This article has been corrected. Please see J Manag Care Spec Pharm, 2018 Jul;24(1):29-38.
Cost-effectiveness of Drugs to Treat Relapsed/Refractory Multiple Myeloma in the United States.
Carlson JJ, Guzauskas GF, Chapman RH, Synnott PG, Liu S, Russo ET, Pearson SD, Brouwer ED, and Ollendorf DA. J Manag Care Spec Pharm. 2018;24(1): 29-38.
The authors would like to make the following corrections to the above article:
Page 31, Table 1: The progression-free survival hazards ratio estimate for PAN+BOR+DEX versus LEN+DEX was corrected. Corrections are shown in bold below:
| Third-line PFS hazardratios vs. LEN-DEX | |||||
| BOR-DEX | 0.93 | 0.58 | 2.04 | LogNormal | Network meta-analysis |
| CFZ-LEN-DEX | 0.69 | 0.54 | 0.87 | LogNormal | Network meta-analysis |
| ELO-LEN-DEX | 0.70 | 0.49 | 0.87 | LogNormal | Network meta-analysis |
| IX-LEN-DEX | 0.74 | 0.40 | 0.84 | LogNormal | Network meta-analysis |
| PAN-BOR-DEX | 0.59 | 0.31 | 1.10 | LogNormal | Network meta-analysis |
| DAR-LEN-DEXa | 0.37 | 0.27 | 0.52 | LogNormal | Network meta-analysis |
| DAR-BOR-DEXa | 0.39 | 0.28 | 0.53 | LogNormal | Network meta-analysis |
Page 31, Table 1: Estimated drug costs were updated. Corrections are shown in bold below:
| Costs | Base Case | Lower | Upper | PSA Distribution | Source |
|---|---|---|---|---|---|
| Drug acquisition and administration costs,c $ | |||||
| Bortezomib 3.5 mg vial | 1,503.00 | 1,202.40 | 1,803.60 | Normal | RED BOOK |
| Bortezomib administration | 111.42 | 89.14 | 133.70 | Normal | CPT 96409 |
| Carfilzomib 60 mg vial | 1,971.50 | 1,577.20 | 2,365.80 | Normal | RED BOOK |
| Carfilzomib administration | 209.24 | 167.39 | 251.09 | Normal | CPT 96360, 96361, 96413 |
| Dexamethasone per mg | 0.32 | 0.26 | 0.39 | Normal | RED BOOK |
| Elotuzumab 300 mg vial | 1,776.00 | 1,420.80 | 2,131.20 | Normal | RED BOOK |
| Elotuzumab 400 mg vial | 2,368.00 | 1,894.40 | 2,841.60 | Normal | RED BOOK |
| Elotuzumab administration | 227.87 | 182.30 | 273.44 | Normal | CPT 96413, 96415, 96417 |
| Ixazomib capsule | 3,006.00 | 2,404.80 | 3,607.20 | Normal | RED BOOK |
| Lenalidomide capsule | 552.98 | 442.38 | 663.58 | Normal | RED BOOK |
| Panobinostat capsule | 1,222.22 | 977.78 | 1,466.67 | Normal | RED BOOK |
| Daratumumab 400 mg vial | 1,850.40 | 1,480.32 | 2,220.48 | Normal | RED BOOK |
| Daratumumab 100 mg vial | 462.60 | 370.08 | 555.12 | Normal | RED BOOK |
| Daratumumab administration | 399.83 | 319.86 | 479.80 | Normal | CPT 96413, 96415, 96417 |
Page 33, Table 2: Comparative estimates in the Third Line section have been changed for PAN+BOR+DEX, as shown in bold below:
TABLE 2.
Comparative Outcomes
| Regimen | Second Line | Third Line (All Comparators) | Third Line (PAN-BOR-DEX Omitted) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Cost, $ | QALYs | ICER | Total Cost, $ | QALYs | ICER | Total Cost, $ | QALYs | ICER | |
| LEN-DEX | 309,997 | 2.59 | Dominated | 281,754 | 2.04 | Dominated | 281,754 | 2.04 | Dominated |
| BOR-DEX | 189,357 | 2.74 | Dominant | 175,315 | 2.16 | Dominant | 175,315 | 2.16 | Dominant |
| IX-LEN-DEX | 622,378 | 3.27 | Dominated | 566,512 | 2.60 | Dominated | 566,512 | 2.60 | Dominated |
| ELO-LEN-DEX | 665,728 | 3.41 | Dominated | 608,651 | 2.71 | Dominated | 608,651 | 2.71 | Dominated |
| CFZ-LEN-DEX | 492,872 | 3.45 | Dominated | 459,868 | 2.74 | Dominated | 459,868 | 2.74 | Dominated |
| PAN-BOR-DEX | 190,876 | 3.23 | 14,598 | ||||||
| DAR-BOR-DEX | 447,182 | 5.29 | 50,704 | 423,119 | 4.38 | 248,762 | 423,119 | 4.38 | 60,359 |
| DAR-LEN-DEX | 845,527 | 5.44 | 2,707,547 | 789,202 | 4.38 | Equal outcomes, higher cost vs. DAR-BOR-DEX | 789,202 | 4.38 | Equal outcomes, higher cost vs. DAR-BOR-DEX |
BOR = bortezomib; CFZ = carfilzomib; CPT = Current Procedural Terminology; DAR = daratumumab; DEX = dexamethasone; ELO = elotuzumab; IX = ixazomib; LEN = lenalidomide; OS = overall survival; PAN = panobinostat; PFS = progression-free survival; QALY = quality-adjusted life-year.
Page 34, Table 3: In the Third Line section, comparative estimates have been changed for PAN+BOR+DEX, as shown in bold below:
| Third Line | LEN-DEX | BOR-DEX | CFZ-LEN-DEX | ELO-LEN-DEX | IX- LEN-DEX | PAN-BOR-DEX | DAR-LEN-DEX | DAR-BOR-DEX |
|---|---|---|---|---|---|---|---|---|
| Total costs, $ | 281,754 | 175,315 | 459,868 | 608,651 | 566,512 | 190,876 | 789,202 | 423,119 |
| Drug acquisition | 237,670 | 121,751 | 401,201 | 541,632 | 516,793 | 131,500 | 707,051 | 344,684 |
| Supportive care | 473 | 1,441 | 1,779 | 2,364 | 2,255 | 411 | 4,579 | 2,403 |
| Administration | 7,365 | 8,113 | 13,394 | – | 3,095 | 22,394 | 21,412 | |
| Progression | 39,261 | 40,175 | 44,318 | 44,105 | 43,298 | 46,744 | 51,708 | 52,014 |
| Adverse event | 4,351 | 4,583 | 4,457 | 7,156 | 4,I66 | 9,127 | 3,469 | 2,607 |
| Total QALYs | 2.04 | 2.16 | 2.74 | 2.71 | 2.60 | 3.23 | 4.38 | 4.38 |
| PFS | 1.00 | 1.07 | 1.37 | 1.36 | 1.30 | 1.69 | 2.28 | 2.35 |
| Progression | 1.03 | 1.09 | 1.37 | 1.36 | 1.30 | 1.54 | 2.10 | 2.03 |
| Total life-years (OS) | 3.25 | 3.44 | 4.37 | 4.32 | 4.14 | 4.93 | 6.97 | 6.71 |
| PFS | 1.55 | 1.64 | 2.12 | 2.09 | 2.00 | 2.41 | 3.52 | 3.38 |
| Progression | 1.70 | 1.79 | 2.25 | 2.23 | 2.14 | 2.52 | 3.44 | 3.33 |
| ICER vs. LEN-DEX | – | -853,800 | 252,293 | 484,168 | 508,021 | Dominant | 216,360 | 60,359 |
Page 35, Figure 1: Third Line graph was updated to reflect a new cost-efffectiveness acceptability curve, as shown below:
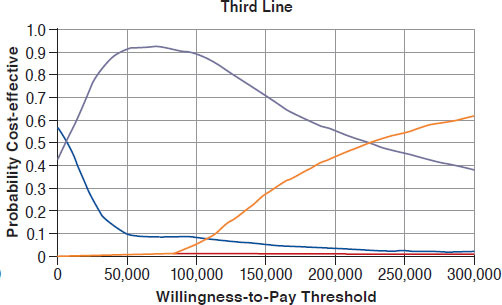
Page 36, Table 4: Changes were nade to drug cost threshold for PAN+BOR+DEX in the Third Line section, as shown below in bold:
TABLE 4.
Drug Cost Thresholds
| Second Line, $ | Third Line, $ | |||||
|---|---|---|---|---|---|---|
| WTP Threshold | 50,000 | 100,000 | 150,000 | 50,000 | 100,000 | 150,000 |
| CFZ+LEN+DEX | 55 | 649 | 1,242 | 0 | 445 | 946 |
| (–906-1,063) | (–68-1,733) | (405 -2,661) | (–938-622) | (–633-1,518) | (–386-2,417) | |
| ELO-LEN-DEX | –69 | 252 | 572 | –126 | 162 | 449 |
| (138-1,272) | (–266-692) | (34-1,032) | ||||
| IX-LEN-DEX | –278 | 127 | 533 | -347 | 19 | 385 |
| (–903-567) | (–294-830) | (84-1,329) | (1046593) | (–502-/69) | (–40-1,180) | |
| PAN-BOR-DEX | 3,459 | 4,344 | 5,229 | |||
| (2,389-5,552) | (2,668-8,242) | (2,792-10,987) | ||||
| DAR-LEN-DEX | –165 | 567 | 1,298 | –293 | 351 | 995 |
| (–779-486) | (–51-1,239) | (614-2,093) | (–902-417) | (–239-1,080) | (338-1,800) | |
| DAR-BOR-DEX | 1,840 | 2,582 | 3,324 | 1,708 | 2,397 | 3,087 |
| (1,495 -2,278) | (2,139-3,050) | (2,674-3,976) | (1,374-2,114) | (1,948-2,959) | (2,479-3,845) | |
Note: Results reflect threshold prices for the first listed drug in each triplet regimen only (all other parameter values held constant).
BOR = bortezomib; CFZ = carfilzomib; DAR = daratumumabt; DEX = dexamethasone; ELO = elotuzumab; IX = ixazomib; LEN = lenalidomide; PAN = panobinostat; WTP = willingness to pay.
Page 36, second column, last paragraph, second sentence, is changed to the following:
“First, the independently modeled PFS and OS curves in the Jakubowiak et al. analysis yielded much more favorable estimates of treatment effect for CFZ+LEN+DEX than those reported in the ASPIRE trial versus LEN+DEX (PFS odds ratio = 0.51 [model] vs. 0.69 [published hazard ratio]; OS hazard ratio = 0.70 [model] vs. 0.79 [published hazard ratio]).”
Page 37, top paragraph, last sentence, is changed to the following:
“Finally, we note that 1 of the findings of the Jakubowiak et al. analysis appears to be counterintuitive, in that CFZ+LEN+DEX patients spend approximately 4 years in the postprogression state in the model versus approximately 3 years for LEN+DEX; however, the postprogression treatment costs for LEN+DEX are reported to be higher.”
While the authors regret these errors, they do not affect the conclusions of the study.


