Abstract
The role of alphaherpesvirus membrane protein internalization during the course of viral infection remains a matter of speculation. To determine the role of internalization of the pseudorabies virus (PRV) gE and gI proteins, we constructed viral mutants encoding specific mutations in the cytoplasmic tail of the gE gene that inhibited internalization of the gE-gI complex. We used these mutants to assess the role of gE-gI endocytosis in incorporation of the proteins into the viral envelope and in gE-mediated spread or gE-promoted virulence. In addition, we report that another viral mutant, PRV 25, which encodes a gE protein defective in endocytosis, contains an additional, previously uncharacterized mutation in the gE gene. We compared PRV 25 to another viral mutant, PRV 107, that does not express the cytoplasmic tail of the gE protein. The gE protein encoded by PRV 107 is also defective in endocytosis. We conclude that efficient endocytosis of gE is not required for gE incorporation into virions, gE-mediated virulence, or spread of virus in the rat central nervous system. However, we do correlate the defect in endocytosis to a small-plaque phenotype in cultured cells.
Pseudorabies virus (PRV) is a neurotropic alphaherpesvirus that causes acute fatal disease in a variety of mammals and birds (13, 39). The viral glycoprotein E (gE) is an important mediator of virulence and spread of PRV in every animal model tested thus far (4, 5, 9, 10, 19, 20, 22, 24, 27, 28, 47). The N-terminal extracellular domain of gE is sufficient to mediate gE-promoted spread in the rat central nervous system (CNS), while the C-terminal cytoplasmic domain of gE is required for gE-mediated virulence (44). The mechanism by which gE accomplishes these two separable functions has not yet been determined. One or both of these functions could involve the glycoprotein I (gI), which binds specifically to gE shortly after synthesis of the proteins (47, 52). Expression of the N-terminal domain of PRV gE is sufficient to mediate binding of gI, suggesting that the gI binding domain is in the extracellular domain of gE (44). Similar findings have been previously made with the feline herpesvirus (FHV) gE homologue (29).
Several groups have begun studying the trafficking and localization of the gE homologues in the alphaherpesvirus family, and they have noted that after expression on the plasma membrane of both transfected and infected cells, the gE protein alone or the gE-gI complex is internalized into the interior of the cell in a manner analogous to receptor-mediated endocytosis (1, 2, 32–34, 43, 49, 50). In some circumstances, gE is required for endocytosis of gI, while in other instances, gI internalizes in the absence of gE (1, 32, 43). The varicella-zoster virus (VZV) gE homologue is targeted either to endosomal compartments or to the trans-Golgi network (TGN) after its internalization from the plasma membrane during transfection studies (1, 32, 33, 35, 49, 50).
In the case of VZV, which is believed to acquire its final envelope in the TGN, endocytosis of proteins from the plasma membrane to the TGN was proposed to be a mechanism for targeting proteins to the site of envelopment (17, 49, 50). In addition, the human cytomegalovirus glycoprotein B is internalized and subsequently incorporated into viral particles (37). Studies using a PRV mutant (PRV 25) expressing a gE protein that lacks the cytoplasmic tail suggested that this may also be the case for PRV (44). The gE protein made by PRV 25 was not internalized and remained stably expressed on the plasma membrane of infected cells. In addition, the protein was not incorporated into viral particles. This finding suggested a direct correlation of the ability of a protein to internalize from the plasma membrane and its ability to be targeted efficiently to the viral envelope.
Several lines of evidence, however, suggest that endocytosis of glycoproteins is not the sole route for incorporation of the proteins into viral particles (43). First, endocytosis of the PRV gE-gI complex is inhibited after 6 h of infection, a time at which the majority of the viral particles are beginning to be made and released. Therefore, only those proteins internalized during the first 6 h of infection would be capable of being targeted to viral particles and would have to be set aside for all subsequent incorporation. However, biotinylation of the cell surface at 4 h postinfection showed that those molecules on the cell surface during the time of biotinylation are not directly targeted to viral particles. In addition, PRV gI is not able to internalize unless it is coexpressed with gE yet can be incorporated into viral particles in the absence of gE (our unpublished observations). This finding suggests that gI need not be internalized to be incorporated. Finally, glycoprotein C (gC) does not internalize at any point postinfection, yet this protein is a major constituent of the PRV viral particle (43).
Endocytosis of cell surface receptors involves the interaction of the cytoplasmic tails of the receptor proteins with intracellular proteins (adaptor complexes) that mediate recruitment of the receptors to clathrin-coated pits for internalization (reviewed in references 25, 30, 45, and 46). One motif in cytoplasmic tails of receptors known to interact with the adaptor complexes is the YXXΦ motif (Y = tyrosine, X = any amino acid, Φ = large hydrophobic residue). Mutation of the tyrosine residue often abrogates internalization of these receptors. The PRV gE cytoplasmic tail contains two potential tyrosine-based internalization motifs beginning with a tyrosine residue at amino acid 478 and one at amino acid 517. To address directly the role of protein endocytosis in incorporation of the protein into the viral particle without large deletions of the cytoplasmic tail of gE, we constructed viral mutants containing single amino acid substitutions at these tyrosine residues. These mutants also allowed us to assess the role of gE endocytosis in gE-promoted spread and gE-mediated virulence of PRV. In conducting these studies, we also found that the mutant gE gene encoded by PRV 25 contains an additional mutation upstream of the engineered stop codon. This additional mutation leads to a shift in the reading frame during translation of the protein such that the engineered stop codon is no longer effective and the protein contains a novel cytoplasmic tail. To determine the effect of this additional mutation on the results obtained with PRV 25, we constructed another viral mutant, PRV 107, that contains only the engineered stop codon after the sequences coding for the transmembrane domain of the protein. This gene does not contain the extra base insertion that is found in the PRV 25 gE gene. PRV 107 encodes an anchored gE protein that no longer expresses the cytoplasmic tail of the protein.
In this report, we show that PRV 107 has most of the phenotypes described for PRV 25, including stable expression of the protein on the plasma membrane, inhibition of endocytosis of the protein, reduced plaque size on Madine-Darby bovine kidney (MDBK) cells, ability to spread to all retinorecipient areas of the rat eye, and decreased virulence of the virus. However, unlike the gE protein produced by PRV 25, the PRV 107 gE protein is efficiently incorporated into viral particles. In further support of this idea, a single mutation in the first tyrosine residue (Y478), but not the second tyrosine residue (Y517), in the gE cytoplasmic tail inhibits endocytosis of the protein. Like the gE protein produced by PRV 107, the gE mutants containing mutations in the tyrosine residues are also efficiently incorporated into the viral envelope. In addition, we report that inhibition of endocytosis of the gE-gI complex leads to a moderate decrease in plaque size when the virus is plated on MDBK cells yet has no effect on the ability of the virus to spread in the rat CNS after eye infection. Finally, we demonstrate that decreasing the amount of gE-gI endocytosis has no effect on gE-mediated virulence in rats.
MATERIALS AND METHODS
Virus strains and cells.
Table 1 lists the viruses used in this study. Wild-type PRV strain Becker (PRV Be) and the isogenic strains PRV 25 and PRV 91 have been previously described (44, 47). All PRV strains were propagated in PK15 (pig kidney) cells. Plaque size phenotypes were analyzed on MDBK cells grown in 1% methocel in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2% fetal bovine serum (FBS). Cells were grown in DMEM supplemented with 10% FBS, while viral infections were performed in DMEM supplemented with 2% FBS.
TABLE 1.
Viruses used in this study
| Virus name | Description |
|---|---|
| PRV Be | Wild type |
| PRV 91 | gE deletion |
| PRV 25 | Frameshift gE |
| PRV 107 | Am457 gE, truncated gE |
| PRV 104 | Y478S gE tail |
| PRV 105 | Y478S + Y517S gE tail |
| PRV 106 | Y517S gE tail |
| PRV 107R | Rescue PRV 107; wild type |
Antisera.
The monoclonal antibody (MAb) specific for gE complexed to gI (MAb 1/14), the polyclonal rabbit PRV-specific antiserum (Rb133), and the polyclonal goat antisera to gC (282) and gB (284) have all been previously described (16, 38, 41, 47). T. Ben-Porat kindly provided the MAb pool to gE (M133, M156, and M138). Rabbit polyclonal antiserum to gE was a generous gift from K. Bienkowska-Szewczyk (University of Gdansk). Alexa-568-conjugated goat anti-mouse immunoglobulin G was purchased from Molecular Probes. Horseradish peroxidase-conjugated donkey anti-rabbit, anti-mouse, and anti-goat immunoglobulin G were purchased from Kirkegaard & Perry Laboratories, Inc.
Construction of mutant viruses.
All oligonucleotide mutagenesis of PRV gE was performed with an Altered Sites kit (Promega).
Mutation of arginine 457 to an amber stop codon.
Site-directed mutagenesis was performed on pRT14 (44), which contains the 1,052-bp BstEII-SphI fragment of the gE gene, using an oligonucleotide that results in the substitution of amino acid 457 of the protein with an amber stop codon (5′-GCTGTGCTCCCGCCGCTAGGCGGCCTCGCGGCCG-3′). The plasmid was sequenced by using Sequenase (United Biochemicals) to confirm the presence of only the desired mutation. The resulting plasmid was named pRS25. The addition of the stop codon created a novel BfaI restriction site in the BamHI-7 restriction fragment of PRV Be viral DNA. A transfer vector, pPH2, was constructed by cloning the SalI-MluI restriction fragment from the Us region of PRV Be into a modified pAlter-1 plasmid. This fragment contains a portion of gD, all of the gE, gI, and Us9 genes, and a portion of the Us2 gene. The 1,052-bp BstEII-SphI restriction fragment from pRS25 containing the site-directed mutation was introduced into pPH2. Transfer of the appropriate fragment was confirmed by restricting the resulting plasmid with BfaI. This plasmid was named pRS33.
Mutation of tyrosine residues to serine residues.
To construct the tyrosine-to-serine amino acid substitutions in the gE tail, the SphI-HindIII fragment of pPH2 was cloned into the pAlter-1 plasmid, creating pRS3. This fragment contains the gE cytoplasmic tail, the entire Us9 gene, and a portion of the Us2 gene. The nucleotide sequences encoding the tyrosine residues in the gE tail at amino acids 478 and 517 were changed individually or in combination to serine residues by using either the single oligonucleotide 5′-CATGCTCTCGCCGGTGAGCACCAGCCTGCCCACG-3′ or 5′-CGGCTACGAGGGGCCGAGCGTGAGCCTGGACGCC-3′, respectively, or by using both oligonucleotides in the same reaction. The resulting plasmids were sequenced to the EagI restriction site in the Us9 gene and were named pRS7 (Y478S), pRS8 (Y517S), and pRS9 (Y478S + Y517S). Mutation of the tyrosine residue at amino acid 478 results in the loss of a BsrGI restriction site, while mutation of tyrosine 517 results in the loss of a BsiWI restriction site. To decrease the amount of sequence that was used in the mutagenesis reactions that would also be used to make recombinant virus, the EagI-MluI restriction fragments containing Us9 and Us2 coding sequences were repaired with wild-type sequences in the following manner. A SphI-SphI restriction fragment containing wild-type sequences encoding the gE tail, the entire Us9 gene, and a portion of the Us2 gene was cloned from PRV Be viral DNA into the SphI restriction site of pSP72 (Promega), creating pGS166. Fragments from pRS7, pRS8, or pRS9 were subcloned into pGS166, using outside XbaI restriction sites contained in the vectors and the EagI sites located in the Us9 gene. Replacement of the wild-type XbaI-EagI restriction fragment in pGS166 with the fragments from pRS7, pRS8, or pRS9 were confirmed by restriction digestion of the plasmids with BsrGI and BsiWI. The resulting plasmids were named pRS13 (Y478S), pRS14 (Y517S), and pRS15 (Y478S + Y517S). The SphI-MluI restriction fragment of each of these plasmids was then subcloned into the transfer vector pPH2, creating pRS28 (Y478S), pRS29 (Y517S), or pRS30 (Y478S + Y517S). Transfer of fragments containing the point mutations was confirmed by restriction digestion of the plasmids with BsrGI and BsiWI.
Construction of mutant viruses.
PRV 91 viral DNA, which has the gE gene deleted, was cotransfected by the calcium phosphate precipitation method into PK15 cells with either pRS33 (amber 457 [Am457]), pRS28 (Y478S), pRS29 (Y517S), or pRS30 (Y478S + Y517S) to enable the formation of recombinant virus. After a complete cytopathic effect was observed, the infected cells were harvested, frozen, thawed, and replated onto PK15 cells to allow plaque formation. Plaques formed by recombinant virus were screened for gE expression by a black plaque assay with monoclonal antiserum against gE. Recombinants that expressed gE protein were picked and purified by four rounds of plaque purification, creating PRV 107 (Am457), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), and PRV 106 (Y517S).
Construction of revertant virus.
PRV 107 revertants were constructed by cotransfection of PRV 107 DNA with a wild-type 1.4-kb StyI restriction fragment that spans the transmembrane domain of gE to the beginning of the Us9 gene. Wild-type recombinants were screened by plaque size phenotypes (large plaques) on MDBK cells. One isolate was plaque purified four times and was named PRV 107R.
Verifying genotypes of recombinant viruses.
The presence or absence of the desired mutations in recombinant viral DNA was confirmed by Southern blot analysis as follows. The amber mutation in PRV 107 creates a novel BfaI restriction site in the BamHI-7 fragment of PRV, which alters a 6,400-bp fragment to 1,400- and 5,000-bp fragments following DNA digestion with BamHI and BfaI. The substitution of tyrosine 478 with serine destroys a BsrGI restriction site in the BamHI-7 fragment of PRV that changes two fragments of 1,280 and 5,077 bp to a 6,400-bp fragment when the DNA is digested with BamHI and BsrGI. The substitution of tyrosine 517 with serine destroys a BsiWI restriction site in the BamHI-7 fragment of PRV that results in the alteration of two fragments of 288 and 1,163 bp to one 1,451-bp fragment when the DNA is digested with BamHI and BsiWI. The presence of both mutations in the double mutant was confirmed. The rescued virus, PRV 107R, had a wild-type restriction pattern.
Cloning of BamHI-7 from PRV 25.
Viral DNA isolated from PRV 25-infected cells was restricted with BamHI, and the approximately 6,400-bp fragment (BamHI-7) was isolated from an agarose gel by using GeneClean (Bio 101). This fragment was ligated into BamHI-restricted pBluescript KS+ (Stratagene) that had also been treated with calf intestinal phosphatase (New England Biolabs). The resulting plasmid was named pRS26.
Black plaque and plaque size analysis.
For black plaque analysis, PK15 cells were infected and overlaid with 1% methocel for 48 h. Plaques were reacted with a 1:1:1 mixture of a gE MAb pool diluted 1:10 as previously described (44).
For plaque size analysis, MDBK cells were infected with sufficient virus to produce approximately 200 plaques per 10-cm2 dish and overlaid with 1% methocel. At 72 h after infection, the plaques were fixed in 2% electron microscopy-grade paraformaldehyde (Electron Microscopy Sciences) 10 min at room temperature and then permeabilized with 0.5% saponin in phosphate-buffered saline containing 3% bovine serum albumin for 3 min at room temperature. Black plaque analysis was performed as described above, using gB-specific polyclonal antiserum 284 to define the outermost border of the plaques. Plaques were measured by using an ocular reticle and a 10× objective on an Olympus inverted microscope.
Indirect immunofluorescence and endocytosis assays.
Indirect immunofluorescence assays were performed on fixed, permeabilized PK15 cells that had been infected for either 4 or 6 h, using a MAb that specifically recognized gE when it was complexed to gI (MAb 1/14) as previously described (43). All endocytosis assays were performed at 4 h postinfection with MAb 1/14 as previously described (43). Single optical sections were taken through the centers of the cells by using a Nikon MRC600 confocal microscope mounted on an Optiphot II which utilizes an argon-krypton laser.
Western blot analysis.
Cell lysates from PK15 cells infected for 16 h were collected in TNX buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100). Virions were isolated from the medium of infected PK15 cells at 14 h postinfection through a sucrose cushion as previously described (43). Virion and cell extracts were electrophoresed through a sodium dodecyl sulfate–8% polyacrylamide gel and transferred to nitrocellulose membranes. Western blot analysis using either a rabbit polyclonal antiserum against gE or goat polyclonal antiserum 282 against gC and enhanced chemiluminescence detection were performed as recommended by the manufacturer of SuperSignal (Pierce).
Animal experiments, tissue processing, and immunohistochemistry.
Adult male Sprague-Dawley rats weighing 200 to 250 g at the time of the experiment were used in this study. Food and water were freely available during the course of the experiment, and the photoperiod was standardized to 14 h of light and 10 h of darkness. Experimental protocols were approved by the Princeton University Animal Welfare Committee and were consistent with the regulations stipulated by the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (Public Law 99-198). The animals were confined to a biosafety level 2 facility, and the experiments were conducted with specific safeguards as described previously (14).
For intraocular injections, 2.5 μl of virus suspension (approximately 108 PFU/ml) was injected into the vitreous humor of the left eye of an anesthetized animal. When symptoms of infection were overt, the animals were sacrificed and exsanguinated, and the brains were removed as described previously (14). Immunohistochemical analysis of coronal brain slices with a rabbit polyclonal antiserum to whole PRV virus (Rb133) has been described previously (14). Tissues were taken for analysis just prior to the estimated time to death.
RESULTS
PRV 25 gE gene contains an additional mutation.
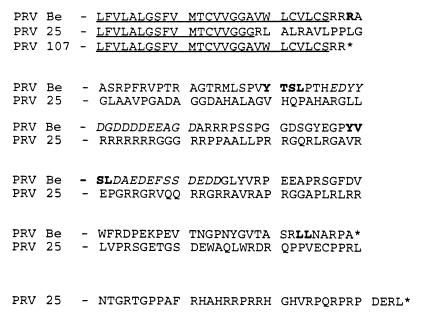
PRV 25 is a previously characterized mutant virus encoding a gE protein designed to contain a stop codon at codon 457 just after the amino acids encoding the gE transmembrane domain (44). Insertion of this stop codon was proposed to create a truncated gE mutant protein that would be anchored in membranes and would not express the cytoplasmic tail of the protein. While conducting further experiments with the plasmid used to make PRV 25 (pRT25), we discovered that this plasmid contained an additional mutation of an extra guanine residue inserted into a string of seven other guanine residues just 33 bases upstream of the engineered stop codon mutation. To confirm the presence of this mutation in PRV 25 viral DNA, the BamHI-7 fragment was cloned from PRV 25 viral DNA, resulting in plasmid pRS26, and portions of the gE gene were sequenced from this plasmid. The extra guanine residue and the engineered stop codon were both confirmed in the viral DNA. The insertion of the extra guanine residue in the gE gene results in a shift of the reading frame in the last portion of the region encoding the gE transmembrane domain during translation of the protein. The resulting frameshifted protein would not terminate at the engineered stop codon or at the natural gE stop codon but would be predicted to continue translating into the downstream sequences encoding the Us9 gene. This would create a novel gE cytoplasmic tail consisting of 167 amino acids as depicted in Fig. 1. A gE-Us9 translational fusion would not be produced, however, as the reading frame would be different from the reading frame of Us9. To test the effects that this extra mutation had on the previous results obtained with PRV 25, we constructed PRV 107, which contains the desired stop codon mutation at amino acid 457, and compared this virus to PRV 25. The mutation in PRV 25 will be referred to as frameshift mutation, and that in PRV 107 will be referred to as Am457.
FIG. 1.
Amino acid comparison of gE proteins. The amino acid sequences of the transmembrane domains and cytoplasmic tails of gE proteins encoded by PRV Be (wild-type), PRV 25 (frameshift), and PRV 107 (Am457) are shown. The transmembrane domain of each protein is underlined. Potential internalization motifs, either tyrosine or dileucine based, are in shown in bold in the wild-type sequence. The two acidic amino acid clusters are shown in italics. An asterisk indicates a stop codon in translation of the protein. PRV 25 contains a shift in reading frame predicted to result in a truncated transmembrane domain and a novel cytoplasmic tail. PRV 107 contains an amber stop codon in place of an arginine residue just after the transmembrane domain and encodes a truncated, anchored gE protein.
Construction of mutant viruses.
At least two types of internalization motifs have been defined in the cytoplasmic tails of cell surface receptors that physically interact with the cellular machinery involved in endocytosis: tyrosine-based motifs and dileucine motifs (21, 45, 46). In addition, stretches of acidic amino acids containing phosphorylated residues have also been implicated in internalization of proteins (21). The PRV gE tail contains two potential tyrosine-based internalization motifs, a single dileucine motif, and two stretches of acidic amino acids, one containing a predicted casein kinase II phosphorylation site as depicted in Fig. 1. Most gE homologs in the alphaherpesvirus family also contain at least one potential tyrosine-based motif. The PRV gE protein is the only homolog containing a traditional potential dileucine-based internalization motif, while the bovine herpesvirus 1, equine herpesvirus 4, and FHV gE homologs contain IL motifs that could lead to endocytosis of the proteins (3, 7, 11, 15, 23, 26, 42, 48). In addition, the stretch of acidic residues is also not completely conserved among homologs. Furthermore, VZV gE protein internalization was inhibited by a single mutation in its tyrosine-based internalization motif. We therefore chose to mutate the tyrosine motifs in PRV gE by changing the tyrosine residues to serine residues. Three viral mutants were constructed; PRV 104 contains a serine codon in place of the tyrosine at codon 478 (Y478S), PRV 105 contains serine codons in place of both tyrosine 478 and tyrosine 517 (Y478S + Y517S), and PRV 106 contains a serine codon in place of tyrosine 517 (Y517S). As PRV 107 also encodes a gE protein defective in endocytosis (see below), we describe its characterization along with these mutants.
As described in detail in Materials and Methods, site-directed mutagenesis was used to create all desired mutations in the gE gene. All resulting plasmids were sequenced to confirm the presence of only the desired mutations. Recombinant virus was made by cotransfection of PRV 91 viral DNA (gE null virus) and plasmids containing the gE gene with the desired mutations. Recombinant viruses were identified by screening for the expression of gE protein, using the black plaque test. All recombinant viruses were purified through four rounds of single-plaque purification. Southern blot analysis was performed on DNA isolated from recombinant virus to confirm the presence of the desired mutations. Revertant virus was made as described above by cotransfecting PRV 107 viral DNA with wild-type restriction fragment of the gE gene. The resulting viruses are described in Table 1.
Expression of gE proteins.
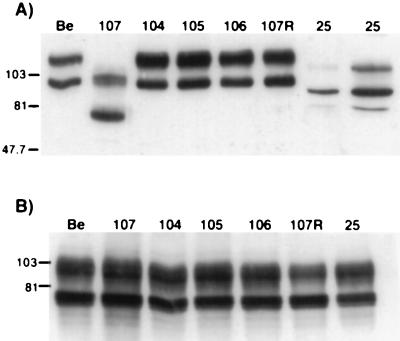
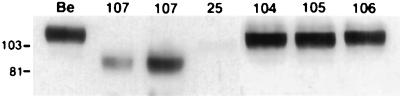
gE proteins were analyzed by Western blot analysis on extracts isolated from infected PK15 cells. Wild-type immature gE protein present in Be-infected cells had a molecular mass of approximately 93 kDa and was further glycosylated to form the 110-kDa mature species, as shown in Fig. 2A. PRV 25 (frameshift) encoded a gE precursor form of approximately 80 kDa and a mature form of approximately 101 kDa as reported previously (44). As noted earlier, the protein was expressed less abundantly than wild-type gE, and six times more total protein from the PRV 25 cell extract was loaded to detect a comparable amount of gE protein. The gE protein made after infection with PRV 107 (Am457) migrated faster than the protein made by PRV 25. The immature form had a molecular mass of approximately 70 kDa and a mature form of 93 kDa. Expression of this protein was similar to expression of wild-type protein. The gE proteins produced by PRV 104 (Y478S), PRV 105 (Y478S + Y517S), PRV 106 (Y517S), and PRV 107R migrated identically to wild-type gE. Equal amounts of protein from each cell extract were also analyzed for gC expression in Fig. 2B for comparison. Identical amounts of both precursor and mature forms of gC were made after infection with each virus. Immunoprecipitations of the proteins showed the same results (data not shown). In addition, we observed that all of the proteins retained the ability to complex with gI (data not shown).
FIG. 2.
Expression of gE proteins. PK15 cells were infected at an MOI of 10 with either PRV Be (wild type), PRV 107 (Am457), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), PRV 106 (Y517S), PRV 107R (revertant PRV 107), or PRV 25 (frameshift) for 16 h prior to preparation of cell lysates. Western blot analysis was performed with either rabbit polyclonal antiserum to gE (A) or goat polyclonal antiserum to gC (B). In lanes 1 to 7 of panel A, 10 μg of total protein was loaded in each lane; 60 μg of total protein was loaded in lane 8. Immature wild-type gE protein has a molecular mass of approximately 93 kDa and a mature form of approximately 110 kDa. Cells infected with PRV 107 contain immature and mature forms of approximately 70 and 93 kDa, respectively. The gE protein produced by PRV 25 has an immature form of 80 kDa and a mature form of 101 kDa. In panel B, 10 μg of total protein was analyzed for each sample. Positions of apparent molecular mass markers are shown on the left in kilodaltons.
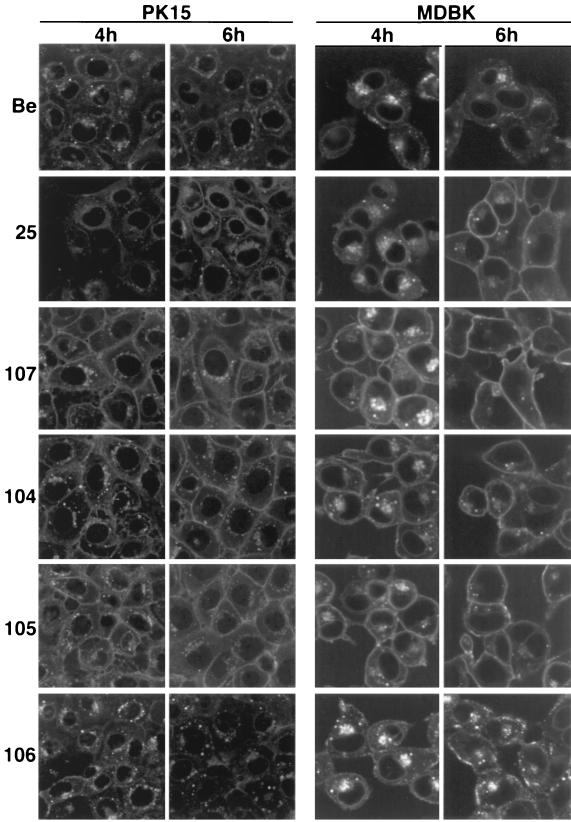
Steady-state localization of gE proteins.
To determine the steady-state localization of the gE proteins produced by PRV mutants in infected cells, indirect immunofluorescence was performed with an antibody that specifically recognized gE complexed with gI (MAb 1/14). This antibody recognizes gE protein found in the endoplasmic reticulum (ER), in the Golgi apparatus, and in the plasma membrane of cells. Infected PK15 or MDBK cells were fixed and permeabilized at either 4 or 6 h postinfection prior to processing. Figure 3 shows infected PK15 cells and infected MDBK cells. In both cell lines, at 4 h postinfection, wild-type (PRV Be) gE-gI complex localized to a perinuclear region reminiscent of the ER and the Golgi apparatus and could also be seen in cytoplasmic vesicles. The complex was barely detectable on the plasma membrane of permeabilized PK15 cells, while almost no gE protein was seen on the plasma membrane of permeabilized MDBK cells. At 6 h postinfection, wild-type gE-gI complex was observed in the interior regions of the cell as noticed above but in greater abundance. In addition, noticeably more of the complex could be detected on the plasma membrane of PK15 cells, while the complex remained virtually undetectable on the surface of MDBK cells. The gE-gI complex made after PRV 25 infection localized predominantly to a perinuclear region at both 4 and 6 h postinfection in both cell lines, as reported previously (44). However, the complex could also be detected in greater abundance on the cell surface of both cell types, especially at 6 h postinfection. The gE-gI complexes formed after infection with PRV 107 (Am457), PRV 104 (Y478S), and PRV 105 (Y478S + Y517S) were localized similarly to wild-type gE-gI complex except considerably more complex accumulated on the plasma membrane of both types of infected cells at 4 and 6 h postinfection. The gE-gI complex formed by PRV 106 (Y517S) infection was also localized to similar structures intracellularly but was difficult to detect the complex on the plasma membrane of cells even at 6 h postinfection. These results indicated that a gE protein lacking the cytoplasmic tail (Am457) or gE proteins containing a single point mutation at tyrosine 478 or a double mutation at tyrosine residues 478 and 517 resulted in a marked localization of the gE-gI complex to the plasma membrane of infected cells. Mutation of tyrosine 517 alone had no effect on localization of the gE-gI complex. Results were similar for the two cell types but more dramatic for the MDBK cells.
FIG. 3.
Steady-state distribution of the gE-gI complex. PK15 and MDBK cells were infected at an MOI of 10 for the times indicated with either PRV Be (wild type), PRV 25 (frameshift), PRV 107 (Am457), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), or PRV 106 (Y517S). The cells were fixed, permeabilized, and reacted with a MAb that specifically recognized gE when it was complexed with gI (MAb 1/14). An Alexa-568-conjugated secondary antibody was used to visualize bound MAb. Confocal sections were taken through the centers of the cells.
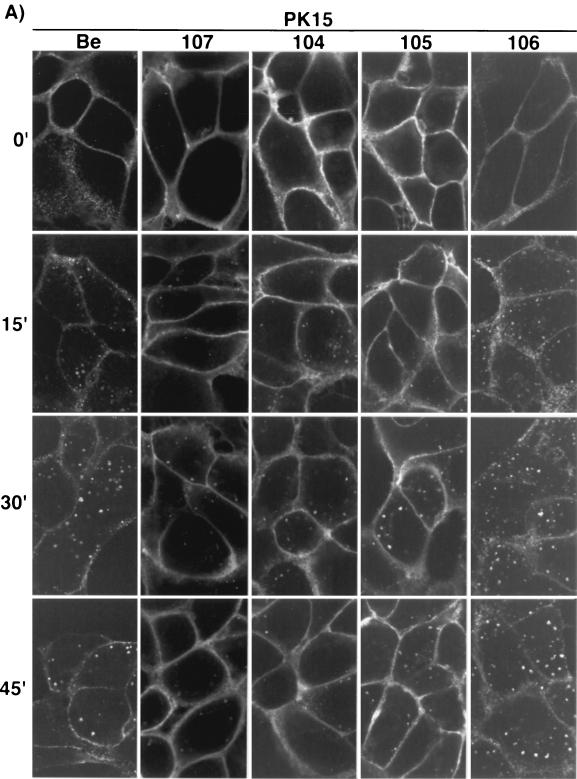
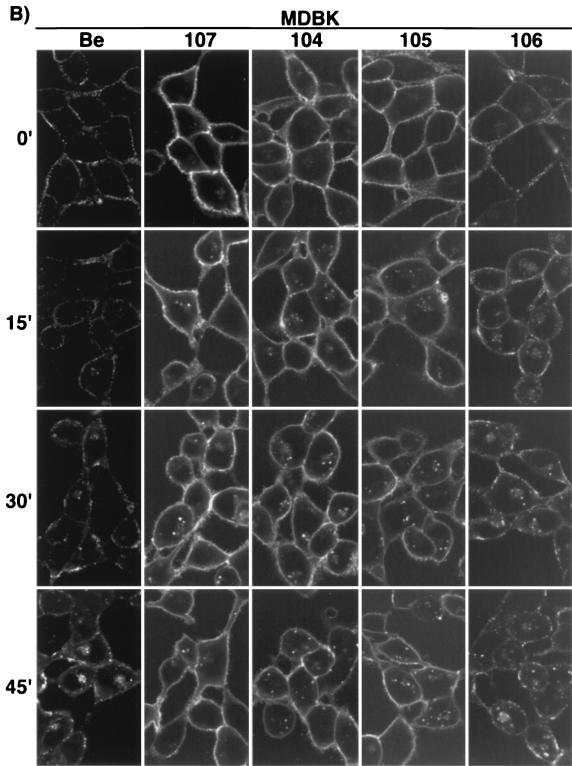
Endocytosis of gE proteins.
To assess the ability of the gE proteins to internalize from the plasma membrane of infected cells, indirect immunofluorescence endocytosis assays using MAb 1/14 were performed with either PK15 (Fig. 4A) or MDBK cells (Fig. 4B) at 4 h postinfection as previously described (43). As shown in Fig. 4A, wild-type gE-gI complex (PRV Be) was seen on the plasma membrane of infected PK15 cells when the cells were not shifted to 37°C. However, after a temperature shift for the indicated times, the complex accumulated in the interior of the cells in cytoplasmic vesicles which became larger and more numerous with increased incubation at 37°C. The internalized vesicles remained scattered throughout the cytosol of the cells. The gE-gI complex formed after infection with PRV 107 was also observed on the plasma membrane of PK15 cells at time zero. However, the complex remained on the cell surface and did not accumulate appreciably in the interior of the cells when the cells were shifted to 37°C, as previously reported for PRV 25 (44). Both PRV 104 (Y478S) and PRV 105 (Y478S + Y517S) gave similar results in the endocytosis assay as PRV 107. The gE-gI complexes formed by both viruses could be found on the plasma membrane of infected cells when the cells were not shifted in temperature (time zero). After a shift to 37°C, some vesicles could be found in the interior of the cells; however, much less of the complex accumulated intracellularly compared to wild-type gE-gI. The plasma membrane of the cells infected with PRV 104 and PRV 105 remained brightly stained even after the cells were shifted to 37°C for up to 45 min. More internalized vesicles were repeatedly seen in PRV 104- or PRV 105-infected PK15 cells than in cells infected with PRV 107. The amount of internalized protein, however, appeared to be much less than that internalized in PRV Be-infected cells. The gE-gI complex formed after infection with PRV 106 (Y517S), was internalized similarly to wild-type gE-gI complex in this assay. Endocytosis assays performed with cells infected with PRV 107R (revertant of PRV 107) yielded data identical to those for cells infected with wild-type virus (data not shown).
FIG. 4.
Endocytosis of the gE mutant-gI complex. PK15 (A) and MDBK (B) cells were infected at an MOI of 10 with either PRV Be (wild type), PRV 107 (Am457), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), or PRV 106 (Y517S) for 4 h prior to an indirect immunofluorescence endocytosis assay as described in Materials and Methods. Briefly, the cells were incubated at 4°C with MAb 1/14, which specifically recognized gE when it was complexed with gI prior to a shift of the cells to 37°C for the indicated times to allow internalization of the protein complex. The cells were then fixed, permeabilized, and reacted with an Alexa-568-conjugated secondary antibody to visualize the bound primary antibody. Confocal sections were taken through the centers of the cells.
The results of endocytosis assays performed in MDBK cells are shown in Fig. 4B. The results are similar to the results obtained for PK15 cells, with a few notable differences. Wild-type gE-gI complex (PRV Be) was difficult to detect on the plasma membrane of the cells, as was also seen with steady-state localization of the complex. The gE-gI complex on the cell surface appeared to be in patches along the membrane. The lack of gE-gI complex on the cell surface of MDBK cells made it difficult to detect internalized protein when it was scattered throughout the cytosol of the cell. In addition, the internalized vesicles in MDBK cells were smaller than those seen in PK15 cells. The complex, however, was easier to detect after 30 to 45 min of temperature shift, when it appeared to accumulate in a region next to the nucleus of the cell. The gE-gI complexes formed after infection with PRV 107, PRV 104, and PRV 105 were virtually indistinguishable from one another and were readily detected on the plasma membrane of the cells. Even after a shift in temperature, the cell surfaces remained brightly stained. As in PK15 cells, some internalized gE-gI complex was seen in infections with all three viral mutants. The localization of the gE-gI complex after PRV 106 infection was similar to wild-type gE-gI complex and was difficult to detect on the cell surface. The complex internalized similarly to wild-type complex.
Taken together, these results suggest that the cytoplasmic tail of gE was required for endocytosis of the protein and that the first tyrosine residue (Y478) was critical for efficient internalization of the protein. Mutation of this residue alone or in combination with tyrosine 517 inhibited endocytosis of the protein. gE protein containing a mutation in only the second tyrosine residue (Y517) internalized as efficiently as wild-type gE-gI. Therefore, PRV 107, PRV 104, and PRV 105 but not PRV 106 encode gE proteins defective in endocytosis.
Incorporation of gE proteins into viral particles.
The gE protein produced by PRV 25 had been previously reported not to be incorporated into viral particles (44). As this protein was also defective in endocytosis (43), there was a direct correlation between the ability of the gE protein to internalize and its incorporation into the viral envelope. We tested the ability of the gE proteins made by PRV 107 (Am457), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), and PRV 106 (Y517S) to be targeted to the virion. Western blot analysis was performed on virion extracts as depicted in Fig. 5. Only the mature gE protein could be seen in virions isolated from PRV Be-infected cells. Mature gE protein from PRV 107-infected cells was also detected in viral particles; however, the protein was approximately twofold less abundant than gE protein in virions isolated from cells infected with wild-type virus. As previously reported, no gE protein could be detected in virions isolated from PRV 25-infected cells even when six times more total protein was loaded on the gel (44). Virions isolated from PRV 104-, PRV 105-, or PRV 106-infected cells contained identical amounts of mature gE protein as virions isolated from PRV Be-infected cells. Although PRV 25 gE protein was not targeted to the virion, the protein produced by PRV 107 was found in viral particles. In addition, gE protein containing mutations in the first (Y478), second (Y517), or both tyrosine motifs was efficiently incorporated into viral particles. We conclude that gE proteins markedly defective in endocytosis are efficiently incorporated into viral particles, showing that there is no correlation between the ability of a protein to internalize and its targeting to the viral envelope.
FIG. 5.
Incorporation of gE proteins into virions. PK15 cells were infected at an MOI of 10 with either PRV Be (wild type), PRV 107 (Am457), PRV 25 (frameshift), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), or PRV 106 (Y517S) for 14 h. Medium was removed from the cells, and released virions were isolated by centrifugation through 30% sucrose. Western blot analysis was performed with a rabbit polyclonal antiserum to gE. The same total volume of extract was loaded in each lane except for lane 3, in which twice as much total protein was analyzed. Positions of apparent molecular mass markers are indicated in kilodaltons on the left.
Plaque size on MDBK cells.
Viruses that lack gE form small plaques on MDBK cells (19, 51). In addition, PRV 25 also forms small plaques on these cells (44). We analyzed plaques formed by the viral mutants on MDBK cells at 72 h postinfection. Plaques formed by wild-type virus had diameters of an average of 1.2 mm (Table 2). Plaques formed by PRV 91 (gE null) were significantly smaller, with diameters of approximately 0.83 mm (69% of the wild-type diameter). We observed that the plaques resulting from infection with PRV 107 (Am457) or PRV 25 (frameshift) were identical to PRV 91 (gE null) plaques in size. We also noted that PRV 104 (Y478S) and PRV 105 (Y478S + Y517S) consistently made plaques that were slightly smaller than plaques formed by wild-type virus measuring approximately 1.11 and 1.02 mm, or 93 and 85%, respectively of the wild-type diameter. However, there was a statistical difference between the plaques formed by either of these viruses and those formed by PRV Be or PRV 91. PRV 106 (Y517S) and PRV 107R made plaques indistinguishable in size from plaques formed by the wild-type virus (PRV Be). Thus, viruses defective in gE endocytosis made small (PRV 25 and PRV 107) or intermediate-size (PRV 104 and PRV 105) plaques, while virus that was not defective in gE endocytosis (PRV 106) formed wild-type-size plaques.
TABLE 2.
Plaque size on MDBK cells
| Virus | Size (mm)a | % Wild type |
Pb with respect to:
|
|
|---|---|---|---|---|
| PRV Be | PRV 91 | |||
| PRV Be | 1.20 ± 0.11 | 100 | ND | <0.005 |
| PRV 91 | 0.83 ± 0.06 | 69 | <0.005 | ND |
| PRV 25 | 0.80 ± 0.07 | 67 | <0.005 | 0.04 |
| PRV 104 | 1.11 ± 0.06 | 93 | <0.005 | <0.005 |
| PRV 105 | 1.02 ± 0.08 | 85 | <0.005 | <0.005 |
| PRV 106 | 1.24 ± 0.07 | 103 | 0.051 | <0.005 |
| PRV 107 | 0.82 ± 0.05 | 68 | <0.005 | 0.278 |
| PRV 107R | 1.24 ± 0.06 | 103 | 0.077 | <0.005 |
Plaque size averages were taken from 40 isolated plaques measured from two independent platings of virus.
Determined by a Student’s t test. Two numbers were considered statistically different if P was <0.005. ND, not determined.
Spread in the rat CNS.
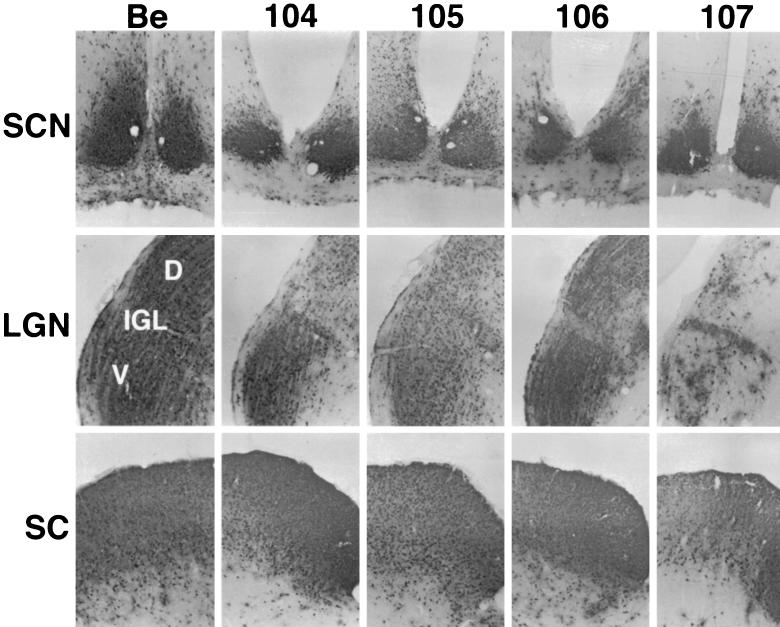
Following intraocular infection with wild-type virus, viral antigen is detected in the visual centers, including the lateral geniculate complex (LGN; dorsal and ventral aspects) and the superior colliculus (SC), as well as the circadian rhythm centers such as the suprachiasmatic nucleus (SCN) and the intergeniculate leaflet (IGL) (10, 47). Infection of the SC and the LGN is dependent on expression of both gE and gI. Viruses lacking one or both of these genes do not spread to these areas but retain the ability to spread to the circadian rhythm centers (47). We determined the ability of the various mutant viruses to infect these areas after intraocular infection to test the role of gE endocytosis in gE-promoted spread of virus. As shown in Fig. 6, wild-type virus (PRV Be) established a robust infection in all of the areas observed: SCN, LGN, IGL, and SC. PRV 104 (Y478S), PRV 105 (Y478S + Y517S), and PRV 106 (Y517S) were identical to wild-type virus; robust staining for viral antigen was observed throughout all of the regions. As described for PRV 25, PRV 107 (Am457) showed strong staining in the SCN, the IGL, and the SC (44). The virus was able to infect all areas of the LGN; however, the dorsal and ventral regions showed consistently less staining than was seen in sections taken from animals infected with wild-type virus. This is different from a gE null virus, which does not show any staining in the dorsal or ventral aspects of the LGN or the SC (47). This finding supports the result obtained with PRV 25 that the N terminus of gE is sufficient for gE-mediated spread to the LGN and the SC. In addition, viruses that are defective in gE endocytosis (PRV 104 and PRV 105) infected all retinorecipient areas as efficiently as wild-type virus. We conclude that efficient endocytosis of gE is not required for gE-promoted spread to rat visual centers upon intraocular infection.
FIG. 6.
Localization of viral antigen in brain sections. The brains from animals taken from infections described in the legend to Fig. 7 were removed and analyzed for viral antigen with a polyvalent rabbit antiserum generated against whole virus particles (Rb133). Serial sections (35 μm) through the coronal plane were cut, processed and mounted on slides. Representative sections containing the SCN, LGN including the dorsal (D) and ventral (V) aspects as well as the IGL, and the SC are pictured.
Virulence of viral mutants.
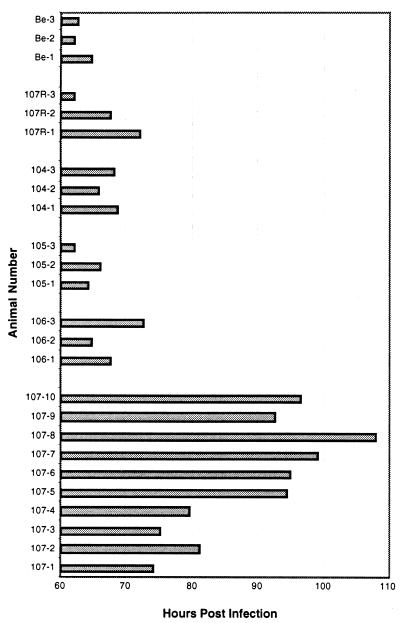
Virulence of PRV can be assessed by determining the mean time to symptoms of imminent death of infected animals. Animals infected with wild-type virus have a mean time to symptoms of imminent death of 72 h postinfection (8). PRV 91 (gE null) and PRV 25 (frameshift) are considerably less virulent, causing death at 110 and 106 h postinfection, respectively (44). This finding suggests that the cytoplasmic tail of gE is required for gE-mediated virulence. Since endocytosis of gE was also directed by the cytoplasmic tail of gE, we tested whether the endocytosis mutants had an effect on virulence of the virus by infecting rats intraocularly and observing them for symptoms. Infections were performed with wild-type virus (PRV Be) and PRV 107R as controls. The mean times to symptoms of imminent death for all of the viruses tested are shown in Table 3. PRV 104 (Y478S), PRV 105 (Y478S + Y517S), and PRV 106 (Y517S) were indistinguishable from PRV Be (63.1 h) and PRV 107R (67.2 h) in this assay, with mean times to death of 67.4, 64.0, 68.2, and 66.0 h postinfection, respectively. There was no statistical difference between PRV Be and each of these viruses. Animals infected with each individual virus all showed signs of death within a close time span to one another, as indicated by the standard deviations. In addition, animals infected with all of these viruses showed severe symptoms of infection typical of a wild-type virus. The mean time to signs of death for PRV 107 (Am457) was approximately 89.5 h. This is statistically different from infections with PRV Be. There was a high degree of variability in the time to symptoms of imminent death for each individual animal infected with PRV 107, as shown in Fig. 7, with animals dying from 74 up to 108 h postinfection. Animals infected with PRV 107 showed markedly less severe symptoms than those infected with wild-type virus. The results obtained with PRV 107 support the conclusion drawn from infections with PRV 25, indicating that the C-terminal cytoplasmic tail of gE is required for gE-mediated virulence. In addition, we conclude that efficient endocytosis of gE does not play a role in virulence promoted by gE, as the mutants defective in gE endocytosis (PRV 104 and PRV 105) were as virulent as wild-type virus. This observation suggests that other functions of gE encoded by the gE tail are responsible for gE-mediated virulence.
TABLE 3.
Mean time to symptoms
| Virus | No. of animals | Mean time (h) to symptoms ± SD | Pa |
|---|---|---|---|
| PRV Be (wild type) | 3 | 63.1 ± 1.5 | NDb |
| PRV 104 (Y478S) | 3 | 67.4 ± 1.5 | 0.022 |
| PRV 105 (Y478S + Y517S) | 3 | 64.0 ± 2.0 | 0.557 |
| PRV 106 (Y517S) | 3 | 68.2 ± 4.01 | 0.110 |
| PRV 107 (Am457) | 10 | 89.5 ± 11.4 | <0.005 |
| PRV 107R (wild type) | 3 | 67.2 ± 5.0 | 0.247 |
P with respect to PRV Be determined by Student’s t test. Two averages were considered significantly different if P was <0.005.
ND, not determined.
FIG. 7.
Distribution of time to symptoms for each infected animal. Animals were infected with either PRV Be (wild type), PRV 104 (Y478S), PRV 105 (Y478S + Y517S), PRV 106 (Y517S), or PRV 107 (Am457) by intraocular injection as described in Materials and Methods. The time at which signs of imminent death were evident for each animal was noted before the animals were sacrificed.
DISCUSSION
These studies were initiated to test the role of gE internalization in known gE functions: plaque size in cultured cells and spread and virulence of PRV during infections of the rat retina. To do so, we created defined mutations in the sequences encoding predicted endocytosis motifs in the gE tail. We predicted that these gE proteins would remain on the cell surface and not be internalized. When we began this work, we discovered that another mutant virus previously characterized by us contained an additional, undescribed mutation (44). This virus, PRV 25, was designed to encode a truncated gE protein by the addition of an engineered stop codon just after the sequences encoding the transmembrane domain of the protein. Due to an unintentional addition of a 1-bp insertion upstream of the engineered stop codon, the gE protein encoded by PRV 25 instead encodes a wild-type gE ectodomain but a novel cytoplasmic tail resulting from a frameshift mutation. This new tail does not contain potential endocytosis motifs, and the protein was not internalized from the plasma membrane of infected cells (43). To clarify the results obtained with PRV 25, we also made another virus, PRV 107, that does encode a truncated, anchored gE protein. This protein was also inhibited in endocytosis but was incorporated into viral particles. To define the role of endocytosis of gE in other gE functions, we characterized PRV 107 along with other viruses encoding gE endocytosis mutants. The cytoplasmic tail of gE was required for endocytosis of the protein. Specifically, tyrosine 478 of the gE cytoplasmic tail was critical in mediating internalization of the protein. Finally, we observed that efficient endocytosis of gE played no role in targeting the protein to the viral envelope, gE-promoted spread in the rat CNS, or gE-mediated virulence. The only phenotype associated with mutants defective in gE internalization was a small plaque size defect in MDBK cells.
The discovery of an additional mutation in the previously described PRV 25 gE gene that led to a frameshift in translation of the protein was surprising. The novel cytoplasmic tail encoded by PRV 25 is predicted to be 167 amino acids long, containing no homology with the cytoplasmic tail encoded by wild-type virus (PRV Be). One would predict that the gE protein made by PRV 25 would migrate slower in a sodium dodecyl sulfate-polyacrylamide gel than PRV Be gE protein. However, immunoprecipitations and Western blot analyses showed that PRV 25 gE migrates faster than wild-type gE protein (44). In concordance with PRV 25 encoding a novel gE cytoplasmic tail, however, is the fact that the gE protein made by PRV 107 migrated faster than PRV 25 gE. The reasons for the anomalous apparent molecular mass of PRV 25 gE remain to be explored. One explanation is that the protein is more ER associated than wild-type gE protein, as evidenced by immunofluorescence. Consequently, the protein may receive inappropriate modifications as a result of being retained in the ER. The native gE cytoplasmic domain contains a DXE signal which has been shown to be an ER export signal (31). The gE protein encoded by PRV 25 does not contain this signal. Nevertheless, the results obtained with PRV 25 in previous studies were informative in that the premise being tested was the importance of the gE tail in gE functions. Although PRV 25 does not express the expected gE protein, the protein made does lack the wild-type gE tail. We have now corroborated these results by constructing PRV 107, which has all of the phenotypes of PRV 25 except gE incorporation into viral particles. Our previous conclusion based on observations made with PRV 25 that the gE tail was required for incorporation of the protein into the virion envelope was therefore incorrect. The protein produced by PRV 25 may not be incorporated into viral membranes due to an additional role played by the novel cytoplasmic tail. Perhaps the novel tail of PRV 25 prevents the protein from being targeted to the site of envelopement or disrupts protein-protein interactions required for incorporation of proteins into the viral envelope.
PRV 107 encodes a gE protein defective in endocytosis, supporting the idea that the cytoplasmic tail of gE is required for internalization of the protein. Both PRV 104 (Y478S) and PRV 105 (Y478S + Y517S) encode gE proteins that are also defective in endocytosis, although there appears to be a greater amount of the gE-gI complex internalized than observed with PRV 107. PRV 106 (Y517S), however, produces a gE protein that is not defective in endocytosis. Thus, we have defined a single amino acid in the gE cytoplasmic tail, tyrosine residue 478, in the membrane-proximal tyrosine motif, that is critical for endocytosis of the protein. Interestingly, the human immunodeficiency virus Env protein also contains two potential tyrosine-based internalization motifs. As for PRV gE, only the membrane-proximal motif mediates internalization of the protein (6, 40). Recent analysis of the crystal structure of the epidermal growth factor receptor or TGN38 tyrosine-based internalization signals complexed with the μ2 subunit of the AP2 adaptor complex has shown that these internalization signals need to be in an extended β-strand conformation to interact with the internalization machinery (36). Perhaps only the membrane-proximal tyrosine motif in the gE tail is in this conformation.
Internalization of gE was not completely inhibited by our mutants, which makes it difficult to determine if the small amount of internalized complex has a function. PRV 107 lacks the gE cytoplasmic tail and presumably is not able to interact with proteins required for internalization of cell surface receptors, yet a few vesicles containing the gE-gI complex still accumulate in the interior of the cell. Olson and Grose (32) have shown that the VZV gI protein can increase internalization of VZV gE when the two proteins are coexpressed. Internalization of VZV gI is directed by a dileucine-type motif in the gI cytoplasmic tail. The anchored gE protein made by PRV 107 does interact with gI, presumably through the N-terminal portion of the protein (data not shown). Although PRV gI contains no previously identified internalization motif, perhaps complex formation facilitates endocytosis of the anchored gE, leading to the residual amount of endocytosis seen with this mutant. In addition, although endocytosis of the gE-gI complex was significantly reduced in PRV 104- or PRV 105-infected cells, more of the complex internalized in these cells than in cells infected with PRV 107, suggesting that other sequences in the cytoplasmic tail were increasing internalization of the protein. As noted earlier, the gE cytoplasmic tail also contains a single dileucine motif and an acidic amino acid patch containing residues that could be phosphorylated. Both of these motifs have been implicated in intracellular targeting of internalized proteins. These motifs may not function as efficiently as the tyrosine-based motif but could still direct endocytosis of the proteins. Alternatively, complex formation with gI could expose other internalization motifs to the cellular endocytosis machinery. Structural analysis has suggested that dimer formation of receptors could strengthen and give specificity to interactions of receptors with the endocytosis machinery (36). We are currently testing the role of these motifs and the role of gI in endocytosis of the gE protein.
In this report, we showed the effects of mutations of the tyrosine residues in endocytosis of the gE-gI complex in both PK15 and MDBK cells. The viral mutants behaved similarly in both cell types, but the effect was more dramatic in MDBK cells. In concordance with lack of internalization of the complex and its subsequent stable expression on the cell surface, the gE-gI complex was localized predominantly on the cell surface when endocytosis of the complex was inhibited. In MDBK cells, wild-type gE-gI complex was almost undetectable on the cell surface, but the proteins defective in endocytosis showed extremely bright cell surface staining. Presumably, endocytosis of the gE-gI complex was efficient in MDBK cells, leading to a lack of the complex on the cell surface. When this retrieval was disrupted, the complex was easily detected on the plasma membrane. Surprisingly, internalization of the complex in MDBK cells was sufficiently efficient to prevent detection of gE-gI on the surface even at 6 h postinfection. As in PK15 cells, endocytosis of the gE-gI complex is also inhibited at 6 h postinfection in MDBK cells, and small amounts of the complex accumulated in the plasma membrane of cells at 8 h postinfection (data not shown). The amount of gE-gI complex on the cell surface appears to have been determined by the amount of the protein that was internalized and by the cell type infected. We are interested in determining the amount of endocytosis that may be occurring in infected neurons in the rat CNS.
Viruses that encoded gE internalization mutants had one notable phenotype: they consistently formed smaller plaques on MDBK cells. It is not clear how endocytosis may be affecting plaque size, but it is of interest that the amount of gE-gI complex on the cell surface of infected MDBK cells is much less than the amount on infected PK15 cells. Inhibition of endocytosis has a more dramatic effect on the distribution of the complex in MDBK cells. Perhaps the concentration of the gE-gI complex on the cell surface is important in directing cell-to-cell spread in tissue culture cells. A change in the amount of the complex on the cell surface could disrupt cell-to-cell spread directed by gE and result in an altered plaque size in MDBK cells.
We tested the role of efficient gE endocytosis in incorporation of the protein into viral particles. We found that all of the endocytosis-defective gE proteins were incorporated. Internalization of glycoproteins to the TGN has been proposed as a mechanism for targeting of the proteins to the site of viral envelopment to ensure incorporation of the proteins into viral particles (17, 49, 50). Clearly, internalization of gE and gI is not required for incorporation of the proteins into particles. However, this does not preclude envelopment of capsids at the TGN or endosomal compartments. Several experiments have shown that VZV gE and gI proteins are targeted to the TGN after internalization of the proteins from the plasma membrane (1, 2, 49, 50). The TGN targeting signal however, could function both after internalization of the complex and in conducting newly synthesized complex to the same intracellular localization. The anchored gE protein produced by PRV 107 is lacking all targeting motifs that may be found in the cytoplasmic domain of the protein; however, newly synthesized protein could still be targeted to the proper intracellular location through its interaction with gI. In addition, the gE-gI complex made by PRV 104 and PRV 105 retains all targeting motifs found in the gE and gI tails with the exception of the internalization motifs. Newly synthesized complex made by these mutants could also be properly localized intracellularly through the action of both the gE and gI cytoplasmic tails. Our data suggest that newly synthesized, rather than retrieved, molecules are incorporated into viral particles. Taken together, the newly synthesized molecules made by these endocytosis mutants could still retain the ability to be properly targeted; however, any protein that is delivered to the plasma membrane would be improperly retained at the cell surface. In this manner, the internalization defective gE-gI complex could still be targeted to the viral envelope.
We also tested the role of efficient gE endocytosis in gE-mediated spread in the rat CNS and in gE-promoted virulence. We found that all viral mutants were able to spread to areas of the rat brain dependent on gE protein for infection. We also found that while a complete lack of the gE tail led to a decreased virulence of the virus, a single mutation that significantly decreased endocytosis of the protein had no effect on virulence. This finding suggests that the decreased virulence of PRV 107 and PRV 25 is not dependent on tyrosine 478 or the efficiency of gE internalization. Therefore, other functions that promote virulence must be encoded by the gE tail. As found with PRV 25, PRV 107 is able to spread in the rat CNS but is still less virulent than wild-type virus (44). This observation supports our hypothesis that the gE tail encodes virulence functions. The gE tail is phosphorylated, presumably on serine residues, and phosphorylation may be a signal that facilitates gE-promoted virulence (12). Perhaps phosphorylation of the gE cytoplasmic tail leads to a signal cascade that activates cellular factors that are detrimental to the health of the host. We are in the process of testing this idea.
In our assays, we can find no obvious role for endocytosis of gE. Endocytosis occurs only during the first 6 h of infection in PK15 and MDBK cells, indicating that endocytosis of the complex must have a role in earlier events of the viral life cycle. One potential role for early endocytosis of gE and gI may occur when the virus uncoats upon entry. Perhaps endocytosis occurs as glycoproteins laterally diffuse into the plasma membrane of cells during fusion of the particle, as shown by Granzow et al. (18). Internalization of the proteins may help pull the viral particle apart and allow efficient release of the tegument and nucleocapsid. Endocytosis may then only serve to increase the efficiency of infection in certain cell types, a phenotype that may not be observed during a high MOI. This hypothesis is being tested by determining the rate of penetration of gE endocytosis mutants compared to wild-type virus. In addition, endocytosis of gE may lead to transcytosis of the protein in some cells types (e.g., polarized cell systems). This could be important at the primary site of infection at mucosal membranes. This possibility is not tested using the intraocular infections of rats used in our studies. Further experiments are necessary to test gE internalization in these functions.
ACKNOWLEDGMENTS
We thank Joe Goodhouse for help and advice with the confocal images. We also thank K. Bienkowska-Szewcyzk and T. Ben-Porat for gE antisera. Many thanks go to members of the Enquist lab for support and critical reading of the manuscript. R.S.T. also sincerely acknowledges P. Husak and G. Smith for invaluable help.
This work was supported by NINDS grant to 1RO133506 to L.W.E. and NIH grant 5T32GM07388 to R.S.T.
REFERENCES
- 1.Alconada A, Bauer U, Baudoux L, Piette J, Hoflack B. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J Biol Chem. 1998;273:13430–13436. doi: 10.1074/jbc.273.22.13430. [DOI] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Audonnet J C, Winslow J, Allen G, Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990;71:2969–2978. doi: 10.1099/0022-1317-71-12-2969. [DOI] [PubMed] [Google Scholar]
- 4.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 5.Banfield B W, Yap G S, Knapp A C, Enquist L W. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J Virol. 1998;72:4580–4588. doi: 10.1128/jvi.72.6.4580-4588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boge M, Wyss S, Bonifacino J S, Thali M. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J Biol Chem. 1998;273:15773–15778. doi: 10.1074/jbc.273.25.15773. [DOI] [PubMed] [Google Scholar]
- 7.Brunovskis P, Velicer L F. The Marek’s disease virus (MDV) unique short region: alphaherpesvirus-homologous, fowlpox virus-homologous, and MDV-specific genes. Virology. 1995;206:324–338. doi: 10.1016/s0042-6822(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 8.Card J P, Dubin J R, Whealy M E, Enquist L W. Influence of infectious dose upon productive replication and transynaptic passage of pseudorabies virus in rat central nervous system. J Neurovirol. 1995;1:349–358. doi: 10.3109/13550289509111024. [DOI] [PubMed] [Google Scholar]
- 9.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 11.Dolan A, Jamieson F E, Cunningham C, Barnett B C, McGeoch D J. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edson C M. Phosphorylation of neurotropic alphaherpesvirus envelope glycoproteins: herpes simplex virus type 2 gE2 and pseudorabies virus gI. Virology. 1993;195:268–270. doi: 10.1006/viro.1993.1372. [DOI] [PubMed] [Google Scholar]
- 13.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1999;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 14.Enquist L W, Card J P. Pseudorabies virus: a tool for tracing neuronal connections. In: Lowenstein P R, Enquist L W, editors. Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 333–348. [Google Scholar]
- 15.Fletcher T M, III, Gray W L. DNA sequence and genetic organization of the unique short (US) region of the simian varicella virus genome. Virology. 1993;193:762–773. doi: 10.1006/viro.1993.1185. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs W, Rziha H J, Lukacs N, Braunschweiger I, Visser N, Lutticken D, Schreurs C S, Thiel H J, Mettenleiter T C. Pseudorabies virus glycoprotein gI: in vitro and in vivo analysis of immunorelevant epitopes. J Gen Virol. 1990;71:1141–1151. doi: 10.1099/0022-1317-71-5-1141. [DOI] [PubMed] [Google Scholar]
- 17.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granzow H, Weiland F, Jons A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs L, Mulder W A, Priem J, Pol J M, Kimman T G. Glycoprotein I of pseudorabies virus (Aujeszky’s disease virus) determines virulence and facilitates penetration of the virus into the central nervous system of pigs. Acta Vet Hung. 1994;42:289–300. [PubMed] [Google Scholar]
- 20.Kimman T G, de Wind N, Oei-Lie N, Pol J M, Berns A J, Gielkens A L. Contribution of single genes within the unique short region of Aujeszky’s disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhausen T, Bonifacino J S, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–95. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 22.Kritas S K, Nauwynck H J, Pensaert M B. Dissemination of wild-type and gC-, gE- and gI-deleted mutants of Aujeszky’s disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995;76:2063–2066. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- 23.Leung-Tack P, Audonnet J C, Riviere M. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain) Virology. 1994;199:409–421. doi: 10.1006/viro.1994.1139. [DOI] [PubMed] [Google Scholar]
- 24.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984;52:198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks M S, Ohno H, Kirchhausen T, Bonifacino J S. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–127. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 26.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 27.Mettenleiter T C, Lukacs N, Rziha H J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985;56:307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettenleiter T C, Zsak L, Kaplan A S, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mijnes J D, Lutters B C, Vlot A C, van Anken E, Horzinek M C, Rottier P J, de Groot R J. Structure-function analysis of the gE-gI complex of feline herpesvirus: mapping of gI domains required for gE-gI interaction, intracellular transport, and cell-to-cell spread. J Virol. 1997;71:8397–8404. doi: 10.1128/jvi.71.11.8397-8404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura N, Balch W E. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 32.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster viral gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson J K, Santos R A, Grose C. Varicella-zoster virus glycoprotein gE: endocytosis and trafficking of the Fc receptor. J Infect Dis. 1998;178(Suppl. 1):S2–S6. doi: 10.1086/514255. [DOI] [PubMed] [Google Scholar]
- 34.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen D J, Evans P R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radsak K, Eickmann M, Mockenhaupt T, Bogner E, Kern H, Eis-Huebinger A, Reschke M. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch Virol. 1996;141:557–572. doi: 10.1007/BF01718317. [DOI] [PubMed] [Google Scholar]
- 38.Robbins A K, Dorney D J, Wathen M W, Whealy M E, Gold C, Watson R J, Holland L E, Weed S D, Levine M, Glorioso J C, Enquist L W. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J Virol. 1987;61:2691–2701. doi: 10.1128/jvi.61.9.2691-2701.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roizman B. Herpesviridae: a brief introduction. In: Knipe D M, Fields B N, editors. Fundamental virology. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1991. pp. 841–847. [Google Scholar]
- 40.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 41.Ryan J P, Whealy M E, Robbins A K, Enquist L W. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J Virol. 1987;61:2251–2257. doi: 10.1128/jvi.61.10.2962-2972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spatz S J, Rota P A, Maes R K. Identification of the feline herpesvirus type 1 (FHV-1) genes encoding glycoproteins G, D, I and E: expression of FHV-1 glycoprotein D in vaccinia and raccoon poxviruses. J Gen Virol. 1994;75:1235–1244. doi: 10.1099/0022-1317-75-6-1235. [DOI] [PubMed] [Google Scholar]
- 43.Tirabassi R S, Enquist L W. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trowbridge I S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3:634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 46.Trowbridge I S, Collawn J F. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 47.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelnik V, Darteil R, Audonnet J C, Smith G D, Riviere M, Pastorek J, Ross L J N. The complete sequence and gene organization of the short unique region of herpesvirus of turkeys. J Gen Virol. 1993;74:2151–2162. doi: 10.1099/0022-1317-74-10-2151. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuckermann F A, Mettenleiter T C, Schreurs C, Sugg N, Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988;62:4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]