Abstract
Gastric cancer (GC) is the remaining concern of cancer-associated health burden. Valuable predictive and prognostic indicators support the early diagnosis and improve outcome. Immune escape and inflammation are important cancer hallmarks. The prognostic and diagnostic value of platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) was reported in some cancers. But these cheap and convenient indexes are far from clinical use. Thus, investigation the alteration of those index on GC is needed to impose the use of those indexes in clinic. The study recruited seventy-seven hospitalized patients newly diagnosed with GC and 90 healthy individuals. The clinical and preclinical data of participants were collected from Hospital Information Management system. This study were approved by the Ethical Committee, Vietnam Military Medical University. The data were analyzed on STATA version 14.0 and GraphPad Prism 8.0. The alteration of immunological system was reported by significantly higher white blood cell count, neutrophils, platelets, PLR, and NLR as well as decreased lymphocytes on GC, compared to healthy individuals. Those indexes were elevated on advanced stage GC, compared to early stage GC. Our receiver operating characteristic curve analysis showed the significant specificity and sensitivity of PLR (cutoff 135.0) and NLR (cutoff 2.0) on GC diagnosis with respective area under receiver operating characteristic curve of 84.74% and 85.17%, P < .0001. Besides, our results reported the tendency of increased PLR and NLR and short time from clinical signs to being diagnosed. PLR and NLR have significant specificity and sensitivity in diagnosis and prognosis of GC.
Keywords: gastric cancer, GC, inflammation, NLR, PLR
1. Introduction
Being at the fifth and fourth position in ranking list regarding morbidity and mortality according to Globocan 2020, gastric cancer (GC) rings the warning bell of preventive and protective need.[1] It is estimating of 1.1 million new cases and 770,000 deaths due to GC in 2020.[1] Meanwhile, the incidence and mortality of GC ranks at sixth and second position according to Globocan 2018.[2] The climbed rank of incidence and dropped rank of mortality suggests the need of development of early diagnosis of GC. By contrast, overall epidemic of GC was steadily reduced in United State, and 5-year survival rate gradually rises from 38.3% in 2007 to 2011 to 42.9% in 2017 to 2021.[3] However, GC with distal metastasis results in only 10% of 5-year survival rate.[3] Noticeably, GC is estimated to increase to 1.8 million new cases and 1.3 million deaths by 2040.[4] Overall survival and 5-year survival rate depending on the stages at diagnosed point. Thus, it is urgent need to promote early diagnosis, reliable prognosis, and effective treatment option to improve benefit for GC patients.
Inflammation is a significant hallmark in tumorigenesis.[5] The association between inflammatory burden and cancer has been reported.[6] Neutrophilia reflects cancer-associated chronic inflammation. Neutrophils (NE) induce carcinogenesis via secreting cancer-promoting cytokines, suppressing cytotoxic T cell activity and thus promoted metastasis.[5] Lymphocytes (LY) dictate malignancy-against immunity, by inducing cytotoxic cell death and preventing the proliferation and migration of malignant cells.[7,8] The concomitance of neutrophilia and lymphocytopenia, resulting in elevated neutrophil-to-lymphocyte ratio (NLR) was reported in many diseases.[5] NLR reflects the immunology balance between inflammation (both acute and chronic) and adaptive immunity.[9] Capturing the detrimental effects of neutrophilia and the beneficial effects of lymphocyte-mediated adaptive immunity, NLR indicated a significant decline in the cell-mediated adaptive immune response.[10] In addition, NLR is increased in various inflammation related diseases such as gastrointestinal diseases, thyroiditis, and infections.[11–15] Besides, NLR can be considered as a robust predictor of cancer severity and mortality, but the normalcy range and age- and disease-adjusted category are needed.[9] The NE and LY count mirror the innate (acute and chronic inflammation) and adaptive immunity, respectively.[16] The significant association between NLR and overall mortality and mortality was reported in cardiovascular disease, but not in cancers.[16] Simultaneous neutrophilia and lymphopenia are associated with poor cancer prognosis.[5] Strong associations between NLR and overall survival was reported in pancreatic cancer, renal cell carcinoma, and mesothelioma.[17] High NLR strongly associated with depth of invasive tumor, older age, male gender, lower 5-year overall survival rate.[18]
Moreover, platelets (PLT) protect tumor cells from immune surveillance, enhance angiogenesis and facilitate tumor metastasis.[19,20] NE and PLT support the immunological escape of metastatic cancer cells.[21] The depletion of PLT resulted in impaired metastasis, while reconstitution of PLT recovered metastasis.[19,20] The elevated number of PLT reflected an increased risk of several cancers.[22] An elevated platelet-to-lymphocyte ratio (PLR) resulting from the increased PLT and/or deceased LY becomes a reliable predictor of several diseases such as thyroiditis, gastrointestinal diseases, type 2 diabetes mellitus, irritable bowel disease, infectious diseases and cancers.[12,23–28] This study aims to investigate the alteration NLR and PLR as a potential predictors of diagnosis and prognosis GC in Vietnamese patients.
2. Materials and methods
2.1. Patient consent and ethical approval
All participants were informed and agreed with the collection and reporting their clinical data in this study. The recruitment and execution of this study were approved by the Ethical Committee, Vietnam Military Medical University. All participants signed the consent agreement form.
2.2. Data collection and study design
This is a cross-sectional descriptive study. Seventy-seven hospitalized patients newly diagnosed with GC (at Military Hospital 103 from June 2020 to September 2022) were recruited for this study. Patient eligibility criteria included confirmation of GC diagnosis, hospitalized patients and agree to participate in the study. Healthy control (HC) group included 90 people without disease detection after examination. These people visited Military Hospital 103 for regular health check. HC eligibility criteria included common laboratory tests in normal range, no disease detection after examination and agree to participate in the study. All participants were explained clearly about the purpose of study and participated voluntarily. The clinical and preclinical data of participants were collected from Hospital Information Management system.
2.3. Statistical analysis
GraphPad Prism version 8.0 (GraphPad Software, Boston, MA) and Stata 14.0 (Stata Software, College Station, TX) were exploited to analyze the data. The difference between more-than-two groups was analyzed by one-way of variance ANOVA if the variance follows the normal distribution or Krukal Wallis, followed by Dunn multiple comparison test if the variance does not follow the normal distribution. The difference between 2 groups was analyzed by unpair t-test. The chi-square (Fisher exact) test was applied to reveal the association between 2 categorical variables. The difference was referred significance *, **, *** if P < .05, P < .01, P < .001, respectively.
3. Results
3.1. The age and gender distribution of study population
The study cohort included 167 participants, 90 HC and 77 GC patients. Among GC group, stage I, II, III, and IV accounted for 15, 12, 27, and 23 patients, respectively. Noticeably, male was prominent in all stage groups with the proportion of 73% in stage I, 91.7% in stage II, 63.0% in stage III and 52.5% in stage IV. The gender distribution is equivalent between GC (66.2%) and HC (61%). HC and GC groups showed male dominance (Table 1).
Table 1.
Age, gender, and stage distribution.
| Stages | Age | Gender | P | Total | |
|---|---|---|---|---|---|
| Male | Female | ||||
| HC | Mean ± SD | 51.1 ± 4 | 52.9 ± 6.8 | 0.042 | 51.8 ± 5.3 |
| n (%) | 55 (61%) | 35 (39%) | 90 (100%) | ||
| I | Mean ± SD | 63.7 ± 9.5 | 65.5 ± 12.3 | 0.107 | 64.2 ± 9.9 |
| n (%) | 11 (73%) | 4 (27%) | 15 (100%) | ||
| II | Mean ± SD | 67.1 ± 5.1 | 52 | 0.026 | 65.8 ± 6.6 |
| n (%) | 11 (91.7%) | 1 (8.3%) | 12 (100%) | ||
| III | Mean ± SD | 66.5 ± 6.2 | 65.6 ± 7.9 | 0.191 | 66.1 ± 6.7 |
| n (%) | 17 (63.0%) | 10 (37.0%) | 27 (100%) | ||
| IV | Mean ± SD | 62.2 ± 9.7 | 62 ± 11.6 | 0.833 | 62.1 ± 10.4 |
| n (%) | 12 (52.2%) | 11 (47.8%) | 23 (100%) | ||
| GC | Mean ± SD | 65 ± 7.8 | 63.5 ± 10.2 | 0.007 | 64.5 ± 8.6 |
| n (%) | 51 (66.2%) | 26 (33.8%) | 77 (100%) | ||
| Total | Mean ± SD | 57.8 ± 9.2 | 57.4 ± 9.8 | 57.7 ± 9.4 | |
| n (%) | 106 (63.5%) | 61 (36.5%) | 167 (100%) | ||
GC = gastric cancer, HC = healthy individuals.
3.2. The comparison of some immunological and hematological indices between GC patients and HC
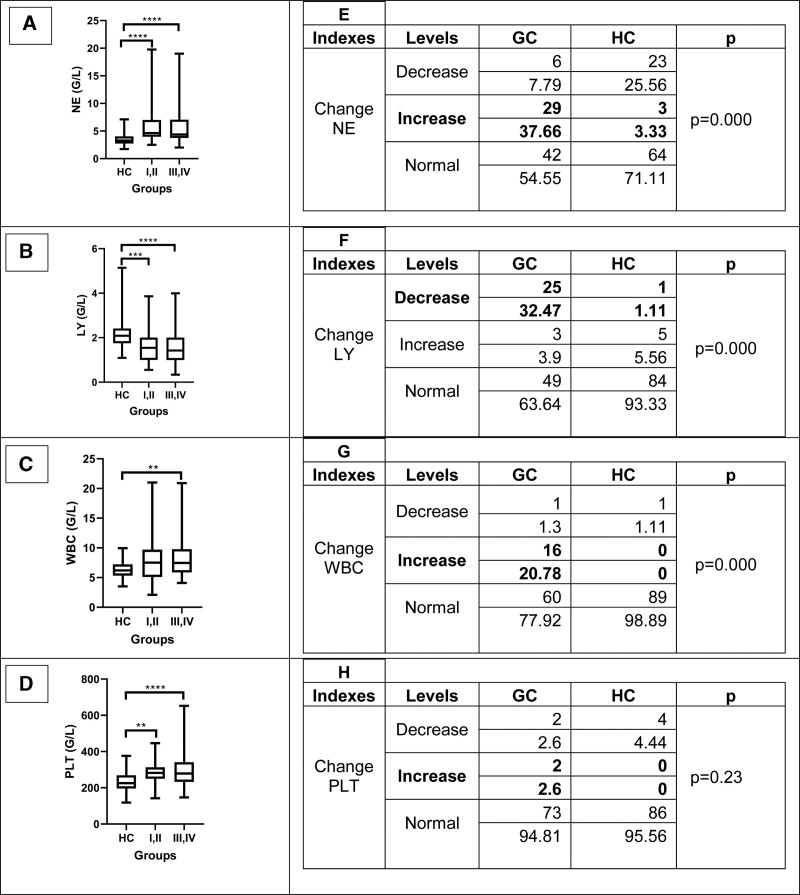
To determine the immunological change on GC, we compared some immunological indices among study cohort. The highest NE was observed in GC stage III to IV, followed by stage I to II and HC. Similar pattern occurred with white blood cell count (WBC). The increase of NE and WBC implied the possible undergoing inflammation and infection during GC progress. The similar pattern was observed with PLT indices that the increased trend from HC to stage I, II to stage III, IV GC (P < .01). By contrast, the LY value was decreased among GC patients, compared to HC (Fig. 1A–D).
Figure 1.
The level distribution of some interesting hematological indices. (A and E) The comparison of NE on HC and GC cohort. (B and F) The comparison of LY on HC and GC cohort. (C and G) The comparison of WBC on HC and GC cohort. (D and H) The comparison of PLT on HC and GC cohort. GC = gastric cancer, HC = healthy individuals, LY = lymphocytes, NE = neutrophils, WBC = white blood cell count.
Besides, we also compared the percentage change of some hematological indexes forwarding cancer-supporting tendency. Our results showed significantly higher proportion of increased NE, WBC, and PLT which were 37.66%, 20.88% and 2.6%, compared to 3.33%, 0% and 0% among HC. In addition, rate of decreased LY was 32.47% in GC group versus 1.11% in HC group (Fig. 1E–H).
To obtain the association between the duration of clinical manifestation prior to firstly diagnosed (C-to-D) with GC and some interest indexes, we determine the C-to-D via survey of medical history. Among 77 GC patients recruited in this study, C-to-D of 11 GC is missing. Among 66 remaining GC, 35/66 (53%), 22/66 (33.3%) and 9/66 (13.6%) realized clinical abnormality within 1 to 3 months, 3 to 6 months and over 6 months before being diagnosed with GC, respectively. In addition, we compared the alteration of LY, NE, PLR, WBC, PLR, and NLR between 3 groups based on C-to-D. In subpopulation with decreased LY, increased WBC, increased PLT and increased NE, most of patients have C-to-D falling in 1 to 3 months, followed by 3 to 6 months. Rarely, patients having C-to-D above 6 months presented decreased LY, increased WBC, increased PLT, and increased NE. Thus, most patients with hematological alteration forwarding cancer promotion appears clinical signs shortly before diagnosed. Besides, our results reported the tendency of highest PLR and NLR in 1 to 3-month C-to-D, followed by 3-6 months and above 6 months (Table 2). The higher PLR and NLR corresponded to shorter C-to-D suggested the prognostic value of PLR and NLR with fast progression of GC.
Table 2.
The association between some hematological indexes and duration from appearance of first clinical symptoms to diagnosis point.
| Months | n | Decreased LY (n) | Increased WBC (n) | Increased NE (n) | Increased PLT (n) | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|
| Pre-diagnosed clinical symptoms | 1–<3 | 35 | 11 | 11 | 15 | 1 | 238 ± 1.8 | 7.3 ± 10.8 |
| 3–≤6 | 22 | 7 | 2 | 9 | 1 | 221.6 ± 101.66 | 5.2 ± 6.4 | |
| >6 | 9 | 3 | 0 | 2 | 0 | 196.4 ± 119.8 | 3.9 ± 4.1 | |
| P | .70 | .166 | .889 | .938 | .608 | .83 |
LY = lymphocytes, NE = neutrophils, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio, PLT = platelets, WBC = white blood cell count.
3.3. The difference of NLR and PLR between GC patients and HC
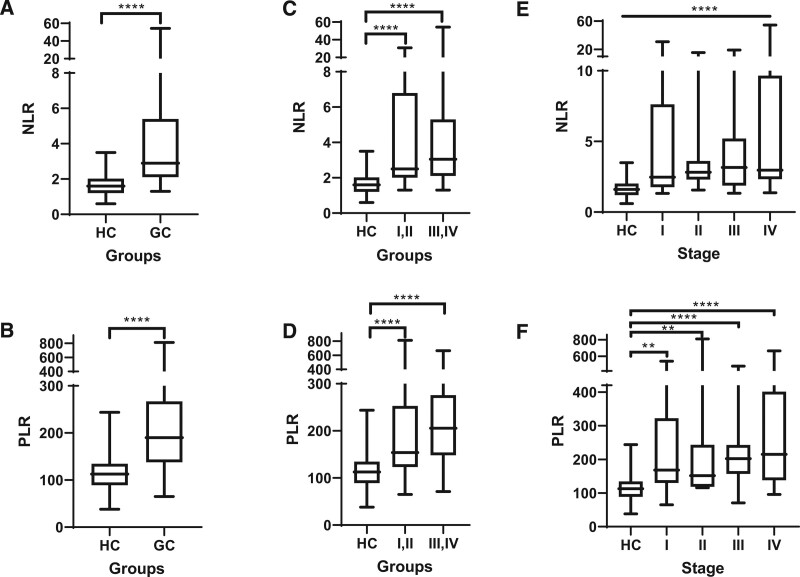
To determine the alteration of NLR and PLR during GC progress, we compared the different data set of HC, GC, and stages of GC. We firstly found that, the value of NLR was significant higher in GC group in comparison with HC (P < .0001, Fig. 2A). The pattern was similar in the PLR value between HC and GC patients (P < .0001, Fig. 2B). Next, we analyzed the value of NLR and PLR in subgroups in different stages of GC. We found the increased tendency of these values from HC cohort to the early stage (I and II) and the late stage (III and IV) of GC patients. These indexes were significantly elevated in early and late stages, compared to HC, (P < .001, Fig. 2C and D). However, we have not found the significant difference of these values between the early stage and the late stage of GC patients. Additionally, we compared NLR and PLR indexes between HC and 4 GC stages. Our results showed the highest average value of NLR and PLR on stage IV GC, followed by stage III, II, I GC and HC (P < .0001, Fig. 2E and F). The PLR value of HC was significantly lower, compared to all 4 stages of GC, but not NLR.
Figure 2.
The comparison of NLR, PLR between HC and GC patients. (A and B) The comparison of NLR, PLR on HC and GC cohort. (C and D) The comparison of NLR, PLR on early and advanced stages of GC patients. (E and F) The comparison of NLR, PLR on different stages of GC patients. GC = gastric cancer, HC = healthy individuals, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio.
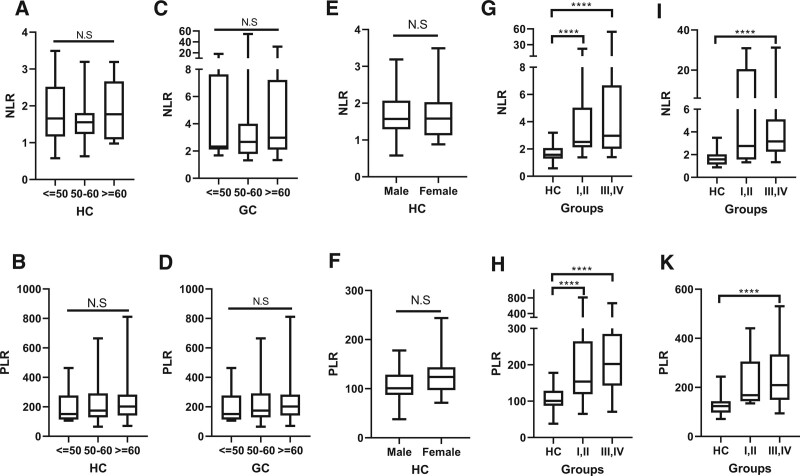
3.4. The distribution of NLR and PLR with age and gender
By age, the participants were categorized into 3 groups: under 50 years old, from 50 to 60 years old and over 60 years old. To determine the interference of age with interest rate, we compared NLR and PLR value between age groups. We have found that, the NLR and PLR value were comparable between different age categories, on both HC and GC population (Fig. 3A–D). In addition, we investigated the association between PLR and gender. The results showed similar value of PLR between male and female both on HC and GC groups (Fig. 3E and F). Thus, our results indicated the independence of NLR and PLR on gender and age. Next, the NLR and PLR value were compared between HC, early and late GC stages according to sub-gender male and female. Among male, NLR and PLR were lowest in HC, followed by stage I, II and stage III, IV GC (Fig. 3G and H). Similarly, among female, NLR and PLR were highest on late stage of GC, followed by early stage of GC and HC (Fig. 3I and K).
Figure 3.
The distribution of NLR and PLR with age and gender. (A and B) NLR and PLR value on different age groups of HC. (C and D) NLR and PLR value on different age groups of GC patients. (E and F) NLR and PLR value on male and female of HC. (G and H) The comparison of NLR, PLR on male GC patients. (I and K) The comparison of NLR, PLR on female GC patients. GC = gastric cancer, HC = healthy individuals, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio.
3.5. The tumor marker level on different stages of GC patients
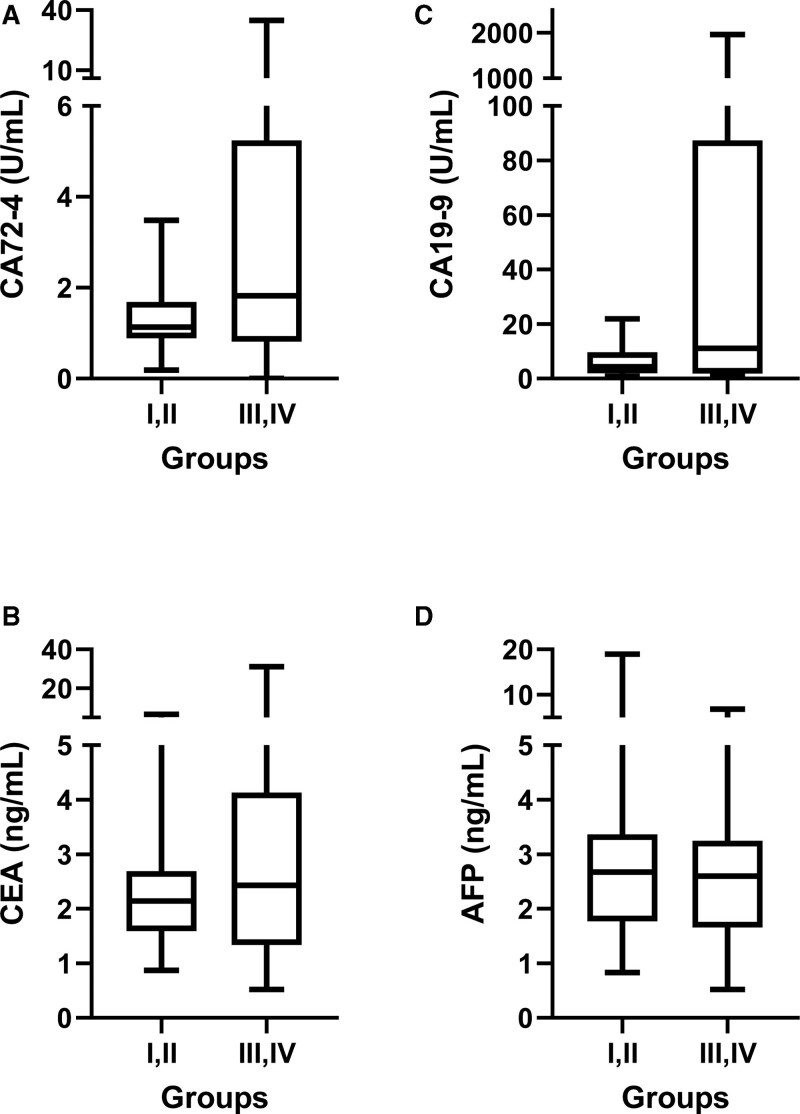
To compare the concentration of some common gastrointestinal tumor markers, such as AFP, CA72-4, CA19-9, and CEA regarding cancer stage, we collected and interpreted the data. The results showed the increased tendency of CA72-4, CA19-9, and CEA in GC patients with late stages, compared to GC patients with early stages, but the difference was not statistically significant (Fig. 4A–C). The AFP concentration was equal between early and late stages of GC patients.
Figure 4.
The tumor marker level on different stages of GC patients. (A) CA72-4; (B) CA19-9; (C) CEA; (D) AFP. GC = gastric cancer.
3.6. Receiver operating characteristic (ROC) curve of NLR and PLR value on GC patients
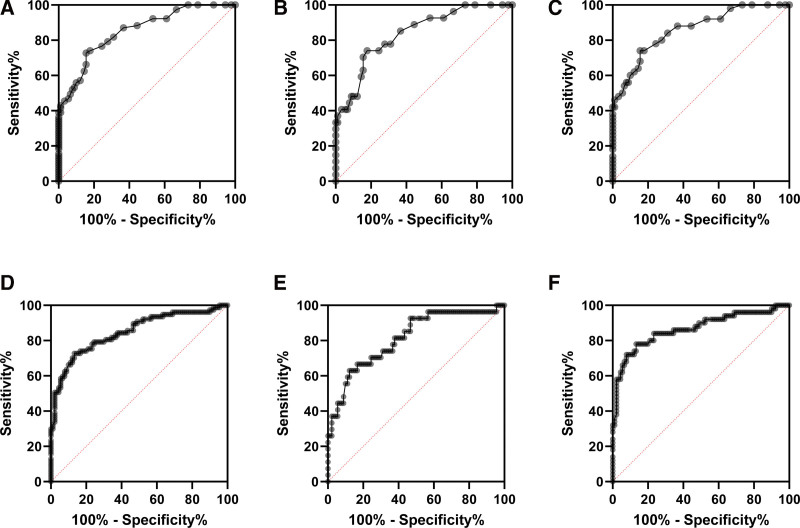
To determine the value of NLR in diagnosis of GC, we executed ROC curve analysis. In our study, area under ROC curve (AUR) of NLR was 85.17, P < .0001. In addition, the cutoff value of 1.95 showed 79.22% sensitivity, (CI 95%: 68.88–86.78%) and 72.2% (CI 95%, 62.20–80.42%) of specificity, the ratio = 2.85. The cutoff value of 2.15 showed 74.03% sensitivity, (CI 95%: 63.26–82.51%) and 82.22% (CI 95%, 73.06–88.75%) of specificity, the ratio = 4.16 (Table 3 and Fig. 5A–C). Thus, NLR index is a significant indicator to diagnose GC with high sensitivity and specificity.
Table 3.
ROC analysis of NLR value in diagnosis of GC patients
| Cutoff of NLR | Sensitivity | Specificity | Likelihood ratio | ||
|---|---|---|---|---|---|
| Mean | CI 95% | Mean | CI 95% | ||
| 1.95 | 79.22 | 68.88–86.78 | 72.22 | 62.20–80.42 | 2.85 |
| 2.05 | 76.62 | 66.05–84.67 | 75.56 | 65.75–83.27 | 3.1 |
| 2.15 | 74.03 | 63.26–82.51 | 82.22 | 73.06–88.75 | 4.16 |
| Area under ROC curve (AUR) | 85.17 | P < .0001 | |||
GC = gastric cancer, NLR = neutrophil-lymphocyte ratio, ROC = receiver operating characteristic.
Figure 5.
Receiver operating characteristic (ROC) curve of NLR and PLR value on GC patients. ROC analysis of NLR value on GC patients (A); on stage I and II of GC patients (B); on stage III and IV of GC patients (C). ROC analysis of PLR value on GC patients (D); on stage I and II of GC patients (E); on stage III and IV of GC patients (F). GC = gastric cancer, HC = healthy individuals, NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio.
To determine the value of PLR in diagnosis of GC, we executed ROC curve analysis. Our analysis of ROC curve showed that, taking the cutoff value of 134.7, the sensitivity and specificity of PLR in diagnosis of GC were 79.22% (CI 95%: 68.88–86.78%), and 75.56 (CI 95%: 65.75– 83.27%), ratio 3.34, respectively (Table 4 and Fig. 5D–F). Besides, at the cutoff value of 136.2, the sensitivity and specificity of PLR were 77.92% (CI 95%: 67.46–85.73), ratio 3.34 and 76.67 (CI 95%: 66.95–84.20%), ratio 3.34, respectively. The AUR was 84.74% with P < .0001, indicating the significant value of PLR in diagnosis of GC.
Table 4.
ROC analysis of PLR value in diagnosis of GC patients.
| Cutoff of PLR | Sensitivity | Specificity | Likelihood ratio | ||
|---|---|---|---|---|---|
| Mean | CI 95% | Mean | CI 95% | ||
| 134.7 | 79.22 | 68.88–86.78 | 75.56 | 65.75–83.27 | 3.24 |
| 135.2 | 77.92 | 67.46–85.73 | 75.56 | 65.75–83.27 | 3.19 |
| 136.2 | 77.92 | 67.46–85.73 | 76.67 | 66.95–84.20 | 3.34 |
| Area under ROC curve (AUR) | 84.74 | P < .0001 | |||
PLR = platelet-lymphocyte ratio, ROC = receiver operating characteristic.
4. Discussion
GC presents two-time higher incidence on male, compared to female.[4] We presented similar gender distribution of male dominance with the male rate was approximately two-time higher than female on GC cohort. The gender distribution was comparable between GC and HC groups. High rate of GC were firstly diagnosed at late stages, presented with stage IV 23/77 (30%), stage III 27/77 (35%), stage II 12/77 (15%) and stage I 15/77 (20%). Meanwhile, advanced GC corresponds to poor prognosis of low 5-year survival rate and short overall survival.[3] Being diagnosed in late stages rules out the curable opportunity by tumor resection surgery. Therefore, development novel markers, indicators is urgent need to early diagnose GC. Follow-up of PLR and NLR might indicate the risk of malignant transformation among patients with benign gastric diseases, such as gastritis, stomach ulcers and polyp.
Inflammation and immune escape are important hallmarks of cancer. NE and PLT induced carcinogenesis while LY control the immunity against cancer.[5,7,8,19,20] Thus, increased NE, decreased LY and increased PLT promote cancer progress (terming cancer-promoting hematological alteration). Increased NE and decreased LY occurred frequently in GC (32.66% and 32.47%, respectively). Our results reported consistently that WBC, NE, and PLT were highest among stage III to IV GC, followed by stage I to II GC and HC. Meanwhile, LY was lower on GC, compared to HC. As the results, the NLR and PLR value were significantly increased on GC, compared to HC. NLR and PLR level on advanced-stage GC was higher than on early-stage GC. Thus, cancer-promoting hematological alteration were associated with GC stages. Moreover, most of GC patients with cancer-promoting hematological alteration of decreased LY, increased NE and increased WBC, increased PLR and increased NLR have short duration from appearing clinical signs to being diagnosed with GC. In details, short C-to-D period corresponded to the prevalence of cancer-promoting hematological alteration. Also, GC with C-to-D falling in 1 to 3 months showed the higher value of PLR and NLR, followed by 3 to 6 months and above 6 months. The short C-to-D might suggest the fast progress of GC. Thus, the shorter C-to-D duration, the higher PLR and NLR value suggested that increased PLR and NLR are warning signs of quick progress of GC. The results also suggest the frequent occurrence of inflammation and immunology escape on GC, and these indexes are predictors of GC progression.
The value of NLR above 3 and below 0.7 were reported abnormally, and 2.3 to 3 is warning sign of pathological state.[29] The wide range of NLR (0.78–3.92) were reported in general population with the higher levels belonging to male and elder.[9] Our results reported differently that NLR and PLR were independent from age and gender. The NLR value of over 4 independently predicted short survival, progression-free survival and disease-free survival.[17] Metastatic and primary brain patients with NLR over 4.7 presented short survival.[30] The value of 5 is NLR cutoff threshold to predict progression free survival and metastatic-mortality of urothelial cancer.[9] On GC patients, significantly higher NLR was observed in undifferentiated adenocarcinoma, compared to differentiated adenocarcinoma.[31] NLR < 4 was correlated and corresponded to significant high rate of successful conversion surgery among stage IV GC.[32] In addition, NLR is valuable marker for predicting the treatment response for oral squamous cell carcinoma.[33] Besides, an elevation of pretreatment PLR predicts poor prognosis of GC, reflecting by short overall survival and disease-free survival, as well as high risk of serosal and lympho node invasion with advanced stages.[34] Referent range of PLR among male and female are 36.63 to 149.13 and 43.36 to 172.68, respectively.[35] PLR is reliable prognostic indicator of pancreatic cancer and predict poor prognosis of colorectal cancer.[36] Moreover, recent study found that increased PLR could be a marker to differentiate between malignant and benign thyroid nodules.[37,38]
Our ROC curve analysis showed the significant specificity and sensitivity of PLR and NLR on GC diagnosis with respective AUR of 84.74% and 85.17%. The diagnostic specificity and sensitivity were 75-80% with the cutoff value of around 2.0 and 135.0, respectively. Lacking referent value of PLR and NLR restricted further analysis their predictive and diagnostic value. But PLR and NLR were significant increased on GC, compared to HC and associated with GC stages. Besides, our results proved the significant value of PLR and NLR in diagnostic GC. Taking the cutoff value of around 2 (NLR) and 135 (PLR), the diagnostic specificity and sensitivity falls in 75-80%, and AUR were about 85%. Available evidence shows that NLR and PLR are valuable markers in diagnosis and prognosis of various diseases. These indices can be calculated from common hematological test. Therefore, NLR and PLR indices are recommended for clinical practice.
However, the study still had limitation such as small sample size and conducted in one hospital. This might affect final statistical analysis and conclusions of the study. Therefore, analysis in bigger cohorts in different institutions will provide more valuable and reliable conclusions.
Taken together, PLR and NLR have significant specificity and sensitivity in diagnosis and prognosis of GC.
Author contributions
Conceptualization: Mai Ly Thi Nguyen, Linh Toan Nguyen, Khac Cuong Bui.
Data curation: Mai Ly Thi Nguyen, Chi Pham, Quoc Vuong Le, Phuong Linh Thi Nham, Van Mao Can, Linh Toan Nguyen, Khac Cuong Bui.
Formal analysis: Mai Ly Thi Nguyen, Chi Pham, Khac Cuong Bui.
Funding acquisition: Linh Toan Nguyen, Khac Cuong Bui.
Investigation: Mai Ly Thi Nguyen, Chi Pham, Quoc Vuong Le, Phuong Linh Thi. Nham, Doanh Hieu Tran, Thanh Son Le, Van Tong Hoang, Van Mao Can, Khac Cuong Bui
Methodology: Mai Ly Thi Nguyen, Linh Toan Nguyen, Khac Cuong Bui.
Project administration: Khac Cuong Bui.
Supervision: Linh Toan Nguyen, Khac Cuong Bui.
Visualization: Khac Cuong Bui.
Writing – original draft: Mai Ly Thi Nguyen, Khac Cuong Bui.
Writing – review & editing: Mai Ly Thi Nguyen, Chi Pham, Quoc Vuong Le, Phuong Linh Thi Nham, Doanh Hieu Tran, Thanh Son Le, Van Tong Hoang, Van Mao Can, Linh Toan Nguyen, Khac Cuong Bui.
Abbreviations:
- AUR
- area under ROC curve
- C-to-D
- the duration of clinical manifestation prior to firstly diagnosed
- GC
- gastric cancer
- HC
- healthy individuals
- LY
- lymphocytes
- NE
- neutrophils
- NLR
- neutrophil-lymphocyte ratio
- PLR
- platelet-lymphocyte ratio
- PLT
- platelets
- ROC
- receiver operating characteristic
- WBC
- white blood cell count.
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 108.02-2019.324. The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Mai Ly Thi Nguyen was funded by Vingroup JSC and supported by the Postdoctoral Scholarship Programme of Vingroup Innovation Foundation (VINIF), Institute of Big Data, code VINIF.2021.STS.25. We appreciate all of patients and individuals participating in this study.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Nguyen MLT, Pham C, Le QV, Nham PLT, Tran DH, Le TS, Hoang VT, Can VM, Nguyen LT, Bui KC. The diagnostic and prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on gastric cancer patients. Medicine 2023;102:31(e34357).
Contributor Information
Mai Ly Thi Nguyen, Email: toannl@vmmu.edu.vn.
Chi Pham, Email: Chipham2412@gmail.com.
Quoc Vuong Le, Email: ltson103@gmail.com.
Phuong Linh Thi Nham, Email: linhpm.hsd@gmail.com.
Doanh Hieu Tran, Email: Drtranhieu103@gmail.com.
Thanh Son Le, Email: ltson103@gmail.com.
Van Tong Hoang, Email: hoangvantong@vmmu.edu.vn.
Van Mao Can, Email: canvanmao@vmmu.edu.vn.
Linh Toan Nguyen, Email: toannl@vmmu.edu.vn.
References
- [1].Section of Cancer Surveillance. Globocan 2020 - global cancer observatory. Global Cancer Observatory 2020; Available at: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf [access date April 20, 2023].
- [2].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Li Y, Feng A, Zheng S, et al. Recent estimates and predictions of 5-year survival in patients with gastric cancer: a model-based period analysis. Cancer Control. 2022;29:10732748221099227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine. 2022;47:101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sit M, Aktas G, Ozer B, et al. Mean platelet volume: an overlooked herald of malignant thyroid nodules. Acta Clin Croat. 2019;58:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [8].Li S, Lan X, Gao H, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017;143:2455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buonacera A, et al. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- [11].Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Rev Assoc Med Bras (1992). 2017;63:1065–8. [DOI] [PubMed] [Google Scholar]
- [12].Balci SB, Aktas G. A comprehensive review of the role of hemogram-derived inflammatory markers in gastrointestinal conditions. Iran J Colorectal Res. 2022;10:75–86. [Google Scholar]
- [13].Khalid A, Ali Jaffar M, Khan T, et al. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS-COV-2 infected patients of Pakistan: a retrospective comparative analysis. Hematology. 2021;26:529–42. [DOI] [PubMed] [Google Scholar]
- [14].Posul E, Yilmaz B, Aktas G, et al. Does neutrophil-to-lymphocyte ratio predict active ulcerative colitis? Wien Klin Wochenschr. 2015;127:262–5. [DOI] [PubMed] [Google Scholar]
- [15].Feng W, Liu Y, Zhu L, et al. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential markers for ulcerative colitis: a retrospective study. BMC Gastroenterol. 2022;22:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Song M, Graubard BI, Rabkin CS, et al. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [18].Szor DJ, Dias AR, Pereira MA, et al. Prognostic role of neutrophil/lymphocyte ratio in resected gastric cancer: a systematic review and meta-analysis. Clinics. 2018;73:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karachaliou N, Pilotto S, Bria E, et al. Platelets and their role in cancer evolution and immune system. Transl Lung Cancer Res. 2015;4:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [20].Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Giannakeas V, Kotsopoulos J, Cheung MC, et al. Analysis of platelet count and new cancer diagnosis over a 10-year period. JAMA Network Open. 2022;5:e2141633–e2141633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Afsin H, Aktas G. Platelet to lymphocyte and neutrophil to lymphocyte ratios are useful in differentiation of thyroid conditions with normal and increased uptake. Ethiop J Health Dev. 2021;35:149–53. [Google Scholar]
- [24].Aktas G. Hematological predictors of novel coronavirus infection. Rev Assoc Med Bras (1992). 2021;67(Suppl 1):1–2. [DOI] [PubMed] [Google Scholar]
- [25].Aktas G, Duman T, Atak BM, et al. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Fam Med Prim Care Rev. 2020;22:107–10. [Google Scholar]
- [26].Atak B, Aktas G, Duman TT, et al. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Rev Assoc Med Bras (1992). 2019;65:38–42. [DOI] [PubMed] [Google Scholar]
- [27].Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio. Diseases. 2023;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Al-Rshaidat MMD, Al-Sharif S, Refaei AA, et al. Evaluating the clinical application of the immune cells’ ratios and inflammatory markers in the diagnosis of inflammatory bowel disease. Pharm Pract (Granada). 2023;21:2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122:474–88. [DOI] [PubMed] [Google Scholar]
- [30].Lianos GD, Alexiou GA, Exarchos C, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in several malignancies: where do we stand? Biomark Med. 2020;14:169–72. [DOI] [PubMed] [Google Scholar]
- [31].Yasui S, et al. Neutrophil-to-lymphocyte ratio is a useful marker for predicting histological types of early gastric cancer. J Clin Med. 2021;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nakamura N, Kinami S, Tomita Y, et al. The neutrophil/lymphocyte ratio as a predictor of successful conversion surgery for stage IV gastric cancer: a retrospective study. BMC Cancer. 2020;20:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tachinami H, Tomihara K, Yamada S-I, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with recurrent oral squamous cell carcinoma treated with nivolumab. Br J Oral Maxillofac Surg. 2023;61:320–6. [DOI] [PubMed] [Google Scholar]
- [34].Zhang X, Zhao W, Yu Y, et al. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: an updated meta-analysis. World J Surg Oncol. 2020;18:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu L, Zou S, Wang C, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc Disord. 2019;19:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang W, Tong Y, Sun S, et al. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol. 2022;12:936206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Atak B, Bakir Kahveci G, Bilgin S, et al. Platelet to lymphocyte ratio in differentiation of benign and malignant thyroid nodules. Exp Biomed Res. 2021;4:148–53. [Google Scholar]
- [38].Deng Y, Zhang J, Zou G, et al. Peripheral blood inflammatory markers can predict benign and malignant thyroid nodules. Int J Endocrinol. 2022;2022:2319660. [DOI] [PMC free article] [PubMed] [Google Scholar]