Abstract
Aims:
With no head-to-head studies comparing the effectiveness of lanadelumab and berotralstat for prevention of hereditary angioedema (HAE) attacks, this network meta-analysis (NMA) aimed to indirectly compare the effectiveness of these treatments.
Materials & methods:
The NMA, using the published data from Phase III trials, was performed using a frequentist weighted regression-based approach following Rücker et al. Efficacy outcomes of interest were HAE attack rate per 28 days and ≥90% reduction in monthly HAE attacks.
Results & conclusion:
In this NMA, lanadelumab 300 mg administered every 2 weeks or every 4 weeks was associated with statistically significantly higher effectiveness versus berotralstat 150 mg once daily (q.d.) or 110 mg q.d. for both efficacy outcomes assessed.
Keywords: berotralstat, comparative effectiveness, hereditary angioedema, indirect treatment comparison, lanadelumab, prophylaxis
Hereditary angioedema (HAE) with C1 inhibitor (C1-INH) deficiency (HAE type I) or dysfunction (HAE type II) is a rare genetic disease associated with recurring, unpredictable episodes of swelling affecting subcutaneous or submucosal tissues [1–3]. HAE attacks can be painful and disfiguring, and laryngeal attacks pose a risk of asphyxiation [1,2]. Furthermore, patients with HAE are often limited in their ability to perform daily activities at work, school or home and report poor health related quality of life, including symptoms of anxiety and depression [4,5].
The underlying pathophysiology of HAE lies in the kallikrein-kinin system. In patients with HAE type I/II, the amount of plasma kallikrein is elevated due to insufficient inhibition by C1-INH. An elevated level of plasma kallikrein in turn leads to overproduction of bradykinin, a potent endogenous vasodilator [2,6]. Elucidation of the underlying pathophysiology led to the development of medications targeting the plasma kallikrein for the treatment or prevention of HAE attacks [3].
HAE treatments can be classified as on-demand treatment, short-term prophylaxis and long-term prophylaxis (LTP). On-demand medications are used to treat HAE attacks as they occur; short-term prophylaxis medications are used to minimize the risk of HAE attacks during situations known to have high risk of HAE attacks (e.g., medical, surgical or dental procedures); and LTP medications are used to prevent or reduce the frequency and/or severity of HAE attacks [7]. Current guidelines for HAE management recommend considering LTP in all patients with HAE type I/II, taking into account several factors including disease activity, patients’ quality of life and patient preference [7–9]. Guideline recommended first-line options for LTP in HAE include newer plasma kallikrein inhibitors lanadelumab and berotralstat as well as plasma-derived C1-INH, which has a longer history of use for LTP in HAE [7–9].
Lanadelumab, a subcutaneously administered fully human monoclonal antibody, is a specific, potent and long-acting inhibitor of active plasma kallikrein [10–12]. Lanadelumab is approved for LTP in patients with HAE aged ≥2 years in the US and in patients with HAE aged ≥12 years in the EU [11,12]. In the Phase III, randomized, double-blind, placebo-controlled HELP trial (NCT02586805), treatment with subcutaneous lanadelumab for 26 weeks significantly reduced HAE attack rate compared with placebo by 87% in the lanadelumab 300 mg every 2 weeks (Q2W) group (p < 0.001), 73% in the lanadelumab 300 mg every 4 weeks (Q4W) group (p < 0.001) and 76% in the lanadelumab 150 mg every 4 weeks group (p < 0.001) [13].
Berotralstat is an oral synthetic small-molecule inhibitor of plasma kallikrein [14]. Berotralstat is approved for LTP in patients with HAE aged ≥12 years in the US and EU [15,16]. In the Phase III, randomized, double-blind, placebo-controlled APeX-2 trial (NCT03485911), berotralstat reduced HAE attack rates compared with placebo by 44% in the berotralstat 150 mg once daily (q.d.) group (p < 0.001) and 30% in the berotralstat 110 mg q.d. group (p = 0.024) [17].
Intravenous plasma-derived C1-INH for LTP of HAE attacks (Cinryze, Takeda) is approved for LTP in patients with HAE aged ≥6 years in the US and EU [18,19]. C1-INH for LTP was assessed in the Phase III, randomized, double-blind, placebo-controlled crossover CHANGE trial (NCT01005888) [20]. In this study, C1-INH 1000 IU twice weekly (BIW) significantly reduced the normalized rate of HAE attacks by 6.47 (95% CI: 4.21, 8.73; p < 0.001) attacks per 12-week crossover period [20].
There are no head-to-head studies comparing the efficacy of lanadelumab and berotralstat for LTP to prevent HAE attacks. In the absence of direct comparative trials, a network meta-analysis (NMA) is a valid approach for indirect treatment comparison (ITC) to estimate relative treatment effects based on published data from different trials [21]. NMAs allow preservation of the randomization from the trials included in the analysis and thus maintain the separation of drug efficacy from placebo effects, and they can partially take account of prognostic characteristics of patients from different trials [21,22].
Although, to our knowledge, there are no trial data directly comparing guideline-recommended first-line options (berotralstat, C1-INH, and lanadelumab) for HAE prophylaxis, an ITC between lanadelumab and C1-INH using the data from the HELP and CHANGE studies has been reported recently [23]. Here, we report the results of indirect comparison between lanadelumab and berotralstat via a frequentist approach to NMAs using a common comparator (placebo).
Materials & methods
NMA overview
A systematic literature review (SLR) was performed to inform the NMA. MEDLINE, Embase, MEDLINE In-Process and The Cochrane Library (including The Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, Cochrane Central Register of Controlled Trials and Health Technology Assessment Database) were searched on 29 June 2017 and updated on 25 July 2018 for publications since database inception to July 2018. Conference proceedings for the 2016–2018 European Academy of Allergy and Clinical Immunology (EAACI), 2016–2018 American College of Allergy, Asthma, and Immunology (ACAAI), 2015–2016 World Allergy Congress (WAC) and 2016 European Society of Immunodeficiency (ESID) congresses, as well as Health Technology Assessment websites (National Institute for Health and Care Excellence, Canadian Agency for Drugs and Technologies in Health Common Drug Review, Scottish Medicines Consortium and All Wales Medicines Strategy Group) were also searched. Studies were included in the SLR if they were randomized controlled trials (RCTs) irrespective of blinding status, non-RCTs, observational studies, single-arm studies, cohort studies (both prospective and retrospective), long-term follow-up studies or systematic reviews and meta-analyses of RCTs/non-RCTs investigating short-term or long-term prophylactic therapies (C1-INH, lanadelumab, attenuated androgens [danazol, stanozolol], oxandrolone, methyltestosterone and testosterone) for HAE prophylaxis in patients with HAE type I/II aged ≥12 years. Case reports, case series, pharmacokinetic studies, economic studies, preclinical studies, reviews, letters or comment articles were excluded. Full inclusion and exclusion criteria are reported in Supplementary Table 1. An updated search was conducted on 26 February 2021 using the inclusion criteria for the 2018 SLR; however, LTP therapies other than C1-INH, lanadelumab and attenuated androgens were also considered.
Following the SLR searches, a feasibility assessment was conducted to determine which RCTs identified by the searches were suitable for inclusion in the NMA. Only studies on treatments in doses that were subsequently approved for use in LTP were included in the NMA; treatments that were not relevant (e.g., did not include LTP-approved doses or were not approved for reimbursement at the time NMA was performed) were excluded. The feasibility assessment focused on study similarity in terms of study design, baseline and disease characteristics and outcome definitions. The availability of data to link networks on outcomes of interest was also evaluated.
According to the US and the EU labeling, the recommended starting dose of lanadelumab is 300 mg Q2W, and a dose reduction to 300 mg Q4W may be considered in patients who are well controlled (stably attack-free on treatment) [11,12]. Accordingly, only these lanadelumab doses were considered for inclusion in the current comparative analysis. Similarly, the recommended dose of berotralstat in the US and the EU is 150 mg q.d. [15,16]. In the US, dose reduction to 110 mg q.d. is recommended in patients with moderate or severe hepatic impairment and those receiving chronic administration of P-glycoprotein or breast cancer resistance protein (BRCP) inhibitors, and may be considered in patients who have a gastrointestinal reaction to berotralstat [15]. Therefore, both doses of berotralstat (150 and 110 mg q.d.) were considered for inclusion in the evidence network.
The baseline characteristics of the populations of the studies included in the evidence network were assessed descriptively, comparing the mean age, percentage of female patients enrolled, baseline HAE attack rate, weight and BMI; only data reported in published literature were used for baseline characteristics assessment.
Outcomes
Outcomes of interest for this NMA were the HAE attack rate per 28 days during treatment with either lanadelumab or berotralstat and the probability of achieving ≥90% reduction in the number of monthly HAE attacks.
NMA methodology: frequentist weighted regression-based approach
For the current NMA, all analyses were conducted through a frequentist weighted regression-based approach to NMA following Rücker et al. [24]. Unlike an ITC following Bucher [25], the Rücker et al. NMA methodology allows multiple pairwise comparisons at one-time, avoiding inflation of alpha-error due to multiple testing issues that would have arisen with the Bucher methodology. The details on the statistical methods used can be found in the Supplementary Methods. Briefly, HAE attack rates per 28 days and the proportions of patients who achieved ≥90% reduction in HAE attack rate from baseline in each study arm from individual studies were extracted from published literature (Table 1). The Rücker NMA methodology was then applied to conduct indirect comparisons between each treatment and its comparators, and to calculate relative and absolute effects.
Table 1. . Data inputs for the network meta-analysis.
| HAE attack rate per 28 days† | Study | Arm 1 | Arm 2 | Rate in arm 1 | Rate in arm 2 | Rate ratio | Ref. |
|---|---|---|---|---|---|---|---|
| HELP | Lanadelumab 300 mg Q2W | Placebo | 0.26 | 1.97 | 0.13 | [13] | |
| HELP | Lanadelumab 300 mg Q4W | Placebo | 0.53 | 1.97 | 0.27 | [13] | |
| HELP | Lanadelumab 300 mg Q2W | Lanadelumab 300 mg Q4W | 0.26 | 0.53 | 0.49 | [13] | |
| APeX-2 | Berotralstat 110 mg q.d. | Placebo | 1.65 | 2.35 | 0.70 | [17] | |
| APeX-2 | Berotralstat 150 mg q.d. | Placebo | 1.31 | 2.35 | 0.56 | [17] | |
| APeX-2 | Berotralstat 110 mg q.d. | Berotralstat 150 mg q.d. | 1.65 | 1.31 | 1.26 | [17] | |
| CHANGE | C1-INH 1000 IU BIW | Placebo | 2.10 | 4.23 | 0.50 | [20] |
| Probability of achieving ≥90% reduction in the monthly HAE attack rate ‡ | Study | Arm 1 | Arm 2 | Proportion in arm 1 | Proportion in arm 2 | ARR § | |
|---|---|---|---|---|---|---|---|
| HELP | Lanadelumab 300 mg Q2W | Placebo | 0.67 | 0.05 | –0.62 | [13] | |
| HELP | Lanadelumab 300 mg Q4W | Placebo | 0.55 | 0.05 | –0.50 | [13] | |
| HELP | Lanadelumab 300 mg Q2W | Lanadelumab 300 mg Q4W | 0.67 | 0.55 | –0.12 | [13] | |
| APeX-2 | Berotralstat 110 mg q.d. | Placebo | 0.10 | 0.08 | –0.02 | [17] | |
| APeX-2 | Berotralstat 150 mg q.d. | Placebo | 0.23 | 0.08 | –0.15 | [17] | |
| APeX-2 | Berotralstat 110 mg q.d. | Berotralstat 150 mg q.d. | 0.10 | 0.23 | 0.13 | [17] | |
| CHANGE | C1-INH 1000 IU BIW | Placebo | 0.18 | 0 | –0.18 | [34] |
| Risk in comparator group ¶ | Placebo | C1-INH 1000 IU BIW | Berotralstat 150 mg q.d. | Berotralstat 110 mg q.d. | Lanadelumab 300 mg Q2W | Lanadelumab 300 mg Q4W | |
|---|---|---|---|---|---|---|---|
| HAE attack rate per 28 days – base case analysis | 2.41 | 4.23 | 2.35 | 2.35 | 1.97 | 1.97 | |
| HAE attack rate per 28 days – steady-state sensitivity analysis | 2.51 | 4.23 | 2.35 | 2.35 | 1.88 | 1.88 | |
| ≥90% reduction in the monthly HAE attack rate | 0.08 | 0.18 | 0.08 | 0.08 | 0.05 | 0.05 |
HAE attack rate per 28 days takes into account the whole observed time horizon (number of events per patient-days at risk).
Probabilities of achieving ≥90% reduction in the number of monthly HAE attacks are estimated as relative reductions in HAE attack rates on treatment compared with prospectively collected HAE attack rates at baseline.
ARR is estimated as proportion in arm 1 subtracted from proportion in arm 2.
For C1-INH, both doses of berotralstat and both doses of lanadelumab, the risk in comparator group corresponds to the data in placebo arm of the corresponding study. For placebo, the risk in comparator group is estimated as the weighted average over the available data.
ARR: Absolute risk reduction; BIW: Twice weekly; C1-INH: C1-inhibitor; HAE: Hereditary angioedema; NMA: Network meta-analysis; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
This analysis exclusively used random-effects models to account for heterogeneity. Meta-regression could not be conducted due to a sparse evidence base that included only three studies.
The results were reported as point estimates along with 95% CIs. For the count outcome of HAE attack rate per 28 days, absolute effects were reported as absolute difference and relative effects were reported as rate ratio (RaR). For the dichotomous outcome of ≥90% reduction in monthly HAE attacks, absolute effects were reported as absolute risk reduction (ARR) and relative effects were reported as risk ratio (RR).
Additionally, treatment rankings via p-score (the frequentist equivalent of surface under cumulative ranking curve values) were calculated for the outcomes of HAE attack rate per 28 days and a ≥90% reduction in the number of monthly HAE attacks. P-score for individual treatment is the average of the probabilities of treatment being better versus the comparator over all pairwise comparisons and represents the rank of this treatment within the range of treatments. P-scores range from 0 to 1, with 1 representing theoretically best and 0 representing theoretically worst treatment (Supplementary Methods).
Based on the 14-day half-life of lanadelumab [26], it is expected that lanadelumab concentration reaches steady state, where the effectiveness of the drug is highest, after 70 days of treatment. To account for potential differences in lanadelumab effectiveness after reaching the steady-state concentration and in the overall study period (inclusive of periods before and after reaching the steady-state concentration), we used the NMA methods described above to perform a sensitivity analysis considering only HAE attack rates during lanadelumab steady state.
All analyses were conducted with statistical software R v3.4.3, using the R package netmeta [27].
Results
Systematic literature review
In the 2018 SLR, 1389 publications were screened, after removing duplications, and 548 full-text articles were assessed for eligibility. After full-text article screening, 467 publications were excluded (exclusion reasons: age unclear, 6; disease, 11; duplicate, 4; language, 8; review/editorial, 16; study design, 135; pediatric, 9; acute/mixed/unclear treatment, 278). An additional one publication identified through bibliographic searching and 17 publications from a conference and HTA website search were also included in the SLR results, for a total of 60 publications reporting data from 10 RCTs and 39 publications reporting data from 28 non-RCTs. Six additional RCTs were identified after the updated manual search in 2021.
The 16 RCTs discovered by the SLR searches and the updated manual search were analyzed in the feasibility analysis for inclusion in the NMA. After feasibility analysis, 12 studies were excluded (not approved for LTP, 5; no placebo comparator arm, 3; not LTP intervention of reimbursement interest, 2; no LTP-approved dosage included, 1; study design, 1) and three studies were included in the evidence network for the NMA base case analysis. One more study was included in an additional sensitivity analysis only. The full network of evidence for the NMA base case reported here included the HELP study (lanadelumab vs placebo) [13], the APeX-2 study (berotralstat vs placebo) [17] and the CHANGE study (C1-INH 1000 IU BIW and placebo; NCT01005888) (Figure 1) [20].
Figure 1. . Evidence network.
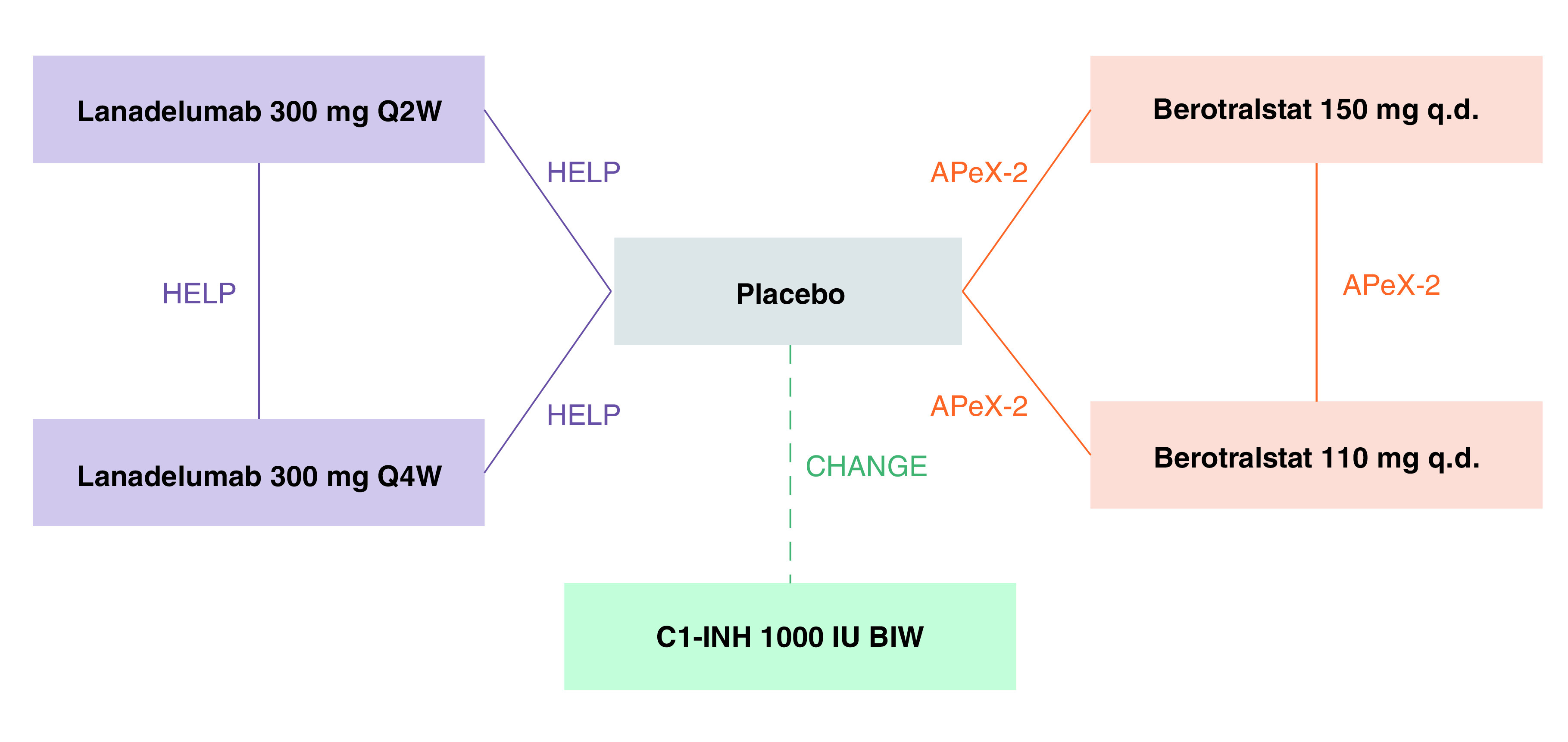
Dashed line represents crossover study design and solid lines represent non crossover study design [13,17,20,33].
BIW: Twice weekly; C1-INH: C1 inhibitor; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
The design of the studies included in the evidence network is summarized in Supplementary Table 2. Briefly, the HELP study randomized 125 patients with HAE type I/II aged ≥12 years who had at least one investigator confirmed HAE attack during the 28-day run-in period to lanadelumab 300 mg Q2W, lanadelumab 300 mg Q4W, lanadelumab 150 mg Q4W or placebo. The double-blind treatment phase lasted 26 weeks [13]. The APeX-2 study randomized 121 patients with a confirmed diagnosis of HAE type I/II aged ≥12 years (US and Canada) or ≥18 years (Europe) who had at least two investigator confirmed HAE attacks during the 56-day run-in period to berotralstat 150 mg q.d., berotralstat 110 mg q.d. or placebo. The double-blind treatment phase lasted 24 weeks, after which the patients continued treatment with berotralstat 110 or 150 mg q.d. for another 24 weeks, for a total of 48 weeks of follow-up. Patients from the placebo group who completed the first 24-week treatment period were re-randomized to berotralstat 110 or 150 mg q.d. for the second 24-week treatment period [17,28]. During the second 24-week treatment period, the patients, investigators and study staff remained blinded to the dose assignment but were informed that all patients would receive active therapy [28]. Because the methodology for this anchored NMA requires the use of a common comparator (placebo), only the data from the first 24 weeks of the APeX-2 trial were used for this analysis. Lastly, the CHANGE study randomized 22 patients with HAE type I/II aged ≥6 years to 12 weeks of C1-INH 1000 IU BIW (n = 11) or placebo (n = 11), followed by 12 weeks of the treatment not received in the first part of the study [20]. On-demand treatment of HAE attacks was permitted in all three studies [13,17,20]. In the HELP study, intravenous C1-INH, icatibant or ecallantide were allowed for on-demand treatment of HAE attacks, following the site investigator’s standard of care [13]. In the APeX-2 study, icatibant, plasma-derived C1-INH, ecallantide or recombinant C1-INH were allowed for on-demand treatment of HAE attacks, following the patients’ usual medical management plan [17]. In the CHANGE study, all patients with acute HAE attacks were eligible for on-demand treatment with open-label C1-INH [20]. The on-demand treatment of HAE attacks in the HELP, APeX-2 and CHANGE studies was not compared in this NMA.
The study included in a sensitivity analysis only was a Phase Ib, randomized, double-blind, placebo-controlled, multiple-ascending-dose trial which included five patients in lanadelumab 300 mg Q2W arm and 13 patients in the placebo arm [26]; this study had less strict inclusion criterion for HAE attack rate at baseline (≥2 HAE attacks per year and ≥1 HAE attack in the previous 6 months) and shorter follow-up (120 days or ~17 weeks) compared with the Phase III studies included in the base case analysis. The results of the sensitivity analysis including this Phase Ib study were aligned with the base case findings (data not shown).
Baseline characteristics
The baseline characteristics as reported in the HELP, APeX-2 and CHANGE studies are summarized in Table 2. Based on the descriptive assessment of mean age, percentage of female patients enrolled, weight, BMI and baseline HAE attack rate, the studies were deemed to have similarity in baseline patient characteristics suitable for an NMA.
Table 2. . Baseline demographic and disease characteristics studies in the evidence network.
| Regimen | HELP [13,35] | APeX-2 [17] | CHANGE [20,23] | |||||
|---|---|---|---|---|---|---|---|---|
| Lanadelumab 300 mg Q2W (n = 27) | Lanadelumab 300 mg Q4W (n = 29) | Placebo (n = 41) | Berotralstat 150 mg q.d. (n = 40) | Berotralstat 110 mg q.d. (n = 41) | Placebo (n = 40) | C1-INH first (n = 11) | Placebo first (n = 11) | |
| Mean (SD) age, years | 40.3 (13.3) | 39.5 (12.8) | 40.1 (16.8) | 40.0 (14.0) | 40.4 (17.5) | 44.5 (14.1) | 41.7 (19.3) | 34.5 (14.8) |
| Mean (SD) body weight, kg | 90.6 (25.2) | 78.5 (16.6) | 76.3 (22.7) | 87.6 (20.4) | 78.8 (21.5) | 84.9 (21.4) | 70.5 (9.3) | 76.3 (25.7) |
| Mean (SD) BMI, kg/m2 | 31.0 (7.8) | 28.1 (5.1) | 27.5 (7.7) | 30.4 (6.7) | 27.5 (7.3) | 29.3 (6.8) | NR | NR |
| Female, n (%) | 15 (55.6) | 19 (65.5) | 34 (82.9) | 23 (57.5) | 30 (73.2) | 27 (67.5) | 9 (81.8) | 11 (100) |
| White, n (%) | 26 (96.3) | 23 (79.3) | 39 (95.1) | 38 (95.0) | 38 (92.7) | 37 (92.5) | 10 (90.9) | 11 (100) |
| HAE type, n (%) | ||||||||
| Type I | 23 (85.2) | 27 (93.1) | 38 (92.7) | NR | NR | NR | 9 (81.8) | 9 (81.8) |
| Type II | 4 (14.8) | 2 (6.9) | 3 (7.3) | NR | NR | NR | 2 (18.2) | 2 (18.2) |
| Mean (SD) run-in/baseline attack rate, attacks/month | 3.5 (2.3) | 3.7 (2.5) | 4.0 (3.3) | 3.1 (1.6) | 3.0 (1.4) | 2.9 (1.1) | 3.8 (2.0)† | |
| Prior use of prophylaxis, n (%) | 14 (51.9) | 20 (69.0) | 24 (58.5) | 30 (75.0) | 32 (78.0) | 29 (72.5) | NR | NR |
Because the CHANGE study has a crossover design, published literature only reports baseline attack rate for C1-INH first and placebo first arms combined.
C1-INH: C1 inhibitor; HAE: Hereditary angioedema; NR: Not reported; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily; SD: Standard deviation.
HAE attack rate per 28 days
Both doses of lanadelumab were associated with a lower rate of HAE attacks per 28 days compared with berotralstat 150 mg q.d. and with berotralstat 110 mg q.d. (Table 3 & Figure 2). Lanadelumab 300 mg Q2W was associated with a 77% lower rate of HAE attacks per 28 days compared with berotralstat 150 mg q.d. (RaR: 0.23 [95% CI: 0.10, 0.55]; p = 0.0009) and 81% lower rate of HAE attacks per 28 days compared with berotralstat 110 mg q.d. (RaR: 0.19 [95% CI: 0.08, 0.43]; p = 0.0001). Lanadelumab 300 mg Q4W was associated with a 52% lower rate of HAE attacks per 28 days versus berotralstat 150 mg q.d. (RaR: 0.48 [95% CI: 0.25, 0.94]; p = 0.0311) and a 62% lower rate of HAE attacks per 28 days versus berotralstat 110 mg q.d. (RaR: 0.38 [95% CI: 0.20, 0.73]; p = 0.0038).
Table 3. . Comparison of hereditary angioedema attack rate per 28 days with lanadelumab 300 mg Q2W and 300 mg Q4W versus other treatment options.
| Comparator, HAE attack rate per 28 days | Berotralstat 150 mg q.d. | Berotralstat 110 mg q.d. | C1-INH 1000 IU BIW | Lanadelumab 300 mg Q2W | Lanadelumab 300 mg Q4W | Placebo |
|---|---|---|---|---|---|---|
| Lanadelumab 300 mg Q2W | ||||||
| Rate ratio (95% CI); p-value | 0.23 (0.10, 0.55); p = 0.0009 | 0.19 (0.08, 0.43); p = 0.0001 | 0.27 (0.11, 0.64); p = 0.0032 | N/A | 0.48 (0.19, 1.20); p = 0.1163 | 0.13 (0.06, 0.29); p < 0.0001 |
| Absolute difference (95% CI); p-value | -1.81 (-2.12, -1.06); p < 0.0001 | -1.90 (-2.16, -1.34); p < 0.0001 | -3.09 (-3.76, -1.52); p = 0.0001 | N/A | -1.02 (-1.6, 0.39); p = 0.1562 | -2.09 (-2.26, -1.71); p < 0.0001 |
| Lanadelumab 300 mg Q4W | ||||||
| Rate ratio (95% CI); p-value | 0.48 (0.25, 0.94); p = 0.0311 | 0.38 (0.20, 0.73); p = 0.0038 | 0.56 (0.29, 1.10); p = 0.0917 | 2.08 (0.83, 5.17); p = 0.1163 | N/A | 0.27 (0.15, 0.47); p < 0.0001 |
| Absolute difference (95% CI); p-value | -1.22 (-1.76, -0.14); p = 0.0268 | -1.46 (-1.88, -0.63); p = 0.0006 | -1.86 (-3.00, 0.42); p = 0.1098 | 1.02 (-0.39, 1.6); p = 0.1562 | N/A | -1.76 (-2.05, -1.28); p < 0.0001 |
| p-score† | 0.4373 | 0.2319 | 0.5407 | 0.9880 | 0.7990 | 0.0032 |
p-score represents the rank of the treatment within the given range of treatments, with p-score of 1 representing the theoretically best treatment and p-score of 0 representing the theoretically worst treatment.
BIW: Twice weekly; C1-INH: C1 inhibitor; N/A: Not available; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
Figure 2. . HAE attack rate per 28 days for (A) lanadelumab 300 mg Q2W and (B) lanadelumab 300 mg Q4W versus other treatment options.
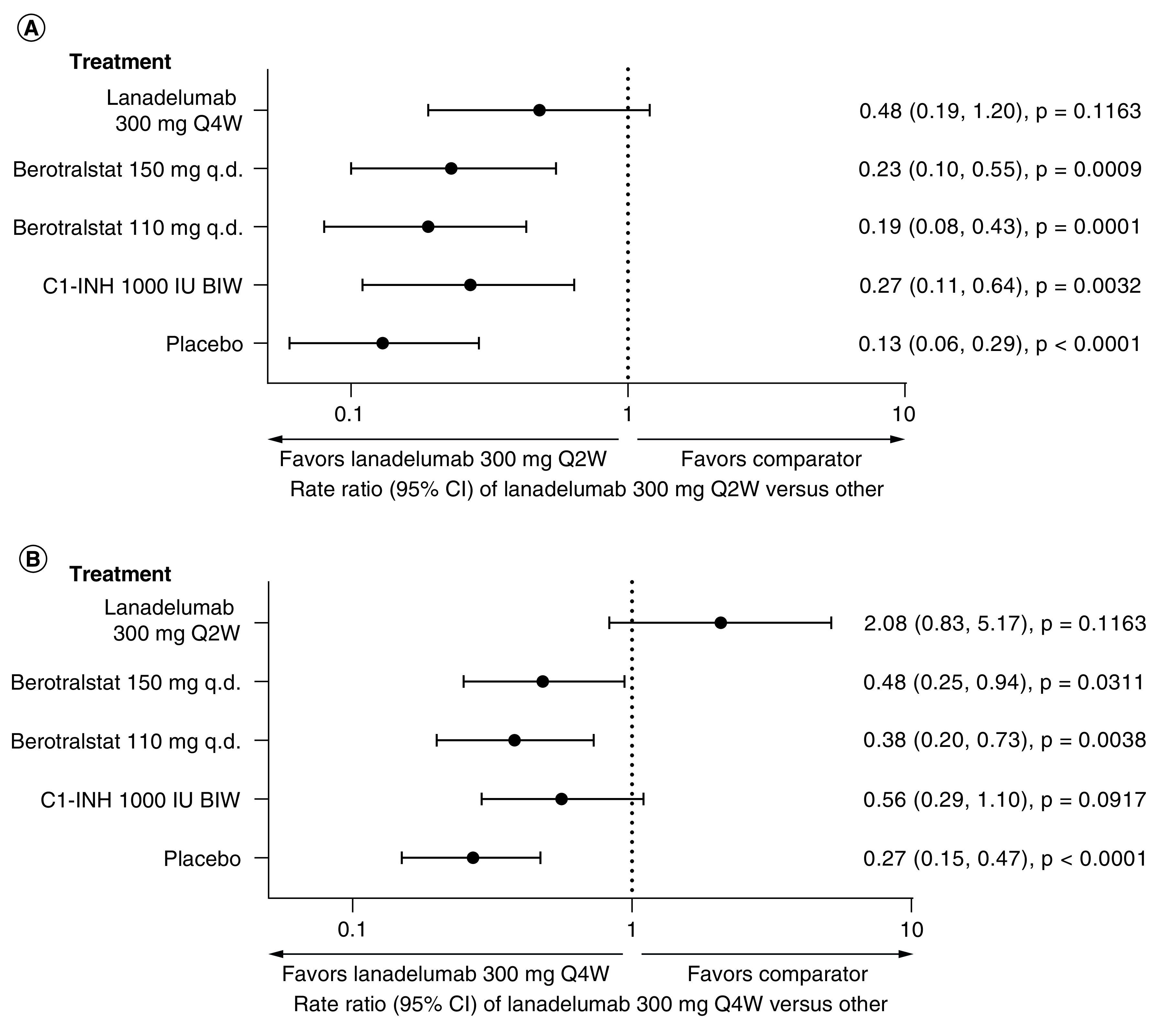
BIW: Twice weekly; C1-INH: C1 inhibitor; HAE: Hereditary angioedema; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
When absolute difference in the rate of HAE attacks per 28 days was analyzed (Table 3), attack rate was lower by 1.81 attacks per 28 days and 1.90 attacks per 28 days with lanadelumab 300 mg Q2W versus berotralstat 150 mg q.d. (absolute difference -1.81 [95% CI: -2.12, -1.06]; p < 0.0001) and berotralstat 110 mg q.d. (absolute difference -1.90 [95% CI: -2.16, -1.34]; p < 0.0001), respectively. Similarly, attack rate was lower by 1.22 attacks per 28 days and 1.46 attacks per 28 days with lanadelumab 300 mg Q4W versus berotralstat 150 mg q.d. (absolute difference -1.22 [95% CI: -1.76, -0.14]; p = 0.0268) and berotralstat 110 mg q.d. (absolute difference -1.46 [95% CI: -1.88; -0.63]; p = 0.0006), respectively.
≥90% reduction in the number of monthly HAE attacks
In this NMA, ≥90% reduction in monthly HAE attack rates was 6.87-times more likely with lanadelumab 300 mg Q2W versus berotralstat 150 mg q.d. (RR: 6.87 [95% CI: 3.62, 10]; p < 0.0001) and 8.5-times more likely with lanadelumab 300 mg Q2W versus berotralstat 110 mg q.d. (RR: 8.5 [95% CI: 5.25, 11.62]; p < 0.0001) (Table 4). Treatment with lanadelumab 300 mg Q2W was estimated to result in 47 additional patients per 100 treated patients with ≥90% reductions in the number of monthly HAE attacks compared with berotralstat 150 mg q.d. (ARR: -0.47 [95% CI: -0.72, -0.21]; p = 0.0004) and in 60 additional patients per 100 treated patients compared with berotralstat 110 mg q.d. (ARR: -0.60 [95% CI: -0.85, -0.34]; p < 0.0001) (Figure 3).
Table 4. . Comparison of the probability of achieving ≥90% reduction in the number of monthly hereditary angioedema attacks with lanadelumab 300 mg Q2W and 300 mg Q4W versus other treatment options.
| Comparator, risk ratio (95% CI); p-value | Berotralstat 150 mg q.d. | Berotralstat 110 mg q.d. | C-INH 1000 IU BIW | Lanadelumab 300 mg Q2W | Lanadelumab 300 mg Q4W | Placebo |
|---|---|---|---|---|---|---|
| Lanadelumab 300 mg Q2W | ||||||
| Absolute risk reduction (95% CI) | -0.47 (-0.72, -0.21) p = 0.0004 | -0.60 (-0.85, -0.34) p < 0.0001 | -0.44 (-0.70, -0.18) p = 0.001 | N/A | -0.12 (-0.31, 0.08) p = 0.2516 | -0.62 (-0.81, -0.42) p < 0.0001 |
| Risk ratio (95% CI) | 6.87 (3.62, 10); p < 0.0001 | 8.5 (5.25, 11.62); p < 0.0001 | 3.44 (2, 4.89); p < 0.0001 | N/A | 1.92 (0.38, 3.38); p = 0.43 | 8.94 (6.38, 11.37); p < 0.0001 |
| Lanadelumab 300 mg Q4W | ||||||
| Absolute risk reduction (95% CI) | -0.35 (-0.62, -0.09) p = 0.0087 | -0.48 (-0.75, -0.22) p = 0.0003 | -0.32 (-0.59, -0.05) p = 0.0185 | 0.12 (-0.08, 0.31) p = 0.2516 | N/A | -0.50 (-0.71, -0.30) p < 0.0001 |
| Risk ratio (95% CI) | 5.38 (2.12, 8.75); p = 0.0004 | 7 (3.75, 10.38); p < 0.0001 | 2.78 (1.28, 4.28); p = 0.0098 | 0.52 (0.3, 2.63); p = 0.43 | N/A | 7.4 (4.84, 10.09); p < 0.0001 |
| p-score† | 0.4606 | 0.1488 | 0.5011 | 0.9747 | 0.8224 | 0.0924 |
p-score represents the rank of the treatment within the given range of treatments, with p-score of 1 representing the theoretically best treatment and p-score of 0 representing the theoretically worst treatment.
BIW: Twice weekly; C1-INH: C1 inhibitor; N/A: Not available; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
Figure 3. . Probability of achieving ≥90% reduction in the monthly HAE attack rate for (A) lanadelumab 300 mg Q2W and (B) lanadelumab 300 mg Q4W versus other treatment options.
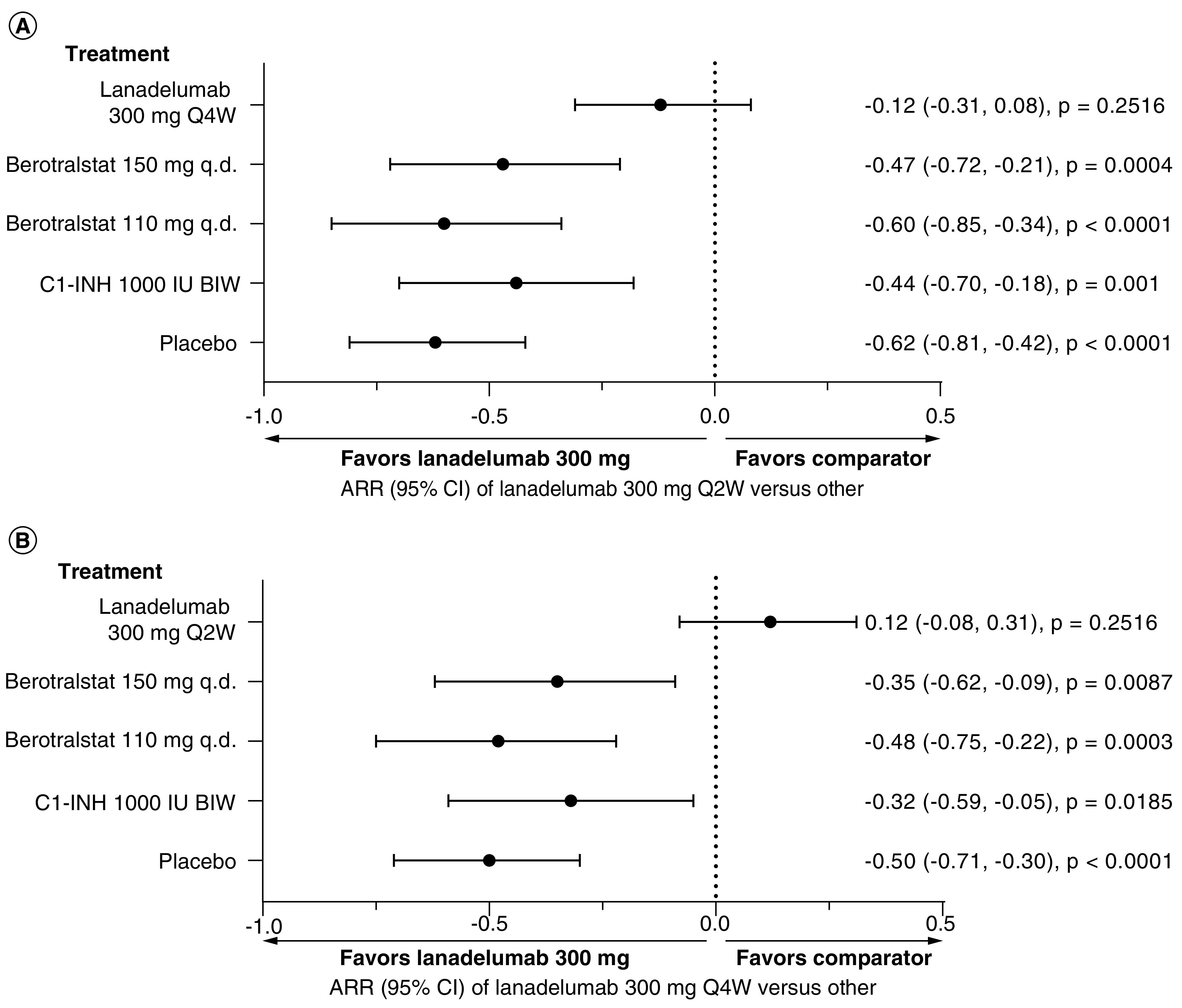
ARR: Absolute risk reduction; BIW, Twice weekly; C1-INH: C1 inhibitor; HAE: Hereditary angioedema; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
Similarly, ≥90% reduction in monthly HAE attack rates was 5.38-times more likely with lanadelumab 300 mg Q4W versus berotralstat 150 mg q.d. (RR: 5.38 [95% CI: 2.12, 8.75]; p = 0.0004) and seven-times more likely with lanadelumab 300 mg Q4W versus berotralstat 110 mg q.d. (RR: 7 [95% CI: 3.75, 10.38]; p < 0.0001) (Table 4). Treatment with lanadelumab 300 mg Q4W was estimated to result in 35 additional patients per 100 treated patients with ≥90% reductions in the number of monthly HAE attacks compared with berotralstat 150 mg q.d. (ARR: -0.35 [95% CI: -0.62, -0.09]; p = 0.0087), and in 48 additional patients per 100 treated patients compared with berotralstat 110 mg q.d. (ARR: -0.48 [95% CI: -0.75, -0.22], p = 0.0003) (Figure 3).
Treatment rankings
The treatment rankings are presented in Tables 3 & 4. For both assessed outcomes (HAE attack rate per 28 days and ≥90% reduction in the number of monthly HAE attacks), lanadelumab 300 mg Q2W had the highest probability of first rank (best outcome) followed by lanadelumab 300 mg Q4W and C1-INH.
Steady-state sensitivity analysis
A steady state sensitivity analysis considered HAE attack rates after lanadelumab reached steady-state concentration (day 70, based on a 14-day half-life of lanadelumab [26]). Data from days 70 to 182 in the HELP study were used for this analysis. In the steady state analysis, lanadelumab 300 mg Q2W and lanadelumab 300 mg Q4W were associated with a statistically significant reduction in HAE attack rate per 28 days versus berotralstat 150 mg q.d. and berotralstat 110 mg q.d.. The findings of steady-state sensitivity analysis were more favorable for lanadelumab versus the base case findings (evaluation from day 0 in the HELP study) (Table 5).
Table 5. . Hereditary angioedema attack rate per 28 days with lanadelumab 300 mg Q2W and 300 mg Q4W at steady state† versus other treatment options.
| Comparator, HAE attack rate per 28 days | Berotralstat 150 mg q.d. | Berotralstat 110 mg q.d. | C1-INH 1000 IU BIW | Lanadelumab 300 mg Q2W | Lanadelumab 300 mg Q4W | Placebo |
|---|---|---|---|---|---|---|
| Lanadelumab 300 mg Q2W | ||||||
| Rate ratio (95% CI); p-value | 0.16 (0.06, 0.46); p = 0.0006 | 0.13 (0.05, 0.36); p = 0.0001 | 0.19 (0.07, 0.54); p = 0.0018 | N/A | 0.47 (0.15, 1.48); p = 0.1991 | 0.09 (0.03, 0.24); p < 0.0001 |
| Absolute difference (95% CI); p-value | -1.97 (-2.21, -1.29); p < 0.0001 | -2.04 (-2.23, -1.5); p < 0.0001 | -3.43 (-3.93, -1.99); p < 0.0001 | N/A | -1 (-1.6, 0.86); p = 0.292 | -2.28 (-2.43, -1.91); p < 0.0001 |
| Lanadelumab 300 mg Q4W | ||||||
| Rate ratio (95% CI); p-value | 0.34 (0.16, 0.72); p = 0.0045 | 0.27 (0.13, 0.56); p = 0.0005 | 0.40 (0.19, 0.84); p = 0.0155 | 2.11 (0.67, 6.6); p = 0.1991 | N/A | 0.19 (0.10, 0.37); p < 0.0001 |
| Absolute difference (95% CI); p-value | -1.55 (-1.97, -0.66); p = 0.0006 | -1.72 (-2.04, -1.03); p < 0.0001 | -2.54 (-3.43, -0.68); p = 0.0074 | 1 (-0.86, 1.6); p = 0.292 | N/A | -2.03 (-2.26, -1.58); p < 0.0001 |
Steady state defined as days 70–182 in the HELP study.
BIW: Twice weekly; C1-INH: C1 inhibitor; HAE: Hereditary angioedema; N/A: Not available; Q2W: Every 2 weeks; Q4W: Every 4 weeks; q.d.: Once daily.
Discussion
The NMA consistently showed that both doses of lanadelumab were associated with statistically significantly higher effectiveness compared with both doses of berotralstat for the outcomes of HAE attack rate per 28 days and ≥90% reduction in the monthly HAE attack rate. Lanadelumab 300 mg Q2W was associated with the first rank (best outcome) for the outcomes of HAE attack rate per 28 days and ≥90% reduction in the number of monthly HAE attacks when ranked versus the other treatments using a p-score. In a sensitivity analysis, HAE attack rates observed during steady state of days 70 to 180 in the HELP study were considered; the results were consistent with the main analysis, with even more favorable outcomes for lanadelumab when only steady state efficacy was considered. Baseline characteristics over the studies were deemed to be comparable. However, covariates such as age, sex and weight could not be included in meta-regression models due to the limited evidence base that included only three studies. The guidance from the Cochrane Collaboration indicates that meta-regression should only be performed when there are ≥10 studies in an analysis [29].
To the extent of our knowledge, direct head-to-head treatment comparisons are absent in HAE. In addition to the difficulties in patient recruitment due to low disease prevalence inherent to rare disease clinical trials, multi-arm trials comparing more than one active treatment face additional difficulties including time required to design a more complex study, potential heterogeneity and loss of efficiency due to heterogeneous settings and operational challenges [30]. In situations where direct comparisons are unavailable, NMAs are a known approach for providing estimates on comparative effectiveness [21]. The results presented here add to a recent NMA that suggested reduced HAE attack rates and extended attack-free intervals in patients with HAE treated with lanadelumab versus intravenous C1-INH [23].
Limitations of this analysis include those that are inherent to any NMA. Although the studies included in the evidence network had broadly similar designs and baseline demographics of the study populations, residual heterogeneity may have influenced the findings. In this respect, NMAs cannot be a substitute for head-to-head comparisons [21]. Because the NMA was performed using published data on the comparator studies, individual-level data analyses were not possible. Due to the rarity of HAE, the HELP, CHANGE and APeX-2 studies contained relatively small sample sizes; furthermore, the follow-up times in these three studies differed. The CHANGE study reported data over two consecutive 12-week treatment periods in a crossover design for a total 24 weeks of follow-up [20]. The HELP study reported data over 26 weeks of double-blind treatment [13]. Outcomes with lanadelumab 300 mg Q2W over a mean of 29.6 months have been since reported in the HELP open-label extension (HELP OLE, NCT02741596) study, which only included a lanadelumab 300 mg Q2W arm [31]. The APeX-2 part 1 reported data over 24 weeks of double-blind treatment, and the APeX-2 part 2 reported data over an additional 24 weeks of blinded active treatment, for a total of 48 weeks of follow-up in APeX-2 parts 1 and 2 [17,28]. Additionally, data from an extended follow-up of the APeX-2 study for up to 96 weeks of exposure to berotralstat in total have been reported up to date [32]. For this analysis, only the 24-week double-blind period was considered for comparability of duration of follow-up (assuming a 2-week tolerance interval). Furthermore, a common comparator (placebo) arm required for the NMA methodology was only included in the first 24 weeks of the APeX-2 study and the 26-week treatment period in the HELP study.
Conclusion
This NMA, subject to its inherent limitations, supports the favorable efficacy of both lanadelumab 300 mg Q2W and lanadelumab Q4W compared with berotralstat 150 mg q.d. and berotralstat 110 mg q.d. in reducing the 28-day HAE attack rate and achieving ≥90% reduction in monthly HAE attacks. Although the APeX-2 and HELP study populations were small, these results reached statistical significance in the NMA. The results of sensitivity analysis that considered only the data from steady state treatment with lanadelumab were consistent with the base case and showed more favorable results for lanadelumab versus base case analysis.
Summary points.
Patients with hereditary angioedema (HAE) suffer from attacks that cause significant morbidity and reduced quality of life.
The impairment from HAE attacks can be reduced with long-term prophylaxis medication.
Lanadelumab and berotralstat have shown significant reductions in HAE attack rates in the Phase III HELP and APeX-2 studies, respectively.
In the absence of direct evidence, a network meta-analysis using frequentist regression weighted-based methodology following Rücker et al. was used to estimate the comparative effectiveness of lanadelumab versus berotralstat over 24 weeks of treatment [24].
The results of this network meta-analysis show that lanadelumab 300 mg every 2 weeks and 300 mg every 4 weeks were associated with statistically significantly higher effectiveness in reducing the rate of HAE attacks per 28 days and a statistically significantly higher probability of achieving a ≥90% reduction in monthly HAE attack rate versus berotralstat 150 mg once daily (q.d.) and 110 mg q.d.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2022-0188
Author contributions
All authors have contributed to the conception or design of the work and/or acquisition, analysis or interpretation of the data; revised the draft carefully for important intellectual content; approved the final version for publishing; and agreed to be accountable for all aspects of work.
Financial & competing interests disclosure
This analysis was funded by Takeda Development Center Americas, Inc., MA, USA. M Watt and D Romanus are employees of and hold stock/stock options in Takeda. M Malmenäs and K Haeussler are employees of ICON plc, which was contracted by Takeda to perform this analysis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Under the direction of authors, Milda Jakutaviciute, PhD, employee of Excel Medical Affairs, provided writing assistance for this manuscript. Editorial assistance in formatting, proofreading, copyediting and fact-checking was also provided by Excel Medical Affairs. Takeda Development Center Americas provided funding to Excel Medical Affairs for support in editing this manuscript. The interpretation of the data was made by the authors independently.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am. J. Manag. Care 24(Suppl. 14), S292–S298 (2018). [PubMed] [Google Scholar]
- 2.Busse PJ, Christiansen SC. Hereditary angioedema. N. Engl. J. Med. 382(12), 1136–1148 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Busse P, Kaplan A. Specific targeting of plasma kallikrein for treatment of hereditary angioedema: a revolutionary decade. J. Allergy Clin. Immunol. Pract. 10(3), 716–722 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Banerji A, Davis KH, Brown TM et al. Patient-reported burden of hereditary angioedema: findings from a patient survey in the United States. Ann. Allergy Asthma Immunol. 124(6), 600–607 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Mendivil J, Murphy R, de la Cruz M et al. Clinical characteristics and burden of illness in patients with hereditary angioedema: findings from a multinational patient survey. Orphanet. J. Rare Dis. 16(1), 94 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan AP, Joseph K. Pathogenesis of hereditary angioedema: the role of the bradykinin-forming cascade. Immunol. Allergy Clin. North Am. 37(3), 513–525 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Maurer M, Magerl M, Betschel S et al. The international WAO/EAACI guideline for the management of hereditary angioedema-the 2021 revision and update. Allergy 77(7), 1961–1990 (2022). [DOI] [PubMed] [Google Scholar]; • The most recent international guidelines on the management of hereditary angioedema.
- 8.Busse PJ, Christiansen SC, Riedl MA et al. US HAEA Medical Advisory Board 2020 guidelines for the management of hereditary angioedema. J. Allergy Clin. Immunol. Pract. 9(1), 132–150.e3 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Betschel S, Badiou J, Binkley K et al. The international/Canadian hereditary angioedema guideline. Allergy Asthma Clin. Immunol. 15, 72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenniston JA, Faucette RR, Martik D et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J. Biol. Chem. 289(34), 23596–23608 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda Pharmaceuticals International AG Ireland Branch. Summary of product characteristics. Takhzyro, INN-lanadelumab. (2023). https://www.ema.europa.eu/en/documents/product-information/takhzyro-epar-product-information_en.pdf
- 12.Takeda Pharmaceutical Company Limited. Highlights of prescribing information. TAKHZYRO® (lanadelumab-flyo) injection, for subcutaneous use. (2023). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761090s010lbl.pdf
- 13.Banerji A, Riedl MA, Bernstein JA et al. HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. J. Am. Med. Assoc. 320(20), 2108–2121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The primary manuscript reporting results from the HELP study.
- 14.Hwang JR, Hwang G, Johri A, Craig T. Oral plasma kallikrein inhibitor BCX7353 for treatment of hereditary angioedema. Immunotherapy 11(17), 1439–1444 (2019). [DOI] [PubMed] [Google Scholar]
- 15.BioCryst Pharmaceuticals, Inc. Highlights of prescribing information. Orladeyo™ (berotralstat) capsules, for oral use. (2023). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214094s000lbl.pdf
- 16.BioCryst Ireland Limited. Summary of product characteristics. Orladeyo, berotralstat. (2023). https://www.ema.europa.eu/en/documents/product-information/orladeyo-epar-product-information_en.pdf
- 17.Zuraw B, Lumry WR, Johnston DT et al. Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled Phase III trial. J. Allergy Clin. Immunol. 148(1), 164–172.e9 (2021). [DOI] [PubMed] [Google Scholar]; •• The primary manuscript reporting results from the APeX-2 study.
- 18.Takeda Manufacturing Austria AG. Summary of product characteristics. Cinryze, INN-Human C1-esterase inhibitor. (2023). https://www.ema.europa.eu/en/documents/product-information/cinryze-epar-product-information_en.pdf
- 19.Takeda Pharmaceuticals U.S.A. Inc. Highlights of prescribing information. CINRYZE (C1 Esterase Inhibitor [Human]) for intravenous use, freeze-dried powder for reconstitution. (2023). https://www.fda.gov/media/75907/download
- 20.Zuraw BL, Busse PJ, White M et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N. Engl. J. Med. 363(6), 513–522 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Jansen JP, Fleurence R, Devine B et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health 14(4), 417–428 (2011). [DOI] [PubMed] [Google Scholar]; • An introduction to network meta-analyses.
- 22.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. Brit. Med. J. 326(7387), 472 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendivil J, Malmenäs M, Haeussler K, Hunger M, Jain G, Devercelli G. Indirect comparison of lanadelumab and intravenous C1-INH using data from the HELP and CHANGE studies: bayesian and frequentist analyses. Drugs R D 21(1), 113–121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports indirect treatment comparison between lanadelumab and C1-INH for the long-term prophylaxis of hereditary angioedema attacks.
- 24.Rücker G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods 3(4), 312–324 (2012). [DOI] [PubMed] [Google Scholar]; •• Methodology paper describes the approach to network meta-analysis used in this analysis.
- 25.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 50(6), 683–691 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Banerji A, Busse P, Shennak M et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. N. Engl. J. Med. 376(8), 717–728 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Rücker G, Krahn U, König J et al. Package ‘Netmeta’. Network Meta-Analysis Using Frequentist Methods. Springer; (2022).https://cran.r-project.org/web/packages/netmeta/netmeta.pdf [Google Scholar]
- 28.Wedner HJ, Aygören-Pürsün E, Bernstein J et al. Randomized trial of the efficacy and safety of berotralstat (BCX7353) as an oral prophylactic therapy for hereditary angioedema: results of APeX-2 through 48 weeks (part 2). J. Allergy Clin. Immunol. Pract. 9(6), 2305–2314.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Cochrane. Chapter 10: analyzing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3. Deeks JJ, Higgins JPT, Altman DG (Eds). John Wiley & Sons, Chichester, UK: (2022). [Google Scholar]
- 30.Day S, Jonker AH, Lau LPL et al. Recommendations for the design of small population clinical trials. Orphanet J. Rare Dis. 13(1), 195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerji A, Bernstein JA, Johnston DT et al. Long-term prevention of hereditary angioedema attacks with lanadelumab: The HELP OLE Study. Allergy 77(3), 979–990 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinaciyan T, Sheridan WP, Desai B, Tomita D, Panovska VG. P053 Sustained reduction in hereditary angioedema (HAE) attack rates following switch to berotralstat: subgroup analysis from APeX-2. Ann. Allergy Asthma Immunol. 127(Suppl. 5), S29 (2021). [Google Scholar]
- 33.Lumry WR, Miller DP, Newcomer S, Fitts D, Dayno J. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy Asthma Proc. 35(5), 371–376 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Bernstein JA, Li HH, Craig TJ et al. Indirect comparison of intravenous vs. subcutaneous C1-inhibitor placebo-controlled trials for routine prevention of hereditary angioedema attacks. Allergy Asthma Clin Immunol 15, 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). TAKHZYRO: EPAR – Public Assessment Report European Medicines Agency; (2018). https://www.ema.europa.eu/en/documents/assessment-report/takhzyro-epar-public-assessment-report_en.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


