Abstract
Objectives
Although postoperative radiotherapy (PORT) could reduce the incidence of local recurrence in patients with IIIA-N2 non-small cell lung cancer (NSCLC), the role of PORT on survival in patients with surgically treated stage IIIA-N2 NSCLC remains controversial. Therefore, this study was designed to evaluate the effect of PORT on survival for patients with surgically treated stage IIIA-N2 NSCLC.
Materials and methods
This study population was chosen from the Surveillance, Epidemiology, and End Results database. The Cox proportional hazards regression analysis was used to determine significant contributors to overall survival (OS) and cancer special survival (CSS) outcomes. To balance baseline characteristics between the non-PORT group and PORT group, propensity score matching (PSM) with 1:1 propensity nearest-neighbor match by 0.001 matching tolerance was conducted by R software. Furthermore, a Kaplan–Meier curve was used to visualize the OS and CSS between the PORT group and non-PORT group survival probability.
Results
Of all evaluated cases, 4511 with IIIA-N2 NSCLC were eligible for inclusion, of which 1920 were enrolled into the PORT group. On univariate analysis and multivariate analysis, sex, age, year of diagnosis, race, histologic type, T stage, PORT, use of chemotherapy, and positive regional nodes were significantly associated with OS and CSS in IIIA-N2 NSCLC (P < 0.05). However, PORT was not significantly associated with OS (univariate HR = 0.92, 95%CI 0.85–0.99, P = 0.02; multivariate HR = 1.01, 95%CI 0.93–1.08, P = 0.91) and CSS (univariate HR = 0.92, 95%CI 0.85–1.01, P = 0.06; multivariate HR = 1.103 95%CI 0.94–1.12, P = 0.56) in IIIA-N2 NSCLC. Meanwhile, after PSM, neither OS nor CSS did differ significantly between the non-PORT group and PORT group (OS HR = 1.08, 95%CI 0.98–1.19, P = 0.12; CSS HR = 1.10, 95%CI 0.99–1.23, P = 0.07).
Conclusion
PORT did not contribute to a survival benefit in patients with surgically treated stage IIIA-N2 NSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-03093-8.
Keywords: Non-small cell lung cancer, Post-operative radiotherapy, Mediastinal involvement, Overall survival, Cancer special survival
Introduction
Lung cancer is the second most commonly diagnosed cancer and the leading cause of cancer-related lethality globally [1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of patients with lung cancer, and one-third have been diagnosed with locally advanced NSCLC [2]. Surgery-based multimodality therapies are one of the available curative treatments for operable advanced NSCLC, especially pathological N2 IIIA NSCLC [3–5]. Notably, the role of PORT in the survival of patients with stage IIIA-N2 NSCLC has caused considerable controversy [3, 4]. It is widely revealed that PORT contributes to a significant benefit in local–regional control in patients with surgically treated stage IIIA-N2 NSCLC [3, 4, 6, 7]. However, the benefit of local–regional control did not stand for an OS advantage. Additionally, PORT naturally increases adverse events, such as cardiopulmonary toxicity [3, 8, 9]. In general, the role of PORT in patients with surgically treated stage IIIA-N2 NSCLC remains controversial [3, 4]. Therefore we aimed to assess the role of PORT on the survival of patients with stage IIIA-N2 NSCLC by a Surveillance, Epidemiology, and End Results Program (SEER) population-based study.
Methods
Data were extracted from the SEER database, a national registry funded by the National Cancer Institute since 1971. This study population was chosen from the SEER Research Plus Database (17 Regs, Nov 2021Sub [2000–2019]) using SEER*stat Version 8.4.0.1 software. Patients who were 18 years old or more diagnosed with primary IIIA-N2 NSCLC were included according to the International Classification of Diseases for Oncology, 3rd edition morphological code including adenocarcinoma (SEER code 8140–8143, 8211, 8230 8250–8255 8323 8480 8481 8490 8550 8570,8572,8574), squamous cell carcinoma (SEER code 8050, 8052, 8070–8078, 8083, 8084, 8123), and others (SEER code 8012–8014, 8046, 8003, 8004, 8022, 8030–8032, 8200, 8240, 8249, 8560). Furthermore, we checked and reclassified the stage IIIA-N2 NSCLC according to the latest 8th Edition of American Joint Committee on Cancer (AJCC) TNM staging system instead of the TNM staging system by SEER [10].
Data extraction, collection, and exclusion
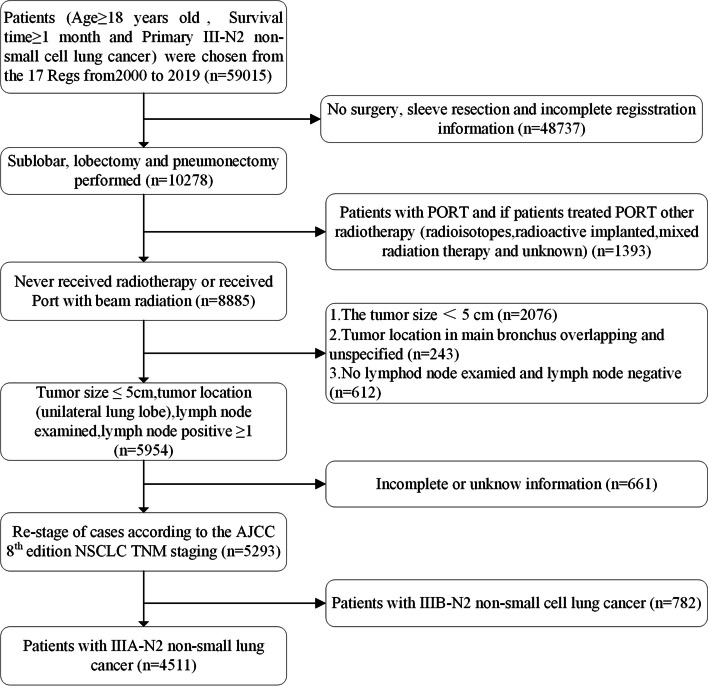
The eligibility criteria of this study and the workflow are presented in Fig. 1. Patients with surgically treated (who perform lobectomy or pneumonectomy) stage IIIA-N2 NSCLC were classified as whether they had a PORT (according to code, received beam radiation after surgery or without radiotherapy). The relevant and complete information was extracted as follows, sex, age, year of diagnosis, race, primary site, laterality, histologic type, tumor size, T stage, surgical procedure, use of radiotherapy, use of chemotherapy, positive regional nodes, presumed survival in months, vital status, and cause of death.
Fig. 1.
Patient selection for this study
Statistical analysis
Categorical variables are presented as numbers and percentages. Chi-square tests and t tests were performed for descriptive analyses. The Cox proportional hazards model was used to determine significant contributors from potential prognostic factors, including age (divided into 2 groups of 65 years old), sex, race, year of diagnosis (divided into 2 periods of 10 years each [2000–2009 2010–2019]), primary site, laterality, histologic type, T stage, use of PORT, use of chemotherapy and positive regional nodes to CSS and OS outcomes. To balance baseline characteristics between non-PORT and PORT groups, R software conducted propensity score matching (PSM) with 1:1 propensity nearest-neighbor match by 0.001 matching tolerance. A Kaplan–Meier curve was used to visualize the OS and CSS between PORT and Non-PORT survival probability. The result visualization by performing R software (Version 3.5.1, package “survival”, “MatchIt”). All P values were 2-sided, with a p value < 0.05 were considered statistically significant.
Results
Patient characteristics
In total, 4511 cases with stage IIIA-N2 NSCLC were eligible for inclusion between 2000 and 2019 (Fig. 1), of which 1920 cases were enrolled into the PORT group (Table 1). The number and proportion of surgically treated stage IIIA-N2 NSCLC following PORT significantly increased over the last two decades. The rate of older (age ≥ 65) patients who received surgically treated stage IIIA-N2 NSCLC following PORT decreased obviously during the last two decades of age.
Table 1.
Characteristics of patients with stage IIIA-N2 NSCLC before PSM
| Characteristic | Non-PORT | PORT | P value |
|---|---|---|---|
| n | 2591 | 1920 | |
| Age, n (%) | < 0.01 | ||
| < 65 | 994 (22%) | 938 (20.8%) | |
| ≥ 65 | 1597 (35.4%) | 982 (21.8%) | |
| Sex, n (%) | 0.87 | ||
| Female | 1400 (31%) | 1032 (22.9%) | |
| Male | 1191 (26.4%) | 888 (19.7%) | |
| Year of diagnosis, n (%) | 0.02 | ||
| 2000–2009 | 1288 (28.6%) | 884 (19.6%) | |
| 2010–2019 | 1303 (28.9%) | 1036 (23%) | |
| Race, n (%) | 0.70 | ||
| White | 2110 (46.8%) | 1554 (34.4%) | |
| Other | 481 (10.7%) | 366 (8.1%) | |
| Primary site, n (%) | 0.01 | ||
| Upper lobe | 1579 (35%) | 1245 (27.6%) | |
| Middle lobe | 125 (2.8%) | 99 (2.2%) | |
| Lower lobe | 887 (19.7%) | 576 (12.8%) | |
| Laterality, n (%) | 0.98 | ||
| Left | 1198 (26.6%) | 886 (19.6%) | |
| Right | 1393 (30.9%) | 1034 (22.9%) | |
| Histologic type, n (%) | < 0.01 | ||
| LUAD | 1742 (38.6%) | 1411 (31.3%) | |
| LUSC | 511 (11.3%) | 308 (6.8%) | |
| Large cell | 85 (1.9%) | 61 (1.4%) | |
| Other | 253 (5.6%) | 140 (3.1%) | |
| T stage, n (%) | 0.03 | ||
| T1 | 989 (21.9%) | 796 (17.6%) | |
| T2 | 1602 (35.5%) | 1124 (24.9%) | |
| Surgery, n (%) | < 0.01 | ||
| Lobectomy | 2187 (48.5%) | 1586 (35.2%) | |
| Sublobectomy | 229 (5.1%) | 242 (5.4%) | |
| Pneumonectomy | 175 (3.9%) | 92 (2%) | |
| Chemotherapy, n (%) | < 0.01 | ||
| No | 1173 (26%) | 282 (6.3%) | |
| Yes | 1418 (31.4%) | 1638 (36.3%) | |
| Regional nodes positive, n (%) | < 0.01 | ||
| 4 | 1745 (38.7%) | 1192 (26.4%) | |
| ≥ 4 | 846 (18.8%) | 728 (16.1%) | |
Survival and high-risk features
On univariate analysis and multivariate analysis, age, year of diagnosis, sex, race, histologic type, T stage, use of chemotherapy, and positive regional nodes were significantly associated with OS and CSS in stage IIIA-N2 NSCLC (P < 0.05, Tables 2 and 3). From multivariate analysis, PORT was not significantly associated with OS (HR = 1.01, 95%CI 0.93–1.08, P = 0.91) and CSS (HR = 1.103 95%CI 0.94–1.12, P = 0.56) in stage IIIA-N2 NSCLC (Tables 2 and 3).
Table 2.
Univariate and multivariate analysis of OS for patients with stage IIIA–N2 NSCLC
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 4511 | ||||
| < 65 | 1932 | Reference | |||
| ≥ 65 | 2579 | 1.43 (1.33–1.54) | < 0.01 | 1.35 (1.25–1.45) | < 0.01 |
| Sex | 4511 | ||||
| Female | 2432 | Reference | |||
| Male | 2079 | 1.34 (1.25–1.44) | < 0.01 | 1.32 (1.23–1.42) | < 0.01 |
| Year of diagnosis | 4511 | ||||
| 2000–2009 | 2172 | Reference | |||
| 2010–2019 | 2339 | 0.67(0.61–0.71) | < 0.01 | 0.69 (0.64–0.75) | < 0.01 |
| Race | 4511 | ||||
| White | 3664 | Reference | |||
| Other | 847 | 0.80 (0.73–0.88) | < 0.01 | 0.82 (0.75–0.91) | < 0.01 |
| Primary site | 4511 | ||||
| Upper lobe | 2824 | Reference | |||
| Middle lobe | 224 | 1.04 (0.88–1.22) | 0.68 | 1.18 (0.99–1.40) | 0.06 |
| Lower lobe | 1463 | 1.06 (0.98–1.14) | 0.17 | 1.07 (0.99–1.16) | 0.08 |
| Laterality | 4511 | ||||
| Left | 2084 | Reference | |||
| Right | 2427 | 0.95 (0.89–1.02) | 0.15 | 0.97 (0.91–1.05) | 0.49 |
| Histologic type | 4511 | ||||
| LUAD | 3153 | Reference | |||
| LUSC | 819 | 1.24 (1.13–1.36) | < 0.01 | 1.10 (1.00–1.21) | 0.05 |
| Large cell | 146 | 1.25 (1.03–1.51) | 0.02 | 1.19 (0.99–1.44) | 0.07 |
| Other | 393 | 1.01 (0.88–1.15) | 0.92 | 0.98 (0.86–1.12) | 0.78 |
| T stage | 4511 | ||||
| T1 | 1785 | Reference | |||
| T2 | 2726 | 1.24 (1.15–1.33) | < 0.01 | 1.23 (1.14–1.32) | < 0.01 |
| Surgery | 4511 | ||||
| Lobectomy | 3773 | Reference | |||
| Sublobectomy | 471 | 1.29 (1.15–1.44) | < 0.01 | 1.38 (1.23–1.54) | < 0.01 |
| Pneumonectomy | 267 | 1.27 (1.10–1.46) | < 0.01 | 0.99 (0.85–1.15) | 0.89 |
| Surg/Rad Seq | 4511 | ||||
| Non-PORT | 2591 | Reference | |||
| PORT | 1920 | 0.92 (0.85–0.99) | 0.02 | 1.01 (0.93–1.08) | 0.91 |
| Chemotherapy | 4511 | ||||
| No | 1455 | Reference | |||
| Yes | 3056 | 0.66 (0.61–0.71) | < 0.01 | 0.72 (0.66–0.78) | < 0.01 |
| Regional nodes positive | 4511 | ||||
| < 4 | 2937 | Reference | |||
| ≥ 4 | 1574 | 1.36 (1.27–1.47) | < 0.01 | 1.48 (1.37–1.59) | < 0.01 |
Table 3.
Univariate and multivariate analysis of CSS for patients with stage IIIA–N2 NSCLC
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 3881 | ||||
| < 65 | 1708 | Reference | |||
| ≥ 65 | 2173 | 1.46 (1.35–1.59) | < 0.01 | 1.37 (1.26–1.49) | < 0.01 |
| Sex | 3881 | ||||
| Female | 2132 | Reference | |||
| Male | 1749 | 1.39 (1.28–1.50) | < 0.01 | 1.37 (1.26–1.49) | < 0.01 |
| Year of diagnosis | 3881 | ||||
| 2000–2009 | 1747 | Reference | |||
| 2010–2019 | 2134 | 0.54 (0.50–0.59) | < 0.01 | 0.56 (0.52–0.61) | < 0.01 |
| Race | 3881 | ||||
| White | 3126 | Reference | |||
| Other | 755 | 0.78 (0.70–0.86) | < 0.01 | 0.81 (0.73–0.90) | < 0.01 |
| Primary site | 3881 | ||||
| Upper lobe | 2421 | Reference | |||
| Middle lobe | 202 | 1.01 (0.84–1.21) | 0.92 | 1.17 (0.97–1.41) | 0.10 |
| Lower lobe | 1258 | 1.04 (0.95–1.13) | 0.44 | 1.06 (0.97–1.160) | 0.18 |
| Laterality | 3881 | ||||
| Left | 1764 | Reference | |||
| Right | 2117 | 0.94 (0.87–1.02) | 0.11 | 0.94 (0.88–1.02) | 0.14 |
| Histologic type | 3881 | ||||
| LUAD | 2777 | Reference | |||
| LUSC | 658 | 1.30 (1.17–1.45) | < 0.01 | 1.17 (1.04–1.30) | 0.01 |
| Large cell | 114 | 1.34 (1.07–1.67) | 0.01 | 1.25 (1.00–1.57) | 0.05 |
| Other | 332 | 1.01 (0.87–1.17) | 0.91 | 1.00 (0.86–1.16) | 0.99 |
| T stage | 3881 | ||||
| T1 | 1532 | Reference | |||
| T2 | 2349 | 1.26 (1.16–1.37) | < 0.01 | 1.260 (1.16–1.37) | < 0.01 |
| Surgery | 3881 | ||||
| Lobectomy | 3259 | Reference | |||
| Sublobectomy | 405 | 1.33 (1.17–1.50) | < 0.01 | 1.45 (1.27–1.64) | < 0.01 |
| Pneumonectomy | 217 | 1.31 (1.12–1.53) | < 0.01 | 0.95 (0.80–1.13) | 0.55 |
| Surg/Rad Seq | 3881 | ||||
| Non-PORT | 2214 | Reference | |||
| PORT | 1667 | 0.92 (0.85–1.01) | 0.06 | 1.03 (0.94–1.12) | 0.56 |
| Chemotherapy | 3881 | ||||
| No | 1180 | Reference | |||
| Yes | 2701 | 0.61 (0.56–0.66) | < 0.01 | 0.70 (0.64–0.77) | < 0.01 |
| Regional nodes positive | 3881 | ||||
| < 4 | 2505 | Reference | |||
| ≥ 4 | 1376 | 1.40 (1.29–1.51) | < 0.01 | 1.51 (1.38–1.64) | < 0.01 |
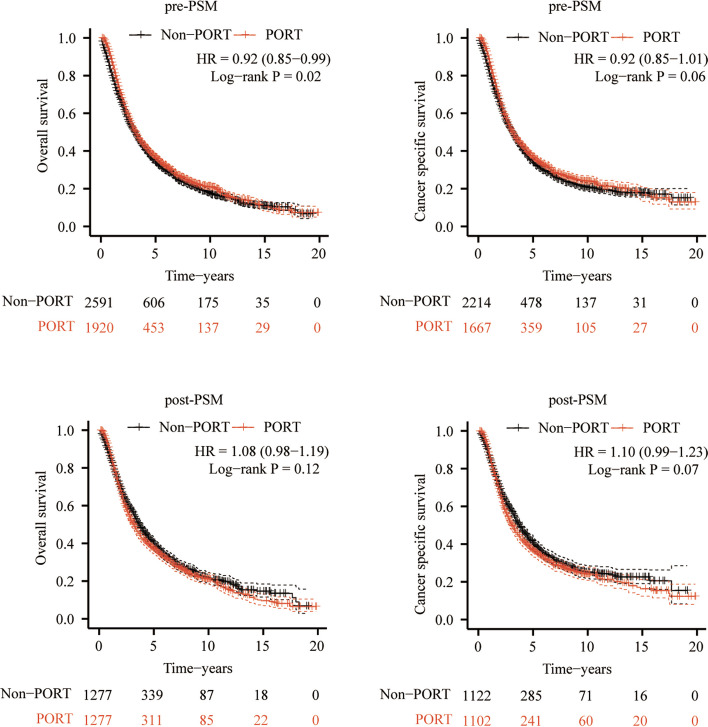
Prior to PSM, PORT might contribute to an OS benefit (HR = 0.92, 95% CI 0.85–0.99, P = 0.02), as shown in Fig. 2A; but CSS did not differ significantly between the non-PORT group and PORT group (HR = 0.92, 95% CI 0.85–1.01, P = 0.06), as shown in Fig. 2B. PSM was conducted to balance baseline characteristics between non-PORT and PORT groups (Supplementary Table S1). Following PSM, neither OS nor CSS did differ significantly between the non-PORT group and PORT group (OS HR = 1.08, 95%CI 0.98–1.19, P = 0.12; CSS HR = 1.10, 95%CI 0.99–1.23, P = 0.07) in unselected patients with surgically treated stage IIIA-N2 NSCLC, as shown in Fig. 2C, D. Furthermore, we found that PORT did not contribute to a survival benefit in patients with stage IIIA-N2 NSCLC diagnosed with adenocarcinoma, squamous, large cell, respectively, respectively (Supplementary Figure S1), and similar results were in patients with stage IIIA-N2 NSCLC underwent lobectomy, pneumonectomy and sublobectomy, respectively (Supplementary Figure S2).
Fig. 2.
Kaplan–Meier curves of the OS and CSS between PORT and non-PORT before and after PSM. A OS curves before PSM. B CSS curves before PSM. C OS curves after PSM. D CSS curves after PSM. OS: overall survival, CSS: cancer-specific survival, PSM: propensity score-matching
Discussion
Although a previous study reported that the use of PORT has declined for surgically treated stage IIIA-N2 NSCLC [11], we found that the number of surgically treated stage IIIA-N2 NSCLC following PORT was still large. However the role of PORT in patients with surgically treated stage IIIA-N2 NSCLC remains controversial [3, 4]. Consequently, there is a compelling need to explore the use of PORT on the survival of patients with stage IIIA-N2 NSCLC.
In previous studies, 20–60% patients with pathological N2 NSCLC had a locoregional recurrence [6, 12]. It is widely accepted that PORT contributes significantly to local–regional control in patients with surgically treated stage IIIA-N2 NSCLC [3, 4, 6, 7]. Therefore, PORT has been considered a strategy to improve outcomes by reducing the risk of local recurrence in patients with surgically treated stage III-N2 NSCLCT [6]. But PORT does not seem to improve OS in patients with surgically treated stage III-N2 NSCLCT [7, 13]. There are some possible reasons for the contradiction. Firstly, the benefit of PORT might be outweighed by itself as PORT naturally increases adverse events, such as cardiopulmonary toxicity [3, 8, 9]. However, several recent studies found that PORT might not contribute to an increase in the hazard for cardiac-related mortality [9, 14, 15]. A SEER population-based study reported no statistically significant difference in cardiac-related mortality between the PORT and non-PORT groups in stage IIIA-N2 NSCLC patients in all periods [9]. In the PORT-C trial, due to modern radiation techniques, such as IMRT, there were no radiotherapy-related grade 4 or 5 adverse events, and only 0.7% of patients had grade 3 radiation pneumonitis. With such low toxic effects, PORT still did not improve OS and DFS for patients with surgically treated stage IIIA-N2 NSCLC compared with the non-PORT group [5]. Secondly, because stage IIIA-N2 NSCLC is a systemic disease, it may be meaningless of local–regional control from PORT for patients with surgically treated stage IIIA-N2 NSCLC. The benefit of local–regional control is not equal to a survival benefit.
Patients with stage IIIA-N2 NSCLC in previous studies mostly were based on the former edition of TNM staging, but the edition of TNM staging have been re-defined. In this study patients were accurately diagnosed with primary IIIA-N2 NSCLC according to the latest 8th edition of TNM staging system, so the results in this study are more consistent with current clinical practice. Besides OS, CSS that only NSCLC-related death other than other causes was censored considered as endpoint was also taken into consideration to minimize the impact of other factors, such as cardiac-related mortality. Furthermore, PSM was performed to reduce the bias caused by selection, meanwhile both Cox multivariate analysis and PSM confirmed that PORT did not significantly improve OS or CSS in patients with IIIA-N2 NSCLC.
This study did not completely deny the application of PORT in surgically treated stage IIIA-N2 NSCLC. Although we do believe that PORT should have a survival benefit in selected patients with unique features, such as multiple N2 stations, a larger number of lymph nodes involvement, a bulky disease and high lymph node ratio (LNR) [16–22], the details or cut-off value of particular features have not yet come to a unified definition. Constructing a risk model, such as patient prognostic scores, might be a more potential candidate to select the proper patients with surgically treated stage IIIA-N2 NSCLC for PORT [12, 20]. Therefore, further studies exploring which patients with surgically treated stage IIIA-N2 NSCLC might optimally have a survival benefit from PORT are required.
There were several limitations in our study. First, potential selection bias cannot be excluded due to its retrospective nature. Second, due to the lack of the related details in SEER database, several important issues were still suspended, such as the PORT timing (concomitant, sequential, or alone) analysis, the N2 surgical resection (clinical N0, limited disease, single station disease, salvage surgery) and the metastatic station number: N2a1 (a single metastatic station with no hilar involvement), N2a2 (a single metastatic station with hilar involvement), and N2b (multiple metastatic stations) [23, 24]. Third, recent treatments, like molecular targeted therapy and cancer immunotherapy, play a vital role in treating patients with surgically treated stage IIIA-N2 NSCLC. Therefore, the impact of molecular targeted therapy and cancer immunotherapy cannot be excluded from this study due to the lack of data in the SEER database.
Generally, we found that PORT did not contribute to a survival benefit in unselected patients with surgically treated stage IIIA-N2 NSCLC. Further prospective randomized controlled trials are needed to confirm the findings.
Supplementary Information
Additional file 1: Supplementary Table S1. Characteristics of patients with stage IIIA-N2 NSCLC after PSM.
Additional file 2: Supplementary Figure S1. PORT did not contribute to a survival benefit in patients with stage IIIA-N2 NSCLC diagnosed with adenocarcinoma, squamous, large cell, respectively, respectively.
Additional file 3: Supplementary Figure S2. Patients with stage IIIA-N2 NSCLC underwent lobectomy, pneumonectomy and sublobectomy, respectively.
Acknowledgments
Animal studies
Not applicable.
Authors’ contributions
Minxia Zhu: Conception and study design, Methodology, Acquisition of data, Analysis and interpretation of data, Visualization, Writing – original draft. Shaomin Li: Methodology, Analysis and interpretation of data, Visualization, Writing – review & editing. Liyue Yuan: Methodology, Acquisition of data, Writing – review & editing. Shiyuan Liu: Conception and study design, methodology, Writing – review & editing. Jianzhong Li: Analysis and interpretation of data, Writing – review & editing. Danjie Zhang: Acquisition of data, Writing – review & editing. Jia Chen: Acquisition of data, Writing – review & editing. Jiantao Jiang: Conception and study design, methodology, Analysis and interpretation of data, Writing – review & editing, Study supervision. Zhengshui Xu: Conception and study design, methodology, Analysis and interpretation of data, Writing – original draft, Writing – review & editing, Study supervision. All authors reviewed the manuscript.
Funding
This work was funded by grants from the Science and Technology Project of Shaanxi Province (Grant serial number: 2023-YBSF-359, 2023-JC-QN-0840).
Availability of data and materials
This study was subject to a Data Use Agreement with NCI. SEER data is publicly available and de-identified.
Declarations
Ethics approval and consent to participate
Ethics approval and consent to participate: not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original article has been updated: “Updated Figure 1 and Supplementary Figure S2.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/3/2024
A Correction to this paper has been published: 10.1186/s12957-023-03263-8
Contributor Information
Jiantao Jiang, Email: jiangjiantao2022@163.com.
Zhengshui Xu, Email: xuzhengshui@xjtu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Ladbury C, Kim J, et al. Postoperative radiation therapy should be used for completely resected stage III-N2 NSCLC in select patients. J Thorac Oncol. 2022;17(2):194–196. doi: 10.1016/j.jtho.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Faivre-Finn C, Edwards JG, Hatton M. Postoperative Radiation Therapy Should Not Be Used for the Therapy of Stage III-N2 NSCLC. J Thorac Oncol. 2022;17(2):197–199. doi: 10.1016/j.jtho.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Hui Z, Men Y, Hu C, et al. Effect of postoperative radiotherapy for patients with pIIIA-N2 non-small cell lung cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-C randomized clinical trial. JAMA Oncol. 2021;7(8):1178–1185. doi: 10.1001/jamaoncol.2021.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu L, Xia B, Ma S. Postoperative radiotherapy for patients with completely resected stage IIIA-N2 non-small cell lung cancer: opt-in or opt-out. Thorac Cancer. 2022;13(5):659–663. doi: 10.1111/1759-7714.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei T, Li J, Zhong H, et al. Postoperative radiotherapy for patients with resectable stage III-N2 non-small cell lung cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:680615. doi: 10.3389/fonc.2021.680615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamasaki N, Kim YH, Iwatsubo S, Nishimura Y, Funada Y. Role of postoperative radiotherapy in patients with completely resected pIIIA-N2 non-small cell lung cancer. Clin Lung Cancer. 2022;23(3):e171–e172. doi: 10.1016/j.cllc.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Men Y, Wang J, et al. Risk of cardiac-related mortality in stage IIIA-N2 non-small cell lung cancer: Analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Thorac Cancer. 2021;12(9):1358–1365. doi: 10.1111/1759-7714.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. 8th ed. Springer International Publishing; 2017. p. 431–56.
- 11.Bekelman JE, Rosenzweig KE, Bach PB, Schrag D. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):492–499. doi: 10.1016/j.ijrobp.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Xie HN, Chen XK, Bi N, Qin JJ, Li Y. Patient prognostic scores and association with survival improvement offered by postoperative radiotherapy for resected IIIA/N2 non-small cell lung cancer: A population-based study. Thorac Cancer. 2021;12(6):760–767. doi: 10.1111/1759-7714.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SF, Mao NQ, Zhao WH, Pan XB. Postoperative radiotherapy in pIIIA-N2 non-small cell lung cancer after complete resection and adjuvant chemotherapy: A meta-analysis. Medicine (Baltimore) 2022;101(28):e29550. doi: 10.1097/MD.0000000000029550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Song J, Long J, Zeng Z, Liu A. Effects of postoperative radiotherapy on cardiovascular-pulmonary disease mortality in patients with stage IIIA-N2 resected NSCLC: analysis of the SEER database. Radiat Oncol. 2021;16(1):184. doi: 10.1186/s13014-021-01912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan H, Liang L, Xie S, Wang C. The impact of order with radiation therapy in stage IIIA pathologic N2 NSCLC patients: a population-based study. BMC Cancer. 2020;20(1):809. doi: 10.1186/s12885-020-07309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao F, Li N, Xu Y, Yang G. Evaluation of postoperative radiotherapy effect on survival of resected stage III-N2 non-small cell lung cancer patients. Front Oncol. 2020;10:1135. doi: 10.3389/fonc.2020.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Ma Z, Yang X, et al. Choice of postoperative radiation for stage IIIA pathologic N2 non-small cell lung cancer: impact of metastatic lymph node number. Radiat Oncol. 2017;12(1):207. doi: 10.1186/s13014-017-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarinshenas R, Ladbury C, McGee H, et al. Machine learning to refine prognostic and predictive nodal burden thresholds for post-operative radiotherapy in completely resected stage III-N2 non-small cell lung cancer. Radiother Oncol. 2022;173:10–18. doi: 10.1016/j.radonc.2022.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Allaeys T, Berzenji L, Lauwers P, et al. Multimodality treatment including surgery related to the type of N2 involvement in locally advanced non-small cell lung cancer. Cancers (Basel). 2022;14(7):1656. [DOI] [PMC free article] [PubMed]
- 20.Zhang CC, Hou RP, Xia WY, et al. Prognostic index for estimating the survival benefit of postoperative radiotherapy in pathologic N2 non-small cell lung cancer: A real-world validation study. Lung Cancer. 2021;156:100–108. doi: 10.1016/j.lungcan.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Zeng WQ, Feng W, Xie L, et al. Postoperative radiotherapy for resected stage IIIA-N2 non-small-cell lung cancer: a population-based time-trend study. Lung. 2019;197(6):741–751. doi: 10.1007/s00408-019-00284-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang CC, Yu W, Zhang Q, Cai XW, Feng W, Fu XL. A decision support framework for postoperative radiotherapy in patients with pathological N2 non-small cell lung cancer. Radiother Oncol. 2022;173:313–318. doi: 10.1016/j.radonc.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Bertoglio P, Ricciardi S, Alì G, et al. N2 lung cancer is not all the same: an analysis of different prognostic groups. Interact Cardiovasc Thorac Surg. 2018;27(5):720–726. doi: 10.1093/icvts/ivy171. [DOI] [PubMed] [Google Scholar]
- 24.Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015;10(12):1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table S1. Characteristics of patients with stage IIIA-N2 NSCLC after PSM.
Additional file 2: Supplementary Figure S1. PORT did not contribute to a survival benefit in patients with stage IIIA-N2 NSCLC diagnosed with adenocarcinoma, squamous, large cell, respectively, respectively.
Additional file 3: Supplementary Figure S2. Patients with stage IIIA-N2 NSCLC underwent lobectomy, pneumonectomy and sublobectomy, respectively.
Data Availability Statement
This study was subject to a Data Use Agreement with NCI. SEER data is publicly available and de-identified.