Abstract
BACKGROUND:
Clinical pharmacy services (CPS) in the primary care setting have been shown to help patients attain treatment goals and improve outcomes. However, the availability of CPS in community-based primary care is not widespread. One reason is that current fee-for-service models offer limited reimbursement opportunities for CPS in the community setting. Furthermore, data demonstrating the value of CPS in this setting are limited, making it difficult for providers to determine the feasibility and sustainability of incorporating CPS into primary care practice.
OBJECTIVES:
To (a) evaluate the association between a pharmacist-led, diabetes collaborative drug therapy management program and patient outcomes, including glycemic control and health care costs, and (b) assess short-term economic outcomes in a primary care setting.
METHODS:
A retrospective cohort analysis was conducted using medical record data. This study was conducted using patients with uncontrolled type 2 diabetes (T2DM), defined as HbA1c ≥ 7.0%. Outcomes were compared between patients referred to a diabetes collaborative care management (DCCM) intervention from 2009-2012 and patients who did not participate in the DCCM program. To illustrate the difference in HbA1c between the 2 cohorts over the follow-up period, mean time adjusted HbA1c values were estimated using a panel-type random effects regression model, with results plotted at 90-day intervals from index date through the end of the study period. To help control for confounding by other factors, multivariate regression models were run. A difference-in-difference model was employed to estimate the effect of the program on resource utilization and all-cause charges.
RESULTS:
A total of 303 DCCM and 394 comparison patients were included. Mean (95% CI) age was 57.4 years (55.963, 58.902) versus 59.9 years (58.613, 61.276; P < 0.001) with 48% and 44% female for DCCM and comparison patients, respectively (P = 0.49). Mean baseline HbA1c was higher for DCCM (10.3%; 10.10, 10.53) than comparison patients (8.4%; 8.26, 8.61; P < 0.001). The greatest reduction in HbA1c was seen for both groups at 9 and 12 months post-index date. At these time points, the mean time adjusted difference in HbA1c between groups was no longer significant. Multivariate modeling identified that the DCCM program was associated with a -0.44% (-0.64, -0.25; P < 0.001) lower HbA1c at follow-up relative to the comparison group controlling for potential confounders, including baseline HbA1c. Change in resource utilization from pre- to post-index date did not differ between groups. However, in the difference-in-difference multivariate analysis the difference in mean all-cause charges from the 12-month pre- to post-index periods DCCM patients experienced a smaller average increase in charges ($250) than comparison patients ($1,341; coefficient = -0.423; 95% CI = -0.779, -0.068).
CONCLUSIONS:
A pharmacist-led diabetes collaborative care management program in a patient-centered primary care setting was associated with significantly better follow-up glycemic control relative to comparison patients. Further, the data suggest that the DCCM program was associated with a less substantial increase in all-cause total costs in patients with uncontrolled T2DM relative to comparison patients, which could translate into reduced costs and improved outcomes to managed care payers.
What is already known about this subject
Clinical pharmacy services are associated with improved glycemic control in patients with type 2 diabetes in a variety of practice settings.
Studies have shown an economic benefit of clinical pharmacy services, although results are not consistent.
What this study adds
A pharmacist-led, diabetes collaborative care management program in a patient-centered primary care setting is associated with improved glycemic control over an 18-month follow-up period relative to comparison patients.
This program was associated with a less substantial increase in all-cause health care costs relative to usual care.
Approximately 9.3% (29.1 million) of the U.S. population has diabetes at an estimated annual cost in the United States of $176 billion in direct medical costs and $69 billion in lost productivity.1,2 These costs are driven in large part by diabetes-related complications, with an annual health care cost for patients with complications approximately $10,000 per year higher than for a patient without complications.3 The overarching goal of diabetes treatment is, therefore, to reduce the risk of diabetes-related complications by achieving and maintaining near normal glycemic control with lifestyle changes and drug therapy.4,5
Treatment goals are facilitated by effective and efficient approaches to managing diabetes at all levels of care, including effective drug therapy management. As health care professionals, clinical pharmacists are specifically trained in the effective and appropriate use of medications to treat disease. Thus, there is significant opportunity in the primary care setting to include clinical pharmacists on the patient care team to manage drug therapy for patients with diabetes. This is particularly true for patients with type 2 diabetes mellitus (T2DM), who often require multiple medications to manage blood glucose and related comorbidities. In this capacity, clinical pharmacists provide drug information and identify patient-specific diabetes drug regimens to optimize outcomes and avoid drug-related problems.
Researchers have generated an abundance of evidence over the past 25 years that demonstrates that clinical pharmacy services (CPS) help patients with T2DM attain treatment goals and improve outcomes in the primary care setting.6-11 For instance, a recent study conducted in a federally qualified health center with a predominately underserved patient population found in patients with hemoglobin A1c (HbA1c) ≥ 9.0% that CPS was associated with a HbA1c reduction from a baseline of 1.5%.10 CPS was associated with a reduction in hospitalizations relative to comparison patients. Another study evaluated diabetes outcomes in patients with HbA1c ≥ 7.0% after a clinical pharmacist was added to the primary care team in an integrated delivery system.11 The patients receiving care from the team with CPS had a 2% point greater reduction in HbA1c than comparison patients. While these studies did not include economic outcomes, other studies conducted in health systems have found that CPS is associated with reduced inpatient/emergency department visits, avoidable drug-related problems, and prescription drug costs.12,13
Few primary care practices include clinical pharmacists on their patient-care teams to deliver CPS. Current fee-for-service payment models lack reimbursement for CPS. This creates uncertainty regarding the feasibility and sustainability of incorporating clinical pharmacists into the primary care delivery team.14
This study aims to contribute to the body of evidence illustrating the value of CPS in diabetes management by examining the impact of a pharmacist-led diabetes management program as part of a patient-centered care team. The setting for this study is 10 university-owned, community-based primary care clinics whose providers treat a diverse population of patients. Of these clinics, 8 have community pharmacies on site, and 3 of these have offered diabetes collaborative care management (DCCM) since 2008.
In this DCCM program, clinical pharmacists work under collaborative practice agreements with clinic physicians and advanced practice clinicians to provide diabetes drug therapy management. A preliminary pre-post analysis of patients with T2DM (mean HbA1c 10.1%) has shown improved glycemic control in DCCM patients with mean HbA1c reduction from enrollment to the last follow-up of 2.0% (P < 0.01).15 However, durability of the intervention has not been previously assessed.
Given the significance of effectively managing patients with chronic diseases, including T2DM, the purpose of this study was to evaluate clinical and economic outcomes associated with pharmacist-led drug therapy management. This study evaluated patients whose diabetes was inadequately controlled (defined for this study as HbA1c ≥ 7.0%) and further contributes to the literature by assessing short-term economic outcomes in a primary care setting.
Methods
Design and Intervention
A retrospective cohort study was conducted to evaluate clinical outcomes in patients with inadequately controlled T2DM, defined by HbA1c ≥ 7.0%, who received DCCM or usual care in a patient-centered primary care setting from 2008 to 2012.
Patients were referred to the DCCM program by the primary care provider at the provider’s discretion. Working under collaborative practice agreement with clinic physicians and advanced practice clinicians, DCCM pharmacists were able to prescribe and modify diabetes medication therapy, adjust insulin dosing, order HbA1c and lipid monitoring tests, and provide diabetes education to patients with uncontrolled T2DM. For patients who agreed to participate in the DCCM program, the intervention included an initial face-to-face visit with the pharmacist and telephonic and in-person follow-up visits every 1 or 2 weeks for dose adjustments, adherence and disease education, and to address patient questions with timing based on patient need. Pharmacists followed patients until goals were met or patients no longer engaged in the program, which occurred on average at 7 months. At this time, patients were released back to their primary care physicians to receive routine diabetes care.
Data Source
Data for this study were obtained from the University of Utah Health Care System Electronic Data Warehouse (EDW) from 2008 to 2012. The EDW comprises electronic medical record (EMR) and patient billing data for 1.4 million patients from 1990 to the present day. The EDW contains data required to assess T2DM outcomes, including biometric and vital sign data, laboratory test orders and results, outpatient prescription drug orders and medication histories, and diagnoses. Encounter and billing data in the EDW, including office visits, emergency department (ED) visits, inpatient stays, procedures, and other health care services delivered in the University Health Care System, support assessment of economic outcomes. Many patients, particularly comparison patients, obtained their prescriptions from non-University Health Care System pharmacies. Therefore, prescription drug dispensing data and amount billed were not included in these analyses.
Study Population
The DCCM cohort included patients aged ≥ 18 years with T2DM who were treated at a community clinic offering the DCCM program (3 of 10 clinics) from 2008 to 2012 and who were referred to the program from 2009 to 2012 (Figure 1A). The date of the patient’s first visit with the clinical pharmacist defined the patient’s study index date. Included patients were treated in the clinic for ≥ 180 days prior to their index date and had follow-up care provided by the clinic as captured by HbA1c monitoring from 3 to 18 months after the index date. A small number of patients participating in the DCCM program with an HbA1c < 7.0% at DCCM enrollment were excluded, since goals for these patients may not have been specifically directed at HbA1c reduction.
FIGURE 1.
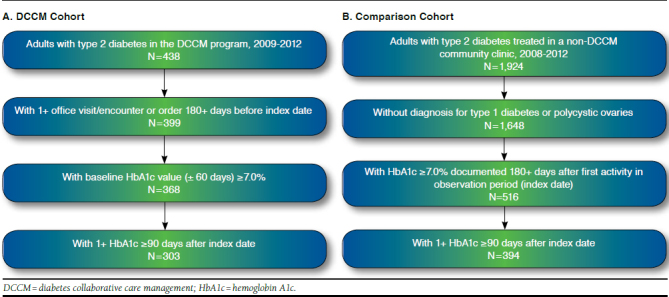
Population Identification Flowchart
Comparison patients were also adults with T2DM treated from 2008 to 2012 by providers at 1 of 5 clinics not offering the DCCM program or from a sixth clinic with a dedicated clinical pharmacist who provides clinical comprehensive medication management services that include T2DM. T2DM diagnoses codes in the EMR were used to identify patients with T2DM to comprise the comparison cohort. Comparison patients were therefore required to have 2 or more International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for T2DM (250.X0 or 250.X2) recorded in the EMR. Because diagnoses may not be recorded, patients with a medication order for any antidiabetic agent were also included (Figure 1B). Patients with a diagnosis of type 1 diabetes at any time or polycystic ovaries (PCO) in the absence of a diagnosis code for diabetes were excluded, since antidiabetic medication may be used for treating PCO. The index dates for the comparison patients were the first HbA1c values after 180 days of activity. This date would indicate diabetes follow-up and monitoring but no DCCM intervention because the program was not available in the comparison clinics. All comparison patients were under ongoing care by a clinic provider as measured by having least 1 HbA1c value documented between 3 months and 18 months after index date. Comparison patients with an HbA1c < 7.0% on index date were excluded. There was no requirement that patients be continuously covered by a given health plan during the study period.
Analysis
The primary independent variable of interest was patient participation in the DCCM program. The primary clinical outcome was glycemic control measured by HbA1c values captured during the 18 months post-index date.
Economic outcomes were identified as the change in medical resource consumption and change in medical charges from the 12-month period prior to the index date to the 12-month period following the index date. Medical care resource consumption was based on administrative data and reported as the number of community clinic office visits, inpatient hospitalizations, outpatient office visits, and ED visits when care was delivered at a university-owned facility. Data for care received outside the university system were not captured. Medical charges as a measure of medical costs were also reported from the university’s perspective and were reported as charges during the 12-month pre-index and 12-month post-index periods for inpatient hospitalizations and outpatient visits. Inpatient and outpatient charges were further combined for all-cause, total medical charges. Upon inspection, it was identified that the data did not discriminate when diabetes was the primary reason for a patient encounter versus other reasons. Thus, economic outcomes were not evaluated separately for diabetes-related care. Patient charges between the 12-month pre- and post-index dates were adjusted to 2012 real dollar values based on the Consumer Price Index.16
Additional independent variables included patient demographic characteristics as well as baseline, clinical, drug treatment, and health care utilization characteristics that could differ by group and influence outcomes. Demographic covariates included age (continuous and < 65 or ≥ 65 years); gender; race (white, black, other, and unknown/not documented); and insurance type (private, Medicaid, Medicare, and self-pay.)
Clinical covariates included in the study were baseline HbA1c (continuous and 7.0%-8.9%, 9.0%-10.9%, and ≥ 11.0%); weight; and body mass index (continuous and < 25, 25- < 30, 30- < 35, 35- < 40, and ≥ 40 kilogram per square meter). Baseline non-HbA1c laboratory and biometric values were the values documented on the index date or within 60 days before or after the index date, with the assumption that the DCCM intervention would have little or no impact on non-HbA1c clinical measures during the first 60 days after the index date. Comorbidities relevant to diabetes were identified with ICD-9-CM codes documented any time pre-index date and included hypertension, dyslipidemia, chronic kidney disease, retinopathy, neuropathy, cerebrovascular disease including stroke, and cardiovascular disease, including myocardial infarction (see Appendix A for ICD-9-CM codes, available in online article).
Diabetes pharmacotherapy information was captured for the 365-day period prior to the index date based on prescription drug orders in the clinic EMR data. Diabetes medication prescriptions were captured by drug class and included alpha-glucosidase inhibitors, biguanides, dipeptidyl peptidase-4 inhibitors, GLP-1 receptor agonists, insulin, thiazolidinediones, meglitinide analogues, and sulfonylureas. Count of antidiabetic classes prescribed the year prior to the index date was captured and categorized as 0 classes, 1 class, 2 classes, 3 classes, or 4+ classes. Health care resource utilization data, as a baseline measure of disease severity, included overall outpatient costs and community clinic encounters during the 6 months prior to the index date.
Statistical Analyses
Clinical Outcomes.
Descriptive statistics (frequency, percentage, mean, and standard deviation [SD]) were used to describe the baseline characteristics of the study cohort stratified by DCCM or comparison group. Independent t-tests and chisquare tests were used for continuous and discrete variables, respectively, to determine if DCCM and comparison patients differed by any patient characteristic.
To illustrate the difference in the variations in HbA1c between the 2 cohorts, mean time adjusted HbA1c values were plotted at 90-day intervals from index date through the end of the study period. A panel-type random effects regression model was employed where HbA1c values were regressed against the DCCM or comparison group indicator, post-index follow-up time, interaction between group and follow-up time, squared follow-up time, and the interaction between group and the squared follow-up time. These time-adjusted HbA1c values were not adjusted for other possible confounding. Results from the random effects regression models were then used to generate plots of adjusted HbA1c against time using the estimated mean HbA1c values at 90-day intervals and the corresponding standard errors and 95% confidence interval (CI) values. HbA1c values likely have a nonlinear association with time. Therefore, follow-up time in months was interacted with the DCCM or the group variable that indicated whether a patient received the intervention or not. To obtain an interpretable mean effect, the follow-up time and its interaction term were mean-centered.17 Thus, DCCM patients were graphically compared with comparison patients at the mean value of the follow-up time.
To control for possible confounding based on differences between DCCM and comparison patients, each patient was assigned a propensity score, a created variable based on measured confounders that represent the probability of receiving an intervention based on a patient’s baseline characteristics.18 Propensity scores are commonly used in comparative effectiveness research studies to reduce the impact of selection bias that can occur when patients are not randomized to treatment. Propensity scores were calculated for this study based on a logistic regression analysis with DCCM participation as the dependent variable. Baseline clinical and demographic characteristics were included as independent variables, and those that were significant at P < 0.05 or that were considered potential confounders were retained in the final propensity score model. The final model included patient age, baseline HbA1c, prior insulin or metformin prescription orders, number of antidiabetic classes previously prescribed, prevalence of retinopathy or chronic kidney disease, number of community clinic visits in the prior 6 months, and 6-month pre-index outpatient costs. Log-likelihood ratio test was used to compare model iterations to ensure that excluding nonsignificant variables did not negatively impact model fit.
Propensity score matching using stratified, radius, and kernel matching (with replacement) were initially explored. Due to the relatively small group of comparison patients identified as eligible for inclusion, these approaches resulted in a high loss of patients and/or did not adequately balance covariates between DCCM and comparison patients. Thus, all comparison patients were retained, and the propensity score was used as a covariate in regression analyses.
Linear regression analyses were used to identify how being in the diabetes intervention affected follow-up HbA1c values relative to comparison patients. Several iterations of the regression model were performed. First, a linear model was estimated to examine the association between participation in the DCCM program and follow-up HbA1c values, controlling only for baseline HbA1c. Second, ordinary least squares (OLS) were used to fit a more complete model, including covariates that were trending towards significance (P < 0.10) or that were believed to be potential confounders based on prior knowledge. The final model controlled for patient age, baseline HbA1c, prior insulin or metformin use, number of antidiabetic classes previously prescribed, prevalence of retinopathy or chronic kidney disease, the number of community clinic visits in the prior 6 months, and 6-month pre-index outpatient costs.
Additional models were based on the initial OLS model, but included propensity scores were explored, including the following: (a) propensity scores included as a covariate to adjust for the likelihood of the patient having received the intervention and reduce treatment effect bias19; (b) stratified analysis with patients stratified by propensity score quintile to help remove imbalance between treatment groups for the variables included in the propensity score model19; (c) weighted propensity scores (pi) to estimate the average causal effect in those most likely to be given the intervention (DCCM patient weighted by 1 and comparison patients by pi /(1-pi); and (d) inverse probability weighting or the inverse of the likelihood of a patient being in the DCCM program to balance representation of DCCM and comparison patients by likelihood of receiving the intervention (DCCM patients as 1/pi and comparison patients as 1/(1 – pi).20 The results of the analysis with a stratified propensity score did not reveal a difference in outcomes by propensity score stratus, suggesting that the propensity score was not contributing to the model. Thus, we present results from the models with the propensity score as a covariate and stratified to illustrate this finding, but the final 2 models using a weighted propensity score are not presented.
This study also evaluated outcomes in a subset of patients with baseline HbA1c ≥ 8.0% in efforts to better balance the cohorts, thereby helping to address concerns that change in HbA1c in the DCCM group may represent the natural tendency for outliers to move towards the mean rather than the effects of the clinical intervention.
Economic Outcomes.
Descriptive statistics (mean, SD) were used to describe resource utilization and charges from the 12-month pre-index period to the 12-month follow-up period. Resource use and charge data were not normally distributed. Therefore, Wilcoxon rank sum tests were used to determine if medical resource use and charges differed between pre-index and post-index dates with DCCM and comparison patients evaluated separately. Additionally, the Wilcoxon-Mann-Whitney rank sum test was employed to examine if changes in charge and usage differed between the DCCM and comparison cohorts.
Economic outcomes were then evaluated for the overall study cohort using multivariable regression models based on a difference-in-difference (DID) estimating method to identify how participation in the DCCM program impacted medical utilization and charges relative to comparison patients pre- and post-index date. The following model was used to analyze the effect of DCCM intervention on medical utilization and charges:
To study medical utilization, the outcome variables employed in the analysis were the average number of inpatient visits; community clinic visits; visits to other outpatient clinics, which are predominantly for specialty care; and ED visits. To analyze charges, 3 outcome variables were used: the aggregate medical charges accrued by patients during the study period, inpatient charges, and all outpatient charges. The parameter α1 captures the effect of being in the DCCM program, while α2 measures the effect of the post-index period. The key DID estimator is α3, which is the coefficient term on the interaction between the DCCM indicator and the post-index period indicator, and captures the difference from pre-index to post-index periods in the average difference in health care utilization or charges for the 2 groups. Mathematically, the parameter can be explained as:
The parameter α4 measures the effect of baseline HbA1c on utilization or charges. Augmented versions of these models included controlling for other covariates such as age, comorbidities, antidiabetic history, and other clinical characteristics.
As utilization is a count with a discrete probability distribution, a negative binomial regression model was utilized. A likelihood ratio test comparing the negative binomial models with Poisson models resulted in chi-square values that suggested alpha is non-zero for all empirical specifications, and the negative binomial is more appropriate model than a Poisson model for this analysis. A zero-inflated model was also considered, but results did not change between the 2 models.
Charge data were skewed because of high utilization by a small number of patients. Therefore, costs were modeled using a generalized linear model (GLM) with a log link and gamma family.21 Gamma regression is a popular statistical model for skewed distributions, such as hospital cost data or for data that can only take on positive values. The model does not assume a normal distribution but rather uses 2 parameters to allow it to be pliable to the shape of the observed distribution.22
All statistical tests were performed at an a priori significance level of 0.05 using Stata SE version 12.0 (StataCorp, College Station, TX). No adjustment was made for multiple comparisons. The protocol for this study was submitted, and approval/waiver of consent was provided by the University of Utah Institutional Review Board (IRB# 00044764).
Results
Population and Baseline Characteristics
Of 438 patients enrolled in the DCCM program from 2009 through 2012, 303 patients were followed for a minimum of 180 days before entering the program, with a baseline HbA1c ≥ 7.0% and at least 1 follow-up HbA1c value documented in the EMR or clinical pharmacist notes from 3 to 18 months after the index date (Figure 1A). Of 1,936 potential comparison patients with indication of T2DM, 394 patients had confirmed T2DM without indication of type diabetes or PCO and EMR activity for at least 180 days before index date. These patients also had an index-date HbA1c ≥ 7.0% and at least 1 follow-up HbA1c value documented in the EMR from 3 to 18 months after the index date (Figure 1B).
Baseline characteristics are provided in Table 1. The mean (95% CI) age of DCCM participants was 57.4 (56.0, 58.9) years and 59.9 (58.6, 61.3) years in the comparison group (P = 0.005). Baseline diabetes control was significantly different between the 2 groups, with DCCM patients having a mean baseline HbA1c of 10.3% (10.10, 10.53) compared with 8.4% (8.26, 8.61) in the comparison group (P < 0.001). The DCCM cohort also had higher rates of several comorbidities than comparison patients, including chronic kidney disease (23.8% vs. 7.6%), coronary heart disease (32.0% vs. 19.5%), and history of stroke (5.6% vs. 1.0%; P < 0.001 for all). In the subset of patients with baseline HbA1c ≥ 8.0%, mean (95% CI) HbA1c was 10.6% (10.44, 10.85) for DCCM (n = 271) and 9.9% (9.65, 10.18; P < 0.001) for comparison patients (n = 171). Comparison patients also continued to differ from the DCCM cohort in this subset regarding prior comorbidities, number of antidiabetic classes, and prior diabetes medications use in this subset of patients.
TABLE 1.
Baseline Characteristics
| Demographics | DCCM Cohort (N = 303) | Comparison Cohort (N = 394) | P Value | ||
|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | ||
| Age | 57.43 | 13.00 | 59.94 | 13.44 | 0.005 |
| 18-44 | 46 | 15.18 | 54 | 13.71 | 0.320 |
| 45-64 | 176 | 58.09 | 191 | 48.48 | |
| 65+ | 81 | 26.73 | 149 | 37.82 | |
| Male | 159 | 52.48 | 219 | 55.58 | 0.494 |
| Race | |||||
| White | 162 | 53.47 | 277 | 70.30 | < 0.001 |
| Black | 10 | 3.30 | 2 | 0.51 | |
| Other | 117 | 38.61 | 71 | 18.02 | |
| Unspecified | 14 | 4.62 | 44 | 11.17 | |
| Baseline HbA1c | |||||
| Mean HbA1c (%) | 10.31 | 1.89 | 8.44 | 1.75 | < 0.001 |
| 7-9% | 89 | 29.37 | 290 | 73.60 | < 0.001 |
| > 9% | 214 | 70.63 | 104 | 26.40 | < 0.001 |
| Subsample: baseline HbA1c ≥ 8% | |||||
| Mean HbA1c (%) | 10.64 | 0.10 | 9.91 | 0.13 | < 0.001 |
| 8-9% | 57 | 21.03 | 67 | 39.18 | < 0.001 |
| > 9% | 214 | 78.97 | 104 | 60.82 | < 0.001 |
| Prior antidiabetic agents | |||||
| Metformin | 242 | 79.87 | 286 | 72.59 | 0.021 |
| Sulphonylurea | 193 | 63.70 | 187 | 47.46 | < 0.001 |
| Thiazolidinedione | 110 | 36.30 | 91 | 23.10 | < 0.001 |
| Insulin | 174 | 57.43 | 90 | 22.84 | < 0.001 |
| DPP-4 | 18 | 5.94 | 31 | 7.87 | 0.346 |
| GLP-1 | 9 | 2.97 | 4 | 1.02 | 0.056 |
| Others | 4 | 1.32 | 6 | 1.52 | 0.838 |
| Prior antidiabetic classes | |||||
| 0 and 1 | 76 | 25.08 | 171 | 43.40 | < 0.001 |
| 2 and 3 | 146 | 48.18 | 188 | 47.72 | |
| 4+ | 81 | 26.73 | 35 | 8.88 | |
| Mean weight (kg) | 95.35 | 25.16 | 99.31 | 26.18 | 0.073 |
| Mean BMI (kg/m 2 ) | 32.54 | 0.51 | 32.33 | 0.54 | 0.778 |
| < 25 | 28 | 9.09 | 35 | 8.80 | 0.891 |
| 25-30 | 85 | 27.95 | 87 | 22.13 | |
| 30-35 | 84 | 27.61 | 102 | 25.87 | |
| 35-40 | 52 | 17.17 | 98 | 24.80 | |
| 40+ | 55 | 18.18 | 72 | 18.40 | |
| Prior comorbidities | |||||
| Hypertension | 216 | 71.29 | 263 | 66.75 | 0.219 |
| Dyslipidemia | 112 | 36.96 | 57 | 14.47 | < 0.001 |
| Chronic kidney disease | 72 | 23.76 | 30 | 7.61 | < 0.001 |
| Retinopathy | 37 | 12.21 | 11 | 2.79 | < 0.001 |
| Neuropathy | 121 | 39.93 | 89 | 22.59 | < 0.001 |
| Cardiovascular disease | 40 | 13.20 | 11 | 2.79 | < 0.001 |
| Stroke | 17 | 5.61 | 4 | 1.02 | < 0.001 |
| Coronary heart disease | 97 | 32.01 | 77 | 19.54 | < 0.001 |
| Myocardial infarction | 14 | 4.62 | 9 | 2.28 | 0.082 |
BMI = body mass index; DCCM = diabetes collaborative care management; HbA1c = hemoglobin A1c; kg = kilogram; kg/m2 = kilogram per square meter; SD = standard deviation.
In the full sample, prescription orders for diabetes medications during the year prior to index date differed between the DCCM and comparison groups (Table 1). While the use of metformin was high in both groups, a greater proportion of DCCM patients were prescribed metformin the year prior to the index date (79.9%) than comparison patients (72.6%; P = 0.021). Insulin was prescribed more frequently at baseline in the DCCM group (57.4%) than in the comparison group (22.8%; P < 0.001). Additionally, the number of diabetes medication classes prescribed the year prior to the index date differed between the groups (P < 0.001 for the distribution). The proportion of DCCM patients prescribed 3 (35.1%) or 4+ (17.5%) classes the year prior to the index date was higher than the proportion of comparison patients (25.6% and 9.1%, respectively).
Glycemic Control
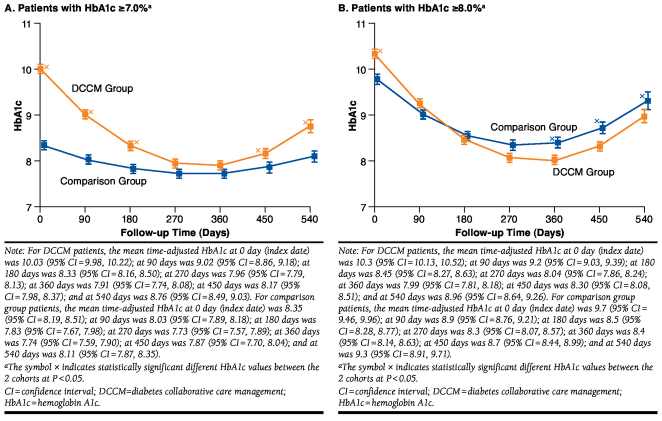
Mean time adjusted HbA1c was plotted at 90-day intervals from the index date through the end of the study period. For both DCCM and comparison patients, HbA1c was lowest at 270 days and gradually rose after 360 days (Figure 2A). The DCCM group had a significantly higher baseline HbA1c, but the gap between the 2 groups narrowed over time, widening slightly between 15 and 18 months. Note that these time-adjusted HbA1c values are not adjusted for other covariates.
FIGURE 2.
Time-Adjusted HbA1c for DCCM Versus Comparison Patients
In the subset of patients with baseline HbA1c ≥ 8.0%, DCCM patients had a higher HbA1c at baseline but, starting around 150 days through the end of the study period (540 days), achieved lower time-adjusted HbA1c levels relative to comparison patients (Figure 2B). Mean HbA1c was the lowest between 270 and 360 days, with a similar increase in HbA1c between 360 and 540 days.
In the linear regression analyses controlling only for baseline HbA1c, participation in the DCCM program was associated with a 0.21% lower HbA1c any time post-index date relative to the comparison group (coefficient [coef] = -0.21, 95% CI = -0.40, -0.02; Table 2A). In the OLS model controlling for baseline characteristics that could confound results but not including a propensity score, the intervention was associated with a 0.44% (coef = -0.44, 95% CI = -0.64, -0.25) lower HbA1c at follow-up relative to the comparison group. Baseline HbA1c was strongly associated with follow-up HbA1c values in these model iterations, implying patients who started at high baseline HbA1c had higher follow-up HbA1c readings. Age, pre-index insulin use, number of antidiabetic classes prescribed prior to index date, and having retinopathy were significantly associated with HbA1c outcomes.
TABLE 2.
Effect on Follow-up HbA1c for Patients with Type 2 Diabetes and Baseline HbA1c ≥ 7.0% Treated in a Pharmacist-Coordinated DCCM Program Versus Comparison Patients (Ordinary Least Squares Regression Analyses)
| Variables | Model 1: Adjustment for Baseline HbA1c | Model 2: Adjustment for All Controls | Model 3: Adjustment for All Controls Plus Propensity Score as Covariate | Model 4: Adjustment for All Controls Plus Propensity Score Quintile Regression | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| DCCM vs. comparison patients | -0.212 (-0.401, -0.023) | 0.028 | -0.440 (-0.635, -0.245) | < 0.001 | -0.442 (-0.641, -0.243) | < 0.001 | -0.437 (-0.637, -0.238) | <0.001 |
| Baseline HbA1c (per %) | 0.532 (0.476, 0.588) | < 0.001 | 0.502 (0.449, 0.554) | < 0.001 | 0.495 (0.325, 0.665) | < 0.001 | 0.493 (0.375, 0.611) | < 0.001 |
| Propensity score | ||||||||
| Covariate | 0.071 (-1.613, 1.755) | 0.934 | ||||||
| Quintile 2 (reference 1) | 0.005 (-0.251, 0.262) | 0.967 | ||||||
| Quintile 3 | 0.083 (-0.361, 0.527) | 0.713 | ||||||
| Quintile 4 | -0.008 (-0.636, 0.619) | 0.979 | ||||||
| Quintile 5 | 0.098 (-0.821, 1.016) | 0.835 | ||||||
| Constant | 3.507 (3.064, 3.951) | < 0.001 | 4.713 (4.054, 5.372) | < 0.001 | 4.746 (3.717, 5.775) | < 0.001 | 4.762 (3.838, 5.686) | < 0.001 |
Note: Models 2, 3, and 4 were controlled for age, prescription orders for antidiabetic agents the year prior to index date, number of antidiabetic classes prescribed prior to index date, prevalence of prior comorbidities, outpatient charges for 6 months prior to index date, and the number of community clinic visits for 6 months prior to index date.
CI = confidence interval; DCCM = diabetes collaborative care management; HbA1c = hemoglobin A1c.
Incorporating a propensity score as a continuous covariate (coef = -0.44; 95% CI = -0.64, -0.24) or stratified in quintile regression (coef = -0.44; 95% CI = -0.64, -0.24) had little additional effect on the DCCM group coefficient. Baseline HbA1c remained highly associated with HbA1c outcomes in these models. In addition to baseline HbA1c, the adjusted models controlled for age, prescription orders for metformin or insulin, the year prior to index date, number of antidiabetic classes prescribed prior to index date, prevalence of retinopathy and chronic kidney disease, outpatient costs 6 months prior to the index date, and the number of community clinic visits 6 months prior to the index date.
Results were similar when evaluating the subset of patients with an HbA1c at baseline of ≥ 8.0%—the DCCM group experienced lower follow-up HbA1c readings of 0.21%, on average, relative to comparison patients (coef = -0.38; 95% CI = -0.62, -0.15) controlling for baseline HbA1c (Table 3). When adjusting for additional covariates, the DCCM group with baseline HbA1c ≥8.0% experienced an average follow-up HbA1c that was 0.58% lower than comparison patients (coef = -0.58; 95% CI = -0.81, -0.34). Including a propensity score as a continuous variable (coef = -0.58; 95% CI = -0.81, -0.34) or in a quintile analyses (coef = -0.58; 95% CI = -0.81, -0.35) had little effect on the identified association. Baseline HbA1c also had a strong statistical association with follow-up HbA1c readings in the subset analyses.
TABLE 3.
Effect on Follow-up HbA1c for Patients with Type 2 Diabetes and Baseline HbA1c ≥ 8.0% Treated in a Pharmacist-Coordinated DCCM Program Versus Comparison Patients (Ordinary Least Squares Regression Analyses)
| Variables | Model 1: Adjustment for Baseline HbA1c | Model 2: Adjusting for All Controls | Model 3: Adjustment for All Controls Plus Propensity Score as Covariate | Model 4: Adjustment for All Controls Plus Propensity Score Quintile Regression | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| DCCM vs. comparison patients | -0.381 (-0.616, -0.146) | 0.002 | -0.577 (-0.810, -0.344) | < 0.001 | -0.575 (-0.811, -0.339) | < 0.001 | -0.579 (-0.812, -0.346) | < 0.001 |
| Baseline HbA1c (per %) | 0.480 (0.408, 0.552) | < 0.001 | 0.473 (0.406, 0.539) | < 0.001 | 0.478 (0.316, 0.641) | < 0.001 | 0.397 (0.267, 0.527) | < 0.001 |
| Propensity score | ||||||||
| Covariate | -0.102 (-2.560, 2.356) | 0.935 | ||||||
| Quintile 2 (reference 1) | 0.192 (-0.184, 0.569) | 0.315 | ||||||
| Quintile 3 | 0.215 (-0.327, 0.757) | 0.437 | ||||||
| Quintile 4 | 0.398 (-0.311, 1.107) | 0.271 | ||||||
| Quintile 5 | 0.852 (-0.241, 1.944) | 0.126 | ||||||
| Constant | 4.215 (3.538, 4.893) | < 0.001 | 5.428 (4.505, 6.352) | < 0.001 | 5.412 (4.382, 6.441) | < 0.001 | 6.158 (4.767, 7.549) | < 0.001 |
Note: Models 2, 3, and 4 were controlled for age, prescription orders for antidiabetic agents the year prior to index date, number of antidiabetic classes prescribed prior to index date, prevalence of prior comorbidities, outpatient charges for 6 months prior to index date, and the number of community clinic visits for 6 months prior to index date.
CI = confidence interval; DCCM = diabetes collaborative care management; HbA1c = hemoglobin A1c.
Economic Outcomes
Unadjusted analyses of medical resource use revealed that utilization generally did not change for inpatient and emergency services in the 12-month period after the index date than the prior 12 months for DCCM patients and comparison patients (Table 4). Exceptions were the increase in the mean (SD) number of office visits per patient in the community clinics (1.24 [6.83] visits; P = 0.003) and outpatient clinics (1.16 visits [6.81]; P < 0.001), which increased significantly in the 12-month post-index period for DCCM patients from the pre-index period. For comparison patients, similar patterns followed. The mean (SD) number of office visits per patient in community clinics (0.75 [4.48] visits; P = 0.011) and outpatient clinics (0.61 [3.93] visits; P = 0.003) increased significantly in the post-index period from the pre-index period for comparison patients. In the unadjusted analyses, the mean difference in the number of per patient visits from the pre- to post-index period differed significantly between DCCM and comparison patients for only outpatient visits (P < 0.001).
TABLE 4.
Health Care Utilization and Charges 12 Months Before and 12 Months After Index Date for DCCM Versus Comparison Patientsa
| DCCM | Comparison | Difference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preperiod | Postperiod | Difference | Z Value b | Preperiod | Postperiod | Difference | Z Value b | Z Value c | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Utilization | |||||||||||||||
| Community | 8.413 | 6.632 | 9.657 | 6.587 | 1.244 | 6.833 | 0.003 | 5.617 | 4.430 | 6.368 | 4.911 | 0.751 | 4.477 | 0.011 | 0.192 |
| Inpatient | 0.152 | 0.606 | 0.145 | 0.655 | -0.007 | 0.686 | 0.547 | 0.033 | 0.179 | 0.041 | 0.244 | 0.008 | 0.298 | 0.990 | 0.559 |
| Outpatient | 3.861 | 6.473 | 5.023 | 6.241 | 1.162 | 6.806 | <0.001 | 1.665 | 3.591 | 2.272 | 4.559 | 0.607 | 3.929 | 0.003 | <0.001 |
| Emergency | 0.337 | 0.999 | 0.406 | 1.517 | 0.069 | 1.276 | 0.640 | 0.025 | 0.157 | 0.063 | 0.340 | 0.038 | 0.351 | 0.080 | 0.780 |
| Charges ($) | |||||||||||||||
| Total | 6,818 | 13,943 | 7,069 | 17,330 | 251 | 18,173 | 0.630 | 2,419 | 6,949 | 3,760 | 13,381 | 1,341 | 14,475 | 0.001 | 0.037 |
| Inpatient | 2,399 | 10,835 | 2,116 | 10,259 | -283 | 12,336 | 0.475 | 545 | 5,090 | 757 | 5,919 | 212 | 7,719 | 0.545 | 0.322 |
| Outpatient | 4,419 | 7,194 | 4,953 | 9,680 | 534 | 10,414 | <0.001 | 1,874 | 3,996 | 3,003 | 11,284 | 1,129 | 11,354 | <0.001 | 0.017 |
a Adjusted to 2012 real U.S. dollars.
b Wilcoxon signed-rank test between periods.
c Wilcoxon rank-sum test between groups.
DCCM = diabetes collaborative care management; SD = standard deviation.
Patient charges between the 12-month pre- and post-index periods, adjusted to 2012 real dollar values, were evaluated based on a comparison of means (Table 4). For DCCM patients, the only change that reached significance was a change in outpatient charges with a mean (SD) increase of $534 ($10,414) in the post-index period (P < 0.001). Total medical charges (SD) were on average $251 ($18,174) higher, and inpatient charges (SD) were on average $283 ($12,336) lower, but the differences were not significant. Outpatient charges (SD) in the comparison group increased by $1,129 (11,354; P < 0.001). Total medical charges were also significantly higher for comparison patients ($1,341 [$14,475]; P < 0.001). The difference in mean charges from the 12-month pre- to post-index periods was significantly different between DCCM and comparison patients for outpatient services and for overall medical charges (P = 0.001), with DCCM patients experiencing smaller increases in charges versus comparison patients during the study period.
A multivariate negative binomial regression analyses was conducted to determine if health care utilization from 12 months before to 12 months after index date differed for DCCM versus comparison patients (Table 5). In the base model, the parameter of interest is the estimated coefficient on the interaction term, which primarily indicated no statistical significant difference in community clinics and inpatient, outpatient, and ED utilization from pre- to post-index date between DCCM and comparison patients. In the augmented model, which controlled for additional covariates such as demographic characteristics (age, race, and gender); comorbidities; antidiabetic history; and other clinical characteristics, the parameter on the interaction term was not significant, implying no difference in utilization from pre- to post-index between the 2 groups.
TABLE 5.
Association Between DCCM Intervention in Post-Index Perioda and Medical Utilization (Negative Binomial Regression)
| Base Model | Augmented Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value | |||
| Inpatient visits | ||||||||
| DCCM intervention in post-index period | -0.245 | -1.283 | 0.792 | 0.643 | -0.342 | -1.361 | 0.677 | 0.510 |
| DCCM group assignment | 1.340 | 0.546 | 2.135 | 0.001 | 0.927 | 0.122 | 1.732 | 0.024 |
| Post-index period (reference pre-index) | 0.216 | -0.618 | 1.051 | 0.611 | 0.257 | -0.563 | 1.076 | 0.539 |
| Baseline HbA1c | 0.089 | -0.041 | 0.219 | 0.178 | 0.070 | -0.064 | 0.204 | 0.306 |
| Other covariatesb | No | Yes | ||||||
| Community visits | ||||||||
| DCCM intervention in post-index period | 0.013 | -0.143 | 0.170 | 0.869 | 0.026 | -0.125 | 0.176 | 0.738 |
| DCCM group assignment | 0.469 | 0.350 | 0.588 | < 0.001 | 0.332 | 0.212 | 0.452 | < 0.001 |
| Post-index period (reference pre-index) | 0.130 | 0.024 | 0.237 | 0.016 | 0.140 | 0.038 | 0.242 | 0.007 |
| Baseline HbA1c | -0.034 | -0.056 | -0.012 | 0.002 | -0.032 | -0.053 | -0.010 | 0.004 |
| Other covariatesb | No | Yes | ||||||
| Outpatient visits | ||||||||
| DCCM intervention in post-index period | -0.022 | -0.365 | 0.320 | 0.899 | 0.007 | -0.332 | 0.345 | 0.969 |
| DCCM group assignment | 0.922 | 0.669 | 1.175 | < 0.001 | 0.820 | 0.559 | 1.082 | < 0.001 |
| Post-index period (reference pre-index) | 0.307 | 0.075 | 0.540 | 0.010 | 0.315 | 0.085 | 0.545 | 0.007 |
| Baseline HbA1c | -0.061 | -0.110 | -0.013 | 0.013 | -0.082 | -0.133 | -0.032 | 0.001 |
| Other covariatesb | No | Yes | ||||||
| Emergency visits | ||||||||
| DCCM intervention in post-index period | -0.729 | -1.673 | 0.215 | -0.130 | -0.692 | -1.632 | 0.247 | 0.149 |
| DCCM group assignment | 2.328 | 1.552 | 3.104 | <0.001 | 2.144 | 1.360 | 2.929 | < 0.001 |
| Post-index period (reference pre-index) | 0.922 | 0.107 | 1.737 | 0.027 | 0.929 | 0.113 | 1.745 | 0.026 |
| Baseline HbA1c | 0.129 | 0.018 | 0.241 | 0.022 | 0.074 | -0.040 | 0.188 | 0.203 |
| Other covariatesb | No | Yes | ||||||
a Variable of interest in this difference-in-difference model is variable representing DCCM group in the post-index period, which is the interaction term between DCCM intervention group assignment and a variable indicating the post-index period.
b Other covariates include age, race, gender, pre-index use of metformin or insulin, number of diabetes classes used pre-index date, and history of retinopathy or chronic kidney disease. See Appendix B (available in online article) for coefficients for other covariates.
CI = confidence interval; DCCM = diabetes collaborative care management; HbA1c = hemoglobin A1c.
A GLM with gamma link and log function to evaluate the effect of the intervention on charges found that, relative to comparison patients, the DCCM group’s change (increase) in charges before and after index was lower (coef = -0.423; 95% CI = -0.779, -0.068), driven by significantly smaller increases in outpatient charges in the DCCM group relative to the comparison group (coef = -0.419; 95% CI = -0.757, -0.081; Table 6). In the augmented models, where additional covariates were included, similar results were observed. DCCM patients had significantly lower increase in overall medical charges (coef = -0.423; 95% CI = -0.768, -0.079) and for outpatient charges (coef = -0.419; 95% CI = -0.747, -0.092) versus comparison patients from pre- to post-index periods. In base and augmented models, baseline HbA1c had a statistically significant negative association with total and outpatient charges. There was no significant difference in inpatient charges from pre- to post-index dates between the intervention and the control group in base and augmented models.
TABLE 6.
Association Between DCCM Intervention in Post-Index Perioda and Medical Charges (GLM Model)
| Base Model | Augmented Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value | |||
| Log (total charges) | ||||||||
| DCCM intervention in post-index period | -0.423 | -0.779 | -0.068 | 0.019 | -0.423 | -0.768 | -0.079 | 0.016 |
| DCCM group assignment | 1.089 | 0.822 | 1.356 | < 0.001 | 0.803 | 0.533 | 1.073 | < 0.001 |
| Post-index period (reference pre-index) | 0.441 | 0.207 | 0.675 | < 0.001 | 0.441 | 0.214 | 0.668 | < 0.001 |
| Baseline HbA1c | -0.122 | -0.171 | -0.074 | < 0.001 | -0.107 | -0.156 | -0.059 | < 0.001 |
| Other covariatesb | No | Yes | ||||||
| Log (inpatient charges) | ||||||||
| DCCM intervention in post-index period | -0.142 | -0.610 | 0.326 | 0.552 | -0.142 | -0.608 | 0.324 | 0.550 |
| DCCM group assignment | 0.609 | 0.257 | 0.962 | 0.001 | 0.436 | 0.071 | 0.801 | 0.019 |
| Post-index period (reference pre-index) | 0.061 | -0.248 | 0.370 | 0.698 | 0.061 | -0.246 | 0.368 | 0.697 |
| Baseline HbA1c | 0.019 | -0.045 | 0.083 | 0.553 | 0.022 | -0.044 | 0.087 | 0.511 |
| Other covariatesb | No | Yes | ||||||
| Log (outpatient charges) | ||||||||
| DCCM intervention in post-index period | -0.419 | -0.757 | -0.081 | 0.015 | -0.419 | -0.747 | -0.092 | 0.012 |
| DCCM group assignment | 1.024 | 0.770 | 1.279 | < 0.001 | 0.750 | 0.493 | 1.007 | < 0.001 |
| Post-index period (reference pre-index) | 0.474 | 0.251 | 0.697 | < 0.001 | 0.474 | 0.258 | 0.690 | < 0.001 |
| Baseline HbA1c | -0.130 | -0.177 | -0.084 | < 0.001 | -0.115 | -0.161 | -0.069 | < 0.001 |
| Other covariatesb | No | Yes | ||||||
a Variable of interest in this difference-in-difference model is variable representing DCCM group in the post-index period, which is the interaction term between DCCM intervention group assignment and a variable indicating the post-index period.
b Other covariates include age, race, gender, pre-index use of metformin or insulin, number of diabetes classes used pre-index date, and history of retinopathy or chronic kidney disease. See Appendix C (available in online article) for coefficients for other covariates.
CI = confidence interval; DCCM = diabetes collaborative care management; GLM = general linear model; HbA1c = hemoglobin A1c.
Discussion
Recognizing the opportunity to positively impact outcomes in patients with T2DM, clinical pharmacists practicing in university-owned community clinic pharmacies manage patients’ diabetes drug therapy according to collaborative practice agreements with clinic primary care providers. At the time of this study, 438 patients had been referred to the DCCM program, and 303 of those with uncontrolled T2DM and sufficient follow-up time to support outcomes analyses were included in this study. Patient diabetes drug therapy management by the pharmacist-led DCCM program was associated with better follow-up glycemic control and cost trends relative to comparison patients.
Time-adjusted estimates of follow-up HbA1c values demonstrated that patients enrolled in the DCCM program experienced significant reductions in HbA1c after entering the DCCM program. By 9 months (270 days) after the index date, the difference in glycemic control identified at baseline between DCCM and comparison patients was no longer observed. While glycemic control deteriorated in both groups after 12 months of follow-up, the similarity in glycemic control was maintained through 15 months. This finding is significant, since the average DCCM patient was discharged from the program after 7 months, suggesting that the benefits are sustained. However, diabetes is a progressive disease and often requires patients to make lifestyle modifications that may be difficult to maintain. Therefore, additional follow-up DCCM encounters with the pharmacist might be warranted to reinforce educational messages and adjust medication therapy.
We also hypothesized that patients receiving the DCCM intervention would have better utilization and cost trends relative to comparison patients. This was observed with respect to medical charges with DCCM having a significantly smaller increase in outpatient and total medical charges in the 12-month post-index period than comparison patients. Utilization analyses were, however, less conclusive. Descriptive results identified that community clinic visits and outpatient specialty clinic visits increased significantly in the post-index period for DCCM patients, while outpatient specialty visits were significantly higher in the post-index period for comparison patients. These findings could reflect a trend for community clinic providers to continue to treat patients who participate in the DCCM program, while similar comparison patients may have been referred to specialists.
Relative to costs, the DCCM cohort’s all-cause total medical charges were higher in the pre- and post-index periods compared with the comparison cohort. However, when controlling for potential confounders, being in the DCCM group was associated with a significantly smaller increase in outpatient and total charges from before to after the index date compared with the comparison group. Baseline HbA1c had a statistically significant negative association with all-cause total medical and outpatient charges. This finding was expected. Other studies have estimated that improving HbA1c by 1% can lead to a significant decrease in costs. One-year savings have been estimated to range from $685 to $950 per patient per year and are greater in patients with poorly controlled T2DM (HbA1c ≥ 10%).22
Other authors have also reported the impact of CPS on glycemic control in patients with T2DM in the ambulatory care setting.10,12,14,24-27 Our study findings were consistent with those studies that identified an HbA1c reduction with CPS from baseline of 0.5% to 2.0% and a difference relative to comparison patients ranging from a 0.2% increase (not significant) to a 1.4% (P < 0.001) reduction.10,12,25,27
Our study is one of a small number that considers economic outcomes in addition to clinical outcomes. Our findings were consistent with Chung et al. (2014), who did not identify a significant difference in inpatient or ED utilization between groups.10 Others have evaluated cost outcomes with diabetes CPS. Cranor et al. (2003) identified a reduction in total annual health care spending of $1,200 to $1,872 per patient versus baseline in patients receiving diabetes-related CPS in the community pharmacy setting.28 Anaya et al. (2008) identified that total costs were over $62,000 less in patients with a diagnosis of diabetes after the implementation of a collaborative practice agreement that included diabetes drug therapy management ($85,184 pre-index vs. $22,259 post-index).13 However, the Anaya et al. and Cranor et al. studies did not include comparison groups.13,28
To help isolate the effect of the DCCM program from underlying trends, this study compared DCCM patients with a cohort of comparison patients with HbA1c ≥ 7.0% treated per usual care. We initially attempted to create a matched cohort to help achieve covariate balance between the DCCM and comparison patients. However, we faced an unanticipated challenge in that matching resulted in considerable loss of patients due to the inability to find suitable matched pairs. Thus, a traditional cohort design with multivariate analysis to control for confounding was used to retain all eligible DCCM and comparison patients. The implication of not matching was that DCCM patients had significantly higher baseline costs and HbA1c than those in the comparison cohort.
Baseline differences between treatment groups are a frequent problem in observational studies.29 This occurs because real-world treatment selection is driven by patient characteristics that influence prescriber expectations of outcomes based on disease severity, treatment effectiveness, or adverse effects. Given this limitation, we evaluated follow-up HbA1c in a subset of patients with a baseline HbA1c of ≥ 8.0%. This step eliminated near-goal patients who may not have been candidates for more aggressive treatment due to age, concerns over hypoglycemia, or other clinical considerations. With this added level of restriction, HbA1c values between groups were more similar than in the overall study cohort but were also statistically different (10.6% in the DCCM group vs. 9.9% in the comparison group; P < 0.001). However, higher baseline HbA1c can lead to larger HbA1c decreases with an intervention. This study evaluated follow-up HbA1c controlling for baseline HbA1c rather than looking only at the change in HbA1c. While this approach may not fully address this limitation, it helps to mitigate the risk of overstating the effect of the intervention on glycemic control.
When adjusting only for baseline HbA1c, follow-up HbA1c values associated with the DCCM intervention was -0.21% overall and -0.38% lower for the subgroup of patients with baseline HbA1c ≥ 8.0% than comparison patients. Because controlling for only baseline HbA1c does not account for other potential confounders, we then employed a series of multivariable regression models, most of which incorporated propensity scores as a further means to statistically control for the differences between the groups. Regression models, including iterations incorporating the propensity score were generally consistent and identified a greater reduction in follow-up HbA1c for DCCM relative to comparison patients, consistently identifying follow-up HbA1c values that were estimated to be 0.44% lower in the DCCM group versus the comparison group overall. This difference was 0.58% in the subset with baseline HbA1c ≥ 8.0%. A reduction in HbA1c of 0.5% is commonly considered to be clinically meaningful; these estimates are within a clinically meaningful range. Thus, the relative reduction in HbA1c with the DCCM intervention was similar if not slightly greater when restricting the cohort and reducing the baseline HbA1c difference. If a high baseline HbA1c was the primary driver of HbA1c reduction observed in the DCCM cohort, the difference in HbA1c reduction between groups would have been expected to be smaller in the restricted cohort. However, restriction did not eliminate the baseline HbA1c difference between groups; thus, the higher baseline HbA1c in the DCCM group may still explain some of the reduction.
This study contributes to the body of evidence regarding the value of CPS in the primary care setting for diabetes by providing data on clinical and economic outcomes related to diabetes collaborative drug therapy management. Having evidence for the value of CPS is important to public and private payers as they consider recognizing pharmacists as providers and/or in expanding reimbursement to cover diabetes-related CPS. Similarly, integrated health systems, including accountable care organizations, need such information when considering how to optimize the role of all team members, including pharmacists, in managing their diabetes population. For either stakeholder, the CPS promise for diabetes care is in optimizing drug therapy and avoiding poor patient outcomes that can result in greater consumption of health care resources.
Limitations
This discussion has touched on several key limitations of this study including possibility of confounding by indication. We used restriction in a subset analysis of HbA1c outcomes in patients with baseline HbA1c ≥ 8.0% and multiple approaches to multivariate analysis to help mitigate this risk. However, the possibility that our study findings are biased due to inadequate control for confounding due to differences in HbA1c, prior diabetes medication use, and comorbidities remains, and results should be interpreted with this limitation in mind. However, this study’s results are in line with the findings of other studies reporting the impact of CPS on glycemic control in patients with T2DM in the ambulatory care setting.10,12,14,24-27
This study is one of a small number that have considered economic outcomes in addition to clinical outcomes. However, baseline differences in cost and variability in costs across the cohort leading to wide CIs were notable and may similarly reflect residual bias, although the economic findings were also consistent with similar studies.10,13,29 Furthermore, administrative costs of the DCCM program were not included. Thus, this study may underestimate costs for the DCCM group. Therefore, the results should be interpreted with caution given the potential bias and limited published data on the economic impact of community clinic- and primary care-based CPS in diabetes. Considering these limitations and the possibility that improved diabetes management associated with the DCCM program could reduce the risk of future diabetes complications, a long-term assessment of outcomes is warranted.
Other limitations include the risk of uncontrolled confounding due to incomplete or unavailable data. For instance, data on patient behaviors that affect outcomes such as compliance with medications, diet, and exercise recommendations, as well as data on health care and medications provided outside our health system, were not available.
Selection bias is also a possibility due to the requirement of having a minimum duration of treatment by community clinic providers and charted HbA1c values. Patients included in this study may have differed from those who were not included based on using the community clinic as a routine source of care. This becomes a bias to the extent that the DCCM patients may be more likely to remain with the community clinic as their primary care provider than comparison patients because of the added care. With more intense monitoring, DCCM patients are also more likely to have HbA1c values identified and recorded in their medical records. Continuity of care and close monitoring could contribute to better patient outcomes independent of DCCM participation.
Conclusions
This study has identified that a pharmacist-led collaborative diabetes management program is associated with better glycemic control and improved all-cause medical cost trends in patients with uncontrolled T2DM treated in a patient-centered primary care setting. These findings contribute to efforts to establish the value of CPS to patients, providers, and payers and provide data for payers who may be considering options for reimbursing for pharmacist-led diabetes drug therapy management.
ACKNOWLEDGMENTS
The authors would like to thank Brian Oberg, MBA, for assistance with data management.
APPENDIX A. ICD-9-CM Codes Used to Identify Diabetes and Comorbidities
| Disease | ICD-9-CM Code |
|---|---|
| Diabetes mellitus | 250.X |
| Hypertension | 401-405 |
| Dyslipidemia | 272.4 |
| Chronic kidney disease | 585.X |
| Retinopathy | 362.X |
| Neuropathy | 356.8 |
| Stroke | 436.0 |
| Myocardial infarction | 410.XX |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
APPENDIX B. Negative Binomial Regression Results on Association Between DCCM Intervention and Health Care Utilization
| Coefficient | Standard Error | 95% CI | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Inpatient utilization | ||||||||
| DCCM in post-index period (interaction term) | -0.342 | 0.520 | -1.361 | 0.677 | 0.510 | |||
| DCCM group assignment (reference comparison) | 0.927 | 0.411 | 0.122 | 1.732 | 0.024 | |||
| Post-index period (reference pre-index period) | 0.257 | 0.418 | -0.563 | 1.076 | 0.539 | |||
| Baseline HbA1c | 0.070 | 0.068 | -0.064 | 0.204 | 0.306 | |||
| Age | -0.002 | 0.010 | -0.021 | 0.018 | 0.863 | |||
| Pre-index diabetes medications | ||||||||
| Metformin | 0.336 | 0.438 | -0.523 | 1.195 | 0.443 | |||
| Insulin use | 0.463 | 0.328 | -0.180 | 1.107 | 0.158 | |||
| Pre-index diabetes medications by number of classes (reference 2-3) | ||||||||
| 0-1 | 0.282 | 0.421 | -0.542 | 1.107 | 0.502 | |||
| 4+ | 0.353 | 0.339 | -0.312 | 1.017 | 0.298 | |||
| Comorbidities | ||||||||
| Retinopathy | 0.086 | 0.440 | -0.777 | 0.949 | 0.845 | |||
| Chronic kidney disease | 1.006 | 0.298 | 0.422 | 1.589 | 0.001 | |||
| Black race (reference white) | -16.336 | 2257.518 | -4440.990 | 4408.318 | 0.994 | |||
| Male (reference female) | 0.358 | 0.260 | -0.151 | 0.868 | 0.168 | |||
| Constant | -4.776 | 1.157 | -7.043 | -2.508 | < 0.001 | |||
| Community clinic visits | ||||||||
| DCCM in post-index period (interaction term) | 0.026 | 0.077 | -0.125 | 0.176 | 0.738 | |||
| DCCM group assignment (reference comparison) | 0.332 | 0.061 | 0.212 | 0.452 | < 0.001 | |||
| Post-index period (reference pre-index period) | 0.140 | 0.052 | 0.038 | 0.242 | 0.007 | |||
| Baseline HbA1c | -0.032 | 0.011 | -0.053 | -0.010 | 0.004 | |||
| Age | 0.004 | 0.002 | 0.001 | 0.007 | 0.004 | |||
| Pre-index diabetes medications | ||||||||
| Metformin | 0.009 | 0.059 | -0.105 | 0.124 | 0.872 | |||
| Insulin use | 0.217 | 0.048 | 0.122 | 0.312 | < 0.001 | |||
| Pre-index diabetes medications by number of classes (reference 2-3) | ||||||||
| 0-1 | -0.098 | 0.056 | -0.207 | 0.011 | 0.078 | |||
| 4+ | 0.021 | 0.059 | -0.094 | 0.136 | 0.723 | |||
| Comorbidities | ||||||||
| Retinopathy | -0.008 | 0.079 | -0.162 | 0.146 | 0.921 | |||
| Chronic kidney disease | 0.179 | 0.058 | 0.066 | 0.292 | 0.002 | |||
| Black race (reference white) | 0.070 | 0.147 | -0.218 | 0.357 | 0.635 | |||
| Male (reference female) | -0.251 | 0.039 | -0.327 | -0.176 | < 0.001 | |||
| Constant | 1.810 | 0.165 | 1.488 | 2.133 | < 0.001 | |||
| Coefficient | Standard Error | 95% CI | P Value | |||||
| Outpatient visits | ||||||||
| DCCM in post-index period (interaction term) | 0.007 | 0.173 | -0.332 | 0.345 | 0.969 | |||
| DCCM group assignment (reference comparison) | 0.820 | 0.133 | 0.559 | 1.082 | <0.001 | |||
| Post-index period (reference pre-index period) | 0.315 | 0.117 | 0.085 | 0.545 | 0.007 | |||
| Baseline HbA1c | -0.082 | 0.026 | -0.133 | -0.032 | 0.001 | |||
| Age | -0.005 | 0.004 | -0.012 | 0.001 | 0.121 | |||
| Pre-index diabetes medications | ||||||||
| Metformin | 0.075 | 0.135 | -0.189 | 0.339 | 0.577 | |||
| Insulin use | 0.131 | 0.109 | -0.083 | 0.345 | 0.231 | |||
| Pre-index diabetes medications by number of classes (reference 2-3) | ||||||||
| 0-1 | 0.147 | 0.131 | -0.109 | 0.404 | 0.261 | |||
| 4+ | 0.039 | 0.132 | -0.220 | 0.298 | 0.769 | |||
| Comorbidities | ||||||||
| Retinopathy | 0.742 | 0.168 | 0.412 | 1.073 | < 0.001 | |||
| Chronic kidney disease | 0.359 | 0.130 | 0.104 | 0.614 | 0.006 | |||
| Black race (reference white) | -0.690 | 0.346 | -1.368 | -0.012 | 0.046 | |||
| Male (reference female) | 0.179 | 0.087 | 0.008 | 0.350 | 0.040 | |||
| Constant | 1.181 | 0.402 | 0.394 | 1.968 | 0.003 | |||
| Emergency department visits | ||||||||
| DCCM in the post-index period (interaction term) | -0.692 | 0.479 | -1.632 | 0.247 | 0.149 | |||
| DCCM group assignment (reference comparison) | 2.144 | 0.400 | 1.360 | 2.929 | < 0.001 | |||
| Post-index period (reference pre-index period) | 0.929 | 0.416 | 0.113 | 1.745 | 0.026 | |||
| Baseline HbA1c | 0.074 | 0.058 | -0.040 | 0.188 | 0.203 | |||
| Age | -0.028 | 0.008 | -0.045 | -0.011 | 0.001 | |||
| Pre-index diabetes medications | ||||||||
| Metformin | -0.530 | 0.391 | -1.296 | 0.235 | 0.175 | |||
| Insulin use | 0.014 | 0.260 | -0.495 | 0.523 | 0.958 | |||
| Pre-index diabetes medications by number of classes (reference 2-3) | ||||||||
| 0-1 | -0.516 | 0.392 | -1.284 | 0.251 | 0.187 | |||
| 4+ | 0.183 | 0.286 | -0.377 | 0.743 | 0.522 | |||
| Comorbidities | ||||||||
| Retinopathy | 0.399 | 0.364 | -0.315 | 1.113 | 0.274 | |||
| Chronic kidney disease | 0.579 | 0.265 | 0.059 | 1.099 | 0.029 | |||
| Black race (reference white) | -1.939 | 1.135 | -4.163 | 0.285 | 0.088 | |||
| Male (reference female) | 0.174 | 0.215 | -0.248 | 0.597 | 0.418 | |||
| Constant | -2.309 | 0.944 | -4.159 | -0.458 | 0.014 | |||
CI = confidence interval; DCCM = diabetes collaborative care management; HbA1c = hemoglobin A1c.
APPENDIX C. Generalized Linear Model: Association Between DCCM Intervention and Health Care Charges
| Coefficient | Standard Error | 95% CI | P Value | ||||
|---|---|---|---|---|---|---|---|
| Total charges | |||||||
| DCCM in the post-index period (interaction term) | -0.423 | 0.176 | -0.768 | -0.079 | 0.016 | ||
| DCCM group assignment (reference comparison) | 0.803 | 0.138 | 0.533 | 1.073 | < 0.001 | ||
| Post-index period (reference pre-index period) | 0.441 | 0.116 | 0.214 | 0.668 | < 0.001 | ||
| Baseline HbA1c | -0.107 | 0.025 | -0.156 | -0.059 | < 0.001 | ||
| Age | 0.009 | 0.003 | 0.002 | 0.016 | 0.012 | ||
| Pre-index diabetes medications | |||||||
| Metformin | 0.161 | 0.135 | -0.103 | 0.424 | 0.233 | ||
| Insulin use | 0.304 | 0.112 | 0.084 | 0.523 | 0.007 | ||
| Pre-index diabetes medications by number of classes (reference 2-3) | |||||||
| 0-1 | -0.222 | 0.128 | -0.472 | 0.029 | 0.082 | ||
| 4+ | -0.118 | 0.137 | -0.386 | 0.151 | 0.390 | ||
| Comorbidities | |||||||
| Retinopathy | 0.521 | 0.184 | 0.160 | 0.883 | 0.005 | ||
| Chronic kidney disease | 0.541 | 0.135 | 0.277 | 0.805 | < 0.001 | ||
| Black race (reference white) | -0.071 | 0.339 | -0.737 | 0.594 | 0.834 | ||
| Male (reference female) | -0.273 | 0.088 | -0.446 | -0.100 | 0.002 | ||
| Constant | 7.100 | 0.378 | 6.359 | 7.841 | < 0.001 | ||
| Inpatient charges | |||||||
| DCCM in the post-index period (interaction term) | -0.142 | 0.237 | -0.608 | 0.324 | 0.550 | ||
| DCCM group assignment (reference comparison) | 0.436 | 0.186 | 0.071 | 0.801 | 0.019 | ||
| Post-index period (reference pre-index period) | 0.061 | 0.157 | -0.246 | 0.368 | 0.697 | ||
| Baseline HbA1c | 0.022 | 0.033 | -0.044 | 0.087 | 0.511 | ||
| Age | 0.001 | 0.005 | -0.008 | 0.010 | 0.862 | ||
| Pre-index diabetes medications | |||||||
| Metformin | 0.082 | 0.182 | -0.275 | 0.438 | 0.653 | ||
| Insulin use | 0.204 | 0.151 | -0.093 | 0.500 | 0.178 | ||
| Pre-index diabetes medications by number of classes (reference 2-3) | |||||||
| 0-1 | 0.163 | 0.172 | -0.175 | 0.501 | 0.345 | ||
| 4+ | 0.238 | 0.185 | -0.125 | 0.600 | 0.198 | ||
| Comorbidities | |||||||
| Retinopathy | 0.196 | 0.249 | -0.292 | 0.683 | 0.432 | ||
| Chronic kidney disease | 0.537 | 0.182 | 0.181 | 0.894 | 0.003 | ||
| Black race (reference white) | -0.728 | 0.458 | -1.627 | 0.171 | 0.113 | ||
| Male (reference female) | 0.109 | 0.119 | -0.125 | 0.342 | 0.363 | ||
| Constant | -0.268 | 0.510 | -1.269 | 0.733 | 0.599 | ||
| Outpatient charges | |||||||
| DCCM in the post-index period (interaction term) | -0.419 | 0.167 | -0.747 | -0.092 | 0.012 | ||
| DCCM group assignment (reference comparison) | 0.750 | 0.131 | 0.493 | 1.007 | < 0.001 | ||
| Post-index period (reference pre-index period) | 0.474 | 0.110 | 0.258 | 0.690 | < 0.001 | ||
| Baseline HbA1c | -0.115 | 0.023 | -0.161 | -0.069 | < 0.001 | ||
| Age | 0.008 | 0.003 | 0.002 | 0.015 | 0.013 | ||
| Pre-index diabetes medications | |||||||
| Metformin | 0.161 | 0.128 | -0.089 | 0.412 | 0.207 | ||
| Insulin use | 0.253 | 0.106 | 0.045 | 0.461 | 0.017 | ||
| Pre-index diabetes medications by number of classes (reference 2-3) | |||||||
| 0-1 | -0.251 | 0.121 | -0.489 | -0.014 | 0.038 | ||
| 4+ | -0.120 | 0.130 | -0.375 | 0.135 | 0.355 | ||
| Comorbidities | |||||||
| Retinopathy | 0.561 | 0.175 | 0.218 | 0.904 | 0.001 | ||
| Chronic kidney disease | 0.493 | 0.128 | 0.242 | 0.743 | < 0.001 | ||
| Black race (reference white) | 0.051 | 0.322 | -0.581 | 0.683 | 0.875 | ||
| Male (reference female) | -0.294 | 0.084 | -0.459 | -0.130 | < 0.001 | ||
| Constant | 7.171 | 0.359 | 6.468 | 7.875 | < 0.001 | ||
CI = confidence interval; DCCM = diabetes collaborative care management; HbA1c = hemoglobin A1c.
Funding Statement
This study was funded in part by the American Association of Clinical Pharmacists. At the time of this study, McAdam-Marx received funding from the National Cancer Institute (award no. KM1CA156723). The other authors declare no conflicts of interest or financial interests in any product or service mentioned in this article, including grants, employment, gifts, stock holdings, or honoraria.
REFERENCES
- 1.National Diabetes Information Clearinghouse. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed May 13, 2015. [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596-615. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-39. [DOI] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352(9131):837-53. [PubMed] [Google Scholar]
- 6.Bojadzievski T, Gabbay RA. Patient-centered medical home and diabetes. Diabetes Care. 2011;34(4):1047-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdam-Marx C, Bouchard J, Aagren M, Conner C, Brixner DI. Concurrent control of blood glucose, body mass, and blood pressure in patients with type 2 diabetes: an analysis of data from electronic medical records. Clin Ther. 2011;33(1):110-20. [DOI] [PubMed] [Google Scholar]
- 9.Patient-Centered Primary Care Collaborative. Defining the medical home: a patient-centered philosophy that drives primary care excellence. 2013. Available at: http://www.pcpcc.org/about/medical-home. Accessed April 18, 2015.
- 10.Chung N, Rascati K, Lopez D, Jokerst J, Garza A. Impact of a clinical pharmacy program on changes in hemoglobin A1c, diabetes-related hospitalizations, and diabetes-related emergency department visits for patients with diabetes in an underserved population. J Manag Care Pharm. 2014;20(9):914-19. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip EJ, Shah BM, Yu J, Chan J, Nguyen LT, Bhatt DC. Enhancing diabetes care by adding a pharmacist to the primary care team. Am J Health Syst Pharm. 2013;70(10):877-86. [DOI] [PubMed] [Google Scholar]
- 12.Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47(6):781-89. [DOI] [PubMed] [Google Scholar]
- 13.Anaya JP, Rivera JO, Lawson K, Garcia J, Luna J Jr., Ortiz M. Evaluation of pharmacist-managed diabetes mellitus under a collaborative drug therapy agreement. Am J Health Syst Pharm. 2008;65(19):1841-45. [DOI] [PubMed] [Google Scholar]
- 14.Edwards HD, Webb RD, Scheid DC, Britton ML, Armor BL. A pharmacist visit improves diabetes standards in a patient-centered medical home (PCMH). Am J Med Qual. 2012;27(6):529-34. [DOI] [PubMed] [Google Scholar]
- 15.Jennings BT, Marx CM. Implementation of a pharmacist-managed diabetes program. Am J Health Syst Pharm. 2012;69(22):1951-53. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Available at: http://www.bls.gov/cpi/. Accessed April 18, 2015.
- 17.Aiken L, West S. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. [Google Scholar]
- 19.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-81. [DOI] [PubMed] [Google Scholar]
- 20.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465-88. [DOI] [PubMed] [Google Scholar]
- 22.Hardin JW, Hilbe JM. Generalized Linear Models and Extensions. 2nd ed. College Station, TX: Stata Press; 2007. [Google Scholar]
- 23.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182-89. [DOI] [PubMed] [Google Scholar]
- 24.Doucette WR, Witry MJ, Farris KB, McDonough RP. Community pharmacist-provided extended diabetes care. Ann Pharmacother. 2009;43(5):882-89. [DOI] [PubMed] [Google Scholar]
- 25.Johnson KA, Chen S, Cheng IN, et al. The impact of clinical pharmacy services integrated into medical homes on diabetes-related clinical outcomes. Ann Pharmacother. 2010;44(12):1877-86. [DOI] [PubMed] [Google Scholar]
- 26.Salvo MC, Brooks AM. Glycemic control and preventive care measures of indigent diabetes patients within a pharmacist-managed insulin titration program vs standard care. Ann Pharmacother. 2012;46(1):29-34. [DOI] [PubMed] [Google Scholar]
- 27.Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-36. [DOI] [PubMed] [Google Scholar]
- 28.Cranor CW, Bunting BA, Christensen DB. The Asheville Project: long-term clinical and economic outcomes of a community pharmacy diabetes care program. J Am Pharm Assoc (Wash). 2003;43(2):173-84. [DOI] [PubMed] [Google Scholar]
- 29.Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther. 2007;82(2):143-56. [DOI] [PMC free article] [PubMed] [Google Scholar]