A 73-year-old patient, ex-smoker (80 pack-year), with a history of Chronic Obstructive Pulmonary Disease (COPD) (moderate grade according to GOLD classification) and three-vessel coronary disease treated by percutaneous coronary intervention (PCI), presented to the Emergency Department (ED) complaining of shortness of breath on exertion (mMRC: ¾) associated with a productive cough with purulent sputum for days. In the ED, a transthoracic ultrasound of the left lower lobe (LLL) was performed as shown in Figure 1. Laboratory testing revealed partially elevated serum C-reactive protein (CRP), which value was 15.6 mg/dl. Subsequently, a chest Computed Tomography (CT) showed some abnormal findings as shown in Figure 2. Quantiferon was negative. Sputum samples for bacterial cultures were also negative. A flexible bronchoscopy was performed where bronchial washings and bronchoalveolar lavage (BAL) were sent for common pathogens, for microscopy for acid fast bacteria (AFB), for mycobacterial culture and for cytological examination, which were all negative, as well. The patient was given empiric treatment with intravenous anti-bacterial medication with ceftriaxone and azithromycin with clinical improvement. A new chest CT in one month and reevaluation were recommended.
Figure 1.
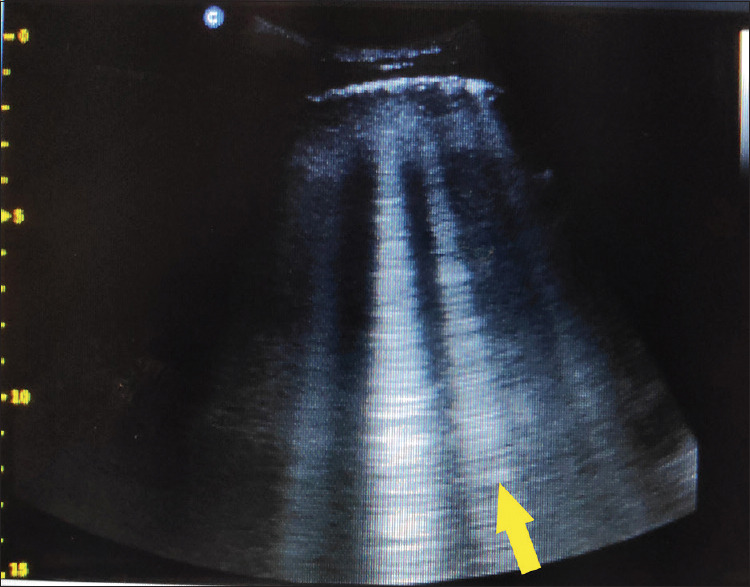
In transthoracic ultrasound of the left lower lobe of the patient, we recognize focally increased number of B-lines (≥3), which are these vertical hyperechoic reverberations (yellow arrow)
Figure 2.
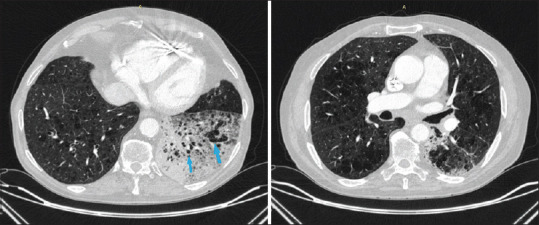
In the chest Computed Tomography (CT), it is reported emphysematous lesions and consolidation with air bronchogram and multiple emphysema cysts (light blue arrows) in the left lower lobe, emphysematous lesions in both lungs
His chest CT re-examination is seen in Figure 3. We recommended performing a Positron Emission Tomography/Computed Tomography (PET/CT), which showed 18fluorodeoxyglucose uptake in the consolidation area (SUVmax: 4.4) A CT-guided fine-needle biopsy (FNB) of the left lower lobe was then performed and a diagnosis of micropapillary lung adenocarcinoma was established.
Figure 3.
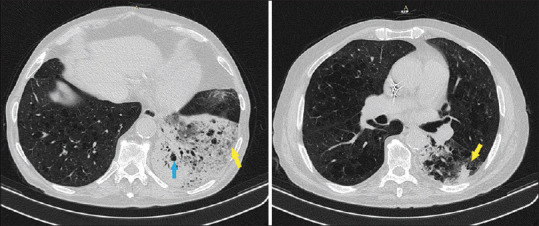
In the chest Computed Tomography (CT), it is reported emphysematous lesions (light blue arrow) and increased consolidation with air bronchogram and multiple emphysema cysts in the left lower lobe. The foci in the lower left lobe was increased (yellow arrows)
Questions
What is recognized in the transthoracic ultrasound of the patient’s LLL?
What is the radiological finding in the chest CT?
What is changed after one month in the chest CT?
Answers
Focally increased number of B-lines (≥3).
Consolidation with air bronchogram and multiple emphysema cysts in the left lower lobe and emphysematous lesions in both lungs.
The foci in the lower left lobe was increased.
DISCUSSION
Lung adenocarcinoma (LADC) is the most common histological subtype of non-small cell lung cancer and it is diagnosed in 38.5% of cases.[1] A type of LADC can mimic the clinical presentation of pneumonia and it is known as pneumonic-type LADC (PLADC). It corresponds to the old term bronchioloalveolar carcinoma (BAC) and it usually appears as non-obstructive focal or diffuse pulmonary consolidation at CT scan.[2] In the 8th Tumor-Node-Metastasis (TNM) staging system, PLADC has been introduced as an uncommon form of lung cancer.[3]
Li et al. conducted a retrospective study and observed that LADC can be visualized in the chest CT scan with 8 different forms. Types I (the most common type) appears as solid nodules. Our case falls into the VII category, which is characterized by diffuse consolidation involving more than half of the affected lobe. In this category, the foci of the lobe appears with multiple nodules or ground glass opacities and the sagging fissure sign. These findings are often considered due to pneumonia. The diagnosis of LADC is made by combining imaging findings with clinical suspicion and clinical data.[4]
Patients with a radiographic image of pneumonia refractory to antimicrobial therapy should undergo an invasive investigation to obtain a biopsy. The aim is to detect cancer at an early stage, when it is still operable.
The outcome of patients treated by lobectomy was significantly better than those treated with chemotherapy, or best supportive care.[5]
For our patient, according to the 8th edition of the TNM classification, PLADC was stage IIIA. Due to the cardiovascular history and the multilobar emphysema identified at CT, the cancer multidisciplinary team (MDT) of our hospital decided chemotherapy and radiotherapy as treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for the images and other clinical information to be reported in the journal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Li D, Shi J, Dong X, Liang D, Jin J, He Y. Epidemiological characteristics and risk factors of lung adenocarcinoma:A retrospective observational study from North China. Front Oncol. 2022;12:892571. doi: 10.3389/fonc.2022.892571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huo JW, Huang XT, Li X, Gong JW, Luo TY, Li Q. Pneumonic-type lung adenocarcinoma with different ranges exhibiting different clinical, imaging, and pathological characteristics. Insights Imaging. 2021;12:169. doi: 10.1186/s13244-021-01114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim W, Lee SM, Lee JB, Seo JB, Kim HK, Kim J, et al. Prognosis for pneumonic-type invasive mucinous adenocarcinoma in a single lobe on CT:Is it reasonable to designate it as clinical T3? Korean J Radiol. 2022;23:370–80. doi: 10.3348/kjr.2021.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, He XQ, Fan X, Luo TY, Huo JW, Huang XT. Computed tomography morphological classification of lung adenocarcinoma and its correlation with epidermal growth factor receptor mutation status:A report of 1075 cases. Int J Gen Med. 2021;14:3687–98. doi: 10.2147/IJGM.S316344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daoud A, Laktineh A, El Zein S, Soubani AO. Unusual presentation of primary lung adenocarcinoma mimicking pneumonia:Case report and literature review. Respir Med Case Rep. 2019;28:100881. doi: 10.1016/j.rmcr.2019.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]


