Abstract
Periodontitis, one of the most common non-communicable diseases, is characterized by chronic oral inflammation and uncontrolled tooth supporting alveolar bone resorption. Its underlying mechanism to initiate aberrant oral barrier immunity has yet to be delineated. Here, we report a unique fibroblast subpopulation activated to guide oral inflammation (AG fibroblasts) identified in a single-cell RNA sequencing gingival cell atlas constructed from the mouse periodontitis models. AG fibroblasts localized beneath the gingival epithelium and in the cervical periodontal ligament responded to the ligature placement and to the discrete application of Toll-like receptor stimulants to mouse maxillary tissue. The upregulated chemokines and ligands of AG fibroblasts linked to the putative receptors of neutrophils in the early stages of periodontitis. In the established chronic inflammation, neutrophils together with AG fibroblasts appeared to induce type 3 innate lymphoid cells (ILC3s) that were the primary source of interleukin-17 cytokines. The comparative analysis of Rag2−/− and Rag2γc−/− mice suggested that ILC3 contributed to the cervical alveolar bone resorption interfacing the gingival inflammation. We propose that AG fibroblasts function as a previously unrecognized surveillant to initiate gingival inflammation leading to periodontitis through the AG fibroblast-neutrophil-ILC3 axis.
Introduction
The oral mucosa is one of the most active barrier tissues in the human body [1, 2] and oral barrier immunity acts as a crucial surveillance system, which must defend from pathogenic organisms, while ensuring sustained tolerance of commensal microbes and harmless antigens [3]. However, once this gingival inflammation progresses, adjacent alveolar bone supporting the cervical area of the dentition is subjected to a localized, but often severe, osteoclastic bone resorption, resulting in tooth loss and disruption of the maxillofacial structure [4]. Periodontitis is not only the most frequent cause of tooth loss in adults [5], but also, globally, ranks among the most significant contributors to poor health and decreased quality of life, imposing substantial economic and healthcare burdens [6]. It is therefore acutely important to elucidate the molecular mechanisms underlying the initiation of gingival inflammation, in order to facilitate the development of effective preventive and therapeutic measures for this debilitating condition.
The postulated pathological framework has been reconstructed from animal models [7, 8], harvested immune cell analyses, and oral microbial associations [4, 9, 10]. For example, the ligature-induced periodontitis mouse model has shown that fully developed periodontal inflammation is closely associated with the abundant recruitment and excessive activation of neutrophils [11] and pathological induction of interleukin (IL)-17–secreting proinflammatory effector CD4+ T helper (Th)17 cells [12]. Based on our current understanding of osteoimmunology, it was hypothesized that these pathological immune cells activate osteoclasts, leading to induction of periodontal alveolar bone resorption [13, 14]. However, the precise cascade of events leading to this disease outcome has not been fully deciphered.
Investigation of the molecular mechanisms underlying interaction between different inflammatory cell fractions in immunological disorders is an intense area of research. Prior studies have shown that chemokines are involved in the trafficking and activation of inflammatory cells during both homeostasis and disease-associated inflammation [14]. These small proteins, which typically contain disulfide cysteine–cysteine (CC) and cysteine–X–cysteine (CXC) molecular signatures, act as ligands for cellular receptors that modulate inflammatory signaling pathways [15]. Such chemokine–receptor networks direct the migration of inflammatory cells, potentially amplifying the resulting tissue damage. However, increasing evidence suggests that in addition to immune cells, barrier tissue stromal cells are involved in innate immune cell regulation [16–19]. We and others have found that oral fibroblasts secrete cytokines and chemokines in response to microbial stimuli or a proinflammatory environment, suggesting a possible mechanism by which they may regulate immune cell function [8, 20]. Therefore, a comprehensive elucidation of chemokine–receptor signaling networks will likely need to include all types of gingival cells.
In the present study, to better understand the pathways contributing to chronic oral inflammation, we constructed the gingival single-cell transcriptomic atlas of the ligature-induced periodontitis mouse model as well as the newly developed mouse model of maxillary topical application (MTA) of pathological oral microbial biofilm component(s), discretely mimicking the initial events of gingival inflammation and degradation. Here we report a novel subpopulation of fibroblasts activated to guide chronic inflammation (AG fibroblasts). Our findings suggest that chemokines and ligands derived from AG fibroblasts bind to and activate receptors involved in neutrophil and lymphocytes. We further show that in the fully developed chronic gingival inflammation of the ligature-induced periodontitis model, Cd4− innate lymphoid cells (ILCs) predominantly produce proinflammatory IL-17 cytokines and provide evidence that type 3 ILCs (ILC3s) are responsible for cervical alveolar bone resorption in mice—a pathological feature consistent with the human periodontitis disease phenotype. Thus, the results from this study identify the AG fibroblast–neutrophil–ILC3 axis as a previously unrecognized mechanism contributing to pathological inflammation in periodontitis.
Results
Alterations in major cell type proportions during periodontitis development
The alveolar bone and gingival tissue surrounding the maxillary second molar of C57BL/6J mice presented healthy connective tissue supporting the dentition with minimal CD45+ immune cell infiltration and well-developed collagen architecture (Figure 1A – figure supplement 1A). The placement of a ligature around the maxillary left second molar[21] induced a localized and small gingival connective tissue degradation and infiltration of CD45+ immune cells near the cervical area (Figure 1B). The noticeable gingival defect and more prominent though still localized CD45+ immune cell infiltration developed after Day 3 following ligature placement (Figure 1C – figure supplement 1A, 1B). On Day 7, the gingival connective tissue was largely degraded, and chronic inflammation was developed (Figure 1D – figure supplement 1A, 1B). Three-dimensional (3D) reconstruction of micro-computed tomography (microCT) images (figure supplement 1C) further revealed a reduction in alveolar bone height starting from Day 3, which progressively increased on Day 7 (figure supplement 1D, 1E). Overall, the observed pattern of periodontal tissue degradation was consistent with that reported in previous studies [22, 23].
Figure 1.
Changes in proportions of major cell types during ligature-induced periodontitis development in mice. (A) Mouse maxillary second molar (M2) has multi-roots and supported by alveolar bone (Bone), periodontal ligament (PDL) and gingiva connective tissue (gCT). The oral barrier immunity is not constitutively activated as evidenced by the lack of CD45+ immune cells. Picrosirius red (PSR) stained collagen fibers connected the root surface and alveolar bone in the PDL and organized as dense parallel bundles in gCT. (B) One day (Day 1) after a ligature (5.0 silk suture) was placed around M2, the cervical PDL and gCT demonstrated a localized connective tissue degradation (*), where CD45+ immune cells clustered (arrows). PSR-stained collagen architecture immediately under the ligature lost the thick collagen bundle structure (*). (C) Day 3 of ligature placement exhibited localized but increased CD45+ immune cell clustering adjacent to the collagen degradation area (*). (D) Day 7 of ligature placement, PDL and gCT tissue degradation increased with inflammatory vascularization (Vas). CD45+ myeloid cells (arrowheads) were observed near the alveolar bone surface and CD45+ lymphocytes (arrows) infiltrated the gCT area. PSR staining lost the typical collagen pattern in gCT and PDL, and a remnant of degraded PDL collagen fiber (white bracket) was attached to the tooth surface. (E) Single-cell RNA sequencing (scRNA-seq) t-distributed stochastic neighbor embedding (t-SNE) projection plots showing the major cell types present within gingival tissue during periodontitis development on Day 0, 1, 4, and 7. Colors indicate cell type, as follows: green, epithelial cells; blue, fibroblasts; pink, endothelial cells; yellow, B cells; red, T cells; and purple, myeloid cells. (F) Proportion plots showing the relative amounts of each major cell type on Days 0, 1, 4, and 7.
Left-side palatal gingiva tissue was harvested from mice on Day 0 (i.e., healthy gingiva without ligature placement) and on Days 1, 4, and 7 after ligature placement, and gingival cells were dissociated for single-cell RNA sequencing (scRNA-seq) [8, 24]. On Days 0 and 1, the major cell types identified included epithelial cells expressing cadherin 1 (Cdh1) [25] and type XVII collagen (Col17a1) [26], fibroblasts expressing type I collagen (Col1a1) [27] and lumican (Lum) [28], B cells expressing membrane spanning 4 domains A1 (Ms4a1) [29] and cluster of differentiation 79A (Cd79a) [30], T cells expressing epsilon subunit of T cell receptor complex (Cd3e) [31] and cluster of differentiation 5 (Cd5) [32], and myeloid cells expressing lysozyme 2 (Lyz2) [33] and integrin subunit alpha M (Itgam) [34] (Figure 1E and F—figure supplement 2). On Days 4 and 7, an additional endothelial cell fraction expressing selectin P (Selp) [35] and selectin E (Sele) [36] emerged (Figure 1E and F—figure supplement 2), suggesting increased inflammatory neovascularization with the progression of periodontal inflammation. The proportion of B cells was increased on Day 1, and the proportion of myeloid cells increased progressively from Day 4 to 7 (Figure 1F). In addition, the proportion of fibroblasts was increased on Day 7 (Figure 1F).
Fibroblasts activated to guide leukocyte migration in periodontitis development
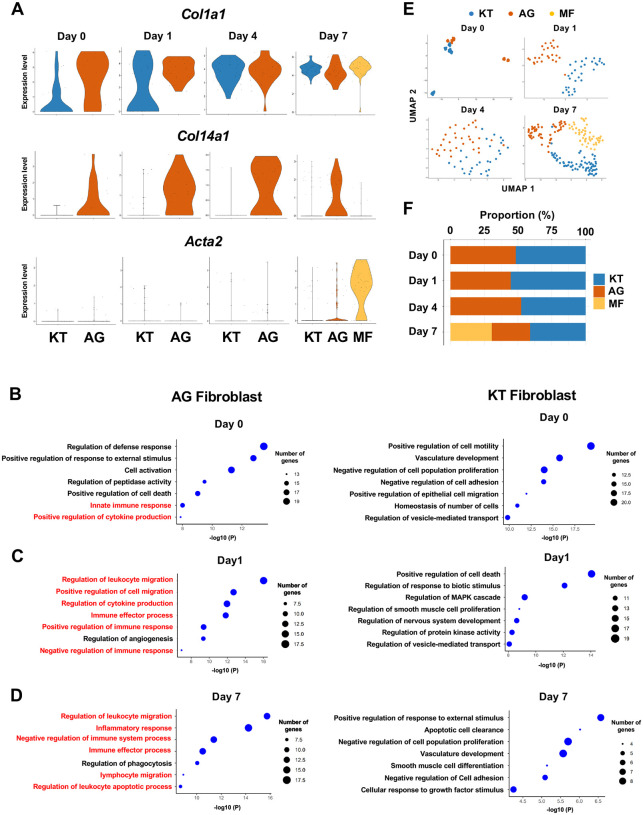
We previously identified two distinct subpopulations of gingival fibroblasts, differentiated by expression of type XIV collagen (Col14a1) [8], and these were also detected in our current scRNA-seq data from Day 0 to 7 (Figure 2A). Gene ontogeny (GO) enrichment analysis of the Col14a1-expressing fibroblast subpopulation revealed expression of major gene clusters related to immune regulation, including “Regulation of leukocyte migration” (Figure 2B). This immune regulatory phenotype appears to be unique to Col14a1-expressing fibroblasts, which are therefore referred to as ‘fibroblasts activated to guide leukocyte migration’ or AG fibroblasts. The other subpopulation of Col1a1-expressing fibroblasts appeared to keep typical fibroblastic features and is thus referred to as KT fibroblasts (Figure 2C). GO analysis suggested that KT fibroblasts primarily maintained connective tissue remodeling but did not express an immune regulatory phenotype. An additional fibroblast subpopulation expressing smooth muscle aortic actin 2 (Acta2) was detected on Day 7, and these were identified as myofibroblasts [37] (MF) (Figure 2A). We found that the proportions of KT and AG fibroblasts were equal on Day 0 and on Days 1 and 4 after ligature placement (Figure 2E, 2F). However, on Day 7, the proportion of AG fibroblasts decreased, and the MF fraction emerged (Figure 2E, 2F).
Figure 2.
Fibroblasts activated to guide leukocyte migration (AG fibroblasts) are one of three fibroblast subpopulations in gingival tissue during periodontitis development. (A) Violin plots showing gene expression levels of type I collagen (Col1a1), type XIV collagen (Col14a1), and smooth muscle aortic actin 2 (Acta2) in gingival fibroblast subpopulations during periodontitis development. AG, AG fibroblasts; KT, ‘keeping typical phenotype’ fibroblasts; MF, myofibroblasts. Gene ontology (GO) enrichment analysis of the biological functions of AG fibroblasts and KT fibroblasts on Day 0 without ligature placement (B) and on Day 1 (C) and Day 7 (D) after ligature placement. Gene clusters related to immune regulation (red) were identified in AG fibroblasts, and these clusters dominate after ligature placement. (E) t-SNE projection plots showing fibroblast subpopulations in gingival tissue during periodontitis development. Colors indicate cell type, as follows: blue, KT fibroblasts; red, AG fibroblasts; and yellow, MFs. (F) Proportion plots showing the relative amounts of each fibroblast subpopulation on Days 0, 1, 4, and 7.
AG fibroblasts and immune surveillance in early periodontitis development
To further characterize AG fibroblasts and their “Regulation of leukocyte migration” phenotype, we analyzed expression of CC motif chemokine ligands (CCLs) and CXC motif chemokine ligands (CXCLs) within fibroblast subpopulations in our scRNA-seq dataset [13]. Results show that AG fibroblasts activated expression of Ccl8, Ccl11, Ccl19, Cxcl1, Cxcl10, and Cxcl12 immediately after ligature placement on Days 1 and 4 (Figure 3A). Chemokine expression levels then decreased on Day 7, suggesting that AG fibroblasts might initiate early leukocyte migration into gingival tissue. Given that activation of Toll-like receptors (TLR) is known to increase chemokine expression, we further assessed expression of TLRs in fibroblast subpopulations. We found that AG fibroblasts displayed temporal activation of Tlr2, Tlr3, and Tlr4 expression on Days 1 and 4 (Figure 3B). Similarly, the TLR downstream genes Myd88, Irak1, Map3k7, and RelA were also expressed on Days 1 and 4 (Figure 3B). These data suggest that AG fibroblasts may guide the establishment of an early inflammatory environment within the gingiva and thereby promote periodontitis pathogenesis.
Figure 3.
AG fibroblasts and immune surveillance in periodontitis development. (A) Dot plots depicting expression levels of the CCL genes Ccl8, Ccl11, Ccl19, Cxcl1, Cxcl9, Cxcl10, and Cxcl12 in gingival fibroblast subpopulations during periodontitis development. (B) Dot plots depicting expression levels of the Toll-like receptor and related genes Tlr2, Tlr3, Tlr4, Myd88, Irak1, Map3k7, and Rela in gingival fibroblast subpopulations during periodontitis development. Upregulation of chemokines and TLR-related molecules is predominantly observed in the AG fibroblast subpopulation. (C) Hematoxylin and eosin (HE) staining and immunohistochemical (IHC) staining for COL14A1 and CXCL12 in periodontal tissue on Day 1; scale bars, 100 μm (HE) and 20 μm, (IHC). Yellow arrows indicate COL14A1- and CXCL12-positive cells in the connective tissue papillae and periodontal ligament (PDL).
To validate the presence of AG fibroblasts, Day 1 gingiva and periodontal tissue histological sections were subjected to immunohistochemistry with antibodies against COL14A1 and CXCL12 (Figure 3C). We detected COL14A1- and CXCL12-positive AG fibroblasts localized near gingival epithelial cells in the connective tissue papillae and free gingiva, as well as in the cervical zone of the periodontal ligament (PDL) space (Figure 3C). We note that this localization pattern of AG fibroblasts appears to be highly suitable for early immune surveillance during periodontitis pathogenesis. Thus, we have hypothesized that AG fibroblasts initially sense the pathological stress including oral microbial stimuli through TLRs and secrete inflammatory signals through chemokine expression.
AG fibroblasts induced by maxillary topical application (MTA) of unmethylated cytidine phosphate guanosine oligonucleotide (CpG ODN) and of Porphyromonas gingivalis lipopolysaccharide (LPS)
To further characterize the behavior of AG fibroblasts, we used the recently developed maxillary topical application (MTA) model without the placement of a ligature. The MTA model applied oral microbial biofilm directly to the maxillary gingiva and held under a custom-made oral appliance (Figure 4A) for 1 hr. Four days after the topical application, mouse maxillary tissue was harvested for histological analysis and for scRNA-seq (Figure 4B) [8]. We previously reported that oral microbial biofilm and its extracellular polymeric substances such as extracellular DNA, but not planktonic microbes, induced initial periodontal tissue reaction in the MTA model [8]. The present study discretely applied ligands of TLR9 and TLR2/4: unmethylated CpG ODN and P. gingivalis LPS, respectively; using the MTA model. CpG ODN induced the expression of cathepsin K (Ctsk) in the periodontal ligament (PDL) and gingival connective tissue (Figure 4C) validating the previous report [8]. Furthermore, there were signs of localized Ctsk+ osteoclastic activities on the surface of alveolar bone (Figure 4C).
Figure 4.
Characterization of AG fibroblasts in the discrete maxillary topical application (MTA) model using CpG ODN (TLR9 ligand) and LPS (TLR2/4 ligand). (A) The MTA model was developed to discretely test the selected oral microbial pathogens. The selected pathogen was topically applied directly on the maxillary gingiva between the molars and covered by a custom fabricated oral appliance for 1 hr. (B) In this study, microbial DNA TLR9 ligand, unmethylated CpG ODN and TLR2/4 ligand P. gingivalis lipopolysaccharide (LPS) were selected in the MTA model. After 1 hr exposure, mice were returned to the vivarium for 4 days and the maxillary tissue was harvested for histology or scRNA-seq. (C) The MTA of CpG ODN developed localized PDL and gCT degradation evidenced by the expression of cathepsin K (Ctsk; arrows). There were signs of localized bone resorption by Ctsk+ osteoclasts (arrowheads) on the surface of alveolar bone (Bone). (D) Gingival cell composition by scRNA-seq of the MTA of CpG ODN or LPS revealed early stage of gingival inflammation, equivalent to Day 1 of the ligature-induced periodontitis model. (E) The fibroblastic gene expression signature revealed the presence of KT and AG fibroblasts by the MTA of CpG ODN, whereas the MTA of LPS induced MF. (F) AG fibroblasts of the MTA of CpG ODN and LPS were activated to express CCL and CXCL chemokines. (G) The MTA of CpG ODN did not upregulate Tlr9, whereas the MTA of LPS increased the expression of Tlr2/4 in AG fibroblasts. However, the both the MTA of CpG ODN and LPS increased the expression of TLR downstream molecules.
The scRNA-seq gingival cell composition of the MTA of CpG ODN and LPS indicated the early periodontitis pattern (Figure 4E) consistent with Day 1 scRNA-seq of the ligature-induced periodontitis (Figure 1F). The MTA of CpG ODN and LPS identified AG fibroblasts along with KT fibroblasts, whereas the MTA of LPS induced the additional MF (Figure 4E). The further analysis demonstrated the expression of CCL and CXCL chemokines by AG fibroblasts activated by the MTA of CpG ODN and LPS (Figure 4F). CpG ODN and LPS are sensed by Toll-Like Receptor 9 (TLR9) and TLR2/4, respectively. The scRNA-seq of MTA of CpG ODN did not capture the transcriptional activation of Tlr9, while the downstream effector genes such as Myd99, Irak1 and RelA were found upregulated in AG fibroblasts (Figure 4G). The MTA of LPS appeared to upregulate Tlr2 and Tlr4 gene transcription of AG fibroblasts and of MF albeit at lesser degrees (Figure 4G). These results indicated that AG fibroblasts were activated by CpG ODN and LPS through the MTA model, while these microbial elements appeared to exert different reactions.
Myeloid cell composition and activity during periodontitis development
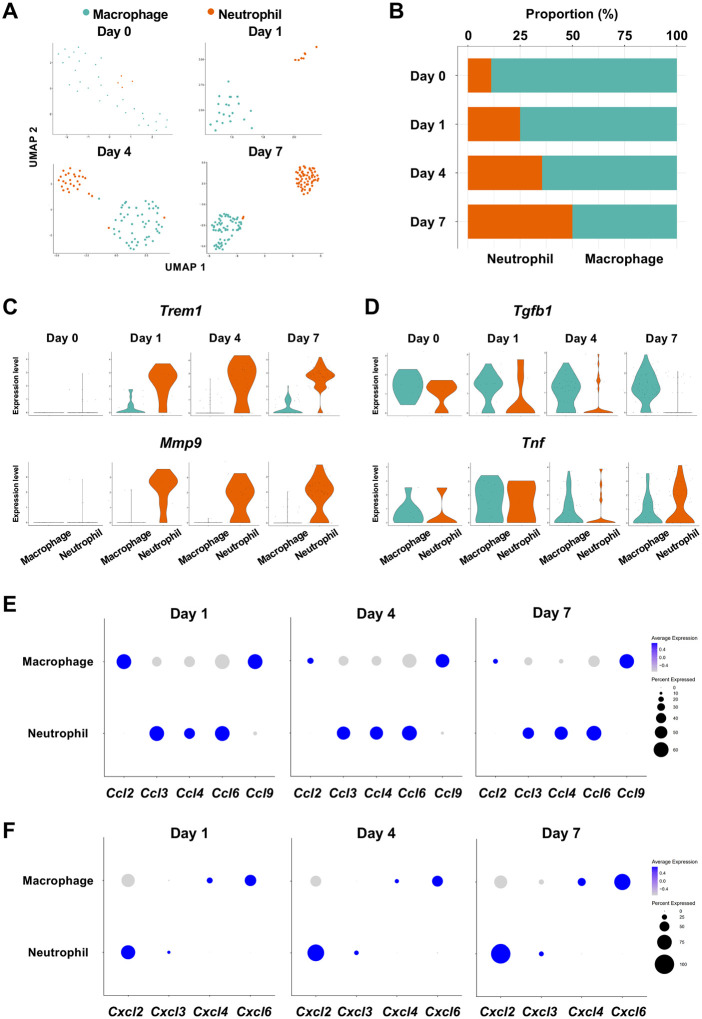
The infiltration of proinflammatory neutrophils into the gingiva has been extensively characterized in this mouse model of periodontitis [11, 38]. Here, we found that macrophages expressing cluster of differentiation 86 (Cd86) [39] and integrin subunit alpha X (Itgax) [40] were predominant on Day 0 in the myeloid cell fraction from healthy gingiva (Figure 5A, 5B—figure supplement 3). In contrast, the proportion of neutrophils expressing CXC motif chemokine receptor (Cxcr2) [41] and G0/G1 switch gene 2 (G0s2) [42] increased after ligature placement and during periodontitis development from Day 1 to 7 (Figure 5A, 5B—figure supplement 3), suggesting continuous neutrophil infiltration into the early and established gingival lesion. Moreover, after ligature placement, gingival neutrophils upregulated the expression of triggering receptor expressed on myeloid cells 1 (Trem1), indicating that these cells are activated and participating in the amplification of inflammatory signals [43, 44] (Figure 5C). Strikingly, the Trem1-expressing activated neutrophils also show upregulation of matrix metalloproteinase 9 (Mmp9; Figure 5C)—a protein associated with extracellular matrix degeneration within gingival tissue [45]. We further detected expression of tumor necrosis factor (Tnf) and transforming growth factor beta 1 (Tgfb1) in both macrophages and neutrophils on Day 0 and after ligature placement (Figure 5D). Collectively, these myeloid cell behaviors are consistent with those reported in prior studies on periodontitis development, thus validating our scRNA-seq data.
Figure 5.
Myeloid cell composition and activity in gingival tissue during in periodontitis development. (A) t-SNE projection plots showing myeloid cell subpopulations in gingival tissue during periodontitis development on Days 0, 1, 4, and 7. Colors indicate cell type, as follows: green, macrophages and red, neutrophils. (B) Proportion plots showing the relative amounts of neutrophils and macrophages on Days 0, 1, 4, and 7. (C) Violin plots showing Trem1 and Mmp9 expression levels in myeloid cells on Days 0, 1, 4, and 7; both genes are upregulated in neutrophils after ligature placement. (D) Violin plots showing Tgfb1 and Tnf expression in myeloid cells on Days 0, 1, 4, and 7; no obvious induction is observed in response to ligature placement. (E) Dot plots depicting expression levels of the C motif chemokine ligand (CCL) genes Ccl2, Ccl3, Ccl4, Ccl6, Ccl9. (F) The expression of CXC motif chemokine ligand (CXCL) genes Cxcl2, Cxcl3, Cxcl4, and Cxcl9. Chemokine expression in myeloid cells was unrelated to progression of gingival inflammation from Day 1 to 7.
Myeloid cells are also known to stimulate other immune cells through the expression of CCL and CXCL chemokines, many of which are associated with periodontitis development [46]. Our scRNA-seq analysis revealed that macrophages expressed Ccl2, Ccl9, Cxcl4, and Cxcl6, and neutrophils expressed Ccl3, Ccl4, Ccl6, Cxcl2, and Cxcl3 throughout periodontitis development (Figure 5E, 5F). These data suggest that chemokines and cytokines produced by macrophages and neutrophils in inflamed tissue may amplify and polarize the immune response toward chronic gingival inflammation.
Role of AG fibroblasts in myeloid cell activation
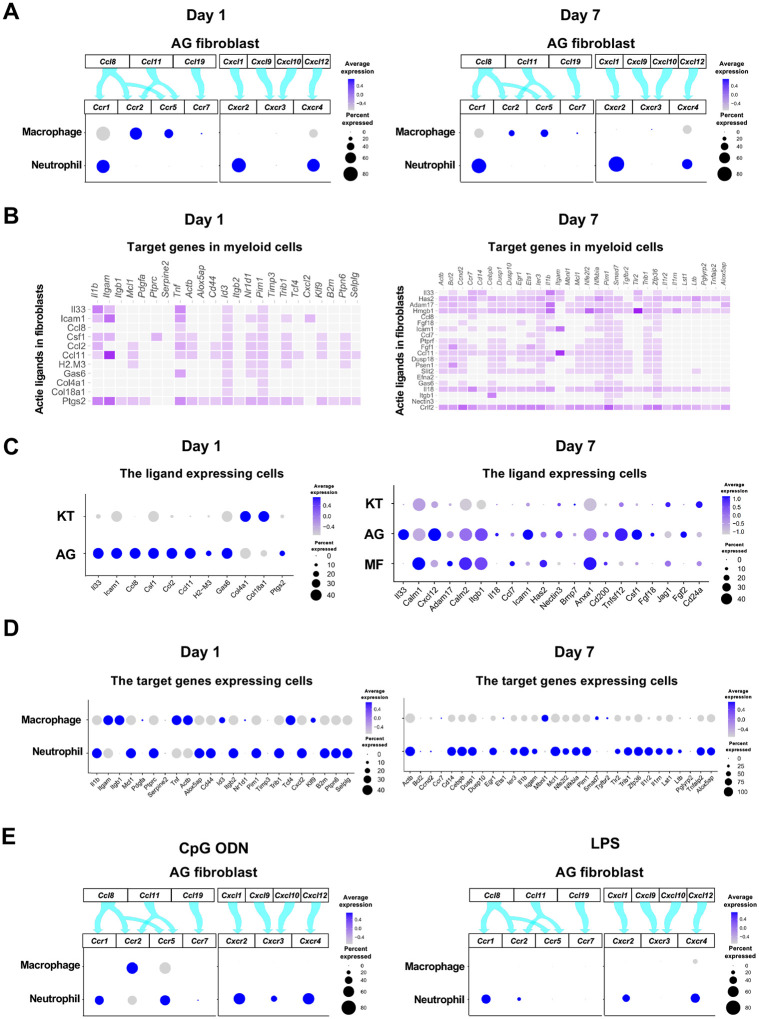
The interaction between chemokine ligands and their receptors has been extensively studied. Here, to evaluate the interaction between chemokine ligands strongly expressed by AG fibroblasts and chemokine receptors in innate immune cells, we matched AG fibroblast-expressed chemokines to expression of their putative receptors in myeloid cells. Results show that AG fibroblasts may regulate macrophages via the expression Ccl8 and Ccl11, which encode chemokines that can interact with CC chemokine receptors (CCRs) encoded by Ccr2 and Ccr5 in macrophages. Similarly, gene expression signatures suggest that AG fibroblast-mediated neutrophil regulation may occur through CCL8–CCR1, CXCL1–CXCR2, and CXCL12–CXCR4 interactions (Figure 6A). Notably, all chemokine–receptor pairs are expressed throughout periodontitis development, although expression of factors mediating the interaction between AG fibroblasts and macrophages was decreased on Day 7.
Figure 6.
Role of AG fibroblasts in myeloid cell activation. (A) Interaction between chemokine ligands expressed by AG fibroblasts and their putative chemokine receptors expressed by myeloid cells during periodontitis development. Dot plots depicting expression levels of the CC chemokine receptor (CCR) and the CXC chemokine receptor (CXCR) genes Ccr1, Ccr2, Ccr5, Ccr7, Cxcr2, Cxcr3, and Cxcr4 in myeloid cell subpopulations on Day 1 and Day 7 following ligature placement. (B) NicheNet ligand–target matrix indicating the regulatory potential between active ligands expressed in fibroblasts and target genes expressed in myeloid cells from the p-EMT program on Day 1 and Day 7. (E) Dot plot depicting expression levels of active ligand genes from panel (B) in fibroblast subpopulations on Day 1 and Day 7. (D) Dot plot depicting expression levels of target genes from panel (B) in myeloid cell subpopulations on Day 1 and Day 7. Results suggest a strong intercellular communication network from AG fibroblasts to neutrophils. (E) Interaction between chemokine ligands expressed by AG fibroblasts and their putative chemokine receptors expressed by myeloid cells in the MTA model of CpG ODN and LPS.
Intercellular communication may occur via other ligand–receptor interactions, which induce downstream target gene expression in recipient cells. Here, we performed NicheNet analysis to identify potential interactions between gingival fibroblasts and myeloid cells, revealing a trend toward increasing ligand–target interactions between fibroblasts and myeloid cells from Day 1 to Day 7 (Figure 6B). On Day 1, ligand expression was present primarily in AG fibroblasts, whereas on Day 7, MFs also exhibited prominent ligand expression (Figure 6C). Analysis of target gene expression revealed that both macrophages and neutrophils expressed receptors capable of interacting with AG fibroblast ligands on Day 1. In contrast, on Day 7, receptor expression was almost exclusively present in neutrophils, suggesting these cells are primarily targeted by MFs and AG fibroblasts at later stages of periodontitis development (Figure 6D).
The chemokine-receptor interaction was also suggested between AG fibroblasts and myeloid cells in the MTA of CpG DON (Figure 6E). However, in the MTA of LPS, macrophage lacked the detectable expression of chemokine receptor and the AG fibroblastic chemokine interaction appeared to be limited to neutrophils (Figure 6E).
Expression of osteoclastogenic cytokines
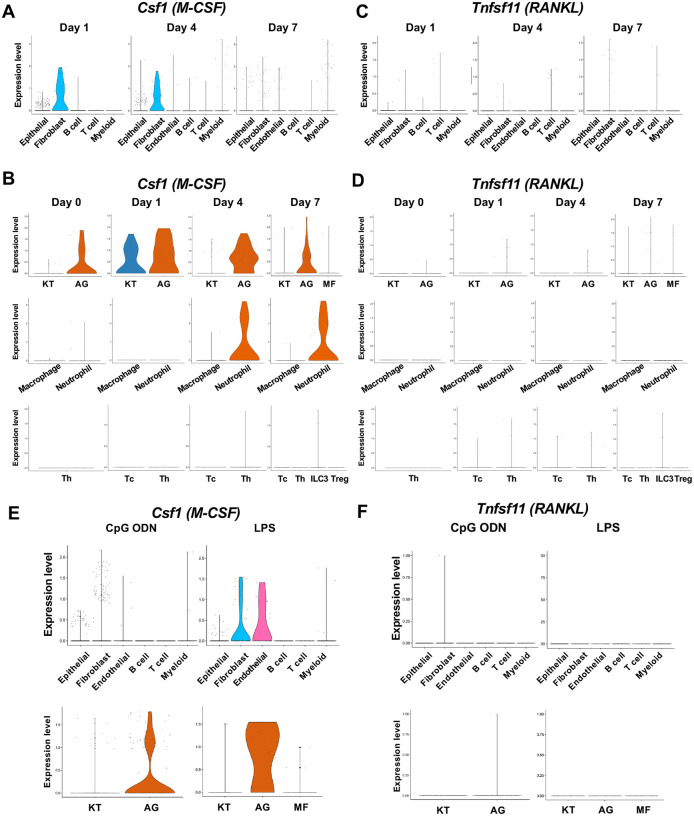
Macrophage-colony stimulating factor (M-CSF), encoded by Csf1, and receptor activator of nuclear factor kappa-B ligand (RANKL), encoded by Tnfsf11, are known to play critical roles in osteoclast induction [47]. We found that in the ligature-induced periodontitis model, the expression of M-CSF was detected in various cell types including fibroblasts (Figure 7A), whereas the RNAKL expression was more restricted to fibroblasts and T cells (Figure 7B). AG fibroblasts consistently expressed M-CSF from Day 0 to Day 7 and neutrophils started to express M-CSF in Day 4 and Day 7 (Figure 7C). The expression of RNAKL was detected in AG fibroblasts but not in myeloid cells (Figure 7D). T cells and type 3 innate lymphoid cells (ILC3; see below) were also shown to express M-CSF and RNAKL (Figure 7C, 7D).
Figure 7.
The cellular source of osteoclastogenic cytokines: M-CSF and RANKL in the ligature-induced model and the MTA model. (A) Violin plots showing expression levels of the macrophage-colony stimulating factor (M-CSF)-encoding gene, Csf1 (M-CSF), in each major cell type from the ligature-induced periodontitis model. (B) Violin plots showing expression levels of Csf1 in fibroblast subpopulations, myeloid cell subpopulations, and T cell subpopulations. (C) Violin plots showing expression levels of the nuclear factor kappa-B ligand (RANKL)-encoding gene, Tnfsf11, in each major cell type. (D) Violin plots showing expression levels of Tnfsf11 in fibroblast subpopulations, myeloid cell subpopulations, and T cell subpopulations. (E) Violin plots of Csf1 expressing cells in the MTA model. (F) Violin plots of Tnfsf11 expressing cells in the MTA model. AG fibroblasts predominantly expressed Tnfsf11.
The MTA of CpG ODN and LPS suggested the AG fibroblasts were one of the predominant cellular sources of M-CSF (Figure 7E). By contrast, the expression of RNAKL was only detected in AG fibroblasts of the MTA of CpG ODN and LPS did not seem to induce RANKL expression in the MTA model (Figure 7F). The osteoclastic activity found in the MTA of CpG ODN (Figure 4D) might be induced by M-CSF and RANKL derived from AG fibroblasts.
Helper T (Th) cells, cytotoxic T (Tc) cells, regulatory T (Treg) cells and innate lymphoid cells (ILCs) in periodontitis development
Our scRNA-seq dataset found that the Day 0 healthy gingiva exclusively contained Th cells expressing Cd4 [48]. However, after ligature placement, Tc cells expressing Cd8 [48] emerged, and on Day 7, Cd4−Zbtb16+ ILCs and Cd4+Foxp3+ Treg cells [49] were also detected (Figure 8A, 8B). The ILCs expressed Nfil3, a basic leucine zipper (bZIP) transcription factor required for ILC development [50] (Figure 8C). However, to our surprise, expression of Rorc, Il17a, and Il17f was detected in ILCs (Figure 8C), indicating that this gingival subpopulation is predominantly composed of type 3 ILCs (ILC3s). ILC3s and Th17 cells share similar regulatory functions. However, the role of ILC3s in the development of periodontitis has not been fully deciphered.
Figure 8.
ILC3s are critical for cervical alveolar bone resorption in the ligature-induced periodontitis development. (A) Proportion plots showing the relative amounts of T cell subpopulations in gingival tissue during periodontitis development. Treg, T regulatory cells; ILC, innate lymphoid cells; Th, T helper cells; Tc, cytotoxic T cells. (B) Violin plots showing expression levels of the T cell marker genes Cd8 (Tc), Cd4 (Th), Zbtb16 (ILC), and Foxp3 (Treg) on Day 7 following ligature placement. (C) Violin plots showing expression levels of Nfil3, Rorγ, Il17a, Il17f, Tbx21, and Gata3 on Day 7 following ligature placement. These gene signatures indicate that gingival ILCs primarily comprise type 3 ILCs (ILC3s). (D) Representative microCT images of the maxilla taken from the lateral view for the ligated side and from the contralateral view for the unligated side. (E) Alveolar bone loss was determined from the total distance between the CEJ and the ABC of the buccal bone or palatal bone at six sites in the ligated side (n = 6). (F) HE staining of the periodontal tissue on Day 7. gCT, gingival connective tissue; Bone, alveolar bone; PDL, periodontal ligament; scale bars, 100 μm. (G) Tartrate-resistant acid phosphatase (TRAP) staining of periodontal tissue from WT mice on Day 7; scale bar, 100 μm. Total number of TRAP-positive cells in a 0.01 mm2-area of the buccal and palatal bone in the cervical PDL site (H) and apical PDL site (H) (n = 6). Significance was determined by ANOVA, with Tukey’s multiple-comparison test. Data are presented as mean values ± SD; p < 0.05 was considered significant.
ILC3s are critical for cervical alveolar bone resorption in the mouse periodontitis model
We thus examined the role of ILC3s in periodontitis pathogenesis by measuring ligature-induced gingival defects and alveolar bone resorption in Rag2−/− mice, which lack functional B, Th, and Tc cells, and Rag2–IL-2 receptor common gamma (Il2rg) double-knockout mice (Rag2γc−/−), lacking all lymphocytes including ILCs. After ligature placement, we found that alveolar bone loss was decreased in Rag2−/− mice and nearly eliminated in Rag2γc−/− mice (Figure 8D). MicroCT image analysis indicated a better perseveration of alveolar bone structure in Rag2γc−/− mice, relative to the other groups (Figure 8E—figure supplement 4A, 4B). However, gingival defects developed similarly in wild-type (WT), Rag2−/−, and Rag2γc−/− mice (figure supplement 4C, 4D).
Histologically, osteoclastic resorption lacunae were observed on the alveolar bone surface at the cervical PDL and tooth apex PDL zones in WT mice (Figure 8F, 8G). In addition, we detected a significant decrease in the number of tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts in the cervical PDL zone of Rag2γc−/− mice (Figure 8H) and in the apical PDL zone of both Rag2−/− and Rag2γc−/− mice (Figure 8I). Collectively, these data suggest that ILC3s, not Th17 cells, are responsible for cervical alveolar bone resorption in the mouse periodontitis model, a pathological phenotype consistent with human disease (figure supplement 4E, 4F).
The role of AG fibroblasts and neutrophils in ILC3 development in periodontitis
Based on our present data, we hypothesize that ILC3s within the gingival tissue play a pathological role in cervical alveolar bone resorption in our mouse model. We therefore aimed to identify the cells that promote ILC3 development in mice. Similar to Th17 cells, ILC3 development was shown to be triggered by IL-6 and IL-23a [51, 52]. A survey of our scRNA-seq data identified epithelial cells, fibroblasts, and myeloid cells as the source of Il6 in gingival tissue (Figure 9A). On Days 1 and 4 following ligature placement, AG fibroblasts primarily expressed Il6, whereas neutrophils became the predominant source on Day 7. We further found that gingival epithelial cells comprise at least four different subpopulations, plus an additional group displaying an epithelial–mesenchymal transition (EMT) phenotype on Day 7; Il6 was expressed by several of these epithelial subpopulations, including the EMT subgroup (Figure 9A—figure supplement 5A–D). Il23a was also detected AG fibroblasts and myeloid cells (Figure 9B), with expression present in all epithelial cell subsets at various points in periodontitis development (Figure 9B—figure supplement 5D).
Figure 9.
The role of AG fibroblasts and neutrophils in ILC3 development in periodontitis. Violin plots showing expression levels of the genes encoding interleukin (IL)-6 (Il6) (A) and IL-23 (Il23a) (B) in each major cell type, fibroblast subpopulations, and myeloid cell subpopulations during periodontitis development. (C) Interaction between chemokine ligands strongly expressed by AG fibroblasts and their putative chemokine receptors expressed by T cells, including ILCs. Dot plots depict gene expression levels of Ccr1, Ccr2, Ccr5, Ccr7, Ccr8, Cxcr2, Cxcr3, and Cxcr4 in T cell subpopulations on Day 7 following ligature placement. (D) Interaction between chemokine ligands strongly expressed by neutrophils and their putative chemokine receptors expressed by T cells. Dot plots depicting gene expression levels of Ccr1, Ccr4, and Ccr5 in T cell subpopulations on Day 7 following ligature placement. (E) NicheNet ligand–target matrix denoting the regulatory potential between active ligands in fibroblasts and target genes in T cells from the p-EMT program on Day 7 following ligature placement. (F) NicheNet ligand–target matrix denoting the regulatory potential between active ligands in myeloid cells and target genes in T cells from the p-EMT program on Day 7 following ligature placement. (G) Dot plot depicting expression levels of active ligand genes from panel (E) in fibroblast subpopulations. (H) Dot plot depicting expression levels of active ligand genes from panel (F) in myeloid cell subpopulations. (I) Dot plot depicting expression levels of target genes from pane (E) in T cell subpopulations. (J) Dot plot depicting expression levels of target genes from panel (F) in T cell subpopulations.
Lastly, we evaluated potential interactions between chemokine ligands expressed by AG fibroblasts and neutrophils and chemokine receptors in innate immune cells. Our data suggest the presence of a chemokine–receptor association between ILC3s and both AG fibroblasts (Figure 9C) and neutrophils (Figure 9D), although interactions with other innate immune cells are also possible. NicheNet analysis further identified potential ligand–target gene associations between lymphocytes, including ILC3s, and both fibroblasts (Figure 9E) and myeloid cells (Figure 9F). Ligand expression was more prominent in AG fibroblasts than in other fibroblast subpopulations (Figure 9G) and elevated in neutrophils relative to macrophages (Figure 9H). Additionally, target gene expression was detected in ILC3s, although it was not specific to these cells (Figure 9I, 9J). Thus, in total, our data suggest a regulatory role for a newly identified AG fibroblast subpopulation in the gingiva, which appears to orchestrate chronic gingival inflammation, at least in the early stages, and to promote alveolar bone resorption via stimulation of neutrophils and ILC3s.
Discussion
The present study revealed that the gene signature of a unique and previously uncharacterized subpopulation of gingival fibroblasts, referred to as AG fibroblasts, could possess the functional capability to serve as an oral immune surveillant and to orchestrate the initiate gingival inflammation. Oral barrier immunity presents a complex interaction between different types of immune cells to protect and maintain the oral environment and structure. Given the frequent exposure to a diverse commensal and pathological microbiome, physical and chemical insults, and dietary and airborne antigens, the oral barrier immune mechanism must resiliently establish the highly tolerant homeostasis [53]. This oral immune homeostasis mechanism is not yet fully understood to date; however, the aberrant oral immune response and gingival chronic inflammation leading to periodontitis have provided an important clue to elucidate the oral barrier immunity.
The previous studies suggested gingival intraepithelial γδT cells [54], a subset of neutrophils [55], macrophages [56], and dendritic cells [57] should serve the candidate immune surveillant in the oral barrier tissue. These immune cells recognize the pathological signals through the TLR sensing mechanism. TLR2−/−, TLR4−/−, TLR2&4−/− [58] and TLR9−/− [59, 60] mice exhibited the reduced alveolar bone loss in various mouse periodontitis models, suggesting that TLRs indeed played an important role in developing discordant oral barrier immunity. However, these studies did not identify the TLR-carrying surveillant cells centrally involved in initiating the chronic gingival inflammation.
It has been suggested that TLR-expressing gingival fibroblasts may regulate innate immune responses [61, 62]. In the present study, we found that not all gingival fibroblasts acquired an immune-sensing capability, but rather, AG fibroblasts represented a distinct fibroblast subpopulation, capable of responding to microbial and tissue damage signals to serve initiate immune surveillance. To test if TLR ligands stimulate the AG fibroblast activation, we applied a newly developed mouse system employing the discrete MTA model [8]. The present study used TLR9 ligand: CpG ODN; and TLR2/4 ligand: LPS. Topical application of these TLR ligands to the maxillary gingival tissue activated AG fibroblasts and increased expression of CC and CXC chemokines (Figure 4F). It was noted that the CpG ODN application did not upregulate Tlr9 expression, while the LPS application increased Tlr2/4 expression. TLR9 mRNA level has not been fully correlated to the chronic inflammatory diseases; however, the pathological activation of TLR9 caused the discordant downstream inflammation [63]. In the present study, the differential expression of the TLR downstream signaling molecules suggested the ligand specific response by AG fibroblasts. In fact, both stimulants increased the expression of myeloid differentiation marker 88 (Myd88), IL1R-associated kinase (Irak), mitogen-activated protein kinase (Map3k7) and NF-kB subunit RelA (Figure 4G), suggesting that the corresponding TLRs were indeed stimulated [64, 65]. The Tlr expression pattern of Day 1 AG fibroblasts was similar to that of the MTA of CpG ODN, whereas the Day 4 AG fibroblasts resembled the Tlr expression pattern of the MTA of LPS (Figure 3B). It is tempting to speculate that microbial DNA/TLR9 activation of AG fibroblasts may initiate the early pathological process followed by the LPS/TRL2/4 stimulation in the more established periodontal inflammation. Taken together, we hypothesize that AG fibroblasts sensitively detect the microbial signals and play a previously unrecognized role in innate immune regulation during the early periodontitis development.
One of the major observations in the present study was that AG fibroblasts clearly upregulated the chemokine expression only after the pathological stimulation. CCL and CXCL chemokines orchestrate the chronic inflammation through the ligation and activation of their putative chemokine receptors in a specific mechanism. For example, neutrophils express a relatively limited number of chemokine receptors—CXCR2, CXCR4, and CCR1 [66, 67]. Therefore, neutrophil migration and trafficking to the affected gingiva as well as its pathological transformation in chronic periodontitis patients [11, 68, 69] may be guided through the specific chemokine-receptor interaction. Consistent with prior studies, we detected the expression of these chemokine receptors in gingival neutrophils, and they were linked to the chemokine ligands expressed by AG fibroblasts—CXCL1, CXCL12, and CCL8, respectively (Figure 6). In addition, neutrophils can respond to various chemo-attractants that modulate and finetune various cellular behaviors, such as migration direction, adhesion strength, and functional heterogeneity [70]. Here, we observed evidence for substantial intracellular communication between AG fibroblasts and neutrophils through ligand–target gene interactions (Figure 6). Although validation of each putative ligand–receptor interaction is beyond the scope of this study, our data suggest that AG fibroblasts support neutrophil trafficking in both early and established periodontitis lesions.
A primary pathological consequence of periodontitis is uncontrolled alveolar bone resorption, leading to tooth loss, which is thought to be mediated by osteoimmune pathways involving IL-17–expressing Th17 cells [71]. Surprisingly, analysis of our scRNA-seq revealed that the expression of Rorc, Il17a, and Il17f was not detected in CD4+ Th cells, but rather, in lymphocytes with ILC characteristics (Figure 8). ILCs share phenotypic and functional features with CD4+ T cells, although they lack antigen-specific T cell receptors [50]. The gingival ILCs expressed Rorc, Il17a, and Il17f, but not Tbx21 and Gata3, indicating that they were type 3 ILCs (ILC3s). Notably, recent clinical studies have reported the presence of ILCs in gingiva from human periodontitis patients [72, 73] and leptin receptor–deficient mice [74], although their pathological contributions have not been fully elucidated.
In the present study, we further explored the role of ILC3s in periodontitis-associated bone loss using Rag2−/− and Rag2γc−/− mice. Rag2−/− mutation prevents V(D)J recombination required for generating immunoglobulin and T cell receptors, resulting in the production of functionally immature B and T cells, including Th17 cells. However, ILCs do not undergo genomic receptor rearrangements and, thus, are unaffected by Rag2−/− mutation. In contrast, Rag2γc−/− mice have the additional Il2rg−/− mutation, which disables common γ chain cytokines (γc). Therefore, in addition to non-functional B and T cells, these animals also have defective γc-dependent ILCs. The ligature-placed Rag2−/− mice exhibited a reduced number of osteoclasts in the apical PDL area, indicative of reduced bone resorption in this root apical region. By contrast, Rag2γc−/− mice demonstrated a significant loss of bone resorption in both the cervical and apical PDL areas (Figure 8). Human periodontitis induces the localized alveolar bone resorption at the cervical PDL zone interfacing the gingival inflammatory legion. In contrast, apical alveolar bone resorption is observed in clinical cases of root canal infection (apical periodontitis). Therefore, our findings suggest that ILC3s, which are differentially impacted by Rag2γc−/− and Rag2−/− mutations, may be primarily responsible for human periodontitis-like cervical alveolar bone resorption near the site of gingival inflammation.
Differentiation of ILC3s and Th17 cells is mediated by similar environmental cues, with IL-6 and IL-23a playing critical roles in both pathways [75, 76]. Our present scRNA-seq data (Figure 9) suggest that AG fibroblasts and neutrophils are the primary cellular sources of Il6 during the early and later stages, respectively, of periodontitis development. In contrast, Il23a was found to be expressed by a variety of cell types, such as AG fibroblasts, myeloid cells, and multiple subsets of epithelial cells, including those with an EMT phenotype [77]. Further, CC and CXC chemokine–receptor associations between ILC3s and both AG fibroblasts and neutrophils appeared to be non-specific. Therefore, our data suggest that rather than a specific trigger, the collective gingival environment, which includes AG fibroblasts, might contribute to ILC3 differentiation.
In conclusion, based on our present findings, we propose that a previously unrecognized AG fibroblast subpopulation in the gingiva can facilitate immune surveillance and participate in the pathological regulation of innate immune cells, such as proinflammatory neutrophils, within oral barrier tissue (Figure 10). Moreover, we hypothesize that ILC3s in the inflamed gingiva play a critical role in pathological alveolar bone resorption in the mouse model of periodontitis and, potentially, in human disease. Thus, the newly proposed AG fibroblast–neutrophil–ILC3 axis may hold valuable clues for unraveling the pathological mechanisms underlying periodontitis development. Moreover, these findings also provide a basis for investigation of new preventive and therapeutic strategies to contain oral barrier inflammation and potentially sever the link between periodontitis and debilitating non-communicative metabolic and cardiovascular diseases.
Figure 10.
Schematic overview of the newly proposed AG fibroblast–neutrophil–ILC3 axis. We propose that periodontal inflammation is initiated by the activation of AG fibroblasts, which secrete chemokines and cytokines that recruit neutrophils to sites of tissue damage. Activated neutrophils and AG fibroblasts, in turn, activate ILC3s, leading to production of proinflammatory IL-17 cytokines. Ultimately, cervical alveolar bone resorption is facilitated by a localized osteoclastogenic environment, induced by activated ILC3s, together with AG fibroblasts, neutrophils, myofibroblasts, and gingival epithelial cells, including those with an epithelial–mesenchymal transition (EMT) phenotype.
Methods
Animal care
All protocols for animal experiments were reviewed and approved by the University of California Los Angeles (UCLA) Animal Research Committee (ARC# 2003–009) and followed the Public Health Service Policy for the Humane Care and Use of Laboratory Animals and the UCLA Animal Care and Use Training Manual guidelines. C57BL/6J WT, Rag2−/−, and Rag2γc−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Animals had free access to regular rodent diet and water and were maintained in standard housing conditions with 12-h light/dark cycles in the Division of Laboratory Animal Medicine at UCLA.
Evaluation of gingival defect and alveolar bone resorption in a ligature-induced mouse model of periodontitis
A silk thread was gently tied around the left maxillary second molar of 8- to 12-week-old female WT, Rag2−/−, and Rag2γc−/− mice under general inhalation anesthesia with isoflurane (Henry Schein, Melville, NY, USA). On Days 1, 3, and 7 after ligature placement, WT mice were randomly chosen and euthanized by 100% CO2 inhalation. WT mice without ligature placement were used as Day 0 pre-periodontitis control. Rag2−/− and Rag2γc−/− mice were euthanized on Day 7. The palatal tissue was digitally photographed, and maxillae were harvested and fixed in 10% buffered formalin (Thermo Fisher Scientific, Waltham, MA, USA). The gingival defect area was measured from digital photographs, using the ImageJ Java-based image-processing program (NIH, Bethesda, MD, USA), and normalized to the circumferential area of the maxillary first molar. Fixed maxillae were subjected to microCT imaging at an energy level of 60 kV and 166 μA, and 3D images were reconstructed from microCT scans (Skyscan 1275: Bruker, Billerica, MA, USA). Alveolar bone loss was assessed at three sites (mesiobuccal cusp, distobuccal cusp, and distal cusp) of the first molar, two sites (mesiobuccal cusp and distobuccal cusp) of the second molar, and one site (buccal cusp) of the third molar by measuring the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) on the buccal and palatal side of the alveolar bone. Total bone loss was calculated from the six-site total CEJ–ABC distance. The bone volume/total volume (BV/TV) ratio, bone surface, trabecular number (Tb.N), and trabecular thickness (Tb.Th) in the buccal side of alveolar bone of the second molar were determined using the proprietary analysis program (CTan: Bruker, Billerica, MA, USA). Statistical analysis to assess differences among multiple experimental groups was performed using one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test; p < 0.05 was considered to be statistically significant.
Evaluation of gingival effect in the maxillary topical application (MTA) model
We have developed a method to apply chemical therapeutic agents topically to the mouse maxillary tissue [24, 78]. We used the maxillary topical application (MTA) model to apply oral microbial components [8]. The present study used the MTA model using 1 μg/ml unmethylated CpG oligonucleotide (CpG ODN: InvivoGen, San Diego, CA) or P. gingivalis lipopolysaccharide (LPS: InvivoGen). First, a custom-made oral appliance was fabricated using clear dental resin, covering the maxillary/palatal tissue between the molars. Mice were anesthetized and placed on a supine position. CpG ODN (3 μl) or LPS (3 μl) was pipetted over the maxillary tissue and the oral appliance was placed to hold the solution with a bite block for 1 hr in an anesthetization chamber. Mice were then transferred to the operation table and the oral appliance and bite block were removed. In general, there was no remaining solution in the mouth. Mice were returned to the cage in the vivarium. On Day 4 of the MTA, mice were euthanized by 100% CO2 inhalation and the maxillary tissue was harvested and fixed with 10% buffered formalin for histological evaluation.
Histological analysis
Fixed maxillae were decalcified in 10% EDTA (Sigma–Aldrich) for 3 weeks and then embedded in paraffin. Histological cross-sections were stained with hematoxylin and eosin (HE) and evaluated on a light microscope. Adjacent paraffin sections (4 μm) were subjected to a heat-induced epitope retrieval procedure and then immunohistochemically stained with polyclonal antibodies to CD45 (#PA5–11671, Thermo Fisher Scientific), COL14A1 (#PA5–49916, Thermo Fisher Scientific), CXCL12 (#PA5–30603, Thermo Fisher Scientific), or Ctsk (PA5–14270, Thermo Fisher Scientific) at the suggested dilution, followed by secondary antibody application, diaminobenzidine staining, and methylene blue counterstaining.
Using maxillary cross-sections of WT, Rag2−/−, and Rag2−/−γc−/− mice, osteoclasts were evaluated by TRAP staining using a commercially available kit (Acid Phosphatase TRAP kit, Sigma–Aldrich), according to the manufacturer’s instructions. TRAP-positive cells were counted in the 0.01-mm2 area. Statistical analysis to assess the differences among multiple experimental groups was performed using one-way ANOVA with Tukey’s multiple-comparison test; p < 0.05 was considered to be statistically significant.
Single cell dissociation from mouse maxillary gingiva
On Days 1, 4, and 7 after ligature placement, and on Day 4 of the MTA of CpG ODN or LPS, mice were euthanized by 100% CO2 inhalation. And maxillary gingival tissues (n = 4 per group) were harvested from freshly isolated mouse maxillae.
Collagenase II treatment:
The tissues were cut into 1-mm pieces and placed immediately into digestion buffer, containing 1-mg/ml collagenase II (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA), 10-units/ml DNase I (Sigma–Aldrich, St. Louis, MO, USA), and 1% bovine serum albumin (BSA; Sigma–Aldrich) in Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies). The tissues were incubated in digestion buffer for 20 min at 37 °C on a shaker at 150 rpm and then passed through a 70-μm cell strainer. The collected cells were pelleted at 1,500 rpm for 10 min at 4 °C and resuspended in phosphate-buffered saline (PBS; Life Technologies), supplemented with 0.04% BSA (Cell suspension A).
Trypsin treatment:
Immediately following collagenase II treatment, tissues were incubated in 0.25% trypsin (Life Technologies) and 10-units/ml DNase I for 30 min at 37 °C on a 150-rpm shaker. Trypsin was neutralized with fetal bovine serum (Life Technologies), and the tissues were passed through a 70-μm cell strainer and washed with DMEM. The collected cells were then pelleted at 1,500 rpm for 10 min at 4 °C and resuspended in PBS with 0.04% BSA (Cell suspension B). Cell suspension A and Cell suspension B were combined in one tube. An equal number of combined cell suspensions A and B from four animals per group were combined for scRNA-seq analysis (10X Genomics, San Francisco, CA, USA).
Cell clustering and Identification
Cell Ranger was used to align reads, generate feature–barcode matrices, and perform clustering and gene expression analyses on the scRNA-seq data, and the output from this program was analyzed using the R-program Seurat (https://satijalab.org/seurat/). Cells with <2,400 genes detected or >1% mitochondrial gene expression were filtered out as low-quality cells. Individual gene counts for each cell were divided by the total gene counts for that cell and multiplied by a scale factor of 10,000; natural-log transformation was then applied to the counts. The FindVariableFeatures function was used to select 2,000 variable genes with default parameters, and the ScaleData function was used to scale and center the counts in the dataset. Principal component analysis and Uniform Manifold Approximation and Projection dimensional reduction were performed on variably expressed genes. The cluster markers were found using the FindAllMarkers function, and cell types were manually annotated based on the cluster markers. Cell types were assigned based on expression of cell marker genes, and gene expression within different cell types was displayed using dot plots and violin plots.
Functional annotation and pathway enrichment analysis
Annotation and visualization of GO terms were performed by Metascape (http://metascape.org/gp/index.html#/main/step1). The top-100 differentially expressed genes in each population were input and filtered with the term “immune”. Filtered genes were then input, and only “biological process” gene sets were retrieved from the GO database.
Ligand–target matrix prediction
NicheNet (v.1.0.0, https://github.com/saeyslab/nichenetr) was used to predict interactions between cell types. In brief, the integrated Seurat object containing each cell subpopulation was input into the NicheNet Seurat wrapper. Sender cells and receiver cells were determined, and interactions between active ligands expressed by sender cells and target receptors expressed by receiver cells were predicted based on information in signaling and ligand–receptor databases.
Supplementary Material
Acknowledgements
We thank Dr. Connie Lee of Division of Periodontology at UCLA School of Dentistry for her guidance on the clinical manifestations of human periodontitis. We also thank Dr. Yunfeng Li of the Translational Pathology Core Laboratory, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA for her immunohistology work. This study was supported by NIH grants R01DE022550, R44DE025524, and C06RR014529 and by SINTX Technologies.
Competing interests
I.N. is a consultant for FUJI FILM Corp and BioVinc LLC and received a research fund from SINTX Technologies, Inc. I.N. and A.H. received a research fund from Maruho Co. Ltd. The other authors declare no competing interests.
Data availability statement
We specify where the data supporting the results of this study can be found and provide hyperlinks to publicly archived datasets analyzed or generated during the study, where applicable. scRNA-seq data are found in NIH Gene Expression Omnibus (GSE228635).
References
- 1.Abusleme L., et al. , The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J, 2013. 7(5): p. 1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y. and Harrison O.J., Homeostatic Immunity and the Microbiota. Immunity, 2017. 46(4): p. 562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moutsopoulos N.M. and Moutsopoulos H.M., The oral mucosa: A barrier site participating in tissue-specific and systemic immunity. Oral Dis, 2018. 24(1–2): p. 22–25. [DOI] [PubMed] [Google Scholar]
- 4.Lamont R.J., Koo H., and Hajishengallis G., The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol, 2018. 16(12): p. 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helal O., et al. , Predictors for tooth loss in periodontitis patients: Systematic review and meta-analysis. J Clin Periodontol, 2019. 46(7): p. 699–712. [DOI] [PubMed] [Google Scholar]
- 6.Peres M.A., et al. , Oral diseases: a global public health challenge. Lancet, 2019. 394(10194): p. 249–260. [DOI] [PubMed] [Google Scholar]
- 7.Lin P., et al. , Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int J Mol Sci, 2021. 22(16): p. 8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo T., et al. , Oral microbial extracellular DNA initiates periodontitis through gingival degradation by fibroblast-derived cathepsin K in mice. Commun Biol, 2022. 5(1): p. 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajishengallis G. and Chavakis T., Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol, 2021. 21(7): p. 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shokeen B., et al. , Effect of Calcium Ion Supplementation on Oral Microbial Composition and Biofilm Formation In Vitro. Microorganisms, 2022. 10(9): p. 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajishengallis G., New developments in neutrophil biology and periodontitis. Periodontol 2000, 2020. 82(1): p. 78–92. [DOI] [PubMed] [Google Scholar]
- 12.Hasiakos S., et al. , Calcium Signaling in T Cells and Chronic Inflammatory Disorders of the Oral Cavity. J Dent Res, 2021. 100(7): p. 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cekici A., et al. , Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000, 2014. 64(1): p. 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokol C.L. and Luster A.D., The chemokine system in innate immunity. Cold Spring Harb Perspect Biol, 2015. 7(5): p. a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes C.E. and Nibbs R.J.B., A guide to chemokines and their receptors. FEBS J, 2018. 285(16): p. 2944–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodzic Z., et al. , IL-33 and the intestine: The good, the bad, and the inflammatory. Cytokine, 2017. 100: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ina K., et al. , Intestinal fibroblast-derived IL-10 increases survival of mucosal T cells by inhibiting growth factor deprivation- and Fas-mediated apoptosis. J Immunol, 2005. 175(3): p. 2000–9. [DOI] [PubMed] [Google Scholar]
- 18.Pinchuk I.V., et al. , PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology, 2008. 135(4): p. 1228–1237, 1237 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H.W., et al. , CCL19-producing fibroblastic stromal cells restrain lung carcinoma growth by promoting local antitumor T-cell responses. J Allergy Clin Immunol, 2018. 142(4): p. 1257–1271 e4. [DOI] [PubMed] [Google Scholar]
- 20.Williams D.W., et al. , Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell, 2021. 184(15): p. 4090–4104 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe T. and Hajishengallis G., Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods, 2013. 394(1–2): p. 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura H., et al. , Analysis of Experimental Ligature-Induced Periodontitis Model in Mice. Methods Mol Biol, 2021. 2210: p. 237–250. [DOI] [PubMed] [Google Scholar]
- 23.Marchesan J., et al. , An experimental murine model to study periodontitis. Nat Protoc, 2018. 13(10): p. 2247–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okawa H., et al. , Mechanism of bisphosphonate-related osteonecrosis of the jaw (BRONJ) revealed by targeted removal of legacy bisphosphonate from jawbone using competing inert hydroxymethylene diphosphonate. Elife, 2022. 11: p. e76207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groeger S. and Meyle J., Oral Mucosal Epithelial Cells. Front Immunol, 2019. 10: p. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamaguchi M., et al. , High Expression of Collagen XVII Compensates for its Depletion Induced by Pemphigoid IgG in the Oral Mucosa. J Invest Dermatol, 2018. 138(8): p. 1707–1715. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi A., et al. , Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc Natl Acad Sci U S A, 2019. 116(2): p. 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lallier T.E., Spencer A., and Fowler M.M., Transcript profiling of periodontal fibroblasts and osteoblasts. J Periodontol, 2005. 76(7): p. 1044–55. [DOI] [PubMed] [Google Scholar]
- 29.Bruno K.F., et al. , Characterization of inflammatory cell infiltrate in human dental pulpitis. Int Endod J, 2010. 43(11): p. 1013–21. [DOI] [PubMed] [Google Scholar]
- 30.Sparger E.E., et al. , Investigation of immune cell markers in feline oral squamous cell carcinoma. Vet Immunol Immunopathol, 2018. 202: p. 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcover A., Alarcon B., and Di Bartolo V., Cell Biology of T Cell Receptor Expression and Regulation. Annu Rev Immunol, 2018. 36: p. 103–125. [DOI] [PubMed] [Google Scholar]
- 32.Fujihashi K., et al. , Immunoregulatory function of CD3+, CD4-, and CD8- T cells. Gamma delta T cell receptor-positive T cells from nude mice abrogate oral tolerance. J Immunol, 1989. 143(11): p. 3415–22. [PubMed] [Google Scholar]
- 33.Cross M., et al. , Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A, 1988. 85(17): p. 6232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubo M., et al. , M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep, 2016. 6: p. 27548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotsch U., et al. , Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes Commun, 1994. 2(1): p. 7–14. [DOI] [PubMed] [Google Scholar]
- 36.Komatsu T., et al. , E-selectin mediates Porphyromonas gingivalis adherence to human endothelial cells. Infect Immun, 2012. 80(7): p. 2570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki H., et al. , Neuronal PAS Domain 2 (Npas2)-Deficient Fibroblasts Accelerate Skin Wound Healing and Dermal Collagen Reconstruction. Anat Rec (Hoboken), 2020. 303(6): p. 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva L.M., Brenchley L., and Moutsopoulos N.M., Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol Rev, 2019. 287(1): p. 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam R.S., et al. , Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. J Immunol, 2014. 193(5): p. 2349–62. [DOI] [PubMed] [Google Scholar]
- 40.Agarbati S., et al. , Prognostic Relevance of Macrophage Phenotypes in High-grade Oral Tongue Squamous Cell Carcinomas. Appl Immunohistochem Mol Morphol, 2021. 29(5): p. 359–365. [DOI] [PubMed] [Google Scholar]
- 41.Hashim A., et al. , Loss of Neutrophil Homing to the Periodontal Tissues Modulates the Composition and Disease Potential of the Oral Microbiota. Infect Immun, 2021. 89(12): p. e0030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F., et al. , Activation of G0S2 is coordinated by recruitment of PML/RARalpha and C/EBPepsilon to its promoter during ATRA-induced APL differentiation. J Leukoc Biol, 2017. 101(3): p. 655–664. [DOI] [PubMed] [Google Scholar]
- 43.Bouchon A., Dietrich J., and Colonna M., Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol, 2000. 164(10): p. 4991–5. [DOI] [PubMed] [Google Scholar]
- 44.Dopheide J.F., et al. , Critical limb ischaemia is characterised by an increased production of whole blood reactive oxygen species and expression of TREM-1 on neutrophils. Atherosclerosis, 2013. 229(2): p. 396–403. [DOI] [PubMed] [Google Scholar]
- 45.Corotti M.V., et al. , Immunolocalization of matrix metalloproteinases-2 and −9 during apical periodontitis development. Arch Oral Biol, 2009. 54(8): p. 764–71. [DOI] [PubMed] [Google Scholar]
- 46.Souto G.R., et al. , Relationship between chemokines and dendritic cells in human chronic periodontitis. J Periodontol, 2014. 85(10): p. 1416–23. [DOI] [PubMed] [Google Scholar]
- 47.Teitelbaum S.L., Bone resorption by osteoclasts. Science, 2000. 289(5484): p. 1504–8. [DOI] [PubMed] [Google Scholar]
- 48.Mahanonda R., et al. , Memory T cell subsets in healthy gingiva and periodontitis tissues. J Periodontol, 2018. 89(9): p. 1121–1130. [DOI] [PubMed] [Google Scholar]
- 49.Wei L., Xu M., and Xiong H., An update of knowledge on the regulatory role of Treg cells in apical periodontitis. Oral Dis, 2021. 27(6): p. 1356–1365. [DOI] [PubMed] [Google Scholar]
- 50.Eberl G., et al. , Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science, 2015. 348(6237): p. aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bielecki P., et al. , Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature, 2021. 592(7852): p. 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell N., et al. , Interleukin 6 Increases Production of Cytokines by Colonic Innate Lymphoid Cells in Mice and Patients With Chronic Intestinal Inflammation. Gastroenterology, 2015. 149(2): p. 456–67 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moutsopoulos N.M. and Konkel J.E., Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol, 2018. 39(4): p. 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., et al. , gammadeltaT cells in oral tissue immune surveillance and pathology. Front Immunol, 2022. 13: p. 1050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fine N., et al. , Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J Dent Res, 2016. 95(8): p. 931–8. [DOI] [PubMed] [Google Scholar]
- 56.Metcalfe S., et al. , Innate Phagocyte Polarization in the Oral Cavity. Front Immunol, 2021. 12: p. 768479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hovav A.H., Dendritic cells of the oral mucosa. Mucosal Immunol, 2014. 7(1): p. 27–37. [DOI] [PubMed] [Google Scholar]
- 58.Lin M., et al. , Different engagement of TLR2 and TLR4 in Porphyromonas gingivalis vs. ligature-induced periodontal bone loss. Braz Oral Res, 2017. 31: p. e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim P.D., et al. , Toll-Like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect Immun, 2015. 83(7): p. 2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crump K.E., et al. , Interplay of Toll-Like Receptor 9, Myeloid Cells, and Deubiquitinase A20 in Periodontal Inflammation. Infect Immun, 2017. 85(1): p. e00814–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian S.J., et al. , Single-Cell RNA Sequencing Identifies New Inflammation-Promoting Cell Subsets in Asian Patients With Chronic Periodontitis. Front Immunol, 2021. 12: p. 711337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naruishi K., Biological Roles of Fibroblasts in Periodontal Diseases. Cells, 2022. 11(21): p. 3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dragasevic S., et al. , Importance of TLR9-IL23-IL17 axis in inflammatory bowel disease development: Gene expression profiling study. Clin Immunol, 2018. 197: p. 86–95. [DOI] [PubMed] [Google Scholar]
- 64.Takeshita F., et al. , Cutting edge: Role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol, 2001. 167(7): p. 3555–8. [DOI] [PubMed] [Google Scholar]
- 65.Takeda K. and Akira S., TLR signaling pathways. Semin Immunol, 2004. 16(1): p. 3–9. [DOI] [PubMed] [Google Scholar]
- 66.Sabroe I., et al. , Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology, 2005. 115(1): p. 90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huston J. 3rd and Muhm J.R., Solitary pulmonary nodules: evaluation with a CT reference phantom. Radiology, 1989. 170(3 Pt 1): p. 653–6. [DOI] [PubMed] [Google Scholar]
- 68.Kolaczkowska E. and Kubes P., Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol, 2013. 13(3): p. 159–75. [DOI] [PubMed] [Google Scholar]
- 69.Tecchio C. and Cassatella M.A., Neutrophil-derived chemokines on the road to immunity. Semin Immunol, 2016. 28(2): p. 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metzemaekers M., Gouwy M., and Proost P., Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol, 2020. 17(5): p. 433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng J., et al. , The Th17/Treg cell balance: crosstalk among the immune system, bone and microbes in periodontitis. J Periodontal Res, 2022. 57(2): p. 246–255. [DOI] [PubMed] [Google Scholar]
- 72.Brown J.L., et al. , Enrichment of Innate Lymphoid Cell Populations in Gingival Tissue. J Dent Res, 2018. 97(12): p. 1399–1405. [DOI] [PubMed] [Google Scholar]
- 73.Dutzan N., et al. , Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol, 2016. 9(5): p. 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Q., et al. , Single-Cell Transcriptomic Atlas of Gingival Mucosa in Type 2 Diabetes. J Dent Res, 2022. 101(13): p. 1654–1664. [DOI] [PubMed] [Google Scholar]
- 75.Klose C.S., et al. , A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature, 2013. 494(7436): p. 261–5. [DOI] [PubMed] [Google Scholar]
- 76.Sawa S., et al. , Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science, 2010. 330(6004): p. 665–9. [DOI] [PubMed] [Google Scholar]
- 77.Wadie K.W., Bashir M.H., and Abbass M.M.S., Epithelial-mesenchymal transition in gingival tissues from chronic periodontitis patients: A case-control study. Dent Med Probl, 2021. 58(3): p. 311–319. [DOI] [PubMed] [Google Scholar]
- 78.Okawa H., et al. , Fluorescent risedronate analogue 800CW-pRIS improves tooth extraction-associated abnormal wound healing in zoledronate-treated mice. Commun Med (Lond), 2022. 2: p. 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We specify where the data supporting the results of this study can be found and provide hyperlinks to publicly archived datasets analyzed or generated during the study, where applicable. scRNA-seq data are found in NIH Gene Expression Omnibus (GSE228635).