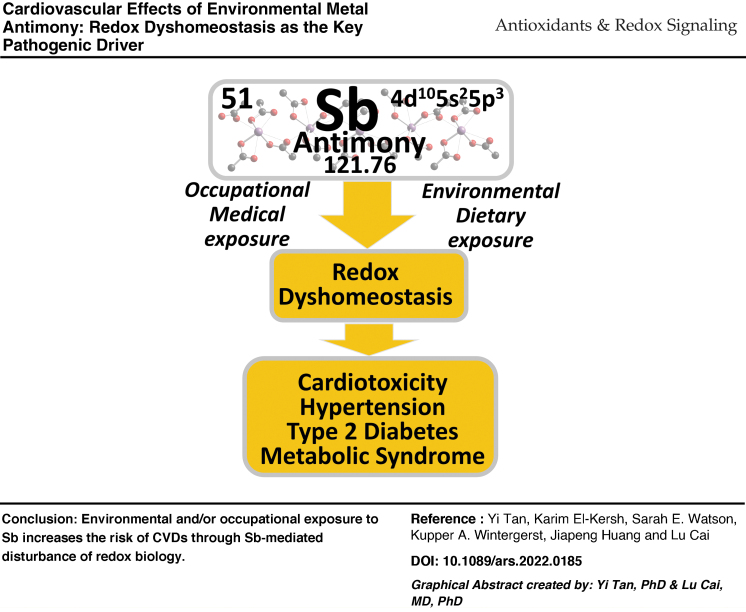
Abstract
Significance:
Cardiovascular diseases (CVDs) are the leading cause of death worldwide, which may be due to sedentary lifestyles with less physical activity and over nutrition as well as an increase in the aging population; however, the contribution of pollutants, environmental chemicals, and nonessential metals to the increased and persistent CVDs needs more attention and investigation. Among environmental contaminant nonessential metals, antimony has been less addressed.
Recent Advances:
Among environmental contaminant nonessential metals, several metals such as lead, arsenic, and cadmium have been associated with the increased risk of CVDs. Antimony has been less addressed, but its potential link to CVDs is being gradually recognized.
Critical Issues:
Several epidemiological studies have revealed the significant deleterious effects of antimony on the cardiovascular system in the absence or presence of other nonessential metals. There has been less focus on whether antimony alone can contribute to the pathogenesis of CVDs and the proposed mechanisms of such possible effects. This review addresses this gap in knowledge by presenting the current available evidence that highlights the potential role of antimony in the pathogenesis of CVDs, most likely via antimony-mediated redox dyshomeostasis.
Future Directions:
More direct evidence from preclinical and mechanistic studies is urgently needed to evaluate the possible roles of antimony in mitochondrial dysfunction and epigenetic regulation in CVDs. Antioxid. Redox Signal. 38, 803–823.
Graphical abstract
Keywords: antimony toxicity, metal cardiotoxicity, redox imbalance, cardiovascular diseases

I. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide due to sedentary lifestyles with less physical activity and/or over nutrition, as well as an increase in the aging population. These CVDs disproportionally affect people living in different communities with greater environmental exposures, to transportation noise, air pollution, and mental stress. Although lifestyle choices such as smoking, diet, and exercise are viewed as major environmental influencers, the contribution of pollutants, environmental chemicals, and nonessential metals to the increased and persistent CVDs needs more attention and investigation.
Exposure to environmental nonessential metals such as arsenic (As), lead (Pb), and cadmium (Cd) has been linked to increased incidence of CVDs (Everson et al, 2021; Swayze et al, 2021; Wang et al, 2022c). For instance, a recent meta-analysis of 350,000 individuals from 37 countries showed that exposure to As, Pb, Cd, and copper (Cu) was positively associated with CVD incidence and mortality rate in a linear-shaped dose–response manner (Guo et al, 2022). However, whether some rare metals such as antimony also contribute to the pathogenesis of CVDs via either direct toxicity or indirect impact by affecting other organs or systems has been less addressed.
Antimony (chemical symbol Sb, its Latin word as stibium; atomic number 51) is a chemical element as a lustrous gray metalloid. More than 10,150 publications regarding Sb have been recorded in PubMed since 1804, but more than half (about 6000) appeared in the last two decades due to the quick economic development as many industries' original material providers. Air pollution and/or environmental contamination derived from the wide application of Sb and its associated products have been gradually addressed (Cheung Chung et al, 2008). In 1974, Science reported the presence of toxic metals As, Sb, Cd, Pb, selenium, and thallium in small inhalable particles from coal-fired power plants (Natusch et al, 1974). It is also used in manufacturing, with occupational exposure to Sb being reported in the 1960s (Taylor, 1966).
Appearing as early as the 1940s, Sb has also been used as medicine to treat schistosomiasis, with recognition of adverse cardiac effects being reported in the same decade, noticed in 141 patients treated with Sb via measuring their electrocardiogram (EKG) (Craddock and Toone, 1947; Tarr, 1947). The cardiovascular effects of Sb were reviewed by Borhani (1981). These authors found that workers exposed to Sb2S3 for up to 2 years exhibited abnormal EKG, mostly in the form of T wave changes.
To date, however, there was no comprehensive review available specifically for the effects of Sb on the cardiovascular system. Although there is consensus that oxidative stress plays a key role in mediating the hazardous effects of environmental metals, the detailed mechanisms responsible for the potential pathogenic role in Sb-induced CVDs are still elusive. Therefore, this review focuses on the involvement of Sb in the pathogenesis of CVDs based on available studies to provide new insights into the potential mechanism responsible for Sb-mediated redox imbalance, that is: oxidative stress.
II. Antimony and Its Potential Toxicity
A. Antimony sources and human exposure
1. Antimony sources
Sb is naturally present in the Earth's crust and can be released into the environment by volcanic eruptions, sea spray, forest fires, and also from anthropogenic sources, particularly coal/refuse combustion, nonferrous metal mining, smelting, and refining (Agency for Toxic Substances and Disease Registry, 2019). Sb naturally exists in the inorganic antimonite [Sb(III)] and antimonate [Sb(V)], mainly in the sulfide form (Sb2S3), although industrially Sb(III) is refined by reduction with carbon or direct reduction of Sb with iron (Fe). For this aspect, a few comprehensive reviews are available (Bolan et al, 2022; Nishad and Bhaskarapillai, 2021; Periferakis et al, 2022; Ye and Jing, 2021). Therefore, we briefly introduce the general information of Sb.
Sb as a metalloid and was known in ancient times by the Arabic name kohl. Sb has been used in cosmetics and as medicine against microbes and parasites. Sb was commercially mined as a principal product or recovered as a by-product during the smelting of base metal ores in nine countries. China was the world's leading producer of primary Sb, accounting for about 60% of world mine production, followed by Russia and then Tajikistan (IndexBox, Inc., 2022), and its use in manufacturing gradually increased from 1900s to 1980s, followed by a rapid increase from the 1990s to 2020s. It is used predominantly in flame retardants, in the form of halogenated Sb compounds, applied in children's clothing, toys, fiberglass, aircrafts, and automobile seat covers. Another large application for metallic Sb is in alloys with Pb and tin, which have improved properties to form hardened alloys used for solders, bullets, plain bearings, and Pb acid batteries (Agency for Toxic Substances and Disease Registry, 2019). New products of Sb sulfide-based nanomaterials used in high-performance sodium-ion batteries are increasing (Ahmad and Mobin, 2020; Choi and Jung, 2020; Wang et al, 2022b), raising concern for greater human exposure.
2. Human exposure to antimony
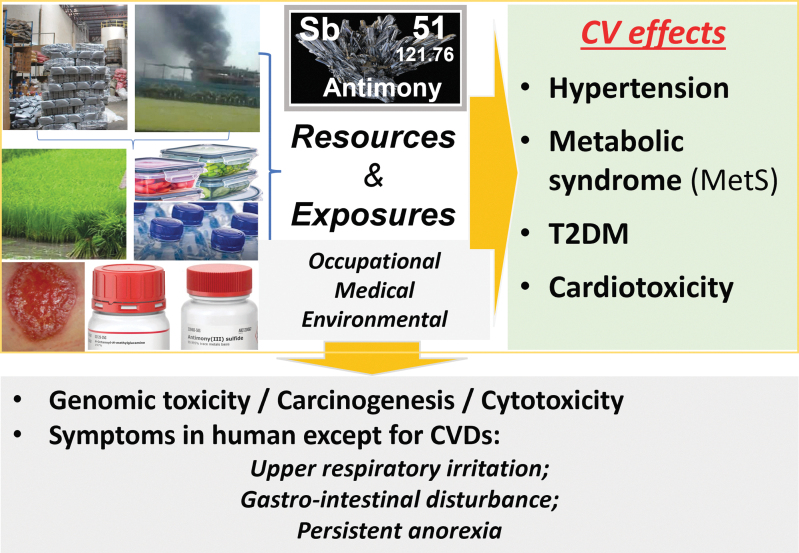
Human exposures to Sb are mainly through three major forms: occupational exposure, medical therapy, and general population exposure due to air pollution and environmental contamination (Fig. 1). Occupational exposure may cause respiratory irritation, pneumoconiosis, spots on the skin, gastrointestinal symptoms, and cancer. Improvements of working conditions have remarkably decreased the incidence of Sb toxicity in the workplace, but Sb-associated cancer risk has increased (Schildroth et al, 2021), suggesting that the Sb effects on human health would not be easily mitigated by just improving working environments.
FIG. 1.
Outline of the key information of this review. Sb exits naturally, but is not an essential metal for human, however, it can be used for military and medical stuffs and in daily-used plastic materials, and it contaminates the environments, including air, soil, plants, and food. Therefore, human is exposed occupationally, medically, and environmentally. The harmful effect of Sb includes CV impact (up, right box) and other non-CV effects (bottom box). CV, cardiovascular; Sb, antimony.
As a therapeutic purpose, the most important application of Sb-based drugs is to treat two parasitic diseases, leishmaniasis and schistosomiasis. Even to date, the Sb(III) forms of potassium antimony tartrate (PAT) and pentavalent antimonials (sodium stibogluconate [SSG], Pentostam; meglumine antimoniate [MA], Glucantime, Table 1) remain in use despite their toxic side effects (Azim et al, 2021; Carvalho et al, 2019; Salari et al, 2022). Quite recently, the use of Sb dithiocarbamate complexes has been studied for their potential antibacterial activity, with promising results, and they have also exhibited a noteworthy antifungal activity (Adeyemi and Onwudiwe, 2020).
Table 1.
Medically Used Sb Compounds
| Chemical name | Commercial names | |
|---|---|---|
| Sb(III) compounds | • PAT • Antimony potassium tartrate trihydrate, or • Potassium antimontarterate • Tartar emetic • Emetic tartar |
|
| Sb(V) compound (pentavalent Sb) | • SSG • SAG • MA |
Pentostam Glucantime |
MA, meglumine antimoniate; PAT, potassium antimony tartrate; SAG, sodium antimony gluconate; Sb, antimony; SSG, sodium stibogluconate.
Exposure to Sb can also occur through exposure to Sb-contaminated environments, and consumption of food and water. In urban settings, Sb is released into the air by the burning of fire retardants and by brake abrasion particles, released during car braking, probably due to which, the elevated Sb serum concentrations in children younger than 6 years in Bucharest were observed (Gaman et al, 2020). In China, there are several places with the highest deposits of Sb, including the Xikuangshan Sb ore field, the Zhazaixi Sb deposit, and the Xiangxi Au-Sb-W deposit. Extensive research has been performed in the past in most of the mining sites of China with the findings of significantly increased Sb contents in the soil, water, and plants of most of the mining areas (Fu et al, 2010; Zeng et al, 2015) and similarly in other places (Periferakis et al, 2022). Sb also accumulates in plants at the aqueous environments.
In any case, direct human contact with contaminated soil is one of the major exposure pathways. Even though Sb is nonessential to plants, it can be taken up through their roots if and when it is available in water-soluble forms. Sb accumulation in plants remains pronounced around the mining areas.
The entry point of Sb into the food chain is through plants, which absorb Sb from contaminated soil. However, the degree of Sb soil contamination is not the sole determining factor, as Sb mobilization in the soil greatly affects its uptake by the local flora and whether the plant is consumed directly by humans, or if it enters the food chain after being consumed by herbivores. Although tap water is an unlikely source of Sb intoxication, the consumption of bottled water is rather different since in the plastics industry, Sb2O3 is used as a catalyst in polyethylene terephthalate production. Due to Sb leaching, the amount of Sb in the water is directly proportional to the duration of plastic water bottle storage, which could be further aggravated by increased storage times of the plastic bottle water and storing temperatures that increase Sb leaching into the water (Shotyk et al, 2006; Zmit and Belhaneche-Bensemra, 2019).
Similarly, the plastics used in food packaging are manufactured by the same process used for water bottle manufacturing. Therefore, packaged food could also be contaminated with Sb, an occurrence that becomes especially pronounced if the plastic container is heated in a microwave oven [further details refer Periferakis et al (2022)].
B. General toxicity of antimony
1. Antimony cytotoxic and antiproliferative activity in cell line in vitro
A recent review (Hadjikakou et al, 2015) systemically discussed Sb anticancer cell proliferative action. Among Sb compounds with sulfur containing ligands, the two polymorphic complexes of the dimethyl dithiocarbamate showed the strongest activity against MCF-7 cells in nanomolar concentrations, which is comparable with those of doxorubicin or tamoxifen and 360 times stronger than that of cisplatin.
The cytotoxic effect of Sb might be demonstrated by its killing effects on the Leishmania, which are obligatory intracellular parasites and grow in the macrophages as the amastigote form of their vertebrate hosts. The Sb killing effects were assessed in various in vitro drug-screening models such as human peripheral blood-derived macrophages (Berman and Wyler, 1980), mouse peritoneal macrophages (Neal and Croft, 1984), and the late human leukemia monocyte THP-1 cell line (Gebre-Hiwot et al, 1992). The toxic levels of drugs on host cells, as determined by the colorimetric methyl tetrazolium assay, were similar to those obtained in the mouse peritoneal macrophage model.
In addition to the cytotoxic effects on the parasite Leishmania, the side effects of Sb on host cells were also reported in primary rat hepatocytes (Hashemzaei et al, 2015), HEK293 cells (Jiang et al, 2016), and acute promyelocytic leukemia cells (Losler et al, 2009; Mann et al, 2006). Recently, new Sb(III) complexes were found with high cytotoxic activity against HL-60 and Jurkat human leukemia cell lines. Two of these complexes [Sb(H2Bz4M)Cl3]·2H2O and [Sb(2Bz4Ph)Cl2] showed higher cytotoxic activity against HL-60 and Jurkat cells, corresponding thiosemicarbazones (Reis et al, 2010).
2. General toxicities of antimony in human
The toxicity of Sb in human was recognized in 1950s (Brieger et al, 1954), with relatively detailed information reported after in the 1960s. Seven men were accidentally exposed to the fume of SbCl3 with symptoms including not only the well-recognized upper respiratory irritation from hydrochloric acid, but also, in five of the men, a slightly delayed onset of gastrointestinal disturbance including abdominal pain and persistent anorexia. Urine Sb measurements revealed Sb concentration in excess of 1 mg/L, while environmental measurements suggest brief exposure of these victims to air containing up to 73 mg Sb/m3 (Taylor, 1966). Later, several other symptoms related to Sb exposure were reported and included respiratory illness (Potkonjak and Pavlovich, 1983), gastrointestinal effects (Taylor, 1966), dermatitis (White et al, 1993), as shown in Figure 1, and cardiovascular effects such as altered EKG and elevated blood pressure (Sundar and Chakravarty, 2010).
However, chronic side effects of long-term usage of Sb-based medicine, including fatal arrhythmias, QT prolongation after correcting for heart rate, and other EKG abnormalities, have been reported (Chulay et al, 1985; Guerin et al, 2002; Sundar and Chakravarty, 2010). In the general population, Sb exposure was also associated with elevated blood pressure (Shiue, 2014). Therefore, acute and chronic toxic effects of Sb seem to include cardiovascular effects, which is the focus in the following sections of this review and the potentially underlying mechanisms are also discussed.
III. Potential Effects of Antimony on CVDs in Human
A. Case reports and epidemiological studies
1. Hypertension
Considering that hypertension-related disease is a major health challenge globally with an estimated 1.56 billion adults affected with hypertension by 2025, environmental factors, such as metals, were evaluated as risk factors (Xu et al, 2020). The first link of Sb to hypertension was a study on the urinary excretion of metals including Sb in five patients with hypertension before and during treatment with hydralazine (Wester, 1975). In this study, Sb was one of a few metals that showed a significantly positive association with hypertension. Consequently, two studies, retrieved from United States National Health and Nutrition Examination Surveys (NHANES) for 2009–2010 (Shiue, 2014) and 2009–2012 (Shiue and Hristova, 2014), showed that urine Sb with other metals (urine cobalt, Pb, and tungsten) was significantly associated with hypertension.
Later, Wang et al (2018) utilized the environmental risk score (ERS) to evaluate the associations between cumulative exposure to a mixture of correlated heavy metals and obesity and comorbidities including hypertension. They found that hypertension was associated with cumulative exposure to heavy metal mixtures including Sb (Wang et al, 2018). This was further confirmed by Swayze et al (2021) who examined the data from the NHANES between 1999 and 2016 and found a linear and positive relationship between urine Sb and hypertension prevalence.
However, other reports showed no correlation between Sb and hypertension even though other metals were found to be related to hypertension. For instance, Xu et al (2020) examined the Census-tract airborne metal concentrations of Sb and other metals (As, Cd, chromium, cobalt, Pb, manganese [Mn], etc.) from the U.S. Environmental Protection Agency 2005 National Air Toxics Assessment database with 47,595 women. Comparing the highest with the lowest quartiles, risk of hypertension was higher among women with higher residential exposure to As, Pb, chromium, cobalt, and Mn, but not other metals including Sb (Xu et al, 2020). Similar results were reported by other studies too (Everson et al, 2021).
These inconsistent results may be related to race/ethnicity (Everson et al, 2021; Xu et al, 2020), pregnancy or even stage of pregnancy (Liu et al, 2021), and many other potential factors, which need to be further explored. However, the direct effect of Sb on hypertension may be supported by an important study from Fernandes et al (2020). In this clinical study as a Phase II, uncontrolled, single-center clinical trial, the safety profile of a standardized MA intralesional therapy was tested among 53 patients, and 86.9% of patients had at least one adverse event. Although laboratory abnormalities were mild, MA was found to directly induce hypertension (Fernandes et al, 2020).
2. Metabolic syndrome and/or type 2 diabetes
In 2016, Menke et al (2016) evaluated the relationship of urine levels of metals including Sb with diabetes prevalence based on 9447 participants in the 1999–2010 NHANES database. After multivariable adjustment, the odds ratios of diabetes associated with the highest quartile of metal, compared with the lowest quartile, were 1.72 for Sb and were also significant for several other metals. Higher quartiles of Sb were also associated with a greater homeostatic model assessment of insulin resistance as the key endpoint of metabolic syndrome (MetS) after adjustment (Menke et al, 2016). By examining data from NHANES (1999–2016, n = 12,636–32,012) for blood or urine metals and MetS including body mass index (BMI), obesity, dyslipidemia, and type 2 diabetes mellitus (T2DM), Swayze et al (2021) reported a curvilinear relationship between urine Sb and obesity, particularly with the moderate group having the highest odds of obesity.
Analyzing the associations of 15 urine metal concentrations with MetS incidence in a prospective cohort of middle-aged women in the United States by ERS and mixed metals including Sb showed significant correlations between Sb-included metal mixture and the risks of MetS in this cohort (Wang et al, 2022c).
Increased fasting glucose was found to be positively associated with plasma Sb and Cu in women, but not in men (Ge et al, 2021), suggesting a potential sex difference. However, a negative association between plasma concentration of Sb and T2DM incidence in both sexes was also reported (Yuan et al, 2018).
There were several studies on the impact of Sb in gestational diabetes mellitus (GDM). One prospective study recruited 2093 pregnant women from the Tongji Maternal and Child Health Cohort and measured metal Sb concentrations in urine samples during early pregnancy. The 95th percentile value of creatinine-corrected Sb concentration in the urine of all pregnant women was 1.33 μg/g. The creatinine-corrected Sb concentrations were significantly higher in women with GDM than those without GDM. After adjustment for potential confounders, for each one natural logarithmic unit increase in Sb concentration, there was a 29% increase in the risk of GDM, suggesting a higher risk of GDM in the pregnant women with higher Sb exposure (Zhang et al, 2019). Sb and four metals also displayed a significantly positive association with GDM based on single-metal models, and multiple-metal models (Wang et al, 2020; Zhang et al, 2020).
3. Cardiovascular diseases
For Sb effects on CVDs, several epidemiological studies are available, mainly from the following two groups: (i) General populations with exposure to either background environment or contaminated environment and (ii) Special populations with exposure either occupationally or medically, which also included clinical observational studies.
In 1171 adults as the representatives of a general population from Spain without clinical CVDs, the association between urine levels of metals and metal mixtures and CVDs was evaluated. In the single-metal model, the hazard ratio of CVD was 1.51 for Sb and 1.31–1.64 for vanadium and chromium. Bayesian kernel machine regression (BKMR) analysis revealed the relationship of Sb in the presence of other metals such as Cu, zinc (Zn), Cd, chromium, and V with CVDs, particularly urine Sb with Cd was found to be significantly associated with increased CVDs (Domingo-Relloso et al, 2019). Another community-based study also revealed the association between Sb and atherosclerotic CVDs in 3081 adults from the Wuhan-Zhuhai cohort in China (Zhu et al, 2020a), and the mild impact of Sb on general CVD risks (Grau-Perez et al, 2022).
Cardiometabolic effects of environmental exposure to Sb were also revealed in prenatal exposure conditions. Kupsco et al (2019) measured 11 metals in second-trimester whole blood in a prospective cohort in Mexico City and analyzed children's (4–6 years old) health status by measuring BMI, percentage of body fat, blood pressure, and plasma hemoglobin A1C, nonhigh-density lipoprotein cholesterol, triglycerides, leptin, and adiponectin. The authors constructed cardiometabolic component scores using age- and sex-adjusted z scores and averaged five scores to create a global risk score. They found that Sb and As were associated with lower leptin in BKMR models, and low essential metals during pregnancy were associated with increased cardiometabolic risk factors in childhood (Kupsco et al, 2019). Consistent with this finding, Onat et al (2021) reported that, compared with the control group, groups with GDM had high levels of Sb and Cd, as well as Pb, but low levels of chromium-III, Zn, and selenium.
These studies suggest that increased blood Sb with other nonessential metals may increase the risk of CVDs directly or by disturbance of the essential metals, which in turn increase CVDs.
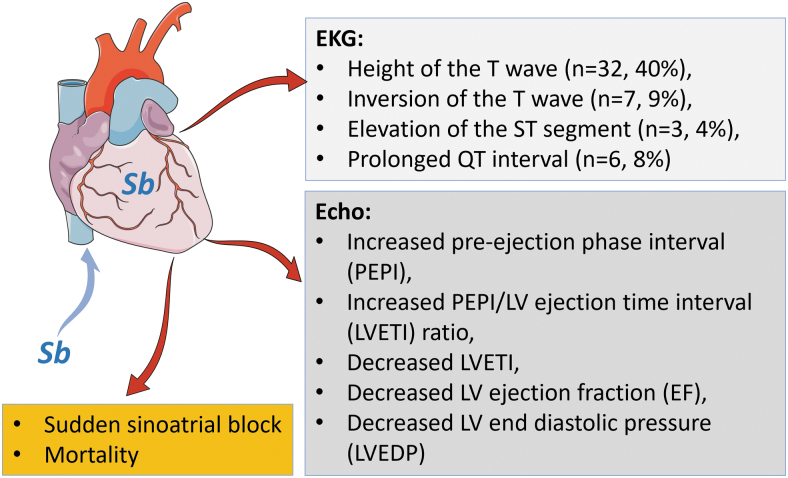
The second group of studies on the effects of Sb on CVDs is based on occupational or medical Sb exposure. In general, significant cardiotoxicity was seen in the individuals exposed to Sb occupationally and the patients with Sb therapy (Fig. 2), for which, the detailed studies and results are discussed below:
FIG. 2.
Short summary of Sb-induced cardiotoxicities. In three boxes, the key cardiotoxicity induced by Sb were summarized as EKG and echo measurements as well as cardiac arrest and mortality rate due to cardiac failure, based on publications (Badr et al, 1978; Daadaa et al, 2021; Frustaci et al, 1999; Gupta, 1990; Pandey et al, 1988; Ribeiro et al, 1999; Rijal et al, 2003; Schnorr et al, 1995; Shiue, 2014; Shrivastava et al, 2007; Thakur et al, 1998). Echo, echocardiographic; EKG, electrocardiogram.
a. Cardiac dysfunction: electrocardiographic abnormalities
In Sb-exposed humans, EKG abnormalities have been reported since the 1940s (Tarr, 1946). Brieger et al (1954) described that among 125 men who were occupationally exposed to Sb2S3 for 8 months to 2 years, 8 died suddenly and 2 died from chronic heart disease. They conducted EKG examinations and showed persistent EKG changes in 12 out of 56 reexamined workers (Brieger et al, 1954).
Cardiac EKG changes were extensively observed in the patients treated with Sb and associated agents. By EKG monitoring, 38 patients with schistosomiasis who received 42 courses of therapy with Sb dimercaptosuccinate, 201 EKGs showed significant abnormalities, similar to those exposed to other Sb compounds but less severe in severity and incidence. Women were found to be more severely affected than men. The presence of hepatic schistosome granuloma had no effect on the degree of EKG changes, but cardiotoxicity from the drug may be increased with liver damage (Waye et al, 1962).
Several cases have been reported since then for SSG-treated patients. The first serial EKGs obtained during 65 courses of SSG treatment for 59 Kenyan patients with leishmaniasis showed that EKG abnormalities developed during 54% of treatment courses in dose- and duration-dependent manner. The most common being flattening and/or inversion of T waves. Minor EKG abnormalities were commonly seen when SSG is used at doses above 20 mg Sb/kg daily for more than 15 days, while life-threatening arrhythmias occurred only at higher doses (Chulay et al, 1985). Cases who were safely treated with SSG with significant EKG abnormalities at relative low dose (10 mg/kg daily) for 21 days in 50 patients from India (Pandey et al, 1988) and for 7 days in a 17-year-old girl who even had idiopathic dilated cardiomyopathy (IDCM) (Gupta, 1990) were also reported.
In contrast to the findings with low-dose SSG, 80 patients with visceral leishmaniasis (VL) were treated with 20 mg SSG/kg for 30 days and many of them showed abnormal EKGs, including decreased T wave 8 (n = 32, 40%), inversion of the T wave (n = 7, 9%), prolonged QT interval (n = 6, 8%), and elevation of the ST segment (n = 3, 4%) (Thakur et al, 1998). In addition, 62 patients with cutaneous leishmaniasis (CL) who were treated with Sb(V) underwent EKG before and immediately after the first cycle of treatment at 15 mg Sb/kg daily. In 12 of 62 patients, corrected QT (cQT) augmentation exceeded 40 ms, 7 of whom developed marked cQT interval prolongation (500 ms) after Sb(V) therapy and none of these patients was on any other drugs that can cause cQT prolongation.
This previously unrecognized cardiac toxicity induced by short-term, low-dose antimonial therapy needs more clinical attention (Ribeiro et al, 1999).
b. Cardiac dysfunction: echocardiographic abnormalities
By echocardiographic examination, the toxic effects of the Sb compound on the heart with diminished contractility of the left ventricular (LV) was evident in 29 patients suffering from schistosomiasis after intravenous (i.v.) injection of PAT at therapeutic doses. At 30 min after dosing PAT, the pre-ejection phase interval (PEPI) and PEPI/LV ejection time interval (LVETI) were increased, the LVETI and ejection fraction were decreased, the LV end-diastolic pressure was raised, and the peak dp/dt and the Vmax were reduced (Badr et al, 1978). In another study with 14 newly diagnosed patients with VL, echo monitoring before, during, and after i.v. SSG therapy at 20 mg/kg daily for 30 days found relatively normal LV function and dimensions in all patients. Pericardial effusion was noted in four patients with heavy parasitemia, but was small, hemodynamically insignificant, and resolved spontaneously (Shrivastava et al, 2007).
The discrepancies between the above two studies have brought many questions regarding the dose, time, patient's age, and sex for further investigation.
c. Cardiac arrest and mortality rate
A sudden sinoatrial block (SAB) type 2 was reported in a young patient who was treated with systemic MA (20 mg/kg daily) for CL. Pretherapeutic investigations did not reveal any significant abnormalities, but on the 10th day of treatment, EKG showed flattened T waves in V3-V4-V5-V6, a cQT prolongation, and an SAB type 2. Four days after interrupting MA, EKG returned to normal with progressively healing cutaneous lesions (Daadaa et al, 2021).
Except for MA, the life-threatening arrhythmias occurred at higher doses of SSG (Chulay et al, 1988), as mentioned above, specifically, one patient died suddenly during the fourth week of treatment with 60 mg Sb/kg daily, and two patients died of measles after 9 or 10 days of treatment with 30 mg Sb/kg daily. QT prolongation and a concave ST segment developed in all three patients who died (Chulay et al, 1985). However, among 80 patients with VL who were treated with 20 mg of SSG/kg for 30 days, about 50% of patients showed EKG abnormalities, 6% suffered cardiac arrhythmia, and 5% of patients died from cardiotoxicity (Thakur et al, 1998).
Rijal et al (2003) reported an outbreak of fatal cardiotoxicity in Nepal among VL patients treated with a newly available batch of generic SSG from April 9 to May 5, 2000. Thirty-six patients treated with this batch died, and 23% of the death was attributed to SSG cardiotoxicity (Rijal et al, 2003). This contrasted with the low total death rate (3.2%) and death from cardiotoxicity (0.8%) observed among 252 patients treated between August 1999 and December 2001 with generic SSG from Albert David Ltd. (Calcutta, India). This outbreak event was thought to be due to the uncontrolled quality of different batches of SSG (Rijal et al, 2003).
A mortality rate study of 1014 men employed between 1937 and 1971 in a Texas Sb smelter consisted primarily of workers of Spanish ancestry and showed a significantly positive trend in mortality rate with increased duration of employment. Except for the elevated mortality rate from lung cancer among Sb workers, the rate for mortality from ischemic heart disease was also increased in two of the three different Spanish-surnamed populations. These data suggested that an increased mortality rate might be caused by Sb-exposure from both malignancy and cardiac disease (Schnorr et al, 1995).
B. Clinical evidence for the cardiac antimony deposition and cardiac effects
By assaying the myocardial and skeletal muscular contents of 32 trace elements in biopsy samples of 13 patients with clinical, hemodynamic, and histologic diagnosis of IDCM, Frustaci et al (1999) demonstrated a large increase (>10,000 times) of Sb and mercury in myocardial but not in muscular samples in all patients with IDCM. Patients with secondary cardiac dysfunction showed a mild increase (≤5 times) of trace elements in the myocardium but normal levels in muscles. In particular, in patients with IDCM, mean Sb was the second highest (12,000 times, 19,260 ng/g vs. 1.5 ng/g), after mercury, when compared with controls (Frustaci et al, 1999).
The level of Sb in the blood was determined before and 1, 2, 4, and 6 h after a single dose of MA intramuscularly at 0.8–9.0 mg/kg body weight among 14 patients, which caused a rapid initial increase at 1 h and a quick decrease up to 4 h, and the peak levels in the blood were dependent on the dose (Fig. 3A) (de Aguiar et al, 2018). Another five patients with VL were treated with SSG or MA by intramuscular injection daily at 10 mg Sb/kg for 30 days, during which blood Sb levels were measured at intervals. The pharmacokinetics of both drugs was remarkably similar, with peak levels of ∼10 mg/L at about 2 h after the initial dose.
FIG. 3.
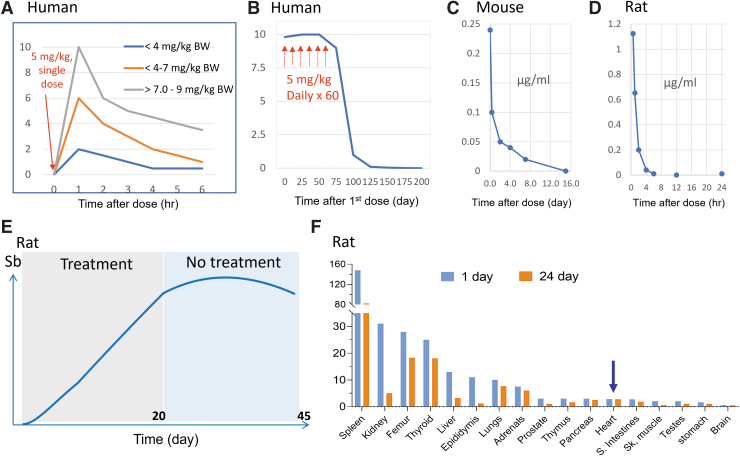
Comparisons of blood Sb levels after single or multiple treatments among species. (A) Mimic eliminating pattern of plasma Sb levels in patients at before and 1, 2, 4, and 6 h after a single intramuscular infiltration of MA to determine the peak and decrease to 6 h with an estimation of the half-life of Sb as ½ h. This mimic time-course curves were roughly made based on the publication (de Aguiar et al, 2018). (B) Total Sb levels in the whole blood of a male patient with CL during and after administration of MA daily at 5 mg/kg body weight for 60 days, which was mimicked based on the publication (Miekeley et al, 2002) to compare with other panels in this figure. (C) The mimic time-course curve of the Sb concentrations in the blood of Schistosoma mansoni-infected mice at different times (0.5 h to 15 days) after a single i.p. injection of 5 mg/kg body weight Sb(III) drug, based on the publication (Molokhia and Smith, 1969). The highest concentration of Sb was at 8 h and quickly decreased to very low beyond 4 days and almost zero at day 16. (D) The mimic time-course of the whole blood Sb levels in male rats after a single i.v. injection of MA (75 mg/kg body weight), showing a sharp fall at (t1/2 = 0.6 h), until 24 h, based on the publication (Coelho et al, 2014). (E) A mimic time-course curve of blood Sb concentrations from 6 male rats treated with MA (300 mg/kg body weight, subcutaneous) during 21 consecutive days (gray box) and an additional 21 days after the last dose (light blue box), based on the publication (Coelho et al, 2014). (F) Concentrations of antimony (μg/g) in rat tissues 24 h and 21 days after a 21-day treatment with MA at the same dose as panel (E) (Coelho et al, 2014). Accumulation of Sb in the heart belongs to the low group of Sb accumulating tissues, indicated by the blue arrow. CL, cutaneous leishmaniasis; i.p., intraperitoneal; i.v., intravenous; MA, meglumine antimoniate.
Most of the Sb was eliminated rapidly, but nadir Sb concentrations increased gradually during treatment from 0.04–0.08 mg/L 24 h after the first dose to 0.19–0.33 mg/L 24 h after the 30th dose (Chulay et al, 1988). In line with this study, Figure 3B is a typical curve model of dynamic profile blood Sb levels after patients with CL received intramuscular injection of MA at 5 mg/kg daily for 60 days (Miekeley et al, 2002). This study showed that the blood Sb quickly increased and remained at high levels during repeated treatment of 60 days, and then sharply decreased once the treatment stopped, reaching very low levels by the 200th day.
To standardize the protocol as a baseline for future analytical antimony from patient plasma, a recent study was developed and validated for the quantitative analysis of antimony in human plasma and peripheral blood mononuclear cells from patients with CL undergoing treatment with MA using the inductively coupled plasma mass spectrometry method, by which the current assay was successfully validated from 25 to 10,000 ng/mL for antimony in human plasma and peripheral blood mononuclear cells (Garay-Baquero et al, 2021). This protocol may serve as a baseline for future analytical designs, aiming to provide a reference method to allow interstudy comparisons (Garay-Baquero et al, 2021).
IV. Experimental Evidence and Preclinical Studies
The above epidemiological studies have shown the association between Sb and various CVDs in humans; however, whether Sb plays a role or other coexisting metals play the pathogenic role remains unclear from these association studies. Although limited clinical studies suggested Sb caused cardiac damages (Fernandes et al, 2020), it remains unclear whether Sb cardiac toxicity is directly or indirectly due to Sb toxic effects on other organs such as the liver that might in turn cause cardiac injury. Therefore, the below section addresses whether Sb has direct toxic effects on the cardiovascular cells, blood vessels, and the heart with in vitro cultured cells and animal models.
A. Cultured cardiac and endothelial cells
The in vitro studies, led by Toraason, were done with the primary cultures of neonatal rat cardiomyocytes isolated from SD rats (Tirmenstein et al, 1995). Cardiac myocytes were exposed to PAT at the dose range of 1–1000 μM for 1 to 24 h, which produced a concentration- and time-dependent depression in chronotropy and an increase in the release of lactate dehydrogenase (LDH, as an assay of cell's release of enzyme into the culture medium after death) and thiobarbituric acid reactive substance (TBARS as lipid peroxidation assay). In the following studies, they confirmed the association between Sb exposure and intracellular calcium dysregulation (Wey et al, 1997) and decreased pyruvate dehydrogenase (PDH) activity and ATP levels in cardiac myocytes (Tirmenstein et al, 1997).
In contrast to the above cytotoxic effects of PAT at the doses of 50 μM or higher, exposure of culture cardiomyocytes to PAT at much low doses such as 5 or 10 μM significantly increased the levels of cellular reduced glutathione (GSH), heme oxygenase-1 (HO-1), cytochrome P450 levels, and even HSP70 and HSP25/27 proteins, all so-called cellular protective molecules. They further revealed that pretreatment of cardiac myocytes with low-dose PAT (0.5–10 μM) protected against a subsequent lethal-dose PAT (200 μM), which was blocked if cells were treated with the protein synthesis inhibitor cycloheximide (Snawder et al, 1999). This is a new protein-synthesized-dependent adaptive mechanism, which may explain why the resistance by parasites to Sb therapy after Sb-compound therapy in clinical settings (Snawder et al, 1999).
There was limited study on the toxic effects of Sb on endothelial cells although endothelia cells are important for vascular diseases. The angiogenesis and cellular apoptosis in the human umbilical vein endothelial cells were examined after treatment with varying doses of MA (100–800 μg/mL) for 24–72 h. For the angiogenesis function of these cells, the most important genes involved in angiogenesis including vascular endothelial growth factor and its receptors (Kdr and Flt-1), neuropilin-1, and hypoxia inducible factor-1α transcription factor as well as antiapoptotic death protein Bcl2 were significantly reduced when compared with the control group. Proteins involving in apoptosis such as P53, Bax, Bak, Apaf-1, and caspases 3, 8, and 9 were all significantly upregulated when compared with the control group. These limited studies showed the toxic effects of MA on endothelial angiogenesis and survival (Khosravi et al, 2019).
B. Preclinical studies with animal models
1. Tissue distribution
The early investigation of the time-course of blood and organ distribution of Sb following exposures in animal models was done by Molokhia and Smith (1969). Sb contents in the blood of Schistosoma mansoni-infected mice were measured at different time intervals (0.5 h to 15 days) after a single intraperitoneal (i.p.) injection of 5 mg/kg body weight Sb(III) drug. In the blood, the highest concentration of Sb was at the 8 h and quickly decreased to very low beyond 4 days and almost zero at day 16, shown in Figure 3C. Coelho et al (2014) showed a similar time-course of whole blood Sb levels in male rats after a single i.v. injection of MA [75 mg Sb(V)/kg body weight]. The sharp fall in Sb blood concentrations (t1/2 = 0.6 h) indicates that almost all Sb given as MA was cleared from the body within 6–12 h of drug injection (Fig. 3D).
However, the time-course of whole-blood Sb after repeated administration of subcutaneous MA at 300 mg Sb(V)/kg body weight daily is different. Initial levels of Sb steadily rose so that at the end of a 21-day course of treatment, Sb attained levels as high as 35–40 μg/g (about 30- to 40-fold higher than an acute single-dose residual level) in the whole blood. Blood levels of Sb in rats euthanized 1 day after the last dose of MA (on day 22) did not differ from the levels of this metalloid in the blood of rats euthanized on day 42, which is illustrated in Figure 3E.
These studies suggested similar to what was seen in humans (Fig. 3A), acute exposure to Sb caused a quick rise of blood Sb but quickly eliminated without high-level residual Sb in the blood chronically (Fig. 3C, D). However, repeated exposures to high-dose Sb could result in high levels and accumulation of Sb in the blood in animals (Fig. 3E), different from humans (Fig. 3B).
The organ distribution of Sb following a single i.p. injection of an Sb in mice was the liver and spleen with the highest levels, followed by alimentary tract organs (colon, duodenum, and stomach), and relative low in all rest tissues, including the heart, as illustrated in Figure 3F (Molokhia and Smith, 1969). Coelho et al (2014) measured Sb concentrations in multiple organs in rats, as illustrated in Figure 3F, and found the highest accumulation of Sb in the spleen and relatively low levels in the heart after a single i.v. injection of MA.
These organ distribution features were confirmed by a later study by Dieter et al (1991) by directly comparing B6C3F1 mice and F344 rats exposed to APT via drinking water for 14 days and by i.p. injections every other day for 90 days. These caused a dose-related increase in residual Sb in the blood, liver, kidney, spleen, and heart of rats and in the liver and spleen of mice (very low levels in other organs). In the rhesus monkeys, tissue Sb concentrations (post-treatment days 55 and 95) were as follows: >1000 ng/g in thyroid, nails, liver, <1000 ng/g in lymph nodes, kidneys, skeletal muscles, heart, and skin, and <200 ng/g in various brain structures, and thymus, stomach, colon, pancreas, and teeth (Friedrich et al, 2012).
In summary, after a single exposure, Sb was eliminated quickly with two phases of elimination at either about 0.6 h or longer than 24 h in all species of mouse, rat, and monkey. However, Sb can accumulate in the body at a higher dose for a longer period after a repeated and high-dose exposure. Although Sb distributes to almost all of organs, the heart is one of the organs accumulating Sb at relatively low levels (Fig. 3).
2. Cardiovascular effects of antimony
In terms of preclinical animal model studies, Alvarez et al (2005) revealed the effects of both Sb(V) and Sb(III) treatment on cardiac function examined by EKG in vivo and single cardiomyocyte contract ability in vitro. In this study, guinea pigs received daily injections of MA as Sb(V) by i.m. injection of 16 mg/kg for 26 days, or PAT as Sb(III) by i.m. injection of 10 mg/kg for 8 days. Treatment with MA prolonged the QT interval of the EKGs (Alvarez et al, 2005), consistently with the most threatening effects of Sb observed in human (EKG changes) with Sb(V) (Chulay et al, 1985; Ortega-Carnicer et al, 1997; Ribeiro et al, 1999). Treatment with PAT was lethal within 2 days in about 50% of the animals.
The survivors showed EKG alterations similar to those described in human: T wave flattening and/or inversion, depression of the ST segment, and elongation of RR and QT intervals. Isolated ventricular cardiomyocytes from these survived animals at the eighth day showed impaired contractile responses to changes in stimulus frequency, elongated action potential (AP), and reduced calcium current (ICa) (Alvarez et al, 2005).
The cardiac toxicity was also studied in dogs by a few studies: A 3-year-old, female bulldog was serologically diagnosed as leishmaniasis and treated with 75 mg/kg MA subcutaneously daily along with other medicines for a week. The dog's general condition worsened, shown by cutaneous ecchymosis, respiratory distress, and finally cardiorespiratory arrest. Histopathological examination showed generalized vasculitis of several internal organs with severe myocarditis (Torrent et al, 2005). Another case was a 4-year-old crossbreed dog, which presented with physical examination and echo feature of pericardial effusion with cardiac tamponade and infected by Leishmania. After the animal was treated with MA at 50 mg/kg subcutaneously every 12 h for 28 days with allopurinol, cardiac tamponade reoccurred at 1 month, and the animal died 4 months later. Histological examination confirmed chronic pericarditis (Sebastian-Marcos et al, 2019).
In contrast to these 2 cases, another study with 28 dogs infected with Leishmania treated with MA at 75 mg/kg subcutaneously twice a day for 60 days showed no cardiac abnormalities by routine and 24-h EKG tracings and serum cardiac troponin I (Luciani et al, 2013). The significant discrepancy might stem from the differences in pretreatment health conditions or other medications.
Studies have demonstrated that As and Sb usually coexist in the natural environment, especially in drinking water and rice, which could result in adverse impacts on human health and animal welfare. Thus, evaluating the toxicity of Sb with As may help elucidate the underlying mechanisms responsible for the synergic effects of Sb and As on CVDs (Ye and Jing, 2021; Fu et al, 2010; Zeng et al, 2015; Losler et al, 2009). Thirty-two adult mice were gavage-fed with As2O3, SbCl3, or both daily for 60 days. As and/or Sb caused histopathological lesions and endoplasmic reticulum (ER) expansion of the heart. The gene expression of ER Ca2+ release channels (RyR2 and IP3R) and CaMKII increased, while the levels of mRNA and protein of SERCA2 downregulated significantly when compared with the controls. As and/or Sb induced ER stress and triggered the ER apoptotic pathway by activating unfolded protein response-associated genes, and apoptosis-related genes.
All these changes in the As+Sb group became more severe than either the As or Sb group, suggesting synergic effects of Sb and As on cardiotoxicity due to calcium homeostasis disturbance (Jiang et al, 2021).
V. Redox Dyshomeostasis as the Key Pathogenic Driving Force
A. Antimony transfer between its active and inactive forms under abiotic redox conditions
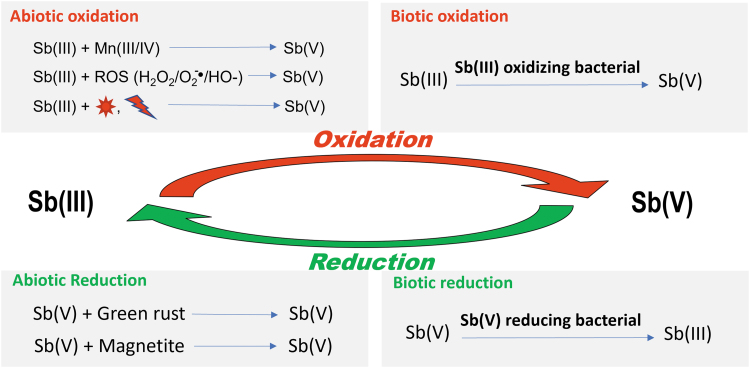
Although Sb stays in either valence III or V states, both often coexist in the environment (Herath et al, 2017; Zhou et al, 2022). Interactions of Sb with environments and/or microorganisms for the reduction and oxidation of Sb by abiotic and/or biotic processes determines the status of Sb in the natural environments and biological system (Deng et al, 2021), as summarized in Figure 4. The following sections discuss the abiotic and biotic (microbial) oxidation of Sb(III) to Sb(V) and reduction of Sb(V) to Sb(III).
FIG. 4.
Conceptual summary of a few models for abiotic and biotic oxidation of Sb(III) and reduction of Sb(V).
 , sunlight;
, sunlight;  , UV or ionizing radiation.
, UV or ionizing radiation.
1. Sb(III) is oxidized into Sb(V)
Although native Sb [i.e., Sb(0)] is uncommon in nature, the use of Sb(0) in munitions can lead to localized areas where Sb(0) is present in soils. The weathering and oxidation of Sb(0) in the projectiles lead to the formation of Sb(III) and Sb(V) species in the form of secondary minerals and dissolved and adsorbed species. The oxidation of Sb(0) in deionized water was rapid, but the oxidation of Sb(0) was more pronounced in the simulated groundwater (Barker et al, 2021; Lewinska and Karczewska, 2019).
In subsurface environments, Sb oxidation takes place in the presence of some metals. For instance, Fe and Mn hydroxides are common minerals. Both natural and synthetic Fe and Mn oxyhydroxides Fe(III) and Mn(III/IV) hydroxides are effective oxidants for Sb(III) and the amorphous Fe and Mn oxyhydroxides present in natural waters and sediments can play a detoxifying role by adsorbing and oxidizing the more toxic Sb(III) into Sb(V) (Belzile et al, 2001).
Sb(III) can be oxidized by reactive oxygen species (ROS): In aqueous system, the oxidation of Sb(III) is caused by ROS, commonly hydroxyl and peroxyl radicals, H2O2, and superoxide radical anion. In fact, the oxidation of Sb(III) to Sb(V) by H2O2 was extensively reported (Elleouet et al, 2005; Kong et al, 2015; Quentel et al, 2004). Quentel et al (2004) reported the oxidation of Sb(III) by H2O2 not at pH <7, but increased with increasing pH above 7. At neutral to mildly acidic pH, Sb(III) is present as Sb(OH)3 which is a weak acid and unreactive with H2O2. However, Sb(OH)4 is highly reactive and even the trace amounts that exist at pH <10 are readily oxidized to Sb(V). This means that Sb(III) oxidation by H2O2 is increased by either elevated H2O2 concentrations or increased alkaline pH values.
Since several metals are able to generate ROS in natural and engineered environments, Sb(III) oxidation is affected by the presence of these metals. For instance, Elleouet et al (2005) reported the effects of Cu(II), Mn(II), Zn(II), and Pb(II) on Sb(III) oxidation by H2O2 under various conditions, showing the oxidation of Sb(III) greatly in the presence of Cu(II), and to a lesser degree by Mn(II) and Pb(II), but not Zn(II).
Except for the above abiotic oxidation, Sb-oxidizing bacteria can oxidize Sb(III) to Sb(V). One of examples is the study by Terry et al (2015) to isolate two Sb(III)-oxidizing bacteria, from microcosms containing sediments from As- and Sb-contaminated mice. Both isolates exhibited relatively high rates of Sb(III) oxidation (Terry et al, 2015). Thus, understanding the biological oxidation mechanism responsible for Sb(III) to Sb(V) may help explore ways to improve the environment and consequently human health (Deng et al, 2021).
In addition, the photo-oxidation of Sb(III) to Sb(V) by marine microalgae with or without transition metals is well studied (Hu et al, 2014; Li et al, 2006). Li et al (2006) found increased conversion ratio of Sb(III) to Sb(V) with increasing algae concentration and irradiation time and different species of marine phytoplankton with different photo-oxidizing abilities. After photoinduced oxidation by marine phytoplankton and transition metals, the ratio of Sb(V) to Sb(III) was increased for six algae, and decreased only for Dunaliella salina. The distribution of Sb in the sunlit surface seawater was greatly affected by the combined effects of marine phytoplankton (main contributor) and transition metals; both synergistic and antagonistic effects were observed.
Hu et al (2014) found that light (sunlight, ultraviolet, simulated sunlight) irradiation initiates the dissolution of Sb2O3 and the dissolved Sb(III) was oxidized to Sb(V), which could be affected by pH, free radical scavengers, dissolved oxygen removal, and the Sb2O3 dosage by the key oxidative components of hydroxyl free radicals and superoxide free radicals.
In addition, oxidation of Sb(III) can result from ROS generated by microbial processes. Bacterial Sb(III) oxidation is a multifactor process (Li et al, 2017). Based on a comparative proteomic study, Li et al (2015) have identified an Sb(III) oxidase AnoA, which catalyzes Sb(III) oxidation with nicotinamide adenine dinucleotide phosphate (NADP+) as a cofactor. AnoA was induced by Sb(III) and significantly affected Sb(III) oxidation and resistance. Heterologous expression of anoA in E. coli remarkably increased the bacterial Sb(III) oxidation rate (Li et al, 2015). Furthermore, two phoB (named as phoB1 and phoB2) and one phoR gene were discovered, and only phoB2 was induced by Sb(III); deletion of phoB2 significantly inhibited the expression of anoA and decreased bacterial Sb(III) oxidation efficiency and Sb(III) resistance.
In contrast, deletion of phoB1 did not affect anoA's expression level and Sb(III) oxidation/resistance, suggesting PhoB2 as the upstream transcription factor of anoA by directly binding to the promoter sequence of anoA.
2. Sb(V) is reduced into Sb(III)
Abiotic reduction of Sb(V) by green rust in a reductive environment was reported by Mitsunobu et al (2008). Suboxic and anoxic environments often contain substantial amounts of Fe(II), which may be present as soluble Fe(II) complexes, and Fe(II) adsorbed on organic and inorganic solid phases. In addition, anoxic environments may also contain S(-II), which is commonly found as dissolved sulfide species and a suite of metal sulfides. Therefore, these Fe (II) and S(-II) species are effective reductants for Sb(V) (Johnson et al, 2021; Mitsunobu et al, 2008).
The microbe-mediated reduction of Sb(V) by the bacterium isolated by Abin and Hollibaugh (2014) established the utilization of Sb(V) as a terminal electron acceptor for anaerobic respiration by native microbial populations in soils and sediments, which was continually adopted by later studies (Zhu et al, 2018). In fact, Sb(V) (e.g., MA and SSG) is used commonly in clinical practices because of its bioreduction from less toxic Sb(V) to more toxic Sb(III) by thiols in both the parasite and host cells (Ferreira Cdos et al, 2003; Hansen et al, 2011; Shaked-Mishan et al, 2001; Yan et al, 2003).
In brief, as illustrated in Figure 4, Sb(III) was oxidized to Sb(V) as a less toxic compound by several methods. Conversely, there are also natural abiotal and biotal ways to reduce Sb(V) to Sb(III), the more toxic form. The mechanisms regarding the development of bacteria's resistance have rapidly advanced in recent years; the readers are referred to the latest reviews and studies (Deng et al, 2021; Rong et al, 2022; Wu et al, 2022; Ye et al, 2022).
B. Antimony-mediated inhibition of genes or proteins induces redox imbalance
1. Generation of ROS in the cell due to Sb interaction with certain proteins leads to their dysfunctions, results in cell redox imbalance
The toxicity of Sb to the cells is derived from its binding to and interaction with cellular thiol-containing enzymes or proteins such as thiol groups of GSH; therefore, Sb binding thiol groups to form thioantimonites is a way to functionally deplete cellular GSH levels, while the GSH system in the mammalian cells and body is a very important defense mechanism against oxidative stress and associated damage. Therefore, this feature might be the most important way for Sb to induce oxidative stress, called redox imbalance, in the cells (Periferakis et al, 2022). The cultured cardiac myocyte exposure to 50 and 100 μM PAT for 4 h induced dose- and time-dependent cell death, along with significantly decreased levels of GSH and protein thiol along with increased levels of oxidized glutathione disulfide (GSSG) (Tirmenstein et al, 1997).
The transformation of Sb(V) to Sb(III) in human whole blood was tested by Lopez et al (2015) and they found a significant decrease in the GSH/GSSG ratio in Sb(V)-treated human blood in vitro as the form of Sb(V) transformed into Sb(III). The exchange rate of GSH between its free and Sb-bound form was pH-dependent, ranging from slow exchange at low pH (such as pH 3.2) to relatively rapid exchange at biological pH.
Thioredoxin reductase (TrxR) and glutathione reductase (GR) are both important enzymes that maintain cellular redox balance. Sb(III) binding to the selenol residue of these enzymes results in strong inhibition of TrxR at submicromolar doses and inhibition of GR at higher concentrations. The selectivity of these complexes for TrxR suggests that metal binding to a selenol residue in the active site of the enzyme leads to functional loss and the subsequent oxidative stress (Parrilha et al, 2014).
A member of the lipoxygenase (LOX) family, 12-LOX, is responsible for the oxygenation of cellular polyunsaturated fatty acids to produce lipid mediators to modulate cell inflammation. 12-LOX and its main product 12-HETE activate cellular NADPH oxidase (NOX) to subsequently generate ROS (Cho et al, 2011; Othman et al, 2013). The mechanism responsible for Sb(III) to form complexes with thioamides involves LOX inhibition and interruption of mitochondrial function, which in turn generates extra ROS, leading to oxidative stress. Sb is able to form complexes not only with the abovementioned enzymes, but also with thiosemicarbazones, ketone complexes, and organoantimony compounds (Hadjikakou et al, 2015, Hadjikakou et al, 2008). Therefore, Sb forms complexes with cellular enzymes, proteins, and others by binding their thiol groups or selenol residuals that would easily change the cellular redox balance, leading to a pathogenic status.
2. Generation of ROS in the cells due to Sb interaction with certain enzymes or kinases causes their dysfunction, results in cell signaling pathway abnormality
In lung adenocarcinoma A549 cells, Su et al (2022) identified mitochondria as the major source of ROS production by Sb exposure, and revealed that Sb exposure decreased the activity of complex I and complex III, the level of -SH and GSH in mitochondria, and the activity of mitochondrial GR, glutathione peroxidase (GPx), and TrxR, but increased the mitochondrial superoxide dismutase (SOD) activity, suggesting the disruption of mitochondrial redox homeostasis (Su et al, 2022). Sb is able to directly inhibit PDH, the basic regulatory enzyme determining the mode of glucose oxidation, that is, anaerobic or aerobic. Exposure of cells to Sb leads to a drop in ATP levels, which is assumed to be caused by the inhibition of PDH by Sb activating the anaerobic glycolysis pathway. Anaerobic glycolysis is way less efficient than aerobic glycolysis and produces less ATP, with resultant mitochondrial dysfunction undesirably generating more ROS (Piao et al, 2017).
In addition, SSG treatment activates some important components of the intracellular signaling pathways, causing an early wave of ROS-dependent parasite killing and a stronger late wave of nitric oxide (NO)-dependent parasite killing (Mookerjee Basu et al, 2006). Mice exposed to PAT showed significantly increased neuronal apoptosis. In vitro, Sb triggered apoptosis in neuronal PC12 cells in a dose-dependent manner. These effects are found to be due to Sb-decreased phosphorylation of protein kinase B (Akt) and mammalian target of rapamycin (mTOR) since an Akt activator protected PC12 cells from autophagy. Similarly, antioxidant N-acetylcysteine (NAC) also prevented Sb-induced Akt/mTOR inhibition and apoptosis (Wang et al, 2019).
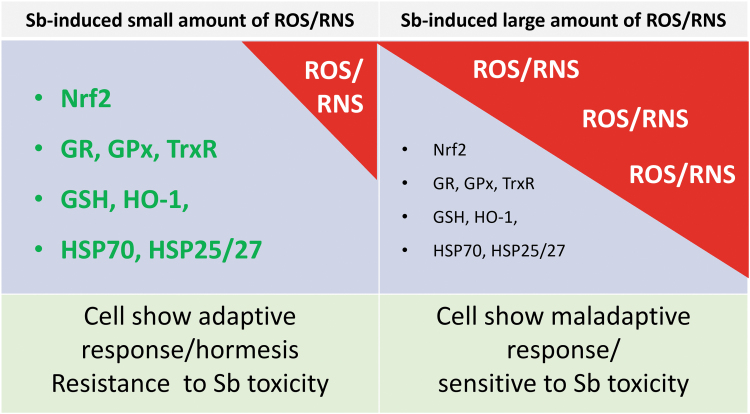
In addition, Sb can change biochemical processes by increasing or decreasing protein function or enzyme activities. Antioxidant activities can be increased by Sb due to its induction of ROS at small amounts to stimulate endogenously defense systems (Gu et al, 2020). As mentioned above, Sb is also able to induce a protective mechanism as an adaptive response since sublethal doses of PAT at 5 or 10 μM, in cultured neonatal rat cardiomyocytes, significantly increased cellular GSH and HO-1 activity after 18 h: 10 μM PAT increased 2.5- and 5.4-fold of GSH and HO-1, respectively. Exposure to low-dose PAT also stimulates the expression of HO-1, HSP70, and HSP25/27 protective proteins, to make the cardiomyocytes resistant to subsequent lethal-dose PAT cytotoxicity (Snawder et al, 1999). As illustrated in Figure 5, a small amount of ROS can stimulate the cellular defense system to become resistant to the subsequent lethal stress-induced toxicity.
FIG. 5.
Sb-induced adaptive or hormetic response or harmful effects. The amounts of Sb-induced ROS and RNS determine the cell sensitivity to the subsequent stress-induced toxicity. When cells are exposed to a small dose of Sb, which induces a small amount of ROS and/or RNS that are not to kill the cell but stimulate the production of cellular response proteins and enzymes, and consequently, the cells become resistant to a subsequent dose of Sb or other stress, as illustrated in the left panel. In contrast, when cells are exposed to a high dose of Sb, which induces a large amount of ROS and/or RNS beyond the capacity of the cellular endogenous defense system, and even exhausted the endogenous defense enzymes and proteins similar to that illustrated in the right panel, these cells will be lethal or become more sensitive to subsequent stress even though these cells still survive. This hypothetic summary was based on publications (Gu et al, 2020; Losler et al, 2009; Snawder et al, 1999; Su et al, 2022). RNS, reactive nitrogen species; ROS, reactive oxygen species.
3. Antimony-induced redox imbalance
Sb can react with vitamins, including vitamins C, D, and E, based on which Sb-related products have been applied and the levels of vitamins C and E in plants, vegetables, and animal and human body fluid (Collins, 1950; Gibson and Taylor, 1945; Katsui et al, 1966; Motsaathebe and Fayemi, 2022; Strong, 1976). We discuss the impact of Sb interaction with vitamins on health. For instance, the lipid peroxidation and LDH release induced by PAT in cultured cardiomyocytes along with decreased GSH could be prevented by pretreatment with vitamin E (Tirmenstein et al, 1995).
This study suggests the importance of redox imbalance in Sb-induced toxicity. It is known that oxidative stress reflects an imbalance between the systemic production of ROS and the ability of a biological system to readily detoxify these ROS or repair the resulting damage. Therefore, the following sections discuss how Sb induces toxicity in different systems by disturbing the redox imbalance, that is: oxidative stress.
a. In plant cells
In the roots and leaves of sunflower and tomato plants, grown in hydroponics with 0, 0.5, or 1 mM of Sb for similar periods, Sb predominantly accumulated in the roots (Espinosa-Vellarino et al, 2020; Ortega et al, 2017). In both cases, Sb reduced biomass production, chlorophyll content, and photosynthesis along with reduced observed antioxidant amounts or activities, such as SOD, ascorbate peroxidase (APX), GR, and peroxidase activity.
In the root of Sb-exposed sunflowers and tomatoes, the S-nitrosoglutathione (GSNO), total S-nitrosothiol, superoxide, and H2O2 levels significantly increased (Espinosa-Vellarino et al, 2020; Ortega et al, 2017). The activity of GSNO reductase in sunflowers was induced mainly by a higher Sb concentration, indicating that Sb caused secondary nitro-oxidative stress. Sb-exposed roots also exhibited elevated NO and ONOO-levels, further supporting the observation that Sb exposure disturbed ROS and reactive nitrogen species metabolism and induced oxidative and nitrosative stress in plants (Espinosa-Vellarino et al, 2020; Ortega et al, 2017).
Similarly, in rice plants, Zhu et al (2020b) reported that exposure to Sb(V) caused oxidative stress, but Sb(III) was more toxic than Sb(V) as judged by a lower shoot biomass and higher production of superoxide. The toxicity of Sb(III) might partially be due to the disturbance of the superoxide dismutation reaction. Sb(V) and Sb(III) both stimulated the accumulation of calcium in the shoots and roots, and calcium significantly correlated with antioxidant systems, suggesting a calcium-induced regulatory mechanism. The activity of GPx was significantly enhanced by Sb(V) and Sb(III), suggesting a role in scavenging H2O2. This study showed that the differences between Sb(V) and Sb(III), and the toxicity of Sb(III) might partially be due to the disturbance of the superoxide dismutation reaction, which resulted in root cell membrane damage (Zhu et al, 2020b).
b. In Leishmania
Mandal et al (2007) studied the contributory role of thiols in the development of Sb unresponsiveness of isolated Leishmania from VL patients who were either Sb responsive or unresponsive. Unresponsive strains showed higher basal levels of thiols and a faster rate of thiol regeneration when compared with sensitive strains. In Sb-resistant strains, H2O2 scavenging activity is higher and Sb-mediated ROS generation was curtailed, a phenomenon that could be reversed by depletion of thiols; this suggests that the higher levels of thiols in Sb-unresponsive strain isolates from VL patients contributed to the Sb resistance. Therefore, Sb antiparasitic action and its side toxicity in host cells might be mediated by its generation of ROS, and the parasites will show resistance once they develop high contents of thiols and antioxidants (Mandal et al, 2007).
In support of the above observation, Leishmania braziliensis with overexpressed APX gene became Sb-resistant. APX is a redox enzyme of the trypanothione pathway that converts H2O2 into water molecules, and therefore, APX-overexpressing clones were not only resistant to Sb (8-fold increase over the parental line), but also to H2O2 (1.8-fold higher than control line); however, the effect was abolished by isoniazid, an antibacterial agent that interacts with APX. This study clearly suggested the importance of APX enzyme in the defense against Sb-therapeutic effects via generating ROS in L. braziliensis lines (Moreira et al, 2018).
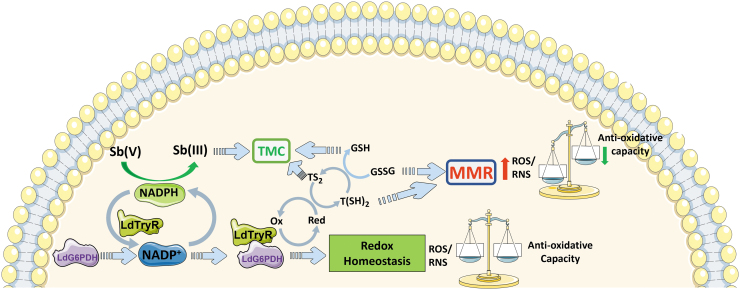
As shown in Figure 6, a novel protein–protein interaction among glucose-6-phosphate dehydrogenase (G6PDH or specific LdG6PDH) and trypanothione reductase (TryR or LdTryR), both localized in the cytosol, of Leishmania donovani has implied an interconnection between central glucose metabolism and thiol metabolism in this parasite, but their interaction is NADPH dependent. The addition of Sb(III) extraneously could severely impair NADPH generation, potentially cause structural changes of the interacting proteins, and lead to apoptotic death of the parasites. However, Sb(III) at a sublethal dose could increase the in vivo interaction between LdG6PDH and LdTryR, which may be a mechanism responsible for the development of Sb resistance in clinical settings. In fact, clinical isolates of Sb-resistant L. donovani to Sb exhibited an enhanced interaction between LdG6PDH and LdTryR and also showed cross resistivity toward As(III).
FIG. 6.
A cartoon representative of the proposed interaction between LdG6PDH and LdTryR, and how its interaction status leads to the redox balance and dyshomeostasis, in Leishmania donovani. This was adopted from the publication (Ghosh et al, 2017). MMR, metalloid-mediated ROS; TMC, thiol metalloid complex.
Thus, these findings propose the metabolism of LdG6PDH and LdTryR as an alternate therapeutic target and provide mechanistic insight about metalloid resistance in VL (Ghosh et al, 2017).
c. In zebrafish
Xia et al (2021) have exposed zebrafish to Sb and examined their embryonic toxicity, identifying an association between embryonic toxicity with increased ROS levels and a significantly decreased GSH and SOD activity. Consistent with this finding, a study by Wang et al (2022a) also reported that in the muscle, the level of malondialdehyde was increased with increasing Sb doses, and catalase was decreased with increasing Sb exposure time, suggesting the importance of redox imbalance or oxidative stress in Sb-induced toxicity in zebrafish.
d. In mammalian cells
Human lung adenocarcinoma A549 cells were treated with SbCl3, which caused autophagy in a dose- and time-dependent manner, which was not related to the classic mechanistic mTOR pathway, but was inhibited by NAC treatment, suggesting that ROS-dependent autophagy mediates Sb-stimulated effects (Zhao et al, 2017). This early study was further confirmed by Su et al (2022) with the same cell line. The level of mitochondrial ROS was significantly increased in Sb-exposed cells, which was prevented by mitochondria-targeted antioxidant mito-TEMPO [(4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy)], suggesting that mitochondria are the major source of ROS production. This suggested that Sb-induced mitochondria dysfunction caused a decrease in ATP level and mitochondrial membrane potential along with decreased activity of complex I and complex III, level of mitochondrial -SH and GSH, and activity of mitochondrial GR, GPx, and TrxR (Su et al, 2022).
In HEK293 (human embryonic kidney cell line) cells, Sb caused a dose-dependent cytotoxicity along with ROS accumulation and nuclear factor NF-E2-related factor 2 (Nrf2) expression and nuclear translocation, and cytotoxicity was aggregated by deletion of Nrf2 gene and prevented by NAC treatment (Jiang et al, 2016).
In the analysis of gene expression changes with ToxTracker assay (Hendriks et al, 2016), the only genes that are responsive to oxidative stress were upregulated by Sb compounds at nontoxic concentrations in C57/Bl6 B4418 wild-type embryonic stem cells. All Sb(III) compounds, Sb powder, and three of the five Sb(V) compounds activated the oxidative stress reporters. The consistent activation of reporters for oxidative stress suggests this mode of Sb action for its toxic responses (Boreiko et al, 2021).
In an investigation on the mechanism by which MA causes DNA damage for BALB/c mice infected by Leishmania and treated with MA (20 mg/kg for 20 days), respectively, both MA and Leishmania infantum infection induced DNA damage in mouse leukocyte and micronucleated cells by the oxidation of nitrogenous bases. Furthermore, MA treatment and L. infantum infection promoted oxidative stress-derived DNA damage, along with increased activities of serum SOD and catalase, but decreased activity of GPx probably as the consequence of GSH depletion (Moreira et al, 2017). The authors further confirmed that MA-induced DNA damages could be prevented by natural antioxidant genistein and vitamin C (ascorbic acid) (de Jesus et al, 2018).
C. Evidence that oxidative stress plays the role in Sb-induced cardiovascular pathogenesis
Although the above studies have clearly indicated the cardiotoxicity of Sb in the models of cultured cardiomyocytes, endothelial cells, and the heart of animal model and even human cardiac functional measurements, the therapeutic studies remain limited. While limited, these prior studies show the importance of oxidative stress in Sb-induced cardiac pathogenesis.
The first study by Tirmenstein et al (1995) used cultured rat neonatal cardiomyocytes with PAT treatment, which induced the release of cardiac LDH and TBARS into the medium. However, these effects were almost completely prevented by the preadministration of vitamin E, and most of the other antioxidants (Tirmenstein et al, 1995). Although this study was an in vitro study without analysis of cardiac function, it is important to confirm the oxidative stress responsible for Sb-induced cardiotoxicity. In fact, the presence of oxidative stress in the heart of an animal exposed to Sb was mirrored by a significant elevation of cardiac lipoperoxidation and protein carbonylation as oxidative endpoints and a decrease in cardiac SOD activity in the heart (Bento et al, 2013).
Alvarez et al (2005) examined the preventive effect of l-carnitine on PAT-induced cardiotoxicity in a series of experiments. Animals were injected (i.m.) daily with saline, 180 mg/kg l-carnitine, and/or 10 mg/kg PAT for 8 days. PAT was lethal to 50% within 2 days, and survivors showed EKG alterations. However, simultaneous or pretreatment with l-carnitine reduced and delayed the Sb-induced mortality rate to less than 10% over 12 days and prevented Sb-induced EKG abnormalities in the survivals. At the end of study, surviving animals were killed and myocytes were isolated for either mechanical or electrical recording experiments. The ventricular myocytes from mice treated by Sb(III) alone showed impaired contractile responses to changes in stimulus frequency, elongated AP, and reduced calcium currents (ICa), all of which were prevented by l-carnitine.
Therefore, l-carnitine has a cardioprotective role against Sb(III)-induced cardiomyopathy (Alvarez et al, 2005). Although l-carnitine may have multiple mechanisms responsible for cardiac protection, it would be most likely to prevent Sb-induced mitochondrial dysfunction and oxidative stress (Montesano et al, 2015; Ringseis et al, 2013; Vacante et al, 2018).
VI. Conclusions and Prospective
Compared with other environmental metals such as As, Pb, and Cd, our knowledge of the biogeochemical cycle of Sb and its potential effects for human health is far less developed. Fortunately, attention to these issues has rapidly increased in the last decades, reflected by significantly more publications cited in PubMed. These works have begun to recognize Sb as an environmental contaminant. Although the studies presented in this review have provided substantial insights into understanding the features of Sb in natural and engineered environments, a comprehensive understanding of the redox processes involved in the redox cycling of Sb and its human health impact has not yet been well developed, suggesting the need for additional research on this topic.
It should be noticed that even though the cardiac toxic effects by EKG measurement in clinical use have been appreciated since the 1940s, research on the effects of occupational, medical, and environmental exposure to Sb on various CVDs is urgently needed.
By this review, we have appreciated that medical and environmental exposure to Sb exists and its risks for various CVDs. However, the molecular mechanisms responsible for Sb-induced cardiac and vascular pathogenesis remain less understood. Su et al (2022) recently use the A549 cell line to define that Sb did not significantly induce cytosolic ROS production by NOX, but rather mitochondrial ROS through mitochondrial damages and dysfunction. However, whether this finding will be the case for Sb-exposed cardiomyopathy or in endothelial cells in vitro and in vivo needs more studies.
DNA methylation is one of the epigenetic mechanisms for gene expression modulation, which can be mechanistically responsible for pathologies caused by exposure to Pb and Sb (Okamoto et al, 2022). They also found that the Pb exposure level was relatively low, and so, Sb might play the major role. In fact, whole blood from adult men without clinically evident CVDs also showed increased DNA methylation and its potential link to metals in the blood, including increased Sb (Riffo-Campos et al, 2018). Therefore, whether these Sb-related DNA methylation changes also affect the cardiovascular system redox balance, leading to the pathogenesis needs more research.
Insufficient sleep and obstructive sleep apnea (OSA) are well linked to the development of several chronic conditions, including CVDs and MetS. The prevalence of short sleep duration was estimated about 37% in the general U.S. population, and OSA affects 12–28 million U.S. adults. Multivariate logistic regression of the association of urine Sb with several sleep disorders, including insufficient sleep and OSA, in adult participants of NHANES 2005–2008, revealed the association of higher urine Sb levels with these sleep disorders (Scinicariello et al, 2017). Therefore, whether the cardiotoxicity in the individuals exposed to Sb medically or occupationally might be also due to these sleeping disorders. Particularly for the potential link to pulmonary arterial hypertension severity as recently reported by the authors of this review (El-Kersh et al, 2022).
Abbreviations Used
- 12-HETE
12-hydroxyeicosatetraenoic acid
- Akt
protein kinase B
- Apaf-1
apoptotic protease activating factor-1
- APX
ascorbate peroxidase
- As
arsenic
- Bak
a member of BCL-2 family members
- Bax
BCL2 associated X protein, a member of BCL-2 family members
- Bcl2
proteins of the B cell lymphoma-2
- BKMR
Bayesian kernel machine regression
- BMI
body mass index
- CaMKII
calmodulin-dependent protein kinase II
- Cd
cadmium
- CL
cutaneous leishmaniasis
- cQT
corrected QT
- Cu
copper
- CV
cardiovascular
- CVDs
cardiovascular diseases
- Echo
echocardiographic
- EKG
electrocardiogram
- ER
endoplasmic reticulum
- ERS
environmental risk score
- Fe
iron
- Flt-1
vascular endothelial growth factor receptor 1
- G6PDH
glucose-6-phosphate dehydrogenase
- GDM
gestational diabetes mellitus
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- GSSG
oxidized glutathione disulfide
- HO-1
heme oxygenase-1
- HSP70
heat shock protein 70
- i.p.
intraperitoneal
- i.v.
intravenous
- IDCM
idiopathic dilated cardiomyopathy
- IP3R
IP3 receptor
- Kdr
VEDF receptor 2
- LDH
lactate dehydrogenase
- LOX
lipoxygenase
- LV
left ventricular
- LVETI
LV ejection time interval
- MA
meglumine antimoniate
- MetS
metabolic syndrome
- Mito-TEMPO
mitochondria-target TEMPO (4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy)
- MMR
metalloid-mediated ROS
- Mn
manganese
- mTOR
mammalian target of rapamycin
- NAC
N-acetylcysteine
- NADP+
nicotinamide adenine dinucleotide phosphate
- NADPH
reduced NADP+
- NHANES
National Health and Nutrition Examination Surveys
- NO
nitric oxide
- NOX
NADPH oxidase
- Nrf2
NF-E2-related factor 2
- ONOO
peroxynitrate
- OSA
obstructive sleep apnea
- PAT
potassium antimony tartrate
- Pb
lead
- PDH
pyruvate dehydrogenase
- PEPI
pre-ejection phase interval
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR2
ryanodine receptor 2
- SAB
sudden sinoatrial block
- Sb
antimony
- SERCA2
sarco (endo)plasmic reticulum calcium-ATPase 2
- SOD
superoxide dismutase
- SSG
sodium stibogluconate
- T2DM
type 2 diabetes mellitus
- TBARS
thiobarbituric acid reactive substance
- TMC
thiol metalloid complex
- TrxR
thioredoxin reductase
- TryR
trypanothione reductase
- VL
visceral leishmaniasis
- Zn
zinc
Authors' Contributions
Y.T.: Reviewing and editing the original and final versions, and graphing the figures. K.E.K.: Reviewing and editing the original versions. S.S.W.: Reviewing and editing the original versions. K.A.W.: Reviewing and editing the original and final versions, contributing to the conceptualization, and support. J.H.: Reviewing and editing the original and final versions, and contributing to the conceptualization, supervision, and support. L.C.: Contributing to the conceptualization, supervision, and support, and writing the original draft and graphing the draft figures. All authors approved the final version.
Author Disclosure Statement
K.E.-K. provided consultative services to Acceleron, Merck, United Therapeutics, served on advisory boards for J&J, Actelion, and United Therapeutics, received institutional research funding from J&J Actelion and United Therapeutics, and participated in a United Therapeutics speaker's bureau (2018–2021).
Funding Information
The authors were supported, in part, by the University of Louisville Executive Vice President for Research and Innovation Internal Grant (Jiapeng Huang and Lu Cai); University of Louisville School of Medicine Basic Grant (Jiapeng Huang and Lu Cai); National Institute of Environmental Health Sciences (P30ES030283 to Kupper A. Wintergerst, Jiapeng Huang and Lu Cai); Gilead Sciences COMMIT COVID-19 RFP Program grant (Gilead IN-US-983-6063 to Jiapeng Huang); National Center for Advancing Translational Sciences grant (1U18TR003787-01 to Jiapeng Huang); the National Heart, Lung, and Blood Institute (R01HL125877, R01HL160927 to Yi Tan, Lu Cai; R01HL158779-01 to Jiapeng Huang); and the National Institute of Allergy and Infectious Diseases (R01AI172873-01 to Jiapeng Huang).
References
- Abin CA, Hollibaugh JT. Dissimilatory antimonate reduction and production of antimony trioxide microcrystals by a novel microorganism. Environ Sci Technol 2014;48:681–688; doi: 10.1021/es404098z [DOI] [PubMed] [Google Scholar]
- Adeyemi JO, Onwudiwe DC. Chemistry and some biological potential of bismuth and antimony dithiocarbamate complexes. Molecules 2020;25:305; doi: 10.3390/molecules25020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Antimony [Online]. 2019. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp23-c5.pdf [Last accessed July 15, 2022]. [PubMed]
- Ahmad K, Mobin SM. Recent progress and challenges in A3Sb2X9-based perovskite solar cells. ACS Omega 2020;5:28404–28412; doi: 10.1021/acsomega.0c04174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, Malecot CO, Gannier F, et al. Antimony-induced cardiomyopathy in guinea-pig and protection by L-carnitine. Br J Pharmacol 2005;144:17–27; doi: 10.1038/sj.bjp.0706030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim M, Khan SA, Ullah S, et al. Therapeutic advances in the topical treatment of cutaneous leishmaniasis: A review. PLoS Negl Trop Dis 2021;15:e0009099; doi: 10.1371/journal.pntd.0009099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr MH, Zayadi GM, Harras FK. Left ventricular dysfunction after intravenous tartar emetic. Egypt J Bilharz 1978;5:9–18. [PubMed] [Google Scholar]
- Barker AJ, Clausen JL, Douglas TA, et al. Environmental impact of metals resulting from military training activities: A review. Chemosphere 2021;265:129110; doi: 10.1016/j.chemosphere.2020.129110 [DOI] [PubMed] [Google Scholar]
- Belzile N, Yu-Wei Chen, Zijian Wang Oxidation of antimony (III) by amorphous iron and manganese oxyhydroxides. Chem Geol 2001;174:379–387; doi: 10.1016/S0009-2541(00)00287-4 [DOI] [Google Scholar]
- Bento DB, De Souza B, Steckert AV, et al. Oxidative stress in mice treated with antileishmanial meglumine antimoniate. Res Vet Sci 2013;95:1134–1141; doi: 10.1016/j.rvsc.2013.08.004 [DOI] [PubMed] [Google Scholar]
- Berman JD, Wyler DJ. An in vitro model for investigation of chemotherapeutic agents in leishmaniasis. J Infect Dis 1980;142:83–86; doi: 10.1093/infdis/142.1.83 [DOI] [PubMed] [Google Scholar]
- Bolan N, Kumar M, Singh E, et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ Int 2022;158:106908; doi: 10.1016/j.envint.2021.106908 [DOI] [PubMed] [Google Scholar]
- Boreiko CJ, Hendriks G, Derr R, et al. Mode of action assessment of the genotoxic properties of antimony and its compounds evaluated in the ToxTracker assay. Mutat Res Genet Toxicol Environ Mutagen 2021;865:503333; doi: 10.1016/j.mrgentox.2021.503333 [DOI] [PubMed] [Google Scholar]
- Borhani NO. Exposure to trace elements and cardiovascular disease. Circulation 1981;63:260A–263A. [PubMed] [Google Scholar]
- Brieger H, Semisch CW, 3rd, Stasney J, et al. Industrial antimony poisoning. Ind Med Surg 1954;23:521–523. [PubMed] [Google Scholar]
- Carvalho SH, Frezard F, Pereira NP, et al. American tegumentary leishmaniasis in Brazil: A critical review of the current therapeutic approach with systemic meglumine antimoniate and short-term possibilities for an alternative treatment. Trop Med Int Health 2019;24:380–391; doi: 10.1111/tmi.13210 [DOI] [PubMed] [Google Scholar]
- Cheung Chung SW, Kwong KP, Yau JC, et al. Dietary exposure to antimony, lead and mercury of secondary school students in Hong Kong. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2008;25:831–840; doi: 10.1080/02652030701697751 [DOI] [PubMed] [Google Scholar]
- Cho KJ, Seo JM, Kim JH. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cells 2011;32:1–5; doi: 10.1007/s10059-011-1021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YC, Jung KW. Recent progress in fabrication of antimony/bismuth chalcohalides for lead-free solar cell applications. Nanomaterials (Basel) 2020;10:2284; doi: 10.3390/nano10112284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulay JD, Fleckenstein L, Smith DH. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg 1988;82:69–72. [PubMed] [Google Scholar]
- Chulay JD, Spencer HC, Mugambi M. Electrocardiographic changes during treatment of leishmaniasis with pentavalent antimony (sodium stibogluconate). Am J Trop Med Hyg 1985;34:702–709; doi: 10.4269/ajtmh.1985.34.702 [DOI] [PubMed] [Google Scholar]
- Coelho DR, Miranda ES, Saint'pierre TD, et al. Tissue distribution of residual antimony in rats treated with multiple doses of meglumine antimoniate. Mem Inst Oswaldo Cruz 2014;109:420–427; doi: 10.1590/0074-0276140030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FD. The reaction between betacarotene and antimony trichloride. Nature 1950;165:817; doi: 10.1038/165817c0 [DOI] [PubMed] [Google Scholar]
- Craddock GB, Toone EC Jr. Electrocardiographic changes occurring during antimony therapy. Va Med Mon (1918) 1947;74:210–214. [PubMed] [Google Scholar]
- Daadaa N, Youssef S, Haggui A, et al. New onset of sinoatrial block in a young patient treated with systemic meglumine antimoniate. Clin Case Rep 2021;9:1797–1798; doi: 10.1002/ccr3.3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aguiar MG, Goncalves JE, Souza MD, et al. Plasma antimony determination during cutaneous leishmaniasis treatment with intralesional infiltration of meglumine antimoniate. Trop Med Int Health 2018;23:1110–1117; doi: 10.1111/tmi.13130 [DOI] [PubMed] [Google Scholar]
- De Jesus LCL, Soares RP, Moreira VR, et al. Genistein and ascorbic acid reduce oxidative stress-derived DNA damage induced by the antileishmanial meglumine antimoniate. Antimicrob Agents Chemother 2018;62:e004560-18; doi: 10.1128/AAC.00456-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R, Chen Y, Deng X, et al. A critical review of resistance and oxidation mechanisms of Sb-oxidizing bacteria for the bioremediation of Sb(III) pollution. Front Microbiol 2021;12:738596; doi: 10.3389/fmicb.2021.738596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter MP, Jameson CW, Elwell MR, et al. Comparative toxicity and tissue distribution of antimony potassium tartrate in rats and mice dosed by drinking water or intraperitoneal injection. J Toxicol Environ Health 1991;34:51–82; doi: 10.1080/15287399109531548 [DOI] [PubMed] [Google Scholar]
- Domingo-Relloso A, Grau-Perez M, Briongos-Figuero L, et al. The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: The Hortega Follow-Up Study. Int J Epidemiol 2019;48:1839–1849; doi: 10.1093/ije/dyz061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kersh K, Danielle Hopkins C, Wu X, et al. Plasma level of antimony correlates with pulmonary arterial hypertension severity. Curr Res Toxicol 2022;3:100080; doi: 10.1016/j.crtox.2022.100080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleouet C, Quentel F, Madec CL, et al. The effect of the presence of trace metals on the oxidation of Sb(III) by hydrogen peroxide in aqueous solution. J Environ Monit 2005;7:1220–1225; doi: 10.1039/b509802e [DOI] [PubMed] [Google Scholar]
- Espinosa-Vellarino FL, Garrido I, Ortega A, et al. Effects of antimony on reactive oxygen and nitrogen species (ROS and RNS) and antioxidant mechanisms in tomato plants. Front Plant Sci 2020;11:674; doi: 10.3389/fpls.2020.00674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson TM, Niedzwiecki MM, Toth D, et al. Metal biomarker mixtures and blood pressure in the United States: Cross-sectional findings from the 1999–2006 National Health and Nutrition Examination Survey (NHANES). Environ Health 2021;20:15; doi: 10.1186/s12940-021-00695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HJ, Da Silva RE, Ramalho DB, et al. Safety profile of meglumine antimoniate intralesional infiltration for cutaneous leishmaniasis. Expert Rev Anti Infect Ther 2020;18:381–387; doi: 10.1080/14787210.2020.1731305 [DOI] [PubMed] [Google Scholar]
- Ferreira CdS, Martins PS, Demicheli C, et al. Thiol-induced reduction of antimony(V) into antimony(III): A comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals 2003;16:441–446; doi: 10.1023/a:1022823605068 [DOI] [PubMed] [Google Scholar]
- Friedrich K, Vieira FA, Porrozzi R, et al. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 2012;75:63–75; doi: 10.1080/15287394.2012.624826 [DOI] [PubMed] [Google Scholar]
- Frustaci A, Magnavita N, Chimenti C, et al. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J Am Coll Cardiol 1999;33:1578–1583; doi: 10.1016/s0735-1097(99)00062-5 [DOI] [PubMed] [Google Scholar]
- Fu Z, Wu F, Amarasiriwardena D, et al. Antimony, arsenic and mercury in the aquatic environment and fish in a large antimony mining area in Hunan, China. Sci Total Environ 2010;408:3403–3410; doi: 10.1016/j.scitotenv.2010.04.031 [DOI] [PubMed] [Google Scholar]
- Gaman L, Delia CE, Luzardo OP, et al. Serum concentration of toxic metals and rare earth elements in children and adolescent. Int J Environ Health Res 2020;30:696–712; doi: 10.1080/09603123.2019.1626353 [DOI] [PubMed] [Google Scholar]
- Garay-Baquero DJ, Rebellon-Sanchez DE, Prieto MD, et al. Inductively coupled plasma mass spectrometry method for plasma and intracellular antimony quantification applied to pharmacokinetics of meglumine antimoniate. Bioanalysis 2021;13:655–667; doi: 10.4155/bio-2021-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Yang A, Huang S, et al. Sex-specific associations of plasma metals and metal mixtures with glucose metabolism: An occupational population-based study in China. Sci Total Environ 2021;760:143906; doi: 10.1016/j.scitotenv.2020.143906 [DOI] [PubMed] [Google Scholar]
- Gebre-Hiwot A, Tadesse G, Croft SL, et al. An in vitro model for screening antileishmanial drugs: The human leukaemia monocyte cell line, THP-1. Acta Trop 1992;51:237–245; doi: 10.1016/0001-706x(92)90042-v [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Saini S, Das S, et al. Glucose-6-phosphate dehydrogenase and trypanothione reductase interaction protects Leishmania donovani from metalloid mediated oxidative stress. Free Radic Biol Med 2017;106:10–23; doi: 10.1016/j.freeradbiomed.2017.02.008 [DOI] [PubMed] [Google Scholar]
- Gibson GP, Taylor RJ. A dynamic method for observing the antimony chloride reaction with vitamin A and related substances. Analyst 1945;70:449–457; doi: 10.1039/an9457000449 [DOI] [PubMed] [Google Scholar]
- Grau-Perez M, Caballero-Mateos MJ, Domingo-Relloso A, et al. Toxic metals and subclinical atherosclerosis in carotid, femoral, and coronary vascular territories: The Aragon Workers Health Study. Arterioscler Thromb Vasc Biol 2022;42:87–99; doi: 10.1161/ATVBAHA.121.316358 [DOI] [PubMed] [Google Scholar]
- Gu J, Yao J, Duran R, et al. Comprehensive genomic and proteomic profiling reveal Acinetobacter johnsonii JH7 responses to Sb(III) toxicity. Sci Total Environ 2020;748:141174; doi: 10.1016/j.scitotenv.2020.141174 [DOI] [PubMed] [Google Scholar]
- Guerin PJ, Olliaro P, Sundar S, et al. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis 2002;2:494–501; doi: 10.1016/s1473-3099(02)00347-x [DOI] [PubMed] [Google Scholar]
- Guo LC, Lv Z, Ma W, et al. Contribution of heavy metals in PM2.5 to cardiovascular disease mortality risk, a case study in Guangzhou, China. Chemosphere 2022;297:134102; doi: 10.1016/j.chemosphere.2022.134102 [DOI] [PubMed] [Google Scholar]
- Gupta P. Electrocardiographic changes occurring after brief antimony administration in the presence of dilated cardiomyopathy. Postgrad Med J 1990;66:1089; doi: 10.1136/pgmj.66.782.1089-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikakou SK, Ozturk II, Banti CN, et al. Recent advances on antimony(III/V) compounds with potential activity against tumor cells. J Inorg Biochem 2015;153:293–305; doi: 10.1016/j.jinorgbio.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Hadjikakou SK, Ozturk, II, Xanthopoulou MN, et al. Synthesis, structural characterization and biological study of new organotin(IV), silver(I) and antimony(III) complexes with thioamides. J Inorg Biochem 2008;102:1007–1015; doi: 10.1016/j.jinorgbio.2007.12.027 [DOI] [PubMed] [Google Scholar]
- Hansen C, Hansen EW, Hansen HR, et al. Reduction of Sb(V) in a human macrophage cell line measured by HPLC-ICP-MS. Biol Trace Elem Res 2011;144:234–243; doi: 10.1007/s12011-011-9079-9 [DOI] [PubMed] [Google Scholar]
- Hashemzaei M, Pourahmad J, Safaeinejad F, et al. Antimony induces oxidative stress and cell death in normal hepatocytes. Toxicol Environ Chem 2015;97:256–265; doi: 10.1080/02772248.2015.1032616 [DOI] [Google Scholar]
- Hendriks G, Derr RS, Misovic B, et al. The extended ToxTracker assay discriminates between induction of DNA damage, oxidative stress, and protein misfolding. Toxicol Sci 2016;150:190–203; doi: 10.1093/toxsci/kfv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath I, Vithanage M, Bundschuh J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ Pollut 2017;223:545–559; doi: 10.1016/j.envpol.2017.01.057 [DOI] [PubMed] [Google Scholar]
- Hu X, Kong L, He M. Kinetics and mechanism of photopromoted oxidative dissolution of antimony trioxide. Environ Sci Technol 2014;48:14266–14272; doi: 10.1021/es503245v [DOI] [PubMed] [Google Scholar]
- IndexBox, Inc. Antimony Ore Market Report: Production, Companies, Trends, and Forecast to 2030—IndexBox; March 30, 2022 [Online]. Available from: https://www.globenewswire.com/en/news-release/2022/03/30/2413214/0/en/Antimony-Ore-Market-Report-Production-Companies-Trends-and-Forecast-to-2030-IndexBox.html [Last accessed: July 15 2022].
- Jiang X, An Z, Lu C, et al. The protective role of Nrf2-Gadd45b against antimony-induced oxidative stress and apoptosis in HEK293 cells. Toxicol Lett 2016;256:11–18; doi: 10.1016/j.toxlet.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Jiang X, Yu W, Wu S, et al. Arsenic (III) and/or Antimony (III) induced disruption of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in mice heart. Ecotoxicol Environ Saf 2021;220:112394; doi: 10.1016/j.ecoenv.2021.112394 [DOI] [PubMed] [Google Scholar]
- Johnson CR, Antonopoulos DA, Boyanov MI, et al. Reduction of Sb(V) by coupled biotic-abiotic processes under sulfidogenic conditions. Heliyon 2021;7:e06275; doi: 10.1016/j.heliyon.2021.e06275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsui G, Ishikawa S, Shimizu M. Reaction products of vitamin A acid with antimony trichloride. J Vitaminol (Kyoto) 1966;12:122–128; doi: 10.5925/jnsv1954.12.122 [DOI] [PubMed] [Google Scholar]
- Khosravi A, Sharifi I, Tavakkoli H, et al. Toxico-pathological effects of meglumine antimoniate on human umbilical vein endothelial cells. Toxicol In Vitro 2019;56:10–18; doi: 10.1016/j.tiv.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Kong L, Hu X, He M. Mechanisms of Sb(III) oxidation by pyrite-induced hydroxyl radicals and hydrogen peroxide. Environ Sci Technol 2015;49:3499–3505; doi: 10.1021/es505584r [DOI] [PubMed] [Google Scholar]
- Kupsco A, Kioumourtzoglou MA, Just AC, et al. Prenatal metal concentrations and childhood cardiometabolic risk using Bayesian kernel machine regression to assess mixture and interaction effects. Epidemiology 2019;30:263–273; doi: 10.1097/EDE.0000000000000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinska K, Karczewska A. Antimony in soils of SW Poland-an overview of potentially enriched sites. Environ Monit Assess 2019;191:70; doi: 10.1007/s10661-019-7214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Q, Li M, et al. Proteomics and genetics for identification of a bacterial antimonite oxidase in Agrobacterium tumefaciens. Environ Sci Technol 2015;49:5980–5989; doi: 10.1021/es506318b [DOI] [PubMed] [Google Scholar]
- Li J, Yang B, Shi M, et al. Abiotic and biotic factors responsible for antimonite oxidation in Agrobacterium tumefaciens GW4. Sci Rep 2017;7:43225; doi: 10.1038/srep43225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Zheng FY, Hong HS, et al. Photo-oxidation of Sb(III) in the seawater by marine phytoplankton-transition metals-light system. Chemosphere 2006;65:1432–1439; doi: 10.1016/j.chemosphere.2006.04.017 [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang M, Rahman ML, et al. Exposure to heavy metals and trace minerals in first trimester and maternal blood pressure change over gestation. Environ Int 2021;153:106508; doi: 10.1016/j.envint.2021.106508 [DOI] [PubMed] [Google Scholar]
- Lopez S, Aguilar L, Mercado L, et al. Sb(V) reactivity with human blood components: Redox effects. PLoS One 2015;10:e0114796; doi: 10.1371/journal.pone.0114796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losler S, Schlief S, Kneifel C, et al. Antimony-trioxide- and arsenic-trioxide-induced apoptosis in myelogenic and lymphatic cell lines, recruitment of caspases, and loss of mitochondrial membrane potential are enhanced by modulators of the cellular glutathione redox system. Ann Hematol 2009;88:1047–1058; doi: 10.1007/s00277-009-0736-4 [DOI] [PubMed] [Google Scholar]
- Luciani A, Sconza S, Civitella C, et al. Evaluation of the cardiac toxicity of N-methyl-glucamine antimoniate in dogs with naturally occurring leishmaniasis. Vet J 2013;196:119–121; doi: 10.1016/j.tvjl.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Mandal G, Wyllie S, Singh N, et al. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology 2007;134:1679–1687; doi: 10.1017/S0031182007003150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KK, Davison K, Colombo M, et al. Antimony trioxide-induced apoptosis is dependent on SEK1/JNK signaling. Toxicol Lett 2006;160:158–170; doi: 10.1016/j.toxlet.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Menke A, Guallar E, Cowie CC. Metals in urine and diabetes in U.S. Adults. Diabetes 2016;65:164–171; doi: 10.2337/db15-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miekeley N, Mortari SR, Schubach AO. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 2002;372:495–502; doi: 10.1007/s00216-001-1213-7 [DOI] [PubMed] [Google Scholar]
- Mitsunobu S, Takahashi Y, Sakai Y. Abiotic reduction of antimony(V) by green rust (Fe(4)(II)Fe(2)(III)(OH)(12)SO(4).3H(2)O). Chemosphere 2008;70:942–947; doi: 10.1016/j.chemosphere.2007.07.021 [DOI] [PubMed] [Google Scholar]
- Molokhia MM, Smith H. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 1969;40:123–128. [PMC free article] [PubMed] [Google Scholar]
- Montesano A, Senesi P, Luzi L, et al. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxid Med Cell Longev 2015;2015:646171; doi: 10.1155/2015/646171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee BASU, J., Mookerjee A, Sen P, et al. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob Agents Chemother 2006;50:1788–1797; doi: 10.1128/AAC.50.5.1788-1797.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira DS, Xavier MV, Murta SMF. Ascorbate peroxidase overexpression protects Leishmania braziliensis against trivalent antimony effects. Mem Inst Oswaldo Cruz 2018;113:e180377; doi: 10.1590/0074-02760180377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira VR, De Jesus LCL, Soares RP, et al. Meglumine antimoniate (glucantime) causes oxidative stress-derived DNA damage in BALB/c mice infected by Leishmania (Leishmania) infantum. Antimicrob Agents Chemother 2017;61:e02360-16; doi: 10.1128/AAC.02360-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motsaathebe PC, Fayemi OE. Electrochemical detection of ascorbic acid in oranges at MWCNT-AONP nanocomposite fabricated electrode. Nanomaterials (Basel) 2022;12:645; doi: 10.3390/nano12040645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natusch DF, Wallace JR, Evans CA, JR. Toxic trace elements: Preferential concentration in respirable particles. Science 1974;183:202–204; doi: 10.1126/science.183.4121.202 [DOI] [PubMed] [Google Scholar]
- Neal RA, Croft SL. An in-vitro system for determining the activity of compounds against the intracellular amastigote form of Leishmania donovani. J Antimicrob Chemother 1984;14:463–475. [DOI] [PubMed] [Google Scholar]
- Nishad PA, Bhaskarapillai A. Antimony, a pollutant of emerging concern: A review on industrial sources and remediation technologies. Chemosphere 2021;277:130252; doi: 10.1093/jac/14.5.463 [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Iwai-Shimada M, Nakai K, et al. Global DNA methylation in cord blood as a biomarker for prenatal lead and antimony exposures. Toxics 2022;10:157; doi: 10.3390/toxics10040157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat T, Demir Caltekin M, Turksoy VA, et al. The relationship between heavy metal exposure, trace element level, and monocyte to HDL cholesterol ratio with gestational diabetes mellitus. Biol Trace Elem Res 2021;199:1306–1315; doi: 10.1007/s12011-020-02499-9 [DOI] [PubMed] [Google Scholar]
- Ortega A, Garrido I, Casimiro I, et al. Effects of antimony on redox activities and antioxidant defence systems in sunflower (Helianthus annuus L.) plants. PLoS One 2017;12:e0183991; doi: 10.1371/journal.pone.0183991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Carnicer J, Alcazar R, De La Torre M, et al. Pentavalent antimonial-induced torsade de pointes. J Electrocardiol 1997;30:143–145; doi: 10.1016/s0022-0736(97)80023-4 [DOI] [PubMed] [Google Scholar]
- Othman A, Ahmad S, Megyerdi S, et al. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: Contribution of NADPH oxidase. PLoS One 2013;8:e57254; doi: 10.1371/journal.pone.0057254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kumar M, Thakur CP. ECG changes in prolonged treatment of kala-azar with antimony compounds. J Assoc Physicians India 1988;36:398–399. [PubMed] [Google Scholar]
- Parrilha GL, Ferraz KS, Lessa JA, et al. Metal complexes with 2-acetylpyridine-N(4)-orthochlorophenylthiosemicarbazone: Cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur J Med Chem 2014;84:537–544; doi: 10.1016/j.ejmech.2014.07.055 [DOI] [PubMed] [Google Scholar]
- Periferakis A, Caruntu A, Periferakis AT, et al. Availability, toxicology and medical significance of antimony. Int J Environ Res Public Health 2022;19:4669; doi: 10.3390/ijerph19084669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao L, Fang YH, Kubler MM, et al. Enhanced pyruvate dehydrogenase activity improves cardiac outcomes in a murine model of cardiac arrest. PLoS One 2017;12:e0185046; doi: 10.1371/journal.pone.0185046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkonjak V, Pavlovich M. Antimoniosis: A particular form of pneumoconiosis. I. Etiology, clinical and X-ray findings. Int Arch Occup Environ Health 1983;51:199–207; doi: 10.1007/BF00377752 [DOI] [PubMed] [Google Scholar]
- Quentel F, Filella M, Elleouet C, et al. Kinetic studies on Sb(III) oxidation by hydrogen peroxide in aqueous solution. Environ Sci Technol 2004;38:2843–2848; doi: 10.1021/es035019r [DOI] [PubMed] [Google Scholar]
- Reis DC, Pinto MC, Souza-Fagundes EM, et al. Antimony(III) complexes with 2-benzoylpyridine-derived thiosemicarbazones: Cytotoxicity against human leukemia cell lines. Eur J Med Chem 2010;45:3904–3910; doi: 10.1016/j.ejmech.2010.05.044 [DOI] [PubMed] [Google Scholar]
- Ribeiro AL, Drummond JB, Volpini AC, et al. Electrocardiographic changes during low-dose, short-term therapy of cutaneous leishmaniasis with the pentavalent antimonial meglumine. Braz J Med Biol Res 1999;32:297–301; doi: 10.1590/s0100-879x1999000300008 [DOI] [PubMed] [Google Scholar]
- Riffo-Campos AL, Fuentes-Trillo A, Tang WY, et al. In silico epigenetics of metal exposure and subclinical atherosclerosis in middle aged men: Pilot results from the Aragon Workers Health Study. Philos Trans R Soc Lond B Biol Sci 2018;373:20170084; doi: 10.1098/rstb.2017.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijal S, Chappuis F, Singh R, et al. Sodium stibogluconate cardiotoxicity and safety of generics. Trans R Soc Trop Med Hyg 2003;97:597–598; doi: 10.1016/s0035-9203(03)80043-3 [DOI] [PubMed] [Google Scholar]
- Ringseis R, Keller J, Eder K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur J Nutr 2013;52:1421–1442; doi: 10.1007/s00394-013-0511-0 [DOI] [PubMed] [Google Scholar]
- Rong Q, Ling C, Lu D, et al. Sb(III) resistance mechanism and oxidation characteristics of Klebsiella aerogenes X. Chemosphere 2022;293:133453; doi: 10.1016/j.chemosphere.2021.133453 [DOI] [PubMed] [Google Scholar]
- Salari S, Bamorovat M, Sharifi I, et al. Global distribution of treatment resistance gene markers for leishmaniasis. J Clin Lab Anal 2022;36(8):e24599; doi: 10.1002/jcla.24599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildroth S, Osborne G, Smith AR, et al. Occupational exposure to antimony trioxide: A risk assessment. Occup Environ Med 2021;78:413–418; doi: 10.1136/oemed-2020-106980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr TM, Steenland K, Thun MJ, et al. Mortality in a cohort of antimony smelter workers. Am J Ind Med 1995;27:759–770; doi: 10.1002/ajim.4700270510 [DOI] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, Feroe AG, et al. Antimony and sleep-related disorders: NHANES 2005–2008. Environ Res 2017;156:247–252; doi: 10.1016/j.envres.2017.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian-Marcos P, Santarelli G, Gomez S, et al. Canine leishmaniasis associated with pericardial effusion in a 4-year-old dog. J Vet Cardiol 2019;23:32–37; doi: 10.1016/j.jvc.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Shaked-Mishan P, Ulrich N, Ephros M, et al. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J Biol Chem 2001;276:3971–3976; doi: 10.1074/jbc.M005423200 [DOI] [PubMed] [Google Scholar]
- Shiue I. Higher urinary heavy metal, arsenic, and phthalate concentrations in people with high blood pressure: US NHANES, 2009–2010. Blood Press 2014;23:363–369; doi: 10.3109/08037051.2014.925228 [DOI] [PubMed] [Google Scholar]
- Shiue I, Hristova K. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3–19% of the population attributable risk for high blood pressure: US NHANES, 2009–2012. Hypertens Res 2014;37:1075–1081; doi: 10.1038/hr.2014.121 [DOI] [PubMed] [Google Scholar]
- Shotyk W, Krachler M, Chen B. Contamination of Canadian and European bottled waters with antimony from PET containers. J Environ Monit 2006;8:288–292; doi: 10.1039/b517844b [DOI] [PubMed] [Google Scholar]
- Shrivastava R, Sinha PR, Singh VP, et al. Echocardiographic evaluation of cardiac status in Indian visceral leishmaniasis patients. Trans R Soc Trop Med Hyg 2007;101:429–432; doi: 10.1016/j.trstmh.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Snawder JE, Tirmenstein MA, Mathias PI, et al. Induction of stress proteins in rat cardiac myocytes by antimony. Toxicol Appl Pharmacol 1999;159:91–97; doi: 10.1006/taap.1999.8739 [DOI] [PubMed] [Google Scholar]
- Strong JW. Combined assays for vitamins A, D (ergocalciferol), and E in multivitamin preparations with separation by reversed-phase partition chromatography. J Pharm Sci 1976;65:968–974; doi: 10.1002/jps.2600650705 [DOI] [PubMed] [Google Scholar]
- Su L, Fang W, Zhao X, et al. Disruption of mitochondrial redox homeostasis as a mechanism of antimony-induced reactive oxygen species and cytotoxicity. Ecotoxicol Environ Saf 2022;237:113519; doi: 10.1016/j.ecoenv.2022.113519 [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J. Antimony toxicity. Int J Environ Res Public Health 2010;7:4267–4277; doi: 10.3390/ijerph7124267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze S, Rotondi M, Kuk JL. The associations between blood and urinary concentrations of metal metabolites, obesity, hypertension, type 2 diabetes, and dyslipidemia among US adults: NHANES 1999–2016. J Environ Public Health 2021;2021:2358060; doi: 10.1155/2021/2358060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr L. Effect of the antimony compounds, fuadin and tartar emetic, on the electrocardiogram; a preliminary report. Bull U S Army Med Dep 1946;5:336–339. [PubMed] [Google Scholar]
- Tarr L. Effect of the antimony compounds, fuadin and tartar emetic, on the electrocardiogram of man; a study of the changes encountered in 141 patients treated for schistosomiasis. Ann Intern Med 1947;27:970–988; doi: 10.7326/0003-4819-27-6-970 [DOI] [PubMed] [Google Scholar]
- Taylor PJ. Acute intoxication from antimony trichloride. Br J Ind Med 1966;23:318–321; doi: 10.1136/oem.23.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry LR, Kulp TR, Wiatrowski H, et al. Microbiological oxidation of antimony(III) with oxygen or nitrate by bacteria isolated from contaminated mine sediments. Appl Environ Microbiol 2015;81:8478–8488; doi: 10.1128/AEM.01970-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur CP, Sinha GP, Pandey AK, et al. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as a first-line drug? An observational study of 80 cases. Ann Trop Med Parasitol 1998;92:561–569; doi: 10.1080/00034989859258 [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Mathias PI, Snawder JE, et al. Antimony-induced alterations in thiol homeostasis and adenine nucleotide status in cultured cardiac myocytes. Toxicology 1997;119:203–211; doi: 10.1016/s0300-483x(97)03628-7 [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Plews PI, Walker CV, et al. A. Antimony-induced oxidative stress and toxicity in cultured cardiac myocytes. Toxicol Appl Pharmacol 1995;130:41–47; doi: 10.1006/taap.1995.1006 [DOI] [PubMed] [Google Scholar]
- Torrent E, Leiva M, Segales J, et al. Myocarditis and generalised vasculitis associated with leishmaniosis in a dog. J Small Anim Pract 2005;46:549–552; doi: 10.1111/j.1748-5827.2005.tb00285.x [DOI] [PubMed] [Google Scholar]
- Vacante F, Senesi P, Montesano A, et al. L-Carnitine: An antioxidant remedy for the survival of cardiomyocytes under hyperglycemic condition. J Diabetes Res 2018;2018:4028297; doi: 10.1155/2018/4028297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yuan Z, Li J, et al. Acute effects of antimony exposure on adult zebrafish (Danio rerio): From an oxidative stress and intestinal microbiota perspective. Fish Shellfish Immunol 2022a;123:1–9; doi: 10.1016/j.fsi.2022.02.050 [DOI] [PubMed] [Google Scholar]
- Wang G, Guo M, Zhao Y, et al. Recent advances in antimony sulfide-based nanomaterials for high-performance sodium-ion batteries: A mini review. Front Chem 2022b;10:870564; doi: 10.3389/fchem.2022.870564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gao D, Zhang G, et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ Int 2020;135:105370; doi: 10.1016/j.envint.2019.105370 [DOI] [PubMed] [Google Scholar]
- Wang X, Karvonen-Gutierrez CA, Herman WH, et al. Metals and risk of incident metabolic syndrome in a prospective cohort of midlife women in the United States. Environ Res 2022c;210:112976; doi: 10.1016/j.envres.2022.112976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Park SK. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ Int 2018;121:683–694; doi: 10.1016/j.envint.2018.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu P, Xu S, et al. Antimony, a novel nerve poison, triggers neuronal autophagic death via reactive oxygen species-mediated inhibition of the protein kinase B/mammalian target of rapamycin pathway. Int J Biochem Cell Biol 2019;114:105561; doi: 10.1016/j.biocel.2019.105561 [DOI] [PubMed] [Google Scholar]
- Waye JD, Donoso E, Spingarn CL, et al. Cardiotoxic effects of antimony dimercaptosuccinate in schistosomiasis with special reference to coexistent hepatic dysfunction. Am J Cardiol 1962;10:829–835; doi: 10.1016/0002-9149(62)90178-9 [DOI] [PubMed] [Google Scholar]
- Wester PO. The urinary excretion of trace elements before and during treatment with hydralazine. Acta Med Scand 1975;197:307–309; doi: 10.1111/j.0954-6820.1975.tb04923.x [DOI] [PubMed] [Google Scholar]
- Wey HE, Richards D, Tirmenstein MA, et al. The role of intracellular calcium in antimony-induced toxicity in cultured cardiac myocytes. Toxicol Appl Pharmacol 1997;145:202–210; doi: 10.1006/taap.1997.8175 [DOI] [PubMed] [Google Scholar]
- White GP JR., Mathias CG, Davin JS. Dermatitis in workers exposed to antimony in a melting process. J Occup Med 1993;35:392–395. [PubMed] [Google Scholar]
- Wu Y, Xiang L, Wang H, et al. Transcriptome analysis of an arsenite-/antimonite-oxidizer, Bosea sp. AS-1 reveals the importance of the type 4 secretion system in antimony resistance. Sci Total Environ 2022;826:154168; doi: 10.1016/j.scitotenv.2022.154168 [DOI] [PubMed] [Google Scholar]
- Xia S, Zhu X, Yan Y, et al. Developmental neurotoxicity of antimony (Sb) in the early life stages of zebrafish. Ecotoxicol Environ Saf 2021;218:112308; doi: 10.1016/j.ecoenv.2021.112308 [DOI] [PubMed] [Google Scholar]
- Xu J, White AJ, Niehoff NM, et al. Airborne metals exposure and risk of hypertension in the Sister Study. Environ Res 2020;191:110144; doi: 10.1016/j.envres.2020.110144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Li F, Ding K, et al. Reduction of pentavalent antimony by trypanothione and formation of a binary and ternary complex of antimony(III) and trypanothione. J Biol Inorg Chem 2003;8:689–697; doi: 10.1007/s00775-003-0468-1 [DOI] [PubMed] [Google Scholar]
- Ye L, Jing C. Environmental geochemistry of thioantimony: Formation, structure and transformation as compared with thioarsenic. Environ Sci Process Impacts 2021;23:1863–1872; doi: 10.1039/d1em00261a [DOI] [PubMed] [Google Scholar]
- Ye L, Zhong W, Zhang M, et al. New mobilization pathway of antimonite: Thiolation and oxidation by dissimilatory metal-reducing bacteria via elemental sulfur respiration. Environ Sci Technol 2022;56:652–659; doi: 10.1021/acs.est.1c05206 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Xiao Y, Yu Y, et al. Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: The Dongfeng-Tongji Cohort. Environ Pollut 2018;237:917–925; doi: 10.1016/j.envpol.2018.01.046 [DOI] [PubMed] [Google Scholar]
- Zeng D, Zhou S, Ren B, et al. Bioaccumulation of antimony and arsenic in vegetables and health risk assessment in the superlarge antimony-mining area, China. J Anal Methods Chem 2015;2015:909724; doi: 10.1155/2015/909724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wang X, Zhang X, et al. Antimony in urine during early pregnancy correlates with increased risk of gestational diabetes mellitus: A prospective cohort study. Environ Int 2019;123:164–170; doi: 10.1016/j.envint.2018.11.072 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li X, Liu X, et al. Association between maternal antimony exposure and risk of gestational diabetes mellitus: A birth cohort study. Chemosphere 2020;246:125732; doi: 10.1016/j.chemosphere.2019.125732 [DOI] [PubMed] [Google Scholar]
- Zhao X, Xing F, Cong Y, et al. Antimony trichloride induces a loss of cell viability via reactive oxygen species-dependent autophagy in A549 cells. Int J Biochem Cell Biol 2017;93:32–40; doi: 10.1016/j.biocel.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Zhou S, Du Y, Feng Y, et al. Stabilization of arsenic and antimony Co-contaminated soil with an iron-based stabilizer: Assessment of strength, leaching and hydraulic properties and immobilization mechanisms. Chemosphere 2022;301:134644; doi: 10.1016/j.chemosphere.2022.134644 [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang B, Xiao L, et al. Mean platelet volume mediated the relationships between heavy metals exposure and atherosclerotic cardiovascular disease risk: A community-based study. Eur J Prev Cardiol 2020a;27:830–839; doi: 10.1177/2047487319830536 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wu M, Gao N, et al. Removal of antimonate from wastewater by dissimilatory bacterial reduction: Role of the coexisting sulfate. J Hazard Mater 2018;341:36–45; doi: 10.1016/j.jhazmat.2017.07.042 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wu Q, Lv H, et al. Toxicity of different forms of antimony to rice plants: Effects on reactive oxidative species production, antioxidative systems, and uptake of essential elements. Environ Pollut 2020b;263:114544; doi: 10.1016/j.envpol.2020.114544 [DOI] [PubMed] [Google Scholar]
- Zmit B, Belhaneche-Bensemra N. Antimony leaching from PET plastic into bottled water in Algerian market. Environ Monit Assess 2019;191:749; doi: 10.1007/s10661-019-7891-4 [DOI] [PubMed] [Google Scholar]