Abstract
Lentivirus Vif proteins are potent regulators of virus infectivity. However, relatively little is known about the functional domains, peptide motifs, or residues of any Vif protein. In this report, we present the first extensive mutagenesis analysis of the 192-amino-acid human immunodeficiency virus type 1 (HIV-1) Vif protein. A large number of scanning missense (mostly alanine substitution) and deletion mutations were introduced into the HIV-1HXB3 vif gene, and the resulting proteins were evaluated for the induction of virus infectivity as well as subcellular localization. The results show that amino acids dispersed throughout Vif’s linear sequence are important for function. However, because many of the inactive proteins also appear to be mislocalized, we suggest that many of them may actually be misfolded rather lacking an intracellular targeting signal. Interestingly, disruptions within an internal region spanning residues 114 to 146 give rise to mutant proteins that either retain function or are inactive but are not substantially mislocalized. We therefore speculate that this region, which harbors two essential cysteine residues and one essential serine residue, may contain aspects of a putative Vif effector domain.
One of the features that distinguishes lentiviruses from prototypic oncoretroviruses is their marked genetic complexity. For example, human immunodeficiency virus type 1 (HIV-1) encodes six accessory/regulatory genes in addition to the structural and enzymatic gag, pol, and env genes that are present in all replication-competent retroviruses. The functions of three of these genes, tat, rev, and vpu, are relatively well established, while those of vif, vpr, and nef remain rather less evident (5, 7).
The consensus model for the function of Vif (viral infectivity factor) is that it acts at a late stage of the virus life cycle, such as assembly or budding, to enhance the infectivity of progeny virions 10- to 100-fold (1, 3, 9, 10, 22, 26, 31). Although the point at which vif-deficient (Δvif) infections are inhibited remains ill defined, current evidence is consistent with the postentry nucleoprotein complexes (often called preintegration complexes) of Δvif viruses being unstable and therefore subject to premature dissolution prior to provirus formation (12, 26). To date, however, the molecular events that take place in virus-producing cells and which predetermine this defect have remained elusive. In particular, biochemical analyses of wild-type and Δvif virions, and their respective producer cells, have failed to reveal any consensus differences in the virion incorporation or processing of the Gag, Pol, and Env proteins (3, 9, 20, 31). Furthermore, even though the Vif protein itself is packaged into virions (4, 9, 14, 15), this appears to be relatively inefficient, correlative with cellular expression levels, and not required for viral infectivity (4, 27).
Consistent with the model that Vif provides a critical function during virus production, confocal microscopy analyses of HIV-1- and feline immunodeficiency virus-infected cells have shown that there is substantial colocalization between Gag and Vif (24). Furthermore, we have recently demonstrated that p55Gag and Vif derived from lysates of HIV-1-infected cells cofractionate in continuous density gradients in the presence of nonionic detergent (23). Importantly, however, coimmunoprecipitation experiments failed to provide evidence to support the idea that Vif and Gag stably interact with each other (23), a finding that appears to contrast with one recent report (2). Based on these observations, we have speculated that Vif and the Gag precursor are independently targeted to a region of the cell where aspects of virion assembly can be regulated. Implicit in this model is the notion that Vif interacts with cellular components in a manner that is essential for its biological activity. Indeed, this hypothesis is supported by other data which suggest that Vif function is subject to a cell species-specific restriction (28) and that Vif acts by suppressing an innate cellular activity which inhibits the infectivity of progeny virions (25).
To understand the function of a given protein at the molecular level, an appreciation of functional domains, motifs, and residues can be of tremendous help. Somewhat surprisingly, an extensive structure-function analysis of the HIV-1 Vif protein has not yet been described. Moreover, the lack of any obvious sequence similarity between Vif and any database entry has not allowed one to predict a precise function for Vif or to identify possible functional motifs. Alignment of lentivirus Vif proteins derived from primate and nonprimate hosts has led to the recognition of a single conserved motif—(S/T)LQ(F/Y/R)LA (18)—that, at least for HIV-1, is important for biological function (33). In the work presented here, we have characterized a large panel of substitution and deletion mutants of the HIV-1 Vif protein by using both a single-cycle functional assay for virus infectivity and biochemical fractionation of virus-producing T cells. Our results show that the conserved domain of Vif is important for the function not only of HIV-1 Vif but also of the Vif protein of simian immunodeficiency virus isolated from rhesus macaques (SIVMAC). We also find that amino acid substitutions distributed throughout HIV-1 Vif are capable of disrupting function and, in many cases, normal localization. Furthermore, we find that Vif does not appear to tolerate the deletion of any region of five or six amino acids except in its carboxy terminus. Based on these findings, we have concluded that HIV-1 Vif cannot be organized into a number of discrete, independently acting functional domains. In particular, it appears that Vif may be folded such that the residues distributed throughout its sequence participate in correct subcellular localization.
MATERIALS AND METHODS
Expression vectors.
The wild-type and vif-deficient HIV-1 proviral expression vectors pIIIB and pIIIB/Δvif, as well as the HIV-1 Vif expression and control vectors pgVif and pgΔVif, have been described elsewhere (28, 29). Missense and deletion mutants of HIV-1 Vif were generated in pgVif by PCR-mediated site-directed mutagenesis, and the integrity of the resulting vif genes was confirmed by automated sequencing. Selected mutated derivatives of HIV-1 vif were rebuilt into proviral clones by replacing the NdeI fragment of pIIIB (positions 5127 to 6404) with that from the pgVif mutant vector. Mutants of SIVMAC Vif were similarly generated in the T7 epitope-tagged vector pgVif:T7SIVMAC (28). The pHIT/G vector expresses the vesicular stomatitis virus G envelope glycoprotein (8).
Functional analysis of Vif mutants.
The biological activities of mutant Vif proteins were determined by using a single-cycle infectivity assay based on the induced expression of the viral transcription trans activator Tat (9, 26). H9 cells were electroporated with pIIIB/Δvif together with pgΔVif (negative control), pgVif (positive control), or a mutated version of pgVif and then incubated at 37°C for 20 h in fresh medium. The cultures were then transferred to centrifuge tubes and centrifuged at 500 × g for 5 min. The cell pellets were washed in phosphate-buffered saline (PBS) and lysed for subsequent Western analysis, while the virus-containing supernatants were filtered through 0.45-μm-pore-size filters and quantitated for p24Gag by enzyme-linked immunosorbent assay; 5 × 105 C8166/HIV-CAT indicator cells were then challenged with normalized quantities of virus. After 24 h, the cells were lysed in 100 mM Tris-HCl (pH 7.8)–0.5% (vol/vol) Triton X-100 (TX-100) for the determination of chloramphenicol acetyltransferase (CAT) activity.
Western analyses and antibodies.
Cell fractions, whole-cell lysates, or immunoprecipitates were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose. The filters were initially hybridized with a mouse monoclonal antibody raised against HIV-1 Vif (319) (29) or HIV-1 p24Gag/CA (p24-3) (24), and bound antibodies were detected by using appropriate horseradish peroxidase-conjugated secondary antibodies raised against mouse or rabbit immunoglobulins, enhanced chemiluminescence, and autoradiography.
Transient infection of H9 cells with HIV-1.
Cells were transiently infected with HIV-1 carrying a wild-type or mutated vif gene as previously described (23). Specifically, initial high-titer stocks of pseudotyped viruses were transiently generated by transfection of 293T monolayers with the relevant pIIIB-based proviral vector and pHIT/G (28). After 24 h, virus-containing supernatants were harvested, clarified by centrifugation at 500 × g for 5 min, filtered through 0.45-μm-pore-size filters, and used to infect 107 H9 cells. After 4 h, the cells were washed in PBS three times to remove input viruses and incubated in fresh medium at 37°C for a further 20 h. The cells were then washed two additional times and incubated in fresh medium for 24 h. At this point, the cultures were centrifuged at 500 × g for 5 min, and the cells were washed in PBS and lysed in preparation for fractionation.
Subcellular fractionation of virus-expressing cells.
Fractionations were performed as described elsewhere (23). Briefly, cells were lysed by incubation in PBS–1% TX-100 for 10 min on ice. Nuclei were then pelleted by centrifugation at 1,000 × g for 10 min at 4°C and lysed in radioimmunoprecipitation assay (RIPA) buffer (0.1% SDS, 1% TX-100, 1% sodium deoxycholate, 150 mM NaCl, 10 mM Tris-HCl [pH 7.5], 1 mM EDTA). The TX-100-soluble and -insoluble fractions of the postnuclear supernatants were separated by centrifugation at 100,000 × g for 60 min in a TLA 100.2 rotor. The resulting pellet was redissolved in 1× RIPA buffer (TX-100-insoluble fraction), and the soluble supernatants were adjusted to 1× RIPA buffer. Importantly, all three fractions were made up to the same final volume.
Alternatively, the postnuclear supernatants were subjected to density gradient centrifugation at 150,000 × g for 2 h in 20 to 60% continuous sucrose gradients. Following collection as 10 1-ml samples, the fractions were diluted fourfold with cold PBS and centrifuged at 100,000 × g as described above, and the pellets were resuspended in gel loading buffer. Alternatively, an aliquot of each fraction was adjusted to 1× RIPA and subjected to immunoprecipitation using a rabbit polyclonal Vif-specific antiserum and protein A-conjugated agarose beads.
RESULTS
Generation and functional analysis of mutant HIV-1 Vif proteins.
A scanning mutagenesis analysis of the HIV-1 Vif protein has not been reported. As an approach to identify critical regions of this protein, and thereby to gain further insight into its mechanism of action, a series of amino acid substitution and in-frame deletion mutations were introduced into the vif gene of HIV-1HXB3 (Tables 1 and 2). Residues that have previously been shown to be critical for function (16, 33), that are conserved among primate lentivirus Vif proteins (18), or that are well conserved between diverse HIV-1 isolates (32) were chosen as the primary sites for disruption. In many cases, a unique SacII restriction site was introduced into the vif gene of the pgVif expression vector such that two or three residues were replaced with alanines. In addition, the use of a single restriction site facilitated the subsequent construction of a series of in-frame deletion mutations.
TABLE 1.
Site-directed mutagenesis of the HIV-1 Vif proteina
| Name | Position | Mutation | Relative infectivity | SD |
|---|---|---|---|---|
| M1 | 5–6 | WQ→AA | 56 | 11 |
| M2 | 12–13 | QV→AA | 115 | 17 |
| M38 | 16–18 | MRI→AAA | 71 | 16 |
| M3 | 22–23 | RL→AA | 86 | 10 |
| M20 | 29–31 | MYI→AAV | 41 | 14 |
| M4 | 33–34 | RK→AA | 65 | 18 |
| M21 | 38–40 | WFY→AAA | 6 | 5 |
| M5 | 43–44 | HY→AA | 5 | 1 |
| M22 | 53–54 | SE→AA | 61 | 22 |
| M6 | 58–59 | PL→AA | 55 | 18 |
| M23 | 69–70 | YW→AA | 3 | 3 |
| M7 | 73–74 | HT→AA | 116 | 23 |
| M24 | 80–81 | HL→AA | 81 | 8 |
| M8 | 86–87 | SI→AA | 58 | 7 |
| M25 | 90–92 | RKK→AAA | 118 | 55 |
| M9 | 97–98 | QV→AA | 73 | 6 |
| M26 | 105–107 | QLI→AAV | 2 | 1 |
| M10 | 111–112 | YF→AA | 7 | 7 |
| M18 | 114 | C→S | 2 | 1 |
| M27 | 121–123 | RNT→AAA | 135 | 7 |
| M11 | 127–128 | RI→AA | 90 | 21 |
| M19 | 133 | C→S | 5 | 1 |
| M28 | 135–136 | YQ→AA | 27 | 4 |
| M12 | 140–141 | NK→AA | 32 | 6 |
| M29 | 144–146 | SLQ→AAA | 7 | 6 |
| M13 | 147–148 | YL→AA | 60 | 4 |
| M39 | 156–158 | PKQ→AAA | 103 | 51 |
| M30 | 157 | K→A | 114 | 23 |
| M40 | 158–160 | QIK→AAA | 91 | 17 |
| M31 | 160 | K→A | 67 | 7 |
| M32 | 161 | P→A | 73 | 16 |
| M33 | 162 | P→A | 99 | 36 |
| M34 | 163 | L→A | 74 | 4 |
| M14 | 161–163 | PPL→AAA | 3 | 1 |
| M35 | 164 | P→A | 37 | 20 |
| M41 | 161–164 | PPLP→APLA | 12 | 13 |
| M36 | 165 | S→A | 33 | 6 |
| M37 | 166 | V→A | 80 | 35 |
| M15 | 169–170 | LT→AA | 58 | 6 |
| M16 | 180–181 | TK→AA | 95 | 11 |
| M17 | 189–190 | MW→AA | 96 | 10 |
| HIV-1/Δvif | 26–192 | NA | 1 | |
| HIV-1 | NA | NA | 100 |
The positions, identities, and nomenclature of the amino acid substitutions that were introduced into the Vif protein of HIV-1HXB3 are listed together with their relative abilities to confer infectivity on a vif-deficient HIV-1. Vif function was determined by cotransfection of H9 cells with pIIIB/Δvif, virus harvest and normalization, and single-cycle infectivity assays using C8166/HIV-CAT indicator cells. The mean infectivity values (and their standard deviations) are set against values of 100 for wild-type Vif and 1 for the negative control (Δvif) following correction for background CAT activity (mock infection). In most cases, the data reflect at least three independent experiments; the exceptions are M24, M27, M39, M30, M41, M36, M16, and M17, which were each tested twice. NA, not applicable.
TABLE 2.
In-frame deletion mutants of HIV-1 Vifa
| Name | Deleted region | Substitution | Relative infectivity |
|---|---|---|---|
| Δ1 | 5–13 | AA | 5 |
| Δ29 | 12–18 | AAA | 5 |
| Δ2 | 12–23 | AA | 5 |
| Δ30 | 16–23 | AA | 8 |
| Δ19 | 22–31 | AAV | 4 |
| Δ3 | 22–34 | AA | 6 |
| Δ20 | 29–34 | AA | 3 |
| Δ4 | 33–44 | AA | 2 |
| Δ21 | 53–59 | AA | 6 |
| Δ5 | 43–59 | AA | 7 |
| Δ6 | 58–74 | AA | 2 |
| Δ7 | 73–87 | AA | 1 |
| Δ22 | 86–92 | AAA | 4 |
| Δ8 | 86–98 | AA | 1 |
| Δ23 | 90–98 | AA | 5 |
| Δ9 | 97–112 | AA | 1 |
| Δ10 | 111–128 | AA | 1 |
| Δ24 | 121–128 | AA | 2 |
| Δ11 | 127–141 | AA | 4 |
| Δ25 | 135–141 | AA | 4 |
| Δ26 | 140–146 | AAA | 5 |
| Δ12 | 140–148 | AA | 2 |
| Δ27 | 144–148 | AA | 4 |
| Δ31 | 147–158 | AAA | 1 |
| Δ13 | 147–163 | AAA | 6 |
| Δ32 | 156–160 | AAA | 2 |
| Δ33 | 158–163 | AAA | 5 |
| Δ14 | 163–170 | AA | 2 |
| Δ15 | 169–181 | AA | 19 |
| Δ28 | 169–190 | AA | 30 |
| Δ16 | 180–190 | AA | 66 |
| HIV-1/Δvif | 26–192 | NA | 1 |
| HIV-1 | NA | NA | 100 |
The positions, introduced amino acids, and nomenclature of the deletions that were introduced into the Vif protein of HIV-1HXB3 are listed together with their relative abilities to confer infectivity on a vif-deficient HIV-1. Vif function was evaluated as for Table 1. NA, not applicable.
To test the various mutant Vif proteins for function, nonpermissive H9 cells (the production of infectious virions from these cells requires Vif) were cotransfected with each of the pgVif-derived vectors and the vif-deficient provirus expression vector pIIIB/Δvif. Importantly, this two-plasmid strategy allowed us to discount any indirect effects on viral infectivity that might have resulted from mutations in vif influencing proviral sequences with other functions, such as the overlapping pol and vpr genes. Resultant viruses were then harvested at 20 h, normalized according to p24Gag content, and used in challenges of C8166/HIV-CAT indicator cells. The positive and negative control viruses were produced by cotransfection of pIIIB/Δvif with pgVif and pgΔVif, respectively; of note, we found that the infectivity of virus produced by cotransfection of pIIIB/Δvif with pgVif was identical to that of wild-type (Vif-expressing) virus produced by cotransfection of pIIIB and a negative control vector (data not shown).
Since the induction of infectivity of HIV-1/Δvif by wild-type Vif ranged from 5- to 20-fold over the negative control in different experiments, and because we wished to compare several different data sets with each other, we assigned arbitrary infectivity values of 1 and 100 to the negative (pIIIB/Δvif plus pgΔVif) and positive (pIIIB/Δvif plus pgVif) controls, respectively. Tables 1 and 2 show the relative activities of each of the mutant Vif proteins, as an average of several separate experiments. The results demonstrate that missense mutations distributed throughout the linear sequence of HIV-1 Vif can disrupt function (summarized in Fig. 1) and that deletion mutations at all tested locations, except in the carboxy-terminal region, can abrogate function (Table 2). Importantly, Western analyses of whole-cell lysates derived from these (cotransfected) virus-producing H9 cultures confirmed that all mutant proteins were expressed and that their relative levels of accumulation did not correlate with activity (data not shown).
FIG. 1.
Site-directed mutagenesis of the HIV-1 Vif protein. The primary amino acid sequence of HIV-1HXB3 Vif is shown together with the missense mutations that were introduced (boxes for multiple substitutions and ovals for single substitutions). Black backgound, 1 to 15% activity compared to wild-type Vif; gray background, 15 to 50% activity; white background, greater than 50% activity (Table 1). In the case of residues 161 to 163, substitution of all three in M14 severely inhibited function whereas changes of the individual amino acids were relatively inconsequential.
One attribute that nonfunctional mutant proteins may display is the ability to suppress the activity of their cognate wild-type protein. Indeed, such dominant negative (also termed trans-dominant) mutants have previously been described for the HIV-1 accessory/regulatory proteins Rev and Tat (17, 21). To determine whether any of our mutant Vif proteins that had less than 15% of wild-type activity had a dominant negative phenotype, we tested the infectivities of viruses derived by cotransfection of H9 cells with pIIIB/Δvif, wild-type pgVif, and each of the mutated pgVif vectors (1 to 5 ratio of wild type to mutated vectors). All viruses produced in this manner had infectivities that were indistinguishable from that of virus produced in the presence of the wild-type protein alone (data not shown). As an additional test of potential dominant negative behavior, the various inactive Vif proteins were also coexpressed in permissive CEM-SS cells (infectious virion production by these cells does not require Vif) together with HIV-1/Δvif; as with the H9 studies, no inhibition of viral infectivity was observed for any of these proteins (data not shown).
Subcellular localization of nonfunctional mutants of Vif.
We have previously shown that Vif and p55Gag are associated with cytoplasmic complexes that are resistant to solubilization with nonionic detergents (23). Consistent with immunofluorescence studies which demonstrated that Vif and Gag colocalize in the cytoplasm of virally infected cells (24), these Vif- and Gag-containing complexes cofractionate in continuous sucrose density gradients (23). Furthermore, it was also noted that Vif and Gag are localized to these complexes independent of each other, most likely via protein-protein interactions. To gain a better appreciation of the molecular bases for some of the loss-of-function phenotypes observed, we decided to determine the patterns of subcellular localization for a subset of our mutant Vif proteins by using a procedure which yields a nuclear sample, as well as TX-100-soluble and -insoluble cytoplasmic fractions (Fig. 2). Hypothetical outcomes for such experiments include the identification of (i) mutations which alter localization and would potentially have affected an intracellular targeting signal or (ii) mutations which do not influence localization and would potentially have disrupted effector function. In addition to analyzing various missense mutants, and since the carboxy terminus has previously been implicated as a membrane targeting signal in HIV-1 Vif (11, 13), we also evaluated the Δ12, Δ13, and Δ14 deletion mutants (Table 2). Because of the very poor transfection efficiencies that can be attained with H9 cells and the significant degree of cell death imparted by electroporation, it proved to be infeasible to perform fractionation studies using cells transiently cotransfected with pIIIB/Δvif- and pgVif-derived vectors (data not shown). We circumvented this problem by introducing the pertinent mutant vif alleles (none of which affected pol or vpr) into pIIIB; this made it possible to generate initial high-titer virus stocks in 293T cells (a permissive cell line) and then to use them to infect H9 cells at high efficiency and render them virus producers amenable to these biochemical studies. Importantly, this strategy also provided an internal standard for fractionation in that the H9 cultures were also expressing Gag.
FIG. 2.
Subcellular localization of Vif and Gag in H9 cells infected with wild-type (WT) or vif mutant viruses. Cells were infected with virus, maintained for 20 h, and separated into nuclear (N), TX-100-insoluble (I), and TX-100-soluble (S) fractions. All samples were analyzed by Western blotting using Gag-specific (upper panels) or Vif-specific (lower panels) monoclonal antibodies. Representative gels for each of the four different fractionation profiles are shown together with the Vif proteins that localized with each of those patterns and their relative activities (Table 1); these gels were obtained with M10, M23, M32, and wild-type Vif. Note that the Gag proteins (in particular, p55Gag and CA) fractionated identically regardless of which Vif protein was coexpressed.
For all of the mutant viruses tested, the fractionation patterns of p55Gag and its processed products were identical to those of wild-type virus (Fig. 2, upper panels; and data not shown). This was to be expected since we have previously shown that coexpression of Vif with Gag does not appear to influence the subcellular localization of Gag (23). When the filters were reprobed with a Vif-specific antibody, four basic fractionation patterns were observed (Fig. 2, lower panels; and data not shown): primary localization to the TX-100-insoluble fraction (right-hand panel), approximately equal division between the soluble and insoluble fractions (center-right panel), predominant localization to the soluble fraction (center-left panel), or almost entire localization to this fraction (left-hand panel). In no cases, however, did we find substantial fractionation with the nuclear pellets.
Wild-type Vif, as described previously, localized mostly to the TX-100-insoluble fraction. Indeed, all functional and partially functional mutants that were examined displayed either this pattern of localization or a relatively even division between this fraction and the TX-100-soluble fraction. In other words, none of the biologically active Vif proteins localized predominantly to the detergent-soluble fraction; we have interpreted this observation as suggesting that targeting to the detergent-insoluble fraction is important for Vif function. In contrast, we identified nonfunctional Vif proteins that fractionated with each of the four different patterns. Because the amino acid alterations that prevent accumulation in the TX-100-insoluble fraction are distributed throughout Vif’s primary sequence, it appears that HIV-1 Vif’s localization signal is conformationally sensitive and may be formed by noncontiguous residues derived from more than one region of the protein. With respect to the proline-rich element at residues 161 to 164 (Fig. 1), even though the M14 and M41 proteins were severely mislocalized, the finding that the more extensive Δ13 mutant (deletion of residues 147 to 163) still localized like wild-type Vif suggests that this region does not mediate targeting to the detergent-insoluble fraction.
Having constructed a number of proviruses that carry disrupted vif genes, we also wished to determine whether the phenotypes of a selection of the mutant Vif proteins were maintained in the context of spreading virus infections. Virus stocks derived from transfected 293T cells were used to challenge nonpermissive H9 cells and permissive CEM-SS cells, and virus production was monitored over time as the accumulation of p24Gag in the culture supernatants. Consistent with the single-cycle cotransfection assays (Table 1), M21, M23, M10, and M29 each failed to support spreading infections in H9 cells whereas M32 appeared to be fully functional; in contrast, all five viruses replicated efficiently in CEM-SS cells (data not shown).
Fractionation using continuous sucrose density gradients.
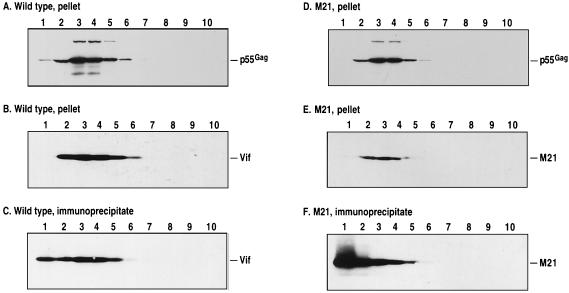
In addition to colocalizing in TX-100-insoluble cytoplasmic complexes, Vif and p55Gag also cosediment in sucrose density gradients (23). Accordingly, and as a more rigorous examination of the subcellular localization of certain mutant Vif proteins, the postnuclear supernatants of H9 cells infected with HIV-1 expressing wild-type Vif or the inactive M21, M5, M29, Δ12, or Δ13 proteins were fractionated on 20 to 60% sucrose gradients. Following fractionation, all samples were subjected to centrifugation at 100,000 × g for 60 min to pellet complexes or adjusted to 1× RIPA buffer, and the Vif proteins were immunoprecipitated with a rabbit polyclonal antiserum. All samples were then analyzed by Western blotting using Vif- or Gag-specific monoclonal antibodies (Fig. 3).
FIG. 3.
Sedimentation of Vif and Gag in continuous sucrose density gradients. Postnuclear supernatants of H9 cells prepared as for Fig. 2 were loaded onto 20 to 60% (wt/vol) sucrose gradients, centrifuged at 150,000 × g for 2 h, and fractionated into 1-ml samples. Each fraction was either subjected to high-speed centrifugation at 100,000 × g for 60 min to pellet complexes (A, B, D, and E) or adjusted to 1× RIPA and the wild-type and M21 Vif proteins were immunoprecipitated with a Vif-specific antiserum raised in rabbit (C and F). All samples were analyzed by Western blotting as for Fig. 2. The identities of the detected proteins are indicated to the right of each panel.
As previously demonstrated (23) and consistent with the results obtained with the three-fraction approach (Fig. 2), the localization of Gag was unaffected by the presence of any Vif protein and, therefore, peaked in fractions 2 to 5 of these gradients (Fig. 3A and D and data not shown). Importantly, these same fractions also contained the majority of the wild type, M5, M29, Δ12 and Δ13 proteins that were recoverable by either high speed centrifugation (Fig. 3B and data not shown) or immunoprecipitation (Fig. 3C and data not shown). That low levels of these proteins were also detected at the tops of the gradients by immunoprecipitation (Fig. 3C) was to be expected since small proportions of these proteins are always present in TX-100-soluble forms (Fig. 2). The analysis of the M21-containing gradient confirmed the mislocalization phenotype of this protein. Specifically, even though the pelleting of fractions revealed a peak of M21 that coincided with Gag (Fig. 3D and E), immunoprecipitation revealed not only that this peak represented a small proportion of the total amount of M21 that was present but also that the majority of this protein was found at the top of the gradient (Fig. 3F). Based on these results, we have concluded that the ability of any given Vif protein to localize to TX-100-insoluble complexes correlates with p55Gag colocalization in density gradients.
Functional analysis of mutated SIVMAC Vif proteins.
On the basis of the above results, we decided to analyze in greater detail the functional importance of two regions of Vif, namely, the 144SLQ and 161PPXP motifs of the HIV-1 protein. The 144SLQ region is well conserved among divergent lentivirus Vif proteins (18) (Fig. 4A) and yields inactive mutant proteins that localize like wild-type Vif when disrupted in the HIV-1 background (mutants M29 and Δ12) (Tables 1 and 2). The proline-rich motif at positions 161 to 164 is conserved among divergent HIV-1 isolates (Fig. 4B), bears some similarity to other proline-rich sequences that mediate protein-protein interactions (30), and can also yield nonfunctional proteins when mutated (M14 and M41) (Table 1). Even though this precise motif is not present in other lentivirus Vif proteins, the HIV-2 and SIVMAC proteins do harbor two closely positioned prolines toward their carboxy termini (Fig. 4B).
FIG. 4.
Sequence alignments of the serine-leucine-glutamine (SLQ) (A) and proline-rich elements (B) of HIV-1 Vif and other lentivirus Vif proteins. The positions at which alanine substitutions were introduced into HIV-1 Vif (A, M29; B, M14 and M41) or SIVMAC Vif (A, SM1; B, SM2) are indicated. SIVAGM/TAN, SIV isolated from a tantalus African green monkey; FIV, feline immunodeficiency virus. Consensus sequences for the proline-rich elements of the A, B, D, and O clades of HIV-1 are also shown.
To evaluate the significance of these two elements in the context of the SIVMAC Vif protein, we constructed the SM1 and SM2 alanine substitution mutant forms of the pgVif:T7SIVMAC vector. The biological activity of each protein was determined by cotransfection of H9 cells with pIIIB/Δvif and infectivity measurements using C8166/HIV-CAT indicator cells (Fig. 5); the pgVif:T7HIV-1 and pgVif:T7SIVMAC vectors served as positive controls, and pgΔVif was used as the negative control (28). The SM1 mutant protein displayed a level of activity that was less than 10% of that of either of the wild-type proteins, whereas the SM2 protein clearly retained wild-type levels of activity. It therefore appears that the SLQ motif of Vif participates in the conserved function of this family of proteins, whereas the carboxy-terminal proline-rich elements of primate lentiviral Vif proteins do not. Given that the M29 and Δ12 mutant variants of HIV-1 Vif both localize identically to the wild-type protein in infected cells (Fig. 2 and 3), we speculate that the SLQ motif may contribute directly to the effector function of Vif.
FIG. 5.
Functional analysis of the SLQ and proline-rich sequences in SIVMAC Vif. The indicated wild-type and mutant Vif expression vectors were cotransfected into H9 cells with pIIIB/Δvif, and the resultant viruses were tested for infectivity in C8166/HIV-CAT indicator cells (Table 1).
DISCUSSION
In an effort to identify functional residues and motifs in the Vif protein of HIV-1, we have generated a number of scanning alanine missense and in-frame deletion mutant proteins. These were all evaluated for function in a trans-activation assay in which a vif-deficient mutant of HIV-1 served as the substrate and infectivities were measured within 24 h of viral challenge. A number of the alanine substitution mutants displayed substantial losses of activity (Table 1 and Fig. 1), whereas all of the deletion mutants, with the exception of carboxy-terminal truncations, were essentially inactive (Table 2). Based on these results, we are currently unable to organize HIV-1 Vif into a straightforward domain structure.
As noted earlier, relatively few mutations in HIV-1 vif have been described. Of those that have been reported, the critical roles played by the cysteine residues at positions 114 and 133 as well as the serine at position 144 are confirmed by our results (16, 33) (Fig. 1); indeed, the importance of 144S is further underscored by the finding that the analogous region in the Vif protein of SIVMAC is essential for activity (Fig. 4 and 5). It has also been reported that the combined action of a number of positively charged amino acids toward Vif’s carboxy terminus contributes to function (2, 11, 13). Although the substitution of the lysines at positions 157 and 160 had no effect on activity (Fig. 1), we did find that the deletion of residues 169 to 190, which removes five basic amino acids, resulted in an ∼70% loss of function (Table 2). Interestingly, a carboxy-terminal truncation of Vif to residue 173 (a deletion of residues 174 to 192) has been shown to have no effect on virus replication in nonpermissive cells when introduced into an infectious molecular clone (19). Although this might appear to be inconsistent with the results of single-cycle studies which have implied that the carboxy terminus participates in Vif function (11, 13) (Table 2), these experimental configurations are not necessarily directly comparable. Thus, the role(s) of positively charged residues in this region remains an open question.
In addition to determining function in terms of the induction of infectivity, we assessed the subcellular localization patterns of many mutant proteins in H9 cells in the presence of all other viral proteins (Fig. 2 and 3). Many of the inactive mutants that were examined mislocalized such that significant amounts of those proteins were present in the TX-100-soluble fraction of a postnuclear supernatant (Fig. 2). The mutations that resulted in mislocalization were scattered throughout Vif’s primary sequence, which suggests that many may have disrupted secondary or tertiary structure as opposed to a specific targeting signal. In contrast, the functional mutants that were tested all localized substantially to the TX-100-insoluble fraction (Fig. 2), a finding which suggests that targeting to this region(s) of the cell may be important for Vif function. In summary, however, we feel that a better understanding of these results requires detailed information on the tertiary structure of Vif.
As discussed, the function of Vif during the late stages of the HIV-1 life cycle remains ill defined at the molecular level, though recent experiments are consistent with the notion that Vif acts to suppress an innate cellular antiviral activity (25). Whatever the precise function of Vif turns out to be, the identification of the residues involved in effector function will be critical to a full appreciation of Vif’s mechanism of action. A potential prediction for such a domain is that its disruption would inhibit function but, perhaps, not affect subcellular localization. Based on the results presented here, the region spanning residues 114 to 146 may fulfill these limited criteria. Specifically, all missense mutations in this region either have full or substantial activity (M27, M11, M28, and M12) or have essentially no activity but still localize to the TX-100-insoluble fraction (M18, M19, M29, and Δ12). These results should aid future structure-function analyses of HIV-1 Vif by providing an initial framework of mutants that can be considered during the design of more targeted mutagenesis studies.
In addition to examining the infectivity phenotypes of our various mutant HIV-1 Vif proteins, we also tested them for potential dominant negative behavior. However, despite numerous attempts, we failed to identify any such mutant. Interestingly, a very recent report from D’Aloja et al. described a variant of the HIV-1 vif gene that does inhibit wild-type virus replication when expressed in trans in either permissive or nonpermissive cells (6). This mutated gene harbors ∼14 amino acid changes with respect to the Vif proteins of typical T-cell line-adapted isolates. The future analysis of the mechanism of inhibition to replication by this protein as well as its phenotype in the context of the assay systems discussed here are of considerable interest.
ACKNOWLEDGMENTS
We thank Laurie Zimmerman for excellent secretarial support.
This work was supported by the Howard Hughes Medical Institute and Public Health Service grant AI38715 from NIAID.
REFERENCES
- 1.Borman A M, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouyac M, Courcoul M, Bertoia G, Baudat Y, Gabuzda D, Blanc D, Chazal N, Boulanger P, Sire J, Vigne R, Spire B. Human immunodeficiency virus type 1 Vif protein binds to the pr55Gag precursor. J Virol. 1997;71:9358–9365. doi: 10.1128/jvi.71.12.9358-9365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouyac M, Rey F, Nascimbeni M, Courcoul M, Sire J, Blanc D, Clavel F, Vigne R, Spire B. Phenotypically Vif− human immunodeficiency virus type 1 is produced by chronically infected restrictive cells. J Virol. 1997;71:2473–2477. doi: 10.1128/jvi.71.3.2473-2477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camaur D, Trono D. Characterization of human immunodeficiency virus type 1 Vif particle incorporation. J Virol. 1996;70:6106–6111. doi: 10.1128/jvi.70.9.6106-6111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen B R. HIV-1 auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 6.D’Aloja P, Olivetta E, Bona R, Nappi F, Pedacchia D, Pugliese K, Ferrari G, Verani P, Federico M. gag, vif, and nef genes contribute to the homologous viral interference induced by a nonproducer human immunodeficiency virus type 1 (HIV-1) variant: identification of novel HIV-1-inhibiting viral protein mutants. J Virol. 1998;72:4308–4319. doi: 10.1128/jvi.72.5.4308-4319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 8.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouchier R A M, Simon J H M, Jaffe A B, Malim M H. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J Virol. 1996;70:8263–8269. doi: 10.1128/jvi.70.12.8263-8269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goncalves J, Jallepalli P, Gabuzda D H. Subcellular localization of the Vif protein of human immunodeficiency virus type 1. J Virol. 1994;68:704–712. doi: 10.1128/jvi.68.2.704-712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalves J, Korin Y, Zack J, Gabuzda D. Role of Vif in human immunodeficiency virus type 1 reverse transcription. J Virol. 1996;70:8701–8709. doi: 10.1128/jvi.70.12.8701-8709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves J, Shi B, Yang X, Gabuzda D. Biological activity of human immunodeficiency virus type 1 Vif requires membrane targeting by C-terminal basic domains. J Virol. 1995;69:7196–7204. doi: 10.1128/jvi.69.11.7196-7204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Wu X, Newman M, Shaw G M, Hahn B H, Kappes J C. The Vif protein of human and simian immunodeficiency viruses is packaged into virions and associates with viral core structures. J Virol. 1995;69:7630–7638. doi: 10.1128/jvi.69.12.7630-7638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X-Y, Sova P, Chao W, Volsky D J. Cysteine residues in the Vif protein of human immunodeficiency virus type 1 are essential for viral infectivity. J Virol. 1994;68:1714–1720. doi: 10.1128/jvi.68.3.1714-1720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malim M H, Böhnlein S, Hauber J, Cullen B R. Functional dissection of the HIV-1 Rev trans-activator-derivation of a trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 18.Oberste M S, Gonda M A. Conservation of amino-acid sequence motifs in lentivirus Vif proteins. Virus Genes. 1992;6:95–102. doi: 10.1007/BF01703760. [DOI] [PubMed] [Google Scholar]
- 19.Ochsenbauer C, Bosch V, Oelze I, Wieland U. Unimpaired function of a naturally occurring C terminally truncated vif gene product of human immunodeficiency virus type 1. J Gen Virol. 1996;77:1389–1395. doi: 10.1099/0022-1317-77-7-1389. [DOI] [PubMed] [Google Scholar]
- 20.Ochsenbauer C, Wilk T, Bosch V. Analysis of vif-defective human immunodeficiency virus type 1 (HIV-1) virions synthesized in ‘non-permissive’ T lymphoid cells stably infected with selectable HIV-1. J Gen Virol. 1997;78:627–635. doi: 10.1099/0022-1317-78-3-627. [DOI] [PubMed] [Google Scholar]
- 21.Pearson L, Garcia J, Wu F, Modesti N, Nelson J, Gaynor R. A transdominant tat mutant that inhibits tat-induced gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci USA. 1990;87:5079–5083. doi: 10.1073/pnas.87.13.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai H, Shibata R, Sakuragi J-I, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon J H M, Carpenter E A, Fouchier R A M, Malim M H. Vif and the p55Gag polyprotein of human immunodeficiency virus type 1 are present in colocalizing membrane-free cytoplasmic complexes. J Virol. 1999;73:2667–2674. doi: 10.1128/jvi.73.4.2667-2674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon J H M, Fouchier R A M, Southerling T E, Guerra C B, Grant C K, Malim M H. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol. 1997;71:5259–5267. doi: 10.1128/jvi.71.7.5259-5267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon J H M, Gaddis N C, Fouchier R A M, Malim M H. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 26.Simon J H M, Malim M H. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J Virol. 1996;70:5297–5305. doi: 10.1128/jvi.70.8.5297-5305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon J H M, Miller D L, Fouchier R A M, Malim M H. Virion incorporation of human immunodeficiency virus type-1 Vif is determined by intracellular expression level and may not be necessary for function. Virology. 1998;248:182–187. doi: 10.1006/viro.1998.9296. [DOI] [PubMed] [Google Scholar]
- 28.Simon J H M, Miller D L, Fouchier R A M, Soares M A, Peden K W C, Malim M H. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 1998;17:1259–1267. doi: 10.1093/emboj/17.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon J H M, Southerling T E, Peterson J C, Meyer B E, Malim M H. Complementation of vif-defective human immunodeficiency virus type 1 by primate, but not nonprimate, lentivirus vif genes. J Virol. 1995;69:4166–4172. doi: 10.1128/jvi.69.7.4166-4172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudol M. The WW module competes with the SH3 domain? Trends Biochem Sci. 1996;21:161–163. [PubMed] [Google Scholar]
- 31.von Schwedler U, Song J, Aiken C, Trono D. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieland U, Hartmann J, Suhr H, Salzberger B, Eggers H J, Kühn J E. In vivo genetic variability of the HIV-1 vif gene. Virology. 1994;203:43–51. doi: 10.1006/viro.1994.1453. [DOI] [PubMed] [Google Scholar]
- 33.Yang X, Gonclaves J, Gabuzda D. Phosphorylation of Vif and its role in HIV-1 replication. J Biol Chem. 1996;271:10121–10129. doi: 10.1074/jbc.271.17.10121. [DOI] [PubMed] [Google Scholar]