Abstract
Background and Objectives
There are no evidence-based guidelines for discussing prognosis in critical neurologic illness, but in general, experts recommend that clinicians communicate prognosis using estimates, such as numerical or qualitative expressions of risk. Little is known about how real-world clinicians communicate prognosis in critical neurologic illness. Our primary objective was to characterize prognostic language clinicians used in critical neurologic illness. We additionally explored whether prognostic language differed between prognostic domains (e.g., survival, cognition).
Methods
We conducted a multicenter cross-sectional mixed-methods study analyzing deidentified transcripts of audio-recorded clinician-family meetings for patients with neurologic illness requiring intensive care (e.g., intracerebral hemorrhage, traumatic brain injury, severe stroke) from 7 US centers. Two coders assigned codes for prognostic language type and domain of prognosis to each clinician prognostic statement. Prognostic language was coded as probabilistic (estimating the likelihood of an outcome occurring, e.g., “80% survival”; “She'll probably survive”) or nonprobabilistic (characterizing outcomes without offering likelihood; e.g., “She may not survive”). We applied univariate and multivariate binomial logistic regression to examine independent associations between prognostic language and domain of prognosis.
Results
We analyzed 43 clinician-family meetings for 39 patients with 78 surrogates and 27 clinicians. Clinicians made 512 statements about survival (median 0/meeting [interquartile range (IQR) 0–2]), physical function (median 2 [IQR 0–7]), cognition (median 2 [IQR 0–6]), and overall recovery (median 2 [IQR 1–4]). Most statements were nonprobabilistic (316/512 [62%]); 10 of 512 prognostic statements (2%) offered numeric estimates; and 21% (9/43) of family meetings only contained nonprobabilistic language. Compared with statements about cognition, statements about survival (odds ratio [OR] 2.50, 95% CI 1.01–6.18, p = 0.048) and physical function (OR 3.22, 95% 1.77–5.86, p < 0.001) were more frequently probabilistic. Statements about physical function were less likely to be uncertainty-based than statements about cognition (OR 0.34, 95% CI 0.17–0.66, p = 0.002).
Discussion
Clinicians preferred not to use estimates (either numeric or qualitative) when discussing critical neurologic illness prognosis, especially when they discussed cognitive outcomes. These findings may inform interventions to improve prognostic communication in critical neurologic illness.
One of the most challenging tasks that clinicians face when caring for patients with critical neurologic illnesses, such as severe stroke, traumatic brain injury (TBI), or other diagnoses, is to discuss prognosis with patients' families. These patients are usually unable to communicate their wishes and families must act as surrogate decision-makers, relying on clinicians' explanation of prognosis to inform decisions around continuation or withdrawal of life-sustaining therapy (WLST).1 WLST is a major cause of death in critical neurologic illness and varies widely by center.2-6 Patient and family characteristics account for some of this variability,7-9 but clinician factors, including communication practices, may also play a role.9-11
Clinicians face several distinct challenges when communicating prognosis in critical neurologic illness. First, there are multiple domains of prognosis, including not just survival but also level of functional independence, motor recovery, and various aspects of cognitive recovery (e.g., memory, personality). Prognosis in each of these domains may inform decisions about WLST.12-14 In addition, prognosis is typically uncertain in the early phase of illness and becomes clearer slowly, over weeks to years.15 These factors may contribute to high rates of family-clinician prognostic discordance in these conditions.16
While there are no evidence-based guidelines around prognostic communication in critical neurologic illness, experts generally recommend that clinicians provide a risk estimate of the most likely outcome (using either numeric estimates or non-numeric estimates like “likely/unlikely”), provide a range of possible outcomes (e.g., best-case, worst-case), and acknowledge prognostic uncertainty.17-19 Our group previously analyzed the general sequence in which clinicians in this cohort addressed each component of a family meeting—for example, delivery of prognosis, assessment of values and preferences, and treatment recommendations—and found variability in the general approach to when and how these components were incorporated into goals of care communication.20 However, it is not yet known what actual words clinicians use to describe prognosis to families of these patients, including whether real-world clinicians use recommended prognostic communication strategies such as best-case/worst-case scenarios or acknowledging prognostic uncertainty. This gap in knowledge is a critical barrier to developing interventions and evidence-based communication guidelines in this patient population.
The primary objective of this study was to characterize the language clinicians use to describe prognosis to families of patients with critical neurologic illness.10 As a secondary objective, we explored how prognostic language differed among different domains of recovery.
Methods
Study Design
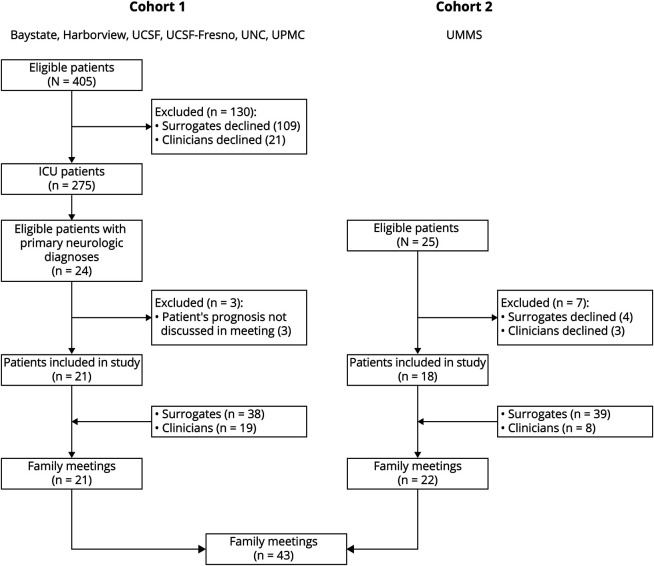
We conducted a multicenter cross-sectional mixed-methods study analyzing deidentified transcripts of audio-recorded clinician-family meetings for patients with critical neurologic illness, pooling transcripts from 2 cohorts (Figure 1). To focus our analysis on prognostic language, we excluded any family meetings wherein prognosis was not discussed (e.g., brief “update” conversations about the current medical plan). Cohort 1 was derived from a previous multicenter study in surrogate decision-makers for patients with acute lung injury admitted to 13 medical and medical-surgical intensive care units (ICUs) at 6 academic medical centers between 2009 and 2012.21 For this study about critical neurologic illness, we restricted inclusion to patients with primary neurologic diagnoses. Cohort 2 was derived from an ongoing single-center study of patients with critical neurologic illness20 in a neuro-ICU at an academic level 1 trauma and comprehensive stroke center in 2019. We combined these 2 cohorts to address selection bias that might have resulted from restricting our cohort to the contemporary recordings from a single neuro-ICU and to demonstrate feasibility of audio-recording sensitive clinician-family meetings at multiple centers.
Figure 1. Flow Diagram Describing Patient Enrollment.
Cohort 1 included a subset of patients with critical neurologic illness from a parent multicenter study at 6 academic centers on clinician-family communication. Cohort 2 included patients from a neuro-trauma ICU at a single academic center. Baystate = University of Massachusetts Chan Medical School—Baystate; Harborview = Harborview Medical Center; ICU = intensive care unit; UCSF = University of California San Francisco; UCSF-Fresno = University of California San Francisco-Fresno; UMMS = University of Massachusetts Chan Medical School; UNC = University of North Carolina Medical Center; UPMC = University of Pittsburgh Medical Center.
Participants
A full description of methods for data collection for cohort 1 has been published previously.21 Briefly, to be eligible, surrogates were included for patients who were18 years or older, who lacked decision-making capacity, who met diagnostic criteria for acute lung injury along with respiratory failure requiring mechanical ventilation, and who had a score of ≥25 on the Acute Physiology Assessment and Chronic Health Evaluation II, predicting 50% chance of long-term severe functional impairment. Exclusion criteria were (1) lack of an English-speaking surrogate decision-maker who was older than 18 years and was able to complete a written questionnaire and (2) being on a waiting list for organ transplantation. Surrogate and clinician participants received financial compensation for their time ($10–20). For our current analysis, we only included patients with a primary neurologic diagnosis. For cohort 2, surrogates were eligible to be included if they were 18 years or older and provided surrogate decision making to an adult critically ill patient with a neurologic diagnosis. Surrogates were excluded if they were non–English-speaking. Participants received no financial compensation.
The audio-recorded clinician-family meetings from both cohorts were professionally transcribed and deidentified before analysis. Surrogates and the clinicians conducting each meeting completed questionnaires collecting demographic characteristics while study personnel abstracted patients' demographic and clinical data from medical records.
Standard Protocol Approvals, Registrations, and Patient Consents
Research staff obtained written or verbal informed consent from all surrogates and health care professionals, as stipulated by the local institutional review boards (IRBs). The IRBs at the University of Massachusetts Chan Medical School (#H00016916) and University of Pittsburgh (#PRO09050285) approved the studies.
Qualitative Analysis
Two coders reviewed all transcripts and marked all prognostic statements made by clinicians, defined as any single characterization of a possible future outcome. Next, the 2 coders independently coded 5 transcripts (12%) and, through consensus, developed an initial codebook assigning each prognostic statement a pair of codes: (1) the domain of prognosis discussed and (2) the type of prognostic language used. The domain-of-prognosis codes were developed inductively through iterative review of data by the 2 coders. Because surrogate decision-makers in critical neurologic illness have described a preference for hearing concrete estimates of risk,22 the coders applied prognostic language codes deductively using a previously published framework that categorized prognostic statements as probabilistic (estimating the likelihood of a future outcome occurring; e.g., “30% of people go home”; or “she'll unlikely be able to eat without a feeding tube”) or nonprobabilistic (characterizing future outcomes without estimating their likelihood; e.g., “this type of stroke is pretty devastating”; “age is not on his side” or “he might have memory gaps”).10 Applying this framework allowed for analysis of a broad spectrum of prognostic statements, including numeric and non-numeric risk estimates (the traditional focus of communications research) as well as less studied prognostic language that conveys a “gist” of prognosis without estimating risk.23 The 2 reviewers then inductively identified subcodes (types of probabilistic statements and types of nonprobabilistic statements) and refined this codebook through iterative review of data and sharing of emerging thematic content between the coders and a third investigator. The coders applied the final codebook to 9 transcripts (21%) in parallel and reached an inter-rater reliability κ of 0.86, with κ > 0.8 indicating excellent inter-rater reliability.24 The 2 coders then reviewed each remaining discrepancy in coding and added further clarification to the codebook. One investigator then coded the remaining transcripts. This qualitative analysis approach was designed to facilitate triangulation and increase trustworthiness of the data.25 Family meetings from cohort 2 were analyzed until data saturation was reached, specifically when consistent relationships between the domain of prognosis and the type of prognostic language had emerged.26,27
In addition, we performed 2 post hoc analyses. During qualitative analysis, it became evident that the most common statements were those about prognostic uncertainty (a nonprobabilistic subcode). As a result, we chose to perform a post hoc analysis of how language about uncertainty differed when clinicians described different domains of prognosis. Finally, we also performed a post hoc analysis to explore surrogate responses to each prognostic statement by clinicians. To achieve this, we applied an existing framework for surrogates' responses that immediately followed each prognostic statement by clinicians (email communication, Douglas B. White, MD, MAS, February 2023). Two coders independently applied this coding framework to 9 transcripts (21%) and achieved a κ of 0.94. One coder coded the remaining 34 transcripts.
Data were analyzed using NVivo qualitative data analysis software (version 12, 2018; QSR International Pty Ltd., Melbourne, Australia).
Quantitative Analysis
We compared the types of prognostic languages clinicians used (probabilistic vs nonprobabilistic and uncertainty-based vs not–uncertainty-based statements) according to the domain of prognosis they were describing. For each domain of prognosis, we calculated the proportion of each type of prognostic statement. We applied binomial logistic regression to model statement type as a function of domain of prognosis, separately for probabilistic and uncertainty-based statements. Pairwise differences between domain-of-prognosis types were expressed as odds ratios (ORs) and 95% CIs. To account for clustering, all models included random effects for study center and for meeting nested within a study center; a random provider effect was not included because provider largely overlapped with both meeting and center.28 Comparisons by domain of prognosis were made before and after adjustment for provider, patient, and surrogate characteristics.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator after signing a data use agreement.
Results
Participants
From cohort 1, 405 ICU patients were identified as eligible for the original study during the study period, for whom 109 surrogates and 21 clinicians declined to participate (enrollment rate 68%). Of 275 ICU patients enrolled, 24 had a primary neurologic diagnosis and were considered for inclusion in our study. Three patients were further excluded because the patient's prognosis was not discussed in the family meeting (n = 21 patients; 21 family meetings). From cohort 2, 25 eligible patients were identified during the study period, for whom surrogates for 4 patients and clinicians for 3 patients declined participation (enrollment rate 72%). Prognosis was discussed in all meetings from cohort 2 (n = 18 patients; 22 family meetings). In total, we analyzed 43 meetings for 39 patients with 78 surrogates and 27 clinicians (Figure 1). Twenty-one clinicians led a single family meeting while 6 clinicians led multiple family meetings (mean 1.6 meetings/clinician).
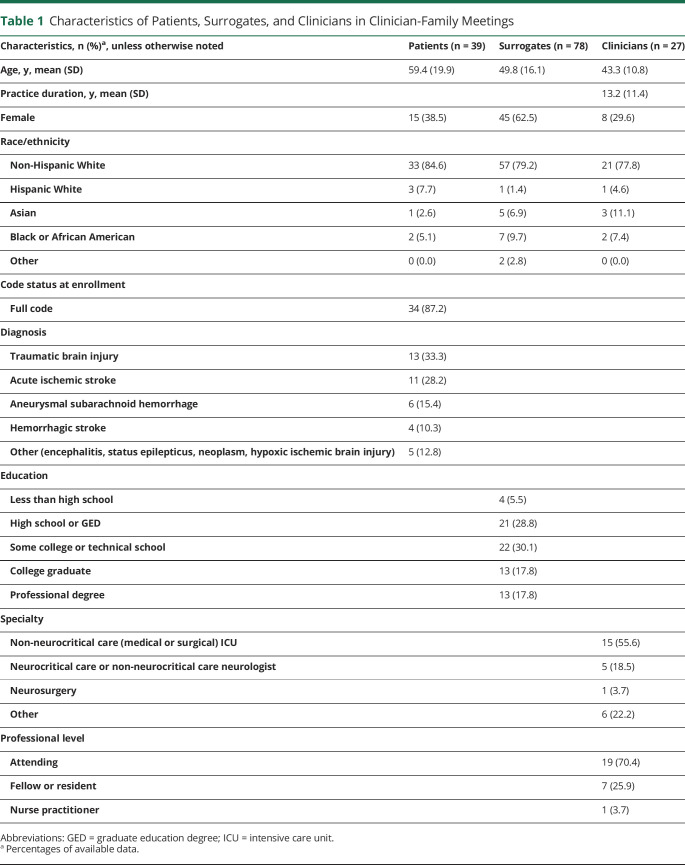
The characteristics of enrolled patients, surrogates, and clinicians are summarized in Table 1. Physicians were trained in internal medicine critical care (37%), critical care surgery (19%), or neurocritical care (15%). The most common neurologic diagnoses were TBI (33%), acute ischemic stroke (28%), and aneurysmal subarachnoid hemorrhage (15%). The average meeting length was 33 minutes (SD 19).
Table 1.
Characteristics of Patients, Surrogates, and Clinicians in Clinician-Family Meetings
Qualitative Analysis
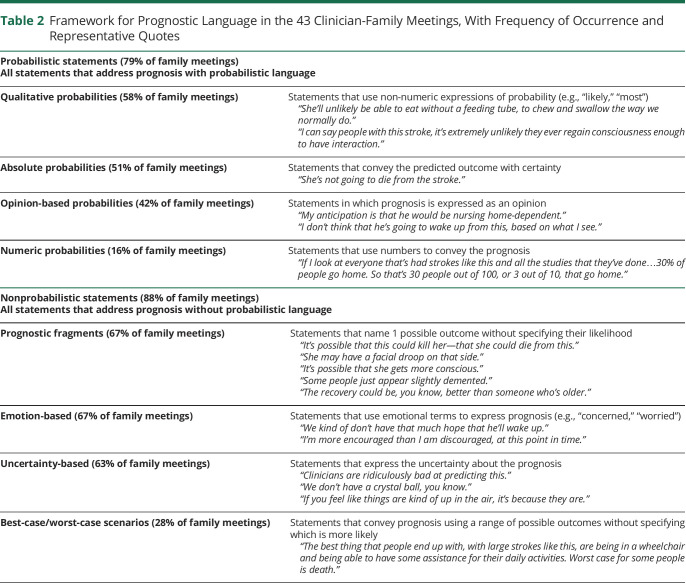
We identified 512 unique prognostic statements. One hundred ninety-six (38%) of all 512 statements were coded as probabilistic because they estimated the likelihood of a future outcome occurring using numbers or qualitative risk expressions. We identified 4 categories of these probabilistic statements: qualitative, absolute, opinion-based, and numeric (Table 2). In qualitative probabilistic statements (75/196 statements [38%]), clinicians used qualitative risk expressions (“likely,” “probably,” “majority”) to estimate the odds of a future outcome occurring. In absolute statements (72/196 statements [37%]), the clinician conveyed the predicted outcome with certainty (e.g., “She's not going to die from the stroke itself”). In opinion-based probabilistic statements (39/196 statements [20%]), the clinician offered a qualitative estimate of the most likely outcome but framed it in terms of their expert opinion: for example, “My expectation is he'll do very well.” In numeric statements (10/196 probabilistic statements [5%]), clinicians used numbers to estimate the odds of a future outcome occurring (e.g., “More than 90% of people with this size stroke at this age are nursing home-dependent”). In summary, the key feature of probabilistic statements was that they provided surrogates with a risk estimate—describing the outcome as likely/unlikely, offering a numeric estimate, or describing an outcome as certain.
Table 2.
Framework for Prognostic Language in the 43 Clinician-Family Meetings, With Frequency of Occurrence and Representative Quotes
The remaining 316 statements (62%) were nonprobabilistic: They characterized outcomes without discussing their likelihood. We identified 4 types of nonprobabilistic statements (Table 2): (1) uncertainty-based, (2) prognostic fragments, (3) emotion-based, and (4) best-case/worst-case scenarios. The most frequent nonprobabilistic statements were uncertainty-based (127/316 total statements [40%] of nonprobabilistic statements; 25% of all statements), which included blanket statements of uncertainty (“I can't tell you anything about personality, higher brain functions”), statements about limited prognostic data (“People don't study intellect that well”), statements that it was too early to prognosticate (“I can't make that call right now”), and statements that prognosis would become clearer as time progressed (“It's kind of a waiting game”). Prognostic fragment statements (100/316 (32%)) named possible outcomes that “might” or “could” occur but did not address their likelihood (e.g., “He could progress. He could also just stabilize and remain the way he is”). Emotion-based statements gave a sense of prognosis using emotional language (e.g., “We're worried…”) and occurred in 68 of 316 nonprobabilistic statements (22%). Finally, best-case/worst-case scenario statements (21/316 [7%]) offered a range of possible outcomes without discussing their likelihood: for example, “Best-case scenario would be that he is able to walk with a walker and able to do some of his activities but needs some assistance…worst case is that he stays in this particular state as he is now for the rest of his life.” None of these best-case/worst-case scenario statements were coupled with a statement about which outcome was most likely. In summary, the key quality of nonprobabilistic statements was that they characterized prognosis in some manner but did not offer a qualitative or quantitative risk estimate.
Probabilistic statements occurred at least once in 79% (34/43) of family meetings while nonprobabilistic statements appeared at least once in 88% (38/43) of meetings. Considering each type of probabilistic statement, qualitative probabilistic statements appeared in 58% of meetings, absolute statements in 51%, opinion-based probabilistic statements in 42%, and numeric statements in 16%. Considering each type of nonprobabilistic statement, emotion-based statements appeared in 67% of meetings, prognostic fragments in 67%, best-case/worst-case statements in 28%, and uncertainty-based statements in 63%. In total, 72% (31/43) of meetings contained both probabilistic and nonprobabilistic statements.
We identified 4 different domains of prognosis (Table 3): (1) survival; (2) physical function (e.g., speech, motor recovery, nursing care requirements); (3) cognition (e.g., behavior, mood, language, identity); and (4) “overall prognosis,” in which the clinician referred to the patient's general prognosis without focusing on any domain of recovery. Survival was discussed in 14 of 43 clinician-family meetings (33%) while physical functional prognosis, cognitive prognosis, and overall prognosis were discussed each in two-thirds or more of the meetings (31 [72%], 30 [70%], and 35 [81%], respectively). Prognosis for survival was discussed a median of 0 times per meeting (interquartile range [IQR] 0–2), prognosis for physical function a median of 2 times per meeting (IQR 0–7), prognosis for cognition a median of 2 times per meeting (IQR 0–6), and overall prognosis a median of 2 times per meeting (IQR 1–4).
Table 3.
Framework for the Domains of Prognosis Discussed in the 43 Clinician-Family Meetings With Frequency of Occurrence and Representative Quotes
Quantitative Analyses
Frequencies of Prognostic Language by Domain of Prognosis
A total of 388 prognostic statements addressed a specific domain of prognosis (survival, cognition, or physical function), as opposed to statements about the overall prognosis. Only these 388 statements were included in our analysis of the type of prognostic language clinicians used to describe each domain of prognosis. Thirty-nine of 43 meetings (91%) contained clinician prognostic language about at least 1 specific domain of prognosis; 27 of 43 meetings (63%) contained statements about at least 2 domains of prognosis; and 9 of 43 meetings (21%) contained statements about all 3 domains.
Association Between Use of Probabilistic Statements and Domain of Prognosis Discussed
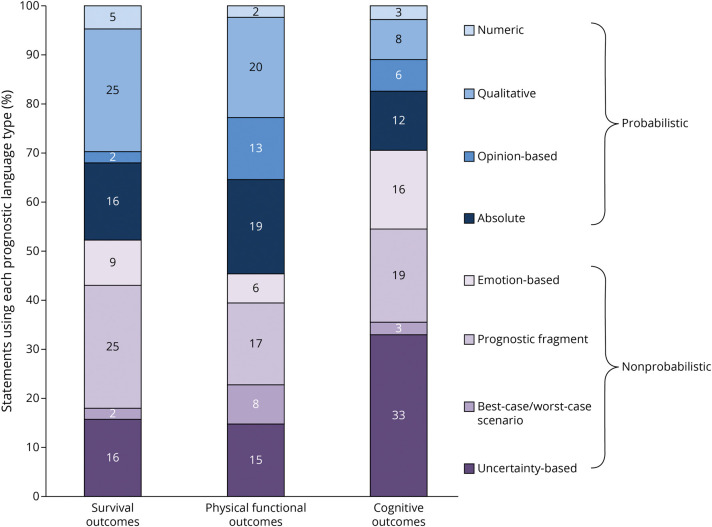
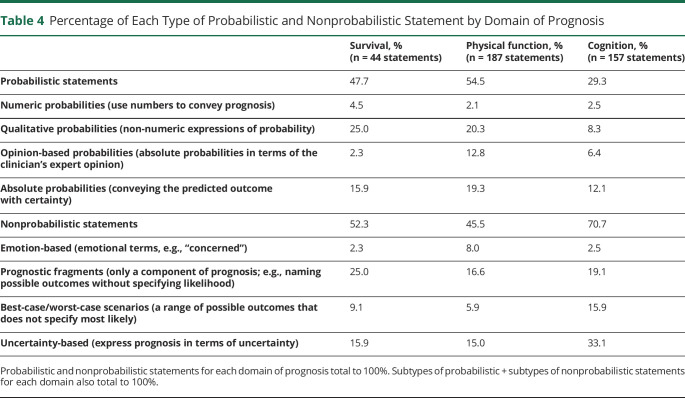
Figure 2 shows the frequency of each type of statement according to the domain of prognosis discussed; 48% of statements about survival and 55% of statements about physical function were probabilistic while 29% of statements about cognition were probabilistic. After adjusting for meeting length, patient age, patient disease, clinician specialty, clinician level of training, clinician years in practice, and maximum family educational level, domain of prognosis remained statistically significantly associated with probabilistic statements compared with nonprobabilistic statements (Table 4, p < 0.001). The independent association of domain of prognosis with clinician, patient, and surrogate characteristics is available in eTable 1 (links.lww.com/WNL/C879). Compared with statements about cognition, statements about survival (OR 2.50, 95% CI 1.01–6.18, p = 0.048) and physical function (OR 3.22, 95% 1.77–5.86, p < 0.001) were more likely to be probabilistic. There was no significant difference in the proportion of probabilistic statements about survival compared with physical function (OR 0.77, 95% CI 0.33–1.83, p = 0.56). In addition, adjusting for cohort (i.e., cohort 1, the earlier recordings, vs cohort 2, the later recordings) did not change the magnitude, direction, or significance level of this analysis.
Figure 2. Breakdown of Probabilistic and Nonprobabilistic Statements by Domain of Prognosis.
In the multivariable analysis, compared with statements describing cognition, statements about survival (OR 2.50, 95% CI 1.01–6.18, p = 0.048) and physical function (OR 3.22, 95% 1.77–5.86, p < 0.001) were more likely to be probabilistic. Statements concerning physical function were less likely to be uncertainty-based than statements about cognition (OR 0.34, 95% CI 0.17–0.66, p = 0.002). There was no significant difference in the proportion of probabilistic statements about survival compared with physical function or the proportion of uncertainty statements about survival compared with cognition or physical function. OR = odds ratio.
Table 4.
Percentage of Each Type of Probabilistic and Nonprobabilistic Statement by Domain of Prognosis
Post Hoc Analysis: Association Between Use of Uncertainty-Based Statements and Domain of Prognosis Discussed
Next, because of the high frequency of statements about prognostic uncertainty, we compared the frequency of uncertainty-based statements (the most frequent subtype of nonprobabilistic statement) across the 3 domains of prognosis. Table 4 summarizes the associations of domain of prognosis with uncertainty-based statements; 33% of statements about cognition were uncertainty-based, compared with 16% of statements about survival and 15% of statements about physical function (p < 0.01). After adjustment for the same covariates as for the multivariable analysis for probabilistic statements (eTable 1, links.lww.com/WNL/C879), except omitting adjustment for provider specialty because of a 0 cell count and its strong association with patient disease, domain of prognosis remained statistically significantly associated with uncertainty-based statements (p = 0.005) (Table 4). Statements about physical function were less likely to be uncertainty-based than statements about cognition (OR 0.34, 95% CI 0.17–0.66, p = 0.002). There was no significant difference in the proportion of uncertainty-based statements about survival compared with cognition (OR 0.34, 95% CI 0.11–1.07, p = 0.07) or compared with physical function (OR 1.01, 95% CI 0.31–3.29, p = 0.98). An additional adjustment for cohort did not have any effect on these results.
Post Hoc Analysis: Surrogate Responses to Clinician Prognostic Statements
Finally, we explored surrogates' responses to both probabilistic and nonprobabilistic prognostic statements by clinicians (eTable 2, links.lww.com/WNL/C880). The most frequent responses by surrogates to both probabilistic and nonprobabilistic clinician prognostic statements were conversation continuers (e.g., “yeah” or “okay”; response to 28% and 34% of statements, respectively), asking a clarifying question about prognosis (response to 27% and 24% of statements, respectively), and proving understanding of prognosis (e.g., restating prognosis in surrogate's own words; response to 14% and 11% of statements, respectively). Other types of responses, such as expressing agreement or disagreement with prognosis or acknowledging or claiming understanding the prognosis (e.g., “I know”; “I understand”), were infrequent for both probabilistic and nonprobabilistic statements (≤8%). Emotional expressions in response to prognostic statements (e.g., crying) were also infrequent (≤3%).
Discussion
In this multicenter mixed-methods study, we found that clinicians used nonprobabilistic language more often than probabilistic language to communicate prognosis to families of patients with critical neurologic illness. In one-fifth of family meetings where prognosis was discussed, nonprobabilistic language was used exclusively. This is contrary to expert recommendations that clinicians avoid overly vague communication and offer some estimate of the patient's most likely outcome.17,29 We also found that clinicians used best-case/worst-case statements, one currently recommended strategy for bracketing the range of possible outcomes,30 in fewer than one-third of family meetings and that none of these ranges were coupled with a statement of the most likely outcome. Explicit acknowledgment of prognostic uncertainty, another recommended communication strategy,17,31 occurred in only two-thirds of the meetings. Clinicians also used variable language to communicate prognosis depending on the domain of prognosis they were describing, favoring nonprobabilistic language, particularly when describing cognitive outcomes as compared with physical function or survival.
Research on prognostic communication in the ICU is essential to help improve clinician communication practices, enhance family understanding of prognosis, and further goal-concordant care. Such research has typically focused on surrogate understanding of prognostic communication strategies using numeric or qualitative estimates of risk.32-34 In our sample, numeric statements occurred in 16% of family meetings and qualitative probabilistic statements in 58% of meetings; these numbers are similar to a previous analysis of family meetings in the general ICU population, which identified numeric prognostic statements in 20% and qualitative probabilistic statements in 72% of family meetings.10 By contrast, in our cohort, clinicians used nonprobabilistic language in 88% of family meetings, compared with 40% of family meetings for the general non-neurologic ICU population.10 Nonprobabilistic language about prognosis is less precise than probabilistic risk estimates and is inconsistent with what many surrogates for patients with critical neurologic illness wish to hear from clinicians.22,31 This type of language has not been rigorously studied in the ICU setting, and its effect on surrogate decision making for these patients is poorly understood.35 Clinicians' tendency to use nonprobabilistic language for patients with critical neurologic illness, and their less frequent use of numeric and qualitative estimates than in general ICU population, may relate to the complexity and uncertainty and, hence, difficulty of prognosticating in the acute phase of critical neurologic illness.36,37 Numerical prognostic statements produce special challenges; while in one small study, families of neurocritically ill patients described preferring to hear numeric prognostic estimates,22 numeric estimates may be misinterpreted by patients and families.23,38 Clinicians might also be wary that data would convey false certainty about prognosis that could lead to self-fulfilling prophecies and premature WLST.39 Yet neurointensivists have been shown to be fairly accurate in estimating the most likely outcomes in intracerebral hemorrhage40; even where reliable population-level prognostic data are not available, experienced physicians may be able to estimate a “most likely” (qualitative probabilistic) scenario to deliver to families. Delivering unnecessarily vague or incomplete descriptions of prognosis may hinder goal-concordant care, may cause surrogates distress,31 and could potentially lead to overtreatment or premature WLST. Our post hoc qualitative analysis of surrogates' responses to clinicians' prognostic statements found that follow-up questions about prognosis were common after both probabilistic and nonprobabilistic statements, but that surrogates rarely directly challenged the prognosis, acknowledged it intellectually, or showed emotion in the face of it. This analysis did not reveal substantive differences between probabilistic and nonprobabilistic language; however, it is not possible to know whether these surrogate behaviors were a direct response to the preceding statement or a delayed response to something that occurred earlier in the meeting. In addition, surrogate responses may not be accurate indicators of important variables such as surrogate understanding of prognosis, satisfaction with prognostic communication, or preparedness for decision making. Given the high prevalence of nonprobabilistic language in the family meetings in our sample, it will be important for future studies to examine why and when clinicians use this language and to directly examine how surrogates for patients with critical neurologic illness interpret nonprobabilistic language and whether surrogates find this language useful in making medical decisions.
In addition, we found that clinicians frequently discussed cognitive prognosis with families. However, they used probabilistic language less when discussing cognition than when discussing prognosis for survival or physical function. Knowledge and beliefs about cognitive prognosis are important to families of patients with critical neurologic illness.41 Poor cognitive prognoses may persuade patients and surrogates to decline life-sustaining therapies.12,42 Yet, despite its importance to patients and families, there are relatively little data on cognitive prognosis in critical neurologic illness.43-45 In stroke, for example, cognitive impairment is mentioned in only a small number of guidelines available for stroke care, and only a small minority of randomized controlled stroke trials have assessed cognitive and mood outcomes.45,46 It is possible that clinicians' tendency to use nonprobabilistic and uncertainty-based language to describe cognitive prognosis reflects this paucity of prognostic data. Our findings underscore the need for more research into predictors of poor cognitive outcomes in survivors of critical neurologic illness.
Prognostic uncertainty is a major challenge to precise prognostic communication in critical neurologic illness. The optimal way to support surrogate decision making in the face of prognostic uncertainty is not known, and the absence of evidence-based strategies for prognostic communication precludes a thorough discussion about the quality of clinician communication in our sample. One qualitative study in critical neurologic illness about surrogates' preferences for prognostication found that surrogates specifically desired to hear numeric prognostic estimates.22 Expert guidelines do not clearly recommend communicating with numerical estimates, but do recommend that clinicians attempt to deliver some estimate of future outcomes (e.g., a probabilistic statement),17,47 for example, by bracketing the range of possible outcomes by describing not just best-case and worst-case scenarios but also most likely scenarios. In our study, clinicians rarely used numbers to discuss prognosis, but also never used best-case/worst-case/most likely statements; they used best-case/worst-case scenarios (without most likely scenarios) in fewer than one-third of family meetings. More research will be needed to identify which communication strategies best facilitate patient-centered care in critical neurologic illness.48
Our study has important strengths and limitations. Among its strengths is that we included patients from multiple centers and with multiple neurologic diagnoses, thereby ameliorating potential bias that stems from single-center research. We recognize that cohort 2 stems from a single neuro-ICU and may, therefore, be over-represented, potentially contributing to selection bias. We attempted to mitigate this bias as best as possible in our quantitative analysis through adjustment for center and cohort. Our qualitative approach was rigorous and systematic and was based on a previously developed coding framework.10 Limitations include that we were unable to adjust for ICU structure, rounding practices, duration between date of admission and date of family meeting, or patient length of stay. Many of our study authors, including the senior author and site principal investigators, are board-certified critical care physicians or neurologists, which may have introduced personal or institutional subjectivity into the analysis. In addition, most clinicians and surrogates in our sample were non-Hispanic White, perhaps, in part, because of the exclusion of participants who did not speak English; hence, our findings may not be generalizable to other racial or ethnic groups. We also identified relatively fewer prognostic statements about survival than statements about overall prognosis, physical function, or cognition, which may suggest fairly high rates of survival with disability in our cohort and may limit the generalizability of our study. While we included patients from multiple centers with many diagnoses, our study may not represent the full diversity of clinical scenarios in critical neurologic illness, and there may be relationships between clinical scenario and clinician communication practices that are unaccounted for in our analysis. Although participants were not aware of the aims of the study, knowledge that conferences were being audiotaped may have influenced clinicians' behaviors, leading them to display their “best” rather than their “usual” behaviors (Hawthorne effect).49 Recordings occurred over a period of a decade, raising the concern that clinician-family communication may have evolved in the interim. However, we found that adjusting for cohort did not change our results. In addition, while guidance for prognostic communication with families has evolved over the past 50 years,50 our review of the limited literature characterizing prognostic communication did not discover evidence that it has changed significantly over the past decade.
Effective communication about prognosis is an important responsibility for clinicians caring for patients with critical neurologic illness. This study reveals wide variation in how clinicians communicate about prognosis to these patients' families and shows that few clinicians offered numeric estimates of risk. Our findings offer a framework for future research into prognostic communication strategies in critical neurologic illness. Future studies should (1) validate our findings in a multicenter cohort with a more diverse patient population, (2) examine the effect of recommended communication strategies (e.g., best-case/worst-case/most likely) on surrogate-clinician prognostic concordance in critical neurologic illness and satisfaction with communication, and (3) test interventions aimed at improving prognostic communication for this population.
Glossary
- ICU
intensive care unit
- IQR
interquartile range
- IRB
institutional review board
- OR
odds ratio
- TBI
traumatic brain injury
- WLST
withdrawal of life-sustaining therapy
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
C. Ge was funded by the American Academy of Neurology Medical Student Research Scholarship (2020). D.B. White was funded by NIH-NHLBI: K24 HL148314. S. Muehlschlegel's research time was funded by NIH/NICHD 5K23HD080971 and NIH/NINR R21NR020231. C.L. Hough was supported by K24HL141526. This project was additionally supported by the University of Massachusetts Chan Medical School Center for Clinical and Translational Science, which is funded by the NIH Clinical and Translational Science Award to the University of Massachusetts Chan Medical School (UL1TR000161). Collection of data occurred under the parent ECALI R01, NIH-NHLBI 5R01HL094553.
Disclosure
A.L. Goss reports no disclosures relevant to the manuscript. C. Ge was funded by the American Academy of Neurology Medical Student Research Scholarship (2020). S. Crawford, K. Goostrey, and P. Buddadhumaruk report no disclosures relevant to the manuscript. C.L. Hough is supported by K24HL141526 and the American Lung Association through payments to her institution. She receives consulting fees from Quantum Leap and the NIH. She receives support for attending Critical Care Reviews meetings. She participates on advisory boards for the NIH, Quantum Leap, and ANSICS. B. Lo receives royalties from Wolters Kluwer for his book, Resolving Ethical Dilemmas. S. Carson reports no disclosures relevant to the manuscript. J. Steingrub reports no disclosures relevant to the manuscript. D.B. White was funded NIH-NHLBI: K24HL148314. Collection of data occurred under the parent ECALI R01, NIH-NHLBI 5R01HL094553. S. Muehlschlegel's research time was funded by NIH/NICHD K23HD080971 and NIH/NINR R21NR020231. Go to Neurology.org/N for full disclosures.
References
- 1.Cai X, Robinson J, Muehlschlegel S, et al. Patient preferences and surrogate decision making in neuroscience intensive care units. Neurocrit Care. 2015;23(1):131-141. doi: 10.1007/s12028-015-0149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56(6):766-772. doi: 10.1212/wnl.56.6.766 [DOI] [PubMed] [Google Scholar]
- 3.Turgeon AF, Lauzier F, Simard J-F, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581-1588. doi: 10.1503/cmaj.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zurasky JA, Aiyagari V, Zazulia AR, Shackelford A, Diringer MN. Early mortality following spontaneous intracerebral hemorrhage. Neurology. 2005;64(4):725-727. doi: 10.1212/01.wnl.0000152045.56837.58 [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Cox M, Lytle B, et al. Early transition to comfort measures only in acute stroke patients. Neurol Clin Pract. 2017;7(3):194-204. doi: 10.1212/cpj.0000000000000358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68(20):1651-1657. doi: 10.1212/01.wnl.0000261906.93238.72 [DOI] [PubMed] [Google Scholar]
- 7.Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson T, Ryser MD, Ubel PA, et al. Withdrawal of life-supporting treatment in severe traumatic brain injury. JAMA Surg. 2020;155(8):723-731. doi: 10.1001/jamasurg.2020.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg A, Soto AL, Knies AK, et al. Predictors of surrogate decision makers selecting life-sustaining therapy for severe acute brain injury patients: an analysis of US population survey data. Neurocrit Care. 2021;35(2):468-479. doi: 10.1007/s12028-021-01200-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. The language of prognostication in intensive care units. Med Decis Making. 2010;30(1):76-83. doi: 10.1177/0272989x08317012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med. 2007;35(2):442-448. doi: 10.1097/01.ccm.0000254723.28270.14 [DOI] [PubMed] [Google Scholar]
- 12.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066. doi: 10.1056/nejmsa012528 [DOI] [PubMed] [Google Scholar]
- 13.Steinberg A, Abella BS, Gilmore EJ, et al. Frequency of withdrawal of life-sustaining therapy for perceived poor neurologic prognosis. Crit Care Explor. 2021;3(7):e0487. doi: 10.1097/cce.0000000000000487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratt AK, Chang JJ, Sederstrom NO. A fate worse than death: prognostication of devastating brain injury. Crit Care Med. 2019;47(4):591-598. doi: 10.1097/ccm.0000000000003647 [DOI] [PubMed] [Google Scholar]
- 15.Creutzfeldt CJ, Longstreth WT, Holloway RG. Predicting decline and survival in severe acute brain injury: the fourth trajectory. BMJ. 2015;351:h3904. doi: 10.1136/bmj.h3904 [DOI] [PubMed] [Google Scholar]
- 16.Kiker WA, Rutz Voumard R, Andrews LIB, et al. Assessment of discordance between physicians and family members regarding prognosis in patients with severe acute brain injury. JAMA Netw Open. 2021;4(10):e2128991. doi: 10.1001/jamanetworkopen.2021.28991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpkin AL, Armstrong KA. Communicating uncertainty: a narrative review and framework for future research. J Gen Intern Med. 2019;34(11):2586-2591. doi: 10.1007/s11606-019-04860-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittenberg E, Ferrell BR, Smith T, Ragan SL, Handzo G. Textbook of Palliative Care Communication. Oxford University Press; 2015. [Google Scholar]
- 19.Brizzi K, Creutzfeldt CJ. Neuropalliative care: a practical guide for the neurologist. Semin Neurol. 2018;38(05):569-575. doi: 10.1055/s-0038-1668074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge C, Goss AL, Crawford S, et al. Variability of prognostic communication in critically ill neurologic patients: a pilot multicenter mixed-methods study. Crit Care Explor. 2022;4(2):e0640. doi: 10.1097/cce.0000000000000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernecoff NC, Curlin FA, Buddadhumaruk P, White DB. Health care professionals' responses to religious or spiritual statements by surrogate decision makers during goals-of-care discussions. JAMA Intern Med. 2015;175(10):1662. doi: 10.1001/jamainternmed.2015.4124 [DOI] [PubMed] [Google Scholar]
- 22.Quinn T, Moskowitz J, Khan MW, et al. What families need and physicians deliver: contrasting communication preferences between surrogate decision-makers and physicians during outcome prognostication in critically ill TBI patients. Neurocrit Care. 2017;27(2):154-162. doi: 10.1007/s12028-017-0427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zier LS, Sottile PD, Hong SY, Weissfield LA, White DB. Surrogate decision makers' interpretation of prognostic information: a mixed-methods study. Ann Intern Med. 2012;156(5):360-366. doi: 10.7326/0003-4819-156-5-201203060-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276-282. doi: 10.11613/bm.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolley EE, Ulin PR, Mack N, Robinson ET, Succop SM. Qualitative Methods in Public Health: A Field Guide for Applied Research. John Wiley & Sons; 2016. [Google Scholar]
- 26.Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59-82. doi: 10.1177/1525822x05279903 [DOI] [Google Scholar]
- 27.O'Reilly M, Parker N. Unsatisfactory Saturation: a critical exploration of the notion of saturated sample sizes in qualitative research. Qual Res. 2012;13(2):190-197. doi: 10.1177/1468794112446106 [DOI] [Google Scholar]
- 28.Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. 2nd ed. Springer; 2010. [Google Scholar]
- 29.Kruser JM, Nabozny MJ, Steffens NM, et al. “Best case/worst case”: qualitative evaluation of a novel communication tool for difficult in-the-moment surgical decisions. J Am Geriatr Soc. 2015;63(9):1805-1811. doi: 10.1111/jgs.13615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson MT, Holloway RG. Palliative care in neurology. Mayo Clin Proc. 2017;92(10):1592-1601. doi: 10.1016/j.mayocp.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 31.Evans LR, Boyd EA, Malvar G, et al. Surrogate decision-makers' perspectives on discussing prognosis in the face of uncertainty. Am J Respir Crit Care Med. 2009;179(1):48-53. doi: 10.1164/rccm.200806-969oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. Chest. 2008;134(4):835-843. doi: 10.1378/chest.08-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman AR, Litton E, Chamberlain J, Ho KM. The effect of prognostic data presentation format on perceived risk among surrogate decision makers of critically ill patients: a randomized comparative trial. J Crit Care. 2015;30(2):231-235. doi: 10.1016/j.jcrc.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 34.Anderson WG, Cimino JW, Ernecoff NC, et al. A multicenter study of key stakeholders' perspectives on communicating with surrogates about prognosis in intensive care units. Ann Am Thorac Soc. 2015;12(2):142-152. doi: 10.1513/annalsats.201407-325oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazaridis C. Withdrawal of life-sustaining treatments in perceived devastating brain injury: the key role of uncertainty. Neurocrit Care. 2019;30(1):33-41. doi: 10.1007/s12028-018-0595-8 [DOI] [PubMed] [Google Scholar]
- 36.Finley Caulfield A, Gabler L, Lansberg MG, et al. Outcome prediction in mechanically ventilated neurologic patients by junior neurointensivists. Neurology. 2010;74(14):1096-1101. doi: 10.1212/wnl.0b013e3181d8197f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holloway RG, Gramling R, Kelly AG. Estimating and communicating prognosis in advanced neurologic disease. Neurology. 2013;80(8):764-772. doi: 10.1212/wnl.0b013e318282509c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holloway RG, Benesch CG, Burgin WS, Zentner JB. Prognosis and decision making in severe stroke. JAMA. 2005;294(6):725-733. doi: 10.1001/jama.294.6.725 [DOI] [PubMed] [Google Scholar]
- 39.Hemphill JC, White DB. Clinical nihilism in neuroemergencies. Emerg Med Clin North Am. 2009;27(1):27-37. doi: 10.1016/j.emc.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang DY, Dell CA, Sparks MJ, et al. Clinician judgment vs formal scales for predicting intracerebral hemorrhage outcomes. Neurology. 2016;86(2):126-133. doi: 10.1212/wnl.0000000000002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schutz REC, Coats HL, Engelberg RA, Curtis JR, Creutzfeldt CJ. Is there hope? Is she there? How families and clinicians experience severe acute brain injury. J Palliat Med. 2017;20(2):170-176. doi: 10.1089/jpm.2016.0286 [DOI] [PubMed] [Google Scholar]
- 42.Zahuranec DB, Anspach RR, Roney ME, et al. Surrogate decision makers' perspectives on family members' prognosis after intracerebral hemorrhage. J Palliat Med. 2018;21(7):956-962. doi: 10.1089/jpm.2017.0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salinas J, Sprinkhuizen SM, Ackerson T, et al. An international standard set of patient-centered outcome measures after stroke. Stroke. 2016;47(1):180-186. doi: 10.1161/strokeaha.115.010898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor A, Lanctôt KL, Bayley M, et al. “Good outcome” isn't good enough. Stroke. 2017;48(6):1688-1690. doi: 10.1161/strokeaha.117.016728 [DOI] [PubMed] [Google Scholar]
- 45.Lees R, Fearon P, Harrison JK, Broomfield NM, Quinn TJ. Cognitive and mood assessment in stroke research: focused review of contemporary studies. Stroke. 2012;43(6):1678-1680. doi: 10.1161/strokeaha.112.653303 [DOI] [PubMed] [Google Scholar]
- 46.Quinn TJ, Richard E, Teuschl Y, et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J. 2021;6(3):I-XXXVIII. doi: 10.1177/23969873211042192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creutzfeldt CJ, Holloway RG. Treatment decisions after severe stroke: uncertainty and biases. Stroke. 2012;43(12):3405-3408. doi: 10.1161/strokeaha.112.673376 [DOI] [PubMed] [Google Scholar]
- 48.Creutzfeldt CJ, Holloway RG, Curtis JR. Palliative care: a core competency for stroke neurologists. Stroke. 2015;46(9):2714-2719. doi: 10.1161/strokeaha.115.008224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267-277. doi: 10.1016/j.jclinepi.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerlin MP, Costa DK, Kahn JM. The society of critical care medicine at 50 years: ICU organization and management. Crit Care Med. 2021;49(3):391-405. doi: 10.1097/ccm.0000000000004830 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator after signing a data use agreement.