Abstract
Monocytes/macrophages (MO/Mφ) are the major target cells for both dengue virus (DV) and bacterial lipopolysaccharide (LPS), and the aim of this study was to define their interactions. We had found that LPS markedly suppressed DV infection of primary human MO/Mφ when it was added to cultures prior to or together with, but not after, viral adsorption. The inhibitory effect of LPS was direct and specific and was not mediated by LPS-induced secretion of cytokines and chemokines such as tumor necrosis factor alpha, interleukin-1β (IL-1β), IL-6, IL-8, IL-12, alpha interferon, MIP-1α, and RANTES. In fact, productive DV infection was not blocked but was just postponed by LPS, with a time lag equal to one viral replication cycle. Time course studies demonstrated that LPS was only effective in suppressing DV infection of MO/Mφ that had not been previously exposed to the virus. At various time points after viral adsorption, the level of unbound viruses that remained free in the culture supernatants of LPS-pretreated cultures was much higher than that of untreated controls. These observations suggest that the LPS-induced suppression of DV replication was at the level of virus attachment and/or entry. Blockade of the major LPS receptor, CD14, with monoclonal antibodies MY4 or MoS39 failed to inhibit DV infection but could totally abrogate the inhibitory effect of LPS. Moreover, human serum could significantly enhance the LPS-induced DV suppression in a CD14-dependent manner, indicating that the “binding” of LPS to CD14 was critical for the induction of virus inhibition. Taken together, our results suggest that LPS blocked DV entry into human MO/Mφ via its receptor CD14 and that a CD14-associated cell surface structure may be essential for the initiation of a DV infection.
Viral hemorrhagic fever poses a serious global health threat (18). Among the causative agents, dengue virus (DV) infection has the highest incidence rate and is the leading cause of viral hemorrhagic fever in the world (18, 25). The clinical manifestations of DV infection range in severity from a self-limited febrile dengue fever to a potentially life-threatening dengue hemorrhagic fever-dengue shock syndrome (DHF-DSS). Although the pathogenesis of DV infection is not well known, a serotype cross-reactive immune response is proposed as one of the risk factors for DHF-DSS (15, 21). Monocytes/macrophages (MO/Mφ) are the major target cells of DV in vivo and in vitro (14, 15) and are responsible for the dissemination of the virus after its initial entry via the mosquito vector. It has been shown that soluble mediators released from DV-infected MO/Mφ exerted prominent influences on the biological properties of endothelial cells and hematopoietic populations (1, 6, 34). Therefore, interaction of DV with MO/Mφ is likely to play a central role in the pathogenesis of dengue illness.
Lipopolysaccharide (LPS) situated in the outer part of the gram-negative bacterial cell wall is the key effector molecule responsible for the pathogenesis of the endotoxic shock that kills millions of people annually (26). LPS can stimulate MO/Mφ to secrete large amounts of pathophysiologically important mediators which act to potentiate cell activation, inflammatory reactions, and vascular modifications (26). In addition, LPS has been shown to modulate virus replication in human MO/Mφ (2, 4, 12, 19–20, 29, 38) and to amplify virally induced, MO/Mφ-mediated immune activation and immunosuppression (9, 27–28).
The myeloid differentiation antigen, CD14, serves as a major binding receptor for LPS (42). This glycoprotein is predominantly expressed on MO/Mφ plasma membrane via a glycosyl-phosphatidylinositol (GPI) anchorage (16). Although LPS by itself can bind to CD14, the binding efficiency is markedly enhanced via complex formation with serum proteins such as the LPS-binding protein (LBP) (13, 17, 33, 35). It has been demonstrated that CD14 increased the sensitivity of the cells to LPS but that CD14 per se did not confer LPS responsiveness (23, 24). In fact, because GPI-linked CD14 lacks an intracellular domain for transducing signals to the cell interior, an additional transmembrane protein with low affinity for LPS has been considered to provide the signal transduction function. This putative molecule is conditionally associated with CD14 by LPS engagement to form the “multimeric LPS receptor” on the surface of MO/Mφ (23–24, 36–37) and may also play an important role in activation of CD14-nonexpressing, LPS-responsive cells (10, 31).
Because both LPS and DV target primarily on the cells of MO/Mφ lineage, we designed an in vitro infection model with primary human MO/Mφ to investigate the interplay among DV, LPS, and MO/Mφ. We have demonstrated for the first time that LPS can block DV infection of human MO/Mφ, probably because LPS can interact with a certain cellular binding structure which is vital for the attachment of the DV particles to the surface membrane receptor(s) of the target cells. Furthermore, this inhibition is unrelated to subsequent MO/Mφ activation or induction of monokines.
MATERIALS AND METHODS
Reagents, cytokines, and MAbs.
Phenol-extracted LPS from Escherichia coli serotype O55:B5 and zymosan A from Saccharomyces cerevisiae were purchased from Sigma (St. Louis, Mo.). Phytohemagglutinin P (PHA) was obtained from Difco (Detroit, Mich.). Recombinant human tumor necrosis factor alpha (rhTNF-α) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) were obtained from R&D (Minneapolis, Minn.) and Genzyme (Boston, Mass.), respectively. All cell culture media were purchased from Life Technologies (Grand Island, N.Y.). Fetal calf serum (FCS) of defined grade and the Pen/Strep solution were obtained from HyClone (Logan, Utah) and Biological Industries (Kibbutz Deit Haemek, Israel), respectively. All cell culture reagents were endotoxin free (<6 pg/ml) as determined by a Limulus amebocyte lysate assay. Monoclonal antibodies (MAbs) against CD11b, CD14, and HLA-DR were purchased from Dako (Glostrup, Denmark). Anti-CD14 MAb MY4 was obtained from Coulter Immunology (Miami, Fla.), and MoS39 was kindly provided by S. M. Goyert at North Shore University Hospital (New York, N.Y.). The MAb 3H5 (D2V env-specific immunoglobulin G1 [IgG1]) was provided by D. J. Gubler of The Centers for Disease Control (Fort Collins, Colo.).
Preparation of virus stocks.
DV strain 16681 (serotype 2) was propagated in C6/36 mosquito cells that were cultured in Dulbecco modified Eagle medium containing 10% FCS, 1.5% Pen/Strep solution, and 2 mM l-glutamine. These cells were infected with DV at a multiplicity of infection (MOI) of 0.0005 PFU per cell. After 2 h of viral adsorption at 28°C, the cell cultures were maintained in 7% CO2-prefilled tissue culture flasks at room temperature for 9 days. Thereafter, the culture supernatants were collected, filtered, and stored at −70°C until use. The virus titer was 2 × 107 PFU/ml as determined by plaque assay on BHK-21 cells (see below).
Isolation and culture of human MO/Mφ.
Human peripheral blood was obtained from healthy donors 22 to 28 years of age. From the same venipuncture, part of the blood was collected for obtaining autologous serum and the remainder was collected in a heparin-containing Vacutainer (Becton Dickinson, Franklin Lakes, N.J.). After the platelet-rich plasma was depleted, the heparinized peripheral blood was underlaid with Histopaque (Sigma) and then subjected to density gradient centrifugation. The peripheral blood mononuclear cells (PBMC) were collected, washed twice with Hanks balanced salt solution (HBSS), and then resuspended in complete alpha minimal essential medium (α-MEM) containing 10% heat-inactivated autologous human serum (ΔAHS) and 2 mM l-glutamine. Approximately 2 × 106 PBMC were plated into 10-mm-diameter tissue culture dishes (Corning) and incubated at 37°C with 7.5% CO2 for 2.5 h to allow monocyte adhesion. The nonadherent cells were removed from the cultures by washing with HBSS three times, and the adherent cells were detached by incubating the cells with an elution buffer (5 mM EDTA in phosphate-buffered saline [PBS] containing 5% FCS) for 15 to 20 min. The nonadherent cell fraction (5 to 10% monocytes, 85 to 90% lymphocytes, and <5% granulocytes) was resuspended in RPMI 1640 supplemented with 10% FCS and used to prepare PHA (10 μg/ml)-stimulated (for 3 days), PBMC-conditioned medium (PHA-CM). The isolated adherent cells were suspended at 4 × 105 cells/ml in the complete α-MEM and then seeded into 24-well (1 ml/well) or 48-well (0.5 ml/well) plastic tissue culture plates (Costar, Cambridge, Mass.). These adherent cells contained more than 95% monocytes, as determined by morphological examination (>96%), nonspecific esterase staining (>95%), and phagocytosis of latex beads (>98%). Moreover, the cells were >90, >95, and >98% positive for CD11b, CD14, and HLA-DR, respectively, as assessed with flow cytometry and immunofluorescence microscopy. The monocytes were allowed to differentiate into 1-week-old MO/Mφ in vitro with a half-change of the culture medium on day 5 after cell isolation.
MO/Mφ activation.
Cultured human MO/Mφ were stimulated with LPS, zymosan, PHA-CM, rhGM-CSF, and rhTNF-α (19) either before, after, or at the same time as DV infection. The levels of MO/Mφ-secreted TNF-α and some other cytokines in the culture supernatants were measured and served as activation markers.
Infection of MO/Mφ with DV.
One- or seven-day-old MO/Mφ were treated with the stimulatory agents, such as LPS, described above or were left untreated and were then used for DV infection. When the treatments were performed prior to infection, the stimuli were usually incubated with the cells for 18 to 24 h or the time period indicated (see Fig. 5). Thereafter, the treated and untreated cultures were extensively washed with incomplete α-MEM three times and then inoculated with DV at an MOI of 3 PFU per cell or according to the experimental design. Viruses were then incubated with the cells in 0.2 to 0.25 ml of α-MEM (serum free) at 37°C with gentle agitation every 15 min. After 2.5 h of adsorption, the unabsorbed viruses were removed, and the cultures were washed twice and replenished with fresh culture medium without the stimulatory agents. When the treatments were performed after infection, the stimulatory agents were added to the cultures immediately after viral adsorption or at the time point (see below). In some experiments, LPS and DV stocks were simultaneously added to the cultures and coincubated for 2.5 h in serum-free α-MEM or α-MEM containing 3% ΔAHS. After viral adsorption, all the cultures were further incubated for 40 to 48 h or the time period indicated. At the end of the incubation, culture supernatants were collected and stored in aliquots at −70°C until use for assays of cytokine secretion and infectious-virus production. The adherent MO/Mφ were harvested in fresh α-MEM by scraping the cell monolayers and then were analyzed for intracellular infectious DV titers.
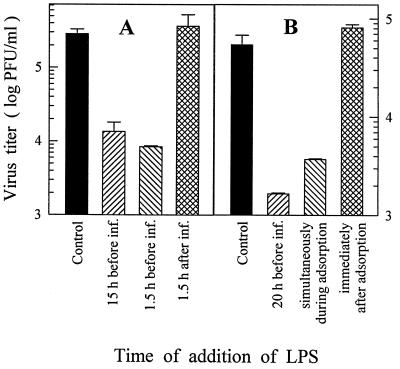
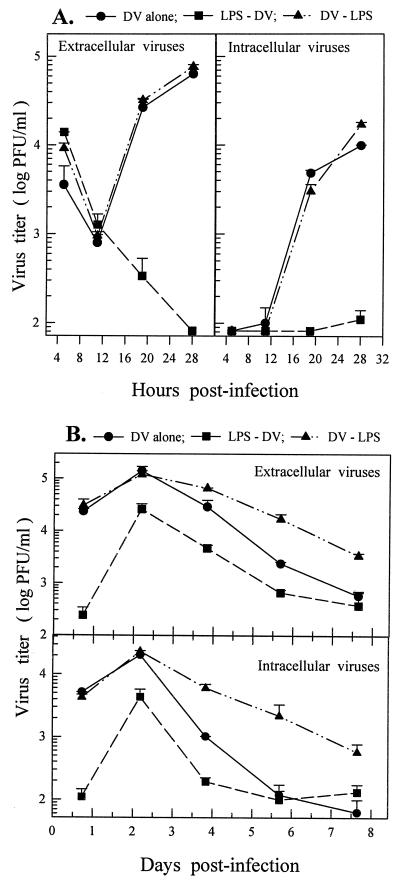
FIG. 5.
Time course of the effectiveness of LPS treatment on reducing DV yields. (A) LPS (5 μg/ml) was added to the 7-day-old MO/Mφ cultures 15 or 1.5 h prior to or 1.5 h after viral adsorption, which was performed in 0.25 ml of serum-free α-MEM. (B) LPS was added to MO/Mφ cultures 20 h before, together with, or immediately after viral adsorption, which was performed in α-MEM supplemented with 3% ΔAHS. The inoculated MOI was 3 PFU per cell. After 44 h of infection, culture supernatants were harvested and assayed to determine the infectious-virus titers. Each experimental point is expressed as the mean ± the SE of results obtained from three independent wells. Control, no LPS treatment.
Blockade of CD14 with MAbs.
Two anti-CD14 MAbs, MY4 and MoS39, that can prevent both LPS-CD14 binding and subsequent MO/Mφ activation (7, 39) were used for cell surface CD14 blocking. The MAbs were added to the cultures at a final concentration of 10 to 40 μg/ml at least 1 h before LPS treatment or DV infection. The blocking effectiveness was determined by evaluating the inhibition of LPS-induced TNF-α release from MO/Mφ.
Assays for cell viability.
Cell viability was examined by both trypan blue dye exclusion test and MTT assay as previously described (27).
DV titration.
The extracellular and intracellular infectious DV titers were determined by plaque assay as the cytopathic effect on confluent monolayers of BHK-21 cells cultured in MEM plus 10% FCS. Intracellular virions were released from the infected cells after three freeze-thaw cycles. When the adherent BHK-21 cells reached 80 to 90% confluence, aliquots of supernatants or cell cryolysates from DV-infected MO/Mφ cultures were inoculated at 10-fold serial dilutions between 10−1 to 10−6. After 2.5 h of viral adsorption, the BHK-21 cell monolayers were overlaid with MEM containing 0.5% agarose (Sigma), 0.5% FCS, and 2 mM l-glutamine. The cultures were incubated at 37°C for 6 days and then counted for plaque formation after fixation with 10% formalin and staining with 0.1% naphthalene black (Sigma).
Assay for cytokines.
The levels of TNF-α and interleukin-6 (IL-6) in the culture supernatants were measured by enzyme immunoassay using commercially available kits purchased from Genzyme (Cambridge, Mass.). The enzyme-linked immunosorbent assay (ELISA) kits used for the detection of IL-8, MIP-1α, and RANTES were purchased from R&D, and those for the measurement of IL-1β, IL-12, and IFN-α were obtained from Biosource (Camarillo, Calif.). The assays were performed according to the instructions of the manufacturers.
Assay for DV binding to hCD14-transfected cells.
70Z/3 murine pre-B cells and CHO cells stably transfected with vector containing human CD14 cDNA (hCD14-70z/3 and hCD14-CHO) or mock-transfected with the vector only (RSV-70z/3 and RSV-CHO) were generous gifts from S. Viriyakosol at The University of California-San Diego. These cells were cultured as previously described (23, 39). The hCD14-expressing cells were further enriched by positive selection with a magnetic cell sorting (MACS) technology as previously described (40). All of the reagents and the apparatus used for MACS were purchased from Miltenyi Biotec (Sunnyvale, Calif.). The purified cells were more than 95% hCD14 positive. Cells were suspended at 105 cells per 100 μl in cold PBS containing 10% FCS and then incubated with DV at different MOIs (2, 5, or 10 PFU per cell) for 30 min on ice with slight shaking. The reaction tubes were gently agitated every 5 min to maximize virus-cell contact. At the end of incubation, the virus-cell mixture was washed to remove unbound viruses, and the absorbed viruses were detected with MAb 3H5 and fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibody. The control groups were tested by the same procedure except that the cells were not exposed to the viruses. The percentages of DV-bound cells and the extent of virus binding were assessed by fluorescence-activated cell sorter (FACS) analysis.
RESULTS
Pretreatment with LPS inhibits DV infection of primary human MO/Mφ.
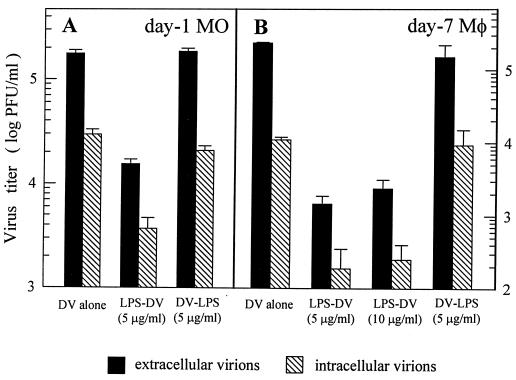
Pretreatment of MO/Mφ with LPS for 20 to 24 h significantly inhibited DV infection, resulting in an approximately 1- to 2-log reduction in both extracellular and intracellular infectious virus yields from either 1- or 7-day-old MO/Mφ (Fig. 1). Although a considerable variability in the extent of inhibition was noted in MO/Mφ obtained from different donors, the virus yields from LPS-pretreated cultures were invariably at a very low level, ranging from <0.05 to 10% of the untreated controls (data not shown). The LPS-induced reduction in virus yield was not due to cell death because >99% of the viable cells could be identified by trypan blue dye exclusion in both LPS-treated and untreated cultures and because the MTT assay revealed similar results. In contrast, however, when LPS was added after infection, such an inhibition was no longer observed (Fig. 1).
FIG. 1.
Effect of LPS treatment on the production of infectious DV by young monocytes (A) and differentiated macrophages (B) cultured for 1 and 7 days, respectively. For each infection experiment, three comparison groups were included. (i) MO/Mφ were infected with DV without any stimulation (DV alone), (ii) LPS (5 or 10 μg/ml) was added to the cultures and incubated for 22 h before DV infection (LPS-DV), and (iii) LPS (5 μg/ml) was added to the cultures 6 h (A) or 12 h (B) after viral adsorption (DV-LPS). The cells were infected with DV at an MOI of 3 PFU per cell. After 42 to 46 h of infection, cells and culture supernatants were collected and assayed for both intracellular and extracellular infectious virus production. For each experimental point, triplicate wells were performed, and the results are given as the mean ± the standard error (SE).
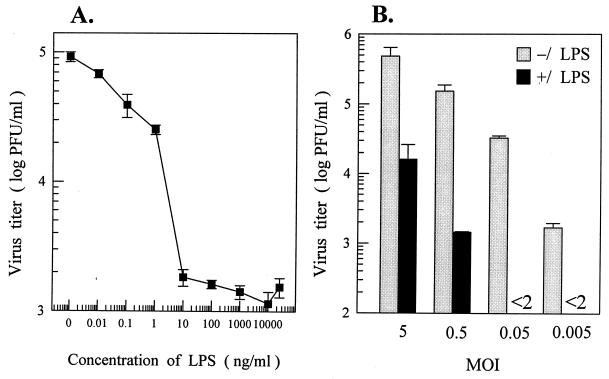
Pretreatment of MO/Mφ with LPS was effective at very low concentrations (10 and 100 pg/ml) in inducing an inhibitory effect upon DV infection, and the maximal inhibition was detectable at a dose of ≥10 ng/ml of LPS (Fig. 2A). The DV yields were proportional to the input MOIs in both LPS-treated and untreated cultures. In addition, the suppressive effect of LPS on DV production was less pronounced with a high-input MOI (Fig. 2B).
FIG. 2.
Dose-response inhibition of DV infection of MO/Mφ by LPS. (A) Seven-day-old cells were left untreated or were treated with increasing concentrations of LPS (10 pg/ml to 25 μg/ml) for 24 h and then infected with DV at an MOI of 3 PFU per cell. (B) Six-day-old MO/Mφ were left untreated or were treated with 5 μg of LPS per ml for 22 h and then infected with DV at an MOI of 5, 0.5, 0.05, or 0.005 PFU per cell. After viral adsorption, cells were incubated with fresh medium without LPS. Culture supernatants were harvested after 44 to 46 h of infection and assayed to determine the infectious-virus titers. Each experimental point is presented as the mean ± the SE of results obtained from three separate wells.
LPS-stimulated monokine secretion and MO/Mφ activation is not responsible for the inhibition of DV infection.
We next assessed the possible roles of several MO/Mφ-derived cytokines and chemokines, including TNF-α, IFN-α, IL-1β, IL-6, IL-8, IL-12, MIP-1α, and RANTES, in mediating the inhibitory effect of LPS. At all time points postinfection, the levels of the mediators were higher in the supernatants of the cultures treated with LPS after DV infection and much lower or even undetectable in the supernatants of the cultures pretreated with LPS or in those without any LPS treatment (data not shown). The apparent lack of inverse correlation between viral yields and cytokine levels indicates that the suppression of DV production was not mediated by the cytokines.
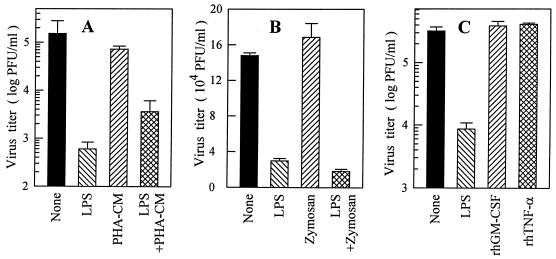
To investigate whether or not MO/Mφ activation is related to DV suppression, we stimulated the cells with several potent MO/Mφ activators. Pretreatment of MO/Mφ with zymosan and PHA-CM had no effect on infectious DV production, as shown in Fig. 3A and B. Interestingly, when MO/Mφ were pretreated with LPS combined with PHA-CM, a partial restoration of the viral yield was observed and the production of cytokines such as TNF-α and IL-6 was also markedly promoted in these cultures (not shown). Similarly, the addition of high doses of rhGM-CSF (20,000 U/ml) or rhTNF-α (20 and 2,000 ng/ml) prior to DV infection had no effect on infectious virus production (Fig. 3C). Taken together, these data clearly exclude the possibility that the inhibitory action of LPS on DV infection is mediated by LPS-induced MO/Mφ activation and/or cytokine secretion.
FIG. 3.
Effects of MO/Mφ activators and stimulatory cytokines on DV infection of MO/Mφ. One-week-old MO/Mφ were left untreated or were treated with LPS (5 μg/ml), zymosan (50 μg/ml), PHA-CM (20% [vol/vol]), rhGM-CSF (20,000 U/ml), or rhTNF-α (20 ng/ml), either alone or in combination, for 20 to 24 h. The cells were then washed and infected with DV at an MOI of 3 PFU per cell. Culture supernatants were harvested after 42 to 46 h of infection and assayed for infectious-virus titers. Each experimental point is expressed as the mean ± the SE.
Pretreatment with LPS results in a delayed but not abortive DV replication.
To elucidate the influence of LPS treatment on the kinetics of DV replication, we studied the short- and long-term growth kinetics of DV in various MO/Mφ cultures. As shown in Fig. 4, no infectious virions were produced or released before 12 h postinfection in all of the cell cultures. Intracellular and extracellular infectious DV became detectable at between 12 and 18 h postinfection (Fig. 4A), peaked at 48 h, and progressively declined thereafter (Fig. 4B). In LPS-pretreated cultures, however, DV production was suppressed and virus production was minimal by 28 h postinfection (Fig. 4A). Nevertheless, there was a subsequent increase in viral yield at about 48 h (Fig. 4B), indicating that the production of infectious DV was delayed rather than blocked by LPS pretreatment. The time lag of the postponed log phase was equal to one replication cycle (about 14 h) of the virus. The failure to achieve one-step growth of DV in these cultures implies that an inefficient initial infection (i.e., virus attachment and penetration) might be induced by LPS pretreatment.
FIG. 4.
Kinetics of DV replication in primary human MO/Mφ. Infection experiments on 7-day-old (A) and 6-day-old (B) MO/Mφ cultures were performed with three comparison groups (DV alone, LPS-DV, and DV-LPS) as described in the legend to Fig. 1. For LPS-DV, LPS at a concentration of 6 μg/ml was incubated with the cells for 22 h prior to infection and for DV-LPS, LPS was added to the cultures immediately after viral adsorption. All cultures were infected with DV at an MOI of 3 PFU per cell and then incubated to follow the short-term (A) and long-term (B) kinetics of extracellular and intracellular infectious virus production. For each time point, three separate wells were prepared and analyzed, and the results are presented as the mean ± the SE.
LPS acts on an early event of viral life cycle to inhibit DV infection.
To understand the stage of viral life cycle at which LPS acted to inhibit DV infection, we analyzed the time course of the effectiveness of LPS treatment. As illustrated in Fig. 5, the production of infectious DV was markedly suppressed when LPS was added to the cultures as early as 1.5 h prior to DV infection. The magnitudes of reduced viral yield were independent of the duration of LPS preincubation. Furthermore, treatment of MO/Mφ with LPS at the same time as DV infection was equally effective in inhibiting DV infection (Fig. 5B). However, if LPS was added to the cultures 1.5 h or even immediately after virus adsorption, the viral yield was unaffected. In 20 separate experiments, LPS treatment was only effective in suppressing DV infection of MO/Mφ that had not been previously exposed to DV. These data strongly suggest that LPS acts at the receptor level or during the early events of virus entry.
To determine whether the blockade of portals for virus entry served as a mechanism by which LPS exerted its inhibitory effect, we measured the titers of unbound viruses that remained free in the culture supernatants at various time points after virus inoculation. The level of unabsorbed viruses in the LPS-pretreated cultures after 1.5 h of virus inoculation was 1.5-fold higher than that in the untreated controls. Such a discrepancy increased with time, and the titer of unbound viruses in the LPS-pretreated cultures after 8 h of virus inoculation was more than fourfold higher than that in the untreated controls (1.4 × 105 and 3.2 × 104 PFU/ml, respectively). These results imply that LPS pretreatment may block DV attachment to the cells.
Role of CD14 in the LPS-mediated suppression of DV infection of MO/Mφ.
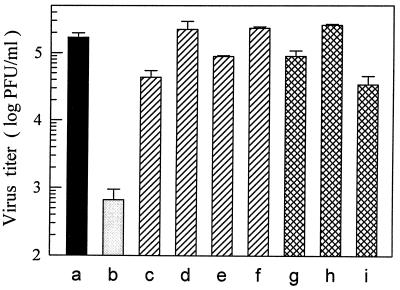
According to the results presented above, it is likely that LPS inhibited DV infection of MO/Mφ by preventing virus entry into the cells. Thus, we investigated whether or not the major LPS binding receptor, CD14, was involved in the LPS-induced inhibition of DV infection. As shown in Fig. 6, blockade of membrane CD14 before LPS treatment with either one of the two anti-CD14 MAbs, MY4 and MoS39, could completely abolish the inhibitory effect of LPS (Fig. 6, columns c to f). On the other hand, MAbs alone had no effect on DV infection (Fig. 6, columns g and h). Furthermore, blockade of CD14 before the coincubation of MO/Mφ with LPS during viral adsorption could also reverse the suppression of the DV yield (Table 1), suggesting that these MAbs acted during an early event of LPS-CD14 interactions to abrogate the suppressive effect of LPS. Moreover, these results also exclude the possibility that LPS alone may directly react with DV to reduce its infectivity in that DV could effectively infect anti-CD14 MAbs-pretreated MO/Mφ even in the presence of LPS during viral adsorption.
FIG. 6.
Blockade of LPS-induced suppression of DV infection by two anti-CD14 MAbs, MY4 and MoS39. One-week-old MO/Mφ were infected with DV at an MOI of 3 PFU per cell without any treatment (a) or were treated with LPS (5 μg/ml) for 20 h prior to DV infection (b). In addition, MO/Mφ were preincubated with 10 or 20 μg of MY4 per ml (c and d, respectively) or with 20 or 40 μg of MoS39 per ml (e and f, respectively) for 2 h before the addition of LPS (i.e., 22 h prior to DV infection). Some cultures were incubated with 10 μg of MY4 per ml for 2 h (g) or 20 μg of MoS39 per ml for 22 h (h) prior to DV infection, without a subsequent LPS treatment. In some cultures, MY4 was added after 2 h of viral adsorption (i). Each experimental point represents the mean ± the SE of results obtained from three separate wells.
TABLE 1.
Effect of human serum on the CD14-dependent inhibition of DV infection of MO/Mφ by LPSa
| Expt no. | Virus titer in PFU/ml (% inhibition) with (+) or without (−):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| −ΔAHS
|
+ΔAHS
|
|||||||
| −LPS | +LPS | +MY4, +LPS | +MoS3, +LPS | −LPS | +LPS | +MY4, +LPS | +MoS39, +LPS | |
| 1 | 2.31 × 105 | 8.46 × 104 (63.4) | 2.20 × 105 (4.7) | 2.46 × 105 (−6.5) | 6.53 × 104 | 3.50 × 103 (94.6) | 5.07 × 104 (22.4) | 6.67 × 104 (−2.1) |
| 2 | 2.74 × 105 | 9.23 × 104 (66.3) | 2.43 × 105 (11.2) | ND | 1.14 × 105 | 1.03 × 104 (90.9) | 1.21 × 105 (−6.1) | ND |
Seven-day-old primary human MO/Mφ obtained from two different donors (i.e., experiments 1 and 2) were used. The cells were infected with DV at an MOI of 3 PFU per cell. Viral adsorption was performed in serum-free α-MEM (−ΔAHS) or α-MEM containing 3% ΔAHS (+ΔAHS), either with or without 5 μg of LPS per ml (+LPS and −LPS, respectively) for 2.5 h. Before coincubation of LPS and DV, some cultures were pretreated overnight with 20 μg of MY4 per ml (+MY4) or 30 μg of MoS39 (+MoS39) per ml. After 44 h of infection, culture supernatants were harvested and assayed to determine the infectious-DV titers. ND, not determined.
To test the possibility that other LPS-binding regions of CD14 not recognized by MY4 and MoS39 may mediate DV infection of human MO/Mφ (39), we performed a binding assay with hCD14-transfected or mock-transfected CHO cells and 70z/3 murine pre-B cells by FACS analysis. There was a dose-dependent binding of DV to all of these cells. For CHO cells, the percentages of fluorescent positive cells after 30 min of incubation were 44.75 (MOI = 2), 51.31 (MOI = 5), and 63.48 (MOI = 10). The levels of virus binding were similar for mock- and hCD14-transfected cells. The lack of increased virus binding to hCD14-transfected cells suggests that the CD14 molecule is not essential for DV binding. Taken together, our observations suggest that CD14 molecule per se is not required for DV infection, although this protein is involved in LPS-mediated blockade for DV entry.
Effect of human serum on the CD14-dependent inhibition of DV infection by LPS.
To investigate whether enhancing the efficiency of LPS “binding” to CD14 would increase the LPS-induced DV inhibition, we examined the role of human serum in this event based on the assumption that some serum factors can accelerate the delivery of LPS to CD14 (13, 17, 33, 35). Coincubation of MO/Mφ with LPS during viral adsorption in the absence of serum resulted in an inhibition rate of viral yield of about 60% (Table 1). However, if autologous human serum was added to the cultures, the inhibition rate was significantly increased to >90%. These results indicate that human serum can augment the inhibitory effect of LPS. Furthermore, the increased loss of viral yield caused by the cooperative actions of LPS and human serum could be completely rescued by the blockade of CD14 with MY4 or MoS39, indicating that the enhancing effect of human serum on the LPS-induced DV suppression is also mediated by CD14.
DISCUSSION
A variety of mechanisms underlie the modulation of LPS in viral infections of human MO/Mφ (2, 4, 12, 19–20, 29, 38). In the present study, we report that LPS suppressed DV infection of MO/Mφ through a novel mechanism that has not been demonstrated in other viral infections. The suppression was LPS specific and was not associated with cell activation. The myeloid antigen CD14 was the key molecule mediating the LPS-induced suppression of DV infection and human serum played a role in the enhancement of such an inhibition.
LPS was known to induce massive MO/Mφ activation and the release of a wide variety of soluble mediators that are responsible for the in vitro antiviral effect of LPS on several viral infections of human MO/Mφ (4, 12, 19–20, 38). However, we failed to observe any relationship between DV yields and the levels of secreted cytokines and chemokines in the MO/Mφ cultures (data not shown). In addition, stimulation of MO/Mφ with other activators such as zymosan, PHA-CM, rhTNF-α, and rhGM-CSF had no inhibitory effect on DV infection, despite the fact that these agents are equally potent in triggering MO/Mφ activation and cytokine secretion (Fig. 3). Furthermore, when LPS was added after viral infection, no reduction in viral yield could be seen from day 1 through day 8 (Fig. 4). Taken together, these results strongly suggest that the suppression of DV infection could be explained neither by MO/Mφ activation nor by the indirect effects of the LPS-induced monokines.
Studies of the short- and long-term kinetics of DV replication in various MO/Mφ cultures demonstrated that LPS-induced suppression of DV production occurred early within the first replication cycle and that the production of infectious viruses was merely postponed but not blocked by LPS (Fig. 4). Such a delay of DV production was not attributable to an LPS-induced cytoplasmic accumulation of infectious virions (5) in that the production of intracellular viruses in LPS-pretreated cultures was also decreased to a similar extent and their production kinetics were parallel to those of extracellular viruses (Fig. 1 and 4). The fact that LPS must be added before cell exposure to DV in order to be effective strongly suggests that LPS acted during an early event in virus entry. Furthermore, the level of unbound viruses that remained free in the supernatants of LPS-pretreated cultures was much higher than that of the untreated controls at various times after viral adsorption, indicating that the LPS-induced suppression may result from a reduced level of virus attachment to the cells. Furthermore, despite the fact that 5 min was sufficient for maximal LPS binding to MO/Mφ and the induction of cytokine synthesis (11), the observation that treatment with LPS even immediately after viral adsorption failed to suppress DV infection further supports the idea that LPS must exert its inhibitory action before virus-cell attachment takes place. In fact, since the time lag of the postponed virus production was equal to the duration for completing one replication cycle of DV, the infectious virions produced during this delayed log phase may be derived from the progeny viruses that reinfected new target cells. This is probably caused by an inefficient initial infection with a lower level of virus entry to achieve one-step growth.
The primary determinant for the establishment of a successful viral infection of target cells is the presence of specific cellular receptors that mediate virus entry (41). Thus, it is possible that LPS suppressed DV infection by masking the cellular receptors for DV on human MO/Mφ, thus making it less accessible. The fact that coincubation of MO/Mφ with LPS during viral adsorption markedly inhibited DV infection further supports the hypothesis that LPS and DV may share and compete for a common cellular receptor. Therefore, we speculated that the major LPS binding receptor CD14 may be exploited by DV for the infection of human MO/Mφ. However, the observations that blockade of this molecule with MY4 or MoS39 did not suppress DV production (Fig. 6) and that the binding assay revealed similar levels of virus binding to mock- and hCD14-transfected cells clearly indicate that the CD14 molecule per se is not essential for DV infection.
Although CD14 by itself does not mediate DV infection, this protein may be involved in the LPS-mediated inhibition of DV entry into human MO/Mφ. Blockade of CD14 with either MY4 or MoS39 before LPS treatment could entirely abrogate the inhibitory action of LPS (Fig. 6). More importantly, blockade with MY4 or MoS39 could restore the virus yield reduced by coincubating LPS during DV adsorption (Table 1), indicating that these MAbs blocked an early event in the process of LPS-CD14 interaction (i.e., LPS-CD14 binding) to ablate the LPS-induced DV suppression. Furthermore, there exist some serum factors, such as LBP, that are not required for LPS-induced MO/Mφ activation but that can make complexes with LPS and accelerate its binding to CD14 (13, 17, 33, 35). Our observation that human serum could enhance the LPS-suppressed DV infection in a CD14-dependent manner further supports the notion that binding of LPS to CD14 alone without subsequent cell activation is sufficient for mediating the inhibition of virus replication.
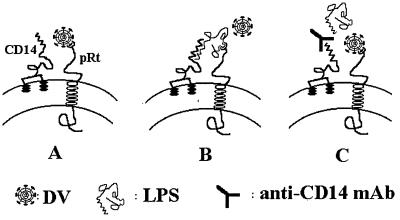
Considerable evidence suggests that the LPS receptor on MO/Mφ is a multiprotein complex containing CD14 and an unidentified, conditionally linked transmembrane signal transducer (10, 23–24, 31–32, 36–37). This putative coreceptor functions in accepting LPS from CD14 with a low binding affinity for LPS. Considering the involvement of CD14 in the LPS-mediated blockade for DV entry, this putative molecule is likely to serve as a receptor for DV entry. Based on this hypothesis, we propose a model for DV entry into human MO/Mφ that is represented schematically in Fig. 7. In the absence of LPS, DV may use this putative structure as its receptor to infect MO/Mφ (Fig. 7A). However, if cells are exposed to LPS before or at the same time as the DV infection, CD14 will first accept LPS due to its high affinity and then deliver LPS to the putative DV binding molecules. As a result, access of DV to the cell is denied, and this results in the inhibition of DV infection (Fig. 7B). Blockade of CD14 with MAbs reduces the efficiency of LPS to interact with the postulated molecules due to their low affinity. Under this circumstance, DV will infect MO/Mφ via this molecule without interference (Fig. 7C). Recently, CD14 has been shown to serve as a multipurpose receptor that recognized a diverse array of bacterial constituents and triggered innate immune responses by cooperating with an associated polyspecific signal transducer (22, 30). Indeed, taxonomically unrelated viral pathogens are also known to share common cellular receptors (3). In the present study, our model raises the possibility that a cell surface structure on MO/Mφ may be targeted both by a bacterial component and a viral pathogen.
FIG. 7.
Proposed model for DV entry into human MO/Mφ. The LPS receptor on the cell surface of human MO/Mφ is a heterodimeric complex containing a GPI-anchored CD14 and an unidentified transmembrane coreceptor, pRt. (A) In the absence of LPS, DV utilizes the pRt for infection. (B) In the presence of LPS, occupancy of the pRt by LPS blocks the access for DV entry. (C) Blockage of CD14 with anti-CD14 MAbs precludes the LPS from occupying the pRt and makes it accessible for DV. pRt, putative transmembrane receptor.
Previous studies have revealed that both LPS-induced MO/Mφ activation and DV infection of human MO/Mφ required a trypsin-sensitive protein on the surface of these cells (8, 37). Together with the findings presented here, it seems clear that structural-biology approaches are warranted in the future to elucidate whether LPS and DV have similar receptor-binding properties or structures and whether they exploit a common or related pathway to manifest their toxicity (32). We provide here a valuable model for the understanding of the interactions between DV and MO/Mφ by way of the interactions between bacterial endotoxin and the CD14 molecules.
ACKNOWLEDGMENTS
The work is supported by grants from National Science Council (NSC86-2314-B-075-055 and NSC86-2314-B002-108-M07) of The Republic of China and grant VGH87-386 from the Veterans General Hospital-Taipei.
We thank Cheng-Po Hu at the Veterans General Hospital (Taipei, Republic of China), Wen Chang at the Academia Sinica (Taipei, Republic of China), Peter S. Tobias at the Scripps Research Institute (La Jolla, Calif.), and Fidel Zavala at the NYU Medical Center (New York, N.Y.) for their helpful discussions. We thank Sanna M. Goyert at North Shore University Hospital (New York, N.Y.) and Suganya Viriyakosol at The University of California (San Diego, Calif.) for providing MAbs and transfected cells. We also thank Ming-Ling Hsu, Miao-Zeng Huang, Yi-Chiuan Chang, Pei-Jun Chen, Hui-Ru Hsieh, and Yueh-Hsia Chiu at the Laboratory of Hematology of The Veterans General Hospital-Taipei for their help and encouragement.
REFERENCES
- 1.Anderson R, Wang S, Osiowy C, Issekutz A C. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol. 1997;71:4226–4232. doi: 10.1128/jvi.71.6.4226-4232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagasra O, Wright S D, Seshamma T, Oakes J W, Pomerantz R J. CD14 is involved in control of human immunodeficiency virus type 1 expression in latently infected cells by lipopolysaccharide. Proc Natl Acad Sci USA. 1992;89:6285–6289. doi: 10.1073/pnas.89.14.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein M S, Tong-Starksen S E, Locksley R M. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Invest. 1991;88:540–545. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas P, Poli G, Kinter A L, Justement J S, Stanley S K, Maury W J, Bressler P J M, Orenstein J M, Fauci A S. Interferon γ modulates the expression of human immunodeficiency virus in persistently infected promonocytic cells by redirecting the production of virions to intracytoplasmic vacuoles. J Exp Med. 1992;176:739–750. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang D-M, Shaio M-F. Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocytes exposed to dengue virus. J Infect Dis. 1994;170:811–817. doi: 10.1093/infdis/170.4.811. [DOI] [PubMed] [Google Scholar]
- 7.Cohen L, Haziot A, Shen D R, Lin X-Y, Sia C, Harper R, Silver J, Goyert S M. CD14-independent responses to LPS require a serum factor that is absent from neonates. J Immunol. 1995;155:5337–5342. [PubMed] [Google Scholar]
- 8.Daughaday C C, Brandt W E, McCown J M, Russell P K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981;32:469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudding L R, Garnett H M. Interaction of strain AD169 and a clinical isolate of cytomegalovirus with peripheral monocytes: the effect of lipopolysaccharide stimulation. J Infect Dis. 1987;155:891–896. doi: 10.1093/infdis/155.5.891. [DOI] [PubMed] [Google Scholar]
- 10.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallay P, Jongeneel C V, Barras C, Burnier M, Baumgartner J-D, Glauser M P, Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993;150:5086–5093. [PubMed] [Google Scholar]
- 12.Gessani S, Testa U, Varano B, Marzio P D, Borghi P, Conti L, Barberi T, Tritarelli E, Martucci R, Seripa D, Peschle C, Belardelli F. Enhanced production of LPS-induced cytokines during differentiation of human monocytes to macrophages: role of LPS receptors. J Immunol. 1993;151:3758–3766. [PubMed] [Google Scholar]
- 13.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halstead S B, O’Rourke E J, Allison A C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977;146:218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 16.Haziot A, Chen S, Ferrero E, Low M G, Silber R, Goyert S M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 17.Heumann D, Gallay P, Barras C, Zaech P, Ulevitch R J, Tobias P S, Glauser M-P, Baumgartner J D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- 18.Johnson K M. Emerging viruses in context: an overview of viral hemorrhagic fevers. In: Morse S S, editor. Emerging viruses—1993. New York, N.Y: Oxford University Press, Inc.; 1993. pp. 46–57. [Google Scholar]
- 19.Kornbluth R S, Oh P S, Munis J R, Cleveland P H, Richman D D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krakauer T, Peters C J. Lipopolysaccharide inhibits the production of lymphocytic choriomeningitis virus in a human monocytic cell line. J Gen Virol. 1993;74:1653–1656. doi: 10.1099/0022-1317-74-8-1653. [DOI] [PubMed] [Google Scholar]
- 21.Kurane I, Ennis F A. Immunity and immunopathology in dengue virus infections. Semin Immunol. 1992;4:121–127. [PubMed] [Google Scholar]
- 22.Kusunoki T, Hailman E, Juan T S-C, Lichenstein H S, Wright S D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J D, Kato K, Tobias P S, Kirkland T N, Ulevitch R J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J D, Kravchenko V, Kirkland T N, Han J, Mackman N, Moriarty A, Leturcq D, Tobias P S, Ulevitch R J. GPI-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci USA. 1993;90:9930–9934. doi: 10.1073/pnas.90.21.9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 27.Nain M, Hinder F, Gong J-H, Schmidt A, Bender A, Sprenger H, Gemsa D. Tumor necrosis factor-α production of influenza A virus-infected macrophages and potentiating effect of lipopolysaccharides. J Immunol. 1990;145:1921–1928. [PubMed] [Google Scholar]
- 28.Panuska J R, Merolla R, Rebert N A, Hoffmann S P, Tsivitse P, Cirino N M, Silverman R H, Rankin J A. Respiratory syncytial virus induces interleukin-10 by human alveolar macrophages. J Clin Invest. 1995;96:2445–2453. doi: 10.1172/JCI118302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomerantz R J, Feinberg M B, Trono D, Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990;172:253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 31.Pugin J, Schurer-Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide (LPS) activation of human endothelial and epithelial cells is mediated by LPS binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Padova F D, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 33.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 34.Shaio M-F, Cheng S-N, Yuh Y-S, Yang K D. Cytotoxic factors released by dengue virus-infected human blood monocytes. J Med Virol. 1995;46:216–223. doi: 10.1002/jmv.1890460309. [DOI] [PubMed] [Google Scholar]
- 35.Tobias P S, Mathison J, Mintz D, Lee J-D, Kravchenko V, Kato K, Pugin J, Ulevitch R J. Participation of lipopolysaccharide-binding protein in lipopolysaccharide-dependent macrophage activation. Am J Respir Cell Mol Biol. 1992;7:239–245. doi: 10.1165/ajrcmb/7.3.239. [DOI] [PubMed] [Google Scholar]
- 36.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 37.Vasselon T, Pironkova R, Detmers P A. Sensitive responses of leukocytes to lipopolysaccharide require a protein distinct from CD14 at the cell surface. J Immunol. 1997;159:4498–4505. [PubMed] [Google Scholar]
- 38.Verani A, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi A G, Vercelli D. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viriyakosol S, Kirkland T N. A region of human CD14 required for lipopolysaccharide binding. J Biol Chem. 1995;270:361–368. doi: 10.1074/jbc.270.1.361. [DOI] [PubMed] [Google Scholar]
- 40.Wang S-Y, Chen L-Y, Hsu M-L, Tzeng C-H, Ho C-H, Ho C-K. Effect of lymphocytes on the production of granulomonopoietic enhancing factor by fully mature macrophages. Stem Cells. 1995;13:435–444. doi: 10.1002/stem.5530130415. [DOI] [PubMed] [Google Scholar]
- 41.Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 42.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]