Abstract
Aims:
Herein, we report safety outcomes for varenicline solution nasal spray (VNS) within the context of clinical trial discontinuation, contrasting those with discontinuation outcomes from topical cyclosporine and lifitegrast clinical trials.
Materials & methods:
1061 subjects were randomized across three clinical trials to receive either VNS 0.06 mg, VNS 0.03 mg, VNS 0.006 mg or vehicle control. Subjects who discontinued from treatment were noted and assigned to their appropriate categories.
Results:
Despite treatment emergent adverse events, 93.5% of subjects receiving VNS completed the treatment period. By comparison, only 80% of subjects in the integrated clinical trials for cyclosporine ophthalmic emulsion and 91% of subjects in the integrated trials for lifitegrast ophthalmic solution completed the full treatment period, respectively.
Conclusion:
In clinical trials, VNS demonstrated improvements in dry eye disease signs and symptoms, was well-tolerated, and had an overall completion rate >93%. Conventional dry eye treatments (e.g., cyclosporine and lifitegrast) noted considerably higher discontinuation rates in their clinical trials.
Keywords: discontinuation, dry eye disease, treatment adherence, TYRVAYA, varenicline solution nasal spray
Plain language summary
What is this article about?
Varenicline solution nasal spray (TYRVAYA®; Oyster Point Pharmaceuticals, Princeton, NJ) is FDA approved for the treatment of signs and symptoms of dry eye disease. It is the first and only nasal spray specifically approved for the treatment of any ocular disorder.
What were the results?
In clinical trials, OC-01 VNS was well tolerated, with over 93% of subjects completing the studies. Ocular adverse events were seen in <5%. Other pharmaceuticals for dry eye disease showed poorer tolerability and higher discontinuation rates in their respective clinical trials.
What do the results of the study mean?
Products with better tolerability may allow for greater treatment adherence, and potentially better outcomes.
Tweetable abstract
Varenicline solution nasal spray displayed excellent safety in clinical trials as well as greater tolerability and lower discontinuation rates than dry eye disease competitors in their respective clinical trials.
Dry eye disease, also known as dry eye syndrome or simply ‘dry eye’, is a chronic condition of the ocular surface. An estimated 38 million patients in the US have dry eye disease, with the reported prevalence being as high as 50% in studies from around the world [1–3]. Dry eye disease is one of the more challenging diseases for optometrists and ophthalmologists to treat, due in part to its complex nature, limited diagnostic applications, and inconsistency of clinical presentation. In a recent international consensus paper on dry eye disease, noted experts defined the condition as follows: “Dry eye is a multifactorial disease characterized by a persistently unstable and/or deficient tear film causing discomfort and/or visual impairment, accompanied by variable degrees of ocular surface epitheliopathy, inflammation and neurosensory abnormalities” [4]. Dry eye disease often presents with bothersome symptoms such as scratchiness, burning, and intermittent blurring of vision, which can negatively impact visual function and productivity. The condition may demonstrate clinically evident disruption or damage to the ocular surface tissues, although there is often little correlation between the symptoms and signs of dry eye disease [5–7]. Historically, conservative management strategies have included ophthalmic lubricants or ‘artificial tears’, as well as punctal occlusion therapy. Data suggest that augmentation of the body's natural tear film is preferable to the continual use of artificial tears, due to the inherent, endogenous proteins, growth factors, anti-microbial and anti-inflammatory elements in natural tears, which are essential for maintenance of normal ocular surface metabolism [8–10]. In more severe or recalcitrant cases, topical pharmaceutical anti-inflammatory agents (i.e., corticosteroid and/or immunomodulatory drugs) may be employed for dry eye disease. Whatever therapeutic option is employed, the ultimate aim of dry eye disease management is to restore the homeostasis of the ocular surface and natural tear film [11].
Topical prescription ophthalmic agents for dry eye disease such as cyclosporine and lifitegrast have been widely available for a number of years. Data from clinical trials and post-marketing surveillance reports indicate these medications are subject to high discontinuation rates among patients [12–16]. Factors that may contribute to medication non-adherence in dry eye disease include a delay between treatment initiation and symptomatic relief, adverse side effects such as ocular burning or blurring upon instillation, and high medication costs [16]. Less common reasons for poor compliance include a lack of manual dexterity, concurrent contact lens wear, or simply an aversion to the instillation of eye drops [17–20].
Varenicline solution nasal spray represents an alternative therapy for the treatment of dry eye disease. Varenicline is a water-soluble acetylcholine receptor agonist that binds with high affinity and selectivity at human α4β2, α4α6β2, α3β4, α3α5β4, and α7 nicotinic acetylcholine receptors [21,22]. When administered into the anterior nasal cavity, varenicline is believed to bind with nicotinic acetylcholine receptors on terminal branches of the trigeminal nerve, opening ligand-gated calcium ion channels and activating the trigeminal-parasympathetic pathway [23]. This pharmacologic neuro-activation is theorized to upregulate production of tears via the innervated lacrimal functional unit, including the conjunctival goblet cells, meibomian glands, primary and accessory lacrimal glands [24]. Recent phase II and phase III clinical studies with varenicline solution nasal spray demonstrated statistically significant improvements in both signs and symptoms of dry eye disease, and was safe and well tolerated [25–27]. In October 2021, varenicline solution nasal spray 0.03 mg was approved by the US FDA as TYRVAYA®, for the signs and symptoms of dry eye disease.
This retrospective integrated safety analysis of the ONSET-1, ONSET-2 and MYSTIC trial data reviews the frequency of adverse events (ocular and non-ocular) and reported adherence/completion rates with other prescription dry eye disease therapies to elucidate and characterize compliance issues with prescription therapeutic management for dry eye disease.
Methods
Study Design
This was a post-hoc, pooled data analysis from the ONSET-1, ONSET-2 and MYSTIC clinical trials. ONSET-1 (clinicaltrials.gov; NCT03636061) was a phase IIb, multicenter, randomized, vehicle-controlled, double-masked study conducted at three centers in USA between 15 August 2018, and 26 September 2018 [25]. MYSTIC (clinicaltrials.gov; NCT03873246) was a phase II, randomized, vehicle-controlled, masked study conducted at a single site in Mexico between 18 February 2019, and 22 November 2019 [26]. ONSET-2 was a phase III, multicenter, randomized, vehicle-controlled, double-masked study (clinicaltrials.gov; NCT04036292) conducted at 22 centers in USA between 23 July 2019, and 10 April 2020 [27]. The clinical study designs from ONSET-1, ONSET-2 and MYSTIC are displayed in Figure 1.
Figure 1. . Study design for ONSET-1, ONSET-2 and MYSTIC clinical trials.

OC-01 represents the pre-approval clinical designation for varenicline solution nasal spray.
Study measures & outcomes
A total of 1,061 subjects were randomized across the three aforementioned clinical trials. 330 received at least one dose of varenicline solution nasal spray 0.06 mg (1.2 mg/ml), 349 received at least one dose of varenicline solution nasal spray 0.03 mg (0.6 mg/ml), and 47 received at least one dose of varenicline solution nasal spray 0.006 mg (0.12 mg/ml); the remaining 335 received vehicle control [25–27]. Note that varenicline solution nasal spray 0.006 mg was only evaluated in the ONSET-1 trial, which explains the comparatively small cohort. Subjects in ONSET-1 and ONSET-2 were followed for 28 days (4 weeks), while subjects in MYSTIC were followed for 84 days (12 weeks). Subjects discontinued from treatment were noted and assigned to their appropriate categories (Table 1).
Table 1. . Completion and discontinuation among subjects in ONSET-1, ONSET-2 and MYSTIC.
| Category | Varenicline solution nasal spray | Vehicle |
|||
|---|---|---|---|---|---|
| 0.006 mg n = 47 n (%) |
0.03 mg n = 349 n (%) |
0.6 mg n = 330 n (%) |
Overall, n = 726 n (%) |
n = 335 n (%) |
|
| Subjects entered the study | 47 | 349 | 330 | 726 | 335 |
| ONSET-1 | 47 (100) | 48 (13.8) | 44 (13.3) | 139 (19.1) | 43 (12.8) |
| ONSET-2 | 0 | 260 (74.5) | 245 (74.2) | 505 (69.6) | 251 (74.9) |
| MYSTIC | 0 | 41 (11.7) | 41 (12.4) | 82 (11.3) | 41 (12.2) |
| Subjects completed treatment period of the study | 47 (100) | 333 (95.4) | 299 (90.6) | 679 (93.5) | 317 (94.6) |
| Subjects discontinued from treatment | 0 | 16 (4.6) | 31 (9.4) | 47 (6.5) | 18 (5.4) |
| Non-fatal adverse event | 0 | 7 (2.0) | 14 (4.2) | 21 (2.9) | 7 (2.1) |
| Protocol violation | 0 | 0 | 0 | 0 | 0 |
| Lost to follow-up | 0 | 6 (1.7) | 5 (1.5) | 11 (1.5) | 7 (2.1) |
| Pregnancy | 0 | 0 | 0 | 0 | 0 |
| Physician decision | 0 | 1 (0.3) | 0 | 1 (0.1) | 0 |
| Subject noncompliance | 0 | 0 | 2 (0.6) | 2 (0.3) | 1 (0.3) |
| Death | 0 | 0 | 0 | 0 | 0 |
| Study terminated by sponsor | 0 | 0 | 0 | 0 | 0 |
| Withdraw by subject | 0 | 2 (0.6) | 9 (2.7) | 11 (1.5) | 3 (0.9) |
| Other reasons | 0 | 0 | 1 (0.3) | 1 (0.1) | 0 |
Results
The most common treatment emergent adverse event (TEAE) reported across the three clinical trials was sneezing. In those receiving varenicline solution nasal spray 0.06 mg, 83.9% reported a sneeze after any administration of the nasal spray; in those receiving varenicline solution nasal spray 0.03 mg, 82.2% reported a sneeze after any administration. In the vehicle control group, 22.4% reported sneezing. In terms of severity however, 98% of subjects rated the associated sneezing as ‘mild’. This included 271 of the 277 (97.8%) subjects treated with varenicline solution nasal spray 0.06 mg, 281 of the 287 (97.9%) subjects treated with varenicline solution nasal spray 0.03 mg, and 75 of the 75 (100%) subjects treated with vehicle control. Other TEAEs associated with varenicline solution nasal spray in >5% of subjects included cough (19.7 and 15.8%, respectively in the 0.06 mg and 0.03 mg groups), throat irritation (16.1 and 12.6%, respectively) and instillation site (nose) irritation (13.3 and 8.3%, respectively). There were no drug-related serious adverse events in any of the studies. A summary of non-ocular TEAEs is shown in Figure 2.
Figure 2. . Notable non-ocular treatment emergent adverse events occurring in subjects in the integrated safety analysis.

OC-01 represents the pre-approval clinical designation for varenicline solution nasal spray.
Ocular TEAEs for varenicline solution nasal spray in clinical trials were 12.7–12.9%) The most commonly reported complaints were reduced visual acuity (3.6–3.7%) and conjunctival hyperemia (3.3–3.7%). TEAEs typically associated with existing topical dry eye therapies such as cyclosporine and lifitegrast (e.g., eye pain, eye irritation and eye pruritis) were <1.0% for varenicline solution nasal spray 0.06 mg, and <0.5% for varenicline solution nasal spray 0.03 mg. The instances of ocular TEAEs with varenicline solution nasal spray were consistent with vehicle control in terms of frequency and severity. A summary of ocular TEAEs is shown in Figure 3.
Figure 3. . Notable ocular treatment emergent adverse events occurring in subjects in the integrated safety analysis.
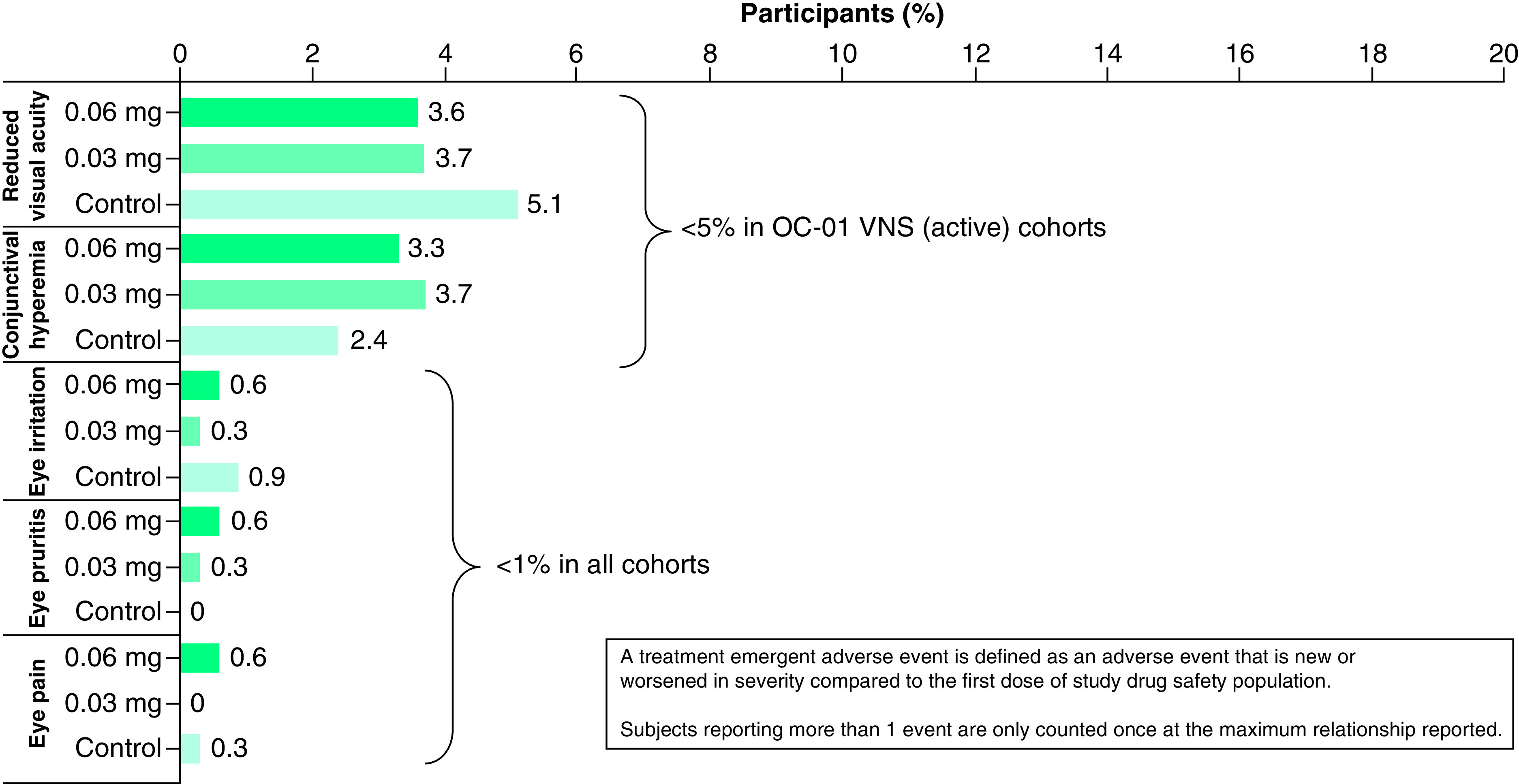
OC-01 represents the pre-approval clinical designation for varenicline solution nasal spray.
VNS: Varenicline solution nasal spray.
In ONSET-1, ONSET-2 and MYSTIC, 93.5% of subjects receiving varenicline solution nasal spray completed the treatment period. The percentage of subjects discontinued from treatment were: 0.006 mg, 0%; 0.03 mg, 4.6%; 0.06 mg, 9.4%; vehicle control, 5.4%. The percentage of subjects who specifically discontinued due to (non-fatal) adverse events were: 0.03 mg, 2.0%; 0.06 mg, 4.2%; vehicle control, 2.1% (Table 1).
Discussion
Compliance and adherence to prescription therapies are critical in the management of dry eye disease patients. Dry eye disease is not only a chronic disorder, but also believed to be progressive in the vast majority of individuals [28]. Hence, poor adherence or treatment discontinuation may potentially result in worsening of the disease state, furthering the complexity of therapeutic intervention necessary for these complex patients. Reasons for non-compliance and early discontinuation of dry eye disease therapies may include delayed symptom improvement after treatment initiation, instillation discomfort, and unanticipated costs [16]. Additionally, research suggests a majority of dry eye patients may use topical ophthalmic agents only when symptomatic, or when it is convenient to do so [29]. Sporadic and inconsistent use of therapy may contribute to discontinuation over time.
More than 93% of subjects treated with varenicline solution nasal spray across the ONSET-1, ONSET-2 and MYSTIC clinical trials completed the study period. This completion rate suggests that a greater overall proportion of patients completed the clinical studies with varenicline solution nasal spray than what was observed in other clinical trial reports with prescription dry eye disease therapies [12–14]. Cyclosporine ophthalmic emulsion 0.05% (Restasis®, Allergan; Irvine, CA) was assessed for safety across three clinical trials, although data is only reported for two of the three. Individual discontinuation rates for topical cyclosporine in protocols -002 and -003 were 20.7 and 19%, respectively [12]. The integrated treatment discontinuation rate for the available studies was 19.8% (58 out of 293 subjects) [12]. Lifitegrast ophthalmic solution 5% (Xiidra®, Novartis; NJ, USA) discontinuation was evaluated across three phase III clinical trials. Individual discontinuation rates for topical lifitegrast reported in OPUS-1, OPUS-2 and OPUS-3 were 4.1, 10.3 and 10.1%, respectively [13]. The integrated treatment discontinuation rate for lifitegrast was 8.9% (89 out of 1066 subjects) [13]. Discontinuation rates for varenicline solution nasal spray 0.03 mg (the commercially available and currently marketed formulation) were: ONSET-1, 4.2% (2 out of 48 subjects); ONSET-2, 3.5% (9 out of 260 subjects); MYSTIC, 12.2% (5 out of 41 subjects). The integrated discontinuation rate across these three trials was 4.6% (16 out of 349 subjects). Table 2.
Table 2. . Treatment discontinuation rates in clinical trials for marketed drug concentrations.
| Drug/Product | Study designation, (n) % | |||
|---|---|---|---|---|
| Cyclosporine ophthalmic emulsion 0.05% |
PROTOCOL-001 (31) |
PROTOCOL-002 (135) |
PROTOCOL-003 (158) |
Integrated (293) |
| REDACTED | (28) 20.7% | (30) 19% | (58) 19.8% | |
| Lifitegrast ophthalmic solution 5% |
OPUS-1 (293) |
OPUS-2 (360) |
OPUS-3 (355) |
Integrated (1,066) |
| (12) 4.1% | (37) 10.3% | (36) 10.1% | (85) 8.9% | |
| Varenicline solution nasal spray 0.03 mg |
ONSET-1 (48) |
MYSTIC (41) |
ONSET-2 (260) |
Integrated (349) |
| (2) 4.2% | (5) 12.2% | (9) 3.5% | (16) 4.6% | |
Ocular TEAEs for varenicline solution nasals spray were reported as low across all clinical trials; this may potentially be expected per the nasal route of administration and hence an “ocular surface-sparing” route of delivery. Ocular TEAEs reported were: reduced visual acuity, 3.7%; conjunctival hyperemia, 3.7%; eye irritation, 0.3%; eye pruritis, 0.3%; eye pain, 0%. Comparatively, clinical study TEAE's reported with topically administered cyclosporine ophthalmic emulsion 0.05% were: burning or stinging, 18.1%; conjunctival hyperemia, 2.0%; visual disturbance, 1.7%; eye pain, 1.0% [12,14]. Lifitegrast ophthalmic solution 5% clinical studies reported: instillation site irritation, 16.1%; instillation site pain, 8.1%; reduced visual acuity, 3.4%; ocular hyperemia, 1.4%; eye pruritis, 1.4% [13].
While adherence rates within rigorously monitored clinical trials may not be predictive of those in clinical practice, they may provide some insight as to factors that may compromise compliance with prescribed therapies for dry eye disease. In the cyclosporine ophthalmic emulsion 0.05% and lifitegrast ophthalmic solution 5% studies, the most notable TEAE was ocular irritation, often described as burning or stinging upon instillation. Not surprisingly, a post-marketing surveillance study by White et al. suggests the most frequently reported side effect of treatment with topical cyclosporine and lifitegrast was burning on instillation, with 21% and 22% of respondents reporting this finding respectively [15]. In an earlier study by White and colleagues, adherence rates for topical cyclosporine and lifitegrast were found to be low during a 12-month period after initial treatment administration, with discontinuation occurring in 70.8% of those receiving cyclosporine and 64.4% of those receiving lifitegrast [16]. It is conceivable that products with greater ocular tolerability may facilitate better adherence to therapy and potentially better outcomes in the management of chronic dry eye disease.
Limitations
Limitations of the adherence rate findings across clinical studies may be greater within the conditions of monitored, randomized, and controlled clinical studies and may not represent real-world findings. Differing factors such as study design, treatment and placebo controls, inclusion and exclusion criteria, patient population baseline demographics and characteristics, and time periods in which the studies were conducted also limit interpretations of comparative outcome findings.
Conclusion
Subjects in the phase II and phase III clinical trials for varenicline solution nasal spray demonstrated excellent adherence to therapy, with an overall completion rate of 93.5%. Adverse event discontinuation rates with varenicline solution nasal spray were low across all treatment groups (<10%), and comparable for the 0.03 mg (4.6%) and vehicle control groups (5.4%) within conditions of the studies. Additionally, these discontinuation rates for varenicline solution nasal spray in the treatment of dry eye disease were notably lower than those for cyclosporine 0.05% ophthalmic emulsion and lifitegrast 5% ophthalmic solution in their respective clinical trials, with values of 19.8% and 8.9%, respectively. Post-market studies are recommended to further assess the real-world adherence/discontinuation rates of varenicline solution nasal spray in the US population.
Summary points.
Varenicline solution nasal spray is FDA approved for the treatment of signs and symptoms of dry eye disease.
The clinical trials involving varenicline solution nasal spray demonstrated very low discontinuation rates, with over 93% of subjects completing the studies.
Other current pharmaceuticals for dry eye disease showed poorer tolerability and higher discontinuation rates in their respective clinical trials.
This research can help to better inform payers and clinicians regarding therapeutic options for dry eye disease, potentially reducing patient complaints while improving treatment adherence.
Acknowledgment
Portions of these data were presented at the Association for Managed Care Pharmacy Annual Meeting; 29 March – 1 April 2022; IL, USA, and the 125th Annual American Optometric Association Congress (Optometry's Meeting); 15–18 June 2022; IL, USA.
Footnotes
Financial & competing interests disclosure
Oyster Point Pharma, Inc. was involved in the study design, data collection, data analysis, and preparation of the manuscript and is the manufacturer/licensee of varenicline solution nasal spray (Tyrvaya®). Oyster Point Pharma, Inc sponsored the phase II and Phase III clinical studies from which these analysis data are obtained. SG Hauswirth consultant/advisor – Allergan, Claris Bio, Dompé, Glaukos, Horizon, Kala, Novartis, NuSight Medical, Ocular Therapeutix, Oyster Point Pharma, Science Based Health, Sight Sciences, TearRestore, and Théa; investigator for Claris Bio, Dompé, NicOx, Oyster Point Pharma, Sylentis, TearRestore, and Tear Solutions. AG Kabat, employee, shareholder – Oyster Point Pharma, Inc. M Hemphill, employee, shareholder – Oyster Point Pharma, Inc. K Somaiya, employee, shareholder – Oyster Point Pharma, Inc. LH Hendrix, employee, shareholder – Oyster Point Pharma, Inc. AA Gibson, employee, shareholder – Oyster Point Pharma, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was conducted in accordance with the principles of Declaration of Helsinki and in compliance with the ICH E6 GCP Consolidated Guideline, ISO 14155:2011, and the applicable US FDA 21 CFR regulations. Before clinical study initiation, the protocol and all amendments, the informed consent form, any other written information given to subjects, and any advertisements planned for subject recruitment was approved by an IRB/IEC (Alpha IRB, CA, USA).
Data sharing statement
The data used to support the primary findings of this study are available at ClinicalTrials.gov (NCT03636061, NCT03873246 and NCT04036292).
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Market Scope. Ophthalmic Comprehensive Reports. 2020 Dry Eye Products Market Report: Global Analysis for 2019 to 2025 (2022). www.market-scope.com/pages/reports/219/2020-dry-eye-products-market-report-a-global-analysis-for-2019-to-2025-october-2020 (Accessed: 18 August 2021). [Google Scholar]
- 2.Paulsen AJ, Cruickshanks KJ, Fischer ME et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 157(4), 799–806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton F, Alves M, Bunya VY et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 15(3), 334–365 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Tsubota K, Pflugfelder SC, Liu Z et al. Defining dry eye from a clinical perspective. Int. J. Mol. Sci. 21(23), 9271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents the most current consensus for dry eye disease management.
- 5.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 23(8), 762–770 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JD, Keith MS, Sudharshan L, Snedecor SJ. Associations between signs and symptoms of dry eye disease: a systematic review. Clin. Ophthalmol. 9, 1719–1730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMonnies CW. Why the symptoms and objective signs of dry eye disease may not correlate. J. Optom. 14(1), 3–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai MC, Cosar CB, Cohen EJ, Rapuano CJ, Laibson PR. The clinical efficacy of silicone punctal plug therapy. Cornea 21(2), 135–139 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Reese V, Youngbar PR. The effect of punctal occlusion on tear lactoferrin in aqueous deficient dry eye patients. Adv. Exp. Med. Biol. 506(Pt B), 1269–1271 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Masoudi S. Biochemistry of human tear film: a review. Exp. Eye Res. 220, 109101 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Craig JP, Nelson JD, Azar DT et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 15(4), 802–812 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Center for Drug Revolution and Research. New Drug Application (NDA) 21-023. Medical Review(s), (2002). www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-023_Restasis_Medr.pdf (Accessed: 6 May 2022). [Google Scholar]
- 13.Center for Drug Revolution and Research. New Drug Application (NDA) 208073Orig1s000. Statistical Review(s), (2016). www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208073Orig1s000StatR.pdf (Accessed: 6 May 2022). [Google Scholar]
- 14.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase III Study Group. Ophthalmology 107(4), 631–639 (2000). [DOI] [PubMed] [Google Scholar]
- 15.White DE, Zhao Y, Jayapalan H et al. Treatment satisfaction among patients using anti-inflammatory topical medications for dry eye disease. Clin. Ophthalmol. 14, 875–883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Offers real-world data on dry eye disease management trends.
- 16.White DE, Zhao Y, Ogundele A et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin. Ophthalmol. 13, 2285–2292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Offers real-world data on dry eye disease management trends.
- 17.Adamson E, Kendall G. Difficulty in eye drop administr ion for people with rheumatoid arthritis. Br. J. Occup. Ther. 79(9), 550–556 (2016). [Google Scholar]
- 18.An JA, Kasner O, Samek DA, Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J. Cataract Refract. Surg. 40(11), 1857–1861 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Davies I, Williams AM, Muir KW. Aids for eye drop administration. Surv. Ophthalmol. 62(3), 332–345 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Sujuan JL, Handa S, Perera C, Chia A. The psychological impact of eyedrops administration in children. J. AAPOS 19(4), 338–343 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J. Pharmacol. Exp. Ther. 342(2), 327–334 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol. Pharmacol. 70(3), 801–805 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem. Senses 25(1), 61–66 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Varenicline nasal spray (Tyrvaya) for dry eye disease. Med. Lett. Drugs Ther. 63(1639), 198–199 (2021). [PubMed] [Google Scholar]
- 25.Wirta D, Torkildsen G, Boehmer B et al. ONSET-1 Phase IIb randomized trial to evaluate the safety and efficacy of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease. Cornea 41(10), 1207–1216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Original report – pivotal clinical data for varenicline solution nasal spray.
- 26.Quiroz-Mercado H, Hernandez-Quintela E, Chiu KH, Henry E, Nau JA. A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (varenicline solution) nasal spray for dry eye disease: the MYSTIC study. Ocul. Surf. 24, 15–21 (2021). [DOI] [PubMed] [Google Scholar]; • Original report – clinical data for varenicline solution nasal spray.
- 27.Wirta D, Vollmer P, Paauw J et al. ONSET-2 Study Group. Efficacy and safety of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease: the ONSET-2 Phase III randomized trial. Ophthalmology 129(4), 379–387 (2022). [DOI] [PubMed] [Google Scholar]; •• Original report – pivotal clinical data for varenicline solution nasal spray.
- 28.Tavakoli A, Flanagan JL. Dry eye disease: an (in)convenient truth. Clin. Exp. Optom. 105(2), 222–229 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Uchino M, Yokoi N, Shimazaki J, Hori Y, Tsubota K, Japan Dry Eye Society. Adherence to eye drops usage in dry eye patients and reasons for non-compliance: a web-based survey. J. Clin. Med. 11(2), 367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Offers real-world data on non-compliance in dry eye disease management.


