Summary
Repeated seizure activity can lead to long-term changes in seizure dynamics and behavior. However, resulting changes in brain-wide dynamics remain poorly understood. This is partly due to technical challenges in precise seizure control and in vivo whole brain mapping of circuit dynamics. Here, we developed an optogenetic kindling model through repeated stimulation of ventral hippocampal CAMKII neurons in adult rats. We then combined fMRI with electrophysiology to track brain-wide circuit dynamics resulting from non-afterdischarge (AD)-generating stimulations, and individual convulsive seizures. Kindling induced widespread increases in non-AD-generating stimulation response and ipsilateral functional connectivity, and elevated anxiety. Individual seizures in kindled animals showed more significant increases in brain-wide activity and bilateral functional connectivity. Onset time quantification provided evidence for kindled seizure propagation from the ipsilateral to contralateral hemisphere. Furthermore, a core of slow-migrating hippocampal activity was identified in both non-kindled and kindled seizures, revealing a novel mechanism of seizure sustainment and propagation.
Keywords: ofMRI, optogenetics, hippocampus, afterdischarge, seizure, kindling, seizure propagation, epilepsy, epileptogenesis, anxiety
eTOC
Choy et al. develop an optogenetic model for epilepsy through repeated stimulations of the hippocampus. Using whole brain functional imaging and electrical recordings, they uncover that kindling results in reorganization of brain-wide circuitry, engaging a larger brain-wide network upon stimulation, elevated anxiety, and a core of slow-migrating hippocampal activities.
Introduction
The hippocampus is a critical region for cognitive and emotional processing. It is also one of the most common sites of seizures, which are characterized by hypersynchronous neuronal activity, high metabolic demand and a collapse of information flow in affected circuits (Adhikari et al., 2010; Gelinas et al., 2016; Pereira de Vasconcelos et al., 1992; Trevelyan et al., 2013). Hippocampal seizure activity, especially when repeated or prolonged, can have long term consequences (epileptogenesis) due to reshaping of normal brain circuits (Gelinas et al., 2016; Leung and Shen, 2006). These circuit changes can eventually affect seizure spread itself, leading to a transition from focal seizures to bilateral tonic-clonic (FBTC) seizures (formerly known as secondarily generalized seizures). This transition in seizure spread can in turn lead to further circuit modifications (Fisher et al., 2017; Wykes et al., 2019). Although various hippocampal circuit changes following prolonged or repeated seizures have been reported, the way in which these changes impact brain circuit function remains elusive (Cavazos et al., 1991; Choy et al., 2014; Leung et al., 2000; Nissinen et al., 2000; Toyoda et al., 2013). Key technological challenges include the need for precise control over repeated seizure generations, and the ability to longitudinally image brain-wide function in vivo.
A long-standing technique for inducing epileptogenesis is known as kindling (Löscher, 2011; McIntyre and Gilby, 2008). Kindling involves sensitization of brain circuits over many days of intermittently induced focal electrographic seizure-like afterdischarges (ADs). The result is reliable induction of FBTC seizures given an initial electrical stimulation. Once the brain is kindled, this state persists for many months and is associated with memory deficits, increased anxiety, and depression, all of which are common comorbidities of epilepsy (Chen et al., 2016; Goddard, 1967; Leung and Shen, 2006). Furthermore, kindled seizures are an excellent model of FTBC seizures because they capture key clinical seizure phenomenology and share similar sensitivity profiles to anti-epileptic drugs (AED) (Löscher, 2011). As such, kindling models are one of the most widely used chronic animal models in AED discovery (Löscher, 2011). Importantly, because kindling is not associated with spontaneous seizures, seizure activity can be precisely controlled, thus reducing confounds (Michalakis et al., 1998). The kindling technique is therefore an ideal method for investigating the impact of repeated hippocampal seizures. More recently, optogenetic methods have been employed for inducing seizures and seizure-like afterdischarges. Optogenetic methods have several advantages over electrical stimulation, including spatiotemporal control of the cell type for seizure initiation and artifact-free electrophysiological recording (Osawa et al., 2013; Weitz et al., 2015). However, while optogenetics has been used to induce repeated seizures, optogenetic kindling leading to a long-term kindled state has not been previously demonstrated (Krook-Magnuson et al., 2015).
Combining electrophysiology with simultaneous functional magnetic resonance imaging (fMRI) provides local neural and brain-wide hemodynamic information that can be acquired in vivo and longitudinally (Carmichael et al., 2012; Duffy et al., 2015; Kim and Ogawa, 2012). Furthermore, by incorporating optogenetic methods with fMRI (ofMRI), localized cell-type activity can be manipulated, and its impacts assessed in an unbiased brain-wide manner. In contrast to common fMRI methods, these measurements from direct stimulation have a known origin, thus removing any ambiguity of directionality and causality. ofMRI is therefore a powerful technique for dissecting functional circuits (Alvarez-Salvado et al., 2014; Duffy et al., 2015; Lee et al., 2010; Moreno et al., 2016; Weitz et al., 2015, 2019). By combining optogenetics, fMRI and electrophysiology, we can for the first time assess changes to brain-wide circuit response associated with repeated seizure activity.
fMRI has been used to visualize both focal and generalized-onset seizures in humans and in animals (Chaudhary et al., 2012; Choy et al., 2010a; Duffy et al., 2015, 2020; Englot et al., 2008; Schridde et al., 2008). However, FBTC seizures have not been imaged in part due to the associated motor activity that leads to motion artifacts. FBTC seizures have the most complex spatiotemporal propagation dynamics of all seizure types, with evidence of both cortical and subcortical circuit involvement (Dabrowska et al., 2019; Handforth and Treiman, 1995). Standard methods of recording seizures, such as electrophysiology and optical imaging, have limited spatial coverage, resulting in a priori selection bias (Durand et al., 2010; Rossi et al., 2017; Schevon et al., 2019). Brain-wide imaging of FBTC seizures would finally allow us to reveal the full complexity of FBTC propagation dynamics (Brodovskaya and Kapur, 2019).
Thusly motivated, we developed a novel kindling model of epileptogenesis that uses cell-type specific, optogenetic stimulations for inducing electrographic seizure-like afterdischarges (AD) by targeting the excitatory neurons in the ventral hippocampus, the region most commonly affected in human epilepsy (SFig. 1) (Bernasconi et al., 2003; Thom et al., 2010). We discovered that with optogenetic kindling, FBTC seizures emerge and can be reliably induced thereafter for many months. Once this particular kindling model was established, we then used simultaneous electrophysiology and fMRI to investigate brain-wide activity. We assessed the impact of the kindling and conducted tests for anxiety and depression (common epilepsy comorbidities), to investigate the relationship between kindling, circuits, and behavior. Furthermore, we directly imaged brain-wide network dynamics of single induced seizures to reveal focal and, for the first time, whole brain time-resolved FBTC seizure propagation in kindled animals (Brodovskaya and Kapur, 2019). In doing so, we identified key features of epileptogenesis, seizure generation, seizure maintenance, and seizure propagation that may have significant implications for therapy development.
Results
Optogenetic Kindling of Ventral Hippocampus
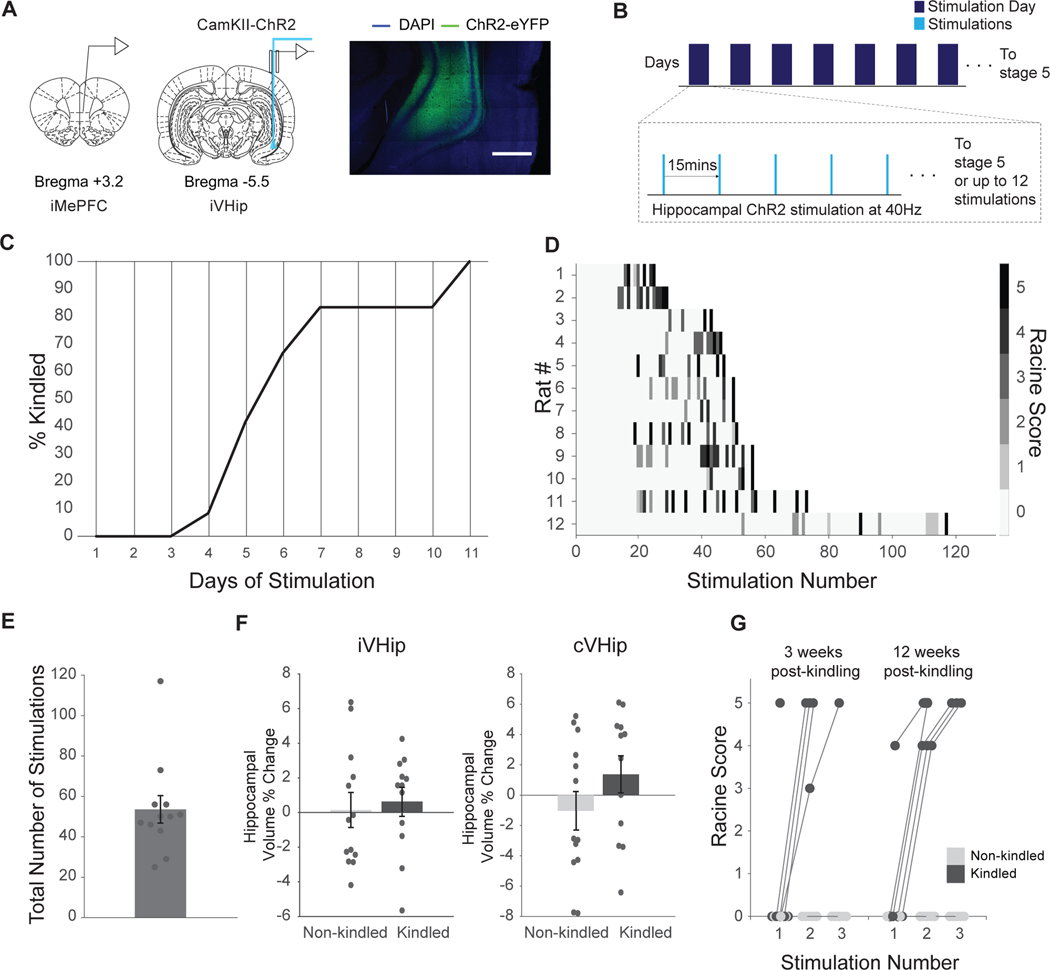
Kindling uses repeated induction of afterdischarges (ADs) to remodel neuronal circuits (Leung and Shen, 2006). We developed a new kindling method and investigated the impact of repeated optogenetically-induced ADs in the ventral hippocampus (VHip), a region in which spontaneous seizures frequently begin in humans and in animal models (Bernasconi et al., 2003; Thom et al., 2010; Toyoda et al., 2013). For optogenetic manipulation, we targeted the ventral hippocampal CAMKII neurons in rats (Fig. 1A,B) (Lothman and Williamson, 1994). Successful kindling was characterized by an evoked generalized motor seizure (Racine stage 5) within the first three stimulations of the day. Twelve rats underwent kindling, and their AD thresholds were determined on day 1. Seven rats had AD thresholds at 1 mW for 10 s, one rat at 2 mW for 10 s, three rats at 4 mW for 10 s and one rat at 8 mW for 12.5 s.
Figure 1: Optogenetic ventral hippocampal kindling persists for three months while there is no change in hippocampal volume.
(A) Left Panel: CamKII cells in the ventral hippocampus were targeted for optogenetic kindling and an optrode was implanted for stimulation and electrophysiology. An electrode was implanted into the ipsilateral medial prefrontal cortex for electrophysiology. Right Panel: Confocal images with localization of ChR2-eYFP expression to the ventral hippocampus. (B) For optogenetic kindling, rats were stimulated every other day with up to 12 stimulations a day or until a Racine stage 5 seizure was observed. Stimulations continued until criterion for kindling was reached: a stage 5 seizure within the first three stimulations of the day. (C) 83 % (10/12) of rats were kindled by day 7, and all were kindled by day 11. (D) Behavior scores for every stimulation in each rat undergoing kindling show successful kindling. (E) Total number of stimulations each rat received to acquire kindling. (F) There is no significant change in hippocampal volumes pre and post in non-kindled and kindled animals. Left panel: ipsilateral hippocampal volume changes, t-test (p = 0.703). Right panel: contralateral hippocampal volume changes, t-test (p = 0.187). (G) A subgroup of rats was tested 3 weeks and then 12 weeks following kindling acquisition to determine if the animals retained the kindling phenotype. Rats were stimulated 3 times. All kindled animals had Racine stage 5 seizures whereas no control rats displayed any seizure-associated Racine behaviors (n = 5 per group), showing persistent change resulting from kindling. All data presented as mean ± s.e.m. A threshold of p < 0.05 was used for determination of statistical significance.
All rats reached kindling criterion by 11 days of stimulations, with 83 % (10 out of 12 rats) reaching kindling criterion by day 7 (Fig. 1C). Mean number of stimulations required was 53.6 (range: 29–117, Fig. 1D), mean number of convulsive seizures (Racine stage 3–5) was 6.75 (range: 2–13), and mean number of generalized seizures (Racine stage 5) was 2.9 (range: 1–9). See Fig. 1E for a visualization of behavioral scores for each stimulation per animal and Supplementary Video 1 for an example of a kindled motor seizure.
Kindling is associated with minimal cell loss in contrast with other common epilepsy models (Choy et al., 2010b; Covolan and Mello, 2000; Dubé et al., 2001). To assess cell loss, we measured hippocampal volumes based on anatomical MRI before and after kindling, and at corresponding timepoints in non-kindled control rats (Sierra et al., 2015). Consistent with other kindling models, we did not detect any overt hippocampal volume changes in either hemisphere (Fig. 1F). The volume change was 0.63 ± 0.82 % vs 0.14 ± 0.96 %, p = 0.7, and 1.37 ± 1.22 % vs −1.03 ± 1.27 %, p = 0.19 for kindled vs. non-kindled in ipsilateral and contralateral hippocampi, respectively.
A key feature of kindling models is that once an animal is kindled, the effects are apparently permanent whereby motor seizures are readily evoked. To confirm that our new kindling procedure resulted in a persistent kindled state, we evaluated subsets of rats at 3 and 12 weeks after kindling, as well similar subsets of non-kindled control rats (n = 5 for each group). Motor seizures (Racine stage 5) were observed in kindled rats at both timepoints, whereas non-kindled rats did not exhibit any overt seizure behavior (Racine stage 0) at either time-point (Fig. 1G). This optogenetic kindling procedure therefore exhibits key features of conventional kindling methods and provides a novel platform for investigating circuit remodeling and FBTC seizures.
Widespread ventral hippocampal circuit remodeling and elevated anxiety after kindling
Kindling induces local structural and functional remodeling, but it remains unclear as to which downstream regions are affected (Cavazos et al., 1991; Goddard et al., 1969). We addressed this challenge by taking an unbiased brain-wide approach. We used simultaneous local field potentials (LFPs) and cerebral blood volume (CBV)-fMRI to evaluate how kindling affects the brain-wide response to VHip CAMKII stimulation. We stimulated VHip CAMKII, the same cell population used for kindling, with a non-AD generating light train at 10 Hz for 5 s. To visualize the brain-wide response, we generated fMRI activation maps using standard general linear model methods to identify voxels that were significantly modulated during stimulation. In non-kindled rats, stimulations resulted in activity that was localized predominantly to the following regions of the ipsilateral hemisphere: dorsal hippocampus (DHip), VHip, septum (Sept), amygdala (Amyg), and medial prefrontal cortex (MePFC) (Fig. 2C, left panel). In contrast, in kindled rats, the same stimulation resulted in activity that extended beyond these regions, even including parts of the contralateral hemisphere (Fig. 2C, right panel, see SFig. 2A for group comparisons at different timepoints). In the kindled rats, we tested whether individual differences in the kindling procedure resulted in differences in regional activity patterns. However, inclusion of the number of stimulations for kindling or the number of stage 5 motor seizures as regressors did not yield any significant voxel-wise relationships beyond noise levels (SFig. 2B). We also compared signal-to-noise ratios (SNR) of fMRI scans between groups to confirm that the increased response was not a consequence of measurement differences. No significant differences between groups were detected (non-kindled SNR = 78.5 ± 3.2, kindled SNR = 84.2 ± 3.88, mean ± s.e.m., independent t-test, p = 0.261).
Figure 2: Hippocampal-kindling results in brain-wide changes in hippocampal activity and heightened anxiety.
(A) Ventral hippocampal CamKII cells were targeted for stimulation with electrodes in ipsilateral ventral hippocampus (iVHip), and ipsilateral medial prefrontal cortex (iMePFC) for LFP recordings. fMRI imaged 27 slices across the brain. (B) Simultaneous LFP and CBV-fMRI were used to measure changes in activity caused by VHip stimulation, both before and after kindling, and in non-kindled control animals (n = 12 in each group). For each scan, a 5 s stimulation delivered at 10 Hz was given once every minute for six cycles. (C) Statistical t-maps for cage-mate, age-matched controls (left panel) and in kindled (right panel) animals with a t-threshold corresponding to p < 0.001. Note that activity in the controls was mostly restricted to iVHip, iDHip, iMePFC, iAmyg, and iSept, whereas activity in the kindled rats included these areas and others. (D) Simultaneously-acquired LFP response in iVHip and iMePFC from a single rat before (left panel) and after kindling (right panel). Blue bar indicates 10-Hz optogenetic stimulation. Note increase in iMePFC LFP response following kindling. LFPs were gradient-artifact corrected and bandpass-filtered at 8–12 Hz. (E) Group analysis of LFP response in non-kindled and kindled rats. Left panel: iVHip LFP response to 10-Hz stimulation did not differ between pre and post conditions in the control and kindled animals (n = 11, 12; respectively, p = 0.35, two-way repeated measures ANOVA). Right panel: iMePFC LFP response to 10-Hz stimulation was increased following kindling when compared to control animals (n = 9, 10; respectively, p = 0.009, two-way repeated measures ANOVA). Post-hoc analysis indicated that there was a 2.9 ± 0.2 fold increase following kindling (paired t-test, p = 0.005). To estimate LFP power, the ratio between LFP power during stimulation and 5 s immediately before stimulation was calculated. For each timepoint per animal, the median of the ratio from 6–18 stimulation blocks was used to assess time-dependent group effects. For each block, normalized power was calculated from the stimulation block relative to the 5 s prior to stimulation onset. Two animals were removed for the prefrontal cortical analysis due to a broken electrode (1 control), or large artifact (1 kindled). Please see SFig. 4 for data from these animals and SFig. 2C for the group analysis including outliers. (F) 10 weeks following fMRI, a subset of animals underwent behavior tests for anxiety and depression. (G) Sucrose preference test. Baseline measurements indicate no preference for either of the two bottles containing water. Test measurements with one bottle containing sucrose water and the other with water indicate both groups preferred the sucrose solution and no differences between the groups were observed (n = 7, 8, t-test, p > 0.05). (H) Forced swim test. No differences were observed between groups following kindling (n = 7,8, t-test, p > 0.05). (I) Open field test. Kindled rats spent significantly less time in the center of the open field than in non-kindled rats indicating an increased anxiety phenotype following kindling (t-test, p = 0.046). All data presented as mean ± s.e.m. A threshold of p < 0.05 was used for determination of statistical significance.
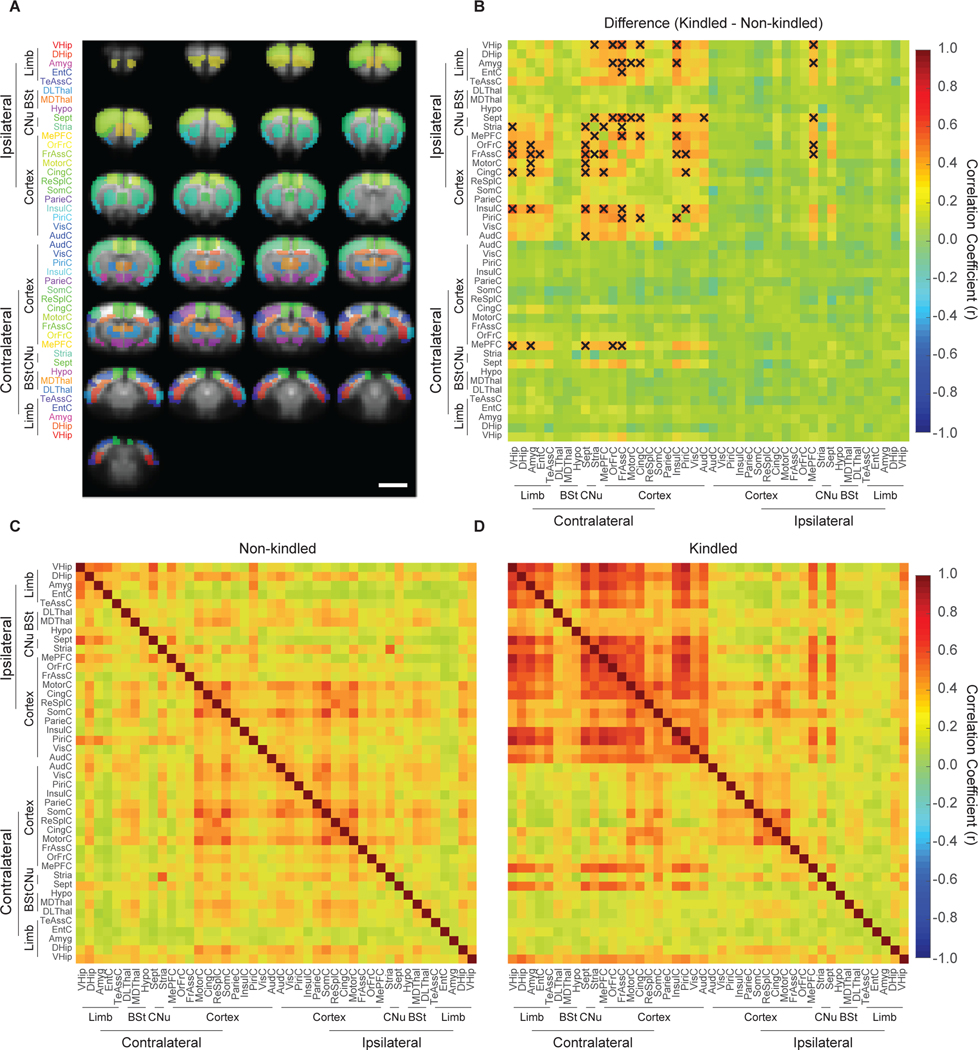
To quantitatively characterize brain-wide differences after kindling, we compared activation volumes from 44 anatomical brain regions between non-kindled and kindled rats (Fig. 3A). We found seven brain regions that differed between non-kindled and kindled groups following adjustment for multiple comparisons: ipsilateral MePFC (27.2 ± 9.0 % vs. 83.3 ± 9.0 %, p = 0.011), contralateral MePFC (1.4 ± 0.7 % vs. 29.2 ± 7.3 %, p = 0.033), ipsilateral frontal association cortex (iFrAssC) (0.24 ± 0.24 % vs. 27.1 ± 5.6 %, p = 0.004), ipsilateral temporal association cortex (iTeAssC) (0.34 ± 0.34 % vs. 26.7 ± 4.8 %, p = 0.0006), ipsilateral orbitofrontal cortex (iOrFrC) (1.4 ± 0.7 % vs. 23.8 ± 3.9 %, p = 0.0005), ipsilateral insular cortex (iInsC) (0.2 ± 0.18 % vs. 13.0 ± 2.7 %, p = 0.0047), and ipsilateral striatum (iStria) (2.6 ± 0.9 % vs. 14.8 ± 2.7 %, p = 0.011). Notably, no significant differences were observed in iVHip or iDHip (17.3 ± 3.0 % vs. 27.8 ± 3.1 %, p = 0.684, 10.1 ± 2.9 % vs. 24.4 ± 5.9 %, p = 0.994). To evaluate changes in connectivity across these regions, we generated functional connectivity matrices for rats in both the kindled and non-kindled groups (Fig. 3). Statistical comparisons between these groups revealed significant increase in functional connectivity between regions in the ipsilateral hemisphere. Most notably, the site of stimulation in the ipsilateral ventral hippocampus (iVHip) showed increased connectivity with the ipsilateral orbitofrontal cortex (iOrFrC), ipsilateral frontal association cortex (iFrAssC), ipsilateral cingulate cortex (iCingC), and ipsilateral insular cortex (iInsulC).
Figure 3: Kindling induces widespread increases in functional connectivity in response to 10-Hz ventral hippocampal stimulation.
(A) Brain-wide CBV-fMRI was segmented according to anatomical regions of interest. (B) Kindling resulted in statistically significant increases in functional connectivity between non-kindled and kindled animals that were predominantly in the ipsilateral hemisphere. (p < 0.05, FDR-corrected for multiple comparisons, significance is denoted by an “X”). (C) Functional connectivity of 10-Hz ventral hippocampal stimulation in non-kindled animals. (D) Functional connectivity of 10-Hz ventral hippocampal stimulation in kindled animals.
We next investigated how kindling impacted regional activations in the ventral hippocampal circuitry by comparing the CBV-fMRI amplitudes between groups (SFig. 3). We chose five regions with the largest activation volumes in the non-kindled group for comparison (SFig. 3B). Following multiple comparisons correction, we did not detect differences in the amplitude of the CBV-fMRI response in iVHip (2.1 ± 0.3 % vs. 3.1 ± 0.4 %, p = 0.122), iSept (1.6 ± 0.3 % vs. 2.5 ± 0.3 %, p = 0.082), or iDHip (0.8 ± 0.2 % vs. 1.2 ± 0.2 %, p = 0.178). However, robust differences were observed in the iMePFC (1.3 ± 0.3 % vs. 4.2 ± 0.6 %, p = 0.0023), and iAmyg (1.1 ± 0.3 % vs.3.0 ± 0.4 %, p = 0.0023). This data shows that optogenetic kindling of the VHip leads to more widespread (SFig. 3A) and intense (SFig. 3B) activity in specific downstream regions, as measured by fMRI during optogenetic iVHip stimulation.
Because CBV-fMRI is only an indirect measure of activity, we next analyzed the simultaneously-acquired LFP to determine if there was a corresponding electrophysiological signal change that would confirm an associated increase in neuronal activity. Following kindling, there was a clear increase in iMePFC response to stimulation, as measured by LFP (Fig. 2D). We quantified this response by calculating the band power during the 5 s stimulation divided by the band power of the 5 s prior to stimulation onset. We used this metric to compare between pre and post kindling and between non-kindled and kindled groups. We did not detect any clear differences in iVHip (Fig. 2E left panel, p = 0.90, n = 11 and 12 non-kindled and kindled animals respectively, 2-way repeated measures ANOVA). However, in iMePFC, we found a significant interaction between group and time. (Fig. 2E right panel, p = 0.009, n = 10 and 9. Reduced number of animals was due to electrode failure [SFig. 4] or removal because of outliers. For full dataset with outliers see SFig. 2C.) Further analysis indicated that there was a 2.9 ± 0.2 fold increase in LFP amplitude following kindling (p = 0.005, paired t-test). Taken together with the fMRI response, this suggests that kindling resulted in increased connectivity of iVHip to iMePFC.
In kindled animals, our unbiased whole brain approach identified the iMEPFC as the region with the greatest change in response (Fig. 3B, SFig. 3A). We also saw that there was an overall elevated brain response. However, it was unclear if all these changes had any long-term consequences on behavior. Because the ventral hippocampus modulates emotional and affective behaviors (Fanselow and Dong, 2010), we investigated whether or not ventral hippocampal kindling resulted in increased anxiety and depression (two of the most common epilepsy comorbidities with controversial mechanistic origins). Both anxiety and depression have been detected following electrical amygdala kindling (Chen et al., 2016). We investigated the long-term behavioral impact of our optogenetic kindling in a subset of animals by conducting behavioral tests 12 weeks following kindling (10 weeks following the second fMRI timepoint) (Fig. 2F). We used forced swim (Fig. 2G) and sucrose preference tests (Fig. 2H) to assess depression, and the open field test to assess anxiety. Neither depression tests indicated that there were clear differences between the non-kindled and kindled animals. For the forced swim test, mean immobility scores were 36.3 ± 0.99 in non-kindled controls and 34.1 ± 1.89 in kindled rats (Fig. 2H, p = 0.303, t-test). For the sucrose preference test, there was no evidence that non-kindled rats differed from kindled rats (Fig. 2G, no significant interaction between group and habituation/sucrose test period, F = 0.48 p = 0.499, two-way repeated measures ANOVA, mean estimates were 96.2 ± 0.9 % vs 83.9 ± 12.8 % for non-kindled and kindled respectively). Both groups increased total volume of liquid consumed when sucrose water was introduced for the test period (non-kindled rats by 32.3 ± 8.0 ml, p = 0.005 and kindled rats by 24.7 ± 6.9 ml, p = 0.011, paired t-test). In contrast, there was evidence for an increased anxiety phenotype in kindled animals. Kindled animals spent less time in the center of the open field arena when compared to the non-kindled rats (Fig. 2I, 12.5 ± 1.5 % vs. 8.0 ± 1.4 % time in center, p=0.046, non-kindled controls vs kindled rats, t-test, n = 8 and 7 respectively). Importantly, both groups of animals traveled a similar distance during the 5-minute test-period (2446 ± 153 cm vs. 2203 ± 197 cm, p = 0.98). We concluded that ventral hippocampal kindling resulted in elevated anxiety but not depression.
We have shown that the optogenetic ventral hippocampal kindling results in an increased response in iMePFC from iVHip stimulation, and increased anxiety as measured by the open field test. Signaling between iVHip and iMePFC has been shown to be an important circuit for anxiety. In a previous study, increased theta coupling (4–12 Hz) between VHip and MePFC has been observed when normal rodents were placed in an anxiogenic area (open field) compared to when they were placed in a safe area (Adhikari et al., 2010). Notably, inhibiting iVHip CAMKII projection terminals in iMePFC abolished this anxiety response (Padilla-Coreano et al., 2016). This all suggests a potential neurobiological basis for this comorbidity (Kanner, 2009). Our study indicates that hippocampal kindling may be a useful model for investigating heightened anxiety arising from dysfunctional signaling between iVHip and iMePFC, and a potential model for investigating epilepsy-associated anxiety.
Brain-wide imaging of single ADs and seizures with simultaneous LFP-fMRI in non-kindled and kindled rats
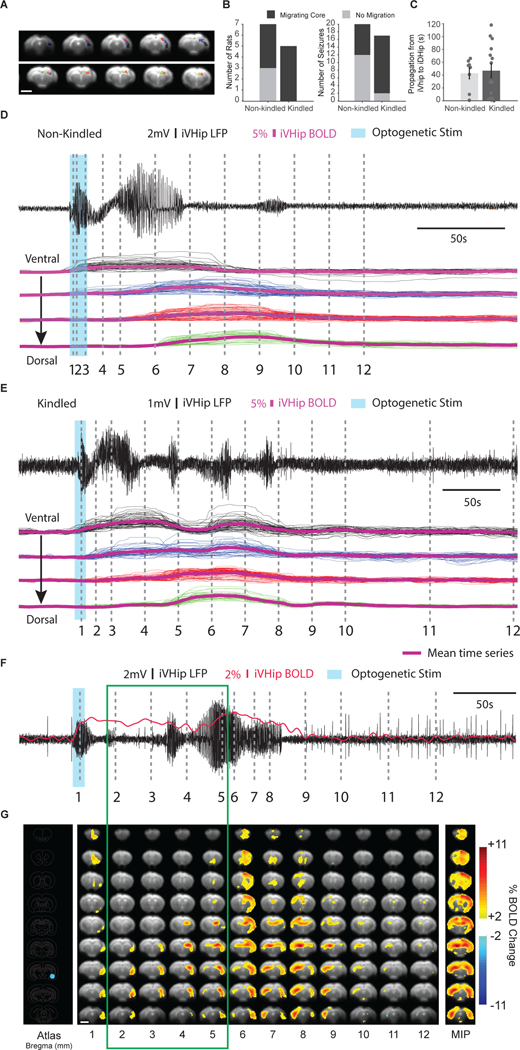
fMRI provides brain-wide information and has been used to visualize both focal and generalized-onset seizures in humans and in animals (Chaudhary et al., 2012; Choy et al., 2010a; Detre et al., 1995; Duffy et al., 2015, 2020; Englot et al., 2008; Schridde et al., 2008). However, the use of fMRI to visualize FBTC seizures has been challenging due to associated motor activity that results in motion artifacts. Sedation or anesthesia is typically required for animal fMRI to limit motion. However, anesthetics can affect seizure activity and associated motor activity (Choy et al., 2010a; Weitz et al., 2015). We have previously demonstrated that we can reliably induce seizures under our dexmedetomidine sedation protocol for imaging (Duffy et al., 2015, 2020), with the seizures retaining similar electrophysiological characteristics in awake and sedated states (SFig. 5 for within-animal electrophysiology during non-kindled seizure in awake and sedated states). When seizures were induced in kindled animals under dexmedetomidine sedation, stereotyped seizure behavior emerged that was reminiscent of that in awake animals, indicating that motor seizure circuits were activated under sedation (see Supplementary Videos 1 and 2 for awake and sedated kindled motor seizures respectively). Therefore, we developed a protocol based on dexmedetomidine sedation and incorporated vecuronium, a short-acting neuromuscular blocker, to abolish motion during imaging of seizures with simultaneous LFP-fMRI (Fig. 4A). As expected, seizure and AD duration estimated from iVHip LFP were longer in kindled animals compared to non-kindled animals (Fig. 4B, 66.2 ± 6.7 s vs. 35.4 ± 5.17 s respectively, p = 0.001).
Figure 4: Kindled seizures gradually propagate to cortex bilaterally whereas non-kindled ADs remain localized.
(A) Seizures were induced and assessed with simultaneous LFP-fMRI. Seizures were induced following a 90s baseline in non-kindled and kindled animals (n = 2–4 per rat, and n = 7 and 5 rats respectively). (B) Induced seizure duration. Seizures in kindled rats were significantly longer than those in the non-kindled rats (t-test, p < 0.001). (C) Examples of regional BOLD and VHip LFP activity from an AD in a non-kindled rat (left), and a seizure induced optogenetically in a kindled rat (right). Brains were automatically segmented into 44 regions using a common atlas (Fig. 5A). Blue bar indicates optogenetic seizure induction period. (D) Probability of regions active in non-kindled and kindled seizures. Probability was calculated by normalizing the number of times the region was active by the total number of seizures (n = 20 and n = 17, respectively). Note that in non-kindled seizures, activated regions were mostly in the ipsilateral hemisphere in contrast to kindled seizures in which bilateral activation was more common. Black circle indicates an 80 % threshold to ensure reliable onset time estimates for regional propagation analysis. (E) Regional propagation of activity in non-kindled and kindled seizures. Regions were sorted from fastest to slowest. White bars indicate ipsilateral regions, and black bars indicate contralateral regions. Left panel: In seizures in non-kindled rats, activated regions were most consistently observed in the ipsilateral hemisphere. Right panel: in contrast, seizures in kindled rats contained activity in both hemispheres. Notably, activity propagated from ipsilateral to contralateral hemisphere. Red labels indicate regions activated in both groups. (F) Onset time comparisons in commonly activated regions. iMePFC was activated significantly faster by 1.28 ± 0.46 s in kindled seizures than in non-kindled seizures (p = 0.044, corrected for multiple comparisons with Bonferroni procedure). A threshold of p < 0.05 was used for determination of statistical significance.
In both kindled and non-kindled animals, the increases in iVHip blood-oxygen-level-dependent (BOLD) signal corresponded to iVHip LFP amplitude increases, showing that BOLD successfully captured local activity changes (SFig. 6). To evaluate single seizure/AD propagation dynamics, we generated voxel-wise maps of % BOLD change from pre-stimulation baseline with a 1 s temporal resolution for every seizure, providing single seizure activity propagation dynamics at an unprecedented spatiotemporal resolution. SFig. 6 shows examples of single ADs and seizures with selected timepoints that capture activity propagation from a non-kindled and a kindled animal (see Supplementary Videos 3 and 4 for complete data sets, respectively). A voxel-wise maximal intensity projection (MIP) over the duration of the scan provides a summary of the regions activated during a seizure. These MIP activity patterns bear striking similarities to those reported for focal and generalized seizures using terminal methods such as 2-deoxyglucose (Dabrowska et al., 2019; Handforth and Treiman, 1995).
Distinct brain-wide propagation dynamics in non-kindled and kindled animals
We next compared the regional BOLD propagation patterns of non-kindled ADs and kindled seizures by calculating regional onset times (n = 20 from seven non-kindled rats, n = 17 from five kindled rats. One non-kindled and two kindled rats lost their head caps and could not be imaged). Using a brain atlas, mean regional responses for 44 ROIs were segmented and calculated for each seizure (Fig. 4C regional dynamics from a non-kindled and kindled rat). Onset time was defined as the time at which BOLD activity first exceeds four standard deviations above the 60 s pre-stimulation baseline, with an additional constraint that the activity must remain above threshold for at least 5 of the subsequent 10 s after this time. We compared the number of activated ROIs and found that more regions were involved in the kindled group than in non-kindled (SFig. 7C, 23.4 ± 2.0 regions vs. 38.8 ± 1.0 regions, n = 20 vs. 17, control vs. kindled, p < 0.0001).
Next, we investigated the frequency of regional activation in non-kindled ADs and kindled seizures using a modified radar plot (Fig. 4D). This visualization of the data set reveals that kindled seizures consistently activated bilaterally, whereas non-kindled ADs preferentially activated the ipsilateral hemisphere. This included bilateral motor cortex (MotorC) activity, which is associated with bilateral limb movement and is a key characteristic of tonic-clonic seizures. This supports the hypothesis that key spatiotemporal dynamics of FBTC seizures were captured in these data (Dabrowska et al., 2019).
To calculate mean regional onset times for investigating activity propagation, only regions that were active in at least 80 % of seizures were used. (See Fig. 4D, n = 16–20 non-kindled and n = 14–17 kindled seizures, 80 % threshold used to obtain reliable onset time estimates. See SFig. 7A,B, for 90 % and 0 % thresholds, respectively). In non-kindled seizures, eight regions were active in at least 80% of the seizures, which were all from the ipsilateral hemisphere (Fig. 4E, left panel, regions were sorted from fastest to slowest): iPiriC, iMePFC, iOrFrC, iVHip, iSept, iInsulC, ipsilateral entorhinal cortex (iEntC), iTeAssC. For kindled seizures, 38 regions from both hemispheres were active in at least 80 % of the seizures (Fig. 4E, right panel). Furthermore, there was a clear pattern of propagation from the ipsilateral to the contralateral hemisphere. Of the first 20 regions with the fastest onset times, 18 of these were in the ipsilateral hemisphere. Of the remaining 18 regions, 14 were in the contralateral hemisphere. Notably, MDThal, a region that has been implicated as a critical node for seizure generalization (Bertram et al., 2001; Zhang and Bertram, 2002), was activated later than many regions of the cortex, suggesting that MDThal activation is downstream of seizure generalization. Statistical comparisons of functional connectivity matrices between the non-kindled ADs and kindled seizures, similar to the analysis performed following 10-Hz VHip stimulation, revealed widespread significant increase in both the ipsilateral and contralateral hemispheres (Fig. 5).
Figure 5: Kindling resulted in brain-wide increases in functional connectivity during seizures/ADs.

(A) Brain-wide BOLD-fMRI was segmented according to anatomical regions of interest. (B) Kindling resulted in statistically significant bilateral increases in functional connectivity during single seizures/ADs when comparing non-kindled and kindled animals. (p < 0.05, FDR-corrected for multiple comparisons, significance is denoted by an “X”). (C) Functional connectivity of optogenetically induced ADs in non-kindled animals. (D) Functional connectivity of optogenetically induced seizures in kindled animals.
We then compared regional onset times of the eight commonly active regions between non-kindled and kindled groups (Fig. 4F). We detected faster onset times in the iMePFC in the kindled seizures (2.8 ± 0.26 s) compared to non-kindled ADs (4.1 ± 0.34 s, Holm’s adjusted p = 0.044), while no consistent differences were observed in other regions (p > 0.3 for all other regions, non-kindled vs. kindled): iVHip (4.2 ± 0.43 s vs. 4.4 ± 0.76 s), iOrFrC (4.2 ± 0.51 s vs. 3.0 ± 0.21 s), iSept (4.6 ± 0.62 s vs. 7.1 ± 1.34 s), iInsC (7.1 ± 3.94 s vs. 6.9 ± 2.33 s), ipsilateral piriform cortex (iPiriC) (3.4 ± 0.32 s vs. 3.4 ± 0.33 s), iEntC (9.8 ± 3.06 s vs. 9.7 ± 5.7 s), and iTeAssC (10.8 ± 2.14 s vs. 8.6 ± 3.46 s). The faster onset time in iMePFC in kindled rats provides further evidence of increased ipsilateral connectivity between iVHip and iMePFC.
Similar network responses to 10-Hz ventral hippocampal stimulation and ventral hippocampal seizure/AD induction
The early network response during seizure/AD induction (SFig. 6, timepoints 1–3) measured with BOLD-fMRI appeared similar to the network response to 10-Hz VHip stimulation measured with CBV-fMRI (Fig. 2C), suggesting a common network response. However, direct comparisons between the two fMRI-modalities are difficult because of their different contrast mechanisms and vascular sensitivities that can result in distinct spatial activity patterns (Zhao et al., 2006). Because of these differences, we instead investigated the regional relationship between the network responses in the two groups. Our aims were to identify distinct temporal components of brain-wide activity during AD and seizures and to confirm persistent circuit changes.
Seizure induction maps were generated for non-kindled and kindled animals (Fig. 6A, n = 7,5; respectively). Visually, there were apparent and striking similarities between the seizure induction networks and those resulting from 10-Hz stimulations (Fig. 2). We quantified regional activation volumes to determine if there were apparent differences between the two groups, but we did not detect any differences following adjustment for multiple comparisons (SFig. 7E). However, we did find that the active voxel count in kindled rats was greater than in non-kindled rats (SFig. 7D, 3562 ± 636 voxels vs 1784 ± 347 voxels, p = 0.025). This suggests a generally larger network response in kindled rats, and that the lack of regional differences may be due to the small numbers of animals or the large number of multiple comparisons involved.
Figure 6: Seizure/AD induction activates underlying ventral hippocampal networks.
(A) Optogenetic seizure/AD induction network activity in non-kindled and kindled animals (n = 7, n = 5; respectively). Group maps from brain-wide BOLD-fMRI depicting normalized percent-active during optogenetic seizure/AD induction. (B) Number of active regions during ventral hippocampal stimulation in non-kindled and kindled animals. For animals that underwent both stimulation and seizure/AD imaging, kindled rats had significantly more active regions during stimulation than non-kindled rats (t-test, p = 0.002). (C) Number of active regions during optogenetic seizure/AD induction in non-kindled and kindled rats. Kindled rats had significantly more active regions during 10-Hz stimulation than in the non-kindled rats (t-test, p = 0.023). (D, E) Distribution of regional conditional probabilities in non-kindled and kindled rats. Regions were grouped from highest to lowest by the probability of that region being activated during the 10-Hz stimulation. This was also used to calculate the conditional probability of that region being active during seizures/ADs, given the likelihood of activity during 10-Hz stimulation.
To further investigate the relationship between the 10-Hz and seizure/AD induction networks, we calculated the conditional probability of a region being active during a seizure/AD, given the probability of it being active during 10-Hz stimulation. First, we performed a robustness test to confirm that reliable differences could be detected between groups for the number of active regions in the 10-Hz and seizure induction networks (Fig. 6B,C for 10-Hz, non-kindled vs kindled: 5.6 ± 1.4 regions vs 16.8 ± 2.6 regions, p = 0.002; for seizure induction 20.6 ± 2.1 regions vs 30.8 ± 3.4 regions, p = 0.023,). Conditional probabilities were calculated by region and group. and displayed in Fig. 6D,E, with regions sorted by activation frequency during 10-Hz stimulation. In non-kindled animals, iVHip and iSept was always active under both conditions.
In kindled animals, iVHip, iMePFC, iOrbFrC, iFrAssC, iSept, iStria, iTeAssC and iAmyg were always active. Overall, the mean regional conditional probability was 0.76 ± 0.11 in non-kindled animals vs 0.89 ± 0.05 in kindled animals. This indicates that regional activation during seizure induction was similar to that of the 10-Hz network that had been assessed 12 weeks earlier. Importantly, these data indicate that initial circuit stimulation is a key component of the observed activity from which seizures arise and suggest that kindling-induced circuit changes persist beyond 3 months.
A migrating core of high amplitude activity in the hippocampus and its role in seizure propagation
This whole brain data provides unbiased single seizure/AD activity propagation dynamics at unprecedented spatiotemporal resolution, thus providing an opportunity for discovering and characterizing new phenomena. We generated 37 individual seizure/AD videos, in the same manner as SFig. 8 and Supplementary Videos 3 and 4, to visualize brain-wide propagation dynamics.
When we examined individual seizure/AD data, we frequently observed a slow migrating core of high amplitude activity that propagates from ventral to dorsal hippocampus. Furthermore, this core was found in both non-kindled and kindled animals, indicating that kindling-induced changes were not required for the core to form and that normal hippocampus already possesses the mechanisms required to sustain it. A migrating core was observed in 15/17 seizures in 5/5 kindled rats and 8/20 ADs in 4/7 non-kindled rats (Fig. 7B). Examples of individual AD data with migrating cores are shown in Fig 7D and SFig. 8A from a non-kindled animal (see Supplementary Video 3 for an example from a different non-kindled animal). An example from a kindled animal is shown in Fig. 7E and SFig. 8B (see Supplementary Videos 5 and 6 for complete time series for a non-kindled AD and kindled seizure, respectively).
Figure 7: A slow migrating hippocampal seizure core occurred frequently in non-kindled and in kindled rats.
(A) Segmentation of ipsilateral hippocampus. Hippocampus was segmented into 4 regions across 10 slices. (B) Migrating cores were observed frequently in both groups of animals. (Left panel) Frequency at individual animal level. (Right panel) Frequency at individual seizure/AD level. (C) Activity propagation time from ventral hippocampus to dorsal hippocampus. Peak-to-peak time from the most ventral to the most dorsal of the segmented regions was used to calculate the time that peak activity was observed in the ventral to dorsal hippocampus. (D, E) Ventral hippocampal LFP recordings and individual hippocampal BOLD voxel time series (D) from a non-kindled rat and (E) from a kindled rat. Dotted lines denote time stamps for corresponding fMRI images (SFig. 8). (F, G) Migrating hippocampal seizure core is the only high amplitude activity in the brain for > 50 s before propagation of activity out of the hippocampus (see Supplementary Video 7 for complete time series data). Single seizure induced in a kindled rat: (timepoint 1) seizure induction; (timepoints 2–5) high amplitude hippocampal activity is the only apparent activity in the brain for ~50 seconds; (timepoint 6) activity propagates to ipsilateral cortex; (timepoints 7–8) activity propagates to contralateral hippocampus; (timepoint 9) activity propagates to contralateral cortex.
Given the migratory nature of the core, we next estimated its propagation speed in the two groups by dividing the hippocampus into four regions, from ventral to dorsal, and calculating peak to peak times in each subregion (Fig. 7A). Propagation speeds of the migrating core were similar in the non-kindled and kindled groups (Fig. 7C) with mean speeds of 0.117 mm/s in non-kindled rats and 0.107 mm/s in kindled rats. Despite similar hippocampal propagation speeds, activity propagation from ipsilateral to contralateral hippocampus was only observed in kindled rats. In seizures with migrating cores, activity propagating from iDHip to cDHip was observed in 0 % (0/8) of non-kindled ADs from 4 rats, vs 53 % (8/15) of kindled seizures from 4 of 5 rats.
Although we observed the migrating core consistently, we sought for more evidence that it could play a key role in seizure propagation. In kindled seizures, widespread activity can generalize quickly so the role of the core is difficult to determine (SFig. 8B). However, we identified a few seizures (three seizures from two kindled rats) in which the migrating hippocampal activity was the only apparent response in the brain for >30 seconds, and cortical activation only emerged after the core had migrated to iDHip. This suggests that the core can play a key role in seizure propagation and sustainment (Fig. 7F,G, see Video 7 for complete time series): activity migrates from iVHip to iDHip (timepoints 2–5) then propagates to ipsilateral cortex (timepoint 6), contralateral hippocampus (timepoints 7–8), and finally to contralateral cortex (timepoint 9).
Discussion
In this paper, we developed a new optogenetic model of hippocampal kindling and used this model to investigate epileptogenesis and changes in seizure/AD dynamics. We first demonstrated that repeated optogenetic stimulations can be used for kindling. Next, we used this model to conduct unbiased brain-wide investigations of ventral hippocampal circuit dynamics. For these investigations, we used two stimulation paradigms: 1) a short and mild 10-Hz stimulation to evaluate underlying functional circuit changes associated with epileptogenesis, and 2) a long and intense 40-Hz stimulation to evaluate seizure/AD circuit dynamics. We found that kindling resulted in chronic and widespread increases in brain-wide response to ventral hippocampal CAMKII neuronal stimulation, with the largest activity changes found in the MePFC. We also found that anxiety behavior was elevated following kindling. Next, we imaged brain-wide dynamics of individual ADs and FBTC seizures to reveal their distinct propagation patterns. Finally, we identified a slowly migrating core of high amplitude activity in the stimulated hippocampus, adding evidence of its role as a novel seizure propagation mechanism.
Optogenetic methods provide improved spatial and temporal targeting compared to electrical or chemical stimulation. We demonstrated that repeated optogenetic stimulations can be used to induce kindling. However, optogenetic kindling likely shares a limitation common to all kindling models in that spontaneous seizures do not readily emerge after kindling. Thus, these models do not fully capture all of the neurobiological changes that underlie epilepsy (Pitkanen and Halonen, 1998). Nevertheless, spontaneous seizures can emerge from kindling if seizures continue to be induced far beyond the fully kindled state. Therefore, kindling is considered to induce circuit changes that are part of a continuum toward epilepsy (Brandt et al., 2004). Furthermore, compared to other common epilepsy models such as pilocarpine or kainic acid, kindling models have a significant advantage in that they minimize cell death and therefore avoid complications from dissociating atrophy and epileptogenic circuit changes (Choy et al., 2010b; Jupp et al., 2012).
We used CBV-fMRI to provide an unbiased brain-wide approach to investigate kindling-induced changes. Overall, we found that the brain-wide response was more intense and widespread in kindled animals, persisted for at least 3 months, was associated with a propensity for FBTC seizures, and correlated with heightened anxiety. Taken together, this within-animal data strongly support the hypothesis that kindling results in the emergence of a sensitized circuit. Further experiments are required to determine how much of the activity changes reflect pre- or post-synaptic remodeling of the circuit.
With this technique, we found that the largest changes to VHip stimulation were in the iMePFC. This increased CBV response was supported and validated by simultaneous LFP recordings, which directly measure neuronal activity. Because we directly stimulated VHip excitatory CAMKII neurons for this experiment, this data indicates that there is an increased excitatory relationship between VHip and MePFC after kindling. The increased iMePFC response, alongside heightened anxiety after kindling, suggest the emergence of a sensitized circuit for anxiety, one of the most common epilepsy comorbidities (Kanner et al., 2012; Keezer et al., 2016; Scott et al., 2018). Notably, VHip and MePFC are two of three nodes of a key anxiety circuit. When an animal enters an anxiolytic environment, theta-coupling (8–12 Hz) between VHip and MePFC increases, and when theta-coupling was disrupted, the associated anxiety behavior was abolished (Adhikari et al., 2010, 2011; Padilla-Coreano et al., 2016). The VHip-MePFC circuit dysfunction has been observed in patients with epilepsy and in other hippocampal kindling models. Moreover, this circuit dysfunction was implicated in cognitive deficits, another common epilepsy comorbidity (Gelinas et al., 2016). Taken together, these data indicate that VHip kindling can be used to model VHip-MePFC circuit dysfunction that may be common in patients with epilepsy. Developing therapies that target VHip-MePFC circuit dysfunction may reduce some of the most common comorbidities associated with epilepsy, and enable effective treatment for conditions that can potentially improve the reduced quality of life caused by seizures (Brandt and Mula, 2016; Chen et al., 2018).
We imaged individual non-kindled ADs and kindled seizures at an unprecedented spatiotemporal resolution to reveal their distinct brain-wide propagation dynamics. We found that the areas recruited in optogenetically-induced ADs and seizures correspond with those found in spontaneous as well as electrically or chemically induced seizures, indicating that optogenetic methods induce similar types of activity (Dabrowska et al., 2019; Englot et al., 2008; Handforth and Treiman, 1995; Toyoda et al., 2013). Furthermore, imaging at this resolution revealed a migrating core of high amplitude in the stimulated hippocampus that we propose as a novel mechanism for seizure propagation. Although kindled seizures are considered to mimic many key aspects of clinical FTBC seizures including pharmacological response, further work is required to determine if such migratory activity occurs in other models and in humans (Klitgaard and Pitkänen, 2003; Löscher, 2011).
The migrating nature of the core indicates that the seizure initiation network is only active during the early stages of seizure. Without imaging the brain from very beginning of seizure initiation, it would likely be difficult to distinguish a migrating core from propagation and expansion of the seizure network (e.g. compare Fig. 7F,G to SFig. 6B). This implies that the migrating core is more easily detectable with electrical or optogenetic seizure induction models versus other models which use local application of chemoconvulsants. Furthermore, we were able to detect the migrating core because we acquired single seizure data at a temporal sampling frequency of 1 s. The speed of the migration suggests that similar or faster sampling frequencies will be necessary to detect the core, and that cross-sectional imaging methods are unlikely to be able to detect core migration. Thus, spatial sampling strategies are also critical for detecting the hippocampal core. Although we took an unbiased whole brain approach, our data indicate that sampling along the longitudinal axis of the hippocampus would be sufficient. A migrating core may have important implications for epilepsy surgery, which requires a seizure onset zone (SOZ) to be reliably localized (Martinez-Vargas et al., 2017; Plummer et al., 2019; Smith et al., 2016).
The mechanisms which underlie the migrating hippocampal core are unclear. Moreover, we do not know if this phenomenon occurs outside of the hippocampus. However, intrinsic hippocampal circuits were sufficient to support this core because it was observed in non-kindled as well as kindled rats. Travelling at ~0.1mm/s, seizure activity propagating at similar speeds has been reported in humans and in animals, and suggests ionic diffusion and inhibitory restraint as potential key mechanisms (Durand et al., 2010; Rossi et al., 2017; Schevon et al., 2012; Zhang et al., 2014). Intriguingly, recent evidence has implicated slow propagating activity as the primary mechanism for sudden unexpected death associated with epilepsy (SUDEP) (Aiba and Noebels, 2015; Ryvlin et al., 2013). However, further studies are required to determine if this activity shares the same underlying mechanisms as the migrating hippocampal core.
When seizures propagated out of the hippocampus, activity followed known axonal pathways, which implicates synaptic mechanisms. Fig. 7G is an example of propagation via synaptic transmission in which seizure activity distinctly propagates from ipsilateral to contralateral hippocampus. Synaptic seizure propagation mechanisms have been previously observed in a mouse model of focal cortical seizures, in which activity propagated to specific regions rather than non-selectively to contiguous regions (Rossi et al., 2017). Rossi et al used widefield calcium imaging and LFP after focal chemoconvulsant application to observe the formation of a stationary activity core which then propagated selectively to homotopically connected cortex. Taken together, these data indicate that FBTC seizures involve both synaptic and non-synaptic mechanisms that evolve over the course of the seizure. Using hippocampal kindling models alongside whole brain imaging may help in developing therapies which target these mechanisms and ultimately improve seizure control.
STAR Methods
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jin Hyung Lee (ljinhy@stanford.com).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals and experimental design
25 adult male Sprague-Dawley rats (Charles River Laboratories) were used in this study. Animal husbandry and experimental protocols were in strict accordance with the National Institutes of Health (NIH) and Stanford University’s Institutional Animal Care and Use Committee (IACUC) guidelines. Animals were housed under environmentally controlled conditions, a 12-hour light-dark cycle with food and water provided ad libitum. For an outline of the experimental design see SFig. 1.
METHOD DETAILS
Surgical procedure for virus injection and implantation of optrodes and electrodes
Briefly, rats were anesthetized using 5 % isoflurane in pure oxygen and then maintained on 2–3 % throughout the duration of the surgical procedure. 2 μl of AAV-5-CAMKIIa-hChR2(H134R)-eYFP was injected into the right ventral hippocampus (AP: −5.6 mm, LR: 5.7 mm, DV: 6 mm from dura) using a using a 33 gauge needle attached to a Hamilton syringe (Duffy et al., 2015; Weitz et al., 2015). A syringe pump (Micro 4, World Precision Instruments, FL) was used to ensure a constant rate (150 nl/min) of administration. MRI compatible carbon fiber optrodes, constructed with 0.22 numerical aperture, 105 μm diameter step-index multimodal optical fiber (ThorLabs, Newton, NJ) as described previously (Duffy et al., 2015), were inserted so that the tip of the electrode and fiber resided just above the injection site. Before implantation, the optrodes were checked to ensure that the percentage of light transmission was greater than 80 % and light transmission to the brain was assumed to be approximately this value. A single brass screw was inserted above the cerebellum to anchor the dental cement and also to serve as ground and reference electrode. A carbon fiber electrode (manufactured as described previously in Duffy et al., 2015) was implanted into the right medial prefrontal cortex (AP: +3.24 mm, LR: +1.25 mm, DV: −3.4 mm from dura at a 10° angle). Finally, the electrode wires were soldered to a DF13 connector (Hirose, Japan) and all components were secured to the skull using light-curable dental cement (Clearfil Liner Bond 2V, Kuraray America, Inc. NY, USA). Buprenorphine sustained release (1 mg/kg, s.c.) was given pre-operatively to alleviate pain and discomfort due to the procedure. Local administration of lidocaine (4 %) and bupivacaine (0.25 %) was also given pre- and post-operatively. To allow time for viral-induced protein expression, experiments were performed at least 6 weeks after the surgical procedure.
Optogenetic kindling
12 rats were kindled using the following procedures and monitored throughout using simultaneous video LFP recordings in their home cages. Animals were connected to a 473 nm (blue light) diode-pumped solid-state laser (Laserglow Technologies, Toronto, Canada) and a 16 channel BrainAmp ExG MR amplifier (Brain Products, Germany) via a fiber-optic and electrical rotary joint (Doric Lenses, Quebec, Canada) (Cardin et al., 2010). Video was recorded using a Logitech C920 HD Pro webcam.
The afterdischarge threshold was first evaluated for each individual animal by gradually increasing stimulation intensity in a step-wise manner to drive electrographic seizures in the absence of behavioral seizures as assessed using the Racine scale. Starting with a 10 s train of 40-Hz stimulation with a pulse width of 7.5 ms at 1 mW, the power was increased 1 mW at a time with an interstimulus interval (ISI) of 1 min up to a maximum of 20 mW. If no afterdischarges were observed, the duration was increased by 2.5 s and power was reset to 1 mW.
After establishing the afterdischarge threshold, kindling commenced. Animals were stimulated up to a maximum of 12 stimulations a day or until the emergence of a stage 5 motor seizure, and stimulated every other day up to a maximum of 12 days of stimulations. Interstimulus interval was 15 minutes. Animals were considered to be kindled if a stage 5 motor seizure was observed within the first three stimulations of the day.
Using the same criterion, the state of kindling was assessed in a subset of animals (five age-matched parallel controls and five kindling rats) at 3- and 12-weeks following kindling by stimulating three times with an ISI of 15 minutes.
Simultaneous LFP-ofMRI data acquisition for assessing brain-wide circuit response to ventral hippocampal neuronal stimulation
To assess brain-wide response to ventral hippocampal neuronal stimulation, CAMKIIα cells in ventral hippocampus were stimulated at 10 Hz and assessed using simultaneous LFP-fMRI. Data acquisition was performed using the 7 T horizontal-bore system (Bruker BioSpec 70/30) at the Stanford Center for Innovation in In-Vivo Imaging (SCi3). An 86 mm diameter 2-channel volume coil (Bruker, MA, USA) was used for RF excitation with a 20 mm single-loop surface coil (Bruker, MA, USA) as the RF receiver. Rats were sedated using a bolus (0.1 mg/kg, s.c.) of dexmedetomidine followed by a continuous infusion (0.05 mg/kg, i.v.) via a 24G cannula inserted into a lateral tail vein. A single bolus of Feraheme (15 mg/kg, i.v., AMAG Pharmaceuticals, Inc., MA, USA) was used for cerebral blood volume (CBV) weighted imaging for the enhanced contrast to noise ratio (Duffy et al., 2015; Mandeville et al., 1998) and microvascular sensitivity (Zhao et al., 2006) that this technique offers in comparison to BOLD fMRI. This increased sensitivity was important for detecting activity and to compensate for reduced signal from a shorter stimulation duration that was necessary to reduce the risk of generating hippocampal ADs. fMRI acquisition was carried out approximately 15 min post contrast agent injection using a 4-shot segmented spiral readout with the following acquisition parameters: TR = 0.75 ms, TE = 9 ms, flip angle = 30, field-of-view = 32 × 32 mm, matrix = 70 × 70, slice thickness = 0.6 mm, number of slices = 30, number of repetitions = 130, number of dummy scans = 4, receiver bandwidth = 500 kHz. For optogenetic stimulation, a block design was used comprising of 5 s on and 55 s off with 10 Hz, 7.5 ms pulse width for stimulation. At the end of each session, atipamazole (0.5 mg/kg, s.c., Zoetis Inc., NJ, USA) was given to reverse the effects of dexmedetomidine. In addition to fMRI, anatomical (fast spin echo) scans were acquired for the assessment of hippocampal volumes with the following parameters: TR = 4,755 ms, TEeff = 29.9 ms, RARE factor = 8, field-of-view = 30 × 30 mm, matrix = 256 × 256, slice thickness = 0.6 mm. For assessing hippocampal volumes, hippocampal volumes were manually drawn on the ipsilateral and contralateral hemispheres for each animal. Baseline and post-kindling measurements were made in non-kindled and kindled animals, and within animal volumes were assessed by normalizing each hippocampal volume to its baseline. An independent t-test was used to assess the differences between the two groups for each hemisphere to determine if overt hippocampal volume loss was associated with kindling.
Simultaneous LFP-ofMRI data acquisition for imaging seizure circuit dynamics
To assess brain-wide seizure circuit dynamics in non-kindled (n = 7) and kindled animals (n = 5), we used simultaneous LFP-BOLD fMRI with optogenetic stimulation of ventral hippocampal CAMKIIα cells. Because motor seizures under the dexmedetomidine sedation protocol results in significant motion, animals were paralyzed and ventilated. For practical reasons, we chose BOLD contrast for this experiment because the signal was easily detectible with BOLD and it was simpler without the need for a contrast agent injection. Three animals could not be imaged due to loss of their implants (non-kindled n = 1, kindled n = 2). Seizures were induced using a 40-Hz pulse train with a 7.5 ms pulse width at the pre-determined afterdischarge threshold as described above.. Anesthesia was induced using 5 % isoflurane and reduced to 2–3% for tail vein cannulation. To maintain sedation and paralysis for imaging, a bolus of dexmedetomidine was injected subcutaneously (0.1 mg/kg, s.c., Dexdomitor, Pfizer, NY, USA) followed by continuous infusion via the tail vein cannula of dexmedetomidine (0.05 mg/kg, i.v) and vecuronium bromide (7.5 mg/kg, Teva Pharmaceuticals USA, Inc, NJ, USA). Isoflurane was slowly withdrawn before imaging start. Animals were maintained at a respiration rate with a range of 60–90 breaths per minute, temperature range of 36–38°C and expired CO2 range of 4–5%.
Data acquisition was performed using the MRI system as described above. For fMRI, data were acquired using a 1-shot EPI readout with the following parameters: TR = 1 s, TE = 16 ms, flip angle = 30, field-of-view = 32 × 32 mm, matrix = 70 × 70, slice thickness = 0.6 mm, number of repetitions = 480, number of dummy scans = 4, receiver bandwidth = 250,000 Hz. Seizure induction began 90 s following the start of imaging. At the end of each session, atipamazole (0.5 mg/kg, s.c.) was given to reverse the effects of dexmedetomidine.
Simultaneous LFP recordings and preprocessing
LFP recordings concurrent with the fMRI data acquisition were carried out using a 16 channel BrainAmp ExG MR amplifier (Brain Products, Germany) with a low pass filter of 1,000 Hz and a sampling rate of 5,000 Hz. Gradient artifacts were removed using the method implemented by Liu et al. using principal component analysis (Liu et al., 2012).
Sucrose preference test
Two freshwater bottles were placed in the home cages of individually housed rats (8 controls and 7 kindling rats) for four days and their locations swapped daily to acclimate the rats to the bottles and to reduce preference for a location. The water bottles were weighed daily (9–10 am). After the acclimatization period, two freshwater bottles - one filled with water and the other filled with 5 % w/v sucrose water- were placed in the cages. The water bottles were weighed and positions were swapped daily (9–10 am) for four days. The identity of the water bottles was blinded for the duration of the study. The volume of water drunk daily and the ratio between water and sucrose water was used to assess the anhedonia.
Open field test
The identity of the animals (8 controls and 7 kindling rats) was blinded for this procedure and only revealed following completion of data processing. Rats were placed individually in a custom-built chamber (1 m × 1 m × 0.3 m) and recorded with a Logitech C920 HD Pro webcam. Automated tracking was performed offline for the first five minutes of the recordings using Viewer3 software (Biobserve GmbH, Germany). Distance travelled and the time in the center of the arena was used to assess anxiety. The center of the arena was defined as a 0.67 m × 0.67 m square center of the 1 m × 1m arena.
Forced swim test
The identity of the animals (8 controls and 7 kindling rats) was blinded for this procedure and only revealed following completion of data processing. Rats were placed individually in a custom-built cylinder (30 cm diameter, 90 cm high) filled up to approximately 60 cm high with water at 23–25 °C. A 10 minute pre-test was performed on the day prior to the test day. Animals were recorded for the duration of the test (5 minutes) using a Logitech C920 HD Pro webcam.
Animals were towel-dried and returned to their home cage. All data was analyzed offline. Animals were scored for immobility and swimming and climbing using the modified FST scoring system for which the dominant behavior at each 5 s epoch was assessed (Slattery and Cryan, 2012). Immobility scores was used to assess behavioral despair.
QUANTIFICATION AND STATISTICAL ANALYSIS
fMRI preprocessing
SFig. 13 provides examples of raw data and a corresponding anatomy scan from a CBV-fMRI and a BOLD-fMRI experiment. All fMRI data was preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm). Data were first smoothed using a Gaussian kernel of 0.5 mm at full-width at half-maximum and motion corrected using a 6-parameter rigid registration. Brain masks were manually drawn on each fMRI dataset and a 12-parameter affine registration was used to align each fMRI dataset to a modality-specific common space i.e. CBV-fMR images were registered to a CBV-fMR image and BOLD-fMR images to a BOLD-fMR image. Mean images for each experiment were generated from the registered data. Modality-specific mean images were used as the base images on which activity was overlaid to enable evaluation of data registration and image distortion. Mean images were used to generate an experiment-specific segmented atlas (for CBV-fMRI see Fig. 3A, for BOLD-fMRI, see Fig. 5A).
fMRI of 10-Hz ventral hippocampal stimulation analysis
A double gamma basis set function was used for signal convolution to the stimulation block. A general linear model (GLM) was used to generate statistical activation maps. For regional analyses, brains were automatically segmented using a common brain atlas that yielded 44 regions after which activation volumes and mean time courses were calculated for individual animals (Fig. 3A). Kindling parameters, such as number of stimulations or stage 5 seizures, could be included as regressors in the GLM to evaluate the significance of their contribution to changes in activation maps (SFig. 2A,B). Custom MATLAB scripts were used to segment scans into regional time series. The signal to noise ratio (SNR) was calculated in individual animals by selecting a single frame and manually drawing an ROI in brain tissue and dividing by the standard deviation of an ROI drawn in the background.
fMRI of single seizures and seizure propagation analysis
All scans were registered to a brain atlas and low pass filtered at 0.1 Hz. All voxels were normalized to their 60 s baseline before stimulation onset to determine percentage BOLD change. For each seizure fMRI, seizure propagation was determined at a region of interest level. The segmented brain atlas was used to define the regions and their time series were extracted using custom MATLAB scripts (Fig. 5A). A mean time course was calculated from all voxels within a given region. Onset time for each region was calculated by determining the time at which the signal reached four standard deviations from baseline (60 s before stimulation onset) with the additional constraint that this signal has to persist above this baseline for at least five of the subsequent 10 s.
Statistical analysis
Values are presented as mean ± s.e.m. Two-way repeated-measures ANOVA was used to analyze hippocampal volumes, VHip and MePFC LFP response and sucrose preference test. Paired t-test was used for post-hoc analysis. Independent t-tests were used to analyze regional activation volumes, SNR, CBV amplitude, regional onset times, forced swim test, open field test (time in center and distance travelled), seizure duration based on LFP, number of activated ROIs during seizures, and migrating core speed. Holm’s Bonferroni method for adjustment of p-values for multiple comparisons was used where indicated.
Functional connectivity analysis
For functional connectivity analysis of a given CBV-fMRI scan, a representative time series was computed for each of the 42 manually annotated anatomical regions by taking the mean signal across all voxels in that region. For each subject, we then computed the functional connectivity matrix consisting of the Pearson correlation coefficient for the time series of each pair of anatomical regions. Group comparisons of these connectivity matrices were performed using independent t-tests with false discovery rate (FDR) correction for multiple comparisons. Potential outliers were identified using a threshold at 1.5 × above and below the interquartile range and removed from further analysis. If any outliers were removed, this is indicated in the manuscript and full datasets with outliers are provided in supplemental materials. To evaluate data normality, Kolmogorov-Smirnov test was used. Statistical analyses were performed using SPSS 21 or custom MATLAB scripts. Statistical significance was set at p < 0.05.
For functional connectivity analysis of a given BOLD-fMRI scan, similar methods were used. However, prior to grouping time series into anatomical region, the scan was first detrended by subtracting a linear fit to the baseline of the scan (first and last 30 seconds), followed by a low-pass filter with a cutoff frequency of 0.1 Hz. Since the BOLD acquisitions suffered from a relatively lower temporal SNR, this filtering was necessary to smooth the signal and attenuate high-frequency noise.
Supplementary Material
A seizure was induced in the ventral hippocampus using optogenetic stimulation. A 15 second, 40 Hz stimulation with a pulse width of 7.5 ms begins at 7 seconds in the video. Following stimulation, the rat displays forelimb clonus and rearing and falling, which are classic stereotyped seizure behaviors under Racine scoring system.
A kindled rat was sedated using dexmedetomidine and a seizure was induced in the ventral hippocampus using optogenetic stimulation. A 40 second, 40 Hz stimulation with at pulse width of 7.5 ms begins at 5 seconds in the video. Following stimulation, the rat displays forelimb clonus and rearing and falling, which are classic stereotyped seizure behaviors under Racine scoring system and similar to those observed in awake kindled rats.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 3C,D.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 3E,F.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 6A,B.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 6C,D.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 6I,J.
Table 1:
Anatomical regions used for fMRI analysis.
| # | Acronym | Region Name |
|---|---|---|
| 1 | Amyg | Amygdala |
| 2 | AudC | Auditory cortex |
| 3 | CingC | Cingulate cortex |
| 4 | DHip | Dorsal hippocampus |
| 5 | DLThal | Dorsolateral thalamus |
| 6 | EntC | Entorhinal cortex |
| 7 | FrAssC | Frontal association cortex |
| 8 | Hypo | Hypothalamus |
| 9 | InsulC | Insular cortex |
| 10 | MDThal | Mediodorsal thalamus |
| 11 | MePFC | Medial prefrontal cortex |
| 12 | MotorC | Motor cortex |
| 13 | OrFrC | Orbitofrontal cortex |
| 14 | ParieC | Parietal cortex |
| 15 | PiriC | Piriform cortex |
| 16 | ReplC | Retrosplenial cortex |
| 17 | Sept | Septum |
| 18 | SomC | Somatosensory cortex |
| 19 | Stria | Striatum |
| 20 | TeAssC | Temporal association cortex |
| 21 | VHip | Ventral hippocampus |
| 22 | VisC | Visual cortex |
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| rAAV5-CaMKII-hChR2(H134R)-EYFP | University of North Carolina vector core | Lot AV4316LM |
| Chemicals, peptides, and recombinant proteins | ||
| Feraheme | AMAG Pharmaceuticals | NDC Code 59338-775-01, 59338-775-10 |
| Experimental models: Organisms/strains | ||
| Sprague-Dawley | Charles River | Strain Code #001; RRID:RGD_734476 |
| Software and algorithms | ||
| MATLAB | MathWorks | RRID:SCR_001622 |
| SPM12 | Ashburner et al., 2014 | RRID:SCR_007037 |
| NiftyReg | Modat et al., 2010, 2014 | RRID:SCR_006593 |
| Other | ||
| 7T Preclinical MRI Scanner | Bruker | BioSpec series; Stanford Center for Innovation in In-Vivo Imaging |
Highlights.
Repeated optogenetic stimulations can reliably achieve kindling.
Kindling results in larger brain-wide network engagement upon stimulation.
Sub-threshold activity and seizures share network nodes.
Seizures show delayed synchronization of migrating core of hippocampal activity.
Acknowledgements
The authors would like to thank all the Lee Lab members for their help with the experiments, discussions, and help with reviewing of the manuscript. This work was supported by NIH/NINDS R01NS087159, NIH/NINDS R01NS091461, NIH/NIA RF1AG047666, NIH/NIMH RF1MH114227, NIH/NIA R01AG064051, and NIH/NINDS DP1NS116783.
Declaration of Interests
J.H.L. is a founder, consultant, and board member of LVIS. J.H.L. and M.C. are inventors on an invention disclosure titled, “Predicting Successful Generation and Inhibition of Seizure-like After discharges and Mapping Their Seizure Networks Using fMRI”.
Abbreviations
- ‘i’
ipsilateral
- ‘c’
contralateral
- ‘ChR2’
channelrhodopsin2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhikari A, Topiwala MA, and Gordon JA (2010). Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron 65, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari A, Topiwala MA, and Gordon JA (2011). Single Units in the Medial Prefrontal Cortex with Anxiety-Related Firing Patterns Are Preferentially Influenced by Ventral Hippocampal Activity. Neuron 71, 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba I, and Noebels JL (2015). Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci. Transl. Med 7, 282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Salvado E., Pallarés V., Moreno A., and Canals S. (2014). Functional MRI of long-term potentiation: imaging network plasticity. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369, 20130152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K, Kiebel S, Kilner J, Litvak V, and Moran R. (2014). SPM12 Manual (Wellcome Trust Centre for Neuroimaging).
- Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, and Arnold DL (2003). Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 126, 462–469. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang D, Scott CA, and Williamson JM (2001). The Midline Thalamus: Alterations and a Potential Role in Limbic Epilepsy. Epilepsia 42, 967–978. [DOI] [PubMed] [Google Scholar]
- Brandt C, and Mula M. (2016). Anxiety disorders in people with epilepsy. Epilepsy Behav. 59, 87–91. [DOI] [PubMed] [Google Scholar]
- Brandt C, Ebert U, and Löscher W. (2004). Epilepsy induced by extended amygdala-kindling in rats: lack of clear association between development of spontaneous seizures and neuronal damage. Epilepsy Res. 62, 135–156. [DOI] [PubMed] [Google Scholar]
- Brodovskaya A, and Kapur J. (2019). Circuits generating secondarily generalized seizures. Epilepsy Behav. 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin J.a, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, and Moore CI (2010). Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc 5, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael DW, Vulliemoz S, Rodionov R, Thornton JS, McEvoy a W., and Lemieux L. (2012). Simultaneous intracranial EEG-fMRI in humans: protocol considerations and data quality. Neuroimage 63, 301–309. [DOI] [PubMed] [Google Scholar]
- Cavazos JE., Golarai G., and Sutula TP. (1991). Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J. Neurosci 11, 2795–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary UJ, Carmichael DW, Rodionov R, Thornton RC, Bartlett P, Vulliemoz S, Micallef C, McEvoy AW, Diehl B, Walker MC, et al. (2012). Mapping preictal and ictal haemodynamic networks using video-electroencephalography and functional imaging. Brain 135, 3645–3663. [DOI] [PubMed] [Google Scholar]
- Chen S-D, Wang Y-L, Liang S-F, and Shaw F-Z (2016). Rapid Amygdala Kindling Causes Motor Seizure and Comorbidity of Anxiety- and Depression-Like Behaviors in Rats. Front. Behav. Neurosci 10, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang S, Wu W, Liu C, Yang X, Zhao H, Wu L, Tan L, Long L, and Xiao B. (2018). Associated and predictive factors of quality of life in patients with temporal lobe epilepsy. Epilepsy Behav. [DOI] [PubMed]
- Choy M, Wells JA, Thomas DL, Gadian DG, Scott RC, and Lythgoe MF (2010a). Cerebral blood flow changes during pilocarpine-induced status epilepticus activity in the rat hippocampus. Exp. Neurol 225, 196–201. [DOI] [PubMed] [Google Scholar]
- Choy M., Cheung KK., Thomas DL., Gadian DG., Lythgoe MF., and Scott RC. (2010b). Quantitative MRI predicts status epilepticus-induced hippocampal injury in the lithium–pilocarpine rat model. Epilepsy Res. 88, 221–230. [DOI] [PubMed] [Google Scholar]
- Choy M, Dubé CM, Patterson K, Barnes SR, Maras P, Blood AB, Hasso AN, Obenaus A, and Baram TZ (2014). A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J. Neurosci 34, 8672–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covolan L, and Mello LE (2000). Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 39, 133–152. [DOI] [PubMed] [Google Scholar]
- Dabrowska N, Joshi S, Williamson J, Lewczuk E, Lu Y, Oberoi S, Brodovskaya A, and Kapur J. (2019). Parallel pathways of seizure generalization. Brain 142, 2336–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Sirven JI, Alsop DC, O’Connor MJ, and French JA (1995). Localization of subclinical ictal activity by functional magnetic resonance imaging: Correlation with invasive monitoring. Ann. Neurol 38, 618–624. [DOI] [PubMed] [Google Scholar]
- Dubé C, Boyet S, Marescaux C, and Nehlig A. (2001). Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp. Neurol 167, 227–241. [DOI] [PubMed] [Google Scholar]
- Duffy BA, Choy M, Chuapoco RMR, Madsen M, and Lee JH (2015). MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like after discharges. Neuroimage 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy BA, Choy MK, and Lee JH (2020). Predicting Successful Generation and Inhibition of Seizure-like Afterdischarges and Mapping Their Seizure Networks Using fMRI. Cell Rep. 30, 2540–2554.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand DM, Park E-H, and Jensen AL (2010). Potassium diffusive coupling in neural networks. Philos. Trans. R. Soc. B Biol. Sci 365, 2347–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ., Mishra AM., Mansuripur PK., Herman P., Hyder F., and Blumenfeld H. (2008). Remote effects of focal hippocampal seizures on the rat neocortex. J. Neurosci 28, 9066–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, and Dong H-W (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, et al. (2017). Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58, 522–530. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Khodagholy D, Thesen T, Devinsky O, and Buzsáki G. (2016). Interictal epileptiform discharges induce hippocampal–cortical coupling in temporal lobe epilepsy. Nat. Med 22, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard GV (1967). Development of epileptic seizures through brain stimulation at low intensity. Nature 214, 1020–1021. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, and Leech CK (1969). A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol 25, 295–330. [DOI] [PubMed] [Google Scholar]
- Handforth A, and Treiman DM (1995). Functional mapping of the early stages of status epilepticus: a 14C-2-deoxyglucose study in the lithium-pilocarpine model in rat. Neuroscience 64, 1057–1073. [DOI] [PubMed] [Google Scholar]
- Jupp B, Williams J, Binns D, Hicks RJ, Cardamone L, Jones N, Rees S, and O’Brien TJ (2012). Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia 53, 1233–1244. [DOI] [PubMed] [Google Scholar]
- Kanner AM (2009). Psychiatric issues in epilepsy: The complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 15, 83–87. [DOI] [PubMed] [Google Scholar]
- Kanner AM., Schachter SC., Barry JJ., Hersdorffer DC., Mula M., Trimble M., Hermann B., Ettinger AE., Dunn D., Caplan R., et al. (2012). Depression and epilepsy: Epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 24, 156–168. [DOI] [PubMed] [Google Scholar]
- Keezer MR, Sisodiya SM, and Sander JW (2016). Comorbidities of epilepsy: current concepts and future perspectives. Lancet. Neurol 15, 106–115. [DOI] [PubMed] [Google Scholar]
- Kim S-G, and Ogawa S. (2012). Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab 32, 1188–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H, and Pitkänen A. (2003). Antiepileptogenesis, neuroprotection, and disease modification in the treatment of epilepsy: focus on levetiracetam. Epileptic Disord. 5 Suppl 1, S9–16. [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Bui A, Lew S, Oijala M, and Soltesz I. (2015). In vivo evaluation of the dentate gate theory in epilepsy. J. Physiol 593, 2379–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim D-S, Fenno LE, Ramakrishnan C, and Deisseroth K. (2010). Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, and Shen B. (2006). Hippocampal CA1 kindling but not long-term potentiation disrupts spatial memory performance. Learn. Mem 13, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L, Ma J, and McLachlan R. (2000). Behaviors induced or disrupted by complex partial seizures. Neurosci. Biobehav. Rev 24, 763–775. [DOI] [PubMed] [Google Scholar]
- Löscher W. (2011). Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20, 359–368. [DOI] [PubMed] [Google Scholar]
- Lothman EW., and Williamson JM. (1994). Closely spaced recurrent hippocampal seizures elicit two types of heightened epileptogenesis: a rapidly developing, transient kindling and a slowly developing, enduring kindling. Brain Res. 649, 71–84. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, and Weisskoff RM (1998). Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn. Reson. Med 39, 615–624. [DOI] [PubMed] [Google Scholar]
- Martinez-Vargas JD, Strobbe G, Vonck K, van Mierlo P, and Castellanos-Dominguez G. (2017). Improved Localization of Seizure Onset Zones Using Spatiotemporal Constraints and Time-Varying Source Connectivity. Front. Neurosci 11, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre DC, and Gilby KL (2008). Mapping seizure pathways in the temporal lobe. Epilepsia 49 Suppl 3, 23–30. [DOI] [PubMed] [Google Scholar]
- Michalakis M, Holsinger D, Ikeda-Douglas C, Cammisuli S, Ferbinteanu J, DeSouza C, DeSouza S, Fecteau J, Racine RJ, and Milgram NW (1998). Development of spontaneous seizures over extended electrical kindling. I. Electrographic, behavioral, and transfer kindling correlates. Brain Res. 793, 197–211. [DOI] [PubMed] [Google Scholar]
- Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC, and Ourselin S. (2010). Fast free-form deformation using graphics processing units. Comput. Methods Programs Biomed 98, 278–284. [DOI] [PubMed] [Google Scholar]
- Modat M., Cash DM., Daga P., Winston GP., Duncan JS., and Ourselin S. (2014). Global image registration using a symmetric block-matching approach. J. Med. Imaging (Bellingham) 1, 024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Morris RGM, and Canals S. (2016). Frequency-Dependent Gating of Hippocampal–Neocortical Interactions. Cereb. Cortex 26, 2105–2114. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Halonen T, Koivisto E, and Pitkänen A. (2000). A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res. 38, 177–205. [DOI] [PubMed] [Google Scholar]
- Osawa S-I, Iwasaki M, Hosaka R, Matsuzaka Y, Tomita H, Ishizuka T, Sugano E, Okumura E, Yawo H, Nakasato N, et al. (2013). Optogenetically induced seizure and the longitudinal hippocampal network dynamics. PLoS One 8, e60928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, and Gordon JA (2016). Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, El Hamdi G, Vert P, and Nehlig A. (1992). An experimental model of generalized seizures for the measurement of local cerebral glucose utilization in the immature rat. II. Mapping of brain metabolism using the quantitative [14C]2-deoxyglucose technique. Dev. Brain Res 69, 243–259. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, and Halonen T. (1998). Prevention of epilepsy. Trends Pharmacol. Sci 19, 253–255. [DOI] [PubMed] [Google Scholar]
- Plummer C., Vogrin SJ., Woods WP., Murphy MA., Cook MJ., and Liley DTJ. (2019). Interictal and ictal source localization for epilepsy surgery using high-density EEG with MEG: a prospective long-term study. Brain 142, 932–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi LF, Wykes RC, Kullmann DM, and Carandini M. (2017). Focal cortical seizures start as standing waves and propagate respecting homotopic connectivity. Nat. Commun 8, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Høgenhaven H, et al. (2013). Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 12, 966–977. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Weiss SA, McKhann G, Goodman RR, Yuste R, Emerson RG, and Trevelyan AJ (2012). Evidence of an inhibitory restraint of seizure activity in humans. Nat. Commun 3, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Tobochnik S, Eissa T, Merricks E, Gill B, Parrish RR, Bateman LM, McKhann GM, Emerson RG, and Trevelyan AJ (2019). Multiscale recordings reveal the dynamic spatial structure of human seizures. Neurobiol. Dis 127, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, and Blumenfeld H. (2008). Negative BOLD with large increases in neuronal activity. Cereb. Cortex 18, 1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, Sharpe L, Thayer Z, Miller LA, Wong T, Parratt K, and Nikpour A. (2018). A qualitative examination and theoretical model of anxiety in adults with epilepsy. Epilepsy Behav. 85, 95–104. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gröhn O, and Pitkänen A. (2015). Imaging microstructural damage and plasticity in the hippocampus during epileptogenesis. Neuroscience 309, 162–172. [DOI] [PubMed] [Google Scholar]
- Slattery DA, and Cryan JF (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc 2012 76 7, 1009. [DOI] [PubMed] [Google Scholar]
- Smith EH., Liou J-Y., Davis TS., Merricks EM., Kellis SS., Weiss SA., Greger B., House PA., McKhann Ii GM., Goodman RR., et al. (2016). The ictal wavefront is the spatiotemporal source of discharges during spontaneous human seizures. Nat. Commun 7, 11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Mathern GW, Cross JH, and Bertram EH (2010). Mesial temporal lobe epilepsy: How do we improve surgical outcome? Ann. Neurol 68, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, and Buckmaster PS (2013). Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J. Neurosci 33, 11100–11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Bruns W, Mann EO, Crepel V, and Scanziani M. (2013). The information content of physiological and epileptic brain activity. J. Physiol 591, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz AJ, Fang Z, Lee HJ, Fisher RSRS, Smith WCWC, Choy M, Liu J, Lin P, Rosenberg M, and Lee JH (2015). Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage 107, 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz AJ, Lee HJ, Choy MK, and Lee JH (2019). Thalamic Input to Orbitofrontal Cortex Drives Brain-wide, Frequency-Dependent Inhibition Mediated by GABA and Zona Incerta. Neuron 104, 1153–1167.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes RC., Khoo HM., Caciagli L., Blumenfeld H., Golshani P., Kapur J., Stern JM., Bernasconi A., Dedeurwaerdere S., and Bernasconi N. (2019). WONOEP appraisal: Network concept from an imaging perspective. Epilepsia 60, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, and Bertram EH (2002). Midline Thalamic Region: Widespread Excitatory Input to the Entorhinal Cortex and Amygdala. J. Neurosci 22, 3277–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Ladas TP, Qiu C, Shivacharan RS, Gonzalez-Reyes LE, and Durand DM (2014). Propagation of epileptiform activity can be independent of synaptic transmission, gap junctions, or diffusion and is consistent with electrical field transmission. J. Neurosci 34, 1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, and Kim S-G (2006). Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: Insights into hemodynamic regulation. Neuroimage 30, 1149–1160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A seizure was induced in the ventral hippocampus using optogenetic stimulation. A 15 second, 40 Hz stimulation with a pulse width of 7.5 ms begins at 7 seconds in the video. Following stimulation, the rat displays forelimb clonus and rearing and falling, which are classic stereotyped seizure behaviors under Racine scoring system.
A kindled rat was sedated using dexmedetomidine and a seizure was induced in the ventral hippocampus using optogenetic stimulation. A 40 second, 40 Hz stimulation with at pulse width of 7.5 ms begins at 5 seconds in the video. Following stimulation, the rat displays forelimb clonus and rearing and falling, which are classic stereotyped seizure behaviors under Racine scoring system and similar to those observed in awake kindled rats.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 3C,D.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 3E,F.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 6A,B.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 6C,D.
Ventral hippocampal LFP recordings are shown above the BOLD fMRI data. The moving vertical line on the LFP shows the corresponding time of the BOLD fMRI map. Note that a 2 % BOLD threshold has been applied for visualization and that the video is at 3x speed (3 frames per second). These data are the complete time series for the data presented in Fig. 6I,J.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the Lead Contact upon request.