Abstract
Acute respiratory distress syndrome (ARDS) is a life-threatening condition, characterized by diffuse inflammatory lung injury. Since the coronavirus disease 2019 (COVID-19) pandemic spread worldwide, the most common cause of ARDS has been the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Both the COVID-19-associated ARDS and the ARDS related to other causes—also defined as classical ARDS—are burdened by high mortality and morbidity. For these reasons, effective therapeutic interventions are urgently needed. Among them, inhaled nitric oxide (iNO) has been studied in patients with ARDS since 1993 and it is currently under investigation. In this review, we aim at describing the biological and pharmacological rationale of iNO treatment in ARDS by elucidating similarities and differences between classical and COVID-19 ARDS. Thereafter, we present the available evidence on the use of iNO in clinical practice in both types of respiratory failure. Overall, iNO seems a promising agent as it could improve the ventilation/perfusion mismatch, gas exchange impairment, and right ventricular failure, which are reported in ARDS. In addition, iNO may act as a viricidal agent and prevent lung hyperinflammation and thrombosis of the pulmonary vasculature in the specific setting of COVID-19 ARDS. However, the current evidence on the effects of iNO on outcomes is limited and clinical studies are yet to demonstrate any survival benefit by administering iNO in ARDS.
Keywords: acute respiratory distress syndrome, COVID-19, inhaled nitric oxide, SARS-CoV-2
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening acute, diffuse, and inflammatory lung injury of different etiologies and it is characterized by hypoxemia and stiff lungs.1 It affects 86 per 100,000 person-years and ∼10%–15% of patients admitted to the intensive care units (ICUs) have ARDS. The mortality rate ranges from 35% to 46% according to the disease severity.2,3
Currently, the clinical diagnosis is based on the Berlin definition, which includes the acute onset of the ARDS, the presence of bilateral lung opacities at chest X-ray or computed tomography scan, the alveolar and interstitial edema—not fully explained by cardiac failure—and the development of hypoxemia.4 At the onset of the lung injury, lung histology is characterized by a diffuse alveolar damage with interstitial edema and inflammation. Nearly a week later, alveolar cell proliferation takes place; some patients enter into a restorative phase, while others progress to a fibrotic stage.1,5,6
Since the coronavirus disease 2019 (COVID-19) pandemic challenged the health care systems worldwide, infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become one of the most common causes of ARDS over the last few years. The urge to decrease mortality and morbidity from COVID-19 has greatly increased the production of clinical research, and new clinical trials were developed to discover effective therapeutic agents.7–10
Among the potential therapeutic agents for ARDS, inhaled nitric oxide (iNO) has been studied in human ARDS since 1993.11 Since then, iNO was used as a rescue therapy in critically ill patients with ARDS who showed a limited response to recommended treatments, such as protective mechanical ventilation and prone positioning.2,12,13
In this review, we aim to describe the biological and pharmacological rationale supporting the use of iNO in ARDS patients. We explored the similarities and differences between classical and COVID-19 ARDS that may unveil the potential of iNO treatment in ARDS. In addition, we reported the clinical evidence available so far on the use of iNO in patients with classical and COVID-19 ARDS.
Methods
Clinical studies on the use of iNO in patients with ARDS published until February 20, 2023, were searched in the Medline database. The Mesh terms “Nitric Oxide,” “Respiratory Distress Syndrome,” and “COVID-19” were used and studies including the definition of ARDS according to the Berlin's4 or the American-European Consensus Conference criteria on ARDS14 were considered. Only two studies, included in a Cochrane meta-analysis,15 did not meet these criteria.16,17 Priority was given to randomized controlled trials (RCTs), meta-analysis, and guidelines. When this evidence was not available, observational studies and case series were considered. Ongoing clinical trials were identified through search on clinicaltrials.gov “Nitric Oxide” was entered as the drug name and “ARDS” was entered as the disease category. RCTs were preferred over other study designs. Preclinical studies were included when helpful to fulfill the aim of the review.
Since the evidence of iNO in COVID-19 ARDS is mainly limited to case series or small observational studies, we widened the study search to studies regarding the administration of iNO to patients with COVID-19, regardless the severity of respiratory failure. We also considered other relevant articles included in the references of other studies when they were not identified in our primary search (i.e., snowballing).
Biology and Pharmacology of NO
NO is a colorless and odorless gas that is endogenously produced from the oxidation of l-arginine and l-citrulline in vascular endothelial cells by the constitutive and inducible NO synthases.18–20 NO diffuses into the vascular smooth muscle cells and activates soluble guanylyl cyclase, which catalyzes the production of cyclic guanosine monophosphate (cGMP). Subsequently, cGMP activates cGMP-dependent protein kinase, which eventually leads to decreased intracellular calcium and thus relaxed vascular smooth muscle tone in precapillary resistance arterioles.21 In addition to this vasodilatory effect, NO has been shown to reduce platelet aggregation,22 smooth muscle cell proliferation,23 and endothelial leukocyte binding.24
When inhaled, NO rapidly diffuses into the smooth muscle cells of lung vessels in the ventilated lung areas and exerts a vasodilation of the pulmonary vasculature. This vasodilatory effect is selective on the vessels in the ventilated areas, since this gas has a short half-life of 2–6 seconds. Indeed, NO is highly reactive and it is rapidly inactivated either by heme moiety scavenging25 or by oxidation to the more stable nitric dioxide and nitric trioxide, which lack vasodilatory properties.26–30
At present, iNO is approved by the Food and Drug Administration only for the treatment of persistent pulmonary hypertension of newborns (PPHN) at the dose of 20 ppm up to 14 days.31 However, it was suggested for the treatment of a variety of other conditions, such as ischemia/reperfusion injury,32 pulmonary hypertension,33 hemolysis-induced vasoconstriction,34 renal failure associated with cardiopulmonary bypass,35 and classical and COVID-19 ARDS.30,36
Classical ARDS
Rationale of iNO in classical ARDS
ARDS is the clinical consequence of the acute lung injury characterized by the histological hallmark of diffuse alveolar damage that is characterized by both the epithelial and the endothelial damages.37 The latter activates the coagulation cascade and pulmonary capillary thrombosis develops, and further contributes to the increase in the right ventricle afterload.5,38 The lung injury undermines the pulmonary gas exchange as it leads to a ventilation/perfusion (V/Q) mismatch.39 A constellation of hypoxic pulmonary vasculature constriction, hypercapnia, acidosis, hemolysis, vasopressors, hypothermia, endothelial dysfunction, vascular thrombosis, airway collapse, and hyperinflation during mechanical ventilation (i.e., airway pressure greater than capillary perfusion pressure) may all determine an increase in pulmonary vascular resistances, which is seen in up to 25% of patients with ARDS.40
In the context of ARDS, systemic vasodilators have been studied.41–44 However, if a systemic vasodilator is administered, such as NO donors (e.g., sodium nitroprusside, nitrates, sildenafil) or prostacyclin, there might be an indiscriminate vasodilation of the whole pulmonary vasculature. Both vasculature of ventilated and nonventilated lung areas would be dilated and there would be an increase in the ventilation-perfusion mismatch leading to worsened respiratory gas exchanges.
Contrarily, iNO may play a role in the treatment of ARDS as it determines selective vasodilation of vessels in ventilated lung units, thus improving the V/Q mismatch. This selective effect is due to the short half-life of iNO.45 Improved V/Q ratio would be associated with better gas exchanges. Moreover, pulmonary vasodilation may decrease pulmonary vascular resistances and reduce the right ventricle workload. In addition, a rapid inactivation of iNO would not affect the systemic vasculature, preventing from the risk of systemic hypotension and subsequent organ hypoperfusion and ischemia.
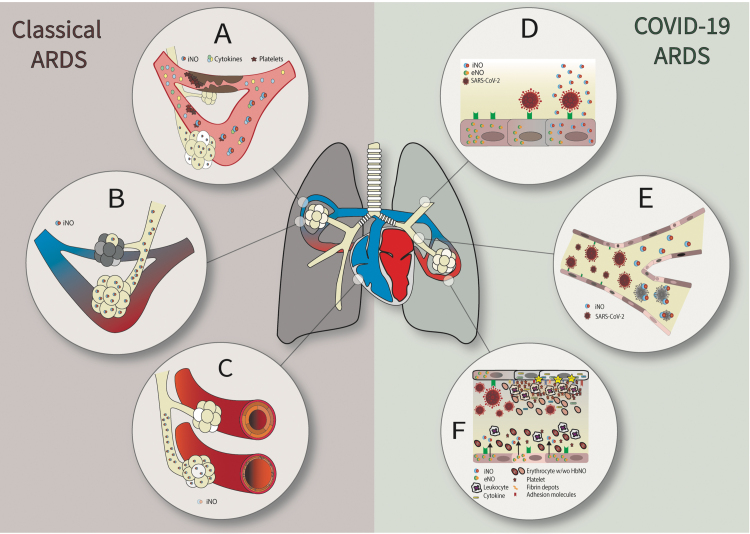
Overall, iNO may have the following potential therapeutic properties in the classical ARDS: (1) enhancement of the V/Q match by diverting blood flow to ventilated lung units and therefore improvement of the respiratory gas exchange, thanks to a selective pulmonary vasodilatory effect11,46,47; (2) offloading of the right ventricle and prevention of its failure, because of pulmonary vasodilation, which determines a reduction in pulmonary arterial pressure (PAP) and then a decrease of right ventricle afterload; and (3) maintenance of patent pulmonary vessels thanks to antiplatelet properties (Fig. 1A–C).
FIG. 1.
iNO properties exerted in classic (left side) and COVID-19 (right side) ARDS. In classic ARDS, iNO exerts the following properties: (A) maintenance of patent pulmonary vessels thanks to antiplatelet properties; (B) enhancement of the V/Q match by diverting blood flow to ventilated lung units and therefore improvement of the respiratory gas exchange, thanks to selective pulmonary vasodilatory effect; (C) offloading of the right ventricle and prevention of its failure, because of pulmonary vasodilation, which determines a reduction in PAP and then a decrease of right ventricle afterload. In COVID-19 ARDS—together with the properties described in classic ARDS—iNO has the following properties: (D) replenishment of the depleted storage of endogenous NO in the presence of inflammatory induced endothelial dysfunction (i.e., eNOS dysfunction); (E) direct viricidal activity against SARS-CoV-2; (F) immune modulation and decreased lung inflammation and prevention of SARS-CoV-2-induced endothelial dysfunction thanks to anti-inflammatory properties. ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; eNOS, endothelial nitric oxide synthase; iNO, inhaled nitric oxide; PAP, pulmonary arterial pressure; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; V/Q, ventilation/perfusion.
Clinical applications of iNO in classical ARDS
iNO was first tested in humans with ARDS in 1993 by Rossaint et al.11 Considering increased pulmonary vascular resistances and intrapulmonary right-to-left shunt key characteristics of ARDS, the authors assumed that iNO would have decreased PAP and not affected the systemic hemodynamics. iNO was administered at 18 and 36 ppm for 40 minutes to 10 consecutive patients. They demonstrated that iNO significantly decreased PAP from 37 ± 3 to 30 ± 2 mmHg (p = 0.008) and intrapulmonary shunting from 36% ± 5% to 31% ± 5% (p = 0.028). As hypothesized, iNO did not alter the systemic arterial pressure, and the cardiac output remained unchanged. Moreover, during iNO administration, the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) improved from 152 ± 15 to 199 ± 23 mmHg (p = 0.008).
The analysis of V/Q distributions through the multiple inert-gas-elimination techniques48 showed a redistribution of pulmonary blood flow from nonventilated lung areas toward ventilated areas, thus confirming an improved ventilation-perfusion match during NO inhalation. The effect of iNO was also compared with the effect of prostacyclin, a systemic vasodilator. As expected, prostacyclin decreased PAP, but also worsened intrapulmonary shunt and gas exchanges.
The authors also tested prolonged administration of iNO in 7 out of 10 patients included in the study. iNO was administered at 5–20 ppm for 3–53 days for 30 min/day and was discontinued when the PaO2/FiO2 rose above 250 mmHg without inhalation of NO. During the intermittent administration of iNO, PAP decreased and PaO2/FiO2 improved consistently, without affecting systemic hemodynamics.
Later, the same group demonstrated that iNO decreases PAP and improves the right ventricle ejection fraction (RVEF) in patients with severe ARDS. Indeed, iNO reduced the mean PAP from 33 ± 2 to 28 ± 1 mmHg (p = 0.008) and increased right ventricular ejection fraction from 28% ± 2% to 32% ± 2% (p = 0.005).49 Similarly, Fierobe et al., in the same year, showed the beneficial effects of iNO on PAP and RVEF in severe ARDS in an independent study.50
In view of the improvement of pulmonary hemodynamics and respiratory gas exchange in patients with ARDS, iNO was tested in 14 RCTs to detect an effect of this treatment on mortality.16,17,50–61 These RCTs have been included into a meta-analysis of 1275 adult and pediatric participants, of whom 654 received iNO.15 A summary of the RCTs is presented in Table 1. iNO was administered at doses ranging from 5 to 80 ppm for a maximum of 30 days. The analysis showed no significant benefit from iNO, neither on long-term mortality, defined as the longest mortality measured in the study (28–90 days) (relative risk [RR] 1.04, 95% confidence interval [CI] 0.9–1.19; I2 statistic = 0%), nor on mortality at 28 days (RR 1.08, 95% CI 0.92–1.27; I2 statistic = 0%). No effect on mortality was observed despite the study population being categorized according to ARDS severity (e.g., moderate and severe).
Table 1.
Randomized Controlled Trials on Inhaled Nitric Oxide Administration in Classical Acute Respiratory Distress Syndrome
| Study name/NCT | Sample size | Treatment group/study population | Control group | NO dose, ppm | Endpoint | Main results |
|---|---|---|---|---|---|---|
| NCT00240487 | 52 children | iNO for the first 4 hours, then no iNO for 4 hours | No iNO for the first 4 hours, then iNO for 4 hours | 10 | Changes in PaO2/FiO2 ratio | ns |
| Bronicki et al.51 | 53 children | iNO until death, ventilator free, or 28 days | Nitrogen | 5 | VFD at 28 days | 14.2 ± 8.1 vs. 9.1 ± 9.5 days (iNO vs. placebo, p = 0.05) |
| Day et al.17 | 24 children | iNO until PEEP decreased to 6 days cmH2O and FiO2 0.5 | Conventional care for 1 day, then iNO | 10 | OI and PVR/SVR | OI and PVR/SVR acutely improved in the first hour of treatment |
| Dellinger et al.52 | 177 adults | iNO for 28 days or until extubation | Nitrogen | 1.25, 5, 20, 40 or 80 | Duration of mechanical ventilation | ns |
| Dobyns et al.53 | 108 children | iNO for 3 days | Air | 10 | Proportion of pts meeting treatment failure criteria (OI ≥40 × 3 hours or ≥25 × 6 hours) | ns |
| Gerlach et al.55 | 40 adults | iNO, daily dose–response analysis | Conventional care | 10 | Dose–response analysis | Peak response at 10 ppm |
| Ibrahim and El-Mohamdy54 | 32 children | iNO at 5 ppm for 18 hours, then 1 ppm for 2 hours + prone position or supine position | Conventional care | 1–5 | Changes in PaO2/FiO2 ratio, OI | Better oxygenation parameters in the prone position iNO group |
| Lundin et al.56 | 180 adults | iNO for 10 min/day, up to 30 days | Conventional care | 2–40 ppm | Reversal of ALI | ns |
| Mehta et al.57 | 14 adults | iNO titrated to best PaO2/FiO2 ratio until PaO2/FiO2 >200 mmHg on FiO2 <0.5 | Conventional care | 5–20 | Changes in mean PAP, PaO2/FiO2 | Reduction of mean PAP at 5–10 ppm and better PaO2/FiO2 at 5–10 ppm |
| Michael et al.58 | 40 adults | iNO at increasing doses every 6 hours for 1 day, then clinically adjusted. | Conventional care | 5–20 | Improvement in oxygenation within 3 days | Better PaO2/FiO2 at 1, 12, 24 hours in the iNO group vs. control group |
| Park et al.59 | 23 adults | iNO or iNO+lung recruitment | Conventional care+lung recruitment | 5 | Oxygenation and hemodynamic parameters | ns |
| Payen et al.60 | 203 adults | iNO | Nitrogen | 10 | Survival and off mechanical ventilation at 28 days | / |
| Schwebel et al.61 | 19 adults | iNO for 17 hours, then at the clinician's discretion | Nitrogen | 10 | Oxygenation and hemodynamic parameters | / |
| Taylor et al.16 | 385 adults | iNO for up to 28 days | Nitrogen | 5 | Days alive and off mechanical ventilation at 28 days | ns |
| Troncy et al.139 | 30 adults | iNO titration | Conventional care | 2.5–40 | Gas exchange | Better gas exchange in iNO group vs. control group in the first day of treatment |
ALI, acute lung injury; iNO, inhaled nitric oxide; ns, not statistically significant; OI, oxygenation index; PaCO2, partial pressure of arterial carbon dioxide; PaO2/FiO2, ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen; PAP, pulmonary arterial pressure; PEEP, positive end expiratory pressure; ppm, part per million; PVR, pulmonary vascular resistances; RCT, randomized controlled trial; SVR, systemic vascular resistances; VFD, ventilator-free days.
Regarding the physiologic parameters, the PaO2/FiO2 increased by 15.91 mmHg after iNO administration at 24 hours and mean PAP was significantly lower by 1.76 mmHg in the iNO group at day 1 compared with control. However, no difference on physiologic parameters was detected from day 2 through day 4. No benefits were reported for ventilator-free days in the iNO-treated group. Therefore, the authors concluded that there is insufficient evidence to suggest iNO for the treatment of ARDS. Furthermore, the same meta-analysis detected a statistically significant increase in renal failure in the iNO groups (RR 1.59, 95% CI 1.17–2.16; I2 statistic = 0%).
Due to the limited evidence of iNO on mortality and the potential harmful effects, the current guidelines of the American Thoracic Society for the treatment of ARDS do not make any recommendation about the use of iNO. Nevertheless, inhaled vasodilators are highlighted as an issue to be addressed in the future iterations of the guidelines.13 Instead, the U.K. Faculty of Intensive Care Medicine guidelines make a weak recommendation against the use of iNO in ARDS.62 However, despite iNO not being part of the routine therapy of patients with ARDS, iNO is considered a “rescue” strategy for severe hypoxemia. The use of iNO in patients with moderate-to-severe ARDS is in <1 out of 10 patients worldwide.63
ARDS is a heterogeneous clinical condition that may affect patients with diverse clinical characteristics and with a potential different immune response to the lung injury. In the meta-analysis by Gebistorf et al., the RCTs included evaluated the role of iNO in the overall population of patients with ARDS.15 However, whether iNO would play a role in the presence of (1) different etiologies of ARDS64; (2) sex65; (3) patient comorbidities66 and organ dysfunctions67,68; (4) management69,70; or (5) limitation of care71 has not been investigated yet. ARDS and patients' characteristics may differently affect the potential therapeutic effect of iNO. Despite iNO having failed to demonstrate a generalizable clinical benefit in ARDS on outcomes, it may still play a role in a selected subgroup of patients.
The current phenotyping of ARDS—which is based on different clinical and biological features within the same definition of ARDS,72 may help to understand the failure of a number of pharmacological clinical trials.73 Indeed, the definition of trial's populations on phenotyping or other patient's characteristics known before randomization, also referred as population enrichment,74,75 may decrease the population heterogeneity and increase the trial sensitivity and clarify which patients may benefit most from different treatments including iNO.9,72,76,77
In addition, other aspects of iNO therapy in ARDS need clarification. Indeed, no consensus on the dose of iNO has been reached. A study by Iotti et al. showed a beneficial effect of iNO on oxygenation in ARDS even at very-low doses (0.5 ppm) and a plateau effect was observed at 5 ppm.78 Similarly, another study showed a peak effect on oxygenation at 10 ppm.55 However, iNO was administered up to 80 ppm in other studies and, so far, the optimal dosing has not been determined. Also, the timing of iNO treatment and its duration are to be explored yet. Particularly, iNO may induce sensitization over time and lower doses may be required to obtain the same improvement on both oxygenation and mean PAP after some days of treatment. Maintaining the same dose showed a deterioration in oxygenation.55
Therefore, ARDS phenotypes—that summarize biological characteristics of the disease and clinical characteristics of the patients—iNO doses, and timing of administration are an open field of research that should be considered in future clinical trials to elucidate the role of iNO in ARDS.
COVID-19 ARDS
Rationale of iNO in COVID-19-associated ARDS
In the context of COVID-19, a worrisome complication of this disease is ARDS. In this condition, the etiology of ARDS is the SARS-CoV-2 infection, which determines pneumonia and, in some cases, progresses to ARDS.
Opposed to classical ARDS, where a linear relationship between lung compliance and hypoxemia is often observed, in COVID-19 ARDS there is a dissociation between severe hypoxemia and lung compliance. This could be explained by a loss of the lung perfusion regulation and hypoxic vasoconstriction, which worsens the V/Q mismatch.79,80 In this context, iNO might play a role in improving the V/Q mismatch, and therefore, the gas exchanges in severely COVID-19 hypoxemic patients.81 Indeed, critically ill COVID-19 patients were shown to have decreased venous erythrocyte levels of 5-α-nitrosyl-hemoglobin (HbNO), a marker of reduced endogenous NO bioavailability and a proxy of severe endothelial dysfunction. In addition, HbNO directly correlated with respiratory gas exchanges in terms of PaO2/FiO2 ratio.82,83
Moreover, as severely hypoxemic patients have high probability of requiring veno-venous extracorporeal membrane oxygenation (V-V ECMO) and the high ICU admission rate of COVID-19 patients may determine a limitation of resources, iNO may serve as an alternative to V-V ECMO or as a bridge to lung healing.7,84
In addition, iNO may be helpful in COVID-19 treatment as it demonstrated to have some antiviral activity.85 This potential effect is supported by an in vitro study, in which SARS-CoV-infected cells were exposed to S-nitroso-N-acetylpenicillamine (SNAP), an NO donor compound. SNAP was able to inhibit SARS-CoV replication.86 Another study confirmed this activity on SARS-CoV-infected cells and also demonstrated that NO produced by stimulation of NOS determined the same antiviral effect.87 A similar effect was observed also in SARS-CoV-2-infected cells exposed to SNAP. Indeed, SNAP inhibited SARS-CoV-2 replication in a dose-dependent manner and delayed or completely prevented the development of a viral cytopathic effect.88 This direct antiviral activity of NO has been demonstrated for a lot of other viruses, both respiratory89 and nonrespiratory viruses.90 Also, NO reacts with other molecules to produce highly reactive species, such as dinitrogen trioxide, peroxynitrite, and nitrogen dioxide (NO2).
These reactive species may have a direct antiviral effect, although oxidative stress and cytotoxicity may be a downside. Interestingly, a trial performed in Beijing in 2002 in six patients affected by severe acute respiratory syndrome by SARS-CoV showed that the administration of iNO up to 30 ppm resulted not only in improved oxygenation and less respiratory support, but also in decreased lung infiltrates compared with controls. Moreover, Moni et al. investigated the effect of iNO on viral clearance in patients with hypoxemic COVID-19. Viral clearance was obtained on day 5 in all the patients treated with iNO and in 72% of controls (p < 0.01).91 These results may support a direct effect of iNO on SARS-CoV-2 along with the pulmonary vasodilatory effect.92
Apart from the antiviral activity, NO may modulate the host immune response during the viral infection, by inducing a suppression of immune cell activity that may be associated with an inadequate immune response to infection. On the contrary, this immune response regulation could decrease the inflammatory-mediated tissue injury.93
Moreover, from a histopathological point of view, pulmonary hyperinflammation is a key element in COVID-19 ARDS. This condition leads to endothelial inflammation, vasculitis, and vascular microthrombi.94 In this situation, iNO may decrease the endothelial damage and the vascular thrombosis thanks to its anti-inflammatory and antiaggregant properties22 and prevention of leukocyte adhesion.24 Indeed, iNO is known to act on the coagulation cascade by increasing the bleeding time through the inhibition of platelet aggregation. This potential adverse effect may be advantageous in a prothrombotic condition as in COVID-19.7,95
Patients with COVID-19 were also found to have lower levels of endogenous NO, compared with healthy controls.82,96,97 This could be determined by endothelial oxidative stress and by inhibition of the angiotensin converting enzyme 2 receptor (ACE-2 receptor) in the lungs by the SARS-CoV-2. In fact, the ACE-2 receptor is involved in a metabolic pathway that enhances NO production and it is vasoprotective. iNO could therefore subsidize the shortage of endogenous NO and exert the beneficial properties above mentioned.98
Lastly, in patients on V-V ECMO that could present hemolysis induced by the extracorporeal therapy, free hemoglobin may deplete endogenous NO and lead to vasoconstriction. Even in this case, iNO could replenish the depleted endogenous NO.
Overall—together with the properties exerted in classic ARDS—iNO may have diverse potential benefits in the treatment of COVID-19 ARDS: (1) direct viricidal activity against SARS-CoV-2; (2) immune modulation and decreased lung inflammation and prevention of SARS-CoV-2-induced endothelial dysfunction thanks to anti-inflammatory properties; and (3) replenishment of the depleted storage of endogenous NO in the presence of inflammatory-induced endothelial dysfunction (i.e., endothelial NO synthase dysfunction) (Fig. 1D–F).
Clinical applications of iNO in COVID-19 ARDS
To date, data on the administration of iNO in COVID-19-associated ARDS are limited and conflicting. The available evidence consists of observational studies, which are summarized in Table 2. All the studies have a low quality of evidence, due to the observational nature and the small sample size, which do not allow generalizability and cause–effect interpretation. RCTs aiming to determine whether iNO improves clinical course, and is safe and feasible in patients with COVID-19, are currently ongoing (NCT04476992, NCT04460183, NCT04383002, NCT04306393).99 So far, no RCT was registered on clinicaltrials.gov with the primary endpoint to detect an effect on mortality by administering iNO in COVID-19 ARDS.
Table 2.
Studies Evaluating Inhaled Nitric Oxide Administration in Patients with Acute Respiratory Distress Syndrome or Severe Pneumonia Associated with Coronavirus Disease 2019
| Study name | Study type | Treatment group/study population | Control group | NO dose, ppm | Endpoint | Main results |
|---|---|---|---|---|---|---|
| Al Sulaiman et al.112 | Multicenter retrospective | 76 pts. receiving iNO | 1:2 propensity score matching | 40 | Change in PaO2, FiO2, PaO2/FiO2 ratio and oxygenation index 24 hours after iNO | Oxygenation parameters significantly improved |
| Poonam et al.109 | Retrospective | 41 pts. iNO started after no response to conventional treatment | N/A | 20–80 | Change in PaO2/FiO2 ratio after iNO | ns |
| Bonizzoli et al.110 | Prospective | 12 pts. iNO started after no response to conventional treatment | N/A | 40 | Right ventricle function, dimension, and PAP before and after iNO | ns |
| Lubinsky et al.103 | Retrospective | 84 pts. iNO started a median of 6 days after intubation, continued for a median of 106 hours | N/A | 10–40 | Change in PaO2/FiO2, OI, VR | ns |
| Laghlam et al.111 | Prospective | 12 pts. iNO for 30 minutes, then iNO+almitrine for 30 minutes, then almitrine for 30 minutes | N/A | 10 | Change in PaO2/FiO2 | ns |
| Lotz et al.100 | Retrospective | 7 pts. iNO administered for 15–30 minutes | N/A | 20 | Pulmonary vasoreactivity, pulmonary shunt fraction, arterial oxygenation | PaO2 from 78.2 to 105.0 after iNO (p = 0.03) |
| Ziehr et al.101 | Retrospective | 12 pts. iNO before prone session | N/A | 20–80 | Change in PaO2/FiO2 ratio, dead-space-to-tidal-volume ratio | PaO2/FiO2 ratio from 136 to 170 (p = 0.003) and dead-space-to-tidal-volume ratio from 0.54 to 0.46 (p = 0.001) after iNO |
| Longobardo et al.104 | Retrospective | 27 pts. | 20 pts. w/ARDS of other causes | 20 | Respiratory parameters | PaO2/FiO2 ratio change greater in control group than in treatment group (47% vs. 3%) |
| Abou-Arab et al.102 | Prospective | 34 pts. iNO administered for 30 minutes whether PaO2/FiO2 <150 | N/A | 10 | Respiratory parameters (PEEP, C,rs, driving pressure, FiO2, PaO2, PaCO2), cor pulmonale at cardiac ultrasound | 65% of pts responders, PaO2/FiO2 significantly lower in responders than nonresponders at baseline |
| Tavazzi et al.108 | Prospective | 16 pts. iNO for 30 minutes | N/A | 20–30 | PaO2/FiO2 increase >20% compared to baseline | 25% pts. responders |
| Cardinale et al.105 | Retrospective | 20 pts. iNO, or almitrine, or both of them | N/A | 10–20 | PaO2/FiO2 increase >20% compared with baseline | No responders in pts. receiving iNO alone or iNO+almitrine. 1 responder received almitrine alone |
| Ferrari et al.106 | Retrospective | 10 pts. iNO after 3.6 days of mechanical ventilation for 30 minutes | N/A | 20 | Improvement in arterial oxygenation | ns |
| Bagate et al.107 | Prospective | 10 pts. iNO alone after 1 prone session | 10 pts. iNO+almitrine for 30 minutes after 1 prone session | 10 | Change in PaO2/FiO2 ratio | PaO2/FiO2 ratio change ns after iNO alone. PaO2/FiO2 ratio from 102 to 180 after iNO+almitrine (p < 0.01) |
| DeGrado et al.140 | Retrospective | 11 pts. iNO in nonresponders to iEPO | N/A | 20–80 | Change in PaO2/FiO2 ratio | ns |
ARDS, acute respiratory distress syndrome; C,rs, compliance of the respiratory system; iEPO, inhaled epoprostenol; PaCO2, partial pressure of carbon dioxide; PAP, pulmonary artery pressure; VR, ventilatory ratio.
In the available studies, the iNO dose ranged from a minimum of 10 ppm to a maximum of 80 ppm and iNO was started at different time points. After intubation, all the patients received conventional protective mechanical ventilation and, in some cases, underwent prone positioning, before iNO. iNO was often used as a rescue therapy and/or as a test for 30 minutes to identify patients who could benefit in terms of respiratory gas exchange.
In all the studies, patients had PaO2/FiO2 below 150 mmHg, consistent with a moderate or severe ARDS, according to the Berlin definition.4
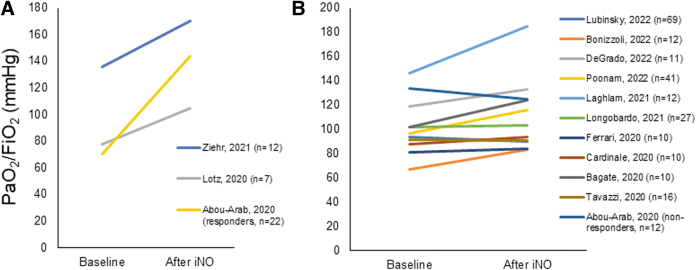
Some studies showed an improvement in arterial oxygenation,100–102 while others did not detect any significant difference (Fig. 2).103–111 Of note, Tavazzi et al. administered iNO at 10 ppm to patients admitted to ICU for COVID-19 pneumonia and a PaO2/FiO2 <150 mmHg. Responders to iNO—defined as patients whose PaO2/FiO2 increased by 20% over 30 minutes after iNO administration—were 65%. Responders had a lower PaO2/FiO2 at baseline compared with nonresponders (70 vs. 134, p < 0.0001, respectively). Interestingly, a trend toward a larger improvement of oxygenation was observed in patients with a dysfunction of the right ventricle compared with patients with normal right ventricle function (PaO2/FiO2 increase 24.1% vs. 3.3%, p = 0.069), suggesting a beneficial effect of NO on right ventricle function. The authors supposed that the limited improvement in oxygenation in patients without right ventricle dysfunction would be a consequence of the endothelial injury, which could lead to a decreased sensitivity to NO.108 The effect of iNO on the right ventricle was also evaluated by Bonizzoli et al. aimed in a small group of patients with COVID-19-associated ARDS. No change in right ventricle function, dimension, and PAP, as determined by cardiac ultrasound, was identified after iNO administration.110 In a multicenter retrospective propensity score-matched study, Al Sulaiman et al. showed that iNO at median doses of 40 (32.5–40.0) ppm was associated with improved oxygenation parameters after 24 hours of treatment.112
FIG. 2.
Changes of PaO2/FiO2 ratio before and after iNO administration in patients with COVID-19 ARDS. (A) Studies that showed a significant change in PaO2/FiO2 ratio after iNO administration. (B) Studies that did not show a significant change in PaO2/FiO2 ratio after iNO administration. Data represent mean or median according to the data presentation in the original study.
Opposite results on oxygenation come from a case–control study that showed a better response rate in patients with ARDS not related to COVID-19 compared with patients with COVID-19-associated ARDS.104 Indeed, PaO2/FiO2 improvement was 3% in patients with COVID-19-associated ARDS and 47% in patients with classical ARDS.
In another study, Bagate et al. found no change of PaO2/FiO2 ratio after iNO treatment, however, when iNO was administered along with almitrine, the PaO2/FiO2 ratio varied from 102 to 180 mmHg (p < 0.01).107 Almitrine, a pulmonary vasoconstrictor, may enhance the iNO effect to divert flow to better ventilated lung areas and thus improving the V/Q matching. However, a similar study found no effect on oxygenation by combining iNO and almitrine.105
Of note, in these studies, iNO was often administered as a “rescue” therapy, after implementing protective mechanical ventilation and prone positioning. However, whether the aim of iNO treatment is the viricidal and anti-inflammatory effect in COVID-19-associated ARDS, it is plausible that an early iNO administration could prevent the progression of the disease. For sure, further investigation is warranted to clarify the correct timing to initiate iNO in COVID-19 ARDS. Indeed, iNO could be considered in spontaneous breathing patients as a therapeutic agent to prevent the need of mechanical ventilation and to speed up the resolution of COVID-19.
In this context, iNO was administered at high doses (160 ppm), twice a day for 30 minutes, in spontaneously breathing patients with COVID-19 and tachypnea. iNO decreased respiratory symptoms and improved oxygenation in hypoxic patients.113,114 iNO was also administered to 20 pregnant patients hospitalized for severe COVID-19 pneumonia. These patients had 63.2% (95% CI 36.2%–95.4%; p < 0.001) more oxygen supplementation-free days compared with pregnant women who did not receive iNO, and no iNO adverse event was reported.115
iNO was also administered as a “rescue” and “bridge” therapy to consent transport of critically ill patients with ARDS from community-based hospitals to tertiary care centers. Indeed, iNO administration during transport seemed feasible and safe in 50 patients, of whom 39 had COVID-19 ARDS.116 Another case series suggested the feasibility of iNO administration during transportation.117
As aforementioned, due to the paucity of data and their limited quality, no efficacy of iNO in COVID-19-associated ARDS can be speculated. RCTs could determine the role of iNO on outcome in this specific subtype of ARDS patients. Results from a multicenter single-blinded RCT, aiming to determine whether iNO may improve arterial oxygenation in patients with hypoxic SARS-CoV-2, are awaited (NCT04306393). Moreover, population enrichment should be considered also in the COVID-19 ARDS population, as preliminary data described different COVID-19 clinical phenotypes that may explain different outcomes and make the study population more homogeneous.118
iNO toxicity and adverse events
Along with the potential benefits of iNO in ARDS patients, the toxicity and adverse effects of iNO should be considered. Indeed, iNO may react with superoxide anion, commonly produced in the presence of inflammation, and generating peroxynitrite, a highly reactive oxidant species, which in turn is able to interfere with mitochondrial respiration and lung surfactant function.119–121 However, these effects have not been well investigated in humans. In addition, iNO is oxidated to NO2, an airway irritant and can lead to pulmonary edema. The exposure limits of NO2 are 5 and 20 ppm, considered immediately dangerous to life or health.122 NO2 levels of 5.6 ppm were reported in a patient receiving intermittent iNO at 160 ppm out of 343 iNO administrations.123,124
Nevertheless, these doses are by far higher than the amount of iNO usually administered in ARDS patients. Measurements of NO2 levels should be performed whether very high doses of iNO are delivered. A potential carcinogenic effect of iNO may be hypothesized as it can alter DNA, although this has never been demonstrated.125,126 iNO promotes the conversion of oxyhemoglobin into methemoglobin (MetHb), impairing the ability of red blood cells to release oxygen to the tissues and can lead to tissue hypoxia. Commonly, there are no clinical implications until MetHb concentrations of 10% and iNO doses up to 40 ppm are not associated with this adverse effect.120 However, treatment with methylene blue, which reduces MetHb to hemoglobin, is prompted when levels of MetHb are above 20% or in case of tissue hypoxia.127
In a meta-analysis of 1363 patients with ARDS enrolled in 10 RCTs, iNO was associated with an increased risk of acute kidney injury compared with placebo (RR 1.4, 95% CI 1.06–1.83).128 In contrast, iNO showed to prevent renal failure associated with cardiopulmonary bypass. Finally, iNO may also have detrimental effects on patients' hemodynamics. Systemic hypotension is more common in patients receiving iNO compared with those treated with placebo129 and it could increase left ventricle filling pressure in patients with concomitant left ventricle heart failure.130 Moreover, rebound increase in pulmonary vascular resistances can be present in up to 25% of patients undergoing abrupt iNO interruption.131 In case of these hemodynamic effects, a careful dose titration, weaning, and/or treatment suspension are necessary. In general, interruption of iNO administration and supportive care or specific treatment is suggested if any of the mentioned adverse effects appear during iNO treatment.
iNO delivery systems and costs of treatment
iNO is commonly administered to the patient through systems that use cylinders containing a mixture of NO and nitrogen. Although these systems are reliable, they are cumbersome, expensive, and require a supply chain and trained health care professionals. This makes iNO treatment expensive and the most expensive drug used in neonatal departments where it is used for PPHN. The average cost of 5 days of iNO treatment for PPHN in the United States is estimated to be $14,000.132 Other iNO delivery strategies are currently under investigation, including electric NO systems,132 chemical-based systems,133 NO-releasing solutions (NCT04337918, NCT04163978), and nanoparticle technology (Table 3).134 NO-releasing solutions and releasing nanoparticles have been studied to treat cutaneous infections, and no application in ARDS of these iNO delivery strategies has been tested so far.
Table 3.
Mechanism of Actions, Advantages, and Potential Drawbacks of Different Nitric Oxide Delivery Systems
| Type of delivery system | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| NO cylindersa | NO is pressurized in cylinders with nitrogen | Reliable, can deliver broad range of NO concentrations | Cumbersome, expensive, require supply chain, and trained personnel, limited to hospitals |
| Electrical NO generationa | High-voltage electricity through two metallic electrodes that ionize air, generating NO, NO2, and ozone. Filter and scavengers remove toxic metallic products, NO2, and ozone | Portable | Risk of release of by-products from air ionization. Dependent on air flow (i.e., the higher the flow, the lower the amount of NO production) |
| Chemical NO generationa | Generation of NO2 from vaporization of dinitrogen tetroxide, then reduction of NO2 through ascorbic acid to obtain NO. | Portable | Requires supply chain |
| Solutions releasing NO | Solutions that release NO when activated by change in pH | Studies currently limited to topical administration to treat cutaneous infections and prevent/treat mild or moderate COVID-19 (NCT04337918, NCT04163978) | |
| Nanoparticles releasing NO | Release of NO from nanoparticles containing NO or precursors | Potential application: topical administration to treat cutaneous infections | |
Commercially available.
COVID-19, coronavirus disease 2019; NO2, nitrogen dioxide.
On the contrary, Yu et al. developed a lightweight portable device, exploiting an economical method that generates NO from room air by pulsed electrical discharge.132 The administration of iNO through the classical delivery system and the portable device is different. Indeed, the former uses a continuous flow, while the latter a pulsed flow. In the case of continuous flow, gas concentration monitors and continuous adaptation of the flow are necessary to maintain the concentration constant and avoid accumulation of iNO in the circuit of the ventilator when there is no ventilator flow. The pulsed NO administration may easily overcome this drawback as a specific dose of NO is delivered at the beginning of each inspiration and it is independent on minute ventilation and gas flow.
In addition, the pulse of NO may minimize the amount of drug used and decrease the costs.135 The new portable device was tested in humans through two exploratory studies that demonstrated its safety, with neither MetHb nor NO2 levels above safety range.29,136 Another electric NO generator has been recently approved by FDA for PPHN.137 Lovich et al. developed an NO chemical generator and currently a device using this technology can deliver NO at 20 ppm.133,138 Further investigation is required to determine the feasibility and safety of these devices in patients with ARDS. In this way, iNO might become more economical and more accessible to patients and clinicians, with less impact on the health care system costs. Moreover, widespread access to iNO might boost the initiation of RCTs to determine whether, and in which patients with ARDS, this drug may benefit the most.
Conclusions
iNO has a strong biological and pharmacological rationale for its use both in classical and COVID-19-associated ARDS. It might improve the ventilation-perfusion match, respiratory gas exchanges, decrease PAPs and prevent or reduce right ventricular failure. In the specific scenario of COVID-19 ARDS, it might be viricidal and decrease the development of pulmonary hyperinflammation and thrombosis. However, so far, data are limited in the subgroup of COVID-19 ARDS. iNO failed to ameliorate clinical outcomes in classical ARDS, although the heterogeneity of ARDS recently highlighted by the study of phenotypes may be a promising field of research to characterize potential ARDS subgroups that may be responders to iNO.
The COVID-19 pandemic has highlighted the urgent need to investigate effective treatments to increase survival in ARDS and that may prevent the progression to the most severe forms of this disease. Therefore, further investigation is needed to demonstrate the theoretical benefits of iNO. Moreover, the timing of iNO treatment initiation, and the optimal duration and dose of iNO, is a field of research that needs to be further explored.
Acknowledgments
The authors of this review would like to dedicate this article to the memory of Warren M. Zapol, MD, a visionary scientist, a tireless mentor, and a scientific father of generations of physicians. His discovery on the therapeutic use of inhaled nitric oxide gas is one of the major contributions he gave to the critical care fields that expanded tremendously since then and the focus of our present review.
Authors' Contributions
S.R.: Investigation, writing—original draft, writing—review and editing, and visualization. M.P.: Visualization and writing—review and editing. M.G., A.M., R.F., and G.F.: Writing—review and editing. L.B.: Supervision, Writing—review and editing. E.R.: Conceptualization, supervision, investigation, visualization, writing—original draft, and writing—review and editing.
Author Disclosure Statement
The authors declare they have no conflicting financial interests.
Funding Information
Lorenzo Berra receives salary support from K23 HL128882/NHLBI NIH as principal investigator for his work on hemolysis and nitric oxide. Lorenzo Berra receives technologies and devices from iNO Therapeutics LLC, Praxair, Inc., Masimo Corp. Lorenzo Berra receives grants from “Fast Grants for COVID-19 research” at Mercatus Center of George Mason University and from iNO Therapeutics LLC. Laboratory work is supported by the Reginald Jenney Endowment Chair at Harvard Medical School, by Sundry Funds at Massachusetts General Hospital, and by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. Emanuele Rezoagli is supported by the Bicocca Starting grant 2020 from the University of Milano-Bicocca with the project titled: “Functional Residual Capacity Assessment using a Wash-In/Wash-Out technique based on a fast main-stream O2 Sensor with nanofluorescenT geometry for severe lung injury (FAST)—COVID and beyond”; by the International Young Investigator Award 2018 from the European Society of Intensive Care Medicine (ESICM) with the project titled: “Role of the exhaled breath condensate as non-invasive monitoring of the lung inflammation during ARDS: a prospective cohort study”; and by the National Merck Sharp & Dohme Corporation Research Award 2017 from the Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI) with the project titled: “Studio della concentrazione di ossido nitrico nell'esalato espiratorio come marcatore di danno polmonare acuto in pazienti adulti con ARDS sottoposti a ventilazione meccanica.”
Reviewed by:
Dean Hess
Adam Wanner
References
- 1. Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med 2017;5(14):282; doi: 10.21037/atm.2017.06.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315(8):788–800; doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 3. Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353(16):1685–1693; doi: 10.1056/NEJMoa050333 [DOI] [PubMed] [Google Scholar]
- 4. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526–2533; doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 5. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377(6):562–572; doi: 10.1056/NEJMra1608077 [DOI] [PubMed] [Google Scholar]
- 6. Gattinoni L, Bombino M, Pelosi P, et al. Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA 1994;271(22):1772–1779. [PubMed] [Google Scholar]
- 7. Rezoagli E, Magliocca A, Bellani G, et al. Development of a critical care response—Experiences from Italy during the coronavirus disease 2019 pandemic. Anesthesiol Clin 2021;39(2):265–284; doi: 10.1016/j.anclin.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. COVID-19 Studies from the World Health Organization Database. Available from: https://clinicaltrials.gov/ct2/who_table [Last accessed: February 20, 2023].
- 9. Horie S, McNicholas B, Rezoagli E, et al. Emerging pharmacological therapies for ARDS: COVID-19 and beyond. Intensive Care Med 2020;46(12):2265–2283; doi: 10.1007/s00134-020-06141-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wendel-Garcia PD, Moser A, Jeitziner M-M, et al. Dynamics of disease characteristics and clinical management of critically ill COVID-19 patients over the time course of the pandemic: An analysis of the prospective, international, multicentre RISC-19-ICU registry. Crit Care 2022;26(1):199; doi: 10.1186/s13054-022-04065-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rossaint R, Falke KJ, López F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 1993;328(6):399–405; doi: 10.1056/NEJM199302113280605 [DOI] [PubMed] [Google Scholar]
- 12. Baxter FJ, Randall J, Miller JD, et al. Rescue therapy with inhaled nitric oxide in critically ill patients with severe hypoxemic respiratory failure (brief report). Can J Anaesth 2002;49(3):315–318; doi: 10.1007/BF03020535 [DOI] [PubMed] [Google Scholar]
- 13. Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017;195(9):1253–1263; doi: 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 14. Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149(3 Pt 1):818–824; doi: 10.1164/ajrccm.149.3.7509706 [DOI] [PubMed] [Google Scholar]
- 15. Gebistorf F, Karam O, Wetterslev J, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev 2016;6:CD002787; doi: 10.1002/14651858.CD002787.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: A randomized controlled trial. JAMA 2004;291(13):1603–1609; doi: 10.1001/jama.291.13.1603 [DOI] [PubMed] [Google Scholar]
- 17. Day RW, Allen EM, Witte MK. A randomized, controlled study of the 1-hour and 24-hour effects of inhaled nitric oxide therapy in children with acute hypoxemic respiratory failure. Chest 1997;112(5):1324–1331; doi: 10.1378/chest.112.5.1324 [DOI] [PubMed] [Google Scholar]
- 18. Fukuto JM. Chemistry of nitric oxide: Biologically relevant aspects. Adv Pharmacol 1995;34:1–15; doi: 10.1016/s1054-3589(08)61078-9 [DOI] [PubMed] [Google Scholar]
- 19. Stuehr DJ, Santolini J, Wang Z-Q, et al. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 2004;279(35):36167–36170; doi: 10.1074/jbc.R400017200 [DOI] [PubMed] [Google Scholar]
- 20. Ichinose F, Roberts JD, Zapol WM. Inhaled nitric oxide. Circulation 2004;109(25):3106–3111; doi: 10.1161/01.CIR.0000134595.80170.62 [DOI] [PubMed] [Google Scholar]
- 21. Steudel W, Hurford WE, Zapol WM. Inhaled nitric oxide: Basic biology and clinical applications. Anesthesiology 1999;91(4):1090–1121; doi: 10.1097/00000542-199910000-00030 [DOI] [PubMed] [Google Scholar]
- 22. Radomski MW, Vallance P, Whitley G, et al. Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc Res 1993;27(7):1380–1382; doi: 10.1093/cvr/27.7.1380 [DOI] [PubMed] [Google Scholar]
- 23. Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 1989;83(5):1774–1777; doi: 10.1172/JCI114081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lefer AM. Nitric oxide: Nature's naturally occurring leukocyte inhibitor. Circulation 1997;95(3):553–554; doi: 10.1161/01.cir.95.3.553 [DOI] [PubMed] [Google Scholar]
- 25. Rimar S, Gillis CN. Selective pulmonary vasodilation by inhaled nitric oxide is due to hemoglobin inactivation. Circulation 1993;88(6):2884–2887; doi: 10.1161/01.cir.88.6.2884 [DOI] [PubMed] [Google Scholar]
- 26. Gibson QH, Roughton FJW. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol 1957;136(3):507–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frostell CG, Blomqvist H, Hedenstierna G, et al. Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology 1993;78(3):427–435; doi: 10.1097/00000542-199303000-00005 [DOI] [PubMed] [Google Scholar]
- 28. Rezoagli E, Ichinose F, Strelow S, et al. Pulmonary and systemic vascular resistances after cardiopulmonary bypass: Role of hemolysis. J Cardiothorac Vasc Anesth 2017;31(2):505–515; doi: 10.1053/j.jvca.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 29. Berra L, Rodriguez-Lopez J, Rezoagli E, et al. Electric plasma–generated nitric oxide: Hemodynamic effects in patients with pulmonary hypertension. Am J Respir Crit Care Med 2016;194(9):1168–1170; doi: 10.1164/rccm.201604-0834LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Signori D, Magliocca A, Hayashida K, et al. Inhaled nitric oxide: Role on the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensive Care Med Exp 2022;10(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Academy of Pediatrics. Committee on Fetus and Newborn. Use of inhaled nitric oxide. Pediatrics 2000;106(2 Pt 1):344–345. [PubMed] [Google Scholar]
- 32. Elzein C, Urbas C, Hughes B, et al. Efficacy of nitric oxide administration in attenuating ischemia/reperfusion injury during neonatal cardiopulmonary bypass. World J Pediatr Congenit Heart Surg 2020;11(4):417–423; doi: 10.1177/2150135120911034 [DOI] [PubMed] [Google Scholar]
- 33. Sadiq HF, Mantych G, Benawra RS, et al. Inhaled nitric oxide in the treatment of moderate persistent pulmonary hypertension of the newborn: A randomized controlled, multicenter trial. J Perinatol 2003;23(2):98–103; doi: 10.1038/sj.jp.7210878 [DOI] [PubMed] [Google Scholar]
- 34. Berra L, Pinciroli R, Stowell CP, et al. Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med 2014;190(7):800–807; doi: 10.1164/rccm.201405-0850OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lei C, Berra L, Rezoagli E, et al. Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med 2018;198(10):1279–1287; doi: 10.1164/rccm.201710-2150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Redaelli S, Magliocca A, Malhotra R, et al. Nitric oxide: Clinical applications in critically ill patients. Nitric Oxide 2022;121:20–33; doi: 10.1016/j.niox.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers 2019;5(1):18; doi: 10.1038/s41572-019-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 1977;296(9):476–480; doi: 10.1056/NEJM197703032960903 [DOI] [PubMed] [Google Scholar]
- 39. Rezoagli E, Laffey JG, Bellani G. Monitoring lung injury severity and ventilation intensity during mechanical ventilation. Semin Respir Crit Care Med 2022;43(3):346–368. [DOI] [PubMed] [Google Scholar]
- 40. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 2001;29(8):1551–1555; doi: 10.1097/00003246-200108000-00009 [DOI] [PubMed] [Google Scholar]
- 41. Torbic H, Szumita PM, Anger KE, et al. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care 2013;28(5):844–848; doi: 10.1016/j.jcrc.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 42. Sawheny E, Ellis AL, Kinasewitz GT. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 2013;144(1):55–62; doi: 10.1378/chest.12-2296 [DOI] [PubMed] [Google Scholar]
- 43. Cornet AD, Hofstra JJ, Swart EL, et al. Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med 2010;36(5):758–764; doi: 10.1007/s00134-010-1754-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leuchte HH, Schwaiblmair M, Baumgartner RA, et al. Hemodynamic response to sildenafil, nitric oxide, and iloprost in primary pulmonary hypertension. Chest 2004;125(2):580–586; doi: 10.1378/chest.125.2.580 [DOI] [PubMed] [Google Scholar]
- 45. Ivy DD, Parker D, Doran A, et al. Acute hemodynamic effects and home therapy using a novel pulsed nasal nitric oxide delivery system in children and young adults with pulmonary hypertension. Am J Cardiol 2003;92(7):886–890; doi: 10.1016/s0002-9149(03)00910-x [DOI] [PubMed] [Google Scholar]
- 46. Hillman ND, Meliones JN, Black DR, et al. In acute lung injury, inhaled nitric oxide improves ventilation-perfusion matching, pulmonary vascular mechanics, and transpulmonary vascular efficiency. J Thorac Cardiovasc Surg 1995;110(3):593–600; doi: 10.1016/S0022-5223(95)70089-7 [DOI] [PubMed] [Google Scholar]
- 47. Pison U, López FA, Heidelmeyer CF, et al. Inhaled nitric oxide reverses hypoxic pulmonary vasoconstriction without impairing gas exchange. J Appl Physiol (1985) 1993;74(3):1287–1292; doi: 10.1152/jappl.1993.74.3.1287 [DOI] [PubMed] [Google Scholar]
- 48. Wagner PD, Saltzman HA, West JB. Measurement of continuous distributions of ventilation-perfusion ratios: Theory. J Appl Physiol 1974;36(5):588–599; doi: 10.1152/jappl.1974.36.5.588 [DOI] [PubMed] [Google Scholar]
- 49. Rossaint R, Slama K, Steudel W, et al. Effects of inhaled nitric oxide on right ventricular function in severe acute respiratory distress syndrome. Intensive Care Med 1995;21(3):197–203; doi: 10.1007/BF01701472 [DOI] [PubMed] [Google Scholar]
- 50. Fierobe L, Brunet F, Dhainaut JF, et al. Effect of inhaled nitric oxide on right ventricular function in adult respiratory distress syndrome. Am J Respir Crit Care Med 1995;151(5):1414–1419; doi: 10.1164/ajrccm.151.5.7735594 [DOI] [PubMed] [Google Scholar]
- 51. Bronicki RA, Fortenberry J, Schreiber M, et al. Multicenter randomized controlled trial of inhaled nitric oxide for pediatric acute respiratory distress syndrome. J Pediatr 2015;166(2):365–369.e1; doi: 10.1016/j.jpeds.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 52. Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: Results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med 1998;26(1):15–23; doi: 10.1097/00003246-199801000-00011 [DOI] [PubMed] [Google Scholar]
- 53. Dobyns EL, Cornfield DN, Anas NG, et al. Multicenter randomized controlled trial of the effects of inhaled nitric oxide therapy on gas exchange in children with acute hypoxemic respiratory failure. J Pediatr 1999;134(4):406–412; doi: 10.1016/s0022-3476(99)70196-4 [DOI] [PubMed] [Google Scholar]
- 54. Ibrahim TS, El-Mohamdy HS. Inhaled nitric oxide and prone position: How far they can improve oxygenation in pediatric patients with acute respiratory distress syndrome? J Med Sci 2007;7:390–395. [Google Scholar]
- 55. Gerlach H, Keh D, Semmerow A, et al. Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: A prospective, randomized, controlled study. Am J Respir Crit Care Med 2003;167(7):1008–1015; doi: 10.1164/rccm.2108121 [DOI] [PubMed] [Google Scholar]
- 56. Lundin S, Mang H, Smithies M, et al. Inhalation of nitric oxide in acute lung injury: Results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med 1999;25(9):911–919; doi: 10.1007/s001340050982 [DOI] [PubMed] [Google Scholar]
- 57. Mehta S, Simms HH, Levy MM, et al. Inhaled nitric oxide improves oxygenation acutely but not chronically in acute respiratory distress syndrome: A randomized, controlled trial. J Appl Res Clin Exp Ther 2001;1:73–84. [Google Scholar]
- 58. Michael JR, Barton RG, Saffle JR, et al. Inhaled nitric oxide versus conventional therapy: Effect on oxygenation in ARDS. Am J Respir Crit Care Med 1998;157(5 Pt 1):1372–1380; doi: 10.1164/ajrccm.157.5.96-10089 [DOI] [PubMed] [Google Scholar]
- 59. Park KJ, Lee YJ, Oh YJ, et al. Combined effects of inhaled nitric oxide and a recruitment maneuver in patients with acute respiratory distress syndrome. Yonsei Med J 2003;44(2):219–226; doi: 10.3349/ymj.2003.44.2.219 [DOI] [PubMed] [Google Scholar]
- 60. Payen D, Vallet B; Group d'étude du NO dans l'ARDS. Results of the French prospective multicentric randomized double-blind placebo-controlled trial on inhaled nitric oxide (NO) in ARDS. Intensive Care Med 1999;25(Suppl 1):166.10193543 [Google Scholar]
- 61. Schwebel C, Beuret P, Perdrix JP, et al. Early inhaled nitric oxide inhalation in acute lung injury: Results of a double-blind randomized study [abstract]. Intensive Care Med 1997;23(Suppl 1):2. [Google Scholar]
- 62. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res 2019;6(1):e000420; doi: 10.1136/bmjresp-2019-000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duggal A, Rezoagli E, Pham T, et al. Patterns of use of adjunctive therapies in patients with early moderate to severe ARDS: Insights from the LUNG SAFE Study. Chest 2020;157(6):1497–1505; doi: 10.1016/j.chest.2020.01.041 [DOI] [PubMed] [Google Scholar]
- 64. Pelosi P, D'Onofrio D, Chiumello D, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 2003;42:48s–56s; doi: 10.1183/09031936.03.00420803 [DOI] [PubMed] [Google Scholar]
- 65. McNicholas BA, Madotto F, Pham T, et al. Demographics, management and outcome of females and males with acute respiratory distress syndrome in the LUNG SAFE prospective cohort study. Eur Respir J 2019;54(4):1900609; doi: 10.1183/13993003.00609-2019 [DOI] [PubMed] [Google Scholar]
- 66. Rezoagli E, McNicholas BA, Madotto F, et al. Presence of comorbidities alters management and worsens outcome of patients with acute respiratory distress syndrome: Insights from the LUNG SAFE study. Ann Intensive Care 2022;12(1):42; doi: 10.1186/s13613-022-01015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rezoagli E, McNicholas B, Pham T, et al. Lung-kidney cross-talk in the critically ill: Insights from the Lung Safe study. Intensive Care Med 2020;46(5):1072–1073; doi: 10.1007/s00134-020-05962-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McNicholas BA, Rezoagli E, Pham T, et al. Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: A secondary analysis of a multicenter observational study. Crit Care Med 2019;47(9):1216–1225; doi: 10.1097/CCM.0000000000003832 [DOI] [PubMed] [Google Scholar]
- 69. Madotto F, Rezoagli E, McNicholas BA, et al. Patterns and impact of arterial CO2 management in patients with acute respiratory distress syndrome: Insights from the LUNG SAFE study. Chest 2020;158(5):1967–1982; doi: 10.1016/j.chest.2020.05.605 [DOI] [PubMed] [Google Scholar]
- 70. Madotto F, Rezoagli E, Pham T, et al. Hyperoxemia and excess oxygen use in early acute respiratory distress syndrome: insights from the LUNG SAFE study. Crit Care 2020;24(1):125; doi: 10.1186/s13054-020-2826-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Madotto F, McNicholas B, Rezoagli E, et al. Death in hospital following ICU discharge: Insights from the LUNG SAFE study. Crit Care 2021;25(1):144; doi: 10.1186/s13054-021-03465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maddali MV, Churpek M, Pham T, et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: An observational, multicohort, retrospective analysis. Lancet Respir Med 2022;10(4):367–377; doi: 10.1016/S2213-2600(21)00461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122(8):2731–2740; doi: 10.1172/JCI60331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shankar-Hari M, Rubenfeld GD. Population enrichment for critical care trials: Phenotypes and differential outcomes. Curr Opin Crit Care 2019;25(5):489–497; doi: 10.1097/MCC.0000000000000641 [DOI] [PubMed] [Google Scholar]
- 75. Ware LB, Matthay MA, Mebazaa A. Designing an ARDS trial for 2020 and beyond: Focus on enrichment strategies. Intensive Care Med 2020;46(12):2153–2156; doi: 10.1007/s00134-020-06232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shah FA, Meyer NJ, Angus DC, et al. A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2021;204(8):891–901; doi: 10.1164/rccm.202108-1908ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sinha P, Calfee CS. Phenotypes in ARDS: Moving towards precision medicine. Curr Opin Crit Care 2019;25(1):12–20; doi: 10.1097/MCC.0000000000000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iotti GA, Olivei MC, Palo A, et al. Acute effects of inhaled nitric oxide in adult respiratory distress syndrome. Eur Respir J 1998;12(5):1164–1171; doi: 10.1183/09031936.98.12051164 [DOI] [PubMed] [Google Scholar]
- 79. Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020;201(10):1299–1300; doi: 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med 2020;46(6):1099–1102; doi: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Alvarez RA, Berra L, Gladwin MT. Home nitric oxide therapy for COVID-19. Am J Respir Crit Care Med 2020;202(1):16–20; doi: 10.1164/rccm.202005-1906ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montiel V, Lobysheva I, Gérard L, et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. EBioMedicine 2022;77:103893; doi: 10.1016/j.ebiom.2022.103893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferrari M, Protti A. Nitric oxide in COVID-19: Too little of a good thing? EBioMedicine 2022;77:103925; doi: 10.1016/j.ebiom.2022.103925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nicholson CJ, Wooster L, Sigurslid HH, et al. Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to Mass General Brigham: The VICE and DICE scores. EClinicalMedicine 2021;33:100765; doi: 10.1016/j.eclinm.2021.100765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sorbo LD, Michaelsen VS, Ali A, et al. High doses of inhaled nitric oxide as an innovative antimicrobial strategy for lung infections. Biomedicines 2022;10:1525; doi: 10.3390/biomedicines10071525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Keyaerts E, Vijgen L, Chen L, et al. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis 2004;8(4):223–226; doi: 10.1016/j.ijid.2004.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Åkerström S, Mousavi-Jazi M, Klingström J, et al. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol 2005;79(3):1966–1969; doi: 10.1128/JVI.79.3.1966-1969.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Akaberi D, Krambrich J, Ling J, et al. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol 2020;37:101734; doi: 10.1016/j.redox.2020.101734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Proud D. Nitric oxide and the common cold. Curr Opin Allergy Clin Immunol 2005;5(1):37–42. [DOI] [PubMed] [Google Scholar]
- 90. Torre D, Pugliese A, Speranza F. Role of nitric oxide in HIV-1 infection: Friend or foe? Lancet Infect Dis 2002;2(5):273–280; doi: 10.1016/S1473-3099(02)00262-1 [DOI] [PubMed] [Google Scholar]
- 91. Moni M, Madathil T, Sathyapalan DT, et al. Clinical efficacy of inhaled nitric oxide in preventing the progression of moderate to severe COVID-19 and its correlation to viral clearance: Results of a pilot study. Infect Microbes Dis 2022;4(1):26–33; doi: 10.1097/IM9.0000000000000079 [DOI] [Google Scholar]
- 92. Chen L, Liu P, Gao H, et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: A rescue trial in Beijing. Clin Infect Dis 2004;39(10):1531–1535; doi: 10.1086/425357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Akaike T, Maeda H. Nitric oxide and virus infection. Immunology 2000;101(3):300–308; doi: 10.1046/j.1365-2567.2000.00142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen W, Pan JY. Anatomical and pathological observation and analysis of SARS and COVID-19: Microthrombosis is the main cause of death. Biol Proced Online 2021;23(1):4; doi: 10.1186/s12575-021-00142-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18(7):1738–1742; doi: 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dominic P, Ahmad J, Bhandari R, et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol 2021;43:101982; doi: 10.1016/j.redox.2021.101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gelzo M, Scialò F, Cacciapuoti S, et al. Inducible nitric oxide synthase (iNOS): Why a different production in COVID-19 patients of the two waves? Viruses 2022;14(3):534; doi: 10.3390/v14030534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Samavati L, Uhal BD. ACE2, much more than just a receptor for SARS-COV-2. Front Cell Infect Microbiol 2020;10:317; doi: 10.3389/fcimb.2020.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hagemo JS, Skulberg AK, Rehn M, et al. Inhaled nitric oxide as temporary respiratory stabilization in patients with COVID-19 related respiratory failure (INOCOV): Study protocol for a randomized controlled trial. PLoS One 2022;17(5):e0268822; doi: 10.1371/journal.pone.0268822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lotz C, Muellenbach RM, Meybohm P, et al. Effects of inhaled nitric oxide in COVID-19-induced ARDS—Is it worthwhile? Acta Anaesthesiol Scand 2021;65(5):629–632; doi: 10.1111/aas.13757 [DOI] [PubMed] [Google Scholar]
- 101. Ziehr DR, Alladina J, Wolf ME, et al. Respiratory physiology of prone positioning with and without inhaled nitric oxide across the coronavirus disease 2019 acute respiratory distress syndrome severity spectrum. Crit Care Explor 2021;3(6):e0471; doi: 10.1097/CCE.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abou-Arab O, Huette P, Debouvries F, et al. Inhaled nitric oxide for critically ill Covid-19 patients: A prospective study. Crit Care 2020;24:645; doi: 10.1186/s13054-020-03371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lubinsky AS, Brosnahan SB, Lehr A, et al. Inhaled pulmonary vasodilators are not associated with improved gas exchange in mechanically ventilated patients with COVID-19: A retrospective cohort study. J Crit Care 2022;69:153990; doi: 10.1016/j.jcrc.2022.153990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Longobardo A, Montanari C, Shulman R, et al. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth 2021;126(1):e44–e46; doi: 10.1016/j.bja.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cardinale M, Esnault P, Cotte J, et al. Effect of almitrine bismesylate and inhaled nitric oxide on oxygenation in COVID-19 acute respiratory distress syndrome. Anaesth Crit Care Pain Med 2020;39(4):471; doi: 10.1016/j.accpm.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ferrari M, Santini A, Protti A, et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care 2020;60:159–160; doi: 10.1016/j.jcrc.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bagate F, Tuffet S, Masi P, et al. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann Intensive Care 2020;10(1):151; doi: 10.1186/s13613-020-00769-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tavazzi G, Marco P, Mongodi S, et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care 2020;24:508; doi: 10.1186/s13054-020-03222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Poonam PBH, Koscik R, Nguyen T, et al. Nitric oxide versus epoprostenol for refractory hypoxemia in Covid-19. PLoS One 2022;17(6):e0270646; doi: 10.1371/journal.pone.0270646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bonizzoli M, Lazzeri C, Cianchi G, et al. Effects of rescue inhaled nitric oxide on right ventricle and pulmonary circulation in severe COVID-related acute respiratory distress syndrome. J Crit Care 2022;153987; doi: 10.1016/j.jcrc.2022.153987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Laghlam D, Rahoual G, Malvy J, et al. Use of almitrine and inhaled nitric oxide in ARDS due to COVID-19. Front Med 2021;8:655763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Al Sulaiman K, Korayem GB, Altebainawi AF, et al. Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: A multicenter cohort study. Crit Care 2022;26(1):304; doi: 10.1186/s13054-022-04158-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wiegand SB, Safaee Fakhr B, Carroll RW, et al. Rescue treatment with high-dose gaseous nitric oxide in spontaneously breathing patients with severe coronavirus disease 2019. Crit Care Explor 2020;2(11):e0277; doi: 10.1097/CCE.0000000000000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Safaee Fakhr B, Di Fenza R, Gianni S, et al. Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia. Nitric Oxide 2021;116:7–13; doi: 10.1016/j.niox.2021.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Valsecchi C, Winterton D, Safaee Fakhr B, et al. High-dose inhaled nitric oxide for the treatment of spontaneously breathing pregnant patients with severe coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol 2022;140(2):195–203; doi: 10.1097/AOG.0000000000004847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Piecek J, Valentino T, Aust R, et al. The use of nitric oxide as a rescue modality for severe adult acute respiratory distress syndrome patients, including COVID-19, in critical care rotor transport: A retrospective community outcome study. Air Med J 202241(5):427–431; doi: 10.1016/j.amj.2022.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Brown CJ, Rubel N, Lai J, et al. Initiation of inhaled nitric oxide by an air transport team in adult coronavirus disease 2019 respiratory failure. Air Med J 2022;41(4):406–410; doi: 10.1016/j.amj.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lusczek ER, Ingraham NE, Karam BS, et al. Characterizing COVID-19 clinical phenotypes and associated comorbidities and complication profiles. PLoS One 2021;16(3):e0248956; doi: 10.1371/journal.pone.0248956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zweier JL, Talukder MAH. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res 2006;70(2):181–190; doi: 10.1016/j.cardiores.2006.02.025 [DOI] [PubMed] [Google Scholar]
- 120. Weinberger B, Laskin DL, Heck DE, et al. The toxicology of inhaled nitric oxide. Toxicol Sci 2001;59(1):5–16; doi: 10.1093/toxsci/59.1.5 [DOI] [PubMed] [Google Scholar]
- 121. Liaudet L, Soriano FG, Szabó C. Biology of nitric oxide signaling. Crit Care Med 2000;28(4 Suppl):N37–N52; doi: 10.1097/00003246-200004001-00005 [DOI] [PubMed] [Google Scholar]
- 122. Medical Management Guidelines for Nitrogen Oxides. Available from: https://wwwn.cdc.gov/tsp/mmg/mmgdetails.aspx?mmgid=394&toxid=69 [Last accessed: February 20, 2023].
- 123. Goldbart A, Golan-Tripto I, Pillar G, et al. Inhaled nitric oxide therapy in acute bronchiolitis: A multicenter randomized clinical trial. Sci Rep 2020;10(1):9605; doi: 10.1038/s41598-020-66433-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: A randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-NO/PPHN Study Group. Pediatrics 1998;101(3 Pt 1):325–334; doi: 10.1542/peds.101.3.325 [DOI] [PubMed] [Google Scholar]
- 125. Arroyo PL, Hatch-Pigott V, Mower HF, et al. Mutagenicity of nitric oxide and its inhibition by antioxidants. Mutat Res 1992;281(3):193–202; doi: 10.1016/0165-7992(92)90008-6 [DOI] [PubMed] [Google Scholar]
- 126. Nguyen T, Brunson D, Crespi CL, et al. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A 1992;89(7):3030–3034; doi: 10.1073/pnas.89.7.3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cefalu JN, Joshi TV, Spalitta MJ, et al. Methemoglobinemia in the operating room and intensive care unit: Early recognition, pathophysiology, and management. Adv Ther 2020;37(5):1714; doi: 10.1007/s12325-020-01282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ruan S-Y, Huang T-M, Wu H-Y, et al. Inhaled nitric oxide therapy and risk of renal dysfunction: A systematic review and meta-analysis of randomized trials. Crit Care 2015;19:137; doi: 10.1186/s13054-015-0880-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Approval Package for: INOMax. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/020845orig1s014.pdf [Last accessed: February 20, 2023].
- 130. Loh E, Stamler JS, Hare JM, et al. Cardiovascular effects of inhaled nitric oxide in patients with left ventricular dysfunction. Circulation 1994;90(6):2780–2785; doi: 10.1161/01.cir.90.6.2780 [DOI] [PubMed] [Google Scholar]
- 131. Christenson J, Lavoie A, O'Connor M, et al. The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med 2000;161(5):1443–1449; doi: 10.1164/ajrccm.161.5.9806138 [DOI] [PubMed] [Google Scholar]
- 132. Yu B, Muenster S, Blaesi AH, et al. Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy. Sci Transl Med 2015;7(294):294ra107; doi: 10.1126/scitranslmed.aaa3097 [DOI] [PubMed] [Google Scholar]
- 133. Lovich MA, Bruno NK, Plant CP, et al. Use of ultra pure nitric oxide generated by the reduction of nitrogen dioxide to reverse pulmonary hypertension in hypoxemic swine. Nitric Oxide 2011;24(4):204–212; doi: 10.1016/j.niox.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 134. Friedman AJ, Han G, Navati MS, et al. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008;19(1):12–20; doi: 10.1016/j.niox.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 135. Gianni S, Berra L, Emanuele R.. Inhaled Nitric Oxide (INO) Administration in Intubated and Non-Intubated Patients: Delivery Systems, Interfaces, Dose Administration, and Monitoring Techniques. Book Therapeutic Applications of Nitric Oxide and Derivatives. Springer; 2023. [in Press]. [Google Scholar]
- 136. Gianni S, Di Fenza R, Araujo Morais CC, et al. A comparison between high dose nitric oxide delivered from pressurized cylinders and nitric oxide produced by an electric generator from air. A safety pilot study. Respir Care 2022;67(2):201–208; doi: 10.4187/respcare.09308 [DOI] [PubMed] [Google Scholar]
- 137. Summary of safety and effectiveness data. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf20/p200044b.pdf [Last accessed: February 20, 2023].
- 138. Approval package. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/202860ong15000SumR.pdf [Last accessed: February 20, 2023].
- 139. Troncy E, Collet JP, Shapiro S, et al. Inhaled nitric oxide in acute respiratory distress syndrome: A pilot randomized controlled study. Am J Respir Crit Care Med 1998;157(5 Pt 1):1483–1488; doi: 10.1164/ajrccm.157.5.9707090 [DOI] [PubMed] [Google Scholar]
- 140. DeGrado JR, Szumita PM, Schuler BR, et al. Evaluation of the efficacy and safety of inhaled epoprostenol and inhaled nitric oxide for refractory hypoxemia in patients with coronavirus disease 2019. Crit Care Explor 2020;2(10):e0259; doi: 10.1097/CCE.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]