Abstract
The Vif protein of human immunodeficiency virus type 1 (HIV-1) is a potent regulator of viral infectivity. Current data posit that Vif functions late in replication to modulate assembly, budding, and/or maturation. Consistent with this model, earlier indirect immunofluorescence analyses of HIV-1-infected cells demonstrated that Vif and Gag colocalize to a substantial degree (J. H. M. Simon, R. A. M. Fouchier, T. E. Southerling, C. B. Guerra, C. K. Grant, and M. H. Malim, J. Virol. 71:5259–5267, 1997). Here, we describe a series of subcellular fractionation studies which indicate that Vif and the p55Gag polyprotein are present in membrane-free cytoplasmic complexes that copurify in sucrose density gradients and are stable in nonionic detergents. Both Vif and Gag are targeted to these complexes independent of each other, and their association with them appears to be mediated by protein-protein interactions. We propose that these complexes may represent viral assembly intermediates and that Vif is appropriately localized to influence the final stages of the viral life cycle and, therefore, the infectivity of progeny virions.
Lentiviruses such as human immunodeficiency virus type 1 (HIV-1) encode a number of genes in addition to the gag, pol, and env genes that are expressed by all replication-competent retroviruses (12, 17). One of these additional genes, vif (viral infectivity factor), is expressed by all known lentiviruses except equine infectious anemia virus (40) and is essential for the pathogenic replication of lentiviruses in vivo (13, 31).
Analyses of vif-deficient (Δvif) HIV-1 infections have shown that such viruses are 10- to 100-fold less infectious than their wild-type counterparts (2, 4, 22, 23, 46, 49) and that the defect can be manifested at an early step of the infection process that may reflect an inappropriate disassembly of postentry viral nucleoprotein complexes (also known as preintegration complexes) prior to DNA integration (28, 49). Taken together with the fact that Vif must be present in virus-producing cells for its effect on infectivity to be exerted (23, 56), this finding has led to the current belief that Vif functions at a late stage of the life cycle, such as assembly, budding, or maturation, to prime progeny virions for productive infection. Consistent with this model, confocal microscopy analyses of HIV-1-infected human cells and feline immunodeficiency virus-infected feline cells have revealed that Vif and the respective Gag polyprotein precursors colocalize to a significant degree (47). Somewhat surprisingly, however, virions that are produced in the absence of Vif have been shown to contain a full complement of processed viral proteins (4, 22, 41, 56)—a finding that has led to the suggestion that Vif may act by modulating the structure or conformation of virion cores or core components. In addition, there has been one report of Vif being able to interact with the p7Gag (nucleocapsid [NC]) portion of p55Gag, although the biological significance of this remains to be ascertained (3).
Two lines of investigation have led to the suggestion that an interaction between Vif and a host cell factor(s) is crucial to its function. First, an examination of various primate lentivirus Vif proteins has revealed that Vif acts in a species-specific manner; for example, the Vif protein of an African green monkey isolate of simian immunodeficiency virus (SIVAGM) is inactive in human cells yet functional in cells derived from African green monkeys (50). Second, it has been known for some time that Vif is required for HIV-1 replication in primary cells and certain cell lines (nonpermissive cells) but is dispensable for replication in certain other cell lines (permissive cells) (18, 19, 23, 46, 52, 56). In other words, the cellular milieu appears to determine whether Vif is necessary for the formation of infectious virions. Interestingly, recent experiments have shown that the fusion of nonpermissive cells to permissive cells results in the formation of heterokaryons that bear the nonpermissive phenotype—a finding which suggests that nonpermissive cells harbor an innate activity that inhibits infectious virion production and that the role of Vif is to suppress this activity (48).
Together, these various studies suggest that Vif functions at the site of retroviral assembly by interacting with a cellular factor(s), and possibly p55Gag, to inhibit a process that would otherwise render progeny virions poorly infectious. To date, relatively little is known about the processes that drive the assembly of HIV-1 cores or how cellular factors might be involved. It is clear that p55Gag encodes several important signals (32), with membrane association being directed by a bipartite signal in the p17Gag (matrix [MA]) component (an amino-terminal myristoyl group and a cluster of positively charged residues) (29, 54, 58, 59) and intermolecular Gag-Gag binding being determined by sequences in MA (9, 11) and both the amino and carboxy termini of p24Gag (capsid [CA]) (10, 14, 44, 55, 57) and NC (15, 34). Consistent with the conclusion that multiple regions of p55Gag contribute to correct HIV-1 core assembly, in vitro studies using purified proteins have revealed that a variety of Gag-derived fragments can form an assortment of tubular and spherical structures that can be visualized by electron microscopy (8, 30, 57). In addition to these self-assembling regions of Gag, cellular components also appear to be required for the normal assembly of spherical particles. In particular, the binding of cyclophilin A to an exposed proline-loop of CA is essential for the production of infectious HIV-1 (38), the assembly of p55Gag into viral cores in a cell-free system requires a detergent-insensitive cellular factor (37), and there have been suggestions that p55Gag may interact with F-actin during assembly (45).
As an approach to dissecting Vif function further, we have initiated biochemical studies to address the localization of Vif and p55Gag in nonpermissive human T cells productively infected with HIV-1. Our results indicate that the majority of Vif and unprocessed p55Gag exist in large, noncytoskeletal complexes that cofractionate in continuous density gradients and are resistant to solubilization by nonionic detergents such as Triton X-100 (TX-100). In contrast to previous reports, however, we were unable to detect associations between Vif and either cellular membranes or p55Gag in this system.
MATERIALS AND METHODS
Cell lines and viruses.
The maintenance of the human T-cell lines C8166, CEM-SS, H9, HUT 78, and H9/hVif and the wild-type and Δvif HIV-1 proviral expression vectors pIIIB and pIIIB/Δvif, respectively, have been described previously (50, 51). For single-cycle infections of T cells with HIV-1, high-titer stocks of pseudotyped viruses were generated by transient cotransfection of 100-mm-diameter subconfluent monolayers of 293T cells with the vesicular stomatitis virus G-protein expression plasmid pHIT/G (21) and either pIIIB or pIIIB/Δvif. After 24 h, virus-containing supernatants were harvested, clarified by centrifugation at 500 × g for 5 min and filtration through 0.45-μm-pore-size filters, and incubated with 10 × 106 T cells for 4 h. The challenged cells were then washed three times in phosphate-buffered saline (PBS) to remove input virus, incubated with fresh medium at 37°C for 20 h, washed a further two times to ensure removal of all input virus, and incubated in fresh medium for a further 24 h. At this time, the cells were pelleted by centrifugation at 500 × g for 5 min, washed in PBS, and lysed for fractionation.
Antibodies and Western analysis.
Samples were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose. The filters were initially hybridized with mouse monoclonal antibodies raised against HIV-1 Vif (319) (51), HIV-1 p24Gag/CA (p24-3) (47), the heterogeneous ribonucleoprotein particle proteins C1 and C2 (hnC1/C2) (4F4) (42), anti-bovine α-tubulin (263-10501; Molecular Probes), or rabbit polyclonal antibodies to calreticulin (Affinity Bioreagents, Inc.) or vimentin (43). Bound antibodies were detected by using appropriate horseradish peroxidase-conjugated secondary antibodies raised against mouse or rabbit immunoglobulins, enhanced chemiluminescence, and autoradiography.
Subcellular fractionation.
Cells were lysed by incubation in PBS containing 1% TX-100 for 10 min on ice (1 × 107 to 5 × 107 cells) or by nitrogen cavitation in the absence of detergents (108 cells) (47). Nuclei were pelleted by centrifugation at 1,000 × g for 10 min at 4°C, lysed in radioimmunoprecipitation assay (RIPA) buffer (0.1% SDS, 1% TX-100, 1% sodium deoxycholate, 150 mM NaCl, 10 mM Tris-HCl [pH 7.5], 1 mM EDTA), and sonicated. The postnuclear supernatant was removed for subsequent analysis, and the TX-100-soluble and -insoluble fractions were separated by centrifugation at 100,000 × g for 60 min, using a TLA 100.2 rotor (Beckman Instruments Inc.). The resulting pellet (TX-100-insoluble fraction) was redissolved in RIPA buffer, and the supernatant (TX-100-soluble fraction) was adjusted to 1× RIPA buffer. All three fractions were finally made up to the same volume.
In some experiments, the postnuclear supernatant was loaded directly onto a 20 to 60% (wt/vol) continuous STE (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA)-buffered sucrose gradient and centrifuged at 150,000 × g for 2 h at 4°C, using an SW41 rotor (Beckman Instruments Inc.). Ten 1-ml fractions were collected, and these were separated into a number of equal fractions for subsequent immunoprecipitation or high-speed pelleting. Pelleting of each fraction was performed by diluting each fraction fourfold in cold PBS and centrifugation at 100,000 × g for 60 min. The density of each fraction was determined with a refractometer (see Fig. 3A for a typical gradient).
FIG. 3.
Fractionation of cytoplasmic extracts of HIV-1-infected H9 cells by using sucrose density gradients. (A) Densities of fractions from a typical sucrose gradient, in this case the gradient analyzed in panel B. H9 cells transiently infected with HIV-1 (B) or HIV-1/Δvif (C) and uninfected H9/hVif cells (D) were lysed with PBS–1% TX-100. In addition, H9/hVif cells were also lysed in the absence of detergent by nitrogen cavitation (E). The postnuclear supernatant from each lysate was loaded onto a 20 to 60% (wt/vol) continuous sucrose gradient and centrifuged at 150,000 × g for 2 h. Ten fractions were harvested (1 = top; 10 = bottom), diluted and centrifuged to pellet the high-molecular-mass complexes. Equivalent amounts from each fraction were resolved on SDS-polyacrylamide gels and analyzed by Western blotting using antibodies specific for CA or Vif.
Immunoprecipitation.
Prior to immunoprecipitation, rabbit polyclonal antibodies raised to HIV-1 Vif or CA (47) or preimmune sera from the same animals were incubated for 1 h at 4°C with the postnuclear supernatant from uninfected H9 cells that had been lysed with PBS–1% TX-100. Agarose beads conjugated to protein A (Gibco BRL Inc.) were blocked with a PBS–1% TX-100–3% nonfat dry milk solution for 1 h at 4°C. The beads were then pelleted, resuspended in PBS–1% TX-100, added to the antibody-containing cell lysate, and incubated for a further 1 h at 4°C to allow the beads to bind to the antibodies. The antibody-coated beads were then pelleted and incubated with a portion of the fractions from density sucrose gradients that had been diluted fourfold with PBS–1% TX-100. After a further 1 h of incubation with shaking at 4°C, the beads were washed three times in PBS–1% TX-100, and the bound proteins were solubilized in gel loading buffer and subjected to Western blot analysis. In some experiments, immunoprecipitated proteins were quantitated by Western blot analysis in parallel with standard curves of recombinant proteins and densitometric scanning of the autoradiographs (22).
RESULTS
Using confocal microscopy and human T cells acutely infected with HIV-1, we previously noted that the Vif and Gag proteins display substantial colocalization within the cytoplasm (47). In an effort to understand the basis for this result at the molecular level, we have initiated a series of biochemical studies. In particular, we are interested in (i) the nature and characterization of the complex or complexes that Vif and Gag are associated with, (ii) the identity and role(s) of the cellular factor(s) in Vif function and the regulation of HIV-1 assembly, and (iii) the possible associations of Vif with membranes and/or the p55Gag precursor.
Fractionation of HIV-1-infected nonpermissive and permissive T cells.
In the first set of experiments, T-cell lines that are either permissive (C8166 and CEM-SS) or nonpermissive (H9 and HUT 78) to Δvif virus replication were infected with high-titer stocks of pseudotyped HIV-1, lysed in PBS–1% TX-100 (a nonionic detergent), and separated by differential centrifugation into nuclear fractions and TX-100-soluble and -insoluble cytosolic fractions. Aliquots of each fraction that corresponded to an equal number of cells were subjected to Western blot analysis (Fig. 1). For each cell line, the majority of unprocessed p55Gag was in the TX-100-insoluble fraction, although significant levels were also present in the other two fractions. Because our previous immunofluorescence-based studies demonstrated that Gag is not localized to the nuclei of virus expressing cells (47), we suspect that the detection of Gag in the nuclear fractions was due to modest association(s) with large organelles and/or cellular debris rather than specific targeting to the nucleus. Most of the fully processed CA was detected in the TX-100-soluble fraction; this probably corresponds to virions that are either bound to cells or in the process of budding. In addition, it appears that different processing intermediates of Gag are associated with the different fractions; the explanation for this awaits further investigation.
FIG. 1.
Fractionation of HIV-1-infected T cells. HIV-1-infected cells were lysed in PBS–1% TX-100 and fractionated by differential centrifugation into nuclear (N), TX-100-soluble (S), and TX-100-insoluble (I) fractions. Aliquots of each fraction corresponding to equivalent numbers of cells were resolved on SDS-polyacrylamide gels, transferred to nitrocellulose, and analyzed by Western blotting using antibodies specific for hnRNP C1/C2, vimentin, α-tubulin, calreticulin, HIV-1 CA, or HIV-1 Vif.
As might have been predicted on the basis of our previous observations (47), the majority of Vif cofractionated with p55Gag in the TX-100-insoluble fractions of CEM-SS, H9, and HUT 78 cells. In contrast, a significant proportion of Vif was also detected in the TX-100-soluble fraction of C8166 cells; we think that this may be related to cellular damage since infection of these cells with HIV-1 results in a significantly more rapid development of cytopathic effects than it does for other cell lines (data not shown). That the fractionation of p55Gag was unaltered in C8166 cells may be consistent with the idea that the subcellular targetings of Vif and Gag occur independent of each other (see below). In addition, and as with Gag, we consider Vif’s association with the nuclear fractions to be a consequence of experimental approach as opposed to bona fide nuclear localization. Importantly, the fractionation of Vif in these experiments was similar to that observed in previous experiments in which Vif-expressing cells were disrupted by nitrogen cavitation in the absence of detergents (47).
To help assess the extent of differential fractionation that was achieved in this analysis, the filters were reprobed with antibodies specific for a nuclear marker (C1/C2), the cytoskeleton (vimentin intermediate filaments or α-tubulin), or an endoplasmic reticulum marker (calreticulin). When T cells are disrupted by nitrogen cavitation in the absence of detergent, the majority of C1/C2 and calreticulin fractionate with the nucleus (47). While the presence of TX-100 did not affect the fractionation of C1/C2, calreticulin no longer purified with the nucleus but instead entered the detergent-soluble fraction; this is presumably because TX-100 treatment, but not nitrogen cavitation, disrupts the endoplasmic reticulum and separates it from the nucleus.
One potential explanation for the fractionation pattern of Vif could be that it is associated with the cytoskeleton (35). However, and in contrast to Vif, the intermediate filament protein vimentin fractionated with the nucleus and the microtubule-associated protein α-tubulin remained in the TX-100-soluble fraction (Fig. 1), thus arguing against such interactions. In fact, immunofluorescence-based analyses of HIV-1-infected H9 cells have indicated that neither Vif nor Gag colocalizes with vimentin (47) or microfilaments (data not shown). Furthermore, we have found that treating such cultures with the cytoskeleton-disrupting drug cytochalasin B (10 μM), which targets actin microfilaments, or colchicine (30 ng/ml) or nocodazole (50 ng/ml), which both target microtubules, has no effect on the fractionation characteristics of Gag or Vif, using the methods discussed here (data not shown). Taken together, these data therefore suggest that Vif is not tightly associated with these cytoskeletal components.
Although we have not specified any of the cellular proteins that are present in the TX-100-insoluble fraction with this panel of antibodies, analysis of the three fractions by gel electrophoresis followed by Coomassie blue staining revealed that they contained similar quantities, yet quite distinct sets, of proteins (data not shown). Moreover, analysis by agarose gel electrophoresis and ethidium bromide staining revealed that the TX-100-insoluble and nuclear fractions each contained approximately equivalent levels of the rRNAs, suggesting that ribosomes were present in these two fractions (data not shown).
Solubilization of Vif and Gag.
The finding that substantial quantities of both Vif and p55Gag are present in a TX-100-insoluble fraction was surprising in the light of two sets of previous data. First, experiments conducted with Vif expressed in rabbit reticulocyte lysates have shown that Vif interacts with canine microsomes in a TX-100-sensitive fashion (27). Second, the bulk of the p55Gag precursor is believed to be localized efficiently to the plasma membrane by virtue of its bipartite membrane targeting signal as a prerequisite to the later stages of core assembly and budding (32). A potential explanation for these discrepancies is (i) Vif and Gag are associated with glycolipid-enriched membrane domains (GEM domains), which are resistant to solubilization by TX-100 (5), or (ii) both proteins are associated with large TX-100-insoluble complexes as well as with membranes which, at least in the case of Vif, are not accurately represented in canine microsomes.
To start addressing these issues, the postnuclear supernatant of a PBS–1% TX-100 lysate of infected H9 cells was treated with a selection of detergents to a final concentration of 1%, or with 1 M NaCl, prior to centrifugation at 100,000 × g for 60 min. Figure 2 shows that the fractionation patterns of both Vif and Gag track each other in that both were solubilized by denaturing detergents (SDS and sodium deoxycholate) but not by zwitterionic {3-([3-cholamidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate (CHAPSO)} or nonionic detergents such as n-octyl β-d-glucopyranoside (octyl glucoside). Because octyl glucoside solubilizes GEM domains (5), the finding that neither Vif nor Gag was solubilized by this detergent indicates that neither protein associates with these membrane domains. The observation that both proteins were solubilized by 1 M NaCl is most consistent with their association with TX-100-insoluble complexes being mediated by protein-protein interactions. Taken together, these data suggest that Vif and p55Gag are associated with high-molecular-mass complexes that are resistant to disruption by nonionic detergents and physiological salt concentrations. At this point, however, one cannot conclude that Vif and Gag are not associated with membranes.
FIG. 2.
Solubilization of Vif and Gag. HIV-1-infected H9 cells were lysed in PBS–1% TX-100, and the nuclei were pelleted by low-speed centrifugation. Various detergents were added to aliquots of the postnuclear supernatant to a final concentration of 1%, or salt was added to a final concentration of 1 M. These were then centrifuged at 100,000 × g to separate soluble (S) from insoluble (I) fractions. Aliquots of the fractions corresponding to equal numbers of cells were examined by Western blot analysis using antibodies specific for calreticulin, HIV-1 CA, or HIV-1 Vif.
Association of Vif and Gag with cytoplasmic complexes.
Rather than restricting the separation of postnuclear supernatants into soluble and insoluble fractions, we next extended this procedure by subjecting these samples to density centrifugation in continuous sucrose density gradients. Interestingly, a similar approach has been used to describe various HIV-1 Gag-containing complexes that are formed in a cell-free system (37) or in virus-producing cells (36). Importantly, the assembly of these complexes is unaltered by either detergent addition or the removal of Gag’s amino-terminal myristoylation signal. Based on such findings and the demonstration in the cell-free system that these complexes can be “chased” into capsid-like structures (37), it has been suggested that these complexes may represent early core assembly intermediates that have not yet engaged the plasma membrane.
A cytoplasmic lysate of productively infected H9 cells was therefore loaded onto a 20 to 60% sucrose gradient, centrifuged at 150,000 × g for 2 h, and harvested as 10 fractions with densities ranging from 1.03 to 1.22 g/ml (Fig. 3A). Each fraction was then diluted and centrifuged at 100,000 × g for 60 min, and the pellets were resuspended in loading buffer for analysis by Western blotting using CA- or Vif-specific antibodies (Fig. 3B). The complexes that contained most of the fully processed CA protein presumably correspond to viral cores and were found at the bottom of the gradient (fractions 8 to 10, density range of 1.19 to 1.22 g/ml). In contrast, the peaks of both Vif and unprocessed p55Gag were found toward the middle of the gradient in fractions 3 and 4 (density range of 1.10 to 1.12 g/ml).
To determine whether the expression of Vif influences the density of these Gag complexes and whether the fractionation of Vif itself is affected by the presence of Gag, we repeated this experiment using both H9 cells infected with HIV-1/Δvif (Fig. 3C) and H9 cells that stably express Vif but no other HIV-1 gene products (Fig. 3D). The results clearly demonstrate that the fractionation pattern of neither protein influences the other and suggest that Vif and p55Gag each independently encode signals that, under the conditions used here, enable them to associate with complexes that are found in fractions with a density of 1.10 to 1.12 g/ml.
Vif is not associated with membranes.
As noted earlier, Vif expressed in vitro has been shown to interact with microsomes (27); moreover, we have also shown that Vif cofractionates with the plasma membrane in Percoll density gradients, though the binding of Vif to membranes was not directly investigated in those studies (47). Since the putative Gag complex assembly intermediates have been shown not to be associated with membranes (36, 37), and since neither TX-100 nor N-octyl glucoside was found to solubilize Vif (Fig. 2), we decided to reexamine Vif’s potential association with membranes in our Vif-expressing H9 cell line. Specifically, it might be expected that the density of complexes that are associated with membranes would increase when the membranes are removed by detergent addition; for example, the density of spherical HIV-1 virions is typically 1.12 to 1.15 g/ml but increases to 1.19 to 1.22 g/ml after removal of the lipid membrane by treatment with TX-100.
We therefore prepared a cytoplasmic extract from H9/hVif cells, using nitrogen cavitation in the absence of detergent, and then subjected it to sucrose density centrifugation as described above. Each fraction was pelleted at 100,000 × g and analyzed by Western blotting (Fig. 3E). Importantly, the profile of Vif was identical to that observed for the same cells—or HIV-1-infected H9 cells—lysed in PBS–1% TX-100 (Fig. 3B and D). In addition, when such an extract was supplemented with 1% TX-100 prior to gradient centrifugation, the pattern of fractionation was not altered (data not shown). Thus, the density of Vif-containing complexes appears to be identical regardless of the presence of 1% TX-100, a result that is most consistent with Vif not being associated with membranes.
To exclude the possibility that p55Gag and/or Vif might be associated with TX-100-insoluble membranes, we lysed infected H9 cells in PBS–1% TX-100 and added octyl glucoside to a final concentration of 1% to half of the postnuclear supernatant. Following density gradient centrifugation of both samples, both Gag and Vif were found to fractionate identically in the presence or absence of octyl glucoside (data not shown), further implying that the Vif- and Gag-containing complexes found in the 1.10- to 1.12-g/ml fractions are not associated with TX-100-insoluble membranes.
Evaluation of Vif-Gag interactions by coimmunoprecipitation.
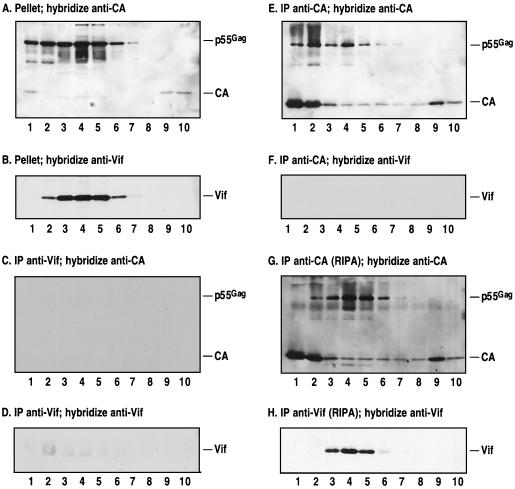
It has previously been reported that Vif can associate with the NC portion of p55Gag and that it can be coimmunoprecipitated from HIV-1-infected cells by using an NC-specific antibody (3). To examine this interaction by the approaches that we have developed, we fractionated an extract of HIV-1-infected H9 cells by sucrose density gradient centrifugation and divided the ensuing fractions into five portions of equal volume. These were then treated in the following ways: (i) dilution with cold PBS–1% TX-100 and pelleting at 100,000 × g (as for Fig. 3B), (ii) immunoprecipitation with a Vif-specific polyclonal rabbit antiserum, (iii) immunoprecipitation with a CA-specific polyclonal rabbit antiserum, (iv) immunoprecipitation with a combination of the preimmune sera that correspond to the Vif- and CA-specific sera (as a control for specificity), and (v) adjustment to 1× RIPA and immunoprecipitation with a combination of Vif- and CA-specific rabbit antisera. All samples were then resuspended in equal volumes of gel loading buffer and subjected to Western analysis using Vif- or CA-specific murine monoclonal antibodies (Fig. 4).
FIG. 4.
Coimmunoprecipitation analysis of Vif-Gag interactions, using density gradient-purified fractions of infected cell lysates. The postnuclear supernatant of HIV-1-infected H9 cells was fractionated on a sucrose density gradient, and equal portions of each fraction were either pelleted at 100,000 × g for 60 min (A and B) or incubated with protein A-conjugated agarose beads bound to rabbit polyclonal antibodies specific for HIV-1 Vif (C, D, and H) or HIV-1 CA (E, F, G, and H) for immunoprecipitation (IP). Aliquots of each sample equivalent to equal numbers of cells were resolved on SDS-polyacrylamide gels and examined by Western blot analysis using murine monoclonal antibodies specific for CA (A, C, E, and G) or Vif (B, D, F, and H).
As demonstrated above, high-speed pelleting showed that the majority of p55Gag (Fig. 4A) and Vif (Fig. 4B) cofractionated toward the middle in the gradient. Analysis of the samples that were initially treated with the Vif-specific antiserum revealed that the immunoprecipitation of Vif was extremely inefficient (compare Fig. 4D with Fig. 4B) and that any coimmunoprecipitation of Gag that may have occurred under these conditions was below the level of detection afforded by this CA-specific monoclonal antibody (Fig. 4C). In contrast, when the samples were treated with RIPA buffer prior to the addition of antibody, a significant proportion of the Vif could be immunoprecipitated (Fig. 4H); in fact, we have found that Vif can be efficiently immunoprecipitated from T-cell lysates only under conditions that result in its solubilization. We have concluded, therefore, that under the nondenaturing conditions used here, the polyclonal Vif-specific antiserum can recognize only a very small proportion of the Vif that is present in these lysates. This suggests that the majority of Vif epitopes either are occluded by other proteins present in the Vif-containing complexes or are unavailable for antibody binding as a consequence of Vif’s tertiary structure.
Unlike Vif, the majority of p55Gag was immunoprecipitated by the CA-specific polyclonal antiserum (Fig. 4E), and this was modestly enhanced by the prior adjustment of the samples to 1× RIPA buffer (Fig. 4G). Of note, fractions 1 and 2 contained a large amount of fully processed CA that was immunoprecipitated but was not pelleted (compare Fig. 4E and G with Fig. 4A); this presumably represents disrupted virion cores that remained at the top of the gradient. Importantly, when the samples that were immunoprecipitated with the CA-specific antiserum were analyzed for the presence of Vif, none could be detected (Fig. 4F). Because this particular result stands in contrast to that of others, we also tried to coimmunoprecipitate Vif with Gag from infected H9 cells by using a methodology described by others, i.e., using a cell lysis buffer that comprised 20 mM HEPES (pH 7.5), 5 mM EDTA, 150 mM NaCl, and 0.2% TX-100. However, and in keeping with our density gradient approach, we were still unable to detect a specific coimmunoprecipitation of Vif with Gag (data not shown).
To pursue the possibility of coimmunoprecipitation further, we also measured the levels of Vif and Gag that could be recovered from the peak gradient fraction (Fig. 4, fraction 4) by pelleting or by immunoprecipitation with the CA-specific polyclonal antiserum or its corresponding preimmune serum. Quantitation was achieved by Western analysis of these samples together with standard curves of purified recombinant His6-tagged CA or Vif (Fig. 5; a longer exposure of lanes 7 and 8 is shown to the right), followed by densitometric scanning of the autoradiographs (Table 1). Consistent with our earlier calculations of the ratio of Vif to Gag in infected cells (22), the high-speed pellet contained approximately half as much Vif as p55Gag. Whereas p55Gag was specifically immunoprecipitated with the CA-specific antiserum (compare lane 7 to lane 8 in the lower panel), this was not the case for Vif; indeed, low levels of Vif were nonspecifically precipitated by both the CA-specific antiserum and the preimmune serum (lanes 9 and 10, upper panel). Accordingly, once a correction for background binding was introduced into these calculations, the ratio of Vif to Gag following Gag-specific immunoprecipitation was essentially zero (Table 1). Taken as a whole, our data therefore appear to be inconsistent with the notion that there is a stable interaction between Vif and p55Gag in HIV-1-expressing cells.
FIG. 5.
Quantitation of Vif and Gag recovered by high-speed centrifugation and immunoprecipitation. The fraction 4 (Fig. 4) samples that had been either pelleted, or immunoprecipitated (IP) with anti-CA or preimmune (pre-imm.) serum were resolved on SDS-polyacrylamide gels together with dilution series of purified recombinant His6-tagged Vif and CA (which served as standard curves). The proteins were visualized by using monoclonal antibodies, chemiluminescence, and autoradiography, and the bands were quantitated by densitometry. Short (lanes 1 to 8) and long (lanes 9 and 10) exposures of the final filters are shown. Note that three times as much sample was loaded on this gel as for the gels shown in Fig. 4.
TABLE 1.
Quantitation of Vif and Gag
| Sample | Vifa (ng/sample) | Gagb (ng/sample) | No. of moleculesc (1014)
|
Vif/Gag ratiod | |
|---|---|---|---|---|---|
| Vif | Gag | ||||
| Pelleted | 5 | 11 | 19 | 36 | 0.5 |
| mImunoprecipitate; anti-CA | <0.032 | 1.5 | <0.1 | 5 | ∼0 |
| Immunoprecipitate; preimmune | <0.032 | <0.032 | <0.1 | <0.1 | ND |
Total amount of protein reactive with the Vif-specific monoclonal antibody, determined for each sample by using the dilution series of recombinant protein as the standard curve (Fig. 5), the calculated relative molecular masses of the native and recombinant proteins, and densitometry.
The total amount of protein (including p24Gag, p55Gag, and the various processing intermediates) reactive with the CA-specific monoclonal antibody, determined for each sample by using the dilution series of recombinant protein as the standard curve (Fig. 5), the calculated relative molecular masses of the native and recombinant proteins, and densitometry.
Calculated by using molecular masses, amounts of each protein, and Avogadro’s constant.
Molar ratio of Gag/Vif calculated as described in footnote c.
DISCUSSION
A consensus model for the function of HIV-1 Vif is that it acts late in the virus life cycle to enhance the infectivity of progeny virions. Consistent with this view, we had previously shown for HIV-1 (and feline immunodeficiency virus) that the Vif and Gag proteins colocalize in productively infected cells. Here, we describe experiments which start to address the underlying basis for colocalization. Using virus-producing cells, we find (i) that the majority of Vif and p55Gag are present in cytoplasmic TX-100-insoluble complexes (Fig. 1); (ii) that any treatment of cytoplasmic extracts that solubilized Vif (in other words, rendered it no longer pelletable by high-speed centrifugation) also solubilized p55Gag (Fig. 2); (iii) that both Vif and p55Gag are found associated with complexes that have a density of 1.10 to 1.12 g/ml in sucrose density gradients (Fig. 3); (iv) that Vif and Gag are targeted to these complexes independent of each other (Fig. 3); (v) that the associations of Vif and Gag with these complexes are likely mediated by protein-protein interactions (Fig. 2); and (vi) that Vif’s association with these complexes is not dependent on the method of cell disruption (Fig. 3).
Because it has been proposed that Vif binds to p55Gag (3), we also subjected these gradient-isolated complexes to coimmunoprecipitation analyses in an effort to explore this interaction further. Surprisingly, however, we were unable to obtain evidence of a specific interaction between Vif and Gag (Fig. 4 and 5). Although the reason for this discrepancy is unclear, and deserves further attention, a possible explanation might be (i) that the fraction of Vif that interacts with p55Gag and is very small and below the level of detection achieved here, though the sensitivity of the Vif-specific monoclonal antibody tends to argue against this possibility; (ii) that the interaction between Vif and Gag is very transient and could not be captured by the methods used here; or (iii) that the polyclonal CA-specific antiserum used in these experiments precludes detection of the Vif-p55Gag interaction whereas the NC-specific monoclonal antibody used by others does not. Our view of this putative interaction is, therefore, that if it does occur, it likely does so with fewer than one molecule of Vif interacting with 50 Gag molecules at any one time (Table 1). Although an interaction between Gag and Vif is not necessarily inconsistent with our previous finding that Vif interfaces with a cellular component(s) in a species-specific manner (50), the mechanistic significance of such an interaction remains to be elucidated. Moreover, the finding that HIV-1 Vif can modulate the infectivity of other retroviruses, including a number of primate lentiviruses and the oncoretrovirus murine leukemia virus (MLV) (50), suggests that a specific interaction between Vif and Gag must be with a region of Gag that is conserved among divergent retroviruses.
Little is known about the trafficking of the Gag and Gag-Pol polyproteins from the site of synthesis at the ribosomes to the plasma membrane, where, for lentiviruses and type C retroviruses, the later stages of assembly and budding occur (1, 26, 32). Recent studies of HIV-1 have suggested that Gag-containing early assembly intermediates may be formed prior to association with the plasma membrane (36, 37); the data presented in this report can be viewed as being consistent with these findings. Although such intermediates have not been detected by electron microscopy, their existence has also been inferred from in vitro assembly studies with purified proteins which demonstrate that membranes are not required for the formation of multisubunit Gag structures (8, 30, 57), from the ability of Gag mutants that are unable to bind membranes to be incorporated into assembled virions in the presence of wild type Gag (58), and from the detection of detergent resistant complexes of Gag in cells infected with MLV (16).
It has previously been shown that HIV-1 Gag possesses a bipartite membrane association signal that consists of an amino-terminal myristoyl residue and a neighboring region of positively charged amino acids. It was therefore unexpected when processed p17Gag (MA) was found to be present in postentry HIV-1 nucleoprotein complexes (7, 20, 25, 39); a conformational switch that results in the myristoyl residue becoming hidden from the surface of MA (53, 60), in conjunction with the phosphorylation of MA (6, 24, 25, 33), is thought to be important for MA’s ability to disengage the membrane and facilitate postentry events. We propose, therefore, that this putative switch is in the membrane-free conformation when unprocessed Gag is present in these putative assembly intermediates. It is also probable that these complexes are associated with cellular proteins: not only does Gag, on its own, fail to assemble into virion-like immature cores, but these assembly intermediates must also traffic to their ultimate site of destination at the plasma membrane.
A molecular model for Vif action remains elusive in the absence of defined Vif interaction partners and the uncertainty that exists regarding the significance of the reported interaction between Vif and Gag (Fig. 5). For example, does Vif transiently bind to virion components to regulate assembly, and/or does it counteract an innate cellular activity that interferes with productive virion formation? The results presented here suggest that HIV-1 Vif and p55Gag are independently associated with TX-100-insoluble cytoplasmic complexes that may represent membrane-free virion assembly intermediates. Vif therefore appears to be appropriately positioned to influence a late step in the virus life cycle and, as a result, to regulate the infectivity of progeny virions. Clearly, a critical future direction of this work will be to purify these complexes further, to identify their components, and to determine the relative stoichiometries of those components. For instance, it will be of interest to determine which host cell proteins are associated with these complexes and whether these vary in relation to the functionality of different Vif proteins in cells of different species (50). Such studies should help not only to identify the relevant cellular cofactors for Vif but also to enhance our mechanistic understanding of Vif function and, more generally, of retrovirus assembly.
ACKNOWLEDGMENTS
We thank Laurie Zimmerman for excellent secretarial support.
This work was supported by the Howard Hughes Medical Institute and Public Health Service grant AI38715 from NIAID.
REFERENCES
- 1.Bolognesi D P, Montelaro R C, Frank H, Schafer W. Assembly of type C oncornaviruses: a model. Science. 1978;199:183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- 2.Borman A M, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouyac M, Courcoul M, Bertoia G, Baudat Y, Gabuzda D, Blanc D, Chazal N, Boulanger P, Sire J, Vigne R, Spire B. Human immunodeficiency virus type 1 Vif protein binds to the pr55Gag precursor. J Virol. 1997;71:9358–9365. doi: 10.1128/jvi.71.12.9358-9365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouyac M, Rey F, Nascimbeni M, Courcoul M, Sire J, Blanc D, Clavel F, Vigne R, Spire B. Phenotypically Vif− human immunodeficiency virus type 1 is produced by chronically infected restrictive cells. J Virol. 1997;71:2473–2477. doi: 10.1128/jvi.71.3.2473-2477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 6.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell S, Vogt V M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon P M, Matthews S, Clark N, Byles E D, Iourin O, Hockley D J, Kingsman S M, Kingsman A J. Structure-function studies of the human immunodeficiency virus type 1 matrix protein, p17. J Virol. 1997;71:3474–3483. doi: 10.1128/jvi.71.5.3474-3483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazal N, Carrière C, Gay B, Boulanger P. Phenotypic characterization of insertion mutants of the human immunodeficiency virus type 1 Gag precursor expressed in recombinant baculovirus-infected cells. J Virol. 1994;68:111–122. doi: 10.1128/jvi.68.1.111-122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chazal N, Gay B, Carrière C, Tournier J, Boulanger P. Human immunodeficiency virus type 1 MA deletion mutants expressed in baculovirus-infected cells: cis and trans effects on the Gag precursor assembly pathway. J Virol. 1995;69:365–375. doi: 10.1128/jvi.69.1.365-375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen B R. HIV-1 auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorfman T, Bukovsky A, Öhagen Å, Höglund S, Göttlinger H G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J Virol. 1994;68:8180–8187. doi: 10.1128/jvi.68.12.8180-8187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorfman T, Luban J, Goff S P, Haseltine W A, Göttlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edbauer C A, Naso R B. Cytoskeleton-associated Pr65gag and retrovirus assembly. Virology. 1983;130:415–426. doi: 10.1016/0042-6822(83)90096-x. [DOI] [PubMed] [Google Scholar]
- 17.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 18.Fan L, Peden K. Cell-free transmission of Vif mutants of HIV-1. Virology. 1992;190:19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- 19.Fisher A G, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo R C, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 20.Fouchier, R. A. M., and M. H. Malim. Nuclear import of human immunodeficiency virus type-1 pre-integration complexes. Adv. Virus Res., in press. [DOI] [PubMed]
- 21.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouchier R A M, Simon J H M, Jaffe A B, Malim M H. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J Virol. 1996;70:8263–8269. doi: 10.1128/jvi.70.12.8263-8269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 25.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 26.Gelderblom H R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991;5:617–638. [PubMed] [Google Scholar]
- 27.Goncalves J, Jallepalli P, Gabuzda D H. Subcellular localization of the Vif protein of human immunodeficiency virus type 1. J Virol. 1994;68:704–712. doi: 10.1128/jvi.68.2.704-712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves J, Korin Y, Zack J, Gabuzda D. Role of Vif in human immunodeficiency virus type 1 reverse transcription. J Virol. 1996;70:8701–8709. doi: 10.1128/jvi.70.12.8701-8709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göttlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross I, Hohenberg H, Huckhagel C, Krausslich H G. N-terminal extension of human immunodeficiency virus capsid protein converts the in vitro assembly phenotype from tubular to spherical particles. J Virol. 1998;72:4798–4810. doi: 10.1128/jvi.72.6.4798-4810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harmache A, Russo P, Guiguen F, Vitu C, Vignoni M, Bouyac M, Hieblot C, Pepin M, Vigne R, Suzan M. Requirement of caprine arthritis encephalitis virus vif gene for in vivo replication. Virology. 1996;224:246–255. doi: 10.1006/viro.1996.0526. [DOI] [PubMed] [Google Scholar]
- 32.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 33.Jacqué J-M, Mann A, Sharova N, Brichacek B, Enslen H, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jowett J B, Hockley D J, Nermut M V, Jones I M. Distinct signals in human immunodeficiency virus type 1 Pr55 necessary for RNA binding and particle formation. J Gen Virol. 1992;73:3079–3086. doi: 10.1099/0022-1317-73-12-3079. [DOI] [PubMed] [Google Scholar]
- 35.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y-M, Yu X-F. Identification and characterization of virus assembly intermediate complexes in HIV-1-infected CD4+ T cells. Virology. 1998;243:78–93. doi: 10.1006/viro.1998.9064. [DOI] [PubMed] [Google Scholar]
- 37.Lingappa J R, Hill R L, Wong M L, Hegde R S. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J Cell Biol. 1997;136:567–581. doi: 10.1083/jcb.136.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luban J. Absconding with the chaperone: essential cyclophilin-Gag interaction in HIV-1 virions. Cell. 1996;87:1157–1159. doi: 10.1016/s0092-8674(00)81811-5. [DOI] [PubMed] [Google Scholar]
- 39.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberste M S, Gonda M A. Conservation of amino-acid sequence motifs in lentivirus Vif proteins. Virus Genes. 1992;6:95–102. doi: 10.1007/BF01703760. [DOI] [PubMed] [Google Scholar]
- 41.Ochsenbauer C, Wilk T, Bosch V. Analysis of vif-defective human immunodeficiency virus type 1 (HIV-1) virions synthesized in ‘non-permissive’ T lymphoid cells stably infected with selectable HIV-1. J Gen Virol. 1997;78:627–635. doi: 10.1099/0022-1317-78-3-627. [DOI] [PubMed] [Google Scholar]
- 42.Piñol-Roma S, Dreyfuss G. Transcription-dependent and transcription-independent nuclear transport of hnRNP proteins. Science. 1991;253:312–314. doi: 10.1126/science.1857966. [DOI] [PubMed] [Google Scholar]
- 43.Pleasure S J, Lee V M, Nelson D L. Site-specific phosphorylation of the middle molecular weight human neurofilament protein in transfected non-neuronal cells. J Neurosci. 1990;10:2428–2437. doi: 10.1523/JNEUROSCI.10-07-02428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reicin A S, Paik S, Berkowitz R D, Luban J, Lowy I, Goff S P. Linker insertion mutations in the human immunodeficiency virus type 1 gag gene: effects on virion particle assembly, release, and infectivity. J Virol. 1995;69:642–650. doi: 10.1128/jvi.69.2.642-650.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-acting present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 46.Sakai H, Shibata R, Sakuragi J-I, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon J H M, Fouchier R A M, Southerling T E, Guerra C B, Grant C K, Malim M H. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol. 1997;71:5259–5267. doi: 10.1128/jvi.71.7.5259-5267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon J H M, Gaddis N C, Fouchier R A M, Malim M H. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 49.Simon J H M, Malim M H. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J Virol. 1996;70:5297–5305. doi: 10.1128/jvi.70.8.5297-5305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon J H M, Miller D L, Fouchier R A M, Soares M A, Peden K W C, Malim M H. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 1998;17:1259–1267. doi: 10.1093/emboj/17.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon J H M, Southerling T E, Peterson J C, Meyer B E, Malim M H. Complementation of vif-defective human immunodeficiency virus type 1 by primate, but not nonprimate, lentivirus vif genes. J Virol. 1995;69:4166–4172. doi: 10.1128/jvi.69.7.4166-4172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sova P, Volsky D J. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with vif-negative human immunodeficiency virus type 1. J Virol. 1993;67:6322–6326. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spearman P, Wang J-J, Heyden N V, Ratner L. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J Virol. 1994;68:3232–3242. doi: 10.1128/jvi.68.5.3232-3242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Poblotzki A, Wagner R, Niedrig M, Wanner G, Wolf H, Modrow S. Identification of a region in the pr55gag polyprotein essential for HIV-1 particle formation. Virology. 1993;193:981–985. doi: 10.1006/viro.1993.1210. [DOI] [PubMed] [Google Scholar]
- 56.von Schwedler U, Song J, Aiken C, Trono D. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Schwedler U K, Stemmler T L, Klishko V Y, Li S, Albertine K H, Davis D R, Sundquist W I. Proteolytic refolding of the HIV-1 capsid protein amino-terminus facilitates viral core assembly. EMBO J. 1998;17:1555–1568. doi: 10.1093/emboj/17.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan X, Yu X, Lee T-H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou W, Resh M D. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]