Abstract
Uric acid (UA) and HDL-cholesterol (HDL-C) level are closely associated to the cardiovascular disease (CVD) morbidity. The UA/HDL-C ratio (UHR), a new parameter combination of serum UA and HDL-C, attracts attention for its association with metabolic and inflammatory conditions. There may exists the association between UHR and arterial stiffness. This study aims to explore the association between the UHR and brachial-ankle PWV (baPWV) and to determine whether or not UHR has effect on arterial stiffness. The present study included a total of 912 Japanese (592 men and 320 women), aged from 24 to 84, received a health medical checkup programme with an automatic waveform analyzer to measure baPWV and various standardized questionnaires in a medical center of Japan. Non-linear regression and threshold effect analysis were conducted to explore the association between UHR and baPWV. It was found that UHR was positively correlated with baPWV after adjusting for multiple confounders. A non-linear relationship (with a inflection point was 14.25) was found between UHR and baPWV. Subgroup analyses showed that the significant association between UHR and baPWV only existed in females group, no fatty liver group and normal BMI groups. This study revealed the nonlinear relationship between UHR and baPWV. A significant correlation between UHR and baPWV existed in females but not in males. Fatty liver status, BMI, and menopausal status may affect the above association.
Keywords: arterial stiffness, baPWV, high density lipoprotein-cholesterol, serum uric acid, UHR
1. Introduction
Cardiovascular disease (CVD) is a group of heart and blood vessel disorders and is the leading cause of death and morbidity worldwide, causing about one-third of all deaths globally and imposing a major public health burden over the past several decades. Arterial stiffness is associated with cardiovascular risk factors, atherosclerotic disease,[1,2] and increased cardiovascular mortality and can be used as powerful determinant for the assessment of cardiovascular risk. Among the various evaluation indicators for arterial stiffness, the brachial–ankle PWV (baPWV) is one of the most representative and noninvasive screening tools applied in clinical practice and is automatically measured using simple separate cuff for each of the 4 limbs by an oscillometric method.[3,4] A meta-analysis demonstrated that high levels of baPWV are closely correlated with increased risk of developing CVD.[5]
Uric acid (UA) is a highly insoluble waste product of endogenous and exogenous purine metabolism in humans, and two-thirds of UA is eliminated by renal excretion. UA is closely associated to various metabolic diseases because it serves as selective antioxidant and free radical scavenger in physiologic conditions, such as gout, hypertriglyceridemia, and diabetes mellitus.[6,7] Dyslipidemia plays an important role on the pathogenesis of CVD. Increased serum concentrations of low-density lipoprotein cholesterol (LDL-C) and triglycerides are recognized as risk factors for CVD, whereas increased high-density lipoprotein cholesterol (HDL-C) is considered to show pleiotropic effects on protecting the cardiovascular system, including reverse cholesterol transport to reduce atherosclerotic burden, anti-inflammation, antioxidation, and vasodilation.[8,9] Low HDL-C and high UA show association with increased risk of CVD and mortality in the general population. The combination of serum UA and HDL-C is proposed as a better predictor of CVD mortality than the single parameter. As one of the combinations, the UA/HDL-C ratio (UHR) has recently attracted attention for its association with metabolic and inflammatory conditions. Evidence showed that increased UHR is suggested to be related to metabolic syndrome,[10] thyroiditis,[11] and hepatic steatosis.[12] UHR can also be used as a strong predictor of diabetes control and metabolic syndrome in patients with diabetes.[13] Studies from different groups of China reported that UHR can serve as a reliable predictor of nonalcoholic fatty liver disease (NAFLD) onset in nonobese[14] or lean Chinese people for UHR level was associated with a significantly increased risk of NAFLD.[15] Elevated UHR levels can be associated with poor blood pressure control. Thus, Aktas and Khalid[16] proposed that the assessment of UHR may be useful in patients with hypertension.
Despite these interesting observations, studies investigating the association between UHR and arterial stiffness are scarce. This study aims to explore the association between UHR and baPWV and determine whether UHR affects arterial stiffness based on previously published data.
2. Materials and methods
This study was a secondary analysis of a cross-sectional study conducted at the Medical Health Checkup Center of Murakami Memorial Hospital (Gifu City, Japan) by Takuya from 2004 to 2012. Raw data were freely available for download and analysis from the “DATADRYAD” database (www.Datadryad.org) without infringing on the authors’ rights for intellectual property or other rights and related ownership of these data were waived by the authors of the original study. We made a definitive statement when we cited the Dryad data package in accordance with the Dryad database policy (Dryad data package: Fukuda T, Hamaguchi M, Kojima T, Ohshima Y, Ohbora A, Kato T, Nakamura N, Fukui M (2014) Data from: Association between serum γ-glutamyltranspeptidase and atherosclerosis: a population-based cross-sectional study. Dryad Digital Repository. Dryad | Data – Association between serum γ-glutamyltranspeptidase and atherosclerosis: a population-based cross-sectional study. https://datadryad.org/stash/dataset/doi:10.5061/dryad.m484p.
In accordance with Takuya’s description,[17] this study reviewed the medical records of 1445 participants who participated in the Japan medical health checkup program at Murakami Memorial Hospital and obtained informed consent from all participants in accordance with the Declaration of Helsinki. A total of 912 participants were received after excluding the following: participants who received medication, including oral contraceptives and hormone replacement therapy; participants who were positive to hepatitis B antigen and/or hepatitis C antibody; pregnant women; and participants with ankle–brachial index (ABI) less than 0.95.
As previously described, the variables included in the database, including γ-glutamyltranspeptidase (γ-GGT), fasting plasma glucose, triglycerides, HDL-C, LDL-C, total cholesterol (TC), alanine aminotransferase (ALT), aspartate transaminase (AST), and UA, were obtained from plasma and serum samples of participants in the fasting state (8 hours fasting). UHR was calculated as serum UA levels (in mg/dL) divided by HDL-C levels (in mg/dL). Weight and height were measured to calculate BMI as weight in kilograms divided by height in meters squared. The Japanese Society of Nephrology equation: eGFR = 194 × Cr−1.094 × age−0.287 (mL/min/1.73 m2) was applied to calculate the estimated glomerular filtration rate (eGFR) for men. The eGFR for women was the eGFR for men multiplied by a correction factor of 0.739. The results of abdominal ultrasonography were applied to diagnose fatty liver by gastroenterologist by 4 criteria (i.e., hepatorenal echo contrast, vascular blurring, deep attenuation, and liver brightness) without reference to other test results of the participants.
Authors provide a detailed description for the measurement of baPWV in the original study. In brief, ECG electrodes on wrists and heart sound microphone on the left edge of the sternal border were placed on subjects, who stayed in the supine position in a quiet room with temperature around 25°C after resting for 5 minutes. Cuffs were wrapped around the arms and ankles and connected with a plethysmographic sensor and an oscillometric pressure sensor, which could determine the volume pulse form and blood pressure, respectively. The path lengths from the suprasternal notch to the ankle (La) and from the suprasternal notch to the brachium (Lb) were then calculated on the basis of the participant’s height, and the delay time from the ascending point of the brachial waveform to the ascending point of each ankle wave form (DTba) was automatically obtained. The authors calculated baPWV (in cm/s) by the pulse wave propagation distance (Lb–La) divided by the pulse wave propagation time (DTba).
A standardized questionnaire for lifestyle factors was applied to acquire information about alcohol consumption, smoking status, and frequency of participation in sports. The total amount of alcohol consumed per week was calculated in grams, and smoking status was categorized into nonsmoker or ex-smoker and current smoker. Regular exercisers were participants who performed any kind of sport regularly at least once a week. Postmenopausal status was defined as more than a year after the cessation of menses.
2.1. Statistical analysis
All statistical analyses were conducted using the commercial statistical software package R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). We analyzed the baseline characteristics of all subjects, which grouped UHR in tertiles. The normality of the data distribution was evaluated using Shapiro–Wilk normality test and kolmogorow–Smirnov normality test in this study. The continuous variables which passed the normality test are expressed as means ± SD while the continuous variables which didn’t passed the normality test are expressed by median (P25, P75) (γGGP and TG). Differences between means and proportions among different groups were determined by one-way ANOVA (normal distribution) and Kruskal–Wallis H (non-normal distribution distribution) test. The categorical variables were expressed as percentages (%) or frequency, the differences of which analyzed by chi-square test. Multiple regression analyses were applied to estimate the independent association between UHR levels and baPWV. Three models adjusted for different degrees were used to provide statistical inference. Generalized additive models and smooth curve fitting were performed to identify nonlinear relationships between UHR levels and baPWV. Additionally, subgroup analyses were applied under stratified linear regression models. P value < .05 indicated a significant difference.
3. Results
3.1. Population characteristics
A total of 1445 participants were included during the study period, and 433 participants (284 men and 149 women) were excluded because they were receiving medication. In addition, 16 men and 10 women were excluded as they tested positive to the hepatitis B antigen and/or positive antihepatitis C antibody. Another 68 women were excluded for receiving hormone replacement therapy (66), taking an oral contraceptive (1), and being on gestational age (1). Additionally, 6 (5 men and 1 woman) participants were excluded due to ABIs < 0.95. Finally, 912 participants (including 592 men and 320 women) were analyzed.
Table 1 summarizes the baseline demographic and clinical characteristics of participants subclassified on the basis of UHR tertiles. The gender distribution, TC level, and postmenopausal status did not differ among 3 subclassified groups. Being female and young age were associated with increased UHR levels. Participants with high UHR levels tended to have high BMI, BP, AST, ALT, γ-GGT, FBS, UA, TC, LDL3, and baPWV. Moreover, an inverse association existed among HDL-C, eGFR, exercise intensity, and smoking status and between amount of alcohol consumption and UHR levels. Besides, obese people (BMI > 26 kg/m2) and individuals with fatty liver tended to have high UHR levels.
Table 1.
Baseline characteristics of participants.
| UHR (tertiles) | T1 | T2 | T3 | P value | P value* |
|---|---|---|---|---|---|
| N | 304 | 304 | 304 | ||
| Age (yr, mean ± SD) | 51.78 ± 9.26 | 51.18 ± 9.43 | 50.43 ± 10.00 | .222 | .223 |
| BMI (kg/m2, mean ± SD) | 21.70 ± 2.58 | 23.11 ± 2.95 | 24.57 ± 3.14 | <.001 | <.001 |
| SBP (mm Hg, mean ± SD) | 115.33 ± 13.83 | 120.99 ± 14.90 | 124.42 ± 14.74 | <.001 | <.001 |
| DBP (mm Hg, mean ± SD) | 71.83 ± 9.24 | 76.99 ± 9.72 | 79.60 ± 9.50 | <.001 | <.001 |
| AST (IU/L, mean ± SD) | 19.01 ± 5.98 | 20.97 ± 8.85 | 22.57 ± 8.73 | <.001 | <.001 |
| ALT (IU/L, mean ± SD) | 16.74 ± 6.49 | 22.43 ± 14.26 | 28.88 ± 17.17 | <.001 | <.001 |
| γGGP (IU/L, median (25P, 75P)) | 14.00 (11.00, 18.25) | 19.00 (14.00, 31.00) | 23.00 (17.00, 33.25) | <.001 | <.001 |
| Fasting glucose (mg/dL, mean ± SD) | 94.78 ± 17.05 | 98.24 ± 12.16 | 101.13 ± 11.65 | <.001 | <.001 |
| Uric acid (mg/dL, mean ± SD) | 3.98 ± 0.90 | 5.35 ± 0.88 | 6.44 ± 1.04 | <.001 | <.001 |
| TC (mg/dL, mean ± SD) | 212.04 ± 35.70 | 207.70 ± 35.98 | 209.72 ± 36.21 | .331 | .258 |
| TG (mg/dL, median (25P, 75P)) | 56.50 (40.00–76.25) | 77.00 (55.00–111.25) | 125.00 (88.75–182.25) | <.001 | <.001 |
| HDL-C (mg/dL, mean ± SD) | 66.62 ± 13.25 | 53.22 ± 8.95 | 40.77 ± 7.03 | <.001 | <.001 |
| LDL-C (mg/dL, mean ± SD) | 121.92 ± 31.14 | 128.24 ± 31.19 | 134.01 ± 31.67 | <.001 | <.001 |
| UHR (%) | 6.08 ± 1.26 | 10.12 ± 1.15 | 16.15 ± 3.49 | <.001 | <.001 |
| eGFR(mL/min/1.73 m2, mean ± SD) | 73.86 ± 12.96 | 70.65 ± 10.79 | 66.73 ± 11.22 | <.001 | <.001 |
| baPWV (cm/s, mean ± SD) | 1368.21 ± 206.99 | 1415.10 ± 225.54 | 1463.94 ± 289.84 | <.001 | <.001 |
| Sex (n, %) | |||||
| Male | 70 (23.03) | 234 (76.97) | 288 (94.74) | <.001 | – |
| Female | 234 (76.97) | 70 (23.03) | 16 (5.26) | ||
| Current smoking (n, %) | |||||
| None | 273 (89.80) | 234 (76.97) | 208 (68.42) | <.001 | – |
| Current | 31 (10.20) | 70 (23.03) | 96 (31.58) | ||
| Ex-smoking (n, %) | |||||
| No | 226 (74.34) | 138 (45.39) | 97 (31.91) | <.001 | – |
| Yes | 78 (25.66) | 166 (54.61) | 207 (68.09) | ||
| Regular exercise (>1 wk) (n, %) | |||||
| No | 222 (75.00) | 238 (79.33) | 259 (86.33) | .002 | – |
| Yes | 74 (25.00) | 62 (20.67) | 41 (13.67) | ||
| Fatty liver (n, %) | |||||
| None | 271 (89.44) | 235 (77.30) | 140 (46.05) | <.001 | – |
| Yes | 32 (10.56) | 69 (22.70) | 164 (53.95) | ||
| Menopausal status (n, %) | |||||
| Post-menopausal | 103 (44.02) | 29 (41.43) | 6 (37.50) | .833 | – |
| No | 131 (55.98) | 41 (58.57) | 10 (62.50) | ||
| Alcohol consumption (n, %) | |||||
| <80 (g/wk) | 253 (84.05) | 199 (65.68) | 197 (66.78) | <.001 | – |
| ≥80, <180 (g/wk) | 24 (7.97) | 55 (18.15) | 45 (15.25) | ||
| ≥180 (g/wk) | 24 (7.97) | 49 (16.17) | 53 (17.97) | ||
| BMI | |||||
| <19 (kg/m2, mean ± SD) | 40 (13.16) | 16 (5.26) | 6 (1.97) | <.001 | – |
| ≥19, <26 (kg/m2, mean ± SD) | 244 (80.26) | 238 (78.29) | 218 (71.71) | ||
| ≥26 (kg/m2, mean ± SD) | 20 (6.58) | 50 (16.45) | 80 (26.32) | ||
ALT = alanine aminotransferase, AST = aspartate transaminase, baPWV = brachial-ankle pulse wave velocity, BMI = body mass index, DBP = diastole pressure, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, SBP = systolic pressure, TC = total cholesterol, TG = triglyceride, UHR = uric acid to high-density lipoprotein cholesterol ratio, γGGT = γ-glutamyltranspeptidase.
P value* was calculated by Kruskal-Wallis H test.
3.2. Association between UHR and baPWV
The effect sizes of association between UHR and baPWV are listed in Table 2. In the crude unadjusted model, a positive association was observed between UHR and baPWV. Similar results were found in models 2 (adjustment for age and sex; β = 6.16, 95% CI = 2.65–9.67) and 3 (adjustment for sex, age, BMI, SBP, DBP, ALT, AST, γ-GTP, and fatty liver status; β = 4.03, 95% CI: 0.76–7.30). We also performed UHR as a tertile categorical variable to explore the sensitivity analysis, it was found the same trend (P for trend was 0.002).
Table 2.
Relationship between UHR and baPWV in different models.
| Exposure | Non-adjusted model β (95% CI) | Adjust I model β (95% CI) | Adjust II model β (95% CI) |
|---|---|---|---|
| UHR | 6.56 (3.19, 9.93) 0.0001 | 6.16 (2.65, 9.67) 0.0006 | 4.03 (0.76, 7.30) 0.0159 |
| UHR (tertiles) | |||
| T1 | Reference | Reference | Reference |
| T2 | 46.89 (8.20, 85.59) 0.0177 | 40.95 (2.40, 79.50) 0.0376 | 26.43 (−6.57, 59.43) 0.1168 |
| T3 | 95.73 (57.04, 134.43) < 0.0001 | 95.14 (52.86, 137.42) < 0.0001 | 59.48 (21.34, 97.62) 0.0023 |
| P for trend | <.001 | <.001 | .002 |
| Gender | |||
| Male | 2.60 (−1.91, 7.11) 0.2584 | 4.03 (0.10, 7.96) 0.0446 | 2.53 (−1.04, 6.10) 0.1650 |
| Female | 20.88 (10.81, 30.95) < 0.0001 | 16.43 (8.10, 24.75) 0.0001 | 11.77 (3.59, 19.95) 0.0051 |
| Fatty liver | |||
| None | 7.33 (2.74, 11.92) 0.0018 | 3.14 (−1.70, 7.98) 0.2047 | 4.80 (0.49, 9.11) 0.0295 |
| Yes | −3.23 (−9.49, 3.03) 0.3126 | 2.08 (−3.94, 8.10) 0.4987 | 2.75 (−2.44, 7.93) 0.2998 |
| Alcohol consumption | |||
| <80 (g/wk) | 8.68 (4.64, 12.72) < 0.0001 | 9.02 (4.70, 13.34) < 0.0001 | 6.70 (2.68, 10.71) 0.0011 |
| ≥80, <180 (g/wk) | 0.40 (−10.34, 11.15) 0.9417 | 0.71 (−9.27, 10.69) 0.8894 | 3.79 (−6.05, 13.63) 0.4523 |
| ≥180 (g/wk) | −2.34 (−10.98, 6.30) 0.5966 | −3.96 (−12.32, 4.40) 0.3548 | −6.64 (−14.54, 1.26) 0.1024 |
| BMI | |||
| <19 (kg/m2) | 14.20 (−2.49, 30.89) 0.1006 | 2.60 (−16.07, 21.27) 0.7860 | 8.61 (−8.98, 26.19) 0.3419 |
| ≥19, <26 (kg/m2) | 8.12 (3.85, 12.40) 0.0002 | 6.65 (2.18, 11.12) 0.0037 | 4.77 (0.74, 8.80) 0.0206 |
| ≥26 (kg/m2) | −0.92 (−7.24, 5.40) 0.7748 | 2.20 (−4.22, 8.62) 0.5035 | 2.36 (−3.39, 8.12) 0.4224 |
Crude model: we did not adjust other covariants.
Minimally adjusted model: we adjusted age and sex.
Fully adjusted model: we adjusted sex; age; BMI; SBP; DBP; ALT; AST; γ-GTP; fatty liver status.
ALT = alanine aminotransferase, AST = aspartate transaminase, baPWV = brachial-ankle pulse wave velocity, BMI = body mass index, CI = confidence interval, DBP = diastole pressure, Ref = reference, SBP = systolic pressure, UHR = uric acid to high-density lipoprotein cholesterol ratio.
In the subgroup analyses stratified by gender, fatty liver status, amount of alcohol consumption, and BMI, the association between UHR and baPWV was no longer significant among males, individuals with fatty liver, medium and high alcohol consumption groups, and lean and obese groups.
3.3. Analyses of nonlinear relationship
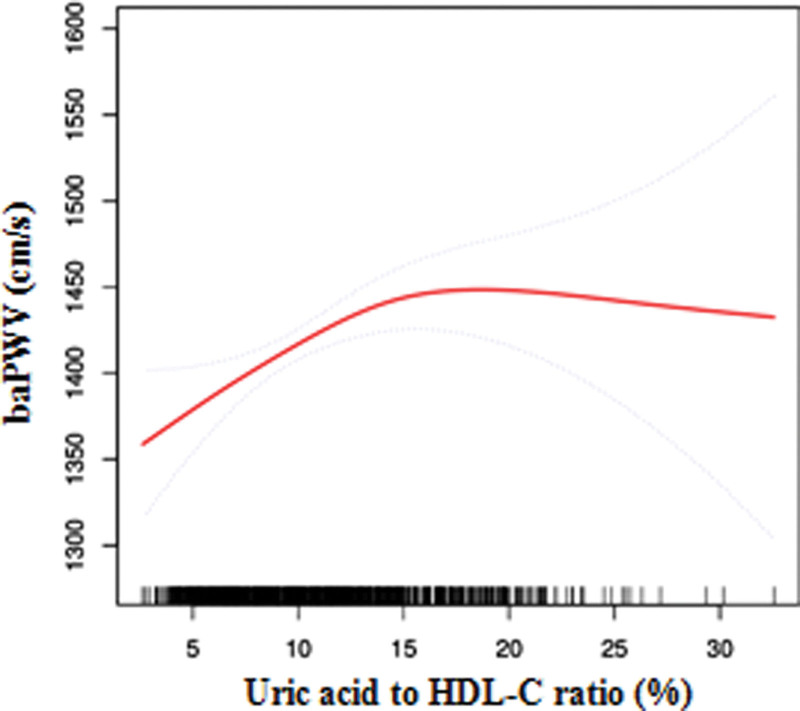
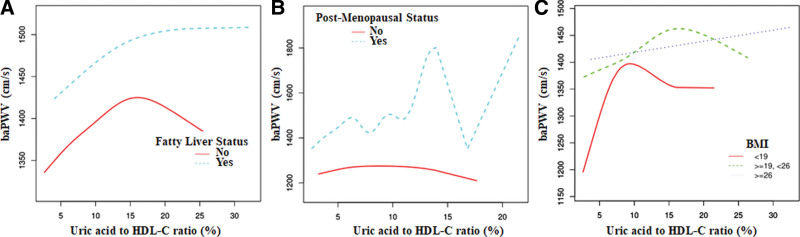
We also conducted generalized additive models and smooth curve fittings to evaluate the associations between UHR and baPWV in the total model and different subgroups. A nonlinear relationship was found between UHR and baPWV. The smooth curve is presented in Figure 1. The inflection point (14.25) was obtained using a two-piecewise linear regression model. For UHR < 14.25%, every 1% increase in UHR was associated with 9.4 cm/s increase in baPWV (95% CI = 4.24–14.56). By comparison, for individuals with UHR > 14.25 mg/dL, a 1% increase in UHR was associated with a 3.1 cm/s decrease in baPWV (95% CI = −9.40 to 3.11). The similar threshold effect of UHR on baPWV was also found in no fatty liver group, premenopausal state group, and nonobese group. Detailed information are presented in Table 3 and Figure 2.
Figure 1.
The non-linear relationship between Uric acid to HDL-C ratio and baPWV. The relationship between AST/ALT and baPWV. Adjusting for sex; age; BMI; SBP; DBP; ALT; AST; γ-GTP; Fatty liver status. (The area between 2 blue dotted lines is expressed as a 95% CI. Each point shows the magnitude of UHR and is connected to form a continuous line). ALT = alanine aminotransferase, AST = aspartate transaminase, baPWV = brachial–ankle PWV, BMI = body mass index, DBP = diastole pressure, HDL-C = high-density lipoprotein cholesterol, SBP = systolic pressure, UHR = UA/HDL-C ratio.
Table 3.
Threshold effect analysis of UHR on baPWV by using two-piecewise linear regression.
| Adjusted ß (95% CI), P value | |
|---|---|
| Fitting by standard linear model | 4.03 (0.76, 7.30) .0159 |
| Fitting by two-piecewise linear model | |
| Inflection point | 14.25 |
| <14.25 (%) | 9.40 (4.24, 14.56) .0004 |
| >14.25 (%) | −3.14 (−9.40, 3.11) .3249 |
| Log likelihood ratio | 0.008 |
| No Fatty Liver | |
| Fitting by standard linear model | 4.80 (0.49, 9.11) .0295 |
| Fitting by two-piecewise linear model | |
| Inflection point | 15.99 |
| <15.99(%) | 9.01 (3.60, 14.42) .0012 |
| >15.99(%) | −14.63 (−30.42, 1.16) .0699 |
| Log likelihood ratio | 0.012 |
| −1.70 (−12.09, 8.70) .7496 | |
| POSR.MENOPAUSAL.STATE | |
| Fitting by standard linear model | −1.70 (−12.09, 8.70) .7496 |
| Fitting by two-piecewise linear model | |
| Inflection point | 5.27 |
| <5.27(%) | 59.12 (4.44, 113.79) .0360 |
| >5.27(%) | −9.96 (−22.54, 2.61) .1229 |
| Log likelihood ratio | 0.021 |
| BMI < 19 | |
| Fitting by standard linear model | 8.61 (−8.98, 26.19) .3419 |
| Fitting by two-piecewise linear model | |
| Inflection point | 6.83 |
| <6.83 (%) | 44.87 (−0.52, 90.27) .0585 |
| >6.83 (%) | −0.86 (−21.31, 19.59) .9345 |
| Log likelihood ratio | 0.060 |
| BMI ≥ 19, <26 | |
| Fitting by standard linear model | 4.77 (0.74, 8.80) .0206 |
| Fitting by two-piecewise linear model | |
| Inflection point | 15.95 |
| <15.95(%) | 9.17 (3.85, 14.49) .0008 |
| >15.95(%) | −8.95 (−20.55, 2.65) .1310 |
| Log likelihood ratio | 0.013 |
Adjusted: Sex; age; BMI; SBP; DBP; ALT; AST; γ-GTP; fatty liver status.
ALT = alanine aminotransferase, AST = aspartate transaminase, baPWV = brachial-ankle pulse wave velocity, BMI = body mass index, CI = confidence interval, DBP = diastole pressure, SBP = systolic pressure, UHR = uric acid to high-density lipoprotein cholesterol ratio.
Figure 2.
The association between serum Uric acid to HDL-C ratio and baPWV according to different subgroup. A. Fatty liver status B. Post-menopausal status. C. BMI. Adjusting for sex; age; BMI; SBP; DBP; ALT; AST; γ-GTP; Fatty liver status. ALT = alanine aminotransferase, AST = aspartate transaminase, baPWV = brachial–ankle PWV, BMI = body mass index, DBP = diastole pressure, HDL-C = high-density lipoprotein cholesterol, SBP = systolic pressure.
3.4. Interaction analyses
As shown in Table 4, interactions were significant for sex (P for interaction = .0327), smoking status (P for interaction were .0452 and .0292 for current smoker and ex-smoker, respectively), alcohol consumption (P for interaction = .0103), and menopausal status for female subjects (P for interaction = .0111), whereas interactions were not statistically significant for exercise status, fatty liver, and BMI (P for interaction = .6190, .5343, and .7205, respectively).
Table 4.
Effect size of UHR on baPWV in prespecified and exploratory subgroup.
| Characteristic | No of participants | Effect size (95% CI) P | P for interaction |
|---|---|---|---|
| Sex | |||
| Male | 592 | 2.34 (−1.21, 5.89) .1970 | .0327 |
| Female | 320 | 11.97 (3.79, 20.15) .0042 | |
| Current smoker | |||
| No | 715 | 5.94 (2.06, 9.81) .0028 | .0452 |
| Yes | 197 | −1.41 (−7.57, 4.75) .6540 | |
| Ex-smoking | |||
| No | 462 | 8.38 (3.21, 13.54) .0015 | .0292 |
| Yes | 450 | 1.18 (−2.94, 5.30) .5744 | |
| Regular exercise (>1 wk) | |||
| No | 719 | 3.65 (0.02, 7.29) .0493 | .6190 |
| Yes | 177 | 1.60 (−5.77, 8.97) .6706 | |
| Fatty liver | |||
| No | 646 | 4.90 (0.56, 9.23) .0270 | .5343 |
| Yes | 265 | 2.87 (−2.01, 7.75) .2495 | |
| Menopausal status | |||
| Postmenopausal | 138 | −2.13 (−15.18, 10.92) .7495 | .0111 |
| No | 182 | 19.00 (8.50, 29.50) .0005 | |
| Alcohol consumption | |||
| <80 (g/wk) | 649 | 6.89 (2.85, 10.92) .0009 | .0103 |
| ≥80, <180 (g/wk) | 124 | 2.69 (−6.54, 11.93) .5676 | |
| ≥180 (g/wk) | 126 | −6.29 (−13.97, 1.40) .1093 | |
| BMI | |||
| <19 (kg/m2, mean ± SD) | 62 | 8.61 (−9.69, 26.90) .3566 | .7205 |
| ≥19, <26 (kg/m2, mean ± SD) | 700 | 4.77 (0.82, 8.73) .0183 | |
| ≥26 (kg/m2, mean ± SD) | 150 | 2.36 (−3.93, 8.66) .4621 | |
Adjusted: Sex; age; BMI; SBP; DBP; ALT; AST; γ-GTP; fatty liver status.
ALT = alanine aminotransferase, AST = aspartate transaminase, baPWV = brachial-ankle pulse wave velocity, BMI = body mass index, CI = confidence interval, DBP = diastole pressure, SBP = systolic pressure, UHR = uric acid to high-density lipoprotein cholesterol ratio.
4. Discussion
In this study, previous data are used to evaluate the associations between UHR and baPWV in a Japanese population. To the best of our knowledge, this study is the first population-based cohort study that has evaluated associations between UHR and arterial stiffness. Results revealed a nonlinear relationship between UHR and baPWV, which follows a nearly inverse U-shaped curve with an inflection point (14.25%). UHR is positively associated with baPWV on the left of the inflection point but is not statistically significant on the right side of the inflection point. Specifically, we found a significant correlation between UHR and baPWV in females but not in males. Other subgroup analyses showed that a significant relationship between UHR and baPWV exists in nonfatty liver, low alcohol consumption, and normal weight (19 kg/m2 ≤ BMI ≤ 26 kg/m2) groups. Inverse U-shaped curves are also observed in nonfatty liver, nonmenopausal status, and nonobese groups.
UHR is a recently introduced physiological indicator that has attracted considerable attention in current research due to its potential impact on physiological processes and the onset of diseases. A study conducted on the Turkish population revealed a significant correlation between UHR and several key indicators of diabetic kidney injury, leading to the proposal of UHR as a diagnostic tool for this condition.[18] In a separate investigation, Chinese researchers focused their efforts on UHR and discovered a connection between visceral adiposity and elevated UHR levels in patients with type 2 diabetes.[19] Additionally, there is emerging evidence suggesting a potential association between UHR and the regulation of ALT levels in Chinese children and adolescents with short stature.[20]
UHR is a marker combining UA and HDL-C. Thus, increased UHR is caused by either decreased serum HDL cholesterol, increased serum UA, or both. UA and HDL-C differ between males and females. Hyperuricemia is UA ≥ 6 mg/dL for women and UA ≥ 8 mg/dL for men, whereas low HDL-C is defined as HDL-C < 40 mg/dL for men or HDL-C < 50 mg/dL for women. Thus, UHR is different between males and females, and we found that UHR and baPWV in men are significantly higher than those in women and that a significantly positive correlation between UHR and baPWV only exists in women. The underlying definitive mechanism of such gender-specific differences remains unknown. Sex hormones for different hormonal milieu may affect the well-regulated balance of 2 main extracellular matrix proteins of the arterial wall, i.e., collagen and elastin, whose dysregulation leads to stiffening of the arterial wall.[21] Estrogen is shown to affect arterial wall remodeling directly by decreasing collagen deposition and increasing elastin production in human arteries.[22] Besides, sex differences relate to the type and levels of sex hormones in vascular biology, and tissue and cellular differences that are responsible for sex-specific responses to various stimuli also exist. For instance, men have less arterial estrogen receptors than women although the human aorta has estrogen and progesterone receptors.[23] Different nitric oxide-mediated vasodilatory effects of estrogen between genders are also observed based on the report that intracoronary injections of estradiol improve endothelial function and coronary flow in women but not in men.[24] Furthermore, sex differences in measures of arterial pulsatility and stiffness are present. The truth that women have shorter aortic length and faster wave travel time also results in achieving the reflected pressure wave in the cardiac cycle fast and increasing baPWV.[25]
Further analysis based on the menopause status of women found a nearly inverse U-shaped relationship between UHR and baPWV with an inflection point in the nonmenopausal status group and irregular UHR–baPWV relationship in the menopausal group. A complex relationship between HDL-C and menopause has been reported, and UA is proposed as an independent predictor of cardiovascular events in postmenopausal women. Moreover, it was multiple and intricate that hormonal and metabolic alterations occur with menopause. The incidence of CVD increases disproportionately in women after menopause, and women who undergo menopause earlier shows poor cardiovascular outcomes.[26,27] The elevation of blood pressure caused by arterial stiffness in women may play a role on the mechanisms underlying the loss of cardiovascular protection with menopause.[28] Furthermore, severe symptoms in menopause are associated with remarkable arterial stiffness, and the association between mortality and arterial stiffness in females is double that in males.[29] Thus, menopause should be considered when evaluating the association between UHR and arterial stiffness.
Fatty liver is the most prevalent chronic liver disease in the world. In recent years, the association between UHR and fatty liver has attracted increasing attention. Zhang et al proposed that UHR is significantly associated with NAFLD and may serve as a novel and reliable marker for NAFLD in lean adults, whereas another study from a Chinese team demonstrated that high UHR values are independently associated with increased risk for NAFLD occurrence in nonobese Chinese individuals with normal blood lipid levels.[14,15] A Japanese study demonstrated the significant association of fatty liver index, a surrogate marker of fatty liver, with baPWV.[30] A recent cross-sectional study conducted on NHANES revealed a significant positive correlation between UHR and the severity of hepatic steatosis, but not fibrosis. The findings demonstrated that elevated UHR levels were independently associated with an increased risk of NAFLD and the severity of liver steatosis.[31] Above reports indicated that an association between UHR and baPWV may exist in fatty liver population. However, our subgroup analysis results showed that the significantly positive correlation between UHR and baPWV only exists in nonfatty liver group but not in the fatty liver group. Our inconsistent results may be due to limited sample size. Further studies are required to validate these findings.
The association between obesity and CVD has been well established, and vascular dysfunction has also been proposed as one of the important factors linking these 2 pathological states. However, conclusions about the relationship between obesity and arterial stiffness are inconsistent. For example, Rodrigues et al reported that BMI is not independently associated with increased aortic stiffness in a Brazilian population.[32] Additionally, Tang et al[33] found that the arterial stiffness measured as baPWV increases with BMI in a middle-aged healthy Chinese population. Our study suggested that BMI has a significant effect on the association between UHR and baPWV. A significantly positive correlation is observed between UHR and baPWV in the normal weight group but not in the lean or obesity group. Given that BMI is associated with UA and adult BMI is negatively related to HDL-C, BMI is speculated to be associated with baPWV. However, the complex mechanism underlying how BMI affects the association between UHR and baPWV is beyond the scope of this study. Based on the second analysis research design, future research should focus on these issues.
This study has limitations. First, this study was cross-sectional and provided limited evidence of associations between exposure and outcome but could not confirm causality. Second, raw data were obtained from a single Japanese population. Thus, applying the results to other ethnic groups should be done with caution. Finally, this study had a small sample size.
In summary, this study revealed a nonlinear relationship between UHR and baPWV. UHR is positively associated with baPWV on the left of the inflection point (14.25%) but is not statistically significant on the right side of the inflection point. Significant correlation between UHR and baPWV exists in females but not in males. Fatty liver status, BMI, and menopausal status may affect the above association. The clinical translation of the study results holds potential implications for UHR as a useful tool, which may contribute to advancements in related medical knowledge and practice.
Acknowledgments
We acknowledge all participant who involved in the original study.
Author contributions
Conceptualization: Xuede Gao.
Data curation: Yudong Ba.
Formal analysis: Yudong Ba, Shuxian Zhang.
Investigation: Haidong Wang, Yanan Li.
Software: Jinxiu Zhuo.
Supervision: Yanan Li.
Validation: Xuede Gao, Shuxian Zhang.
Writing – original draft: Haidong Wang.
Writing – review & editing: Jianhua Sun.
Abbreviations:
- ABI
- ankle–brachial index
- ALT
- alanine aminotransferase
- AST
- aspartate transaminase
- baPWV
- brachial–ankle PWV
- CVD
- cardiovascular disease
- eGFR
- glomerular filtration rate
- HDL-C
- high-density lipoprotein cholesterol
- NAFLD
- nonalcoholic fatty liver disease
- TC
- total cholesterol
- UA
- uric acid
- UHR
- UA/HDL-C ratio
- γ-GGT
- γ-glutamyltranspeptidase
HW, YB, XG, and JZ equally contributed to the study.
The original study had been approved the Medical Health Checkup Center at Murakami Memorial Hospital, Gifu, Japan.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Wang H, Ba Y, Gao X, Zhuo J, Li Y, Sun J, Zhang S. Association between serum uric acid to high density lipoprotein-cholesterol ratio and arterial stiffness in a Japanese population. Medicine 2023;102:31(e34182).
Contributor Information
Haidong Wang, Email: wanghaidong7924046@163.com.
Yudong Ba, Email: bayudong1006@126.com.
Xuede Gao, Email: dygaoxd@126.com.
Jinxiu Zhuo, Email: 0532zjx@163.com.
Yanan Li, Email: liyanan8808@126.com.
Jianhua Sun, Email: sjh740409@126.com.
References
- [1].Townsend N, Kazakiewicz D, Lucy Wright F, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19:133–43. [DOI] [PubMed] [Google Scholar]
- [2].Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12. [DOI] [PubMed] [Google Scholar]
- [3].Sugawara J, Tanaka H. Brachial-ankle pulse wave velocity: myths, misconceptions, and realities. Pulse (Basel). 2015;3:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Munakata M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10:49–57. [DOI] [PubMed] [Google Scholar]
- [5].Ato D. Brachial-ankle pulse wave velocity, cardio-ankle vascular index, and prognosis. Vasc Health Risk Manag. 2018;14:321–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borghi C, Agabiti-Rosei E, Johnson RJ, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. 2020;80:1–11. [DOI] [PubMed] [Google Scholar]
- [7].Katsiki N, Dimitriadis GD, Mikhailidis DP. Serum uric acid and diabetes: from pathophysiology to cardiovascular disease. Curr Pharm Des. 2021;27:1941–51. [DOI] [PubMed] [Google Scholar]
- [8].Nagao M, Nakajima H, Toh R, et al. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J Atheroscler Thromb. 2018;25:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Manandhar B, Cochran BJ, Rye KA. Role of high-density lipoproteins in cholesterol homeostasis and glycemic control. J Am Heart Assoc. 2020;9:e013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kocak MZ, Aktas G. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras (1992). 2019;65:9–15. [DOI] [PubMed] [Google Scholar]
- [11].Kurtkulagi O, Tel BMA, Kahveci G, et al. Hashimoto’s thyroiditis is associated with elevated serum uric acid to high density lipoprotein-cholesterol ratio. Rom J Intern Med. 2021;59:403–8. [DOI] [PubMed] [Google Scholar]
- [12].Kosekli MA, Kurtkulagii O. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Rev Assoc Med Bras (1992). 2021;67:549–54. [DOI] [PubMed] [Google Scholar]
- [13].Aktas G, Kocak MZ. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23:1098–102. [DOI] [PubMed] [Google Scholar]
- [14].Zhu W, Liang A, Shi P, et al. Higher serum uric acid to HDL-cholesterol ratio is associated with onset of non-alcoholic fatty liver disease in a non-obese Chinese population with normal blood lipid levels. BMC Gastroenterol. 2022;22:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang YN, Wang QQ, Chen YS, et al. Association between serum uric acid to HDL-cholesterol ratio and nonalcoholic fatty liver disease in lean Chinese adults. Int J Endocrinol. 2020;2020:5953461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aktas G, Khalid A. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. 2022;134:297–302. [DOI] [PubMed] [Google Scholar]
- [17].Fukuda T, Hamaguchi M. Association between serum γ-glutamyltranspeptidase and atherosclerosis: a population-based cross-sectional study. BMJ Open. 2014;4:e005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aktas G, Yilmaz S, Kantarci DB, et al. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgrad Med. 2023;135:519–23. [DOI] [PubMed] [Google Scholar]
- [19].Sun H, Su H, Zheng R, et al. Serum uric acid to high-density lipoprotein cholesterol ratio is associated with visceral fat in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2023;16:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li G, Zhao Q, Zhang X, et al. Association between the uric acid to high density lipoprotein cholesterol ratio and alanine transaminase in Chinese short stature children and adolescents: a cross-sectional study. Front Nutr. 2023;10:1063534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. [DOI] [PubMed] [Google Scholar]
- [22].Natoli AK, Medley TL, Ahimastos AA, et al. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension. 2005;46:1129–34. [DOI] [PubMed] [Google Scholar]
- [23].Campisi D, Cutolo M, Carruba G, et al. Evidence for soluble and nuclear site I binding of estrogens in human aorta. Atherosclerosis. 1993;103:267–77. [DOI] [PubMed] [Google Scholar]
- [24].Forte P, Kneale BJ, Milne E, et al. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension. 1998;32:730–4. [DOI] [PubMed] [Google Scholar]
- [25].Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. [DOI] [PubMed] [Google Scholar]
- [26].Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–76. [DOI] [PubMed] [Google Scholar]
- [27].Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas. 2018;108:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laucyte-Cibulskiene A, Vickiene A, Ryliskyte L, et al. Should we calculate arterial stiffness gradient in middle-aged women with increased cardiovascular risk? Blood Press. 2019;28:199–205. [DOI] [PubMed] [Google Scholar]
- [29].Yang R, Zhou Y, Li C, et al. Association between pulse wave velocity and hot flashes/sweats in middle-aged women. Sci Rep. 2017;7:13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Iwasaki Y, Shiina K, Matsumoto C, et al. Correlation of the Fatty Liver Index with the pathophysiological abnormalities associated with cardiovascular risk markers in Japanese Men without any history of cardiovascular disease: comparison with the Fibrosis-4 score. J Atheroscler Thromb. 2021;28:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xie Y, Huang K, Zhang X, et al. Association of serum uric acid-to-high-density lipoprotein cholesterol ratio with non-alcoholic fatty liver disease in American adults: a population-based analysis. Front Med (Lausanne). 2023;10:1164096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rodrigues SL, Baldo MP, Lani L, et al. Body mass index is not independently associated with increased aortic stiffness in a Brazilian population. Am J Hypertens. 2012;25:1064–9. [DOI] [PubMed] [Google Scholar]
- [33].Tang B, Luo F, Zhao J, et al. Relationship between body mass index and arterial stiffness in a health assessment Chinese population. Medicine (Baltimore). 2020;99:e18793. [DOI] [PMC free article] [PubMed] [Google Scholar]