ABSTRACT
Introduction:
Right ventricular dysfunction (RVD) is a key component in the process of risk stratification in patients with acute pulmonary embolism (PE). Echocardiography remains the gold standard for RVD assessment, however, measures of RVD may be seen on CTPA imaging, including increased pulmonary artery diameter (PAD). The aim of our study was to evaluate the association between PAD and echocardiographic parameters of RVD in patients with acute PE.
Methods:
Retrospective analysis of patients diagnosed with acute PE was conducted at large academic center with an established pulmonary embolism response team (PERT). Patients with available clinical, imaging, and echocardiographic data were included. PAD was compared to echocardiographic markers of RVD. Statistical analysis was performed using the Student’s t test, Chi-square test, or one-way analysis of variance (ANOVA); P < 0.05 was considered statistically significant.
Results:
270 patients with acute PE were identified. Patients with a PAD >30 mm measured on CTPA had higher rates of RV dilation (73.1% vs 48.7%, P < 0.005), RV systolic dysfunction (65.4% vs 43.7%, P < 0.005), and RVSP >30 mmHg (90.2% vs 68%, P = 0.004), but not TAPSE ≤1.6 cm (39.1% vs 26.1%, P = 0.086). A weak increasing linear relationship between PAD and RVSP was noted (r = 0.379, P = 0.001).
Conclusions:
Increased PAD in patients with acute PE was significantly associated with echocardiographic markers of RVD. Increased PAD on CTPA in acute PE can serve as a rapid prognostic tool and assist with PE risk stratification at the time of diagnosis, allowing rapid mobilization of a PERT team and appropriate resource utilization.
KEY WORDS: Echocardiography, pulmonary embolism, right ventricular dysfunction
INTRODUCTION
Acute pulmonary embolism (PE) is a common cause of morbidity and mortality, resulting in an estimated 12.3 deaths per 100,000 people annually in the United States.[1] The incidence of acute venous thromboembolism (VTE) overall is estimated at 1-2 per 1000 people in the United States,[2,3] with an incidence of PE of 121 per 100,000 people annually.[4]
Assessment of right ventricular dysfunction (RVD) is a key component of risk stratification in acute PE.[5,6] RVD refers to a state of filling or contraction abnormality without reference to signs or symptoms or right heart failure.[7] The development of RVD due to acute PE is likely multi-factorial, due to direct mechanical obstruction from clot burden and release of vasoactive mediators (such as thromboxane A2) leading to an increase in pulmonary vascular resistance.[8] The presence of RVD has been associated with increased short term mortality in clinically stable patients,[9] and may be associated with higher rates of chronic thromboembolic disease.[10,11]
Transthoracic echocardiogram (TTE) remains the gold standard to evaluate RV size and function, however, it may not always readily available and requires skilled image acquisition and interpretation. Even when available, obtaining high quality imaging in certain circumstances such as critically ill patients[12] and obese patients[13] can be challenging. Echocardiographic assessment of RVD in the setting of acute PE includes measures of RV size and function, and estimation of right heart pressures using the simplified Bernoulli equation,[14] CT Pulmonary Angiography (CTPA) is the imaging modality of choice for evaluating patients with acute PE,[5] and can also provide valuable information on RVD.
The most widely used CT measure of RVD is an elevated RV/LV ratio, which has been associated with increased in a 5-fold increase in PE related mortality,[15] and has shown good agreement with echocardiographic measures of RVD.[16] However, RV/LV measurement should ideally be used measured using reformatted 4-chamber views to compensate for the cardiac axis relative to the patient, which may be cumbersome. Increased PAD on CTPA is a simple measurement which has been well described in detecting pulmonary hypertension.[17,18] Increased PAD has been associated with increased short-term adverse outcomes in acute PE;[19] however, the association between echocardiographic measures of RVD, PE risk stratification, and PAD is less well described.[20,21,22,23]
In this study, our aim was to evaluate if increased PAD diameter measured on CTPA is associated with echocardiographic parameters of RVD in patients presenting with an acute PE. We also aimed to assess for inter-reader correlation between radiologists and non-radiologists to establish utility by non-radiologists, and to compare performance of PAD compared to RV/LV ratio on CT imaging to detect echocardiographic RVD.
METHODS
We retrospectively reviewed 366 consecutive patients with acute PE collected from September 2017 to June 2019 in the Temple University Hospital Pulmonary Embolism Response Team (PERT) registry (approved review board protocol 26021). Patients with available clinical, imaging and echocardiographic data were included. The PAD measurement was obtained from official radiology reports (measured by a radiologist) if available or measured by 2 non-radiologist readers after formal instruction and acceptable inter-reader agreement demonstration. Sectra PACS workstation IDS7 V19.3 imaging software was used for PAD measurement by both radiologists and non-radiologists. The PAD was measured in cross sectional view at the level of continuity with the right main PA [Figure 1]. Increased PAD was defined as a PAD >30 mm. The following echocardiographic parameters of RVD were collected from formal echocardiography reports: RV dilation (graded as present or not present), RV systolic dysfunction (graded as present or not present), RV systolic pressure (RVSP), and tricuspid annular plane systolic excursion (TAPSE). Elevated RVSP was defined as >30 mmHg. Reduced TAPSE was defined as ≤1.6 cm. Statistical analysis was performed using the Student’s t test, Chi-square test or one-way analysis of variance (ANOVA). Pearson correlation coefficient (PCC) and paired t test statistical analysis was used to measure inter-reader agreement between radiologist and non-radiologists for the PA diameter measurements. P value <0.05 was considered to be statistically significant. It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research due to the retrospective nature of the study.
Figure 1.
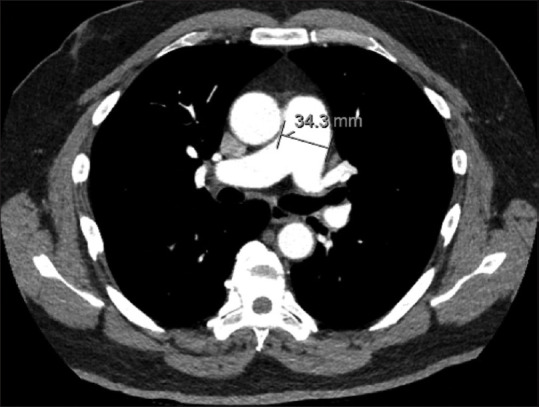
PA diameter measurement on CTPA
RESULTS
366 patients with an acute PE were initially evaluated within the study period. Of those, 270 patients had a PE diagnosed by CTPA who also had echocardiograms available for full analysis.
Using a sample of the overall cohort (n = 18), two thoracic radiologists (MK and CD) were assessed for inter-reader agreement of PAD measurement and compared to two pulmonologist readers (RA and PR). Inter-reader agreement between the two chest radiologists (Pearson correlation coefficient 0.89, P < 0.05) and between the readers and the radiologists was excellent (Pearson correlation coefficient 0.79–0.88, P < 0.05).
Patient demographics, comorbidities, treatment, and outcomes are listed in Table 1. Patients were divided into two groups: patients with increased PAD (n = 110) and those without (n = 160). Patients in the increased PAD group had a significantly higher BMI (p = 0.001), and a lower mean SpO2 on presentation (93.4% vs 88.8%, P = 0.001).
Table 1.
Demographics
| PAD ≤30 mm (n=160) | PAD >30 mm (n=110) | P | |
|---|---|---|---|
| Mean PA diameter, mm (SD) | 26.5 (± 2.7) | 34.1 (±3.2) | |
| Age, years (range) | 57.3 (18.4-99.2) | 60.6 (22.4-91.6) | 0.134 |
| Gender n (%) | |||
| Male | 89 (55.6) | 50 (45.5) | 0.100 |
| Female | 71 (44.4) | 60 (54.5) | |
| BMI (range) | 32.0 (17.5-64.02) | 35.0 (19.5-55.0) | 0.001 |
| Comorbidities | |||
| Hypothyroidism, n (%) | 16 (10) | 6 (5) | 0.180 |
| Prior DVT, n (%) | 31 (19) | 30 (27) | 0.127 |
| Prior PE, n (%) | 32 (20) | 20 (18) | 0.736 |
| Prior malignancy, n (%) | 34 (21) | 12 (11) | 0.026 |
| Diabetes Mellitus, n (%) | 35 (22) | 31 (28) | 0.236 |
| COPD, n (%) | 24 (15) | 17 (16) | 0.894 |
| Recent surgery, n (%) | 28 (18) | 12 (11) | 0.134 |
| Anticoagulation prior to admission, n (%) | 22 (14) | 12 (11) | 0.489 |
| Previous IVC filter, n (%) | 3 (2) | 7 (6) | 0.055 |
| CKD, n (%) | 10 (6) | 6 (5) | 0.786 |
| ESRD, n (%) | 3 (2) | 5 (5) | 0.207 |
| SPO2 on admission, % (range) | 93.4 (80-100) | 88.8 (70-100) | 0.001 |
| Initial BNP, pg/ml | 260.4 | 558.7 | 0.07 |
| Initial Troponin I, ng/ml | 0.192 | 0.201 | 0.85 |
| Treatment, n (%) | |||
| No AC | 4 (2.5) | 0 (0) | 0.004 |
| AC only | 124 (77.5) | 70 (63.6) | |
| Reperfusion therapies | 32 (20) | 40 (36.4) | |
| Inpatient Mortality, n (%) | 7 (4.3) | 10 (9) | 0.14 |
Patients with increased PAD also received reperfusion therapies in addition to anticoagulation (including catheter directed therapy, systemic thrombolysis, mechanical thrombectomy, or surgical thrombectomy) more often when compared to patients without PA enlargement (20% vs 36.7%, P = 0.004). There was no difference in mean length of stay (9.5 vs 10.7 days, P = 0.661). There was a trend toward increased mortality but did not reach statistical significance (4% vs 9%, P = 0.14).
Patients were risk stratified according to European Society of Cardiology (ESC) guidelines as low risk, intermediate-low risk, intermediate-high risk, and high risk for early death.[5] Increased PAD was associated with an increased incidence of intermediate-high and high risk PE compared to those without (9% vs 6% and 40% vs 26%, respectively) [Figure 2].
Figure 2.
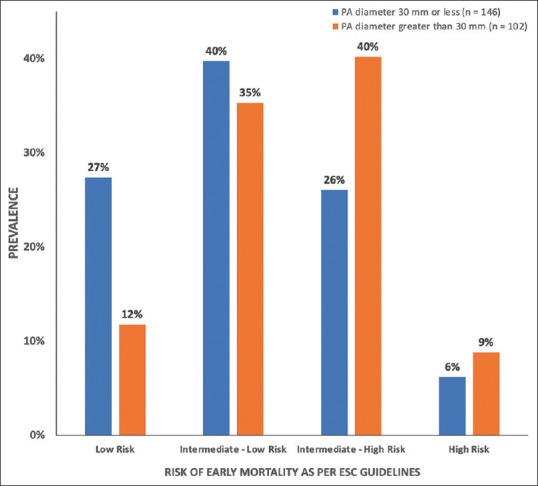
Risk of early (in-hospital or 30 day) death as per ESC guideline classification
The median time interval between CTPA and formal echocardiogram reporting was 17 hours. Values for RVSP were available in 46.6% (n = 126) and TAPSE in 57.7% (n = 156) of echocardiograms. Patients with a PAD >30 mm measured on CTPA had higher rates of RV dilation (73.1% vs 48.7%, P < 0.005), RV systolic dysfunction (65.4% vs 43.7%, P < 0.005), and RVSP >30 mmHg (90.2% vs 68%, P = 0.004), but not TAPSE <1.6 cm (39.1% vs 26.1%, P = 0.086) [Table 2].
Table 2.
Echocardiographic parameters
| PAD ≤30 | PAD >30 | P | |
|---|---|---|---|
| RV Dilation, n (%) | 78 (48.7) | 79 (73.1) | <0.005 |
| RV systolic dysfunction, n (%) | 70 (43.7) | 72 (65.4) | <0.005 |
| RVSP >30 mmHg, n (%) | 51/75 (68) | 46/51 (90.2) | 0.004 |
| TAPSE ≤1.6 cm, n (%) | 24/92 (26.1) | 25/64 (39.1) | 0.086 |
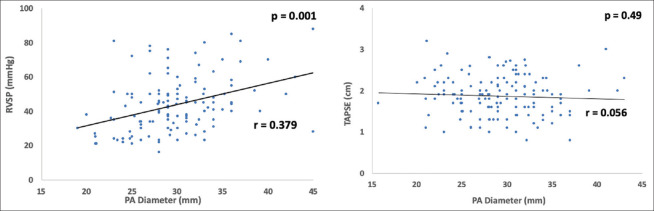
A weak increasing linear relationship between PAD and RVSP was noted (r = 0.379, P = 0.001), but no relationship with TAPSE was found (r = 0.056, P = 0.49) [Figure 3].
Figure 3.
Correlation between the PA diameter and the right ventricular systolic pressure and TAPSE
A subset of patients for whom RV/LV ratio (n = 250) was available were studied for performance of RV/LV ratio to predict echocardiographic parameters of RVD. Receiver operator curves (ROC) were used to assess for predictive value of RV/LV ratio and PAD [Table 3, Figure 4]. PAD appeared to have a similar predictive value to RV/LV ratio to identify patients with echocardiographic RVD.
Table 3.
ROC
| RV/LV >0.9 AUC | PAD ≥30 AUC | |
|---|---|---|
| RV Dilation | 0.6473 | 0.6388 |
| RV systolic dysfunction | 0.6451 | 0.6346 |
| RVSP >30 mmHg | 0.6398 | 0.6275 |
| TAPSE ≤1.6 cm | 0.5349 | 0.5176 |
Figure 4.
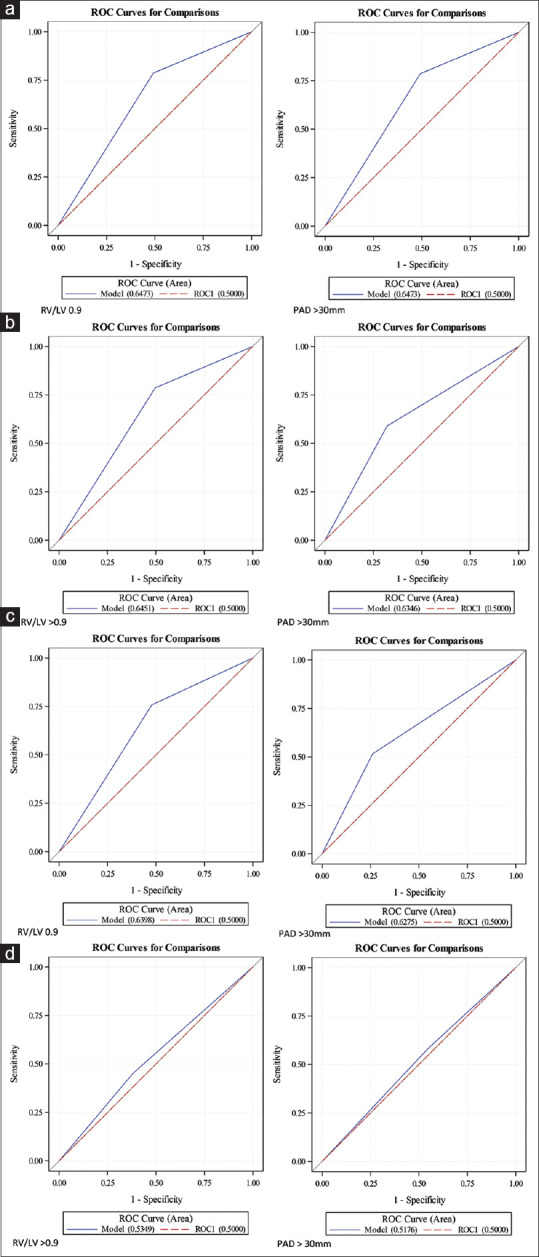
ROC curves (a) RV dilation; (b) RV systolic dysfunction; (c) RVSP; (d) TAPSE
DISCUSSION
Our study demonstrated a strong association between increased PAD >30 mm on CTPA with echocardiographic markers of RVD; RV dilation, RV systolic dysfunction and RVSP >30 mmHg in the setting of acute PE. We also found a weak increasing linear relationship between increasing PAD and RVSP. Additionally, we established excellent inter-reader agreement among radiologists and pulmonologists for PAD measurement. We found that increased PAD was associated with higher incidence of intermediate-high and high risk PE. Increased PAD was associated with increased hypoxia on presentation and higher rates of reperfusion interventions. PAD appeared to have a similar predictive value to RV/LV ratio to identify patients with echocardiographic RVD.
Our cohort is the largest to assess the relationship between PAD and echocardiographic RVD in the context of acute PE to our knowledge [N = 80,[23] N = 118,[20] N = 113,[21] N = 190[22]]. The association between PAD and echocardiographic findings of RVD has previously been investigated with mixed results;[20,21,22,23] however, the definition of RVD used varies widely. Previous studies have defined RVD as a composite of RV hypokinesis, septal wall paradoxical movement, RV dilation, an increased RV-LV diameter ratio (>0.9), increased velocity of the jet of tricuspid regurgitation and decreased TAPSE in variable combinations.[20,21,23] PASP >50 mmHg has also been studied without an association with mean PAD;[22] however, this measurement is limited by the presence or absence of a tricuspid regurgitant jet. Given a lack of standardized definition of RVD, our study aimed to use commonly reported parameters of RVD, and studied each parameter separately to increase utility in the event that a particular echocardiographic measurement could not be obtained.
Our study highlighted the ease and reliability of PAD measurement by non-radiology trained physicians. Previous studies have variably used radiologist only[23] and both radiologist and pulmonologist readers,[20] however, have not assessed for inter-reader agreement. High levels of inter-reader agreement further increases the utility of PAD as a rapid screening tool in real-world practice. While other CT measures of RVD using PAD have been studied, chiefly RV/LV ratio,[15] but also including PA to aortic diameter ratio[21,22] and clot burden,[24,25] PAD measurement is the most feasible and clinically reproducible, and does not require reformatting imaging for accurate measurements. As computer aided detection software continues to develop,[26,27,28] there may be a role for automated PAD reporting in the future.
The clinical utility of PAD is not to replace formal echocardiography, but to alert clinicians for potential RVD at time of the PE diagnosis. An increased PAD >30 mm could prompt early assessment and risk stratification by a multidisciplinary PERT team and may serve as a trigger for echocardiogram. An average delay of 17 hours between CTPA and formal echocardiography in our cohort further highlights the utility of PAD measurement to bridge this gap. Previous data in patients with acute PE identified an average delay of 21 hours before formal echocardiography could be obtained, in line without findings.[29]
There were several limitations to our study. This was a single center, retrospective review. There was a mean delay of 17 hours between echocardiography and CTPA. Our study demonstrated a positive association between mean PAD and RV systolic function, size and RVSP, but not with TAPSE. TAPSE has been shown to correlate well with other echocardiographic markers of RVD in the setting of acute PE.[30] Previous studies of PAD in acute PE did not study or did not establish a relationship with reduced TAPSE.[20,21,22,23] As TAPSE could only be measured in 57.7% of echocardiograms obtained, it is likely our study was underpowered to find a significant difference. We did not assess for pre-existing chronic PH or RVD. Echocardiography data was obtained from formal echocardiogram reports but were not re-interpreted for the purpose of our study. A cut off of >30 mm (dichotomous cut off) was chosen due to ease of recall but remains broadly in line with other data.[20,22] CTPA were not ECG gated, thus PA pulsatility was not accounted for.
CONCLUSIONS
PAD correlates with echocardiographic measures of RVD in the setting of acute PE and may be able to identify intermediate and high risk PE patients at time of PE diagnosis. PAD measurement by non-radiologist physicians appears to be accurate.
Abbreviations
CTPA = Computed tomography pulmonary angiography
PE = Pulmonary embolism
PERT = Pulmonary embolism response team
RV = Right ventricle
RVD = Right ventricular dysfunction
RVSP = Right ventricular systolic pressure
TAPSE = Tricuspid Annulus Plane Systolic Excursion
VTE = Venous thromboembolism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Evan Carabelli, Brooke Heyman, Ryan Cobb, Eno Essian, Eneida Harrison, Ali Noory, Samantha Pettigrew, Dianna Gaballa.
REFERENCES
- 1.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States:Evidence of overdiagnosis. Arch Intern Med. 2011;171:831–7. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, et al. The worcester venous thromboembolism study. J Gen Intern Med. 2006;21:722–7. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism:A 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Venous thromboembolism in adult hospitalizations-United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012;61:401–4. [PubMed] [Google Scholar]
- 5.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. doi:10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 6.Triantafyllou GA, O'Corragain O, Rivera-Lebron B, Rali P. Risk stratification in acute pulmonary embolism:The latest algorithms. Semin Respir Crit Care Med. 2021;42:183–98. doi: 10.1055/s-0041-1722898. [DOI] [PubMed] [Google Scholar]
- 7.François H, Ramona D, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II. Circulation. 2008;117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 8.Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism:The pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48:23–33. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism:A systematic review. Eur Heart J. 2008;29:1569–77. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 10.Hsu C-H, Lin C-C, Li W-T, Chang H-Y, Chang W-T. Right ventricular dysfunction is associated with the development of chronic thromboembolic pulmonary hypertension but not with mortality post-acute pulmonary embolism. Medicine (Baltimore) 2019;98:e17953. doi: 10.1097/MD.0000000000017953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens H, Fang W, Clements W, Bloom J, McFadyen J, Tran H. Risk Stratification of acute pulmonary embolism and determining the effect on chronic cardiopulmonary complications:The REACH study. TH Open. 2020;4:e45–50. doi: 10.1055/s-0040-1708558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vignon P, Mentec H, Terré S, Gastinne H, Guéret F. Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest. 1994;106:1829–34. doi: 10.1378/chest.106.6.1829. [DOI] [PubMed] [Google Scholar]
- 13.Finkelhor RS, Moallem M, Bahler RC. Characteristics and impact of obesity on the outpatient echocardiography laboratory. Am J Cardiol. 2006;97:1082–4. doi: 10.1016/j.amjcard.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 14.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults:A report from the American Society of Echocardiography:Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Meinel FG, Nance JW, Jr, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, et al. Predictive value of computed tomography in acute pulmonary embolism:Systematic review and meta-analysis. Am J Med. 2015;128:747–59.e2. doi: 10.1016/j.amjmed.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Henzler T, Roeger S, Meyer M, Schoepf UJ, Nance JW, Jr, Haghi D, et al. Pulmonary embolism:CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 2012;39:919–26. doi: 10.1183/09031936.00088711. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y, Wan C, Tian P, et al. CT-base pulmonary artery measurementin the detection of pulmonary hypertension. Medicine (Baltimore) 2014;93:e256. doi: 10.1097/MD.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan RT, Kuzo R, Goodman LR, Siegel R, Haasler GB, Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998;113:1250–6. doi: 10.1378/chest.113.5.1250. [DOI] [PubMed] [Google Scholar]
- 19.Lyhne MD, Schultz JG, MacMahon PJ, Haddad F, Kalra M, Tso DM, et al. Septal bowing and pulmonary artery diameter on computed tomography pulmonary angiography are associated with short-term outcomes in patients with acute pulmonary embolism. Emerg Radiol. 2019;26:623–30. doi: 10.1007/s10140-019-01709-9. [DOI] [PubMed] [Google Scholar]
- 20.İn E, Aydın AM, Özdemir C, Sökücü SN, Dağlı MN. The efficacy of CT for detection of right ventricular dysfunction in acute pulmonary embolism, and comparison with cardiac biomarkers. Jpn J Radiol. 2015;33:471–8. doi: 10.1007/s11604-015-0447-9. [DOI] [PubMed] [Google Scholar]
- 21.Jia D, Zhou X, Hou G. Estimation of right ventricular dysfunction by computed tomography pulmonary angiography:A valuable adjunct for evaluating the severity of acute pulmonary embolism. J Thromb Thrombolysis. 2017;43:271–8. doi: 10.1007/s11239-016-1438-0. [DOI] [PubMed] [Google Scholar]
- 22.Sanal S, Aronow WS, Ravipati G, Maguire GP, Belkin RN, Lehrman SG. Prediction of moderate or severe pulmonary hypertension by main pulmonary artery diameter and main pulmonary artery diameter/ascending aorta diameter in pulmonary embolism. Cardiol Rev. 2006;14:213–4. doi: 10.1097/01.crd.0000181619.87084.8b. [DOI] [PubMed] [Google Scholar]
- 23.Seon HJ, Kim KH, Lee WS, Choi S, Yoon HJ, Ahn Y, et al. Usefulness of computed tomographic pulmonary angiography in the risk stratification of acute pulmonary thromboembolism. Circ J. 2011;75:428–36. doi: 10.1253/circj.cj-10-0361. [DOI] [PubMed] [Google Scholar]
- 24.Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism:Comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415–20. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 25.Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, et al. Severity of acute pulmonary embolism:Evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol. 2003;13:29–35. doi: 10.1007/s00330-002-1515-y. [DOI] [PubMed] [Google Scholar]
- 26.Wittenberg R, Berger FH, Peters JF, Weber M, van Hoorn F, Beenen LF, et al. Acute pulmonary embolism:Effect of a computer-assisted detection prototype on diagnosis--An observer study. Radiology. 2012;262:305–13. doi: 10.1148/radiol.11110372. [DOI] [PubMed] [Google Scholar]
- 27.Wittenberg R, Peters JF, Sonnemans JJ, Prokop M, Schaefer-Prokop CM. Computer-assisted detection of pulmonary embolism:Evaluation of pulmonary CT angiograms performed in an on-call setting. Eur Radiol. 2010;20:801–6. doi: 10.1007/s00330-009-1628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahiji K, Kligerman S, Jeudy J, White C. Improved accuracy of pulmonary embolism computer-aided detection using iterative reconstruction compared with filtered back projection. AJR Am J Roentgenol. 2014;203:763–71. doi: 10.2214/AJR.13.11838. [DOI] [PubMed] [Google Scholar]
- 29.Filopei J, Acquah SO, Bondarsky EE, Steiger DJ, Ramesh N, Ehrlich M, et al. Diagnostic accuracy of point-of-care ultrasound performed by pulmonary critical care physicians for right ventricle assessment in patients with acute pulmonary embolism. Crit Care Med. 2017;45:2040–5. doi: 10.1097/CCM.0000000000002723. [DOI] [PubMed] [Google Scholar]
- 30.Park J-H, Kim JH, Lee J-H, Choi SW, Jeong JO, Seong IW. Evaluation of right ventricular systolic function by the analysis of tricuspid annular motion in patients with acute pulmonary embolism. J Cardiovasc Ultrasound. 2012;20:181–8. doi: 10.4250/jcu.2012.20.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]