Abstract
Aims:
We compared the effectiveness of teclistamab versus real-world physician’s choice of therapy (RWPC) in triple-class exposed relapsed/refractory multiple myeloma.
Materials & methods:
MajesTEC-1 eligibility criteria were applied to the RWPC cohort. Baseline covariate imbalances were adjusted using inverse probability of treatment weighting. Overall survival, progression-free survival and time to next treatment were compared.
Results:
After inverse probability of treatment weighting, baseline characteristics were similar between cohorts (teclistamab, n = 165; RWPC, n = 364 [766 observations]). Teclistamab treated patients had numerically better overall survival (hazard ratio [HR]: 0.82 [95% CI: 0.59–1.14]; p = 0.233) and significantly greater progression-free survival (HR: 0.43 [0.33–0.56]; p < 0.0001) and time to next treatment (HR: 0.36 [0.27–0.49]; p < 0.0001) versus the RWPC cohort.
Conclusion:
Teclistamab offered clinical benefit over RWPC in triple-class exposed relapsed/refractory multiple myeloma.
Keywords: B-cell maturation antigen, bispecific antibody, comparative effectiveness, indirect treatment comparison, MajesTEC-1
Plain language summary
What is this article about?
This article looked at the outcomes of patients with triple-class exposed relapsed/refractory multiple myeloma (MM), who were treated with the bispecific antibody teclistamab in the MajesTEC-1 trial and analyzed how they compared with outcomes of similar patients from the nationwide deidentified electronic health record derived Flatiron Health multiple myeloma cohort database (real-world physician’s choice cohort).
What were the results?
Patients treated with teclistamab in the MajesTEC-1 study had statistically better progression-free survival (hazard ratio [HR]: 0.43 [95% CI: 0.33–0.56]; p < 0.0001) and time to next treatment (HR: 0.36 [0.27–0.49]; p < 0.0001) and numerically better overall survival (HR: 0.82 [0.59–1.14]; p = 0.233), compared with patients in the real-world physician’s choice cohort. Any imbalances in baseline characteristics of prognostic significance in the two groups of patients were adjusted to ensure statistical comparability.
What do the results of the study mean?
These findings highlight the clinical benefit of teclistamab relative to other therapies for relapsed/refractory MM, which is particularly relevant for physicians caring for patients who are triple-class exposed and have limited remaining treatment options.
Multiple myeloma (MM) is an incurable disease [1]. Despite an increasing incidence over the last 15 years, improvements in treatment have led to higher overall survival (OS) rates [1,2]. However, most patients eventually relapse, and the disease typically becomes increasingly refractory to existing treatments [3].
Treatment for MM often involves sequential lines of therapy (LOTs), with three of the most commonly used classes of agents comprising immunomodulatory drugs (IMiD), proteasome inhibitors and anti-CD38 monoclonal antibodies (mAbs) [4]. Once patients are exposed to all three of these therapies (i.e., triple-class exposed [TCE]), treatment options are limited, and patients tend to have poor outcomes, with an OS of ∼12 months and a progression-free survival (PFS) of ∼4 months [5,6]. Newer, off-the-shelf options include selinexor and belantamab mafodotin, but response rates are approximately 30% or lower [7,8]. Chimeric antigen receptor T-cell (CAR-T) therapies such as ciltacabtagene autoleucel and idecabtagene vicleucel have demonstrated substantial improvements in response rates (97.9 and 73%, respectively) and OS (OS not reached and 19.4 months, respectively) in this population [9,10]. However, in the context of limitations around patient access and waiting times for CAR-T therapy, as well as subsets of patients who may require alternative therapies, there remains an unmet need for new treatments for patients with TCE relapsed/refractory multiple myeloma (RRMM).
Teclistamab is the first bispecific antibody targeting B-cell maturation antigen × CD3 approved for the treatment of TCE RRMM [11,12]. Teclistamab was evaluated in the multicohort Phase I/II MajesTEC-1 (NCT03145181/NCT04557098) trial in patients with RRMM who had received ≥3 prior LOTs and were TCE [13]. At the time that MajesTEC-1 started, there were no approved therapies in patients with TCE RRMM to serve as a comparator. Selinexor was approved in the US for penta-drug refractory RRMM and idecabtagene vicleucel and belantamab mafodotin were under review. In the absence of randomized controlled trials comparing treatments in this patient population, indirect comparisons have been used to estimate differences between treatments [14–19]. Here we performed an indirect treatment comparison to assess the comparative effectiveness of teclistamab versus real-world physician’s choice of therapy (RWPC) in patients with TCE RRMM.
Materials & methods
Patient population
Detailed descriptions of data sources, study designs, outcomes and analysis methods are described in the Appendix. Adjusted indirect treatment comparisons between teclistamab and RWPC were conducted using individual patient-level data (IPD) from the MajesTEC-1 trial (for teclistamab) and the nationwide de-identified electronic health record–derived Flatiron Health multiple myeloma cohort database (for the RWPC cohort). IPD from MajesTEC-1 patients treated with teclistamab 1.5 mg/kg weekly (n = 165; clinical cut-off of 16 March 2022) were included and compared with data from patients with TCE RRMM who had ≥2 documented clinical visits on or after 1 January 2011, in the Flatiron Health database; participants included in the present analysis initiated eligible LOTs between February 2016 and August 2021. The Flatiron Health database is a longitudinal database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction [20,21]. During the study period, the de-identified data originated from approximately 280 US cancer clinical (∼800 sites of care). Key MajesTEC-1 eligibility criteria were applied to the RWPC cohort (Figure 1), including a diagnosis of MM using International Myeloma Working Group (IMWG) criteria, prior exposure to ≥3 prior LOTs (including a PI, an IMiD and an anti-CD38 mAb), receipt of a subsequent therapy after becoming TCE and documented evidence of disease progression on or within 12 months of the last LOT. Given that Flatiron Health’s multiple myeloma registry is retrospective, it was possible to include participants in the current analysis at the earliest LOT initiated after all key eligibility criteria were met. This differed from MajesTEC-1, in which participants may have received additional LOTs between the time at which they first met all eligibility criteria and the time at which they were enrolled into the clinical trial. To account for this difference, participants in the RWPC cohort who received multiple subsequent therapies after meeting eligibility criteria contributed multiple observations (corresponding to all eligible LOTs) to the current analysis, provided they met eligibility criteria at the beginning of each LOT. In such cases, a participant had multiple index dates (one for each eligible LOT). For MajesTEC-1, the index date was defined as date of first teclistamab dose. In the RWPC cohort, the index date was defined as the start of each eligible LOT.
Figure 1. . Participant selection.
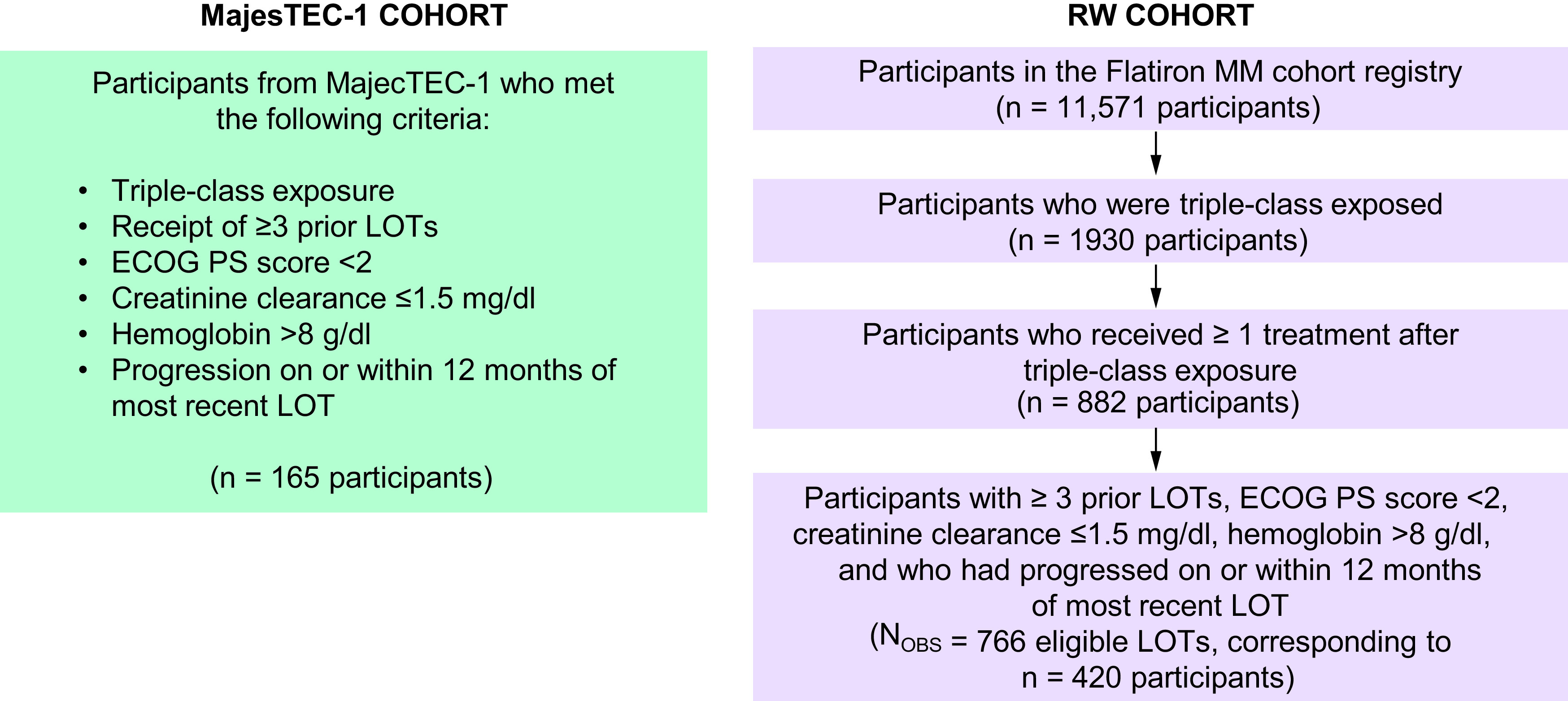
ECOG PS: Eastern Cooperative Oncology Group performance status; LOT: Line of therapy; MM: Multiple myeloma; NOBS: Number of observations; RW: Real world.
End points
The comparative effectiveness of teclistamab versus RWPC was determined for OS, PFS, and time to next treatment (TTNT). OS was defined as the time from the index date to the date of death. In MajesTEC-1, if the patient was alive or the vital status was unknown, then the patient’s data were censored at the date the patient was last known alive. For the RWPC cohort, if the patient was alive, the censoring date was the date of last follow-up. In MajesTEC-1, PFS was defined as the duration from the index date to the date of disease progression or death due to any cause, whichever occurred first. For patients who had not progressed and were alive at the data cut-off, data were censored at the last disease evaluation before the start of any subsequent antimyeloma therapy. PFS was evaluated according to IMWG criteria and was adjudicated by an independent review committee. In the RWPC cohort, PFS was defined as the duration from index date to the date of progression, switch to subsequent treatment or death due to any cause, whichever occurred first. For patients who had not developed an event of interest, data were censored at the date of the last follow-up. In both data sources, TTNT was defined as the time from index date to the initiation of the next therapy line or death. Patients who were still alive and did not initiate a new LOT at the cut-off were censored at last date known to be alive.
Statistical analysis
To reweight the RWPC cohort to align with the MajesTEC-1 population to adjust for imbalances between patient populations in baseline characteristics of prognostic significance, we used the propensity-score based method of inverse probability treatment weighting (IPTW) (with average treatment effect in the treated [ATT] weighting). To identify and rank-order prognostic factors, a pool of prognostic variables was identified by consulting studies from a review of the literature conducted to identify clinical outcomes in patients with TCE RRMM, as well as input from clinical experts. Clinical experts were consulted to provide input on the most important factors that should be adjusted for in the analyses. These top-ranked variables were considered as the primary analysis. The remaining factors were adjusted for as a sensitivity analysis and were ranked in order of importance based on a previous analysis [15]. Population differences between the MajesTEC-1 and RWPC cohorts were assessed using standardized mean differences (SMDs), where an SMD between 0 and 0.1 was considered a small difference, an SMD >0.1 and ≤0.2 was a moderate difference and >0.2 was a substantial difference. For both clinically important and strong prognostic covariates, mode value was used to impute missingness; the only variable requiring imputation of missingness in both cohorts was International Staging System (ISS) stage.
The base case analysis weighted patients on the following factors: refractory status, time to progression on last LOT, cytogenetic risk status, ISS stage, number of prior LOT, years since MM diagnosis, age, and hemoglobin. The fully adjusted scenario weighted patients on prior stem cell transplant, Eastern Cooperative Oncology Group performance status, race, sex and type of MM, in addition to the base case variables. A scenario analysis was conducted to investigate the impact on the treatment effect estimates, balance of participant populations and effective sample size when adjusting for additional covariates in the analyses. Several sensitivity analyses were performed, including multivariable regressions including a binary treatment indicator and covariates for adjustment in the model, IPTW with ATT weighting and a complete case analysis in which observations with missing values for covariates of interest were excluded.
Kaplan–Meier estimates were used to estimate survival curves and the median time to events. For time-to-event outcomes, a weighted Cox proportional hazards model was used to estimate the hazard ratio (HR) and its 95% CI. All statistical analyses were carried out with SAS 9.4 (SAS Institute, NC, USA) and R version 3.6.1 and 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). A significance level of 0.05 was used for all comparisons with no adjustment for multiplicity.
Results
Patient characteristics
The MajesTEC-1 cohort included data from 165 patients, while the unadjusted population of the RWPC cohort included 420 unique patients, corresponding to 766 eligible LOTs (between February 2016 and August 2021). The estimated median follow-up was 14.1 months in MajesTEC-1 and 18.2 months in the RWPC cohort. Prior to reweighting, most base case variables showed substantial differences (SMD >0.2) between cohorts. The MajesTEC-1 population had a higher proportion of patients who were triple- or quad-refractory, had ISS stage I disease, had standard risk cytogenetics, had prior stem cell transplant, who were white and who were aged <65 years. In contrast, the RWPC cohort had a greater proportion of patients with <6 years since MM diagnosis and who were aged ≥75 years. After IPTW, the baseline characteristics were balanced between the two cohorts (Table 1). Considering all factors, the mean SMD reduced from 0.24 prior to weighting to 0.11 after weighting. The fully adjusted scenario showed a further improvement in the overall balance, with a reduced mean SMD of 0.03.
Table 1. . Differences in baseline characteristics between patient populations.
| Variable, % | Unadjusted comparison | Adjusted comparison (Primary analysis) |
Fully adjusted comparison |
|
|---|---|---|---|---|
| MajesTEC-1 (n = 165) | RWPC cohort NOBS = 766 |
RWPC cohort ESS = 326 |
RWPC cohort ESS = 195 |
|
| Refractory status | ||||
| Penta-refractory† | 30.3 | 27.5 | 32.7 | 31.7 |
| Triple- or quad-refractory‡ | 47.3 | 39.3 | 46.4 | 46.8 |
| Other | 22.4 | 33.2 | 20.9 | 21.5 |
| Time to progression on last LOT, >4 mo | 61.8 | 59.3 | 60.0 | 61.3 |
| Cytogenetic risk | ||||
| High§ | 23.0 | 21.3 | 22.6 | 21.2 |
| Standard | 66.7 | 53.3 | 66.7 | 67.1 |
| Unknown | 10.3 | 25.5 | 10.7 | 11.7 |
| ISS stage | ||||
| Stage I¶ | 53.3 | 38.8 | 52.6 | 52.8 |
| Stage II¶ | 34.5 | 32.0 | 35.7 | 35.1 |
| Stage III¶ | 12.1 | 29.2 | 11.8 | 12.0 |
| Number of prior LOT >4 | 52.7 | 54.7 | 56.0 | 56.1 |
| Time since MM diagnosis, ≥6 years | 50.9 | 27.0 | 52.3 | 51.6 |
| Age ≥65 years | 47.8 | 64.2 | 48.1 | 48.4 |
| Hemoglobin ≥12 g/dl | 24.8 | 32.9 | 24.2 | 25.0 |
| Prior HSCT | 81.8 | 32.4 | 39.5 | 81.1 |
| ECOG performance status 1 | 66.7 | 68.8 | 65.7 | 61.8 |
| Race | ||||
| White | 81.2 | 73.0 | 69.3 | 80.0 |
| Black/African–American | 12.7 | 11.4 | 15.6 | 14.0 |
| Not reported/other | 6.1 | 15.7 | 15.1 | 6.0 |
| Male sex | 58.2 | 50.1 | 53.5 | 56.1 |
| Type of MM | ||||
| IgG | 55.2 | 60.4 | 64.3 | 57.5 |
| Light chain | 21.8 | 15.7 | 15.4 | 20.7 |
| Other | 23.0 | 23.9 | 20.3 | 21.7 |
Refractory to ≥2 IMiDs, 2 PIs and an anti-CD38 monoclonal antibody.
Refractory to 2 IMiDs and 1 PI; or 2 PIs and 1 IMiD; or 2 IMiDs and 2 PIs.
≥1 del17p, t(14;16) or t(4;14).
ISS stage was imputed for two observations in the MajesTEC-1 cohort.
ECOG: Eastern Cooperative Oncology Group; ESS: Effective sample size; HSCT: Hematopoietic stem cell transplantation; IMiD: Immunomodulatory drug; ISS: International Staging System; LOT: Line of therapy; MM: Multiple myeloma; mo: Months; NOBS: Number of observation; PI: Proteasome inhibitor; RWPC: Real-world physician’s choice.
Treatment regimens used in the Flatiron Health database
The most common RWPC therapies, used as either monotherapy or in combination with other treatments, were dexamethasone (79.1%), daratumumab (30.6%), pomalidomide (24.4%) and carfilzomib (24.4%). Most patients were prescribed combination regimens (Table 2); the two regimens prescribed to the largest proportion of patients were dexamethasone with elotuzumab and pomalidomide (5.7%) and daratumumab with dexamethasone and pomalidomide (5.1%). Although the Flatiron Health database includes newer agents such as belantamab mafodotin and selinexor, few patients received these therapies (for example, only 1.2% of patients received belantamab mafodotin) due to the timing of data collection (eligible LOTs had to be received between February 2016 and August 2021).
Table 2. . Treatments in the real-world physician’s choice of therapy cohort (≥9 patients; Flatiron Health database).
| Treatment regimen, n (%) | n = 766 |
|---|---|
| Dexamethasone, elotuzumab, pomalidomide | 44 (5.7%) |
| Daratumumab, dexamethasone, pomalidomide | 39 (5.1%) |
| Clinical study drug | 37 (4.8%) |
| Carfilzomib, dexamethasone | 32 (4.2%) |
| Carfilzomib, dexamethasone, pomalidomide | 32 (4.2%) |
| Carfilzomib, cyclophosphamide, dexamethasone | 30 (3.9%) |
| Carfilzomib, daratumumab, dexamethasone | 21 (2.7%) |
| Dexamethasone, pomalidomide | 18 (2.3%) |
| Bortezomib, daratumumab, dexamethasone | 15 (2.0%) |
| Bortezomib, dexamethasone, selinexor | 14 (1.8%) |
| Daratumumab, dexamethasone, lenalidomide | 14 (1.8%) |
| Dexamethasone, isatuximab-Irfc, pomalidomide | 14 (1.8%) |
| Dexamethasone, selinexor | 13 (1.7%) |
| Dexamethasone, elotuzumab, lenalidomide | 11 (1.4%) |
| Bortezomib, daratumumab, dexamethasone, pomalidomide | 10 (1.3%) |
| Daratumumab, dexamethasone | 10 (1.3%) |
| Belantamab mafodotin | 9 (1.2%) |
| Carfilzomib, dexamethasone, lenalidomide | 9 (1.2%) |
| Clinical study drug, dexamethasone | 9 (1.2%) |
Percentages are calculated with the number of participants in the all-treated analysis set as denominator (n = 766). Participants can be counted in more than one regimen or combination if they have received more than one combination in their treatment before progression or death.
Comparative effectiveness: primary analysis
In the primary analysis of the RWPC cohort, median OS was 14.46 months (95% CI: 12.29–18.56) after IPW-ATT adjustment compared with 18.27 months (95% CI: 15.08–NR) in patients treated with teclistamab. Median PFS was 3.65 months (95% CI: 3.09–4.30) after IPW-ATT adjustment in the RWPC cohort compared with 11.30 months (95% CI: 8.77–17.15) with teclistamab, while median TTNT was 4.90 months (95% CI: 4.07–5.88) after IPW-ATT adjustment in the RWPC cohort and was not reached in the teclistamab cohort from MajesTEC-1. Compared with the RWPC cohort, patients treated with teclistamab had numerically better OS (HR [95% CI]: 0.82 [0.59–1.14]; p = 0.233) and significantly greater PFS (HR [95% CI]: 0.43 [0.33–0.56]; p < 0.0001) and TTNT (HR [95% CI]: 0.36 [0.27–0.49]; p < 0.0001) (Table 3 & Figure 2).
Table 3. . Primary adjusted analysis and fully adjusted model for overall survival, progression-free survival and time to next treatment.
| Outcome/analysis | Median (95% CI) MajesTEC-1 |
Median (95% CI) RWPC |
HR (95% CI) | p-value |
|---|---|---|---|---|
| OS | ||||
| Unadjusted | 18.27 (15.08–NR) | 13.83 (12.32–15.67) | 0.77 (0.58–1.02) | 0.070 |
| Primary analysis | 18.27 (15.08–NR) | 14.46 (12.29–18.56) | 0.82 (0.59–1.14) | 0.233 |
| Fully adjusted model | 18.27 (15.08–NR) | 13.67 (11.30–18.92) | 0.79 (0.54–1.15) | 0.220 |
| PFS | ||||
| Unadjusted | 11.30 (8.77–17.15) | 3.91 (3.48–4.30) | 0.42 (0.33–0.53) | <0.0001 |
| Primary analysis | 11.30 (8.77–17.15) | 3.65 (3.09–4.30) | 0.43 (0.33–0.56) | <0.0001 |
| Fully adjusted model | 11.30 (8.77–17.15) | 3.38 (2.92–4.30) | 0.41 (0.31–0.55) | <0.0001 |
| TTNT | ||||
| Unadjusted | NR (12.68–NR) | 5.19 (4.63–5.75) | 0.34 (0.26–0.45) | <0.0001 |
| Primary analysis | NR (12.68–NR) | 4.90 (4.07–5.88) | 0.36 (0.27–0.49) | <0.0001 |
| Fully adjusted model | NR (12.68–NR) | 4.40 (3.55–5.72) | 0.37 (0.26–0.51) | <0.0001 |
HR: Hazard ratio; NR: Not reached; OS: Overall survival; PFS: Progression-free survival; RWPC: Real-world physician’s choice; TTNT: Time to next treatment.
Figure 2. . Unadjusted and adjusted (ATT weighted) Kaplan–Meier plots.
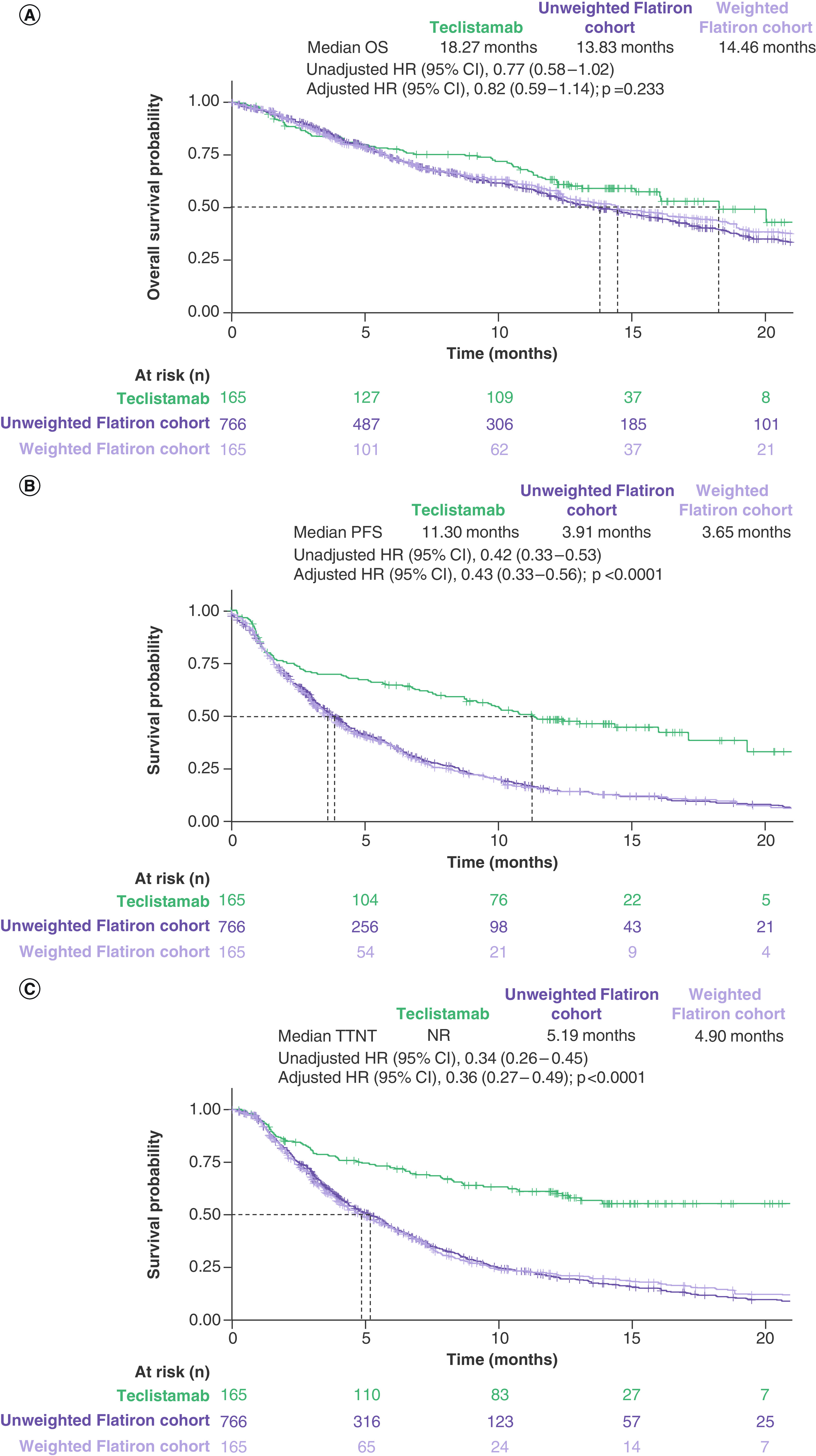
(A) OS, (B) PFS and (C) TTNT. The number of patients at risk is the sum of weights for the cohort-weighted physician’s choice of therapy cohort. Dashed lines indicate median values. Adjusted HRs and adjusted curves reflect IPTW with ATT weights.
†TTNT defined as time to next treatment or death, whichever comes first.
ATT: Average treatment effect in the treated; HR: Hazard ratio; IPTW: Inverse probability of treatment weighting; OS: Overall survival; PFS: Progression-free survival; TTNT: Time to next treatment.
Similar results were obtained across sensitivity analyses for OS (IPTW with ATT weights: HR [95% CI] = 0.84 [0.61–1.17], p = 0.305; multivariable regression: HR [95% CI] = 0.84 [0.61–1.16], p = 0.295; complete case: HR [95% CI] = 0.82 [0.57–1.18], p = 0.286), PFS (IPTW with ATT weights: HR [95% CI] = 0.45 [0.34–0.59], p < 0.0001; multivariable regression: HR [95% CI] = 0.41 [0.31–0.54], p < 0.0001; complete case: HR [95% CI] = 0.42 [0.32–0.56], p < 0.0001) and TTNT (IPTW with ATT weights: HR [95% CI] = 0.36 [0.27–0.49], p < 0.0001; multivariable regression: HR [95% CI] = 0.33 [0.24–0.44], p < 0.0001; complete case: HR [95% CI] = 0.36 [0.26–0.50], p < 0.0001).
Comparative effectiveness: fully adjusted model
Outcomes for the fully adjusted model were generally consistent with the adjusted primary analysis for each outcome (Table 3). Median OS, PFS and TTNT were shorter in the RWPC cohort compared with patients treated with teclistamab, with hazard ratios consistently in favor of teclistamab (OS HR [95% CI]: 0.79 [0.54–1.15]; p = 0.220; PFS HR [95% CI]: 0.41 [0.31–0.55]; p < 0.0001; TTNT HR [95% CI]: 0.37 [0.26–0.51]; p < 0.0001).
Discussion
Randomized controlled trials are the gold standard for assessing safety and efficacy of novel clinical interventions; however, such trials are not feasible in all situations, including in disease states that lack a standard of care or clinical equipoise [22]. In the absence of an appropriate comparator, indirect treatment comparisons, which use an external control arm from a real-world data source (such as the Flatiron Health database) and statistical methods to control for baseline differences in patient populations, can be performed to assess the relative clinical benefits conferred by new agents. These methodologies are also necessary for regulatory approvals. However, such comparisons can be biased if there are significant differences in the characteristics of the populations being compared, so it is imperative to ensure that nonrandomized populations are well balanced across all prognostic variables. The adjusted comparisons reported here represent valuable evidence regarding the comparative effectiveness of teclistamab compared with RWPC.
In the current study, we used IPTW to compare time-to-event outcomes with teclistamab (as assessed in MajesTEC-1) versus RWPC (as assessed in the de-identified Flatiron Health database). We found numerically better OS and significantly greater PFS and TTNT with teclistamab versus RWPC in patients with TCE RRMM who received ≥3 prior LOTs. These results were observed with the primary analysis as well as in sensitivity analyses and the fully adjusted model, which included adjustment for five additional covariates. The consistency of the results across all sensitivity analyses suggests that teclistamab represents a promising new treatment option for patients with TCE RRMM.
The robustness of our findings was further enhanced by the strength of the RWPC control arm provided by the Flatiron Health database. The long-term median follow-up in the RWPC cohort was 18.2 months, comprising patients in the US setting, which aligned with the population from MajesTEC-1. The Flatiron Health database has been recognized as having a regional population distribution similar to that of the US census and the National Program of Cancer Registries [20]. Another key advantage of the Flatiron Health database is that it includes a wide range of baseline clinical factors and longitudinal treatment sequences that enhanced the ability to accurately derive patient characteristics and survival outcomes [20]. The Flatiron population has also been used as a comparator for a clinical trial population in an analysis comparing outcomes in patients treated with ciltacabtagene autoleucel versus those treated with RWPC in which the RWPC cohort was restricted to patients who had adequate organ function, which is a general requirement for patients enrolled in clinical trials [15]. Additionally, the survival outcomes observed in the RWPC cohort were consistent with those reported in other real-world cohorts of patients with RRMM [23–25], further strengthening the validity of the current findings. Median OS, PFS and TTNT in the unadjusted RWPC cohort in our study were 13.83 months (95% CI: 12.32–15.67), 3.91 months (95% CI: 3.48–4.30) and 5.19 months (95% CI: 4.63–5.75), respectively. Comparatively, median OS in the retrospective MAMMOTH study (data cut-off: 2018) was 9.3 months (95% CI: 8.1–10.6) and median PFS was 3.4 months (95% CI: 2.8–4.0) for patients who received a subsequent treatment after becoming refractory to an index regimen containing an anti-CD38 mAb [23].
Our study does have some limitations. The Flatiron Health database lacks information on response outcomes and whether patients included in the analysis received treatments prior to database entry. Due to the timing of data collection, only a few patients received recently approved agents such as belantamab mafodotin, selinexor or CAR-T therapies. As with any nonrandomized study, the potential for residual confounding cannot be excluded. However, imbalances in important prognostic factors were able to be adjusted for using IPD from both cohorts, and rigorous statistical methods were applied to ensure appropriate adjustment for confounding bias due to imbalances in baseline characteristics between the cohorts. To ensure that the most important clinical factors were balanced between the two populations, an evidence-informed process was used to select the covariates for adjustment, and clinical experts were consulted at multiple stages of the analysis to ensure clinical validity of the chosen covariates. Of all prognostic factors identified a priori, data for total plasmacytomas (including extramedullary plasmacytomas) were not available and lactate dehydrogenase was not used due to a high percentage of missing data in the RWPC cohort. Although 26% of patients in the Flatiron Health database had missing cytogenetic risk data, cytogenetic risk was balanced after adjustment and imputation (as for all other baseline characteristics). In addition, patients in MajesTEC-1 may have had access to better supportive care and been more regularly and strictly monitored for factors such as disease progression and adherence than those in the RWPC cohort. This difference is especially relevant for PFS outcomes; the level of monitoring could have improved outcomes in MajesTEC-1, and progression data were more likely to be missing for participants in the RWPC cohort than in MajesTEC-1. To address this, PFS outcomes considered the start of subsequent treatment as a progression event in the RWPC cohort, as the start of a new LOT may have been more reliably reported than progression, but this may have led to an overestimation of the time to progression for the RWPC cohort. Alternatively, the RW PFS definitions may have misclassified progression for participants who initiated a new LOT for reasons other than progression; however, lack of efficacy is most often the reason for initiating a new LOT at this late stage in a patient’s treatment journey.
Conclusion
Teclistamab showed improved effectiveness for OS, PFS and TTNT compared with RWPC in patients with TCE RRMM who received ≥3 prior LOTs. The results of sensitivity analyses and the fully adjusted model analysis were consistent with the adjusted primary analysis. These findings highlight the clinical benefit of teclistamab in patients with TCE RRMM who have limited treatment options.
Summary points.
Teclistamab is the first bispecific antibody targeting B-cell maturation antigen × CD3 approved for the treatment of triple-class exposed (TCE) relapsed/refractory multiple myeloma (RRMM). Teclistamab was evaluated in the multicohort Phase I/II MajesTEC-1 (NCT03145181/NCT04557098) trial in patients with TCE RRMM who had received ≥3 prior lines of therapy.
Trials in patients with TCE RRMM often use a single-arm design due to the lack of a standard of care and a relatively small number of potential patients.
An external control arm of patients treated with the real-world physician’s choice of therapy (RWPC) was created using data from the Flatiron Health database; key eligibility criteria from MajesTEC-1 were applied.
Baseline covariates were adjusted using inverse probability treatment weighting, and outcomes were analyzed as time-to-event data using inverse probability treatment weighting adjusted Kaplan–Meier estimates and a weighted Cox proportional hazards model.
Patients treated with teclistamab had numerically better overall survival and significantly greater progression-free survival and time to next treatment compared with those treated with RWPC.
Results using the fully adjusted model, which adjusted for five additional baseline covariates, were consistent with the primary analysis.
In the absence of head-to-head trials comparing teclistamab with other treatments, this study demonstrated that teclistamab offers clinical benefit over RWPC in patients with TCE RRMM who received ≥3 prior lines of therapy.
Supplementary Material
Acknowledgments
Portions of the work described in this paper were presented at the 19th International Myeloma Society (IMS) Annual Meeting, CA, USA (August 2022).
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2022-0186
Author contributions
A Krishnan, AK Nooka, A Chari, AL Garfall, TG Martin and SZ Usmani: interpretation of data, revising work for important intellectual content, final approval. S Nair, X Lin, K Qi, A Londhe, L Pei, E Ammann, R Kobos, J Smit, T Parekh, A Marshall and M Slavcev: design of the work, acquisition, analysis and interpretation of data; drafting and revising the work for important intellectual content; final approval.
Financial & competing interests disclosure
The study was funded by Janssen Global Services, LLC. A Krishnan reports leadership with Sutro Biopharma, has stock/other ownership interests with Bristol-Myers Squibb, has served in a consulting/advisory role for Janssen, Bristol-Myers Squibb, Sanofi, Regeneron, Pfizer, and GlaxoSmithKline, has served on speakers' bureau for Takeda, Bristol-Myers Squibb, and GlaxoSmithKline Canada, and has received research funding from Janssen. AK Nooka has served in a consulting/advisory role for Amgen, Janssen Oncology, Bristol-Myers Squibb, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, and Secura Bio, reports travel, accommodations, and expenses from GlaxoSmithKline, has received honoraria from Amgen, Janssen Oncology, Bristol-Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, and Secura Bio, and has received research funding from Amgen, Janssen Oncology, Takeda, Bristol-Myers Squibb/Celgene, Arch Oncology, and GlaxoSmithKline. A Chari has served in a consulting/advisory role for Amgen, Janssen Oncology, Seattle Genetics, Karyopharm Therapeutics, Genzyme, Oncopeptides, Takeda, Antengene, GlaxoSmithKline, Secura Bio, Shattuck Labs, Genentech, AbbVie, and Bristol-Myers Squibb/Celgene, and has received research funding from Celgene, Janssen, Seattle Genetics, Takeda, and Pharmacyclics. AL Garfall has served in a consulting/advisory role for Janssen Oncology, GlaxoSmithKline, Bristol-Myers Squibb, and Amgen, has patent applications in the field of CAR-T cell therapy, has stock and other ownership interests in Cabaletta Bio, and has received research funding from Novartis, Tmunity Therapeutics, Inc., Janssen Oncology, and CRISPR Therapeutics. TG Martin has served in a consulting/advisory role for Juno Therapeutics and GlaxoSmithKline, and has received research funding from Sanofi, Amgen, and Janssen Oncology. S Nair, X Lin, K Qi, A Londhe, E Ammann, T Parekh and A Marshall are employed by and have stock/other ownership interests in Janssen/Johnson & Johnson. L Pei, R Kobos and M Slavcev are employed by and have stock/other ownership interests in Janssen. J Smit is employed by, has stock/other ownership interests in, and reports travel, accommodations, expenses from Johnson & Johnson/Janssen. SZ Usmani has served in a consulting/advisory role for Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GlaxoSmithKline, Karyopharm Therapeutics, AbbVie, SkylineDX, Merck, Oncopeptides, Genentech, Gilead Sciences, and Bristol-Myers Squibb/Celgene, has served on speakers' bureau for Takeda, Amgen, Janssen Oncology, Sanofi, and Bristol-Myers Squibb/Celgene, and has received research funding from Celgene, Array BioPharma, Janssen Oncology, Pharmacyclics, Sanofi, Bristol-Myers Squibb, Amgen, Seattle Genetics, Merck, Skyline Diagnostics, and GlaxoSmithKline. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial and medical writing support was provided by Corey Eagan, MPH, Claire Line, PhD, and Linda Wychowski, PhD, of Eloquent Scientific Solutions, and was funded by Janssen Global Services, LLC.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Data sharing statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at www.janssen.com/clinical-trials/transparency. The data for the real-world physicians' practice cohort were originated by Flatiron Health, Inc. These de-identified data may be made available upon request and are subject to a license agreement with Flatiron Health; interested researchers should contact DataAccess@flatiron.com to determine licensing terms.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Stalker ME, Mark TM. Clinical management of triple-class refractory multiple myeloma: a review of current strategies and emerging therapies. Curr. Oncol. 29, 4464–4477 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2020. American Cancer Society, GA, USA: (2020). www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. Multiple myeloma. Blood 111, 2962–2972 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Multiple myeloma. Version 5.2022. www.nccn.org/guidelines/guidelines-detail?category=1&id=1445 [DOI] [PubMed]; •• Guidelines for the treatment of multiple myeloma are provided.
- 5.Dhanasiri S, Hollier-Hann G, Stothard C, Dhanda DS, Davies FE, Rodriguez-Otero P. Treatment patterns and outcomes in triple-class exposed patients with relapsed and refractory multiple myeloma: findings from the multinational ITEMISE study. Clin. Ther. 43, 1983–1996.e3 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Mateos MV, Weisel K, De Stefano V et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 36, 1371–1376 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonial S, Lee HC, Badros A et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, Phase II study. Lancet Oncol. 21, 207–221 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Chari A, Vogl DT, Gavriatopoulou M et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 381, 727–738 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Munshi NC, Anderson LD Jr, Shah N et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Martin T, Usmani SZ, Berdeja JG et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. 41(6), 1265–1274 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen Biologics B.V. TECVAYLI. Summary of Product Characteristics. European Medicines Agency, 1–49. www.ema.europa.eu/en/documents/product-information/tecvayli-epar-product-information_en.pdf [Google Scholar]
- 12.Janssen Biotech, Inc. TECVAYLI (teclistamab-cqyv). Janssen Biotech, Inc, PA, USA: (2022). [Google Scholar]
- 13.Moreau P, Garfall AL, van de Donk NWCJ et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 495–505 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The results of Phase I/II of the MajesTEC-1 trial are presented.
- 14.Costa LJ, Hari P, Berdeja JG et al. Meta-analysis of ciltacabtagene autoleucel versus physician's choice therapy for the treatment of patients with relapsed or refractory multiple myeloma. Curr. Med. Res. Opin. 38(10), 1759–1767 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Martin T, Krishnan A, Yong K et al. Comparative effectiveness of ciltacabtagene autoleucel in CARTITUDE-1 versus physician's choice of therapy in the Flatiron Health multiple myeloma cohort registry for the treatment of patients with relapsed or refractory multiple myeloma. EJHaem 3, 97–108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Flatiron versus cilta-cel indirect comparison.
- 16.Martin T, Usmani SZ, Schecter JM et al. Matching-adjusted indirect comparison of efficacy outcomes for ciltacabtagene autoleucel in CARTITUDE-1 versus idecabtagene vicleucel in KarMMa for the treatment of patients with relapsed or refractory multiple myeloma. Curr. Med. Res. Opin. 37, 1779–1788 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Shah N, Mojebi A, Ayers D et al. Indirect treatment comparison of idecabtagene vicleucel versus conventional care in triple-class exposed multiple myeloma. J. Comp. Eff. Res. 11(10), 737–749 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Weisel K, Krishnan A, Schecter JM et al. Matching-adjusted indirect treatment comparison to assess the comparative efficacy of ciltacabtagene autoleucel in CARTITUDE-1 versus belantamab mafodotin in DREAMM-2, selinexor-dexamethasone in STORM Part 2, and melphalan flufenamide-dexamethasone in HORIZON for the treatment of patients with triple-class exposed relapsed or refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 22, 690–701 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Weisel K, Martin T, Krishnan A et al. Comparative efficacy of ciltacabtagene autoleucel in CARTITUDE-1 vs physician's choice of therapy in the long-term follow-up of POLLUX, CASTOR, and EQUULEUS clinical trials for the treatment of patients with relapsed or refractory multiple myeloma. Clin. Drug Investig. 42, 29–41 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv (2020). www.medrxiv.org/content/10.1101/2020.03.16.20037143v2 [Google Scholar]
- 21.Birnbaum B, Nussbaum N, Seidl-Rathkopf K et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv (2020). https://arxiv.org/abs/2001.09765 [Google Scholar]
- 22.Joseph NS, Kaufman JL, Dhodapkar MV et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J. Clin. Oncol. 38, 1928–1937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi UH, Cornell RF, Lakshman A et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33, 2266–2275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Real-world study of relapsed/refractory multiple myeloma (RRMM).
- 24.Usmani S, Ahmadi T, Ng Y et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist 21, 1355–1361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Real-world study of RRMM.
- 25.Kumar SK, Dimopoulos MA, Kastritis E et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia 31, 2443–2448 (2017). [DOI] [PubMed] [Google Scholar]; • Real-world study of RRMM.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


