Abstract
Gibberellin (GA) plays a major role in controlling Brassica rapa stalk development. As an essential negative regulator of GA signal transduction, DELLA proteins may exert significant effects on stalk development. However, the regulatory mechanisms underlying this regulation remain unclear. In this study, we report highly efficient and inheritable mutagenesis using the CRISPR/Cas9 gene editing system in BraPDS (phytoene desaturase) and BraRGL1 (key DELLA protein) genes. We observed a loss-of-function mutation in BraRGL1 due to two amino acids in GRAS domain. The flower bud differentiation and bolting time of BraRGL1 mutants were significantly advanced. The expression of GA-regulatory protein (BraGASA6), flowering related genes (BraSOC1, BraLFY), expansion protein (BraEXPA11) and xyloglucan endotransferase (BraXTH3) genes was also significantly upregulated in these mutants. BraRGL1-overexpressing plants displayed the contrasting phenotypes. BraRGL1 mutants were more sensitive to GA signaling. BraRGL1 interacted with BraSOC1, and the interaction intensity decreased after GA3 treatment. In addition, BraRGL1 inhibited the transcription-activation ability of BraSOC1 for BraXTH3 and BraLFY genes, but the presence of GA3 enhanced the activation ability of BraSOC1, suggesting that the BraRGL1-BraSOC1 module regulates bolting and flowering of B. rapa through GA signal transduction. Thus, we hypothesized that BraRGL1 is degraded, and BraSOC1 is released in the presence of GA3, which promotes the expression of BraXTH3 and BraLFY, thereby inducing stalk development in B. rapa. Further, the BraRGL1-M mutant promoted the flower bud differentiation without affecting the stalk quality. Thus, BraRGL1 can serve as a valuable target for the molecular breeding of early maturing varieties.
Introduction
Gene editing techniques are useful in studies investigating gene function and crop enhancement strategies. CRISPR/Cas9 is an emerging, rapidly evolving, and powerful gene-editing technology. Compared with established gene editing technologies, such as zinc finger nucleases and transcription activator-like effector nucleases [1, 2], CRISPR/Cas9 technology is increasingly favored by researchers due to its advantages including simplicity of use, cost-effectiveness, fast operation, targeted mutation, simultaneous editing of multiple target genes, homozygous mutants in the T0 generation, high specificity, and easy mutation detection [3]. To date, the CRISPR/Cas9 system has been established in numerous plants, including Arabidopsis [4], tobacco [5], sorghum [6], wheat [7], rice [3], Zea mays [8], tomato [9], cucumber [10], banana [11], chrysanthemum [12], kiwifruit [13], Brassica carinata [14], switch grass [15]. To address the low efficiency of CRISPR/Cas9 at multi-gene or multi-site editing, researchers combined tRNA and gRNA to form a polycistronic gene, and established tandem two or more sgRNAs on the same expression vector, thus generating a large number of sgRNAs carrying the correct targeting sequence in order to greatly improve mutation efficiency [16]. This system has been utilized successfully in rice, corn, wheat, and other crops [16–18], which has greatly promoted the genetic research of plants and improved crop varieties.
Brassica vegetables are important agricultural and horticultural crops. However, only a few instances of effective genome editing in Brassica vegetables have been documented [14, 19–22]. One of these cases was BcPME37c gene knockout in ‘Youqing 49’ [14, 19–22]. A genome-wide triplication event that occurred in Brassica rapa during evolution produced multicopy genes or numerous substantially related homologous genes [23]. However, research on gene function and molecular breeding in B. rapa is severely constrained due to the difficulty of its genetic transformation compared to that in other Brassica species. Brassica oleracea and B. rapa have a close genetic association. The efficient editing of the cabbage genome by Ma et al. serves as a model for the development of an effective gene editing technology system in B. rapa [24].
Stalks are the main food product of Caixin. Bolting (stem thickening and elongation) and flowering are important stalk developmental traits, both of which are directly related to plant yield and quality [25]. Exogenous gibberellin (GA3) treatment advances the timing of bolting and flowering in Caixin because GA is the primary regulator of these processes [26, 27] by acting through the GA signaling pathway. As a negative regulator of GA signal transduction, DELLA protein is a key factor in modulating the GA response [28]. DELLA proteins are distinguished by a GRAS domain and a DELLA/TVHYNP motif at the N-terminus [29]. DELLA proteins work as nuclear-localized transcriptional regulators, and their accumulation is heavily reliant on the concentrations of GA present within the cell. Increased GA concentrations encourage the polyubiquitination of DELLAs by the 26S proteasome and the GIBBERELLIN INSENSITIVE DWARF1 (GID1) receptor [29–31].
One of the most important functions of DELLA is to regulate plant height. Many DELLA mutants have been identified and most of them are insensitive to GA signals and exhibit dwarfing and delayed flowering, including gai in Arabidopsis [32], Rht in wheat [33], sln1d in barley [34]. Another phenotype of DELLA mutants is a GA-sensitive slender form, whose product does not appear to be able to repress, often referred to as a loss-of-function mutation, including rga and rgl in Arabidopsis [35, 36], slr in rice [37], and sln1c in barley [34]. The molecular mechanism of the DELLA protein in B. rapa may be studied thanks to advancements in studies on its function and GA signal transduction pathways in model plants [38]. We previously isolated five DELLA family genes (RGA1, RGL1, RGA2, RGL2, and RGL3) from Caixin and examined their expression levels in two distinct cultivars. Only one of these, BraRGL1 (BraA02g017510.3.5C), showed significantly varied expression levels at the two-true-leaf stage, suggesting that it may play a role in how various types of early bud differentiation processes affect bolting and flowering [39]. However, its specific functions have not yet been verified.
Vegetative and reproductive growth are synchronous during stalk formation, which is different from the development of Arabidopsis, thereby making the stalk development in Caixin special and complex. Therefore, the establishment of an efficient genome-editing system for Caixin is of great significance for exploring the regulatory mechanisms involved in stalk development. Here, we achieved efficient editing of two genes (BraPDS and BraRGL1) and characterized the function of BraRGL1. We observed that the loss of function of BraRGL promoted early bud differentiation and bolting and was more sensitive to GA3. Protein–protein interaction analyses showed that BraRGL1 interacted with BraSOC1, and exogenous GA3 treatment weakened this interaction. In addition, we determined that the RGL1-SOC1 module regulated bolting and flowering in Caixin by controlling the expression of cell elongation and flower-related genes. These findings provide theoretical and technical support for us to further exploration of the regulatory mechanisms of stem development in B. rapa.
Results
Analysis of the BraPDS mutation efficiency and types
PDS encodes a key enzyme involved in carotenoid production. The albino phenotype caused by its disruption is straightforward to identify [5]. Therefore, we first selected BraPDS as a target to examine the effectiveness of genome modification using the tRNA-processing system in Caixin. Approximately 1800 explants were transformed using an Agrobacterium-mediated method with a vector containing the sgRNA-BraPDS-1234 cassette, and 22 T0 lines were generated. Among them, 16 lines exhibited the completely albino or mosaic albino phenotype, resulting in 72.72% knockout efficiency (Figure S1, Table S1; see online supplementary material). Full-length sequences of BraPDS gene in three albino buds and one albino plant (M1, M2, M3, and M4) were amplified by PCR, and the results were immediately sequenced. Four of the transgenic lines were heterozygous or chimeric mutants with overlapping peaks at the target site of BraPDS gene. We investigated the mutation types and frequencies by performing TA cloning and Sanger sequencing of the PCR products of the four lines. A total of 32 clones were randomly selected for sequencing, among which only four clones had the same wild type (WT) sequence, and all the other clones (87.5%) presented mutations at the target site (Figure S2a, see online supplementary material). All mutations were short insertions or deletions, with the 1 bp insertion being the most prevalent mutation type (Figure S2a and S3, see online supplementary material). The highest mutation frequency of the four target sites was site 4, with up to 71.88%, followed by site 2 with 50.0%, site 3 with a mutation frequency of 6.25%, and no mutagenesis was detected at site 1 (Table S1, see online supplementary material).
The mutated nucleotide sequences were translated into amino acid sequences to investigate mutations at the translational level (Figure S2b, see online supplementary material). All nine mutation types resulted in frameshift mutations that finally caused premature translation termination and gave rise to proteins with only 134, 257, and 260 amino acids in length (Figure S2b, see online supplementary material). The Phytoene desaturase domains of BraPDS were destroyed by each of the aforementioned mutations (Figure S2b, see online supplementary material). The loss of BraPDS gene function in M4 plants caused not only the albino phenotype, but also pollen development defects (Figure S1e, see online supplementary material). No off-target mutation was detected at the top two ranking off-targets (Figure S4, see online supplementary material). These findings suggest that CRISPR/cas9-induced target mutations are highly efficient and specific in B. rapa.
Analysis of the BraRGL1 mutation efficiency and types
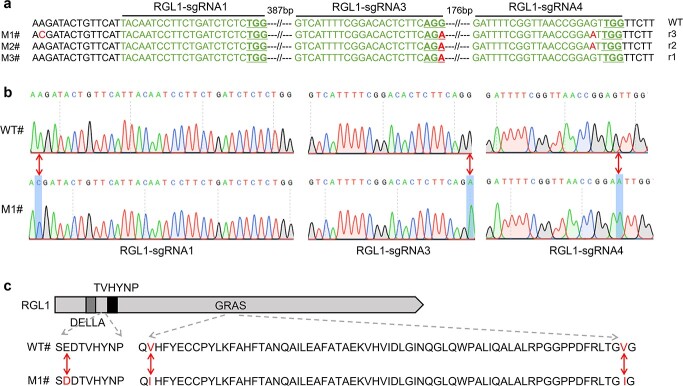
Our earlier research suggested that BraRGL1 might be crucial for bolting and flowering in Caixin in various cultivar variations [39]. We further investigated the biological function of BraRGL1 by knocking out the BraRGL1. Approximately 2000 explants were transformed to generate 19 T0-positive lines. Full-length of BraRGL1 gene in all positive strains was amplified by PCR, and the products were directly sequenced. Twelve transgenic lines were homozygous or heterozygous mutants with base substitutions at target sites and flanking sequences in BraRGL1, resulting in 63.15% knockout efficiency (Table S1, see online supplementary material). Three strains (M1, M2, and M3) were randomly selected for cloning and sequencing. Half of these clones were consistent with the WT, whereas the other half presented mutations at the target sites and their flanking sequences. All mutation types were base substitutions and mainly occurred at target sites 3 and 4. No mutagenesis was detected at site 2 (Figure 1a and b; Table S1, see online supplementary material), which differed from the BraPDS gene modification.
Figure 1.
Mutagenesis types of BraRGL1-mutated lines. a Nucleotide sequence alignment of target sites in wild type (WT) and three BraRGL1 mutants (M1, M2, and M3). The PAM sequence is underlined. Green indicates the target sequences; red indicates mutated bases. b Chromatogram of WT and three homozygous mutants at target sites and flanking sequences in BraRGL1. c Protein sequence alignment between WT and M1 mutants. Red arrows represent substitutions.
Translation of the mutated DNA sequences into amino acid sequences and all three nucleotide substitutions resulted in amino acid mutations (Figure 1c). Notably, there was a mutation site between the DELLA and the TVHYNP domains where glutamate (E) was mutated to aspartic acid (D). And there were two amino acid mutations in the GRAS region of the C-terminus, two valines (V) mutated to isoleucine (I). The number of amino acids between the DELLA and TVHYNP domains is essential for GA signal reception. The GRAS domain is a functional structural region and controls DELLA protein activity [40, 41]. Considering the importance of these three mutation sites, we selected the M1 mutants for further analysis. The genotypes of the M1 offspring (T0 line) were examined to confirm the heritability of the mutations. All 36 T1 descendants of M1 were sequenced, 15 of which were wild-type and the others were heterozygous, and they were identical to those found in the T0 generation (Figure 1a and b). These findings suggest that mutations caused by the tRNA-processing system are inheritable in B. rapa. No off-target mutation was identified at the top two ranking off-targets (Figure S4, see online supplementary material).
BraRGL1 mutation accelerates flower bud differentiation and bolting
We used paraffin sections to investigate the stem tip structure of ‘youlv501’ and ‘youqing 80 day’ two varieties in our previous studies [39]. Neither type differentiated the flower buds completely at the two-true-leaf stage and three-true-leaf stage, but the ‘youlv501’ variety did so at the four-true-leaf stage. Among the six BraDELLA genes, only the expression level of BraRGL1 showed a substantial difference at the two-true-leaf stage, suggesting that BraRGL1 may be involved in the early bud differentiation of different varieties. To further characterize the biological function of BraRGL1, we observed the stem tip structure of BraRGL1 mutants at the three-true-leaf stage using paraffin sections. WT plants did not initiate flower bud differentiation and were in the leaf primordium stage in the three-true-leaf stage, whereas BraRGL1 mutants had differentiated flower primordium (Figure 2a). Further statistics on the bolting and flowering phenotypes showed that the flowering time of BraRGL1 mutants was significantly earlier than that of WT (Figure 2b and c), and the expression levels of GA-regulated gene (BraGASA6: BraA02g023240.3.5C), flowering-related genes (BraSOC1: BraA05g005290.3.5C) and (BraLFY: BraA02g045080.3.5C), expansion gene (BraEXPA11: BraA07g016390.3.5C), and xyloglucan endotransferase gene (BraXTH3: BraA07g008170.3.5C) were significantly upregulated in the stem tip of BraRGL1 mutant (Figure 2e). These findings suggest that the mutation in BraRGL1 accelerates early flower bud differentiation, thereby encouraging bolting and flowering.
Figure 2.
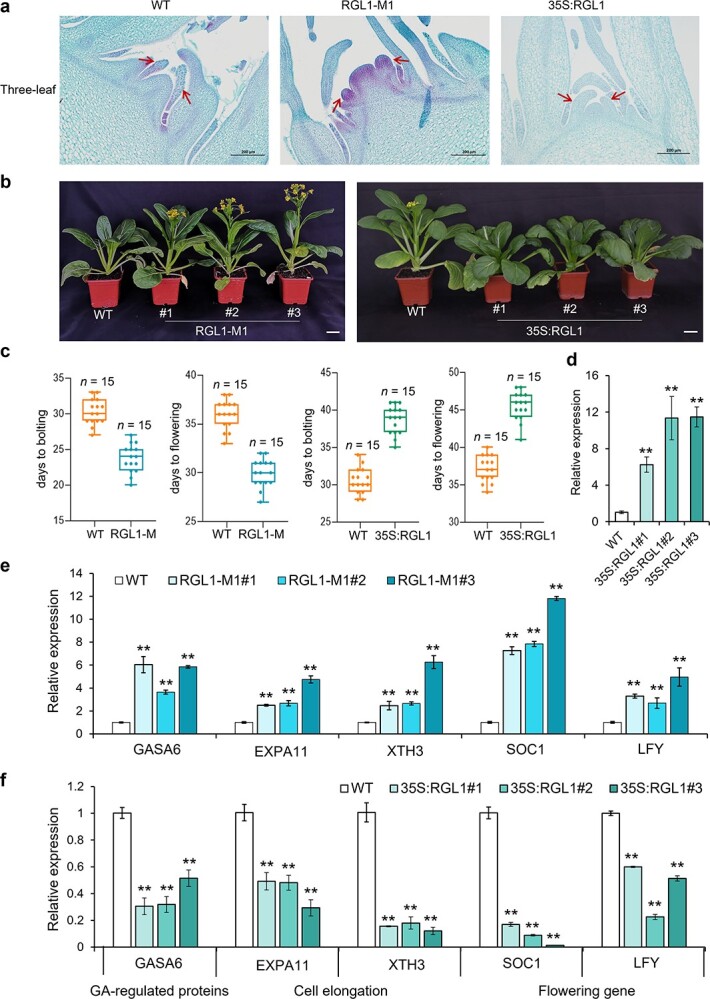
BraRGL1 negatively regulates the expression of bolting- and flowering-related genes to control bolting. a Stem tip longitudinal structures of BraRGL1-M knockout lines 35S:BraRGL1 overexpression lines in the three-true-leaf stag. The red arrow points to the leaf or flower primordium. Scale bar = 200 μm. b Phenotypic of the WT, BraRGL1-M, and 35S:BraRGL1 lines. Scale bar = 5 cm. c Quantification of bolting and flowering time in BraRGL1-M and 35S:BraRGL1 lines. The number of studied accessions for each line is given above the graph. d Relative expression of BraRGL1 in 35S:BraRGL1 lines. e Relative expression of GA-regulated protein (BraGASA6), flowering-related genes (BraSOC1 and BraLFY), and expansion-related genes (BraEXPA11 and BraXTH3) in BraRGL1-M knockout lines at bolting stage compared to the levels in WT. f Relative expression levels of bolting- and flowering-related genes at the bolting stage in the 35S:BraRGL1 overexpression lines compared with that in WT. Data are presented as the mean ± standard deviation (n = 3). Significant deviations from the control determined using Student’s t-test (e and f) (**P < 0.01).
To further validate our experimental results in the BraRGL1 mutants, we generated BraRGL1 overexpression lines under the control of the CaMV 35S promoter. The bolting and flowering time of 35S:BraRGL1 lines were significantly delayed, and the expression levels of BraRGL1 in the stem tip were significantly increased, while the expression levels of bolting and flowering-related genes were significantly decreased (Figure 2b–d and f). These results further confirmed that BraRGL1 negatively regulates bolting- and flowering-related genes to control bolting.
BraRGL1 loss-of-function mutants are more sensitive to GA signaling
GA signal transduction is negatively regulated by the BraRGL1 proteins and the BraRGL1 loss of function mutation weakens its inhibitory effect, thereby accelerating the bolting. Subcellular localization analysis revealed that BraRGL1 was localized in the nucleus (Figure 3a). Expression levels of the GA-regulated protein BraGASA6 were significantly increased in BraRGL1 mutants (Figure 2e), suggesting that its loss of function may regulate GA signal transduction. We previously showed that BraRGL1 interacts with BraGIDb in the presence of GA3 (200 mg/L) [39]. To further explore whether the BraRGL1 mutation affects the sensitivity to GA signaling, we determined the degree of interaction BraRGL1 and BraRGL1-M with BraGID1b in the presence of GA3 (100 mg/L). BraRGL1 and BraGID1b showed weak interactions, while BraRGL1-M presented strong interactions with BraGID1b (Figure 3b). The addition of a dose gradient of GA3 further confirmed the results (Figure S5, see online supplementary material). These findings indicated that BraRGL1-M was more sensitive to GA3 signaling. The sensitivity of BraRGL1 mutant to GA signaling was further verified by hypocotyl elongation experiments. Seeds of the WT and BraRGL1 mutants were sown on seeding medium with or without GA3 (100 mg/L). On the third day after sowing, there was no significant difference in the hypocotyl length of WT on the medium with or without GA3, whereas the hypocotyl length of BraRGL1 mutant on GA3-containing seeding medium was significantly longer than that of the control (without GA3) and WT (Figure 3c and d). These results further confirmed that BraRGL1-M mutants were more sensitive to GA3 signaling.
Figure 3.
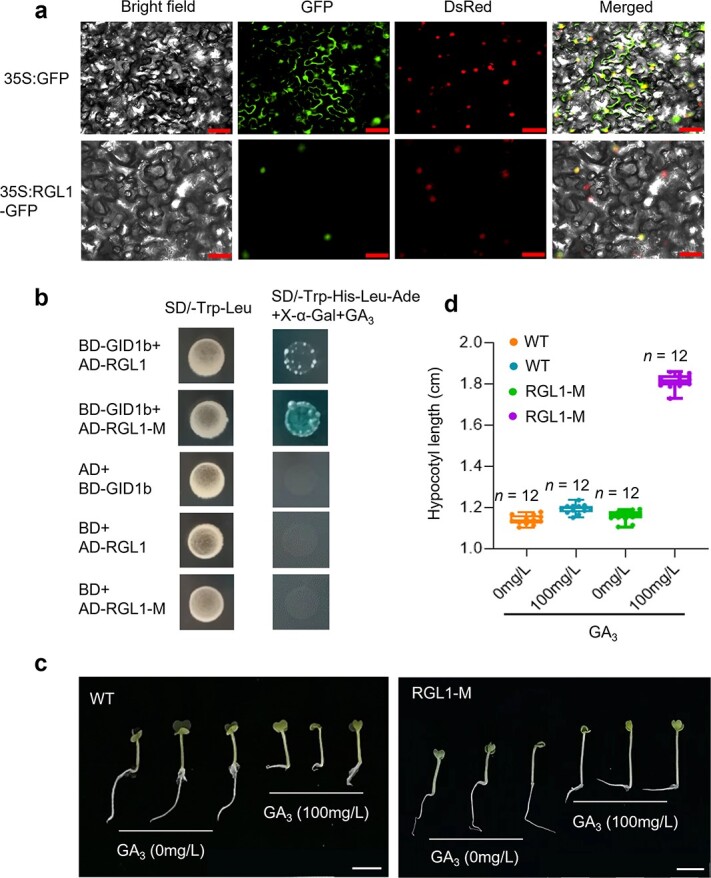
GA3 sensitivity of the BraRGL1 mutants. a Subcellular localization of BraRGL1 in Nicotiana benthamiana. DsRed was used to stain the nuclei. Scale bar = 50 μm. b Detection of interactions between BraRGL1 proteins and BraGID1b after treatment with 100 mg/L GA3. BraRGL1-M represents the mutated protein. AD and BD represent empty pGADT7 and pGBKT7, respectively. SD/−Trp-Leu means medium lacked tryptophan and leucine; SD/−Trp-His-Leu-Ade means medium lacked tryptophan, histidine, leucine, and adenine. c Sensitivity of BraRGL1 mutants to GA3 determined using a hypocotyl elongation assay with 100 mg/L GA3 treatment. Scale bar = 1 cm. d Quantification of hypocotyl lengths, as in Figure 2. n, number of hypocotyls.
GA3 attenuates the interaction between BraRGL1 and BraSOC1
BraRGL1-M mutants exhibit an early bolting and flowering phenotype because they are more sensitive to GA, indicating GA affects bolting and flowering through BraRGL1 proteins, but the underlying molecular mechanism is not clear. DELLA is thought to lack a DNA-binding domain (DBD), indicating that it might play its negative regulatory effects through interacting with other transcription factors [42, 43]. In our previous study, the flowering-promoting factor BraSOC1 positively regulated bolting and flowering by upregulating BraEXPA11, BraXTH3, and BraLFY upon exogenous GA3 treatments [44]. In the present study, BraRGL1 negatively regulated the expression of these genes, thus affecting bolting and flowering. Therefore, we hypothesized that BraRGL1 interacts with BraSOC1 to control bolting. We tested this hypothesis by performing a yeast two-hybrid (Y2H) assay between the BraRGL1 and BraSOC1 proteins. We discovered that BraRGL1 and BraSOC1 interacted to create heterologous dimers (Figure 4a). A bimolecular fluorescence complementation (BiFC) assay was performed in vivo to confirm results of the Y2H assay. The association between BraRGL1 and BraSOC1 was validated by GFP fluorescence in the nuclei of plant cells (Figure 4b). We further examined the interaction of BraRGL1 and BraSOC1 after GA3 treatment and observed that GA3 significantly attenuated the GFP signal (Figure 4c). In addition, GA3 administration drastically reduced the 35S: RGL1-GFP fluorescence (Figure 4c). Combined with the signal transduction mechanism of GA3 in model plants, we speculate that increased GA3 concentration resulted in the degradation of DELLA protein and the release of BraSOC1 from the BraRGL1 and BraSOC1 dimer.
Figure 4.
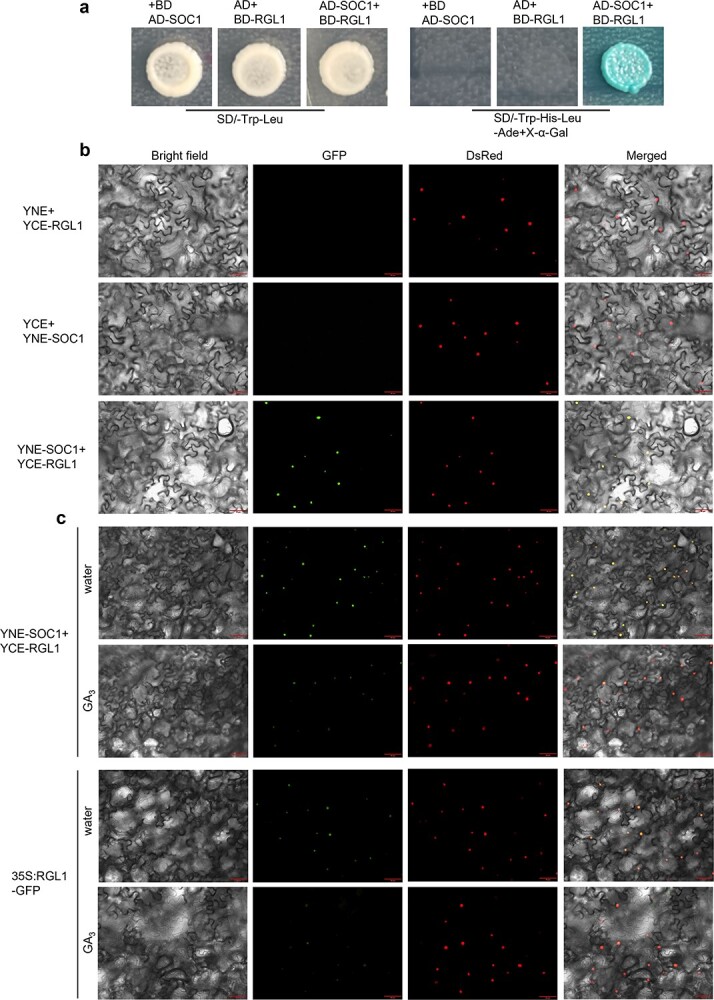
BraRGL1 interacts with BraSOC1. a Yeast two-hybrid assay for protein–protein interactions between BraRGL1 and BraSOC1. AD and BD represent empty pGADT7 and pGBKT7, respectively. SD/−Trp-Leu means medium lacked tryptophan and leucine; SD/−Trp-His-Leu-Ade means medium lacked tryptophan, histidine, leucine, and adenine. b The interaction between BraRGL1 fusing to C-termini of YFP and BraSOC1 fusing to N-termini of YFP was detected by bimolecular fluorescence complementation assay. c Bimolecular fluorescence and 35S: RGL1-GFP fluorescence after GA3 treatment. Leaves were sprayed with water or GA3 1 h before observation of the signals. DsRed was used to stain the nuclei. Scale bar = 50 μm.
BraRGL1 inhibits the transcriptional regulation of BraSOC1
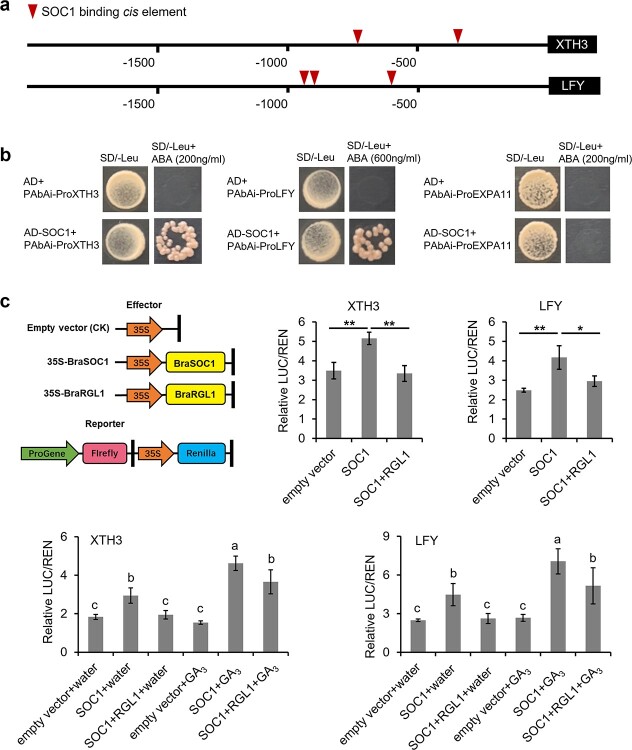
We further identified the target genes regulated by the BraRGL1 and BraSOC1 interactions by analyzing the promoter regions of BraEXPA11, BraXTH3, and BraLFY. The promoter regions of BraXTH3 and BraLFY contained two and three SOC1-binding cis-elements, respectively, whereas the promoter region of BraEXPA11 did not (Figure 5a). These elements were located 720 bp and 368 bp upstream of the BraXTH3 start codon and 959, 928, and 677 bp upstream of the BraLFY start codon (Figure 5a). We next performed Y1H assays to assess potential BraSOC1 binding to the target genes promoter. BraSOC1 bound to the both BraXTH3 and BraLFY promoter fragments containing SOC1-binding cis-elements but did not interact with the BraEXPA11 promoter (Figure 5b; , Figure S6, see online supplementary material). We further verified their transcriptional regulation of BraRGL1 and BraSOC1 by using a dual luciferase assay. BraSOC1 bound to the promoters of BraXTH3 and BraLFY to induce their transcription, whereas the presence of BraRGL1 inhibited this transcriptional capacity of BraSOC1 (Figure 5c), indicating that BraSOC1 regulates the expression levels of these two genes by interacting with BraRGL1. In addition, GA3 enhanced the transcriptional activation capacity of BraSOC1 (Figure 5c), indicating that increased GA3 concentrations resulted in the release of BraSOC1 from the BraRGL1 and BraSOC1 dimer, which upregulates the expression of BraXTH3 and BraLFY.
Figure 5.
Validation of BraSOC1 and BraRGL1 regulation of BraXTH3 and BraLFY. a Location of the SOC1-binding elements in the promoter of BraXTH3 and BraLFY. b Yeast one-hybrid assays identify the interaction of BraSOC1 with the promoter of BraXTH3 and BraLFY. AD represents empty pGBKT7. SD/−L means medium lacking leucine. c Dual luciferase assay to detect BraSOC1, BraRGL1 and their interaction regulate the transcription of BraXTH3 and BraLFY. Empty vector was used as the negative control. Leaves were sprayed with water or GA3 1 h before determination of luciferase activity. Data are presented as the mean ± standard deviation (n = 3). Student’s t test was used to identify significant differences compared to the control (*P < 0.05 and **P < 0.01).
We further examined the interaction between BraRGL1-M and BraSOC1, and the effect of variation in the interaction intensity on downstream gene transcription. Y2H assay showed that the interaction intensity of BraRGL1-M with BraSOC1 is stronger than that of BraRGL1 with BraSOC1 (Figure S7, see online supplementary material). However, DLR assay showed that BraSOC1’s transcriptional activation ability on downstream genes did not change significantly in the presence of BraRGL1-M (Figure S8, see online supplementary material), that is, BraRGL1-M could not inhibit BraSOC1’s transcriptional activation ability on downstream genes, indicating that BraRGL1-M may have lost its inhibitory function. This is consistent with the phenotype of BraRGL1-M mutant with early bolting.
BraRGL1-M mutants advance flower bud differentiation without affecting stalk quality
The stalk is not only the main part of the product organ but also the nutrient storage organ in Caixin. Carbohydrates were the main nutrient component in Caixin, and soluble sugars and vitamin C in stalks were significantly higher than those in the leaves [45]. To test whether early flower bud differentiation of BraRGL1-M mutants would reduce the quality of stalks, we determined the growth indicators and nutritional indicators in BraRGL1-M mutants. The plant height of the BraRGL1-M mutants was slightly higher than that of the WT, while the stem diameter was slightly lower than that of the WT, but the difference was not significant (Figure 6a and b), and there were no significant differences in fresh and dry weight between BraRGL1-M mutants and WT (Figure 6c and d). In addition, the contents of soluble sugar and nitrate in the BraRGL1-M mutants were slightly higher than those in the WT, while soluble protein and vitamin C contents were slightly lower than those in the WT, but the difference was not significant (Figure 6e–h). These results indicated that BraRGL1-M mutants promoted flower bud differentiation without affecting the stalk quality, which shortened the growth cycle of Caixin while maintaining yield, providing a scientific basis for further breeding of excellent early maturing varieties.
Figure 6.
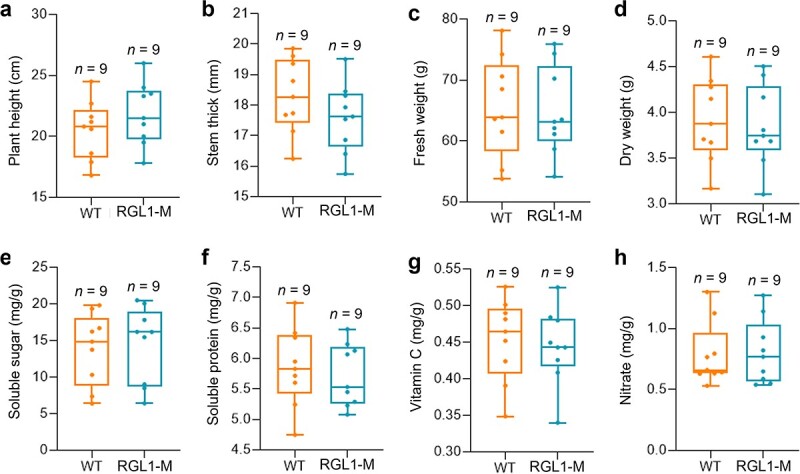
Determination of growth and nutritional indicators of WT and BraRGL1 mutants. a Plant height. b Stem thick. c Fresh weight. d Dry weight. e Soluble sugar content. f Soluble protein content. g Vitamin C content. h Nitrate content. n, number of plants.
Discussion
The CRISPR/Cas9 system has several advantages, enabling almost all genes to be edited; however, the gene targeting efficiency is exhibited in a species-dependent or cell-type-dependent manner [46]. Earlier reports in Fastcycling B. oleracea DH1012 showed only 10% editing efficiency in the GA4 gene [19], which is far lower than the mutation efficiency of 91.6% in rice [47], 89% in Arabidopsis [48], 87.5% in tobacco [49], 87.5% in petunia [50], and 86.4% in poplars [51]. BoPDS and BoSRK high-efficiency mutagenesis (68% and 100%, respectively) were achieved using the CRISPR/Cas9 gene editing system based on endogenous tRNA processing in B. oleracea [24]. In this study, we achieved efficient inheritable mutagenesis (72.72% and 63.15%) in ‘youlv501’, which was higher than the mutation efficiency of 20%–56% of the three sgRNAs in ‘Youqing 49’ [22]. These results indicate that the CRISPR/Cas9 gene editing system with endogenous tRNA processing is suitable for efficient mutagenesis in B. rapa, providing an important technical strategy for gene function identification and functional gene mining of B. rapa and other Brassica vegetables.
Five DELLA genes have been identified in Arabidopsis: GAI, RGA, RGL1, RGL2, and RGL3 [52, 32]. GAI and RGA inhibit stem elongation and flower development [34, 37]. RGL2 is a major negative regulator of seed germination [35]. RGL1 can enhance the roles of RGA and RGL2 in flower development [53]. RGL3 plays a positive regulatory role in stress resistance [54]. These results suggest that they have redundant and distinct purposes [55, 56]. In this study, flower bud differentiation and bolting time of BraRGL1 loss-of-function mutants were significantly advanced, and overexpressed plants showed opposite phenotypes, suggesting that BraRGL1 is a key factor regulating bolting and flowering in B. rapa.
A crucial regulating mechanism in the GA signaling pathway is GA-induced DELLA degradation [30, 31]. GID1 acquires the ability to interact with DELLA by binding to active GAs, enabling further interaction with the F box protein. DELLA is polyubiquitinated by E3 ubiquitin ligase SCFSLY1/GID2 and is finally degraded by the 26S proteasome. GID2 in rice and SLY1 in Arabidopsis bind to DELLA proteins in the presence of GA and promote DELLA protein degradation, thereby activating the GA response [57, 58]. Accordingly, our study showed that BraRGL1 interacted with BraGID1b in the presence of GA3. In addition, the interaction between BraRGL1-M and BraGID1b was stronger under the same concentration of GA3 treatment, and hypocotyl of the BraRGL1-M mutant was significantly extended. This is consistent with the idea that DELLA loss-of-function mutants are more sensitive to GA [34–37]. DELLA and TVHYNP domains are critical GA signal sensing domains, but amino acid sequences are not conserved. Therefore, GA-sensitive BraRGL1-M mutation may be attributed to the substitution of two amino acids in GA signal suppression region (GRAS domain), which may lead to the loss of DELLA protein inhibitor function [40, 41].
Because DELLA is thought to lack a DBD, intermediate proteins that mediate DELLA/DNA interactions are thought to be required for the activation of DELLA target genes [42, 43]. DELLA and FLC directly interact with each other and probably function in a large complex to repress the target gene expression, thus regulating flowering transition in Arabidopsis [59]. In this study, BraRGL1 was found to directly interacts with BraSOC1. BraSOC1 is crucial for controlling bolting and stem elongation in Caixin [44], indicating that the potential bolting and flowering mechanism of B. rapa may be different from that of Arabidopsis with particularity and complexity. In addition, GA induces the rapid degradation of DELLA proteins by 26S proteasome, resulting in a reduction in the interaction strength of the protein dimers [43, 60]. The bimolecular fluorescence complementation assay when imposing GA3 supported the idea that increased GA levels promoted the GID1 receptor-mediated ubiquitination degradation of DELLA proteins, thereby releasing BraSOC1. Therefore, the BraRGL1-BraSOC1 module regulates the bolting and flowering by modulating the GA signal transduction pathway.
Increased cell expansion may be the cause of stalk elongation of Caixin [44]. BraEXPA11 and BraXTH3 are the key factors involved in cell expansion [61, 62]. EXPA11 enabling cell wall expansion by reducing the viscosity of polysaccharides between cell walls [63]. XTH3 regulates cell wall relaxation through cleaving xyloglucan chains [64]. GA3 treatment decreased the expression of the BraRGL1 genes and increased the expression of BraSOC1, BraGASA4, BraEXPA11, and BraXTH3 [39, 44]. GA stimulates the expression of EXPA and XTH to encourage cell wall relaxation and elongation [65]. LFY is a flowering factor that acts downstream of SOC1 in Arabidopsis [66]. In this study, in addition to BraLFY, we also identified another target gene of BraSOC1, BraXTH3, which is closely related to the function of BraSOC1 in regulating stem elongation [44]. We determined that BraSOC1 binds directly to the BraXTH3 and BraLFY promoters. Although BraEXPA11 showed a consistent expression pattern with BraSOC1, BraXTH3, and BraLFY in BraRGL1 mutants and overexpressed plants, there was no SOC1 binding site, suggesting indirect regulation. However, this conclusion needs to be confirmed by further experiments, as we did not examine other copies of BraEXPA11. DELLA interacts with PIF3 and PIF4 and blocks their DNA-binding, whereas GA-induced DELLA degradation promotes the activation of PIF3 and PIF4 to target genes, thus promoting Arabidopsis hypocotyl cell extension [43, 67]. In this study, the presence of BraRGL1 inhibited the activation ability of BraSOC1 for BraXTH3 and BraLFY transcription, whereas GA3 enhanced the activation ability of BraSOC1, suggesting that the BraRGL1-BraSOC1 module regulates the bolting and flowering through controlling the expression of BraXTH3 and BraLFY. Based on these findings, we hypothesized that the increase of GA3 content causes the degradation of BraRGL1 protein, which releases BraSOC1, thus upregulating the expression of BraXTH3 and BraLFY genes, finally promoting bolting and flowering in B. rapa (Figure 7).
Figure 7.
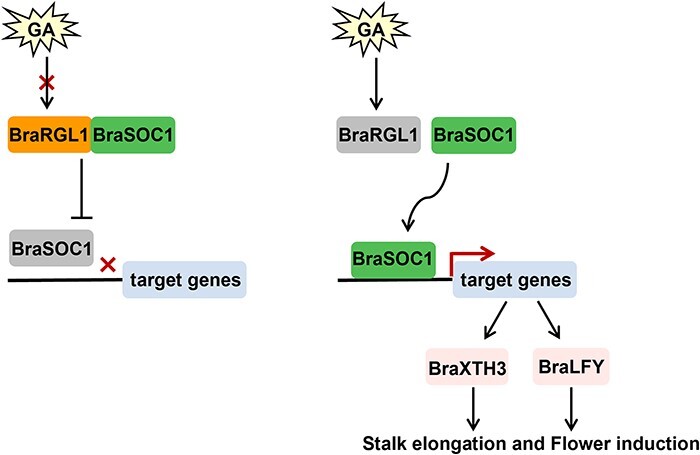
Schematic model of the BraRGL1-mediated GA pathway regulating bolting and flowering in Brassica rapa. Increased GA3 concentration results in BraRGL1 degradation and the release of BraSOC1 from the BraRGL1 and BraSOC1 dimer, which upregulates the expression of BraXTH3 and BraLFY, thus promoting bolting and flowering.
There have been many studies on GA-induced ubiquitination degradation of DELLA protein, but the specific mechanism of DELLA protein action remains unclear, such as how DELLA protein interacts with downstream genes and how it inhibits transcriptional activity of downstream genes. In this study, BraRGL1-M had stronger interaction with BraSOC1, while BraRGL1-M did not inhibit the transcriptional activation of BraSOC1 (Figs ure S9 and ure S10, see online supplementary material). This indicated that BraRGL1-M only lost its inhibitory function but did not lose the ability of protein interaction. In addition, rga-1 and sln1c accumulate mutated DELLA proteins, but they also lack repressive function [68]. These results indicated that the inhibition effect of DELLA protein on downstream genes may not be realized through simple protein accumulation or interaction. In addition, it was reported that the inhibition effect of DELLA protein on downstream genes may be related to protein phosphorylation and ubiquitination [60], which also indicated the complexity of the mechanism of DELLA protein and interacting proteins and that is what we will tackle next.
Early flower bud differentiation can significantly shorten the growth cycle of B. rapa, which is of great economic significance. In this study, although flower bud differentiation was advanced in BraRGL1-M mutants, growth and nutrition indices were not affected (Figure 6). This indicates that BraRGL1-M mutants may have applications in breeding. The gene TaDEP1 that controls inflorescence formation, spike grain growth, and grain yield of wheat was knocked out using CRISPR/Cas9 technology to shorten inflorescence internode length, increase grain number per spike, and increase grain yield [69]. Shi et al. obtained maize varieties with high yield and drought tolerance by knocking out the ARGOS, which regulates ethylene biosynthesis and signal transduction [70]. Using the CRISPR/Cas9 system to knock out the tomato flowering inhibitory gene SP5G can quicken tomato blossoming and enhance the compact limited growth habit, leading to rapid fruit ripening [71]. In addition, the stability and inheritance of mutations are crucial for the generation of mutants using the CRISPR/Cas9 system [13, 19]. In the present study, the CRISPR/Cas9 system-induced genomic mutations in B. rapa were stable and inheritable, which laid the foundation for further segregation of excellent early maturing varieties.
In summary, we demonstrated that the CRISPR/Cas9 system based on endogenous tRNA processing is an effective tool for studying gene function in B. rapa, achieving high efficiency and inheritable mutagenesis of multiple targets. BraRGL1 plays a crucial biological role in the early flower bud differentiation of B. rapa. BraRGL1 and BraSOC1 interact to regulate the expression of BraXTH3 and BraLFY, thereby controlling the bolting and flowering. These findings expand our understanding of the regulatory mechanisms that underlie bolting and flowering in B. rapa. In addition, BraRGL1-M mutant promoted flower bud differentiation without affecting the stalk quality, which provides a scientific basis for the further application of breeding strategies to control this important trait.
Materials and methods
Vector construction
The target sites of BraPDS and BraRGL1 were designed as described by CRISPR-GE (http://skl.scau.edu.cn/) [72]. Four optimal target sites were selected according to sequence, position, positive and negative strands, GC content, potential off-target sites, and valuation information of the candidate target sites. Complementary oligos of the target sequences were synthesized, and double-stranded DNA fragments were formed after denaturation (95°C for 5 min) and renaturation (room temperature for 1 h). Four target sites were then cloned into four sites (BbsI, BsaI, BsmBI, and BfuAI) of the tRNA-sgRNA vectors to generate At6–26::tRNA-sgRNA-BraPDS-1234 and At6–26::tRNA-sgRNA-BraRGL1–1234 expressing cassettes. Finally, gene editing vectors of BraPDS and BraRGL1, respectively, were created by cloning the expressing cassettes into the BamHI and EcoRI sites of the pCACas9 vector (Figure S9, see online supplementary material).
Agrobacterium-mediated transformation in B. rapa
Caixin (B. rapa ssp. Chinensis var. parachinensis) is a varietas of pak choi originally from South China. A highly inbred line ‘youlv501’ from our laboratory was used in this study for genetic transformation. Caixin was subjected to cotyledon transformation using a previously reported procedure [44] (Figure S10, see online supplementary material). Briefly, three days after emergence, the cotyledons of sterile seedlings were removed. The cotyledon explants were subsequently pre-cultured medium to initiate callus growth for 3 days. The preincubated cotyledons were then transferred to the co-cultivation medium in the dark for 3 days after being infected with liquid medium containing Agrobacterium (GV3101 strain containing corresponding plasmids, OD600 = 0.6) for 10 min. After inhibition of Agrobacterium for 7 days, the explants were placed in selection medium [co-cultivation medium with 4 mg/L phosphinothricin (PPT) or 10 mg/L Kanamycin (Kan)]. The PPT-resistant shoots were placed in the rooting medium upon reaching a height of 2–3 cm.
Mutation detection
Genomic DNA was extracted from the leaves of individual PPT-resistant plants. DNA from the positive transgenic plants served as the template for PCR amplification using gene-specific primers, and the PCR products were directly sequenced for indel detection. PCR amplicons with double peaks were ligated into the pMD19-T vector, and eight monoclones were randomly selected for further sequencing to determine the mutation pattern.
Off-target evaluation
Potential off-target sites were predicted using the CRISPR-GE system (http://skl.scau.edu.cn/) [72]. Two potential off-target sites with the highest off-target risk were selected for further confirmation, and they contained less than or equal to 4-bp mismatches in the 12-bp seed sequence with the target sites. Using gene-specific primers, three putative off-target regions were cloned and the PCR results were examined using Sanger sequencing. DNAMAN software was used to examine sequencing outcomes.
The generation and identification of 35S: BraRGL1 lines
To create the 35S: BraRGL1 overexpression vector, the full-length CDS of BraRGL1 without stop codons was cloned into the XbaI and BamHI sites of the pBI121-GFP vector. The resulting construct was introduced into the GV3101 strain of Agrobacterium, which subsequently used the cotyledon method to convert it into Caixin. Genomic DNA was extracted from the Kan-resistant plants using the CTAB method. Specific primers were used to amplify the target gene, and the resulting PCR products were sequenced and aligned.
Quantitative reverse transcription PCR (qRT–PCR) analysis
Total RNA was extracted from the stem tips of WT and BraRGL1 transgenic plants, and qRT–PCR analyses were carried out using ChamQ SYBR Color qPCR Master Mix under the following PCR conditions: 5 min at 95°C, 40 cycles of 10 s at 95°C, 30 s at 55°C, 20 s at 72°C. The internal reference gene for gene expression analysis was glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Subcellular localization
Full-length coding sequences without stop codons of BraRGL1 were cloned into the AgeI site of the pEAQ-EGFP vector and fused with green fluorescent protein (GFP). Young tobacco leaves were invaded by the GV3101 strain for 2 days, which included nuclear localization signal (NLS-DsRed) and the necessary constructs. A laser-scanning confocal microscope was used to detect GFP fluorescence at 448 nm. At 550 nm, DsRed was observed to represent the nucleus.
Histological analysis of the BraRGL1 mutants
The stem tips (5 mm) of WT and BraRGL1 mutant plants were collected and immersed in FAA fixative solution (70% alcohol: acetic acid: formaldehyde = 90:5:5) for 20 min before being vacuum pumped and incubated at 4°C. The materials were dehydrated in 70% alcohol for two days before being embedded in paraffin. The slices were then stained with a reddish-green dye so that the cell structure could be seen.
Yeast two-hybrid assay (Y2H)
A combination of stem and leaf cDNA from WT and BraRGL1 mutant plants was used to amplify the full-length coding sequences of BraRGL1, BraRGL1 mutant gene (BraRGL1-M), and BraGID1b. To create yeast two-hybrid vectors, the full-length CDSs of BraRGL1 and BraRGL1-M were cloned into EcoRI and BamHI sites of pGADT7 vector, and the full-length CDS of BraGID1b were cloned into EcoRI and BamHI sites of pGBKT7 vector. Yeast strain Y2Hgold was transformed with the recombinant vector to produce fusion proteins. Diploids were selected on SD/−Trp-Leu medium and interactions were validated on SD/−Trp-Leu-His-Ade medium with X-a-Gal. The sensitivity of BraRGL1 and BraRGL1-M to GA3 was validated by the interaction of BraRGL1 and BraRGL1-M with BraGID1b in the presence and absence of GA3.
Hypocotyl elongation assay
The sterile seeds of WT and BraRGL1 mutants were seeded on seeding medium with or without 0.3 mM GA3 (MS medium containing 1/2 MS, 1% sucrose and 0.6% agar [pH 5.8]). On the third day after sowing, the hypocotyl elongation was observed and photographed. Hypocotyl length was measured using Image J software. According to the hypocotyl elongation of the WT and BraRGL1 mutants, the sensitivity of BraRGL1 mutants to GA3 was verified.
Bimolecular fluorescence complementation (BiFC) assay
To create fusion proteins, the full-length CDSs of BraRGL1 and BraSOC1 without stop codons were cloned into BamHI and SalI sites at the N- or C-termini of the pSPYNE-35S and pSPYCE-35S vectors, respectively. The recombinant vectors were transferred into the Agrobacterium strain GV3101. Young tobacco leaves also harbored the Agrobacterium strain carrying DsRed and the recombinant plasmid. DsRed protein and GFP fluorescence were observed at 550 and 448 nm, respectively, after two days of incubation.
Yeast one-hybrid assay (Y1H)
Full-length CDS of BraSOC1 (BraA05g005290.3.5C) was cloned into pGADT7 as a prey vector. The promoter fragments containing the SOC1-binding cis-elements of the target genes (BraA07g016390.3.5C, BraEXPA11; BraA07g008170.3.5C, BraXTH3, and BraA02g045080.3.5C, BraLFY) were individually cloned into HindIII and KpnI sites of the pAbAi bait vectors. The linearized pAbAi constructs were transformed into the Y1H Gold yeast strain and incubated on SD/−Trp medium at 30°C for three days. Positive clones were collected and inoculated on the SD medium lacking Leu (SD/−Leu), with or without aureobasidin A at the selected concentration. After 2–3 days, the binding activity of BraSOC1 to the target genes was evaluated.
Dual-luciferase assay (DLR)
Full-length coding sequences of BraRGL1 and BraSOC1 were cloned into BamHI and EcoRI sites of pGreenII 62-SK vector. The promoter fragments containing the SOC1-binding cis-elements of BraEXPA11, BraXTH3, and BraLFY were cloned into KpnI and NcoI sites of pGreenII0800-LUC vector, respectively. Young tobacco leaves were infiltrated with the effector and reporter of the GV3101 Agrobacterium strain for 3 days. Firefly LUC and Renilla LUC (REN) activity was measured using the Dual-Luciferase® Reporter Assay System (Promega, Madison, USA).
Growth and phytochemical determination
The plants were harvested 37–39 days after sowing, and fresh and dry weights (1 h at 105°C and 48 h at 75°C before determination.) were measured (nine biological replicates per treatment). A ruler was used to measure plant height (cm), a Vernier caliper was used to measure stem thickness (mm), and an electronic balance was used to measure dry and fresh weights.
Distilled water (10 mL) and fresh frozen samples (0.5 g) were incubated in a boiling water bath for 30 min. Then, 5 mL of vitriol, 0.5 mL of anthrone ethyl acetate and 1.9 mL of distilled water were combined with 0.1 mL of the supernatant. After cooling, the soluble sugar content was measured using a UV spectrophotometer at 630 nm [73].
Fresh frozen tissue (0.2 g) was added to 5 mL of distilled water. The supernatant was centrifuged, then diluted with the same amount of distilled water before adding 4 mL of Coomassie brilliant blue G-250 solution. The soluble protein content was measured using a UV spectrophotometer at 595 nm [74].
Fresh frozen samples (0.5 g) were crushed into pulp with 1 mL of 15% potassium ferrocyanide, 1 mL of 30% zinc sulfate, and 3 mL of 1% oxalic acid. Phosphate-acetic acid (1 mL), 5% vitriol (2 mL), and ammonium molybdate (4 mL) were combined with 10 mL of extraction solution. After 15 min, vitamin C content was determined at 500 nm using a UV–visible spectrophotometer [75].
Freshly frozen tissue (0.2 g) was boiled for 30 minutes after soaking in 10 mL of distilled water. After the extract was filtered, 0.1 mL of the extraction solution containing 0.1 mL of salicylic and sulfuric acid (5%) and 9.5 mL of NaOH (8%) was added. A UV spectrophotometer was used to detect the nitrate content at 410 nm [76].
Supplementary Material
Contributor Information
Yudan Wang, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Shiwei Song, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Yanwei Hao, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Changming Chen, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Xi Ou, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Bin He, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Jiewen Zhang, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Zhehao Jiang, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Chengming Li, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Shuaiwei Zhang, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Wei Su, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Riyuan Chen, Key Laboratory of Horticultural Crop Biology and Germplasm Innovation in South China, Ministry of Agriculture, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32072656, 31972481), the Key-Area Research and Development Program of Guangdong Province, China (2020B0202010006), and the China Agriculture Research System of MOF and MARA.
Author contributions
Y.W., X.O., B.H., J.Z., Z.J., C.L., and S.Z. performed the research; S.S., W.S., and R.C. designed the research. Y.W., S.S., and Y.H. analysed the data, and Y.W. wrote the manuscript. S.S., Y.H., C.C., W.S., and R.C. assisted with revising the manuscript. All authors have read and approved the final version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Conflict of interest statement
The authors declare no conflicts of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
References
- 1. Xiong JS, Ding J, Li Y. Genome-editing technologies and their potential application in horticultural crop breeding. Hortic Res. 2015;2:15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sprink T, Metje J, Hartung F. Plant genome editing by novel tools: TALEN and other sequence specific nucleases. Curr Opin Biotechnol. 2015;32:47–53. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Zhang J, Wei Pet al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol J. 2014;12:797–807. [DOI] [PubMed] [Google Scholar]
- 4. Feng Z, Mao Y, Xu Net al. Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc Natl Acad Sci U S A. 2014;111:4632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nekrasov V, Staskawicz B, Weigel Det al. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:691–3. [DOI] [PubMed] [Google Scholar]
- 6. Jiang W, Zhou H, Bi Het al. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013;41:e188–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Cheng X, Shan Qet al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol. 2014;32:947–51. [DOI] [PubMed] [Google Scholar]
- 8. Liang Z, Zhang K, Chen Ket al. Targeted mutagenesis in Zea mays using TALENs and the CRISPR/Cas system. J Genet Genomics. 2014;41:63–8. [DOI] [PubMed] [Google Scholar]
- 9. Xu C, Park SJ, Eck JVet al. Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev. 2016;30:2048–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu B, Li D, Liu Xet al. Engineering non-transgenic Gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant. 2017;10:1575–8. [DOI] [PubMed] [Google Scholar]
- 11. Kaur N, Alok A, Shivani et al. CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome. Funct Integr Genomics. 2018;18:89–99. [DOI] [PubMed] [Google Scholar]
- 12. Kishi-Kaboshi M, Aida R, Sasaki K. Generation of gene-edited Chrysanthemum morifolium using multicopy transgenes as targets and markers. Plant Cell Physiol. 2017;58:216–26. [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Wang S, Li Det al. Optimized paired-sgRNA/Cas9 cloning and expression cassette triggers high-efficiency multiplex genome editing in kiwifruit. Plant Biotechnol J. 2018;16:1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchner TW, Niehaus M, Debener Tet al. Efficient generation of mutations mediated by CRISPR/Cas9 in the hairy root transformation system of Brassica carinata. PLoS One. 2017;12:e0185429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Merrick P, Zhang Zet al. Targeted mutagenesis in tetraploid switchgrass (Panicum virgatum L.) using CRISPR/Cas9. Plant Biotechnol J. 2018;16:381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie K, Minkenberg B, Yang Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci. 2015;112:3570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qi W, Zhu T, Tian Zet al. High-efficiency CRISPR/Cas9 multiplex gene editing using the glycine tRNA-processing system-based strategy in maize. BMC Biotechnol. 2016;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W, Akhunova A, Chao Set al. Optimizing multiplex CRISPR/Cas9-based genome editing for wheat. bioRxiv. 2016;051342. preprint: not peer reviewed. [Google Scholar]
- 19. Lawrenson T, Shorinola O, Stacey Net al. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 2015;16:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braatz J, Harloff HJ, Mascher Met al. CRISPR-Cas9 targeted mutagenesis leads to simultaneous modification of different Homoeologous gene copies in Polyploid oilseed rape (Brassica napus). Plant Physiol. 2017;174:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang H, Wu J-J, Tang Tet al. CRISPR/Cas9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci Rep. 2017;7:7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiong X, Liu W, Jiang Jet al. Efficient genome editing of Brassica campestris based on the CRISPR/Cas9 system. Mol Gen Genomics. 2019;294:1251–61. [DOI] [PubMed] [Google Scholar]
- 23. Cai X, Chang L, Zhang Tet al. Impacts of allopolyploidization and structural variation on intraspecific diversification in Brassica rapa. Genome Biol. 2021;22:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma C, Zhu C, Zheng Met al. CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic Res. 2019;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang X, Lei Y, Guan Het al. Transcriptomic analysis of the regulation of stalk development in flowering Chinese cabbage (Brassica campestris) by RNA sequencing. Sci Rep. 2017;7:15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song S, Lei Y, Huang Xet al. Crosstalk of cold and gibberellin effects on bolting and flowering in flowering Chinese cabbage. J Integr Agric. 2019;18:992–1000. [Google Scholar]
- 27. Dahanayake SR, Galwey NW. Effects of interactions between low-temperature treatments, gibberellin (GA3) and photoperiod on flowering and stem height of spring rape (Brassica napus var. annua). Ann Bot. 1999;84:321–7. [Google Scholar]
- 28. Richards DE, King KE, Ait-ali Tet al. How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:67–88. [DOI] [PubMed] [Google Scholar]
- 29. Sun T. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book. 2008;6:e0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harberd NP, Belfield E, Yasumura Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell. 2009;21:1328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun TP. Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol. 2010;154:567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peng J, Carol P, Richards DEet al. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng J, Richards DE, Hartley NMet al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature. 1999;400:256–61. [DOI] [PubMed] [Google Scholar]
- 34. Chandler PM, Marion-Poll A, Ellis Met al. Mutants at the Slender1 locus of barley cv Himalaya. Molecular and physiological characterization. Plant Physiol. 2002;129:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee S, Cheng H, King KEet al. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002;16:646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wen CK, Chang C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell. 2002;14:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikeda A, Ueguchi-Tanaka M, Sonoda Yet al. Slender Rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tyler L, Thomas SG, Hu Jet al. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004;135:1008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guan H, et al. Identification of DELLA genes and key stage for GA sensitivity in bolting and flowering of flowering Chinese cabbage. Int J Mol Sci. 2021;22:12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun X, Frearson N, Kirk Cet al. An E. coli expression system optimized for DELLA proteins. Protein Expr Purif. 2008;58:168–74. [DOI] [PubMed] [Google Scholar]
- 41. Wang F, Zhu D, Huang Xet al. Biochemical insights on degradation of Arabidopsis DELLA proteins gained from a cell-free assay system. Plant Cell. 2009;21:2378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida H, Hirano K, Sato Tet al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc Natl Acad Sci U S A. 2014;111:7861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lucas M, Davière JM, Rodríguez-Falcón Met al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–4. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Huang X, Huang Xet al. BcSOC1 promotes bolting and stem elongation in flowering Chinese cabbage. Int J Mol Sci. 2022;23:3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Su W, Chen W, Song Set al. Distribution of main nutrients and nitrate in organs of vegetable heart products. J Changjiang Veg. 2014;358:40–3. [Google Scholar]
- 46. Jain S, Shukla S, Yang Cet al. TALEN outperforms Cas9 in editing heterochromatin target sites. Nat Commun. 2021;12:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miao J, Guo D, Zhang Jet al. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013;23:1233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mao Y, Zhang H, Xu Net al. Application of the CRISPR–Cas system for efficient genome engineering in plants. Mol Plant. 2013;6:2008–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao J, Wang G, Ma Set al. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol Biol. 2015;87:99–110. [DOI] [PubMed] [Google Scholar]
- 50. Zhang B, Yang X, Yang Cet al. Exploiting the CRISPR/Cas9 system for targeted genome mutagenesis in petunia. Sci Rep. 2016;6:20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Port F, Bullock SL. Augmenting CRISPR applications in drosophila with tRNA-flanked sgRNAs. Nat Methods. 2016;13:852–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sánchez-Fernández R, Ardiles-Díaz W, Montagu MVet al. Cloning of a novel Arabidopsis thaliana RGA-like gene, a putative member of the VHIID-domain transcription factor family. J Exp Bot. 1998;49:1609–10. [Google Scholar]
- 53. Cheng H, Qin L, Lee Set al. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development. 2004;131:1055–64. [DOI] [PubMed] [Google Scholar]
- 54. Wild M, Achard P. The DELLA protein RGL3 positively contributes to jasmonate/ethylene defense responses. Plant Signal Behav. 2013;8:e23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wild M, Davière JM, Cheminant Set al. The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell. 2012;24:3307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hirano K, Ueguchi-Tanaka M, Matsuoka M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008;13:192–9. [DOI] [PubMed] [Google Scholar]
- 57. Sasaki A, et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science. 2003;299:1896–8. [DOI] [PubMed] [Google Scholar]
- 58. McGinnis KM, Thomas SG, Soule JDet al. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell. 2003;15:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li M, An F, Li Wet al. DELLA proteins interact with FLC to repress flowering transition. J Integr Plant Biol. 2016;58:642–55. [DOI] [PubMed] [Google Scholar]
- 60. Li K, Yu R, Fan LMet al. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat Commun. 2016;7:11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shi H, Wang LL, Sun LTet al. Cell division and endoreduplication play important roles in stem swelling of tuber mustard (Brassica juncea Coss. Var. tumida Tsen et Lee). Plant Biol. 2012;14:956–63. [DOI] [PubMed] [Google Scholar]
- 62. Kou E, Huang X, Zhu Yet al. Crosstalk between auxin and gibberellin during stalk elongation in flowering Chinese cabbage. Sci Rep. 2021;11:3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marowa P, Ding A, Kong Y. Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016;35:949–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tenhaken R. Cell wall remodeling under abiotic stress. Front Plant Sci. 2014;5:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park J, Nguyen KT, Park Eet al. DELLA proteins and their interacting RING finger proteins repress gibberellin responses by binding to the promoters of a subset of gibberellin-responsive genes in Arabidopsis. Plant Cell. 2013;25:927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee J, Oh M, Park Het al. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 2008;55:832–43. [DOI] [PubMed] [Google Scholar]
- 67. Feng S, Martinez C, Gusmaroli Get al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Muangprom A, Thomas SG, Sun T-Pet al. A novel dwarfing mutation in a green revolution gene from Brassica rapa. Plant Physiol. 2005;137:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang X, Qian Q, Liu Zet al. Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet. 2009;41:494–7. [DOI] [PubMed] [Google Scholar]
- 70. Shi J, Gao H, Wang Het al. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J. 2017;15:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Soyk S, Müller NA, Park SJet al. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat Genet. 2017;49:162–8. [DOI] [PubMed] [Google Scholar]
- 72. Zhu Q, Liu Y, Ma Xet al. A protocol for CRISPR/Cas9-based multi-gene editing and sequence decoding of mutant sites in plants. Sci Sin Vitae. 2018;48:783–94. [Google Scholar]
- 73. Song SW, Liao GX, Liu HCet al. Effect of ammonium and nitrate ratio on nutritional quality of Chinese kale. Adv Mater Res. 2012;461:13–6. [Google Scholar]
- 74. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 75. Shyamala B, Jamuna P. Nutritional content and antioxidant properties of pulp waste from Daucus carota and Beta vulgaris. Malays J Nutr. 2010;16:397–408. [PubMed] [Google Scholar]
- 76. Cataldo DA, Haroon M, Schrader LEet al. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal. 1975;6:71–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.