Abstract
Organoids are established through in vitro 3D culture, and they can mimic the structure and physiological functions of organs or tissues in vivo. Organoids have attracted much attention in recent years. They can provide a reliable technology platform for cancer research and treatment and are a valuable preclinical model for academic research and personalized medicine. A number of studies have confirmed that organoids have great application prospects in new drug development, drug screening, tumour mechanism research, and precision medicine. In this review, we mainly focus on recent advances in the application of organoids in cancer research. We also discussed the opportunities and challenges facing organoids, hoping to indicate directions for the development of organoids in the future.
Keywords: Organoid, Cancer, Precision Medicine, Drug screening, Organoid Biobank
Background
Organoids are a model based on a three-dimensional (3D) cell culture system in vitro that is highly similar to source tissues or organs in vivo [1–5]. Organoids were named the 2017 Technology of the Year in Life Sciences by the journal Nature Methods. Although organoids are not real human organs, they can simulate real organs in terms of structure and function and can simulate the interactions and spatial position relationships between cells and between cells and their surrounding matrix [6, 7].
Organoids are widely used in cancer research [3, 6, 8–14]. The emergence of organoid models has made it possible to culture certain cancerous tissues in vitro. Compared with the traditional 2D cell culture model, 3D cultured organoids contain a variety of cell types, can form functional “micro-organs”, and can be better used to simulate the development process and physiological and pathological state of organ tissues. Organoids preserve the genetic, phenotypic, and behavioural characteristics of the tumour tissue of origin and have become the most relevant models for drug discovery, tracking treatment response, and personalized medicine [15–23]. It has broad application prospects in basic cancer research and clinical diagnosis and treatment.
Organoids are currently divided into two categories: tissue-derived organoids and pluripotent stem cell-derived organoids [6, 17, 19, 24]. Patient-derived tumour organoids (PDTOs) are commonly used in disease modelling, research and drug screening of tumour patients. Tumour organoids can be obtained by culturing biopsied, aspirated, or surgically resected tissue in Matrigel for several weeks. Tumour organoids maintain the heterogeneity of the source tumours and patients; moreover, the shape and size of organoids are basically uniform among individuals. Thus, they provide a good technical platform for research on tumour pathogenesis, drug screening, precision medicine and other fields [25–28]. In clinical work, before treating cancer patients, organoids derived from tumour tissues can be used to screen sensitive drugs and provide individualized treatment options for cancer treatment.
In this review, we mainly focused on recent advances in the application of organoids in cancer research, especially in precision cancer therapy. We also discussed the opportunities and challenges facing organoids, hoping to point out the direction for future organoid development.
History of organoids
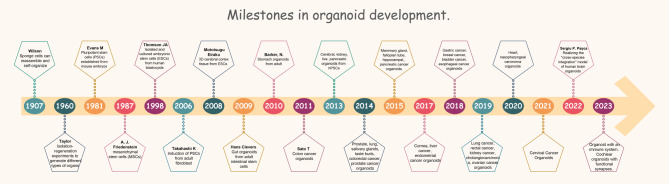
The earliest events related to organoid development date back to 1907. Professor H. V. Wilson discovered that mechanically separated sponge cells can reassemble and self-organize into new sponge organisms with normal functions. This research demonstrated that adult organisms can successfully develop into new organisms without external assistance and without starting from a specific anatomical stage [29]. This could be considered the source of the development of organoid technology. In 1960, Professor Taylor, A. C. conducted isolation-regeneration experiments to generate different types of organs from isolated amphibian pro-nephros and chicken embryos [30]. In 1961, Verney, E. L. observed the differentiation of embryoid bodies in vitro [31]. In 1981, scientists first isolated pluripotent stem cells (PSCs) from mouse embryos. Stem cell research has grown rapidly since then [32, 33]. In 1987, scientists began to improve cell culture conditions by simulating the microenvironment in vivo. Li et al. demonstrated that mammary epithelial cells formed 3D ducts and lumens when cultured in extracellular matrix (ECM) extracts of Engelbreth-Holm-Swarm (EHS) mouse sarcoma [34]. In the same year, a study found that alveolar type II epithelial cells were able to maintain their differentiation capacity in the presence of ECM stroma, highlighting the importance of cell-matrix interactions in tissue maintenance and differentiation [35]. In 1987, A. J. Friedenstein discovered mesenchymal stem cells (MSCs). In 1998, scientists isolated and cultured embryonic stem cells from human blastocysts for the first time [36]. From 2006 to 2007, Thomson et al. successfully prepared human induced pluripotent stem cells (induced pluripotent stem cells, iPSCs), which is of great significance to stem cell and organoid research [37–39]. Today, most types of nontumor-derived human organoids can be developed from embryonic stem cells (ESCs), MSCs or iPSCs [40]. The rapid progress of stem cell research has brought new vitality to organoid research.
The milestone moment was in 2009, when the Hans Clevers team in the Netherlands used a single leucine-rich repeat-containing receptor 5+ (LGR5+) intestinal stem cell to culture cells in vitro to establish an intestinal organoid with an intestinal crypt-villus structure, which opened a new era of organoid technology development [41]. As early as 2007, Hans Clevers discovered that the stem cells of the small intestine and colon were LGR5 + cells [42]. In 2009, the experimental team cultured crypt cells isolated from mouse intestines in a 3D Matrigel culture system containing ENR (EGF, Noggin, R-spondin). They found that under the cultivation of this culture system, crypt cells can form microstructures similar to the crypt-villi-like complexes of the intestines. Using this culture system to culture a single LGR5 + intestinal stem cell isolated and identified earlier, it was found that organoids with the abovementioned special structure can also be formed, and the organoid still has LGR5 + intestinal stem cells [41]. Therefore, the model could well simulate the morphology, structure and function of the small intestine in vivo. The successful establishment of the small intestine organoid system has opened a new chapter in organoid research.
Since 2009, organoids of the stomach, retina, brain, liver, kidney, pancreas, breast, and fallopian tubes have been successfully grown [43–47]. In 2011, Hans Clevers established tumour organoids using organoid technology. Based on the culture conditions of mouse colonic crypts, they cultivated organoids of the human small intestine and colon by adjusting the culture environment. After further optimization and adjustment, tumour organoid models were established from colon adenoma and adenocarcinoma and Barrett’s oesophagus [48]. In 2014, a study was the first to successfully grow six prostate cancer organoids using tumour samples from patients with metastatic prostate cancer. Tumour samples were largely consistent with the genetic signature of the corresponding organoids. In addition, organoids were successfully grown from a patient’s circulating tumour cells [10]. In 2015, Hans Clevers’ team used CRISPR/Cas9 technology to create colorectal cancer (CRC) organoids. In the same year, he also reported tumour organoids established from 20 CRC patient samples. High-throughput sequencing analysis found that the gene change profile of organoids was highly consistent with the results of large-scale mutation analysis of CRC [49]. These organoids could be used for large-scale drug screening to detect some genetic changes associated with drug sensitivity, which is of great significance for individualized precision medicine. In 2015, Tuveson, D.A. successfully developed a new model system for cultivating normal and cancerous pancreatic cells in the laboratory, which could establish organoid models from normal and tumour mice and human pancreatic tissue [50]. The results of this study provide a new platform for the study of the molecular mechanism of pancreatic cancer. In 2017, Meritxell Huch reported the successful cultivation of the first human primary liver cancer organoids [15]. The effect of 29 anticancer drugs, including existing drugs and new drugs in development, was tested using liver cancer organoids, and the preliminary results were encouraging. This research achievement was a milestone in the development history of liver cancer research.
In 2018, Hans Clevers developed a method for long-term culture and maintenance of breast epithelial organoids, and the success rate of breast cancer organoid culture was over 80%. Their team established more than 100 breast cancer primary tumour and metastatic tumour organoids and used them for analysis of gene expression and drug sensitivity experiments [51]. The study confirmed that breast cancer organoids maintained the histopathological characteristics of the primary tumour well. In 2019, Professor Smith, J.J. established 65 tumour organoids from patients with primary, metastatic or recurrent rectal cancer. The study found that rectal cancer organoids retained the molecular characteristics of their origin tumours, and the results of in vitro drug sensitivity experiments correlated with the clinical responses of tumour patients [6]. Therefore, the findings confirmed that this rectal cancer organoid can be used as an in vitro platform for drug sensitivity experiments. In September 2020, Lee J. et al. successfully produced heart organoids that could beat autonomously [52]. In 2022, Sergiu P. Pașca’s team discovered that transplanting human cortical organoids into rat brains can lead to normal development, realizing the “cross-species integration” model of human brain organoids for the first time (Fig. 1) [53].
Fig. 1.
Milestones in organoid development
Organoid formation, culture, identification, and storage
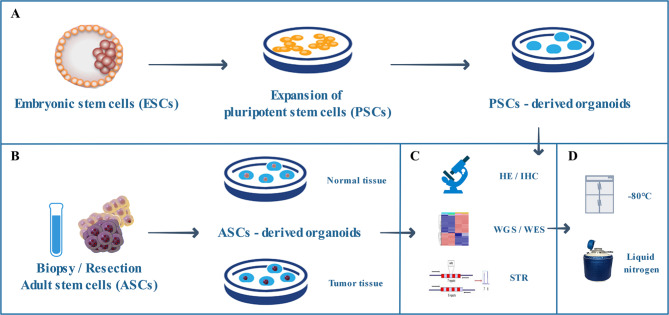
According to the source of stem cells, organoids are mainly divided into two types: organoids derived from pluripotent stem cells (PSCs), in other words, either embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), and organoids derived from organ-specific adult stem cells (ASCs), which are tissue-specific resident stem cells (Fig. 2A, B) [54]. Stem cell-derived organoids form through self-organization. They serve as models of organ development, function, and disease and have potential applications in drug development and personalized medicine. However, stem cell self-organization is difficult to control in the absence of external guidance, leading to a general lack of reproducibility in most organoids. Researchers are constantly exploring culture methods that can produce uniform and stable organoid models for better application in clinical research (Fig. 2).
Fig. 2.
Organoid formation, culture, identification, and storage. A PSC-derived organoids. B ASC-derived organoids. C Identification of organoids. D Storage of organoids. PSCs: pluripotent stem cells; ASCs: adult stem cells
N. Gjorevski et al. used in situ photopatterned hydrogel mechanics and hydrogel microfabrication techniques to control the self-organization process of intestinal stem cells and established intestinal organoids with well-defined shapes, sizes, and cell distributions [55]. In October 2022, a bioengineering device that can be used for long-term organoid culture was reported in the journal Nature Methods; this device can evenly diffuse nutrients and oxygen to grow larger, better and more complex organoids [56]. This simple, flexible, and automatable platform made it possible to grow tissue-specific organoids on this platform for extended periods of time.
To obtain human pluripotent stem cell (hPSC)-derived gastrointestinal (GI) organoids, hPSCs need to be de novo differentiated to generate isolated spheroids. In June 2022, a method for simple, reproducible, and scalable small intestinal organoid production based on cryopreserved hPSC-derived mid-hindgut endoderm monolayer aggregation was reported [57]. This approach could remove a significant hurdle in the adoption of hPSC-derived GI organoid technology. This approach could also be used to generate gastric antrum and colon organoids. This research achievement made it possible to ship the starting material to other laboratories around the world, thereby significantly accelerating the use of human gastrointestinal organoids throughout medical research. In October 2022, Chen released important innovations in the high-throughput culture of liver organoids. The team developed a method to generate novel liver organoids by micropatterning [58]. This technique enabled the generation of homogeneous liver organoids, providing a powerful tool for liver drug screening and hepatotoxicity assessment.
Tumour organoids are established by 3D tissue culture of tumour stem cells in tumour tissues, which can simulate the characteristics of tumours in vivo and the heterogeneity of tumour cells. Cancer stem cells are also considered to be the producers of the entire tumour microenvironment [59, 60]. Currently, successfully cultured tumour organoids include those of colon cancer, gastric cancer, pancreatic cancer, prostate cancer, breast cancer, endometrial cancer, ovarian cancer, and kidney cancer. Circulating tumour cells (CTCs) are cells derived from primary tumours or tumour metastases. After shedding, CTCs enter the peripheral blood of patients through the blood system or lymphatic system, which is related to tumour invasion and metastasis [61, 62]. Therefore, organoids derived from CTCs are of great value for the study of tumour invasion and metastasis mechanisms. CTC-derived organoids have the advantages of a higher success rate, shorter generation time and better suitability for gene editing. Drug susceptibility testing of CTC-derived organoids can predict treatment outcomes in matched patients. However, CTC-derived organoids have only been established in a few cancers [62].
Most tumour organoid cultures currently use Matrigel hydrogel as a culture substrate [63]. Matrigel is a colloidal protein mixture secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells and is produced by Corning Life Sciences [64]. The adjustment of tumour organoid induction medium composition is the most critical part of tumour organoid culture. Because tumour patients have individual differences, the induction factors of tumour organoids are not fixed, which also makes the development of commercial tumour organoid culture medium extremely difficult. The induction medium is the basal medium Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) supplemented with various factors, and these factor components are key to the formation of tumour organoids. The various factors that need to be added to the medium must be explored and verified [65, 66]. These factors generally include the following four categories: (1) Wnt signalling pathway activators, which mainly promote angiogenesis and include Wnt signalling ligands and the GR5 ligand Rspo-1; (2) ligands of tyrosine receptor kinases, which mainly stimulate epithelial cell proliferation and include epidermal growth factor (EGF), recombinant fibroblast growth factor 10 (FGF10), and nerve growth factor (NGF); (3) TGF-β signalling pathway inhibitors, which mainly inhibit epithelial cell differentiation and include noggin, A83-01; and (4) ROCK inhibitors, which mainly maintains stem cell pluripotency and includes Y-27,632. The culture conditions of tumour organoids from different sources are different (Table 1) [67].
Table 1.
Culture conditions for different tumor organoids
| Colorectal Cancer | Gastric Cancer | Breast Cancer | Pancreatic Cancer | Cholangiocarcinoma | Lung Cancer | Hepatocellular Cancer | Bladder Cancer | Esophagus Cancer | Thyroid Cancer | Prostate Cancer | Cervical Cancer | Ovarian Cancer | Head and Neck Cancer | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | Advance DMEM/F12 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| HEPES 10 mM | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Glutamax 2 mM | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Antibiotics/antimycotics (1%) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Cytokines | rhR-Spondin-1 (ng/ml) | 500 | 250 | 1000 | 250 | 500 | 500 | 250 | 125 | 250 | 100 | ||||||
| rhNoggin (ng/ml) | 100 | 1000 | 100 | 100 | 100 | 25 | 100 | 100 | 40 | ||||||||
| rmNoggin (ng/ml) | 100 | 100 | 100 | ||||||||||||||
| rhFGF basic (ng/ml) | 12.5 | 20 | 1 | 50 | 5 | ||||||||||||
| rhFGF-7 (ng/ml) | 5 | 25 | 25 | 25 | |||||||||||||
| rhFGF-10 (ng/ml) | 50 | 20 | 100 | 100 | 100 | 100 | 100 | 10 | 100 | 10 | |||||||
| rhIGF (ng/ml) | 100 | ||||||||||||||||
| rhEGF (ng/ml) | 50 | 5 | 50 | 50 | 10 | 50 | 20 | 50 | 50 | 50 | |||||||
| rmEGF (ng/ml) | 50 | 50 | 50 | 50 | |||||||||||||
| rhWnt-3a (ng/ml) | 50 | 50 | 25 | 50 | 25 | 50 | 50 | 20 | |||||||||
| rhVEGF121 (ng/ml) | 10 | ||||||||||||||||
| rhNeuregulin 1 (nM) | 5 | ||||||||||||||||
| rhHGF (ng/ml) | 25 | ||||||||||||||||
| Additive | N-2 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| N-21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | ||
| Small Molecular Compounds | Wnt-C59 | 100 | |||||||||||||||
| Y-27,632 (µM) | 5 | 10 | 5 | 10 | 10 | 10 | 10 | 10 | 10 | ||||||||
| A83-01 (nM) | 500 | 500 | 500 | 500 | 500 | 500 | 5000 | 500 | 5 | 5000 | 500 | 5000 | 500 | 500 | 500 | ||
| Dihydrotestosterone (DHT) (nM) | 0.1/1 | ||||||||||||||||
| Primocin | 1:100v/v | ||||||||||||||||
| Gastrin I (Human) (nM) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||||||||||
| Forskolin (µM) | 10 | 10 | 10 | 1 | |||||||||||||
| Dexamethasone (nM) | 10 | 10 | 3 | ||||||||||||||
| SB 202,190 | 500 nM | 10 mM | 10 µM | 10 µM | 1 µM | ||||||||||||
| Prostaglandin E2 (µM) | 500 | 1 | |||||||||||||||
| Beta-Estradiol (nM) | 100 | ||||||||||||||||
| Wnt Surrogate (nM) | 0.5 | ||||||||||||||||
| CHIR 99,021 (µM) | 0.3 | 3 | |||||||||||||||
| Nicotinamide (mM) | 10 | 5 | 10 | 10 | 5 | 10 | 10 | 10 | 0.1 | 10 | 2.5 | ||||||
| N-Acetylcysteine (mM) | 1.25 | 1 | 1 | 1.25 | 1 | 1 | 1 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1 | 1.25 | |||
| References | [79] | [82] | [136] | [51] | [50] | [170] | [102] | [171] | [15] | [172] | [149] | [173] | [10] | [174] | [175] | [176] | |
After the organoid is established, it needs to be identified, and there are many methods to choose from (Fig. 2C) [15, 68]. According to the purpose of the research, the cost, and the feasibility of the identification method, the appropriate identification method can be selected. In the early stage of organoid culture, haematoxylin-eosin (HE) staining and immunohistochemistry are recommended to preliminarily determine the similarity between the cultured organoid and the source tissue [69]. If the morphological verification is qualified, expansion of the culture of organoids should be considered. Whole exome sequencing (WES) is then performed to identify the overlap rate of the characteristic genes between the organoid and the source tissue [70, 71]. If more accurate and comprehensive verification is needed, transcriptome sequencing, whole genome sequencing (WGS), and DNA methylation sequencing can be considered to analyse whether special targets, signalling pathways, or metabolic pathways are retained and to select qualified organoid models. To eliminate the misleading effect of cross-contamination on drug screening, short tandem repeats (STR) can be used to identify and verify that organoids are not contaminated by other cell lines or organoids during passaging [72]. Optimal growth organoids can be resuspended in cryopreservation medium and frozen at -80 °C or in liquid nitrogen (Fig. 2D) [73].
Technological revolution in organoid models
To meet the different needs in cancer research, a number of important technologies and methods have been derived from the application of organoids. For example, researchers have established organoid biobanks for high-throughput and rapid screening of sensitive drugs. Organoid biobanks provide a powerful platform for discovering and screening anticancer drugs. To study the influence of immune cells on cancer cells, researchers have used cell coculture and chip technology to simulate the tumour immune microenvironment in vivo. Technological innovation has promoted the wider application of organoids in the field of cancer research.
Organoid biobanks
Although the research results in tumours in recent years are extensive, these studies consume considerable manpower, material and financial resources to conduct research on each individual sample tissue. How to conduct high-throughput sample research and verification is an urgent problem in current cancer research.
With the widespread application of organoid technology, its clinical value has become increasingly prominent. Since organoids can be directly derived from diseased tissue (Patient Derived Organoid, PDO), it is feasible to further establish an organoid biobank [49, 74]. An established organoid biobank can provide drug susceptibility data for drug development research and help research in individualized medicine and regenerative medicine. It is also of great value to carry out related research on the dynamic occurrence, development and treatment of living tumours [75]. The main body of the tumour organoid biobank is organoids derived from tumour patients. Many organoids can be used at any time to carry out targeted scientific experimental research.
In 2015, Hans Clevers et al. established the first colorectal cancer organoid biobank. They successfully cultivated 22 colorectal cancer organoids from 27 surgically resected colorectal cancer samples, with an overall success rate of 90%. These organoids can be frozen after passage, and the survival rate after resuscitation can reach more than 80% [49]. Since then, several colorectal cancer biobanks have been established [76–83]. In 2017, the Hans Clevers team completed a large-scale breast cancer organoid project and established the world’s first breast cancer organoid biobank [51]. In 2018, Prof. Yan reported the establishment of a gastric cancer organoid biobank with associated genomic data, which provided a useful resource for studying gastric cancer cell biology and precision cancer therapy [74]. In 2022, Demyan L published an article on pancreatic cancer organoids in the journal Annals of Surgery. The researchers collected tumour samples from 117 pancreatic cancer patients, of which 16% were black, 9% were Asian, and 7% were Hispanic or Latino. This was the largest and most ethnically diverse pancreatic ductal adenocarcinoma (PDAC) organoid biobank reported thus far [26]. In August 2022, researchers established a library of 19 well-characterized and well-annotated paediatric rhabdomyosarcoma (RMS) organoids, which included all major subtypes of RMS. This was the first tumour organoid bank of pure mesenchymal malignancy (sarcoma) origin [84].
The establishment of tumour organoid biobanks is conducive to promoting the research progress of tumour molecular backgrounds and tumour biological behaviours (such as clinicopathological characteristics, drug response, etc.) and promoting the development of precision tumour treatment.
Organoid immune cell coculture models
Suppression and reprogramming of the immune system play a key role in tumour initiation and progression [85–87]. The purpose of immunotherapy is to reactivate antitumour immune cells and overcome the immune escape mechanism of tumours. Immunotherapy works in several ways to help the immune system recognize tumours, initiate an immune response, or boost an existing immune response to fight tumour cells [12, 88]. As the tumour mutational burden varies among different tumours, tumour cells exhibit different immunogenicities, which leads to significant differences in immune responses. In recent years, the use of a patient’s own immune system to eliminate cancer cells in cancer treatment has achieved great success. However, a pressing problem in the field is the lack of suitable models to test and screen for this treatment. The emergence of organoids has ignited new hope for tumour immunotherapy.
A major limitation of organoids for testing the efficacy of immunotherapies is the lack of immune cell components. Immune cells in organoids are very different from those in the tumour immune microenvironment in vivo. At present, researchers are developing more complex organoid combination models, such as organoid immune cell coculture models [59, 60]. Organoid immune cell coculture models are mainly divided into two types. (1) One method is to retain and expand endogenous immune cells in tumour organoids or to add exogenous immune cells (autologous or allogeneic) [59, 89]. (2) Another method is the organoid chip, which can coculture tumour organoids and immune cells in a continuously perfused chamber by combining microfluidic technology and can reproduce the structural characteristics of the tumour microenvironment [90, 91].
Considering that tumour neoantigens may not bring the desired immune response, some scientists are trying to activate immune cells in vitro and then apply them to patients. In vitro activation of immune cells can be achieved using organoids. In September 2018, the journal Cell published a study by Professor Dijkstra KK [92]. They established patient-derived tumour organoids and simultaneously extracted peripheral blood lymphocytes from patients with colon or lung cancer. By coculturing CD8+ T lymphocytes with tumour organoids to activate immune cells, T cells with antitumour activity were screened. The treated killer T cells showed a good tumour killing effect in in vitro cell experiments. The key tool for this was tumour organoids. Without tumour organoids, it was impossible to produce specific killer T lymphocytes. Although this study demonstrated that organoid-induced T cells kill tumours in vitro, their role in vivo remains unclear. Therefore, large-scale clinical studies are needed to verify these findings [92]. Alternatively, circulating lymphocytes or tumour-infiltrating lymphocytes (TILs) are collected, screened, modified, expanded, and activated in vitro and then reinjected back into the patient for cancer treatment. Michie J and Schnalzger TE studied chimeric antigen receptor T-cell (CAR-T) and natural killer cell (NK cell)-mediated cytotoxicity in tumour organoid models, confirming that organoids are an effective platform for evaluating CAR cell efficacy and tumour specificity [93, 94].
Michie J established human epidermal growth factor receptor-2 (HER2)-expressing colorectal cancer organoids and added anti-HER2 CAR-T cells for treatment [93]. CAR-T cells alone caused only weak killing, while the combination of CAR-T cells and the apoptosis antagonist birinapant significantly increased the death of tumour organoids. This combination therapy could also initiate potent tumour-killing effects. Colorectal cancer organoids were also used to track CAR-mediated cytotoxicity in NK cells in a study by the Schnalzger TE research group. Using a colorectal cancer organoid model, the researchers demonstrated efficient targeting of CAR-NK cells against the ubiquitous epithelial antigen epithelial cell adhesion molecule (EpCAM) [94]. Specific CAR-NK cells effectively killed tumour organoids expressing EGFRvIII but not normal tissue organoids. PDTOs could be used to monitor and enhance the recruitment and infiltration of immune cells into tumour tissues and serve as a platform to evaluate the efficiency of tumour-specific T-cell killing.
Recently, Carine Bouffi successfully developed a new generation of complex intestinal organoids that include key elements of a functional immune system. This is the first in vivo organoid containing a functional immune system that scientists have developed thus far [95]. These results indicated that organoids have broad application prospects in tumour immunotherapy.
Organoids-on-chip
Organoids-on-chip technology combines organoids and chips and integrates the advantages of organoids and organ chips [96]. An organ physiological microsystem is built on a chip, which can be used to predict the body’s response to drugs or different external stimuli. Mature organoid chips can almost simulate the microenvironment of the human body. Studies have shown that organoids-on-chips have great advantages over ordinary organoid models. In August 2022, the Food and Drug Administration (FDA) approved the world’s first new drug based entirely on organoid-on-a-chip research and obtained preclinical data to enter clinical trials (NCT04658472). This milestone means that in vitro drug testing through the organoid-on-chip platform will replace the traditional in vitro model and is increasingly used in the development of new drugs [97].
The organoid-on-a-chip is constructed directly from human tissue. Thus, the drug effect can be more fully evaluated before clinical trials, and inappropriate drugs can be excluded, thereby improving the success rate of drug development. Organoids-on-chip address the shortcomings of traditional organoid applications, such as the lack of immune cells and vascular structures, and these advantages are crucial for research in the field of tumours [96]. Organoids-on-chip integrate microfluidic technology, which can control the time of exogenous substance addition [98]. Shirure et al. established a tumour organoid-on-a-chip that can mimic angiogenesis of endothelial cells and support the construction of breast cancer patient-derived organoid blood vessels [99]. Although this process was distinct from vascularization during embryogenesis, it could also help characterize the tumour microenvironment in vivo and maintain organoid growth. Microengineering techniques incorporated in organoid chips could assist organoids in mimicking the various types of mechanical forces experienced during real development, which are integral to regulating organ development and maturation. The platform mimicked the intraluminal flow and rhythmic contractions of the stomach in vivo by using a peristaltic pump attached to a pipette [100].
Organoids can simulate the interaction between different tissues and different organs by using microfluidic arrays to coculture organoids from different tissue sources. Jin Y et al. established a multichamber microchip device for culturing hepatocytes and human endothelial cells. By simulating blood circulation in vivo using a laboratory rocker, vascularized liver organoids were generated after 21 days [101]. The researchers also cocultured liver, intestinal, and gastric organoids and allowed the different culture chambers to communicate through media flow. The results showed that paracrine factors produced by intestinal organoids reduced the expression of bile acid synthase in liver organoids, revealing the interactions between physiological organs [101].
Organoids-on-chip play an important role in the fields of new drug development, personalized treatment and regenerative medicine by combining organoid technology and organ chip technology. However, due to technical limitations, organoids-on-chips still have many limitations. With the development of technology and the integration of multiple disciplines, it is believed that organoids-on-chip can better contribute to human health in the future.
Application of organoids in cancer research
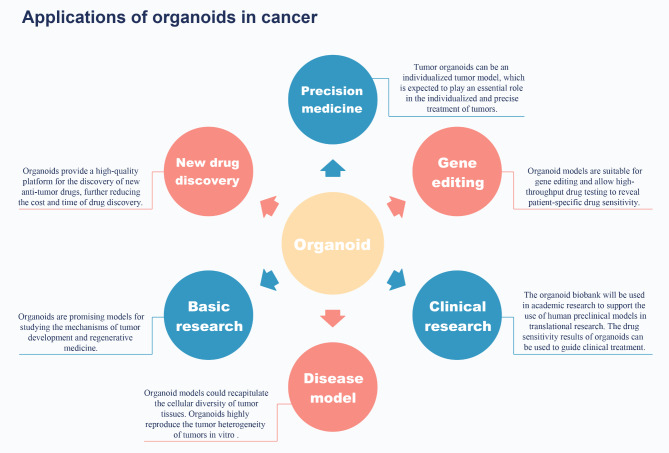
Organoids are of great value in cancer research. Organoids play an important role in disease models, precision medicine, new drug discovery, gene editing, and basic and clinical research (Fig. 3).
Fig. 3.
The application of organoids in cancer is mainly reflected in six aspects: precision medicine, gene editing, clinical research, disease modelling, basic research, and new drug discovery
Organoids are promising models in vitro, and they provide a platform for basic and clinical research on cancer. In February 2018, Science published a study using tumour organoid models to predict tumour responsiveness to anticancer drugs. It was found that the tumour organoid model predicted patient response to drugs with an overall sensitivity of 100%, a specificity of 93%, a positive predictive value of 88%, and a negative predictive value of 100% [80]. In short, we can know ahead of time which treatments are not working for patients, freeing up valuable time to try treatments that might work.
Tumour organoids are most commonly used in drug sensitivity screening to guide individualized precision medicine approaches for cancer treatment [102, 103]. Drug sensitivity testing of tumour organoids is similar to that of cell lines cultured in vitro. Both chemotherapy drugs and most targeted drugs can be tested, and both single drugs and drug combinations can be examined. However, not all chemotherapeutic drugs are suitable for drug sensitivity testing with tumour organoids. These drugs include (1) chemotherapeutic drugs that need to be metabolized in vivo to produce antitumour active ingredients, such as capecitabine and irinotecan. They also include (2) certain targeted drugs, such as antiangiogenic targeted drugs (axitinib), that act through vascular endothelial growth factor receptor (VEGFR)-1/2/3 in vascular endothelial cells, as tumour organoids cultured in vitro do not contain vascular structures. Another drug class that is not suitable is (3) immunotherapy drugs, such as immune checkpoint inhibitors, as their function requires the participation of the tumour immune microenvironment in vivo.
Organoids are of great value for the development of new drug discovery [104, 105]. Using organoids in the early stages of drug development can help identify those drugs that work for most patients and even for tumours with specific mutations. Additionally, using organoids from healthy tissue can identify side effects of drugs on the organ [106, 107]. One of the main reasons for drug failure in clinical trials is liver toxicity, and hepatocyte organoids can be used to test the liver toxicity of potential new drugs [108]. However, the use of organoid drug sensitivity screening to guide clinical treatment is in its infancy, and a large number of clinical studies are still needed to confirm its reliability and safety.
Organoids can be genetically engineered using CRISPR‒Cas9 gene editing technology to study the effects of specific oncogenic mutations controlled by alleles. In vitro models of colorectal cancer were induced by introducing mutations in knowledge representation for autonomous systems (KRAS), adenomatous polyposis coli (APC), recombinant mothers against decapentaplegic homologue 4 (SMAD4), and tumour protein p53 (TP53) in wild-type intestinal organoids [109, 110]. Stephen Meltzer cultivated a gastro-oesophageal junction (GEJ) organoid from healthy human tissue and used CRISPR‒Cas9 gene editing technology to knock out two key tumour suppressor genes, TP53 and Cyclin Dependent Kinase Inhibitor 2 A (CDKN2A), thus revealing the mechanism of early GEJ tumour development [111].
Organoids have been used in different tumour types (Table 2). Herein, we describe the current status of organoids in the field of tumour research according to the time sequence in which tumour organoids were successfully established.
Table 2.
The time of the first report of different tumor organoids
| Sources of Tumor Organoids | First Report Time | Publication | References |
|---|---|---|---|
| Colon | 2011 | Gastroenterology | [48] |
| Prostate | 2014 | Cell | [10] |
| Pancreas | 2015 | Cell | [50] |
| Brain | 2016 | Cancer Research | [122] |
| Liver | 2017 | Nature Medicine | [15] |
| Endometrium | 2017 | Nature Cell Biology | [133] |
| Stomach | 2018 | Science | [80] |
| Breast | 2018 | Cell | [51] |
| Bladder | 2018 | Cell | [20] |
| Esophagus | 2018 | Nature Communications | [149] |
| Lung | 2019 | Nature Communications | [152] |
| Rectal | 2019 | Nature Medicine | [79] |
| Kidney | 2019 | Cell Death & Disease | [158] |
| Biliary tract | 2019 | Cell Reports | [102] |
| Ovary | 2019 | Nature Medicine | [11] |
Gut
Colon cancer organoids were the first successfully established tumour organoids. In 2011, these organoids were first reported by Professor Hans Clevers, the originator of organoids [48]. Since then, colon cancer organoids have become an important model for studying the evolution of colon cancer. In 2017, Toshiro Sato confirmed that LGR5 + tumour cells have the characteristics of tumour stem cells, revealing their self-renewal and differentiation capabilities [112]. Knockout of LGR5 + cells leads to transient tumour regression, but KRT20 + tumour cells quickly differentiate into LGR5 + tumour cells. These cells exert the characteristics of tumour stem cells and continue to promote tumour growth. Mark Schmitt’s research using colorectal cancer organoids has revealed the direct impact of dying cancer cells on adjacent tumour epithelial cells and elucidated the molecular mechanisms that lead to treatment resistance through paracrine effects [113]. The research results also create new opportunities for the treatment of tumour drug resistance. Using colorectal cancer organoids, Dmitrieva-Posocco O demonstrated that β-hydroxybutyrate (BHB) increased HOPX expression and inhibited the growth of patient-derived colorectal cancer organoids [114]. This study provides a new target for the treatment of colorectal cancer and has important clinical value.
The rectum and colon are highly similar in structure, but there are still some differences in molecular biological characteristics. Therefore, it is more suitable to establish rectal cancer organoids for rectal cancer research. In 2019, Smith, JJ established 65 tumour organoids from patients with primary, metastatic or recurrent rectal cancer. The established rectal cancer organoids retained the molecular characteristics of the source tumours, and the results of in vitro drug sensitivity experiments correlated with the clinical responses of tumour patients [79]. Guoqiang Hua found similar histopathological features between primary rectal cancer and rectal cancer organoids, and organoids showed a sensitivity of 78% and specificity of 92% in predicting the clinical response of patients. The study elucidated the strong correlation between the chemoradiotherapy sensitivity of patient-derived tumour organoids and the clinical responses observed in large cohort studies [77]. In short, colorectal cancer organoids are one of the earliest and most mature organoids and have been widely used in clinical and basic research on colorectal cancer. Thus, important research results are emerging continuously, and these organoid systems present good application prospects.
Prostate
Prostate cancer is a malignant tumour with high incidence in older men. Gao D’s research was the first to report that organoids derived from human prostate tumours can be grown in the laboratory. The researchers successfully generated six prostate cancer organoids and one organoid derived from circulating tumour cells. The tissue structure of these prostate cancer organoids was highly similar to that of the metastatic samples from which they originated [10]. The research results were published in Cell in 2014. In 2016, Hans Clevers published a culture protocol for generating 3D prostate organoids, which can be obtained in approximately 2 weeks. The prostate cancer organoids established according to this protocol highly imitate the characteristics of tumours in vivo and can be widely used in molecular mechanism research and drug screening for prostate cancer [115].
Zhang Z found that cancer-associated fibroblasts (CAFs) promote anti-androgen resistance in prostate organoids. Following blockade of the NeuReGulin-1/human epidermal growth factor receptor-3 (NRG1/HER3) axis using antibodies, tumours were resensitized to antiandrogen therapy [116]. Sawyers CL used mouse prostate organoids to study the biological characteristics of wild-type forkhead box protein A1 (FOXA1) and mutant FOXA1. Studies have confirmed that mutations in FOXA1 alter its function, disrupt the normal luminal epithelial differentiation program, and further promote prostate cancer progression [117]. In the future, it is hoped that prostate cancer organoids will bring new breakthroughs in the diagnosis and treatment of prostate cancer.
Pancreas
Pancreatic cancer is one of the tumours with the worst treatment effect and prognosis among digestive system tumours. Pancreatic cancer organoids were first established by the team of Professor Boj SF [10]. The research results were published in Cell in 2015. The researchers extracted normal and tumour tissues from mouse and human pancreases, respectively, and successfully established normal and tumour pancreatic organoids by modifying the previous method of culturing gastrointestinal tumour organoids. The success rate of creating pancreatic cancer organoids was approximately 80%. This study opened a new chapter in organoid research in pancreatic cancer.
In April 2021, Cell Stem Cell published two articles on pancreatic cancer organoids [118, 119]. Both studies used pluripotent stem cells (PSCs) that differentiated into pancreatic progenitor cells (PP) and then formed pancreatic organoids in Matrigel. The same oncogene mutations were then manipulated to induce the formation of pancreatic cancer organoids. The Harvard study created both pancreatic ductal and acinar organoids. These two studies provide detailed strategies for the establishment of pancreatic cancer organoids. In October 2021, Barbara T. Grünwald used pancreatic ductal carcinoma organoids to reveal the role of different mesenchymal subtypes (deserted, reactive, and intermediate) in tumour development and response to drug therapy [120]. They established pancreatic ductal carcinoma organoids and cocultured them with CAFs from different stromal subtypes. The results showed that reactive tumour stroma could promote tumour cell proliferation and shorten disease-free survival. This study clarified the mechanism of pancreatic cancer chemotherapy resistance and provided a new direction for the treatment of pancreatic cancer [120]. In the same year, Jorgensen, C. published a study revealing the role of the laminin-integrin α3/α6 signalling pathway in the establishment and survival of pancreatic organoids, establishing a more ideal pancreatic cancer organoid. It can better reproduce the characteristics of the tumour microenvironment and is more suitable for basic and translational research on pancreatic cancer [121].
At present, the establishment scheme of pancreatic cancer organoids has been continuously improved, and an increasing number of organoids are being used in clinical and basic research on pancreatic cancer. Pancreatic cancer organoids have brought new hope to overcome difficult problems, such as pancreatic cancer chemotherapy resistance.
Brain
Brain tumours are among the most aggressive and least survivable types of human tumours. In 2016, the team of Rich, JN reported brain tumour organoids for the first time [122]. The researchers created tumour organoids in tissue culture from glioblastoma. The biological characteristics of patient tumour-derived organoids are similar to those of parental tumours. Since then, brain tumour organoids have opened a new chapter in brain tumour research.
Brain tumour organoids have been widely used in clinical and basic research [123–127]. In 2018, Shan Bian established a brain tumour organoid model. The researchers claim that this model can reproduce the complex structure of the human brain and the occurrence of brain tumours in vitro and can be used for clinical drug screening. The study successfully screened 18 single-gene mutations and 15 mutation combinations that are considered to be critical for brain tumorigenesis using brain tumour organoid models [128]. Davy, A focused on the role of dihydrofolate reductase (DHFR) in brain tumours. The authors used brain tumour organoids and found that methotrexate can inhibit DHFR, reduce the self-renewal ability of brain tumour organoids, and inhibit the tumorigenic ability of brain tumour initiating cells (BTIC) [129]. In conclusion, brain tumour organoids provide a platform for rapid screening of oncogenes and screening of effective drugs in vitro, bringing new hope to the research of brain tumours.
Liver
Liver cancer is one of the most common malignant tumours. Humans have been devoted to research on liver cancer for more than half a century. There is an urgent need to identify the underlying mechanism of liver cancer progression and explore new therapeutic drugs. A study published in the journal Nature Medicine in 2017 brought new hope to liver cancer research [15]. Researchers successfully grew the first human primary liver cancer organoid model in the laboratory and used it to test 29 different cancer drugs. This research achievement was regarded as a major milestone in the development of liver cancer research [15]. In 2018, Nuciforo, S used patient biopsy tissue to construct liver cancer organoids for drug sensitivity testing [130]. The researchers used tumour organoids to conduct drug sensitivity tests on sorafenib and found that organoids from different patients had different sensitivities to sorafenib. The results of the study confirmed that the organoid model derived from the biopsy tissue was highly consistent with the parental tumour tissue, which can provide a tool for the development of individualized precision therapy [130].
In 2019, researchers used CRISPR gene editing technology to alter the genes of normal human liver organoids to explore the role of a certain gene mutation in tumour formation. Using this approach, the researchers found that BAP1 mutations altered organoid behavioural traits, such as faster growth and easier fusion. These features resemble more aggressive malignancies [131]. In early 2023, Bin Li reported using tumour organoids derived from liver cancer patients to screen 419 FDA-approved drugs and found that loratadine could inhibit the growth of liver cancer organoids [132]. This study used tumour organoid models to confirm that desloratadine may be a new type of anticancer drug, and NMT1 is expected to become a potential biomarker and therapeutic target for liver cancer.
Endometrium
Endometrial cancer is a malignant tumour that occurs in the endometrium. It is one of the three major tumours of the female reproductive system and accounts for 20-30% of these tumours. In 2017, Turco, MY established three-dimensional cultures of normal and decidualized human endometrium by adapting the conditions used to establish human adult stem cell-derived organoid cultures. The researchers also grew organoids from malignant endometrium for the first time [133]. These organoids are capable of long-term expansion, providing an in vitro model for the study of endometrial cancer. In the same year, Girda, E reported the establishment process of endometrial cancer patient-derived organoids, which were used to screen various drugs, including endocrine therapy drugs. Studies have confirmed that organoids are very similar to the source tumours in histomorphology and immunohistochemical expression [134]. Therefore, in vitro drug susceptibility testing using endometrial cancer patient-derived organoids is feasible. In 2019, Boretto, M reported the establishment of organoids extracted from low- to high-grade endometrial cancer patients for in vitro drug screening [17]. In the future, these organoids may replace patient-derived xenograft (PDX) mouse models, significantly reducing the overall cost associated with in vivo models [17, 135].
Stomach
Gastric cancer is a common malignant tumour of the digestive tract. Gastric cancer organoids were first established by Vlachogiannis G., and the research was published in Science in 2018 [80]. The genotypes of the gastric cancer patient-derived organoids established by the researchers were highly similar to the original patient tumours, and the molecular analysis of the tumour organoids highly matched the drug screening results [80]. These research results provide a new in vitro model for clinical and basic research on gastric cancer. In August, a research team established a biobank of gastric organoids. The library consists of genetically engineered gastric organoids carrying various mutations and 37 patient-derived organoids. This study details the characterization of human gastric cancer organoids derived from patients with different tumour subtypes. The results revealed the key pathways of gastric carcinogenesis, emphasizing the important value of genotype-phenotype screening strategies for an in-depth understanding of gastric cancer [136]. In December, the team of Professor Yan, H.H.N. reported the establishment of a primary gastric cancer organoid biobank, which contained normal, abnormal hyperplasia, cancer and lymph node metastasis samples from 34 patients [74]. Tumour organoids are highly similar to the source tumour in terms of morphological characteristics, genome profile and transcriptome profile and can be used for large-scale drug screening. Thus, they can serve as high-quality resources for molecular mechanism research and individualized precision treatment of gastric cancer. Since 2018, gastric cancer organoids have been gradually applied in basic and clinical research on gastric cancer, and research results have continued to emerge.
In 2019, human gastric cancer organoids were established by the team of Seidlitz, T. team. These organoids simulated the typical characteristics and pathway variations of human gastric cancer and confirmed the application value of gastric cancer organoids in studying molecular mutation patterns and drug sensitivity [137]. The study found that trastuzumab can be used to target organoids carrying HER2 mutations, and palbociclib can be used to target organoids carrying CDKN2A deletion mutations. Gastric cancer organoids can be used not only to identify effective drugs but also to determine which drugs are resistant. Thus, they can be used to reveal drugs that only cause side effects but have little therapeutic effect and should therefore be avoided in clinical treatment [137]. In addition to being used in drug screening, gastric cancer organoids can also be combined with technologies such as CRISPR/Cas9 editing to explore the characteristic phenotypes and functions of different mutation types of gastric cancer. In 2021, Yuan-Hung Lo used primary human gastric organoids combined with gene editing technology to establish the first genetic model of ARID1A mutations, and its multiomics analysis revealed many characteristic phenotypes of ARID1A mutant gastric cancer [138]. The results of the study found that the loss of ARID1A caused mucinous metaplasia, inhibited Wnt/β-Catenin pathway activity, and promoted tumorigenesis in primary human gastric organoids [138]. Human tumour organoids edited by CRISPR/Cas9 technology provide a valuable platform for studying human gastric cancer development. Gastric cancer organoids have good application prospects in the future and are effective tools for research on the molecular mechanisms of gastric cancer occurrence and evolution, biomarker identification, drug screening, and preclinical evaluation of individualized drug strategies [139].
Breast
In January 2018, Hans Clevers first reported a method for long-term culture and maintenance of breast epithelial organoids, and the success rate of breast cancer organoid culture was over 80% [51]. The team successfully established more than 100 breast cancer primary tumour and metastatic tumour organoids. They also conducted in-depth histopathological and gene sequencing analysis on the organoids and conducted in-depth research on the drug sensitivity of breast cancer organoids. Breast cancer organoids well maintain the characteristics of the original tumour in histopathological morphology. In February of the same year, an article published in Nature Methods introduced how to use the breast cancer susceptibility gene (BRCA) mutation mouse model of breast cancer to establish organoids and used this model for therapeutic drug research [140]. The research team that published the article is from the Netherlands, and Hans Clevers is also on the list of authors. The P53 and BRCA1/2 genes are well-known tumour suppressor genes, and their mutations can cause tumour syndromes, one of which is breast cancer. This study described the establishment method of P53/BRCA gene mouse breast cancer organoids and confirmed the application value of breast cancer organoids in breast cancer research.
Breast cancer organoids provide a good model for molecular mechanism research and clinical drug screening of breast cancer. Cong, M used breast cancer organoids to explore the molecular mechanism of metastasis suppressor 1 (MTSS1) [141]. Xiaolong Wang used patient-derived organoids to demonstrate that targeting circRNA-CREIT could serve as a promising therapeutic strategy for chemotherapy-resistant triple-negative breast cancer [14]. Chen Ping established a breast cancer organoid biobank that contains tumour tissues of different disease stages and molecular subtypes [142]. Patient-derived breast cancer organoids can serve as a diagnostic platform to support and guide drug therapy in advanced breast cancer. There are several different molecular subtypes of breast cancer, and there are significant differences in the genomic variation among the various subtypes, which is the basis for the choice of treatment options in advanced breast cancer. Breast cancer organoids provide a good platform for the in-depth exploration of the differential characteristics of different subtypes, which is of great significance in breast cancer precision medicine research.
Bladder
Bladder cancer is a malignant tumour originating from the urothelium of the bladder, and it ranks first in the incidence of urinary system tumours. In 2018, the team of Professor Michael M. Shen successfully cultivated bladder cancer organoids for the first time, and the research results were published in the journal Cell [20]. Researchers isolated tumour cells from bladder cancer of 22 patients and cultured them into organoids with a diameter of approximately 1 mm in vitro [20]. These tumour organoids shared the same molecular and genetic characteristics as the patient’s actual tumour. The researchers also plan to conduct clinical trials to test the accuracy of bladder cancer organoids in predicting treatment response.
Bladder cancer organoids are increasingly used for tumour biology research and drug screening [143–147]. Tan, P reported organoid-based small molecule drug screening for bladder cancer for the first time [144]. The study found that the Sirtuin1 (SIRT1) activator SRT1720 significantly inhibited the growth of mouse and human bladder cancer organoids. Another research team reported the use of patient-derived bladder cancer organoids to evaluate CAR-T-cell-mediated cytotoxicity against bladder cancer cells [148]. The research team successfully cultivated two main subtypes of bladder cancer organoids using the self-developed bladder cancer organoid culture system. Immunofluorescence staining experiments confirmed that the staining results of molecular markers of bladder cancer organoids and their source tumour tissues were consistent, which indicated that the biomarkers in cancer tissues were well reproduced on bladder cancer organoids. To evaluate the application of bladder cancer organoids in testing the killing effect of CAR-T cells, researchers cocultured bladder cancer organoids with mucin 1 (MUC1)-CAR-T cells. The results showed that the expression levels of interleukin-2 (IL-2), tumour necrosis factor α (TNF-α), and interferon γ (IFN-γ) were all significantly increased. These results fully demonstrated the potential application value of bladder cancer organoids in evaluating the killing effect of CAR-T cells [148].
Oesophagus
In 2018, a research team reported the successful establishment of 10 oesophageal adenocarcinoma organoid models [149]. In this study, the medium of gastric cancer organoids was used to culture oesophageal cancer organoids. However, only 10 of the 31 samples were successfully established as organoids, and 9 of them could be passaged for more than half a year. The author analysed the possible reason that most of the experiments failed and found that it was due to an insufficient amount of initial tumour cells. Genomic analysis revealed that oesophageal cancer organoids are as polyclonal as the original tumour. A more interesting finding is that organoids are not static during subculture. Additionally, some subclones gradually become dominant clones because they are better adapted to the environment, resulting in changes in the genomics of organoids [149]. This discovery is of great value to the study of tumour evolution and tumour drug resistance. This study confirmed the potential value of using organoids for individualized tumour drug sensitivity screening and demonstrated the application prospects of tumour organoids in oesophageal cancer research. The 3D oesophageal cancer organoid model has also been used to study the molecular mechanism of oesophageal cancer [150, 151]. Professor Tang, Q. ‘s team used oesophageal cancer organoids to find that the knockdown of the Rab11-FIP1 gene led to increased organoid size. Loss of Rab11-FIP1 increases tumour cell invasion, partly through mutated p53 but also in an independent manner [151]. The researchers cultured organoids from stem cells in the patient’s own normal tissue and then used CRISPR/Cas9 technology to knock out two key tumour suppressor genes (TP53 and CDKN2A) in the organoids. Knocking out these genes made the cells more cancerous, and they rapidly grew into malignancies. This model may be used to further our understanding of the early occurrence and progression of gastroesophageal junction carcinogenesis and reveal its potential therapeutic strategies [111].
Lung
In 2019, Kim, M. first reported lung cancer organoids [152]. In this study, different types of primary lung cancer tissue and normal bronchial tissue were used as samples for organoid culture, and then the cultured organoids were compared with the primary tissue for histology, morphology, and genomics. Studies have confirmed that lung cancer organoids are highly consistent with primary tumour tissues. Lung cancer organoids were used for drug sensitivity tests of docetaxel, erlotinib, crizotinib, and olaparib, and finally, the PDX model was used to verify the drug screening results of lung cancer organoids [152]. Drug screening using lung cancer organoids is highly consistent with gene sequencing results, and the results are more accurate and intuitive. Afterwards, several laboratories successively reported the successful establishment of lung cancer organoids and conducted related research [153–156]. In January 2023, Yilong Wu published the largest research cohort of lung cancer organoids in the world. The main aim of the study was to predict the efficacy of locally advanced or metastatic lung cancer using patient-derived organoids [157]. The researchers established 212 lung cancer organoids from 107 patients, with a success rate of 81.5%. Based on the accuracy of the drug sensitivity test results of lung cancer organoids to predict clinical efficacy, the results confirm that lung cancer organoids have the ability to predict the effectiveness of treatment options. Therefore, lung cancer organoids are a promising tool for precision medicine.
Kidney
Renal cell carcinoma (RCC) is a malignant tumour originating from the epithelium of the urinary tubules of the renal parenchyma. In 2019, Bonci D. first reported the establishment of kidney cancer organoids and established 10 kidney cancer organoids from 15 samples [158]. For the first time, this study confirmed the future prospects and application value of kidney cancer organoids.
In 2020, a study reported the establishment of the first childhood kidney cancer organoid biobank, providing an impetus for childhood kidney cancer research [159]. Single-cell RNA sequencing analysis revealed that organoids from different patients had distinct cell populations and distinct cell subpopulations within the same organoid. Thus, Wilms tumour organoids retained the cellular heterogeneity of the disease. The organoids were highly similar to their corresponding tumour tissues. They retained the epigenetic characteristics of the original tumour, and serial passages did not disturb the genetic stability of organoids. The researchers used childhood renal cancer organoids for drug sensitivity testing, and the results showed that childhood renal cancer organoids can be used for high-throughput drug testing to reveal patient-specific drug sensitivity [159]. In 2022, Xue, W. established 7 strains of kidney clear cell carcinoma organoids and conducted a drug sensitivity test on toripalimab (a PD-1 inhibitor), confirming that tumour organoids are a promising and reliable preclinical model for predicting the response to immunotherapy in RCC [160]. In the same year, Weiren Huang’s study also confirmed that patient-derived RCC organoids were valuable preclinical models for academic research and personalized medicine [161].
Challenges and future perspectives
As a 3D cell culture model, organoids are highly similar to human organs in structure and function and have the characteristics of cell proliferation and differentiation, self-renewal, self-assembly, long-term culture, and genetic stability [162]. A number of studies have confirmed that organoids have a high degree of clinical relevance and can efficiently carry out drug sensitivity testing for patients, making individualized precise tumour treatment possible [6, 163]. Compared with established cell lines, cell line-derived xenograft (CDX) and PDX models, tumour organoids are irreplaceable as disease models (Table 3). However, we must also acknowledge that the development of organoids is still at an early stage, and there are still many limiting factors restricting the application of this technology.
Table 3.
Comparison of advantages and disadvantages of common cancer models
| Cell model | Animal model | Organoid model | |||||
|---|---|---|---|---|---|---|---|
| Primary cells | Cell lines | Cell-derived xenograft | Patient-derived xenograft | ||||
| Methods | Cells isolated directly from animal or human tissue. | Cells that converge in function, metabolism, and morphology. Infinite proliferation and immortalization. | The tumour cells cultured in vitro were inoculated subcutaneously into immunodeficient mice. | Patient-derived tumour tissue was implanted into immunodeficient mice. | Derived from embryonic stem cells or induced pluripotent stem cells (iPSCs). Derived from tumour tissue of patients. | ||
| Advantages | Similar characteristics to animal or human cells. | Less interference factors, easy synchronization, easier control of experimental conditions and easy gene manipulation. | The effect on the host is similar. Tumour morphology, growth rate, drug sensitivity, and death time of animals were very similar. | Preserve the microenvironment of parental tumour growth. High tumour similarity. Preserve tumour heterogeneity. | Simulate the complexity of tumour microenvironments. High plasticity. The cultivation time is short. There are no ethical issues. | ||
| Disadvantages | Poor uniformity. The proliferative ability is low and cannot be passaged. The transfection efficiency is low. | Partial or complete loss of the characteristics of primary cells. Mutations may occur during long-term passage. | The growth rate is fast, the proliferation ratio is high, and the volume doubling time is short, which is significantly different from human tumours. | The in vivo microenvironment cannot be fully simulated. Model building takes a long time. The success rate of model building is low. | Lack of innate immune cells. No endocrine and neural regulation. The technology is not yet mature. | ||
The reproducibility and consistency of organoid culture are the first problems to be addressed that largely affect the development of organoids. At present, there is no standardized process for organoid harvesting, culture, cryopreservation and recovery, and there is a lack of established industry standards. There are many human factors involved in the establishment of organoids, resulting in differences in the organoids established by different researchers. Even organoids created by the same researcher in different batches varied in shape and function [164]. To address this problem, it is necessary to continuously explore the optimal cultivation conditions and formulate recognized standards. It is also necessary to improve the automation level of the organoid establishment process and reduce the interference of human factors. In addition, organoid identification technology is important for ensuring good repeatability and consistency. However, the current observation of organoids is mainly focused on morphology, and technology to detect various indicators of living organoids in real time is still relatively lacking.
Currently, mouse-derived extracellular matrix (ECM) substitutes and foetal bovine serum are commonly used as culture media. Matrigel is difficult to apply to many human treatments because it contains exogenous components. Compared with the human physiological environment, organoids lack connective tissue, blood vessels and immune cells [162, 164]. To address this issue, the main methods used by researchers are techniques such as coculture and microfluidics. In 2020, the journal Nature Protocol published a protocol for the coculture of tumour organoids and immune cells, which can simulate some characteristics of the tumour microenvironment and the interactions between tumours and immune cells [165]. Although some studies have reported the combined application of organoids and microfluidic technology, microfluidic technology is still immature. Moreover, engineering control of the organoid culture process is still an urgent problem to be addressed. In addition, the diameter of existing organoids is approximately 100–500 μm. Although this size range has a certain degree of scale effect, it is still difficult to simulate real tissues and organs. If larger organoids are to be produced, the vascularization of organoids is also a problem that needs to be addressed.
Although patient-derived tumour organoids can reflect some tumour heterogeneity, patient-derived tumour organoids cannot fully reproduce the tumour microenvironment [166]. Some tumours, such as gastric cancer, have high tumour heterogeneity. Thus, gastric cancer organoids cannot fully reflect the heterogeneity of gastric cancer tissues in the body, which will reduce the efficiency of drug screening for gastric cancer organoids [164]. The current method to address this issue is to take tumour tissues at multiple points to build organoids, but this still cannot fully reflect the heterogeneity of tumours in vivo. As a new type of drug screening model, organoids are more cost effective than PDXs but are still more expensive than cell lines. Compared to the culture of cancer cell lines, the cultivation of organoids requires more time and resources. Organoids do not have an absolute advantage over other models in any aspect, so comprehensive judgement should be used to decide the best method.
Recently, a study reported by Professor Carine Bouffi was the first attempt to establish intestinal organoids in humanized mice. The team successfully obtained intestinal organoids with immune cells, opening the era of next-generation organoids [95]. From the 16th week, human intestinal organoids already have the typical signs of embryonic intestinal immune development, such as the accumulation and partitioning of T cells and B cells and the infiltration of granulocytes and plasma cells. This study overcomes the limitation of organoids lacking an immune microenvironment and opens a new platform in the field of immunotherapy of organoids. It is expected to bring new opportunities for tumour immunotherapy in the future.
Although there are many difficulties in the road ahead, we still have hope for the future of organoid research. Researchers are paying more attention to organoids than ever before. It is believed that in the near future, industry standards for various organoids will emerge. The combination of organoids and new technologies has continuously broken through application limitations and brought novel advancements to research. Examples include the combination of organoids with in vivo real-time imaging technology, 3D bioprinting technology, and the “Human Cell Atlas (HCA)” [167–169]. As a highly simulated disease model, organoids are expected to continue to make new progresses in precision medicine, regenerative medicine and other fields. We have reason to expect that organoids will play a greater role in the field of biological and clinical medical research in the future.
Conclusion
The development of organoid technology has provided a new cancer model. Tumour organoids can simulate the structure and function of tumours in vivo to the greatest extent and are more suitable for in vitro tumour biology and treatment-related research. With the continuous deepening and progress of organoid research, the current bottlenecks that need to be overcome will be addressed; such solutions include further optimizing conditions to improve the timeliness of detection, improve the success rate of culture, simulate organoid vascularization, and simulate the tumour immune microenvironment. The response of organoids to drugs is highly correlated with patient clinical data, providing a reliable model for cancer research and treatment. In the future, organoids will complement other models and technologies and will open up new horizons for new drug development, drug screening, tumour mechanism research, and personalized precision therapy.
Acknowledgements
Not applicable.
Author contributions
ZF and PJ L wrote the manuscript. FY D was responsible for figures and tables drawing. LS and LP L supervised this work. All authors read and approved the final manuscript.
Funding
This paper was supported by the National Natural Science Foundation of China (82203854); Key Research and Development Program of Shandong Province (No.2019JZZY010104; 2021CXGC011104); Special Foundation for Taishan Scholars Program of Shandong Province (No.ts20190978); Academic promotion programme of Shandong First Medical University (No. 2019QL021).
Data Availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Abbreviations
Three-dimensional (3D); Patient-derived tumor organoids (PDTOs); Extracellular Matrix (ECM); Engelbreth-Holm-Swarm (EHS); Mesenchymal stem cells (MSC); Induced Pluripotent Stem Cells (iPSCs); EGF, Noggin, R-spondin (ENR); Colorectal cancer (CRC); Embryonic stem cells (ESCs); Adult stem cells (ASCs); Human pluripotent stem cell (hPSC); Circulating tumor cells (CTCs); Patient Derived Organoid (PDO); Rhabdomyosarcomas (RMS); Tumor-infiltrating lymphocytes (TILs); Food and Drug Administration (FDA); β-hydroxybutyrate (BHB); Cancer-associated fibroblasts (CAFs).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhen Fang, Peijuan Li and Fengying Du these authors contributed equally to this article.
Contributor Information
Liang Shang, Email: shangl20@yeah.net.
Leping Li, Email: docfli@163.com.
References
- 1.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–87. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 2.Dart A. Organoid 2.0. Nat Rev Cancer. 2019;19(3):126–7. doi: 10.1038/s41568-019-0108-x. [DOI] [PubMed] [Google Scholar]
- 3.Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364(6444):952–5. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 4.Tenreiro MF, Branco MA, Cotovio JP, Cabral JMS, Fernandes TG, Diogo MM. Advancing organoid design through co-emergence, assembly, and bioengineering. Trends Biotechnol. 2023:S0167-7799(0122)00349 – 00343. [DOI] [PubMed]
- 5.Beydag-Tasoz BS, Yennek S, Grapin-Botton A. Towards a better understanding of diabetes mellitus using organoid models. Nat Rev Endocrinol. 2023;19(4):232–48. doi: 10.1038/s41574-022-00797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauve CG, Adileh M, Wasserman I, Marco MR, Kim AS, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25(10):1607–14. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 8.Dekkers JF, Alieva M, Cleven A, Keramati F, Wezenaar AKL, van Vliet EJ, Puschhof J, Brazda P, Johanna I, Meringa AD, et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat Biotechnol. 2023;41(1):60–9. doi: 10.1038/s41587-022-01397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minoli M, Cantore T, Hanhart D, Kiener M, Fedrizzi T, La Manna F, Karkampouna S, Chouvardas P, Genitsch V, Rodriguez-Calero A, et al. Bladder cancer organoids as a functional system to model different disease stages and therapy response. Nat Commun. 2023;14(1):2214. doi: 10.1038/s41467-023-37696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176–87. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopper O, de Witte CJ, Lohmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838–49. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Jiao D, Liu A, Wu K. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol. 2022;15(1):58. doi: 10.1186/s13045-022-01278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z, Li K, Qin K, Liang J, Shi M, Ma Y, Zhao S, Liang H, Han D, Shen B, et al. The LINC00623/NAT10 signaling axis promotes pancreatic cancer progression by remodeling ac4C modification of mRNA. J Hematol Oncol. 2022;15(1):112. doi: 10.1186/s13045-022-01338-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Wang X, Chen T, Li C, Li W, Zhou X, Li Y, Luo D, Zhang N, Chen B, Wang L, et al. CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J Hematol Oncol. 2022;15(1):122. doi: 10.1186/s13045-022-01345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarro LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–35. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, Zhang T, Pipalia NH, Chen HJ, Witherspoon M, et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 2017;23(7):878–84. doi: 10.1038/nm.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–51. doi: 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- 18.Weber C. Organoids test drug response. Nat Cell Biol. 2018;20(6):634. doi: 10.1038/s41556-018-0116-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21(11):1364–71. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, et al. Tumor evolution and drug response in patient-derived Organoid models of bladder Cancer. Cell. 2018;173(2):515–28. doi: 10.1016/j.cell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T. Organoids for Drug Discovery and Personalized Medicine. Annu Rev Pharmacol Toxicol. 2019;59:447–62. doi: 10.1146/annurev-pharmtox-010818-021108. [DOI] [PubMed] [Google Scholar]
- 22.van Tienderen GS, Li L, Broutier L, Saito Y, Inacio P, Huch M, Selaru FM, van der Laan LJW, Verstegen MMA. Hepatobiliary tumor organoids for personalized medicine: a multicenter view on establishment, limitations, and future directions. Cancer Cell. 2022;40(3):226–30. doi: 10.1016/j.ccell.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Seidlitz T, Koo BK, Stange DE. Gastric organoids-an in vitro model system for the study of gastric development and road to personalized medicine. Cell Death Differ. 2021;28(1):68–83. doi: 10.1038/s41418-020-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20(7):377–88. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillen KP, Fujita M, Butterfield AJ, Scherer SD, Bailey MH, Chu Z, DeRose YS, Zhao L, Cortes-Sanchez E, Yang CH, et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3(2):232–50. doi: 10.1038/s43018-022-00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demyan L, Habowski AN, Plenker D, King DA, Standring OJ, Tsang C, St Surin L, Rishi A, Crawford JM, Boyd J, et al. Pancreatic Cancer patient-derived Organoids can predict response to Neoadjuvant Chemotherapy. Ann Surg. 2022;276(3):450–62. doi: 10.1097/SLA.0000000000005558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong TL, Loh JJ, Lu S, Yan HHN, Siu HC, Xi R, Chan D, Kam MJF, Zhou L, Tong M, et al. ADAR1-mediated RNA editing of SCD1 drives drug resistance and self-renewal in gastric cancer. Nat Commun. 2023;14(1):2861. doi: 10.1038/s41467-023-38581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beshiri M, Agarwal S, Yin JJ, Kelly K. Prostate organoids: emerging experimental tools for translational research. J Clin Invest. 2023;133(10):e169616. doi: 10.1172/JCI169616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson HV. A New Method by which sponges may be artificially reared. Science. 1907;25(649):912–5. doi: 10.1126/science.25.649.912. [DOI] [PubMed] [Google Scholar]
- 30.Weiss P, Taylor AC. Reconstitution of Complete Organs from single-cell suspensions of Chick embryos in Advanced Stages of differentiation. Proc Natl Acad Sci U S A. 1960;46(9):1177–85. doi: 10.1073/pnas.46.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce GB, Jr, Verney EL. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer. 1961;14:1017–29. doi: 10.1002/1097-0142(196109/10)14:5<1017::AID-CNCR2820140516>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Evans M. Origin of mouse embryonal carcinoma cells and the possibility of their direct isolation into tissue culture. J Reprod Fertil. 1981;62(2):625–31. doi: 10.1530/jrf.0.0620625. [DOI] [PubMed] [Google Scholar]
- 33.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987;84(1):136–40. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shannon JM, Mason RJ, Jennings SD. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim Biophys Acta. 1987;931(2):143–56. doi: 10.1016/0167-4889(87)90200-X. [DOI] [PubMed] [Google Scholar]
- 36.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 40.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3(5):519–32. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 42.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 43.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 45.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 49.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–45. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–38. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al. A living biobank of breast Cancer Organoids captures Disease Heterogeneity. Cell. 2018;172(1–2):373–86. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Sutani A, Kaneko R, Takeuchi J, Sasano T, Kohda T, Ihara K, Takahashi K, Yamazoe M, Morio T, et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun. 2020;11(1):4283. doi: 10.1038/s41467-020-18031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, Li MY, Birey F, Yang X, Saw NL, et al. Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022;610(7931):319–26. doi: 10.1038/s41586-022-05277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D Organoid Systems. Trends Mol Med. 2017;23(5):393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Gjorevski N, Nikolaev M, Brown TE, Mitrofanova O, Brandenberg N, DelRio FW, Yavitt FM, Liberali P, Anseth KS, Lutolf MP. Tissue geometry drives deterministic organoid patterning. Science. 2022;375(6576):eaaw9021. doi: 10.1126/science.aaw9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hillion K, Mahe MM. Redesigning hydrogel geometry for enhanced organoids. Nat Methods. 2022;19(11):1347–8. doi: 10.1038/s41592-022-01656-3. [DOI] [PubMed] [Google Scholar]
- 57.Pitstick AL, Poling HM, Sundaram N, Lewis PL, Kechele DO, Sanchez JG, Scott MA, Broda TR, Helmrath MA, Wells JM, et al. Aggregation of cryopreserved mid-hindgut endoderm for more reliable and reproducible hPSC-derived small intestinal organoid generation. Stem Cell Reports. 2022;17(8):1889–902. doi: 10.1016/j.stemcr.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang S, Xu F, Jin M, Wang Z, Xu X, Zhou Y, Wang J, Gu L, Fan H, Fan Y, et al. Development of a high-throughput micropatterned agarose scaffold for consistent and reproducible hPSC-derived liver organoids. Biofabrication. 2022;15(1):1. doi: 10.1088/1758-5090/ac933c. [DOI] [PubMed] [Google Scholar]
- 59.Chakrabarti J, Koh V, So JBY, Yong WP, Zavros Y. A Preclinical Human-Derived Autologous Gastric Cancer Organoid/Immune Cell Co-Culture Model to predict the efficacy of targeted therapies. J Vis Exp. 2021(173):1. [DOI] [PubMed]
- 60.Chakrabarti J, Holokai L, Syu L, Steele N, Chang J, Dlugosz A, Zavros Y. Mouse-derived gastric organoid and Immune Cell co-culture for the study of the Tumor Microenvironment. Methods Mol Biol. 2018;1817:157–68. doi: 10.1007/978-1-4939-8600-2_16. [DOI] [PubMed] [Google Scholar]
- 61.Praharaj PP, Bhutia SK, Nagrath S, Bitting RL, Deep G. Circulating tumor cell-derived organoids: current challenges and promises in medical research and precision medicine. Biochim Biophys Acta Rev Cancer. 2018;1869(2):117–27. doi: 10.1016/j.bbcan.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z, Hu E, Shen H, Tan J, Zeng S. The functional and clinical roles of liquid biopsy in patient-derived models. J Hematol Oncol. 2023;16(1):36. doi: 10.1186/s13045-023-01433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye S, Boeter JWB, Mihajlovic M, van Steenbeek FG, van Wolferen ME, Oosterhoff LA, Marsee A, Caiazzo M, van der Laan LJW, Penning LC, et al. A chemically defined hydrogel for Human Liver Organoid Culture. Adv Funct Mater. 2020;30(48):2000893. doi: 10.1002/adfm.202000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S, Min S, Choi YS, Jo SH, Jung JH, Han K, Kim J, An S, Ji YW, Kim YG, et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat Commun. 2022;13(1):1692. doi: 10.1038/s41467-022-29279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jamaluddin MFB, Ghosh A, Ingle A, Mohammed R, Ali A, Bahrami M, Kaiko G, Gibb Z, Filipe EC, Cox TR, et al. Bovine and human endometrium-derived hydrogels support organoid culture from healthy and cancerous tissues. Proc Natl Acad Sci U S A. 2022;119(44):e2208040119. doi: 10.1073/pnas.2208040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dye BR, Decker JT, Hein RFC, Miller AJ, Huang S, Spence JR, Shea LD. Human lung Organoid Culture in Alginate with and without Matrigel to Model Development and Disease. Tissue Eng Part A. 2022;28(21–22):893–906. doi: 10.1089/ten.tea.2022.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang E, Xiang K, Zhang Y, Wang X-F. Patient-derived organoids (PDOs) and PDO-derived xenografts (PDOXs): new opportunities in establishing faithful pre-clinical cancer models. J Natl Cancer Cent. 2022;2(4):263–76. doi: 10.1016/j.jncc.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inglebert M, Dettwiler M, Hahn K, Letko A, Drogemuller C, Doench J, Brown A, Memari Y, Davies HR, Degasperi A, et al. A living biobank of canine mammary tumor organoids as a comparative model for human breast cancer. Sci Rep. 2022;12(1):18051. doi: 10.1038/s41598-022-21706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Chen Y, Zhang Y, Peng X, Wu M, Chen L, Zhan X. Clinical value and influencing factors of establishing stomach cancer organoids by endoscopic biopsy. J Cancer Res Clin Oncol. 2023;149(7):3803–10. doi: 10.1007/s00432-022-04296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derouet MF, Allen J, Wilson GW, Ng C, Radulovich N, Kalimuthu S, Tsao MS, Darling GE, Yeung JC. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep. 2020;10(1):14514. doi: 10.1038/s41598-020-71589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engel RM, Jarde T, Oliva K, Kerr G, Chan WH, Hlavca S, Nickless D, Archer SK, Yap R, Ranchod P, et al. Modeling colorectal cancer: a bio-resource of 50 patient-derived organoid lines. J Gastroenterol Hepatol. 2022;37(5):898–907. doi: 10.1111/jgh.15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo X, She J, Xu T, Zhou Y, Xu C, Jiang J, Li T, Liu H, Shen H, Yin B, et al. Establishment and characterization of organoids from a patient with adenomyoepithelioma of the breast. Bioengineered. 2021;12(2):11578–85. doi: 10.1080/21655979.2021.1974809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maru Y, Tanaka N, Itami M, Hippo Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol Oncol. 2019;154(1):189–98. doi: 10.1016/j.ygyno.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, Chan D, Chan AS, Ma S, Lam KO, et al. A Comprehensive Human gastric Cancer Organoid Biobank captures Tumor Subtype Heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23(6):882–897e811. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 75.Li H, Liu H, Chen K. Living biobank-based cancer organoids: prospects and challenges in cancer research. Cancer Biol Med. 2022;19(7):965–82. doi: 10.20892/j.issn.2095-3941.2021.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan HHN, Siu HC, Ho SL, Yue SSK, Gao Y, Tsui WY, Chan D, Chan AS, Wong JWH, Man AHY, et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut. 2020;69(12):2165–79. doi: 10.1136/gutjnl-2019-320019. [DOI] [PubMed] [Google Scholar]
- 77.Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, Xia F, Fu G, Deng Y, Pan M, et al. Patient-derived Organoids Predict Chemoradiation responses of locally advanced rectal Cancer. Cell Stem Cell. 2020;26(1):17–26e16. doi: 10.1016/j.stem.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, Schipper L, Hoes L, Vis DJ, van de Haar J, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019;11(513):1. doi: 10.1126/scitranslmed.aay2574. [DOI] [PubMed] [Google Scholar]
- 79.Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25(10):1607–14. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–6. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schutte M, Risch T, Abdavi-Azar N, Boehnke K, Schumacher D, Keil M, Yildiriman R, Jandrasits C, Borodina T, Amstislavskiy V, et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun. 2017;8:14262. doi: 10.1038/ncomms14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, et al. A colorectal tumor Organoid Library demonstrates progressive loss of Niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18(6):827–38. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Geevimaan K, Guo JY, Shen CN, Jiang JK, Fann CSJ, Hwang MJ, Shui JW, Lin HT, Wang MJ, Shih HC, et al. Patient-derived Organoid serves as a platform for personalized chemotherapy in Advanced Colorectal Cancer Patients. Front Oncol. 2022;12:883437. doi: 10.3389/fonc.2022.883437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meister MT, Groot Koerkamp MJA, de Souza T, Breunis WB, Frazer-Mendelewska E, Brok M, DeMartino J, Manders F, Calandrini C, Kerstens HHD, et al. Mesenchymal tumor organoid models recapitulate rhabdomyosarcoma subtypes. EMBO Mol Med. 2022;14(10):e16001. doi: 10.15252/emmm.202216001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu H, Lyu X, Yi M, Zhao W, Song Y, Wu K. Organoid technology and applications in cancer research. J Hematol Oncol. 2018;11(1):116. doi: 10.1186/s13045-018-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seo HR, Han HJ, Lee Y, Noh YW, Cho SJ, Kim JH. Human pluripotent stem cell-derived alveolar organoid with macrophages. Int J Mol Sci. 2022;23(16):1. doi: 10.3390/ijms23169211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sachdeva UM, Shimonosono M, Flashner S, Cruz-Acuna R, Gabre JT, Nakagawa H. Understanding the cellular origin and progression of esophageal cancer using esophageal organoids. Cancer Lett. 2021;509:39–52. doi: 10.1016/j.canlet.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szeto GL, Finley SD. Integrative approaches to Cancer Immunotherapy. Trends Cancer. 2019;5(7):400–10. doi: 10.1016/j.trecan.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shek D, Chen D, Read SA, Ahlenstiel G. Examining the gut-liver axis in liver cancer using organoid models. Cancer Lett. 2021;510:48–58. doi: 10.1016/j.canlet.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 90.Rajasekar S, Lin DSY, Abdul L, Liu A, Sotra A, Zhang F, Zhang B. IFlowPlate-A customized 384-Well plate for the culture of Perfusable vascularized Colon organoids. Adv Mater. 2020;32(46):e2002974. doi: 10.1002/adma.202002974. [DOI] [PubMed] [Google Scholar]
- 91.Cherne MD, Sidar B, Sebrell TA, Sanchez HS, Heaton K, Kassama FJ, Roe MM, Gentry AB, Chang CB, Walk ST, Synthetic Hydrogel A, et al. VitroGel((R)) ORGANOID-3, improves Immune cell-epithelial interactions in a tissue chip co-culture model of human gastric organoids and dendritic cells. Front Pharmacol. 2021;12:707891. doi: 10.3389/fphar.2021.707891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL, Kaing S, Kelderman S, et al. Generation of Tumor-Reactive T cells by co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell. 2018;174(6):1586–1598e1512. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michie J, Beavis PA, Freeman AJ, Vervoort SJ, Ramsbottom KM, Narasimhan V, Lelliott EJ, Lalaoui N, Ramsay RG, Johnstone RW, et al. Antagonism of IAPs enhances CAR T-cell efficacy. Cancer Immunol Res. 2019;7(2):183–92. doi: 10.1158/2326-6066.CIR-18-0428. [DOI] [PubMed] [Google Scholar]
- 94.Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Roder J, Darvishi T, Wels WS, Farin HF. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38(12):e100928. doi: 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bouffi C, Wikenheiser-Brokamp KA, Chaturvedi P, Sundaram N, Goddard GR, Wunderlich M, Brown NE, Staab JF, Latanich R, Zachos NC, et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat Biotechnol. 2023;41(6):824–31. doi: 10.1038/s41587-022-01558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park SE, Georgescu A, Huh D. Organoids-on-a-chip. Science. 2019;364(6444):960–5. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rumsey JW, Lorance C, Jackson M, Sasserath T, McAleer CW, Long CJ, Goswami A, Russo MA, Raja SM, Gable KL, et al. Classical complement pathway inhibition in a “Human-On-A-Chip” model of autoimmune demyelinating neuropathies. Adv Ther (Weinh) 2022;5(6):1. doi: 10.1002/adtp.202200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Demers CJ, Soundararajan P, Chennampally P, Cox GA, Briscoe J, Collins SD, Smith RL. Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development. 2016;143(11):1884–92. doi: 10.1242/dev.126847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, Aft R, Fields RC, George SC. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip. 2018;18(23):3687–702. doi: 10.1039/C8LC00596F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee KK, McCauley HA, Broda TR, Kofron MJ, Wells JM, Hong CI. Human stomach-on-a-chip with luminal flow and peristaltic-like motility. Lab Chip. 2018;18(20):3079–85. doi: 10.1039/C8LC00910D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jin Y, Kim J, Lee JS, Min S, Kim S, Ahn D-H, Kim Y-G, Cho S-W. Vascularized liver organoids generated using Induced hepatic tissue and dynamic liver-specific Microenvironment as a drug testing platform. Adv Funct Mater. 2018;28(37):1801954. doi: 10.1002/adfm.201801954. [DOI] [Google Scholar]
- 102.Saito Y, Muramatsu T, Kanai Y, Ojima H, Sukeda A, Hiraoka N, Arai E, Sugiyama Y, Matsuzaki J, Uchida R, et al. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27(4):1265–1276e1264. doi: 10.1016/j.celrep.2019.03.088. [DOI] [PubMed] [Google Scholar]
- 103.Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun. 2018;9(1):5167. doi: 10.1038/s41467-018-07594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weeber F, Ooft SN, Dijkstra KK, Voest EE. Tumor Organoids as a pre-clinical Cancer Model for Drug Discovery. Cell Chem Biol. 2017;24(9):1092–100. doi: 10.1016/j.chembiol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 105.Costamagna G, Comi GP, Corti S. Advancing Drug Discovery for neurological Disorders using iPSC-Derived neural organoids. Int J Mol Sci. 2021;22(5):1. doi: 10.3390/ijms22052659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, Koike H, Maezawa M, Zhang RR, Dunn A, et al. High-Fidelity Drug-Induced Liver Injury screen using human pluripotent stem cell-derived Organoids. Gastroenterology. 2021;160(3):831–846e810. doi: 10.1053/j.gastro.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brancato V, Oliveira JM, Correlo VM, Reis RL, Kundu SC. Could 3D models of cancer enhance drug screening? Biomaterials. 2020;232:119744. doi: 10.1016/j.biomaterials.2019.119744. [DOI] [PubMed] [Google Scholar]
- 108.Harrison SP, Baumgarten SF, Verma R, Lunov O, Dejneka A, Sullivan GJ. Liver organoids: recent developments, Limitations and potential. Front Med (Lausanne) 2021;8:574047. doi: 10.3389/fmed.2021.574047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, Watanabe T, Kanai T, Sato T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256–62. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 110.Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521(7550):43–7. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 111.Zhao H, Cheng Y, Kalra A, Ma K, Zheng Y, Ziman B, Tressler C, Glunde K, Shin EJ, Ngamruengphong S, et al. Generation and multiomic profiling of a TP53/CDKN2A double-knockout gastroesophageal junction organoid model. Sci Transl Med. 2022;14(673):eabq6146. doi: 10.1126/scitranslmed.abq6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T, Sato T. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545(7653):187–92. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- 113.Schmitt M, Ceteci F, Gupta J, Pesic M, Bottger TW, Nicolas AM, Kennel KB, Engel E, Schewe M, Callak Kirisozu A, et al. Colon tumour cell death causes mTOR dependence by paracrine P2 × 4 stimulation. Nature. 2022;612(7939):347–53. doi: 10.1038/s41586-022-05426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dmitrieva-Posocco O, Wong AC, Lundgren P, Golos AM, Descamps HC, Dohnalova L, Cramer Z, Tian Y, Yueh B, Eskiocak O, et al. beta-hydroxybutyrate suppresses colorectal cancer. Nature. 2022;605(7908):160–5. doi: 10.1038/s41586-022-04649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, Clevers H. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11(2):347–58. doi: 10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Z, Karthaus WR, Lee YS, Gao VR, Wu C, Russo JW, Liu M, Mota JM, Abida W, Linton E, et al. Tumor Microenvironment-Derived NRG1 promotes Antiandrogen Resistance in prostate Cancer. Cancer Cell. 2020;38(2):279–96. doi: 10.1016/j.ccell.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adams EJ, Karthaus WR, Hoover E, Liu D, Gruet A, Zhang Z, Cho H, DiLoreto R, Chhangawala S, Liu Y, et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature. 2019;571(7765):408–12. doi: 10.1038/s41586-019-1318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Breunig M, Merkle J, Wagner M, Melzer MK, Barth TFE, Engleitner T, Krumm J, Wiedenmann S, Cohrs CM, Perkhofer L, et al. Modeling plasticity and dysplasia of pancreatic ductal organoids derived from human pluripotent stem cells. Cell Stem Cell. 2021;28(6):1105–1124e1119. doi: 10.1016/j.stem.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang L, Desai R, Conrad DN, Leite NC, Akshinthala D, Lim CM, Gonzalez R, Muthuswamy LB, Gartner Z, Muthuswamy SK. Commitment and oncogene-induced plasticity of human stem cell-derived pancreatic acinar and ductal organoids. Cell Stem Cell. 2021;28(6):1090–1104e1096. doi: 10.1016/j.stem.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grunwald BT, Devisme A, Andrieux G, Vyas F, Aliar K, McCloskey CW, Macklin A, Jang GH, Denroche R, Romero JM, et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell. 2021;184(22):5577–5592e5518. doi: 10.1016/j.cell.2021.09.022. [DOI] [PubMed] [Google Scholar]
- 121.Below CR, Kelly J, Brown A, Humphries JD, Hutton C, Xu J, Lee BY, Cintas C, Zhang X, Hernandez-Gordillo V, et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat Mater. 2022;21(1):110–9. doi: 10.1038/s41563-021-01085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hubert CG, Rivera M, Spangler LC, Wu Q, Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE, Rich JN. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas recapitulates the hypoxic gradients and Cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 2016;76(8):2465–77. doi: 10.1158/0008-5472.CAN-15-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klein E, Hau AC, Oudin A, Golebiewska A, Niclou SP. Glioblastoma Organoids: Pre-Clinical Applications and Challenges in the context of Immunotherapy. Front Oncol. 2020;10:604121. doi: 10.3389/fonc.2020.604121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Azzarelli R, Ori M, Philpott A, Simons BD. Three-dimensional model of glioblastoma by co-culturing tumor stem cells with human brain organoids. Biol Open. 2021;10(2):1. doi: 10.1242/bio.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clark CC, Yoo KM, Sivakumar H, Strumpf K, Laxton AW, Tatter SB, Strowd RE, Skardal A. Immersion bioprinting of hyaluronan and collagen bioink-supported 3D patient-derived brain tumor organoids. Biomed Mater. 2022;18(1):1. doi: 10.1088/1748-605X/aca05d. [DOI] [PubMed] [Google Scholar]
- 126.Chen CC, Li HW, Wang YL, Lee CC, Shen YC, Hsieh CY, Lin HL, Chen XX, Cho DY, Hsieh CL, et al. Patient-derived tumor organoids as a platform of precision treatment for malignant brain tumors. Sci Rep. 2022;12(1):16399. doi: 10.1038/s41598-022-20487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eichmuller OL, Corsini NS, Vertesy A, Morassut I, Scholl T, Gruber VE, Peer AM, Chu J, Novatchkova M, Hainfellner JA, et al. Amplification of human interneuron progenitors promotes brain tumors and neurological defects. Science. 2022;375(6579):eabf5546. doi: 10.1126/science.abf5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15(8):631–9. doi: 10.1038/s41592-018-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fawal MA, Jungas T, Davy A. Inhibition of DHFR targets the self-renewing potential of brain tumor initiating cells. Cancer Lett. 2021;503:129–37. doi: 10.1016/j.canlet.2021.01.026. [DOI] [PubMed] [Google Scholar]
- 130.Nuciforo S, Fofana I, Matter MS, Blumer T, Calabrese D, Boldanova T, Piscuoglio S, Wieland S, Ringnalda F, Schwank G, et al. Organoid Models of Human Liver Cancers derived from Tumor needle biopsies. Cell Rep. 2018;24(5):1363–76. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Artegiani B, van Voorthuijsen L, Lindeboom RGH, Seinstra D, Heo I, Tapia P, Lopez-Iglesias C, Postrach D, Dayton T, Oka R, et al. Probing the tumor suppressor function of BAP1 in CRISPR-Engineered Human Liver Organoids. Cell Stem Cell. 2019;24(6):927–943e926. doi: 10.1016/j.stem.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 132.Tan XP, He Y, Yang J, Wei X, Fan YL, Zhang GG, Zhu YD, Li ZQ, Liao HX, Qin DJ, et al. Blockade of NMT1 enzymatic activity inhibits N-myristoylation of VILIP3 protein and suppresses liver cancer progression. Signal Transduct Target Ther. 2023;8(1):14. doi: 10.1038/s41392-022-01248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Girda E, Huang EC, Leiserowitz GS, Smith LH. The Use of Endometrial Cancer patient-derived Organoid Culture for Drug Sensitivity Testing is feasible. Int J Gynecol Cancer. 2017;27(8):1701–7. doi: 10.1097/IGC.0000000000001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Song Y, Fazleabas AT. Endometrial organoids: a rising star for Research on Endometrial Development and Associated Diseases. Reprod Sci. 2021;28(6):1626–36. doi: 10.1007/s43032-021-00471-z. [DOI] [PubMed] [Google Scholar]
- 136.Nanki K, Toshimitsu K, Takano A, Fujii M, Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, et al. Divergent routes toward wnt and R-spondin Niche Independency during Human gastric carcinogenesis. Cell. 2018;174(4):856–869e817. doi: 10.1016/j.cell.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 137.Seidlitz T, Merker SR, Rothe A, Zakrzewski F, von Neubeck C, Grutzmann K, Sommer U, Schweitzer C, Scholch S, Uhlemann H, et al. Human gastric cancer modelling using organoids. Gut. 2019;68(2):207–17. doi: 10.1136/gutjnl-2017-314549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lo YH, Kolahi KS, Du Y, Chang CY, Krokhotin A, Nair A, Sobba WD, Karlsson K, Jones SJ, Longacre TA, et al. A CRISPR/Cas9-Engineered ARID1A-Deficient human gastric Cancer Organoid Model reveals essential and nonessential modes of Oncogenic Transformation. Cancer Discov. 2021;11(6):1562–81. doi: 10.1158/2159-8290.CD-20-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pang MJ, Burclaff JR, Jin R, Adkins-Threats M, Osaki LH, Han Y, Mills JC, Miao ZF, Wang ZN. Gastric organoids: progress and remaining Challenges. Cell Mol Gastroenterol Hepatol. 2022;13(1):19–33. doi: 10.1016/j.jcmgh.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duarte AA, Gogola E, Sachs N, Barazas M, Annunziato S, Velds JRdR, Blatter A, Houthuijzen S, van de Ven JM. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat Methods. 2018;15(2):134–40. doi: 10.1038/nmeth.4535. [DOI] [PubMed] [Google Scholar]
- 141.Cong M, Wang Y, Yang Y, Lian C, Zhuang X, Li X, Zhang P, Liu Y, Tang J, Yang Q, et al. MTSS1 suppresses mammary tumor-initiating cells by enhancing RBCK1-mediated p65 ubiquitination. Nat Cancer. 2020;1(2):222–34. doi: 10.1038/s43018-019-0021-y. [DOI] [PubMed] [Google Scholar]
- 142.Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, Lei JH, Wang L, Bi J, Shao N, et al. Patient-derived Organoids can Guide Personalized-Therapies for patients with advanced breast Cancer. Adv Sci (Weinh) 2021;8(22):e2101176. doi: 10.1002/advs.202101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Medle B, Sjodahl G, Eriksson P, Liedberg F, Hoglund M, Bernardo C. Patient-derived bladder Cancer Organoid Models in Tumor Biology and Drug Testing: a systematic review. Cancers (Basel) 2022;14(9):1. doi: 10.3390/cancers14092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tan P, Wang M, Zhong A, Wang Y, Du J, Wang J, Qi L, Bi Z, Zhang P, Lin T, et al. SRT1720 inhibits the growth of bladder cancer in organoids and murine models through the SIRT1-HIF axis. Oncogene. 2021;40(42):6081–92. doi: 10.1038/s41388-021-01999-9. [DOI] [PubMed] [Google Scholar]
- 145.Meijer RP. Urothelial cancer organoids: a tool for bladder cancer research. Pathologe. 2021;42(Suppl 2):165–9. doi: 10.1007/s00292-021-00988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mastri M, Ramakrishnan S, Shah SD, Karasik E, Gillard BM, Moser MT, Farmer BK, Azabdaftari G, Chatta GS, Woloszynska A, et al. Patient derived models of bladder cancer enrich the signal of the tumor cell transcriptome facilitating the analysis of the tumor cell compartment. Am J Clin Exp Urol. 2021;9(6):416–34. [PMC free article] [PubMed] [Google Scholar]
- 147.Kong J, Lee H, Kim D, Han SK, Ha D, Shin K, Kim S. Network-based machine learning in colorectal and bladder organoid models predicts anti-cancer drug efficacy in patients. Nat Commun. 2020;11(1):5485. doi: 10.1038/s41467-020-19313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu L, Li Z, Mei H, Li W, Chen D, Liu L, Zhang Z, Sun Y, Song F, Chen W, et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin Transl Immunology. 2021;10(2):e1248. doi: 10.1002/cti2.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li X, Francies HE, Secrier M, Perner J, Miremadi A, Galeano-Dalmau N, Barendt WJ, Letchford L, Leyden GM, Goffin EK, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun. 2018;9(1):2983. doi: 10.1038/s41467-018-05190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ebbing EA, van der Zalm AP, Steins A, Creemers A, Hermsen S, Rentenaar R, Klein M, Waasdorp C, Hooijer GKJ, Meijer SL, et al. Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc Natl Acad Sci U S A. 2019;116(6):2237–42. doi: 10.1073/pnas.1820459116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tang Q, Lento A, Suzuki K, Efe G, Karakasheva T, Long A, Giroux V, Islam M, Wileyto EP, Klein-Szanto AJ, et al. Rab11-FIP1 mediates epithelial-mesenchymal transition and invasion in esophageal cancer. Embo Rep. 2021;22(2):e48351. doi: 10.15252/embr.201948351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10(1):3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Z, Qian Y, Li W, Liu L, Yu L, Liu X, Wu G, Wang Y, Luo W, Fang F, et al. Human lung adenocarcinoma-derived Organoid Models for Drug Screening. iScience. 2020;23(8):101411. doi: 10.1016/j.isci.2020.101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen JH, Chu XP, Zhang JT, Nie Q, Tang WF, Su J, Yan HH, Zheng HP, Chen ZX, Chen X, et al. Genomic characteristics and drug screening among organoids derived from non-small cell lung cancer patients. Thorac Cancer. 2020;11(8):2279–90. doi: 10.1111/1759-7714.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi R, Radulovich N, Ng C, Liu N, Notsuda H, Cabanero M, Martins-Filho SN, Raghavan V, Li Q, Mer AS, et al. Organoid cultures as preclinical models of Non-Small Cell Lung Cancer. Clin Cancer Res. 2020;26(5):1162–74. doi: 10.1158/1078-0432.CCR-19-1376. [DOI] [PubMed] [Google Scholar]
- 156.Kim SY, Kim SM, Lim S, Lee JY, Choi SJ, Yang SD, Yun MR, Kim CG, Gu SR, Park C, et al. Modeling clinical responses to targeted therapies by patient-derived organoids of Advanced Lung Adenocarcinoma. Clin Cancer Res. 2021;27(15):4397–409. doi: 10.1158/1078-0432.CCR-20-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang HM, Zhang CY, Peng KC, Chen ZX, Su JW, Li YF, Li WF, Gao QY, Zhang SL, Chen YQ, et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: a real-world study. Cell Rep Med. 2023;4(2):100911. doi: 10.1016/j.xcrm.2022.100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grassi L, Alfonsi R, Francescangeli F, Signore M, De Angelis ML, Addario A, Costantini M, Flex E, Ciolfi A, Pizzi S, et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019;10(3):201. doi: 10.1038/s41419-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calandrini C, Schutgens F, Oka R, Margaritis T, Candelli T, Mathijsen L, Ammerlaan C, van Ineveld RL, Derakhshan S, de Haan S, et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun. 2020;11(1):1310. doi: 10.1038/s41467-020-15155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xue Y, Wang B, Tao Y, Xia J, Yuan K, Zheng J, Zhai W, Xue W. Patient-derived organoids potentiate precision medicine in advanced clear cell renal cell carcinoma. Precis Clin Med. 2022;5(4):pbac028. doi: 10.1093/pcmedi/pbac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li Z, Xu H, Yu L, Wang J, Meng Q, Mei H, Cai Z, Chen W, Huang W. Patient-derived renal cell carcinoma organoids for personalized cancer therapy. Clin Transl Med. 2022;12(7):e970. doi: 10.1002/ctm2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li M, Izpisua Belmonte JC. Organoids - Preclinical Models of Human Disease. N Engl J Med. 2019;380(6):569–79. doi: 10.1056/NEJMra1806175. [DOI] [PubMed] [Google Scholar]
- 163.Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, et al. A patient-derived Glioblastoma Organoid Model and Biobank recapitulates Inter- and intra-tumoral heterogeneity. Cell. 2020;180(1):188–204e122. doi: 10.1016/j.cell.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Foo MA, You M, Chan SL, Sethi G, Bonney GK, Yong WP, Chow EK, Fong ELS, Wang L, Goh BC. Clinical translation of patient-derived tumour organoids- bottlenecks and strategies. Biomark Res. 2022;10(1):10. doi: 10.1186/s40364-022-00356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN, Voest EE. Tumor organoid-T-cell coculture systems. Nat Protoc. 2020;15(1):15–39. doi: 10.1038/s41596-019-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H, Egan DA, et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature. 2018;556(7702):457–62. doi: 10.1038/s41586-018-0024-3. [DOI] [PubMed] [Google Scholar]
- 167.Roper J, Tammela T, Akkad A, Almeqdadi M, Santos SB, Jacks T, Yilmaz OH. Colonoscopy-based colorectal cancer modeling in mice with CRISPR-Cas9 genome editing and organoid transplantation. Nat Protoc. 2018;13(2):217–34. doi: 10.1038/nprot.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Rourke KP, Loizou E, Livshits G, Schatoff EM, Baslan T, Manchado E, Simon J, Romesser PB, Leach B, Han T, et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat Biotechnol. 2017;35(6):577–82. doi: 10.1038/nbt.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roper J, Tammela T, Cetinbas NM, Akkad A, Roghanian A, Rickelt S, Almeqdadi M, Wu K, Oberli MA, Sanchez-Rivera FJ, et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol. 2017;35(6):569–76. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Seino T, Kawasaki S, Shimokawa M, Tamagawa H, Toshimitsu K, Fujii M, Ohta Y, Matano M, Nanki K, Kawasaki K, et al. Human pancreatic tumor Organoids Reveal loss of stem cell niche factor dependence during Disease Progression. Cell Stem Cell. 2018;22(3):454–467e456. doi: 10.1016/j.stem.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 171.Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Bottinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38(4):1. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mullenders J, de Jongh E, Brousali A, Roosen M, Blom JPA, Begthel H, Korving J, Jonges T, Kranenburg O, Meijer R, et al. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci U S A. 2019;116(10):4567–74. doi: 10.1073/pnas.1803595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sondorp LHJ, Ogundipe VML, Groen AH, Kelder W, Kemper A, Links TP, Coppes RP, Kruijff S. Patient-derived papillary thyroid Cancer Organoids for Radioactive Iodine Refractory Screening. Cancers (Basel) 2020;12(11):1. doi: 10.3390/cancers12113212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lohmussaar K, Oka R, Espejo Valle-Inclan J, Smits MHH, Wardak H, Korving J, Begthel H, Proost N, van de Ven M, Kranenburg OW, et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell. 2021;28(8):1380–1396e1386. doi: 10.1016/j.stem.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 175.Nanki Y, Chiyoda T, Hirasawa A, Ookubo A, Itoh M, Ueno M, Akahane T, Kameyama K, Yamagami W, Kataoka F, et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;10(1):12581. doi: 10.1038/s41598-020-69488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Driehuis E, Spelier S, Beltran Hernandez I, de Bree R, Clevers MW, Oliveira H. Patient-derived Head and Neck Cancer Organoids recapitulate EGFR expression levels of respective tissues and are responsive to EGFR-Targeted photodynamic therapy. J Clin Med. 2019;8(11):1. doi: 10.3390/jcm8111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.