Summary:
Wound healing complications present a significant burden on both patients and health-care systems, and understanding wound healing principles is crucial across medical and surgical specialties to help mitigate such complications. One of these longstanding principles, specifically delayed primary closure (DPC), described as mechanically closing a wound after several days of secondary intention healing, lacks clear consensus on its definition, indications, and outcomes. This practical review examines wound healing fundamentals, focusing on DPC, its execution, indications, and comparative outcomes. A PubMed literature search was conducted to retrieve studies on DPC. Inclusion criteria included comparative studies assessing outcomes and complications between DPC and other closure techniques, as well as articles investigating DPC’s underlying physiology. Twenty-three comparative studies met inclusion criteria. DPC wounds have significantly higher partial pressure of oxygen, higher blood flow, and higher rates of collagen synthesis and remodeling activity, all of which help explain DPC wounds’ superior mechanical strength. DPC seems most beneficial in contaminated wounds, such as complicated appendectomies, postcardiac surgery wounds, and complicated abdominal wall reconstructions, where it has been associated with lower rates of surgical site infections. This practical review provides an evidence-based approach to DPC, its physiology, technique, and indications. Based on the existing literature, the authors recommend that DPC wounds should be dressed in saline/betadine soaks, changed and irrigated daily, with delayed closure lasting between 3 and 5 days or until the infection has resolved. The clearest indications for DPC are in the context of contaminated abdominal surgery and sternal wound dehiscence post cardiac surgery.
Takeaways
Question: How can delayed primary closure (DPC) improve wound healing and reduce complications?
Findings: This comprehensive analysis of 23 comparative studies showcases DPC’s superior mechanical strength, with increased oxygen, blood flow, and collagen synthesis, and its benefits in contaminated wounds, particularly in abdominal surgery and sternal wound dehiscence post cardiac surgery.
Meaning: DPC offers a more effective and evidence-based approach to wound healing in certain surgical scenarios, reducing the risk of infection and promoting better outcomes.
INTRODUCTION
Wounds can generally be described as a disruption in the integrity of the skin and underlying soft tissue resulting from external force or trauma. This damage to the epidermis, dermis, and hypodermis—our body’s primary defense against foreign pathogens—puts our internal organs at risk. To counter this disruption, the body initiates a complex, immune-mediated response that triggers the wound healing process, aiming to repair and restore the body’s largest organ.
The concept of wounds has been known for millennia, with some of its earliest descriptions found in the Smith papyrus dating back to approximately 1700 BC.1 Ancient civilizations in Egypt, India, and Greece recognized the potential dangers of untreated wounds and implored practices that parallel contemporary techniques, including foreign body removal, wound irrigation, and various forms of closure.1 Our understanding of the physiological, biochemical, molecular, and pathophysiological processes of wound healing has grown since those initial writings necessitating regular reviews of the ever-evolving literature.2–8
Wound healing complications impose a significant burden on health-care systems with the cost estimates for treatment ranging from $28 billion to almost $100 billion.9 Although wound healing is often associated with the field of plastic surgery, its widespread impact and substantial costs across various surgical and medical specialties make a comprehensive understanding of its principles imperative.
Wound healing is broadly classified into three categories: primary, secondary, and tertiary [otherwise known as delayed primary closure (DPC)].10 Primary intention healing refers to the process where the wound edges are mechanically brought together, often using sutures, staples, or topical adhesives and adjuncts. Secondary intention healing takes place when the wound edges cannot be approximated due to the defect’s size, and the wound heals via granulation tissue growth and contraction. Tertiary intention, or DPC, a technique mandated during the Korean War, World Wars, and the Vietnam War,11 occurs when the wound is initially left open and later approximated, allowing for primary healing but in a delayed manner. Although the former two categories of healing are well established, there remains ambiguity surrounding DPC, with no clear consensus on its exact definition, indications, or outcomes.
To that end, the goal of this practical review was to provide a thorough examination of the fundamentals of wound healing, focusing on DPC; its execution; indications; and a concise, practical presentation of its published comparative outcomes.
METHODS
A literature search was conducted via the National Library of Medicine (PubMed) to retrieve all existing studies on DPC. The search strategy included the terms “delayed primary healing,” “delayed primary closure,” and “wound” as both keywords and MeSH terms. The terms were combined with Boolean terms and and or. There were no restrictions to the types of studies or year of publication. Only English and French articles were included. All the search entries were reviewed by two independent reviewers (H.E. and S.A.).
Due to the lack of consensus on when DPC should be performed over other closure techniques, the inclusion criteria comprised any comparative study that assessed the outcomes and complications between DPC and other closure techniques. Moreover, to provide the reader with the necessary basic science principles of wound healing and DPC, we included articles that investigated the underlying physiology of DPC.
Data extracted included number of patients, age, method of DPC and primary closure, and duration of DPC. The primary outcomes were surgical site infection (SSI), wound dehiscence, and length of hospital stay.
RESULTS
The initial search strategy yielded 563 articles, which underwent title and abstract screening. Sixty-one articles met the inclusion/exclusion criteria and underwent full-text review for inclusion in our qualitative analysis of outcomes and indications. Twenty-one comparative articles were found and included for our analysis of outcomes. A reference search found two additional relevant studies, which were also included in the analysis. Moreover, 20 noncomparative studies were included to provide a comprehensive practical review of DPC.
DPC Techniques
DPC is defined in the literature as mechanically closing a wound after allowing several days of healing by secondary intention.12 However, an analysis of the literature shows that many variations of DPC exist, with no current gold standard on how it should be performed. The literature described variations for three factors consisting of (1) the number of days before primarily closing the wound (delay duration), (2) the type of irrigation/dressing used in the interval to wound re-approximation, and (3) the frequency of interval irrigation/dressing changes. Of the 23 comparative studies included, 18 studies specified the duration of delay before primarily closing the wound. Among these studies, the delay most frequently used was 3–5 days (n = 13/17; 72.2%). A total of 18 studies specified the type of irrigation/dressing used with saline soaks/irrigation found to be used most often (n=9/18; 50%) followed by betadine soaks/irrigation (n = 3/18; 16.6%) and vacuum assisted closure dressings (n = 2/18; 11.1%). Finally, 18 studies also reported the frequency of irrigation/dressing change, with daily changes reported most frequently (n = 12/18; 66.7%), two of which performed it after postoperative day (POD) 3, but the remaining nine began immediately on POD 1. See Table 1 for details.
Table 1.
Overview of Delayed Primary Closure Techniques in Reviewed Studies
| Closure Delay (d) | Irrigation/Dressing | Frequency of Change | |
|---|---|---|---|
| Agrawal et al19 | NS | Saline soaks | Daily |
| Ahmad et al13 | 4 | Saline irrigation | Daily |
| Ayuso et al26 | 4–6 | VAC dressing | Every 2 days |
| Baksi et al14 | 10 | Saline irrigation | Daily |
| Briggs et al27 | 3 | NS | NS |
| Chatwiriyacharoen et al28 | 5 | Betadine soaks | Daily |
| Chiang et al15 | 5 | Betadine soaks | Daily |
| Cohn et al16 | 10 | Saline soaks | Daily after POD 3 |
| Duttaroy et al17 | Variable | Saline soaks | Daily after POD 3 |
| Fleck et al29 | 10 | VAC dressing | Every 2 days |
| Inyang et al20 | 5 | Saline soaks | Every 2 days after POD 3 |
| Jenkinson et al30 | 2 | None | None |
| Khan et al21 | 3–5 | None | None |
| Lahat et al31 | 3 | None | None |
| McGreal et al22 | 4 | Betadine irrigation | Daily |
| Ogawa et al18 | 7 | Saline irrigation | Daily |
| Pettigrew et al32 | 5 | None | None |
| Siribumrungwong et al25,33 | NS | NS | NS |
| Tofigh et al23 | 2–5 | Saline irrigation | Daily |
| Tsang et al24 | 4 | Saline soaks | Daily |
| Yousafzai etal34 | NS | NS | NS |
| Xiaowei et al35 | 3 | NS | NS |
NS, not specified.
Outcomes
Appendicitis/Gangrenous or Perforated Hollow Viscus
The vast majority of comparative literature on DPC is in the context of abdominal wounds, specifically wounds post complicated/perforated appendectomy or gangrenous/perforated hollow viscus. A total of 16 studies comparatively assessed the effect of DPC versus primary closure on complicated appendectomy/perforated hollow viscus surgery. Of the 16 studies, 14 were randomized controlled trials (RCTs) (level of evidence I) and two were retrospective cohort studies (level of evidence III). SSI was reported in all 16 studies. Five RCTs and one retrospective study with a total of 449 participants found significantly reduced SSIs associated with DPC compared with primary closure.13–18 Seven RCTs with a total of 681 participants showed no difference in SSI between DPC and primary closure,19–24 whereas only one study (retrospective cohort) with 128 participants showed that primary closure led to significantly lower rates of SSI compared with DPC.25 Finally, two RCTs with a total of 84 participants showed higher level SSIs associated with DPC compared with those related to primary closure; however, the significance was not reported in the original studies.28,31
The second most frequently reported outcome was length of hospital stay (LOS), which was reported in 10 studies. Seven studies with a total of 512 patients showed no statistical significance in LOS between patients undergoing primary closure and DPC.14,16,18,20,22,23,31 Two other studies with a total of 147 patients showed that DPC was associated with significantly shorter LOS,15,17 whereas one study of 100 participants showed DPC was associated with a significantly longer LOS.21
Four studies reported on wound dehiscence. Three of these, reporting on 157 patients, showed no significant difference in wound dehiscence between patients undergoing DPC and primary closure,14,19,20 whereas only one study on 77 patients showed that patients undergoing DPC had significantly less wound dehiscence compared with their counterparts who underwent primary closure.17
Sternal Infections/Dehiscence
Two studies comparatively assessed DPC and primary/secondary healing. Fleck et al investigated the difference between DPC and primary closure in patients with sternal dehiscence post cardiac surgery. The study showed that although 36% of patients who underwent primary closure developed infections, there were no instances of infection in the DPC group (P < 0.001).29 A more recent study by Yousafzai et al compared DPC with secondary healing and showed that DPC was associated with an average hospitalization of 5.1 days compared with 36.7 days in those who were subject to healing by secondary intention (P < 0.001). It also showed that those who underwent DPC had a more cosmetic linear scar than their counterparts who healed by secondary intention.34
Others
Other comparative studies that assess DPC versus primary closure were found in procedures including abdominal wall reconstruction, C-sections, suturing following dog bites, and open fractures. Ayuso et al compared vacuum assisted closure-assisted DPC and primary closure in patients undergoing abdominal wall reconstruction and found a significantly lower rate of infection (P = 0.09), wound dehiscence (P = 0.005), and overall wound complications (P = 0.02) in the former group compared with the latter group.26 Another pilot study showed similar results, where patients who had abdominal wall reconstruction after dirty/infected hernia repairs had lower rates of complications if they underwent a delayed staged repair compared with their counterparts who underwent primary repair and closure in one stage.36 On the other hand, in an RCT of 120 patients with dog bites, Xiaowei et al found that there was no significant difference in infection rates between DPC and primary closure; however, there was a significantly better cosmetic outcome associated with primary closure (P < 0.05).35 Similarly, Briggs et al showed no difference in infection rates between DPC and primary closure in patients undergoing C-sections.27 Finally, Jenkinson et al found that DPC was associated with a significantly higher rate of infections compared with primary closure in patients with Gustillo Anderson types I, II, and IIIa open fractures.30
DISCUSSION
The goal of this practical review is to provide the reader with practical, evidence-based guidelines on what DPC is, its physiology, how to perform it, and the comparative evidence of its outcomes and indications.
Physiology of Wound Healing and DPC
Wound healing is a complex process that occurs in response to tissue damage. It involves a series of overlapping stages, including inflammation, proliferation, and remodeling, each of which is governed by specific cells and cytokines.3,4,6,37
The first stage of wound healing is the inflammatory phase. Typically lasting between 2 and 5 days, there are several cells implicated in this early stage. Mast cells are largely responsible for the characteristic signs of inflammation, the rubor (redness), calor (heat), tumor (swelling), and dolor (pain) observed in the early days; however, it is the neutrophils, monocytes, and macrophages that govern the inflammatory phase and lay the foundation for the subsequent stages.38 The initiation of the inflammatory phase is marked by the adhesion of neutrophils to the vascular endothelial cells surrounding the wound (margination) and permeation through the cell junctions (diapedesis) to efficiently migrate to the wound site (chemotaxis). In addition to clearing the wound of infection and debris, these initial inflammatory cells release proinflammatory cytokines and growth factors to signal the recruitment and activation of fibroblasts and epithelial cells in anticipation of the next phase in healing3,37
The second stage of wound healing is proliferation, which typically lasts from 3 days to 3 weeks. During this stage, endothelial cells and fibroblasts migrate to the wound site to further angiogenesis and provide structural support (by replacing the interim fibrin matrix with a collagen-based matrix), respectively. The proliferation of myofibroblasts that induce wound contraction marks the conclusion of the second stage and the commencement of the final stage.39
The final stage of wound healing is remodeling and occurs on the order of several months. During this stage, deposition and organization of collagen fibers allows for the strengthening and consolidation of newly formed tissue. Collagen production persists for approximately 4 weeks after the injury, which is then followed by replacement of type III collagen with type I collagen over the following year, during which tensile strength increases (reaching a maximum of 80% of the original tensile strength at 90 days).40,41 The wound site may also continue to contract during this stage, which helps reduce the final size of the scar.3,37,42
Each stage of wound healing is essential, and disruption or attenuation of any of these processes can result in delayed wound healing. Delayed wound healing is most often the result of infection but can also be due to inadequate blood supply. Decreased perfusion can arise because of various factors such as diabetes and autoimmune disease, other comorbidities, certain medications such as glucocorticoid steroids, and smoking and alcoholism.43
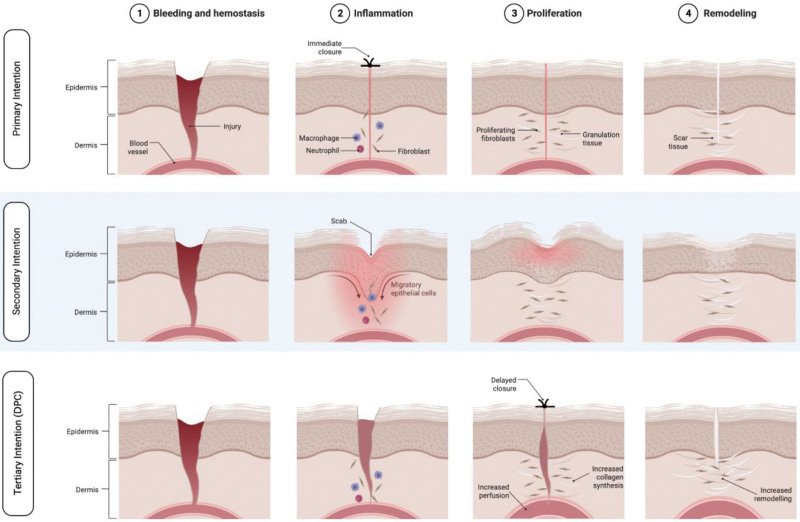
Delayed wound healing is different from delayed primary wound healing or DPC, in which the latter is done intentionally for therapeutic purposes. DPC has been shown to result in pronounced increases in wound strength compared with primary closure wounds in the intermediate and long-term in animal studies. Specifically, Fogdestam found that DPC wounds were significantly stronger than primarily closed wounds in rats when assessed 20 and 60 days post closure.44 Other studies were then published showing that DPC wounds have significantly higher partial pressure of oxygen, higher blood flow, and higher rates of collagen synthesis and remodeling activity, all of which help explain DPC wounds’ superior mechanical strength45–47 (Fig. 1).
Fig. 1.
Different stages of wound healing in primary, secondary, and tertiary healing (DPC). Created with Biorender.com.
How to Perform DPC
Due to the many variations of DPC that exist in the literature, the authors of this article have reviewed all the different technical variations and provide our suggested method based on our current understanding of wound healing mechanisms and previously published outcomes. The practice of assessing bacterial count to determine the optimal time for wound closure traces back to French surgeons in World War I and is based on the idea that wounds with greater contamination are best closed in a delayed manner. Although informative, the use of quantitative bacteriology has fallen out of favor due to its impracticality and improved sterile conditions.48,49 We suggest that DPC wounds be dressed in saline or dilute betadine soaks (<10%), which should be changed and irrigated daily to mechanically remove the infection and wound debris.15,50 The delay in closure should last between 3 and 5 days, which is in line with most clinical and biochemical animal studies. However, an important stipulation must be made in that signs of infections should be eradicated before closure, which may correspond to a closure delay of more than 5 days.
Indications
To provide evidence-based recommendations on the indications for DPC, we need to assess the available comparative evidence of DPC versus other types of closure. The strongest indication for DPC, according to our review, is in the context of complicated appendectomies/perforated hollow viscus. These wounds are often considered contaminated wounds, lending to their high risk of infection, and hence, could benefit from DPC.51 It is worth noting that some older systematic reviews showed no significant advantage of DPC compared with primary closure on reducing SSI in complicated appendectomies and perforated hollow viscus surgery33,52; however several more recent systematic reviews and meta analyses demonstrated a reduction in SSI in patients who received DPC.53,54 DPC has also been found to have beneficial effects in managing sternal wound dehiscence and infections. Although only two comparative studies were found, both found significant advantages of DPC over primary closure and healing by secondary intention. Similarly, DPC has been found to reduce wound complications post abdominal wall reconstruction in two recent studies published in 2022.26,36
DPC may also be indicated in patients who have a high risk of wound dehiscence. Patients with comorbidities that affect wound healing such as diabetes, smoking, and long-term glucocorticoid use could benefit from the use of DPC.55 One study published in the gynecologic oncology literature shows that patients who underwent DPC due to one or more existing comorbidities had no wound dehiscence compared with 27.3% dehiscence in the group that underwent primary closure.55 However, it is important to note that in addition to the study’s small sample size (DPC, n=6) resulting in its exclusion from our comparative analysis, the statistical significance was not reported, further limiting its interpretation. Nevertheless, it does shed some light on the possible benefits of DPC in highly comorbid patients and more importantly paves the way for future studies to better investigate this domain.
Contrary to historical to belief, there was no strong evidence supporting DPC over primary closure in open fractures. Although few comparative studies exist in this domain, and remaining conscious of possible selection bias, many noncomparative studies have found that primary closure of open fractures is safe and not associated with higher risks of infection.56–58
CONCLUSIONS
In conclusion, this practical review provides an evidence-based approach to DPC, its physiology, technique, and indications. Although there are mixed results from various studies, most of the studies show that DPC is most beneficial in the context of contaminated wounds, such as complicated appendectomies, postcardiac surgery wounds, and complicated abdominal wall reconstruction wounds, where it has been associated with lower rates of SSI. DPC may also be useful in patients with comorbidities affecting wound healing, although more research is needed to confirm this. Our review suggests that DPC wounds should be dressed in saline or dilute betadine soaks, changed, and irrigated daily, and delayed closure should last between 3 and 5 days or, if signs of infection, until the infection has resolved. Moreover, it is important to note that although all the studies included were comparative, a significant proportion were not RCTs, which affects the level of evidence of our conclusion. The decision to use DPC over secondary intention should be decided on a case-by-case basis, taking in to account the extent of tissue damage, the risk of infection and other patient-specific factors.
DISCLOSURES
Dr. Janis receives royalties from Thieme and Springer Publishing. All the authors have no financial interest to declare in relation to the content of this article.
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Chhabra S, Chhabra N, Kaur A, et al. Wound healing concepts in clinical practice of OMFS. J Maxillofac Oral Surg. 2017;16:403–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broughton G, II, Janis JE, Attinger CE. A brief history of wound care. Plast Reconstr Surg. 2006; 117(7 Suppl):6s–11s. [DOI] [PubMed] [Google Scholar]
- 3.Broughton G, II, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006; 117(7 Suppl):12s–34s. [DOI] [PubMed] [Google Scholar]
- 4.Broughton G, II, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006; 117(7 Suppl):1e-S–32e-S. [DOI] [PubMed] [Google Scholar]
- 5.Janis J, Harrison B. Wound healing: part II. Clinical applications. Plast Reconstr Surg. 2014;133:383e–392e. [DOI] [PubMed] [Google Scholar]
- 6.Janis JE, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2014;133:199e–207e. [DOI] [PubMed] [Google Scholar]
- 7.Janis JE, Kwon RK, Lalonde DH. A practical guide to wound healing. Plast Reconstr Surg. 2010;125:230e–244e. [DOI] [PubMed] [Google Scholar]
- 8.Teot L, Ohura N. Challenges and management in wound care. Plast Reconstr Surg. 2021;147:9s–15s. [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27–32. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Abbas AK, Fausto N, et al. Robbins and Cotran Pathologic Basis of Disease, Professional Edition e-Book. Elsevier health sciences; 2014. [Google Scholar]
- 11.Meakins JL. What’s past is prologue: delayed primary closure. Am J Surg. 1984;148:698–699. [DOI] [PubMed] [Google Scholar]
- 12.Ozgok Kangal MK, Regan JP. Wound Healing. Treasure Island, Fla.: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 13.Ahmad M, Ali K, Latif H, et al. Comparison of primary wound closure with delayed primary closure in perforated appendicitis. J Ayub Med Coll Abbottabad. 2014;26:153–157. [PubMed] [Google Scholar]
- 14.Baksi A, Chatterjee S, Ray U, et al. A randomized trial analyzing the effects of primary versus delayed primary closure of incision on wound healing in patients with hollow viscus perforation. Turk J Surg. 2020;36:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang RA, Chen SL, Tsai YC. Delayed primary closure versus primary closure for wound management in perforated appendicitis: a prospective randomized controlled trial. J Chin Med Assoc. 2012;75:156–159. [DOI] [PubMed] [Google Scholar]
- 16.Cohn SM, Giannotti G, Ong AW, et al. Prospective randomized trial of two wound management strategies for dirty abdominal wounds. Ann Surg. 2001;233:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duttaroy DD, Jitendra J, Duttaroy B, et al. Management strategy for dirty abdominal incisions: primary or delayed primary closure? A randomized trial. Surg Infect (Larchmt). 2009;10:129–136. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa K, Nitta H, Masuda T, et al. Efficacy of delayed primary closure with intrawound continuous negative pressure and irrigation treatment after surgery for colorectal perforation. Acute Med Surg. 2021;8:e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal V, Joshi MK, Gupta AK, et al. Wound outcome following primary and delayed primary skin closure techniques after laparotomy for non-traumatic ileal perforation: a randomized clinical trial. Indian J Surg. 2017;79:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inyang AW, Usang UE, Talabi AO, et al. Primary versus delayed primary closure of laparotomy wounds in children following typhoid ileal perforation in Ile-Ife, Nigeria. Afr J Paediatr Surg. 2017;14:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan KI, Mahmood S, Akmal M, et al. Comparison of rate of surgical wound infection, length of hospital stay and patient convenience in complicated appendicitis between primary closure and delayed primary closure. J Pak Med Assoc. 2012;62:596–598. [PubMed] [Google Scholar]
- 22.McGreal GT, Joy A, Manning B, et al. Antiseptic wick: does it reduce the incidence of wound infection following appendectomy? World J Surg. 2002;26:631–634. [DOI] [PubMed] [Google Scholar]
- 23.Tofigh AM, Family S. Primary versus delayed primary skin closure in operated patients due to perforated peptic ulcer disease: a randomized controlled clinical trial. Langenbecks Arch Surg. 2022;407:1471–1478. [DOI] [PubMed] [Google Scholar]
- 24.Tsang TM, Tam PK, Saing H. Delayed primary wound closure using skin tapes for advanced appendicitis in children. A prospective, controlled study. Arch Surg. 1992;127:451–453. [DOI] [PubMed] [Google Scholar]
- 25.Siribumrungwong B, Srikuea K, Thakkinstian A. Comparison of superficial surgical site infection between delayed primary and primary wound closures in ruptured appendicitis. Asian J Surg. 2014;37:120–124. [DOI] [PubMed] [Google Scholar]
- 26.Ayuso SA, Elhage SA, Aladegbami BG, et al. Delayed primary closure (DPC) of the skin and subcutaneous tissues following complex, contaminated abdominal wall reconstruction (AWR): a propensity-matched study. Surgical Endoscopy. 2022;36:2169–2177. [DOI] [PubMed] [Google Scholar]
- 27.Briggs R, Chari RS, Mercer B, et al. Postoperative incision complications after cesarean section in patients with antepartum syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP): does delayed primary closure make a difference? Am J Obstet Gynecol. 1996;175:893–896. [DOI] [PubMed] [Google Scholar]
- 28.Chatwiriyacharoen W. Surgical wound infection post surgery in perforated appendicitis in children. J Med Assoc Thai. 2002;85:572–576. [PubMed] [Google Scholar]
- 29.Fleck TM, Koller R, Giovanoli P, et al. Primary or delayed closure for the treatment of poststernotomy wound infections? Ann Plast Surg. 2004;52:310–314. [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson RJ, Kiss A, Johnson S, et al. Delayed wound closure increases deep-infection rate associated with lower-grade open fractures: a propensity-matched cohort study. J Bone Joint Surg Am. 2014;96:380–386. [DOI] [PubMed] [Google Scholar]
- 31.Lahat G, Tulchinsky H, Goldman G, et al. Wound infection after ileostomy closure: a prospective randomized study comparing primary vs. delayed primary closure techniques. Tech Coloproctol. 2005;9:206–208. [DOI] [PubMed] [Google Scholar]
- 32.Pettigrew RA. Delayed primary wound closure in gangrenous and perforated appendicitis. Br J Surg. 1981;68:635–638 [DOI] [PubMed] [Google Scholar]
- 33.Siribumrungwong B, Noorit P, Wilasrusmee C, et al. A systematic review and meta-analysis of randomised controlled trials of delayed primary wound closure in contaminated abdominal wounds. World J Emerg Surg. 2014;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yousafzai SM, Ugurlucan M, Awan A, et al. Cost and clinical effectiveness of aggressive surgical debridement and delayed primary closure of infected cardiac surgical wounds. J Wound Care. 2022;31:148–153. [DOI] [PubMed] [Google Scholar]
- 35.Xiaowei Z, Wei L, Xiaowei H, et al. Comparison of primary and delayed wound closure of dog-bite wounds. Vet Comp Orthop Traumatol. 2013;26:204–207. [DOI] [PubMed] [Google Scholar]
- 36.Hackenberger PN, Eiferman D, Janis JE. “Delayed-immediate” hernia repairs in infected wounds: clinical and economic outcomes. Am Surg. [Published online ahead of print Apr29, 2022]. [DOI] [PubMed] [Google Scholar]
- 37.Schultz GS, Chin GA, Moldawer L, et al. Principles of wound healing. In: Fitridge R, Thompson M, eds. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. University of Adelaide Press; 2011. [PubMed] [Google Scholar]
- 38.Wang PH, Huang BS, Horng HC, et al. Wound healing. J Chin Med Assoc. 2018;81:94–101. [DOI] [PubMed] [Google Scholar]
- 39.Desmoulière A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diegelmann RF. Analysis of collagen synthesis. Methods Mol Med. 2003;78:349–358. [DOI] [PubMed] [Google Scholar]
- 41.Carlson MA, Longaker MT. The fibroblast‐populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12:134–147. [DOI] [PubMed] [Google Scholar]
- 42.Almadani YH, Vorstenbosch J, Davison PG, et al. Wound healing: a comprehensive review. Semin Plast Surg. 2021;35:141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fogdestam I. A biomechanical study of healing rat skin incisions after delayed primary closure. Surg Gynecol Obstet. 1981;153:191–199. [PubMed] [Google Scholar]
- 45.Fogdestam I, Jensen FT, Nilsson SK. Delayed primary closure. Blood-flow in healing rat skin incisions. Scand J Plast Reconstr Surg. 1981;15:81–85. [DOI] [PubMed] [Google Scholar]
- 46.Fogdestam I, Niinikoski J. Delayed primary closure. Tissue gas tensions in healing rat skin incisions. Scand J Plast Reconstr Surg. 1981;15:9–14. [DOI] [PubMed] [Google Scholar]
- 47.Danielsen CC, Fogdestam I. Delayed primary closure: collagen synthesis and content in healing rat skin incisions. J Surg Res. 1981;31:210–217. [DOI] [PubMed] [Google Scholar]
- 48.Krizek TJ, Robson MC. Evolution of quantitative bacteriology in wound management. Am J Surg. 1975;130:579–584. [DOI] [PubMed] [Google Scholar]
- 49.Shulman G, Petro JA, Hallgren EH. Quantitative bacteriology in wound care. Am Surg. 1979;45:374–377. [PubMed] [Google Scholar]
- 50.Lammers RL, Fourré M, Callaham ML, et al. Effect of povidone-iodine and saline soaking on bacterial counts in acute, traumatic, contaminated wounds. Ann Emerg Med. 1990;19:709–714. [DOI] [PubMed] [Google Scholar]
- 51.Costa ACD, Santa-Cruz F, Ferraz AAB. What’s new in infection on surgical site and antibioticoprophylaxis in surgery? Arq Bras Cir Dig. 2021;33:e1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry MC, Moss RL. Primary versus delayed wound closure in complicated appendicitis: an international systematic review and meta-analysis. Pediatr Surg Int. 2005;21:625–630. [DOI] [PubMed] [Google Scholar]
- 53.Bhangu A, Singh P, Lundy J, et al. Systemic review and meta-analysis of randomized clinical trials comparing primary vs delayed primary skin closure in contaminated and dirty abdominal incisions. JAMA Surg. 2013;148:779–786. [DOI] [PubMed] [Google Scholar]
- 54.Tang S, Hu W, Hu L, et al. Primary versus delayed primary incision closure in contaminated abdominal surgery: a meta-analysis. J Surg Res. 2019;239:22–30. [DOI] [PubMed] [Google Scholar]
- 55.Podder AR, Jyothi GS. Wound dehiscence and role of delayed primary closure in gynaecological oncology. Indian J Gynecol Oncol. 2018;16:23. [Google Scholar]
- 56.Scharfenberger AV, Alabassi K, Smith S, et al. Primary wound closure after open fracture: a prospective cohort study examining nonunion and deep infection. J Orthop Trauma. 2017;31:121–126. [DOI] [PubMed] [Google Scholar]
- 57.Moola FO, Carli A, Berry GK, et al. Attempting primary closure for all open fractures: the effectiveness of an institutional protocol. Can J Surg. 2014;57:E82–E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajasekaran S. Early versus delayed closure of open fractures. Injury. 2007;38:890–895. [DOI] [PubMed] [Google Scholar]