Abstract
L-carnitine, through its antioxidant potential, plays a significant role in reducing ROS production in male genital tract; therefore, fundamental improvements in spermatogenesis process and sperm structural and functional parameters in seminal plasma can be observed by treatment with L-carnitine. A literature search was performed using PubMed (including Medline) from the database earliest inception to 2021. Eligibility criteria included studies on protective effects of L-carnitine against damages to the male reproductive system. Based on the findings of the current study, L-carnitine has an effective potential to protect testis and improve conventional and functional sperm parameters against ROS-induced damages by sperm cryopreservation, busulfan treatment, and radiation.
Keywords: Busulfan, L-carnitine, Male fertility, Radiation, Sperm cryopreservation
Introduction
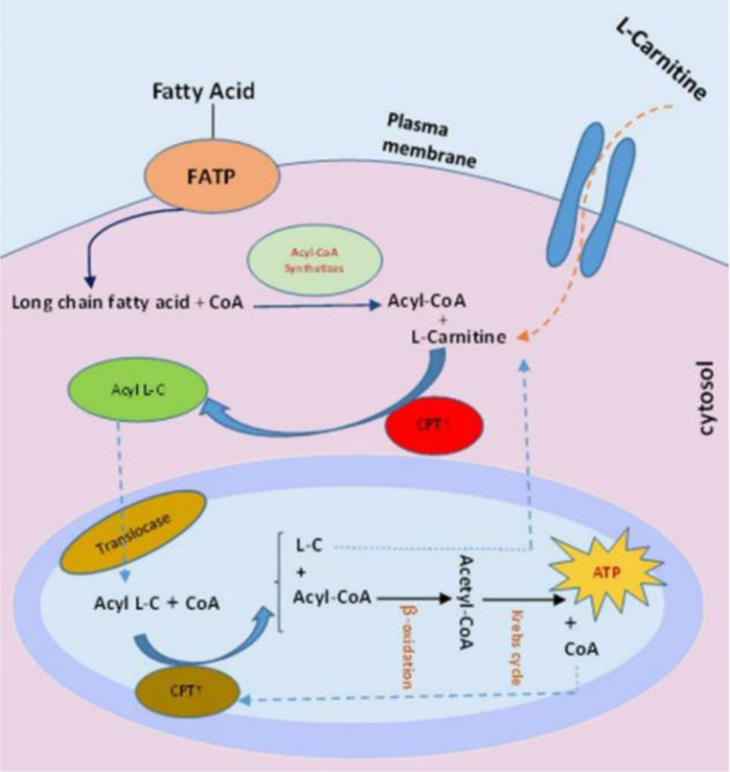
L-carnitine as a hydrophilic quaternary ammonium cation can be obtained from exogenous sources such as poultry, fish, and meat or endogenous sources like organs that are metabolically active such as kidney, brain, liver, and skeletal, cardiac, and reproductive systems (1–4). Exogenous dietary sources provide nearly 75% of human body's L-carnitine reserves, while only 25% of L-carnitine is synthesized de novo from essential amino acids of methionine and lysine (5). In human body’s cells, L-carnitine as an essential component has a significant role in transferring long-chain fatty acids through internal membrane of mitochondria for β-oxidation process and energy production (1, 2, 6). Besides, it can modulate the acyl-coA/coA ratio and reduce the acyl groups toxicity by excreting carnitine esters (7, 8) (Figure 1). L-carnitine has also anti-oxidant and antiradical properties that can protect the cell membrane, mitochondria, and DNA integrity against oxidative damages (9–11).
Figure 1.
The role of L-carnitine in sperm metabolism (CPT1: carnitine palmitoyltransferase 1, CPT2: carnitine palmitoyltransferase 2)
In male human genital tract, L-carnitine is concentrated in the epididymis, spermatozoa, and testis. In epididymal fluid, L-carnitine concentration is at the highest level, approximately 2000 times higher than in the blood (12). Epithelial cells of epididymis absorb L-carnitine from the blood and secrete it in epididymal lumen by a specific active transport system. Then, L-carnitine goes through the spermatozoa by passive diffusion and it is amalgamated in free and acetylated forms (13, 14). According to previous studies, L-carnitine positively affects spermatogenesis and sperm maturation and its metabolism (15). The high concentration of L-carnitine in testicular tissues (half of the level in rat epididymis) and acetyl-L-carnitine in developing testicular tissue (especially primary spermatocytes) reflects its role in spermatogenesis (10). Investigations on beneficial impacts of L-carnitine on spermatogenesis were related to its efficacy on post-injury recovery of spermatogenesis, although the exact mechanism has not been discovered yet. However, antiapoptotic properties of L-carnitine may account for such effects. Histological examinations of testicular tissue showed that germinal epithelium reorganization in seminiferous tubules was more rapid with L-carnitine treatment (16). In addition, according to previous studies, L-carnitine affects spermatogenesis through stimulation of glucose uptake by Sertoli cells that supply essential energy for epithelial germ cell development. Post gonadal sermiogenesis occurs in testicles and sperm maturation in epididymis, where sperm cells come into contact with significant concentration of L-carnitine to acquire motility. In fact, L-carnitine plays a significant role in spermatozoa energy metabolism and provides the main fuel for sperm cell motility, so that the onset of sperm motility is associated to an increment in the concentration of free L-carnitine in epididymal lumen. Furthermore, L-carnitine in epididymis increases sperm viability by stabilizing sperm plasma membrane through inhibition of oxygen consumption and cellular efflux of enzymes and reduction of spontaneous acrosome reaction (10). In addition to L-carnitine contribution to spermatogenesis, it can protect germ cells as an antiapoptotic factor by interfering with extrinsic Fas-FAS-L–mediated pathway (through intervening with Fas-triggered apoptotic signals and preventing ceramide generation) and intrinsic mitochondrial-dependent pathway (by inactivating caspases 3, 7, and 8) that are common effectors of apoptosis. Also, L-carnitine protects sperm cells against apoptosis by eliminating acetyl-CoA that is a potent toxic mediator (16). Besides, L-carnitine has effects on spermatogenesis, and as an antioxidant agent, it exerts protective effects on sperm cells, fertilization, implantation, and pregnancy rate through oxidative stress reduction, removing free radicals, and prevention of ROS production (17, 18). Indeed, L-carnitine can maintain sperm cellular membrane and DNA integrity through decreasing OS-induced lipid peroxidation and DNA oxidation (19–23). Since oxidative stress can alter the sperm epigenetic profile through affecting sperm DNA, L-carnitine can also modulate the expression of the epigenetically regulated genes, especially genes involved in DNA methylation by reducing ROS and consequent oxidative stress (24, 25). Additionally, L-carnitine positively affects sperm mitochondrial structure, metabolism, oxidative stress, and apoptosis. It potently protects sperm mitochondria against electron transport chain-induced ROS through some mechanisms including radical scavenging, iron ions chelating, and increasing expression of mitochondrial antioxidants such as superoxide dismutase (SOD), glutathione (GSH), catalase (CAT), and glutathione peroxidase (GPx), maintains sperm mitochondrial membrane and DNA integrity, and finally modulates the sperm cell apoptotic signaling. Furthermore, the role of L-carnitine in mitochondrial metabolism is connected with the import of longchain fatty acid through the mitochondrial membrane for oxidation (26).
In this review, protective impacts of L-carnitine alone in preventing male reproductive damages were studied including abnormal sperm and conventional and functional parameters, sperm cryopreservation-induced injuries, and testicular dysfunction caused after cancer treatment by radiation and busulfan; in the following three session of the review, the below-mentioned questions were addressed: 1) how L-carnitine can maintain sperm fertility potential by improving sperm conventional and functional parameters?, 2) how L-carnitine can preserve and maintain male fertility after cancer treatment?, and 3) how L-carnitine can protect sperm functional parameters after freezing–thawing?
How L-carnitine can maintain sperm fertility potential by improving sperm conventional and functional parameters?
L-carnitine with its antioxidant property has a vital role in sperm metabolism and optimizes sperm conventional and functional parameters in seminal plasma. Many studies which have investigated L-carnitine’s protective effects are summarized below (Table 1) (14, 27–32).
Table 1.
Summary of the selected studies on protective impact of L-carnitine on sperm parameters
| Authors | Patients | Treatment | Parameters | Results |
|---|---|---|---|---|
| Lenzi et al. (2004) | 60 infertile patients | Groups:
|
Sperm parameters (sperm concentration, motility, and morphology) | Effective increase following LC and LAC treatment in sperm motility, particularly in groups who had lower baseline levels |
| Aliabadi et al. (2012) | 30 male mice | Groups:
Treatment groups:
Positive control group:
|
|
|
| Poveda et al. (2013) | 60 infertile men | Groups:
|
Semen parameters (sperm count, motility, and morphology) |
|
| Haje and Naoom (2015) | 128 infertile men with idiopathic oligoasthenozoospermia (iOA) | Groups:
|
|
|
| Jihad Manssor et al. (2019) | Infertile patients |
|
Semen parameters (volume, sperm concentration, motility, morphology, and viability) |
|
| Zhang et al. (2020) | 693 idiopathic Oligoasthenoteratozoospermia(iOAT) |
|
|
|
| Wei et al. (2021) | 621 idiopathic asthenozoospermic men |
|
|
|
In a meta-analysis in 2021, Wei et al. analyzed seven articles including 621 patients. They evaluated the effective potential of L-carnitine/L-acety-l-carnitine (LC/LAC) in idiopathic asthenozoospermic men. The results of this study show that LC/LAC could result in sperm normal morphology and enhanced motility in comparison with the placebo group (27). In another study by Jihad Manssor et al. in 2019, many infertile men were treated with L-carnitine (500 mg) once daily for four months. In this research, the results of sperm analysis, pre and post treatment, indicated a significant increase in sperm motility, concentration, and the reduction of dead and abnormal sperm following L-carnitine treatment (28). In the same year, a systematic review and meta-analysis also evaluated combined L-carnitine and L-acetyl carnitine efficacy in idiopathic oligoasthenoteratozoospermic men. In this study, 7 randomized controlled trials, including 693 patients, were analyzed. The comparison of assessed parameters in these trials, such as sperm total motility, sperm count, sperm forward motility, semen volume, normal sperm morphology, and the number of pregnancies between two groups treated with L-carnitine+L-acetyl carnitine and placebo showed that the rate of sperm forward motility, sperm total motility, and the number of pregnancies have significantly increased. However, in other semen parameters, there was no significant difference between two groups (29).
Furthermore, to clarify the role of L-carnitine in male fertility, in a randomized controlled trail in 2015, 20 couples with idiopathic oligoasthenozoospermia were admitted for treatment with L-carnitine (1000 mg/day) for 3 to 6 months. The results showed the improvement of sperm parameters (sperm count, motility, and normal morphology) and ICSI outcomes in comparison with the control group (30). Lenzi et al. also conducted a randomized trial to assess the effect of combined L-carnitine and L-acetyl-carnitine treatment in sixty infertile oligoastheno-teratozoospermic men (aged 20–40 years). Patients were divided to two groups of combined treatment of L-carnitine (2 g/d) and L-acetyl-carnitine (1 g/d) and placebo. The study design was 2 months of wash-out, 6 months of therapy, and 2 months of follow-up. The result of this study showed that even though combined L-carnitine and L-acetyl carnitine treatment improves all sperm parameters, the greatest sperm motility improvement (both total and forward motility) was observed in patients with lower baseline levels of motile sperm (14). In addition, an animal research by Aliabadi et al. in 2013 showed that adding L-carnitine or L-acetyl-carnitine to culture media containing testicular sperm extracted of testis from 30 mature male mice can improve sperm motility and chromatin quality (31). In another randomized trial in 2013, the effect of spermotrend (1 g tablet every 8 hr), maca extract (1 g every 12 hr), and L-carnitine (1 g tablet every 12 hr) on semen parameters of infertile men was evaluated. In this study, semen analysis was performed initially, and 30 days, 60 days, and 90 days after treatment. The results of this study showed that taking all three oral supplements can improve the semen parameters of infertile patients, but L-carnitine can significantly increase the concentration of the sperm (p<0.05) (32).
How L-carnitine can preserve male fertility after cancer treatment?
Protective impact of L-carnitine against busulfan-induced male infertility: Following the significant growth of cancer incidence rate, the number of people undergoing chemotherapy is increasing. Busulfan (1,4-butanediol dimethanesulfonate, C6-H14O6S2) as a chemotherapy drug, is utilized for treating various types of cancer and before bone marrow transplantation (33, 34). It belongs to the class of alkyl sulfonate that functions as a cell cycle alkylating antineoplastic agent (35). Busulfan works on DNA molecule through substituting alkyl groups for hydrogen atoms, resulting in DNA alkylation and formation of guanine-adenine crosslinks. Cellular machinery cannot repair these busulfan-induced DNA damages and interferes with DNA replication and transcription of RNA, which prevents further cell division and ultimately leads to cytotoxic, mutagenic, and carcinogenic effects. Busulfan with having two methanesulfonate groups, attached to two ends of a butane chain, hydrolyzes and releases extremely reactive positively charged carbonium ions, which react with the guanine molecules in cell's DNA, resulting in DNA interstrand crosslinks. Another inhibitory effect of busulfan is its binding to the cysteine molecules of histone proteins, leading to DNA-protein binding. In addition, busulfan can interact with the glutathione and sulfhydryl groups and disrupt the cellular redox equilibrium, resulting in increased oxidative stress in cancer cells (36, 37). Besides positive therapeutic effects, busulfan has some undesirable side effects particularly in cells or tissues with high proliferative activities (38). Animal experiments have demonstrated that busulfan can induce significant cytotoxic and apoptotic effects on reproductive glands (size and weight), germ cells in spermatogenic process (sperm production and maturation) (39), and epididymal sperm (40).
These harmful effects include temporary or permanent dose-dependent changes in normal semen parameters, changes in testis biochemical and structural characteristics, and apoptosis-related gene expression and testosterone level, which finally result in spermatogenesis disorders and male infertility (34). According to multiple studies, sperm analysis of all animals that received busulfan indicated that busulfan can significantly reduce sperm concentration, motility, viability, normal morphology, and DNA integrity (39, 41–43). Also, treatment with busulfan as a cytotoxic agent can cause abnormal apoptosis in spermatogonial cell lines, leading to a pathological condition. Findings showed that weight and size of rat testis after treatment with busulfan decreased, which may be related to structural changes following busulfan exposure. Stereological and histological studies exhibited that, after busulfan treatment, some large vacuoles were observed in the semi-niferous tubules in the rat testis and the size of seminiferous tubules was reduced, which was linked to disorganization and reduction of height of germinal epithelium following depletion of spermatogenic lineage cells (spermatogonia, spermatocytes, and round elongated spermatid). Furthermore, immunofluorescence analysis of spermatogenic cell specific markers indicated that C18-4 and GC-1, markers of spermatogonial stem cells, decreased more than markers of other spermatogenic cells following busulfan treatment, indicating that type A and B spermatogonial stem cells were more vulnerable to busulfan (39). It was found that the spermatogenic impairment could be induced by decreasing expression of ckit, as a survival factor, in spermatogonia or enhancing CK18, as a death factor, in Sertoli cells (42, 44–50). Studies on rat testis presented that, in addition to spermatogenic cells, the quantity of supporting Sertoli and Leydig cells declined significantly in the rats exposed to busulfan in comparison with the control group. However, spermatogonia loss was higher than Sertoli and Leydig cell loss after busulfan exposure (39) and because the status of spermatic germ cells depends on Leydig and Sertoli cells function, apoptosis of these supporting cells can also endanger vitality of spermatic germ cells (46). Reduction of serum testosterone levels in busulfan-administered mice compared to the control group can also confirm the decrease of Leydig cells following busulfan treatment (44, 45).
Furthermore, biochemical analysis of testes revealed that in busulfan-treated rat testis, activity of antioxidant enzymes of superoxide dismutase (SOD), SOD/total protein (TP), catalase, catalase/TP, and thiol significantly decreased and, conversely, the level of malondialdehyde (MDA) as a lipid peroxidation marker significantly increased. These findings suggested that busulfan can significantly induce ROS creation and reduce antioxidants, leading to oxidative stress and lipid peroxidation in testicular tissue, which, in turn, can result in germ cell death, spermatogenesis impairment, and infertility (49).
Evidence shows that the level of ROS is closely related to increased levels of apoptotic events in busulfan-exposed spermatogonial stem cells. In fact, busulfan, through interaction with glutathione and sulfhydryl groups and disruption of balance between ROS and antioxidant, increases oxidative stress and causes extensive intracellular damages to proteins, nucleic acids, and lipid molecules; moreover, it is crucial for cell survival, which compromises plasma membrane, DNA and mitochondrial integrity, and eventually leads to the apoptosis. In agreement with these findings, busulfan-induced ROS activated the ERK/p38 pathway, which enhanced the expression of p53 levels, as an apoptosis-related gene (39, 43).
Additionally, busulfan can decrease Bcl-2 gene expression (as an antiapoptotic gene) and increase Bax gene expression (as a proapoptotic gene) in epididymal sperm of busulfan treated mice, which are related to increased oxidative stress as a result of decreased enzymatic antioxidants of GPx and SOD and increased level of lipid peroxidation in sperm cell (50).
Antioxidant treatment can improve the detrimental effects of busulfan on the male reproductive system (51) due to its potential in attenuating free radicals and reducing oxidative stress and apoptotic process. Accordingly, antioxidants can reduce the DNA damage and lipid peroxidation in testicular cells during treatment with busulfan. L-carnitine with antioxidant and antiapoptotic properties has a protective effect against busulfan-induced infertility. Investigations have revealed that generation of free radicals can be decreased or prevented by L-carnitine through its free radical scavenging efficacy (superoxide anion and hydrogen peroxide) and metal chelating activities, respectively, culminating in reduction of lipid peroxidation and DNA and protein oxidation rate (52). In agreement with these findings, Abd-Elrazek and Ahmed-Farid found that L-carnitine significantly decreased busulfan-induced elevated oxidative stress markers (MDA, glutathione disulfide (GSSG), nitric oxide (NO), and 8-hydroxyguanosine (8-HdG)) and significantly increased busulfan-induced glutathione (GSH) with antioxidant activity (53). Also, another study has shown that L-carnitine is effective in attenuation of MDA level as a lipid peroxidation end-product, and increase of SOD level, as a main antioxidant enzyme found in the male reproductive system (54). In fact, L-carnitine can improve the imbalance between oxidant (ROS) and antioxidant species caused by busulfan, and decrease oxidative stress through ROS reduction and increase of antioxidants such as GSH and SOD. On the other hand, L-carnitine can reduce the expression of caspase-3, an apoptosis-related protein, that is increased following busulfan treatment (55). As a result, L-carnitine with antioxidant and antiapoptotic properties can reduce adverse effects of busulfan on plasma membrane, DNA and mitochondrial integrity through reduction of lipid peroxidation, DNA as well as protein oxidation and decrease busulfan-induced apoptosis. Furthermore, it was well known that L-carnitine can also maintain the sperm plasma membrane against oxidative stress caused by toxic materials such as busulfan via eliminating the toxic acetyl-CoA and substituting fatty acids in cell membrane (13). Measuring cell energy parameters shows that L-carnitine plays a fundamental role in transporting long-chain fatty acids across the inner mitochondrial membrane, resulting in β-oxidation and ATP production (53). Therefore, L-carnitine can increase motility and viability of sperm through maintaining sperm plasma membrane flexibility and supplying energy.
L-carnitine has protective effects on sperm parameters; Abd-Elrazek and Ahmed-Farid demonstrated that L-carnitine can improve testis weight (8, 16, 49), which may be related to structural changes in testis following reorganization and elevation of height of germinal epithelium, increase seminiferous tubules size, and also increase interstitial volume through reduction of ROS level and spermatogenic apoptosis. In contrast, Dehghani et al. did not observe any significant effect of L-carnitine on improvement of rat testis weight following busulfan exposure. A summary of the studies on protective impact of L-carnitine against busulfan-induced male infertility is presented in table 2 (53, 56).
Table 2.
Summary of the selected studies on protective impact of L-carnitine against busulfan-induced male infertility
| Authors | Species | Treatment | Dosage and groups | Parameters | Results |
|---|---|---|---|---|---|
| Dehghani et al. (2012) | 20 adult male rats (180±20 gr) |
|
|
|
|
| Abd-Elrazek and Ahmed-Farid (2017) | 20 adult male rats (180±20 g) |
|
|
|
|
Effective potential of L-carnitine in improving radiation-induced testicular dysfunction: Today, radiation is increasingly used in industry, medicine, agriculture, military, and scientific research, often causing undesirable effects on people who have been exposed to it. Therefore, there is a need to study the radiation injuries to identify appropriate strategies to reduce these effects and recover the damages. The harmful impacts of radiation on cells are mainly exerted by free radicals like hydroxyl, superoxide, and hydrogen peroxide (57), which can damage lipids, nucleic acids, and proteins, leading to cellular dysfunction and even apoptosis (58–60). One of ROS-induced long-term side effects of radiation is the damage to reproductive system, resulting in infertility. Testis as one of the most radiosensitive organs has many cells with various degrees of sensitivity to radiation, so that exposure of the testis to radiation can induce apoptosis of germ cells and particularly affect the division of spermatogonia to preleptotene spermatocytes. Sertoli and Leydig cells as testicular supporting cells have more resistance to apoptosis induced by radiation (61).
Morphologically, light and electron microscopic examinations of irradiated testes have revealed marked disorganization, desquamation, and vacuolization of the germ cells of rats’ seminiferous tubules, as well as formation of multinucleated giant cells in the germinal epithelium following spermatogenesis arrest, compared to the unirradiated testes. It was observed that the type and extent of these changes vary at different exposure intervals using radiation. In addition, Leydig cells illustrated shrunken pyknotic nuclei. Besides, some marked pathologic changes were detected in Sertoli cells by electron microscopy. Also, investigations showed that radiation could change the location and morphology of Sertoli cell nucleus. It could also increase nuclear invaginations, dilation of smooth endoplasmic reticulum cisterna, mitochondrial swelling, and the number and size of lipid droplets in these cells. In above examinations, some irregular round empty spaces were evident between Sertoli cells. It was also seen that basement membrane of seminiferous tubules of irradiated rats is either severely folded or thickened. Based on previous studies, these post-radiation histopathologic changes in testis can be reduced by administrating L-carnitine during radiation exposure. Aktos et al. in histopathological evaluation of rat testis after irradiation observed disorganization in the stratification of spermatogenic cells, arrest in spermatogenesis, and vacuolization in the germinal epithelium. Pretreatment of irradiated rat testis with L-carnitine in their study showed that L-carnitine has radioprotective effects against radiation-induced acute histopathological and biochemical testicular damage (62). Also, Kanter et al. in 2010 showed that pretreatment with L-carnitine in rats, one day before radiation exposure, considerably decreased the radiation-induced germ cell apoptosis and morphological changes in the irradiated testis (63).
In a study by Topcu-Tarladacalisir et al. in 2009, it was revealed that L-carnitine improved the spermatogenic recovery following irradiation in rats (64). In addition to animal studies, data obtained from men with idiopathic oligoasthenospermia subjected to radiation has also shown that sperm parameters can be improved following L-carnitine treatment due to its antioxidant and antiapoptotic impacts on the testis (55). While radiation induces ROS production that has a key role in induction of DNA double-strand breaks and activation of apoptosis signaling pathways, recent studies have shown that treatment with L-carnitine and its derivatives as potent antioxidants can inhibit DNA damage and activate DNA-repair genes through ROS reduction (65). Furthermore, in mice testicular tissue, pretreatment with L-carnitine can inhibit apoptosis related to genes such as FasL, implicated in programmed cell death, and Cyclin D2, involved in cell cycle regulation, and oncogenic transformation and differentiation which are activated following radiation.
Recently, Soliman and Aldhahrani, in their immunohistochemistry findings, showed that γ-irradiation incremented caspase-9 expression and reduced Bcl-2 expression and L-carnitine protected γ-irradiated mice following changes in caspase-9 and Bcl-2, as apoptotic factors. They showed that all altered testicular anti-oxidants and mRNA expression of apoptotic, pro-apoptotic, and anti-apoptotic genes were improved by preadministration of L-carnitine in γ-irradiated mice, providing evidence for the protective effects of L-carnitine against testicular oxidative stress as well as apoptosis caused by γ-irradiation at biochemical, molecular, and cellular levels (66). L-carnitine can also improve expression of some proinflammatory cytokines including TNF-α, IL1-β, and IFN-γ, which increase after radiation (19, 65). Since there is a correlation between ROS and expression of these proinflammatory cytokines, it seems that L-carnitine’s suppressive effect on radiation-induced cytokine expression may be explained by L-carnitine antioxidant effects (67). In line with the findings of previous research, Famularo et al. in 2004 reported that L-carnitine can down-regulate proinflammatory cytokines (68).
Moreover, Ahmed et al. indicated that the expression of aromatase enzyme, androgen-binding protein (ABP), and cholesterol side chain cleavage enzyme (CYP450SCC) in reproductive system can be affected by radiation. ABP, a glycoprotein secreted by Sertoli cells in the seminiferous tubules, regulates spermatogenesis through maintenance of higher androgen levels in epididymis and testis. Also, aromatase, an enzyme encoded by the CYP19 gene, that is responsible for biosynthesis of estrogen (essential for male fertility) in Sertoli, Leydig, and spermatogenic line cells and CYP450SCC which is involved in biosynthesis of testosterone from cholesterol have important roles in spermatogenesis process. Studies have revealed that radiation exposure decreases expression of aromatase and suppresses expression of CYP450SCC and ABP, whereas L-carnitine treatment prior and during radiation exposure can inhibit these suppressions and increase reduced expression of aromatase mRNA (69).
Moreover, radiation can alter activation of some peroxidation biomarkers. Many studies have demonstrated that radiation decreases activities of plasma antioxidants including SOD, catalase and GSH, and increases plasma MDA, resulting in lipid peroxidation in different systems of human body, particularly reproductive system (70). On the other hand, biochemical analyses for investigating the protective effect of L-carnitine against free oxygen radicals induced by radiation showed that increased plasma level of MDA following radiation, as a lipid peroxidation biomarker, can be decreased significantly by L-carnitine before radiation exposure. Also, pretreatment with L-carnitine can modulate the decreased plasma level of SOD, catalase and GSH, as antioxidant defense mechanisms, which are effective in scavenging of radiation-induced free radicals. The potential of L-carnitine to reduce and eliminate free radicals is related to the suppression of hydroxyl radical production in a chemical reaction, the Fenton reaction, possibly through chelating the iron ions necessary for the hydroxyl radical generation (71). A summary of the studies on effective potential of L-carnitine in improving testicular radiation-induced dysfunction is presented in table 3 (62–64, 66, 69).
Table 3.
Summary of the selected studies on effective potential of L-carnitine in improving testicular radiation-induced dysfunctions
| Authors | Species and number | Radiation damages | Radiation dose | Treatment | Results |
|---|---|---|---|---|---|
| Topcu-Tarladacalisir et al. (2009) | 42 Wistar albino male rats |
Germ cell depletion and disorganization, spermatogenesis arrest, multinucleated giant cells formation, and germinal epithelium vacuolization | 10 dose of Gy γ-irradiation | LC (200 mg/kg) | LC enhanced the ratio of regeneration in seminiferous tubules and increased the recovery of spermatogenic cells after irradiation in rats |
| Kanter et al. (2010) | 18 Wistar albino male rats |
Germinal cell disorganization and desquamation, sperm count reduction in seminiferous tubule and an increment in TUNEL-positive cells in irradiated rats | 10 dose of Gy γ-irradiation | LC (200 mg/kg) | Pretreatment with LC, 24 hours before exposure to γ radiation, reduced radiation-induced germ cell apoptosis and significant testis histopathological changes |
| Ahmed et al. (2014) | 24 Swiss male mice |
Suppression of ABP expression and CYP450SCC mRNA, down-regulation of aromatase mRNA expression, up-regulation of FasL and cyclin D2 mRNA expression, up-regulation of TNF-α, IL1-β, and IFN-γ mRNA expressions | 0.1 doses of Gy/day (10 days) | LC (10 mg/kg) | LC decreased the apoptosis in testicular tissue and normalized the changes in the expression of testicular genes and sperm abnormalities in comparison with irradiated mice |
| Aktoz et al. (2017) | 30 Wistar albino male rats |
Spermatogenesis arrest, disorganization in the stratification of spermatogenic germ cells and vacuolization in the germinal epithelium | 20 doses of Gy γ-irradiation | LC (300 mg/kg) | LC has radioprotective effects against radiation-induced acute histopathological and biochemical testicular damage |
| Soliman and Aldhahrani (2020) | 28 Swiss male mice |
Induced testicular oxidative stress, changes in antioxidant activities, testicular dysfunction, and changes in apoptosis-associated genes (c-jun, c-fos, Bcl-xL, caspase-3, and BAX) | 0.1 doses of Gy/day (10 days) | L-carnitine LC (10 mg/kg) |
|
How L-carnitine can protect sperm functional parameters after freezing–thawing?: Cryopreservation is a practical method to maintain male fertility, which presently has a routine use in the field of reproductive medicine. Despite the benefits of sperm cryopreservation, its significant and detrimental impacts on sperm structural and functional parameters are common post-thawing consequences, including reduction of sperm maturity, decrease of sperm concentration, normal morphology, motility, and viability and disruption of sperm plasma, mitochondrial membrane, acrosome, and DNA integrity (51, 58). Therefore, despite different advances in cryopreservation methods, the functional sperm cells’ recovery rate after thawing must be improved. Investigations have demonstrated that most of such detrimental consequences are linked to elevated ROS throughout sperm freezing–thawing process. Normally, there is a balance between antioxidant scavenging activities and ROS production in male reproductive tract. Disturbance of this balance leads to oxidative stress. Cryopreservation induces excessive generation of ROS and causes sperm cell damage with extreme sensitivity to ROS owing to limited cytoplasm, low antioxidant capacity, and higher level of polyunsaturated fatty acids (PUFA) in plasma membrane of sperm cells.
Sperm OS-induced cryodamages are mostly related to lipid peroxidation, DNA, and protein oxidation. Loss of plasma membrane integrity and flexibility results in lipid peroxidation of PUFA in sperm plasma membrane as well as sperm mitochondrial ROS-induced damage; all these consequences culminate in ATP depletion and decreased sperm motility and viability as the most vulnerable sperm parameters due to insufficient axonemal phosphorylation during cryopreservetion, and the final outcome would be decreased male fertility potential. In addition, lipid peroxidation leads to generation of derived aldehydes and malondialdehyde that can react with proteins and nucleic acids, causing further cell damage. Also, free radicals can damage sperm DNA through oxidation of purine and pyrimidine bases, breakage of DNA single and double strands, protamine deficiency, chromosomal rearrangement and gene mutation and the detrimental consequences are cell damage, cell apoptosis, and impaired fetus development. OS-induced protein oxidation following cryopreservation can reduce cellular enzyme efficiency, lower ROS-scavaging potential, and ultimately cause cell damage, particularly in sperm cells due to lack of their antioxidant systems to protect against oxidative injuries (72–76).
Experimental evidence revealed that one of the most important strategies to reduce OS-induced sperm damage during sperm cryopreservation is to use antioxidants at appropriate concentration in cryomedium. In fact, antioxidants can protect sperm cells through decreasing ROS production and increasing ROS removal that, in turn, subsequently reduce lipid peroxidation and DNA and protein oxidation. They can also regulate sperm mitochondrial protein synthesis, improve mitochondrial membrane and acrosomal integrity, and increase sperm survival rate (74, 75).
Due to high concentration of L-carnitine in epididymis and its potential in stabilizing sperm plasma membrane and increasing sperm survival, and reduction of sperm intracellular L-carnitine level following cryopreservation, (77) some studies have suggested that adding L-carnitine to cryomedium can compensate its loss in sperm cell and improve sperm parameters during freeze-thaw process; all these features can be explained by L-carnitine’s antioxidant property as a ROS scavenger and its role in sperm metabolism as an energy production facilitator.
L-carnitine significantly increases sperm motility and viability during cryopreservation by reducing plasma membrane lipid peroxidation through inhibition of ROS formation, chelating the iron ions required for the hydroxyl radical formation, and scavenging free radicals; therefore, it is effective in improving sperm plasma membrane flexibility. Also, La-carnitine acts as a carrier for translocating long chain fatty acids across the inner mitochondrial membrane for β-oxidation in Krebs cycle and ATP production for sperm motility (78, 79).
Furthermore, L-carnitine as a potent antioxidant can decrease oxidation of purine and pyrimidine bases, breakage of DNA single and double strands, DNA fragmentation and protamine deficiency and maintain DNA integrity during cryopreservation by ROS reduction (78). Also L-carnitine with antioxidant and antiapoptotic properties can decrease cryodamages to sperm mitochondrial DNA and membrane through reduction of ROS and increase of mitochondrial antioxidants. Furthermore, it can improve the potential of mitochondria in ATP production and increase motility with transfer of long-chain fatty acid across mitochondrial inner membrane.
The function of L-carnitine in cryopreservation has been demonstrated in various human (65, 80–82) and animal studies (Table 4) (79, 83–86). Zhang et al. assessed protective effects of L-carnitine on human semen specimens of 37 asthenozoospermic and 33 normozoospermic men during freezing–thawing procedure. In this study, viability, motility, mitochondrial membrane potential, and DNA fragmentation index of sperm cells were analyzed in fresh and frozen–thawed semen, in both experimental (with cryomedium containing L-carnitine) and control groups. The results of this research demonstrated that supplementation of the cryopreservation medium with L-carnitine before freezing yields a considerable enhancement in post-thaw sperm parameters and reduces sperm cryodamage in both the normozoospermic and asthenozoospermic semen samples, compared to the control group.
Table 4.
Summary of the selected animal studies on cryoprotective effect of L-carnitine on freeze–thaw induced sperm damages
| Authors | Patients | Treatment | Freezing and thawing method | Freezing media | Results |
|---|---|---|---|---|---|
| Namik et al. (2000) | 35 infertile men |
|
|
TEST-yolk buffer containing glycerol (Irvine Scientific, US) |
|
| Zhang et al. (2000) |
|
L-carnitine (LC) (1.0 g/l) |
|
Quinn’s Advantage Sperm Freezing Medium, (SAGE BioPharma, US) |
|
| Banihani et al. (2013) | 22 infertile men | L-carnitine (LC) 0.5 mg/ml |
|
TEST-yolk buffer (Irvine Scientific, US) |
|
| Aliabadi et al. (2017) | 30 healthy men |
|
|
(Life Global, US) |
|
| Ghorbani et al. (2021) | 20 healthy men |
|
|
Solution containing 0.5 mol/L of sucrose and 5% human serum albumin (HSA, Sigma-Aldrich, US) |
|
| Chavoshi Nezhad et al. (2021) | 30 oligospermic men men |
|
|
Human Sperm Preservation Medium (HSPM) |
|
It should be noted, for sperm parameters such as DNA fragmentation index and viability, the cryoprotective effectiveness of L-carnitine in asthenozoospermic specimens was superior to that of normozoospermic specimens (82). Similarly, in another study, assessment of the effects of L-carnitine on sperm conventional and functional parameters, protamine deficiency and DNA fragmentation before and after freezing in oligospermic men showed that L-carnitine can improve sperm motility and reduce ROS, protamine deficiency, and DNA fragmentation in pre and post-freezing stages (78). Contrary to previous studies, an evaluation by Duru et al. in 2000 about the effects of acetyl-L-carnitine on plasma membrane and sperm motility in semen samples of 41 men before freezing-thawing revealed that acetyl-L-carnitine could not prevent cryodamage to the membrane or motility integrity in human spermatozoa of subfertile men (87).
Improvement of these sperm parameters following L-carnitine supplementation in cryopreservation can be explained by its antioxidant property and crucial role in fatty acid metabolism and energy production in sperm (74). In fact, L-carnitine, through transporting fatty acid long-chain acyl groups across sperm mitochondrial inner membrane, can facilitate β-oxidation, cellular ATP production, improve sperm flagellar movement, and ultimately sperm motility (84).
Assessment of 8-hydroxy-guanosine (oh8G) as an indicator of oxidative DNA damage has displayed that ROS-induced DNA oxidation during the freezing procedures can be decreased after adding L-carnitine to the cryomedium (81). It was also observed that MDA levels as a lipid peroxidation marker is reduced after adding L-carnitine to freezing media which is associated to its potential to chelate free ferrous ions, prevent superoxide ion generation, and also scavenge excessive ROS. Besides, increased phosphatidylserine (PS) as an apoptotic marker could be reduced using L-carnitine through its stabilizing effect on sperm plasma membrane (83).
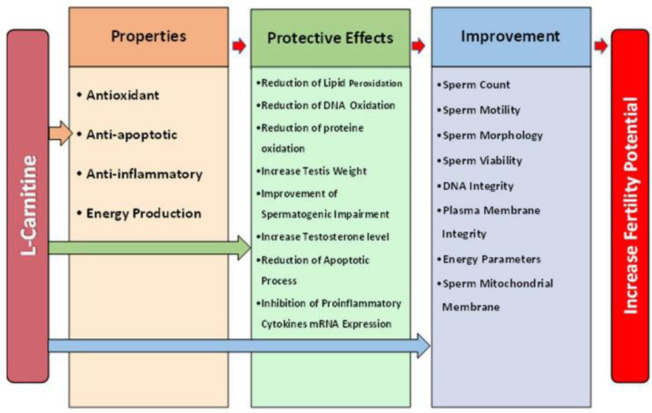
A summary of the studies on cryoprotective effects of L-carnitine on freeze–thaw induced sperm damages is presented in table 4 (79, 83, 84) and table 5 (31, 81, 82, 87, 88). A summary of the protective effects of L-carnitine on male reproductive system is presented in figure 2.
Table 5.
Summary of the selected human studies on cryoprotective effect of L-carnitine on freeze–thaw induced sperm damages
| Authors | Species and number | Treatment | Dosage | Freezing and thawing media and procedures | Results |
|---|---|---|---|---|---|
| Parmornsupornvichit et al. (2013) | 24 healthy cats of various breeds, aged between 1–7 years | L-carnitine (LC) | Groups: i) 0 mM LC (control), ii) 12.5 mM LC, and iii) 25 mM LC |
|
|
| Sarıözkan et al. (2014) | 10 sexually mature male New Zealand white rabbits |
|
|
Freezing: cooling semen samples from 37 to 5°C, in a cold cabinet, maintaining at 5°C, and then examining after 0, 6, 12, and 24 hr of liquid storage |
|
| Fattah et al. (2016) | 12 adult male roosters | L-carnitine (LC) | Groups: Beltsville without LC (control), Beltsville with 0.5 mM (L0.5), 1 mM (L1), 2 mM (L2), 4 mM (L4), and 8 mM (L8) LC |
|
|
Figure 2.
Summary of protective effects of L-carnitine on male reproductive system
Conclusion
L-carnitine with antioxidant, anti-inflammatory, and antiapoptotic properties along with a crucial role in sperm metabolism can optimize sperm conventional and functional parameters in seminal plasma, particularly in asthenozoospermic men. In addition, treatment with L-carnitine at appropriate concentration, before or during radiation and busulfan exposure, can reduce testis structural, biochemical, and genetic alterations caused by radiation and busulfan therapy. It can also modulate apoptotic events, lipid peroxidation, and ultimately improve testicular and sperm quality and quantity. Furthermore, supplementation of the cryomedium with L-carnitine before freezing improves post-thaw sperm parameters and reduce the sperm cryodamage.
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
Funding:
None.
References
- 1. D’Antona G, Nabavi SM, Micheletti P, Di Lorenzo A, Aquilani R, Nisoli E, et al. Creatine, L-carnitine, and ω 3 polyunsaturated fatty acid supplementation from healthy to diseased skeletal muscle. Biomed Res Int. 2014; 2014: 613890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li LY, Lu DL, Jiang ZY, Limbu SM, Qiao F, Chen LQ, et al. Dietary L-carnitine improves glycogen and protein accumulation in Nile tilapia via increasing lipid-sourced energy supply: an isotope-based metabolic tracking. Aquac Rep. 2020; 17: 100302. [Google Scholar]
- 3. Talenezhad N, Mohammadi M, Ramezani-Jolfaie N, Mozaffari-Khosravi H, Salehi-Abargouei A. Effects of L-carnitine supplementation on weight loss and body composition: a systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin Nutr ESPEN. 2020; 37: 9– 23. [DOI] [PubMed] [Google Scholar]
- 4. Askarpour M, Hadi A, Symonds ME, Miraghajani M, Sadeghi O, Sheikhi A, et al. Efficacy of L-carnitine supplementation for management of blood lipids: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2019; 29( 11): 1151– 67. [DOI] [PubMed] [Google Scholar]
- 5. Fielding R, Riede L, Lugo JP, Bellamine A. L-carnitine supplementation in recovery after exercise. Nutrients. 2018; 10( 3): 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. AbuMoh’d MF, Obeidat G, Alsababha W. Effect of oral supplementation with L-carnitine on performance time in a 5000 m race and responses of free fatty acid and carnitine concentrations in trained-endurance athletes. Montenegrin J Sport Sci Med. 2021; 10( 2): 5– 11. [Google Scholar]
- 7. Sawicka AK, Renzi G, Olek RA. The bright and the dark sides of L-carnitine supplementation: A systematic review. J Int Soc Sports Nutr. 2020; 17( 1): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mardanshahi T, Rezaei N, Zare Z, Shafaroudi MM, Mohammadi H. Effects of l-carnitine on the sperm parameters disorders, apoptosis of spermatogenic cells and testis histopathology in diabetic rats. Int J Reprod Biomed. 2019; 17( 5): 325– 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Odo S, Tanabe K, Yamauchi M. A pilot clinical trial on L-carnitine supplementation in combination with motivation training: effects on weight management in healthy volunteers. Food Nutr Sci. 2013; 04( 02): 222– 31. [Google Scholar]
- 10. Agarwal A, Said TM. Carnitines and male infertility. Reprod Biomed Online. 2004; 8( 4): 376– 84. [DOI] [PubMed] [Google Scholar]
- 11. Omar MI, Pal RP, Kelly BD, Bruins HM, Yuan Y, Diemer T, et al. Benefits of empiric nutritional and medical therapy for semen parameters and pregnancy and live birth rates in couples with idiopathic infertility: a systematic review and meta-analysis. Eur Urol. 2019; 75( 4): 615– 25. [DOI] [PubMed] [Google Scholar]
- 12. Micic S, Lalic N, Djordjevic D, Bojanic N, Bogavac-Stanojevic N, Busetto GM, et al. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia. 2019; 51( 6): e13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mongioi L, Calogero AE, Vicari E, Condorelli RA, Russo GI, Privitera S, et al. The role of carnitine in male infertility. Andrology. 2016; 4( 5): 800– 7. [DOI] [PubMed] [Google Scholar]
- 14. Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, et al. A placebo-controlled double-blind randomized trial of the use of combined L-carnitine and L-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004; 81( 6): 1578– 84. [DOI] [PubMed] [Google Scholar]
- 15. Aboul-Naga AM, Hamam ET, Awadalla A, Shokeir AA. The protective role of L-carnitine on spermatogenesis after cisplatin treatment during prepubertal period in rats: a pathophysiological study. Life Sci. 2020; 258: 118242. [DOI] [PubMed] [Google Scholar]
- 16. Chiu MN, Blackman MR, Wang C, Swerdloff RS. The role of carnitine in the male reproductive system. Ann N Y Acad Sci. 2004; 1033: 177– 88. [DOI] [PubMed] [Google Scholar]
- 17. Cabral REL, Mendes TB, Vendramini V, Miraglia SM. Carnitine partially improves oxidative stress, acrosome integrity, and reproductive competence in doxorubicin-treated rats. Andrology. 2018; 6( 1): 236– 46. [DOI] [PubMed] [Google Scholar]
- 18. Tsunoda S, Kimura N, Fujii J. Oxidative stress and redox regulation of gametogenesis, fertilization, and embryonic development. Reprod Med Biol. 2013; 13( 2): 71– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed SDH, Ahsan S, Iqbal T, Burney SIA. Relationship of seminal free L-carnitine with functional spermatozoal characteristics: Results from an observational study conducted in a tertiary care hospital of Karachi, Pakistan. J Pak Med Assoc. 2017; 67( 2): 280– 4. [PubMed] [Google Scholar]
- 20. Khaw SC, Wong ZZ, Anderson R, Martins da Silva S. L-carnitine and L-acetylcarnitine supplementation for idiopathic male infertility. Reprod Fertil. 2020; 1( 1): 67– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Luca MN, Colone M, Gambioli R, Stringaro A, Unfer V. Oxidative stress and male fertility: Role of antioxidants and inositols. Antioxidants (Basel). 2021; 10( 8): 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Majzoub A, Agarwal A. Systematic review of anti-oxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018; 16( 1): 113– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobori Y, Suzuki K, Iwahata T, Shin T, Sadaoka Y, Sato R, et al. Improvement of seminal quality and sexual function of men with oligoasthenoteratozoospermia syndrome following supplementation with L-Arginine and Pycnogenol®. Arch Ital Urol Androl. 2015; 87( 3): 190– 3. [DOI] [PubMed] [Google Scholar]
- 24. Jiang XH, Jiang C, Yu L, Li XL, Zuo T, Gu PF, et al. Supplementation with l-carnitine rescues sperm epigenetic changes in asthenospermic male semen with altered acetyl-l-carnitine levels. Reprod Dev Med. 2020; 4( 3): 146– 55. [Google Scholar]
- 25. Choucair F, Saliba E, Jaoude IA, Hazzouri M. Antioxidants modulation of sperm genome and epigenome damage: Fact or fad? Converging evidence from animal and human studies. Middle East Fertil Soc J. 2018; 23( 2): 85– 90. [Google Scholar]
- 26. Modanloo M, Shokrzadeh M. Analyzing mitochondrial dysfunction, oxidative stress, and apoptosis: potential role of L-carnitine. Iran J Kidney Dis. 2019; 13( 2): 74– 86. [PubMed] [Google Scholar]
- 27. Wei G, Zhou Z, Cui Y, Huang Y, Wan Z, Che X, et al. A meta-analysis of the efficacy of L-carnitine/L-acetyl-carnitine or N-acetyl-cysteine in men with idiopathic asthenozoospermia. Am J Mens Health. 2021; 15( 2):15579883211011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jihad Manssor1 AR, Al–Mahdawi1 ZMM, Hadi2 AM. The effect of L-Arginine of treatment for infertile men on semen parameters. Tikrit J Pure Sci. 2019; 24( 5): 1– 4. [Google Scholar]
- 29. Zhang X, Cui Y, Dong L, Sun M, Zhang Y. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: a systematic review and meta-analysis. Andrologia. 2020; 52( 2): e13470. [DOI] [PubMed] [Google Scholar]
- 30. Haje M, Naoom K. Combined tamoxifen and L-carnitine therapies for the treatment of idiopathic male infertility attending intracytoplasmic sperm injection: a randomized controlled trial. Int J Infertil Fetal Med. 2015; 6( 1): 20– 4. [Google Scholar]
- 31. Aliabadi E, Mehranjani MS, Borzoei Z, Talaei-Khozani T, Mirkhani H, Tabesh H. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality. Iran J Reprod Med. 2012; 10( 2): 77– 82. [PMC free article] [PubMed] [Google Scholar]
- 32. Poveda C, Rodriguez R, Chu EE, Aparicio LE, Gonzales IG, Moreno CJ. A placebo-controlled double-blind randomized trial of the effect of oral supplementation with spermotrend, maca extract (lepidium meyenii) or L-carnitine in semen parameters of infertile men. Fertil Steril. 2013; 100( 3): S440. [Google Scholar]
- 33. Patel JN, Hamadeh I, Zhang Q, Brown T, Steuerwald NM, Hamilton A, et al. Busulfan pharmacogenetics and drug exposure in cancer patients undergoing hematopoietic stem cell transplantation. Blood. 2017; 130( Suppl 1): 3215. [Google Scholar]
- 34. Zangoie R, Eshraghi H, Shirian S, Kadivar A, Nazari H, Aali E. Melatonin synergistically enhances protective effect of atorvastatin against busulfan-induced spermatogenesis injuries in a rat model. Comp Clin Path. 2020; 29( 1): 161– 6. [Google Scholar]
- 35. Houot M, Poinsignon V, Mercier L, Valade C, Desmaris R, Lemare F, et al. Physico-chemical stability of busulfan in injectable solutions in various administration packages. Drugs R D. 2013; 13( 1): 87– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jung SW, Kim HJ, Lee BH, Choi SH, Kim HS, Choi YK, et al. Effects of Korean red ginseng extract on busulfan-induced dysfunction of the male reproductive system. J Ginseng Res. 2015; 39( 3): 243– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salehinezhad F, Eshraghi H, Kadivar A, Shirian S, Asghari A, Aali E, et al. Amelioration effects of vitamin E, melatonin, L-carnitine, and atorvastatin, on destructive effects of busulfan in the testes of male rats: A gene expression evaluation. Kafkas Univ Vet Fak Derg. 2019; 25( 5): 709– 16. [Google Scholar]
- 38. Xu J, Zhang X, Sun X, Lv Q, Zhang Y. Red-fleshed apple anthocyanin extracts attenuate male reproductive system dysfunction caused by busulfan in mice. Front Nutr. 2021; 8: 632483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li B, He X, Zhuang M, Niu B, Wu C, Mu H, et al. Melatonin ameliorates busulfan-induced spermatogonial stem cell oxidative apoptosis in mouse testes. Antioxid Redox Signal. 2018; 28( 5): 385– 400. [DOI] [PubMed] [Google Scholar]
- 40. Vahdati A. Busulfan induces apoptotic and cytotoxic effects on testis and epididymal sperm of adult male mouse following low dose treatment. Int J Biosci. 2015; 6( 5): 70– 8. [Google Scholar]
- 41. Moloody-tappe M, Shahrooz R, Razi M, Zarei L. The effect of CoQ10 on testicular tissue in rats treating with busulfan: sperm quality and histological changes histological analyses. Iran J Veterin Surg. 2018; 13( 1): 29– 38. [Google Scholar]
- 42. Bahmanpour S, Namavar Jahromi B, Koohpeyma F, Keshavarz M, Bakhtari A. Effects of different doses and time-dependency of busulfan on testes parameters and spermatogenesis in a rat model: a quantitative stereological study. J Adv Med Sci Appl Technol. 2019; 3( 3): 155– 62. [Google Scholar]
- 43. Mirhoseini M, Saki G, Hemadi M, Khodadadi A, Asl JM. Melatonin and testicular damage in busulfan treated mice. Iran Red Crescent Med J. 2014; 16( 2): e14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dehghani F, Sotoude N, Bordbar H, Panjeshahin MR, Karbalay-Doust S. The use of platelet-rich plasma (PRP) to improve structural impairment of rat testis induced by busulfan. Platelets. 2019; 30( 4): 513– 20. [DOI] [PubMed] [Google Scholar]
- 45. Vafaei A, Mohammadi S, Fazel A, Soukhtanloo M, Pour AM, Beheshti F. Effects of carob (Ceratonia siliqua) on sperm quality, testicular structure, testosterone level and oxidative stress in busulfan-induced infertile mice. Pharm Sci. 2018; 24( 2): 104– 11. [Google Scholar]
- 46. Chen Z, Liu M, Hu JH, Gao Y, Deng C, Jiang MH. Substance P restores spermatogenesis in busulfan-treated mice: A new strategy for male infertility therapy. Biomed Pharmacother. 2021; 133: 110868. [DOI] [PubMed] [Google Scholar]
- 47. Ohira T, Saito T, Ando R, Tamura K, Hoshiya T. Systemic histopathology of infant rats exposed to busulfan. J Toxicol Pathol. 2014; 27( 1): 25– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao X, Liu Z, Gao J, Li H, Wang X, Li Y, et al. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology. 2020; 440: 152489. [DOI] [PubMed] [Google Scholar]
- 49. Ahar NH, Khaki A, Akbari G, Novin MG. The effect of Busulfan on body weight, Testis weight and MDA enzymes in male rats. Int J Women’s Heal Reprod Sci. 2014; 2( 5): 316– 9. [Google Scholar]
- 50. Nasimi P, Tabandeh MR, Vahdati A, Khatamsaz S. Busulfan induces oxidative stress- and Bcl-2 family gene-related apoptosis in epididymal sperm and testis of adult male mice Busulfan as a re-presentative chemotherapeutic agent. Physiol Pharmacol. 2015; 19( 3): 208– 15. [Google Scholar]
- 51. Hakemi SG, Sharififar F, Haghpanah T, Babaee A, Eftekhar-Vaghefi SH. The effects of olive leaf extract on the testis, sperm quality and testicular germ cell apoptosis in male rats exposed to busulfan. Int J Fertil Steril. 2019; 13( 1): 57– 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006; 78( 8): 803– 11. [DOI] [PubMed] [Google Scholar]
- 53. Abd-Elrazek AM, Ahmed-Farid OAH. Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia. 2018; 50( 1). [DOI] [PubMed] [Google Scholar]
- 54. Fathizadeh H, Milajerdi A, Reiner Ž, Amirani E, Asemi Z, Mansournia MA, et al. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Metab Disord. 2020; 19( 2): 1879– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mutomba MC, Yuan H, Konyavko M, Adachi S, Yokoyama CB, Esser V, et al. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS Lett. 2000; 478( 1–2): 19– 25. [DOI] [PubMed] [Google Scholar]
- 56. Dehghani F, Hassanpour A, Poost-pasand A, Noorafshan A, Karbalay-doust S. Protective effects of L-carnitine and homogenized testis tissue on the testis and sperm parameters of busulfan-induced infertile male rats. Iran J Reprod Med. 2013; 11( 9): 693– 704. [PMC free article] [PubMed] [Google Scholar]
- 57. Ozmen HK, Erdemci B, Askin S, Sezen O. Carnitine and adiponectin levels in breast cancer after radiotherapy. Open Med (Wars). 2017; 12: 189– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Virmani A, Diedenhofen A. The possible mechanisms involved in the protection strategies against radiation-induced cellular damage by carnitines. Int J Clin Med. 2015; 06( 02): 71– 80. [Google Scholar]
- 59. Khan HA, Alhomida AS. A review of the logistic role of l-carnitine in the management of radiation toxicity and radiotherapy side effects. J Appl Toxicol. 2011; 31( 8): 707– 13. [DOI] [PubMed] [Google Scholar]
- 60. Seifried HE, Anderson DE, Sorkin BC, Costello RB. Free radicals: the pros and cons of anti-oxidants. Executive summary report. J Nutr. 2004; 134( 11): 3143S– 63S. [DOI] [PubMed] [Google Scholar]
- 61. Qu N, Itoh M, Sakabe K. Effects of chemotherapy and radiotherapy on spermatogenesis: the role of testicular immunology. Int J Mol Sci. 2019; 20( 4): 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aktoz T, Caloglu M, Yurut-Caloglu V, Yalcin O, Aydogdu NU, Nurlu D, et al. Histopathological and biochemical comparisons of the protective effects of amifostine and l-carnitine against radiation-induced acute testicular toxicity in rats. Andrologia. 2017; 49( 9). [DOI] [PubMed] [Google Scholar]
- 63. Kanter M, Topcu-Tarladacalisir Y, Parlar S. Anti-apoptotic effect of l-carnitine on testicular irradiation in rats. J Mol Histol. 2010; 41( 2–3): 121– 8. [DOI] [PubMed] [Google Scholar]
- 64. Topcu-Tarladacalisir Y, Kanter M, Uzal MC. Role of L-carnitine in the prevention of seminiferous tubules damage induced by gamma radiation: a light and electron microscopic study. Arch Toxicol. 2009; 83( 8): 735– 46. [DOI] [PubMed] [Google Scholar]
- 65. Düzenli U, Altun Z, Olgun Y, Aktaş S, Pamukoğlu A, Çetinayak HO, et al. Role of N-acetyl cysteine and acetyl-l-carnitine combination treatment on DNA-damage-related genes induced by radiation in HEI-OC1 cells. Int J Radiat Biol. 2018; 95( 3): 298– 306. [DOI] [PubMed] [Google Scholar]
- 66. Soliman MM, Elshazly SA, Aldhahrani A. Gamma-irradiation-induced testicular oxidative stress and apoptosis: Mitigation by L-carnitine. J Biochem Mol Toxicol. 2020; 34( 11): e22565. [DOI] [PubMed] [Google Scholar]
- 67. Elshazly SA, Ahmed MM, Hassan HE, Ibrahim ZS. Protective effect of L-carnitine against yrays irradiation-induced tissue damage in mice. Am J Biochem Mol Biol. 2012; 2( 3): 120– 32. [Google Scholar]
- 68. Famularo G, Simone CDE, Trinchieri V. Carnitines and its congeners a metabolic pathway to the regulation of immune response and inflammation. Ann N Y Acad Sci. 2004; 133: 132– 8. [DOI] [PubMed] [Google Scholar]
- 69. Ahmed MM, Ibrahim ZS, Alkafafy M, El-Shazly SA. L-carnitine protects against testicular dysfunction caused by gamma irradiation in mice. Acta Histochem. 2014; 116( 6): 1046– 55. [DOI] [PubMed] [Google Scholar]
- 70. Dokmeci D, Akpolat M, Aydogdu N, Uzal C, Doganay L, Turan FN. The protective effect of L-carnitine on ionizing radiation-induced free oxygen radicals. Scand J Lab Anim Sci. 2006; 33( 2): 75– 83. [Google Scholar]
- 71. Mansour HH. Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res. 2006; 54( 3): 165– 71. [DOI] [PubMed] [Google Scholar]
- 72. Palomar Rios A, Molina Botella I. Description and outcomes of current clinical techniques for sperm cryopreservation. Eur Med J. 2019; 5: 79– 92. [Google Scholar]
- 73. Rios A, Botella IM. Causes and impact of cryopreservation-associated damage on different parameters of human spermatozoa and its clinical impact. Reprod Health. 2019; 5( 1): 100– 9. [Google Scholar]
- 74. Liu X, Xu Y, Liu F, Pan Y, Miao L, Zhu Q, et al. The feasibility of antioxidants avoiding oxidative damages from reactive oxygen species in cryopreservation. Front Chem. 2021; 9: 648684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, Esmaeili V, et al. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018; 37( 3): 327– 39. [DOI] [PubMed] [Google Scholar]
- 76. Makary S, Abdo M, Fekry E. Oxidative stress burden inhibits spermatogenesis in adult male rats: Testosterone protective effect. Can J Physiol Pharmacol. 2018; 96( 4): 372– 81. [DOI] [PubMed] [Google Scholar]
- 77. Bahmyari R, Zare M, Sharma R, Agarwal A, Halvaei I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: a systematic review and meta-analysis. Andrologia. 2020; 52( 3): e13514. [DOI] [PubMed] [Google Scholar]
- 78. Chavoshi Nezhad N, Vahabzadeh Z, Allahveisie A, Rahmani K, Raoofi A, Rezaie MJ, et al. The effect of L-carnitine and Coenzyme Q10 on the sperm motility, DNA fragmentation, chromatin structure and oxygen free radicals during, before and after freezing in oligospermia men. Urol J. 2021; 18( 3): 330– 6. [DOI] [PubMed] [Google Scholar]
- 79. Sariözkan S, Ozdamar S, Türk G, Cantürk F, Yay A. In vitro effects of l-carnitine and glutamine on motility, acrosomal abnormality, and plasma membrane integrity of rabbit sperm during liquid-storage. Cryobiology. 2014; 68( 3): 349– 53. [DOI] [PubMed] [Google Scholar]
- 80. Aliabadi E, Jahanshahi S, Talaei-Khozani T, Banaei M. Comparison and evaluation of capacitation and acrosomal reaction in freeze-thawed human ejaculated spermatozoa treated with L-carnitine and pentoxifylline. Andrologia. 2018; 50( 2). [DOI] [PubMed] [Google Scholar]
- 81. Banihani S, Agarwal A, Sharma R, Bayachou M. Cryoprotective effect of l-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia. 2014; 46( 6): 637– 41. [DOI] [PubMed] [Google Scholar]
- 82. Zhang W, Li F, Cao H, Li C, Du C, Yao L, et al. Protective effects of L-carnitine on astheno- and normozoospermic human semen samples during cryopreservation. Zygote. 2016; 24( 2): 293– 300. [DOI] [PubMed] [Google Scholar]
- 83. Fattah A, Sharafi M, Masoudi R, Shahverdi A, Esmaeili V, Najafi A. L-Carnitine in rooster semen cryopreservation: Flow cytometric, biochemical and motion findings for frozen-thawed sperm. Cryobiology. 2017; 74: 148– 53. [DOI] [PubMed] [Google Scholar]
- 84. Manee-In S, Parmornsupornvichit S, Kraiprayoon S, Tharasanit T, Chanapiwat P, Kaeoket K. L-carnitine supplemented extender improves cryopreserved-thawed cat epididymal sperm motility. Asian-Australas J Anim Sci. 2014; 27( 6): 791– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gibb Z, Lambourne SR, Quadrelli J, Smith ND, Aitken RJ. L-carnitine and pyruvate are prosurvival factors during the storage of stallion spermatozoa at room temperature. Biol Reprod. 2015; 93( 4): 104. [DOI] [PubMed] [Google Scholar]
- 86. Khan IM, Cao Z, Liu H, Khan A, Rahman SU, Khan MZ, et al. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm cryotolerance in farm animals. Front Vet Sci. 2021; 8: 6091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duru NK, Morshedi M, Schuffner A, Oehninger S. Semen treatment with progesterone and/or acetyl-L-carnitine does not improve sperm motility or membrane damage after cryopreservation-thawing. Fertil Steril. 2000; 74( 4): 715– 20. [DOI] [PubMed] [Google Scholar]
- 88. Ghorbani F, Nasiri Z, Koohestanidehaghi Y, Lorian K. The antioxidant roles of L-carnitine and N-acetyl cysteine against oxidative stress on human sperm functional parameters during vitrification. Clin Exp Reprod Med. 2021; 48( 4): 316– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]