Abstract
The anterior pituitary controls key biological processes, including growth, metabolism, reproduction, and stress responses through distinct cell types that each secrete specific hormones. The anterior pituitary cells show a remarkable level of cell type plasticity that mediates the shifts in hormone-producing cell populations that are required to meet organismal needs. The molecular mechanisms underlying pituitary cell plasticity are not well understood. Recent work has implicated the pituitary stem cell populations and specifically, the mRNA binding proteins of the Musashi family in control of pituitary cell type identity. In this study we have identified the target mRNAs that mediate Musashi function in the adult mouse pituitary and demonstrate the requirement for Musashi function in vivo. Using Musashi RNA immunoprecipitation, we identify a cohort of 1184 mRNAs that show specific Musashi binding. Identified Musashi targets include the Gnrhr mRNA, which encodes the gonadotropin-releasing hormone receptor (GnRHR), and the Fshb mRNA, encoding follicle-stimulating hormone (FSH). Reporter assays reveal that Musashi functions to exert repression of translation of the Fshb mRNA, in addition to the previously observed repression of the Gnrhr mRNA. Importantly, mice engineered to lack Musashi in gonadotropes demonstrate a failure to repress translation of the endogenous Gnrhr and Fshb mRNAs during the estrous cycle and display a significant heterogeneity in litter sizes. The range of identified target mRNAs suggests that, in addition to these key gonadotrope proteins, Musashi may exert broad regulatory control over the pituitary proteome in a cell type–specific manner.
Keywords: Musashi, Gnrhr, Fshb, gonadotrope, estrous cycle, pituitary
The anterior pituitary controls diverse physiological processes (1, 2) through the production of hormones from specialized secretory cell types: follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from gonadotropes, growth hormone (GH) from somatotropes, adrenocorticotropin (ACTH) from corticotropes, thyroid-stimulating hormone (TSH) from thyrotropes, and prolactin (PRL) from lactotropes. The levels of specific hormones produced reflect the ability of the pituitary to utilize plasticity in gene and protein expression in the distinct hormone-producing cell populations. This plasticity allows the pituitary to meet changing organismal demands by switching the production of specific hormones as necessary. The regulatory mechanisms governing pituitary cell plasticity are not fully understood and may involve recruitment of immature adult tissue stem cells, proliferation of existing mature hormone-producing cells, and/or transdifferentiation of one hormone-producing cell type into another hormone-producing cell type (3). A key example of pituitary cell plasticity is in the reproducible transcriptomic and protein changes that occur during each estrous cycle within the gonadotrope population in females (4).
The phenotypic plasticity in the gonadotrope cell population supports a midcycle LH/FSH surge and an early estrous rise in serum FSH. These surges in production and secretion of LH and FSH are dependent on cyclic changes in gonadal steroids, alterations in hypothalamic gonadotropin-releasing hormone (GnRH) secretion, and/or changes in TGFß signaling, among other paracrine and circulating factors (5-7). On a basic level, FSH is required for the stimulation of ovarian follicles that produce estradiol, which in turn exerts an indirect positive feedback effect on the GnRH-producing hypothalamic neurons (8) and a direct positive feedback on gonadotropes, which primes these cells to produce LH (8-16). A critical event in gonadotrope remodeling is the production of GnRH receptors (GnRHR) (3, 8, 10, 17-21), the expression of which varies with the developmental and reproductive state (22-25) and determines the gonadotrope responsiveness to GnRH (14, 17-21, 26). In rodent models, GnRHRs are produced 1 to 2 days before the designated period of positive estrogen feedback to the kisspeptin/GnRH neurons. Alterations in GnRH secretion (changes in pulsatility or a surge in secretion, for example) then evoke the midcycle LH surge (16, 19, 20, 27-31). After the positive feedback period and during the surge, the gonadotrope population is remodeled anew with increases in levels of Lhb and Fshb mRNA (14, 17, 20, 30, 32-39).
In addition to the extensive gene transcriptional control that mediates pituitary development and function, growing evidence suggests an important role for posttranscriptional regulation of mRNA translation in the adaptation to altered physiological and pathological cues. Our recent work has revealed a role for the sequence-specific RNA binding protein Musashi family members (Musashi1 and Musashi2) in control of pituitary mRNA translation (40-42). Outside the pituitary, the Musashi proteins have been demonstrated to function within the stem and progenitor cell populations of multiple tissues, where they act to oppose cell differentiation and maintain the self-renewal capacity of the stem/progenitor cells (43). The adult human pituitary expresses the highest levels of the Musashi1 and Musashi2 genes (Msi1 and Msi2) of all nongonadal tissues, and we have observed that in the mouse pituitary, the Musashi1 and Musashi2 proteins are not only present in the SOX2-positive stem and progenitor cells as expected, but surprisingly, are also present in the mature hormone-producing cell types (40). This observation suggested a role for Musashi-mediated control of mRNA translation within the hormone-producing pituitary cell populations. Consistent with this, we have shown that Musashi exerts translational repression of several key pituitary mRNAs, including those encoding GnRHR (42), the hormones thyroid-stimulating hormone (TSHß) and prolactin (PRL) (41), and the POU family lineage specification transcription factor Pou1f1 (40). We hypothesize that Musashi may function to maintain the plasticity of differentiated hormone-producing cell lineages. However, the full range of Musashi target mRNAs in the pituitary has not been characterized, and the requirement for Musashi to exert target mRNA translational control within the mammalian pituitary in vivo has not been established.
Here, we have employed an unbiased RNA immunoprecipitation and sequencing (RIPseq) strategy to identify Musashi target mRNAs in the pituitary. We have identified 1184 mRNAs that were specifically enriched in Musashi immunoprecipitates and which are implicated in a range of biological processes including amino acid transport, cellular homeostasis, Rab signaling, unfolded protein response, endocrine processes, and pregnancy. Because of the multiple gonadotrope-relevant targets identified in our RIPseq study, we performed a validation of our findings using a gonadotrope-specific Msi1 and Msi2 gene deletion mouse model and we demonstrate an in vivo requirement for Musashi function to repress translation of the Gnrhr and Fshb mRNAs during the estrous cycle. We propose that Musashi exerts influence over the translation of a broad range of distinct pituitary mRNA populations, including gonadotropes, to control pituitary hormone production in response to altered organismal demands.
Methods
Animals
The use of animals was approved by the University of Arkansas for Medical Sciences Animal Care and Use Committee. The mice used in these studies were maintained on a 14-hour light/10-hour dark cycle at 27 °C. The lights are on from 06:00 to 20:00. All nonbreeding mice were fed a standard diet (crude protein ≥ 18%, crude fat ≥ 5%, crude fiber ≤ 5%; LabDiet, 5V5R). All breeder mice were fed a breeder diet (crude protein ≥ 18%, crude fat ≥ 8%, crude fiber ≤ 5%; LabDiet, 5V5 M). Food and water were provided ad libitum. Mice were weaned at 21 days of age and housed at no more than 5 animals per cage.
For the RNA immunoprecipitation studies (RIPseq), pituitaries from adult, sighted FVB males and mixed-cycle females (FVB.129P2-Pde6b + Tyrc-ch/Ant, The Jackson Laboratory) were collected and placed into Dulbecco's phosphate-buffered saline (Gibco) with Protector RNase inhibitor (Sigma) and stored at −20 °C. Once all pituitaries were collected, 6 pituitaries per sex per experimental group were pooled and lysed in radioimmunoprecipitation (RIPA) buffer (Sigma) containing a protease inhibitor cocktail (Thermo Scientific).
A gonadotrope-specific Msi1 and Msi2 knockout animal model (Gon-Msi-null) was created by crossing Msi1 floxed and Msi2 floxed mice (Msi1/2flox/flox, a gift from Dr. Christopher Lengner) with mice bearing a Gnrhr-internal ribosomal entry site (IRES)-Cre (GRIC) driver (44). The combination of Cre recombinase and Msi1/2flox/flox results in the deletion of the transcriptional start site and exons 1 and 2 for Msi1, and deletion of exons 1 to 4 for Msi2 (45), resulting in a gonadotrope-Msi-null mouse line. The earliest detection of GRIC-Cre expression in the pituitary occurs on embryonic day 12.75, and expression occurs in cells committed to the gonadotrope lineage, with some expression also reported in male germ cells (46). Given the potential for extra-pituitary Cre expression in males, studies involving the Gon-Msi-null line were limited to females.
For gonadotrope cell purification, a floxed fluorescent reporter transgene (mT/mG or Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, Stock 007576, The Jackson Laboratory) was introduced into the gonadotrope-Msi-null mouse line. The addition of the mT/mG construct drives expression of membrane-targeted tdTomato before Cre excision, and expression of membrane-targeted EGFP after Cre excision (47). Therefore, all nongonadotropes express red fluorescence (tdTomato) and all gonadotropes express green fluorescence (EGFP).
Immunoprecipitation
Musashi1 and Musashi 2 were immunoprecipitated from lysate from 6 adult 8- to 10-week-old control mouse pituitaries that was equally split between Musashi1 antibody (Abcam, ab52865; RRID: AB_881168) plus Musashi 2 antibody (Abcam, ab114107; RRID: AB_10858464) or control rabbit IgG (Millipore Sigma PP64; RRID: AB_97852) as per the kit supplier's protocol (Invitrogen Dynabeads Protein A IP kit, Fisher Scientific, catalog # 10006). The protein was degraded, and RNA was isolated, precipitated and utilized for next-generation RNA sequencing (RNAseq). The immunoprecipitations were performed in 6 separate experiments (3 for male pituitaries, 3 for female pituitaries).
RNAseq and Bioinformatics
Libraries for RNAseq were prepared using the TruSeq Stranded Total RNA Library Prep Gold kit (Illumina Inc) according to the supplier's protocol. The resulting libraries were sequenced on a NovaSeq 6000 using an SP flow cell, which generated an average of 72.34 M reads/sample, with read length of 101 bp paired ends. RNA reads were checked for quality of sequencing using FastQC, v0.11.8. The adaptors and low-quality bases (Q < 20) were trimmed to a minimum of 36 base pairs using Trimmomatic, v0.39 (48). Reads that passed quality control were aligned to the reference genome, Mus musculus GRCm39.104 using STAR, version 2.7.1a (49). Raw counts were obtained from BAM files using Subread's featuresCount function (vs 2.0.0) and transformed to log2 counts per million (CPM) (50). Low expressed genes were filtered out and libraries were normalized by trimmed mean of M values (51). Differential expression and linear modeling were performed using the R package, Limma, using the voomWithQualityWeights function (52). Genes with an FDR P value < .1 and absolute fold change (FC) > 2 were considered significant. One male sample showed no enrichment of mRNAs and was discarded prior to bioinformatics analysis. Note, male and female samples (IgG or Musashi1/2 immunoprecipitations) were combined for analyses as the 2 male and 3 female sample sets did not provide sufficient statistical rigor to allow sex-separated differential analyses. GO term analysis was performed on enriched gene lists using the topGO R package. GO terms belonging to Biological Processes were queried with the parentchild algorithm and Fisher statistic (53).
To determine the relative abundance of the Musashi RIPseq targets in the pituitary, we re-analyzed previously published male (54) and female mouse (40) scRNA-seq raw data sets. The datasets were downloaded from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) database with the accession number GSE120410 and GSE153045, respectively. Using Seurat (v4.3.0) (55) in R (v4.2.3) (56), the raw counts were preprocessed to filter low-quality cells, cell doublets, and cells with a high percentage of mitochondrial genes according to the original thresholds. The male and female data sets were then SCTransformed to perform normalization and variance stabilization by using the function SCTransform, followed by merging both data sets together and fetching to retrieve the normalized feature expression. Function geom_jitter from the package ggplot2 (v3.4.1) was used to visualize the expression of each feature.
Real-Time Quantitative Polymerase Chain Reaction Analyses
Additional sets of female pituitaries were immunoprecipitated as described above for the RNAseq experiments. However, after immunoprecipitation and RNA precipitation, the samples were analyzed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) for the indicated target mRNA content relative to the housekeeping transcript peptidylprolyl isomerase A/cyclophilin A (Ppia) with the QuantStudio 12k Flex system (Applied Biosystems) (57) and each qRT-PCR was repeated in duplicate. Musashi1 and Musashi2 protein in the Musashi1/2 immunoprecipitation was confirmed by Western blot (vs control IgG immunoprecipitation, data not shown). The same method was used for the quantification of Gnrhr, Fshb, and Lhb mRNAs in the gonadotrope-specific Musashi knockout female pituitaries. Primers used for qRT-PCR are listed in Table 1.
Table 1.
qPCR primers
| Gene name | IDT Assay ID | Ref Seq # | Forward sequence (5′ to 3′) | Reverse sequence (5′ to 3′) |
|---|---|---|---|---|
| Atf4 | Mm.PT.58.32663810.g | NM_009716 | GGCTATGGATGATGGCTTGG | ACAGAGCATCGAAGTCAAACTC |
| Avpr1b | Mm.PT.58.12605492 | NM_011924 | ACGAGAATGCCCCTAATGAAG | TGTTGAAGCCCATATAGATCCAG |
| Cdh1 | Mm.PT.58.43112087 | NM_009864 | ACAGCACATATGTAGCTCTCATC | CGTTGTCATTGACGTCTAACAG |
| Cldn4 | Mm.PT.58.11569298.g | NM_009903 | CACTCAGCCTACACGTTACTC | CACTCAGCACACCATGACTT |
| Creb3l2 | Mm.PT.58.42926069 | NM_178661 | AGTACTGTTGGAGCTTCAGC | AAAGGACGATCAGATGGAGTTAG |
| Cx3cl1 | Mm.PT.58.8767901 | NM_009142 | ATCCGCTATCAGCTAAACCAG | TGATCCAGATGCTTCATGGC |
| Cxcl2 | Mm.PT.58.8366748 | NM_001012477 | GCGCTCTGCATCAGTGA | ACAGTTTGGAGTGTTGAGGAT |
| Cxcl14 | Mm.PT.58.21980826 | NM_019568 | CAAGTGGTACAATGCCTGGA | CTGCTGAAGTCTCTCAACTGG |
| Ppia | Custom oligo (58) | NM_008907 | TGGTCTTTGGGAAGGTGAAAG | TGTCCACAGTCGGAAATGGT |
| Ddx6 | Mm.PT.58.730224 | NM_001110826 | CAATCTTGTTTGCACTGATCTGT | CAAGATGACCAAAGCGACCT |
| Foxj1 | Mm.PT.58.7661191 | NM_008240 | ATCACGGACAACTTCTGCTAC | GCACTTTGATGAAGCACTTGT |
| Foxo3 | Mm.PT.58.7873180 | NM_019740 | CTGAAGGATCACTGAGGAAAGG | GATCACTGTCCACTTGCTGAG |
| Foxp2 | Mm.PT.58.29755951 | NM_053242 | CGAACGTCTTCAAGCGATGA | CGACATGGTGACACTAGACAC |
| Fshb | Custom oligo (59) | GTGCGGGCTACTGCTACACT | CAGGCAATCTTACGGTCTCG | |
| Gata2 | Mm.PT.58.13913016.g | NM_008090 | CCTCTACTACAAGCTGCACAA | TCTTGGATTTGCTGGACATCT |
| Ghsr | Mm.PT.58.9588879 | NM_177330 | ACCAGAACCACAAACAGACAG | CAGGCTCGAAAGACTTGGAAA |
| Gjd2 | Mm.PT.58.6160898 | NM_010290 | AGCAGCACTCCACTATGATTG | GTTGCACACAAACATGGTCTG |
| Gnrhr | Custom Oligo (60) | NM_001003685.1 | GGCTGCCTCTTCATCCCCCT | CGTTCTCAGCCGAGCTCTTGGG |
| Insig1 | Mm.PT.58.9805181 | NM_153526 | CCCTGATTTCCTCTATATCCGTTC | ACTCTGAACCATGTGCTGAAG |
| Lhb | Custom Oligo (61) | TGTCCTAGCATGGTCCGAGT | AGGAAAGGAGACTATGGGGTCTA | |
| Msi1 | Mm.PT.58.31248964 | NM_008629(1) | CCTGCAAGATGTTCATCGGA | TGAAAGTGACGAAGCCGAAA |
| Msi2 | Custom Oligo (62) | GCGATGCTGATGTTCGACAA | TCTCCACAACGTCTTCATTCTCA | |
| Norad | Mm.PT.58.43986704.g | NR_024329 | CCTCTTGAACATGGAACTGCTA | AGACCTCATCCTGTGAACCT |
| Nr5a1 | Mm.PT.58.11115555 | NM_139051 | GAGTTAGTGCTCCAGTTGCAT | CTTTACGAGGCTGTGGTTGT |
| Ormdl1 | Mm.PT.58.10595788 | NM_145517 | GGACTACGGAGTACAGTTTACATC | TGGGAATAAGTACACTCAGCAG |
| Ormdl3 | Mm.PT.56a.42805026 | NM_025661 | CTGTCGTCTGGACCCTCA | TGAACTGGACCCCGTAGT |
| Pitx1 | Mm.PT.58.9006855 | NM_011097 | TGAGCATGAGAGAGGAGATCG | CCTTGCACAGGTCCAACT |
| Prop1 | Mm.PT.58.8919461 | NM_008936 | CTCAGTGAAGCCAGAATCCAG | GCAAGAAGCCAGAGAAGGT |
| Rab21 | Mm.PT.58.45878817 | NM_024454 | GCAGAATCGTATGCAGAGTCT | TTTTGCTCTCTCATCCACCTG |
| Rab27a | Mm.PT.58.7083493 | NM_023635 | GTGCTGTGTGGAAATAAGAGTG | GCTTATGTTTGTCCCGTTGG |
| Rab2a | Mm.PT.58.11823848 | NM_021518 | CGAGAGCATGGACTTATCTTCA | ATTTTAATGCCGTTTGCCTCA |
| Rab3d | Mm.PT.58.42963574 | NM_031874 | GACAATGCCCAGGTAATCCT | CACCTGCTTCACATTGATATTCTC |
| Rxra | Mm.PT.58.5331319 | NM_011305 | GAAGCGTACTGCAAACACAAG | CCCGATGAGCTTGAAGAAGA |
| Sox9 | Mm.PT.58.41323084 | NM_011448 | ACTCCCCACATTCCTCCT | CCCCTCTCGCTTCAGATCA |
| Sprtn | Mm.PT.56a.29073822 | NM_001111141 | GAAGACAAAAGCAGACAAGCAG | CGTCTCCAAGAACATAGCCTT |
| Tbx19 | Mm.PT.58.23718192 | NM_032005 | ATCCTGTCTGTACCCCACA | AGAATTGTTCCTGAGGTGCTG |
| Yap1 | Mm.PT.58.10478408 | NM_001171147 | TGACAACCAATAGTTCCGATCC | CCTGTATCCATTTCATCCACACT |
Abbreviation: IDT, Integrated DNA Technologies, Inc.
Fshb 3′ UTR Reporter Cloning
The 3′ untranslated region (UTR) of the murine Fshb gene (NM_008045) was cloned into the pmiRGLO vector using geneblock primers (Integrated DNA Technologies, Inc.) encompassing the full-length mRNA 3′ UTR with the addition of a 5′ NheI and a 3′ SalI restriction site. After digestion of the double-stranded geneblock primer with NheI and SalI, the recovered 3′ UTR fragment was cloned into NheI/SalI digested pmiRGLO. The resulting plasmid was designated pmiRGLO Fshb 3′ UTR. The integrity of the cloned 3′ UTR was validated by DNA sequencing of the final pmiRGLO plasmid. The pmiRGLO 552 bp Pou1f1 3′ UTR plasmid has been previously described (40).
Luciferase Reporter Assays
NIH3T3 cells (ATCC CRL-1658) were co-transfected with the indicated pmiRGLO 3′ UTR reporter plasmid along with either wild-type MSI1-eGFP, MSI1-bm-eGFP (which is mutated within the RNA recognition motif [RRM] to abrogate target RNA association) or eGFP (peGFP N1 empty vector control) plasmids as described previously (40, 63-65). Luciferase activity was determined in quadruplicate after 24 hours, using the Dual-Luciferase Reporter Assay System (Promega, E2920) and Turner Biosystems luminometer (Promega) according to the supplier’s protocol. Data are expressed as relative luciferase activity (FLuc/RLuc). All tests were repeated on at least 3 separate occasions, unless otherwise indicated.
Tail Snip and Organ Genotyping of Transgenic Animals
Routine genotyping was performed on tail snip samples of newly weaned 21-day-old mice. These samples were collected by slicing a small portion off the end of each mouse tail using a clean, disposable razor blade. Genomic DNA was extracted from tail snip samples using the Maxwell 16 Mouse Tail DNA Kit Purification Kit (Promega, AS1120) and used to detect genes of interest by polymerase chain reaction (PCR). Cre recombinase was detected with the following primers: Forward (5′-CGT ACT GAC GGT GGG AGA AT-3′) and Reverse (5′-CCC GGC AAA ACA GGT AGT TA-3′). The primer sequences used for detection of floxed Msi1 were: Forward (5′-CGG ACT GGG AGA GGT TTC TT-3′) and Reverse (5′-AGC TCC CCT GAT TCC TGG T-3′); and for detection of floxed Msi2: Forward (5′-TCT CCT TGT TGC GCT CAG TA-3′) and Reverse (5′-GCT CGG CTG ACA AAG AAA GT-3′). The housekeeping gene, T-cell receptor delta (TCR-Δ), was detected using the primers: Forward (5′-CAA ATG TTG CTT GTC TGG TG-3′) and Reverse (5′-GTC AGT CGA GTG CAC AGT TT-3′). The detection of Rosa TdTomato was detected with the primers: 5′-CGA GGC GGA TCA CAA GCA ATA-3′, 5′-CTC TGC TGC CTC CTG GCT TCT-3′ and 5′-TCA ATG GGC GGG GGT CGT T-3′. For organ genotyping, excision of Msi1 was revealed through the primers: Forward (5′- AGG GAG GAA GGA CTA GGG AG-3′) and Reverse (5′- CGG CGG CTT GTA CTG ATA AC-3′), and excision of Msi2 with the primers: Forward (5′-CCT GTC TGG TTG CTT CCT CG-3′) and Reverse (5′-GAG CCA ACT CGC TAG CTT G-3′).
The PCR conditions for detection of Cre recombinase were: Denaturation stage: 94 °C for 3 minutes; Annealing stage (30 cycles): 94 °C for 1 minute, 63 °C for 1 minute, 72 °C for 1 minute; Extension stage: 72 °C for 10 minutes, and 4 °C hold. For detection of floxed Msi1 and excised Msi1, the following condition were used: Denaturation stage: 95 °C for 4 minutes; Annealing stage (45 cycles): 95 °C for 1 minute, 60 °C for 1 minute, 72 °C for 1 minute; Extension stage: 72 °C for 7 minutes, and 4 °C hold. Floxed Msi2 primers were detected with the following protocol: Denaturation stage: 95 °C for 4 minutes; Annealing stage (40 cycles): 95 °C for 30 second, 58 °C for 30 second, 72 °C for 40 second; Extension stage: 72 °C for 7 minutes, and 4 °C hold. Excision of Msi2 was detected with the following PCR conditions: Denaturation stage: 95 °C for 4 minutes; Annealing stage (40 cycles): 95 °C for 30 second, 58 °C for 30 second, 72 °C for 40 second; Extension stage: 72 °C for 2 minutes, and 10 °C hold. The detection of the Rosa TdTomato was done using the following PCR protocol: Denaturation stage: 94 °C for 3 minutes; Annealing stage (35 cycles): 94 °C for 30 second, 61 °C for 1 minute, 72 °C for 30 second; Extension stage: 72 °C for 2 minutes, and 10 °C hold.
Female deletion mutants and littermate controls from the gonadotrope-Msi-null mouse line were used for extensive organ genotyping to validate the specificity of the knockout. The purpose of the genotyping was to determine if there was Cre-recombinase-mediated deletion of Msi1 and Msi2 outside of the pituitary. The deletion of Msi1 and Msi2 was analyzed using primers for the recombined Msi1 and Msi2 alleles. Three tissues representing each tier of the HPG axis (pituitary, hypothalamus, and ovary) were collected from 3 Gon-Msi-null females and 3 Controls and genotyped using the primer sets mentioned above.
Fluorescence-Activated Cell Sorting
Whole pituitaries from 6 Control and 6 Gon-Msi-null females between 2 and 4 months of age were collected at diestrus 0900 and dispersed as described above. These animals carry the Cre-dependent reporter transgene, which causes all cells without Cre to fluoresce red, and all Cre-expressing cells (gonadotropes, in our study) to fluoresce green. After dispersion, the gonadotropes were sorted via fluorescence-activated cell sorting (FACS) as previously described (66). RNA was purified from the gonadotropes for qRT-PCR of Msi1 and Msi2 (primers in Table 1).
Estrous Cycle Studies
All female mice used for these studies were between 2 and 4 months of age. Vaginal smears were collected daily to identify the stage of the estrous cycle of adult female mice. Sterile glass pipettes were used to flush the vaginal opening with phosphate buffer saline (PBS) (67). The flush containing vaginal fluid was placed in a 24-well tray and observed under a light microscope. The stage of the cycle was determined by cytology. Smears were collected daily through 2 full estrous cycles to ensure all experimental females (Control and Gon-Msi-null) were cycling.
Control and Gon-Msi-null females were euthanized during one of the following time points: Diestrus: 09:00, Proestrus: 09:00, 18:30, 19:00, 19:30, 20:00, 20:30, 21:30, Estrus: 09:00, and Metestrus: 09:00. Preliminary measurements of LH secretion during the surge period dictated the collection times. Following isoflurane anesthesia and decapitation, whole pituitaries were collected in 150 µL ice-cold radioimmunoprecipitation (RIPA) buffer (Sigma, R0278) with 10 µg/mL protease inhibitors (ThermoFisher Scientific, 78425) and homogenized with pellet pestles. From this homogenate, 30 µL was pulled and stored at −20 °C for RNA extraction. The remaining lysate was incubated overnight at 4 °C, centrifuged at 14 000 rpm for 20 minutes at 4 °C, and the supernatant was stored at −20 °C for protein analysis. Trunk blood was collected and left on ice for 1 hour, then centrifuged at 4600 rpm for 20 minutes at 4 °C, and serum was stored at −20 °C. At least 7 females were used for each experimental group and each stage of the cycle.
Detection of Pituitary Hormones
Pituitary GnRHR protein expression was measured by enzyme-linked immunosorbent assay (ELISA; MyBiosource, MBS9311067, RRID:AB_2941662) as previously described (42). Pituitary content (1:200 dilution) and serum levels (1:10 dilution) of LH and FSH were measured by a multiplex assay (68-71), using the rat pituitary magnetic bead panel kit assay (Millipore Sigma, Milliplex RPTMAG-86k, RRID:AB_2716840) and the Luminex 200 system. Samples were run in duplicate and averaged. All pituitary protein content measurements were normalized to total pituitary protein as measured by BCA assay. Pituitary mRNA (Gnrhr, Lhb, Fshb), protein (GnRHR, LH, FSH), and serum LH and FSH values come from the same animals in most cases.
Breeding Studies
A breeding study was set up to compare the experimental breeding cages: pairing a CreGnrhr+/− Msi1/2fl/fl TdTomato+/− (Gon-Msi-null) female with a Msi1/2fl/fl TdTomato+/− (no Cre, Control) male; with control breeding cages: pairing a control female with a control male. For each condition, 4 breeding cages were set up. Males and females were paired between 2 and 4 months of age. The breeding pairs were kept together for an average of 295 days, when the average age of the female breeders was 370 days. From each breeding cage, data on the number of pups per litter and the number of days between litters was collected.
Statistical Analysis
Results were analyzed using the GraphPad Prism software and statistical analysis was determined by t test or Mann-Whitney nonparametric test when comparing 2 groups or by one-way or two-way analysis of variance (ANOVA) followed by either Fisher's LSD, Tukey, or Šídák's multiple comparisons tests when comparing multiple groups. The F-test for population variance was performed in R code. The specific statistical tests used, as well as the sample sizes for each study, are indicated in the individual figure legends. The level of significance was determined as follows: **P < .01; ***P < .001.
Results
Unbiased Identification of Musashi Targets in the Mouse Pituitary
To identify pituitary target mRNAs of the Musashi family of RNA binding proteins (Musashi1 and Musashi2), we performed RNA immunoprecipitation (IP) followed by RNA sequencing (RNAseq) of recovered targets (RIPseq). To select for mRNAs with high-confidence association with Musashi and to minimize potential false positives, the IPs were done in the absence of crosslinking. For each sample, 6 individual pituitaries were combined, lysed and aliquoted into 2 equal volumes for either a Musashi antibody IP or a matched IP with control IgG (Fig. 1A). For the Musashi IP, we combined both Musashi1 and Musashi2 specific antibodies to ensure a full representation of possible Musashi targets. Each IP was repeated in triplicate for both males and females. One male sample set failed to show any mRNA enrichment in either the Musashi or IgG immunoprecipitation and was discarded from further analyses. All subsequent bioinformatic analyses were performed on the 5 combined male and female datasets.
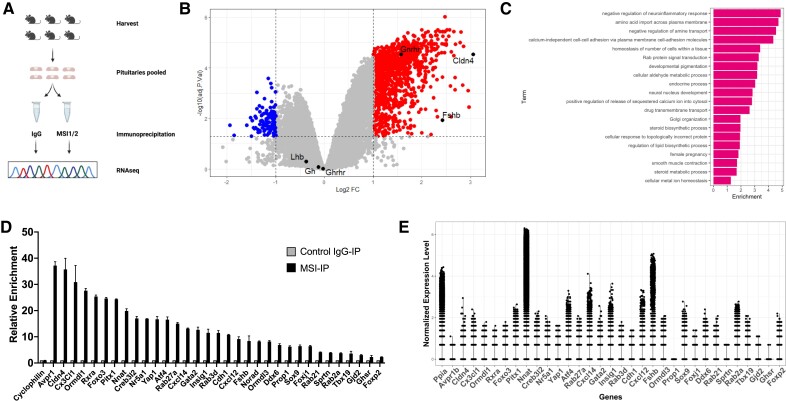
Figure 1.
Identification of Musashi mRNA targets in the adult mouse pituitary. (A) Schematic representation of the experimental strategy employed. Pituitaries were collected and split into 2 aliquots per sex and subjected to immunoprecipitation with either control IgG or a combination of Musashi1 and Musashi2 antibodies. Bound mRNA was recovered and sequenced. (B) Volcano plot showing differential recovery of mRNAs in Musashi1/2 IP over IgG control IP. RNA sequencing data from males and females were pooled prior to bioinformatic analysis. mRNAs are displayed based on log2 fold change (logFC) and adj P value. The 1184 mRNAs enriched more than 2-fold (logFC > 1) in Musashi1/2 immunoprecipitations with adj P value < 0.1 are displayed in the top right section, while mRNAs significantly underrepresented in Musashi1/2 IPs are shown in the top left section. (C) Top GO terms for enriched mRNAs in the Musashi1/2 RIPseq experiment. mRNAs that were ≥2-fold enriched (adj P value <0.1) in the Musashi1/2 IP were analyzed for gene ontology. Enriched GO terms were calculated using the topGO algorithm (53). Enrichment values in the x-axis represent the number of genes belonging to each GO term present in a given list divided by the number which would be expected by chance. (D) Validation of increased enrichment of 32 candidate Musashi target mRNAs. Pituitaries from 6 female mice were lysed, separated into 2 even aliquots and immunoprecipitated with total RNA recovered. Target mRNA enrichment in Musashi1/2 IP (MSI-IP) vs control IgG IP (IgG-IP) was assessed in duplicate using quantitative real-time polymerase chain reaction and normalized to cyclophilin using the ΔΔ cycle threshold method. Enrichment ranged from 37.19-fold (Avpr1) to 2.26-fold (Foxp2). (E) Relative expression of Musashi RIPseq target mRNAs in the adult pituitary. Previously published male and female scRNAseq pituitary datasets (see Methods) were re-analyzed to determine the relative abundance of the Musashi mRNA target mRNAs validated in Fig. 1D. Figure 1A was created with Biorender.com.
Using a threshold of >2-fold enrichment (log2FC > 1) in the Musashi IP over the IgG control IP, we identified 1184 significantly enriched (adj P value < .1) Musashi target mRNAs in the mouse pituitary (Fig. 1B). A previously validated gonadotrope-specific Musashi target, Gnrhr mRNA (42) was found in the Musashi IP-enriched mRNA pool as were the Ddx3x, Ddx6, Eif4g2, Leprot, and Ywhags mRNAs, which have been previously identified as a Musashi targets in a number of other tissues and cell types (45, 72, 73). In addition, 2 well characterized Musashi targets, the Cdkn1a mRNA (encoding the cell cycle control protein, p21) and the Numb mRNA (encoding the Notch inhibitor protein, Numb) (74, 75) were also significantly enriched over the IgG controls (adj P value < .05) but were below the >2-fold threshold we employed here (1.9-fold and 1.3-fold enriched, respectively). By contrast, mRNAs with no prior characterized Musashi interaction (eg, the cyclophilin mRNA (Ppia) (42)) or no consensus Musashi binding elements (MBEs) (eg, the LH-beta mRNA (Lhb), the growth hormone releasing hormone receptor mRNA (Ghrhr) or the growth hormone mRNA (Gh) were not enriched (Supplementary Table S1 (76)) indicating that the differential mRNA recovery reflected true biological interactions of target mRNAs with Musashi1 and Musashi2 proteins in the pituitary. Analysis of the recovered mRNAs revealed that the mRNA regulatory 3′ untranslated regions (3′ UTRs) of the Musashi targets tended to be longer than those preferentially recovered in the IgG IP controls (Supplementary Fig. S1A (76)) and the mRNA 3′ UTRs of the mRNAs not enriched in the Musashi IPs, have a higher proportion lacking MBEs (Supplementary Fig. S1B (76)). The 1184 mRNAs enriched as Musashi targets (Supplementary Table S2 (76)) have a bias toward containing MBEs in the 5′ portion of the 3′ UTR over those preferentially recovered in the IgG IP controls, resulting in a more uniform distribution of MBEs across the length of their 3′ UTRs (Supplementary Fig. S1C (76)). We note there is a strong correlation between enrichment, as indicated by log2 fold change (log2FC) and the number of MBEs per kilobase of mRNA, with a greater enrichment for those Musashi target mRNAs with a higher density of MBEs in their 3′ UTRs (Supplementary Fig. S1D (76)). Of the 1184 target mRNAs, 24 appeared to not contain consensus MBEs (Supplementary Table S3 (76)). Upon closer inspection, however, we note that the sequence for these 24 mRNA 3′ UTRs were mostly incomplete, as listed in the ENSEMBL database, as they lacked the canonical AAUAAA or AUUAAA polyadenylation hexanucleotide motif necessary for polyadenylation (poly[A] tail addition) of the mature mRNA (77). Re-query of the NCBI Reference Sequence (Refseq) database for the same 24 mRNA mRNAs revealed longer 3′ UTR sequences that included a polyadenylation hexanucleotide and one or more consensus MBEs in the 3′ UTRs of 19/24 of the mRNAs (Supplementary Table S3 (76)). One of the mRNA 3′ UTRs was incomplete in both ENSEMBL and RefSeq databases while 4 appeared complete but lacked consensus MBEs. Nonetheless, these findings demonstrate that Musashi IP-enriched target mRNAs are positively correlated with the presence of MBEs in their 3′ UTRs.
We performed gene ontology analyses to identify biological processes that may be regulated by the proteins encoded by the 1184 high-confidence Musashi target mRNAs. The 20 most significantly enriched processes of the Musashi target mRNAs are illustrated graphically in Fig. 1C. These include negative regulation of neuroinflammation, amine transport, cell adhesion, homeostasis of number of cells in a tissue, Rab protein signal transduction, endocrine processes, calcium signaling, Golgi organization, steroid and lipid biosynthesis, unfolded protein response, and female pregnancy. The individual mRNAs identified in each gene ontology category are listed in Supplementary Table S4 (76). Given the role of the pituitary in hormone secretion, control of metabolism, and reproduction, the proteins encoded by Musashi target mRNAs are consistent with a role in regulation of these functions.
Validation of Pituitary Musashi Target mRNAs
To validate the interaction of Musashi with target mRNAs, we utilized a similar Musashi and control IgG IP strategy on pituitaries isolated from an additional cohort of animals followed by qPCR for candidate mRNAs. We analyzed genes represented in the enriched pathways after gene ontology (Fig. 1C) as well as other candidates potentially relevant to pituitary function. These Musashi target mRNAs span a range of fold enrichments in the Musashi RIPseq dataset (Supplementary Table S1 (76)). As shown in Fig. 1D, the selected Musashi target mRNA candidates identified by RIPseq (Supplementary Table S2 (76)) were confirmed in the Musashi IPs relative to either control IgG or to mRNA levels of the cyclophilin control gene (Ppia). Confirmed mRNAs include those encoding the lineage specification transcription factors (Pitx1, Sox9, Foxj1, Prop1, Gata2, and Nr5a1); the beta subunit of FSH (Fshb); chemokine ligands (Cxcl12, Cxcl14, and Cx3cl1); receptors (Ghsr, Rxra, and Avpr1b); a master negative regulator of cholesterol and fatty acid synthesis (Insig1); negative regulators of sphingolipid synthesis (Ormdl1 and Ormdl3); an enhancer of the secretory capacity of hormone-producing cells (Creb3l2); a transcriptional mediator of the unfolded protein response (Atf4); a tight junction protein (Cldn4 that was the most enriched Musashi1/2 target mRNA); a regulator of mRNA translation (Ddx6); a proteolipid implicated in brain development (Nnat); and a regulatory long noncoding RNA (lncRNA, Norad). Of note, the enrichment seen in Fig. 1D is not simply due to transcript abundance as abundant pituitary transcripts such as Nnat and Fshb are not the most enriched in the Musashi immunoprecipitations, while the most enriched transcript (Avpr1b) is one of the least abundant transcripts analyzed (Fig. 1E). We conclude that Musashi-bound mRNA enrichment is not simply due to transcript abundance but rather must reflect differential affinity for individual mRNAs.
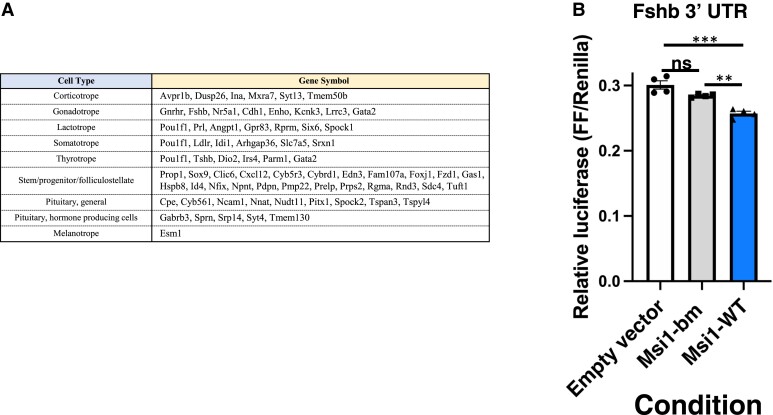
We have previously studied the function of the anterior pituitary in control of reproduction and the potential role of Musashi in this process (42). These data indicated that Musashi interacts specifically with the Gnrhr mRNA 3′ UTR and exerts translational repression in mRNA reporter assays. Prior pituitary single cell RNA sequencing has reported mRNAs showing cell lineage-restricted expression in the mouse or rat (54, 78, 79). Here we cross-reference those mRNAs with the high-confidence RIPseq Musashi target mRNAs identified in this study. Some of these target mRNAs are broadly expressed across pituitary cell types, while others have a more restricted cell type expression (Fig. 2A), including mRNA targets in gonadotropes. Here we have focused on one of these, the mRNA encoding the beta subunit of FSH, a major gonadotrope product and one of the 2 gonadotropins required for steroidogenesis and gametogenesis (80). Since Musashi1 and Musashi2 generally act in a functionally redundant manner (45, 81-83) and both isoforms are expressed to similar levels in the mouse pituitary (Supplementary Fig. S1E (76)), we have employed Musashi1 in all subsequent mRNA reporter assays. The 1140 nucleotide mouse Fshb (mFshb) mRNA 3′ UTR contains 6 MBEs and was cloned downstream of a firefly luciferase mRNA. The effect of Musashi1 upon mRNA translational control of the firefly luciferase reporter was assessed in NIH3T3 cells. NIH3T3 cells lack endogenous Musashi expression and display Musashi-dependent mRNA translational repression upon ectopic expression of mammalian Musashi1 (74). When co-expressed with wild-type Musashi1, the mFshb mRNA 3′ UTR directed a modest but reproducible repression of reporter mRNA translation (Fig. 2B). We note that the level of repression exerted by Musashi1 is comparable to the magnitude of repression exerted by individual microRNAs (miRNAs) which is often less than 20% (84) and working alone or in concert with other posttranscriptional regulators, this level of modulation allows for the fine tuning of mRNA translational outputs. No translational repression was observed with the RNA binding mutant form of Musashi1, indicating that the translational repression directed by the mFshb mRNA 3′ UTR was Musashi1-dependent.
Figure 2.
Musashi-target lineage restricted mRNAs. (A) Musashi target mRNAs with pituitary cell lineage-restricted expression. Prior pituitary single cell RNA sequencing has reported mRNAs showing cell lineage-restricted expression in the mouse or rat (54, 78, 79). Here we cross reference those mRNAs with mRNAs enriched >2-fold in Musashi IPs (Supplementary Table S2 (76)). (B) Musashi exerts repression via the Fshb 3′ UTR. NIH3T3 cells were co-transfected with the Fshb mRNA 3′ UTR controlling Firefly luciferase reporter translation and either eGFP alone (peGFPN1), eGFP-Musashi1 (peGFP Msi1), or eGFP linked to an RNA binding mutant form of Musashi1 (peGFP Msi1-bm). Firefly luciferase enzyme activity values were normalized to control Renilla luciferase enzyme activity values (expressed from the same plasmid, FF/Renilla). Five separate experiments were performed with each condition measured in quadruplicate. Values that differ significantly after one-way ANOVA are indicated, ** (P value < 0.01), *** (P value < 0.001). A representative experiment is shown.
In Vivo Identification of Musashi Regulation of Gonadotrope mRNA Translation
We next asked if Musashi-directed repression of Gnrhr and Fshb mRNA translation occurs in vivo. To this end, Cre-LoxP technology was used to generate a mouse model in which both paralogs of vertebrate Musashi (Msi1 and Msi2) are deleted specifically in pituitary gonadotropes (Supplementary Fig. S2A (76)). To create this gonadotrope-specific Musashi deletion model, animals bearing a Gnrhr-internal ribosomal entry site-Cre (GRIC) driver were crossed with a floxed Msi1 and floxed Msi2 (Msi1/2flox/flox) mouse line, designated Gon-Msi-null (44, 45). All mice also carried a Cre reporter to allow FACS purification and isolation of the gonadotrope population away from other cells of the anterior pituitary (see “Methods”). In the resulting offspring, the Cre recombinase drives the deletion of Msi1 and Msi2 in gonadotropes. This was confirmed by both tissue genotyping (Supplementary Fig. S2B-S2E (76)) and purified gonadotrope qRT-PCR (Supplementary Fig. S2F (76)). Although Gon-Msi-null females appear to have a slight decrease in the proportion of gonadotropes (as calculated from FACS experiments), this is not statistically significant (Supplementary Fig. S2G (76)).
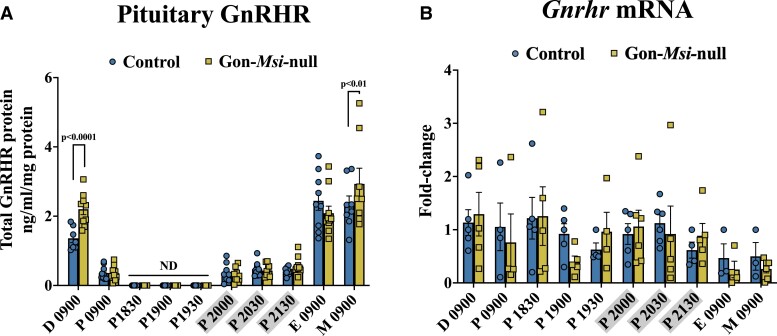
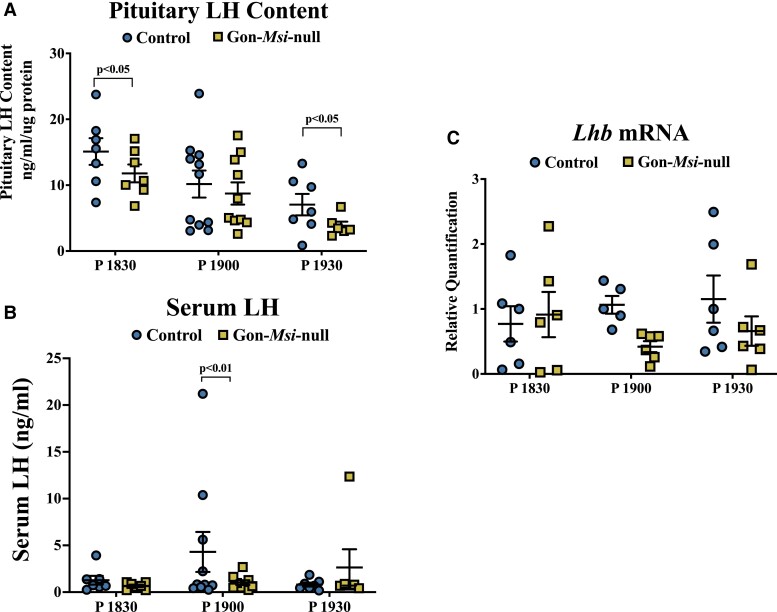
To determine the role of Musashi in gonadotropes in vivo, we collected pituitaries and sera from Control and Gon-Msi-null females throughout the estrous cycle. A large survey of timepoints was performed because we recognize that Musashi might differentially regulate our targets at different points in the cycle. GnRHR protein levels were measured in whole pituitaries from Control and Gon-Msi-null female mice (Fig. 3). GnRHR expression was significantly higher in Gon-Msi-null females when compared with controls in the morning of metestrus (Control: 2.34 ± 0.25 ng/mL/mg protein; Msi-null: 2.93 ± 0.45 ng/mL/mg protein, P value < .01) as well as in the morning of diestrus (Control: 1.36 ± 0.12 ng/mL/mg protein; Msi-null: 2.20 ± 0.16 ng/mL/mg protein, P value < .0001). To determine if the changes in GnRHR protein expression were simply a reflection of changes in gene expression, Gnrhr mRNA was also measured via qRT-PCR (Fig. 3). No significant differences in Gnrhr mRNA expression were observed between Controls and Gon-Msi-null pituitaries through the estrous cycle. Since the levels of Gnrhr mRNA were indistinguishable between Control and Gon-Msi-null animals on the mornings of metestrus and diestrus, but the mutant animals had elevated GnRHR protein, these findings show that loss of Musashi results in increased translation of the Gnrhr mRNA and indicate that in control animals, Musashi represses Gnrhr mRNA during metestrus and diestrus.
Figure 3.
GnRHR protein and mRNA expression through the estrous cycle in Control and Gon-Msi-null females. (A) Pituitary GnRHR content (measured by enzyme-linked immunosorbent assay [ELISA] and normalized to total protein) is shown for each stage of the estrous cycle as well as for time points bracketing the proestrous LH surge (n = 7-10 pituitaries assayed/group/timepoint). GnRHR protein was not detectable (ND) at 18:30, 19:00, and 19:30 on the evening of proestrus. (B) Gnrhr mRNA was measured by qRT-PCR, and fold-change in Gon-Msi-null Gnrhr levels are shown relative to controls and normalized to Ppia mRNA (n = 3-6 pituitaries analyzed/group/timepoint). For both protein and mRNA, two-way ANOVAs followed by Fisher's LSD tests were used to determine significant differences within timepoints between Control and Gon-Msi-null groups (significance = P value < 0.05). Timepoints that are grayed on the x-axes occurred during the dark cycle.
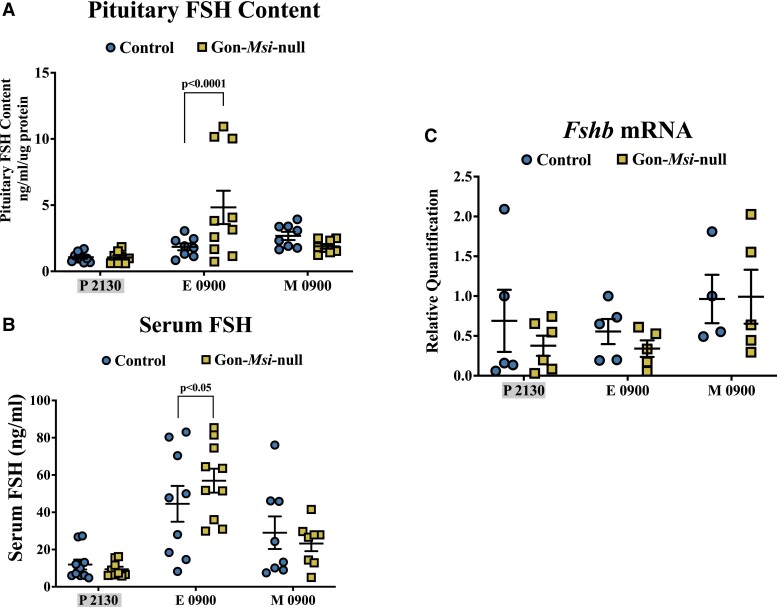
To determine if Musashi also represses Fshb mRNA translation in vivo, pituitary FSH content and serum FSH levels were measured in Control and Gon-Msi-null female mice throughout the estrous cycle (Fig. 4, Supplementary Fig S3 (76)). The pituitary content of FSH was significantly higher in Gon-Msi-null females during the morning of estrus (Control: 1.85 ± 0.26 ng/mL/ug protein; Msi-null: 4.83 ± 1.26 ng/mL/µg protein, P < .0001), which in our animals is during the secondary rise in FSH (peak serum FSH). At the same timepoint, Gon-Msi-null females have significantly higher serum FSH (Control: 44.52 ± 9.60 ng/mL; Msi-null: 56.94 ± 6.43 ng/mL, P = .05). The levels of Fshb mRNA in the pituitary were also measured at different stages of the estrous cycle (Fig. 4, Supplementary Fig. S3 (76)). In contrast to FSH protein and serum levels, Fshb mRNA was not changed during the time of the secondary rise in FSH (Fig. 4). Together, these results indicate that Musashi exerts translational repression of the Fshb mRNA in vivo.
Figure 4.
FSH protein pituitary stores, serum levels, and Fshb mRNA in Control and Gon-Msi-null females. Blood and pituitary samples were collected from Control (circles) and Gon-Msi-null (squares) females at Proestrus (P) 21:30, Estrus (E) 09:00, and Metestrus (M) 09:00, timepoints that bracket the secondary rise in serum FSH in our mice. (A) Pituitary and (B) serum content of FSH were determined by Milliplex rat pituitary assay (n = 8-11). (C) Pituitary Fshb mRNA was quantified with qPCR and normalized to Ppia (n = 4-6). Significant differences were determined by two-way ANOVA followed by Fisher's LSD test. Significant changes (P value < 0.05) are shown between groups within timepoints only. Data from additional estrous cycle timepoints can be found in Supplementary Fig. S3 (76). Timepoints that are grayed on the x-axes occurred during the dark cycle.
By contrast to Gnrhr and Fshb, the Lhb mRNA is not predicted to be a direct Musashi target as the Lhb mRNA 3′ UTR lacks MBEs and was not enriched in the MSI RIPseq (Supplementary Table S1 (76)). We measured LH pituitary content and serum levels in Control and Gon-Msi-null female mice throughout the estrous cycle (Fig. 5, Supplementary Fig. S3 (76)). Gon-Msi-null females have significantly lower circulating levels of LH than the control mice at Proestrus1900, 1 hour before lights-off and during the time of the preovulatory LH surge in this mouse model (Control: 4.30 ± 2.14 ng/mL; Msi-null: 1.02 ± 0.23 ng/mL; P value < .01). Gon-Msi-null females also show significantly lower pituitary LH content than their littermate controls 30 minutes before (18:30) and 30 minutes after (19:30) the surge. No significant changes in Lhb mRNA levels were observed (Fig. 5, Supplementary Fig. S3 (76)). The data indicate that LH is also affected by the loss of Musashi, although this must necessarily be through an indirect mechanism(s).
Figure 5.
LH protein pituitary stores, serum levels, and Lhb mRNA in Control and Gon-Msi-null females. Blood and pituitary samples were collected from control (circles) and Gon-Msi-null (squares) females at Proestrus (P) 18:30, 19:00, and 19:30, timepoints that bracket the LH surge in this line of mice. (A) Pituitary and (B) serum content of LH were determined by Milliplex rat pituitary assay (n = 6-11). (C) Pituitary Lhb mRNA was quantified with qPCR and normalized to Ppia mRNA (n = 5-6). Significant differences were determined by two-way ANOVA followed by Fisher LSD test. Significant changes (P value < 0.05) are shown between groups within timepoints only. Data from additional estrous cycle timepoints can be found in Supplementary Fig. S3 (76).
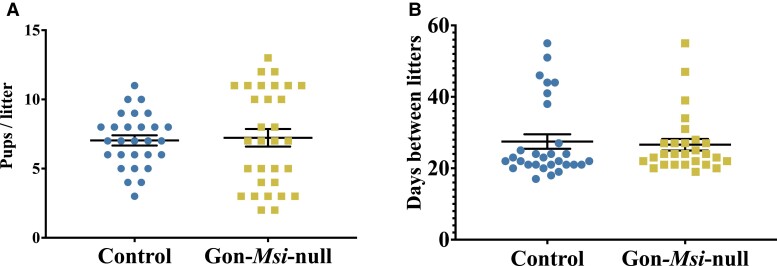
We next determined the effect of the changes in GnRHR, FSH, and LH upon the female reproductive system. No changes in estrous cyclicity were observed (data not shown). Though the average number of pups per littler and average number of days between litters were not statistically different, the Gon-Msi-null females showed a higher variance in litter sizes (mean: 7.226, standard error of the mean: 0.6337) compared to controls (mean: 7.036, standard error of the mean: 0.3690) (Fig. 6A and 6B, F value = 3.264, P value = .00264).
Figure 6.
Characterization of the reproductive phenotype of Gon-Msi-null females. (A) Data collected from breeding studies with control breeding cages (pairing a control female with a control male) and mutant breeding cages (pairing a mutant female with a control male). For each condition, 4 breeding cages were set up. The number of pups per litter (test of variance in Gon-Msi-null litter size vs control litter size, F value = 3.264, P value = 0.00264) and (B) the number of days between litters. Each dot (control) or square (mutant) represents a value collected from a single litter. Differences were determined by Student t tests, * = P value < 0.05.
Discussion
Our prior work suggested a role for Musashi in modulating adult pituitary function, although few pituitary mRNA targets of Musashi had been characterized (40-42), and the requirement for Musashi function in vivo within the pituitary had not been established. Here, we identify in vivo Musashi target mRNAs in the adult pituitary and demonstrate that Musashi exerts translational repression of the gonadotrope Gnrhr and Fshb mRNAs in female mice.
Comparison of Pituitary mRNA Targets and Musashi Targets Identified in Other Tissue Types
A number of prior studies have employed crosslinking and RIPseq or RNA editing methodologies to identify Musashi1 or Musashi2 target mRNAs in human or murine cells (45, 72, 73, 82, 85-89). It has been noted that there is considerable variation in recovered Musashi target mRNAs between different cell and tissue types (73, 85). We similarly observed cell type variations in recovered Musashi mRNA targets between the distinct cell populations of the pituitary (Fig. 2A). Cross referencing of our 1184 high-confidence pituitary target mRNAs to the datasets returning the largest number of either Musashi1 (45, 72, 82) or Musashi2 target mRNAs (73) revealed 112 target mRNAs (9.4%) shared with the prior Musashi1 datasets and 173 mRNAs (14.5%) shared with the Musashi2 datasets. When combined, 53 of these mRNAs (4.4%) were common to both the Musashi1 and Musashi2 datasets (Supplementary Table S5 (76)). Gene ontology analysis indicated that these 53 shared mRNAs encode proteins involved in a range of cellular processes including regulation of translational initiation, stress granule assembly and the endoplasmic reticulum unfolded protein response (Supplementary Table S6 (76)). It is likely that these represent common core functions modulated by Musashi1 and/or Musashi2 across cell types, and that each cell type has additional mRNA targets and specific regulatory processes targeted by Musashi superimposed over these core functions.
Musashi Targets Pituitary mRNAs Critical for Development and Hormone Secretion
The Musashi target mRNAs identified within the pituitary (Supplementary Table S2 (76)) overwhelmingly contained consensus MBEs in their 3′ UTRs and included several previously characterized Musashi target mRNAs. As anticipated, mRNAs lacking MBEs in their 3′ UTR were not recovered in the Musashi immunoprecipitations. The qPCR validation of target mRNA recovery in independently prepared samples confirmed association with Musashi1 and/or Musashi2 in the adult pituitary (Fig. 1D).
The identified Musashi target mRNAs encode proteins associated with the regulation and performance of numerous pituitary functions. These functions include intracellular protein trafficking and control of protein secretion, control of lipid biosynthesis and metabolism, response to lipid bilayer stress and unfolded proteins, and regulation of reproduction (Fig. 1C). We specifically noted several mRNAs encoding proteins associated with pituitary stem cell and progenitor cell functions, as well as lineage specification transcription factors implicated in ventral gonadotrope development. Musashi-associated mRNAs also include those encoding the transcription factors PITX1, GATA2, and SF-1 (encoded by the Nr5a1 mRNA) that are vital for the development of gonadotropes and thyrotropes (90). Additional critical gonadotrope regulatory factors encoded by mRNAs identified as Musashi targets include the Fshb mRNA as well as the Gnrhr mRNA, as anticipated from our previous study (42).
Notably, about 76% of murine mRNA 3′ UTRs contain MBEs (91); however, only 7.3% (1184/16 186) of the total mRNAs identified by RNAseq showed high-confidence association with Musashi in immunoprecipitations (Fig. 1B). This observation suggests that factors other than the MBE consensus sequence make important contributions to functional Musashi association. Such factors may include mRNA 3′ UTR structures that favor presentation of MBEs for Musashi interaction (92), proximity of nearby MBEs (93, 94) and/or occlusion of MBEs by other RNA binding proteins or miRNAs.
Musashi Regulates Translation of Gnrhr and Fshb mRNAs In Vivo
We created a novel mouse model in which both Musashi gene paralogs, Msi1 and Msi2, are specifically deleted in gonadotropes and identified the functional consequences of loss of Musashi in vivo. Consistent with our in vitro findings, Gon-Msi-null females showed a significant increase in GnRHR protein levels, but not in Gnrhr mRNA expression, compared to controls during the morning of metestrus and diestrus (Fig. 3). These data provide key evidence that Musashi acts to repress translation of the Gnrhr mRNA in vivo. Despite the increased GnRHR protein content in Gon-Msi-null females, the deletion of Musashi in the gonadotropes led to significantly decreased levels of peak pituitary LH stores (at P1830, Fig. 5). The consequence of this reduction was a lack of surge-level secretion in Gon-Msi-null mice. The LH deficiency is presumably not directly resultant from the lack of Musashi, since the Lhb mRNA 3′ UTR lacks MBEs and is not a direct target of Musashi binding (Supplementary Table S1 (76)). Ongoing studies seek to functionally bridge between our identified Musashi targets and the observed drop in LH pituitary stores. We further note that the number of pups per litter shows significant variance in the Gon-Msi-null female mutants compared to control animals (Fig. 6), suggesting an underlying compromising deficit in the Gon-Msi-null females that may reflect a reduced number of ovarian follicles in these animals. We hypothesize that in this fecund FVB strain, the residual 36% of serum LH that is observed in the Gon-Msi-null females at the time of the LH surge, is sufficiant to induce ovulation. Indeed, it has been reported in female mice and rats that ovulation occurs even when LH levels are reduced to 10% to 20% of normal at the time of the surge (95, 96).
The gonadotrope deletion of Musashi also affected FSH levels and the Gon-Msi-null females had significantly higher FSH content in the pituitary and FSH secretion, during the morning of estrus, compared to controls. Notably, the increase in FSH protein during the morning of estrus was not due to changes in Fshb mRNA expression, suggesting that like GnRHR, the increase in FSH protein synthesis is due to loss of posttranscriptional control. Although dysregulated GnRHR protein levels may also contribute to the altered FSH synthesis and secretion in the Gon-Msi-null females, FSH secretion is less sensitive to GnRH signaling than LH secretion (97). More likely, the elevated FSH protein synthesis reflects a lack of Fshb mRNA translation repression due to loss of Musashi. Consistent with this hypothesis, we observe that the Fshb mRNA interacts directly with Musashi in vivo and is subject to Musashi-dependent translational repression in mRNA reporter assays (Fig. 2B). Taken together, these experiments support our hypothesis that in addition to the Gnrhr mRNA, Musashi represses translation of the Fshb mRNA in vivo. Of note, our data indicate the repression of Gnrhr mRNA translation during metestrus and diestrus and repression of Fshb mRNA translation during estrus, suggesting that Musashi functions to fine tune target mRNA translation at multiple points during the estrous cycle in a mRNA-specific manner. Consequently, it will be necessary to assess individual Musashi target mRNAs on a case-by-case basis for altered translational control across distinct phases of the estrous cycle. Moreover, since Musashi can direct translational activation of target mRNAs in a context-dependent manner (98), it is possible that some of the Musashi target mRNAs identified in the pituitary in general, and in gonadotropes specifically, may be activated rather than repressed. Future experiments will be necessary to resolve these issues.
Considerations for Data Interpretation and Limitations of the Study
The present study utilized an in vivo conditional knockout mouse model to validate the molecular findings, particularly Musashi-mediated translational repression of Fshb and Gnrhr. All of our sample collections were terminal. Had we performed serial tail bleeds, we might have been able to characterize the LH surge in this model and check for alterations in LH pulsatility. For example, the samples we collected during the presumed timing of the LH surge in controls were quite variable. Not all control mice were surging at the time of collection. We do note that no Gon-Msi-null mice surged during this timepoint, and only one was found to have surge-level secretion 30 minutes later. However, we cannot definitively say we have altered surge-level secretion until we have collected serial samples across the full span of the surge.
Given that each timepoint represents a snapshot of hormone levels for an individual mouse, there is quite a bit of variability at certain timepoints. Again, this is to be expected in the nonstimulated, intact, cycling mouse, and our sample numbers were guided by power analyses. However, even with this variability, we are able to see significant changes in the model, and our in vivo findings regarding FSHß protein production and upregulation of GnRHR fit the in vitro findings.
Previous studies have suggested that peak FSH secretion in mice may occur around 02:00 to 04:00 on the morning of Estrus. We did not sample between 21:30 on Proestrus and 09:00 on Estrus, so we cannot be certain that we have measured the peak of the secondary rise in FSH. We do note here that, in a model not yet published from our group, we did sample Estrus 04:00 and found FSH levels to be higher than Proestrus 24:00 but still lower than Estrus 09:00, again indicating that the timing of these hormonal events may be model/facility specific.
Finally, we are limited in our ability to correlate the changes in Musashi targets with effects upon reproduction. Our retrospective study of breeding and litter numbers point to reproductive dysfunction in the population, but not complete infertility. Here we report significant heterogeneity in litter size in the mutants (Fig. 6). The level of subfertility slowed breeding and collection of mice for the study which took 2 years to complete. Future studies of this line should include steroid hormone and inhibin measurements to determine if and/or how ovarian feedback has been altered.
Conclusions
In the studies reported here, we identify a diverse range of Musashi target mRNAs in the adult mouse pituitary that exhibit both general pan-pituitary expression as well as pituitary cell type–restricted expression. We identify a number of potential biological roles for the proteins encoded by these mRNA toward defining the mechanisms by which Musashi regulates pituitary function. Together, the data presented here characterize the role Musashi plays in vivo to fine tune control of gonadotrope protein expression in females. We have discovered that Musashi exerts repression of both Gnrhr and Fshb mRNAs at multiple stages within the estrous cycle, indicating that Musashi facilitates gonadotrope remodeling and coordination of gonadotropin synthesis during the estrous cycle. Our analysis further shows additional gonadotrope Musashi targets, including vital lineage specification factors. In addition to the control of gonad function, FSH has been recently implicated in paracrine actions upon the extra-gonadotrope populations of the pituitary and in mediating inter-organ communication through effects upon hepatic lipid metabolism (99). The holistic impact of Musashi regulation of gonadotrope mRNAs, and of the additional pituitary target mRNAs identified in this study, will be critical areas for future investigation.
Acknowledgments
We wish to thank Dr. Daniel J. Bernard for providing us with the GRIC mice. We also thank Dr. Charles O’Brien for designing the genotyping primers to detect deleted Msi1.
Abbreviations
- FACS
fluorescence-activated cell sorting
- FC
fold change
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- GnRHR
gonadotropin-releasing hormone receptor
- GRIC
Gnrhr-internal ribosomal entry site-Cre
- IP
immunoprecipitation
- LH
luteinizing hormone
- MBE
Musashi binding element
- miRNA
microRNA
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- RIP-seq
RNA-immunoprecipitation and sequencing
- UTR
untranslated region
Contributor Information
Ana Rita Silva Moreira, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Juchan Lim, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Alicja Urbaniak, Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Jewel Banik, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Katherine Bronson, Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Alex Lagasse, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Linda Hardy, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Anessa Haney, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Melody Allensworth, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Tiffany K Miles, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Allen Gies, Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Stephanie D Byrum, Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA; Department of Biomedical Informatics, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA; Arkansas Children's Research Institute, Arkansas Children's Hospital, Little Rock, AR 72202, USA.
Ania Wilczynska, Bit.bio, The Dorothy Hodgkin Building, Babraham Research Campus, Cambridge CB22 3FH, UK.
Ulrich Boehm, Department of Experimental Pharmacology, Center for Molecular Signaling, Saarland University School of Medicine, Homburg 66421, Germany.
Michael Kharas, Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
Christopher Lengner, Department of Biomedical Sciences, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA 19146, USA.
Melanie C MacNicol, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA; Department of Biomedical Informatics, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Gwen V Childs, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Angus M MacNicol, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Angela K Odle, Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
Funding
This work was supported by the National Institutes of Health R01HD093461 (to A.M.M., G.V.C., and M.C.M.), R01DK113776 (to G.V.C., A.M.M., and M.C.M.), R01HD087057 (to G.V.C. and A.M.M.) and R01DK127723 (to G.V.C., A.M.M., and M.C.M.), a fellowship from the National Science Foundation OIA-1946391 (to J.B.), a UAMS Development Enhancement Award (DEAP) (to G.V.C. and A.M.M.), the Sturgis Charitable Trust (to A.M.M., G.V.C. and M.C.M., and A.O.), a WPRCI Team Science Award (A.M.M.), an Arkansas Breast Cancer Research Project award (A.M.M.), a Barton Pilot Award (A.M.M., S.D.B., and M.C.M.), a UAMS TRI Data Scholar award (M.C.M., UL1 TR003107, KL2 TR003108, and TL1 TR003109) and a Mentored Career Development Award (KL2 TR003108 to A.U.). The UAMS Bioinformatics Core Facility is supported by the Winthrop P. Rockefeller Cancer Institute and the National Institutes of Health P20GM121293.
Disclosures
G.V.C. is a member of the Endocrinology Editorial Board.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
The RNAseq data described in this study have been deposited in the Gene Expression Omnibus under accession number GSE199098. Go to https://urldefense.proofpoint.com/v2/url?u=https-3A__www.ncbi.nlm.nih.gov_geo_query_acc.cgi-3Facc-3DGSE199098&d=DwIBAg&c=27AKQ-AFTMvLXtgZ7shZqsfSXu-Fwzpqk4BoASshREk&r=dZZEevHpMAUI1uLm27Y4zMzKeai_60o5nCzUFripuNo&m=KXct0JwmIYrlaAmK41HxpL_xsNnEhMZGNeB_wBx9I8c&s=hXd50nJ33UE9sqvd4z_i5QCn4LFvKxbaA8LSDwUu74&e=
References
- 1. Davis SW, Ellsworth BS, Perez Millan MI, et al. Pituitary gland development and disease: from stem cell to hormone production. In: Current Topics in Developmental Biology. Vol. 106. 1st ed. Elsevier; 2013:1-47. [DOI] [PMC free article] [PubMed]
- 2. Perez-Castro C, Renner U, Haedo MR, Stalla GK, Arzt E. Cellular and molecular specificity of pituitary gland physiology. Physiol Rev. 2012;92(1):1‐38. [DOI] [PubMed] [Google Scholar]
- 3. Childs GV, MacNicol AM, MacNicol MC. Molecular mechanisms of pituitary cell plasticity. Front Endocrinol (Lausanne). 2020;11:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Odle AK, Akhter N, Syed MM, et al. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front Endocrinol (Lausanne). 2017;8:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kauffman AS. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front Neurosci. 2022;16:953252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz NB, Channing CP. Evidence for ovarian “inhibin”: suppression of the secondary rise in serum follicle stimulating hormone levels in proestrous rats by injection of porcine follicular fluid. Proc Natl Acad Sci U S A. 1977;74(12):5721‐5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Besecke LM, Guendner MJ, Sluss PA, et al. Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology. 1997;138(7):2841‐2848. [DOI] [PubMed] [Google Scholar]
- 8. Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149(11):5328‐5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149(8):4168‐4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56(2):293‐302. [DOI] [PubMed] [Google Scholar]
- 11. Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol. 2001;22(2):69‐106. [DOI] [PubMed] [Google Scholar]
- 12. McDevitt MA, Glidewell-Kenney C, Jimenez MA, et al. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290(1-2):24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh SP, Wolfe A, Ng Y, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol Reprod. 2009;81(3):488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alim Z, Hartshorn C, Mai O, et al. Gonadotrope plasticity at cellular and population levels. Endocrinology. 2012;153(10):4729‐4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ko C, Gieske MC, Hudgins-Spivey S, Bridges P, Lee S. Estrogen in female reproductive axis: positive estrogen feedback to pituitary. Korean J Reprod Med. 2007;34(4):207‐217. [Google Scholar]
- 16. Yasin M, Dalkin AC, Haisenleder DJ, Kerrigan JR, Marshall JC. Gonadotropin-releasing hormone (GnRH) pulse pattern regulates GnRH receptor gene expression: augmentation by estradiol. Endocrinology. 1995;136(4):1559‐1564. [DOI] [PubMed] [Google Scholar]
- 17. Childs GV. Gonadotropes and lactotropes. In: Neill J, Knobil E, eds. Physiology of Reproduction. Elsevier Press; 2006:1483‐1579. [Google Scholar]
- 18. Clarke IJ. Multifarious effects of estrogen on the pituitary gonadotrope with special emphasis on studies in the ovine species. Arch Physiol Biochem. 2002;110(1-2):62‐73. [DOI] [PubMed] [Google Scholar]
- 19. Clarke IJ, Tobin VA, Pompolo S, Pereira A. Effects of changing gonadotropin-releasing hormone pulse frequency and estrogen treatment on levels of estradiol receptor-alpha and induction of Fos and phosphorylated cyclic adenosine monophosphate response element binding protein in pituitary gonadotropes: studies in hypothalamo-pituitary disconnected ewes. Endocrinology. 2005;146(3):1128‐1137. [DOI] [PubMed] [Google Scholar]
- 20. Clay CM, Cherrington BD, Navratil AM. Plasticity of anterior pituitary gonadotrope cells facilitates the Pre-ovulatory LH surge. Front Endocrinol (Lausanne). 2020;11:616053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lloyd JM, Childs GV. Changes in the number of GnRH-receptive cells during the rat estrous cycle: biphasic effects of estradiol. Neuroendocrinology. 1988;48(2):138‐146. [DOI] [PubMed] [Google Scholar]
- 22. Janjic MM, Stojilkovic SS, Bjelobaba I. Intrinsic and regulated gonadotropin-releasing hormone receptor gene transcription in mammalian pituitary gonadotrophs. Front Endocrinol (Lausanne). 2017;8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kakar SS, Grantham K, Musgrove LC, Devor D, Sellers JC, Neill JD. Rat gonadotropin-releasing hormone (GnRH) receptor: tissue expression and hormonal regulation of its mRNA. Mol Cell Endocrinol. 1994;101(1-2):151‐157. [DOI] [PubMed] [Google Scholar]
- 24. Schirman-Hildesheim TD, Bar T, Ben-Aroya N, Koch Y. Differential gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of the female rat across the estrous cycle. Endocrinology. 2005;146(8):3401‐3408. [DOI] [PubMed] [Google Scholar]
- 25. Schirman-Hildesheim TD, Ben-Aroya N, Koch Y. Daily GnRH and GnRH-receptor mRNA expression in the ovariectomized and intact rat. Mol Cell Endocrinol. 2006;252(1-2):120‐125. [DOI] [PubMed] [Google Scholar]
- 26. Savoy-Moore RT, Schwartz NB, Duncan JA, Marshall JC. Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science. 1980;209(4459):942‐944. [DOI] [PubMed] [Google Scholar]
- 27. Childs GV, Odle AK, MacNicol MC, MacNicol AM. The importance of leptin to reproduction. Endocrinology. 2021;162(2):bqaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall JE, Schoenfeld DA, Martin KA, Crowley WF. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab. 1992;74(3):600‐607. [DOI] [PubMed] [Google Scholar]
- 29. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133(2):931‐934. [DOI] [PubMed] [Google Scholar]
- 30. Kerrigan JR, Yasin M, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit messenger ribonucleic acid expression in gonadotropin-releasing hormone (GnRH)-deficient female rats: effects of GnRH, galanin, GnRH-associated peptide, neuropeptide-Y, and thyrotropin-releasing hormone. Biol Reprod. 1995;53(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 31. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21(5):1175‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33(3):559‐584. [DOI] [PubMed] [Google Scholar]
- 33. Childs GV. Division of labor among gonadotropes. Vitam Horm. 1995;50:215‐286. [DOI] [PubMed] [Google Scholar]
- 34. Childs GV, Unabia G, Rougeau D. Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) beta-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LH beta, FSH beta, and/or growth hormone. Endocrinology. 1994;134(2):990‐997. [DOI] [PubMed] [Google Scholar]
- 35. Stamatiades GA, Carroll RS, Kaiser UB. GnRH-A key regulator of FSH. Endocrinology. 2019;160(1):57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ortolano GA, Haisenleder DJ, Dalkin AC, et al. Follicle-stimulating hormone beta subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology. 1988;123(6):2946‐2948. [DOI] [PubMed] [Google Scholar]
- 37. Qiao S, Nordstrom K, Muijs L, et al. Molecular plasticity of male and female murine gonadotropes revealed by mRNA sequencing. Endocrinology. 2016;157(3):1082‐1093. [DOI] [PubMed] [Google Scholar]
- 38. Shupnik MA, Gharib SD, Chin WW. Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol. 1989;3(3):474‐480. [DOI] [PubMed] [Google Scholar]
- 39. Zmeili SM, Papavasiliou SS, Thorner MO, Evans WS, Marshall JC, Landefeld TD. Alpha and luteinizing hormone beta subunit messenger ribonucleic acids during the rat estrous cycle. Endocrinology. 1986;119(4):1867‐1869. [DOI] [PubMed] [Google Scholar]
- 40. Allensworth-James M, Banik J, Odle A, et al. Control of the anterior pituitary cell lineage regulator POU1F1 by the stem cell determinant musashi. Endocrinology. 2021;162(3):bqaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Allensworth-James ML, Odle AK, Lim J, et al. Metabolic signalling to somatotrophs: transcriptional and post-transcriptional mediators. J Neuroendocrinol. 2020;32(11):e12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Odle AK, Benes H, Melgar Castillo A, et al. Association of Gnrhr mRNA with the stem cell determinant musashi: a mechanism for leptin-mediated modulation of GnRHR expression. Endocrinology. 2018;159(2):883‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fox RG, Park FD, Koechlein CS, Kritzik M, Reya T. Musashi signaling in stem cells and cancer. Annu Rev Cell Dev Biol. 2015;31(1):249‐267. [DOI] [PubMed] [Google Scholar]
- 44. Wen S, Schwarz R Jr, Niculescu D, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701‐2711. [DOI] [PubMed] [Google Scholar]
- 45. Li N, Yousefi M, Nakauka-Ddamba A, et al. The Msi family of RNA-binding proteins function redundantly as intestinal oncoproteins. Cell Rep. 2015;13(11):2440‐2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107(37):16372‐16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593‐605. [DOI] [PubMed] [Google Scholar]
- 48. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30(15):2114‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-Seq aligner. Bioinformatics. 2013;29(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liao Y, Smyth GK, Shi W. Featurecounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923‐930. [DOI] [PubMed] [Google Scholar]
- 51. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-Seq data. Genome Biol. 2010;11(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-Sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alexa A, Rahnenfuhrer J. topGO: Enrichment Analysis for Gene Ontology. R package version 2.46.0. Published 2021. Accessed 4 January 2022. https://bioconductor.org/packages/release/bioc/html/topGO.html
- 54. Cheung LYM, George AS, McGee SR, et al. Single-cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology. 2018;159(12):3910‐3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hao YH, Hao S, Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573‐3587.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. R: A Language and Environment for Statistical Computing. [Computer Program]. R Foundation for Statistical Computing; 2022.
- 57. Odle AK, Haney A, Allensworth-James M, Akhter N, Childs GV. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: somatotropes develop normally in the absence of circulating leptin. Endocrinology. 2014;155(11):4316‐4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gahete MD, Cordoba-Chacon J, Salvatori R, Castano JP, Kineman RD, Luque RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol. 2010;317(1-2):154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li Y, Schang G, Boehm U, Deng C-X, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem. 2017;292(6):2301‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Torrealday S, Lalioti MD, Guzeloglu-Kayisli O, Seli E. Characterization of the gonadotropin releasing hormone receptor (GnRHR) expression and activity in the female mouse ovary. Endocrinology. 2013;154(10):3877‐3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Babwah AV, Navarro VM, Ahow M, et al. GnRH neuron-specific ablation of galphaq/11 results in only partial inactivation of the neuroendocrine-reproductive axis in both male and female mice: in vivo evidence for Kiss1r-coupled galphaq/11-independent GnRH secretion. J Neurosci. 2015;35(37):12903‐12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang S, Li N, Yousefi M, et al. Transformation of the intestinal epithelium by the MSI2 RNA-binding protein. Nat Commun. 2015;6(1):6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. MacNicol AM, Hardy LL, Spencer HJ, MacNicol MC. Neural stem and progenitor cell fate transition requires regulation of Musashi1 function. BMC Dev Biol. 2015;15(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. MacNicol MC, Cragle CE, MacNicol AM. Context-dependent regulation of Musashi-mediated mRNA translation and cell cycle regulation. Cell Cycle. 2011;10(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arumugam K, MacNicol MC, Wang Y, et al. Ringo/cyclin-dependent kinase and mitogen-activated protein kinase signaling pathways regulate the activity of the cell fate determinant Musashi to promote cell cycle re-entry in Xenopus oocytes. J Biol Chem. 2012;287(13):10639‐10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Odle AK, Allensworth-James ML, Akhter N, et al. A sex-dependent, tropic role for leptin in the somatotrope as a regulator of POU1F1 and POU1F1-dependent hormones. Endocrinology. 2016;157(10):3958‐3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;48(1):A.4I.1-A.4I.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kadam KM, Mande PV, Gawas N, Ahire S, Khole LV. Autoantibodies to heat-shock protein, HSPA5, and epitope spreading: high-dose dexamethasone therapy rescues ovarian function in experimental autoimmune ovarian insufficiency mouse model. Am J Reprod Immunol. 2016;75(5):580‐593. [DOI] [PubMed] [Google Scholar]
- 69. Barsoum IB, Kaur J, Ge RS, Cooke PS, Yao HH-C. Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. FASEB J. 2013;27(7):2657‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chu A, Zhu L, Blum ID, et al. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154(8):2924‐2935. [DOI] [PubMed] [Google Scholar]
- 71. Brothers KJ, Wu S, DiVall SA, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12(3):295‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uren PJ, Vo DT, de Araujo PR, et al. RNA-binding protein musashi1 is a central regulator of adhesion pathways in glioblastoma. Mol Cell Biol. 2015;35(17):2965‐2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nguyen DTT, Lu Y, Chu KL, et al. HyperTRIBE uncovers increased MUSASHI-2 RNA binding activity and differential regulation in leukemic stem cells. Nat Commun. 2020;11(1):2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Imai T, Tokunaga A, Yoshida T, et al. The neural RNA-binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol Cell Biol. 2001;21(12):3888‐3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21(WAF-1). Mol Cell Neurosci. 2006;31(1):85‐96. [DOI] [PubMed] [Google Scholar]
- 76. Silva Moreira AR, Lim J, Urbaniak A, et al. Data from: Musashi exerts control of gonadotrope target mRNA translation during the mouse estrous cycle. Dryad. Deposited June 29, 2023. 10.5061/dryad.bcc2fqzfj [DOI] [PMC free article] [PubMed]
- 77. Tian B, Graber JH. Signals for pre-mRNA cleavage and polyadenylation. Wiley Interdiscip Rev RNA. 2012;3(3):385‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fletcher PA, Smiljanic K, Maso Previde R, et al. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne). 2019;10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ho Y, Hu P, Peel MT, et al. Single-cell transcriptomic analysis of adult mouse pituitary reveals sexual dimorphism and physiologic demand-induced cellular plasticity. Protein Cell. 2020;11(8):565‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McDonald R, Sadler C, Kumar TR. Gain-of-Function genetic models to study FSH action. Front Endocrinol (Lausanne). 2019;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Arumugam K, Wang Y, Hardy LL, MacNicol MC, MacNicol AM. Enforcing temporal control of maternal mRNA translation during oocyte cell-cycle progression. EMBO J. 2010;29(2):387‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Matalkah F, Jeong B, Sheridan M, Horstick E, Ramamurthy V, Stoilov P. The Musashi proteins direct post-transcriptional control of protein expression and alternate exon splicing in vertebrate photoreceptors. Commun Biol. 2022;5(1):1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sakakibara S, Nakamura Y, Yoshida T, et al. RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci U S A. 2002;99(23):15194‐15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bennett CG, Riemondy K, Chapnick DA, et al. Genome-wide analysis of Musashi-2 targets reveals novel functions in governing epithelial cell migration. Nucleic Acids Res. 2016;44(8):3788‐3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Duggimpudi S, Kloetgen A, Maney SK, et al. Transcriptome-wide analysis uncovers the targets of the RNA-binding protein MSI2 and effects of MSI2's RNA-binding activity on IL-6 signaling. J Biol Chem. 2018;293(40):15359‐15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen H-Y, Wang ML, Laurent B, et al. Musashi-1 promotes stress-induced tumor progression through recruitment of AGO2. Theranostics. 2020;10(1):201‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li M, Li A-Q, Zhou S-L, Lv H, Wei P, Yang W-T. RNA-binding protein MSI2 isoforms expression and regulation in progression of triple-negative breast cancer. J Exp Clin Cancer Res. 2020;39(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Makhov P, Bychkov I, Faezov B, et al. Musashi-2 (MSI2) regulates epidermal growth factor receptor (EGFR) expression and response to EGFR inhibitors in EGFR-mutated non-small cell lung cancer (NSCLC). Oncogenesis. 2021;10(3):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kelberman D, Dattani MT. The role of transcription factors implicated in anterior pituitary development in the aetiology of congenital hypopituitarism. Ann Med. 2006;38(8):560‐577. [DOI] [PubMed] [Google Scholar]
- 91. MacNicol MC, Cragle CE, Arumugam K, Fosso B, Pesole G, MacNicol AM. Functional integration of mRNA translational control programs. Biomolecules. 2015;5(3):1580‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang J, Lan L, Wu X, Xu L, Miao Y. Mechanism of RNA recognition by a Musashi RNA-binding protein. Curr Res Struct Biol. 2022;4:10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zearfoss NR, Deveau LM, Clingman CC, et al. A conserved three-nucleotide core motif defines Musashi RNA binding specificity. J Biol Chem. 2014;289(51):35530‐35541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Iwaoka R, Nagata T, Tsuda K, et al. Structural insight into the recognition of r(UAG) by Musashi-1 RBD2, and construction of a model of musashi-1 RBD1-2 bound to the minimum target RNA. Molecules. 2017;22(7):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Greig F, Weisz J. Preovulatory levels of luteinizing hormone, the critical period and ovulation in rats. J Endocrinol. 1973;57(2):235‐245. [DOI] [PubMed] [Google Scholar]
- 96. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Das N, Kumar TR. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Mol Endocrinol. 2018;60(3):R131‐R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Charlesworth A, Wilczynska A, Thampi P, Cox LL, MacNicol AM. Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation. EMBO J. 2006;25(12):2792‐2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Qiao S, Alasmi S, Wyatt A, et al. Intra-pituitary follicle-stimulating hormone signaling regulates hepatic lipid metabolism in mice. Nat Commun. 2023;14(1):1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
The RNAseq data described in this study have been deposited in the Gene Expression Omnibus under accession number GSE199098. Go to https://urldefense.proofpoint.com/v2/url?u=https-3A__www.ncbi.nlm.nih.gov_geo_query_acc.cgi-3Facc-3DGSE199098&d=DwIBAg&c=27AKQ-AFTMvLXtgZ7shZqsfSXu-Fwzpqk4BoASshREk&r=dZZEevHpMAUI1uLm27Y4zMzKeai_60o5nCzUFripuNo&m=KXct0JwmIYrlaAmK41HxpL_xsNnEhMZGNeB_wBx9I8c&s=hXd50nJ33UE9sqvd4z_i5QCn4LFvKxbaA8LSDwUu74&e=