation-time. These might be the maintenance of the proper function of the entirety of the NO-system. There was the release of the NO by its own[29,38-40,47,48], counteraction of the adverse effects of L-NAME (i.e., L-NAME-hypertension[38] and pro-thrombotic[40] effect), counteraction of the adverse effects of L-arginine (i.e., L-arginine-hypotension[39] and anti-thrombotic[40] effect). These suggested a modulatory effect (thereby, counteraction of NOS-blockade and counteraction of NOS-over-stimulation)[29,38-40,47,48] controlling vasomotor tone and the activation of the Src-Caveolin-1-eNOS pathway[47,48]. Thrombocyte function was maintained (i.e., without interfering with coagulation pathways)[40-42]. Counteraction of all adverse effects of NSAIDs[30] includes acting as membrane stabilizers and counteracted leaky gut [26] and acting as free radical scavengers, particularly in vascular studies[11,13-16,21,22,26,35-37]. The therapy effect on NSAIDs is widespread, including both nonspecific NSAIDs[26,41,42,57-61] and specific NSAID[62]. In particular, there BPC 157 may cure stomach lesions that regularly were perforated or about to perforate[58-60]. Furthermore, the healing of the fistula may be an important analogy for the perforated lesions healing, as “two sides” wounds[63], and BPC 157 may cure both external[64-66], and internal[67-69] fistulas in rats.
Summarizing, the findings presented in the rats with perforated stomachs and occlusion/occlusion-like syndrome might demonstrate how the initial cytoprotective effect (stomach epithelium maintenance) is translated to the systemic or generalized cytoprotection to many other organs (organoprotection) where the vascular endothelium might be the possible target, as it had been long-ago envisaged[34]. The local effect of the BPC 157 therapy in the stomach (i.e., immediately restored stomach vessels (vessels filled/reappeared, “run” toward the defect), counteracted bleeding and contracted perforated defect, and later, complete healing (day 7), and fewer adhesions[10]), might be, at the same time, consistently translated into the rapid beneficial effect on the whole syndrome. There, BPC 157 therapy application counteracted all organs lesions, progressive venous/arterial thrombosis/stasis, peripherally and centrally, along with the ECG disturbances, intracranial (superior sagittal sinus) hypertension, portal and caval hypertension, and aortal hypotension eliminated/attenuated[10-24]. Thus, the particularly activated azygos vein (direct blood flow delivery), and minor vessel upgrading might provide a consistent basis for the demonstrated pleiotropic beneficial effect as cytoprotection endothelial maintenance→epithelial maintenance maxim as general cytoprotective (organoprotective) effect. This may implement and reorganize blood flow and instantly attenuate the consequences of maintained perforation occlusion/occlusion-like syndrome-induced vascular and multiorgan failure[10-24]. Along with the activated azygos vein, the congested inferior caval vein and superior mesenteric vein were quickly reversed to the normal vessel presentation. These highlight the inferior caval vein-superior caval vein via azygos vein as rescuing pathway, portal and caval hypertension eliminated, aortal hypotension attenuated, thrombosis almost annihilated, as congestion in the lung, liver, kidney, and gastrointestinal tract markedly attenuated or even eliminated[10-24]. Note, BPC 157 might specifically maintain the function of thrombocytes[42], without affecting coagulation pathways, as shown by aggregometry and thromboelastometry studies. There, BPC 157, given with aspirin, clopidogrel, or cilostazol in rats, counteracted their inhibitory effects on aggregation activated by arachidonic acid, ADP, collagen, and arachidonic acid/PGE1[42]. These findings might be responsible for the attenuation of the bleeding from the perforated defect, and lack of hemorrhage in other organs (i.e., brain and lung) and thrombosis, peripherally and centrally, attenuated/eliminated[10-24]. These particular points might be along with the beforementioned particular modulatory effect on NO-system function (either NO-reduction or NO-overload can be harmful), opposing mentioned both NO-reduction (L-NAME-adverse effects) and NO-overload (L-arginine-adverse effects)[29,38-40]. Besides, BPC 157 therapy has particular wound healing capabilities (thereby, a distinctive effect on all four major events in clot formation and dissolution) as a cytoprotective agent’s essential effect that realized the healing process for ruptured blood vessels as a whole[5]. Thus, BPC 157 therapy might have this innate distinctive effect on all four major events in clot formation and dissolution and the therapy effects might be used in distinctive ways depending on the given injury and time of agent application[5,41]. As mentioned, these combined effects (attenuated/eliminated bleeding/attenuated/eliminated thrombosis) occurred as consistent findings commonly seen in the BPC 157 therapy of the counteraction of the occlusion/occlusion-like syndromes (intracerebral and intraventricular, and internal organs hemorrhage/progressive thrombosis, peripherally and centrally)[10-24]. This might accord with the evidence that BPC 157 therapy might reverse heart failure as a whole, including arrhythmias and thrombosis[3,4] which was also noted in the rats with stomach perforation.
Thereby, the BPC 157 therapy given into the perforated defect initiates a consistent chain of events verified by immediate counteraction of the gross brain swelling, and attenuated intracranial (superior sagittal sinus) hypertension as the upgraded venous system, adequately resolving the anatomical imbalance in venous drainage, which would thereby not occur[10-24]. Centrally, evidencing rapid counteraction of such venous and intracranial hypertension[14-17,20-24] reveals successful resolution, ability to drain venous blood adequately for a given cerebral blood inflow without rising venous pressures, and harmful inability resolved. As specifically shown in the maintained intra-abdominal hypertension studies[20], the three interconnected body cavities[14-17,20-24] require the venous system upgraded so that these might act peripherally (i.e., perforated defect), centrally (i.e., huge intraventricular hemorrhage), and then peripherally and centrally, and centrally and peripherally. Otherwise, if the venous system is not adequately upgraded, the disturbances may be rapidly transmitted through the venous system from the periphery to centrally, and from centrally to the periphery. Consequently, given the upgraded venous system, rats with BPC 157 therapy may smoothly sustain without major harm even the conditions of severe grade III and grade IV intra-abdominal hypertension[20]. Likely, the BPC 157 therapy’s effect in the rats with perforated stomachs might also effectively operate and might accommodate the particular central/peripheral and peripheral/central equation[10-24] to counteract perforated defect[10]. Note, with BPC 157 therapy into the perforated defect, upon volume application instead of volume-induced dilatation as regular finding in the rats with the perforated stomach, the whole stomach reacted with marked shrinking. This finding might be a sign of the rapid smooth muscle stomach function recovery and might occur along with noted smooth muscle function recovery (i.e., various sphincters to counteract esophagitis development[36,57,70-74], urinary incontinence[35,67,75], pupil dysfunction, miosis[76] and mydriasis[76] (in particular in glaucoma therapy[19]). Likewise, the rapid smooth muscle stomach function might be along with the recovery as rapid adaptation following injury (i.e., short bowel rats)[77]. Thus, as proof of the concept, peripherally and centrally, BPC 157 therapy might rapidly overwhelm Virchow triad circumstances, which are otherwise commonly presented in the rats with perforated stomach occlusion/occlusion-like syndrome as in the rats with occlusion/occlusion like syndromes induced with major vessel(s) occlusion or application of other similar noxious procedures[10-24].
By doing so, the pleiotropic lesions counteraction in the studies of the perforated stomach and other vascular occlusion/occlusion-like syndromes[10-24], was particular cytoprotection/organoprotection background of the cytoprotective agents’ innate activity. This cytoprotection/organoprotection background of the cytoprotective agent might be also in the previous non-vascular studies since there was a particular counteraction of the lesions in the brain[57-62,78,79], lung[80-83], liver[57-62,79,84-86], kidney[87-89] and gastrointestinal tract[26,57-62,73], and in particular, the counteraction of the heart arrhythmias and infarction[83,90-95]. Together, these findings might suggest a particular regulatory role.
Finally, we envisaged the perforated defect as direct damage, and prototype lesion that as such should be the core hallmark in cytoprotection studies[10]. This might be further indicative for additional implementation of the cytoprotection concept into the whole occlusion/occlusion-like syndrome[10-24], and demonstration of the therapeutic potential of the stable gastric pentadecapeptide BPC 157, in particular, and cytoprotective agents in general. In conclusion, it might be the cytoprotection phenomenon with application in distinctive injuries, analogous to, if not identical with, that which might occur during major vessel occlusion and application of other alike noxious procedures[10-24]. Furthermore, it might be that in both cases, the same agent - pentadecapeptide BPC 157, also suggested being the novel cytoprotection mediator as native and stable in human gastric juice, easily applicable[1-6]-might be responsible for the effect. The investigated and counteracted lesions all attenuated/eliminated might provide full support[10-24]. The attenuated/eliminated lesions, brain swelling, and lesions, heart dysfunction, lung lesions, liver and kidney failure, gastrointestinal lesions, widespread arterial and venous thrombosis, and vessel failure, each as the ultimate outcome of the tightly interconnected network, suggests that stomach perforation-induced occlusion/occlusion syndrome was resolved in all cause-consequence terms. The additional support might provide the counteraction of the severe intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension, and ECG disturbances fully counteracted as well. Finally, BPC 157 was found in human fetuses and adult in various tissues (i.e., gastrointestinal mucosa, lung bronchial epithelium, the epidermal layer of the skin, and kidney glomeruli) (in situ hybridization and immunostaining)[5,96]. Consequently, as evidenced by the resolution of the complex occlusion/occlusion-like syndrome[10-24], BPC 157 may have a regulatory physiologic role in bodily functions. Similar importance was suggested also for other species (i.e., birds[72] and insects[97,98])based on the alike beneficial effects. A very safe BPC 157 profile supports a LD1 not achieved in toxicological studies and clinical trials (ulcerative colitis, phase II) without adverse effects (for review see[1-6,29,30,96]). This favorable point was recently confirmed in a large study conducted by Xu and collaborators[99]. Finally, very recently, the essential point, rapid functional improvement of minor vessels to take over the function of disabled major vessels, reorganize blood flow, and compensate failed vessel function when confronted with major vessel occlusion and vascular failure was supplemented with Fourier transform infrared spectroscopy evidence[100]. Illustratively, there was an increased capability of the rat thoracic aorta to function even in the worst circumstances due to the rapid change in the lipid contents and protein secondary structure conformation produced instantly by BPC 157 therapy[100].
CONCLUSION
Together, these findings, providing firm evidence of the suited models (i.e., perforated stomach-occlusion/occlusion-like syndrome)[1-6,29,30,96] may be suggestive for further BPC 157 therapy application. Besides, such an experimental design strongly emphasizes the need for patients to receive timely treatment, providing that in general with full medical care life-threatening situations rarely occur.
ARTICLE HIGHLIGHTS
Research background
Taking stomach perforation as a prime direct injury regularly used in cytoprotection studies, as a new insight in resolving the full extent of the cytoprotection issue, there is the rapid progression of severe occlusion/occlusion-like syndrome considerably complicating the course following stomach perforation in rats. Otherwise, such occlusion/occlusion-like syndrome may suffer rats with permanent occlusion of the major vessel(s), veins and/or arteries, peripherally and centrally, and rats who underwent similar noxious procedures that all severely affect endothelium function. There were cause-consequence lesions in the brain (intracerebral and intraventricular hemorrhage), heart (congestion), lung (hemorrhage), congestion in the liver, kidney, and gastrointestinal tract, arrhythmias, vessel failure (congested inferior caval and superior mesenteric vein, collapsed azygos vein), and blood pressure disturbances (intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension), and widespread thrombosis, peripherally and centrally.
Research motivation
In occlusion/occlusion-like syndromes, stable gastric pentadecapeptide BPC 157 was recognized as counteracting therapy, and has already presented in recent reviews (Curr Med Chem 2023; 30: 1568-1573; World J Gastroenterol 2022; 28: 23-46). In the rats with perforated stomachs, and subsequent occlusion/occlusion-like syndrome, we emphasized the cytoprotection potential of the stable gastric BPC 157 as native and stable in gastric juice, thought to play the role of cytoprotection mediator maintaining stomach mucosa integrity. As a particular clue recognized also in the counteraction of stomach perforation-induced occlusion/occlusion-like syndromes, there was activation of collateral pathways depending on the injury, locally (raised vessel presentation; less bleeding, defect contraction) and systemically. Accordingly, BPC 157 therapy induced direct blood delivery from the inferior caval vein to the superior caval vein via the azygos vein. Application of the BPC 157 therapy (10 µg/kg and 10 ng/kg) into the perforated defect, induced immediate shrinking and contraction of the whole stomach. Simultaneously, there were attenuated/eliminated intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension. Thrombosis, peripherally (inferior caval vein, portal vein, abdominal aorta) and centrally (superior sagittal sinus) BPC 157 therapy markedly reduced/annihilated, and the severe lesions in the brain, heart (congestion and arrhythmias), lung (hemorrhage and congestion) and marked congestion in the liver, kidney and gastrointestinal tract markedly reduced. In conclusion, with stomach perforation severe occlusion/occlusion-like syndrome, and rapid progression peripherally and centrally, the rapid counteraction should be by BPC 157 therapy.
Research objectives
The first objective was stomach perforation presentation in rats as severe occlusion/occlusion-like syndrome, peripherally and centrally. The second objective was rapid counteraction by BPC 157 therapy.
Research methods
In deeply anesthetized rats underwent calvariectomy, stomach perforation was performed and rats received BPC 157 therapy or saline into the defect in the stomach. Then, in addition to assessing shrinking and contraction/enlargement of the whole stomach, the assessment (gross and microscopy, volume) was as in the occlusion/occlusion-like studies and included all points regularly analyzed. Assessed were swollen brain, failed peripheral vessels (azygos vein, superior mesenteric vein, portal vein, inferior caval vein) and heart, other organs lesions (i.e., stomach, defect closing or widening); intracranial (superior sagittal sinus), portal and caval hypertension, aortal hypotension; clots peripherally and centrally; ECGs; and bleeding time from the perforation(s).
Research results
BPC 157 effects accord with the beneficial effect noted before in the healing of the perforated defect (raised vessel presentation; less bleeding, defect contraction) and in the occlusion/occlusion-like syndromes. In rats with perforated stomachs, BPC 157 therapy (10 µg/kg and 10 ng/kg) given into the perforated defect, induced immediate shrinking and contraction of the whole stomach (unlike saline-induced immediate stomach considerable enlargement). There were cause-consequence lesions all eliminated/attenuated by BPC 157 therapy (i.e., direct blood flow delivery via azygos vein). There were eliminated/attenuated lesions in the brain (i.e., swelling, hemorrhage), heart (congestion), lung (hemorrhage), congestion in the liver, kidney, and gastrointestinal tract, arrhythmias, and blood pressure disturbances (intracranial (superior sagittal sinus), portal and caval hypertension, and aortal hypotension). Counteracted was major vessel failure (i.e., reversal of congested inferior caval vein and superior mesenteric vein, collapsed azygos vein), and eliminated widespread thrombosis, peripherally and centrally.
Research conclusions
Stomach perforation occurred as severe occlusion/occlusion-like syndrome, peripherally and centrally. Rapid counteraction was by BPC 157 therapy. Thereby, further BPC 157 therapy may be warranted.
Research perspectives
Stomach perforation as severe occlusion/occlusion-like syndrome needs immediate additional medical attention. There, BPC 157, as a prototype cytoprotective agent, should be also considered as further therapy.
Footnotes
Institutional animal care and use committee statement: This research was reviewed and approved by the Local Ethic Committee (Approval No. 380-59-10106-17-100/290).
Conflict-of-interest statement: Authors declare no conflict of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 19, 2023
First decision: May 23, 2023
Article in press: June 19, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo W, China; Stambolija V, Croatia S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
Contributor Information
Luka Kalogjera, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Ivan Krezic, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Ivan Maria Smoday, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Hrvoje Vranes, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Helena Zizek, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Haidi Yago, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Katarina Oroz, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Vlasta Vukovic, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Ivana Kavelj, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Luka Novosel, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Slavica Zubcic, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Ivan Barisic, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Lidija Beketic Oreskovic, Division of Oncology and Radiotherapy, University Hospital for Tumors, Sestre milosrdnice University Hospital Centre, Zagreb 10000, Croatia.
Sanja Strbe, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Marko Sever, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Ivica Sjekavica, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Anita Skrtic, Department of Pathology, School of Medicine, Zagreb 10000, Croatia.
Alenka Boban Blagaic, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia.
Sven Seiwerth, Department of Pathology, School of Medicine, Zagreb 10000, Croatia.
Predrag Sikiric, Department of Pharmacology, School of Medicine, Zagreb 10000, Croatia. sikiric@mef.hr.
Data sharing statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- 1.Sikiric P, Skrtic A, Gojkovic S, Krezic I, Zizek H, Lovric E, Sikiric S, Knezevic M, Strbe S, Milavic M, Kokot A, Blagaic AB, Seiwerth S. Cytoprotective gastric pentadecapeptide BPC 157 resolves major vessel occlusion disturbances, ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome. World J Gastroenterol. 2022;28:23–46. doi: 10.3748/wjg.v28.i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sikiric P, Gojkovic S, Knezevic M, Tepes M, Strbe S, Vukojevic J, Duzel A, Kralj T, Krezic I, Zizek H, Oroz K, Vranes H, Smoday IM, Kalogjera L, Vlainic J, Kokot A, Jurjevic I, Blagaic AB, Skrtic A, Seiwerth S. Stable Gastric Pentadecapeptide BPC 157: Prompt Particular Activation of Collateral Pathways. Curr Med Chem. 2023;30:1568–1573. doi: 10.2174/0929867329666221005111553. [DOI] [PubMed] [Google Scholar]
- 3.Sikiric P, Udovicic M, Barisic I, Balenovic D, Zivanovic Posilovic G, Strinic D, Uzun S, Sikiric S, Krezic I, Zizek H, Yago H, Gojkovic S, Smoday IM, Kalogjera L, Vranes H, Sola M, Strbe S, Koprivanac A, Premuzic Mestrovic I, Mestrovic T, Pavic P, Skrtic A, Blagaic AB, Lovric Bencic M, Seiwerth S. Stable Gastric Pentadecapeptide BPC 157 as Useful Cytoprotective Peptide Therapy in the Heart Disturbances, Myocardial Infarction, Heart Failure, Pulmonary Hypertension, Arrhythmias, and Thrombosis Presentation. Biomedicines. 2022;10 doi: 10.3390/biomedicines10112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staresinic M, Japjec M, Vranes H, Prtoric A, Zizek H, Krezic I, Gojkovic S, Smoday IM, Oroz K, Staresinic E, Dretar V, Yago H, Milavic M, Sikiric S, Lovric E, Batelja Vuletic L, Simeon P, Dobric I, Strbe S, Kokot A, Vlainic J, Blagaic AB, Skrtic A, Seiwerth S, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 and Striated, Smooth, and Heart Muscle. Biomedicines. 2022;10 doi: 10.3390/biomedicines10123221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seiwerth S, Milavic M, Vukojevic J, Gojkovic S, Krezic I, Vuletic LB, Pavlov KH, Petrovic A, Sikiric S, Vranes H, Prtoric A, Zizek H, Durasin T, Dobric I, Staresinic M, Strbe S, Knezevic M, Sola M, Kokot A, Sever M, Lovric E, Skrtic A, Blagaic AB, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 and Wound Healing. Front Pharmacol. 2021;12:627533. doi: 10.3389/fphar.2021.627533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vukojevic J, Milavić M, Perović D, Ilić S, Čilić AZ, Đuran N, Štrbe S, Zoričić Z, Filipčić I, Brečić P, Seiverth S, Sikirić P. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen Res. 2022;17:482–487. doi: 10.4103/1673-5374.320969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–767. [PubMed] [Google Scholar]
- 8.Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–443. [PubMed] [Google Scholar]
- 9.Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228–236. doi: 10.1016/s0016-5085(85)80176-1. [DOI] [PubMed] [Google Scholar]
- 10.Bilic Z, Gojkovic S, Kalogjera L, Krezic I, Malekinusic D, Knezevic M, Sever M, Lojo N, Kokot A, Kasnik K, Kralj T, Vukojevic J, Siroglavic M, Peklic M, Drmic D, Milavic M, Sikiric S, Skorak I, Brizic I, Hriberski K, Kubat M, Vladic J, Boban Blagaic A, Tvrdeic A, Skrtic A, Seiwerth S, Sikiric P. Novel insight into Robert's cytoprotection: complex therapeutic effect of cytoprotective pentadecapeptide pentadecapeptide BPC 157 in rats with perforated stomach throughout modulation of nitric oxide-system. Comparison with L-arginine, ranitidine and pantoprazole therapy and L-N(G)-nitro-L-arginine methyl ester worsening. J Physiol Pharmacol. 2021;72 doi: 10.26402/jpp.2021.6.11. [DOI] [PubMed] [Google Scholar]
- 11.Vukojević J, Siroglavić M, Kašnik K, Kralj T, Stanćić D, Kokot A, Kolarić D, Drmić D, Sever AZ, Barišić I, Šuran J, Bojić D, Patrlj MH, Sjekavica I, Pavlov KH, Vidović T, Vlainić J, Stupnišek M, Seiwerth S, Sikirić P. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol. 2018;106:54–66. doi: 10.1016/j.vph.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Gojkovic S, Krezic I, Vrdoljak B, Malekinusic D, Barisic I, Petrovic A, Horvat Pavlov K, Kolovrat M, Duzel A, Knezevic M, Kasnik Kovac K, Drmic D, Batelja Vuletic L, Kokot A, Boban Blagaic A, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J Gastrointest Pathophysiol. 2020;11:1–19. doi: 10.4291/wjgp.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolovrat M, Gojkovic S, Krezic I, Malekinusic D, Vrdoljak B, Kasnik Kovac K, Kralj T, Drmic D, Barisic I, Horvat Pavlov K, Petrovic A, Duzel A, Knezevic M, Mirkovic I, Kokot A, Boban Blagaic A, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J Hepatol. 2020;12:184–206. doi: 10.4254/wjh.v12.i5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knezevic M, Gojkovic S, Krezic I, Zizek H, Vranes H, Malekinusic D, Vrdoljak B, Knezevic T, Horvat Pavlov K, Drmic D, Staroveski M, Djuzel A, Rajkovic Z, Kolak T, Lovric E, Milavic M, Sikiric S, Barisic I, Tepes M, Tvrdeic A, Patrlj L, Strbe S, Sola M, Situm A, Kokot A, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Complex Syndrome of the Complete Occlusion of the End of the Superior Mesenteric Vein, Opposed with the Stable Gastric Pentadecapeptide BPC 157 in Rats. Biomedicines. 2021;9 doi: 10.3390/biomedicines9081029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knezevic M, Gojkovic S, Krezic I, Zizek H, Malekinusic D, Vrdoljak B, Knezevic T, Vranes H, Drmic D, Staroveski M, Djuzel A, Rajkovic Z, Kolak T, Lovric E, Milavic M, Sikiric S, Tvrdeic A, Patrlj L, Strbe S, Sola M, Situm A, Kokot A, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Occluded Superior Mesenteric Artery and Vein. Therapy with the Stable Gastric Pentadecapeptide BPC 157. Biomedicines. 2021;9 doi: 10.3390/biomedicines9070792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knezevic M, Gojkovic S, Krezic I, Zizek H, Malekinusic D, Vrdoljak B, Vranes H, Knezevic T, Barisic I, Horvat Pavlov K, Drmic D, Staroveski M, Djuzel A, Rajkovic Z, Kolak T, Kocman I, Lovric E, Milavic M, Sikiric S, Tvrdeic A, Patrlj L, Strbe S, Kokot A, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Occlusion of the Superior Mesenteric Artery in Rats Reversed by Collateral Pathways Activation: Gastric Pentadecapeptide BPC 157 Therapy Counteracts Multiple Organ Dysfunction Syndrome; Intracranial, Portal, and Caval Hypertension; and Aortal Hypotension. Biomedicines. 2021;9 doi: 10.3390/biomedicines9060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gojkovic S, Krezic I, Vranes H, Zizek H, Drmic D, Horvat Pavlov K, Petrovic A, Batelja Vuletic L, Milavic M, Sikiric S, Stilinovic I, Samara M, Knezevic M, Barisic I, Sjekavica I, Lovric E, Skrtic A, Seiwerth S, Sikiric P. BPC 157 Therapy and the Permanent Occlusion of the Superior Sagittal Sinus in Rat: Vascular Recruitment. Biomedicines. 2021;9 doi: 10.3390/biomedicines9070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vukojević J, Vrdoljak B, Malekinušić D, Siroglavić M, Milavić M, Kolenc D, Boban Blagaić A, Batelja L, Drmić D, Seiverth S, Sikirić P. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020;10:e01726. doi: 10.1002/brb3.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kralj T, Kokot A, Zlatar M, Masnec S, Kasnik Kovac K, Milkovic Perisa M, Batelja Vuletic L, Giljanovic A, Strbe S, Sikiric S, Balog S, Sontacchi B, Sontacchi D, Buljan M, Lovric E, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 Therapy of Rat Glaucoma. Biomedicines. 2021;10 doi: 10.3390/biomedicines10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tepes M, Gojkovic S, Krezic I, Zizek H, Vranes H, Madzar Z, Santak G, Batelja L, Milavic M, Sikiric S, Kocman I, Simonji K, Samara M, Knezevic M, Barisic I, Lovric E, Strbe S, Kokot A, Sjekavica I, Kolak T, Skrtic A, Seiwerth S, Boban Blagaic A, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 Therapy for Primary Abdominal Compartment Syndrome in Rats. Front Pharmacol. 2021;12:718147. doi: 10.3389/fphar.2021.718147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barisic I, Balenovic D, Udovicic M, Bardak D, Strinic D, Vlainić J, Vranes H, Smoday IM, Krezic I, Milavic M, Sikiric S, Uzun S, Zivanovic Posilovic G, Strbe S, Vukoja I, Lovric E, Lozic M, Sever M, Lovric Bencic M, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 May Counteract Myocardial Infarction Induced by Isoprenaline in Rats. Biomedicines. 2022;10 doi: 10.3390/biomedicines10020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strbe S, Gojkovic S, Krezic I, Zizek H, Vranes H, Barisic I, Strinic D, Orct T, Vukojevic J, Ilic S, Lovric E, Muzinic D, Kolenc D, Filipčić I, Zoricic Z, Marcinko D, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Over-Dose Lithium Toxicity as an Occlusive-like Syndrome in Rats and Gastric Pentadecapeptide BPC 157. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smoday IM, Petrovic I, Kalogjera L, Vranes H, Zizek H, Krezic I, Gojkovic S, Skorak I, Hriberski K, Brizic I, Kubat M, Strbe S, Barisic I, Sola M, Lovric E, Lozic M, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P. Therapy Effect of the Stable Gastric Pentadecapeptide BPC 157 on Acute Pancreatitis as Vascular Failure-Induced Severe Peripheral and Central Syndrome in Rats. Biomedicines. 2022;10 doi: 10.3390/biomedicines10061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gojkovic S, Krezic I, Vranes H, Zizek H, Drmic D, Batelja Vuletic L, Milavic M, Sikiric S, Stilinovic I, Simeon P, Knezevic M, Kolak T, Tepes M, Simonji K, Strbe S, Nikolac Gabaj N, Barisic I, Oreskovic EG, Lovric E, Kokot A, Skrtic A, Boban Blagaic A, Seiwerth S, Sikiric P. Robert's Intragastric Alcohol-Induced Gastric Lesion Model as an Escalated General Peripheral and Central Syndrome, Counteracted by the Stable Gastric Pentadecapeptide BPC 157. Biomedicines. 2021;9 doi: 10.3390/biomedicines9101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikiric P, Hahm KB, Blagaic AB, Tvrdeic A, Pavlov KH, Petrovic A, Kokot A, Gojkovic S, Krezic I, Drmic D, Rucman R, Seiwerth S. Stable Gastric Pentadecapeptide BPC 157, Robert's Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye's Stress Coping Response: Progress, Achievements, and the Future. Gut Liver. 2020;14:153–167. doi: 10.5009/gnl18490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JM, Lee HJ, Sikiric P, Hahm KB. BPC 157 Rescued NSAID-cytotoxicity Via Stabilizing Intestinal Permeability and Enhancing Cytoprotection. Curr Pharm Des. 2020;26:2971–2981. doi: 10.2174/1381612826666200523180301. [DOI] [PubMed] [Google Scholar]
- 27.Sikiric P, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, Drmic D, Stupnisek M, Misic M, Vuletic LB, Pavlov KH, Barisic I, Kokot A, Peklic M, Strbe S, Blagaic AB, Tvrdeic A, Rokotov DS, Vrcic H, Staresinic M, Seiwerth S. Novel Cytoprotective Mediator, Stable Gastric Pentadecapeptide BPC 157. Vascular Recruitment and Gastrointestinal Tract Healing. Curr Pharm Des. 2018;24:1990–2001. doi: 10.2174/1381612824666180608101119. [DOI] [PubMed] [Google Scholar]
- 28.Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, Sever M, Zoricic I, Zoricic Z, Batelja L, Ziger T, Luetic K, Vlainic J, Rasic Z, Bencic ML. Stress in Gastrointestinal Tract and Stable Gastric Pentadecapeptide BPC 157. Finally, do we have a Solution? Curr Pharm Des. 2017;23:4012–4028. doi: 10.2174/1381612823666170220163219. [DOI] [PubMed] [Google Scholar]
- 29.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Aralica G, Stupnisek M, Suran J, Barisic I, Dzidic S, Vrcic H, Sebecic B. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126–1135. doi: 10.2174/13816128113190990411. [DOI] [PubMed] [Google Scholar]
- 30.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Aralica G, Safic H, Suran J, Rak D, Dzidic S, Vrcic H, Sebecic B. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76–83. doi: 10.2174/13816128130111. [DOI] [PubMed] [Google Scholar]
- 31.Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D. Revised Robert's cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224–1234. doi: 10.2174/138161210790945977. [DOI] [PubMed] [Google Scholar]
- 32.Drmic D, Samara M, Vidovic T, Malekinusic D, Antunovic M, Vrdoljak B, Ruzman J, Milkovic Perisa M, Horvat Pavlov K, Jeyakumar J, Seiwerth S, Sikiric P. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24:5462–5476. doi: 10.3748/wjg.v24.i48.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, Grgic T, Strbe S, Zukanovic G, Crvenkovic D, Madzarac G, Rukavina I, Sucic M, Baric M, Starcevic N, Krstonijevic Z, Bencic ML, Filipcic I, Rokotov DS, Vlainic J. Brain-gut Axis and Pentadecapeptide BPC 157: Theoretical and Practical Implications. Curr Neuropharmacol. 2016;14:857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo S, Usadel KH. Cytoprotection - organoprotection by somatostatin: gastric and hepatic lesions. Experientia. 1982;38:254–256. doi: 10.1007/BF01945097. [DOI] [PubMed] [Google Scholar]
- 35.Sucic M, Luetic K, Jandric I, Drmic D, Sever AZ, Vuletic LB, Halle ZB, Strinic D, Kokot A, Seiwerth RS, Zoricic I, Blagaic AB, Seiwerth S, Sikiric P. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur J Pharmacol. 2019;861:172593. doi: 10.1016/j.ejphar.2019.172593. [DOI] [PubMed] [Google Scholar]
- 36.Belosic Halle Z, Vlainic J, Drmic D, Strinic D, Luetic K, Sucic M, Medvidovic-Grubisic M, Pavelic Turudic T, Petrovic I, Seiwerth S, Sikiric P. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology. 2017 doi: 10.1007/s10787-017-0358-8. [DOI] [PubMed] [Google Scholar]
- 37.Luetic K, Sucic M, Vlainic J, Halle ZB, Strinic D, Vidovic T, Luetic F, Marusic M, Gulic S, Pavelic TT, Kokot A, Seiwerth RS, Drmic D, Batelja L, Seiwerth S, Sikiric P. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology. 2017;25:255–264. doi: 10.1007/s10787-017-0330-7. [DOI] [PubMed] [Google Scholar]
- 38.Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, Turković B, Rotkvić I, Mise S, Zoricić I, Konjevoda P, Perović D, Jurina L, Separović J, Hanzevacki M, Artuković B, Bratulić M, Tisljar M, Gjurasin M, Miklić P, Stancić-Rokotov D, Slobodnjak Z, Jelovac N, Marović A. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol. 1997;332:23–33. doi: 10.1016/s0014-2999(97)01033-9. [DOI] [PubMed] [Google Scholar]
- 39.Turkovic B, Sikiric P, Seiwerth S, Mise S, Anic T, Petek M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology. 2004;126:287. [Google Scholar]
- 40.Stupnisek M, Kokot A, Drmic D, Hrelec Patrlj M, Zenko Sever A, Kolenc D, Radic B, Suran J, Bojic D, Vcev A, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 Reduces Bleeding and Thrombocytopenia after Amputation in Rats Treated with Heparin, Warfarin, L-NAME and L-Arginine. PLoS One. 2015;10:e0123454. doi: 10.1371/journal.pone.0123454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stupnisek M, Franjic S, Drmic D, Hrelec M, Kolenc D, Radic B, Bojic D, Vcev A, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb Res. 2012;129:652–659. doi: 10.1016/j.thromres.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 42.Konosic S, Petricevic M, Ivancan V, Konosic L, Goluza E, Krtalic B, Drmic D, Stupnisek M, Seiwerth S, Sikiric P. Intragastric Application of Aspirin, Clopidogrel, Cilostazol, and BPC 157 in Rats: Platelet Aggregation and Blood Clot. Oxid Med Cell Longev. 2019;2019:9084643. doi: 10.1155/2019/9084643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212–221. doi: 10.1016/j.ejphar.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 44.Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066–19077. doi: 10.3390/molecules191119066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985) 2011;110:774–780. doi: 10.1152/japplphysiol.00945.2010. [DOI] [PubMed] [Google Scholar]
- 46.Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, Mu N, Gu J, Zhang W, Wang Y, Zhang Y. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485–2499. doi: 10.2147/DDDT.S82030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh MJ, Lee CH, Chueh HY, Chang GJ, Huang HY, Lin Y, Pang JS. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci Rep. 2020;10:17078. doi: 10.1038/s41598-020-74022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JS. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl) 2017;95:323–333. doi: 10.1007/s00109-016-1488-y. [DOI] [PubMed] [Google Scholar]
- 49.Kang EA, Han YM, An JM, Park YJ, Sikiric P, Kim DH, Kwon KA, Kim YJ, Yang D, Tchah H, Hahm KB. BPC157 as Potential Agent Rescuing from Cancer Cachexia. Curr Pharm Des. 2018;24:1947–1956. doi: 10.2174/1381612824666180614082950. [DOI] [PubMed] [Google Scholar]
- 50.Huang BS, Huang SC, Chen FH, Chang Y, Mei HF, Huang HY, Chen WY, Pang JS. Pentadecapeptide BPC 157 efficiently reduces radiation-induced liver injury and lipid accumulation through Kruppel-like factor 4 upregulation both in vivo and in vitro. Life Sci. 2022;310:121072. doi: 10.1016/j.lfs.2022.121072. [DOI] [PubMed] [Google Scholar]
- 51.Wu H, Wei M, Li N, Lu Q, Shrestha SM, Tan J, Zhang Z, Wu G, Shi R. Clopidogrel-Induced Gastric Injury in Rats is Attenuated by Stable Gastric Pentadecapeptide BPC 157. Drug Des Devel Ther. 2020;14:5599–5610. doi: 10.2147/DDDT.S284163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XY, Qu M, Duan R, Shi D, Jin L, Gao J, Wood JD, Li J, Wang GD. Cytoprotective Mechanism of the Novel Gastric Peptide BPC157 in Gastrointestinal Tract and Cultured Enteric Neurons and Glial Cells. Neurosci Bull. 2019;35:167–170. doi: 10.1007/s12264-018-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang JL, Jing X, Tian X, Qin MC, Xu ZH, Wu DP, Zhong ZG. Neuroprotective Properties of Panax notoginseng Saponins via Preventing Oxidative Stress Injury in SAMP8 Mice. Evid Based Complement Alternat Med. 2017;2017:8713561. doi: 10.1155/2017/8713561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer M, Deigendesch N, Wittig H, Scheurer E, Lenz C. Tissue sample analysis for post mortem determination of brain edema. Forensic Sci Int. 2021;323:110808. doi: 10.1016/j.forsciint.2021.110808. [DOI] [PubMed] [Google Scholar]
- 55.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 56.Lane JS, Todd KE, Lewis MP, Gloor B, Ashley SW, Reber HA, McFadden DW, Chandler CF. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery. 1997;122:288–294. doi: 10.1016/s0039-6060(97)90020-9. [DOI] [PubMed] [Google Scholar]
- 57.Vitaic S, Stupnisek M, Drmic D, Bauk L, Kokot A, Klicek R, Vcev A, Luetic K, Seiwerth S, Sikiric P. Nonsteroidal anti-inflammatory drugs-induced failure of lower esophageal and pyloric sphincter and counteraction of sphincters failure with stable gatric pentadecapeptide BPC 157 in rats. J Physiol Pharmacol. 2017;68:265–272. [PubMed] [Google Scholar]
- 58.Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, Zoricic I, Sever M, Seiwerth S, Sikiric P. Effects of Diclofenac, L-NAME, L-Arginine, and Pentadecapeptide BPC 157 on Gastrointestinal, Liver, and Brain Lesions, Failed Anastomosis, and Intestinal Adaptation Deterioration in 24 Hour-Short-Bowel Rats. PLoS One. 2016;11:e0162590. doi: 10.1371/journal.pone.0162590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilic S, Drmic D, Zarkovic K, Kolenc D, Brcic L, Radic B, Djuzel V, Blagaic AB, Romic Z, Dzidic S, Kalogjera L, Seiwerth S, Sikiric P. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol. 2011;667:322–329. doi: 10.1016/j.ejphar.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 60.Ilic S, Drmic D, Franjic S, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V, Filipovic M, Djakovic Z, Stambolija V, Blagaic AB, Zoricic I, Gjurasin M, Stupnisek M, Romic Z, Zarkovic K, Dzidic S, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88:535–542. doi: 10.1016/j.lfs.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V, Ivica M, Boban Blagaic A, Zoricic Z, Anic T, Zoricic I, Djidic S, Romic Z, Seiwerth S, Sikiric P. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736) J Physiol Pharmacol. 2010;61:241–250. [PubMed] [Google Scholar]
- 62.Drmic D, Kolenc D, Ilic S, Bauk L, Sever M, Zenko Sever A, Luetic K, Suran J, Seiwerth S, Sikiric P. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol. 2017;23:5304–5312. doi: 10.3748/wjg.v23.i29.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sikiric P, Drmic D, Sever M, Klicek R, Blagaic AB, Tvrdeic A, Kralj T, Kovac KK, Vukojevic J, Siroglavic M, Gojkovic S, Krezic I, Pavlov KH, Rasic D, Mirkovic I, Kokot A, Skrtic A, Seiwerth S. Fistulas Healing. Stable Gastric Pentadecapeptide BPC 157 Therapy. Curr Pharm Des. 2020;26:2991–3000. doi: 10.2174/1381612826666200424180139. [DOI] [PubMed] [Google Scholar]
- 64.Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, Brcic L, Rokotov DS, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203–212. doi: 10.1016/j.ejphar.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 65.Skorjanec S, Kokot A, Drmic D, Radic B, Sever M, Klicek R, Kolenc D, Zenko A, Lovric Bencic M, Belosic Halle Z, Situm A, Zivanovic Posilovic G, Masnec S, Suran J, Aralica G, Seiwerth S, Sikiric P. Duodenocutaneous fistula in rats as a model for "wound healing-therapy" in ulcer healing: the effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and L-arginine. J Physiol Pharmacol. 2015;66:581–590. [PubMed] [Google Scholar]
- 66.Skorjanec S, Dolovski Z, Kocman I, Brcic L, Blagaic Boban A, Batelja L, Coric M, Sever M, Klicek R, Berkopic L, Radic B, Drmic D, Kolenc D, Ilic S, Cesarec V, Tonkic A, Zoricic I, Mise S, Staresinic M, Ivica M, Lovric Bencic M, Anic T, Seiwerth S, Sikiric P. Therapy for unhealed gastrocutaneous fistulas in rats as a model for analogous healing of persistent skin wounds and persistent gastric ulcers: stable gastric pentadecapeptide BPC 157, atropine, ranitidine, and omeprazole. Dig Dis Sci. 2009;54:46–56. doi: 10.1007/s10620-008-0332-9. [DOI] [PubMed] [Google Scholar]
- 67.Rasic D, Zenko Sever A, Rasic F, Strbe S, Rasic Z, Djuzel A, Duplancic B, Boban Blagaic A, Skrtic A, Seiwerth S, Sikiric P, Sever M. Stable Gastric Pentadecapeptide BPC 157 Heals Established Vesicovaginal Fistula and Counteracts Stone Formation in Rats. Biomedicines. 2021;9 doi: 10.3390/biomedicines9091206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grgic T, Grgic D, Drmic D, Sever AZ, Petrovic I, Sucic M, Kokot A, Klicek R, Sever M, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 heals rat colovesical fistula. Eur J Pharmacol. 2016;780:1–7. doi: 10.1016/j.ejphar.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 69.Baric M, Sever AZ, Vuletic LB, Rasic Z, Sever M, Drmic D, Pavelic-Turudic T, Sucic M, Vrcic H, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci. 2016;148:63–70. doi: 10.1016/j.lfs.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 70.Petrovic I, Dobric I, Drmic D, Sever M, Klicek R, Radic B, Brcic L, Kolenc D, Zlatar M, Kunjko K, Jurcic D, Martinac M, Rasic Z, Boban Blagaic A, Romic Z, Seiwerth S, Sikiric P. BPC 157 therapy to detriment sphincters failure-esophagitis-pancreatitis in rat and acute pancreatitis patients low sphincters pressure. J Physiol Pharmacol. 2011;62:527–534. [PubMed] [Google Scholar]
- 71.Dobric I, Drvis P, Petrovic I, Shejbal D, Brcic L, Blagaic AB, Batelja L, Sever M, Kokic N, Tonkic A, Zoricic I, Mise S, Staresinic M, Radic B, Jakir A, Babel J, Ilic S, Vuksic T, Jelic I, Anic T, Seiwerth S, Sikiric P. Prolonged esophagitis after primary dysfunction of the pyloric sphincter in the rat and therapeutic potential of the gastric pentadecapeptide BPC 157. J Pharmacol Sci. 2007;104:7–18. doi: 10.1254/jphs.fp0061322. [DOI] [PubMed] [Google Scholar]
- 72.Petrovic I, Dobric I, Drvis P, Shejbal D, Brcic L, Blagaic AB, Batelja L, Kokic N, Tonkic A, Mise S, Baotic T, Staresinic M, Radic B, Jakir A, Vuksic T, Anic T, Seiwerth S, Sikiric P. An experimental model of prolonged esophagitis with sphincter failure in the rat and the therapeutic potential of gastric pentadecapeptide BPC 157. J Pharmacol Sci. 2006;102:269–277. doi: 10.1254/jphs.fp0060070. [DOI] [PubMed] [Google Scholar]
- 73.Becejac T, Cesarec V, Drmic D, Hirsl D, Madzarac G, Djakovic Z, Bunjevac I, A Zenko Sever A, Sepac A, Batelja Vuletic L, Stancic Rokotov D, Seiwerth S, Sikiric P. An endogeous defensive concept, renewed cytoprotection/adaptive cytoprotection: intra(per)-oral/intragastric strong alcohol in rat. Involvement of pentadecapeptide BPC 157 and nitric oxide system. J Physiol Pharmacol. 2018;69 doi: 10.26402/jpp.2018.3.11. [DOI] [PubMed] [Google Scholar]
- 74.Djakovic Z, Djakovic I, Cesarec V, Madzarac G, Becejac T, Zukanovic G, Drmic D, Batelja L, Zenko Sever A, Kolenc D, Pajtak A, Knez N, Japjec M, Luetic K, Stancic-Rokotov D, Seiwerth S, Sikiric P. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J Gastroenterol. 2016;22:9127–9140. doi: 10.3748/wjg.v22.i41.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jandric I, Vrcic H, Jandric Balen M, Kolenc D, Brcic L, Radic B, Drmic D, Seiwerth S, Sikiric P. Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med Sci Monit Basic Res. 2013;19:93–102. doi: 10.12659/MSMBR.883828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kokot A, Zlatar M, Stupnisek M, Drmic D, Radic R, Vcev A, Seiwerth S, Sikiric P. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol. 2016;771:211–219. doi: 10.1016/j.ejphar.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Sever M, Klicek R, Radic B, Brcic L, Zoricic I, Drmic D, Ivica M, Barisic I, Ilic S, Berkopic L, Blagaic AB, Coric M, Kolenc D, Vrcic H, Anic T, Seiwerth S, Sikiric P. Gastric pentadecapeptide BPC 157 and short bowel syndrome in rats. Dig Dis Sci. 2009;54:2070–2083. doi: 10.1007/s10620-008-0598-y. [DOI] [PubMed] [Google Scholar]
- 78.Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, Sever M, Holjevac J, Radic B, Turudic T, Kokot A, Patrlj L, Rucman R, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64:597–612. [PubMed] [Google Scholar]
- 79.Ilic S, Brcic I, Mester M, Filipovic M, Sever M, Klicek R, Barisic I, Radic B, Zoricic Z, Bilic V, Berkopic L, Brcic L, Kolenc D, Romic Z, Pazanin L, Seiwerth S, Sikiric P. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J Physiol Pharmacol. 2009;60 Suppl 7:107–114. [PubMed] [Google Scholar]
- 80.Grabarevic Z, Tisljar M, Artukovic B, Bratulic M, Dzaja P, Seiwerth S, Sikiric P, Peric J, Geres D, Kos J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J Physiol Paris. 1997;91:139–149. doi: 10.1016/s0928-4257(97)89478-8. [DOI] [PubMed] [Google Scholar]
- 81.Stancic-Rokotov D, Slobodnjak Z, Aralica J, Aralica G, Perovic D, Staresinic M, Gjurasin M, Anic T, Zoricic I, Buljat G, Prkacin I, Sikiric P, Seiwerth S, Rucman R, Petek M, Turkovic B, Kokic N, Jagic V, Boban-Blagaic A. Lung lesions and anti-ulcer agents beneficial effect: anti-ulcer agents pentadecapeptide BPC 157, ranitidine, omeprazole and atropine ameliorate lung lesion in rats. J Physiol Paris. 2001;95:303–308. doi: 10.1016/s0928-4257(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 82.Stancic-Rokotov D, Sikiric P, Seiwerth S, Slobodnjak Z, Aralica J, Aralica G, Perovic D, Anic T, Zoricic I, Buljat G, Prkacin I, Gjurasin M, Rucman R, Petek M, Turkovic B, Ivasovic Z, Jagic V, Staresinic M, Boban-Blagaic A. Ethanol gastric lesion aggravated by lung injury in rat. Therapy effect of antiulcer agents. J Physiol Paris. 2001;95:289–293. doi: 10.1016/s0928-4257(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 83.Udovicic M, Sever M, Kavur L, Loncaric K, Barisic I, Balenovic D, Zivanovic Posilovic G, Strinic D, Uzun S, Batelja Vuletic L, Sikiric S, Skrtic A, Drmic D, Boban Blagaic A, Lovric Bencic M, Seiwerth S, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 Therapy for Monocrotaline-Induced Pulmonary Hypertension in Rats Leads to Prevention and Reversal. Biomedicines. 2021;9 doi: 10.3390/biomedicines9070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sever AZ, Sever M, Vidovic T, Lojo N, Kolenc D, Vuletic LB, Drmic D, Kokot A, Zoricic I, Coric M, Vlainic J, Poljak L, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur J Pharmacol. 2019;847:130–142. doi: 10.1016/j.ejphar.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 85.Sikiric P, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Rotkvic I, Turkovic B, Jagic V, Mildner B, Duvnjak M. Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci. 1993;53:PL291–PL296. doi: 10.1016/0024-3205(93)90589-u. [DOI] [PubMed] [Google Scholar]
- 86.Prkacin I, Separovic J, Aralicia G, Perovic D, Gjurasin M, Lovric-Bencic M, Stancic-Rokotov D, Staresinic M, Anic T, Mikus D, Sikiric P, Seiwerth S, Mise S, Rotkvic I, Jagic V, Rucman R, Petek M, Turkovic B, Marovic A, Sebecic B, Boban-Blagaic A, Kokic N. Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine. J Physiol Paris. 2001;95:315–324. doi: 10.1016/s0928-4257(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 87.Vranes H, Krezic I, Gojkovic S, Zizek H, Durasin T, Petrovic A, Horvat Pavlov K, Batelja L, Blagaic Boban A, Seiwerth S, Sikriic P. In hydornephrosis-rats, BPC 157 exerts a strong anti-ulcer effect in both stomach and duodenum along with marked kidney recovery. FASEB J . 2020;34:1. [Google Scholar]
- 88.Drmic D, Sucic M, Zenko Sever A, Kolenc D, Suran J, Seiwerth S, Sikiric P. BPC 157 counteracts gastric lesions after bilateral nephrectomy and attenuates deleterious course in rats. FASEB J. 2015;29:628.10. [Google Scholar]
- 89.Drmic D, Sucic M, Zenko Sever A, Kolenc D, Andrijasevic V, Suran J, Seiweth S, Sikriic P. Attenuation of the deleterious course and gastric lesions after bilateral nephrectomy in rats, NO-system relation. BPC 157, L-arginine, L-NAME. FASEB J . 2016;30:720.2. [Google Scholar]
- 90.Lozic M, Stambolija V, Krezic I, Dugandzic A, Zivanovic-Posilovic G, Gojkovic S, Kovacevic J, Vrdoljak L, Mirkovic I, Kokot A, Petrovic A, Pavlov KH, Drmic D, Suran J, Blagaic AB, Seiwerth S, Sikiric P. In relation to NO-System, Stable Pentadecapeptide BPC 157 Counteracts Lidocaine-Induced Adverse Effects in Rats and Depolarisation In Vitro. Emerg Med Int. 2020;2020:6805354. doi: 10.1155/2020/6805354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strinic D, Belosic Halle Z, Luetic K, Nedic A, Petrovic I, Sucic M, Zivanovic Posilovic G, Balenovic D, Strbe S, Udovicic M, Drmic D, Stupnisek M, Lovric Bencic M, Seiwerth S, Sikiric P. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017;186:66–79. doi: 10.1016/j.lfs.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Zivanovic-Posilovic G, Balenovic D, Barisic I, Strinic D, Stambolija V, Udovicic M, Uzun S, Drmic D, Vlainic J, Bencic ML, Sindic A, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur J Pharmacol. 2016;793:56–65. doi: 10.1016/j.ejphar.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 93.Barisic I, Balenovic D, Klicek R, Radic B, Nikitovic B, Drmic D, Udovicic M, Strinic D, Bardak D, Berkopic L, Djuzel V, Sever M, Cvjetko I, Romic Z, Sindic A, Bencic ML, Seiwerth S, Sikiric P. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept. 2013;181:50–66. doi: 10.1016/j.regpep.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Balenovic D, Bencic ML, Udovicic M, Simonji K, Hanzevacki JS, Barisic I, Kranjcevic S, Prkacin I, Coric V, Brcic L, Coric M, Brcic I, Borovic S, Radic B, Drmic D, Vrcic H, Seiwerth S, Sikiric P. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regul Pept. 2009;156:83–89. doi: 10.1016/j.regpep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Lovric-Bencic M, Sikiric P, Hanzevacki JS, Seiwerth S, Rogic D, Kusec V, Aralica G, Konjevoda P, Batelja L, Blagaic AB. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J Pharmacol Sci. 2004;95:19–26. doi: 10.1254/jphs.95.19. [DOI] [PubMed] [Google Scholar]
- 96.Seiwerth S, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, Drmic D, Stupnisek M, Misic M, Vuletic LB, Pavlov KH, Barisic I, Kokot A, Japjec M, Blagaic AB, Tvrdeic A, Rokotov DS, Vrcic H, Staresinic M, Sebecic B, Sikiric P. BPC 157 and Standard Angiogenic Growth Factors. Gastrointestinal Tract Healing, Lessons from Tendon, Ligament, Muscle and Bone Healing. Curr Pharm Des. 2018;24:1972–1989. doi: 10.2174/1381612824666180712110447. [DOI] [PubMed] [Google Scholar]
- 97.Tlak Gajger I, Smodiš Škerl MI, Šoštarić P, Šuran J, Sikirić P, Vlainić J. Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157. Biology (Basel) 2021;10 doi: 10.3390/biology10090891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tlak Gajger I, Ribarić J, Smodiš Škerl M, Vlainić J, Sikirić P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J Vet Pharmacol Ther. 2018;41:614–621. doi: 10.1111/jvp.12509. [DOI] [PubMed] [Google Scholar]
- 99.Xu C, Sun L, Ren F, Huang P, Tian Z, Cui J, Zhang W, Wang S, Zhang K, He L, Zhang C, Hao Q, Zhang Y, Li M, Li W. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul Toxicol Pharmacol. 2020;114:104665. doi: 10.1016/j.yrtph.2020.104665. [DOI] [PubMed] [Google Scholar]
- 100.Gamulin O, Oroz K, Coric L, Krajacic M, Skrabic M, Dretar V, Strbe S, Talapko J, Juzbasic M, Krezic I, Lozic M, Stambolija V, Zizek H, Jurca I, Jurjevic I, Blagaic AB, Skrtic A, Seiwerth S, Sikiric P. Fourier Transform Infrared Spectroscopy Reveals Molecular Changes in Blood Vessels of Rats Treated with Pentadecapeptide BPC 157. Biomedicines. 2022;10 doi: 10.3390/biomedicines10123130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.