Abstract
We aimed to present our 20-year experience of using the deep inferior epigastric vessels as recipient vessels for free scapular flaps phalloplasty and evaluate the outcomes. Penile reconstruction was performed using a free scapular flap between 2000 and 2020 by the same surgical team. Deep inferior epigastric vessels were used in all the cases. The surgical techniques and outcomes were described. Overall, 73 patients used the deep inferior epigastric artery (DIEA) as the recipient artery. Regarding the recipient veins, 2 veins were anastomosed in 72 (98.6%) patients, 1 deep inferior epigastric vein (DIEV) was used in 1 patient, 2 DIEV in 14, 1 DIEV + superficial inferior epigastric vein (SIEV) in 13, 1 DIEV + superficial circumflex iliac vein (SCIV) in 38, great saphenous vein (GSV) + SCIV in 4, and GSV + SIEV in 3. The mean age and body mass index of the study cohort was 28 years and 24.3 kg/m2, respectively. The shortest follow-up time was 7 months. Eleven patients had flap-related complications. Three patients were readmitted to the operating room within 24 hours, and 2 of them underwent salvage procedures with venous revision. Two patients lost the entire flap. One patient with 3-cm distal portion necrosis required surgical intervention. Three patients experienced urethral necrosis. DIEA is a suitable receptor artery for inflow. The DIEV, SIEV, and SCIV are available options for venous drainage according to the patient anatomical characteristics. The GSV can be an excellent backup for outflow and salvage procedures.
Keywords: deep inferior epigastric vessels, free scapular flap phalloplasty, penile reconstruction, superficial circumflex iliac vein, superficial inferior epigastric vein
1. Introduction
Phalloplasty is a technically challenging procedure that requires multiple stages and deals with various complications at each stage.[1–3] Phalloplasty aims to construct an esthetically pleasing phallus with a functional urethra.[4,5] Currently, the most widely used method of penile reconstruction is using a skin flap, including a free flap and a pedicled flap.[2,3,6,7] For penile reconstruction with a free flap, a good microsurgical technique is necessary to achieve a favorable outcome.[8–10] Many other factors such as vessel diameter, wall thickness, pedicle length, and vascular variation can significantly affect the difficulty and duration of microsurgery and outcomes.[11] Therefore, detailed preoperative planning and intraoperative selection of appropriate vessels for anastomosis are necessary for the successful completion of the procedure.
Many studies have focused on penile reconstruction with different donor sites; however, there is limited literature concerning the recipient vessels.[2,3] In free flap phalloplasty, most microsurgical technical difficulties and flap compromises are related to the conditions of the recipient vessels.[11,12] For example, the mismatching caliber of the 2 vessels or thin vessel walls can make the anastomosis much difficult and subsequently increase perioperative complications. Therefore, choosing appropriate recipient vessels can minimize such complications.
This study presents our 20-year experience of using deep inferior epigastric vessels as recipient vessels for free scapular flap phalloplasty and evaluates the microsurgical outcomes, with an aim of sharing the surgical techniques and lessons learned to provide reference to surgeons with relatively less experience.
2. Methods
Between 2000 and 2020, 73 patients with penile trauma (n = 19), micropenis (n = 42), self-amputation (n = 5), and genital ambiguity (n = 7) underwent phalloplasty using a free scapular flap under the same surgical team. Patients using deep inferior epigastric artery (DIEA)/deep inferior epigastric vein (DIEV) as the recipient vessels were included in the study.
Free scapular flap phalloplasty technique was introduced previously. The procedure was conducted in 3 stages as follows: penile and urethral reconstruction, urethral anastomosis, and penile prosthesis implantation. Complications were divided into 2 groups according to the time of occurrence and then recorded: perioperative complications, which included an unplanned return to the operating room for vascular insufficiency within 48 hours and early complications, which included partial or complete flap loss.
All patients signed an informed consent form, and the medical ethics review board approved the study.
2.1. Flap preparation
Preoperatively, all patients were instructed to stop smoking for 4 weeks before the date of surgery, and their weight was controlled at a body mass index < 30 kg/m2. The skin of the scapular flap was verified to be healthy, with no signs of inflammation. The anatomical distribution of the circumflex scapular artery and DIEA was examined and marked using Doppler ultrasonography. The scapular flap and recipient vessels were dissected in the lateral position. The procedures for penile (re)construction and blood vessel anastomosis were performed in the supine position. Surgeons were divided into 2 groups based on the dissection site used to prepare the donor and recipient sites (Fig. 1).
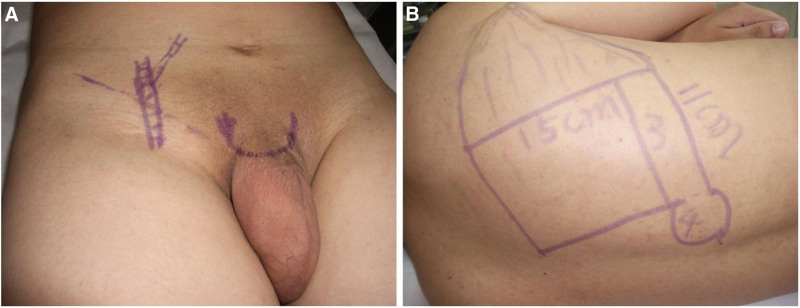
Figure 1.
Preoperative photographs. (A) Preoperative design of the recipient site. (B) Preoperative scapular flap design.
2.2. Donor site dissection
The flap was elevated with the transverse and descending cutaneous branches of the circumflex scapular artery and 2 vena comitantes. The dissection was started from the distal and medial margins of the flap toward the trilateral foramen because the distal and medial superficial fascia do not contain important structures and are far from the vascular pedicle.[13] Thereafter, the trilateral foramen was readily identified, and the vascular pedicle was carefully cut down. A 3 × 10 cm2 triangular subcutaneous tissue around the pedicle was preserved to wrap the pedicle and later sutured to the recipient area. The donor site was directly closed. Any residual defect was covered with a full-thickness skin graft in case closure could not be performed.
2.3. Recipient site dissection
A suprapubic C-shaped incision was made. The incision extended from C to the middle of the inguinal ligament, and a 2 to 3 cm incision extended upward. The superficial circumflex iliac vein (SCIV) or superficial inferior epigastric vein (SIEV), which could be identified in the superficial fascia of the lower abdomen, was carefully preserved. The anterior rectus sheath fascia was incised, and the deep inferior epigastric vessels could be identified when the lateral border of the rectus abdominis muscle was elevated. The deep inferior epigastric vessels were isolated to the navel to obtain a sufficient length (5 cm) for a comfortable anastomosis. The motor intercostal nerve branches were carefully preserved. The vessels were then cut distally and transposed to the recipient site through the opening of the fascia. The incision was closed immediately, and the fascia was carefully reapproximated with 3-0 absorbable thread, leaving a 3-cm tension-free hole for the pedicle to pass through.
2.4. Vascular anastomosis
The circumflex scapular artery and the DIEA were anastomosed together. Venous anastomosis was performed between the 2 vena comitantes, DIEV, and SIEV.
The circulation of the grafted flap was observed for 30 minutes to ensure successful vascular anastomosis. A compressive dressing of penile shaft and suction drain was routinely used to prevent postoperative hematoma. Patients were transferred to a recovery room maintained at 25°C, and the daily rehydration volume was maintained to a minimum of 3000 mL, monitored for 2 days. Subsequently, the patients were transferred to the general ward and prescribed bed rest for 1 week. The penis was raised by approximately 45° during this period. Antibiotics are routinely administered to prevent infection (Fig. 2).
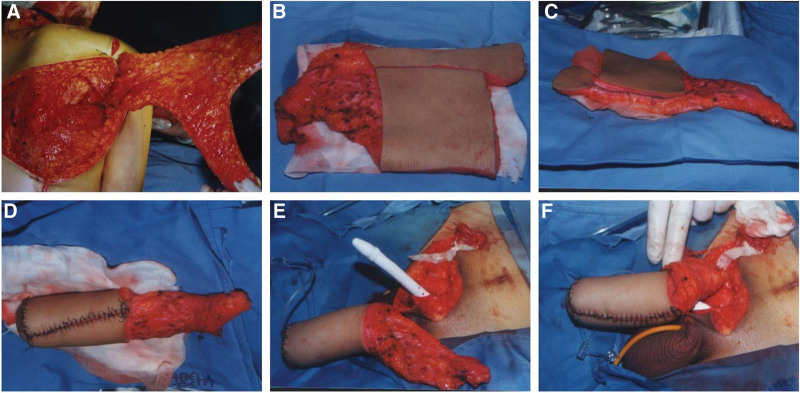
Figure 2.
Intraoperative photographs of phalloplasty with scapular flap. (A) The scapular flap was raised. (B) The vascular pedicle was cut off. (C) The inner flap was sutured to form the urethra. (D) The outer flap was wrapped around the neourethra to form the shaft. (E) The penile prosthesis was inserted routinely and immediately before flap anastomosis. (F) Vascular anastomosis is complete.
3. Results
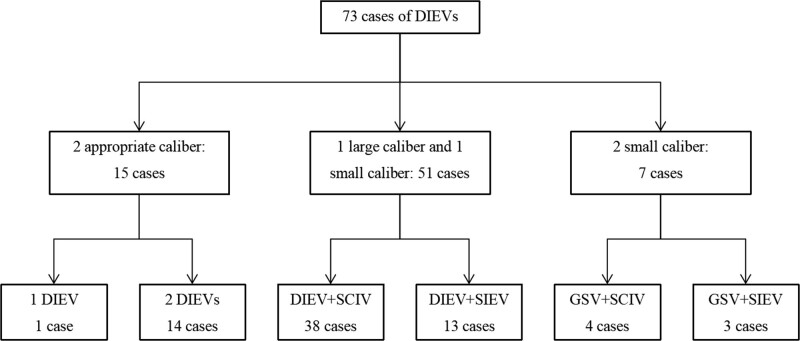
Between 2000 and 2020, 73 patients underwent free scapular flap phalloplasty. In all patients, the deep inferior epigastric vessels were used as the recipient vessels. The mean age was 28 years, and the mean body mass index was 24.3 kg/m2. The shortest follow-up time was 7 months. In this study, 72 (98.6%) patients had 2 veins anastomosed. Figure 3 shows the recipient veins used in this study.
Figure 3.
The recipient veins used in this study.
Eleven patients experienced flap-related complications. Three patients were returned to the operating room because of venous congestion within 24 hours. One of these patients suffered active bleeding of the penile shaft flap postoperatively—the blood and clots accumulated in the anastomotic area. After evacuating hematoma, we found intravascular thrombosis in both veins (1 DIEV + 1 SCIV), which rendered them useless. Consequently, the great saphenous vein (GSV) was dissected and transposed cranially to the recipient area for anastomosis, and the flap was salvaged. In the other 2 patients, no active bleeding was found, but fluid and blood clots accumulated around the anastomotic vessels, and intravascular thrombosis was noted. After anastomotic revision, 1 flap could be salvaged. One patient experienced flap loss after 6 hours of traveling home while in a sitting position on postoperative day 12. Two patients eventually experienced total flap loss. No arterial compromise was observed. There were 4 cases of partial necrosis, of which 3 were easily repaired without interventions; 1 case of distal portion necrosis required surgical debridement and secondary revision with the groin flap after 6 months. In the early stage of this study, 2 patients experienced urethral necrosis because the flaps were not large enough, leading to tension closure. The urethral flaps were lost despite removing the suture to relieve the tension. The remaining 1 case of urethral flap necrosis that occurred on postoperative day 5 might be related to pedicle compression. Table 1. shows the details of perioperative and early complications.
Table 1.
Details of perioperative and early complications.
| Patients | Veins | Postoperative day | Description | Intervention | Outcome |
|---|---|---|---|---|---|
| 1 | DIEV + SCIV | 1 | Active bleeding, 2 vein thrombosis | Revised vein and re-anastomosis with GSV | Flap salvage |
| 2 | DIEV + SIEV | 1 | 1 vein thrombosis | Revised vein and re-anastomosis | Flap salvage |
| 3 | DIEV + SIEV | 1 | 2 vein thrombosis | Revised vein and re-anastomosis | Flap loss |
| 4 | DIEV + SCIV | 13 | 6 h of traveling home | N/A | Flap loss |
| 5 | DIEV + SCIV | 6 | N/A | Surgical debridement | Distal portion loss |
| 6–9 | DIEV + SCIV | 5,5,6 | Partial flap blackening | Dressing change | No flap loss |
| 10–11 | 1DIEV + SCIV 1DIEV + SIEV |
5, 7 | Infection Secretion outflow |
Debridement | Urethral flaps loss |
DIEV = deep inferior epigastric vein, GSV = great saphenous vein, SCIV = superficial circumflex iliac vein, SIEV = superficial inferior epigastric vein.
This study involved a total of 73 patients undergoing penile reconstruction with scapular flap between 2000 and 2020, among them 66 were followed for at least 24 months. All these 66 follow-up patients were able to urinate while standing either after urethra anastomosis or the fistula revision. Table 2. demonstrates the details of the long-term complications (Fig. 4).
Table 2.
Long-term (24 mo) complications of 66 patients.
| Urologic | Prosthesis | ||||
|---|---|---|---|---|---|
| Urethral fistula | Urethra necrosis | Urethral strictures | Exposure | Infection | Uncomfortable |
| 6/66 | 2/66 | 4/66 | 14/45 | 2/45 | 5/45 |
45 of 66 patients underwent prosthesis implantation.
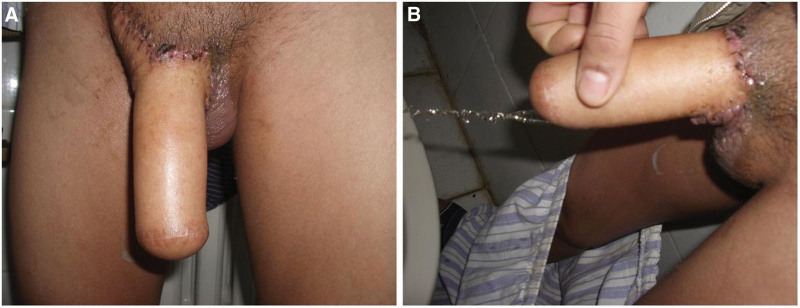
Figure 4.
Follow-up photographs. (A) Six months after the first stage of phalloplasty. (B) Standing urination after urethral anastomosis.
4. Discussion
Deep inferior epigastric vessels can be a reliable recipient in free flap phalloplasty. In our study, we observed 11 flap-related complications, 3 perioperative complications, and 8 early complications. However, excluding 2 cases of unreasonable preoperative design, 2 cases related to poor postoperative posture, and 3 cases of spontaneous healing, which could be avoided as we learned from our failure, only 4 complications (two of them were salvaged) could be attributed to anastomotic problems. In this study, we share our experiences of recipient vessel selection in terms of what we learned from failed cases and describe some technical modifications to reduce the risk of vascular insufficiency.
4.1. Advantages and disadvantages of DIEA/DIEV
Since 2000, the authors have used deep inferior epigastric vessels as receptor vessels in free scapular flap phalloplasty. It has obvious advantages, including consistent anatomy, abundant inflow (a direct branch of the external iliac artery), suitable caliber, and sufficient length that allows for transposition to a superficial location. However, its vena comitantes have some disadvantages: vascular variation, potentially smaller caliber, and potential for abdominal wall hernia formation. In the authors’ practice, 3 DIEVs were observed intraoperatively: the most common condition was 1 large caliber and 1 small caliber; 2 vessels with appropriate caliber; or 2 vessels with small caliber, which occurs occasionally. The latter type requires the use of a GSV or its branch to solve this potential problem, which will be discussed later. During vessel dissection, the anterior rectus sheath is incised, and the vascular bundle is transposed from the opening of the anterior rectus sheath to the superficial recipient location. This opening, even if small, can lead to late development of abdominal wall hernia. Some surgeons advocate using mesh grafts to reinforce the anterior rectus sheath to avoid abdominal wall hernia; however, we considered this as unnecessary. In the authors’ practice, after reapproximating the 2 fascia layers and continuously suturing with 3-0 absorbable thread, only 1 patient suffered hernia that was easily repaired with mesh graft.
4.2. Selection of recipient vessels
As mentioned above, the consistent anatomy, abundant inflow, suitable caliber, and sufficient length make the DIEA a reliable and easy-to-use option as the recipient artery. Therefore, the authors prefer using this artery when performing free flap phalloplasty. Some surgeons prefer to use the femoral artery,[14] which has a sufficient diameter and inflow. Another benefit of using the femoral artery is that through 1 incision, access to good inflow as well as exposure to all the aforementioned vessels such as SIEV, SCIV, GSV, and various unnamed branches can be obtained. However, compared with the end-to-end anastomosis of the DIEA, an arteriotomy of the main artery of the lower extremity for end-to-side anastomosis has greater technical requirements and higher relative risk. Therefore, we recommend novices to use the DIEA as the recipient vessel. The femoral artery can be a good alternative when the DIEA is not available.
The use of the DIEV is more complicated because of its wide variation. Therefore, we opted for different recipient vessels for anastomosis according to the specific characteristics of the 2 DIEVs. Two recipient veins are routinely anastomosed to minimize the risk of insufficient outflow in free flap phalloplasty.[15,16] The combination of a superficial and a deep vein system, which showed distinct advantages in some studies, is preferred.[16,17] Generally, any superficial vein such as the GSV and its branches with adequate length and caliber can be used as a recipient vein. The SCIV should take precedence over the SIEV because it is longer and larger. However, when the branch veins are unusable or the anastomotic veins cannot be salvaged, the GSV can always be harvested with sufficient length to allow a safe, large-caliber, and tension-free venous anastomosis. In most cases, DIEVs appear as 1 large-caliber vessel and 1 small vessel, so the larger 1 and a SCIV can be used for 2 venous outflows. DIEVs of 2 appropriate sizes and 2 small sizes are uncommon. Therefore, we anastomosed directly to the former and used a GSV and SCIV or SIEV for the latter.
4.3. Minimize the complications
Most of the anastomotic complications were related to venous thrombosis because of its low-flow, low-pressure nature.[18] In this study, all 3 takebacks were due to postoperative hematoma, which led to venous thrombosis within 24 hours. During the surgical exploration, 1 patient was found to have active bleeding, and 2 patients had old hemorrhage and clots around the anastomotic vessels. A rational explanation is that the accumulated fresh or old hemorrhage and clots put pressure on the anastomosed vessels, slowing the venous outflow, leading to thrombosis. Therefore, we modified their techniques to minimize venous compromise as follows: hemostasis should be performed strictly and carefully, and 2 drainages should be placed immediately and routinely post-operation. The first one is half-tube drainage without suction for the anastomotic area, which can drain the liquid promptly without affecting the anastomotic vessels. The second one is negative pressure drainage, inserted from the tip to the root of the neophallus to drain the penile shaft fluid and prevent it from accumulating in the anastomotic area. All the drainage was removed on postoperative day 5 when the subcutaneous tunnels had been formed, preventing the pulling out action from disturbing the anastomotic blood vessel. Proper compressive dressing of the penile shaft will help venous reflow. Phalloplasty free flaps are highly sensitive to venous outflow because of the tubular structure; therefore, any flap swelling postoperatively can compromise the outflow, because there is limited room for expansion. Hence, moderate external compression is beneficial for venous outflow; we believe the pressure should be lower than blood pressure. In our center, the pressure degree is judged by an experienced doctor and the state of the flap is observed closely to adjust the pressure postoperatively.
Phalloplasty is different from other flap reconstructions because of its unique functional and anatomical characteristics. To ensure the movement, only the root of the penis was fixed in front of the pubis. However, this structure increases the risk of vascular insufficiency. Four patients experienced partial flap necrosis, and one of them had 3-cm distal part necrosis that was debrided surgically and repaired with a groin flap 6 months later. A modification of the proposed technique aims to increase the contact area between the reconstructed penis and the recipient area. A 3 × 10 cm2 triangular subcutaneous tissue around the pedicle was preserved to wrap the pedicle and later sutured to the recipient area, which protected the pedicle and increased the blood supply to the penile flap. According to the triangular tissue flap thickness, the triangular subcutaneous pedicle can be sutured through the tunnel, the principle is not to compress the pedicle. We use this technique for phalloplasty with other flaps as well.
Furthermore, the vascular pedicle can get compressed when the patient bends down, especially if the recipient veins pass through the inguinal ligament, such as the branch of the GSV. Therefore, prolonged sitting in the early stages after surgery is not recommended. In this study, 1 patient lost the whole flap after a long trip home; thus, the patients were instructed to avoid prolonged sitting for a month after the procedure.
4.4. Limitations
This study had some limitations. First, this is a retrospective observational study that covers a long study period. Second, the free scapular flap is an uncommon phalloplasty method, and the length and caliber of the circumflex scapular vessels are very suitable for anastomosis. Third, the study does not include a control group. Commonly used flaps, such as the radial forearm flap and latissimus dorsi flap, could be included in the control group to compare the applicability and stability of the recipient vessels. Finally, the study included a small sample size. A prospective series comparing different free flap phalloplasty techniques is advocated to improve this study further.
5. Conclusion
DIEA is a suitable receptor artery for inflows. The DIEV, SIEV, and SCIV are available choices for venous drainage. According to the patient anatomical characteristics, choosing veins with appropriate caliber, sufficient wall thickness, and vascular length for anastomosis will greatly reduce the technical difficulties of microsurgery and the occurrence of complications. The GSV can be an excellent backup option for drainage vessels and salvage procedures. Careful hemostasis, good drainage, close postoperative observation, and timely treatment of complications can further increase the success rate of these procedures.
Acknowledgment
Informed consent was obtained from the patient for publication of this care report details.
Author contributions
Data curation: Shuyuan Li.
Formal analysis: Shuyuan Li.
Methodology: Shuyuan Li, Sisi Luo.
Project administration: Shuyuan Li.
Resources: Sisi Luo.
Software: Ning Ma.
Supervision: Zhe Yang, Ning Ma, Yang-Qun Li.
Writing – original draft: Shuyuan Li, Sisi Luo, Yang-Qun Li.
Writing – review & editing: Shuyuan Li, Sisi Luo, Yang-Qun Li.
Abbreviations:
- DIEA
- deep inferior epigastric artery
- DIEV
- deep inferior epigastric vein
- GSV
- great saphenous vein
- SCIV
- superficial circumflex iliac vein
- SIEV
- superficial inferior epigastric vein
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
All patients signed an informed consent form, and the medical ethics review board approved the study.
The authors have no funding and conflicts of interest to disclose.
SL and SL contributed equally to this work.
How to cite this article: Li S, Luo S, Yang Z, Ma N, Li Y-Q. Deep inferior epigastric vessels for free scapular flap phalloplasty. Medicine 2023;102:31(e34603).
Contributor Information
Shuyuan Li, Email: liyangqundoctor@126.com.
Sisi Luo, Email: rachel_sisi@126.com.
Zhe Yang, Email: yangzhe@psh.pumc.edu.cn.
Ning Ma, Email: 171677184@qq.com.
References
- [1].Esmonde N, Bluebond-Langner R, Berli JU. Phalloplasty flap-related complication. Clin Plast Surg. 2018;45:415–24. [DOI] [PubMed] [Google Scholar]
- [2].Yao A, Ingargiola MJ, Lopez CD, et al. Total penile reconstruction: a systematic review. J Plast Reconstr Aesthet Surg. 2018;71:788–806. [DOI] [PubMed] [Google Scholar]
- [3].Morrison SD, Shakir A, Vyas KS, et al. Phalloplasty: a review of techniques and outcomes. Plast Reconstr Surg. 2016;138:594–615. [DOI] [PubMed] [Google Scholar]
- [4].Gilbert DA, Horton CE, Terzis JK, et al. New concepts in phallic reconstruction. Ann Plast Surg. 1987;18:128–36. [DOI] [PubMed] [Google Scholar]
- [5].Hage JJ, De Graaf FH. Addressing the ideal requirements by free flap phalloplasty: some reflections on refinements of technique. Mircosurg. 1993;14:592–8. [DOI] [PubMed] [Google Scholar]
- [6].Kropp B, Cohn JE, Wang W, et al. Free tissue transfer penile reconstruction. Semin Plast Surg. 2019;33:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Namba Y, Watanabe T, Kimata Y. Flap combination phalloplasty in female-to-male transsexuals. J Sex Med. 2019;16:934–41. [DOI] [PubMed] [Google Scholar]
- [8].Doornaert M, Hoebeke P, Ceulemans P, et al. Penile reconstruction with the radial forearm flap: an update. Handchir Mikrochir Plast Chir. 2011;43:208–14. [DOI] [PubMed] [Google Scholar]
- [9].Monstrey S, Hoebeke P, Selvaggi G, et al. Penile reconstruction: is the radial forearm flap really the standard technique? Plast Reconstr Surg. 2009;124:510–8. [DOI] [PubMed] [Google Scholar]
- [10].Falcone M, Blecher G, Anfosso M, et al. Total phallic reconstruction in the genetic male. Eur Urol. 2021;79:684–91. [DOI] [PubMed] [Google Scholar]
- [11].Evans BCD, Evans GRD. Microvascular surgery. Plast Reconstr Surg. 2007;119:18e–30e. [DOI] [PubMed] [Google Scholar]
- [12].Danker S, Annen AW, Cylinder I, et al. Technical description and microsurgical outcomes in phalloplasty using the deep inferior epigastric artery and locoregional veins. Plast Reconstr Surg. 2020;146:196e–204e. [DOI] [PubMed] [Google Scholar]
- [13].Mayou BJ, Whitby D, Jones BM. The scapular flap—an anatomical and clinical study. Br J Plast Surg. 1982;35:8–13. [DOI] [PubMed] [Google Scholar]
- [14].Ascha M, Massie JP, Morrison SD, et al. Outcomes of single stage phalloplasty by pedicled anterolateral thigh flap versus radial forearm free flap in gender confirming surgery. J Urol. 2018;199:206–14. [DOI] [PubMed] [Google Scholar]
- [15].Ichinose A, Terashi H, Nakahara M, et al. Do multiple venous anastomoses reduce risk of thrombosis in free-flap transfer? Efficacy of dual anastomoses of separate venous systems. Ann Plast Surg. 2004;52:61–3. [DOI] [PubMed] [Google Scholar]
- [16].Riot S, Herlin C, Mojallal A, et al. A systematic review and meta-analysis of double venous anastomosis in free flaps. Plast Reconstr Surg. 2015;136:1299–311. [DOI] [PubMed] [Google Scholar]
- [17].Hanasono MM, Kocak E, Ogunleye O, et al. One versus two venous anastomoses in microvascular free flap surgery. Plast Reconstr Surg. 2010;126:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plast Reconstr Surg. 2000;105:2374–80. [DOI] [PubMed] [Google Scholar]